Published online Oct 14, 2024. doi: 10.3748/wjg.v30.i38.4232
Revised: September 10, 2024
Accepted: September 13, 2024
Published online: October 14, 2024
Processing time: 194 Days and 19.3 Hours
Composite tumors are neoplasms comprising two distinct, yet intermingling, cell populations. This paper reports a rare phenomenon where early gastric signet-ring cell carcinoma (SRCC) and gastric mucosa-associated lymphoid tissue (MALT) lymphoma coexist within the same lesion.
A 40-year-old woman presented to the West China Hospital for examination, which revealed a whitish, shallow, and uneven mucosal lesion in the stomach. The lesion was diagnosed as a poorly differentiated adenocarcinoma, including SRCC with atypical lymphoid hyperplasia associated with Helicobacter pylori infection, based on histopathological examination of the biopsy specimen. The lesion was excised using segmental gastrectomy. However, histological exami
It is uncommon for gastric SRCC and MALT lymphoma to coexist without distinct borders. Surgical resection is effective for these lesions.
Core Tip: This paper reports a rare case of an early composite tumor involving the simultaneous occurrence of gastric signet-ring cell carcinoma (SRCC) and mucosa-associated lymphoid tissue lymphoma. A “composite tumor” is a unique phenomenon that combines two distinct tumor types into a single lesion without clear borders. When signet-ring cells are present in gastric B-cell lymphoma, it is crucial to carefully distinguish between lymphoma-associated signet-ring cell changes and gastric SRCCs. The former exhibits inconspicuous epithelial cell atypia and is mostly negative for p53 on immunohistochemistry, with positive E-cadherin expression, whereas the latter exhibits the opposite pattern.
- Citation: Jia YF, Chen FF, Yang L, Ye YX, Gao YZ, Zhang WY, Yang JL. Early gastric composite tumor comprising signet-ring cell carcinoma and mucosa-associated lymphoid tissue lymphoma: A case report. World J Gastroenterol 2024; 30(38): 4232-4238
- URL: https://www.wjgnet.com/1007-9327/full/v30/i38/4232.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i38.4232
Among the most common cancers worldwide, gastric cancer is the third largest cause of cancer-related death[1]. Notably, specific gastric cancer lesions may contain components of signet-ring cell carcinoma (SRCC). However, the incidence of SRCC, a distinctive subtype of gastric cancer, has consistently increased, accounting for 35% to 45% of the incidence of gastric adenocarcinoma[2]. Pathological classification of SRCC of the stomach corresponds to the low adhesion type classified by the World Health Organization, diffuse type by the Lauren classification, undifferentiated type classified by the Nakamura classification, and poorly differentiated adenocarcinoma classified by the Japan Gastric Cancer Association[3]. Mucosa-associated lymphoid tissue (MALT) lymphomas occur in organs outside the lymph nodes, such as the lungs, salivary glands, and gastrointestinal tract. Gastric MALT lymphomas account for 40% to 50% of primary gastric malignant lymphomas[4]. Although there have been reports of SRCC combined with MALT lymphoma, most of these have described two lesions that existed independently[5-9]. Herein, we report a composite tumor involving early gastric SRCC and MALT lymphoma, where the two histological patterns lack a distinct boundary.
A 40-year-old woman came to West China Hospital for a routine physical examination.
The patient's medical history was unremarkable, with no recent weight loss or symptoms like abdominal pain, vomiting, nausea, or diarrhea.
The patient had a medical history of appendectomy 30 years previously and a cesarean section 5 years ago.
The patient denied using alcohol, smoking cigarettes, or abusing any illicit drugs and had no personal history of peptic ulcer, family history of gastric cancer, or MALT lymphoma.
The patient appeared well on physical examination, with a body mass index of 20 kg/m2. An examination of the abdomen revealed a midline cesarean section scar and an oblique surgical scar from the prior appendectomy on the right lower abdomen. No abdominal tenderness or masses were observed. There was no palpable spleen or liver, and no nodes were palpable.
Hematological tests suggested normocytic anemia [112 g/L (normal, > 115 g/L)]. Laboratory investigations, including liver and kidney function tests, and tumor markers, such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9, showed normal results. However, serum levels of pepsinogen (PG) 2 reached 31.2 ug/L (normal, 3–15 ug/L), and the PG1/2 ratio was low [4.04 (normal, 7-20)]. The 13C-urea breath test was positive.
Abdominal contrast-enhanced computed tomography revealed no significant thickening or mass shadow in the gastric wall, but enlargement of the hepatoduodenal ligament and para-abdominal aortic lymph nodes was evident.
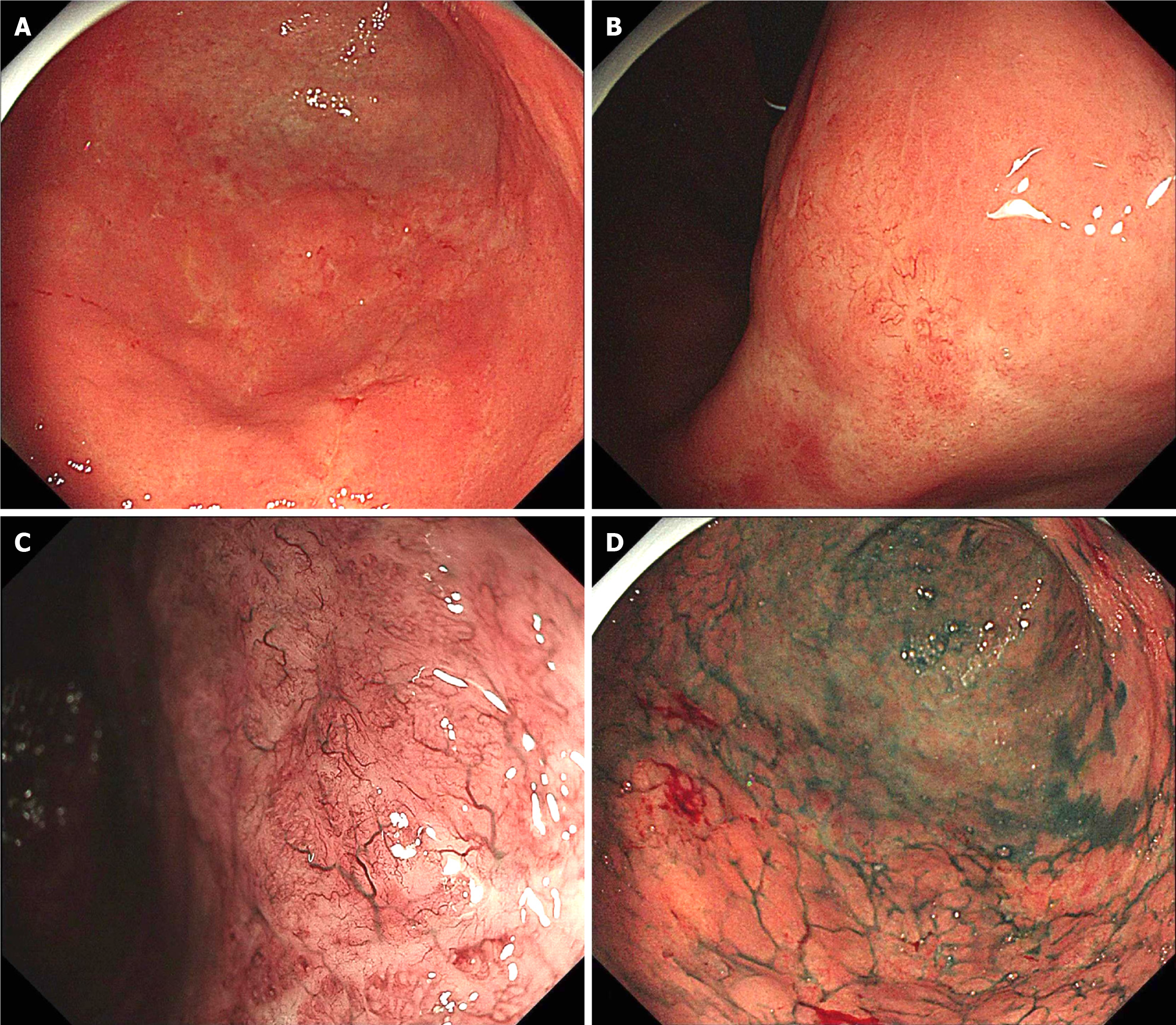
Endoscopy of the upper gastrointestinal tract revealed a whitish, shallow, and uneven mucosal lesion (type IIc according to the Paris endoscopic classification) with minimal spontaneous bleeding. The lesion was located in the lower part of the stomach, including the angular notch, the greater curve of the gastric antrum, and the junction of the gastric antrum and body (Figure 1A and B). Magnifying endoscopy with narrow-band imaging (ME-NBI) revealed an irregular and partially absent microsurface along with tree-like appearance (TLA) microvessels (Figure 1C). The demarcation line, which may have distinguished the boundary between the gastric cancer and normal mucosa, was poorly defined. Indigo carmine chromoendoscopy revealed a poorly demarcated lesion with irregular margins (Figure 1D). Biopsy of the lesion subsequently confirmed it to be a poorly differentiated adenocarcinoma, including SRCC with atypical lymphoid hyperplasia (ALH), and was positive for Helicobacter pylori (H. pylori) infection in the gastric antral junction. In addition, endoscopic biopsy specimens of the posterior wall of the gastric antrum revealed ALH and follicular colonization, and immunohistochemical staining confirmed B-cell predominance. Distal gastrectomy with D2 Lymphadenectomy was performed after informed consent was obtained from the patient.
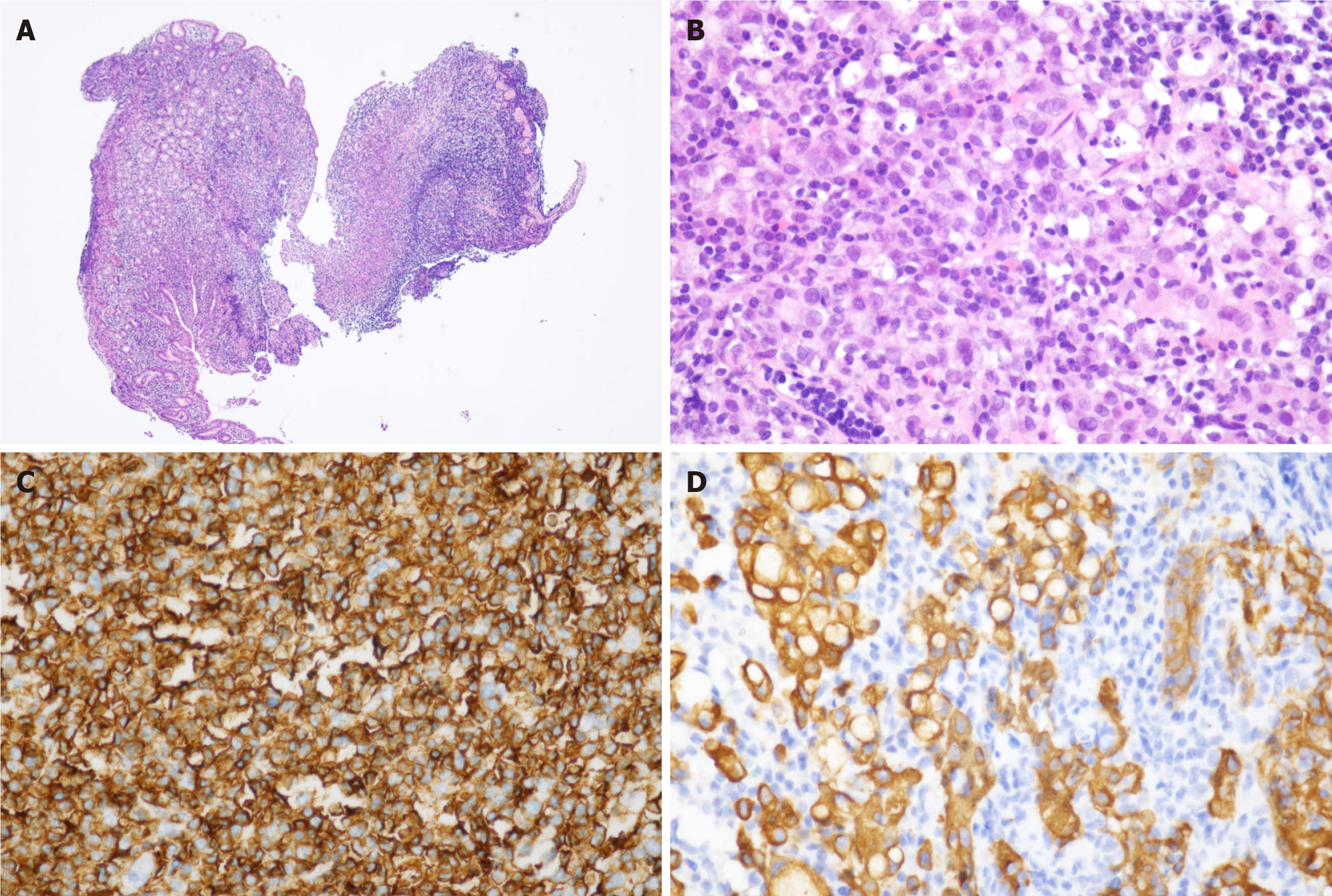
Postoperative pathological examination revealed no residual cancer tissue at the surgical margin. The resected specimen was a single lesion situated at the junction between the gastric antrum and body on the anterior wall, involving the lesser curvature of the stomach. Macroscopically, a superficial elevated (0-IIa) type and superficial depressed (0-IIc) type early gastric cancer, measuring 4.0 cm × 5.0 cm, was observed. The lesion was characterized by slight mucosal protrusions and scattered small erosions with clear boundaries. Histologically, numerous lymphoid cells diffusely infiltrated within the laminae propria mucosae. The lymphoid cells had small to medium-sized, slightly irregular nuclei with moderately dispersed chromatin and inconspicuous nucleoli. Few reactive lymph follicles were scattered. These tumorous lymphocytes infiltrated around lymph follicles in a marginal zone distribution and spread out to form confluent areas (Figure 2A). Simultaneously, multifocal atypical epithelial cells were observed. These atypical epithelial cells were isolated or arranged in small aggregates without well-formed glands. Some atypical cells presented a signet-ring cell appearance, characterized by a central globoid droplet of cytoplasmic mucin with an eccentrically placed nucleus (Figure 2B). Partial signet-ring cells formed delicate microtrabecular pattern. Immunohistochemical analyses revealed gastric lymphoid hyperplasia and heterotopic epithelial cells. The atypical lymphoid cells were positive for CD20, CD79a, p53 (partially), and negative for CD3, CD5, CD43, CD10, cyclin D1 and CD23. The Ki-67 index was 10%-20% (Figure 2C). Atypical epithelial cells were positive for CEA, pan-cytokeratin (pan-CK), p53 (partially), and Ki-67 (partially; Figure 2D). Monoclonal immunoglobulin heavy chain (IGH) and immunoglobulin Kappa light chain (IGK) gene rearrangements were detected. Moreover, pathology results for the lymph node tissue removed by surgery exhibited no tumor metastasis. Overall, the pathology examination revealed that the single lesion was an early-stage SRCC with MALT extranodal marginal zone B-cell lymphoma. Both tumors invaded within mucosal lamina propria.
The final diagnosis was T1N0M0 SRCC of the stomach coexisting with MALT lymphoma.
In addition to the standard quadruple anti-H. pylori therapy, as described in the diagnostic workup, the patient underwent distal gastrectomy with D2 lymphadenectomy and was administered intraperitoneal chemotherapy with 0.5 g of 5-fluorouracil and 1 mL of carboplatin during surgery. Intraoperative gastroscopy revealed smooth and normal residual gastric mucosa.
The patient made a good recovery and was discharged on the 10th postoperative day. At the six-month follow-up, there was no evidence of recurrence, and no additional adjuvant therapy was required.
Herein, we report a rare case of gastric SRCC complicated by MALT lymphoma. In a review of previous cases, it was found that in 2014, George et al[6] documented a mixed lesion of poorly differentiated gastric SRCC and MALT lymphoma due to gastrointestinal bleeding. However, the patient had lymph node metastases when the lesion was discovered. In a separate study, Suwa et al[7] described a case involving gastric MALT lymphoma coexisting with early poorly differentiated adenocarcinoma, which included SRCC as part of the adenocarcinoma component. However, these two lesions were independent of one another and existed in different locations in the stomach. Additionally, Yacoub et al[8] reported collision tumors that comprise a well-differentiated gastric cancer adjacent to MALT lymphoma, with 10% SRCC. In this case, such a “collision tumor” represents the parallel development of two different pathological types of tumors, and it is believed that H. pylori is the cause of simultaneous occurrence. Distinguishing our case from the above-mentioned cases is that we report a composite tumor with SRCC and MALT lymphoma at an early stage. Composite tumors are characterized by the coexistence of two tumors with ill-defined boundaries. The corresponding collision tumor was composed of two tumors with different histological forms and clear margins[9].
Furthermore, the occurrence and development of MALT lymphoma are related to various signaling pathways, the most representative of which is the nuclear factor-κB (NF-κB) signaling pathway[10]. In particular, in the case of H. pylori infection, the bacteria bind to gastric epithelial cells via adhesion molecules, and nearby epithelial and immune cells recognize H. pylori through both membrane and intracellular receptors, triggering apoptosis and NF-κB-dependent inflammatory responses. Chronic H. pylori infection not only induces persistent inflammation in the gastric mucosa and stimulates lymphocyte proliferation, but also leads to genetic alterations. These genetic changes are primarily characterized by chromosomal translocations, resulting in the formation of fusion genes such as AP12-MALT1, BCL10-IGH, and BIRC3-MALT1 in B cells, which subsequently activate the NF-κB signaling pathway[10,11]. More interestingly, a single-cell analysis of SRCC demonstrated significant enrichment of the NF-κB signaling pathway in SRCC cells[12]. These studies indicate that the involvement of the immune response, particularly through the NF-κB signaling pathway, may play a crucial role in the coexistence of MALT lymphoma and SRCC under H. pylori infection. However, further research with larger sample sizes and more comprehensive studies is needed to elucidate the specific mechanisms underlying these conditions.
Our case report provides additional information for the early identification and study of concomitant SRCC and MALT lymphoma, especially regarding endoscopic manifestations and pathological features. Previous studies have shown that ME-NBI has a significantly higher sensitivity and specificity for gastric tumors than white light endoscopy[13]. We encourage using ME-NBI for comprehensive observation in cases where the coexistence of two distinct tumor types is suspected. The endoscopic findings of superficial flat-type early-stage gastric SRCC are mainly based on the destruction of the glands and the presence of typical microvessels found under ME-NBI. Previous studies have summarized the typical microvessels into three types: Wavy microvessels, corkscrew pattern, and raimon vessels[14,15]. On the other hand, “intersection traffic” and “pebble” signs are characteristics of the microsurface pattern of MALT lymphoma. Additionally, MALT lymphoma microvascular morphology frequently exhibits TLA[16]. In this case, we report that ME-NBI revealed typical TLA-like microvascular structures; however, the pathological result of the biopsy was SRCC. This discrepancy may be attributed to MALT usually accumulating in the submucosal layer rather than in the mucosal layer, making MALT rarely diagnosed on biopsy[17].
Signet-ring cells represent a morphological description of cellular proliferation under various conditions. They can be observed in mucous-producing epithelial tumors and various cell types, including endocrine cells, histiocytes, lymphoid cells, melanocytes, and squamous cells. This wide distribution presences span both reactive processes and benign or malignant tumors. Signet-ring cells have been identified in some cases of primary gastric B-cell lymphoma[18]. Notably, Zamboni et al[19] reviewed the surgical and biopsy specimens of 70 cases of MALT lymphoma and found signet-ring cell change (SCC) in 20 of the 70 cases of MALT lymphoma. They characterized SCC by an abundant pale cytoplasm and a small peripheral nucleus with insignificant nucleoli, without heterotypic or mitotic activity. The cells in SCC had high positive for E-cadherin expression but negative p53 and Ki-67 results. In contrast, cells in SRCC exhibited strong positivity for p53 and a lack of or weak positivity for E-cadherin. Routine immunohistochemical staining for p53, Ki-67, and E-cadherin can help distinguish between SCC and SRCC[18]. In the present case, the expression of pan-CK, CEA, p53, and Ki-67 in heterotopic epithelial cells, combined with general morphology, supported the diagnosis of SRCC rather than SCC in MALT lymphoma. When two types of tumors are present simultaneously, the diagnosis can only be confirmed after surgery. Although the occurrence of early gastric SRCC and MALT lymphoma is uncommon, early detection and timely intervention can yield a favorable prognosis. This underscores the importance of gastroenterologists being vigilant during routine gastroscopic procedures, devoting close attention to suspicious lesions, conducting multiple biopsies, and capturing lesions as much as possible.
In summary, we report a rare case of an early composite tumor, including gastric SRCC and MALT lymphoma. For suspected lesions, detailed observation with ME-NBI should be incorporated into endoscopic examination, with multiple biopsies to evaluate. It is crucial to distinguish SCC observed in gastric B-cell lymphoma from gastric SRCC, as SCC typically exhibit minimal epithelial atypia, are p53-negative, and show positive E-cadherin expression, which contrasts with the pattern in gastric SRCC. Surgical resection is effective for early-stage SRCC combined with MALT lymphoma.
| 1. | Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol. 2023;20:338-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 342] [Reference Citation Analysis (1)] |
| 2. | Mariette C, Carneiro F, Grabsch HI, van der Post RS, Allum W, de Manzoni G; European Chapter of International Gastric Cancer Association. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer. 2019;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 3. | Kushima R. The updated WHO classification of digestive system tumours-gastric adenocarcinoma and dysplasia. Pathologe. 2022;43:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Tanaka T, Matsuno Y, Torisu T, Shibata H, Hirano A, Umeno J, Kawasaki K, Fujioka S, Fuyuno Y, Moriyama T, Esaki M, Kitazono T. Gastric microbiota in patients with Helicobacter pylori-negative gastric MALT lymphoma. Medicine (Baltimore). 2021;100:e27287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Mróz A, Kiedrowski M, Malinowska M, Sopyło R. Collision tumour of the stomach--adenocarcinoma and neuroendocrine carcinoma: case report and review of the literature. Pol J Pathol. 2009;60:94-97. [PubMed] |
| 6. | George SA, Junaid TA. Gastric marginal zone lymphoma of mucosa-associated lymphoid tissue and signet ring cell carcinoma, synchronous collision tumour of the stomach: a case report. Med Princ Pract. 2014;23:377-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Suwa T, Uotani T, Inui W, Ando T, Tashiro K, Kasahara M. A case of signet ring cell carcinoma and mucosa-associated lymphoid tissue lymphoma of the stomach diagnosed simultaneously via magnifying endoscopy with narrow-band imaging. Clin J Gastroenterol. 2021;14:453-459. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Yacoub H, Ben Safta N, Abdelaali ZEI, Ben Rejeb S, Bellakhal S, Jomni MT. Gastric Collision Tumor of MALT Lymphoma and Signet Ring Cell Adenocarcinoma: a Rare Preoperative Diagnosis. J Gastrointest Cancer. 2021;52:1098-1101. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Fukui H, Takada M, Chiba T, Kashiwagi R, Sakane M, Tabata F, Kuroda Y, Ueda Y, Kawamata H, Imura J, Fujimori T. Concurrent occurrence of gastric adenocarcinoma and duodenal neuroendocrine cell carcinoma: a composite tumour or collision tumours ? Gut. 2001;48:853-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Du MQ. MALT lymphoma: A paradigm of NF-κB dysregulation. Semin Cancer Biol. 2016;39:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Du MQ. MALT lymphoma: Genetic abnormalities, immunological stimulation and molecular mechanism. Best Pract Res Clin Haematol. 2017;30:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Zhao W, Jia Y, Sun G, Yang H, Liu L, Qu X, Ding J, Yu H, Xu B, Zhao S, Xing L, Chai J. Single-cell analysis of gastric signet ring cell carcinoma reveals cytological and immune microenvironment features. Nat Commun. 2023;14:2985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 13. | Ezoe Y, Muto M, Horimatsu T, Minashi K, Yano T, Sano Y, Chiba T, Ohtsu A. Magnifying narrow-band imaging versus magnifying white-light imaging for the differential diagnosis of gastric small depressive lesions: a prospective study. Gastrointest Endosc. 2010;71:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Wang W, Yang Y, Xu Q, Wang S, Zhang L, Yu R, Han J, Cao J. Superficial Flat-Type Early-Stage Gastric Signet Ring Cell Carcinoma in the Atrophic Background Mucosa: Two Case Reports. J Gastrointest Cancer. 2023;54:677-681. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Wu XW, Zhuang Q, Wang J, Chen DF, Dong ZX, Qian YQ, Lu LG, Wan XJ, Zhou H. [Differences of endoscopic features between undifferentiated-typed early gastric cancer and gastric mucosa-associated lymphoid tissue lymphoma]. Zhonghua Xiaohuaneijing Zazhi. 2021;38:894-900. [DOI] [Full Text] |
| 16. | Nonaka K, Ishikawa K, Shimizu M, Sakurai T, Nakai Y, Nakao M, Yoshino K, Arai S, Kita H. Education and Imaging. Gastrointestinal: gastric mucosa-associated lymphoma presented with unique vascular features on magnified endoscopy combined with narrow-band imaging. J Gastroenterol Hepatol. 2009;24:1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Violeta Filip P, Cuciureanu D, Sorina Diaconu L, Maria Vladareanu A, Silvia Pop C. MALT lymphoma: epidemiology, clinical diagnosis and treatment. J Med Life. 2018;11:187-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Wang K, Weinrach D, Lal A, Musunuri S, Ramirez J, Ozer O, Keh P, Rao MS. Signet-ring cell change versus signet-ring cell carcinoma: a comparative analysis. Am J Surg Pathol. 2003;27:1429-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Zamboni G, Franzin G, Scarpa A, Bonetti F, Pea M, Mariuzzi GM, Menestrina F. Carcinoma-like signet-ring cells in gastric mucosa-associated lymphoid tissue (MALT) lymphoma. Am J Surg Pathol. 1996;20:588-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |