Published online Oct 7, 2024. doi: 10.3748/wjg.v30.i37.4090
Revised: August 23, 2024
Accepted: September 12, 2024
Published online: October 7, 2024
Processing time: 170 Days and 7.2 Hours
Helicobacter pylori (H. pylori) colonizes the human stomach and many studies have discussed the mechanisms of H. pylori infection leading to gastric diseases, including gastric cancer. Additionally, increasing data have shown that the infection of H. pylori may contribute to the development of extra-gastric diseases and tumors. Inflammation, systemic immune responses, microbiome disorders, and hypergastrinemia caused by H. pylori infection are associated with many extra-gastric malignancies. This review highlights recent discoveries; discusses the relationship between H. pylori and various extra-gastric tumors, such as colorectal cancer, lung cancer, cholangiocarcinoma, and gallbladder carcinoma; and explores the mechanisms of extra-gastric carcinogenesis by H. pylori. Overall, these findings refine our understanding of the pathogenic processes of H. pylori, provide guidance for the clinical treatment and management of H. pylori-related extra-gastric tumors, and help improve prognosis.
Core Tip: Apart from gastric diseases, several studies have revealed an association between Helicobacter pylori (H. pylori) and extra-gastric diseases, including cancers. Inflammation, systemic immune responses, microbiome disorders, and hypergastrinemia caused by H. pylori may change the tumor microenvironment and eventually contribute to extra-gastric carcinogenesis. However, the research in this field remains controversial. We summarized the effects of H. pylori infection on the occurrence of extra-gastric tumors and their possible mechanisms, hoping to provide insights for clinical treatment and management of H. pylori-related extra-gastric tumors, as well as help improve prognosis.
- Citation: Zhao SQ, Zheng HL, Zhong XT, Wang ZY, Su Y, Shi YY. Effects and mechanisms of Helicobacter pylori infection on the occurrence of extra-gastric tumors. World J Gastroenterol 2024; 30(37): 4090-4103
- URL: https://www.wjgnet.com/1007-9327/full/v30/i37/4090.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i37.4090
Helicobacter pylori (H. pylori) is a gram-negative, helical, microaerophilic, flagellated bacterium that has evolved unique features to survive in the host gastric microenvironment[1]. The infection rate of H. pylori varies across different countries and regions[1,2], and recent data show that the global prevalence of H. pylori in adults has decreased from 52.6% in 1990 to 43.9% in 2015-2022, which can be attributed to a global public health campaign aimed at decreasing the prevalence of H. pylori[3]. Although the global prevalence of H. pylori infection in adults has declined over the last three decades, rising antibiotic resistance remains a significant public health concern[4]. H. pylori infection is the main cause of gastric mucosal injuries such as chronic non-atrophic gastritis, which can progress to intestinal metaplasia, dysplasia, and finally, gastric cancer[2,5]. As a class I carcinogen identified by the World Health Organization in 1994, H. pylori is the primary etiological factor of non-cardia gastric cancer, with approximately nine in 10 cases attributed to H. pylori infection[2,6]. In addition to gastric diseases, increasing data have shown that the infection of H. pylori may contribute to the development of extra-gastric diseases; including ocular, neurodegenerative, cardiovascular, and obstetric disorders[7-11]. It may also affect the tumor microenvironment and potentially undermine the efficacy of tumor immunotherapies, which have recently become a hot spot[12,13].
In recent years, clinical epidemiology has focused on whether H. pylori infection increases the risk of extra-gastric tumors and explored its mechanisms in establishment and progression of extra-gastric tumors, such as colorectal cancer (CRC), pancreatic cancer, and esophageal cancer; however, it remains unclear and divergences exists[14-19]. This review focuses on the effects of H. pylori infection on the occurrence of extra-gastric tumors and their possible mechanisms.
In recent years, clinical epidemiology has focused on whether H. pylori infection increases the risk of extra-gastric tumors, but the underlying molecular mechanisms are still unclear. One important potential mechanism is the possible involvement of various virulence factors of H. pylori in the occurrence and development of extra-gastric tumors by interfering with cell signaling pathways. H. pylori-related gastric mucosal diseases are closely related to the pathogenicity of various virulence factors, especially vacuolating cytotoxin A (VacA) and cytotoxin-associated gene A (CagA). These virulence factors alter host cell responses and signaling pathways by causing epithelial cell damage, triggering disorders of cell proliferation and apoptosis, disturbing multiple host cell signaling cascades, and inducing host immune-inflammatory responses[20-22]. In addition, H. pylori displays complex tolerance mechanisms to accomplish immune evasion and long-term colonization and then establishes chronic inflammation in the stomach[23,24]. For example, VacA can inhibit the activation and proliferation of T cells and B cells, and induce apoptosis in macrophages via interferon-β signaling inhibition to significantly lead to immunosuppression[25]. Specific masking mechanisms have been found during H. pylori infection, such as lipopolysaccharide (LPS) modifications or flagellin mutations that cannot be detected by toll-like receptor: TLR4[26] and TLR5[27] to evade immune system recognition. In addition, reactive oxygen and nitrogen species produced during H. pylori infection can further damage host cells and lead to DNA damage[28]. The long-lasting state of chronic inflammation and oxidative stress induced by H. pylori ultimately promotes gastric carcinogenesis. The effects of H. pylori virulence factors on the stomach are relatively clear, but how do they produce extra-gastric effects?
Virulence factors in extracellular vesicles, including CagA and VacA, from H. pylori-infected cells and H. pylori outer membrane vesicles provide theoretical evidence for the possible influence of H. pylori virulence factors on extra-gastric tumor development[29,30]. With the secretion of extracellular vesicles into the blood, these extracellular vesicles can be transported to other organs and tissues, where they play a carcinogenic role by interfering with cell signaling pathways; inducing an inflammatory response, and promoting oxidative stress. Extracellular vesicles with H. pylori virulence factors found in extra-gastric diseases indicate a potential role of H. pylori in the development of extra-gastric tumors[29,31,32].
In addition, the effects of H. pylori infection on the host immune system may play a role in extra-gastric tumor development. H. pylori infection elicits robust immune responses during its colonization and survival in the gastric mucosa; including immune recognition, innate and adaptive immune responses, and immune tolerance; which are mixtures characterized by T helper (Th): Th1 and Th17, and regulatory T cell (Treg) responses[33,34]. These immunoregulatory effects between H. pylori and gastric mucosa maintain the coexistence of H. pylori and its host. In summary, in view of the possibility that abnormal regulation of the local immune response may interfere with normal immune surveillance and anti-tumor responses, H. pylori infection may increase the risk of extra-gastric tumor development by affecting the tumor immune microenvironment of the host.
Human microbiota consists of a set of dynamic microbial communities that inhabit different parts of the body. The co-evolution of microbiota with the host causes microbiota to play a profound role in promoting human health, and the disturbance of the human microbiota, especially intestinal microbiota, can cause or exacerbate many diseases[35]. The interaction between the intestinal microbiota and the host is related to the occurrence and development of various cancers[36]. H. pylori infection can alter the diversity and abundance of the intestinal microbiota[37], and its effects on the intestinal microbiota may be a possible cause for the development of cancers in distant organs and tissues. Therefore, the interaction between H. pylori and intestinal microbiota may contribute to the carcinogenic potential of H. pylori beyond the stomach.
Moreover, H. pylori infection causes hypergastrinemia[38]. Gastrin is a peptide hormone that is synthesized and released by G cells in the gastric antrum. H. pylori elevates the pH on the surface of the gastric epithelium by decom
The virulence factors of H. pylori secreted into the bloodstream in the form of extracellular vesicles, regulation of the tumor immune microenvironment by H. pylori, hypergastrinemia and gastrointestinal microbiota disturbance caused by H. pylori infection suggest that H. pylori infection may affect the occurrence and development of extra-gastric tumors. In this section, we summarize the effects of H. pylori on the incidence of extra-gastric tumors and investigate the potential mechanisms with the aim of highlighting the comprehensive effects of H. pylori on the human body.
CRC is the third most common cancer worldwide and imposes a huge burden on the imminent future[40]. Given that colorectal adenomas are premalignant lesions that may develop into CRC through the adenoma-to-carcinoma sequence[41], in this section we summarize the potential pathogenesis of H. pylori infection in colorectal adenoma and CRC, aiming to provide insights for clinical diagnostic and therapeutic strategies. As previously indicated, H. pylori infection is associated with an increased risk of colorectal adenoma and CRC[42-44].
Intestinal homeostasis encompasses the dynamic equilibrium of the intestinal mucosa, immune barrier, and intestinal environment, which includes intestinal microbiota, nutrients, and metabolites[45]. If the dynamic equilibrium is disrupted, the disturbance of intestinal homeostasis may lead to damage in the mucosal immune barrier and an imbalance in intestinal microbiota. This can result in abnormal intestinal development, function, and even colonic diseases; including inflammation and tumor[46]. A recent study found that H. pylori infection promoted colorectal carcinogenesis by altering intestinal homeostasis; including the intestinal mucosa, immune barrier, intestinal microbiota, and metabolites[47].
H. pylori infection alters intestinal mucosal barrier: Integrity of the mucosal barrier is important for the body to resist pathogenic invasion. Research has shown that alterations in tight and adherence junctions in the gastrointestinal epithelium were significantly affected after in vitro H. pylori infection, showing decreased expression of a series of junctional molecules and glycosylation of MUC1[48]. In addition, intestinal goblet cells emerge as pivotal regulators of intestinal health, owing to their roles in providing a protective mucus layer that covers the intestine, sensing changes in the local environment, and shaping gut immunity[49]. Recent research has reported that in C57BL/6 and Apc mutant mice, the maturational states of goblet cells were distinctly affected by H. pylori infection, with a switch to less differentiated cells. Significantly lower expression of Atoh1 was also found in stem cells of both the small intestine and colon in H. pylori infected mice, indicating a skewed differentiation of stem cells into unspecialized colonocytes rather than into goblet cells[47]. H. pylori induced pro-carcinogenic signal transducer and activator of transcription-3 signaling of intestinal epithelial cells and the loss of goblet cells[47], all of which demonstrated that H. pylori exert a detrimental impact on mucus-producing goblet cells, thereby potentially fostering carcinogenesis.
H. pylori infection alters intestinal immune barrier: The intestinal immune barrier is composed mainly of intestine-associated lymphoids tissue and diffuse immune cells. The immune system of the intestinal mucosa can recognize antigens such as bacteria, viruses, and toxins; induce humoral and cellular immunity to effectively eliminate antigens; resist the invasion of pathogenic microorganisms; resist allergic reactions; and suppress immune responses[50]. H. pylori infection induced an H. pylori-specific pro-inflammatory immune response in the small intestine and colon of infected mice, which is characterized by the loss of Treg cells and their differentiation into Foxp3+/IL-17+ T cells[47]. Altered T cell homeostasis caused by H. pylori in the intestine may be a key event during colorectal carcinogenesis, driving tumor development and progression. In addition, enhanced indoleamine 2,3-dioxygenase activity and a significantly high rate of kynurenine/tryptophan ratio in patients with H. pylori seropositive CRC suggested that H. pylori may support immune tolerance, leading to cancer development[51]. In summary, H. pylori infection may promote the occurrence and development of CRC by affecting the intestinal immune homeostasis.
H. pylori infection alters intestinal microbial barrier: Intestinal flora mainly includes mucosal and intestinal cavity flora, which forms a multilevel intestinal microbial barrier and plays a pivotal role in the metabolic, physiological, and immunological systems of the human body. H. pylori infection can affect the colonic microbiota by interacting with the host’s immune system or by altering the local gastric environment where gastric acidity is changed[37,52]. Several studies have demonstrated the compositional changes in the gut microbiota of H. pylori-infected patients[52]. Although some results have been inconsistent, this is a possible mechanism. Furthermore, changes in Akkermansia spp. and Ruminococcus spp. in the gut bacterial community have been reported in mouse models, indicating that H. pylori alters the microbiota of the lower gastrointestinal tract, favors the mucus-degrading microbiota, and induces a pro-inflammatory and pro-carcinogenic microbiota signature[47].
The enteric virome is a component of the gut microbiome that interacts with bacteria and other microbial agents[53]. The virome coexists with other components of the microbiota and host in dynamic equilibrium, jointly maintaining intestinal homeostasis and function. Recent studies have demonstrated that alterations in the colonic virome diversity can disrupt intestinal microbial homeostasis, cause intestinal diseases, and promote CRC growth and invasiveness[54,55]. In healthy Western adults, phages or bacteriophages represent the vast majority of enteroviruses, accounting for 97.7%[56]. Recent studies have highlighted the effect of H. pylori on intestinal phages in CRC development. Using shotgun viral metagenomic sequencing to characterize the viral community in mice, researchers have discovered a complex phage-bacterial infection network associated with H. pylori-promoted CRC. Notably, the relative abundance of temperate phages increased in H. pylori-infected Apc+/1638N mice at 12 weeks post-infection, whereas virulent phages became dominant at 24 weeks. The expansion of temperate phages, potentially induced by the activation of prophages triggered by H. pylori infection, may contribute to the development of CRC in mice by interacting with the bacterial community[55].
H. pylori infection affects intestinal metabolism: (1) H. pylori-induced hypergastrinemia may contribute to the development of CRC: Increased gastrin release has been reported to potentiate the carcinogenic effects of colorectal adenoma and CRC by overexpressing proinflammatory and growth factors[57]. The proliferative effect of chronic hypergastrinemia may further increase mutation susceptibility and result in the development of adenomas[58]. Gastrin precursors, known to promote colon epithelial cell proliferation and survival, have been reported to contribute to angiogenesis by stimulating the expression of the proangiogenic factor and vascular endothelial growth factor (VEGF) via the PI3K/AKT pathway[59]. Similarly, the induction of VEGF-A transcription and translation may play a role in the carcinogenic effects of gastrin[60]. Furthermore, studies have shown feed-forward mechanisms, whereby gastrin and cholecystokinin-2 receptor expression were upregulated during inflammation. Gastrin increases the expression of inflammatory mediators such as COX-2 and IL-8 via multiple pathways, thus strengthening the pro-inflammatory function of immunocytes[57,61].
H. pylori infection is an important cause of hypergastrinemia[62], which suggests that H. pylori may promote hypergastrinemia to the occurrence and development of CRC by promoting gastrin secretion. And it has been reported that H. pylori commonly infects patients with CRC and leads to hypergastrinemia and COX-2 expression, both of which are probably responsible for the initiation and promotion of CRC[39]. These findings suggest that H. pylori-induced alterations in gastrin and its precursors and derivatives may contribute to the development of CRC;
And (2) H. pylori acts synergistically with metabolic disease to promote CRC development: Interestingly, one pers
In conclusion, a growing body of evidence suggests that H. pylori infection may increase the risk of CRC. However, the relationship between H. pylori infection and CRC is complex and variable, and the exact underlying mechanisms are not fully understood. Further clinical and experimental studies are required to elucidate the underlying mechanisms and their associations.
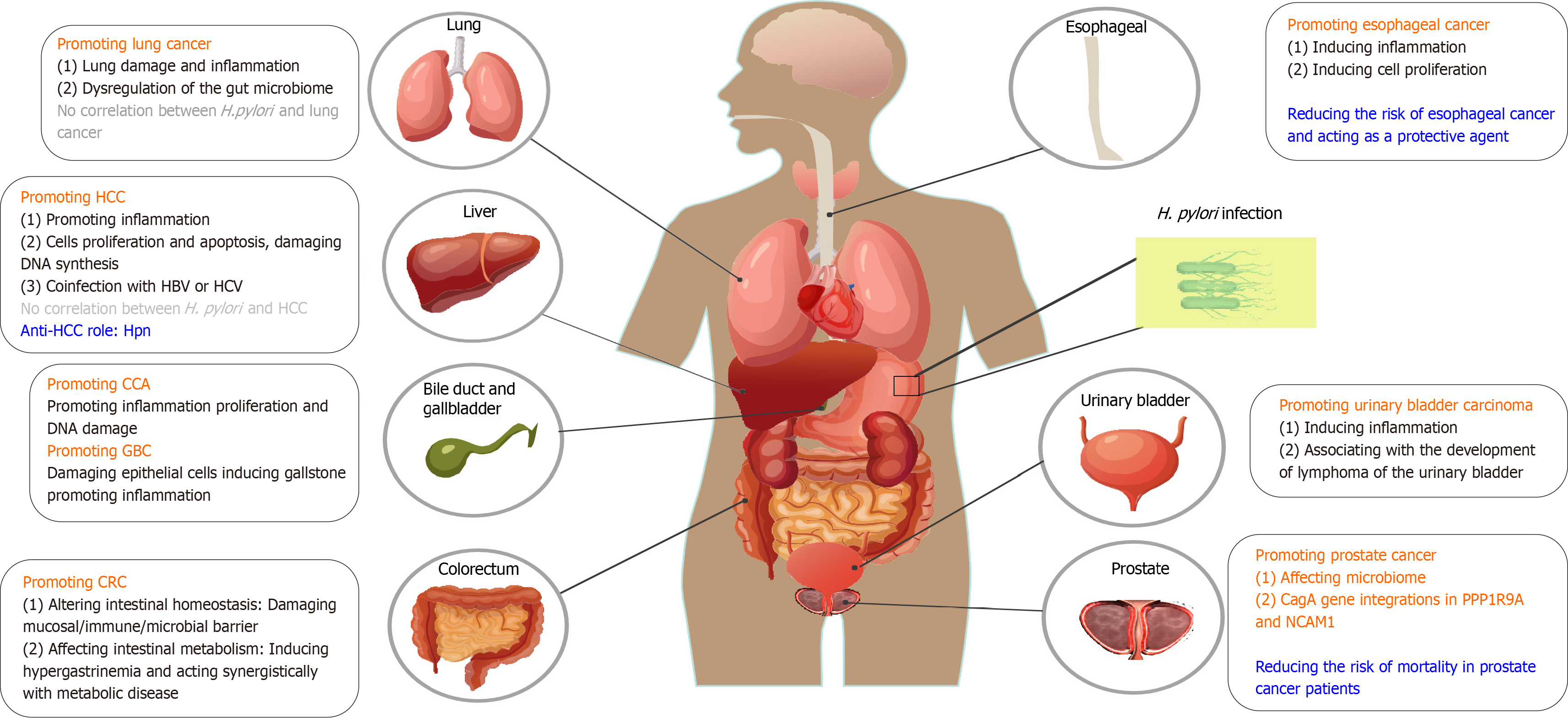
Lung cancer was the second most commonly diagnosed cancer and the leading cause of cancer-related mortality in 2020[66]. An increasing number of studies have revealed an association between H. pylori infection and respiratory diseases and lung cancer. The seroprevalence of H. pylori, especially CagA expression, was significantly higher in patients with lung cancer than in healthy controls[67]. In fact, H. pylori has been detected in the bronchoalveolar lavage of patients with lung cancer using real-time polymerase chain reaction[68]. Specific H. pylori biomarkers, such as CagA, VacA, HP1564 and Catalase, are significantly associated with an increased risk of lung cancer; whereas not all H. pylori strains are associated with it, indicating the role of specific antigens or virulence factors in lung carcinogenesis[69].
H. pylori infection increases the risks of lung damage and inflammation. The oral cavity is a reservoir for H. pylori[70] and gastroesophageal reflux plays a role in the transmission of H. pylori to the lungs. The gastroesophageal reflux of H. pylori urease proteins may provide an antigenic trigger for the initiation of pulmonary granuloma[71]. In addition, H. pylori VacA has been detected in the human lung, where it exhibited cytotoxic effects and stimulated the secretion of IL-8 and IL-6 in targeted airway epithelial cells, suggesting its role in the pathogenesis of lung inflammation[72]. Another study has found an increasing secretion of the inflammatory factors like tumor necrosis factor α, IL-1β, IL-6, and IL-8, as well as an increasing p65 nuclear factor-kappa B (NF-κB) protein expression both in mice and in vitro with H. pylori infection and VacA treatment. This indicated that H. pylori infection promotes inflammatory factor secretion and induces lung injury through the activation of NF-κB signaling via VacA exotoxin[73]. Additionally, chronic and subclinical tracheobronchial aspiration in H. pylori infected individuals, together with the effects of smoking and air pollution, may synergistically initiate and sustain an inflammatory response within the lung epithelium. This inflammatory state could potentially facilitate malignant transformation and tumor growth[74].
H. pylori can promote lung cancer by affecting the gut microbiome. Research has found that disorders of the gut microbiome had an impact on the progression of lung cancer and that the interconnection between the lung and gut microbiomes is particularly important. This interrelation between the two organ systems called the gut-lung axis[75,76]. Dysregulation of the gut microbiome caused by H. pylori infection is associated with the exacerbation of chronic respiratory diseases such as chronic obstructive pulmonary disease, suggesting that H. pylori has a negative effect on lung growth[77]. However, H. pylori-specific effects on the lung microbiome remain unclear, and whether it plays a role in increasing susceptibility to lung cancer through microbiome disorders remains to be elucidated.
In conclusion, the correlation between H. pylori and lung cancer, as well as its underlying mechanisms, remain to be precisely elucidated, and the relevant explanation remains controversial. Few studies have investigated the association between H. pylori and lung cancer. However, there are opposing conclusions from these studies[78]. Considering the small sample size, the fact that existing studies are all retrospective, and mixed factors such as smoking habits and air pollution, the association between H. pylori infection and lung cancer should be interpreted cautiously[78]. Hence, a more comprehensive investigation of the precise relationship between H. pylori infection and lung cancer is warranted. Such research would provide insights into the prevention and treatment strategies of lung cancer.
H. pylori may be associated with Cholangiocarcinoma (CCA) carcinogenesis through the induction of biliary epithelial cell inflammation and proliferation[79]. H. pylori CagA-positive (CagA+) strain DNA was detected more frequently in bile samples of CCA patients than in cholelithiasis patients, and was not detected in the control group. Moreover, patients with CCA and H. pylori-PCR-positive liver tissue presented with a significantly higher inflammatory grade and change in the proliferation of biliary cells in the portal zone around the bile ducts than patients with a non-infected liver[79], suggesting that H. pylori infection may be involved in the severity of hepatobiliary diseases. In addition, another study found that H. pylori infection induced CCA development via inflammation-mediated DNA damage. Specifically, elevated high-mobility group box 1, proliferating cell nuclear antigen levels and increased IL-8 mRNA expression were observed in the liver tissues of H. pylori-infected hamsters, indicating that H. pylori infection may contribute to CCA development, particularly when combined with other carcinogenic agents[80].
H. pylori may also be involved in gallbladder carcinoma (GBC) development. H. pylori isolated from the gallbladder damages human gallbladder epithelial cells in vitro, specifically causing obvious vacuoles, cell rupture necrosis, and even cell death[81]. Moreover, it is interesting to note that H. pylori may act as a gallstone disease lithogenic factor[82] and there is a higher presence of H. pylori in patients with cholelithiasis than non-infected group[83]. Confirmed by histopathological data, H. pylori infection in patients with CCA and GBC implies an association with bile duct stone formation[84,85], which provides new insights into the effects of H. pylori infection in liver diseases. Moreover, it is noted that the co-infection of H. pylori with other bacteria like Helicobacter bilis and Helicobacter hepaticus resulted in more aggressive inflammation in gallbladder mucosa[79,83].
H. pylori may be associated with the carcinogenesis of hepatocellular carcinoma (HCC) through the induction of epithelial cell inflammation, proliferation, and apoptosis. Research has shown that H. pylori affects the replication of hepatocytes primarily by inducing apoptosis with a compensatory increase in DNA synthesis to maintain the balance of cell loss[86]. Analogously, H. pylori infection might also increase the risk of transforming growth factor-β1-mediated tumorigenesis by disturbing the balance between apoptosis and proliferation of hepatocytes[87]. In addition, portal vein thrombosis and HCC development were found to increase with H. pylori infection through increased inflammatory markers and vascular mediators such as nitric oxide and VEGF, which may lead to HCC development[88].
In terms of the combination of the role of bacteria and viruses, there is a significantly higher risk of developing HCC and hepatic fibrosis in the presence of hepatitis C virus infection along with H. pylori[89,90], and similar results were reported in studies of patients infected with hepatitis B virus[91], indicating the role of co-infection with H. pylori and virus in the development of HCC. Thus, it is meaningful to elucidate the relationship between chronic liver disease and H. pylori infection; and whether there is a link to the development of cancer, especially in patients at high risk of severe hepatic impairment.
An increasing number of clinical and experimental studies have found associations between H. pylori and liver diseases, such as nonalcoholic fatty liver disease and HCC[90,92-95]. However, no correlations were identified[96-98]. Histidine-rich protein (Hpn), a nickel-affinity protein from H. pylori, was found to suppress cell growth and induce apoptosis in HCC by suppressing ubiquitin-specific peptidase 5 expression and activating P14ARF-P53 signaling, suggesting an anti-HCC role for Hpn[99]. Thus, despite increasing evidence on the correlation between H. pylori infection and the pathogenesis of liver diseases, definitive conclusions cannot be drawn.
Esophageal cancer is the eighth most commonly diagnosed cancer and the sixth most common cause of cancer-related deaths globally, and squamous cell carcinoma is a major burden[100]. The controversy surrounding the role of H. pylori in Barrett’s esophagus (BE), esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC) persists, suggesting that H. pylori acts as a protective agent or contributes to these diseases. This contention arises partly because of the considerable heterogeneity and improper handling of potential confounding factors[101-103].
H. pylori infection may offer protection against gastroesophageal reflux disease (GERD) and its associated neoplastic complications such as BE and EAC. This protective effect may be associated with H. pylori-induced gastritis and the subsequent reduction in stomach acidity[104]. A meta-analysis of 72 studies indicated that H. pylori infection was associated with a lower risk of BE, while another study indicated that the correlation was stronger in the CagA+ subgroup[102,105]. In addition, H. pylori preferentially induces apoptosis in Barrett's-derived cancer cells compared to normal cells, suggesting that H. pylori may protect against the development of dysplasia in the Barrett's epithelium in patients with GERD[106]. Whether H. pylori actually protects against these esophageal diseases is still under debate. Some studies indicated that neither H. pylori infection nor H. pylori infection by CagA+ strains reduce the risk of BE[107] and that no significant correlation between H. pylori infection and ESCC in the general population has been observed[103]. Studies on the mechanisms of H. pylori infection and esophageal cancer suggest that this protective effect may be overemphasized[101].
Gastrin induced by H. pylori infection, especially amidated gastrin, plays an important role in the neoplastic progression of BE via the activation of several signaling pathways to maintain chronic inflammation, and certain molecular alterations support the pathophysiology of H. pylori-related GERD-BE-EAC sequence[108,109]. In addition, cytokine IL-8 release was significantly increased via the p38 mitogen-activated protein kinase (MAPK) signaling pathway, and the expression of Src homology-2 domain-containing phosphatase mRNA declined sharply in the CagA+H. pylori group, indicating that CagA+H. pylori filtrates could induce the proliferation of Ec109 cells in vitro[110]. In another study, researchers also observed that LPS-TLR4 signaling was associated with the activation of extracellular regulated protein kinase and p38 MAPK signaling pathways[111], indicating that H. pylori may play a role in the LPS-induced development of ESCC. Moreover, it is interesting to find that H. pylori infection may cause changes in the esophageal microbiota, and research in this area is considered imperative[37].
Several studies have focused on the relationship between the microbiome composition and prostate cancer (PCa) risk, demonstrating that certain bacteria may be associated with cancer development and altered responses to treatment[112]. A study provides molecular evidence for the presence of H. pylori DNA in the prostate tissue of patients with benign prostatic hyperplasia and PCa[113]. More importantly, research has found a marked increase in the integration of viral and microbial sequences in prostate tumor DNA; in particular, H. pylori CagA gene integration in the PPP1R9A and NCAM1 genes was found, which may be a contributing factor to prostate tumorigenesis[114]. There is still a dearth of coverage on this theme; conversely, we have noticed that H. pylori infection is associated with a reduced risk of mortality in patients with PCa receiving androgen deprivation therapy[115]. Therefore, relevant research is warranted.
Inflammation in the urinary bladder and pelvis was observed when H. pylori was transurethrally inoculated into the mouse urinary tract[116] and the disappearance of mucosa-associated lymphoid tissue lymphoma of the urinary bladder after treatment for H. pylori was found as well[117], indicating that chronic antigenic stimulation of infectious agents might be associated with the development of this malignancy. However, direct evidence for this correlation is lacking. Further studies are required to confirm the role of H. pylori infection in urinary bladder carcinogenesis.
In this review, we discuss the relationship between H. pylori infection and the development of extra-gastric tumors, as shown in Figure 1 and Table 1. Through exosomes or other forms, H. pylori virulence factors play an important role in promoting inflammation, regulating host immune responses, inducing abnormal proliferation and apoptosis[1] and may finally contribute to the onset of extra-gastric tumors. Currently, it has been reported that H. pylori affects the occurrence and development of some inflammatory diseases through exosomes, such as atherosclerosis, liver fibrosis, and Alzheimer’s disease[30]; but in fact; there is no literature directly reporting that H. pylori affects the occurrence and development of extra-gastric tumors through exosomes. This may be because studies on exosomes of H. pylori infected cells are relatively new and limited. For the study of extra-gastric cancer caused by H. pylori exosomes, in vivo experiments in mice may require a longer period and a larger exosome; and the dose and experimental details, such as how to measure the role of key exosome molecules; need to be explored. In addition, it puts forward higher requirements for manpower, material resources, experimental technology, operating conditions, and determination of experimental endpoints. These may be the main reasons for the lack of research on extra-gastric tumors caused by H. pylori exosomes.
| Cancers affected by H. pylori | Roles of H. pylori | Effects and mechanisms of H. pylori infection | Results |
| CRC | Damaging mucosal barrier | Decreased expression of junctional molecules and the glycosylation of MUC1 in gastrointestinal epithelium[48] | Affecting the assembly and function of the adherent junctions and breaking the mucosal barrier that protects the epithelial cells from the degradative enzymes |
| Inducing goblet cells switch to less differentiated ones and a lower expression of Atoh[49] | A loss of goblet cells | ||
| Damaging immune barrier | Inducing loss of Treg cells and their differentiation into Foxp3+ IL-17A+ T cells[47] | Inducing a pro-inflammatory immune response | |
| Enhanced indoleamine 2,3-dioxygenase activity and increased ratio of the kynurenine to tryptophan low serum nitrite levels[51] | Enhancing systemic immune tolerance | ||
| Damaging microbial barrier | Increasing Akkermansia spp and Ruminococcus spp[47] | Altering the microbiota of the lower gastrointestinal tract, favoring mucus-degrading microbiota and inducing a pro-inflammatory and pro-carcinogenic microbiota signature | |
| Elevating relative abundance of temperate phages[55] | Expanding virulence by phage-mediated horizontal gene transfer and contributing to the development of CRC | ||
| Inducing hypergastrinemia | Promoting the secretion of gastrin and COX-2 expression[39] | Contribute to the initiation and promotion of CRC | |
| Acting synergistically with metabolic disease | Combination with elevated HbA1c or diabetes mellitus[63,64] | Increasing the risk of colorectal adenoma | |
| Lung cancer | Promoting lung cancer | Gastroesophageal reflux of H. pylori urease may trigger for the initiation of pulmonary granuloma[71] | Inducing the risk of lung damage and inflammation |
| Promoting inflammatory factor secretion and inducing lung injury through the activation of NF-κB signaling via VacA exotoxin[72] | |||
| Acting synergically with the effects of smoking or air-pollution to establish and perpetuate an inflammatory reaction[74] | |||
| Inducing dysregulation of the gut microbiome[77] | May increase susceptibility to lung cancer | ||
| No clear evidence of a causal association between H. pylori infection and respiratory diseases | A limited number of underpowered studies reporting contrasting results[78] | The correlation between H. pylori and lung cancer as well as its underlying mechanisms, remains to be elucidated with precision | |
| CCA and GBC | Associating with the carcinogenesis of CCA | Enhancing inflammation, promoting proliferation of biliary cells, inducing inflammation-mediated DNA damage[79] | Contributing to the development of CCA |
| Involving in GBC development | Damaging human gallbladder epithelial cells[81] | Contributing to the development of GBC | |
| Acting as a gallstone disease lithogenic factor[82] | |||
| inducing more aggressive inflammation in in gallbladder mucosa with other bacteria[79,83] | |||
| HCC | The relationship between H. pylori infection and risk of HCC is still controversial | Inducing epithelial cell inflammation, proliferation and disturbance of apoptosis and DNA synthesis[86] | Leading to HCC development |
| Coinfection with HBV or HCV[86] | |||
| No associations between H. pylori and liver diseases[96-98] | No correlations with HCC or playing an anti-HCC role | ||
| Hpn, a nickel-affinity protein from H. pylori can suppress cell growth and induce apoptosis of HCC[99] | |||
| Esophageal cancer | The relationship between H. pylori infection and risk of Esophageal cancer is still controversial | Gastrin maintains chronic inflammation and certain pathophysiology molecular alterations[108,109] | May contribute to esophageal cancer development |
| Increased IL-8 via p38 MAPK pathway and the declined expression of SHP-2 mRNA to induce cell proliferation[110], LPS-TLR4 signaling associating with the activation of ERK and p38 MAPK pathways[110] | |||
| Heterogeneity and improper handling of potential confounding factors[101-103] | Arguing that whether H. pylori is a protective agent or a cause of esophageal cancer | ||
| PCa | The relationship between H. pylori infection and risk of prostate cancer is still controversial | Affecting human microbiome[112] | May contribute to the development of prostate cancer |
| CagA gene integrations in PPP1R9A and NCAM1 genes[113] | |||
| Associating with a reduced risk of mortality in PCa patients receiving androgen deprivation therapy[113] | Negative correlations with prostate cancer | ||
| Urinary bladder carcinoma | Associating with the development of urinary bladder carcinoma | Inducing inflammation in the urinary bladder and pelvis[116], associating with the development of mucosa-associated lymphoid tissue lymphoma of the urinary bladder[116] | May contribute to urinary bladder carcinoma but lacking of direct evidence and further studies are needed to confirm |
Epithelial cells, immune cells, and microbiota constitute the mucosal, immune, and microbial barriers in some organs and tissues, such as the stomach, intestine, and lungs. These barriers jointly maintain homeostasis of these organs and the host body. Notably, damage to the dynamic equilibrium by H. pylori infection leads to the breakdown of homeostasis, which induces diseases and cancers in related organs and tissues. Disturbances in the microbiome are frequently observed in patients with cancer, especially colorectal and PCas. This phenomenon may be attributed to an increased systemic inflammatory response or the ability of certain bacteria to alter the host immune response, making it more favorable for tumor growth[1,55,118,119]. There is growing evidence of the impact of H. pylori infection on the host microbiota, including bacteria and viruses that play a direct or indirect role in the progression of extra-gastric diseases. However, there remains a lack of data depicting the functional profiles of host microbial species and revealing the specific mechanisms by which microbes support cancer development[120]. With advances in technology, the integration of spatial transcriptomics with viral metagenomics is promising for studying the spatial distribution of viruses and virus-host cell interactions[54].
Several studies have revealed an association between H. pylori and extra-gastric tumors. Inflammation response, systemic immune responses, microbiome disorders, and hypergastrinemia caused by H. pylori infection may contribute to this process. Although this review highlights the relationship between H. pylori infection and tumorigenesis beyond the stomach, the underlying mechanism remains unclear. However, it should be noted that not all people infected with H. pylori develop tumors. Occurrence of tumors is relative to the heterogeneity of pathogenic substances of H. pylori strains and population heterogeneity, such as lifestyle habits. It is anticipated that these findings will prompt clinicians to focus on H. pylori infections and offer new strategies for the management of extra-gastric tumors.
We would like to give the heartfelt thanks to all the people who have ever helped in this paper. We are also grateful to all those who devoted much time to reading this thesis and gave us much advice, which will benefit us in our later study.
| 1. | Baj J, Forma A, Sitarz M, Portincasa P, Garruti G, Krasowska D, Maciejewski R. Helicobacter pylori Virulence Factors-Mechanisms of Bacterial Pathogenicity in the Gastric Microenvironment. Cells. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 2. | Malfertheiner P, Camargo MC, El-Omar E, Liou JM, Peek R, Schulz C, Smith SI, Suerbaum S. Helicobacter pylori infection. Nat Rev Dis Primers. 2023;9:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 401] [Article Influence: 200.5] [Reference Citation Analysis (1)] |
| 3. | Chen YC, Malfertheiner P, Yu HT, Kuo CL, Chang YY, Meng FT, Wu YX, Hsiao JL, Chen MJ, Lin KP, Wu CY, Lin JT, O'Morain C, Megraud F, Lee WC, El-Omar EM, Wu MS, Liou JM. Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer Between 1980 and 2022. Gastroenterology. 2024;166:605-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 166] [Article Influence: 166.0] [Reference Citation Analysis (0)] |
| 4. | Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology. 2018;155:1372-1382.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 823] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 5. | He J, Hu W, Ouyang Q, Zhang S, He L, Chen W, Li X, Hu C. Helicobacter pylori infection induces stem cell-like properties in Correa cascade of gastric cancer. Cancer Lett. 2022;542:215764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8187] [Article Influence: 8187.0] [Reference Citation Analysis (2)] |
| 7. | Ezzati Amini E, Moradi Y. Association between helicobacter pylori infection and primary open-angle glaucoma: a systematic review and meta-analysis. BMC Ophthalmol. 2023;23:374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Wei BR, Zhao YJ, Cheng YF, Huang C, Zhang F. Helicobacter pylori infection and Parkinson's Disease: etiology, pathogenesis and levodopa bioavailability. Immun Ageing. 2024;21:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Sun L, Zheng H, Qiu M, Hao S, Liu X, Zhu X, Cai X, Huang Y. Helicobacter pylori infection and risk of cardiovascular disease. Helicobacter. 2023;28:e12967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Masaadeh AH, Mathias PC, Ford BA, Bosch DE. Helicobacter pylori Exposure in Nausea and Vomiting of Pregnancy Increases Risk of Preterm Delivery. Infect Dis Obstet Gynecol. 2023;2023:6612268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Pellicano R, Ianiro G, Fagoonee S, Settanni CR, Gasbarrini A. Review: Extragastric diseases and Helicobacter pylori. Helicobacter. 2020;25 Suppl 1:e12741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Oster P, Vaillant L, Riva E, McMillan B, Begka C, Truntzer C, Richard C, Leblond MM, Messaoudene M, Machremi E, Limagne E, Ghiringhelli F, Routy B, Verdeil G, Velin D. Helicobacter pylori infection has a detrimental impact on the efficacy of cancer immunotherapies. Gut. 2022;71:457-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 123] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 13. | Deng R, Zheng H, Cai H, Li M, Shi Y, Ding S. Effects of helicobacter pylori on tumor microenvironment and immunotherapy responses. Front Immunol. 2022;13:923477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 14. | Boyuk B, Ozgur A, Atalay H, Celebi A, Ekizoglu I, Aykurt E. Helicobacter pylori infection coexisting with intestinal metaplasia is not associated with colorectal neoplasms. Prz Gastroenterol. 2019;14:133-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Siddheshwar RK, Muhammad KB, Gray JC, Kelly SB. Seroprevalence of Helicobacter pylori in patients with colorectal polyps and colorectal carcinoma. Am J Gastroenterol. 2001;96:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | de Martel C, Llosa AE, Friedman GD, Vogelman JH, Orentreich N, Stolzenberg-Solomon RZ, Parsonnet J. Helicobacter pylori infection and development of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:1188-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Venerito M, Kohrs S, Wex T, Adolf D, Kuester D, Schubert D, Peitz U, Mönkemüller K, Malfertheiner P. Helicobacter pylori infection and fundic gastric atrophy are not associated with esophageal squamous cell carcinoma: a case-control study. Eur J Gastroenterol Hepatol. 2011;23:859-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 18. | Cook MB, Dawsey SM, Diaw L, Blaser MJ, Perez-Perez GI, Abnet CC, Taylor PR, Albanes D, Virtamo J, Kamangar F. Serum pepsinogens and Helicobacter pylori in relation to the risk of esophageal squamous cell carcinoma in the alpha-tocopherol, beta-carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev. 2010;19:1966-1975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Xu W, Zhou X, Yin M, Gao J, Weng Z, Xu C. The relationship between Helicobacter pylori and pancreatic cancer: a meta-analysis. Transl Cancer Res. 2022;11:2810-2822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 20. | Salvatori S, Marafini I, Laudisi F, Monteleone G, Stolfi C. Helicobacter pylori and Gastric Cancer: Pathogenetic Mechanisms. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 119] [Reference Citation Analysis (1)] |
| 21. | Sharndama HC, Mba IE. Helicobacter pylori: an up-to-date overview on the virulence and pathogenesis mechanisms. Braz J Microbiol. 2022;53:33-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 126] [Article Influence: 42.0] [Reference Citation Analysis (1)] |
| 22. | Wang H, Zhao M, Shi F, Zheng S, Xiong L, Zheng L. A review of signal pathway induced by virulent protein CagA of Helicobacter pylori. Front Cell Infect Microbiol. 2023;13:1062803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 23. | Sijmons D, Guy AJ, Walduck AK, Ramsland PA. Helicobacter pylori and the Role of Lipopolysaccharide Variation in Innate Immune Evasion. Front Immunol. 2022;13:868225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 24. | Zhang X, Zhang K, Yan L, Wang P, Zhao F, Hu S. The role of toll-like receptors in immune tolerance induced by Helicobacter pylori infection. Helicobacter. 2023;28:e13020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Chauhan N, Tay ACY, Marshall BJ, Jain U. Helicobacter pylori VacA, a distinct toxin exerts diverse functionalities in numerous cells: An overview. Helicobacter. 2019;24:e12544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 26. | Schmidinger B, Petri K, Lettl C, Li H, Namineni S, Ishikawa-Ankerhold H, Jiménez-Soto LF, Haas R. Helicobacter pylori binds human Annexins via Lipopolysaccharide to interfere with Toll-like Receptor 4 signaling. PLoS Pathog. 2022;18:e1010326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 27. | Pachathundikandi SK, Tegtmeyer N, Backert S. Masking of typical TLR4 and TLR5 ligands modulates inflammation and resolution by Helicobacter pylori. Trends Microbiol. 2023;31:903-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Butcher LD, den Hartog G, Ernst PB, Crowe SE. Oxidative Stress Resulting From Helicobacter pylori Infection Contributes to Gastric Carcinogenesis. Cell Mol Gastroenterol Hepatol. 2017;3:316-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 29. | Wang C, Li W, Shao L, Zhou A, Zhao M, Li P, Zhang Z, Wu J. Both extracellular vesicles from helicobacter pylori-infected cells and helicobacter pylori outer membrane vesicles are involved in gastric/extragastric diseases. Eur J Med Res. 2023;28:484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 30. | González MF, Díaz P, Sandoval-Bórquez A, Herrera D, Quest AFG. Helicobacter pylori Outer Membrane Vesicles and Extracellular Vesicles from Helicobacter pylori-Infected Cells in Gastric Disease Development. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 31. | Park AM, Tsunoda I. Helicobacter pylori infection in the stomach induces neuroinflammation: the potential roles of bacterial outer membrane vesicles in an animal model of Alzheimer's disease. Inflamm Regen. 2022;42:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 32. | Qiang L, Hu J, Tian M, Li Y, Ren C, Deng Y, Jiang Y. Extracellular vesicles from helicobacter pylori-infected cells and helicobacter pylori outer membrane vesicles in atherosclerosis. Helicobacter. 2022;27:e12877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 33. | Wang L, Cao ZM, Zhang LL, Dai XC, Liu ZJ, Zeng YX, Li XY, Wu QJ, Lv WL. Helicobacter Pylori and Autoimmune Diseases: Involving Multiple Systems. Front Immunol. 2022;13:833424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 34. | Kronsteiner B, Bassaganya-Riera J, Philipson C, Viladomiu M, Carbo A, Abedi V, Hontecillas R. Systems-wide analyses of mucosal immune responses to Helicobacter pylori at the interface between pathogenicity and symbiosis. Gut Microbes. 2016;7:3-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science. 2021;371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 743] [Article Influence: 185.8] [Reference Citation Analysis (0)] |
| 36. | Park EM, Chelvanambi M, Bhutiani N, Kroemer G, Zitvogel L, Wargo JA. Targeting the gut and tumor microbiota in cancer. Nat Med. 2022;28:690-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 298] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 37. | Chen CC, Liou JM, Lee YC, Hong TC, El-Omar EM, Wu MS. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes. 2021;13:1-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (3)] |
| 38. | Duan S, Rico K, Merchant JL. Gastrin: From Physiology to Gastrointestinal Malignancies. Function (Oxf). 2022;3:zqab062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Hartwich A, Konturek SJ, Pierzchalski P, Zuchowicz M, Labza H, Konturek PC, Karczewska E, Bielanski W, Marlicz K, Starzynska T, Lawniczak M, Hahn EG. Helicobacter pylori infection, gastrin, cyclooxygenase-2, and apoptosis in colorectal cancer. Int J Colorectal Dis. 2001;16:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N, Bray F. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 926] [Article Influence: 463.0] [Reference Citation Analysis (1)] |
| 41. | Hu KC, Wu MS, Chu CH, Wang HY, Lin SC, Liu SC, Liu CC, Su TH, Chen CL, Liu CJ, Shih SC. Synergistic Effect of Hyperglycemia and Helicobacterpylori Infection Status on Colorectal Adenoma Risk. J Clin Endocrinol Metab. 2017;102:2744-2750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Chen YS, Xu SX, Ding YB, Huang XE, Deng B. Helicobacter pylori Infection and the risk of colorectal adenoma and adenocarcinoma: an updated meta-analysis of different testing methods. Asian Pac J Cancer Prev. 2013;14:7613-7619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Wu Q, Yang ZP, Xu P, Gao LC, Fan DM. Association between Helicobacter pylori infection and the risk of colorectal neoplasia: a systematic review and meta-analysis. Colorectal Dis. 2013;15:e352-e364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Sonnenberg A, Genta RM. Helicobacter pylori is a risk factor for colonic neoplasms. Am J Gastroenterol. 2013;108:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 45. | Mitson-Salazar A, Medzhitov R. Environmental sensing mechanisms in intestinal homeostasis. J Allergy Clin Immunol. 2022;150:577-579. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 46. | Wang X, Ding C, Li HB. The crosstalk between enteric nervous system and immune system in intestinal development, homeostasis and diseases. Sci China Life Sci. 2024;67:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Ralser A, Dietl A, Jarosch S, Engelsberger V, Wanisch A, Janssen KP, Middelhoff M, Vieth M, Quante M, Haller D, Busch DH, Deng L, Mejías-Luque R, Gerhard M. Helicobacter pylori promotes colorectal carcinogenesis by deregulating intestinal immunity and inducing a mucus-degrading microbiota signature. Gut. 2023;72:1258-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 48. | Zhang C, Zhang H, Yu L, Cao Y. Helicobacter pylori dwelling on the apical surface of gastrointestinal epithelium damages the mucosal barrier through direct contact. Helicobacter. 2014;19:330-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Gustafsson JK, Johansson MEV. The role of goblet cells and mucus in intestinal homeostasis. Nat Rev Gastroenterol Hepatol. 2022;19:785-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 304] [Reference Citation Analysis (0)] |
| 50. | Spencer J, Bemark M. Human intestinal B cells in inflammatory diseases. Nat Rev Gastroenterol Hepatol. 2023;20:254-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 51. | Engin AB, Karahalil B, Karakaya AE, Engin A. Helicobacter pylori and serum kynurenine-tryptophan ratio in patients with colorectal cancer. World J Gastroenterol. 2015;21:3636-3643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Iino C, Shimoyama T. Impact of Helicobacter pylori infection on gut microbiota. World J Gastroenterol. 2021;27:6224-6230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (3)] |
| 53. | Cadwell K. Expanding the role of the virome: commensalism in the gut. J Virol. 2015;89:1951-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Tun HM, Peng Y, Massimino L, Sin ZY, Parigi TL, Facoetti A, Rahman S, Danese S, Ungaro F. Gut virome in inflammatory bowel disease and beyond. Gut. 2024;73:350-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 28.0] [Reference Citation Analysis (103)] |
| 55. | Luo S, Ru J, Mirzaei MK, Xue J, Peng X, Ralser A, Mejías-Luque R, Gerhard M, Deng L. Gut virome profiling identifies an association between temperate phages and colorectal cancer promoted by Helicobacter pylori infection. Gut Microbes. 2023;15:2257291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 56. | Gregory AC, Zablocki O, Zayed AA, Howell A, Bolduc B, Sullivan MB. The Gut Virome Database Reveals Age-Dependent Patterns of Virome Diversity in the Human Gut. Cell Host Microbe. 2020;28:724-740.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 385] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 57. | Colucci R, Blandizzi C, Tanini M, Vassalle C, Breschi MC, Del Tacca M. Gastrin promotes human colon cancer cell growth via CCK-2 receptor-mediated cyclooxygenase-2 induction and prostaglandin E2 production. Br J Pharmacol. 2005;144:338-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Georgopoulos SD, Polymeros D, Triantafyllou K, Spiliadi C, Mentis A, Karamanolis DG, Ladas SD. Hypergastrinemia is associated with increased risk of distal colon adenomas. Digestion. 2006;74:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Bertrand C, Kowalski-Chauvel A, Do C, Résa C, Najib S, Daulhac L, Wang TC, Ferrand A, Seva C. A gastrin precursor, gastrin-gly, upregulates VEGF expression in colonic epithelial cells through an HIF-1-independent mechanism. Int J Cancer. 2010;126:2847-2857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Ellrichmann M, Ritter PR, Schrader H, Schmidt WE, Meier JJ, Schmitz F. Gastrin stimulates the VEGF-A promotor in a human colon cancer cell line. Regul Pept. 2010;165:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Chao C, Hellmich MR. Gastrin, inflammation, and carcinogenesis. Curr Opin Endocrinol Diabetes Obes. 2010;17:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | D'Onghia V, Leoncini R, Carli R, Santoro A, Giglioni S, Sorbellini F, Marzocca G, Bernini A, Campagna S, Marinello E, Vannoni D. Circulating gastrin and ghrelin levels in patients with colorectal cancer: correlation with tumour stage, Helicobacter pylori infection and BMI. Biomed Pharmacother. 2007;61:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Upala S, Jaruvongvanich V, Riangwiwat T, Jaruvongvanich S, Sanguankeo A. Association between Helicobacter pylori infection and metabolic syndrome: a systematic review and meta-analysis. J Dig Dis. 2016;17:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 64. | Butt J, Epplein M. Helicobacter pylori and colorectal cancer-A bacterium going abroad? PLoS Pathog. 2019;15:e1007861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 65. | Ko HJ, Lin YC, Chen CC, Chen MJ, Wu MS, Liu CJ, Huang CT, Yang HW, Shih SC, Yu LY, Kuo YC, Wang HY, Hu KC. Helicobacter pylori infection and increased diabetes prevalence were the risks of colorectal adenoma for adults: A systematic review and meta-analysis (PRISMA-compliant article). Medicine (Baltimore). 2021;100:e28156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 66. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64681] [Article Influence: 16170.3] [Reference Citation Analysis (177)] |
| 67. | Gocyk W, Nikliński T, Olechnowicz H, Duda A, Bielański W, Konturek PC, Konturek SJ. Helicobacter pylori, gastrin and cyclooxygenase-2 in lung cancer. Med Sci Monit. 2000;6:1085-1092. [PubMed] |
| 68. | Samareh-Fekri M, Hashemi Bajgani SM, Shafahi A, Asadi-Zarandi M, Mollaie H, Jamali Paghalhe A. Detection of Helicobacter pylori in the Bronchoalveolar Lavage of Patients with Lung Cancer Using Real-Time PCR. Jundishapur J Microbiol. 2016;9:e32144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Yoon HS, Shu XO, Cai H, Zheng W, Wu J, Wen W, Courtney R, Shidal C, Waterboer T, Blot WJ, Cai Q. Associations of lung cancer risk with biomarkers of Helicobacter pylori infection. Carcinogenesis. 2022;43:538-546. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 70. | Zhang L, Chen X, Ren B, Zhou X, Cheng L. Helicobacter pylori in the Oral Cavity: Current Evidence and Potential Survival Strategies. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 71. | Herndon B, Quinn T, Wasson N, Nzabi M, Molteni A. Urease and Helicobacter spp. antigens in pulmonary granuloma. J Comp Pathol. 2013;148:266-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 72. | Nakashima S, Kakugawa T, Yura H, Tomonaga M, Harada T, Hara A, Hara S, Nakano M, Yamasaki E, Sakamoto N, Ishimatsu Y, Isomoto H, Gochuico BR, Suffredini AF, Mukae H, Kurazono H, Hirayama T, Moss J, Kohno S. Identification of Helicobacter pylori VacA in human lung and its effects on lung cells. Biochem Biophys Res Commun. 2015;460:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 73. | Chen M, Huang X, Gao M, Yang Z, Fang Z, Wei J, Wu B. Helicobacter pylori promotes inflammatory factor secretion and lung injury through VacA exotoxin-mediated activation of NF-κB signaling. Bioengineered. 2022;13:12760-12771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 74. | GonzÁlez I, Araya P, Rojas A. Helicobacter Pylori Infection and Lung Cancer: New Insights and Future Challenges. Zhongguo Fei Ai Za Zhi. 2018;21:658-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 75. | Santacroce L, Charitos IA, Ballini A, Inchingolo F, Luperto P, De Nitto E, Topi S. The Human Respiratory System and its Microbiome at a Glimpse. Biology (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 76. | Zhang D, Li S, Wang N, Tan HY, Zhang Z, Feng Y. The Cross-Talk Between Gut Microbiota and Lungs in Common Lung Diseases. Front Microbiol. 2020;11:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 77. | Ananya FN, Ahammed MR, Fahem MM, Kafle S, Viswanathan M, Desai D, Akku R, Khan F, Hernandez TE, Bala SK, Gulati S, Martin N, Yatzkan GD, Pérez-Fernández J. Association of Intestinal Microbial Dysbiosis With Chronic Obstructive Pulmonary Disease. Cureus. 2021;13:e19343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 78. | Durazzo M, Adriani A, Fagoonee S, Saracco GM, Pellicano R. Helicobacter pylori and Respiratory Diseases: 2021 Update. Microorganisms. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 79. | Boonyanugomol W, Chomvarin C, Sripa B, Bhudhisawasdi V, Khuntikeo N, Hahnvajanawong C, Chamsuwan A. Helicobacter pylori in Thai patients with cholangiocarcinoma and its association with biliary inflammation and proliferation. HPB (Oxford). 2012;14:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 80. | Dangtakot R, Intuyod K, Chamgramol Y, Pairojkul C, Pinlaor S, Jantawong C, Pongking T, Haonon O, Ma N, Pinlaor P. CagA(+) Helicobacter pylori infection and N-nitrosodimethylamine administration induce cholangiocarcinoma development in hamsters. Helicobacter. 2021;26:e12817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 81. | Chen DF, Hu L, Yi P, Liu WW, Fang DC, Cao H. Helicobacter pylori damages human gallbladder epithelial cells in vitro. World J Gastroenterol. 2008;14:6924-6928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 82. | Cen L, Wu J, Zhu S, Pan J, Zhou T, Yan T, Shen Z, Yu C. The potential bidirectional association between Helicobacter pylori infection and gallstone disease in adults: A two-cohort study. Eur J Clin Invest. 2023;53:e13879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 83. | Zhou D, Zhang Y, Gong W, Mohamed SO, Ogbomo H, Wang X, Liu Y, Quan Z. Are Helicobacter pylori and other Helicobacter species infection associated with human biliary lithiasis? A meta-analysis. PLoS One. 2011;6:e27390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 84. | Yakoob J, Khan MR, Abbas Z, Jafri W, Azmi R, Ahmad Z, Naeem S, Lubbad L. Helicobacter pylori: association with gall bladder disorders in Pakistan. Br J Biomed Sci. 2011;68:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Cherif S, Rais H, Hakmaoui A, Sellami S, Elantri S, Amine A. Linking Helicobacter pylori with gallbladder and biliary tract cancer in Moroccan population using clinical and pathological profiles. Bioinformation. 2019;15:735-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 86. | Ito K, Yamaoka Y, Yoffe B, Graham DY. Disturbance of apoptosis and DNA synthesis by Helicobacter pylori infection of hepatocytes. Dig Dis Sci. 2008;53:2532-2540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Ki MR, Goo MJ, Park JK, Hong IH, Ji AR, Han SY, You SY, Lee EM, Kim AY, Park SJ, Lee HJ, Kim SY, Jeong KS. Helicobacter pylori accelerates hepatic fibrosis by sensitizing transforming growth factor-β1-induced inflammatory signaling. Lab Invest. 2010;90:1507-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | Abdel-Razik A, Mousa N, Elhelaly R, Elzehery R, Hasan AS, Abdelsalam M, Seif AS, Tawfik AM, El-Wakeel N, Eldars W. Helicobacter pylori as an Initiating Factor of Complications in Patients With Cirrhosis: A Single-Center Observational Study. Front Med (Lausanne). 2020;7:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 89. | Attallah AM, Albannan MS, Ghaly MF, Sallam SE, Amer MM, Attia AA. Prevalence of Helicobacter pylori infection in patients with chronic hepatitis C. J Genet Eng Biotechnol. 2022;20:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 90. | Madala S, MacDougall K, Surapaneni BK, Park R, Girotra M, Kasi A. Coinfection of Helicobacter pylori and Hepatitis C Virus in the Development of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. J Clin Med Res. 2021;13:530-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 91. | Wang J, Chen RC, Zheng YX, Zhao SS, Li N, Zhou RR, Huang Y, Huang ZB, Fan XG. Helicobacter pylori infection may increase the risk of progression of chronic hepatitis B disease among the Chinese population: a meta-analysis. Int J Infect Dis. 2016;50:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 92. | Heydari K, Yousefi M, Alizadeh-Navaei R, Lotfi P, Sheydaee F, Raei M, Vahdatinia A, Hessami A, Rafati S, Moosazadeh M, Ghasemian R, Salehi F, Massoudi H, Ghaffari-Saravi F, Rismantab S. Helicobacter pylori Infection and Non-alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Turk J Gastroenterol. 2022;33:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 93. | Zhou D, Guan WB, Wang JD, Zhang Y, Gong W, Quan ZW. A comparative study of clinicopathological features between chronic cholecystitis patients with and without Helicobacter pylori infection in gallbladder mucosa. PLoS One. 2013;8:e70265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 94. | Mekonnen HD, Fisseha H, Getinet T, Tekle F, Galle PR. Helicobacter pylori Infection as a Risk Factor for Hepatocellular Carcinoma: A Case-Control Study in Ethiopia. Int J Hepatol. 2018;2018:1941728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 95. | Makkar R, Butt J, Huang WY, McGlynn KA, Koshiol J, Pawlita M, Waterboer T, Freedman ND, Murphy G. Seropositivity for Helicobacter pylori and hepatobiliary cancers in the PLCO study. Br J Cancer. 2020;123:909-911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 96. | Cai O, Huang Z, Li M, Zhang C, Xi F, Tan S. Association between Helicobacter pylori Infection and Nonalcoholic Fatty Liver Disease: A Single-Center Clinical Study. Gastroenterol Res Pract. 2018;2018:8040262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 97. | Fan N, Peng L, Xia Z, Zhang L, Wang Y, Peng Y. Helicobacter pylori Infection Is Not Associated with Non-alcoholic Fatty Liver Disease: A Cross-Sectional Study in China. Front Microbiol. 2018;9:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 98. | Okushin K, Tsutsumi T, Ikeuchi K, Kado A, Enooku K, Fujinaga H, Moriya K, Yotsuyanagi H, Koike K. Helicobacter pylori infection and liver diseases: Epidemiology and insights into pathogenesis. World J Gastroenterol. 2018;24:3617-3625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 99. | Liu Y, Wang WM, Zou LY, Li L, Feng L, Pan MZ, Lv MY, Cao Y, Wang H, Kung HF, Pang JX, Fu WM, Zhang JF. Ubiquitin specific peptidase 5 mediates Histidine-rich protein Hpn induced cell apoptosis in hepatocellular carcinoma through P14-P53 signaling. Proteomics. 2017;17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 100. | Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, Laversanne M, Ferlay J, Arnold M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates From GLOBOCAN 2020. Gastroenterology. 2022;163:649-658.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 556] [Article Influence: 185.3] [Reference Citation Analysis (0)] |
| 101. | Kountouras J, Doulberis M, Papaefthymiou A, Polyzos SA, Vardaka E, Tzivras D, Dardiotis E, Deretzi G, Giartza-Taxidou E, Grigoriadis S, Katsinelos P. A perspective on risk factors for esophageal adenocarcinoma: emphasis on Helicobacter pylori infection. Ann N Y Acad Sci. 2019;1452:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 102. | Erőss B, Farkas N, Vincze Á, Tinusz B, Szapáry L, Garami A, Balaskó M, Sarlós P, Czopf L, Alizadeh H, Rakonczay Z Jr, Habon T, Hegyi P. Helicobacter pylori infection reduces the risk of Barrett's esophagus: A meta-analysis and systematic review. Helicobacter. 2018;23:e12504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 103. | Gao H, Li L, Zhang C, Tu J, Geng X, Wang J, Zhou X, Jing J, Pan W. Systematic Review with Meta-analysis: Association of Helicobacter pylori Infection with Esophageal Cancer. Gastroenterol Res Pract. 2019;2019:1953497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 104. | Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1413-1417, 1417.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 105. | Vaezi MF, Falk GW, Peek RM, Vicari JJ, Goldblum JR, Perez-Perez GI, Rice TW, Blaser MJ, Richter JE. CagA-positive strains of Helicobacter pylori may protect against Barrett's esophagus. Am J Gastroenterol. 2000;95:2206-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 106. | Jones AD, Bacon KD, Jobe BA, Sheppard BC, Deveney CW, Rutten MJ. Helicobacter pylori induces apoptosis in Barrett's-derived esophageal adenocarcinoma cells. J Gastrointest Surg. 2003;7:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 107. | Ferrández A, Benito R, Arenas J, García-González MA, Sopeña F, Alcedo J, Ortego J, Sainz R, Lanas A. CagA-positive Helicobacter pylori infection is not associated with decreased risk of Barrett's esophagus in a population with high H. pylori infection rate. BMC Gastroenterol. 2006;6:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 108. | Liu FX, Wang WH, Wang J, Li J, Gao PP. Effect of Helicobacter pylori infection on Barrett's esophagus and esophageal adenocarcinoma formation in a rat model of chronic gastroesophageal reflux. Helicobacter. 2011;16:66-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 109. | Abdel-Latif MM, Windle H, Terres A, Eidhin DN, Kelleher D, Reynolds JV. Helicobacter pylori extract induces nuclear factor-kappa B, activator protein-1, and cyclooxygenase-2 in esophageal epithelial cells. J Gastrointest Surg. 2006;10:551-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 110. | Ying GX, Wen Sheng LI, Xia ZL, Tao WH. CagA+ H. pylori filtrate induces cytokine IL-8 secretion by esophageal squamous carcinoma EC 109 cells via a p38 pathway. Indian J Pathol Microbiol. 2014;57:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 111. | Zu Y, Ping W, Deng T, Zhang N, Fu X, Sun W. Lipopolysaccharide-induced toll-like receptor 4 signaling in esophageal squamous cell carcinoma promotes tumor proliferation and regulates inflammatory cytokines expression. Dis Esophagus. 2017;30:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 112. | Ohadian Moghadam S, Momeni SA. Human microbiome and prostate cancer development: current insights into the prevention and treatment. Front Med. 2021;15:11-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 113. | Al-Marhoon MS, Ouhtit A, Al-Abri AO, Venkiteswaran KP, Al-Busaidi Q, Mathew J, Al-Haddabi I, Shareef O, Aquil S, Rahman K, Al-Hashmi I, Gupta I, Ganguly SS. Molecular Evidence of Helicobacter Pylori Infection in Prostate Tumors. Curr Urol. 2015;8:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 114. | Banerjee S, Alwine JC, Wei Z, Tian T, Shih N, Sperling C, Guzzo T, Feldman MD, Robertson ES. Microbiome signatures in prostate cancer. Carcinogenesis. 2019;40:749-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 115. | Liu JM, Wu CT, Hsu RJ, Hsu WL. Association between Helicobacter pylori infection and mortality risk in prostate cancer patients receiving androgen deprivation therapy: A real-world evidence study. Cancer Med. 2021;10:8162-8171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 116. | Isogai H, Isogai E, Kimura K, Fujii N, Yokota K, Oguma K. Helicobacter pylori induces inflammation in mouse urinary bladder and pelvis. Microbiol Immunol. 1994;38:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 117. | van den Bosch J, Kropman RF, Blok P, Wijermans PW. Disappearance of a mucosa-associated lymphoid tissue (MALT) lymphoma of the urinary bladder after treatment for Helicobacter pylori. Eur J Haematol. 2002;68:187-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 118. | Garbas K, Zapała P, Zapała Ł, Radziszewski P. The Role of Microbial Factors in Prostate Cancer Development-An Up-to-Date Review. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 119. |
Archibugi L, Signoretti M, Capurso G. The Microbiome and Pancreatic Cancer: An Evidence-based Association?
J Clin Gastroenterol. 2018; |
| 120. | Kustrimovic N, Bombelli R, Baci D, Mortara L. Microbiome and Prostate Cancer: A Novel Target for Prevention and Treatment. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |