Published online Aug 28, 2024. doi: 10.3748/wjg.v30.i32.3755
Revised: July 11, 2024
Accepted: August 2, 2024
Published online: August 28, 2024
Processing time: 220 Days and 17.1 Hours
Primary hyperparathyroidism (PHPT)-induced acute pancreatitis (AP) during pregnancy has rarely been described. Due to this rarity, there are no diagnostic or treatment algorithms for pregnant patients.
To determine appropriate diagnostic methods, therapeutic options, and factors related to maternal and fetal outcomes for PHPT-induced AP in pregnancy.
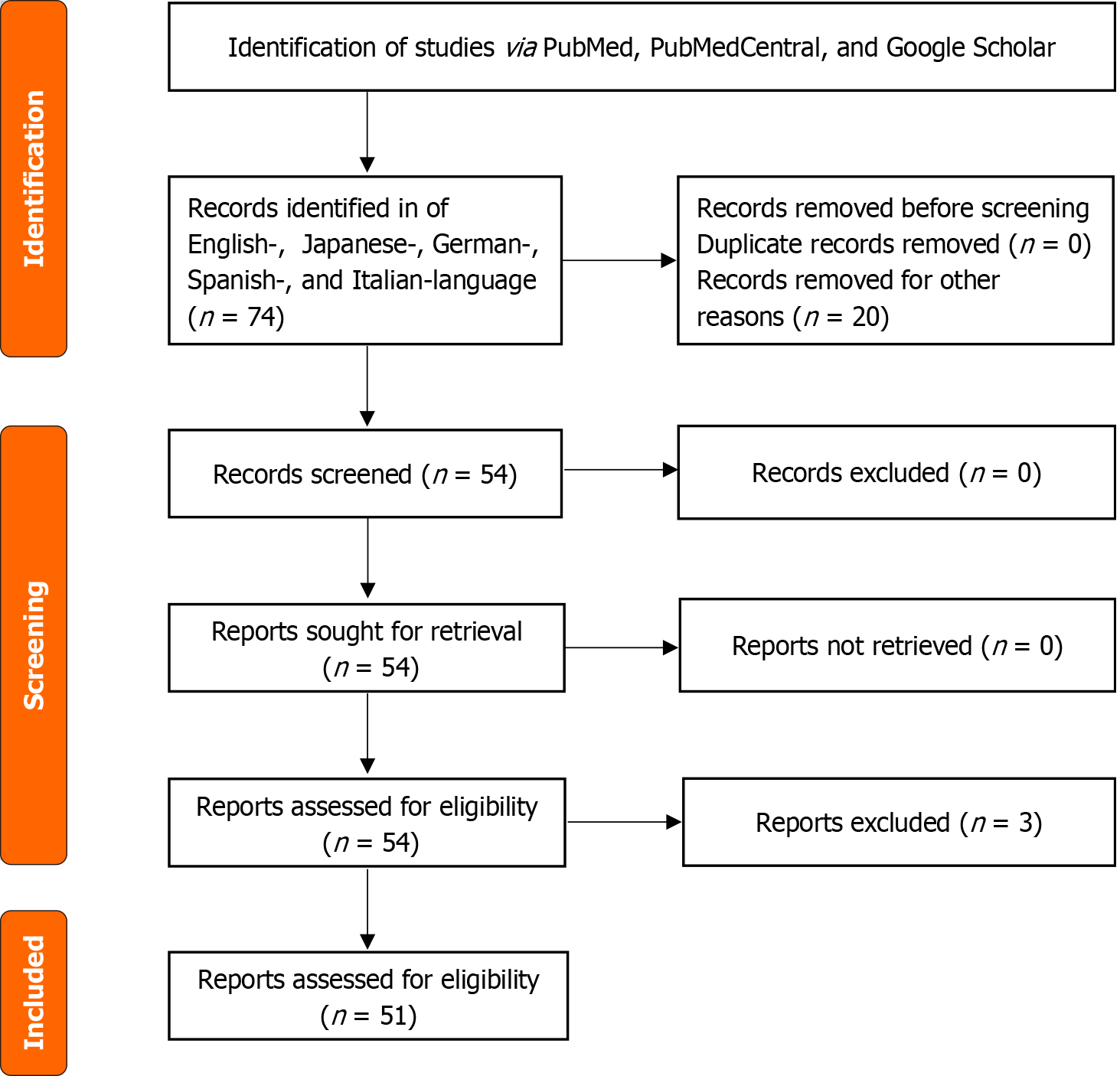
A literature search of articles in English, Japanese, German, Spanish, and Italian was performed using PubMed (1946-2023), PubMed Central (1900-2023), and Google Scholar. The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) protocol was followed. The search terms included “pancreatite acuta,” “iperparatiroidismo primario,” “gravidanza,” “travaglio,” “puerperio,” “postpartum,” “akute pankreatitis,” “primärer hyperparathyreoidismus,” “Schwangerschaft,” “Wehen,” “Wochenbett,” “pancreatitis aguda,” “hiperparatiroidismo primario,” “embarazo,” “parto,” “puerperio,” “posparto,” “acute pancreatitis,” “primary hyperparathyroidism,” “pregnancy,” “labor,” “puerperium,” and “postpartum.” Additional studies were identified by reviewing the reference lists of retrieved studies. Demographic, imaging, surgical, obstetric, and outcome data were obtained.
Fifty-four cases were collected from the 51 studies. The median maternal age was 29 years. PHPT-induced AP starts at the 20th gestational week; higher gestational weeks were seen in mothers who died (mean gestational week 28). Median values of amylase (1399, Q1-Q3 = 519-2072), lipase (2072, Q1-Q3 = 893-2804), serum calcium (3.5, Q1-Q3 = 3.1-3.9), and parathormone (PTH) (384, Q1-Q3 = 123-910) were reported. In 46 cases, adenoma was the cause of PHPT, followed by 2 cases of carcinoma and 1 case of hyperplasia. In the remaining 5 cases, the diagnosis was not reported. Neck ultrasound was positive in 34 cases, whereas sestamibi was performed in 3 cases, and neck computed tomography or magnetic resonance imaging was performed in 9 cases (the enlarged parathyroid gland was not localized in 3 cases). Surgery was the preferred treatment during pregnancy in 33 cases (median week of gestation 25, Q1-Q3 = 20-30) and postpartum in 12 cases. The timing was not reported in the remaining 9 cases, or surgery was not performed. AP was managed surgically in 11 cases and conservatively in 43 (79.6%) cases. Maternal and fetal mortality was 9.3% (5 cases). Surgery was more common in deceased mothers (60.0% vs 16.3%; P = 0.052), and PTH values tended to be higher in this group (910 pg/mL vs 302 pg/mL; P = 0.059). Maternal mortality was higher with higher serum lipase levels and earlier delivery week. Higher calcium (4.1 mmol/L vs 3.3 mmol/L; P = 0.009) and PTH (1914 pg/mL vs 302 pg/mL; P = 0.003) values increased fetal/child mortality, as well as abortions (40.0% vs 0.0%; P = 0.007) and complex deliveries (60.0% vs 8.2%; P = 0.01).
If serum calcium is not tested during admission, definitive diagnosis of PHPT-induced AP in pregnancy is delayed, while early diagnosis and immediate intervention lead to excellent maternal and fetal outcomes.
Core Tip: Primary hyperparathyroidism (PHPT)-induced acute pancreatitis (AP) in pregnancy is extremely rare. Definitive diagnosis of PHPT-induced AP in pregnancy is delayed if serum calcium is not tested during admission. PHPT-induced AP starts at the 20th gestational week. Maternal and fetal mortality was 9.3%. Maternal mortality was higher with higher serum lipase levels and earlier delivery week. Higher calcium and PTH values increased fetal/child mortality, abortions, and complex deliveries Early diagnosis and immediate intervention lead to excellent maternal and fetal outcomes.
- Citation: Augustin G, Lai Q, Cigrovski Berkovic M. Primary hyperparathyroidism-induced acute pancreatitis in pregnancy: A systematic review with a diagnostic-treatment algorithm. World J Gastroenterol 2024; 30(32): 3755-3765
- URL: https://www.wjgnet.com/1007-9327/full/v30/i32/3755.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i32.3755
Acute pancreatitis (AP) during pregnancy is uncommon but can lead to severe maternal and fetal adverse outcomes. The most prominent causes of AP, due to the increasing parturition age, rising overweight/obesity rates, and dietary fat content, include cholelithiasis and dyslipidemia-hypertriglyceridemia[1]. Primary hyperparathyroidism (PHPT) in preg-nancy is rare, with a reported incidence of 1.4%[2]. Maternal and fetal/neonatal complications occur in 67% and 80% of untreated cases, respectively[3]. Maternal complications include nephrolithiasis, AP, hyperemesis gravidarum, preeclampsia, and hypercalcemic crises. Fetal complications include intrauterine growth restriction, preterm delivery, and a 3-fold to 5-fold increased risk of miscarriage. There is a direct relationship between the degree of hypercalcemia and miscarriage risk, with miscarriage being more common in patients with serum calcium greater than 2.85 mmol/L[3,4].
PHPT during pregnancy is often overseen because specific signs and symptoms related to hypercalcemia, especially if not severe, can be mistaken for normal pregnancy. Hyperemesis gravidarum is the most common symptom, and nephrolithiasis and AP are important hypercalcemia-related complications. Diagnosis of PHPT requires elevated serum calcium values (serum ionized calcium or calcium adjusted for albumin) and parathormone (PTH), either elevated or non-suppressed for the level of calcemia[3]. The management of PHPT-induced AP, due to lack of strong evidence, is still elusive and further complicates decision-making on the best approach to AP during pregnancy and puerperium.
Although four “systematic” reviews collecting 15 to 26 cases were published, there are still no recommendations and guidelines for this condition during pregnancy. This is the largest systematic review of PHPT-induced AP in pregnancy (54 cases) of literature published over 55 years. The core of this review is summarized using knowledge on the topic, focusing on the diagnostic approach, type, and timing of interventions, and a proposed diagnostic-treatment algorithm.
Following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) and Meta-Analysis of Observational Studies in Epidemiology guidelines[5], we performed a systematic review of articles in English, Japanese, German, Spanish, and Italian using PubMed, PubMed Central, and Google Scholar (Figure 1). The search items included “pancreatite acuta,” “iperparatiroidismo primario,” “gravidanza,” “travaglio,” “puerperio,” “postpartum,” “akute pankreatitis,” “primärer hyperparathyreoidismus,” “Schwangerschaft,” “Wehen,” “Wochenbett,” “Wochenbett,” “pancreatitis aguda,” “hiperparatiroidismo primario,” “embarazo,” “parto,” “puerperio,” “posparto,” “acute pancreatitis,” “primary hyperparathyroidism,” “pregnancy,” “labor,” “puerperium,” and “postpartum.” Additional studies were identified by reviewing the reference lists of retrieved studies. Articles in languages other than English were translated using the websites deepl.com and Google Translate.
We included all cases and case series identified as having a PHPT-induced AP during pregnancy or puerperium (Supplementary material). Exclusion criteria were: (1) AP of other causes; (2) Patients not fulfilling the definition of pregnancy or puerperium; and (3) Incomplete data or unavailable full-text articles. The primary outcome was to identify associations of demographic data (age), obstetric data (trimester of pregnancy, parity), maternal risk factors (e.g., previous pancreatitis episodes, miscarriage), serum calcium and PTH levels, and pancreatitis severity, diagnostic tools for diagnosing AP (ultrasound, abdominal computed tomography [CT], abdominal magnetic resonance imaging [MRI]/magnetic resonance cholangiopancreatography) and PHPT (neck ultrasound, 99 mTc-sestamibi scintigraphy, neck CT/MRI), therapeutic intervention related to AP and PHPT (surgical, pharmacological), timing of surgery with maternal and fetal outcomes. The study is exempt from ethics approval because the data was procured from previously published studies.
A data extraction form was prepared and piloted to determine if changes were required before extracting data from the full review. Two authors (Augustin G and Cigrovski Berkovic M) independently performed data extraction following the PRISMA guidelines for data extraction and quality assessment. In addition, the examiners assessed the studies’ methodologies according to the tool for evaluating the methodological quality of case reports and case series described by Murad et al[6].
The data extracted were as follows: Publication year, maternal age, obstetric history (parity, the number of prior Cesarean sections), gestational age at presentation or postpartum day at presentation, symptoms, diagnostic modalities, treatment approaches, gestation age at delivery and delivery type, and maternal/fetal outcome. The data collected were available from the included studies. However, several characteristics were missing from more than half of the data.
Categorical data are reported as absolute numbers with percentages, and continuous data as medians with first-third quartile (Q1-Q3) ranges. Data were pooled, and descriptive statistics were produced from the dataset. A pseudo-individual participant data meta-analysis (pseudo-Individual Participant Data Meta-Analysis [IPDMA]) was conducted to synthesize clinical study evidence. IPDMA represents a specific type of systematic review in which, rather than extracting aggregate data from study publications, the original research data are obtained directly from the researchers responsible for each study. These data can then be re-analyzed centrally and combined in meta-analyses. Overall, pseudo-IPDMA analyses can improve the quality of data and the type of analyses that can produce more reliable results. For this reason, this method is considered the ‘gold standard’ of systematic review. In this specific setting, as all variables of interest were available from the articles, a pseudo-IPDMA was created. In other terms, the authors of the original articles were not directly recontacted, but all the information available from the papers was adequate for creating a pseudo-individual baseline and outcomes dataset. Categorical variables were compared with the χ2 or Fischer’s exact test, and the continuous variables with the Mann-Whitney test. Multivariable logistic regression analyses were also realized. Odds ratios (OR) and 95% confidence interval (95%CI) were reported. P < 0.05 was considered statistically significant. All analyses were performed using SPSS 27.0 (SPSS Inc., Chicago, IL, United States).
This study included 54 cases from 51 studies published between 1968 and 2023. All of the studies are case reports or small case series.
All reported cases had PHPT-induced AP during pregnancy or immediately after delivery. The median maternal age was 29 (Q1-Q3 = 26-32) years. Median values of amylase (1399 IU/L, Q1-Q3 = 519-2072 IU/L), lipase (2072 IU/L, Q1-Q3 = 893-2804 IU/L), serum calcium (3.5 mmol/L, Q1-Q3 = 3.1-3.9), and PTH (384 pg/mL, Q1-Q3 = 123-910 pg/mL) were reported.
In 41 cases, imaging for identifying the cause of hyperparathyroidism was reported. Neck ultrasounds identified the cause of PHPT in 34 cases (82.9%), rarely by 99 mTc-sestamibi scintigraphy (n = 3) and CT/MRI (n = 1). In 3 cases, the imaging was negative. Neck surgery was performed during pregnancy in 33 cases (median gestational week = 25, Q1-Q3 = 20-30). In 12 cases, surgery was performed after delivery. The timing was not reported in the remaining 9 cases, or surgery was not performed. In 46 cases, an adenoma was identified, followed by 2 cases of carcinoma and 1 of hyperplasia. In the remaining 5 cases, no histological characteristics of the cause of hyperparathyroidism were specified, nor surgery was performed. In 5 (9.3%) cases, maternal death was reported. Two groups were compared according to the maternal outcome (Table 1). No statistical differences were found between the groups regarding the mother’s age, number of previous pregnancies, number of previous deliveries, and pregnancy week in which AP was diagnosed.
| Variables | Entire cohort, n = 54, 100.0% | Mother alive, n = 49, 90.7% | Mother died, n = 5, 9.3% | P value |
| Age in years | 29 (26-32) | 29 (27-32) | 26 (24-33) | 0.25 |
| Previous pregnancies | 2 (1-2) | 2 (1-2) | 2 (1-2) | 0.56 |
| Previous deliveries | 1 (0-1) | 1 (0-1) | 1 (1-2) | 0.35 |
| Pancreatitis occurrence in gestational week | 27 (20-33) | 26 (20-33) | 28 (21-40) | 0.38 |
| Pancreatitis postpartum | 4 (7.4) | 3 (6.1) | 1 (20.0) | 0.33 |
| Surgical management | 11 (20.4) | 8 (16.3) | 3 (60.0) | 0.052 |
| Amylase in IU/L | 1399 (519-2072) | 1415 (510-2006) | 1200 (642-3091) | 0.66 |
| Lipase in IU/L | 2072 (893-2804) | 2072 (869-2521) | 3320 (855-6997) | 0.31 |
| Calcium in mmol/L | 3.5 (3.1-3.9) | 3.5 (3.1-3.8) | 3.1 (2.5-4.7) | 0.73 |
| Phosphorus in mmol/L | 0.7 (0.7-0.7) | 0.7 (0.7-0.7) | 0.7 (0.7-0.8) | 0.50 |
| PTH in pg/mL | 384 (123-910) | 302 (122-910) | 910 (541-5665) | 0.059 |
| Abortion | 2 (3.7) | 1 (2.0) | 1 (20.0) | 0.18 |
| Complex delivery | 7 (13.0) | 6 (12.2) | 1 (20.0) | 0.52 |
| Delivery occurrence week | 36 (36-38) | 36 (36-39) | 36 (23-37) | 0.09 |
| Fetal/child death | 5 (9.3) | 4 (8.2) | 1 (20.0) | 0.40 |
Although not statistically significant, the indication to manage AP surgically merged the statistical significance with a greater number of patients requiring surgery in the group of mothers experiencing death (60.0% vs 16.3%; P = 0.052). No differences between the groups were reported regarding serum calcium, phosphorus, amylase, and lipase levels reported at the time of AP. PTH values were higher in the group with maternal death, although only merging statistical relevance (910 pg/mL vs 302 pg/mL; P = 0.059). No differences were observed in terms of the number of abortions, complex deliveries, and fetal/child deaths. Delivery tended to be during earlier gestation in cases of maternal death, but the difference did not reach statistical significance (P = 0.09).
A total of 53 children were born: Twins in 1 case and abortion in 2. There were 21 vaginal deliveries and 21 cesarean sections (unknown type of delivery for additional cases). A higher number of vaginal deliveries compared to cesarean sections was observed before 2000 (38.1% vs 19.0%). The median child gestational age at delivery was 36 (Q1-Q3 = 36-38). The child’s sex was reported in 30 cases: 20 females and 10 males. The child’s weight was reported in 30 cases, with a median weight of 2583 gr (Q1-Q3 = 2083-3010 gr). The Apgar score at birth was reported in 22 cases with a median of 8 (Q1-Q3 = 6-9).
Fetal mortality was 9.3% (5 cases): Two miscarriages and three child deaths after the delivery. Two groups were compared according to the outcome of the fetus/child (Table 2). No statistical differences were observed in the two groups regarding the mother’s age, number of previous pregnancies, and number of previous deliveries. The pregnancy week in which the AP was diagnosed was earlier for the group with fetal/child death, although not statistically significant (16 weeks vs 28 weeks; P = 0.08). The need to manage AP surgically did not correlate with the risk of fetal/child death.
| Variables | Entire cohort, n = 54, 100.0% | Child alive, n = 49, 90.7% | Fetal/child death, n = 5, 9.3% | P value |
| Age in years | 29 (26-32) | 29 (26-32) | 28 (28-36) | 0.77 |
| Previous pregnancies | 2 (1-2) | 2 (1-2) | 2 (1-7) | 0.98 |
| Previous deliveries | 1 (0-1) | 1 (0-1) | 1 (1-5) | 0.52 |
| Pancreatitis occurrence week | 27 (20-33) | 28 (22-33) | 16 (13-26) | 0.08 |
| Pancreatitis post-partum | 4 (7.4) | 4 (8.2) | 0 (-) | 1.00 |
| Surgical management | 11 (20.4) | 10 (20.4) | 1 (20.0) | 0.73 |
| Amylase in IU/L | 1399 (519-2072) | 1500 (510-2072) | 1113 (752-2004) | 0.89 |
| Lipase in IU/L | 2072 (893-2804) | 2072 (869-2808) | 1415 (751-5664) | 0.95 |
| Calcium in mmol/L | 3.5 (3.1-3.9) | 3.3 (3.1-3.8) | 4.1 (3.8-4.7) | 0.009 |
| Phosphorus in mmol/L | 0.7 (0.7-0.7) | 0.7 (0.7-0.7) | 0.7 (0.5-0.8) | 0.89 |
| PTH in pg/mL | 384 (123-910) | 302 (122-910) | 1914 (910-3330) | 0.003 |
| Abortion | 2 (3.7) | 0 (-) | 2 (40.0) | 0.007 |
| Complex delivery | 7 (13.0) | 4 (8.2) | 3 (60.0) | 0.01 |
| Delivery week | 36 (36-38) | 36 (36-39) | 34 (19-39) | 0.12 |
| Mother death | 5 (9.3) | 4 (8.2) | 1 (20.0) | 0.40 |
No differences between the groups were reported regarding serum phosphorus, amylase, and lipase levels at the time of AP. Calcium (4.1 mmol/L vs 3.3 mmol/L; P = 0.009) and PTH (1914 pg/mL vs 302 pg/mL; P = 0.003) values were statistically higher in the group with fetal/child death. Miscarriages (40.0% vs 0.0%; P = 0.007) and complex deliveries (60.0% vs 8.2%; P = 0.01) increased fetal/child mortality. A delivery week and the mother’s death did not influence fetal mortality. In detail, only 1 case of contemporary death of the mother and the fetal abortion was reported. In the other 4 cases of the mother’s death, the child survived.
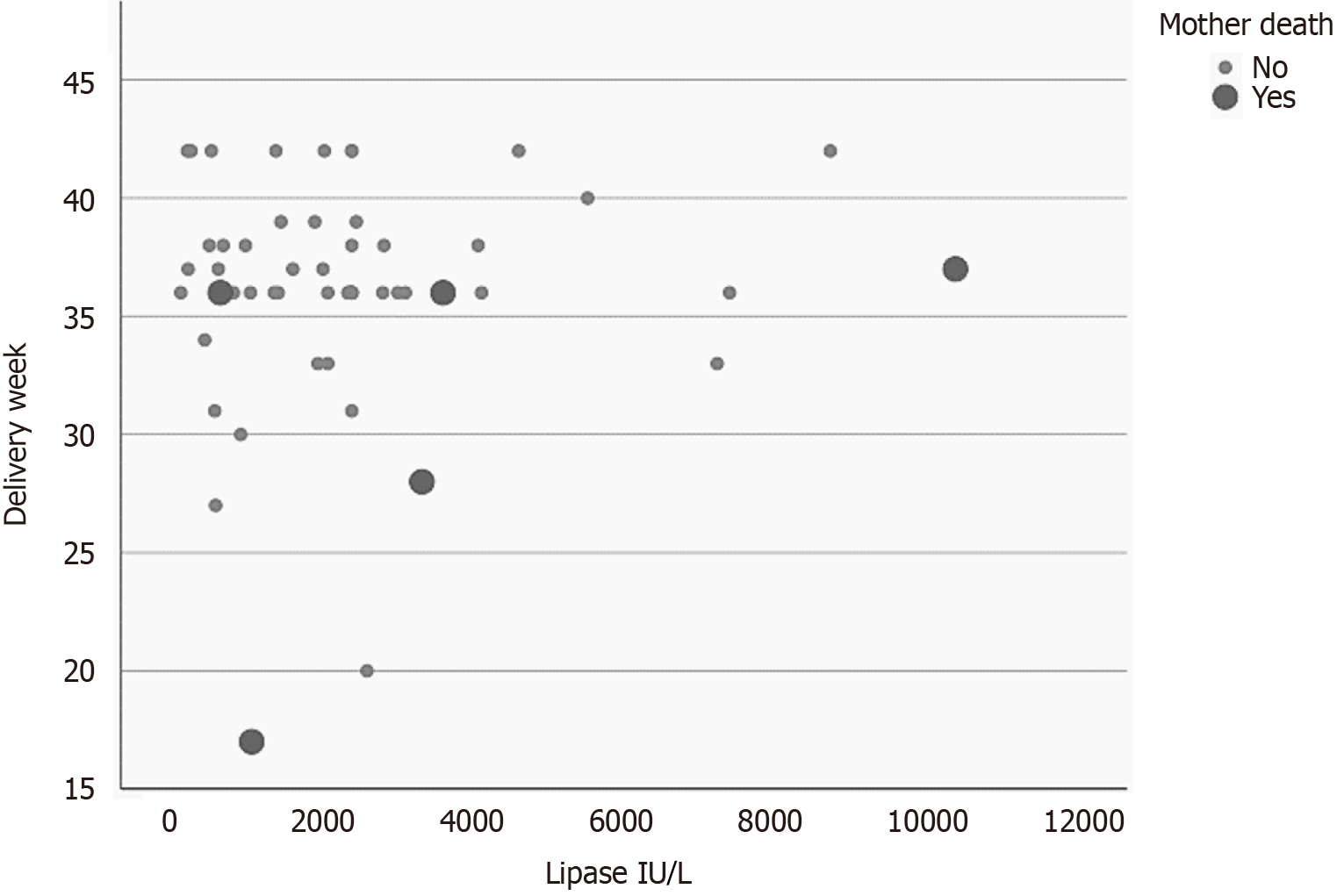
A multivariable logistic regression model analyzed the risk of maternal mortality according to the pseudo-IPDMA data (Table 3). The value of serum lipase at the time of AP (OR = 1.001, 95%CI: 1.00-1.001; P = 0.03) and the delivery week (OR = 0.81, 95%CI: 0.66-0.99; P = 0.04) were the only independent parameters. The number of previous pregnancies and deliveries only merged the statistical relevance (P = 0.051 for both). A plot correlating maternal mortality to the values of serum lipase and delivery week is presented in Figure 2.
| Variables | Beta | SE | Wald | OR | Lower | Upper | P value |
| Mother death | |||||||
| Lipase in IU/L | 0.001 | < 0.001 | 4.63 | 1.001 | 1.00 | 1.001 | 0.03 |
| Delivery week | -0.21 | 0.10 | 4.46 | 0.81 | 0.66 | 0.99 | 0.04 |
| Previous pregnancies | -2.61 | 1.34 | 3.80 | 0.07 | 0.01 | 1.01 | 0.051 |
| Previous deliveries | 3.22 | 1.65 | 3.80 | 25.12 | 0.98 | 64.68 | 0.051 |
| Constant | 4.15 | 3.29 | 1.59 | 63.37 | - | - | 0.21 |
| Fetal/child death | |||||||
| Complex delivery | 5.25 | 2.31 | 5.18 | 11.31 | 2.07 | 17.67 | 0.02 |
| Pancreatitis occurrence in pregnancy week | -0.25 | 0.13 | 3.84 | 0.78 | 0.61 | 1.00 | 0.05 |
| Constant | 1.32 | 1.81 | 0.53 | 3.73 | - | - | 0.47 |
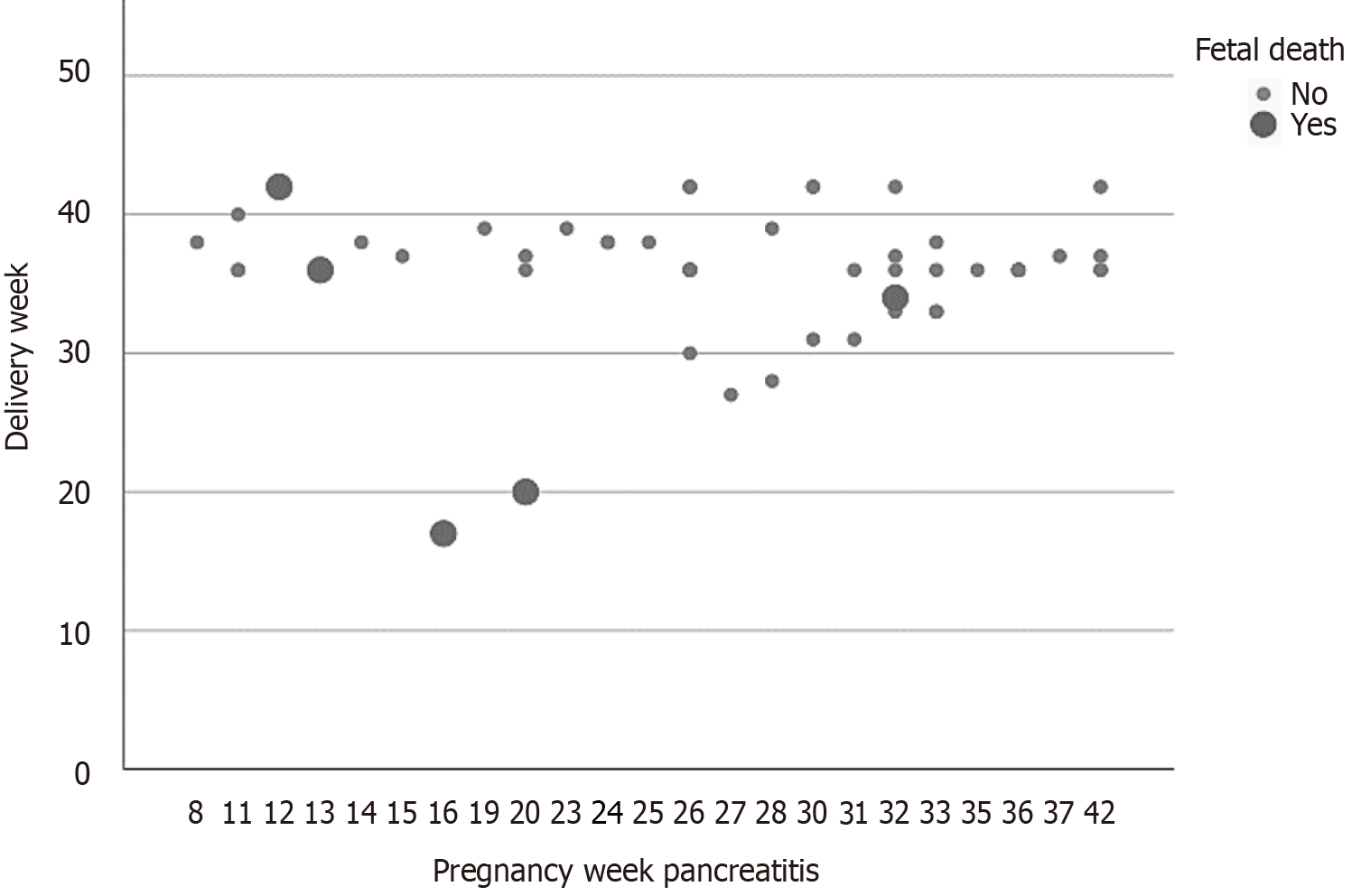
The complex delivery was the only independent risk factor for fetal/child mortality or miscarriage (OR = 11.31, 95%CI: 2.07-17.67; P = 0.02). Only gestational week of AP occurrence merged statistical relevance (OR = 0.78, 95%CI: 0.61-1.00; P = 0.05) (Table 3). A plot correlating fetal/child death to the pregnancy week at the time of AP diagnosis is presented in Figure 3.
PHPT arises from the overproductive parathyroid adenoma or, less often, parathyroid hyperplasia, or carcinoma, characterized by signs and symptoms of hypercalcemia[7]. It occurs mainly sporadic in women over 50 years and is usually asymptomatic. In 3%-5%, it occurs at a younger age as a part of multiple endocrine neoplasia syndromes[8,9]. Hence, although PHPT in pregnant women should prompt investigation of syndromic hypercalcemia and genetic testing, it is rarely done in clinical practice. Most nonpregnant PHPT patients have serum calcium from 1 to 1.5 mg/dL (0.25 to 0.375 mmol/L) above the upper normal limit, accompanied by an elevated or inappropriately normal (nonsupressed) PTH level. By contrast, calcium levels in pregnant women with PHPT are reportedly much higher, and those greater than 2.85 mmol/L correlate with a higher probability of maternal and fetal complications[3,10,11]. The clinical manifestations of PHPT are diverse and include hypercalcemia-related gastrointestinal, neuromuscular, renal, and psychological signs and symptoms, which can be overseen during pregnancy and, therefore, postpone the correct diagnosis or calcium level determination[12]. Due to its rarity, PHPT-induced AP during pregnancy is a diagnostic and therapeutic challenge[1].
Hence, a case series of PHPT during pregnancy reported from a single center between 2000 and 2015 suggested calcemia < 2.85 mmol/L could be managed conservatively in the absence of AP or other hypercalcemia-related symptoms[9]. According to the guidelines for PHPT management in the nonpregnant population, surgery is the preferred treatment for: (1) Symptomatic disease; (2) Calcium levels > 0.25 mmol/L (> 1 mg/dL) above the upper limit; or (3) Complications such as nephrolithiasis, osteoporosis or AP[8]. Due to the lack of randomized controlled trials, there are no clear recommendations for the management of PHPT during pregnancy or PHPT-induced AP. Currently, the main practice is to operate PHPT during pregnancy in case of persistent hypercalcemia above 2.75 mmol/L or symptomatic hypercalcemia. However, some advocate surgery in the second trimester regardless of serum calcium levels[13-18]. Conservative treatment options are limited and lack clear guidance. They rely on intravenous fluids and forced diuresis with furosemide, calcitonin subcutaneous (limited use due to tachyphylaxis), and cinacalcet (lacking long-term safety data). By contrast, intravenous bisphosphonate use is debatable due to potentially negative effects on fetal bone development[14].
A further issue is the assessment of AP severity during pregnancy and its relation to treatment decisions. Different scoring systems in nonpregnant populations rely on imaging, such as CT, commonly avoided during pregnancy. Several recent articles described the case of PHPT-induced AP in pregnancy and made a “review of the literature.” Unfortunately, all four reviews have less than half of the cases we collected. In 2014, Lee et al[19] collected 15 cases with a small number of analyzed parameters. There were no conclusions that help in dealing with this pathology except that it is rare, and clinicians should have a high level of suspicion. Liu et al[20] have “literature review” in their title but without a formal systematic or any type of review. It is not even a “narrative” review because many statements do not have references for statement confirmation. The best review was by Kongmalai[21] in 2021, but it had significant limitations. The first was that it collected only 23 cases. The second is a limited number of analyzed parameters with a minimal number of conclusions. In the most recent (2023) review, Zhou et al[22] collected 26 cases, while there were only 16 cases from 2000 onwards in the supplementary online material. Again, only several parameters were analyzed. The authors did a narrative review without the data/percentages from their collection of cases of pregnancy. For example, we do not know which imaging method has the best accuracy, sensitivity, and specificity for diagnosing PHPT in pregnancy. A similar goes to the treatment with a single conclusion based on 2 (carcinoma) cases. In conclusion, neither of these four reviews made a detailed analysis of laboratory, diagnostic, or therapeutic parameters. The most important drawback is the lack of diagnostic, treatment, or diagnostic-treatment algorithms. Therefore, within this review, it is presently the largest systematic review of PHPT-induced AP in pregnancy and postpartum. It proposes optimal diagnostic workups and treatment strategies, all of which are summarized in the diagnostic treatment algorithm.
PHPT-induced AP in pregnancy is exceedingly rare. We collected 54 cases, the highest collection of this pathology in pregnancy. The overall incidence of AP in pregnancy is estimated to be approximately 1 in 1000-10000 pregnancies[1,23]. The incidence of PHPT-induced AP cannot be determined due to the extreme rarity and unknown data on other causes of AP in pregnancy in these periods and countries.
Most AP cases result from parathyroid adenoma. The symptomatology starts after 20 weeks of gestation, similar to other, more frequent causes of AP (gallstones, hypertriglyceridemia)[1]. Physiologically, PTH during pregnancy falls to a low-normal range during the first trimester and rises to a mid-normal range by term. This follows the findings that AP occurs after 20 weeks, although adenoma PTH secretion is probably completely independent. Increasing intra-abdominal pressure as the pregnancy advances probably does not increase the probability of AP as in biliary AP due to biliary compression.
Clinical presentation of PHPT-induced AP included a spectrum of symptoms ranging from nausea, vomiting, hyperemesis gravidarum, acute abdomen, preeclampsia, hypertension, and behavioral changes to loss of consciousness. Confirmed PHPT before conception was described in 2 cases, while possible, undiagnosed PHPT presenting as AP, hypercalcemia and/or nephrolithiasis, all suggestive of undiagnosed PHPT, emerged in four and seven reported pregnancies, respectively.
PHPT-related AP was mostly diagnosed during pregnancy, beginning with 20 weeks of gestation. In 4 cases, previous pregnancies were complicated by AP, leading to multiple miscarriages. Mean calcium levels were 3.5 mmol/L, and the mean PTH was 384 pg/mL. Data on vitamin D (25-OH-D) were reported in 13 cases, and an average value was 52.81 nmol/L. A neck ultrasound was the first imaging method, and it detected parathyroid pathology in 34 patients (62.96%). Other imaging methods were 99 mTc-sestamibi scintigraphy (positive in 8/10 cases) and neck CT/MRI (positive in 8/10 cases). Ultrasound-negative but 99 mTc-sestamibi scintigraphy-positive finding was reported in 3 (5.55%) and ultrasound-negative but CT/MRI-positive finding of overactive parathyroid gland in 1 (1.85%) patients, respectively. In 3 patients, no imaging modality was useful in detecting the overactive parathyroid gland.
Histopathology confirmed parathyroid adenoma in 46 cases, followed by 2 cases of carcinoma and 1 case of hyperplasia. In the remaining 5 cases, no histological characteristics of the cause of hyperparathyroidism were specified, nor was surgery performed. Six studies reported AP severity (according to Glasgow, Ranson, or Balthazar criteria). Twenty-five cases involved abdominal ultrasound, while in 16 studies, abdominal CT and MRI/MRCP were performed. Workup for multiple endocrine neoplasia was done in 3 cases and reported negative.
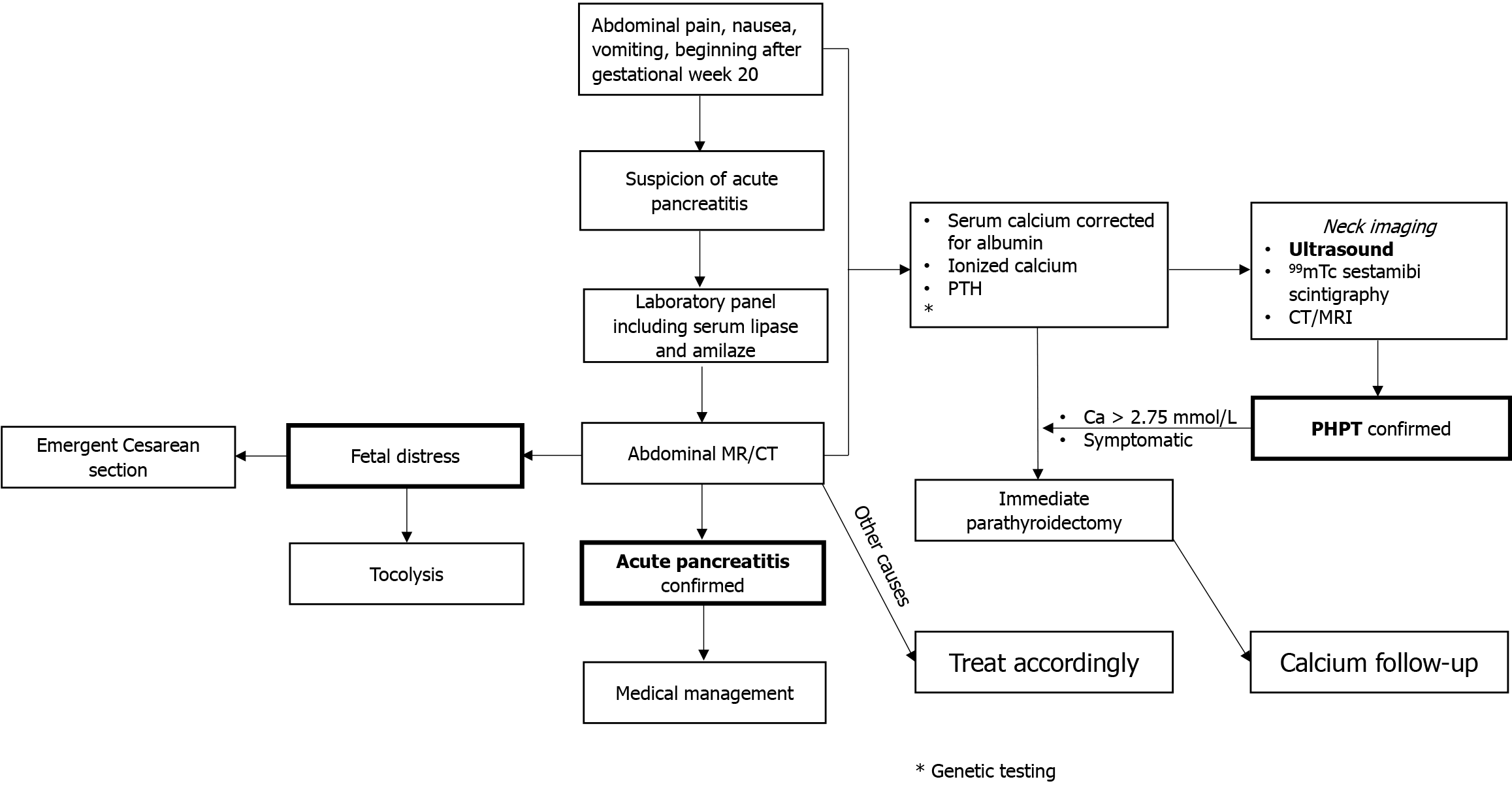
While there is no clear international consensus on the management of PHPT in pregnancy, a historical consensus is a parathyroidectomy in the second trimester for patients whose calcium persists above 2.75 mmol/L[24-26]. However, this approach is not evidence-based, and many of the complications and risks of severe hypercalcemia may occur in the first trimester, such as hyperemesis gravidarum, renal calculi, AP, and hypercalcemic crisis[13,14,27]. Moreover, prolonged hypercalcemia increases the risk of renal calculi, renal insufficiency, and osteoporosis. Neck surgery was performed during pregnancy in 33 cases (median week = 25, Q1-Q3 = 20-30). In 12 cases, surgery was performed after delivery. The timing was not reported in the remaining 9 cases, or surgery was not performed. Conservative treatment with a eucalcemic diet and aggressive hydration often fails[28]. Moreover, guidelines on pharmacotherapy are still lacking. However, calcitonin, cinecalcet, and intravenous bisphosphonates (zolendronate) have been used. Still, the results are either modest due to tachyphylaxis (calcitonin) or insufficient long-term safety data (cinacalcet and intravenous bisphosphonates). Therefore, symptomatic cases (AP) and patients with calcium levels above 2.75 mmol/L should undergo parathyroidectomy (Figure 4). The treatment of AP should not differ from other causes of AP and should not be influenced by pregnancy.
A total of 53 children were born. In 1 case, a delivery of twins was reported, while 2 cases of miscarriages were described. The type of delivery was described in 42 cases: 21 vaginal deliveries and 21 cesarean sections. A major number of vaginal deliveries vs cesarean sections was observed before the year 2000 (38.1% vs 19.0%). The median child gestational age at delivery was 36 weeks (Q1-Q3 = 36-38). The child’s sex was reported in 30 cases: 20 females and 10 males. The child’s weight was reported in 30 cases, with a median weight of 2583 gr (Q1-Q3 = 2083-3010 gr). The Apgar score at birth was reported in 22 cases: The median value was 8 (Q1-Q3 = 6-9). Regarding children’s complications, the most reported was transient hypocalcemia. In short, the earlier treatment of PHPT and resolution of AP, the higher proportion of term pregnancies delivered vaginally.
Maternal outcome: Maternal mortality due to PHPT-induced AP is 9.3%, while maternal mortality from other causes of AP during pregnancy dropped to 2.8%[23,29,30]. PHPT is a single-cause condition resulting in AP. With the cause elimination, the AP resolves. AP resolution decreases the risk of maternal mortality. This is confirmed by the fact that the value of serum lipase at the time of AP (OR = 1.001, 95%CI: 1.00-1.001; P = 0.03) and the delivery week (OR = 0.81, 95%CI: 0.66-0.99; P = 0.04) were the only independent parameters increasing maternal mortality. As for post-parathyroidectomy complications, hungry bone syndrome was described in 3 and transient hypocalcemia in 2 cases.
Neonatal outcome: Neonatal survival is excellent, with 9.3% mortality. Outcomes of other causes of AP are improving but are still 12.3%[1,28-30]. The median weight was 2583 gr (Q1-Q3 = 2083-3010 gr), and the median Apgar score at birth was 8 (Q1-Q3 = 6-9). PHPT is a single-cause condition of hypercalcemia resulting in AP. The AP and hypercalcemia were resolved by eliminating the cause (PHPT treatment). AP resolution decreases the risk of fetal mortality. This is confirmed by the fact that higher calcium (4.1 mmol/L vs 3.3 mmol/L; P = 0.009) and PTH (1914 pg/mL vs 302 pg/mL; P = 0.003) values increase fetal mortality. Transient hypocalcemia might occur; therefore, calcium serum levels should be monitored.
Limitations of the study: Despite the highest number of cases collected, these were published over 55 years. Older studies have limited value today, primarily in diagnostic and partly management aspects. PTH essays differ significantly over 55 years. Older essays also measured inactive PTH units, so false values could be presented. Conservative treatment is of limited use, with no randomized controlled trials and long-term safety data and no reliable comparison between the outcomes related to treatment options: Conservative vs operative.
PHPT-induced AP patients have serum calcium levels above 3.5 mmol/L. Symptomatic PHPT cases and patients with calcium levels above 2.75 mmol/L should undergo semi-urgent parathyroidectomy. PHPT-induced AP starts at the 20th gestational week. Parathyroidectomy immediately normalizes serum calcium values, leading to AP resolution. Maternal and fetal survival is excellent with AP caused by PHPT, although transient hypocalcemia (maternal and fetal) can be expected.
| 1. | Mądro A. Pancreatitis in Pregnancy-Comprehensive Review. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 2. | Kelly TR. Primary hyperparathyroidism during pregnancy. Surgery. 1991;110:1028-33; discussion 1033. [PubMed] |
| 3. | McCarthy A, Howarth S, Khoo S, Hale J, Oddy S, Halsall D, Fish B, Mariathasan S, Andrews K, Oyibo SO, Samyraju M, Gajewska-Knapik K, Park SM, Wood D, Moran C, Casey RT. Management of primary hyperparathyroidism in pregnancy: a case series. Endocrinol Diabetes Metab Case Rep. 2019;2019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Ali DS, Dandurand K, Khan AA. Primary Hyperparathyroidism in Pregnancy: Literature Review of the Diagnosis and Management. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Brooke BS, Schwartz TA, Pawlik TM. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021;156:787-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 393] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 6. | Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1008] [Cited by in RCA: 1533] [Article Influence: 219.0] [Reference Citation Analysis (0)] |
| 7. | Bilezikian JP, Cusano NE, Khan AA, Liu JM, Marcocci C, Bandeira F. Primary hyperparathyroidism. Nat Rev Dis Primers. 2016;2:16033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 8. | Wilhelm SM, Wang TS, Ruan DT, Lee JA, Asa SL, Duh QY, Doherty GM, Herrera MF, Pasieka JL, Perrier ND, Silverberg SJ, Solórzano CC, Sturgeon C, Tublin ME, Udelsman R, Carty SE. The American Association of Endocrine Surgeons Guidelines for Definitive Management of Primary Hyperparathyroidism. JAMA Surg. 2016;151:959-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 612] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 9. | Rigg J, Gilbertson E, Barrett HL, Britten FL, Lust K. Primary Hyperparathyroidism in Pregnancy: Maternofetal Outcomes at a Quaternary Referral Obstetric Hospital, 2000 Through 2015. J Clin Endocrinol Metab. 2019;104:721-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Bilezikian JP, Brandi ML, Eastell R, Silverberg SJ, Udelsman R, Marcocci C, Potts JT Jr. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99:3561-3569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 795] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 11. | Silva BC, Cusano NE, Bilezikian JP. Primary hyperparathyroidism. Best Pract Res Clin Endocrinol Metab. 2018;32:593-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 12. | Shifrin A. Advances in Diagnosis and Management of Primary Hyperparathyroidism During Pregnancy. In: Advances in Treatment and Management in Surgical Endocrinology. Amsterdam: Elsevier, 2020: 125-127. |
| 13. | Latif A, Gastelum AA, Farhan K, Jagadesh S, Mutnuri S. Treatment approach for primary hyperparathyroidism in pregnancy. Proc (Bayl Univ Med Cent). 2020;34:191-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Kort KC, Schiller HJ, Numann PJ. Hyperparathyroidism and pregnancy. Am J Surg. 1999;177:66-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Australia's Mothers and Babies 2015-in Brief. Canberra: Australian Institute of Health, 2017. |
| 16. | Hong MK, Hsieh CT, Chen BH, Tu ST, Chou PH. Primary hyperparathyroidism and acute pancreatitis during the third trimester of pregnancy. J Matern Fetal Med. 2001;10:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Kondo Y, Nagai H, Kasahara K, Kanazawa K. Primary hyperparathyroidism and acute pancreatitis during pregnancy. Report of a case and a review of the English and Japanese literature. Int J Pancreatol. 1998;24:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Inabnet WB, Baldwin D, Daniel RO, Staren ED. Hyperparathyroidism and pancreatitis during pregnancy. Surgery. 1996;119:710-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Lee CC, Chao AS, Chang YL, Peng HH, Wang TH, Chao A. Acute pancreatitis secondary to primary hyperparathyroidism in a postpartum patient: a case report and literature review. Taiwan J Obstet Gynecol. 2014;53:252-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Liu Y, Wang JN, Huang Y, Zhu YH, Liu RL, Xu CF, Li X. Acute pancreatitis and preeclampsia induced by parathyroid adenoma in pregnancy: a case report and literature review. Int J Clin Exp Med. 2016;9:22652-22655. |
| 21. | Kongmalai T. Recurrent Acute Pancreatitis in Pregnancy Caused by Parathyroid Hyperplasia: A Case Report and Literature Review. J Med Assoc Thai. 2021;. [DOI] [Full Text] |
| 22. | Zhou Y, Wang Q, Zou L, Wu X, Yang A. Parathyroid carcinoma-related severe acute pancreatitis during pregnancy: a case report and literature review. Gastroenterol Rep (Oxf). 2023;11:goac087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Niu C, Zhang J, Liu H, Zhu K, Okolo PI 3rd. Maternal and fetal outcomes of acute pancreatitis in pregnancy: a population-based study. Eur J Gastroenterol Hepatol. 2023;35:1354-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Brodsky JB, Cohen EN, Brown BW Jr, Wu ML, Whitcher C. Surgery during pregnancy and fetal outcome. Am J Obstet Gynecol. 1980;138:1165-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 149] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Beck AJ, Reddy VM, Sulkin T, Browne D. Management of severe and symptomatic primary hyperparathyroidism in the first trimester of unplanned pregnancy. Endocrinol Diabetes Metab Case Rep. 2022;2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Diaz-Soto G, Linglart A, Sénat MV, Kamenicky P, Chanson P. Primary hyperparathyroidism in pregnancy. Endocrine. 2013;44:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Sharma SG, Levine SN, Yatavelli RK, Shaha MA, Nathan CAO. Parathyroidectomy in First Trimester of Pregnancy. J Endocr Soc. 2020;4:bvaa015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Malekar-Raikar S, Sinnott BP. Primary hyperparathyroidism in pregnancy-a rare cause of life-threatening hypercalcemia: case report and literature review. Case Rep Endocrinol. 2011;2011:520516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Tang M, Xu JM, Song SS, Mei Q, Zhang LJ. What may cause fetus loss from acute pancreatitis in pregnancy: Analysis of 54 cases. Medicine (Baltimore). 2018;97:e9755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Hughes DL, Hughes A, White PB, Silva MA. Acute pancreatitis in pregnancy: meta-analysis of maternal and fetal outcomes. Br J Surg. 2021;109:12-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |