Published online Aug 21, 2024. doi: 10.3748/wjg.v30.i31.3628
Revised: July 18, 2024
Accepted: July 23, 2024
Published online: August 21, 2024
Processing time: 145 Days and 18.5 Hours
This editorial comments on the manuscript by Chang et al, focusing on the still elusive interplay between epigenetic regulation and autophagy in gastrointestinal diseases, particularly cancer. Autophagy, essential for cellular homeostasis, exhibits diverse functions ranging from cell survival to death, and is particularly implicated in physiological gastrointestinal cell functions. However, its role in pathological backgrounds remains intricate and context-dependent. Studies underscore the dual nature of autophagy in cancer, where its early suppressive effects in early stages are juxtaposed with its later promotion, contributing to chemoresistance. This discrepancy is attributed to the dysregulation of autopha
Core Tip: Understanding the intricate interplay between autophagy, epigenetics, and non-coding RNA (ncRNA) regulation in gastrointestinal (GI) cancers is crucial for devising effective therapeutic interventions. Autophagy exhibits a dual role in cancer progression, impacting treatment response rates, and its modulation through epigenetic alterations and ncRNA regulation offers promising avenues for targeted therapies. Developing strategies to manipulate autophagy, particularly via epigenetic mechanisms and nanocarrier-based ncRNA delivery systems, holds immense potential to enhance the effecti
- Citation: Ramoni D, Carbone F, Montecucco F. Navigating the autophagic landscape: Epigenetic modulation in gastrointestinal cancer. World J Gastroenterol 2024; 30(31): 3628-3634
- URL: https://www.wjgnet.com/1007-9327/full/v30/i31/3628.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i31.3628
Chang et al[1] emphasize a fundamental subject to understand human pathology and review the research progress of autophagy in benign and malignant gastrointestinal (GI) diseases. Autophagy, a catabolic process involving the consumption and recycling of proteins and organelles, plays a crucial role in maintaining cellular homeostasis. It is worth noting that autophagy itself is not a mechanism of cell death. However, it has been observed to accompany cell death induced by conditions such as nutrient starvation or hypoxia[2]. This process has been extensively implicated in the growth, development, and various physiological functions of the GI tract, including mucosal barrier integrity[3], secretion[4], and cell regeneration across different GI cell types[5].
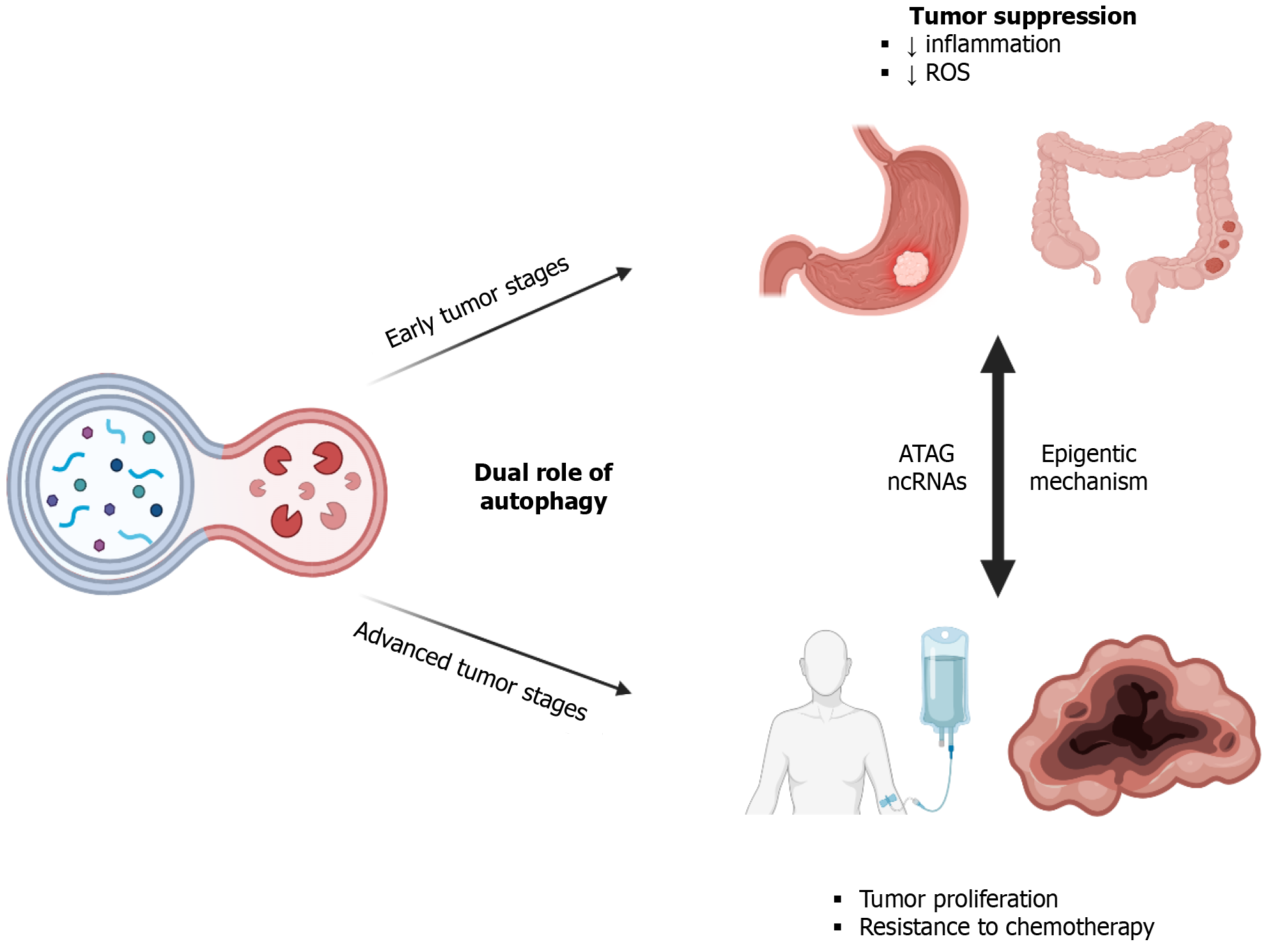
In pathological contexts, however, the role of autophagy may vary widely. This discrepancy could stem from differences in autophagic activity levels or the influence of other contributing factors, although the precise mechanisms remain poorly understood. Notably, evidence suggests that while autophagy may impede the progression of GI tumors in early stages, it could also confer resistance to chemotherapy in advanced stages[6] (Figure 1). The underlying reasons for these dual effects remain elusive, but emerging evidence points toward the involvement of epigenetic changes, which may modulate various genes at different stages of cancer, thereby influencing responses to major therapeutic interventions. Given the significant recurrence and mortality rates associated with GI malignancies, a deeper comprehension of this interplay holds promise for the development of novel therapeutic strategies.
Autophagy plays a complex and multifaceted role in cancer development and progression, exhibiting both tumor-suppressive and tumor-promoting effects at different stages of the disease. This duality can lead to varying responses to anticancer drugs in a phase-dependent manner[7]. The reasons behind the variability of the same phenomenon at different stages of the disease are still under investigation, but some hypotheses have been proposed. Varying responses are related not only to regulation by oncogenes and tumor suppressors, but also to heterogeneity that is driven both by cell-specific and tissue-specific factors. To date, researchers have identified approximately 40 autophagy-related genes (ATGs) implicated in the formation of autophagosomes, double-membrane structures responsible for degrading damaged organelles[8,9]. The dysregulation of these genes, whether through over- or under-expression, directly influences tumor proliferation. Notably, studies have revealed the significance of the ATG BECN1, encoding Beclin 1, a crucial molecule for formation and maturation of autophagosomes. Specifically, it has been demonstrated that the E3 ubiquitin ligase CUL3 (cullin 3) can interact with BECN1, a widely recognized tumor-suppressor gene. This interaction mediates the K48-linked ubiquitination of BECN1 at sites K53, K185, and K270, leading to its subsequent proteasomal degradation via the ubiquitin-proteasome pathway. The Kelch-BTB domain protein, KLHL38, is essential for the recognition and interaction between CUL3 and BECN1. Through the degradation of BECN1, the CUL3-KLHL38 complex inhibits autophagic flux by downregulating BECN1, thereby promoting cell proliferation and predicting an unfavorable prognosis in various spectra of human malignancies[10]. Additionally, investigations have highlighted the tumor-suppressive functions of other key autophagy genes, including the ultraviolet radiation resistance-associated gene (UVRAG) and Bax interacting factor-1 (Bif-1), which interact with BECN1 in its tumor-suppressive capacity. Bif-1 serves as a key activator of class III phos
Autophagy, though inherently a cellular response to both internal and external stimuli, can become intricately intertwined with the life cycle of tumor cells, affording them protection against the cytotoxic effects of chemotherapy[15,16]. Moreover, autophagy can sequester organelles within the cell, hindering the expression of pro-apoptotic proteins and shielding the tumor from apoptosis. Notably, inhibition of autophagy appears to mitigate chemoresistance in tumor cells, whereas its overexpression inversely impacts chemotherapy response rates. Consequently, autophagy inhibition itself exerts a pro-apoptotic influence on GI tumor cells, underscoring the intimate interplay between autophagy and apoptosis in either promoting or suppressing tumorigenesis[17].
In addition to other factors contributing to poor therapeutic responses observed in GI cancers, epigenetic modifications emerge as critical factors warranting consideration. Epigenetic modifications encompass chemical alterations of DNA or chromatin that do not alter the primary DNA sequence, thereby inducing phenotypic changes[18]. Key modifications in the pathophysiology of GI malignancies include epigenetic mechanisms such as histone modification and DNA methylation. Furthermore, the regulation of gene expression by non-coding RNAs (ncRNAs)[19,20], functional RNA molecules that are not translated into proteins, but wield considerable influence over DNA methylation and chromatin organization, modulate the expression of numerous genes. However, while the pivotal role of ncRNAs in the path
ncRNAs, particularly microRNAs (miRNAs), exert regulatory control over autophagy flux[21,22], targeting gene expression at both chromatin organization levels, either co- or post-transcriptionally, differing from other epigenetic modifications. This modulation can significantly impact treatment response rates, depending on whether autophagy is inhibited or overexpressed[23]. Moreover, the presence of these regulatory ncRNAs has been detected in whole blood, indicating their potential for intercellular transfer[24]. Extensive investigations have focused on the association between aberrant ncRNA expression and autophagy in the progression and resistance of GI tumors[25,26], with some ncRNAs explored for diagnostic and prognostic purposes[27,28].
MiRNAs have been classified as oncogenes (called oncomiRs) or tumor-suppressor miRNAs, and both target mRNA of ATGs rather than ATG proteins directly. Multiple instances illustrate the modulation of different cancers by miRNAs. Among the most important, the oncogenic miR-423-3p activates BECN1-dependent autophagy, promoting gastric tumor progression by reducing the expression of Bcl-2-interacting mediator of cell death (Bim). Specifically, Bim inhibits autophagy by recruiting Beclin-1 to microtubules, mediated through the bridging of Beclin-1 and Dynein light chain LC8[29]. Studies have demonstrated that autophagy triggered by the miR-423-3p-Bim pathway relies on Beclin-1[30]. When Bim is overexpressed, it significantly curtails the proliferation and invasion of gastric cancer cells, accompanied by a reduction in microtubule-associated protein LC3 levels. This suggests that the miR-423-3p-Bim pathway could be a regulator of autophagy in cancer cells. On the other hand, ATG7, which is similar to E1 ubiquitin-activating enzymes, is crucial for the maturation stage of autophagy. The deletion of ATG7 prevents the proliferation of gastric cancer cells driven by miR-423-3p[30].
Moreover, both in vitro and in vivo studies have shown that miR-181a plays a role in regulating autophagy in gastric malignancies by targeting ATG5, contributing to drug response. Overexpression of miR-181a significantly reduces autophagic activity. Since ATG5 is crucial for the formation of autophagosomes, its reduction leads to impaired autophagy. Introducing a miR-181a mimic significantly lowers ATG5 mRNA, while inhibiting endogenous miR-181a significantly increases ATG5[31]. Many chemotherapy and targeted therapy drugs can induce autophagy, which appears to act as a survival mechanism for cancer cells. Blocking this protective autophagy could prevent drug resistance[32]. In fact, overexpression of miR-181a appears to counteract cisplatin resistance and enhance chemosensitivity[31].
In esophageal carcinoma, the tumor-suppressor miR-382 inhibits the mTOR signaling pathway and promotes autophagy processes, inducing apoptosis and suppressing tumor proliferation and invasion[33]. Additionally, miR-30d inhibits cell proliferation in hepatocellular carcinoma by reducing autophagy and targeting ATG5 and BECN1[34], while acting similarly in colon cancer cell[35]. MiR-372 suppresses pancreatic cancer cell proliferation and invasion by directly targeting the autophagy-initiating kinase ULK1. The activity of the ULK1 complex is inhibited by mTOR signaling, and miR-372 induces the loss of phosphorylation in ULK1, potentially through the inhibition of autophagic cell death, thereby playing a tumor-suppressor role[36]. Furthermore, miR-183 enhances autophagy and UVRAG-induced apoptosis in colorectal cancer, with its inhibition suppressing tumor growth in vivo[37].
Understanding the role of epigenetic regulation in autophagy is crucial to identify specific pharmacological targets and develop potential antitumor treatments. Consequently, the debate persists regarding whether inducing or inhibiting autophagy should be prioritized in cancer therapy. In this context, two approaches can be considered. One strategy involves inhibiting the cytoprotective functions of autophagy to enhance the response rate of chemotherapeutic agents. Transcriptional suppression of ATGs like those mentioned above may be considered a potential strategy to induce chemosensitivity by inhibiting autophagy in GI cancer cells. Another method is pharmacological autophagy inhibition using approved agents like quinolones, which effectively hinder autophagosome-lysosome fusion[38]. Alternatively, autophagic cell death can be triggered through autophagy induction, impacting ATGs via certain chemotherapeutic drugs, such as docetaxel, 5-fluorouracil (5-FU), and paclitaxel. 5-FU directly activates AMPK and p53[39], while docetaxel and paclitaxel directly increase the expression of BECN1[40,41]. Based on these observations, targeting epigenetic regulation of autophagy is pivotal to counteract or reverse chemoresistance in gastrointestinal cancer.
For instance, doxorubicin resistance in hepatocellular carcinoma cells is observed following autophagy activation consequent to the downregulation of miR-223[42]. Conversely, inhibition of autophagy and sensitization of gastric cancer cells to docetaxel are achieved through the upregulation of miR-361-5p[43]. Additionally, epigenetic alterations that activate autophagy, consequently leading to 5-FU resistance in colon-rectal cancer, include the upregulation of miR-125b[44], as well as the downregulation of miR-361[45]. Instead, oxaliplatin resistance in colon-rectal cancer is induced by the activation of autophagy through the downregulation of miR-409-3p by inhibiting Beclin-1[46] and miR-27b-3p[47].
Despite significant improvements in understanding the mechanisms and implications of autophagy in cancer, numerous gaps in knowledge remain. A principal gap is the heterogeneity of autophagy regulation across different cancers. This heterogeneity is not fully understood and poses a challenge in developing universal autophagy-targeting therapies. Autophagy can be either upregulated or downregulated in various cancers, impacting tumor progression and treatment responses differently. Another critical gap is the bidirectional influence between autophagy and the tumor microenvi
Among potential therapeutic strategies, regulating autophagy should be considered as one approach for future therapy. In addition to previously reported miRNA manipulation, innovative technology aimed at enhancing treatment effectiveness involves the use of nanocarriers designed to encapsulate various drugs and deliver them efficiently. Scientists develop nanoparticles to deliver miRNA in hepatocellular carcinoma cells, demonstrating an increased suppressive effect of miR-26a[49]. Similarly, the utilization of protamine sulfate-nanodiamond nanoparticles to delivering miRNA-203 into esophageal cancer cells demonstrates the enhanced efficacy of this delivery system[50]. Recently, polymeric nanoparticles were developed for the release of miR-204-5p in colon cancer cells, observing efficient inhibitory effects on cell proliferation[51]. Utilizing nanoparticles as delivery systems for miRNA modulation of autophagy presents a promising therapeutic approach for gastrointestinal cancer therapy.
Autophagy significantly influences the efficacy of several chemotherapy drugs in GI cancers, either enhancing or hindering therapy responses depending on the cellular stage it impacts. A comprehensive understanding of the mechanisms governing autophagy, particularly regarding epigenetic phenomena and ncRNA regulation, holds the potential to advance the development of therapeutic strategies that improve the response rate of malignancies with poor prognoses.
| 1. | Chang YF, Li JJ, Liu T, Wei CQ, Ma LW, Nikolenko VN, Chang WL. Morphological and biochemical characteristics associated with autophagy in gastrointestinal diseases. World J Gastroenterol. 2024;30:1524-1532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Reference Citation Analysis (0)] |
| 2. | Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5421] [Cited by in RCA: 5293] [Article Influence: 311.4] [Reference Citation Analysis (0)] |
| 3. | Wu Y, Tang L, Wang B, Sun Q, Zhao P, Li W. The role of autophagy in maintaining intestinal mucosal barrier. J Cell Physiol. 2019;234:19406-19419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Naama M, Bel S. Autophagy-ER stress crosstalk controls mucus secretion and susceptibility to gut inflammation. Autophagy. 2023;19:3014-3016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Yang Y, Gomez M, Marsh T, Poillet-Perez L, Sawant A, Chen L, Park NR, Jackson SR, Hu Z, Alon N, Liu C, Debnath J, Guan JL, Davidson S, Verzi M, White E. Autophagy in PDGFRα+ mesenchymal cells is essential for intestinal stem cell survival. Proc Natl Acad Sci U S A. 2022;119:e2202016119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Ávalos Y, Canales J, Bravo-Sagua R, Criollo A, Lavandero S, Quest AF. Tumor suppression and promotion by autophagy. Biomed Res Int. 2014;2014:603980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 7. | Shafabakhsh R, Arianfar F, Vosough M, Mirzaei HR, Mahjoubin-Tehran M, Khanbabaei H, Kowsari H, Shojaie L, Azar MEF, Hamblin MR, Mirzaei H. Autophagy and gastrointestinal cancers: the behind the scenes role of long non-coding RNAs in initiation, progression, and treatment resistance. Cancer Gene Ther. 2021;28:1229-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Tamargo-Gómez I, Fernández ÁF, Mariño G. Pathogenic Single Nucleotide Polymorphisms on Autophagy-Related Genes. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Dupont N, Nascimbeni AC, Morel E, Codogno P. Molecular Mechanisms of Noncanonical Autophagy. Int Rev Cell Mol Biol. 2017;328:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Li X, Yang KB, Chen W, Mai J, Wu XQ, Sun T, Wu RY, Jiao L, Li DD, Ji J, Zhang HL, Yu Y, Chen YH, Feng GK, Deng R, Li JD, Zhu XF. CUL3 (cullin 3)-mediated ubiquitination and degradation of BECN1 (beclin 1) inhibit autophagy and promote tumor progression. Autophagy. 2021;17:4323-4340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 11. | Xie T, Li SJ, Guo MR, Wu Y, Wang HY, Zhang K, Zhang X, Ouyang L, Liu J. Untangling knots between autophagic targets and candidate drugs, in cancer therapy. Cell Prolif. 2015;48:119-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer. 2020;19:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 1091] [Article Influence: 218.2] [Reference Citation Analysis (0)] |
| 13. | Bhutia SK, Mukhopadhyay S, Sinha N, Das DN, Panda PK, Patra SK, Maiti TK, Mandal M, Dent P, Wang XY, Das SK, Sarkar D, Fisher PB. Autophagy: cancer's friend or foe? Adv Cancer Res. 2013;118:61-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 14. | Katheder NS, Khezri R, O'Farrell F, Schultz SW, Jain A, Rahman MM, Schink KO, Theodossiou TA, Johansen T, Juhász G, Bilder D, Brech A, Stenmark H, Rusten TE. Microenvironmental autophagy promotes tumour growth. Nature. 2017;541:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 358] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 15. | Cicchini M, Karantza V, Xia B. Molecular pathways: autophagy in cancer--a matter of timing and context. Clin Cancer Res. 2015;21:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Zada S, Hwang JS, Ahmed M, Lai TH, Pham TM, Elashkar O, Kim DR. Cross talk between autophagy and oncogenic signaling pathways and implications for cancer therapy. Biochim Biophys Acta Rev Cancer. 2021;1876:188565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Mowers EE, Sharifi MN, Macleod KF. Autophagy in cancer metastasis. Oncogene. 2017;36:1619-1630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 375] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 18. | Sui X, Zhu J, Zhou J, Wang X, Li D, Han W, Fang Y, Pan H. Epigenetic modifications as regulatory elements of autophagy in cancer. Cancer Lett. 2015;360:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Baylin SB, Jones PA. Epigenetic Determinants of Cancer. Cold Spring Harb Perspect Biol. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 836] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 20. | Cheng Y, He C, Wang M, Ma X, Mo F, Yang S, Han J, Wei X. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduct Target Ther. 2019;4:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 478] [Cited by in RCA: 699] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 21. | Zhou C, Liang Y, Zhou L, Yan Y, Liu N, Zhang R, Huang Y, Wang M, Tang Y, Ali DW, Wang Y, Michalak M, Chen XZ, Tang J. TSPAN1 promotes autophagy flux and mediates cooperation between WNT-CTNNB1 signaling and autophagy via the MIR454-FAM83A-TSPAN1 axis in pancreatic cancer. Autophagy. 2021;17:3175-3195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 22. | Gao M, Li C, Xu M, Liu Y, Liu S. LncRNA UCA1 attenuates autophagy-dependent cell death through blocking autophagic flux under arsenic stress. Toxicol Lett. 2018;284:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Chowdhury SG, Karmakar P. Revealing the role of epigenetic and post-translational modulations of autophagy proteins in the regulation of autophagy and cancer: a therapeutic approach. Mol Biol Rep. 2023;51:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 24. | Makarova J, Turchinovich A, Shkurnikov M, Tonevitsky A. Extracellular miRNAs and Cell-Cell Communication: Problems and Prospects. Trends Biochem Sci. 2021;46:640-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 25. | Maleki M, Golchin A, Javadi S, Khelghati N, Morovat P, Asemi Z, Alemi F, Vaghari-Tabari M, Yousefi B, Majidinia M. Role of exosomal miRNA in chemotherapy resistance of Colorectal cancer: A systematic review. Chem Biol Drug Des. 2023;101:1096-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Talebian S, Daghagh H, Yousefi B, Ȍzkul Y, Ilkhani K, Seif F, Alivand MR. The role of epigenetics and non-coding RNAs in autophagy: A new perspective for thorough understanding. Mech Ageing Dev. 2020;190:111309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Shan C, Chen X, Cai H, Hao X, Li J, Zhang Y, Gao J, Zhou Z, Li X, Liu C, Li P, Wang K. The Emerging Roles of Autophagy-Related MicroRNAs in Cancer. Int J Biol Sci. 2021;17:134-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | Zhu X, Tian X, Yu C, Shen C, Yan T, Hong J, Wang Z, Fang JY, Chen H. A long non-coding RNA signature to improve prognosis prediction of gastric cancer. Mol Cancer. 2016;15:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 29. | Luo S, Garcia-Arencibia M, Zhao R, Puri C, Toh PP, Sadiq O, Rubinsztein DC. Bim inhibits autophagy by recruiting Beclin 1 to microtubules. Mol Cell. 2012;47:359-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 30. | Kong P, Zhu X, Geng Q, Xia L, Sun X, Chen Y, Li W, Zhou Z, Zhan Y, Xu D. The microRNA-423-3p-Bim Axis Promotes Cancer Progression and Activates Oncogenic Autophagy in Gastric Cancer. Mol Ther. 2017;25:1027-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Zhao J, Nie Y, Wang H, Lin Y. MiR-181a suppresses autophagy and sensitizes gastric cancer cells to cisplatin. Gene. 2016;576:828-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 32. | Mele L, Del Vecchio V, Liccardo D, Prisco C, Schwerdtfeger M, Robinson N, Desiderio V, Tirino V, Papaccio G, La Noce M. The role of autophagy in resistance to targeted therapies. Cancer Treat Rev. 2020;88:102043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 33. | Feng J, Qi B, Guo L, Chen LY, Wei XF, Liu YZ, Zhao BS. miR-382 functions as a tumor suppressor against esophageal squamous cell carcinoma. World J Gastroenterol. 2017;23:4243-4251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Fu XT, Shi YH, Zhou J, Peng YF, Liu WR, Shi GM, Gao Q, Wang XY, Song K, Fan J, Ding ZB. MicroRNA-30a suppresses autophagy-mediated anoikis resistance and metastasis in hepatocellular carcinoma. Cancer Lett. 2018;412:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 35. | Zhang R, Xu J, Zhao J, Bai J. Mir-30d suppresses cell proliferation of colon cancer cells by inhibiting cell autophagy and promoting cell apoptosis. Tumour Biol. 2017;39:1010428317703984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 36. | Chen H, Zhang Z, Lu Y, Song K, Liu X, Xia F, Sun W. Downregulation of ULK1 by microRNA-372 inhibits the survival of human pancreatic adenocarcinoma cells. Cancer Sci. 2017;108:1811-1819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Huangfu L, Liang H, Wang G, Su X, Li L, Du Z, Hu M, Dong Y, Bai X, Liu T, Yang B, Shan H. miR-183 regulates autophagy and apoptosis in colorectal cancer through targeting of UVRAG. Oncotarget. 2016;7:4735-4745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 38. | Kurdi A, Cleenewerck M, Vangestel C, Lyssens S, Declercq W, Timmermans JP, Stroobants S, Augustyns K, De Meyer GRY, Van Der Veken P, Martinet W. ATG4B inhibitors with a benzotropolone core structure block autophagy and augment efficiency of chemotherapy in mice. Biochem Pharmacol. 2017;138:150-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 39. | Tang JC, Feng YL, Liang X, Cai XJ. Autophagy in 5-Fluorouracil Therapy in Gastrointestinal Cancer: Trends and Challenges. Chin Med J (Engl). 2016;129:456-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Wang Q, He WY, Zeng YZ, Hossain A, Gou X. Inhibiting autophagy overcomes docetaxel resistance in castration-resistant prostate cancer cells. Int Urol Nephrol. 2018;50:675-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Xi G, Hu X, Wu B, Jiang H, Young CY, Pang Y, Yuan H. Autophagy inhibition promotes paclitaxel-induced apoptosis in cancer cells. Cancer Lett. 2011;307:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 42. | Zhou Y, Chen E, Tang Y, Mao J, Shen J, Zheng X, Xie S, Zhang S, Wu Y, Liu H, Zhi X, Ma T, Ni H, Chen J, Chai K, Chen W. miR-223 overexpression inhibits doxorubicin-induced autophagy by targeting FOXO3a and reverses chemoresistance in hepatocellular carcinoma cells. Cell Death Dis. 2019;10:843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 43. | Tian L, Zhao Z, Xie L, Zhu J. MiR-361-5p suppresses chemoresistance of gastric cancer cells by targeting FOXM1 via the PI3K/Akt/mTOR pathway. Oncotarget. 2018;9:4886-4896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 44. | Yu X, Shi W, Zhang Y, Wang X, Sun S, Song Z, Liu M, Zeng Q, Cui S, Qu X. CXCL12/CXCR4 axis induced miR-125b promotes invasion and confers 5-fluorouracil resistance through enhancing autophagy in colorectal cancer. Sci Rep. 2017;7:42226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 45. | Zhang L, Li B, Zhang B, Zhang H, Suo J. miR-361 enhances sensitivity to 5-fluorouracil by targeting the FOXM1-ABCC5/10 signaling pathway in colorectal cancer. Oncol Lett. 2019;18:4064-4073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Tan S, Shi H, Ba M, Lin S, Tang H, Zeng X, Zhang X. miR-409-3p sensitizes colon cancer cells to oxaliplatin by inhibiting Beclin-1-mediated autophagy. Int J Mol Med. 2016;37:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 47. | Sun W, Li J, Zhou L, Han J, Liu R, Zhang H, Ning T, Gao Z, Liu B, Chen X, Ba Y. The c-Myc/miR-27b-3p/ATG10 regulatory axis regulates chemoresistance in colorectal cancer. Theranostics. 2020;10:1981-1996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 48. | Camuzard O, Santucci-Darmanin S, Carle GF, Pierrefite-Carle V. Autophagy in the crosstalk between tumor and microenvironment. Cancer Lett. 2020;490:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 49. | Cai C, Xie Y, Wu L, Chen X, Liu H, Zhou Y, Zou H, Liu D, Zhao Y, Kong X, Liu P. PLGA-based dual targeted nanoparticles enhance miRNA transfection efficiency in hepatic carcinoma. Sci Rep. 2017;7:46250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Cao M, Deng X, Su S, Zhang F, Xiao X, Hu Q, Fu Y, Yang BB, Wu Y, Sheng W, Zeng Y. Protamine sulfate-nanodiamond hybrid nanoparticles as a vector for MiR-203 restoration in esophageal carcinoma cells. Nanoscale. 2013;5:12120-12125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Zheng B, Chen L, Pan CC, Wang JZ, Lu GR, Yang SX, Xue ZX, Wang FY, Xu CL. Targeted delivery of miRNA-204-5p by PEGylated polymer nanoparticles for colon cancer therapy. Nanomedicine (Lond). 2018;13:769-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |