Published online Jan 21, 2024. doi: 10.3748/wjg.v30.i3.252
Peer-review started: August 31, 2023
First decision: October 29, 2023
Revised: November 8, 2023
Accepted: December 18, 2023
Article in press: December 18, 2023
Published online: January 21, 2024
Processing time: 139 Days and 20 Hours
Ulcerative colitis (UC) is an inflammatory condition with frequent relapse and recurrence. Evidence suggests the involvement of SLC6A14 in UC pathogenesis, but the central regulator remains unknown.
To explore the role of SLC6A14 in UC-associated pyroptosis.
Quantitative real-time polymerase chain reaction (qRT-PCR), immunoblotting, and immunohistochemical were used to assess SLC6A14 in human UC tissues. Lipopolysaccharide (LPS) was used to induce inflammation in FHC and NCM460 cells and model enteritis, and SLC6A14 levels were assessed. Pyroptosis markers were quantified using enzyme-linked immunosorbent assay, Western blotting, and qRT-PCR, and EdU incubation, CCK-8 assays and flow cytometry were used to examine proliferation and apoptosis. Mouse models of UC were used for verification.
SLC6A14 was increased and correlated with NLRP3 in UC tissues. LPS-induced FHC and NCM460 cells showed increased SLC6A14 levels. Reducing SLC6A14 increased cell proliferation and suppressed apoptosis. Reducing SLC6A14 decreased pyroptosis-associated proteins (ASC, IL-1β, IL-18, NLRP3). NLRP3 overexpression counteracted the effects of sh-SLC6A14 on LPS-induced FHC and NCM460 cell pyroptosis. SLC6A14 improved the mucosa in mice with dextran sulfate sodium-induced colitis.
SLC6A14 promotes UC pyroptosis by regulating NLRP3, suggesting the therapeutic potential of modulating the SLC6A14/NLRP3 axis.
Core Tip: Ulcerative colitis (UC) is an inflammatory condition associated with frequent relapse and recurrence. Dysregulation of intestinal epithelial cells (IECs) and mucosal barrier impairment contribute to sustained inflammation in UC. Hence, an in-depth exploration of the triggers and mechanisms of IEC death could result in efficacious therapeutic options for UC patients. Here, we demonstrated the close involvement of SLC6A14 in promoting pyroptosis in the context of UC by upregulating NLRP3 expression. These findings indicate the potential of targeting SLC6A14/NLRP3 axis-mediated pyroptosis as a promising therapeutic strategy for treating UC. Our research provides valuable insights into the mechanisms driving UC pathogenesis and offers a possible direction for developing innovative treatments to alleviate the impact of this chronic inflammatory disorder.
- Citation: Gu Q, Xia H, Song YQ, Duan J, Chen Y, Zhang Y, Chen HP, Zhang L. SLC6A14 promotes ulcerative colitis progression by facilitating NLRP3 inflammasome-mediated pyroptosis. World J Gastroenterol 2024; 30(3): 252-267
- URL: https://www.wjgnet.com/1007-9327/full/v30/i3/252.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i3.252
Ulcerative colitis (UC) is an inflammatory condition associated with frequent relapse and recurrence. Its prevalence is increasing in developing nations as a result of increased consumption of Western diets[1,2]. UC is typified by ulceration and inflammation of the colonic and rectal mucosa, leading to symptoms such as pain, diarrhea, and bleeding[3,4]. UC has a complex etiology involving genetic, environmental, and immunological elements[5]. Dysregulation of intestinal epithelial cells (IECs) and mucosal barrier impairment contribute to sustained inflammation[6,7]. The death of IECs disrupts the balance between intestinal microorganisms and the host, the regulation of mucosal immunity, nutrient absorption, and the integrity of the mucosal barrier, culminating in recurrent, prolonged colitis[8,9]. Hence, an in-depth exploration of the triggers and mechanisms of IEC death could result in efficacious therapeutic options for UC patients.
Recent investigations underscore the vital role of NLRP3, the NLR family pyrin domain containing 3, inflammasome in UC[10,11]. This is activated in conditions of tissue damage, pathogen infection, and oxidative stress[12-14]. NLRP3 interacts with ASC through its N-terminal PYD domain, upregulating pro-IL-1β and pro-IL-18 and thus augmenting inflammation[15,16]. Consequently, NLRP3, in conjunction with IL-1β, IL-18, and ASC, has emerged as a prospective therapeutic target for ameliorating pyroptosis and mitigating inflammatory responses in UC.
The solute carrier (SLC) transporter family has been recognized as a significant regulator of ferroptosis and diverse cancers[17]. SLC6A14, SLC family 6 member 14, belongs to the SLC transporter family and may contribute to UC[18,19]. In this study, we investigated SLC6A14, which is upregulated in various colonic disorders, including UC, to evaluate its role in modulating IEC pyroptosis within the context of UC by using human IECs and murine models. Our findings revealed robust SLC6A14 expression in UC patients, experimental colitis models, and pyroptosis-induced cell models. Importantly, a positive correlation between SLC6A14 and NLRP3 expression was identified. Downregulating SLC6A14 reduced the levels of pyroptosis-associated proteins, including NLRP3, and the production of IL-18 and IL-1β. Mechanistically, SLC6A14 was a positive regulator of NLRP3, a central figure in inflammasome-driven pyroptosis. The promotion of pyroptosis by SLC6A14 by targeting NLRP3 was verified in LPS-treated IECs and a UC mouse model, indicating the potential of SLC6A14 in the treatment of UC.
Colorectal mucosal biopsies were procured from two groups: healthy individuals (n = 29) and patients diagnosed with UC (n = 55). These biopsies were obtained during endoscopic examinations conducted at the Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital [Lunshen (Yan)2022-380]. The study protocol was granted ethics approval by the Ethics Committee of the same institution and was performed in compliance with the Declaration of Helsinki (2013 revision). Prior to the extraction of the tissue samples, written informed consent was provided by each participant. The collected tissue specimens were promptly cryopreserved using liquid nitrogen for subsequent analysis.
Hematoxylin and eosin (HE) staining was performed using established protocols. In summary, murine and human colonic samples were initially fixed (4% paraformaldehyde) and embedded in paraffin before being sectioned (5 μm). The sections were stained with hematoxylin to visualize the nuclei, and eosin was used to reveal cytoplasmic structures.
Immunohistochemical (IHC) staining was performed as previously described using a DAB kit (Gene Tech, Shanghai, China). In summary, colonic tissue sections were treated overnight at 4 °C with an anti-SLC6A14 antibody (Abcam Cat# ab254786, RRID:AB_3073883) at a 1:200 dilution. Then, a subsequent incubation was conducted with an appropriate HRP-conjugated secondary antibody for 1 h at 37 °C. The reactions were visualized after incubation with DAB (brown) with hematoxylin counterstaining (purple). The prepared tissue sections were subsequently observed and imaged by phase-contrast microscopy (Leica, Germany). An evaluation of these images was performed by two independent blinded pathologists, and the slides were assessed by multiplying the staining intensity (ranging from grades 0 to 5, with 0 indicating negative and 5 indicating strong positivity) by the corresponding positivity score (ranging from 0 to 5, where 0% to 100% was indicated by the scores).
The normal colon epithelial cell lines FHC and NCM460 were sourced from the National Collection of Authenticated Cell Cultures (Shanghai, China). FHC and NCM460 cells were grown in RPMI-1640 medium (Gibco, United States) with 10% fetal bovine serum (Gibco). To establish a cellular model of colitis, the cells were incubated with 10 ng/mL lipopolysaccharide (LPS) for 6 h[20,21]. The cells were grown in 6-well plates at 37 °C and 5% CO2.
To downregulate SLC6A14, shRNA sequences targeting SLC6A14 and the negative control (NC) shRNA were obtained from GenePharma (Shanghai, China). Additionally, the NLRP3 overexpression plasmid pcDNA4.0-NLRP3 and the empty pcDNA4.0 plasmid were procured from Synbio Technologies Co. Ltd.™ (Suzhou, China). Cells were grown to 70% confluence and transfected with the plasmids using Lipofectamine 2000 (Thermo Fisher Scientific, United States) according to the provided directions. RNA was isolated after 48 h, and protein was isolated after 72 h.
SLC6A14 protein levels in cells and patient biopsy tissues were analyzed by Western blotting. Proteins were extracted from the samples using RIPA buffer containing protease and phosphatase inhibitors, and concentrations were assessed using a BCA kit (Thermo Fisher). Aliquots (30 μg) were separated on 10%-12% SDS-PAGE gels and transferred to PVDF membranes (Millipore, Bedford, MA, United States). Primary antibodies against SLC6A14 (Thermo Fisher Scientific Cat# PA5-87998, RRID:AB_2804576, 1:1500), NLRP3 (Abcam Cat# ab263899, RRID:AB_2889890, 1:1000), ASC (Abcam Cat# ab283684, RRID:AB_3073880, 1:800), pro-IL-18 (Proteintech Cat# 10663-1-AP, RRID:AB_2123636, 1:1000), IL-1β (Abcam Cat# ab254360, RRID:AB_2936299, 1:1000), IL-18 (Abcam Cat# ab207324, RRID:AB_3073881, 1:1000), and GAPDH (Abcam Cat# ab9485, RRID:AB_307275, 1:1000) were used to probe the blots overnight at 4 °C, after which the blots were incubated with secondary antibodies for 60 min at room temperature. Bands were visualized using the ECL Western Blotting Detection System (Amersham, United Kingdom) and quantified using ImageJ. The internal reference was GAPDH. Three separate experiments were conducted.
RNA was extracted from the samples using TRIzol reagent (Invitrogen, United States). cDNA was reverse transcribed using the Bestar qPCR RT kit (DBI Bioscience, #2220, Germany). To examine SLC6A14, the following primer sequences were used: forward primer 5’-TGCACCTGCTACCAGTCAAG-3’ and reverse primer 5’-GTCCATGGTTCACTCCCTCG-3’. The GAPDH primers were forward, 5’-GACAGTCAGCCGCATCTTCT-3’ and reverse, 5’-GCGCCCAATACGACCAAATC-3’. To assess SLC6A14 expression, Bestar qPCR MasterMix (DBI Bioscience, #2043) was used. GAPDH was used as the internal reference. The 2−ΔΔCt method was used to quantify gene expression according to established pro
Cell proliferation was assessed using CCK-8 assays (Dojindo Laboratories, Japan). Cells (2 × 103/well) were plated in 96-well plates and grown at 37 °C for 2 h. Absorbances at 450 nm were measured every 24 h for 72 h.
Cellular proliferation was assessed using an EdU assay kit (Ribobio, China). Cells were seeded on confocal plates at 10 × 105 cells per well. Next, the cultures were treated with 50 μM EdU solution for 120 min at 37 °C, after which they were fixed (4% formaldehyde, 30 min) and permeabilized (0.1% Triton X-100, 20 min) before the nuclei were stained with Hoechst and the samples were evaluated by fluorescence microscopy.
Apoptosis was assessed using flow cytometry. FHC and NCM460 cells were transfected and grown to 90% confluence in 6-well plates. The cells were harvested and treated with 10 μL of reagent from the Annexin V-FITC/PI apoptosis kit (Lianke Biotech, China) (10-15 min, room temperature, away from light). The cells were evaluated on a FACSCalibur flow cytometer (BD Biosciences, United States).
Culture supernatants and standards were incubated in precoated enzyme-linked immunosorbent assay (ELISA) plates for 2 h at 37 °C. This was followed by two washes and probing with an HRP-conjugated secondary antibody (1 h, 37 °C). After further rinsing, the plates were incubated with the chromogenic solution (10-15 min, room temperature, away from light). After the addition of the stop solution, the absorbances at 450 nm were measured. The specific antibodies used were anti-human IL-1β (Abcam, ab214025), anti-human IL-18 (Abcam, ab215539), anti-mouse IL-1β (Abcam, ab197742), and anti-mouse IL-18 (Abcam, ab216165). All assays were performed in triplicate.
Kunming (KM) mice (male, 20 ± 2 g) were sourced from the Experimental Animal Center of Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, China. All animal experiments were performed with the approval of the Animal Care and Use Committee of Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital (Lunshen (Yan)2022-380). The animals were maintained at a temperature of 22-24 °C with 20% humidity and a 12-h light/dark cycle. They were provided unrestricted access to standard feed and water. After one week of acclimatization, 48 mice were randomly assigned to four groups, with each group containing 12 mice. In Group 1, the mice were provided regular drinking water. Groups 2 to 4 were given drinking water containing 3% dextran sulfate sodium (DSS) (MW 36 kDa to 50 kDa, MP Biomedicals LLC, Santa Ana, CA, United States) for seven days to induce UC. Group 3 received 100 μL of control lentivirus (LV-sh-CTRL, obtained from GenePharma, Suzhou, China) via tail vein injection twice per week, while Group 4 was injected with 100 μL of the SLC6A14 knockdown lentivirus (LV-sh-SLC6A14, obtained from GenePharma) via tail vein injection twice per week. The mice were euthanized using 1% sodium pentobarbital (adminis
The animals were subjected to daily evaluations to gauge UC severity, and body weights, observable rectal bleeding, and stool consistency were assessed. A comprehensive disease activity index (DAI) score was assessed using an established method to quantify disease severity. The DAI score was determined by combining three individual scores: the weight loss rate score, the stool trait score, and the occult blood score. These three scores were added together and then divided by three to yield the final DAI score. This systematic approach provides an accurate representation of the overall disease severity experienced by the mice.
Colon lengths were measured, and a sample (0.5 cm) was collected and fixed (10% formalin, 24 h). Following fixation, the tissue was paraffin-embedded and sectioned (5-μm sections) before undergoing HE staining. Each sample was meticulously evaluated at a magnification of 100×.
All statistical analyses were performed using GraphPad Prism 9.0 (GraphPad, United States). The data are presented as the mean ± SD. Group differences were analyzed by two-way analysis of variance (ANOVA), followed by Bonferroni post hoc test. For nonparametric data, the Kruskal-Wallis test was used, and continuous variables were analyzed using one-way ANOVA. P values < 0.05 were considered statistically significant.
To examine the role of SLC6A14 as a diagnostic biomarker for UC, we examined SLC6A14 protein expression. Histopathological analysis revealed that UC samples showed typical colonic inflammation, which was particularly noticeable in UC biopsies compared to healthy adjacent tissue. IHC analysis of UC samples revealed increased SLC6A14 protein levels, which led to higher histological scores (Figure 1A and E). Additionally, a comprehensive investigation was performed using a dataset containing 55 UC tissues and 29 normal tissues, and SLC6A14 mRNA levels were quantified by quantitative real-time polymerase chain reaction. Significant increases in the mRNA expression of SLC6A14 were observed in UC tissues relative to control tissues (P < 0.01, Figure 1B). Notably, these expression levels were further confirmed by Western blot analysis (Figure 1C and D).
To explore the correlation between SLC6A14 and pyroptosis, we examined NLRP3 protein levels in the tissues of UC patients. The results showed substantial upregulation of the NLRP3 protein in UC tissues compared to their normal counterparts (Figure 1F and G). Moreover, our investigation revealed a positive correlation between SLC6A14 expression and NLRP3 expression (Figure 1H). These findings indicate a pronounced increase in SLC6A14 expression in the context of UC.
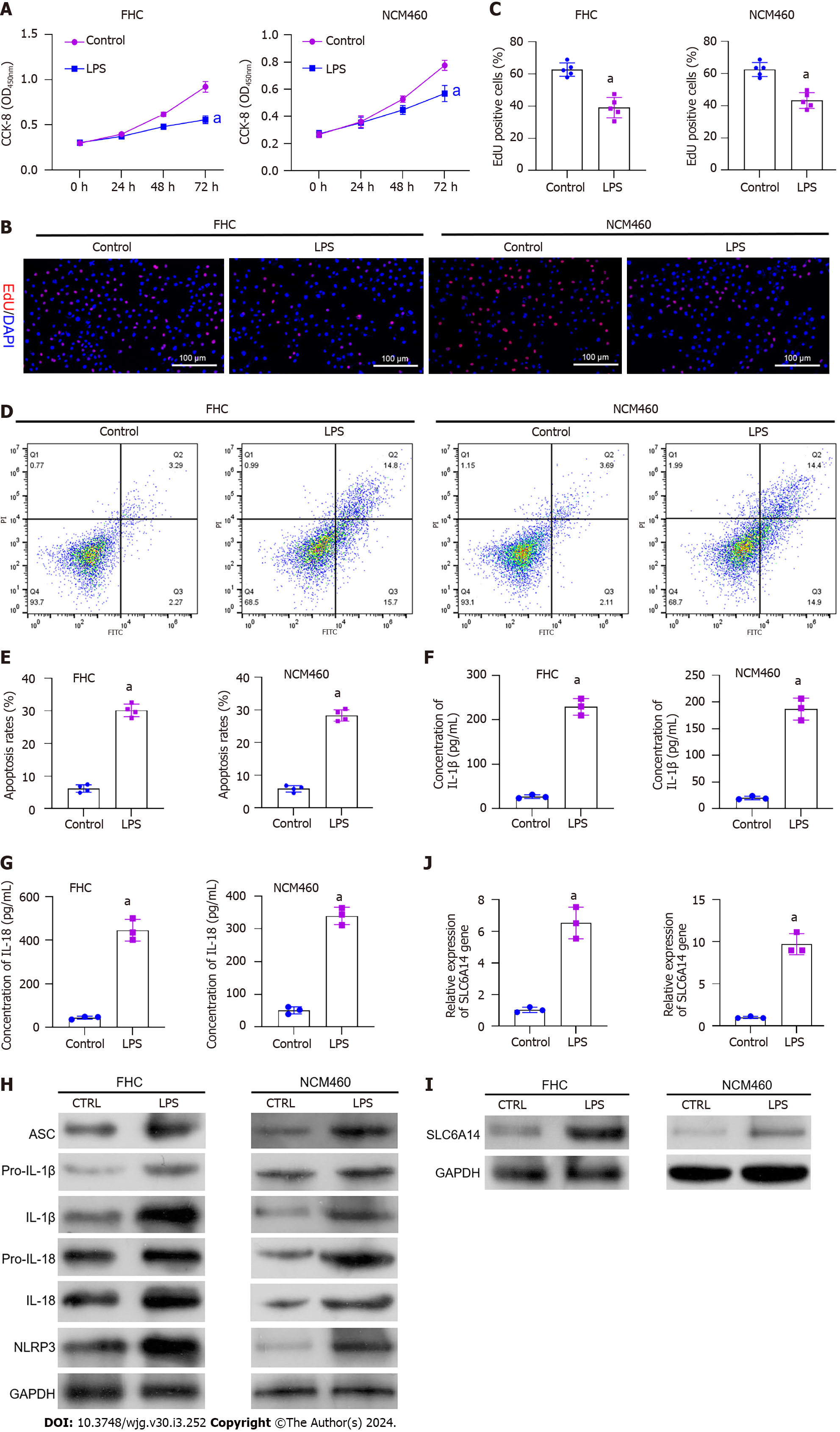
To mimic UC-induced inflammation, we treated FHC and NCM460 cells, which are normal IECs, with 10 ng/mL LPS. This stimulation reduced cell proliferation (Figure 2A-C). In parallel, flow cytometry indicated an increase in cell death following LPS exposure (Figure 2D and E). Moreover, LPS increased inflammatory factor levels in the cells. Pyroptosis has been shown to enhance inflammation, and increased levels of IL-1β and IL-18 were observed after LPS exposure (Figure 2F and G). Subsequently, we used Western blot analysis to assess key effector proteins associated with pyroptotic pathways, including ASC, pro-IL-1β, pro-IL-18, IL-1β, IL-18, and NLRP3. Increases in these proteins were observed after LPS stimulation of FHC cells (Figure 2H and Supplementary Figure 1). Notably, SLC6A14 levels in LPS-treated IECs were markedly increased relative to those in the controls (Figure 2I and J). These findings suggest that LPS induces pyroptosis in FHC and NCM460 cells, underscoring the potential involvement of SLC6A14 in this process.
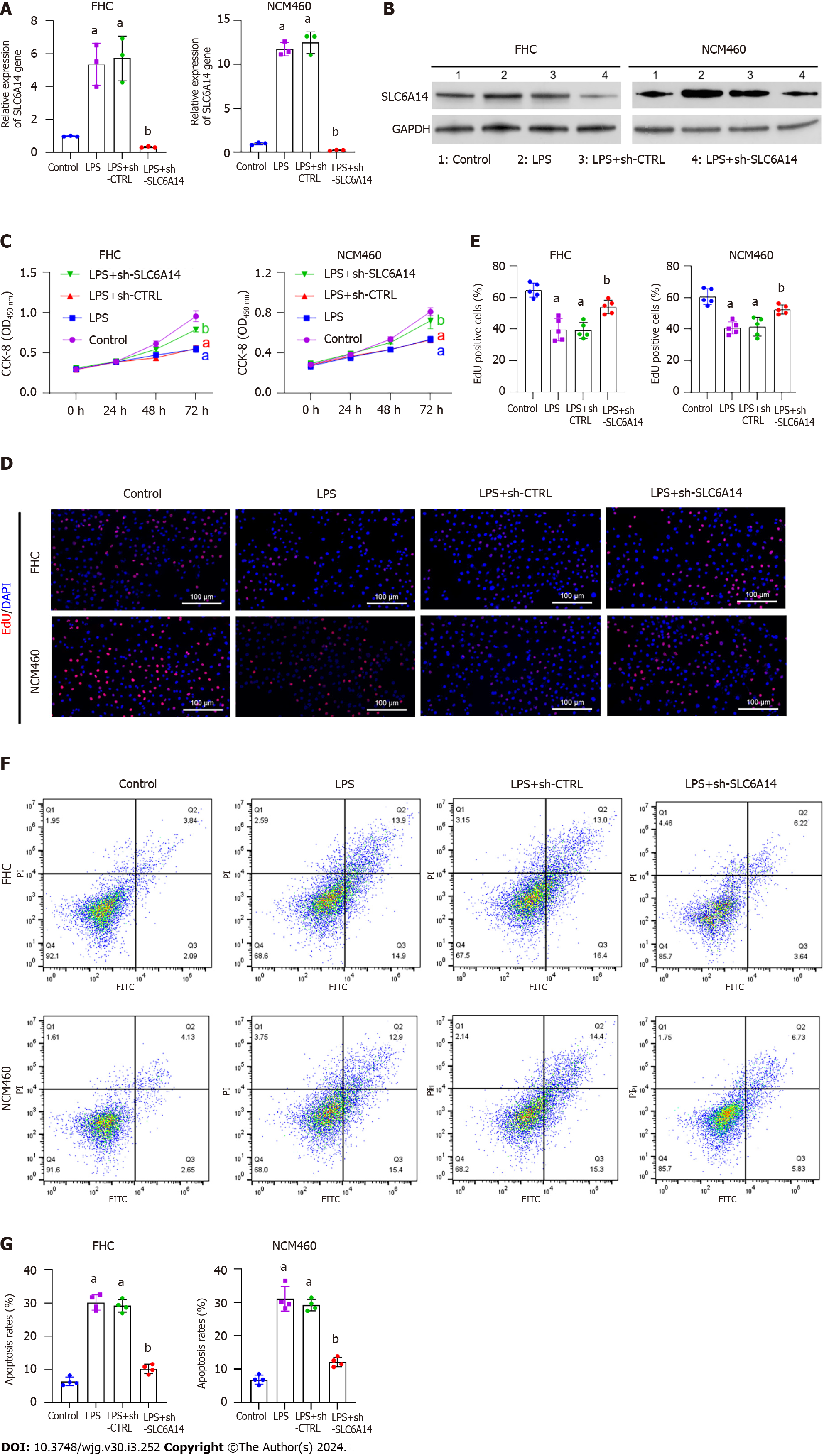
Given the close association between proinflammatory cytokine production and intestinal inflammation, we aimed to determine whether decreasing SLC6A14 expression could mitigate inflammation in the LPS-stimulated epithelial cell model. We introduced the sh-SLC6A14 plasmid into FHC and NCM460 cells to knockdown SLC6A14 expression. Subsequently, the cells were subjected to LPS stimulation (10 ng/mL) for 24 h. As shown in Figure 3A and B, there was a significant reduction in SLC6A14 expression in the LPS + sh-SLC6A14 group. This observation demonstrated the efficacy of the synthetic sh-SLC6A14 vector. Subsequently, we revealed that SLC6A14 downregulation increased cell proliferation (Figure 3C-E). Moreover, flow cytometry showed that suppressing SLC6A14 expression mitigated apoptosis induced by LPS (Figure 3F and G). Collectively, these findings suggested that suppressing SLC6A14 expression enhanced proliferation and reduced apoptosis in FHC and NCM460 cells subjected to LPS stimulation.
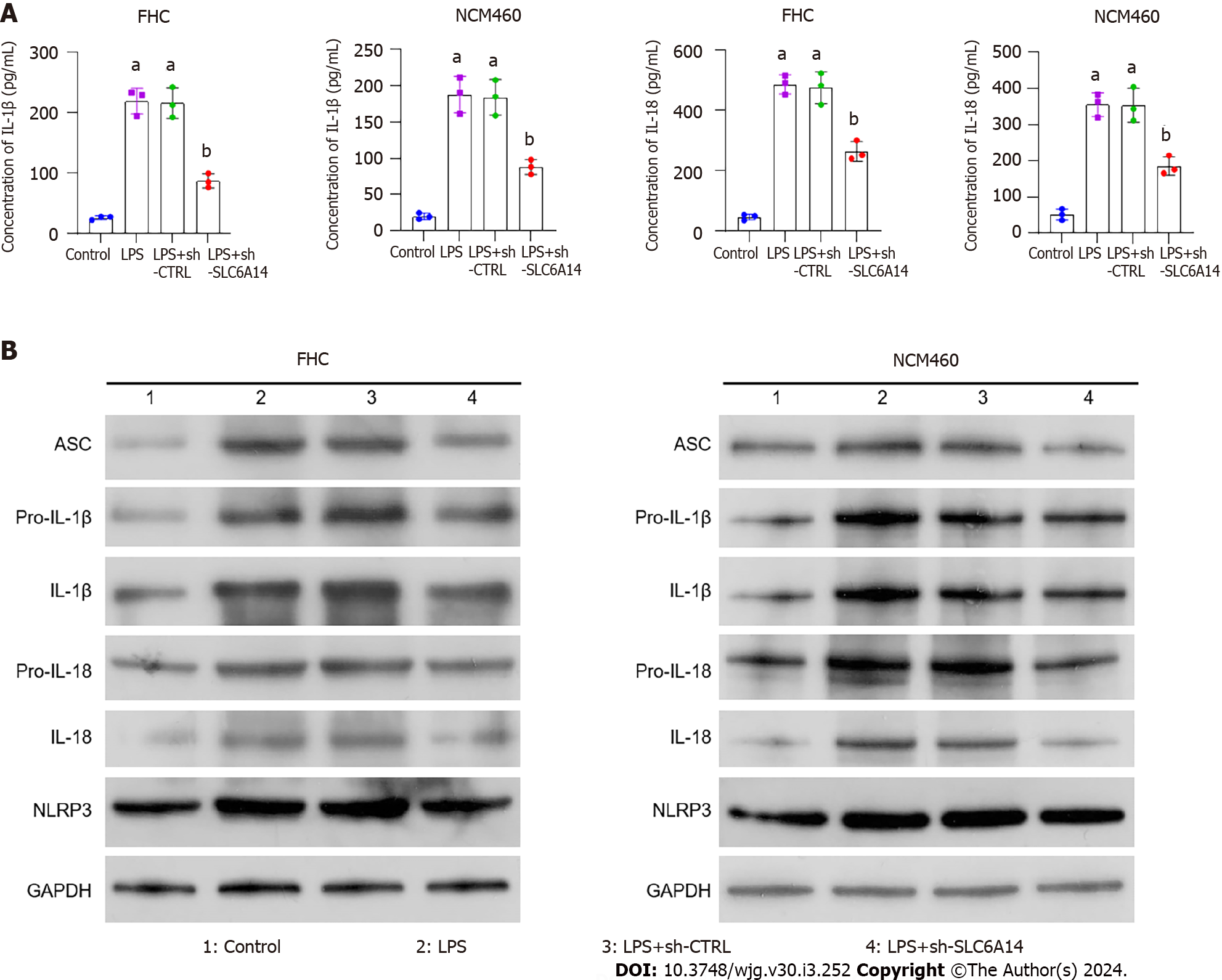
Our subsequent experiments revealed that SLC6A14 could promote the secretion of the pyroptosis-associated proteins IL-1β and IL-18 by LPS-induced IECs (Figure 4A). In addition, Western blotting showed that reducing SLC6A14 expression inhibited key pyroptotic effector proteins (Figures 4B and Supplementary Figure 2). These findings suggest that SLC6A14 can amplify LPS-induced inflammatory cytokine secretion.
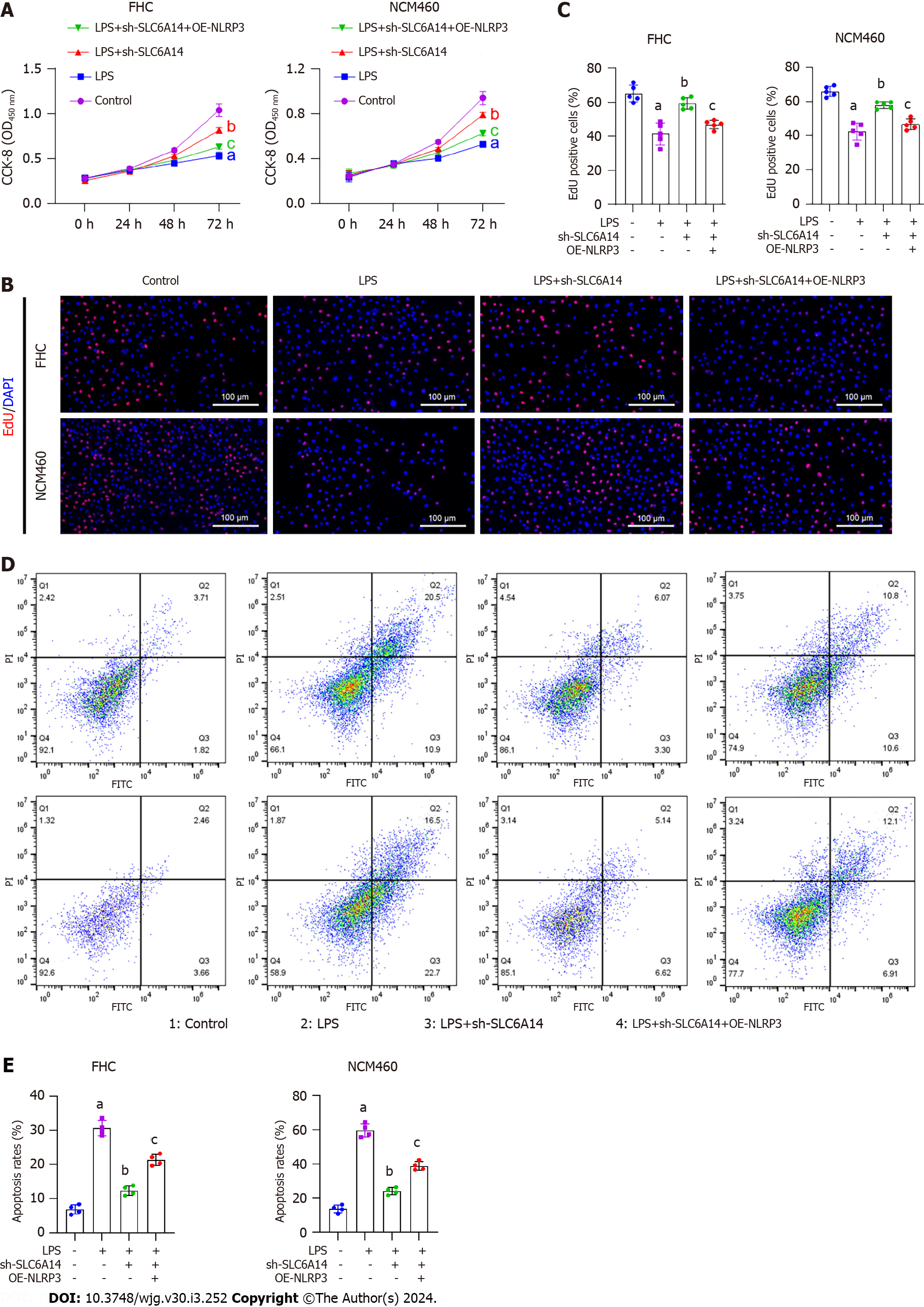
The EdU and CCK-8 assay results showed that upregulating NLRP3 counteracted the inhibitory effects of SLC6A14 knockdown induced by the sh-SLC6A14 plasmids on the proliferation of LPS-treated FHC and NCM460 cells (Figure 5A-C). Flow cytometry further revealed that NLRP3 upregulation mitigated the impact of SLC6A14 downregulation on apoptosis in LPS-induced FHC cells (Figure 5D and E). Moreover, ELISA revealed that NLRP3 overexpression reversed the SLC6A14-induced suppression of IL-18 and IL-1β production in both cell lines after LPS treatment (Figure 6A). Finally, Western blotting showed that increasing NLRP3 expression increased the levels of ASC, IL-1β, IL-18, and NLRP3. This effect abrogated the inhibitory effect of SLC6A14 downregulation of these protein levels (Figure 6B and Supplementary Figure 3). Taken together, these results demonstrate the involvement of SLC6A14 in NLRP3-mediated pyroptosis.
The colons of UC mice were markedly shorter than those of the controls. The downregulation of SLC6A14 mitigated the reduction in colon length induced by DSS (Figure 7A). DSS-treated animals and those that received a combination of DSS and the control vector exhibited greater fluctuations in weight relative to the controls. Furthermore, animals in the DSS and DSS plus vector groups experienced more substantial weight loss relative to those in the DSS plus SLC6A14 vector group (Figure 7B). An increase in the DAI was observed in the DSS-treated groups, as indicated by markedly increased DAI scores compared with those in the controls. Notably, lower DAI scores were observed in the DSS plus SLC6A14 vector group relative to the DSS-only group (Figure 7C). Additionally, histopathological analysis revealed that downregulating SLC6A14 significantly alleviated colonic inflammation induced by DSS and reduced epithelial crypt numbers, mucosal barrier disruption, and inflammatory cell infiltration (Figure 7D). Intriguingly, DSS dramatically induced SLC6A14 expression, while SLC6A14 expression was markedly decreased in DSS plus SLC6A14 knockdown mice compared to other DSS-treated mice (Figure 7E and F). It was also found that SLC6A14 downregulation reduced cytokine production. Thus, our results revealed that downregulating SLC6A14 ameliorated pyroptosis induced by activation of the NLRP3 inflammasome after DSS treatment (Figure 7G). Additionally, SLC6A14 transfection reduced NLRP3 levels in the colons of mice following DSS treatment (Figures 7H and Supplementary Figure 4). In summary, our findings suggest that the downregulation of SLC6A14 effectively protected colon tissue integrity and mitigated the morphological changes caused by DSS, suggesting its potential for treating UC.
UC is associated with chronic inflammation in the colonic mucosa, and new forms of treatment are urgently needed[23-25]. Recent studies focusing on the pathogenesis of UC highlight the involvement of an aberrant immune response and dysregulated inflammation in UC development[26,27]. Invading pathogens and threats are identified by PAMPs and DAMPs associated with the innate immune response[28,29]. Intracellular inflammasome complexes respond to PAMPs and DAMPs, triggering inflammation[30]. NLRP1, NLRP3, NLRC4, and AIM2 are well-documented inflammasome components and receptors. There is strong evidence of the involvement of the NLRP3 inflammasome in inflammatory bowel diseases (IBDs), including Crohn's disease and UC[31,32].
Consistently, recent strategies to suppress chronic inflammation have targeted pyroptosis, offering a novel approach to managing IBD. For instance, L38 exerted positive therapeutic effects on a DSS-induced UC mouse model by inhibiting NLRP3 inflammasome activation and pyroptosis[33]. Similarly, mesalazine and corticosteroids have been shown to attenuate pyroptosis in IECs[34]. These approaches highlight the potential of targeting pyroptosis to alleviate inflammation in the context of IBD. There is a close link between UC progression and increased levels of IL-1β and IL-18, suggesting that overproduction of these cytokines by cells such as macrophages can worsen this condition[35]. However, NLRP3 activation stimulates IL-18 production and enhances intestinal barrier integrity. Intriguingly, the administration of exogenous recombinant IL-18 has been shown to alleviate the inflammatory symptoms of UC resulting from DSS administration[36]. This dual function of NLRP3 emphasizes the importance of selectively targeting macrophages rather than IECs for the effective management of UC[37].
Given the complex and sometimes contradictory aspects associated with NLRP3 activation in different cell types in the context of colitis, it is evident that further research is needed to elucidate the role of pyroptosis in UC. This would help guide the development of targeted therapeutic strategies to modulate the inflammatory response and effectively treat the disease. In our study, we treated IECs with LPS to create a model of intestinal inflammation. By analyzing the levels of key pyroptosis-associated factors, we observed the upregulation of these markers, indicating that LPS effectively induced pyroptosis in colonic epithelial cells. Additionally, our investigation of SLC6A14 expression in the context of LPS-induced colonic epithelial cells revealed the significant upregulation of SLC6A14. This finding is consistent with recent microarray expression data[38].
The SLC transporter family, which includes proteins such as SLC7A11, SLC3A2, and SLC25A28, is linked with a variety of metabolic disorders, especially those of the liver. SLC6A14 in particular has been shown to be upregulated in different colonic diseases, including ulcerative colitis[18]. Studies using microarrays of colonic tissue from UC patients and normal tissue showed a noticeable increase in SLC6A14 mRNA expression in UC cases[39]. SLC6A14 is an efficient transporter of amino acids that is associated with various intracellular activities. Leucine, which is one of its substrates, is critical for activating the mTOR signaling pathway in tumor cells. Moreover, SLC6A14 contributes to cellular glutathione synthesis by using glycine as a substrate. Multiple studies have showed the critical involvement of SLC6A14 in UC. Zhang et al[40] suggested that SLC6A14 was a biomarker of UC in tissue biopsies and might offer a novel target for gene therapy in UC. Similarly, Li et al[41] identified a potential regulatory pathway involving NEAT1-miR-342-3p/miR-650-SLC6A14 in UC. More recently, Chen et al[18] demonstrated that knockdown of SLC6A14 blocked ferroptosis and that SLC6A14 promoted ferroptosis in epithelial cells through C/EBPβ-PAK6 signaling in UC. In the context of our study, we showed that downregulating SLC6A14 effectively blocked NLRP3 activation, resulting in notable alleviation of colitis. This suggests a potential therapeutic strategy for managing colitis by targeting SLC6A14 to modulate the NLRP3 pathway and pyroptosis. These findings highlight the complexity of SLC6A14 in inflammatory conditions and UC.
We also examined the role of SLC6A14 in UC pathogenesis using a UC mouse model. These mice showed severe damage to colonic tissue, increased oxidative stress and cytokine production, and NLRP3 inflammasome activation. These findings indicate that DSS-induced UC led to the activation of the inflammatory caspase-mediated NLRP3 inflammasome. NLRP3 activation increased cytokine production and induced pyroptosis. Notably, our findings suggest that DSS induced pyroptosis, which further aggravated colon tissue damage. Our investigations revealed that downregulating SLC6A14 inhibited IEC pyroptosis in UC, which was mediated by the NLRP3 pathway. Interestingly, our results also indicated a positive association between the increase in SLC6A14 expression and pyroptosis in UC tissue samples. This evidence suggests that SLC6A14 actively promotes pyroptosis in the context of UC by regulating the NLRP3 pathway. Consequently, our findings highlight SLC6A14 as a prospective new therapeutic target that could be used to mitigate cellular damage during the course of UC. Our study contributes to our knowledge of UC pathogenesis and identifies a potential strategy for therapeutic intervention.
In summary, the results demonstrated the close involvement of SLC6A14 in promoting pyroptosis in the context of UC by upregulating NLRP3 expression. These findings indicate the potential of targeting SLC6A14/NLRP3 axis-mediated pyroptosis as a promising therapeutic strategy for treating UC. Our research provides valuable insights into the mechanisms driving UC pathogenesis and offers a possible direction for developing innovative treatments to alleviate the impact of this chronic inflammatory disorder.
Ulcerative colitis (UC) is an inflammatory condition associated with frequent relapse and recurrence. Dysregulation of intestinal epithelial cells (IECs) and mucosal barrier impairment contribute to sustained inflammation in UC. Hence, an in-depth exploration of the triggers and mechanisms of IEC death could result in efficacious therapeutic options for UC patients.
Evidence suggests the involvement of SLC6A14 in UC pathogenesis, but the central regulator remains unknown.
We aimed to explore the role of SLC6A14 in UC-associated pyroptosis.
Quantitative real-time polymerase chain reaction (qRT-PCR), immunoblotting, and IHC assessed SLC6A14 in human UC tissues. LPS induced FHC and NCM460 cell inflammation, modeling enteritis; SLC6A14 levels were assessed. Pyroptosis markers were quantified using enzyme-linked immunosorbent assay, Western blotting, and qRT-PCR, while EdU incubation, CCK-8 assay and flow cytometry examined proliferation and apoptosis, respectively. Mouse models of UC were used for verification.
SLC6A14 was elevated, correlating with NLRP3 in UC tissues. LPS-induced FHC and NCM460 cells showed increased SLC6A14. Reduced SLC6A14 boosted cell proliferation, suppressed apoptosis. Lower SLC6A14 Led to decreased pyroptosis-associated proteins (ASC, IL-1β, IL-18, NLRP3). NLRP3 overexpression counteracted sh-SLC6A14 effects on LPS-induced FHC and NCM460 cell pyroptosis. SLC6A14 improved murine dextran sulfate sodium colitis mucosa.
SLC6A14 promotes UC pyroptosis via NLRP3 upregulation, indicating therapeutic potential through SLC6A14/NLRP3 axis modulation.
We demonstrated the close involvement of SLC6A14 in promoting pyroptosis in the context of UC by upregulating NLRP3 expression. These findings underline the potential significance of targeting the SLC6A14/NLRP3 axis-mediated pyroptosis as a promising therapeutic strategy for addressing UC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Castelucci P, Brazil; Chiba T, Japan S-Editor: Gao CC L-Editor: A P-Editor: Yu HG
| 1. | Du L, Ha C. Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol Clin North Am. 2020;49:643-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 357] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 2. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1547] [Article Influence: 119.0] [Reference Citation Analysis (5)] |
| 3. | Kaenkumchorn T, Wahbeh G. Ulcerative Colitis: Making the Diagnosis. Gastroenterol Clin North Am. 2020;49:655-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 4. | Eisenstein M. Ulcerative colitis: towards remission. Nature. 2018;563:S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 5. | Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2482] [Article Influence: 310.3] [Reference Citation Analysis (2)] |
| 6. | Parikh K, Antanaviciute A, Fawkner-Corbett D, Jagielowicz M, Aulicino A, Lagerholm C, Davis S, Kinchen J, Chen HH, Alham NK, Ashley N, Johnson E, Hublitz P, Bao L, Lukomska J, Andev RS, Björklund E, Kessler BM, Fischer R, Goldin R, Koohy H, Simmons A. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature. 2019;567:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 559] [Article Influence: 93.2] [Reference Citation Analysis (0)] |
| 7. | Soderholm AT, Pedicord VA. Intestinal epithelial cells: at the interface of the microbiota and mucosal immunity. Immunology. 2019;158:267-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 8. | Kang L, Fang X, Song YH, He ZX, Wang ZJ, Wang SL, Li ZS, Bai Y. Neutrophil-Epithelial Crosstalk During Intestinal Inflammation. Cell Mol Gastroenterol Hepatol. 2022;14:1257-1267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Zhou C, Li L, Li T, Sun L, Yin J, Guan H, Wang L, Zhu H, Xu P, Fan X, Sheng B, Xiao W, Qiu Y, Yang H. SCFAs induce autophagy in intestinal epithelial cells and relieve colitis by stabilizing HIF-1α. J Mol Med (Berl). 2020;98:1189-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Lv Q, Xing Y, Liu J, Dong D, Liu Y, Qiao H, Zhang Y, Hu L. Lonicerin targets EZH2 to alleviate ulcerative colitis by autophagy-mediated NLRP3 inflammasome inactivation. Acta Pharm Sin B. 2021;11:2880-2899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 11. | Qu S, Fan L, Qi Y, Xu C, Hu Y, Chen S, Liu W, Si J. Akkermansia muciniphila Alleviates Dextran Sulfate Sodium (DSS)-Induced Acute Colitis by NLRP3 Activation. Microbiol Spectr. 2021;9:e0073021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 149] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 12. | Shao BZ, Xu ZQ, Han BZ, Su DF, Liu C. NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol. 2015;6:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 642] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 13. | Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 925] [Cited by in RCA: 2369] [Article Influence: 394.8] [Reference Citation Analysis (0)] |
| 14. | Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17:588-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 1201] [Article Influence: 171.6] [Reference Citation Analysis (0)] |
| 15. | Larabi A, Barnich N, Nguyen HTT. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy. 2020;16:38-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 541] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 16. | Xu Y, Shen J, Ran Z. Emerging views of mitophagy in immunity and autoimmune diseases. Autophagy. 2020;16:3-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 358] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 17. | Lin L, Yee SW, Kim RB, Giacomini KM. SLC transporters as therapeutic targets: emerging opportunities. Nat Rev Drug Discov. 2015;14:543-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 579] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 18. | Chen Y, Yan W, Chen Y, Zhu J, Wang J, Jin H, Wu H, Zhang G, Zhan S, Xi Q, Shi T, Chen W. SLC6A14 facilitates epithelial cell ferroptosis via the C/EBPβ-PAK6 axis in ulcerative colitis. Cell Mol Life Sci. 2022;79:563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Lu JJ, Maimaiti M, Liu H, Liu WD, Hui WJ, Huang XL, Gao F. Potential Biomarkers Associated with Differential Manifestations of Ulcerative Colitis (UC) in Uyghur and Han Population in China. J Inflamm Res. 2021;14:7431-7441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Wei YY, Fan YM, Ga Y, Zhang YN, Han JC, Hao ZH. Shaoyao decoction attenuates DSS-induced ulcerative colitis, macrophage and NLRP3 inflammasome activation through the MKP1/NF-κB pathway. Phytomedicine. 2021;92:153743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 21. | Liu C, Yan X, Zhang Y, Yang M, Ma Y, Xu Q, Tu K, Zhang M. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J Nanobiotechnology. 2022;20:206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 133] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 22. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 133914] [Article Influence: 5579.8] [Reference Citation Analysis (1)] |
| 23. | Kucharzik T, Koletzko S, Kannengiesser K, Dignass A. Ulcerative Colitis-Diagnostic and Therapeutic Algorithms. Dtsch Arztebl Int. 2020;117:564-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 24. | Lee SD. Health Maintenance in Ulcerative Colitis. Gastroenterol Clin North Am. 2020;49:xv-xvi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Cheng J, Zhang Y, Ma L, Du W, Zhang Q, Gao R, Zhao X, Chen Y, Jiang L, Li X, Li B, Zhou Y. Macrophage-Derived Extracellular Vesicles-Coated Palladium Nanoformulations Modulate Inflammatory and Immune Homeostasis for Targeting Therapy of Ulcerative Colitis. Adv Sci (Weinh). 2023;10:e2304002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 26. | Yang J, Guan X, He S, Ge L, Gao Q, Wu X. FTY720 attenuates acute colitis via colonic T cells reduction and inhibition of M1 macrophages polarization independent of CCR2-mediated monocytes input. Int Immunopharmacol. 2023;123:110731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 27. | Furfaro F, Ragaini E, Peyrin-Biroulet L, Danese S. Novel Therapies and Approaches to Inflammatory Bowel Disease (IBD). J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 28. | Zindel J, Kubes P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu Rev Pathol. 2020;15:493-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 535] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 29. | Remick BC, Gaidt MM, Vance RE. Effector-Triggered Immunity. Annu Rev Immunol. 2023;41:453-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 93] [Reference Citation Analysis (0)] |
| 30. | Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1529] [Cited by in RCA: 2485] [Article Influence: 276.1] [Reference Citation Analysis (0)] |
| 31. | Xue JC, Yuan S, Hou XT, Meng H, Liu BH, Cheng WW, Zhao M, Li HB, Guo XF, Di C, Li MJ, Zhang QG. Natural products modulate NLRP3 in ulcerative colitis. Front Pharmacol. 2023;14:1265825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Wu H, Zeng R, Qiu X, Chen K, Zhuo Z, Guo K, Xiang Y, Yang Q, Jiang R, Leung FW, Lian Q, Sha W, Chen H. Investigating regulatory patterns of NLRP3 Inflammasome features and association with immune microenvironment in Crohn's disease. Front Immunol. 2022;13:1096587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Li N, Jiang X, Zhang R, Ye N, Tang M, Cai X, Su K, Peng J, Zhang X, Zhao M, Wu W, Ye H. Discovery of Triazinone Derivatives as Novel, Specific, and Direct NLRP3 Inflammasome Inhibitors for the Treatment of DSS-Induced Ulcerative Colitis. J Med Chem. 2023;66:13428-13451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 34. | Chen K, Shang S, Yu S, Cui L, Li S, He N. Identification and exploration of pharmacological pyroptosis-related biomarkers of ulcerative colitis. Front Immunol. 2022;13:998470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 35. | Sharma BR, Kanneganti TD. Inflammasome signaling in colorectal cancer. Transl Res. 2023;252:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 48] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 36. | Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 719] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 37. | Shao X, Sun S, Zhou Y, Wang H, Yu Y, Hu T, Yao Y, Zhou C. Bacteroides fragilis restricts colitis-associated cancer via negative regulation of the NLRP3 axis. Cancer Lett. 2021;523:170-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 38. | Xu M, Tao J, Yang Y, Tan S, Liu H, Jiang J, Zheng F, Wu B. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis. 2020;11:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 39. | Wang S, Liu W, Wang J, Bai X. Curculigoside inhibits ferroptosis in ulcerative colitis through the induction of GPX4. Life Sci. 2020;259:118356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 40. | Zhang J, Wang X, Xu L, Zhang Z, Wang F, Tang X. Investigation of Potential Genetic Biomarkers and Molecular Mechanism of Ulcerative Colitis Utilizing Bioinformatics Analysis. Biomed Res Int. 2020;2020:4921387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Li Y, Tang M, Zhang FJ, Huang Y, Zhang J, Li J, Wang Y, Yang J, Zhu S. Screening of ulcerative colitis biomarkers and potential pathways based on weighted gene co-expression network, machine learning and ceRNA hypothesis. Hereditas. 2022;159:42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |