Published online Jul 14, 2024. doi: 10.3748/wjg.v30.i26.3247
Revised: May 28, 2024
Accepted: June 20, 2024
Published online: July 14, 2024
Processing time: 73 Days and 23.3 Hours
Multiple endocrine neoplasias (MENs) are a group of hereditary diseases invol
We present the case of a patient in whom MEN1 was detected early. A middle-aged male with recurrent abdominal pain and diarrhea was admitted to the hos
For patients who present with gastrointestinal symptoms accompanied by hyper
Core Tip: Multiple endocrine neoplasia type 1 (MEN1) mainly affects the parathyroid glands, gastrointestinal tract, pancreas and pituitary gland and manifests as parathyroid adenomas, gastrinomas, insulinomas and pituitary tumors. As a rare disease, MEN1 is easily missed in clinical practice. We analyzed a case of MEN in a patient who had gastrointestinal symptoms as the main manifestation, and this report provides a reference for MEN1 clinical diagnosis and treatment.
- Citation: Yuan JH, Luo S, Zhang DG, Wang LS. Early detection of multiple endocrine neoplasia type 1: A case report. World J Gastroenterol 2024; 30(26): 3247-3252
- URL: https://www.wjgnet.com/1007-9327/full/v30/i26/3247.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i26.3247
As an autosomal dominant genetic disease, multiple endocrine neoplasia type 1 (MEN1) has a low incidence, diverse clinical manifestations, and a complex prognosis, and MEN1 is easily misdiagnosed; therefore, early diagnosis and treatment are very important[1,2]. In this case report, the diagnosis and treatment of a patient with MEN1 who ma
A 51-year-old male was admitted to the hospital on June 8, 2022, due to a 1-year history of subxiphoid pain and watery diarrhea.
One year prior, the patient developed subxiphoid pain accompanied by yellow watery stools 3-4 times per day without significant weight loss. No obvious abnormalities were found on gastrointestinal endoscopy at other hospitals. Symptoms improved after oral omeprazole, but discomfort recurred after withdrawal of the drug.
There was no specific past medical history.
The patient denied a significant personal and family history.
There were no significant positive signs on physical examination.
Laboratory investigations revealed no obvious abnormalities in routine blood work or examinations of liver, kidney, and thyroid function, tumor markers, and feces. The laboratory test results during hospitalization were as follows: Blood calcium, 2.63 mmol/L (reference value 2.05-2.55); blood phosphorus, 0.71 mmol/L (0.8-1.5); parathyroid hormone, 80.80 pg/mL (15-65); calcitonin, 21.7 pg/mL (< 18); 24-hour urinary calcium, 8.62 mmol/24 h (2.5-7.5); 25-hydroxytotal vitamin D, 48.4 nmol/L (75-250); gastrin, 281 pg/mL (13.00-15.00); and gastrin-17, 34.06 pmol/L (1.00-15.00). Human chromogranin A, anti-inner factor antibody immunoglobulin G, anti-parietal cell antibody immunoglobulin G, pituitary prolactin, human growth hormone, glycated hemoglobin, blood cortisol and adrenocorticotropic hormone at 8:00 am and 24-hour urinary vanillylmandelic acid were normal.
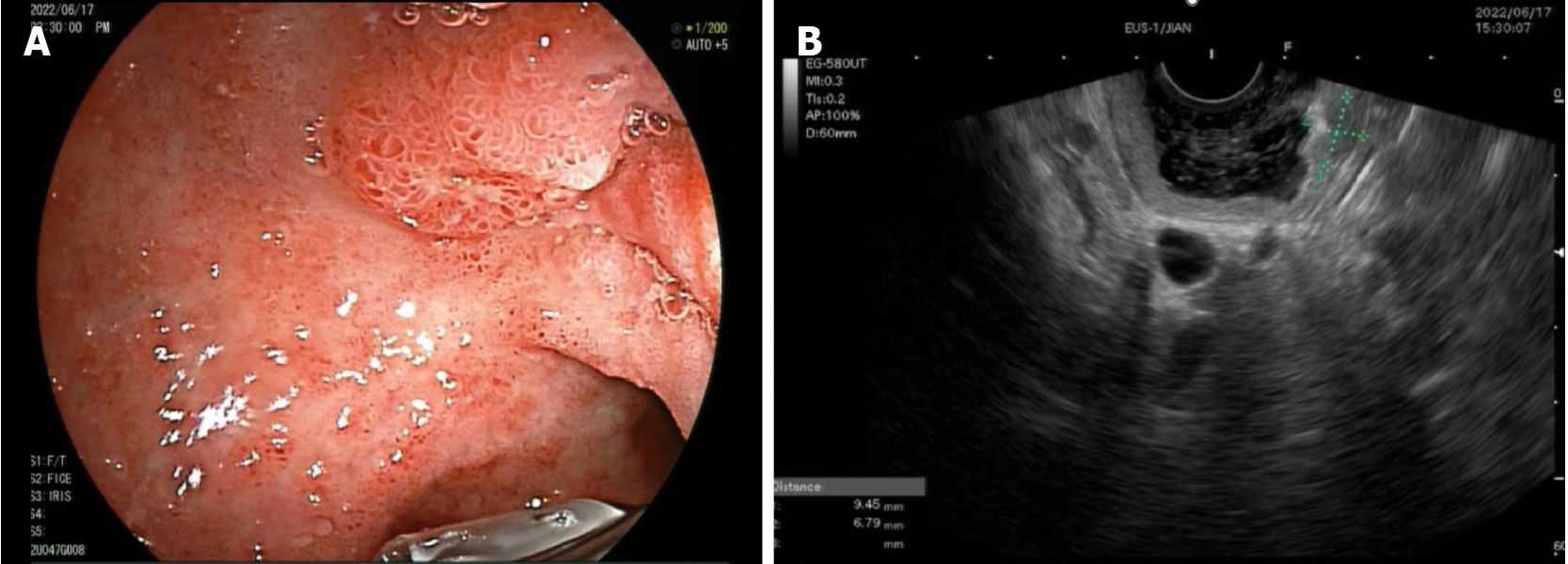
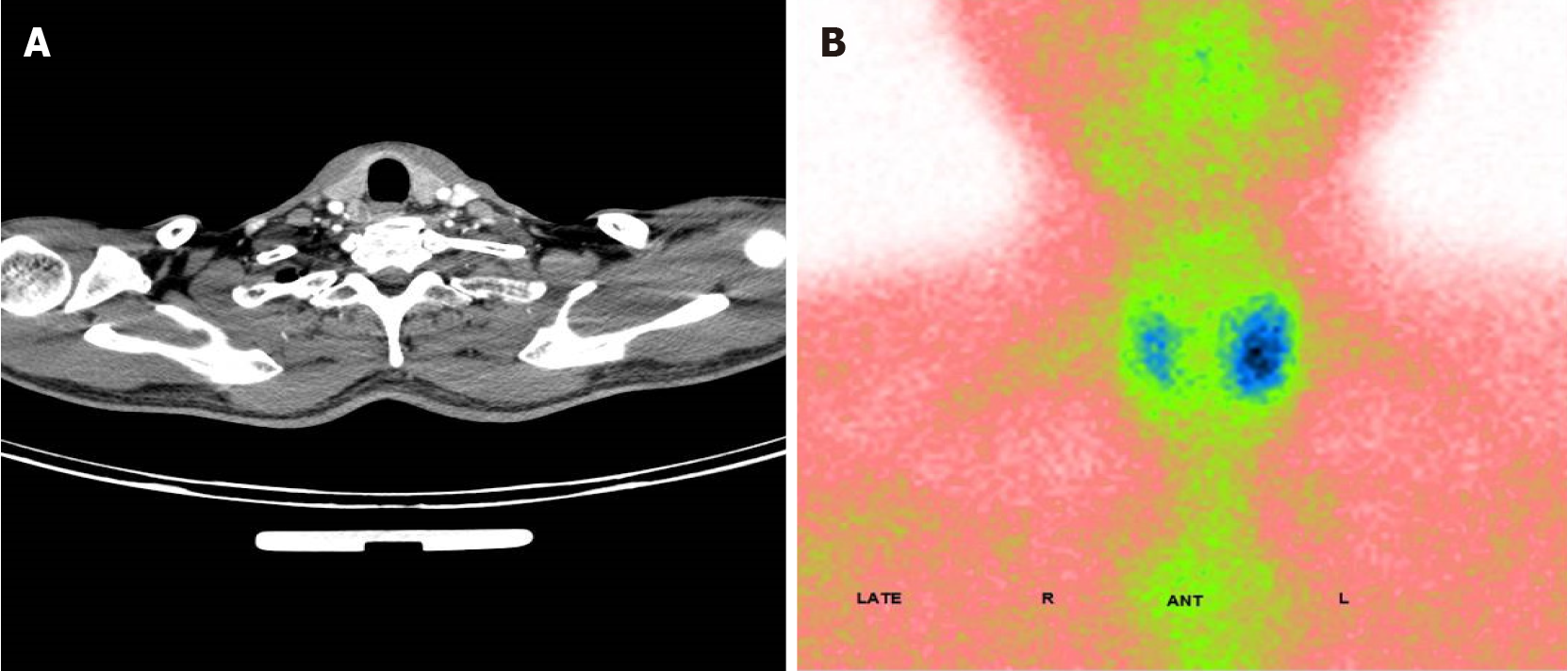
After admission, enhanced computed tomography (CT) of the abdomen showed marked enhancement of the mucosa of the stomach, duodenum and jejunum, and a nodule was observed on the medial wall of the duodenal bulb. Further gastroscopy revealed a bulge in the duodenal bulb with surface ulcer formation, and a deep biopsy was performed (Figure 1A). Ultrasound endoscopy revealed a hypoechoic lesion in the duodenal bulb, approximately 9.4 mm × 6.7 mm in cross-section, with inhomogeneous internal echogenicity (Figure 1B); the lesion protruded into the lumen and originated from the submucosa. Endoscopic biopsy revealed atypical cell nests in the local mucosal layer and submucosal layer, which were considered to indicate micronodular hyperplasia of neuroendocrine cells. Immunohistochemistry results were as follows: CK (+), Syn (+), CD56 (-), CgA (+), Ki67 (approximately 2%+), and p53 (-). Urological ultrasound revealed a stone in the left kidney. Thyroid and parathyroid ultrasound revealed a hypoechoic lesion in the left thyroid gland, nearly 43 mm × 10 mm in size, for which origination from the parathyroid gland could not be excluded. Enhanced CT revealed a blood-rich nodule in the posterior left lobe of the thyroid gland and in the medial left common carotid artery, with a size of 17 mm × 11 mm (Figure 2A). Emission CT indicated a hyperfunctional lesion change (Figure 2B). A magnetic resonance imaging scan of the pituitary showed no abnormalities.
Sequencing of the MEN1 gene showed detection of heterozygous variants. MLPA technology to detect large segment deletions/insertions in the MEN1 gene did not detect mutations.
The patient underwent left inferior parathyroidectomy on June 23, 2022, with a postoperative pathological diagnosis consistent with parathyroid adenoma. Watery diarrhea persisted after surgery. Then, combined laparoscopic and gastroscopic duodenal bulb resection of the mass was performed.
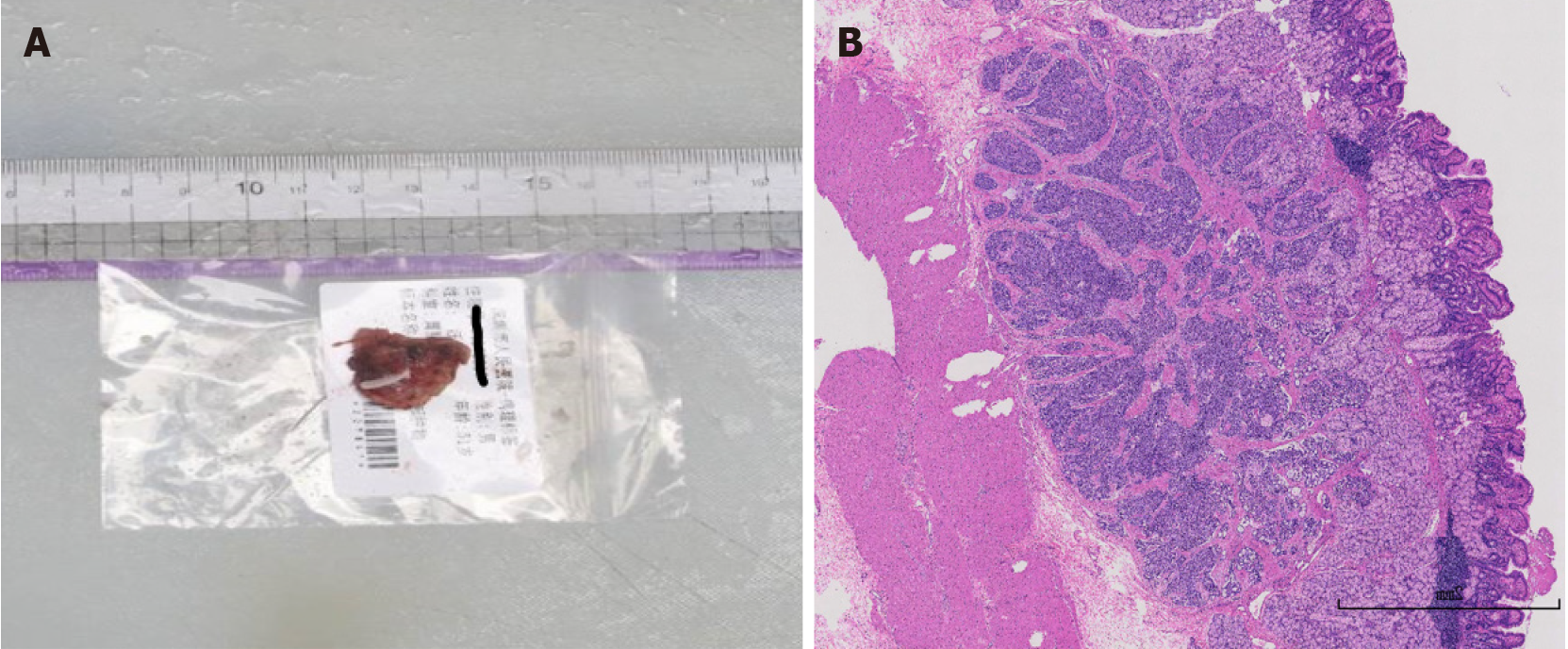
Postoperative pathological analysis revealed a neuroendocrine tumor, G1, located in the mucosal and submucosal layers, with a maximum diameter of approximately 0.6 cm, and no tumor was observed at the margins of the incision or basal incision (Figure 3). Immunohistochemistry results were as follows: CK (+), CD56 (+), Syn (+), CgA (+), SSTR2 (2+), and Ki-67 (approximately 2%+). No tumor metastasis was detected in the lymph nodes of the gastric wall (0/1). After discharge following the procedure, the patient’s symptoms of abdominal pain or diarrhea were significantly improved compared with before the procedure.
MENs are a group of hereditary diseases involving multiple endocrine glands. MEN can be divided into MEN1 and MEN2[1]. MEN1 mainly affects the parathyroid glands, gastrointestinal tract, pancreas and pituitary gland and manifests as parathyroid adenomas, gastrinomas, insulinomas and pituitary tumors. As a rare disease, MEN1 is easily missed in clinical practice[2]. In this case, we analyzed the diagnosis of MEN1 in a patient with gastrointestinal symptoms as the main manifestation, and this report provides a reference for the clinical diagnosis and treatment of MEN1.
The characteristics of this patient were as follows: (1) A middle-aged male with a chronic course; (2) Recurrent abdominal pain, diarrhea, and symptoms relieved by oral proton pump inhibitors (PPIs). Symptoms recurred after the discontinuation of PPIs, and gastrointestinal endoscopy in the hospital did not reveal obvious pathological changes; (3) Admission examination findings suggested hypercalcemia and hypophosphatemia. Further examination revealed elevated parathyroid hormone, calcitonin and urinary calcium levels. Parathyroid enhanced CT revealed blood-rich nodules, and the findings of emission CT of the parathyroid glands suggested hyperfunctioning parathyroid lesions; (4) Gastroscopy at our hospital revealed duodenal bulging and ulceration. Ultrasonographic endoscopy revealed submu
Gastrinoma manifests as excessive gastric acid secretion and thus recurrent peptic ulcers, epigastric pain and diarrhea and is known as Zollinger-Ellison syndrome. Hereditary gastrinoma is associated with MEN1[3]. Research has shown that 20%-30% of cases of Zollinger-Ellison syndrome are caused by MEN1, while approximately 60% of MEN-1 patients will develop gastrinoma[4]. Gastrinoma also manifests as gastroesophageal reflux disease, such as acid reflux and heartburn. Due to atypical early symptoms, gastrinoma can easily be missed and misdiagnosed[5]. Studies have shown the time from the appearance of gastrointestinal symptoms to the final diagnosis of gastrinoma usually takes more than 5 years[6]. Diagnosis is based on the clinical manifestations of hypergastrinemia, elevated serum gastrin, endoscopic findings, imaging evidence and pathological analysis findings. In this patient, symptoms improved after the use of PPIs and recurred after their discontinuation, which indicates the possibility of gastrinoma. Postoperative pathological analysis findings suggested a neuroendocrine tumor. Serum gastrin was elevated more than 10-fold, so the possibility of gastrinoma was high. The patient was diagnosed with MEN1 only 1 year after the onset of symptoms in this case, and timely treatment prevented further progression.
A greater percentage of parathyroid tumors than gastrinomas occur in MEN1. Studies have shown that nearly 90% of MEN1 patients have parathyroid gland involvement, resulting in primary hyperparathyroidism[7]. Generally, MEN1 causes mild symptoms of hypercalcemia, and affected individuals can even be asymptomatic, while the incidence of hypercalcemic crisis is low[8]. Sometimes, hypercalcemia is found only during laboratory tests, which is an obstacle to early detection. In this case, the patient’s blood biochemistry results suggested hypercalcemia. Further examination revealed a renal stone and parathyroid gland nodules, which are key for the diagnosis of a parathyroid adenoma. For patients with gastrinoma combined with hypercalcemia or a parathyroid tumor, we need to consider whether MEN1 is possible. The mechanism by which gastrinoma develops in MEN1 has still not been elucidated. Hackeng et al[9] proposed the hypothesis of a parathyroid-intestinal axis and suggested that the hypercalcemia induced by parathyroid tumor may promote gastrinoma development by affecting calcium-sensing receptors, which promote the proliferation of gastri
MEN1 is an autosomal dominant disorder linked to the MEN1 gene on chromosome 11q13. Deletions or mutations in the MEN1 gene result in a shortening of the encoded protein Menin. Altered length Menin cannot be transferred to the nucleus, resulting in a loss of function[10]. It has been reported that a proportion of patients diagnosed with MEN1 do not present with mutations in the MEN1 gene, and their diagnosis is mainly based on clinical criteria[11]. de Laat et al[12] showed that the clinical manifestations of patients with mutation-positive vs mutation-negative MEN1 differed. Patients with mutation-negative MEN1 later developed MEN1-related clinical symptoms, with a better MEN1 prognosis. In this case, heterozygous variants of unknown significance were detected in the whole exon by gene sequencing, which indicated a high probability of MEN1 when combined with the clinical manifestations. However, attention should be given to identifying MEN1-like syndrome or sporadic coincidence of two neuroendocrine tumors.
In this case, the patient presented with nonspecific gastrointestinal symptoms, hypercalcemia and elevated serum gastrin and was ultimately diagnosed with MEN1. The time from symptom onset to definitive diagnosis of MEN1 was only 1 year. MEN1 can manifest different clinical symptoms and is easily misdiagnosed. Therefore, for patients with gastrointestinal symptoms, especially the recurrence of symptoms after PPI discontinuation, it is necessary to be alert to the possibility of MEN1 to achieve early diagnosis and early treatment and to improve the outcomes.
We would like to express our gratitude to the patient for permission of case report.
| 1. | Al-Salameh A, Baudry C, Cohen R. Update on multiple endocrine neoplasia Type 1 and 2. Presse Med. 2018;47:722-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Al-Salameh A, Cadiot G, Calender A, Goudet P, Chanson P. Clinical aspects of multiple endocrine neoplasia type 1. Nat Rev Endocrinol. 2021;17:207-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 3. | Epelboym I, Mazeh H. Zollinger-Ellison syndrome: classical considerations and current controversies. Oncologist. 2014;19:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Effraimidis G, Knigge U, Rossing M, Oturai P, Rasmussen ÅK, Feldt-Rasmussen U. Multiple endocrine neoplasia type 1 (MEN-1) and neuroendocrine neoplasms (NENs). Semin Cancer Biol. 2022;79:141-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103:153-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 985] [Article Influence: 109.4] [Reference Citation Analysis (1)] |
| 6. | Gibril F, Jensen RT. Zollinger-Ellison syndrome revisited: diagnosis, biologic markers, associated inherited disorders, and acid hypersecretion. Curr Gastroenterol Rep. 2004;6:454-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi ML; Endocrine Society. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97:2990-3011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 878] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 8. | Song A, Yang Y, Liu S, Nie M, Jiang Y, Li M, Xia W, Wang O, Xing X. Prevalence of Parathyroid Carcinoma and Atypical Parathyroid Neoplasms in 153 Patients With Multiple Endocrine Neoplasia Type 1: Case Series and Literature Review. Front Endocrinol (Lausanne). 2020;11:557050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Hackeng WM, Dreijerink KMA, Offerhaus GJA, Brosens LAA. A Parathyroid-Gut Axis: Hypercalcemia and the Pathogenesis of Gastrinoma in Multiple Endocrine Neoplasia 1. Mol Cancer Res. 2021;19:946-949. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Norton JA, Krampitz G, Jensen RT. Multiple Endocrine Neoplasia: Genetics and Clinical Management. Surg Oncol Clin N Am. 2015;24:795-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Cebrián A, Ruiz-Llorente S, Cascón A, Pollán M, Díez JJ, Picó A, Tellería D, Benítez J, Robledo M. Mutational and gross deletion study of the MEN1 gene and correlation with clinical features in Spanish patients. J Med Genet. 2003;40:e72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | de Laat JM, van der Luijt RB, Pieterman CR, Oostveen MP, Hermus AR, Dekkers OM, de Herder WW, van der Horst-Schrivers AN, Drent ML, Bisschop PH, Havekes B, Vriens MR, Valk GD. MEN1 redefined, a clinical comparison of mutation-positive and mutation-negative patients. BMC Med. 2016;14:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |