Published online Jan 14, 2024. doi: 10.3748/wjg.v30.i2.158
Peer-review started: October 25, 2023
First decision: November 12, 2023
Revised: November 23, 2023
Accepted: December 14, 2023
Article in press: December 14, 2023
Published online: January 14, 2024
Processing time: 78 Days and 15.4 Hours
Tumor budding (TB) has emerged as a promising independent prognostic biomarker in colorectal cancer (CRC). The prognostic role of TB has been exten
To analyze the relationship between TB categories and clinicopathological characteristics and assess their prognostic value in stage III-IV CRC to further refine the prognostic risk stratification of stage III-IV CRC.
The clinical data of 547 CRC patients were collected for this retrospective study. Infiltration at the front edge of the tumor buds was counted according to the 2016 International Tumor Budding Consensus Conference guidelines.
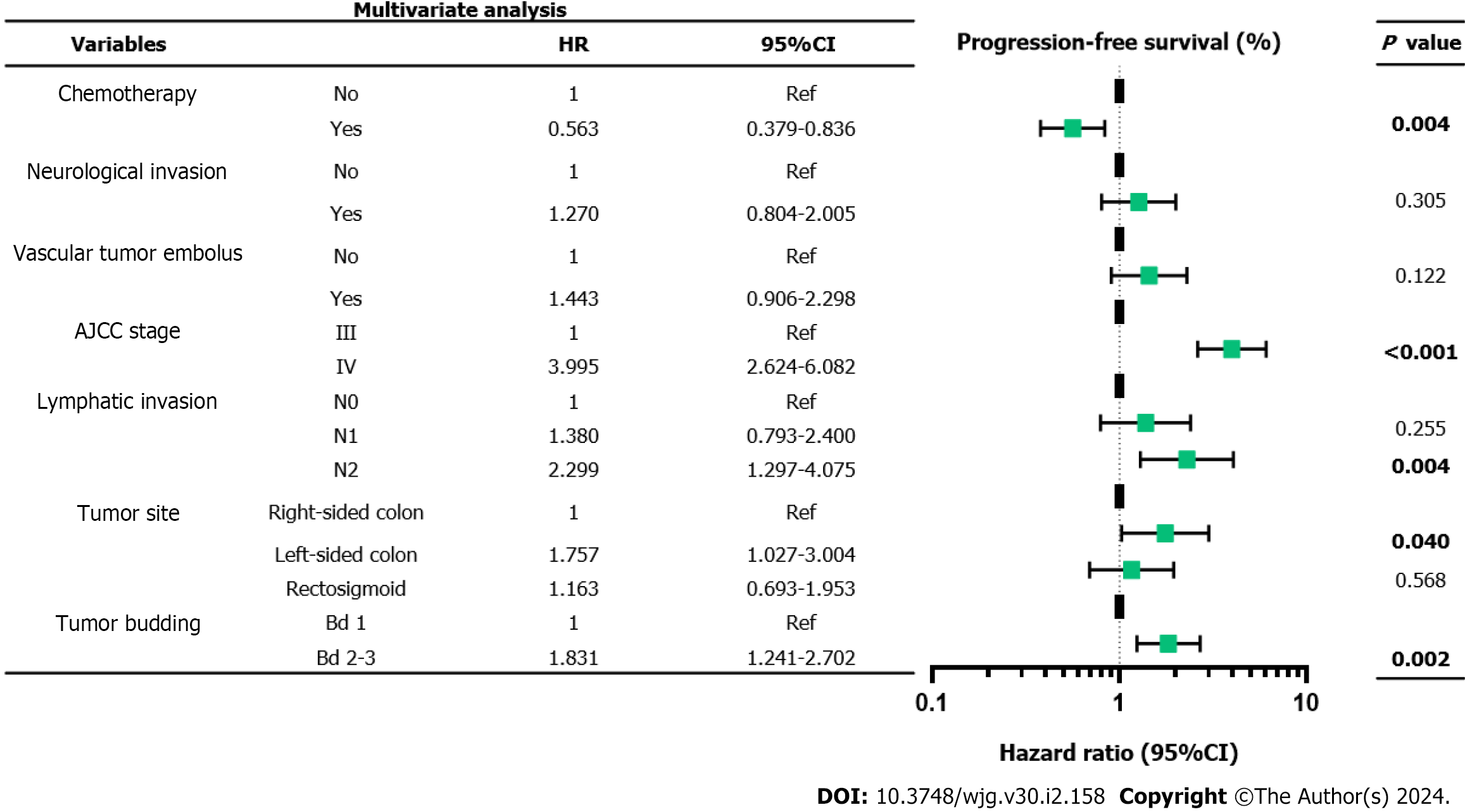
Multivariate Cox proportional hazards regression analysis demonstrated that chemotherapy (P = 0.004), clinical stage IV (P < 0.001), ≥ 4 regional lymph node metastases (P = 0.004), left-sided colonic cancer (P = 0.040), and Bd 2-3 (P = 0.002) were independent prognostic factors in patients with stage III-IV CRC. Moreover, the density of tumor infiltrating lymphocytes was higher in Bd 1 than in Bd 2-3, both in the tumor stroma and its invasive margin.
TB has an independent predictive prognostic value in patients with stage III-IV CRC. It is recommended to complete the TB report of stage III-IV CRC cases in the standardized pathological report to further refine risk stratification.
Core Tip: This study included 547 colorectal cancer (CRC) patients. Tumor budding (TB) was evaluated independently by two pathologists and re-evaluated by a third pathologist when the results were inconsistent, ensuring a high level of reliability. The 2016 International Tumor Budding Consensus Conference recommendations were followed to evaluate TB in patients with stage III-IV CRC, thereby investigating its impact on patient prognosis. TB has predictive prognostic value for progression-free survival and overall survival in patients with stage III-IV CRC. It is recommended to complete the TB report of stage III-IV CRC cases in the standardized pathological report to further refine risk stratification.
- Citation: Luo YH, Yan ZC, Liu JY, Li XY, Yang M, Fan J, Huang B, Ma CG, Chang XN, Nie X. Association of tumor budding with clinicopathological features and prognostic value in stage III-IV colorectal cancer. World J Gastroenterol 2024; 30(2): 158-169
- URL: https://www.wjgnet.com/1007-9327/full/v30/i2/158.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i2.158
Colorectal cancer (CRC) is the third most common malignant tumor in the world[1]. The occurrence and development of CRC represent a complex multifactorial process[2]. Despite significant advancements in medical technology both domestically and internationally, the incidence of CRC has continued to rise in recent years. This trend can be attributed to improvements in people's living standards as well as changes in lifestyle and dietary habits. There were an estimated 1.93 million new CRC cases diagnosed, and 0.94 million CRC-related deaths in 2020 worldwide. China is expected to have the highest estimated number of new CRC cases over the next 20 years. The number of new CRC cases is expected to increase from 0.56 million (2020) to 0.91 million (2040) in China[3].The tumor-node-metastasis (TNM) staging system published simultaneously by the Union for International Cancer Control and The American Joint Committee on Cancer (AJCC) remains a widely accepted standard for classifying malignant tumors[4]. The prognosis of patients with CRC is often influenced by the time gap between cancer detection and treatment initiation. Notably, even patients with identical stage of disease may exhibit diverse clinical outcomes, suggesting the limited predictive value of TNM stage in estimating CRC prognosis. Tumor budding (TB), defined as a single cancer cell of up to four cancer cells at the tumor invasive margin, has emerged as a promising independent prognostic biomarker in CRC[5].
TB reflects an invasive growth pattern with metastatic potential that plays a key role in the tumor microenvironment (TME) and epithelial-mesenchymal transition (EMT). The prognostic role of TB has been extensively studied and currently affects clinical decision making in patients with stage I and II CRC[6,7]. However, existing prognostic studies on TB in stage III CRC have been confined to small retrospective cohort studies. Consequently, this study investigated the correlation among TB categories, clinicopathological features, and prognosis in stage III-IV CRC to further enhance the precision and individualization of treatment through refined prognostic risk stratification.
Tumor-infiltrating lymphocytes (TILs) are a heterogeneous group of cells that leave the blood and migrate to the tumor regions with antigenic effects. They are also an important part of the TME and are generally considered to protect the host against tumor development. It also plays a vital role in inhibiting cancer cell invasion and distant metastasis[8]. The interaction between TB and the immune system is known as the attack-defense model[9]. TB reflects an aggressive disease phenotype; however, TILs can regulate immune function and improve the body's ability to kill tumors. Therefore, in our study, we also discussed the relationship between TB and TILs based on the idea that the combination of TB and TILs can effectively predict the prognosis of stage III-IV CRC patients and analyzed the changes in TILs in cases of different TB levels to preliminarily explore the correlation between TB and the immune microenvironment.
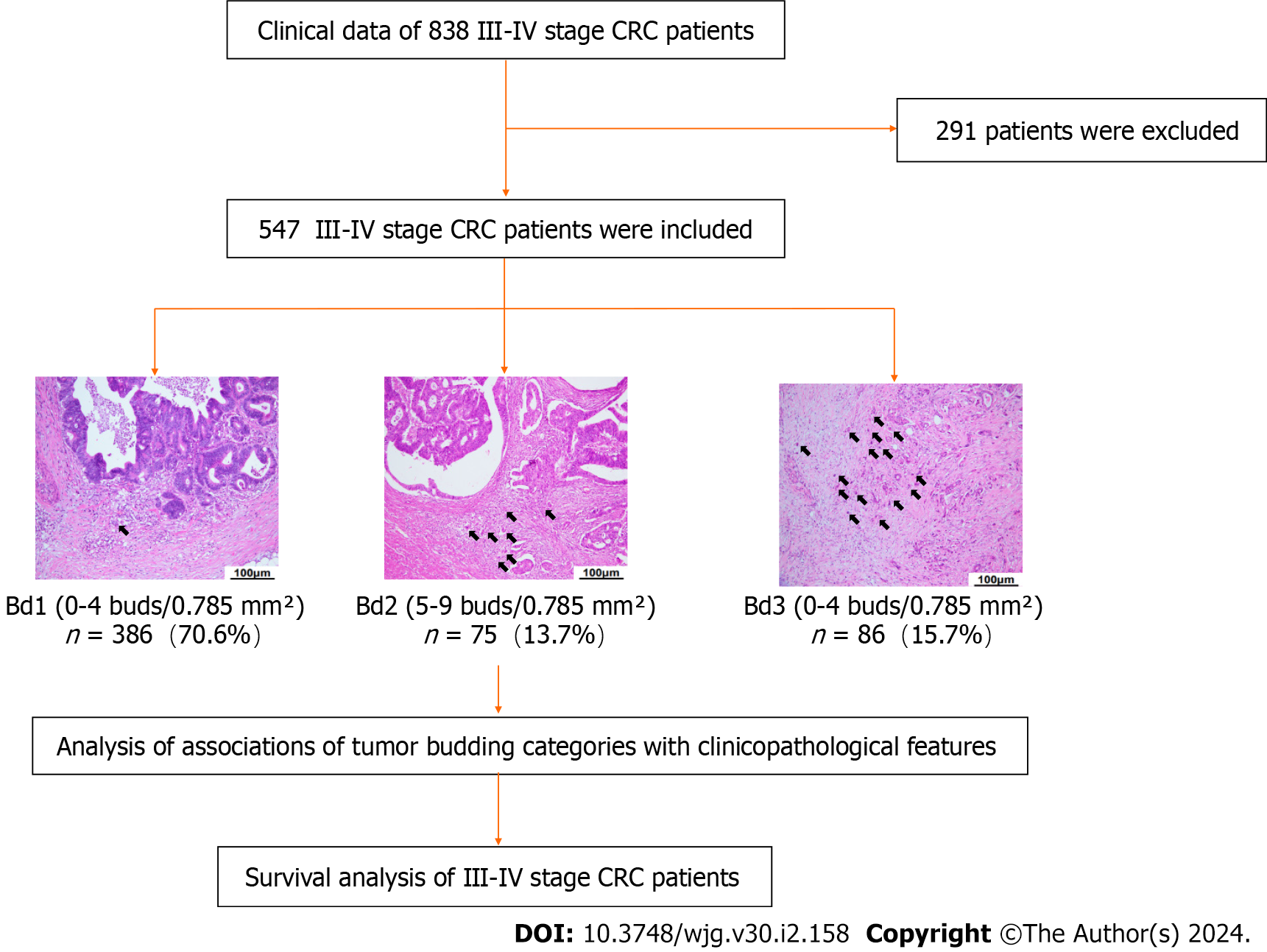
We retrospectively analyzed the medical records and clinical data of 838 patients with stage III-IV resectable CRC pathologically diagnosed at Union Hospital, Tongji Medical College of Huazhong University of Science and Technology (Wuhan City, Hubei Province, China) from January 1, 2015, to December 31, 2018 (Figure 1). A total of 547 patients with CRC were enrolled, and their TB categories and clinicopathological features were analyzed.
The primary objective of this study was to investigate the relationship between TB and progression-free survival (PFS), defined as the time from the beginning of radical surgery to relapse or death, whichever occurred first. The secondary objective was to determine the association between TB and overall survival (OS), defined as the time from the start of radical surgery until death from any cause. The survival time and prognosis of the patients were followed up until the end of the study. The follow-up period was 61 mo. This study was approved by the review committee of the affiliated institution of Tongji Medical College of Huazhong University of Science and Technology (2018-S377), and consent was obtained from all patients participating in the study.
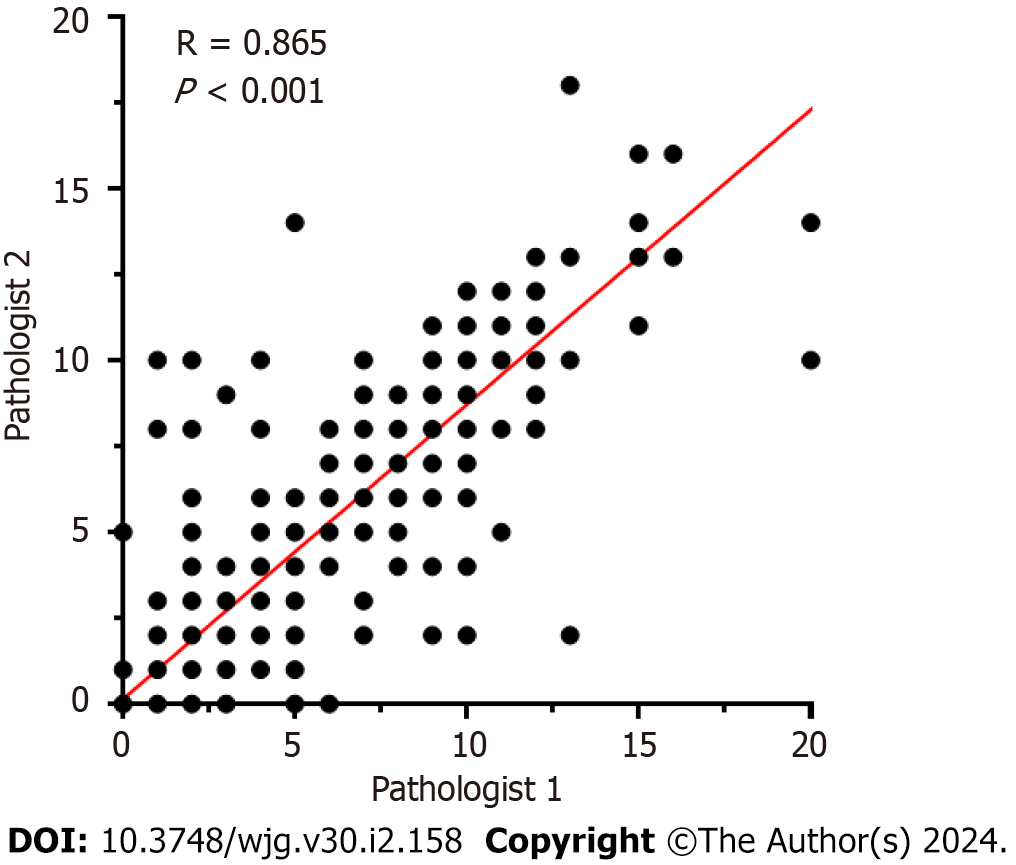
TB was assessed on scanned hematoxylin and eosin (H&E)-stained tissue sample slides (Union Hospital, Wuhan, China) and scored at medium power (10 × to 20 × magnification) in a single hotspot field area normalized to 0.785 mm2 at the invasive front according to the 2016 International TB Consensus Conference (ITBCC 2016). TB was defined as a single tumor cell or a cell cluster of up to four tumor cells at the invasive margin. Tumor buds were counted independently by two pathologists (Yue-Hao Luo and Zhe-Cheng Yan), and any discrepancies were resolved by a third expert (Xiao-Na Chang). The TB scoring categories were Bd 1 (0-4 buds: Low), Bd 2 (5-9 buds: Intermediate), and Bd 3 (≥ 10 buds: High).
All statistical analyses were performed using the statistical software SPSS version 27.0, with two-sided statistical testing, and P < 0.05 was considered statistically significant. Clinicopathological features and KRAS, NRAS, BRAF mutation status of the patients were descriptively analyzed. The χ2 test (or Fisher’s exact test, if appropriate) was used to evaluate the relationship between the TB categories and clinicopathological features. Spearman correlation analysis was used to evaluate the consistency between the TB categories evaluated by the two pathologists (Figure 2). Kaplan-Meier survival analysis was used to compare PFS and OS between patients with TB and those with other clinicopathological features. Cox proportional hazard regression models were used to estimate hazard ratios (HRs) and 95% confidence interval (CI). The association of baseline parameters with PFS and OS was first examined using univariate Cox analysis, and variables with P < 0.05 were entered into a Cox regression multivariate model. After normal distribution detection of TILs in the tumor stroma and at the TB of the tumor invasion front was conducted, it was found that both groups of data were biased. The Mann-Whitney U test was used to evaluate the relationship between TILs in the tumor stroma and TILs at TB of the tumor invasion front with Bd 1 and Bd 2-3, respectively.
The clinical data of 838 CRC patients were retrospectively collected. The TB of 547 CRC patients was assessed. The frequency of Bd 1, Bd 2, and Bd 3 were 70.6%, 13.7%, and 15.7%, respectively. The clinicopathological characteristics are listed in Table 1. Patients with KRAS, NRAS, BRAF mutations are listed in Table 2. TB categories were correlated with vascular tumor embolus (P = 0.004), neurological invasion (P < 0.001), AJCC stage III-IV (P = 0.006), and lymph node metastasis (LNM) (P = 0.019), but they were not significantly correlated with age, sex, chemotherapy, radiotherapy or tumor site (P > 0.05).
| Characteristics | Total, n = 547 | Bd 1, n = 386 | Bd 2-3, n = 161 | P value |
| Sex | 0.514 | |||
| Male | 317 (58.0) | 224 (58.0) | 93 (57.8) | |
| Female | 230 (42.0) | 162 (42.0) | 68 (42.2) | |
| Age in yr | 0.141 | |||
| < 60 | 276 (50.5) | 201 (52.1) | 75 (46.6) | |
| ≥ 60 | 271 (49.5) | 185 (47.9) | 86 (53.4) | |
| Chemotherapy | 0.055 | |||
| Yes | 319 (58.3) | 234 (60.6) | 85 (52.8) | |
| No | 228 (41.7) | 152 (39.4) | 76 (47.2) | |
| Radiotherapy | 0.119 | |||
| Yes | 32 (5.9) | 26 (6.7) | 6 (3.7) | |
| No | 515 (94.1) | 360 (93.3) | 155 (96.3) | |
| Perineural invasion | < 0.001 | |||
| Yes | 171 (31.3) | 100 (25.9) | 71 (44.1) | |
| No | 376 (68.7) | 286 (74.1) | 90 (55.9) | |
| Vascular invasion | 0.004 | |||
| Yes | 152 (27.8) | 94 (24.4) | 58 (36.0) | |
| No | 395 (72.2) | 292 (75.6) | 103 (64.0) | |
| Clinical stages | 0.006 | |||
| 3 | 436 (79.7) | 319 (82.6) | 117 (72.7) | |
| 4 | 111 (20.3) | 67 (17.4) | 44 (27.3) | |
| Lymphatic invasion | 0.019 | |||
| N0 | 134 (24.5) | 105 (27.2) | 29 (18.0) | |
| N1 | 258 (47.2) | 183 (47.4) | 75 (46.6) | |
| N2 | 155 (28.3) | 98 (25.4) | 57 (35.4) | |
| Tumor location | 0.458 | |||
| Right-sided colon | 167 (30.5) | 124 (32.1) | 43 (26.7) | |
| Left-sided colon | 136 (24.9) | 93 (24.1) | 43 (26.7) | |
| Rectosigmoid | 244 (44.6) | 169 (43.8) | 75 (46.6) |
| Characteristics | Total, n = 112 | Bd 1, n = 87 | Bd 2-3, n = 25 | P value |
| KRAS | 0.064 | |||
| Wild | 53 (47.3) | 45 (51.7) | 8 (32.0) | |
| Mutant | 59 (52.7) | 42 (48.3) | 17 (68.0) | |
| NRAS | 0.275 | |||
| Wild | 107 (95.5) | 82 (94.3) | 25 (100.0) | |
| Mutant | 5 (4.5) | 5 (5.7) | 0 (0.0) | |
| BRAF | 0.597 | |||
| Wild | 106 (94.6) | 82 (94.3) | 24 (96.0) | |
| Mutant | 6 (5.4) | 5 (5.7) | 1 (4.0) |
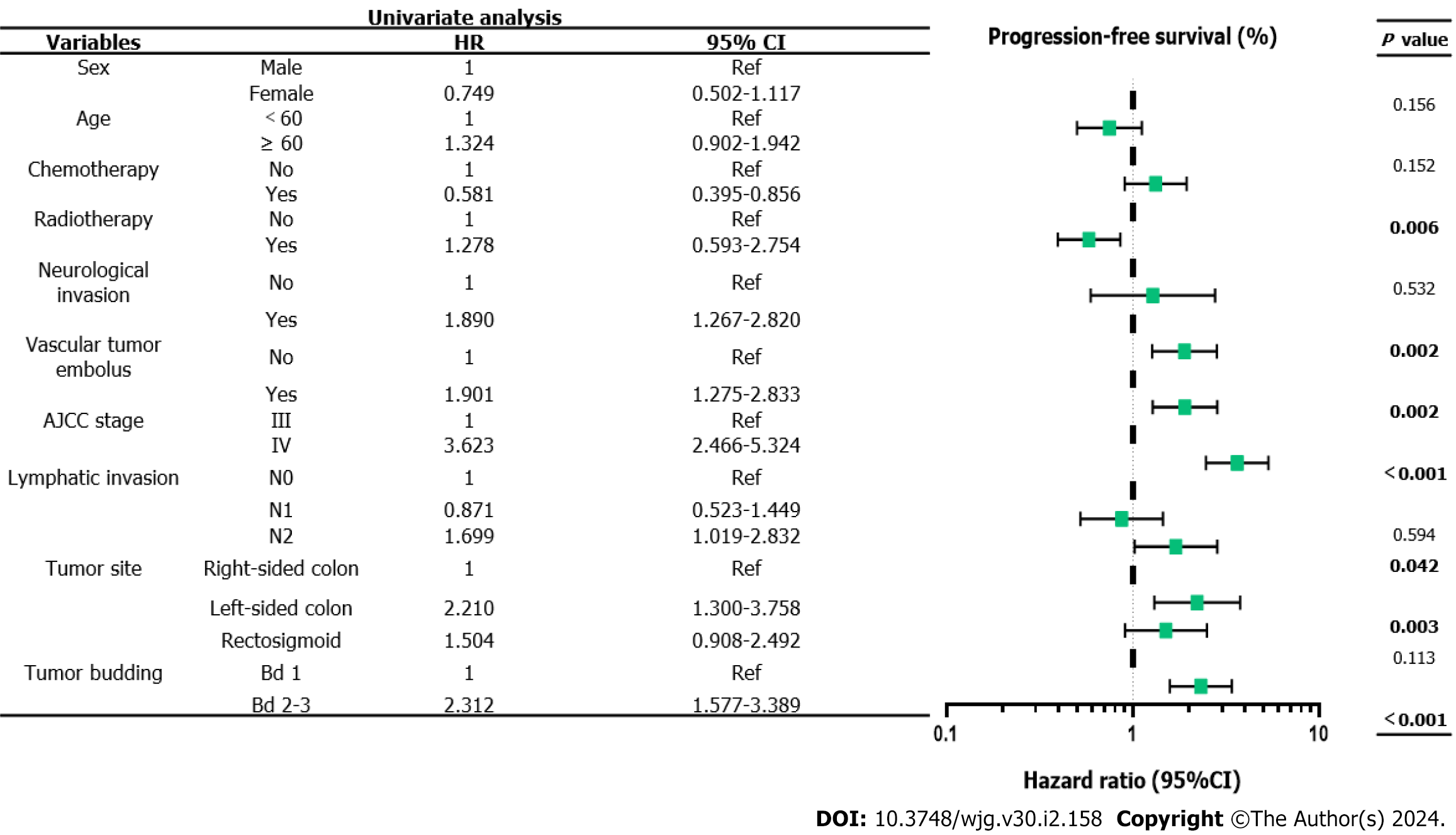
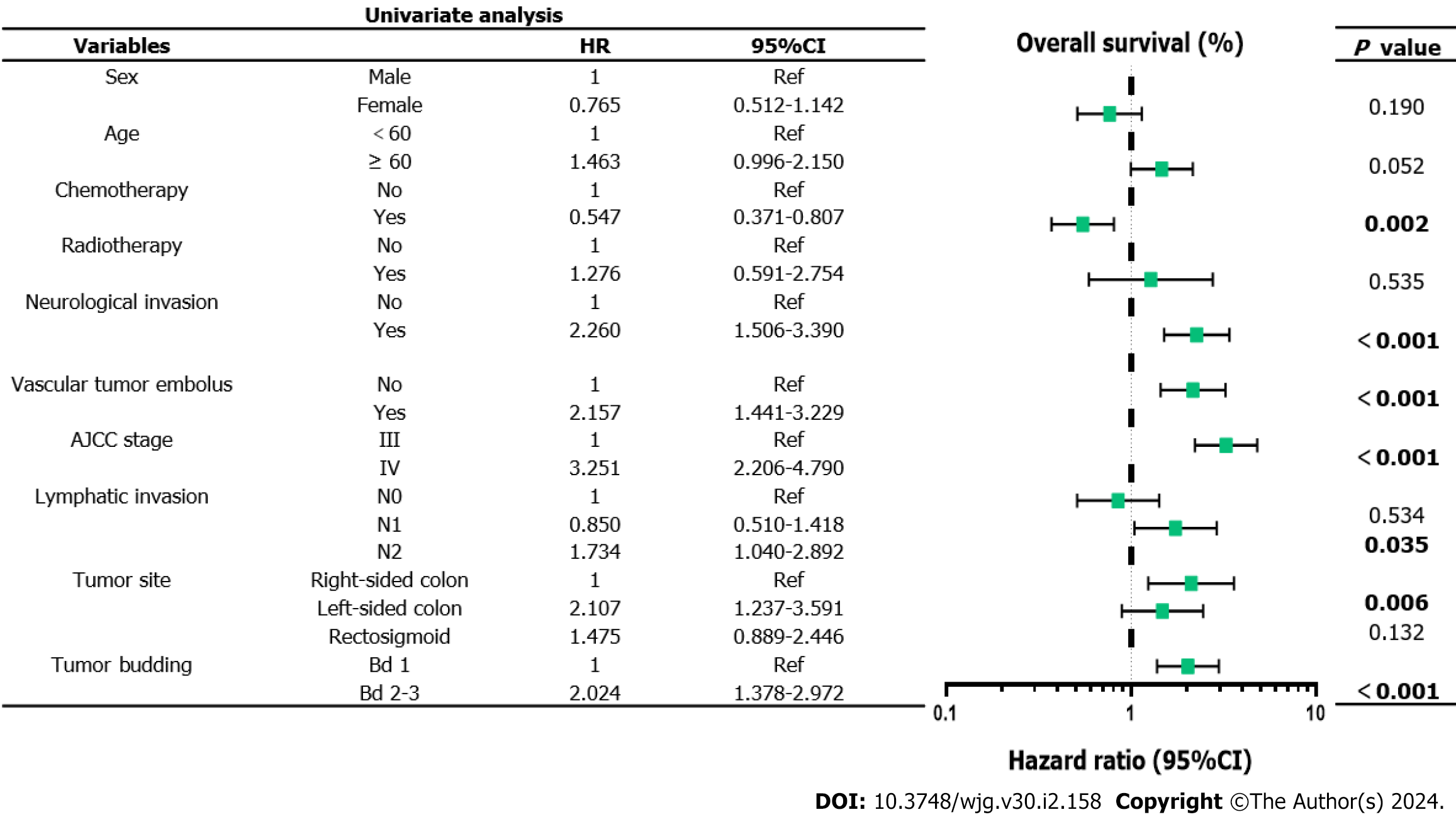
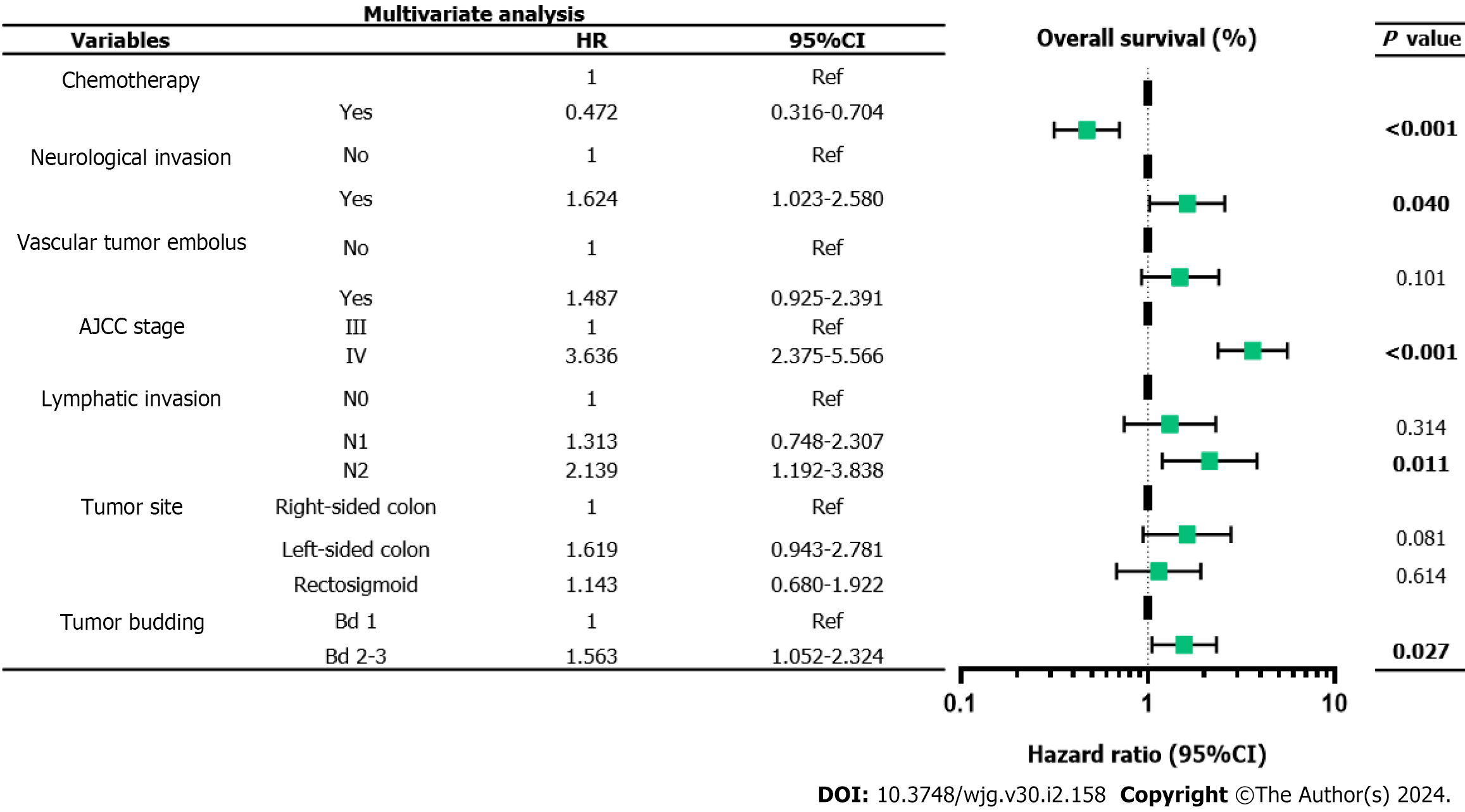
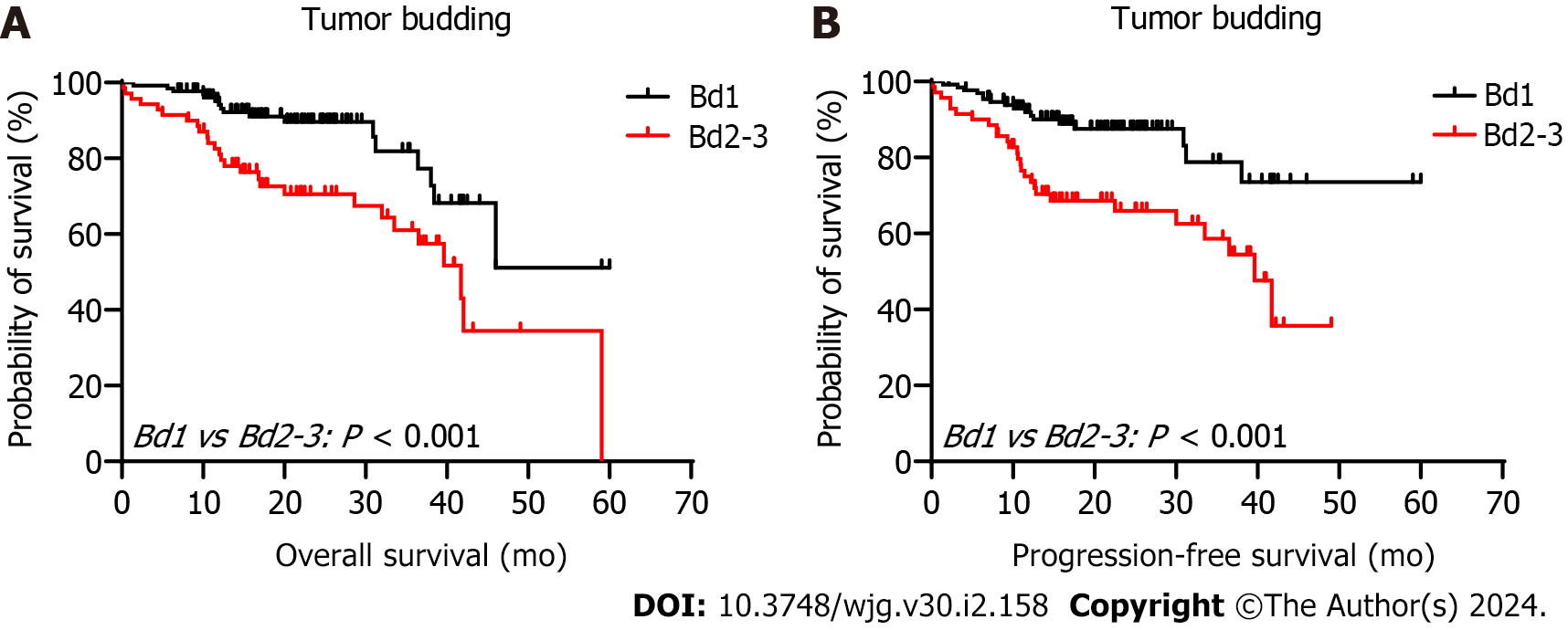
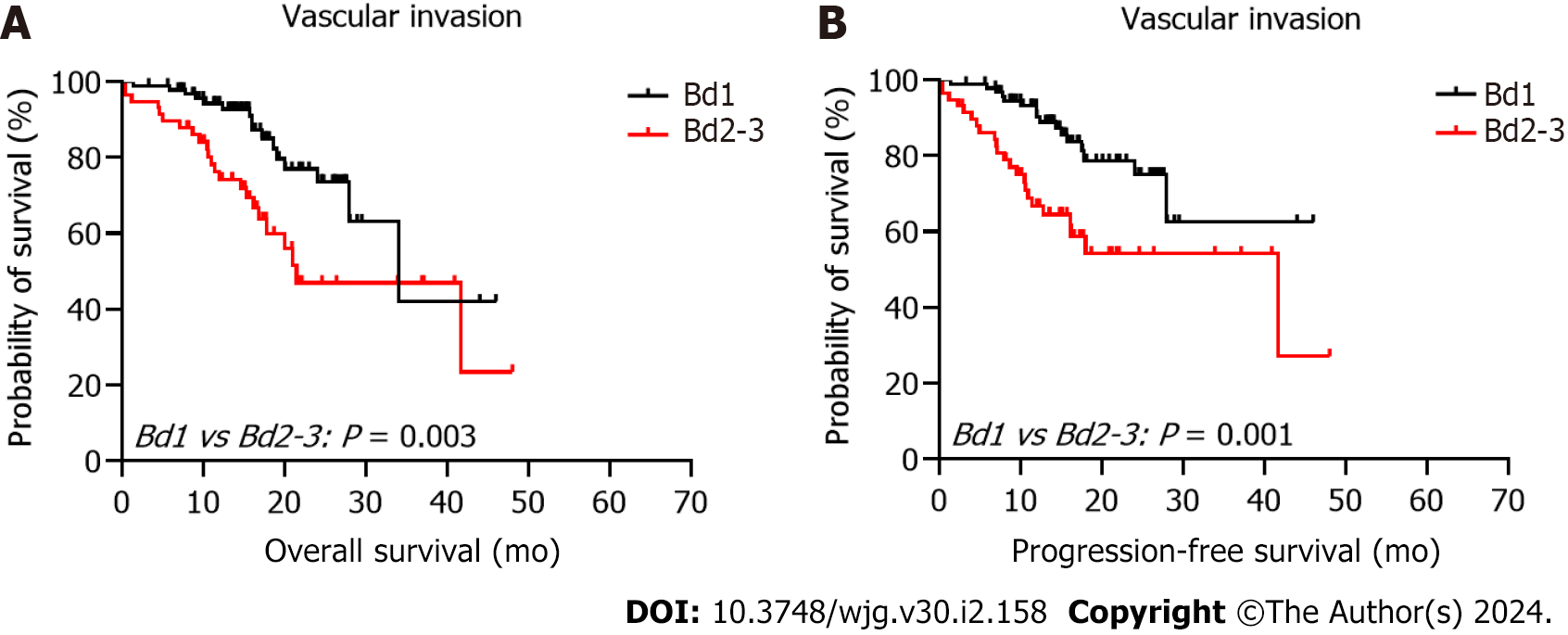
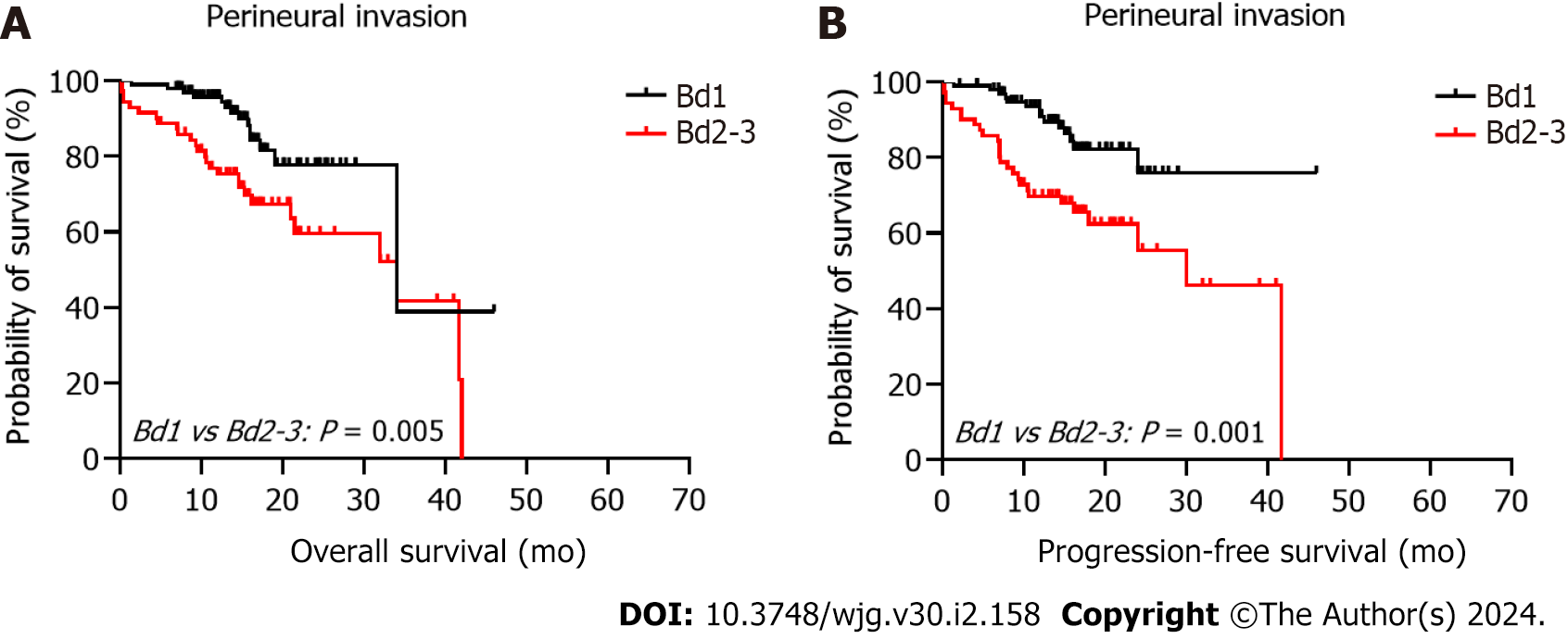
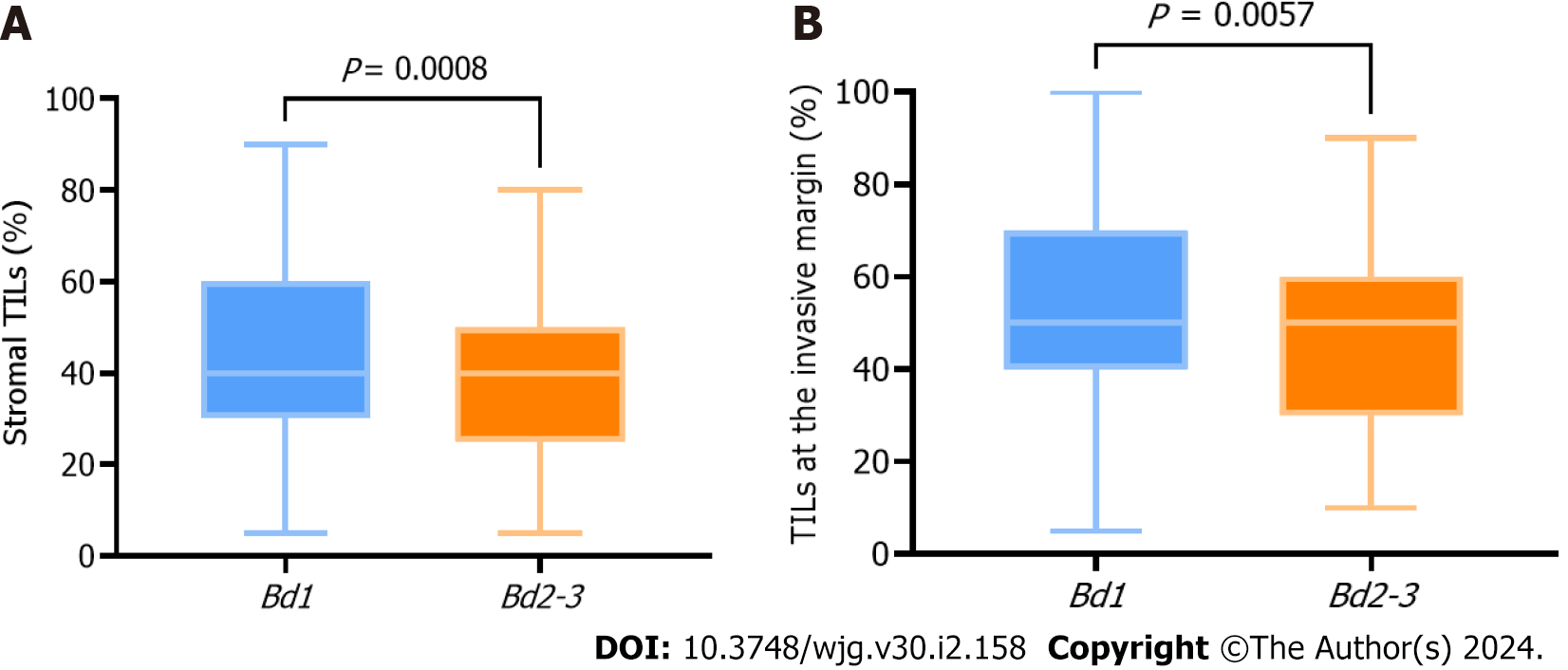
The 5-year PFS and 5-year OS rates of Bd 1 vs Bd 2-3 were 72% vs 45% (P < 0.001) and 49% vs 17% (P < 0.001), respectively. In univariable analysis, Bd 2-3 was associated with shorter PFS (HR: 2.312; 95%CI: 1.577 to 3.389; P < 0.001) (Figure 3) and OS (HR: 2.024, 95%CI: 1.378 to 2.972; P = 0.002) (Figure 4). Moreover, neurological invasion (HR: 1.890, 95%CI: 1.267 to 2.820; P = 0.002), vascular tumor embolus (HR: 1.901, 95%CI: 1.275 to 2.833; P = 0.002), AJCC stage IV (HR: 3.623, 95%CI: 2.466 to 5.324; P < 0.001), N2 (number of lymph node metastases ≥ 4) (HR: 1.699, 95%CI: 1.019 to 2.832; P = 0.042), and left-sided colon cancer (HR: 2.210, 95%CI: 1.300 to 3.758; P = 0.003) were associated with shorter PFS, and the prognosis of patients with chemotherapy was better (HR: 0.581, 95%CI: 0.395 to 0.856; P = 0.006). The other clinicopathological variables were not correlated with PFS (P > 0.05). Multivariate Cox proportional hazards regression analysis demonstrated that AJCC stage IV, N2 (number of lymph node metastases ≥ 4), left-sided colon cancer, and Bd 2-3 were independent risk factors for stage III-IV CRC. Similarly, chemotherapy (P = 0.004) was an independent protective factor (Figures 5 and 6). Kaplan-Meier survival analysis (Figure 7) showed that patients with Bd 1 had a better prognosis than those with Bd 2-3. Meanwhile, when further grouping, we found that patients with Bd 1 had longer PFS and OS than those with Bd 2-3 in the invasion area (perineural and vascular invasions), and the difference was statistically significant (Figures 8 and 9). The density of TILs was higher in Bd 1 than in Bd 2-3, both in the stromal area and invasive margin (Figure 10).
As early as 1954, Imai first reported the phenomenon of TB in various solid tumors and its correlation with prognosis of disease[10]. Subsequent studies have further demonstrated that TB serves as an independent prognostic biomarker in CRC and is observed in a variety of solid tumors, including tongue, larynx, esophagus, stomach, breast, and intrahepatic cholangiocarcinomas. It has been shown to be predictive of cancer progression[11-14].
Currently, high-grade TB is an independent predictor of LNM to determine subsequent surgical treatment for stage I CRC[6,15]. In particular, Bd 3 is a strong adverse prognostic factor used to identify those stage II CRC patients with high recurrence rates and high mortality to guide adjuvant treatment[16]. In the new 2017 TNM and 2019 World Health Organization classifications, TB is regarded as an important complementary biological marker for predicting the prognosis of CRC[17]. Although the prognostic impact of TB on stage I and II CRC is clear, the application of this criterion in clinical practice for treatment decision-making in stage III-IV CRC is limited. This is mainly due to the lack of relevant research data in this context[18]. In this study, 547 patients with stage III-IV CRC were evaluated for TB and TILs according to the ITBCC 2016 scoring system. Combined with patient history, clinicopathological data and patient prognosis, the association between TB and various clinicopathological features and TILs, as well as the prognostic significance, were analyzed. These results suggest that TB independently affects the prognostic outcome of stage III-IV CRC.
The results of our study are consistent with the conclusion reached by Akabane et al[18] who retrospectively collected clinicopathological data from 237 patients with stage III CRC in a single center. This study showed that Bd 1 patients showed significantly better disease-free survival. The importance of TB in adjuvant treatment decision-making for stage III CRC has been emphasized. Notably, our study showed that TB was an independent adverse prognostic biomarker for PFS and OS in stage III-IV CRC. A single-center retrospective study of 589 patients with stage III CRC reported by Yamadera et al[19] used a binary classification system similar to that of Akabane et al[18] but the cut-off value was different (0-9 buds: Low; ≥ 10 buds: High). The results showed that among patients with stage III CRC, the Bd1 group had better survival after adjuvant chemotherapy. Rogers et al[16] conducted a meta-analysis of 34 studies with a total of 7821 CRC patients at various stages, and found that TB was significantly correlated with LNM (odds ratio [OR]: 4.94, 95%CI: 3.96-6.17; P < 0.00001), 5-year PFS (OR: 5.50, 95%CI: 3.64-8.29; P < 0.00001) and 5-year OS (OR: 4.51, 95%CI: 2.55-7.99; P < 0.0001) . It was concluded that TB was a risk factor for predicting LNM at 5 years, PFS at 5 years, and OS at 5 years in CRC patients, and its inclusion in CRC staging facilitated further risk stratification; however, there was a lack of studies on stage III-IV CRC in a meta-analysis by Rogers et al[16] and the definition and evaluation criteria of TB varied greatly between different studies. Similarly, Graham et al[20] evaluated 553 CRC patients at various stages and found that high-grade TB was an independent predictor of poor disease-specific Survival. Therefore, TB could also be a pathological marker that is clinically practical to further refine risk classification and guide decision-making in stage III-IV CRC.
Interestingly, our study also found that the Bd 1 group had a higher proportion of TILs at the tumor invasive margin, which may be associated with better prognosis. This is consistent with the findings of Nearchou et al[21], who found the deletion of TILs in high-grade TB by exploring the effects of different lymphocyte distribution patterns and TB quantity and density on prognosis in stage II CRC through automatic imaging analysis and machine learning workflow.
During the development of cancer, EMT promotes cancer development by empowering mesenchymal phenotypes associated with highly aggressive tumor cells[22]. TB demonstrates the dynamic process of EMT; that is, tumor cells in the aggressive front acquire an interstitial phenotype. This increases the malignant potential of vascular invasion and distant metastasis, and consequently, metastases to lymph nodes or distant parenchymal organs through the vasculature[23-25].
E-cadherin is an intercellular adhesion protein and pivotal regulatory factor in this process[26,27]. Numerous studies have claimed that in different solid tumors, the expression of E-cadherin is reduced or absent on the cell surface of the tumor invasion front, especially in TB[28-31]. This loss is usually accompanied by a decrease in β-catenin expression, indicating that the WNT pathway in cells may be activated. Hence, budding tumor cells can degrade the extracellular matrix (ECM) and invade and migrate through the surrounding stroma. In addition, it has been found that the expression of laminin 5γ2 is usually increased in response to tumor invasion[32]. In the gene ontology study, RNA sequencing data from the TB region and the corresponding central tumor region were compared and analyzed. The expression of genes involved in integrin-mediated cell adhesion, migration, cytoskeleton rearrangement and ECM degradation were found to be significantly different between the two regions[33]. Koelzer et al[34] found that there is an immune escape mechanism in EMT, in which tumor cells lose the expression of their major histocompatibility complex, allowing them to evade recognition and attack by the immune system.
Finally, our study included a substantial number of 547 CRC patients. TB was evaluated independently by two pathologists and re-evaluated by a third pathologist when the results were inconsistent, ensuring a high level of reliability in the TB assessment. Furthermore, the ITBCC 2016 recommendations were followed to evaluate TB specifically in patients with stage III-IV CRC, thereby investigating its impact on patient prognosis.
However, this study had some limitations. First, this study was a retrospective analysis conducted at a single center, which may introduce some degree of bias as the study population may not represent all CRC patients in China. Second, using a single index as a risk factor still has some drawbacks, which can be solved by constructing a prognosis model with a multi-index multidimensional association algorithm. Furthermore, the accuracy of the assessment results based on TILs on H&E slides must be improved to provide better medical strategies and achieve individualized precision treatment for patients with CRC. Future efforts should focus on utilizing artificial intelligence techniques, such as digital pathology methods, to enhance the accuracy and reproducibility of TB and TIL assessments. Additionally, integrating TB with the immunoscore could refine the risk stratification of stage III-IV CRC.
TB has an independent predictive prognostic value for PFS and OS in patients with stage III-IV CRC. It is recommended to complete the TB report of stage III-IV CRC cases in the standardized pathological report to further refine risk stratification.
Tumor budding (TB) is a novel prognostic biomarker that may influence clinical treatment decisions in stage I-II colorectal cancer (CRC) patients. This study analyzed the relationship between TB categories and clinicopathological characteristics and assess their prognostic value in stage III-IV CRC to further refine the prognostic risk stratification of stage III-IV CRC. Additionally, we analyzed changes in tumor-infiltrating lymphocytes (TILs) in patients with different TB categories and initially explored the correlation between TB and the tumor immune microenvironment.
To explore the association of TB with clinicopathological features and prognostic value in stage III-IV CRC.
This study analyzed the relationship between TB categories and clinicopathological characteristics and assess their prognostic value in stage III-IV CRC to further refine the prognostic risk stratification of stage III-IV CRC.
This study included a substantial number of 547 CRC patients. TB was evaluated independently by two pathologists and re-evaluated by a third pathologist when the results were inconsistent, ensuring a high level of reliability in the TB assessment. Furthermore, the 2016 International TB Consensus Conference recommendations were followed to evaluate TB specifically in patients with stage III-IV CRC, thereby investigating its impact on patient prognosis.
Multivariate Cox proportional hazards regression analysis demonstrated that chemotherapy, clinical stage IV, ≥ 4 regional lymph node metastases, left-sided colonic cancer, and Bd 2-3 were independent prognostic factors in patients with stage III-IV CRC. Moreover, the density of TILs was higher in Bd 1 than in Bd 2-3, both in the tumor stroma and its invasive margin.
TB has an independent predictive prognostic value for progression-free survival and overall survival in patients with stage III-IV CRC. It is recommended to complete the TB report of stage III-IV CRC cases in the standardized pathological report to further refine risk stratification.
A multicenter prospective study with large samples should be conducted in the future, and constructing a prognosis model with a multi-index multidimensional association algorithm with other prediction models is needed to further verify its reliability.
We thank all the patients and their family members involved in the study without which the advancements described herein would have not been made possible.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jeong KY, South Korea; Lim YC, Brunei Darussalam S-Editor: Li L L-Editor: Filipodia P-Editor: Zhao S
| 1. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2912] [Article Influence: 364.0] [Reference Citation Analysis (3)] |
| 2. | Murphy N, Moreno V, Hughes DJ, Vodicka L, Vodicka P, Aglago EK, Gunter MJ, Jenab M. Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol Aspects Med. 2019;69:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 3. | Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14:101174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 687] [Cited by in RCA: 1354] [Article Influence: 338.5] [Reference Citation Analysis (5)] |
| 4. | Basile D, Broudin C, Emile JF, Falcoz A, Pagès F, Mineur L, Bennouna J, Louvet C, Artru P, Fratte S, Ghiringhelli F, André T, Derangère V, Vernerey D, Taieb J, Svrcek M; for PRODIGE investigators, GERCOR, Fédération Française de Cancerologie Digestive, and UNICANCER. Tumor budding is an independent prognostic factor in stage III colon cancer patients: a post-hoc analysis of the IDEA-France phase III trial (PRODIGE-GERCOR). Ann Oncol. 2022;33:628-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, El Zimaity H, Fléjou JF, Hansen TP, Hartmann A, Kakar S, Langner C, Nagtegaal I, Puppa G, Riddell R, Ristimäki A, Sheahan K, Smyrk T, Sugihara K, Terris B, Ueno H, Vieth M, Zlobec I, Quirke P. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017;30:1299-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 729] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 6. | Cappellesso R, Luchini C, Veronese N, Lo Mele M, Rosa-Rizzotto E, Guido E, De Lazzari F, Pilati P, Farinati F, Realdon S, Solmi M, Fassan M, Rugge M. Tumor budding as a risk factor for nodal metastasis in pT1 colorectal cancers: a meta-analysis. Hum Pathol. 2017;65:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Ueno H, Ishiguro M, Nakatani E, Ishikawa T, Uetake H, Matsuda C, Nakamoto Y, Kotake M, Kurachi K, Egawa T, Yasumasa K, Murata K, Ikawa O, Shinji S, Murotani K, Matsui S, Teramukai S, Tomita N, Sugihara K; SACURA Study Group. Prospective Multicenter Study on the Prognostic and Predictive Impact of Tumor Budding in Stage II Colon Cancer: Results From the SACURA Trial. J Clin Oncol. 2019;37:1886-1894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 8. | Lugli A, Karamitopoulou E, Panayiotides I, Karakitsos P, Rallis G, Peros G, Iezzi G, Spagnoli G, Bihl M, Terracciano L, Zlobec I. CD8+ lymphocytes/ tumour-budding index: an independent prognostic factor representing a 'pro-/anti-tumour' approach to tumour host interaction in colorectal cancer. Br J Cancer. 2009;101:1382-1392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Lugli A, Zlobec I, Berger MD, Kirsch R, Nagtegaal ID. Tumour budding in solid cancers. Nat Rev Clin Oncol. 2021;18:101-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 10. | IMAI T. Growth patterns in human carcinoma. Their classification and relation to prognosis. Obstet Gynecol. 1960;16:296-308. [PubMed] |
| 11. | Berg KB, Schaeffer DF. Tumor budding as a standardized parameter in gastrointestinal carcinomas: more than just the colon. Mod Pathol. 2018;31:862-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Voutsadakis IA. Prognostic role of tumor budding in breast cancer. World J Exp Med. 2018;8:12-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Beer A, Reber A, Paireder M, Schoppmann SF, Heber S, Schiefer AI. Tumor cell budding in preoperative biopsies of esophageal and gastroesophageal junction carcinoma independently predicts survival in a grade-dependent manner. Surgery. 2022;172:567-574. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Kosaka H, Ishida M, Ueno M, Komeda K, Hokutou D, Iida H, Hirokawa F, Matsui K, Sekimoto M, Kaibori M. Tumor budding may be a promising prognostic indicator in intrahepatic cholangiocarcinoma: A multicenter retrospective study. Ann Gastroenterol Surg. 2023;7:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 15. | Bosch SL, Teerenstra S, de Wilt JH, Cunningham C, Nagtegaal ID. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. 2013;45:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 297] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 16. | Rogers AC, Winter DC, Heeney A, Gibbons D, Lugli A, Puppa G, Sheahan K. Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br J Cancer. 2016;115:831-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 17. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2441] [Article Influence: 488.2] [Reference Citation Analysis (3)] |
| 18. | Akabane S, Shimizu W, Takakura Y, Kochi M, Taguchi K, Nakashima I, Sato K, Hattori M, Egi H, Sentani K, Yasui W, Ohdan H. Tumor budding as a predictive marker for 5-fluorouracil response in adjuvant-treated stage III colorectal cancer. Int J Clin Oncol. 2021;26:1285-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Yamadera M, Shinto E, Kajiwara Y, Mochizuki S, Okamoto K, Hase K, Yamamoto J, Ueno H. Differential Survival Benefits of 5-Fluorouracil-Based Adjuvant Chemotherapy for Patients With Microsatellite-Stable Stage III Colorectal Cancer According to the Tumor Budding Status: A Retrospective Analysis. Dis Colon Rectum. 2019;62:1316-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Graham RP, Vierkant RA, Tillmans LS, Wang AH, Laird PW, Weisenberger DJ, Lynch CF, French AJ, Slager SL, Raissian Y, Garcia JJ, Kerr SE, Lee HE, Thibodeau SN, Cerhan JR, Limburg PJ, Smyrk TC. Tumor Budding in Colorectal Carcinoma: Confirmation of Prognostic Significance and Histologic Cutoff in a Population-based Cohort. Am J Surg Pathol. 2015;39:1340-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Nearchou IP, Lillard K, Gavriel CG, Ueno H, Harrison DJ, Caie PD. Automated Analysis of Lymphocytic Infiltration, Tumor Budding, and Their Spatial Relationship Improves Prognostic Accuracy in Colorectal Cancer. Cancer Immunol Res. 2019;7:609-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 22. | Derynck R, Weinberg RA. EMT and Cancer: More Than Meets the Eye. Dev Cell. 2019;49:313-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 23. | Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher's conceptual friend and foe. Am J Pathol. 2009;174:1588-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 400] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 24. | Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1356] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 25. | Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2456] [Cited by in RCA: 2573] [Article Influence: 160.8] [Reference Citation Analysis (0)] |
| 26. | Zlobec I, Lugli A, Baker K, Roth S, Minoo P, Hayashi S, Terracciano L, Jass JR. Role of APAF-1, E-cadherin and peritumoral lymphocytic infiltration in tumour budding in colorectal cancer. J Pathol. 2007;212:260-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Kohler I, Bronsert P, Timme S, Werner M, Brabletz T, Hopt UT, Schilling O, Bausch D, Keck T, Wellner UF. Detailed analysis of epithelial-mesenchymal transition and tumor budding identifies predictors of long-term survival in pancreatic ductal adenocarcinoma. J Gastroenterol Hepatol. 2015;30 Suppl 1:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Jensen DH, Dabelsteen E, Specht L, Fiehn AM, Therkildsen MH, Jønson L, Vikesaa J, Nielsen FC, von Buchwald C. Molecular profiling of tumour budding implicates TGFβ-mediated epithelial-mesenchymal transition as a therapeutic target in oral squamous cell carcinoma. J Pathol. 2015;236:505-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 29. | Attramadal CG, Kumar S, Boysen ME, Dhakal HP, Nesland JM, Bryne M. Tumor Budding, EMT and Cancer Stem Cells in T1-2/N0 Oral Squamous Cell Carcinomas. Anticancer Res. 2015;35:6111-6120. [PubMed] |
| 30. | Lee SJ, Choi SY, Kim WJ, Ji M, Lee TG, Son BR, Yoon SM, Sung R, Lee EJ, Youn SJ, Park SM. Combined aberrant expression of E-cadherin and S100A4, but not β-catenin is associated with disease-free survival and overall survival in colorectal cancer patients. Diagn Pathol. 2013;8:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Nakagawa Y, Ohira M, Kubo N, Yamashita Y, Sakurai K, Toyokawa T, Tanaka H, Muguruma K, Shibutani M, Yamazoe S, Kimura K, Nagahara H, Amano R, Ohtani H, Yashiro M, Maeda K, Hirakawa K. Tumor budding and E-cadherin expression are useful predictors of nodal involvement in T1 esophageal squamous cell carcinoma. Anticancer Res. 2013;33:5023-5029. [PubMed] |
| 32. | Katayama M, Sekiguchi K. Laminin-5 in epithelial tumour invasion. J Mol Histol. 2004;35:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Zhou B, Zong S, Zhong W, Tian Y, Wang L, Zhang Q, Zhang R, Li L, Wang W, Zhao J, Chen X, Feng Y, Zhai B, Sun T, Liu Y. Interaction between laminin-5γ2 and integrin β1 promotes the tumor budding of colorectal cancer via the activation of Yes-associated proteins. Oncogene. 2020;39:1527-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Koelzer VH, Herrmann P, Zlobec I, Karamitopoulou E, Lugli A, Stein U. Heterogeneity analysis of Metastasis Associated in Colon Cancer 1 (MACC1) for survival prognosis of colorectal cancer patients: a retrospective cohort study. BMC Cancer. 2015;15:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |