Published online Jan 14, 2024. doi: 10.3748/wjg.v30.i2.128
Peer-review started: November 6, 2023
First decision: November 30, 2023
Revised: December 5, 2023
Accepted: December 28, 2023
Article in press: December 28, 2023
Published online: January 14, 2024
Processing time: 66 Days and 20.8 Hours
Emerging evidence and perspectives have pointed towards the heart playing an important role in hepatorenal syndrome (HRS), outside of conventional under
Core Tip: There is emerging evidence to suggest that the heart plays an important role in advanced liver disease and contributes significantly to hepatorenal syndrome (HRS) progression. It is now increasingly agreed upon that circulatory dysfunction in HRS is at least in part due to cardiac impairment, which can exist prior to kidney dysfunction in cirrhotic patients who develop HRS. There are numerous pathophysiological mechanisms which may co-exist in both hepatorenal and cardiorenal syndrome pathways, and treatments which ameliorate kidney dysfunction in HRS are likely to also address the mechanisms which lie within this intricate hepatocardiorenal syndrome entity that is being postulated.
- Citation: Wu HHL, Rakisheva A, Ponnusamy A, Chinnadurai R. Hepatocardiorenal syndrome in liver cirrhosis: Recognition of a new entity? World J Gastroenterol 2024; 30(2): 128-136
- URL: https://www.wjgnet.com/1007-9327/full/v30/i2/128.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i2.128
Hepatorenal syndrome (HRS) is a condition that occurs during decompensated liver disease defined by volume-unresponsive kidney dysfunction in the absence of circulatory shock, identified structural kidney disease, and nephrotoxins[1]. Our current understanding of the condition recognizes the liver as the centerpiece of various patho
Whilst there has been recognition on the negative impact of liver dysfunction upon the cardiovascular system, understanding the exact mechanisms which lead to this have not been fully established[6-8]. Ongoing advancement of laboratory technology and discovery of biomarkers certainly allowed for a more precise and closer monitoring of cardiac and circulatory function in HRS[9]. It is thought that cirrhotic cardiomyopathy occurs in HRS settings in the absence of previous cardiac co-morbidities because of blunted contractile responsiveness to stress stimuli and altered diastolic relaxation with electrophysiological abnormalities[10].
The former mainstream opinion in medicine views HRS and cardiorenal syndrome (CRS) as two separate conditions with distinct pathophysiological pathways. One with acute or chronic liver disease and the other with acute or chronic heart failure leading to kidney complications (in the case of CRS, acute or chronic kidney disease may also result in cardiac dysfunction)[11]. Evidence building from previous clinical observations is gradually pointing towards a significant relationship which connects between the liver, heart, and kidneys mechanistically in disease[12].
Throughout this review, we aim to explore the cardiac contribution towards circulatory changes and dysfunction in HRS, and whether there is indeed an undervalued element of CRS within the HRS pathway. We will also specifically explore the role of inflammation and endothelial dysfunction in the HRS pathway, given previous suggestions of its significance towards cardiorenal interactions in HRS. The temporality (prior onset) of cardiorenal interactions in HRS will be reviewed. With a substantial number of patients who may receive portosystemic shunting in advanced liver disease, we will also discuss the dynamics of cardiorenal interaction following treatment. Advancing our understanding in the intertwined relationship across the liver-heart-kidney connection is important to inform clinicians on the diagnostic and therapeutic implications within this complex setting.
Circulatory function in the early stages of cirrhotic liver disease is characterized by maintenance of its homeostasis through development of a hyperdynamic circulation[13]. This involves an increased cardiac output, heart rate and plasma volume. Because there is a normal or increased cardiac output in the initial phase of cirrhosis, general opinion considers the heart to be largely intact throughout the early disease processes[11,14]. The progression of circulatory dysfunction in cirrhosis is thought to be mainly led by systemic vascular resistance and arterial underfilling in the form of central hypovolemia[15]. It is now increasingly acknowledged that hemodynamic derangement typically occurs in later stages of cirrhosis because progressive decline in hemodynamic status in cardiac afterload is no longer responded to by an increase in cardiac output[11,14,15]. Previous longitudinal studies have demonstrated that baseline cardiac function markers such as cardiac output and stroke volume were significantly lower in cirrhotic patients who subsequently developed HRS, and these markers further decreased with HRS progression[16].
What is significant in linking the pathophysiology that exist between the heart and liver and kidney dysfunction here is that neurohormonal activation is the cornerstone pathway which result in the development of both CRS and HRS[17,18]. In the case of CRS, numerous factors in cardiac dysfunction from a low cardiac output to diuretic treatment may result in heightened neurohormone levels and adverse consequences for the kidneys with their downstream[18]. Decline in estimated glomerular filtration rate and impairment in sodium and water excretion would be expected[19]. Furthermore, deterioration in the hemodynamics and function of the kidneys may occur with elevated right-sided cardiac pressure, leading to increased congestion in the kidney venous system[20]. The heart is a key driving factor for higher neurohormonal activity in later stages of cirrhosis progressing to HRS, considering abnormal cardiac output in the face of unaltered systemic vascular resistance[21,22]. This is supported by seminal results from a study by Krag et al[23], which found patients with cirrhosis who developed kidney failure during spontaneous bacterial peritonitis had a reduced cardiac output compared to those without kidney failure. Following treatment and resolution of spontaneous bacterial peritonitis, an even lower cardiac output was observed amongst patients with kidney failure. This is important to support the claim that abnormalities in cardiac inotropic and chronotropic function is an instrumental component of circulatory dysfunction in HRS, given current evidence point out irregulated neurohormonal activation as a key mechanism in HRS-related cardiac dysfunction[17,18,23].
Nevertheless, there have been more recent observations that it is an increased cardiac output, rather than low cardiac output, that is the instigator for progression to HRS[9,24-26]. Using dobutamine stress echocardiography to monitor patients with cirrhosis, Koshy et al[25] indicated that it is not the hyperdynamic cardiac output at rest, but rather an inability to increment this cardiac output during periods of physiological stress such as where there is infection and hemodynamic stress, that predisposes to the development of HRS. They noted that an impaired cardiac reserve (defined by a change in cardiac reserve < 25% with low-dose dobutamine), was associated with a 4-fold risk of progressing to HRS[25]. The investigators ultimately concluded that a hyperdynamic resting cardiac function may in fact represent patients encroaching on their resting state cardiac reserve[25]. This may have therapeutic implications because the use of β-blockers in this patient group can be harmful. Further study is required before we can verify the pathophysiological claims from this stance more confidently.
Whilst hemodynamic and neurohormonal dysregulation has been primarily thought of as the main pathways of HRS manifestation and circulatory dysfunction in HRS, there is increasing evidence to suggest that systemic inflammation and endothelial dysfunction have a major role in this scenario by triggering splanchnic arterial vasodilation and other pathways[11,27,28]. Inflammatory response and endothelial dysfunction also lead to derangements of other organs such as the heart in decompensated liver disease and HRS[18,27]. The pathophysiological hallmarks of liver cirrhosis are associated with inflammation. Increased macrophage activation, pro-inflammatory cytokines, systemic oxidative stress and activated circulating monocytes and neutrophils are observed in cirrhotic disease[18,27]. In decompensated liver disease, such as when spontaneous bacterial peritonitis is present, it has been described that the degree of inflammation and endothelial dysfunction correlates with the severity of hepatic, cardiac and kidney dysfunction[29]. Commonly it is bacterial infections which precipitate the progression to HRS. The development of an aggressive inflammatory response is stimulated by translocation of bacteria and pathogen-associated molecular patterns from the intestine[30]. Animal models of cirrhosis suggest these profound inflammatory responses, especially the build-up of oxidative stress and tumor necrosis factor-α, may result in β-receptor signaling alterations and cardiac systolic function impairment[31]. It has been demonstrated that serum levels of lipopolysaccharide-binding protein are independently associated with the severity of left ventricular diastolic dysfunction (LVDD)[32,33]. Lipopolysaccharide-binding protein is a marker of bacterial endotoxin exposure[32]. Coupled with convincing evidence demonstrating the instrumental role played by endothelial dysfunction in CRS, such observations are important to indicate that inflammation is a very plausible pathophysiological process linking the liver-heart-kidney connection together in HRS.
It is logical to assume that cardiac dysfunction would precede the manifestations of HRS in cirrhosis here given the postulations of how cardiac dysfunction may mechanistically contribute towards kidney dysfunction in cirrhosis. The original observational study by Ruiz-del-Arbol et al[34] have found that cirrhotic patients who develop HRS already presented with clinical features of cardiac dysfunction in the form of low stroke volume, for example, prior to kidney function decline. In their cohort of patients, Ruiz-del-Arbol et al[34] noted that a low cardiac output and increased plasma-renin activity at baseline appeared to be the only independent predictors for HRS development. Krag et al[23] found that there were more patients with low cardiac index at baseline (defined as < 1.5 L/min/m2 measured by gated myocardial perfusion imaging) who developed HRS compared to those with higher cardiac index levels. Albeit significant additional cardiovascular stress during the procedure, the general direction of evidence have pointed towards liver transplantation reversing these aspects of cardiac dysfunction which are commonly observed in HRS, resulting in improved cardiac performance and restored hemodynamics post-transplantation[35,36]. These observations highlighted a temporal pattern of cardiac and kidney dysfunction in HRS, suggesting perhaps there is pathophysiological involvement of the heart in the manifestation of HRS and cardiac dysfunction is not simply just the consequence of a HRS-associated complication[23,37].
Given there could be cardiac contribution towards a myriad of pathophysiological effects in HRS as outlined from our review, it would be prudent to systematically evaluate and monitor cardiac function in advanced liver disease patients, including for those without previously known cardiac impairment. In cirrhotic disease and early HRS, abnormalities in left ventricular systolic function is usually identified late and often only diagnosed when individuals display a blunted response during hemodynamic or pharmacologic stress[38]. Therefore, an abnormal left ventricular systolic function may be clinically utilized as a specific prognostic indicator to determine at-risk subgroups of cirrhotic patients who are at higher risk of developing HRS[16]. The advent of more recently developed imaging methods may allow for better identification of subclinical cardiac dysfunction in HRS. For one, 2-dimensional speckle-tracking echocardiography assesses left ventricular regional myocardial function and global longitudinal strain (GLS) through tracking natural acoustic markers such as speckles[39,40]. This technique is likely to be less dependent on cardiac preload or afterload in comparison to standard echocardiography. A previous study by Altekin et al[41] using 2-dimensional speckle-tracking echocardiography has revealed that a reduced longitudinal systolic function is common amongst cirrhosis patients despite a normal ejection fraction.
A very recent study by Danielsen et al[9] utilized magnetic resonance imaging to assess cardiac function and peripheral blood flow in patients across a spectrum of liver disease, including those with HRS. The investigators characterized renal blood flow in their study where despite a high cardiac output in patients with HRS, they demonstrated lower renal artery flow compared to non-HRS groups[9]. These findings indicate that even with a hyperdynamic resting cardiac output there was inadequate kidney perfusion in early stages of HRS triggered by activation of the renin-angiotensin-aldosterone system[9]. It confirms the pathophysiological link of cardiac dysfunction being manifested during periods of physiological stress in cirrhosis resulting in progression to HRS. Moreover, contrary to updated consensus guidance that GLS is a prognostically accurate marker of systolic dysfunction in subclinical myocardial dysfunction in HRS, this study by Danielsen et al[9] concluded no significant differences were found in GLS in HRS patients with different levels of cardiac output.
Although it remains debatable, diastolic dysfunction could be a useful marker for prognostication in cirrhotic cardiomyopathy and HRS, which is often overlooked compared to systolic dysfunction within the clinical setting[42-44]. Ruíz-del-Árbol et al[43] investigated LVDD and its relationship with circulatory function and prognosis in cirrhotic patients with portal hypertension and normal serum creatinine levels. Monitoring 80 patients prospectively with conventional and tissue Doppler echocardiography as well as their systemic and hepatic hemodynamics and the activity of endogenous vasoactive systems, Ruíz-del-Árbol et al[43] noted 37 patients out of the 80 presenting with LVDD in which 14 of these 37 patients went on to develop HRS. They concluded that patient survival was associated with the extent of LVDD, and LVDD occurs simultaneously with changes in cardiac structure and function and is associated with an impairment of effective arterial blood volume in cirrhosis. Ultimately, LVDD demonstrated to be a sensitive marker for progression from cirrhosis to HRS and mortality[43]. These conclusions were concurred in the study from Premkumar et al[44], where their group additionally found that the degree of LVDD is significantly associated with health-related quality of life outcomes in cirrhotic patients.
Whilst guidelines are constantly revised and optimized focusing on the utilization of serum pro-brain natriuretic peptide (pro-BNP) as a screening marker for acute and chronic heart failure, there is emerging discussions over the past decade on its clinical utility to project cirrhosis severity, the presence of cirrhotic cardiomyopathy, and HRS[45,46]. Kapoor et al[47] performed a prospective observational study for 53 cirrhosis patients who underwent 2-dimensional Doppler echocardiography, and had their serum pro-BNP and troponin T levels measured. This study showed that diastolic dysfunction is highly prevalent in cirrhotic patients (56.6% of cohort), and that serum pro-BNP as well as QTc intervals were also significantly raised in those with diastolic dysfunction[47]. Moreover, this group of patients were also more likely to progress to HRS, as well as developing other complications of cirrhosis such as spontaneous bacterial peritonitis and hepatic encephalopathy compared to those without cardiac dysfunction[47]. Overall, despite encouraging developments in research and updated findings, further studies are imperative to inform clinicians on which cardiac testing and imaging methods are cost-effective and clinically reliable to reflect cardiac function in cirrhosis and HRS.
Many patients with decompensated liver disease may receive transjugular intrahepatic shunting (TIPS) to treat portal hypertension[48,49]. The changes in hepatocardiorenal interactions following TIPS are of interest as immediately after the procedure, blood from the splanchnic bed and portal system is unloaded into the systemic circulation[48,49]. The kidney’s hemodynamic status is typically not affected unless there is change in the state of kidney perfusion with renal veins draining directly into the systemic circulation[50]. TIPS have shown efficacy on improving kidney function in patients across all stages of kidney status, where there is evidence of increased urinary sodium excretion indicative of improved kidney perfusion, and improved creatinine clearance[51]. Better kidney perfusion following TIPS is thought to be due to the mediating effects from cardiorenal interplay, which enhances cardiac inotropic function resulting in increased central blood volume and subsequently kidney perfusion[52]. Another positive effect which can result from TIPS to improve kidney function is its ability to improve endothelial function which follows the amelioration of systemic inflammation[53-55]. This is achieved from lowering pressures and shear stress in the portal system and preventing the intestinal translocation of bacteria[55]. More studies are needed to further distinguish the hepatocardiorenal interactions before TIPS and following TIPS in advanced liver disease.
In terms of other medical treatments, beyond its utility as a potent plasma and volume expander, the anti-inflammatory properties of albumin and its role in improving endothelial dysfunction during decompensated liver disease and HRS has further supported the notion that inflammation and endothelial dysfunction are key pathophysiological processes in the proposed hepatocardiorenal syndrome entity[56]. Rat studies have shown the infusion of albumin increases cardiac contractility through counteracting myocardial oxidative stress and inflammation[31]. It has been found that in spontaneous bacterial peritonitis, albumin instead of hydroxyethyl starch increased systemic vascular resistance and the left ventricular stroke work index[57]. The incidence of HRS and mortality rate more than halved in cirrhotic patients with spontaneous bacterial peritonitis if albumin is added to antibiotic treatment[58].
The use of cardiac inotropes to reverse cardiac and kidney dysfunction in HRS have been considered. There are reports of successful use to reverse kidney dysfunction in refractory HRS, which indicates the contributive role of cardiorenal pathways within the HRS setting[59,60]. Otherwise, β-agonists have been thought of as being unsuitable in this setting, given there is downregulation of β-adrenergic receptors in cirrhosis[61,62]. It remains to be clarified what is the optimized strategy in prescribing cardiac inotrope therapy in HRS. There are other emerging treatment options with potential benefits in heart failure such as empagliflozin, urodilatin and urocortin which are currently investigated for use in HRS[63-67]. Serelaxin, which is a recombinant human relaxin-2 with cardioprotective effects in acute heart failure, has been evaluated in a phase II randomized-controlled trial[67]. The investigators noted significantly improved kidney perfusion in patients with decompensated liver disease who were prescribed serelaxin compared to the control group.
It is concurred from the majority of published evidence that there is a positive long-term cardiac outlook for patients with HRS following liver transplantation[36]. An important factor would be the individual’s pre-existing cardiac status prior to transplantation, although cardiac dysfunction in cirrhosis does not appear to be linearly associated with severity of liver disease[43,68,69]. Whilst international consensus on an optimal strategy of assessing pre-transplant cardiac status remains desirable, it is now established that cirrhotic patients with cardiac risk factors selected for liver transplant should undergo a rigid evaluation for operative risk. There is significant cardiovascular stress associated with this major operation, particularly for those with pre-existing cardiac dysfunction due to cirrhosis, hence an accurate patient selection for liver transplantation and precise investigation for degree of cirrhotic cardiomyopathy is vital[70]. Pre-transplant screening for cirrhotic cardiomyopathy should be conducted independently of Child-Pugh or model for end-stage liver disease classifications. Results from electrocardiography may demonstrate early indications of cirrhotic cardiomyopathy in potential transplant candidates from the presence of QT interval prolongation. QT interval prolongation is considered the earliest sign of cirrhotic cardiomyopathy, with the prevalence of QT interval prolongation amongst cirrhotic patients shown to be reaching 60% in those with Child Pugh class C (compared with 25% in Child Pugh class A vs 51% in Child Pugh class B)[71,72]. Otherwise, the American Association for the Study of Liver Diseases currently recommends that it is mandatory for all liver transplant candidates to undergo transthoracic echocardiography as the minimum pre-transplant cardiac investigation[73].
Changes in cardiac preload and afterload due to fluid infusion and clamping of the hepatic vein would result in tremendous stress towards a post-transplant patient’s cardiovascular homeostasis, irrespective of pre-existing cardiac status[74]. Meticulous management to ensure appropriate responses in myocardial contractility is important, and prompt fluid management with cardiac monitoring through transesophageal echocardiography and/or pulmonary artery catheterization are often required in the early post-transplant scenario[75]. There remains much uncertainty as to the optimal timing to restore to restore hemodynamics during the post-transplant phase, but it is expected that a progressive correction of portal hypertension and hyperdynamic status would ensue with a transplanted liver[76]. Previous studies have reported a high prevalence of post-transplant subtle, sub-clinical cardiac complications such as diastolic function deterioration and ventricular dysfunction[77,78]. Nevertheless, the prognostic ability of these events to predict short- and long-term cardiac and overall clinical outcomes remain in discussion due to a relative paucity of data currently. Further work to address this is needed.
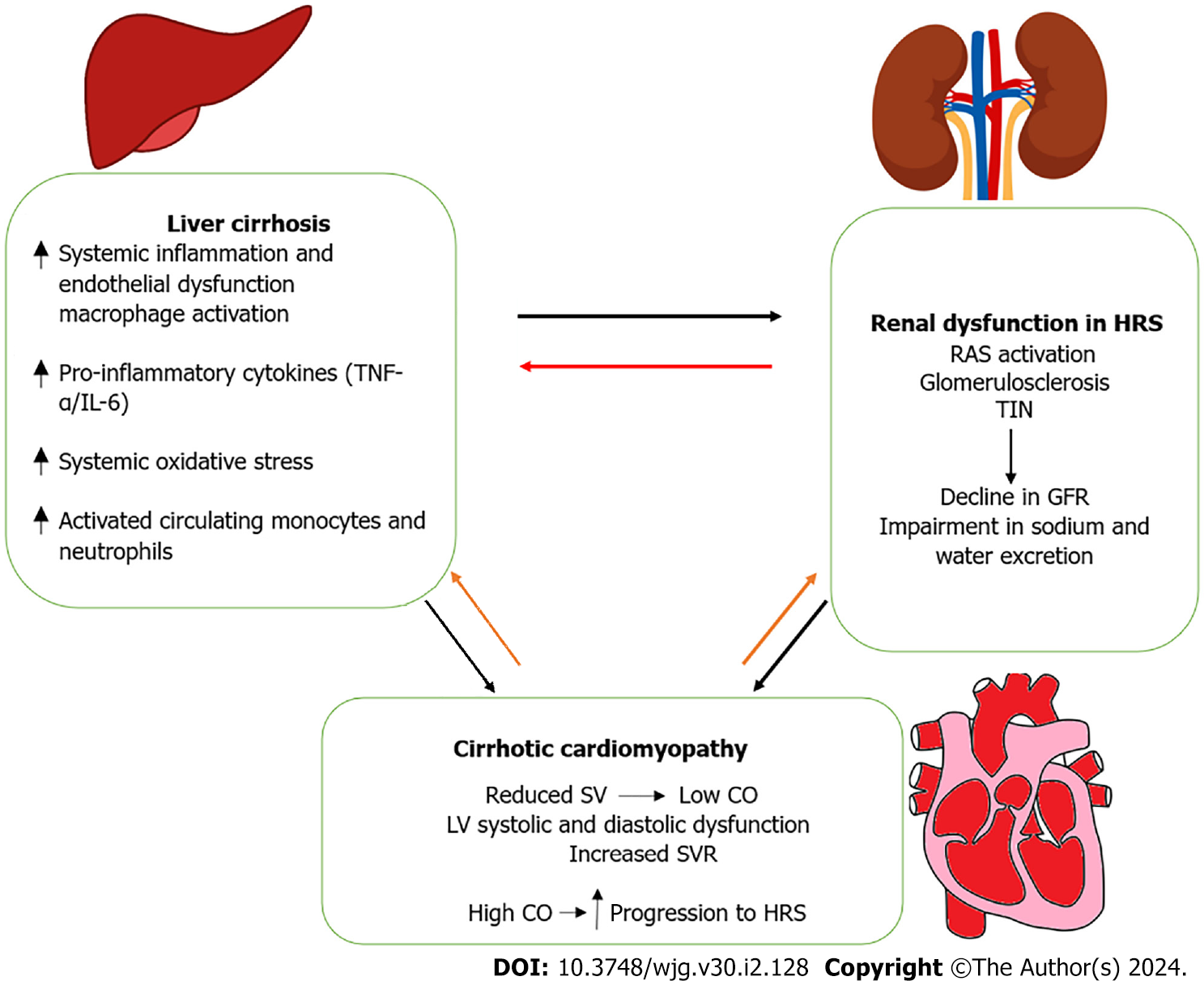
Our historical understanding of HRS and the mechanisms affecting circulatory dysfunction is being challenged, with the emergence of data and hypotheses which consider the extent of the heart’s involvement and contributory role in cirrhosis and HRS. Although the impact of the heart in HRS may result in varying levels of disease severity and clinical presentations, it is now increasingly established that circulatory dysfunction in HRS is at least in part due to cardiac impairment; that cardiac dysfunction commonly exist prior to kidney dysfunction in cirrhotic patients who develop HRS; that numerous cardinal pathophysiological pathways such as neurohormonal activation, inflammation and endothelial dysfunction are interlinked between HRS and CRS; and that treatments which are known to improve kidney dysfunction in HRS may also address pathophysiological mechanisms within the liver-heart-kidney connection (Figure 1).
Despite our increased knowledge, insight and appreciation into the existence of a hepatocardiorenal syndrome entity, there are still many unknowns in this novel model that requires further investigation (Table 1). We are still not at a stage where there is clarity on defining the criteria of hepatocardiorenal syndrome or even cirrhotic cardiomyopathy itself. The cardiac and circulatory disturbances within the HRS definition which are discussed in our review could instead represent a hepatic form of CRS, for example. In this scenario, the liver affects the kidneys primarily via cardiorenal pathways. Addressing the unknowns in this topic would be challenging due to the current scarcity of data and importantly, given the pathophysiological interactions which occur in HRS and CRS are still not entirely understood. There should also be consideration on how other metabolic pathways (i.e., lipid and cholesterol metabolism) could potentially affect the liver-heart-kidney connection, given prior evidence of these being risk factors for disease in each organ[79,80]. We anticipate research efforts to hopefully provide further answers on current knowledge gaps, and to optimize the diagnostic and treatment approach for this patient population.
| What is currently known | Remaining knowledge gaps |
| Pathophysiological mechanisms behind the onset and progression of HRS[1-5] | Exact timing and sequelae of cirrhotic cardiomyopathy in the setting of HRS[10-25] |
| Targeted prevention and interventional strategies to prevent the onset and terminate the progression of HRS[1-5] | Potential pathophysiological mechanisms that interlink the onset and progression of cardiac dysfunction in HRS[11,18,23,27-34,37] |
| Optimal screening markers and imaging modalities in the clinical setting to diagnose cardiac disease in cirrhosis and HRS, and to prognosticate outcomes[9,38-47] | |
| How can specific interventions like volume expansion using albumin and TIPS improve HRS-associated cardiac dysfunctions?[31,48-58] |
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: European Renal Association; American Society of Nephrology; International Society of Nephrology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Nakeep S, Egypt; Zhao P, China S-Editor: Wang JJ L-Editor: A P-Editor: Yu HG
| 1. | Francoz C, Durand F, Kahn JA, Genyk YS, Nadim MK. Hepatorenal Syndrome. Clin J Am Soc Nephrol. 2019;14:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 2. | Ginès P, Solà E, Angeli P, Wong F, Nadim MK, Kamath PS. Hepatorenal syndrome. Nat Rev Dis Primers. 2018;4:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (3)] |
| 3. | Simonetto DA, Gines P, Kamath PS. Hepatorenal syndrome: pathophysiology, diagnosis, and management. BMJ. 2020;370:m2687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (1)] |
| 4. | Bloom S, Kemp W, Lubel J. Portal hypertension: pathophysiology, diagnosis and management. Intern Med J. 2015;45:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Arroyo V, Guevara M, Ginès P. Hepatorenal syndrome in cirrhosis: pathogenesis and treatment. Gastroenterology. 2002;122:1658-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Cogger VC, Fraser R, Le Couteur DG. Liver dysfunction and heart failure. Am J Cardiol. 2003;91:1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Lee H, Lee YH, Kim SU, Kim HC. Metabolic Dysfunction-Associated Fatty Liver Disease and Incident Cardiovascular Disease Risk: A Nationwide Cohort Study. Clin Gastroenterol Hepatol. 2021;19:2138-2147.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 315] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 8. | Ren Z, Wesselius A, Stehouwer CDA, Brouwers MCGJ. Cardiovascular Implications of Metabolic Dysfunction-Associated Fatty Liver Disease. Endocrinol Metab Clin North Am. 2023;52:459-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 9. | Danielsen KV, Wiese S, Busk T, Nabilou P, Kronborg TM, Petersen CL, Hove JD, Møller S, Bendtsen F. Cardiovascular Mapping in Cirrhosis From the Compensated Stage to Hepatorenal Syndrome: A Magnetic Resonance Study. Am J Gastroenterol. 2022;117:1269-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Izzy M, VanWagner LB, Lin G, Altieri M, Findlay JY, Oh JK, Watt KD, Lee SS; Cirrhotic Cardiomyopathy Consortium. Redefining Cirrhotic Cardiomyopathy for the Modern Era. Hepatology. 2020;71:334-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (2)] |
| 11. | Kazory A, Ronco C. Hepatorenal Syndrome or Hepatocardiorenal Syndrome: Revisiting Basic Concepts in View of Emerging Data. Cardiorenal Med. 2019;9:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (2)] |
| 12. | Krag A, Gluud LL. Cross-talk between the liver, heart and kidney - another piece in the puzzle. J Gastrointestin Liver Dis. 2014;23:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Carvalho MVH, Kroll PC, Kroll RTM, Carvalho VN. Cirrhotic cardiomyopathy: the liver affects the heart. Braz J Med Biol Res. 2019;52:e7809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Kaur H, Premkumar M. Diagnosis and Management of Cirrhotic Cardiomyopathy. J Clin Exp Hepatol. 2022;12:186-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Dourakis SP, Geladari E, Geladari C, Vallianou N. Cirrhotic Cardiomyopathy: The Interplay Between Liver and Cardiac Muscle. How Does the Cardiovascular System React When the Liver is Diseased? Curr Cardiol Rev. 2021;17:78-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Ginès P, Moreira V, Milicua JM, Jiménez W, Arroyo V. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 383] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 17. | Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol. 2006;1:1066-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012;60:1031-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 19. | Ronco C, McCullough PA, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House A, Katz NM, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P. Cardiorenal syndromes: an executive summary from the consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol. 2010;165:54-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Mukherjee M, Sharma K, Madrazo JA, Tedford RJ, Russell SD, Hays AG. Right-Sided Cardiac Dysfunction in Heart Failure With Preserved Ejection Fraction and Worsening Renal Function. Am J Cardiol. 2017;120:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Clementi A, Virzì GM, Battaglia GG, Ronco C. Neurohormonal, Endocrine, and Immune Dysregulation and Inflammation in Cardiorenal Syndrome. Cardiorenal Med. 2019;9:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Patel KP, Katsurada K, Zheng H. Cardiorenal Syndrome: The Role of Neural Connections Between the Heart and the Kidneys. Circ Res. 2022;130:1601-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 23. | Krag A, Bendtsen F, Henriksen JH, Møller S. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut. 2010;59:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 250] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 24. | Gaskari SA, Honar H, Lee SS. Therapy insight: Cirrhotic cardiomyopathy. Nat Clin Pract Gastroenterol Hepatol. 2006;3:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Koshy AN, Farouque O, Cailes B, Testro A, Ramchand J, Sajeev JK, Han HC, Srivastava PM, Jones EF, Salehi H, Teh AW, Lim HS, Calafiore P, Gow PJ. Impaired Cardiac Reserve on Dobutamine Stress Echocardiography Predicts the Development of Hepatorenal Syndrome. Am J Gastroenterol. 2020;115:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Cailes B, Farouque O, Majumdar A, Koshy AN. Cardiac Dysfunction in the Pathogenesis of Hepatorenal Syndrome. Am J Gastroenterol. 2023;118:179-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Matyas C, Haskó G, Liaudet L, Trojnar E, Pacher P. Interplay of cardiovascular mediators, oxidative stress and inflammation in liver disease and its complications. Nat Rev Cardiol. 2021;18:117-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 28. | Adebayo D, Wong F. Pathophysiology of Hepatorenal Syndrome - Acute Kidney Injury. Clin Gastroenterol Hepatol. 2023;21:S1-S10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 29. | Navasa M, Follo A, Filella X, Jiménez W, Francitorra A, Planas R, Rimola A, Arroyo V, Rodés J. Tumor necrosis factor and interleukin-6 in spontaneous bacterial peritonitis in cirrhosis: relationship with the development of renal impairment and mortality. Hepatology. 1998;27:1227-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 295] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 30. | Fasolato S, Angeli P, Dallagnese L, Maresio G, Zola E, Mazza E, Salinas F, Donà S, Fagiuoli S, Sticca A, Zanus G, Cillo U, Frasson I, Destro C, Gatta A. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Bortoluzzi A, Ceolotto G, Gola E, Sticca A, Bova S, Morando F, Piano S, Fasolato S, Rosi S, Gatta A, Angeli P. Positive cardiac inotropic effect of albumin infusion in rodents with cirrhosis and ascites: molecular mechanisms. Hepatology. 2013;57:266-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Virzì GM, Zhang J, Nalesso F, Ronco C, McCullough PA. The Role of Dendritic and Endothelial Cells in Cardiorenal Syndrome. Cardiorenal Med. 2018;8:92-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Karagiannakis DS, Vlachogiannakos J, Anastasiadis G, Vafiadis-Zouboulis I, Ladas SD. Frequency and severity of cirrhotic cardiomyopathy and its possible relationship with bacterial endotoxemia. Dig Dis Sci. 2013;58:3029-3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Ruiz-del-Arbol L, Urman J, Fernández J, González M, Navasa M, Monescillo A, Albillos A, Jiménez W, Arroyo V. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38:1210-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 307] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 35. | Gadano A, Hadengue A, Widmann JJ, Vachiery F, Moreau R, Yang S, Soupison T, Sogni P, Degott C, Durand F. Hemodynamics after orthotopic liver transplantation: study of associated factors and long-term effects. Hepatology. 1995;22:458-465. [PubMed] |
| 36. | Zardi EM, Zardi DM, Chin D, Sonnino C, Dobrina A, Abbate A. Cirrhotic cardiomyopathy in the pre- and post-liver transplantation phase. J Cardiol. 2016;67:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P; Acute Dialysis Quality Initiative (ADQI) consensus group. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 592] [Cited by in RCA: 657] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 38. | Arroyo V, Fernandez J, Ginès P. Pathogenesis and treatment of hepatorenal syndrome. Semin Liver Dis. 2008;28:81-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 39. | Marwick TH, Leano RL, Brown J, Sun JP, Hoffmann R, Lysyansky P, Becker M, Thomas JD. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging. 2009;2:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 392] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 40. | Jansen C, Nordmann P, Cremonese C, Praktiknjo M, Chang J, Lehmann J, Thomas D, Nickenig G, Weber M, Stöhr E, Öztürk C, Zachoval C, Hammersting C, Strassburg CP, Meyer C, Trebicka J. Change of Left Ventricular Myocardial Contractility in Speckle Tracking Echocardiography After Transjugular Intrahepatic Portosystemic Shunt Predicts Survival. Front Gastroenterol. 2022;1:860800. [DOI] [Full Text] |
| 41. | Altekin RE, Caglar B, Karakas MS, Ozel D, Deger N, Demir I. Evaluation of subclinical left ventricular systolic dysfunction using two-dimensional speckle-tracking echocardiography in patients with non-alcoholic cirrhosis. Hellenic J Cardiol. 2014;55:402-410. [PubMed] |
| 42. | Somani PO, Contractor Q, Chaurasia AS, Rathi PM. Diastolic dysfunction characterizes cirrhotic cardiomyopathy. Indian Heart J. 2014;66:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Ruíz-del-Árbol L, Achécar L, Serradilla R, Rodríguez-Gandía MÁ, Rivero M, Garrido E, Natcher JJ. Diastolic dysfunction is a predictor of poor outcomes in patients with cirrhosis, portal hypertension, and a normal creatinine. Hepatology. 2013;58:1732-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 44. | Premkumar M, Devurgowda D, Vyas T, Shasthry SM, Khumuckham JS, Goyal R, Thomas SS, Kumar G. Left Ventricular Diastolic Dysfunction is Associated with Renal Dysfunction, Poor Survival and Low Health Related Quality of Life in Cirrhosis. J Clin Exp Hepatol. 2019;9:324-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Bayes-Genis A, Docherty KF, Petrie MC, Januzzi JL, Mueller C, Anderson L, Bozkurt B, Butler J, Chioncel O, Cleland JGF, Christodorescu R, Del Prato S, Gustafsson F, Lam CSP, Moura B, Pop-Busui R, Seferovic P, Volterrani M, Vaduganathan M, Metra M, Rosano G. Practical algorithms for early diagnosis of heart failure and heart stress using NT-proBNP: A clinical consensus statement from the Heart Failure Association of the ESC. Eur J Heart Fail. 2023;25:1891-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 91] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 46. | Ge PS, Runyon BA. Role of plasma BNP in patients with ascites: advantages and pitfalls. Hepatology. 2014;59:751-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 47. | Kapoor N, Mehta V, Singh B, Karna R, Kumar S, Kar P. Prevalence of cirrhotic cardiomyopathy and its relationship with serum pro-brain natriuretic peptide, hepatorenal syndrome, spontaneous bacterial peritonitis, and mortality. Indian J Gastroenterol. 2020;39:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Han G, Qi X, He C, Yin Z, Wang J, Xia J, Yang Z, Bai M, Meng X, Niu J, Wu K, Fan D. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with symptomatic portal hypertension in liver cirrhosis. J Hepatol. 2011;54:78-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 49. | Rajesh S, George T, Philips CA, Ahamed R, Kumbar S, Mohan N, Mohanan M, Augustine P. Transjugular intrahepatic portosystemic shunt in cirrhosis: An exhaustive critical update. World J Gastroenterol. 2020;26:5561-5596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 50. | Lotterer E, Wengert A, Fleig WE. Transjugular intrahepatic portosystemic shunt: short-term and long-term effects on hepatic and systemic hemodynamics in patients with cirrhosis. Hepatology. 1999;29:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Ponzo P, Campion D, Rizzo M, Roma M, Caviglia GP, Giovo I, Rizzi F, Bonetto S, Saracco GM, Alessandria C. Transjugular intrahepatic porto-systemic shunt in cirrhotic patients with hepatorenal syndrome - chronic kidney disease: Impact on renal function. Dig Liver Dis. 2022;54:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 52. | Busk TM, Bendtsen F, Poulsen JH, Clemmesen JO, Larsen FS, Goetze JP, Iversen JS, Jensen MT, Møgelvang R, Pedersen EB, Bech JN, Møller S. Transjugular intrahepatic portosystemic shunt: impact on systemic hemodynamics and renal and cardiac function in patients with cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2018;314:G275-G286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 53. | Deltenre P, Zanetto A, Saltini D, Moreno C, Schepis F. The role of transjugular intrahepatic portosystemic shunt in patients with cirrhosis and ascites: Recent evolution and open questions. Hepatology. 2023;77:640-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 45] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 54. | Hartl L, Rumpf B, Domenig O, Simbrunner B, Paternostro R, Jachs M, Poglitsch M, Marculescu R, Trauner M, Reindl-Schwaighofer R, Hecking M, Mandorfer M, Reiberger T. The systemic and hepatic alternative renin-angiotensin system is activated in liver cirrhosis, linked to endothelial dysfunction and inflammation. Sci Rep. 2023;13:953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 55. | Fakhritdinovna JM. Hepatocardiorenal Syndrome. Asian J Pharm Bio Res. 2022;11:82-97. |
| 56. | Garcia-Martinez R, Andreola F, Mehta G, Poulton K, Oria M, Jover M, Soeda J, Macnaughtan J, De Chiara F, Habtesion A, Mookerjee RP, Davies N, Jalan R. Immunomodulatory and antioxidant function of albumin stabilises the endothelium and improves survival in a rodent model of chronic liver failure. J Hepatol. 2015;62:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 57. | Fernández J, Monteagudo J, Bargallo X, Jiménez W, Bosch J, Arroyo V, Navasa M. A randomized unblinded pilot study comparing albumin versus hydroxyethyl starch in spontaneous bacterial peritonitis. Hepatology. 2005;42:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 58. | Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, Ginès P, Rodés J. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1003] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 59. | Mocarzel L, Lanzieri P, Nascimento J, Peixoto C, Ribeiro M, Mesquita E. Hepatorenal syndrome with cirrhotic cardiomyopathy: case report and literature review. Case Reports Hepatol. 2015;2015:573513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 60. | Mocarzel LO, Bicca J, Jarske L, Oliveira T, Lanzieri P, Gismondi R, Ribeiro ML. Cirrhotic Cardiomyopathy: Another Case of a Successful Approach to Treatment of Hepatorenal Syndrome. Case Rep Gastroenterol. 2016;10:531-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Theocharidou E, Krag A, Bendtsen F, Møller S, Burroughs AK. Cardiac dysfunction in cirrhosis - does adrenal function play a role? A hypothesis. Liver Int. 2012;32:1327-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Muciño-Bermejo MJ. Mechanisms of kidney dysfunction in the cirrhotic patient: Non-hepatorenal acute-on-chronic kidney damage considerations. Ann Hepatol. 2020;19:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Gao Y, Wei L, Zhang DD, Chen Y, Hou B. SGLT2 Inhibitors: A New Dawn for Recurrent/Refractory Cirrhotic Ascites. J Clin Transl Hepatol. 2021;9:795-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | ElBaset MA, Salem RS, Ayman F, Ayman N, Shaban N, Afifi SM, Esatbeyoglu T, Abdelaziz M, Elalfy ZS. Effect of Empagliflozin on Thioacetamide-Induced Liver Injury in Rats: Role of AMPK/SIRT-1/HIF-1α Pathway in Halting Liver Fibrosis. Antioxidants (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 65. | Carstens J, Grønbaek H, Larsen HK, Pedersen EB, Vilstrup H. Effects of urodilatin on natriuresis in cirrhosis patients with sodium retention. BMC Gastroenterol. 2007;7:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 66. | Hey P, Sinclair M. Upcoming pharmacological and interventional therapies for the treatment of physical frailty and sarcopenia. In: Tandon P, Montano-Loza A. Frailty and Sarcopenia in Cirrhosis. Switzerland: Springer, 2020: 211-232. |
| 67. | Snowdon VK, Lachlan NJ, Hoy AM, Hadoke PW, Semple SI, Patel D, Mungall W, Kendall TJ, Thomson A, Lennen RJ, Jansen MA, Moran CM, Pellicoro A, Ramachandran P, Shaw I, Aucott RL, Severin T, Saini R, Pak J, Yates D, Dongre N, Duffield JS, Webb DJ, Iredale JP, Hayes PC, Fallowfield JA. Serelaxin as a potential treatment for renal dysfunction in cirrhosis: Preclinical evaluation and results of a randomized phase 2 trial. PLoS Med. 2017;14:e1002248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 68. | Merli M, Calicchia A, Ruffa A, Pellicori P, Riggio O, Giusto M, Gaudio C, Torromeo C. Cardiac dysfunction in cirrhosis is not associated with the severity of liver disease. Eur J Intern Med. 2013;24:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 69. | Darstein F, König C, Hoppe-Lotichius M, Grimm D, Knapstein J, Mittler J, Zimmermann A, Otto G, Lang H, Galle PR, Zimmermann T. Preoperative left ventricular hypertrophy is associated with reduced patient survival after liver transplantation. Clin Transplant. 2014;28:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 70. | Torregrosa M, Aguadé S, Dos L, Segura R, Gónzalez A, Evangelista A, Castell J, Margarit C, Esteban R, Guardia J, Genescà J. Cardiac alterations in cirrhosis: reversibility after liver transplantation. J Hepatol. 2005;42:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 204] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 71. | Zardi EM, Abbate A, Zardi DM, Dobrina A, Margiotta D, Van Tassell BW, Afeltra A, Sanyal AJ. Cirrhotic cardiomyopathy. J Am Coll Cardiol. 2010;56:539-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 72. | Bernardi M, Maggioli C, Dibra V, Zaccherini G. QT interval prolongation in liver cirrhosis: innocent bystander or serious threat? Expert Rev Gastroenterol Hepatol. 2012;6:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 73. | American Association for the Study of Liver Diseases. Evaluation for Liver Transplantation in Adults. [cited 15 September 2023]. Available from: https://www.aasld.org/practice-guidelines/evaluation-adult-liver-transplant-patient. |
| 74. | Aggarwal S, Kang Y, Freeman JA, Fortunato FL Jr, Pinsky MR. Postreperfusion syndrome: hypotension after reperfusion of the transplanted liver. J Crit Care. 1993;8:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 159] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 75. | Izzy M, Fortune BE, Serper M, Bhave N, deLemos A, Gallegos-Orozco JF, Guerrero-Miranda C, Hall S, Harinstein ME, Karas MG, Kriss M, Lim N, Palardy M, Sawinski D, Schonfeld E, Seetharam A, Sharma P, Tallaj J, Dadhania DM, VanWagner LB. Management of cardiac diseases in liver transplant recipients: Comprehensive review and multidisciplinary practice-based recommendations. Am J Transplant. 2022;22:2740-2758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 76. | Manning MW, Kumar PA, Maheshwari K, Arora H. Post-Reperfusion Syndrome in Liver Transplantation-An Overview. J Cardiothorac Vasc Anesth. 2020;34:501-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 77. | Koshy AN, Gow PJ, Han HC, Teh AW, Jones R, Testro A, Lim HS, McCaughan G, Jeffrey GP, Crawford M, Macdonald G, Fawcett J, Wigg A, Chen JWC, Gane EJ, Munn SR, Clark DJ, Yudi MB, Farouque O. Cardiovascular mortality following liver transplantation: predictors and temporal trends over 30 years. Eur Heart J Qual Care Clin Outcomes. 2020;6:243-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 78. | Altieri MH, Liu H, Lee SS. Cardiovascular events after liver transplantation: MACE hurts. Rev Cardiovasc Med. 2022;23:91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Tauseef A, Zafar M, Rashid B, Thirumalareddy J, Chalfant V, Farooque U, Mirza M. Correlation of Fasting Lipid Profile in Patients With Chronic Liver Disease: A Descriptive Cross-Sectional Study in Tertiary Care Hospital. Cureus. 2020;12:e11019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 80. | Liu H, Naser JA, Lin G, Lee SS. Cardiomyopathy in cirrhosis: From pathophysiology to clinical care. JHEP Rep. 2024;6:100911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |