Published online Apr 28, 2024. doi: 10.3748/wjg.v30.i16.2272
Revised: March 13, 2024
Accepted: April 8, 2024
Published online: April 28, 2024
Processing time: 92 Days and 0.7 Hours
The magnetic compression technique has been used to establish an animal model of tracheoesophageal fistula (TEF), but the commonly shaped magnets present limitations of poor homogeneity of TEF and poor model control. We designed a T-shaped magnet system to overcome these problems and verified its effectiveness via animal experiments.
To investigate the effectiveness of a T-shaped magnet system for establishing a TEF model in beagle dogs.
Twelve beagles were randomly assigned to groups in which magnets of the T-shaped scheme (study group, n = 6) or normal magnets (control group, n = 6) were implanted into the trachea and esophagus separately under gastroscopy. Operation time, operation success rate, and accidental injury were recorded. After operation, the presence and timing of cough and the time of magnet shedding were observed. Dogs in the control group were euthanized after X-ray and gastroscopy to confirm establishment of TEFs after coughing, and gross specimens of TEFs were obtained. Dogs in the study group were euthanized after X-ray and gastroscopy 2 wk after surgery, and gross specimens were obtained. Fistula size was measured in all animals, and then harvested fistula specimens were examined by hematoxylin and eosin (HE) and Masson trichrome staining.
The operation success rate was 100% for both groups. Operation time did not differ between the study group (5.25 min ± 1.29 min) and the control group (4.75 min ± 1.70 min; P = 0.331). No bleeding, perforation, or unplanned magnet attraction occurred in any animal during the operation. In the early postoperative period, all dogs ate freely and were generally in good condition. Dogs in the control group had severe cough after drinking water at 6-9 d after surgery. X-ray indicated that the magnets had entered the stomach, and gastroscopy showed TEF formation. Gross specimens of TEFs from the control group showed the formation of fistulas with a diameter of 4.94 mm ± 1.29 mm (range, 3.52-6.56 mm). HE and Masson trichrome staining showed scar tissue formation and hierarchical structural disorder at the fistulas. Dogs in the study group did not exhibit obvious coughing after surgery. X-ray examination 2 wk after surgery indicated fixed magnet positioning, and gastroscopy showed no change in magnet positioning. The magnets were removed using a snare under endoscopy, and TEF was observed. Gross specimens showed well-formed fistulas with a diameter of 6.11 mm ± 0.16 mm (range, 5.92-6.36 mm), which exceeded that in the control group (P < 0.001). Scar formation was observed on the internal surface of fistulas by HE and Masson trichrome staining, and the structure was more regular than that in the control group.
Use of the modified T-shaped magnet scheme is safe and feasible for establishing TEF and can achieve a more stable and uniform fistula size compared with ordinary magnets. Most importantly, this model offers better controllability, which improves the flexibility of follow-up studies.
Core Tip: The magnetic compression technique has been successfully used to establish animal models of tracheoesophageal fistula (TEF) in beagle dogs. However, for TEF, use of the common circular magnet shape is associated with poor homogeneity and poor controllability of model. In this study, we used a modified T-shaped magnet system to establish a TEF, and the results showed this approach could obtain a more stable and uniform fistula size compared with standard magnets. Most importantly, the proposed model offers better controllability, which improves the flexibility of subsequent studies.
- Citation: Zhang MM, Mao JQ, Shen LX, Shi AH, Lyu X, Ma J, Lyu Y, Yan XP. Optimization of tracheoesophageal fistula model established with T-shaped magnet system based on magnetic compression technique. World J Gastroenterol 2024; 30(16): 2272-2280
- URL: https://www.wjgnet.com/1007-9327/full/v30/i16/2272.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i16.2272
Tracheoesophageal fistula (TEF) is a rare condition in clinic that is difficult to treat. Methods for treatment of TEF include endoscopic treatment and surgical treatment[1-4], and no uniform and standardized surgical method has been developed. Good animal models hold great significance for studying the occurrence and development of human diseases, pathophysiological conditions, and prognosis after treatment. In small animals, TEF can generally be established by puncture injury[5], while larger animal models of TEF, such as those in pigs and dogs, are generally established by surgical operation[6,7]. The operation for establishing TEF animal models via the surgical method is complicated and time-consuming.
With the magnetic compression technique, upon placement of magnets in the empty organs where anastomosis is to occur, the magnets are attracted together, and the compressed tissue undergoes pathological changes of ischemia, necrosis, and shedding[8], resulting in the establishment of a new channel. The magnetic compression technique can be used for vascular anastomosis[9-11], digestive anastomosis[12-15] and ureteral anastomosis[16,17]. Five years ago, we first proposed application of the magnetic compression technique for establishing animal models of TEF in beagle dogs. Subsequent animal experiments fully verified the feasibility of the method and its advantages of a simple operation, little trauma, and high success rate[18]. However, in continued research, we found that this method is limited by poor controllability of model establishment time and time-limited requirements for subsequent therapeutic intervention.
To overcome the above shortcomings, we developed an improved T-shaped magnet design scheme on the basis of our previous research, and in the present study, we investigated the feasibility of this scheme for establishing a TEF model compared with the use of ordinary shaped magnets in beagle dogs.
The experimental protocol was approved by the Committee for Ethics of Animal Experiments of Xi’an Jiaotong University (license No. XJTUAE2023-2207). Twelve beagles (6 males and 6 females), > 1 year old and weighing 10-15 kg, were obtained from the Laboratory Animal Center of the Xi’an Jiaotong University (Xi’an, China). The research protocol and all experimental procedures were strictly in accordance with the Guidelines for the Care and Use of Experimental Animals, issued by the Xi’an Jiaotong University Medical Center. We fully safeguarded animal welfare and minimized animal suffering in this research.
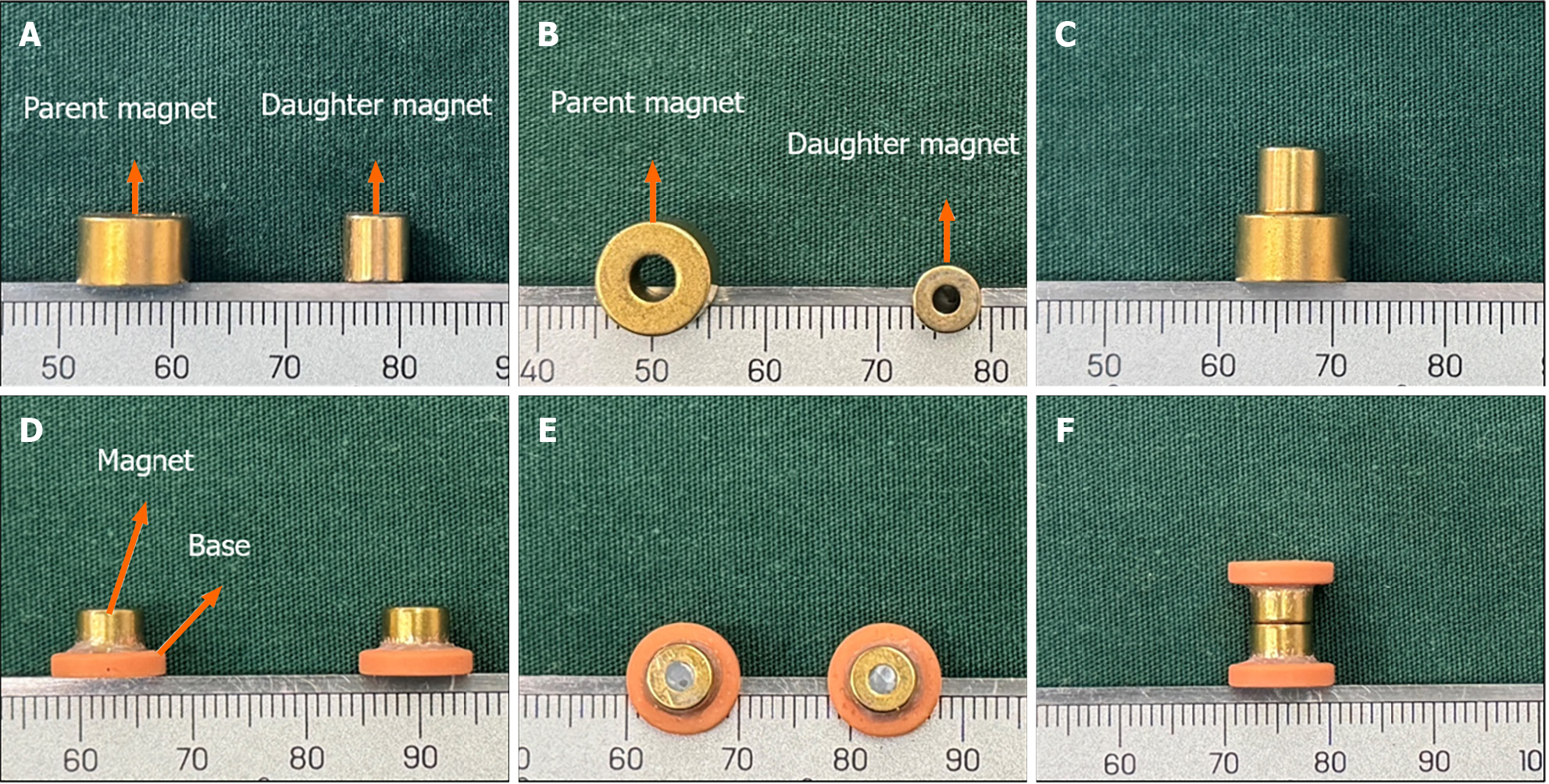
The control group used cylindrical magnets. The magnet system of the control group consisted of two parts: The daughter magnet and the parent magnet. The daughter magnet was a cylindrical magnet with a diameter of 6 mm, a height of 6 mm, and a central hole with a diameter of 2 mm. The parent magnet also was a cylinder with a diameter of 9 mm, a height of 6 mm, and a central hole with a diameter of 3 mm. Both the daughter magnet and the parent magnet were fabricated from N50 sintered NdFeb, with saturation magnetization in the height direction and nitriding coating on the surface (Figure 1A-C).
A composite magnet design scheme was used in the study group. The T-shaped magnet comprised a magnet part and a base part. The magnet part was a cylindrical magnet with a diameter of 6 mm, a height of 6 mm, and a central hole with a diameter of 2 mm. The magnet was fabricated from N50 sintered NdFeb material with saturation magnetization in the height direction and titanium nitride coating on the surface. The base was a 3D-printed plastic ring with an outer diameter of 10.0 mm, an inner diameter of 6.5 mm, and a height of 2.0 mm. One end of the magnet was inserted into the central hole of the plastic ring and fixed firmly with a binder (Figure 1D-F). The daughter magnet in the study group was identical in size and structure to the parent magnet. The maximum magnetic force between the daughter and parent magnets was 11 N in the study group and 10 N in the control group.
The 12 beagles were randomly divided into two groups: the study group (3 males and 3 females) and the control group (3 males and 3 females). The modified T-shaped magnet scheme was applied in the study group, and normal cylindrical magnets were used in the control group.
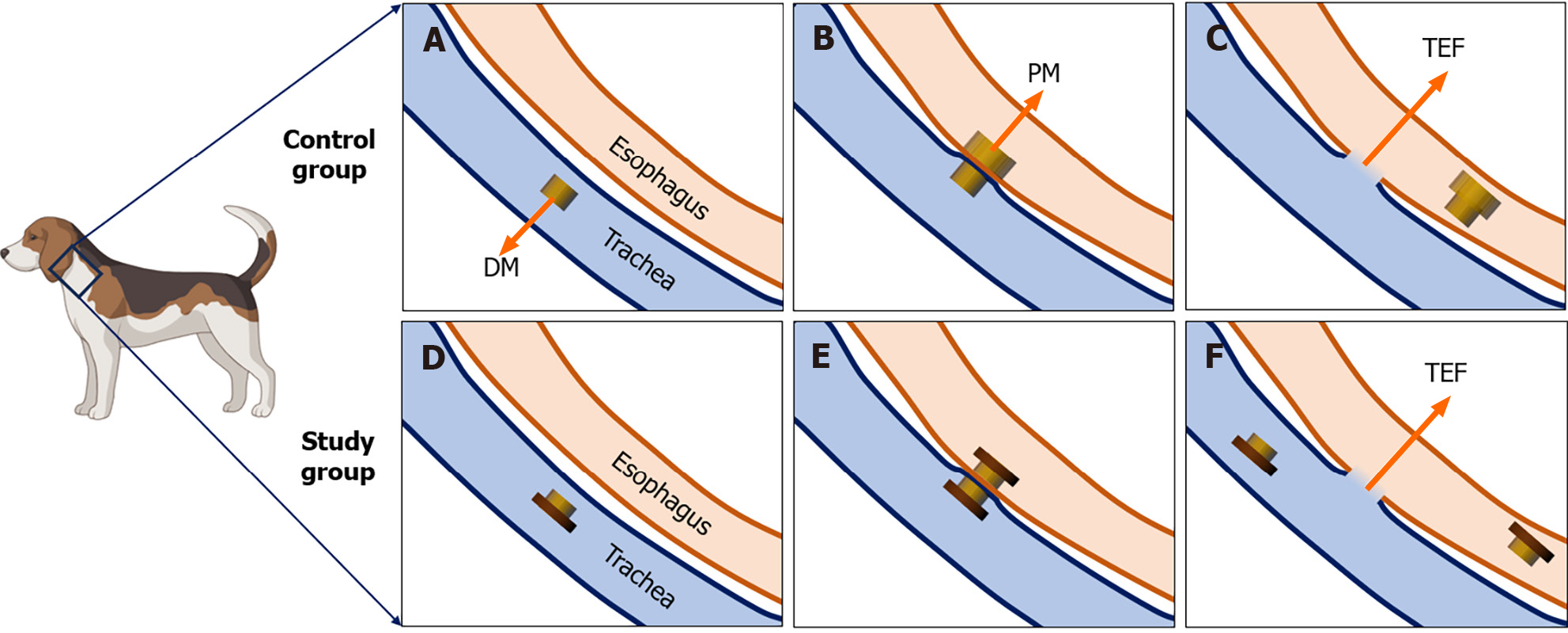
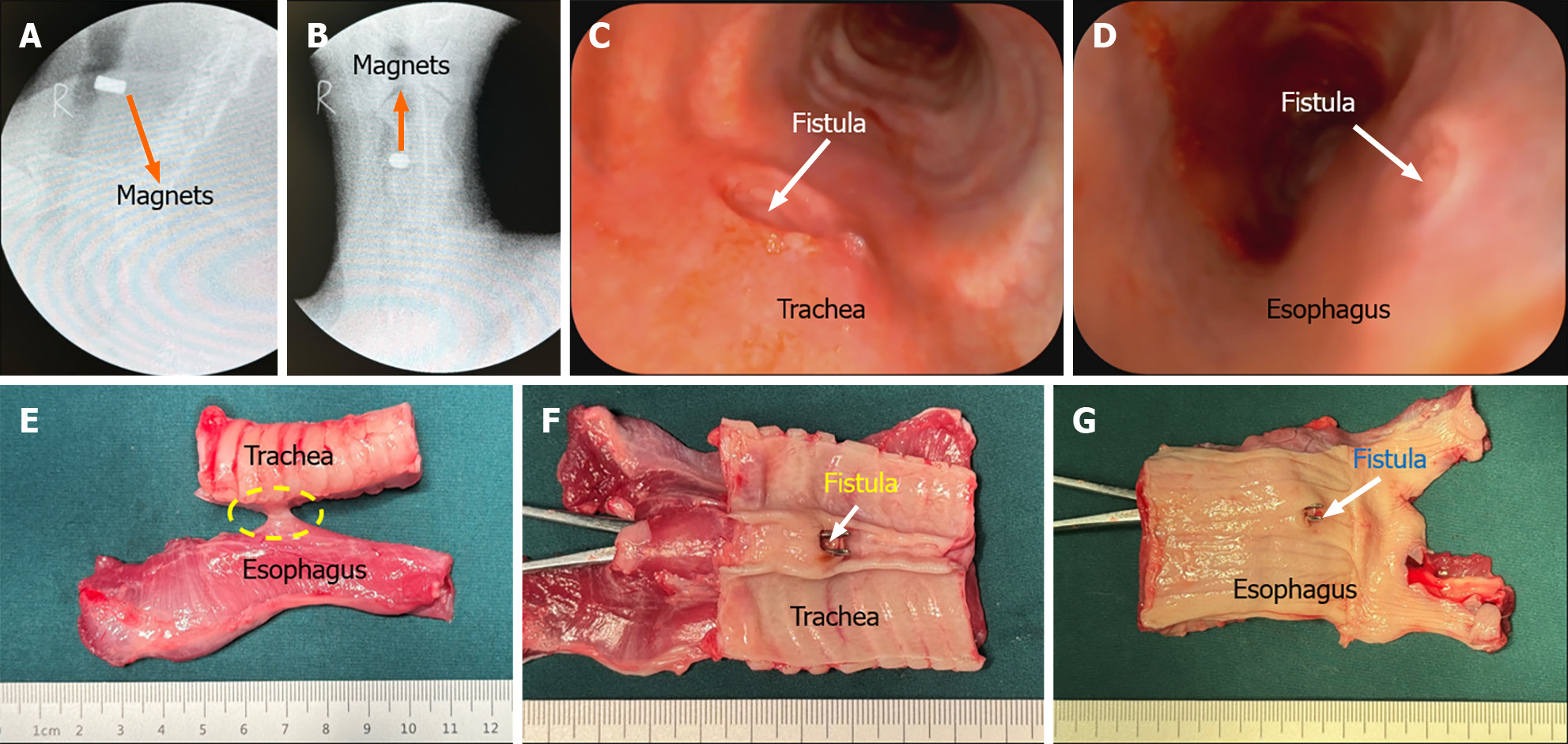
The beagles were fasted for 12 h before surgery. For the operation, they were anesthetized by intravenous injection of 3% pentobarbital sodium (1 mL/kg) and fixed in the supine position. Once loss of the paw withdrawal reflex was confirmed, the dogs were placed on an operating table in the supine position. The procedure and method for magnet placement were the same as those described in our previous article[18]. Briefly, the glottis was sufficiently exposed, and the daughter magnet was inserted into the trachea (Figure 2A). Then the parent magnet was inserted into the cervical esophagus, and the parent magnet and daughter magnet automatically attracted together (Figure 2B). Neck X-ray and gastroscopy were used to confirm the target location and accurate coupling of the parent and daughter magnets. After a period of time, the parent magnet and daughter magnet are expected to fall off into the distal esophagus, and TEF can be established (Figure 2C). The T-shaped magnet scheme components were inserted into the trachea and esophagus in the study group using the same method as the control group (Figure 2D and E). TEF formation was observed after magnet removal under endoscopy (Figure 2F).
The operation time, operation success rate, and occurrence of accidental injury were recorded. Dogs in the control group were euthanized after X-ray and gastroscopy to confirm the establishment of TEF after coughing, and gross specimens of TEF were obtained. Dogs in the study group were euthanized after X-ray and gastroscopy at 2 wk after surgery, and gross specimens of TEF were obtained.
All dogs were managed in a single cage after emergence from anesthesia. Pethidine hydrochloride (1 mg/kg) was injected intramuscularly every 12 h for 3 d after operation for analgesia. After the operation, the general conditions of the experimental dogs, including the presence and timing of cough and the time at which the magnets left the neck were observed. X-ray examinations are usually performed every other day beginning on the fourth day after surgery to determine whether the magnet has departed from its initial position. Of course, if the dog has a cough during the observation period, X-ray examination is performed immediately to determine whether the magnet has moved from its initial position.
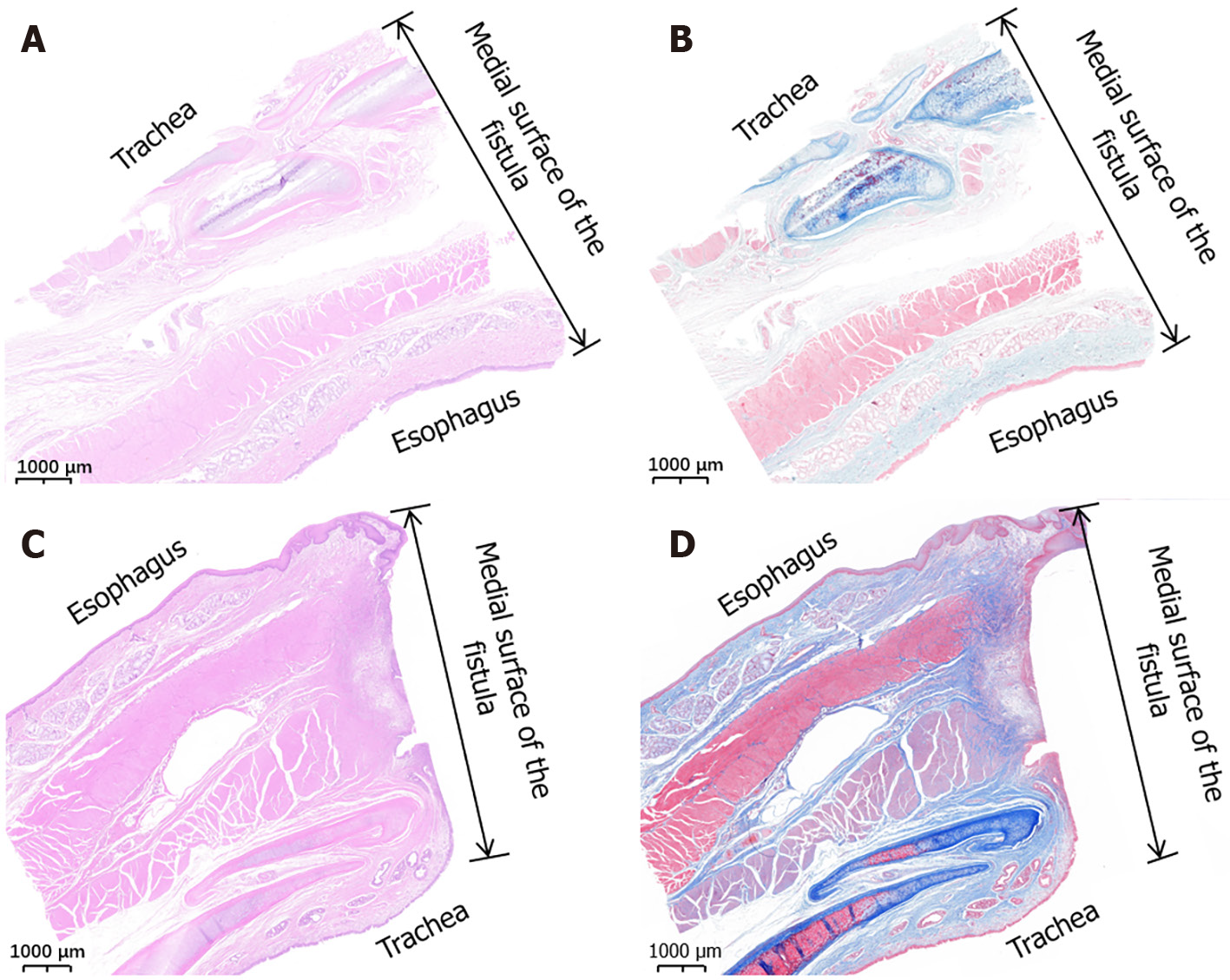
All harvested TEFs were visually inspected and measured before processing for pathological examination by hematoxylin and eosin (HE) and Masson trichrome staining.
For fixation, the TEF specimens were soaked overnight in 10% formalin. After fixation, each specimen was embedded in paraffin, and 4-μm-thick sections were prepared from the anastomosis. The sections were stained with HE and Masson trichrome stains and examined under a bright-field microscope.
All data were analyzed using SPSS statistical 20.0 software. The normality of the study variables was assessed by Shapiro-Wilk test. Normally distributed quantitative data are presented as mean ± SD values, while data that did not conform to a normal distribution are described as median values. Differences between the study and control groups were determined using an independent sample t-test or a nonparametric test, with P < 0.05 indicating a significant difference.
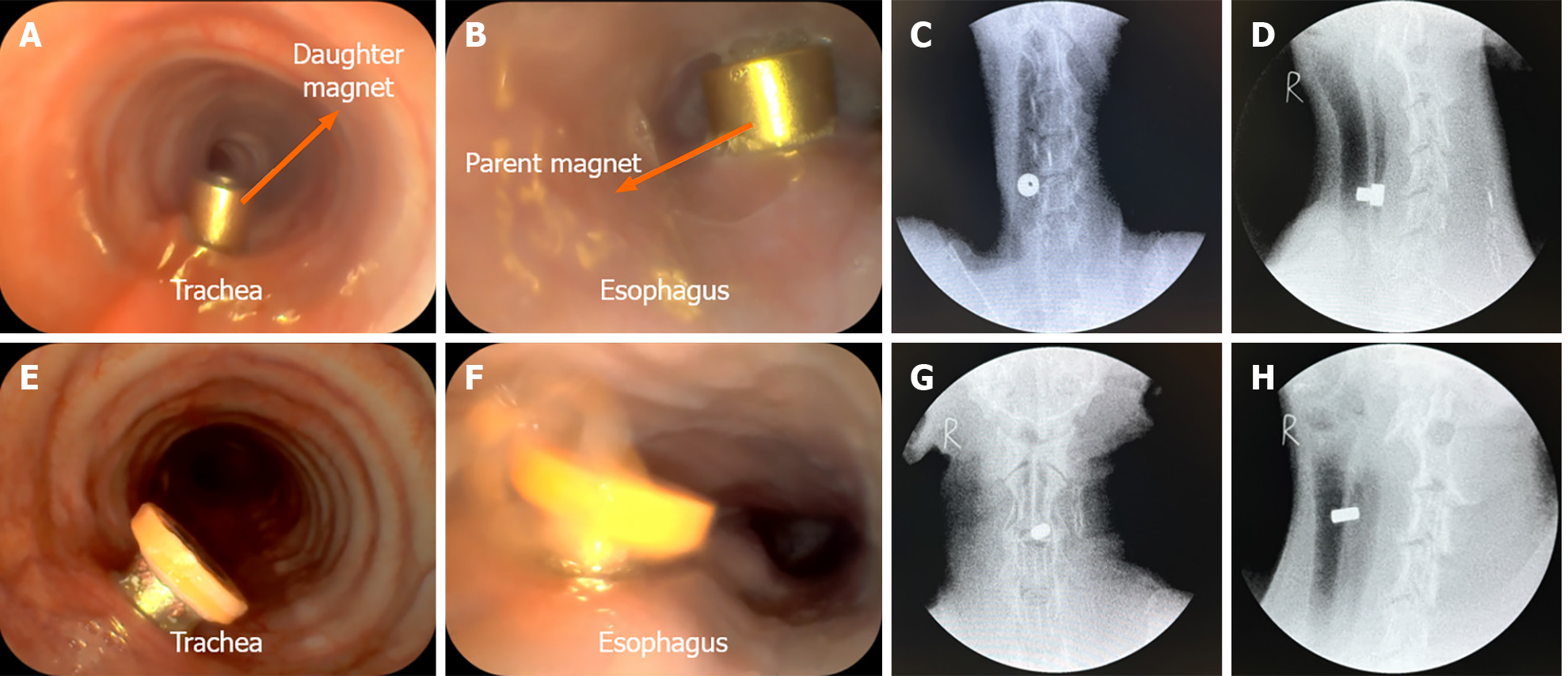
The magnet placement operations were successful in all 12 dogs (Figure 3A, B, E, and F), and no bleeding, asphyxia, or injury of the trachea and esophagus occurred during the operation. The operation time did not differ significantly between the study group (5.25 min ± 1.29 min; range, 4.00-7.50 min) and the control group (4.75 min ± 1.70 min; range, 3.50-8.00 min; P = 0.331). Cervical X-ray showed that the magnets attracted well (Figure 3C, D, G, and H). The operation success rate was 100% in both groups.
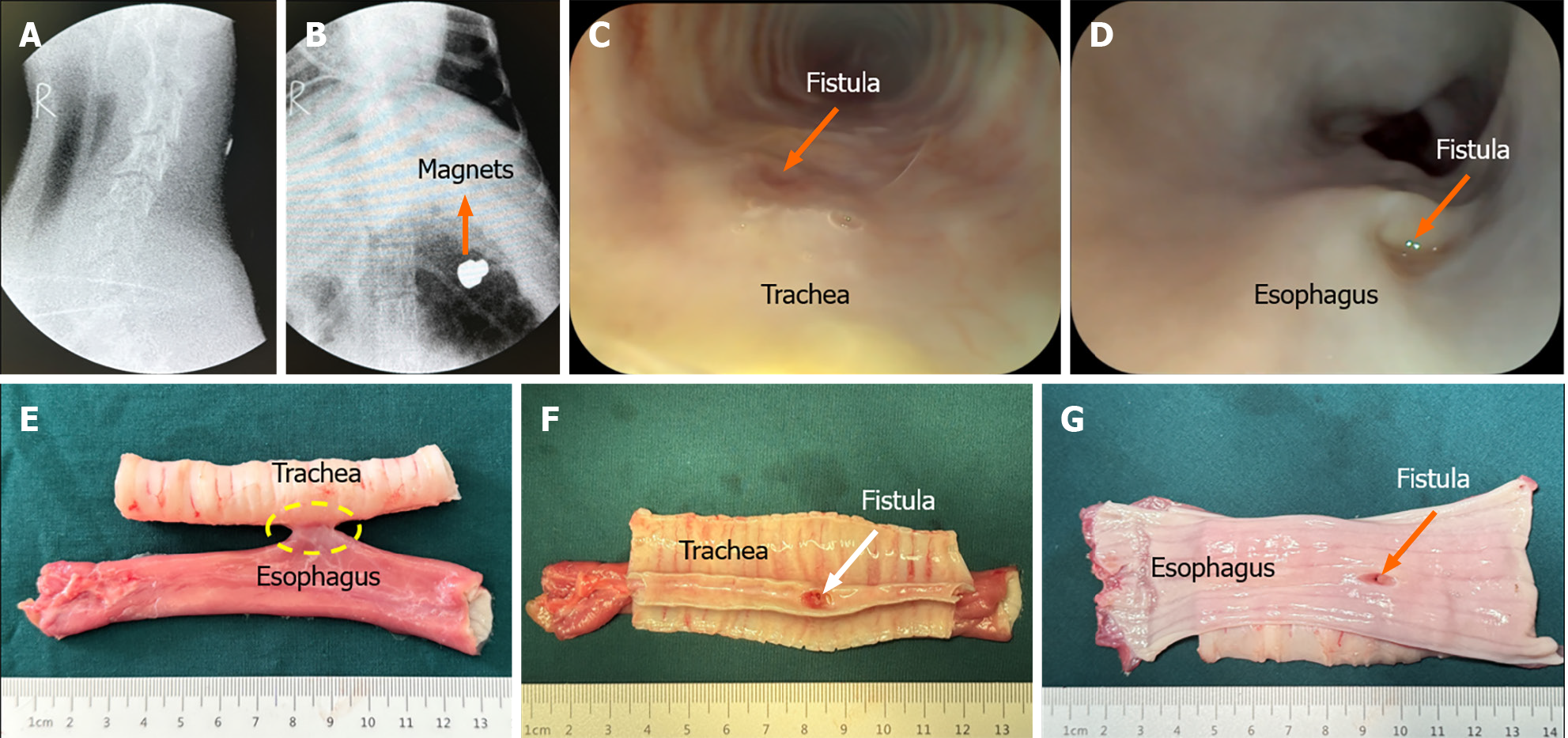
None of the animals exhibited cough after recovery from anesthesia, and all dogs ate generally well after surgery. The dogs in the control group developed coughs 6-9 d after surgery. X-ray examination indicated that the magnets had left the esophagus and entered the stomach (Figure 4A and B). Further gastroscopy showed the TEF formation (Figure 4C and D), and thus, the animals were euthanized for collection of gross specimens of TEF (Figure 4E-G). None of the dogs in the study group had obvious coughs after operation. X-ray examination of the neck indicated that the magnets remained in good position (Figure 5A and B). The magnets were removed under gastroscopy 2 wk after operation, and the TEF was well formed (Figure 5C and D). The dogs in the study group were euthanized, and the gross specimens of TEF were obtained (Figure 5E-G).
In the gross specimens, the esophagus and trachea showed normal morphology in both the study group and the control group. Except for the fistula between the trachea and esophagus, no obvious tissue adhesion was observed between the trachea and esophagus, and no obvious thickening of the mucosa of the trachea was observed. The shape and patency of fistulas in the study group were better than those in the control group. The diameter of fistulas in the study group was 6.11 mm ± 0.16 mm (range: 5.92-6.36 mm), which was greater and showed less variability in shape than that in the control group of 4.94 mm ± 1.29 mm (range, 3.52-6.56 mm; P < 0.001). Histological observation showed that the trachea and esophageal mucosa converged along the medial surface of the fistula (Figure 6).
In addition to inducing digestive tract anastomosis, the magnetic compression technique can also be used to create “therapeutic” fistulas. Uygun et al[19-21] reported experimental studies on the use of the magnetic compression technique in gastrostomy[19], colostomy[20], and cystostomy[21] in rats. The essence of endoscopic gastrointestinal bypass anastomosis is also enterostomy[22-26]. Although animal models generated using the magnetic compression technique represent pathological ostomy, the histopathological changes are the same.
The results of the present study indicate that the magnetic compression technique can be applied to establish a canine TEF model in a minimally invasive manner, and that the T-shaped magnet scheme offers an improved system compared with traditional round magnets. Compared with the control procedure, the newly developed procedure using the T-shaped magnet scheme showed distinct advantages, such as after the establishment of the TEF, the magnets remained at the fistula, where they would not cause serious complications, such as coughing and lung infection in the short term. At the time of further interventional treatment, the magnets can be removed from the fistula. In comparison, a disadvantage of the control procedure is that after the formation of TEF, an interventional operation must be performed as soon as possible to prevent serious lung infection caused by aspiration, which can lead to death of the animal. In general, the newly developed approach provides researchers with more flexible experimental arrangements than the control procedure. The magnet used in this study has a unit price of $10, which is a low-cost experimental consumable.
In this study, with the use of the T-shaped magnet scheme for esophageal and tracheal compression, the size of the fistula was equal to the outer diameter of the magnet, and the base of the magnet was larger than the diameter of the fistula. Therefore, after the formation of the fistula, the magnets would not fall off by themselves as in the control group, but rather would stay in the TEF. Such a design has two effects: First, it avoids aspiration by the experimental dogs, which would accelerate lung infection and death; and secondly, retention of the magnets in the fistula for a period of time is beneficial to the size of the fistula and the stability of the internal surface tissue of the fistula. In the preparation of an animal model of rectovaginal fistula, previous studies found that the fistula has a tendency to self-heal in the animal model, and thus, it is necessary to insert foreign bodies in the fistula mouth to promote the stability of the fistula[27,28]. The T-shaped magnet scheme introduced in this study can play exactly such a role.
In the present study, the time at which gross specimens of TEF were obtained was inconsistent between the study group and the control group. The dogs in the control group were euthanized and killed immediately after the establishment of TEF, because in the early period the TEF was established, the dogs would cough violently after eating and drinking, which was painful to the experimental animals. Therefore, to reduce the suffering of animals during the experiment and follow the ethical norms for animal experiments, we euthanized these dogs immediately after confirming TEF establishment. In the study group, the specially designed T-shaped magnet scheme avoided the onset of such coughing, allowing us to extend the time to obtain specimens. According to our research results from 5 years ago, the time for the magnetic compression technique to establish TEF is 4-6 d[18]. The control group in this study showed that TEF was established 6-9 d after surgery. Therefore, the TEF should be established within about 10 d in the study group. Therefore, we assumed that 2 wk is enough time for TEF establishment in the study group. Accordingly, we chose to euthanize the dogs in the study group at 2 wk to obtain TEF specimens. Of course, the purpose of our design of T-shaped magnets was to provide researchers with more flexible options for further treatment of TEF. Thus, anything longer than 10 d is appropriate.
One limitation of the present study is the small number of experimental animals. Additionally, the observation time after TEF formation was shorter for the control group than the study group, and the observation time could be appropriately extended if effective methods could be applied to reduce the choking and pain of the control animals. The anatomical characteristics of the trachea and esophagus in pigs are more similar to those in humans, and thus, an animal model of TEF that is more similar to human TEF may be obtained if experimental pigs are adopted.
To date, few reports have described the establishment of animal models of TEF. In one example, a rat model of TEF was prepared by injecting mitomycin into pregnant rats[29]. For larger animals, surgical methods have been required, and a pig TEF model was established by surgical methods[6]. A disadvantage of this model is that the operation is complicated and traumatic, which is not conducive to the next stage of intervention surgery. The successful establishment of a TEF model in a larger animal by applying the magnetic compression technique is beneficial to the development of intervention and treatment measures.
The present study demonstrated the feasibility of establishing TEF using the magnetic compression technique and revealed the advantages of using a T-shaped magnet scheme rather than traditional cylindrical magnets.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade A
Scientific Significance: Grade B
P-Reviewer: Trebol J, Spain S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
| 1. | Holmquist A, Wendt M, Papatziamos G, Svensson J, Wester T, Burgos CM, Gahm C. Endoscopic Chemocauterization with Trichloroacetic Acid for Congenital or Recurrent Tracheoesophageal Fistula in Children with Esophageal Atresia: Experience from a Tertiary Center. J Pediatr Surg. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Patkowski D, Toczewski K, Ergun E. Novel Left-Sided Thoracoscopic Approach to Recurrent Tracheoesophageal Fistula and Post-Fistula Tracheal Diverticula. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Seebauer CT, Völkl M, Kunkel J, Künzel J, Kühnel T, Hofmann HS, Bohr C. Tracheoesophageal Fistula Closure in a Pediatric Patient Using a Supraclavicular Artery Island Flap. Plast Reconstr Surg Glob Open. 2023;11:e5250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Dai J, He W, Liu X, Jiang G. Use of an autologous dermal flap and rib cartilage graft in the repair of a large tracheoesophageal fistula with tracheal stenosis. Interdiscip Cardiovasc Thorac Surg. 2023;37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Wang F, Li Z, Lyu FJ, Gao J, Lin J, Liu J, Chen X, Shan J, Wu J. The therapeutic effect of stem cells from human exfoliated deciduous teeth on a rat model of tracheal fistula. Stem Cell Res Ther. 2022;13:310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Wagner HJ, Stinner B, Barth P, Klose KJ. Are covered stents really effective at closing esophagotracheal fistulas? Results of an animal study. Cardiovasc Intervent Radiol. 2000;23:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Yang G, Zhou YA, Bai GZ, Han Y, Li WM, Wang J, Li XF, Yan XL. Effect of cauterizing esophageal mucosa in "double-patch" treatment for acquired benign tracheoesophageal fistula/bronchogastric stump fistula. J Surg Res. 2017;209:1-7. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Zhang M, Lyu X, Zhao G, An Y, Lyu Y, Yan X. Establishment of Yan-Zhang's staging of digestive tract magnetic compression anastomosis in a rat model. Sci Rep. 2022;12:12445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Lee WG, Evans LL, Harrison MR. Beyond the gut: spectrum of magnetic surgery devices. Front Surg. 2023;10:1253728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Hornok Z, Kubiak R, Csukas D, Ferencz A, Cserni T. Esophageal Magnetic Anastomosis Device (EMAD) to Simplify and Improve Outcome of Thoracoscopic Repair for Esophageal Atresia with Tracheoesophageal Fistula: A Proof of Concept Study. J Pediatr Surg. 2023;58:1489-1493. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Klima U, MacVaugh H 3rd, Bagaev E, Maringka M, Kirschner S, Beilner J, Haverich A. Magnetic Vascular Port in minimally invasive direct coronary artery bypass grafting. Circulation. 2004;110:II55-II60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Gagner M, Krinke T, Lapointe-Gagner M, Buchwald JN. Side-to-side duodeno-ileal magnetic compression anastomosis: design and feasibility of a novel device in a porcine model. Surg Endosc. 2023;37:6197-6207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 13. | Zhang M, He S, Sha H, Xue H, Lv Y, Yan X. A novel self-shaping magnetic compression anastomosis ring for treatment of colonic stenosis. Endoscopy. 2023;55:E1132-E1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (1)] |
| 14. | Krishnan N, Pakkasjärvi N, Kainth D, Danielson J, Verma A, Yadav DK, Goel P, Anand S. Role of Magnetic Compression Anastomosis in Long-Gap Esophageal Atresia: A Systematic Review. J Laparoendosc Adv Surg Tech A. 2023;33:1223-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Gagner M, Abuladze D, Koiava L, Buchwald JN, Van Sante N, Krinke T. First-in-Human Side-to-Side Magnetic Compression Duodeno-ileostomy with the Magnet Anastomosis System. Obes Surg. 2023;33:2282-2292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 16. | Ünal E, Çiftçi TT, Akinci D. Magnetic Compression Anastomosis of Benign Short-Segment Ureteral Obstruction. J Vasc Interv Radiol. 2024;35:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 17. | Shlomovitz E, Copping R, Swanstrom LL. Magnetic Compression Anastomosis for Recanalization of Complete Ureteric Occlusion after Radical Cystoprostatectomy. J Vasc Interv Radiol. 2023;34:1640-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Gao Y, Wu RQ, Lv Y, Yan XP. Novel magnetic compression technique for establishment of a canine model of tracheoesophageal fistula. World J Gastroenterol. 2019;25:4213-4221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 19. | Uygun I, Okur MH, Cimen H, Keles A, Yalcin O, Ozturk H, Otcu S. Magnetic compression gastrostomy in the rat. Pediatr Surg Int. 2012;28:529-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Uygun I, Okur MH, Arayici Y, Keles A, Ozturk H, Otcu S. Magnetic compression ostomy for simple tube colostomy in rats--magnacolostomy. Adv Clin Exp Med. 2012;21:301-305. [PubMed] |
| 21. | Uygun I, Okur MH, Cimen H, Keles A, Yalcin O, Ozturk H, Otcu S. Magnetic compression ostomy as new cystostomy technique in the rat: magnacystostomy. Urology. 2012;79:738-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Ryou M, Cantillon-Murphy P, Azagury D, Shaikh SN, Ha G, Greenwalt I, Ryan MB, Lang JH, Thompson CC. Smart Self-Assembling MagnetS for ENdoscopy (SAMSEN) for transoral endoscopic creation of immediate gastrojejunostomy (with video). Gastrointest Endosc. 2011;73:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | van Hooft JE, Vleggaar FP, Le Moine O, Bizzotto A, Voermans RP, Costamagna G, Devière J, Siersema PD, Fockens P. Endoscopic magnetic gastroenteric anastomosis for palliation of malignant gastric outlet obstruction: a prospective multicenter study. Gastrointest Endosc. 2010;72:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Cho YB, Park JH, Chun HK, Park CM, Kim HC, Yun SH, Lee WY. Multimedia article. Natural orifice transluminal endoscopic surgery applied to sigmoidectomy in survival animal models: using paired magnetic intra-luminal device. Surg Endosc. 2011;25:1319-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Toselli L, Martinez-Ferro M, Cervio G, Kwiat D, Imamura-Ching J, Graves CE, Gaston B, Harrison M. Magnetic Compression Anastomosis (Magnamosis) for Functional Undiversion of Ileostomy in Pediatric Patients. J Laparoendosc Adv Surg Tech A. 2017;27:1314-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Leroy J, Perretta S, Diana M, Wall J, Lindner V, Harrison M, Marescaux J. An original endoluminal magnetic anastomotic device allowing pure NOTES transgastric and transrectal sigmoidectomy in a porcine model: proof of concept. Surg Innov. 2012;19:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Aungst MJ, Fischer JR, Bonhage MR, Albright TS, Noel KA, Wright J. Rectovaginal fistula model in the New Zealand white rabbit. Int Urogynecol J. 2010;21:885-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Roshanravan R, Ghahramani L, Hosseinzadeh M, Mohammadipour M, Moslemi S, Rezaianzadeh A, Safarpour AR, Rahimikazerooni S, Hosseini SV. A new method to repair recto-vaginal fistula: Use of human amniotic membrane in an animal model. Adv Biomed Res. 2014;3:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Diez-Pardo JA, Baoquan Q, Navarro C, Tovar JA. A new rodent experimental model of esophageal atresia and tracheoesophageal fistula: preliminary report. J Pediatr Surg. 1996;31:498-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 94] [Article Influence: 3.2] [Reference Citation Analysis (0)] |