Published online Apr 28, 2024. doi: 10.3748/wjg.v30.i16.2220
Revised: January 24, 2024
Accepted: April 1, 2024
Published online: April 28, 2024
Processing time: 124 Days and 3.7 Hours
Several features of drug-induced mucosal alterations have been observed in the upper gastrointestinal tract, i.e., the esophagus, stomach, and duodenum. These include pill-induced esophagitis, desquamative esophagitis, worsening of gastroesophageal reflux, chemotherapy-induced esophagitis, proton pump inhibitor-induced gastric mucosal changes, medication-induced gastric erosions and ulcers, pseudomelanosis of the stomach, olmesartan-related gastric mucosal inflammation, lanthanum deposition in the stomach, zinc acetate hydrate tablet-induced gastric ulcer, immune-related adverse event gastritis, olmesartan-asso-ciated sprue-like enteropathy, pseudomelanosis of the duodenum, and lanthanum deposition in the duodenum. For endoscopists, acquiring accurate knowledge regarding these diverse drug-induced mucosal alterations is crucial not only for the correct diagnosis of these lesions but also for differential diag-nosis of other conditions. This minireview aims to provide essential information on drug-induced mucosal alterations observed on esophagogastroduodenoscopy, along with representative endoscopic images.
Core Tip: Various lesions associated with medication use are detected during esophagogastroduodenoscopy, including pill-induced esophagitis, desquamative esophagitis, deteriorating gastroesophageal reflux, chemotherapy-induced esophagitis, proton pump inhibitor-induced gastric mucosal changes, medication-induced gastric erosions and ulcers, pseudomelanosis of the stomach, olmesartan-related gastric mucosal inflammation, lanthanum deposition in the stomach, zinc acetate hydrate tablet-induced gastric lesions, immune-related adverse event gastritis, olmesartan-associated sprue-like enteropathy, duodenal pseudomelanosis, and lanthanum deposition. Endoscopists must diagnose these mucosal alterations by acquiring pertinent knowledge regarding medication-induced lesions, concomitant with inquiries concerning patient medication history.
- Citation: Iwamuro M, Kawano S, Otsuka M. Drug-induced mucosal alterations observed during esophagogastroduodenoscopy. World J Gastroenterol 2024; 30(16): 2220-2232
- URL: https://www.wjgnet.com/1007-9327/full/v30/i16/2220.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i16.2220
The primary purpose of screening esophagogastroduodenoscopy (EGD) is to comprehensively examine the esophagus, stomach, and duodenum to detect neoplasms. Furthermore, EGD provides invaluable information for disease diagnosis, assessment of disease state, and treatment planning in symptomatic patients. This procedure can reveal a spectrum of conditions, including cancer, and can also enable the identification of mucosal changes attributed to medications taken by the patient[1,2]. Although gastric and duodenal ulcers caused by nonsteroidal anti-inflammatory drugs (NSAIDs) have long been known as drug-induced upper gastrointestinal lesions[3,4], the advent of various medications on the market has led to the emergence of new types of mucosal injuries and alterations. Despite the inclusion of information on some drug-induced upper gastrointestinal mucosal lesions in the package inserts of medications, not all prescribing physicians are acquainted with these conditions due to their infrequency. Therefore, endoscopists should acquire accurate know-ledge regarding diverse drug-induced mucosal alterations for appropriate diagnosis. This knowledge is also crucial for the differential diagnosis of other conditions, including neoplastic lesions. Herein, we review articles associated with drug-induced mucosal alterations in the esophagus, stomach, and duodenum, and present endoscopic images of representative lesions detected on EGD.
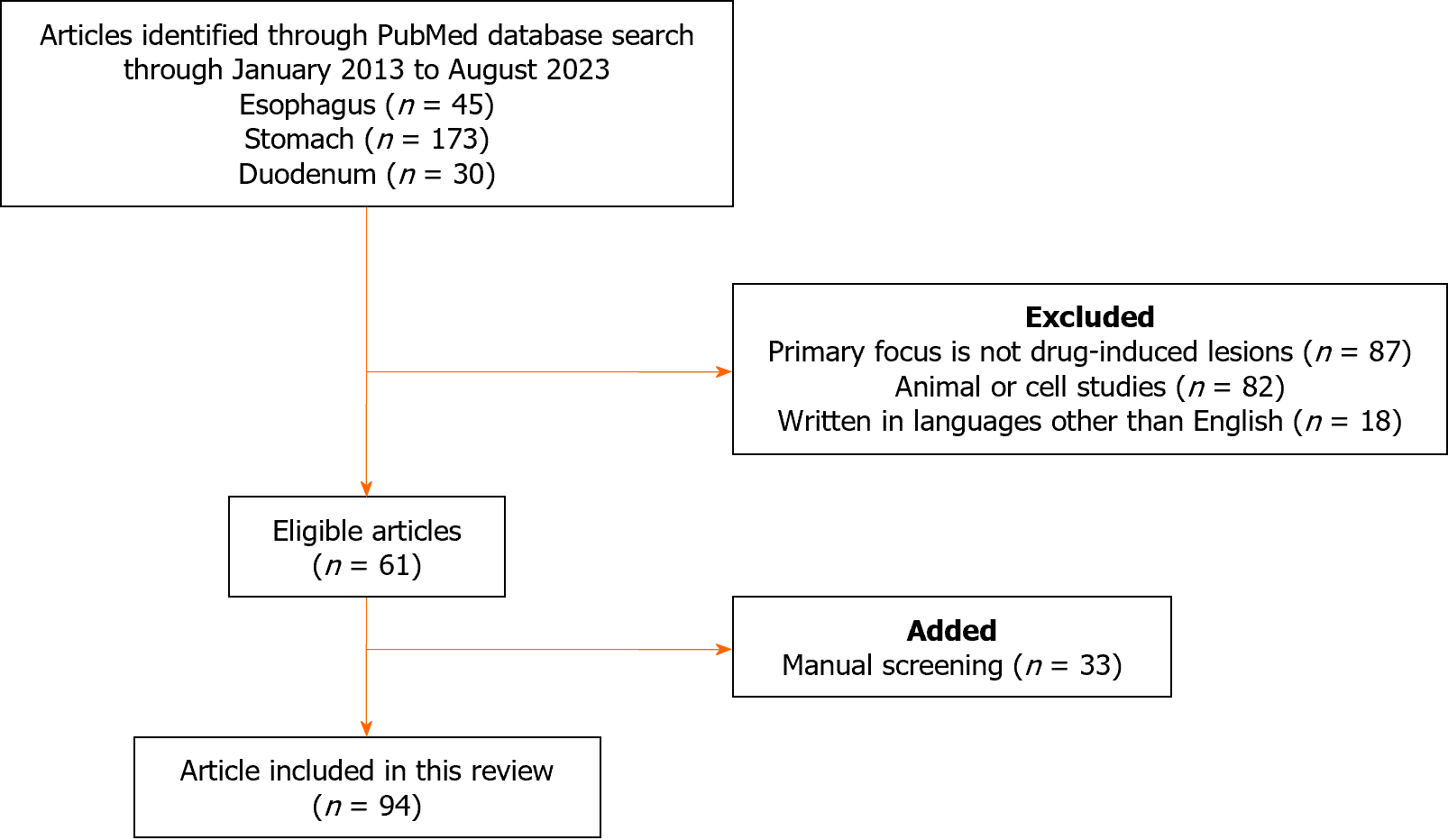
We conducted a systematic search of the PubMed database to retrieve all peer-reviewed articles published between January 1, 2013, and August 3, 2023, without imposing any study design filters. To augment our search results, we manually screened additional relevant articles using a reference list of selected publications that met our eligibility criteria. Our search used the keywords “drug-induced” and “esophagus”, “stomach”, or “duodenum”, and was performed by the principal investigator Iwamuro M. The inclusion criteria were as follows: (1) Peer-reviewed articles describing cases of drug-induced upper gastrointestinal tract lesion; and (2) Review articles, original articles, case series, and case reports. Articles were excluded if they: (1) Did not focus primarily on drug-induced upper gastrointestinal tract lesion; (2) Were animal or cell studies; (3) Were letters, editorials, or correction notices; or (4) Were written in languages other than English. All the eligible articles were evaluated.
Figure 1 presents a flow diagram summarizing the identification, screening, eligibility, and exclusion processes of the literature search. The keywords “drug-induced” and “esophagus” yielded 45 papers of which 19 were excluded for the following reasons: Not primarily focused on drug-induced upper gastrointestinal tract lesion (n = 14); animal or cell studies (n = 2); and written in languages other than English (n = 3). The keywords “drug-induced” and “stomach” yielded 173 papers, of which 149 papers were excluded for the following reasons: Not primarily focused on drug-induced upper gastrointestinal tract lesion (n = 65); animal or cell studies (n = 72); and studies written in languages other than English (n = 12). The keywords “drug-induced” and “duodenum” yielded 30 articles, of which 19 were excluded due to the following reasons: Not primarily focused on drug-induced upper gastrointestinal tract lesion (n = 8); animal or cell studies (n = 8); and studies written in languages other than English (n = 3). Finally, 61 articles were retrieved from the initial PubMed search after applying the exclusion criteria. After a manual screening, 33 additional articles were deemed relevant and included. A total of 94 articles were reviewed in detail.
Virtually all drugs may cause adverse events, including those involving the digestive tract, and various changes in the gastrointestinal mucosa due to different drugs have been reported. Drugs reported in two or more papers are presented in Table 1. In the subsequent sections, we elucidate the discernible categories of drug-induced mucosal alterations accompanied by illustrative EGD images.
| Esophagus | Stomach | Duodenum | |
| NSAIDs | Warfarin | PPIs | Olmesartan |
| Bisphosphonates | DOACs | NSAIDs | Iron tablets |
| Iron tablets | SSRIs | Steroids | Diuretics |
| Doxycycline | Benzodiazepine | Bisphosphonates | Lanthanum carbonate |
| Tetracycline | Phenytoin | Iron tablets | |
| Ciprofloxacin | Pinaverium | Doxycycline | |
| Clindamycin | Ascorbic acid | Diuretics | |
| Amoxicillin | L-arginine | Olmesartan | |
| Metronidazole | Opiates | Lanthanum carbonate | |
| Rifaximin | 5-fluorouracil | Zinc acetate | |
| Potassium chloride | Bleomycin | Immune checkpoint inhibitors | |
| Antihypertensives | Dactinomycin | ||
| Nitrates | Methotrexate | ||
| Quinidine | Cytarabine | ||
| Acetaminophen | Vincristine | ||
| Colchicine |
Given that tablets are ingested in a supine posture or preceding sleep, accompanied by inadequate water intake, the entrapment of medication within the esophagus may result in the release of deleterious agents, imparting noxious constituents capable of inflicting damage to the esophageal wall. The mucosal injury to the esophagus due to the retention of such medications is also referred to as pill-induced esophagitis[5-19]. Esophageal injury can be caused by over hundred distinct substances consumed in the form of oral pharmaceuticals. Principal contributors include antibiotics, notably tetracycline and doxycycline, along with other agents such as bisphosphonates[20], NSAIDs, potassium chloride[21], and iron pills. Acetaminophen, warfarin, colchicine, ascorbic acid, L-arginine, pinaverium, antihypertensives, and antiarrhythmic agents may also induce esophagitis. These pharmaceutical agents are believed to exert a corrosive effect on the esophageal mucosa, thereby instigating processes that lead to inflammation, irritation, erosion, and ulceration within the esophagus. Pill-induced esophagitis manifests as dysphagia, pain during swallowing, thoracic discomfort, heartburn, and general esophageal irritation. To attenuate the risk of esophageal injury, it is imperative for patients to ingest medications with a copious volume of plain water and concurrently adopt an upright posture (either sitting or standing) for a minimum of 30 min following the intake of the medication.
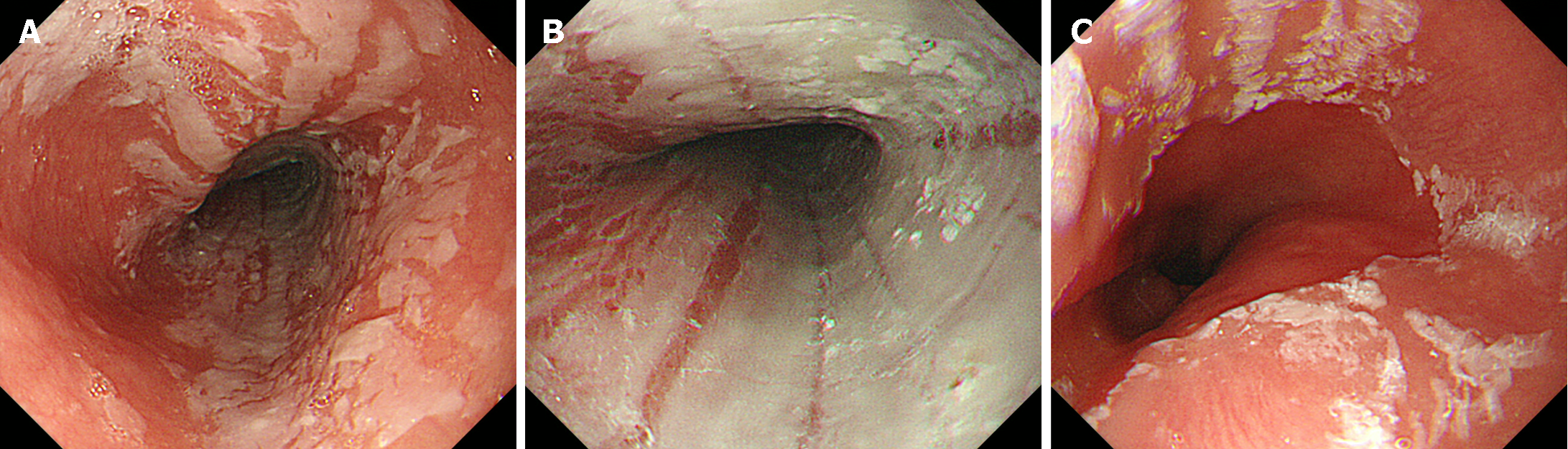
Desquamative esophagitis, also known as esophagitis dissecans superficialis, or sloughing esophagitis, is an infrequent, unique endoscopic finding characterized by mucosal sloughing into the esophageal lumen. Desquamative esophagitis occurs in patients taking direct oral anticoagulants, which are commonly prescribed for the prevention and treatment of blood clots. While dabigatran is frequently implicated[22-26], rivaroxaban, apixaban, and edoxaban can also induce this condition. A typical appearance is depicted in Figure 2, illustrating the presence of diffuse white membranous deposits in the mid to distal esophagus. Endoscopic biopsy of the white membranous deposits reveals a degenerated squamous epithelium accompanied by inflammatory cell infiltration[26]. A previous study found that the use of psychoactive agents, particularly selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors, was prevalent in patients with desquamative esophagitis[27]. Other medications, such as benzodiazepines, opioids, and antiepileptic agents, similarly contribute to the occurrence of desquamative esophagitis[8,28]. Such esophageal mucosal injuries are believed to occur through a mechanism similar to that of pill-induced esophagitis, in which damage arises from the retention of medication in the esophagus. Therefore, for prevention, it is crucial to take medication with a full glass of water while in an upright position to ensure smooth passage into the stomach.
In gastroesophageal reflux, the primary precipitant of mucosal injury is the refluxed gastric acid. However, various medications may exacerbate or trigger the onset of gastroesophageal reflux[13,14]. Nitrates such as nitroglycerin are commonly used to treat conditions such as angina by relaxing and dilating blood vessels. This relaxation effect is not specific to the blood vessels in the heart, but also extends to other smooth muscles, including the lower esophageal sphincter (LES), allowing stomach acid to flow back into the esophagus. Calcium channel blockers, anticholinergic medications, sedatives, tranquilizers, and theophylline may also relax the LES and contribute to acid reflux. In symptomatic individuals, it is imperative not only to administer proton pump inhibitors (PPIs), but also to evaluate the potential exacerbating effects of pharmacological agents on gastroesophageal reflux. Therefore, it is important to promptly discontinue or modify medication accordingly.
Chemotherapy-induced esophagitis refers to inflammation and irritation of the esophagus, which occurs as a side effect of chemotherapy drugs. These potent medications used to treat cancer can inadvertently damage the esophageal lining, leading to a range of symptoms and complications[8,11,18]. Drugs such as 5-fluorouracil, bleomycin, dactinomycin, methotrexate, cytarabine, and vincristine have been identified as causative agents.
PPIs, a class of medications that reduce stomach acid production, are commonly prescribed to treat conditions such as gastroesophageal reflux disease and peptic ulcers. Although PPIs are generally regarded as safe with a low incidence of adverse effects, emerging evidence suggests that their long-term use can elicit diverse endoscopic and histopathological alterations in the gastric mucosa[29,30]. These include multiple white and flat elevated lesions, fundic gland polyps, hyperplastic polyps, cobblestone-like mucosa, black spots, and a white globe appearance.
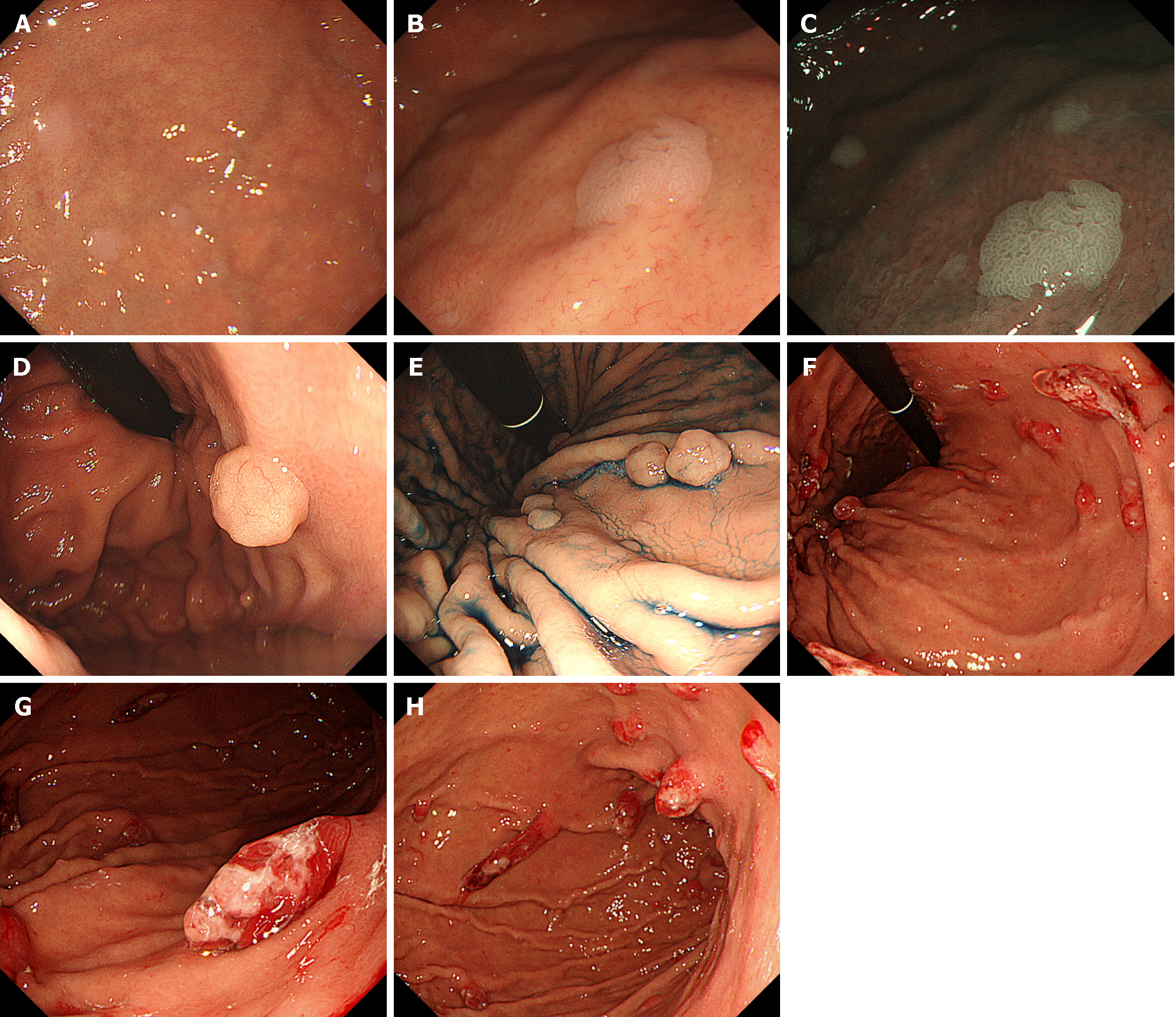
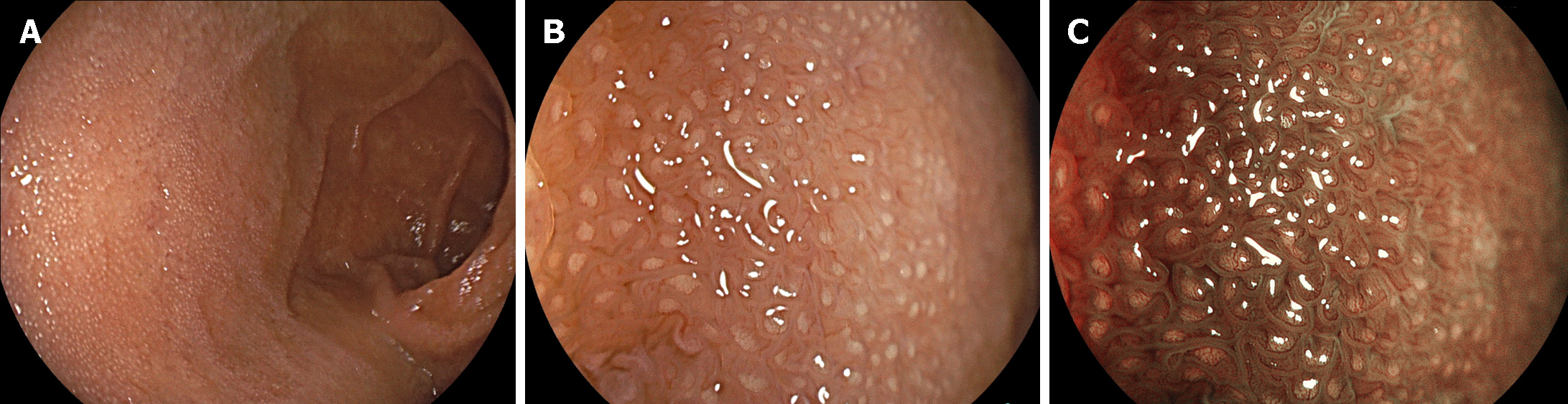
The term “multiple white and flat elevated lesions” was proposed in 2011 to describe a new type of polyp associated with PPI use that was observed in the gastric cardia, fornix, or corpus[31-34] (Figure 3A-C). These lesions manifest as circumscribed and sharply demarcated areas characterized by a whitish appearance, exhibiting a round morphology and slight elevation of the mucosa with a smooth surface. Multiple white and flat elevated lesions were more easily identified on narrow band imaging than on normal white-light observation. Pathologically, a straight, enlarged, and hyperplastic foveolar epithelium was observed, which is a typical feature of this lesion.
Fundic gland polyps are one of the most prevalent types of gastric polyps, with an estimated incidence ranging from approximately 2% to 11%, albeit subject to variation among diverse populations (Figure 3D and E)[35]. Notably, their occurrence tends to diminish in patients with Helicobacter pylori infection, but conversely increases in individuals undergoing PPI therapy. Fundic gland polyps reportedly regress after cessation of PPIs in some patients[36-40].
Several studies have explored the potential association between PPI use and the development of hyperplastic polyps in the stomach (Figure 3F-H). Some studies suggest that the long-term use of PPIs may be associated with an increased risk of gastric polyps[41]. The frequency of hyperplastic polyps exhibited a propensity for elevation among individuals testing positive for Helicobacter pylori. Similar to fundic gland polyps, hyperplastic polyps reportedly regress in some patients following the discontinuation of PPIs[36,42].
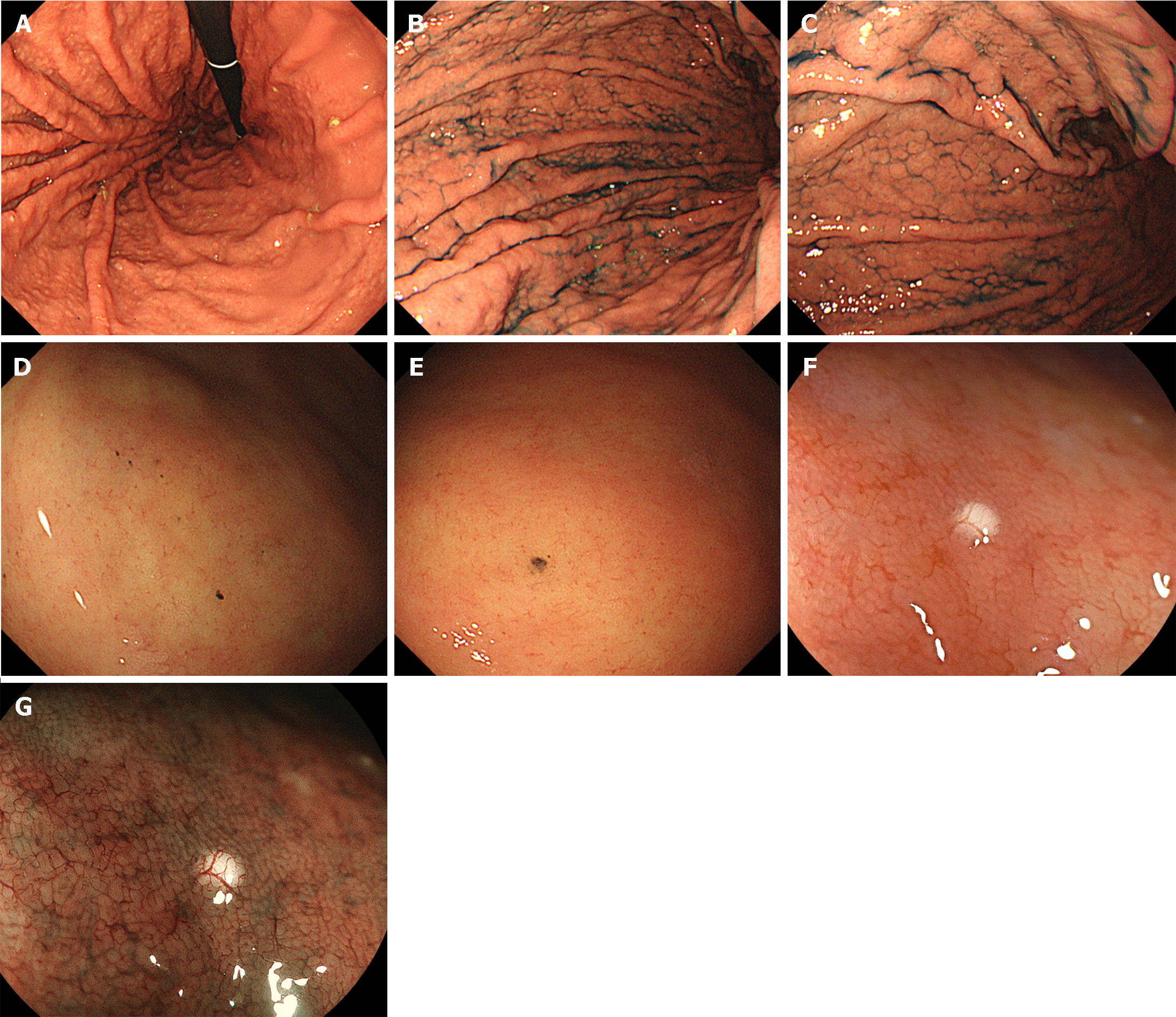
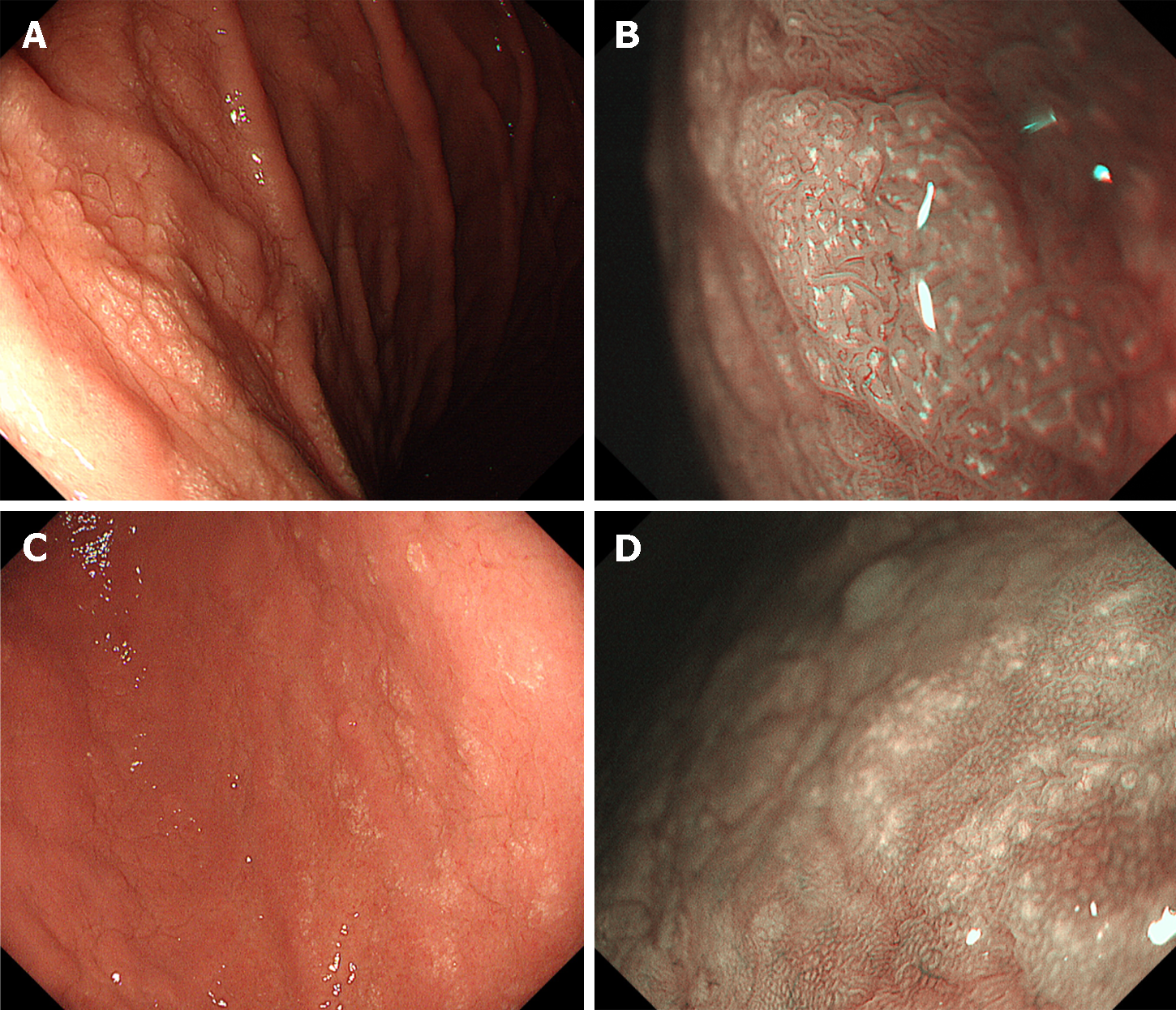
Cobblestone-like mucosa refers to the manifestation of numerous, approximately 3-5 mm-sized, irregular, elevated mucosal lesions in the gastric body[34,43,44] (Figure 4A-C). This distinctive mucosal pattern has a similar coloration as that of contiguous mucosa and is typically discerned as interspersed among the gastric folds. This represents a histopathological alteration attributable to prolonged PPI use. The histopathological characterization of the cobblestone-like mucosa involves the presence of parietal cell protrusions and cystic dilatation of the fundic glands, with these changes particularly accentuated in non-atrophic gastric regions.
Prolonged usage of PPIs induces the formation of black spots in the gastric mucosa, which are discerned as diminutive, dark, dot-like lesions on EGD (Figure 4D and E)[45]. Histopathologically, these spots are characterized by the entrapment of brownish substances within the dilated lumina of the expanded fundic gland cysts. The cystic dilatation of fundic gland cysts induced by the use of PPIs is strongly posited as a key etiological factor contributing to the development of black spots.
The term “white globe appearance” is defined as a small (≤ 1 mm) white globe-shaped feature located beneath the gastric epithelium, observed during magnifying endoscopic observation with narrow band imaging[46]. This feature is associated with early gastric cancers and is often detected near the demarcation line. It indicates cancers with a differentiated component. Conversely, a white globe appearance has also been noted in the gastric mucosa of non-cancer patients with autoimmune gastritis or during PPI use[47,48] (Figure 4F and G).
Gastric mucosal damage caused by NSAIDs has long been recognized. The mechanism involves several complex interactions[1,3,4,49]. NSAIDs inhibit cyclooxygenase and subsequently reduce the synthesis of prostaglandins, which play a protective role in maintaining the integrity of the gastric mucosa. NSAIDs cause vasoconstriction and thereby reduce the blood flow, which compromises the delivery of oxygen and nutrients to the gastric mucosa, resulting in mucosal damage. Some NSAIDs have direct toxic effects on the gastric mucosa. Epidemiological investigations have shown that the relative risk for the development of gastrointestinal complications escalates in patients concomitantly administered with corticosteroids and NSAIDs[50,51]. Similar to pill-induced esophagitis, bisphosphonates, iron tablets, and doxycycline directly irritate the gastric mucosa due to the chemical properties of the drug and its direct contact with the lining of the stomach.
Pseudomelanosis is an infrequent and benign pathological condition in which a dark pigment accumulates within macrophages located in the lamina propria. Unlike melanosis coli, the onset of gastric pseudomelanosis is unrelated to laxative use, but is thought to be associated with diuretics, beta-blockers, and iron supplementation. While gastric pseudomelanosis induces alterations in mucosal coloration, patients are devoid of accompanying clinical symptoms and do not manifest mucosal damage such as erosions or ulcers[52-55]. Deemed a benign condition, a diagnosis of gastric pseudomelanosis does not necessarily mandate any modification in the prescribed medication.
Olmesartan, an angiotensin II receptor antagonist commonly used to treat hypertension, induces enteropathy with sprue-like symptoms. Although infrequent, olmesartan has been reported to induce lymphocytic, collagenous, or chronic gastritis[18,56-59].
Lanthanum carbonate is used for the therapeutic management of hyperphosphatemia, primarily in patients with chronic renal insufficiency. White lesions are characteristic endoscopic features indicative of gastric lanthanum deposition[60-67]. These whitish deposits are easily discernible through narrow band or blue laser imaging. We have elucidated that the endoscopic manifestations of gastric lanthanum deposition vary between mucosa with and without atrophy. In non-atrophic mucosa, lanthanum was initially deposited on the posterior wall to the greater curvature of the gastric body, presenting as diffuse white lesions, the extent of which increased over time (Figure 5A and B). The susceptibility of the posterior wall to the greater curvature of the gastric body suggests that the active ingredient of the orally ingested lanthanum remains in prolonged contact with this region. Conversely, in atrophic mucosa, particularly with intestinal metaplasia, lanthanum deposition manifested as circular or granular white lesions (Figure 5C and D), and the extent of lanthanum deposition increased concurrently with the expansion of the intestinal metaplasia. The increased permeability of lanthanum in areas with intestinal metaplasia compared to that in normal mucosa may facilitate its deposition on the gastric mucosa. We speculate that the multifocal occurrence and mosaic-like distribution of intestinal metaplasia result in the circular or granular appearance of lanthanum deposition. Confirming a history of ingestion of lanthanum carbonate is essential for diagnosing this condition.
The pathological significance of lanthanum deposition in the human gastric mucosa remains unclear. To date, there have been no reports of health impairments associated with gastric lanthanum deposition, suggesting that the diagnosis of gastric lanthanum deposition does not necessarily mandate the discontinuation of lanthanum carbonate intake. However, the long-term prognosis of this condition is currently unknown, and ongoing follow-up of individual cases is desirable.
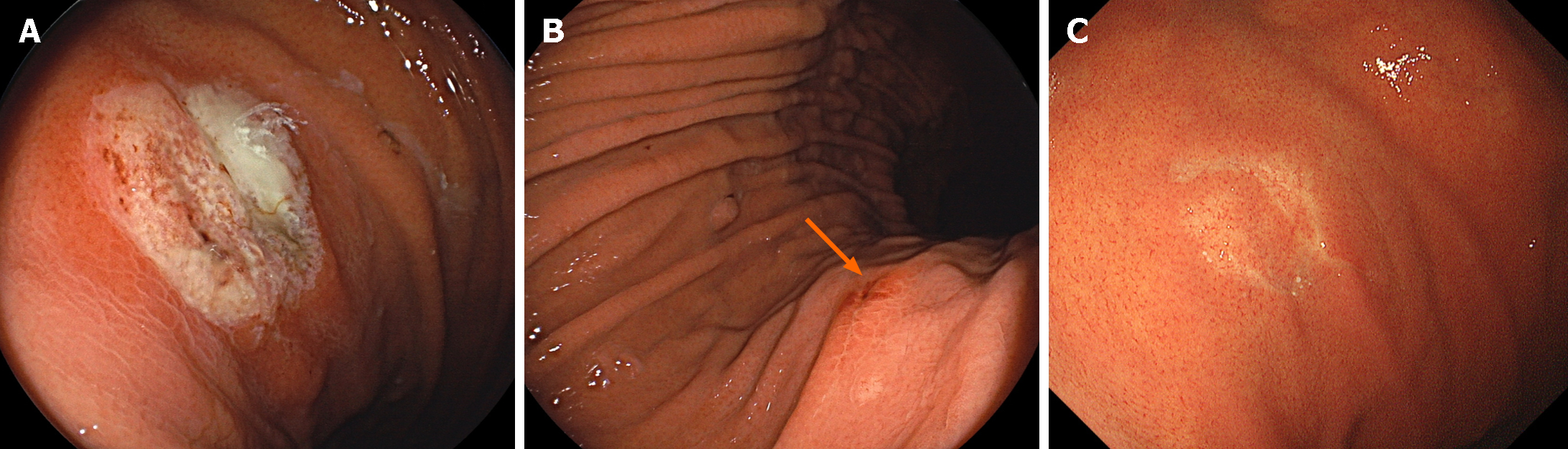
Zinc acetate tablets are used to treat zinc deficiency and Wilson’s disease. We found that approximately two-thirds of the patients subjected to oral administration of zinc acetate tablets manifested gastric mucosal injuries characterized by mucosal erythema, erosions, white patches, and ulcers[68] (Figure 6). Localization occurred predominantly in the middle third region, followed by the upper third region. Owing to the potential occurrence of hemorrhagic gastric ulcers, patients undergoing oral administration of zinc acetate hydrate should be monitored for gastric mucosal damage.
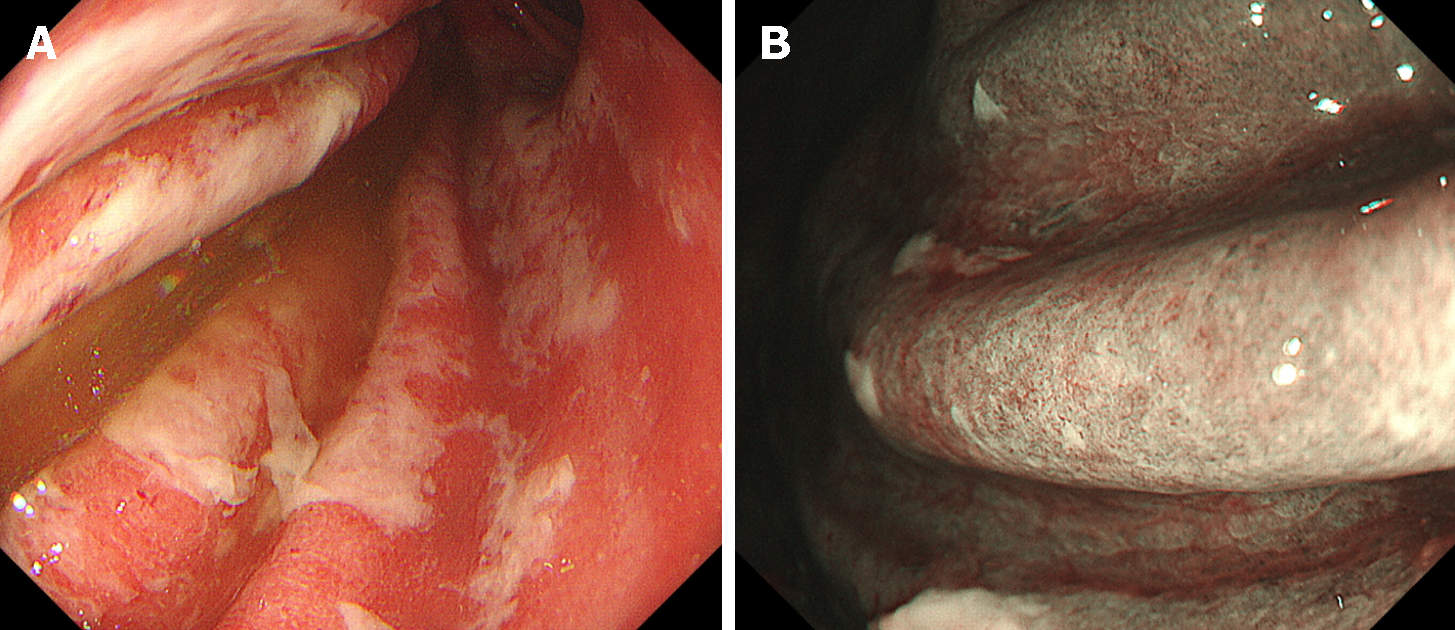
Immune checkpoint inhibitors can alleviate T-cell deactivation by reinstating the immune response against tumor cells. However, systemic activation of immune cells simultaneously induces self-reactive T cells in organs other than the tumor, potentially leading to the onset of immune-related adverse event (irAE) in various organs. Among the immune checkpoint inhibitor-induced gastrointestinal injuries, irAE colitis is well recognized[69]. Although the incidence of irAE gastritis is presumed to be lower than that of irAE colitis, endoscopic features of erythema, white exudates, and friable mucosa have been documented (Figure 7)[70-77]. The destruction of the glandular structure is visible upon magnifying observation with narrow band imaging[78]. If such lesions are observed after the administration of immune checkpoint inhibitors, the possibility of irAE gastritis should be considered. In irAEs, the prompt cessation of the causative agent does not consistently lead to rapid symptom amelioration and often necessitates the administration of steroids.
Olmesartan-associated sprue-like enteropathy denotes a condition associated with the usage of olmesartan, an angiotensin II receptor blocker. Sprue-like enteropathy is characterized by symptoms resembling those of celiac disease, such as chronic diarrhea, weight loss, and malabsorption of nutrients. Unlike in celiac disease, these symptoms persist even with a gluten-free diet. Villous atrophy, crypt hyperplasia, and inflammation are discernible in biopsied specimens[79-81]. Consequently, if a patient presents with persistent diarrhea, weight loss, and malabsorption, particularly when using olmesartan, duodenal biopsy, along with EGD, is essential for the evaluation of these characteristic pathological features and for diagnosis. Discontinuation of olmesartan typically resolves the symptoms and mucosal changes.
A black to dark brown pigmentation can be observed in the duodenum, termed duodenal pseudomelanosis (Figure 8)[54,82-85]. This condition is often observed in patients with chronic diseases such as hypertension, chronic kidney failure, and diabetes. A history of oral iron supplementation, antihypertensive agents (hydralazine), diuretics (thiazides, furosemide), and beta-blockers was noted in most cases. As mentioned in the section on gastric pseudomelanosis, this condition is considered benign and a change in oral medications is not necessarily required.
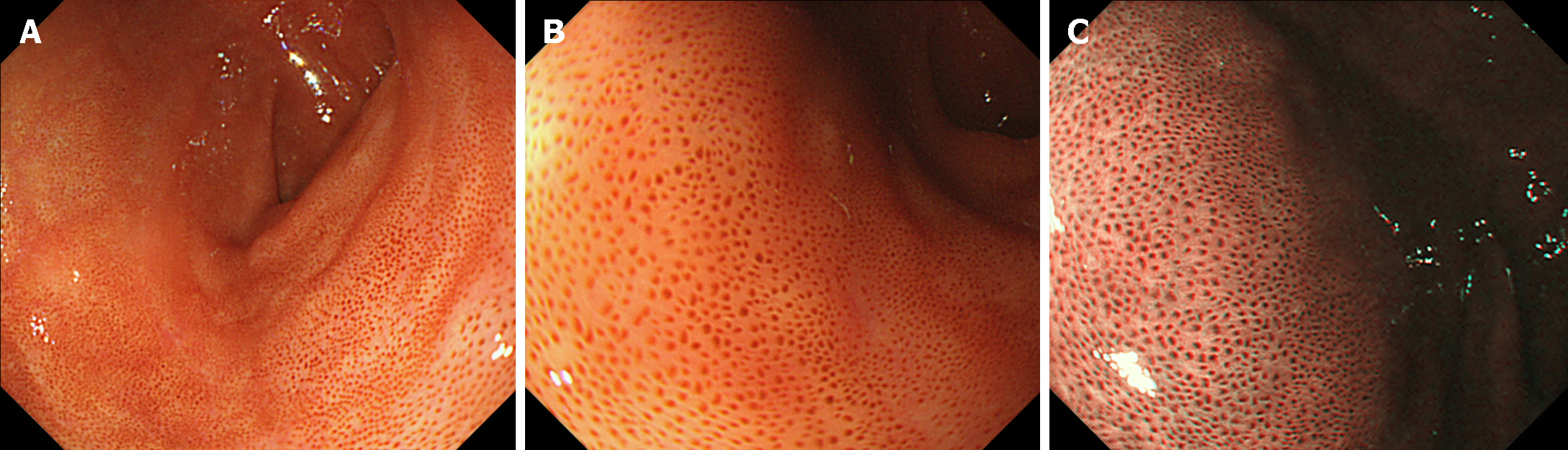
Lanthanum deposition in the duodenum refers to the accumulation of lanthanum in patients with chronic kidney disease taking lanthanum carbonate as a phosphate binder to treat elevated phosphate levels. The representative endoscopic feature is the presence of whitish discoloration of the villi, displaying numerous pinpoint or dot-like white deposits (Figure 9)[86-88]. Although this discoloration is a notable finding, the clinical significance of lanthanum deposition in the duodenum is still not fully understood, and its presence does not necessarily indicate pathology or adverse effects.
Diagnosing drug-induced mucosal alterations in the upper gastrointestinal tract is important for several reasons. First, in cases where a specific drug is identified as causing alterations in the esophageal, gastric, and duodenal mucosa, reassessment of treatment strategies is imperative. Discontinuation of the causative medication is generally recommended for patients presenting with symptoms or displaying evident mucosal damage, such as ulcers. If discontinuation of the causative agent proves challenging, dose reduction or transitioning to a medication with similar effects should be considered. Additionally, in the presence of lesions such as ulcers or erosions, acid-suppressing agents and mucosal protective agents may be administered. Second, establishing a diagnosis enables discerning whether gastrointestinal symptoms are attributable to a particular drug, prevents unnecessary examinations aimed at excluding other diseases, and facilitates the identification of appropriate interventions. In conclusion, the diagnosis of drug-induced upper gastrointestinal tract lesion is crucial for ensuring patient safety and facilitating appropriate medical management. Understanding the characteristic endoscopic images presented in this paper and conducting a thorough diagnosis will enable the implementation of suitable treatments and preventive measures.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu C, China S-Editor: Wang JJ L-Editor: A P-Editor: Chen YX
| 1. | Bordin DS, Livzan MA, Gaus OV, Mozgovoi SI, Lanas A. Drug-Associated Gastropathy: Diagnostic Criteria. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (2)] |
| 2. | Joo MK, Park CH, Kim JS, Park JM, Ahn JY, Lee BE, Lee JH, Yang HJ, Cho YK, Bang CS, Kim BJ, Jung HK, Kim BW, Lee YC; Korean College of Helicobacter Upper Gastrointestinal Research. Clinical Guidelines for Drug-Related Peptic Ulcer, 2020 Revised Edition. Gut Liver. 2020;14:707-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Nguyen TNM, Sha S, Chen LJ, Holleczek B, Brenner H, Schöttker B. Strongly increased risk of gastric and duodenal ulcers among new users of low-dose aspirin: results from two large cohorts with new-user design. Aliment Pharmacol Ther. 2022;56:251-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Bjarnason I, Scarpignato C, Holmgren E, Olszewski M, Rainsford KD, Lanas A. Mechanisms of Damage to the Gastrointestinal Tract From Nonsteroidal Anti-Inflammatory Drugs. Gastroenterology. 2018;154:500-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 311] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 5. | Bordea MA, Pirvan A, Sarban C, Margescu C, Leucuta D, Samasca G, Miu N. Pill -Induced Erosive Esophagitis in Children. Clujul Med. 2014;87:15-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Abdi S, Masbough F, Nazari M, Abbasinazari M. Drug-induced esophagitis and helpful management for healthcare providers. Gastroenterol Hepatol Bed Bench. 2022;15:219-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Hu SW, Chen AC, Wu SF. Drug-Induced Esophageal Ulcer in Adolescent Population: Experience at a Single Medical Center in Central Taiwan. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Mastracci L, Grillo F, Parente P, Unti E, Battista S, Spaggiari P, Campora M, Valle L, Fassan M, Fiocca R. Non gastro-esophageal reflux disease related esophagitis: an overview with a histologic diagnostic approach. Pathologica. 2020;112:128-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Gore RM, Levine MS. Diseases of the Upper GI Tract. 2018 Mar 21. In: Diseases of the Abdomen and Pelvis 2018-2021: Diagnostic Imaging - IDKD Book [Internet]. Cham (CH): Springer; 2018–. [PubMed] [DOI] [Full Text] |
| 10. | Panarelli NC. Other Forms of Esophagitis: It Is Not Gastroesophageal Reflux Disease, So Now What Do I Do? Surg Pathol Clin. 2017;10:765-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Grossi L, Ciccaglione AF, Marzio L. Esophagitis and its causes: Who is "guilty" when acid is found "not guilty"? World J Gastroenterol. 2017;23:3011-3016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (2)] |
| 12. | McGettigan MJ, Menias CO, Gao ZJ, Mellnick VM, Hara AK. Imaging of Drug-induced Complications in the Gastrointestinal System. Radiographics. 2016;36:71-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Domagała-Rodacka R, Cibor D, Szczeklik K, Rodacki T, Mach T, Owczarek D. Gastrointestinal tract as a side-effect target of medications. Przegl Lek. 2016;73:652-658. [PubMed] |
| 14. | Drug-induced lesions of the oesophageal mucosa. Prescrire Int. 2015;24:210-211, 213. [PubMed] |
| 15. | Kim SH, Jeong JB, Kim JW, Koh SJ, Kim BG, Lee KL, Chang MS, Im JP, Kang HW, Shin CM. Clinical and endoscopic characteristics of drug-induced esophagitis. World J Gastroenterol. 2014;20:10994-10999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (2)] |
| 16. | Dağ MS, Öztürk ZA, Akın I, Tutar E, Çıkman Ö, Gülşen MT. Drug-induced esophageal ulcers: case series and the review of the literature. Turk J Gastroenterol. 2014;25:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Almashat SJ, Duan L, Goldsmith JD. Non-reflux esophagitis: a review of inflammatory diseases of the esophagus exclusive of reflux esophagitis. Semin Diagn Pathol. 2014;31:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Allard FD, Stelow EB. Review of Drug-induced Injury in Mucosal Biopsies From the Tubular Gastrointestinal Tract. Adv Anat Pathol. 2019;26:151-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Kwak HA, Hart J. The Many Faces of Medication-Related Injury in the Gastrointestinal Tract. Surg Pathol Clin. 2017;10:887-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Costa MS, Gravito-Soares E, Gravito-Soares M, Figueiredo P. Severe drug-induced oesophagitis in a young male patient. BMJ Case Rep. 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Maesaka K, Tsujii Y, Shinzaki S, Yoshii S, Hayashi Y, Iijima H, Nakamoto K, Ohtani T, Sakata Y, Takehara T. Successful treatment of drug-induced esophageal ulcer in a patient with chronic heart failure: A case report. Medicine (Baltimore). 2018;97:e13380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Araki T, Hayashi K, Sonoda Y, Honda T, Imamura Y, Koide Y, Hamada H, Nakao K. Dabigatran-induced esophagitis with full circumferential blue pigmentation. DEN Open. 2024;4:e271. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Lin S, Wang Y, Zhang L, Guan W. Dabigatran must be used carefully: literature review and recommendations for management of adverse events. Drug Des Devel Ther. 2019;13:1527-1533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Cuadros Martínez M, Froilán Torres C, Gonzalo Bada N. Symptomatic exfoliative esophagitis induced by dabigatran. Rev Esp Enferm Dig. 2018;110:743-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Zhou Y, Su Y, Li Z, Wu C, Sun W, Wang C. Analysis of the clinical characteristics of dabigatran-induced oesophagitis. Eur J Hosp Pharm. 2023;30:e24-e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 26. | Toya Y, Nakamura S, Tomita K, Matsuda N, Abe K, Abiko Y, Orikasa S, Akasaka R, Chiba T, Uesugi N, Sugai T, Matsumoto T. Dabigatran-induced esophagitis: The prevalence and endoscopic characteristics. J Gastroenterol Hepatol. 2016;31:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Hart PA, Romano RC, Moreira RK, Ravi K, Sweetser S. Esophagitis Dissecans Superficialis: Clinical, Endoscopic, and Histologic Features. Dig Dis Sci. 2015;60:2049-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Purdy JK, Appelman HD, McKenna BJ. Sloughing esophagitis is associated with chronic debilitation and medications that injure the esophageal mucosa. Mod Pathol. 2012;25:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Shinozaki S, Osawa H, Hayashi Y, Sakamoto H, Miura Y, Lefor AK, Yamamoto H. Changes in gastric morphology during long-term use of vonoprazan compared to proton pump inhibitors. Singapore Med J. 2022;63:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Kim GH. Proton Pump Inhibitor-Related Gastric Mucosal Changes. Gut Liver. 2021;15:646-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 31. | Adachi K, Mishiro T, Okada M, Kinoshita Y. Prevalence of Multiple White and Flat Elevated Lesions in Individuals Undergoing a Medical Checkup. Intern Med. 2018;57:1213-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Hasegawa R, Yao K, Kanemitsu T, Arima H, Hirase T, Hiratsuka Y, Takeda K, Imamura K, Ohtsu K, Ono Y, Miyaoka M, Hisabe T, Ueki T, Tanabe H, Ohta A, Nimura S. Association between occurrence of multiple white and flat elevated gastric lesions and oral proton pump inhibitor intake. Clin Endosc. 2024;57:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Majima K, Muraki Y, Shimamoto T. Multiple White and Flat Elevated Lesions Observed in the Stomach: A Prospective Study of Clinical Characteristics and Risk Factors. Intern Med. 2018;57:2613-2619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Kiso M, Ito M, Boda T, Kotachi T, Masuda K, Hata K, Sasaki A, Kawamura T, Yoshihara M, Tanaka S, Chayama K. Endoscopic findings of the gastric mucosa during long-term use of proton pump inhibitor - a multicenter study. Scand J Gastroenterol. 2017;52:828-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Martin FC, Chenevix-Trench G, Yeomans ND. Systematic review with meta-analysis: fundic gland polyps and proton pump inhibitors. Aliment Pharmacol Ther. 2016;44:915-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 36. | Iwamuro M, Shiraha H, Okada H. Gastric polyps' regression after potassium-competitive acid blocker cessation. J Gen Fam Med. 2022;23:358-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Tanaka M, Kataoka H, Yagi T. Proton-pump inhibitor-induced fundic gland polyps with hematemesis. Clin J Gastroenterol. 2019;12:193-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Takeda T, Asaoka D, Tajima Y, Matsumoto K, Takeda N, Hiromoto T, Okubo S, Saito H, Aoyama T, Shibuya T, Sakamoto N, Hojo M, Osada T, Nagahara A, Yao T, Watanabe S. Hemorrhagic polyps formed like fundic gland polyps during long-term proton pump inhibitor administration. Clin J Gastroenterol. 2017;10:478-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Fukuda M, Ishigaki H, Sugimoto M, Mukaisho KI, Matsubara A, Ishida H, Moritani S, Itoh Y, Sugihara H, Andoh A, Ogasawara K, Murakami K, Kushima R. Histological analysis of fundic gland polyps secondary to PPI therapy. Histopathology. 2019;75:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Ochiai Y, Kikuchi D, Ito S, Takazawa Y, Hoteya S. Large Fundic Gland Polyp Associated with Long-Term Proton Pump Inhibitor Administration Mimicking Gastric-Type Neoplasm. Case Rep Gastroenterol. 2021;15:123-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Hongo M, Fujimoto K; Gastric Polyps Study Group. Incidence and risk factor of fundic gland polyp and hyperplastic polyp in long-term proton pump inhibitor therapy: a prospective study in Japan. J Gastroenterol. 2010;45:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 42. | Nikaido M, Miyamoto S, Utsumi T, Shimizu T, Nakanishi Y, Kumagai K, Teramura M, Setoyama T, Seno H. Gastric Hyperplastic Polyps Can Shrink After Discontinuation of Proton Pump Inhibitors: A Case Series Compared With Continuation of Proton Pump Inhibitors. J Clin Gastroenterol. 2022;56:e216-e221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Hatano Y, Haruma K, Kamada T, Shiotani A, Takahari K, Matsumoto M, Uchida O. Factors Associated with Gastric Black Spot, White Flat Elevated Mucosa, and Cobblestone-Like Mucosa: A Cross-Sectional Study. Digestion. 2018;98:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Takahari K, Haruma K, Ohtani H, Kiyoto S, Watanabe A, Kamada T, Manabe N, Hatano Y. Proton Pump Inhibitor Induction of Gastric Cobblestone-like Lesions in the Stomach. Intern Med. 2017;56:2699-2703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Hatano Y, Haruma K, Ayaki M, Kamada T, Ohtani H, Murao T, Manabe N, Mori H, Masaki T, Shiotani A. Black Spot, a Novel Gastric Finding Potentially Induced by Proton Pump Inhibitors. Intern Med. 2016;55:3079-3084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Doyama H, Yoshida N, Tsuyama S, Ota R, Takeda Y, Nakanishi H, Tsuji K, Tominaga K, Tsuji S, Takemura K, Yamada S, Katayanagi K, Kurumaya H, Iwashita A, Yao K. The "white globe appearance" (WGA): a novel marker for a correct diagnosis of early gastric cancer by magnifying endoscopy with narrow-band imaging (M-NBI). Endosc Int Open. 2015;3:E120-E124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Iwamuro M, Tanaka T, Kanzaki H, Kawano S, Kawahara Y, Okada H. Two Cases of White Globe Appearance in Autoimmune Atrophic Gastritis. Case Rep Gastrointest Med. 2018;2018:7091520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Miwa W, Hiratsuka T, Sato K, Fujino T, Kato Y. Marked reduction in the number of white globe appearance lesions in the noncancerous stomach after exchanging vonoprazan for esomeprazole treatment: a follow-up case report. Clin J Gastroenterol. 2021;14:1046-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Cheng H, Huang H, Guo Z, Chang Y, Li Z. Role of prostaglandin E2 in tissue repair and regeneration. Theranostics. 2021;11:8836-8854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 159] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 50. | Piper JM, Ray WA, Daugherty JR, Griffin MR. Corticosteroid use and peptic ulcer disease: role of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;114:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 372] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 51. | Aalykke C, Lauritsen K. Epidemiology of NSAID-related gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15:705-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Ibrahim M, Herman M. A Rare Finding of Gastric Pseudomelanosis in Chronic Kidney Disease: A Case Report. Cureus. 2023;15:e38933. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 53. | Qureshi NU, Younus MF, Alavi K, Sheikh MY. Gastric and Duodenal Pseudomelanosis: An Extended Unusual Finding in a Patient with End Stage Kidney Disease. Case Rep Gastrointest Med. 2016;2016:2861086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Wael Mohamed M, Althahabi R, Abubaker F, Sharif O. An Incidental Finding of Gastric and Duodenal Pseudomelanosis: A Case Report. Case Rep Gastroenterol. 2022;16:264-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Samiullah S, Bhurgri H, Babar A, Samad F, Choudhary MM, Demyen M. Peppered and rare - Gastric and Duodenal Pseudomelanosis: A case series. Pak J Med Sci. 2017;33:757-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 56. | Seminerio J, McGrath K, Arnold CA, Voltaggio L, Singhi AD. Medication-associated lesions of the GI tract. Gastrointest Endosc. 2014;79:140-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Ma C, Park JY, Montgomery EA, Arnold CA, McDonald OG, Liu TC, Salaria SN, Limketkai BN, McGrath KM, Musahl T, Singhi AD. A Comparative Clinicopathologic Study of Collagenous Gastritis in Children and Adults: The Same Disorder With Associated Immune-mediated Diseases. Am J Surg Pathol. 2015;39:802-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 58. | Rubio-Tapia A, Herman ML, Ludvigsson JF, Kelly DG, Mangan TF, Wu TT, Murray JA. Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc. 2012;87:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 307] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 59. | Shenbagaraj L, Swift G. Olmesartan-associated severe gastritis and enteropathy. BMJ Case Rep. 2018;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Iwamuro M, Urata H, Tanaka T, Okada H. Review of the diagnosis of gastrointestinal lanthanum deposition. World J Gastroenterol. 2020;26:1439-1449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Goto K, Ogawa K. Lanthanum Deposition Is Frequently Observed in the Gastric Mucosa of Dialysis Patients With Lanthanum Carbonate Therapy: A Clinicopathologic Study of 13 Cases, Including 1 Case of Lanthanum Granuloma in the Colon and 2 Nongranulomatous Gastric Cases. Int J Surg Pathol. 2016;24:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 62. | Rothenberg ME, Araya H, Longacre TA, Pasricha PJ. Lanthanum-Induced Gastrointestinal Histiocytosis. ACG Case Rep J. 2015;2:187-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Haratake J, Yasunaga C, Ootani A, Shimajiri S, Matsuyama A, Hisaoka M. Peculiar histiocytic lesions with massive lanthanum deposition in dialysis patients treated with lanthanum carbonate. Am J Surg Pathol. 2015;39:767-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Hoda RS, Sanyal S, Abraham JL, Everett JM, Hundemer GL, Yee E, Lauwers GY, Tolkoff-Rubin N, Misdraji J. Lanthanum deposition from oral lanthanum carbonate in the upper gastrointestinal tract. Histopathology. 2017;70:1072-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 65. | Yasunaga C, Haratake J, Ohtani A. Specific Accumulation of Lanthanum Carbonate in the Gastric Mucosal Histiocytes in a Dialysis Patient. Ther Apher Dial. 2015;19:622-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Nishida S, Ota K, Hattori K, Iwatsubo T, Kawaguchi S, Kojima Y, Takeuchi T, Maeda T, Sakaguchi M, Higuchi K. Investigation of the clinical significance and pathological features of lanthanum deposition in the gastric mucosa. BMC Gastroenterol. 2020;20:396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Yabuki K, Haratake J, Hisaoka M. Lanthanum Deposition in the Gastroduodenal Mucosa of Dialysis Patients. J UOEH. 2019;41:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 68. | Iwamuro M, Tanaka T, Kuraoka S, Hamada K, Abe M, Kono Y, Kanzaki H, Kawano S, Kawahara Y, Okada H. Zinc Acetate Dihydrate Tablet-associated Gastric Lesions. Intern Med. 2022;61:1931-1938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 69. | Xiang Z, Li J, Zhang Z, Cen C, Chen W, Jiang B, Meng Y, Wang Y, Berglund B, Zhai G, Wu J. Comprehensive Evaluation of Anti-PD-1, Anti-PD-L1, Anti-CTLA-4 and Their Combined Immunotherapy in Clinical Trials: A Systematic Review and Meta-analysis. Front Pharmacol. 2022;13:883655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 70. | Yip RHL, Lee LH, Schaeffer DF, Horst BA, Yang HM. Lymphocytic gastritis induced by pembrolizumab in a patient with metastatic melanoma. Melanoma Res. 2018;28:645-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Rao BB, Robertson S, Philpott J. Checkpoint Inhibitor-Induced Hemorrhagic Gastritis with Pembrolizumab. Am J Gastroenterol. 2019;114:196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 72. | Gonzalez RS, Salaria SN, Bohannon CD, Huber AR, Feely MM, Shi C. PD-1 inhibitor gastroenterocolitis: case series and appraisal of 'immunomodulatory gastroenterocolitis'. Histopathology. 2017;70:558-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 73. | Ferrian S, Liu CC, McCaffrey EF, Kumar R, Nowicki TS, Dawson DW, Baranski A, Glaspy JA, Ribas A, Bendall SC, Angelo M. Multiplexed imaging reveals an IFN-γ-driven inflammatory state in nivolumab-associated gastritis. Cell Rep Med. 2021;2:100419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 74. | Rovedatti L, Lenti MV, Vanoli A, Feltri M, De Grazia F, Di Sabatino A. Nivolumab-associated active neutrophilic gastritis. J Clin Pathol. 2020;73:605-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 75. | Obeidat A, Silangcruz K, Kozai L, Wien E, Fujiwara Y, Nishimura Y. Clinical Characteristics and Outcomes of Gastritis Associated With Immune Checkpoint Inhibitors: Scoping Review. J Immunother. 2022;45:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 76. | Iwamuro M, Tanaka T, Kono Y, Kawano S, Okada H. Multiple White Plaques in the Esophagus: A Possible Case of Esophageal Mucosal Alteration Associated With Immune-Related Adverse Events of Immune Checkpoint Inhibitors. Cureus. 2022;14:e32710. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 77. | Nishimura Y, Yasuda M, Ocho K, Iwamuro M, Yamasaki O, Tanaka T, Otsuka F. Severe Gastritis after Administration of Nivolumab and Ipilimumab. Case Rep Oncol. 2018;11:549-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 78. | Johncilla M, Grover S, Zhang X, Jain D, Srivastava A. Morphological spectrum of immune check-point inhibitor therapy-associated gastritis. Histopathology. 2020;76:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 79. | Bashari DR. Severe Sprue-Like Enteropathy and Colitis due to Olmesartan: Lessons Learned From a Rare Entity. Gastroenterology Res. 2020;13:150-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 80. | Santos-Antunes J, Coelho R, Cardoso H, Macedo G. Drug-induced small-bowel mucosal atrophy. Endoscopy. 2015;47 Suppl 1 UCTN:E8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 81. | Nielsen JA, Steephen A, Lewin M. Angiotensin-II inhibitor (olmesartan)-induced collagenous sprue with resolution following discontinuation of drug. World J Gastroenterol. 2013;19:6928-6930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Oduru O, Jo Gumm A, Sigurdsson L, Cook S, O'Connell DM. Pseudomelanosis Duodeni in a Child With Chronic Diarrhea. J Pediatr Gastroenterol Nutr. 2023;76:e45. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 83. | Kanda N, Izumi Y. A case of pseudomelanosis duodeni that disappeared after discontinuation of iron pills. Gastrointest Endosc. 2023;97:597-598. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 84. | Cheema HI, Raghavapuram S, Boston I, Augustine T, Tharian B. A Case of Duodenal "Spot" Diagnosis. Cureus. 2022;14:e25500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 85. | Lopez G, D'Ercole M, Ferrero S, Croci GA. Duodenal Pseudomelanosis: A Literature Review. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 86. | Ching D, Houghton D, Slim Z, Kumarasinghe MP. Duodenal pigment deposition in a patient with chronic renal failure. Pathology. 2020;52:729-731. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 87. | Iwamuro M, Urata H, Tanaka T, Kawano S, Kawahara Y, Okada H. Frequent Involvement of the Duodenum with Lanthanum Deposition: A Retrospective Observational Study. Intern Med. 2019;58:2283-2289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 88. | Iwamuro M, Tanaka T, Urata H, Kimoto K, Okada H. Lanthanum phosphate deposition in the duodenum. Gastrointest Endosc. 2017;85:1103-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |