Published online Apr 7, 2024. doi: 10.3748/wjg.v30.i13.1899
Peer-review started: January 4, 2024
First decision: January 17, 2024
Revised: January 29, 2024
Accepted: March 13, 2024
Article in press: March 13, 2024
Published online: April 7, 2024
Processing time: 89 Days and 15.2 Hours
Population of patients with inflammatory bowel disease (IBD) is burdened by various extraintestinal manifestations which substantially contribute to greater morbidity and mortality. Growth-differentiation factor-15 (GDF-15) is often over-expressed under stress conditions, such as inflammation, malignancies, heart failure, myocardial ischemia, and many others.
To explore the association between GDF-15 and IBD as serum concentrations of GDF-15 were shown to be an independent predictor of poor outcomes in multiple diseases. An additional aim was to determine possible associations between GDF-15 and multiple clinical, anthropometric and laboratory parameters in patients with IBD.
This cross-sectional study included 90 adult patients diagnosed with IBD, encompassing both Crohn’s disease (CD) and ulcerative colitis (UC), and 67 healthy age- and sex-matched controls. All patients underwent an extensive workup, including colonoscopy with subsequent histopathological analysis. Disease activity was assessed by two independent gastroenterology consultants specialized in IBD, employing well-established clinical and endoscopic scoring systems. GDF-15 serum concentrations were determined following an overnight fasting, using electrochemiluminescence immunoassay.
In patients with IBD, serum GDF-15 concentrations were significantly higher in comparison to the healthy controls [800 (512-1154) pg/mL vs 412 (407-424) pg/mL, P < 0.001], whereas no difference in GDF-15 was found between patients with CD and UC [807 (554-1451) pg/mL vs 790 (509-956) pg/mL, P = 0.324]. Moreover, multiple linear regression analysis showed that GDF-15 levels predict CD and UC severity independent of age, sex, and C-reactive protein levels (P = 0.016 and P = 0.049, respectively). Finally, an association between GDF-15 and indices of anemia was established. Specifically, negative correlations were found between GDF-15 and serum iron levels (r = -0.248, P = 0.021), as well as GDF-15 and hemoglobin (r = -0.351, P = 0.021). Accordingly, in comparison to IBD patients with normal hemoglobin levels, GDF-15 serum levels were higher in patients with anemia (1256 (502-2100) pg/mL vs 444 (412-795) pg/mL, P < 0.001).
For the first time, we demonstrated that serum concentrations of GDF-15 are elevated in patients with IBD in comparison to healthy controls, and the results imply that GDF-15 might be involved in IBD pathophysiology. Yet, it remains elusive whether GDF-15 could serve as a prognostic indicator in these patients.
Core Tip: Serum concentrations of growth-differentiation factor-15 (GDF-15) exhibit a significant elevation in inflammatory bowel disease (IBD) patients compared to healthy controls, irrespective of Crohn’s disease or ulcerative colitis diagnosis. GDF-15 levels independently predict disease severity and demonstrate an association with anemia indices, indicating its potential as a biomarker for IBD pathophysiology. Further exploration is nonetheless warranted to determine the prognostic value of GDF-15 in predicting outcomes for patients with IBD.
- Citation: Tonkic A, Kumric M, Akrapovic Olic I, Rusic D, Zivkovic PM, Supe Domic D, Sundov Z, Males I, Bozic J. Growth differentiation factor-15 serum concentrations reflect disease severity and anemia in patients with inflammatory bowel disease. World J Gastroenterol 2024; 30(13): 1899-1910
- URL: https://www.wjgnet.com/1007-9327/full/v30/i13/1899.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i13.1899
Inflammatory bowel disease (IBD) represents a group of chronic inflammatory disorders that affect the gastrointestinal tract, primarily manifesting as Crohn’s disease (CD) and ulcerative colitis (UC)[1]. Even though patients with IBD in general exhibit lower prevalence of traditional cardiovascular risk factors, such as hypertension, obesity and dyslipidemia, ample data suggests that the cardiovascular risk by which these patients are burdened exceeds that of the general population[2-6]. Moreover, the population of patients with IBD is burdened by various other extraintestinal manifestations, such as anemia, arthritis, and cancer, which substantially contribute to greater morbidity and mortality[7-10].
Growth-differentiation factor-15 (GDF-15) is a divergent member of the transforming growth factor-β super family[11]. It has been consistently demonstrated that GDF-15 is weakly expressed in all tissue types (except for placenta) under normal physiological states, where it plays a role in cell growth, signal transduction, and apoptosis regulation[11]. On the other hand, GDF-15 is often over-expressed under stress conditions, such as inflammation, malignancies, heart failure, myocardial ischemia and many others[12-15]. In fact, serum concentrations of GDF-15 were shown to predict poor outcomes in conditions with very variegated pathogenesis, for instance colorectal cancer and heart failure[14,15]. Although the exact function of GDF-15 is still not completely understood, it seems that the main function of overexpression in the aforementioned conditions is to maintain cell and tissue homeostasis[16]. Importantly, recent research indicated that serum levels of GDF-15 are associated with subclinical indices of atherosclerosis development in patients with rheumatoid arthritis (RA)[17]. On the other hand, although the data concerning the role of GDF-15 in IBD is limited, recent research indicated a possible association between GDF-15 and indices of iron homeostasis/erythropoiesis in patients with UC[18,19].
Therefore, the aim of the present study was to establish whether serum levels of GDF-15 are higher in patients with IBD in comparison to healthy controls. Moreover, we aimed to determine possible associations between GDF-15 and multiple clinical, anthropometric and laboratory parameters in patients with IBD.
The present cross-sectional study was conducted in the Laboratory for Cardiometabolic Research, University of Split School of Medicine and Department of Gastroenterology, University Hospital of Split in the period from January 2022 to January 2023.
The study was conducted in accordance with the ethical principles defined by the Declaration of Helsinki and its amendments, as well as the Good Clinical Practice guidelines from the International Conference on Harmonisation. Ethical approval for the present study was issued by the Ethics Committee of the University Hospital of Split (Class: 500-03/21-01/186; No: 2181-147/01/06/M.S.-21-02; Date: 22 December 2021). Prior to study inclusion, each participant was informed about the goal, procedures, and course of this study and has signed the written informed consent.
We consecutively enrolled 90 patients with IBD, 42 of which were diagnosed with UC and 48 with CD. Patients were recruited from the outpatient clinic of the Department of Gastroenterology, University Hospital of Split. In addition, we recruited 67 healthy volunteers with the purpose of forming a control group. The principal inclusion criteria, i.e., IBD diagnosis, was established in the accordance with contemporary guidelines of the European Crohn’s and Colitis Organization (ECCO) and the European Society of Gastrointestinal and Abdominal Radiology[20,21]. Specifically, we included patients who fulfilled the following criteria: disease duration of at least one year and stable disease activity in the past three months. The following exclusion criteria were applied: Age under 18 or over 65; established cardiovascular or cerebrovascular disease (heart failure, myocardial infarction, stroke, and peripheral artery disease); history of significant renal, pulmonary, or liver disease; diabetes mellitus; arterial hypertension; autoimmune/chronic inflammatory disorders (other than IBD); history of malignancy; use of corticosteroids in the last 3 months and acute inflammation. The same exclusion criteria applied to the control group. Subjects from the control group were additionally screened for irritable bowel syndrome symptoms according to the Rome IV criteria, as well as other abdominal symptoms suggestive of lactose and/or gluten intolerance[21].
Physical examination and relevant items from past medical history were obtained from all participants. Height and weight were measured using calibrated medical scale with integrated altitude meter (Seca, Birmingham, United Kingdom) body mass index was calculated by dividing the value of body mass and the squared value of height. The hip and waist circumferences were measured at standard positions while patients were standing. Waist-to-hip ratio was calculated by dividing the two. Office blood pressure was measured in seating position, following the principles outlined in the contemporary guidelines for management of hypertension[22].
Blood samples for the present analysis were obtained after an overnight fast by an experienced laboratory technician. A maximum of 25 mL of blood was obtained from the cubital vein using a sterile disposable needle. Sampled blood was either immediately analyzed, or aliquoted and stored at -80 °C for subsequent analysis of biomarkers, including GDF-15. GDF-15 serum concentrations were determined using an electrochemiluminescence immunoassay on Cobas e8000 analyzer (Elecsys, Roche Diagnostics). The reported sensitivity for GDF-15 was 400 pg/mL, with a linear range of 400-20000 pg/mL. The inter-assay coefficient of variability was 5%. Fecal calprotectin (FC) concentrations were measured by turbidimetric method (Beckman Coulter AU 680). Reported sensitivity for FC was 15 μg/g, with a linear range of 20-1500 μg/g, and intra-assay coefficient of variability < 20%. The rest of biochemical analyses were conducted by standard laboratory methods by an experienced biochemist. All biochemical analyses in the same certified institutional laboratory, using standard operating procedures, with the biochemist being blinded to the participant’s assignment to the IBD or control group.
Disease activity was assessed by two independent gastroenterology consultants specialized in IBD, using well-established clinical and endoscopic scoring systems. In case of score difference between the two, a consensus was made. For CD activity, simple endoscopic score for CD (SES-CD), and CD activity index[23,24]. For patients with UC, we reported Mayo score/disease activity index for UC (Mayo/DAI) and UC endoscopic index of severity (UCEIS)[23,24]. In light of the latest recommendations by ECCO, we used endoscopic index scores (UCEIS and SES-CD, respectively) in assessment of association between GDF-15 and disease severity, whereas other clinical index scores were descriptively reported[20]. Anemia was defined as Hgb < 130 g/L for male, and Hgb < 120 g/L for female patients.
MedCalc version 20.113 (MedCalc Software BV, Ostend, Belgium) and GraphPad Prism version 9.4.1 (GraphPad, La Jolla, CA, United States) were used for statistical data analysis and visual representation of data. Qualitative data was presented as whole number (n) and percentage (%), with the Chi-squared (χ2) test being used for the comparison of categorical variables. Quantitative data was expressed as mean ± SD or median and interquartile range, depending on data distribution. Accordingly, quantitative variables were compared with either Welch’s t-test or Mann-Whitney U test. In light of non-normal distribution of the main parameter of interest, Spearman’s rank correlation analysis was used to establish the association between GDF-15 and multiple clinical, laboratory and anthropometric variables. To ascertain that GDF-15 levels differ between IBD and control group independently of the possible confounders, we conducted multiple logistic regression analysis. Covariates for the above-noted analysis were age, sex, C-reactive protein (CRP), low-density lipoprotein (LDL)-cholesterol and albumin levels. Finally, multiple linear regression analysis was used to determine whether GDF-15 serum concentrations might predict disease activity independent of age, sex, and CRP levels. Variance inflation factor was used for detection of multicollinearity in linear regression analysis. Statistical significance was set at P < 0.05 for all comparisons.
The sample size was determined based on the analysis of GDF-15 serum levels in a pilot study involving 10 IBD patients and 10 control subjects. Our calculations, with a power of 90% and a type I error of 0.05, indicate that 40 subjects are required to detect a significant difference in GDF-15 serum levels.
In comparison to the control group, patients with IBD were more likely to have a positive family history of IBD (P = 0.003), and less likely to have a positive family history of cardiovascular disease (CVD) (P = 0.041). Additionally, patients with IBD had higher CRP levels (P < 0.001), but lower serum iron (P = 0.009), total cholesterol (P = 0.008), LDL-cholesterol (P < 0.001), and albumin levels (P < 0.001) compared to the control group. No significant differences were noted in other variables of interest. The baseline characteristics of patients are summarized in Table 1.
| Parameter | Control group (n = 67) | IBD group (n = 90) | P value1 |
| Age, yr | 38.5 ± 12.3 | 41.2 ± 15.8 | 0.195 |
| Male sex, n (%) | 43 (64.2) | 53 (58.9) | 0.503 |
| Body mass index, kg/m2 | 24.7 ± 2.8 | 23.8 ± 4.2 | 0.070 |
| Waist-to-hip ratio | 0.91 ± 0.36 | 0.87 ± 0.08 | 0.368 |
| Hypertension, n (%) | 1 (1.5) | 6 (6.7) | 0.122 |
| Dyslipidemia, n (%) | 2 (3.0) | 7 (7.8) | 0.203 |
| Family history of IBD, n (%) | 3 (4.5) | 16 (17.8) | 0.012 |
| Family history of CRC, n (%) | 9 (13.4) | 17 (18.9) | 0.365 |
| Family history of CVD, n (%) | 31 (46.3) | 29 (32.2) | 0.074 |
| Smoking, n (%) | 12 (18.5) | 17 (18.9) | 0.947 |
| C-reactive protein, mg/L | 0.7 (0.4-1.6) | 1.6 (0.7-3.8) | < 0.001 |
| Serum iron, μmol/L | 18.1 ± 6.7 | 15.2 ± 7.8 | 0.009 |
| Albumins, g/L | 43.9 ± 2.5 | 40.4 ± 4.9 | < 0.001 |
| Serum urate levels, mmol/L | 296.1 ± 76.0 | 275.5 ± 70.9 | 0.064 |
| Fasting blood glucose, mmol/L | 5.1 ± 0.7 | 5.2 ± 1.6 | 0.691 |
| Total cholesterol, mmol/L | 5.2 ± 1.2 | 4.7 ± 1.3 | 0.008 |
| LDL-C, mmol/L | 3.3 ± 1.1 | 2.7 ± 1.1 | < 0.001 |
| HDL-C, mmol/L | 1.4 ± 0.3 | 1.4 ± 0.4 | 0.409 |
| Triglycerides, mmol/L | 1.2 ± 0.6 | 1.4 ± 1.1 | 0.158 |
Compared to patients with UC, those with CD exhibited a higher prevalence of smoking (P < 0.001), extraintestinal manifestations (P < 0.001), and a greater likelihood of undergoing IBD-related surgery previously (P < 0.001). Furthermore, CD patients showed higher FC levels (P = 0.009) but lower levels of albumin (P < 0.001), total cholesterol (P = 0.005), LDL-cholesterol (P < 0.001), and high-density lipoprotein-cholesterol (P = 0.045). A comprehensive comparison of relevant basic characteristics, laboratory parameters, and disease features between patients with CD and UC is delineated in Table 2.
| Parameter | Crohn’s disease (n = 48) | Ulcerative colitis (n = 42) | P value1 |
| Basic characteristics | |||
| Age, yr | 40.7 ± 16.0 | 42.7 ± 15.9 | 0.567 |
| Male sex, n (%) | 31 (64.6) | 22 (52.4) | 0.243 |
| Body mass index, kg/m2 | 23.2 ± 3.6 | 23.9 ± 4.9 | 0.459 |
| Waist-to-hip ratio | 0.86 ± 0.08 | 0.86 ± 0.08 | 0.495 |
| Hypertension, n (%) | 2 (4.2) | 4 (9.5) | 0.312 |
| Dyslipidemia, n (%) | 3 (6.2) | 4 (9.5) | 0.565 |
| Family history of IBD, n (%) | 10 (20.8) | 6 (14.3) | 0.420 |
| Family history of CRC, n (%) | 10 (20.8) | 7 (16.7) | 0.616 |
| Family history of CVD, n (%) | 12 (28.6) | 17 (35.4) | 0.491 |
| Smoking, n (%) | 16 (33.3) | 1 (2.4) | < 0.001 |
| Laboratory parameters | |||
| C-reactive protein, mg/L | 1.9 (0.6-7.6) | 1.4 (0.8-2.2) | 0.153 |
| Serum iron, μmol/L | 14.6 ± 7.6 | 16.5 ± 7.9 | 0.247 |
| Albumins, g/L | 39.5 ± 5.5 | 41.6 ± 3.6 | 0.018 |
| Fasting blood glucose, mmol/L | 4.9 ± 0.8 | 5.6 ± 2.5 | 0.100 |
| Total cholesterol, mmol/L | 4.4 ± 1.2 | 5.1 ± 1.3 | 0.005 |
| LDL-C, mmol/L | 2.3 ± 0.9 | 3.1 ± 1.1 | < 0.001 |
| HDL-C, mmol/L | 1.3 ± 0.4 | 1.4 ± 0.4 | 0.045 |
| Triglycerides, mmol/L | 1.7 ± 1.5 | 1.2 ± 0.7 | 0.079 |
| Disease characteristics | |||
| Disease duration, yr | 7 (3-14) | 9 (5-13) | 0.397 |
| IBD-related surgery, n (%) | 20 (41.7) | 0 (0.0) | < 0.001 |
| ExtraintestiN/Al manifestations, n (%) | 27 (56.3) | 7 (16.7) | < 0.001 |
| SES-CD | 10 (5-13) | N/A | N/A |
| CDAI | 55 (34-84) | N/A | N/A |
| UCEIS | N/A | 5.0 (1.5-6.5) | |
| Mayo/DAI | N/A | 3 (2-5) | N/A |
| Fecal calprotectin, μg/g | 232 (80-589) | 85 (10-246) | 0.009 |
| Therapy, n (%) | |||
| Aminosalicylates | 22 (45.8) | 23 (54.8) | 0.421 |
| DMARDs | 18 (37.5) | 17 (40.5) | 0.826 |
| MonocloN/Al antibodies | 36 (75.0) | 32 (76.2) | 0.896 |
In comparison to the healthy age and sex-matched control group, GDF-15 serum concentrations were significantly higher in patients with IBD (P < 0.001) (Table 3). Additionally, multiple logistic regression analysis revealed that GDF-15 serum levels predict the presence of IBD independently of age, sex, CRP, albumin, and LDL serum levels (odds ratio: 1.17, 95% confidence interval: 1.05-1.19, P < 0.001). Serum concentrations of GDF-15 did not exhibit a significant difference between patients with CD and UC (P = 0.324) (Table 3).
| Parameter | Study groups | P value1 | |
| GDF-15, pg/mL | Control group (n = 67) | IBD group (n = 90) | |
| 412 (407-424) | 800 (512-1154) | < 0.001 | |
| Crohn’s disease (n = 48) | Ulcerative colitis (n = 42) | ||
| 807 (554-1451) | 790 (509-956) | 0.324 | |
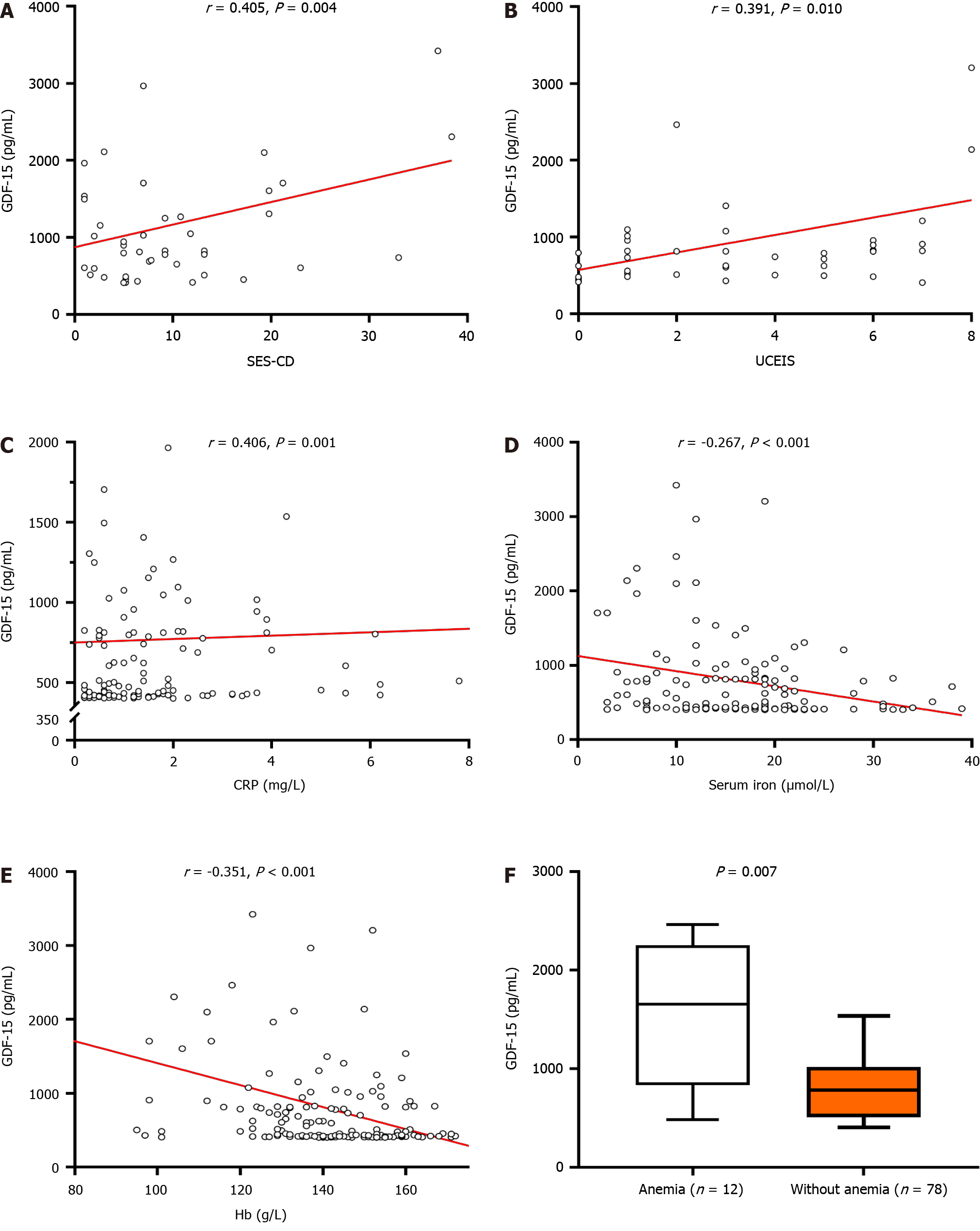
Significant correlations were noted between GDF-15 serum levels and IBD endoscopic disease activity indices. Specifically, GDF-15 serum levels in patients with CD were found to correlate with SES-CD (r = 0.405, P = 0.004) (Figure 1A), whereas in UC, a positive correlation was noted between GDF-15 levels and UCEIS (r = 0.391, P = 0.010) (Figure 1B). Moderate positive correlation was also found between CRP and GDF-15 in the total studied population (r = 0.406, P < 0.001) (Figure 1C). Nonetheless, it is worth noting that multiple linear regression analysis showed that GDF-15 levels predict CD and UC severity independent of age, sex, and CRP levels (P = 0.016 and P = 0.049, respectively).
On the other hand, negative correlations were found between GDF-15 and serum iron levels (r = -0.248, P = 0.021), as well as GDF-15 and hemoglobin (r = -0.351, P = 0.021) (Figure 1D and E). Accordingly, in comparison to IBD patients with normal hemoglobin levels, GDF-15 serum levels were higher in patients with anemia [1256 (502-2100) pg/mL vs 444 (412-795) pg/mL, P < 0.001] (Figure 1F). However, when analyses for UC and CD were conducted separately, the difference in GDF-15 with respect to the presence of anemia [859.5 (502.0-2464.0) pg/mL vs 787.0 (531.0-940.3) pg/mL, P = 0.396], as well as correlations with the aforementioned variables (serum iron and hemoglobin), were lost (r = -0.147, P = 0.359 and r = 0.003, P = 0.984, respectively).
Finally, we performed a correlation analysis between serum concentrations of GDF-15 and multiple anthropometric and laboratory variables. Significant correlations with serum GDF-15 were observed for albumins (r = -0.338), total cholesterol (r = -0.196) and LDL-cholesterol (r = -0.216). The results of the analysis are presented in Table 4.
To the best of our knowledge, this is the first study in which serum concentrations of GDF-15 were compared between patients with IBD and healthy age and sex-matched controls, and the only report in which the association between GDF-15 and IBD severity was explored.
There is a paucity of data concerning the role of GDF-15 in IBD, especially in human subjects. In a recent study, Yamamoto et al[18] reported that GDF-15 serum levels are significantly higher in CD patients with low skeletal muscle mass index (SMI) in comparison to high SMI, even after adjusting for possible confounders. Accordingly, negative correlation was established between GDF-15 and SMI. A possible explanation of this association is that GDF-15 may promote muscle wasting. Aside from the direct catabolic effects, it has been reported that the binding of GDF-15 to the receptors in the brainstem may lead to the loss of appetite and concurrent weight loss[25-30]. Although Yamamoto et al[18] did not have a control group, GDF-15 levels were similar to that of our CD population, and significantly higher than in healthy controls from our study or from previous reports[31,32]. In contrast to our results, a recent study failed to demonstrate a difference in GDF-15 serum levels between patients with UC and healthy controls[19]. The probable cause of the disparity is the fact that our study included significantly higher proportion of severe UC cases (approximately 30% vs approximately 10%). Since patients in the above-noted study were appropriately matched with controls, and as control subjects seem to be concordant with ours in terms of age and sex distribution, another possible explanation of conflicting results is ethnicity difference. Specifically, all our patients were of European ancestry, in contrast to the study by Ramasamy et al[19] that included Indian population exclusively. Although a conclusive answer on why the GDF-15 levels is elevated in IBD regardless of the CRP levels cannot be reached with the current study design, in line with the available data in other autoimmune disorders, we hypothesized that the elevated GDF-15 reflect its protective role in IBD. For instance, preclinical studies demonstrated that mice deficient in GDF-15 exhibited a more pronounced systemic inflammatory response, marked by increased levels of IL-6, IL-12, tumour necrosis factor alpha, and interferon-gamma in the serum, as well as enhanced local inflammatory response characterized by increased T-cell infiltration and upregulation of CXCR3 in a model of membrane glomerulonephritis[33].
Positive correlation between GDF-15 and endoscopic indices of CD and UC is concordant with previous reports in other chronic diseases[34]. In patients with RA, multiple authors demonstrated a positive correlation between the disease severity and the GDF-15 serum levels[17,35]. Brown et al[35] even demonstrated that applying GDF-15 in algorithms may aid in predicting response to hematopoietic stem cell transplantation, the presence of severe form, and joint erosions in RA. A preliminary report in patients with spondyloarthritis is in line with the data from RA studies, as a pilot study showed a moderate correlation between GDF-15 and multiple indices of spondyloarthritis severity[36]. Of note, in idiopathic inflammatory myopathy, GDF-15 serum levels correlated with the extent of myocardial injury[37]. Unfortunately, in previously conducted studies that measured GDF-15 serum levels in IBD patients, an association between disease severity and GDF-15 was not explored.
As anemia is a well-established extraintestinal manifestation of IBD, the presence of higher GDF-15 levels in anemic patients, as well as negative correlations of GDF-15 with both hemoglobin and serum iron, deserve particular attention. However, inferring about causative relation between any biomarker and anemia in IBD is challenging owing to its dual pathophysiology: Iron deficiency anemia develops as a result of chronic blood loss in IBD, whereas chronic inflammation underlies the development of anemia of chronic disease[38]. Previous research has indicated that GDF-15 levels correlate with anemia severity in patients with cancer, and that GDF-15 is in fact a negative regulator of hepcidin, a central regulator of iron homeostasis[39-41]. However, a recent study failed to demonstrate a correlation between GDF-15 and hepcidin in patients with UC[19]. In addition, the authors did not find any difference in the GDF-15 serum levels between anemic and non-anemic UC patients, which is in line with our sub-analysis on UC patients, but not on the overall IBD population. The authors argued that lack of change reflects insufficient anemia severity needed to induce GDF-15 secretion, which is also consistent with our data, i.e., negative correlation between GDF-15 and hemoglobin levels. Significant correlations in CD but not UC, are challenging to interpret in the absence of data for comparison and limited sample size, but since hemoglobin, serum iron and GDF-15 levels in our study were similar between UC and CD patients, we argue that there is a possibility that pathophysiological mechanisms underlying anemia in our UC and CD population may not be completely concordant.
Although GDF-15 has been shown to be a very successful prognostic indicator in multiple diseases, especially those of cardiovascular origin, current study design prevents us from making inferences about prognostic value of GDF-15 in IBD population. Yet, in light of the fact that serum concentrations of GDF-15 were associated with disease severity and anemia in patients with IBD, both of which could potentially contribute to morbidity and mortality, GDF-15 might serve as a predictor of poor outcomes beyond traditional risk factors in IBD population, providing a strong basis to explore such association in future studies. Of important note, the pathophysiological processes that underlie the relationship between GDF-15 and CVD are still elusive, rendering the interpretation difficult[33]. In fact, as GDF-15 appears to produce both beneficial and adverse effects depending on the microenvironment, it remains unclear whether GDF-15 offers protective or detrimental role.
The present study has several limitations. Cross-sectional design prevents us from establishing causality. Furthermore, relapsing, and remitting nature of IBD alongside non-constant secretion of GDF-15 further impedes the establishment of causality by single point measurement. Nonetheless, it is worth noting that patients were equally distributed with respect to disease activity in both UC and CD. The study might benefit from concurrent measurement of serum hepcidin and soluble transferrin receptor concentrations. Finally, although sample size was somewhat limited, we aimed to create IBD and control groups devoid of any other pathologies that might violate the assumptions about the role of GDF-15 in IBD.
For the first time, we demonstrated that serum concentrations of GDF-15 are elevated in patients with IBD in comparison to age and sex-matched healthy controls independently of the factors that might affect GDF-15 levels. Moreover, in patients with both CD and UC, a positive correlation was found between GDF-15 and endoscopic disease activity indices, whereas no significant difference in GDF-15 serum levels was found between CD and UC groups. In addition, an association between the serum iron and hematological parameters of anemia with GDF-15 serum levels was established in patients with CD. Overall, although the present results implicate that GDF-15 might be involved in pathophysiology of IBD and its extraintestinal manifestations, currently there is insufficient data to establish whether serum GDF-15 levels might predict outcomes in patients with IBD. Exploring molecular pathways associating GDF-15 and IBD, and adequately powered prospective studies that assess crude outcomes represent the two crucial tools required to put these premises to the test. Finally, given the well-established role of GDF-15 in predicting cardiovascular outcomes, future research needs to elucidate whether GDF-15 explains paradoxically worse cardiovascular outcomes in patients with IBD.
Although commonly perceived as disease of the gastrointestinal system, inflammatory bowel disease can affect other organ systems, including cardiovascular, potentially leading to increased morbidity and mortality. Growth-differentiation factor-15 (GDF-15) is often over-expressed in stress conditions, including inflammation, malignancies, heart failure, and myocardial ischemia. In fact, elevated serum concentrations of GDF-15 have been linked to poor outcomes in conditions with diverse pathogenesis, such as colorectal cancer and heart failure.
As serum concentrations of GDF-15 were shown to be an independent predictor of poor outcomes in diverse ailments, we aimed to explore whether such association is present in the setting of inflammatory bowel disease (IBD) and its consequences. Establishing the role of GDF-15 in IBD might be relevant since poor long-term outcomes in IBD population are currently not fully elucidated.
To establish whether serum levels of GDF-15 in patients with IBD are different then in the healthy controls. Furthermore, we aimed to establish whether GDF-15 level correlate with disease severity, thus providing a rationale for the future assessment of its prognostic role in IBD. Finally, we investigated if association between indices of anemia and GDF-15 serum levels exists in this population.
In this cross-sectional study, patients with IBD and healthy age- and sex-matched participants underwent an extensive diagnostic workup. IBD group also underwent colonoscopy with subsequent histopathological analysis, and the disease activity was assessed using well-established clinical and endoscopic scoring systems. GDF-15 serum concentrations were determined using electrochemiluminescence immunoassay.
The principal findings of the present study reveal significantly elevated levels of GDF-15 in patients with IBD compared to the control group, and these levels increase with greater disease severity. Since no similar data have been previously published, the reasons behind this observation remain elusive. Nevertheless, considering the independent association between GDF-15 and indices of anemia, it is plausible that pathophysiological changes in anemia and iron metabolism might, to some extent, explain the observed difference.
This study marks the first demonstration of significantly elevated serum concentrations of GDF-15 in patients with IBD. While mechanistic studies and prospective trials are essential for firm conclusions, these preliminary findings suggest that exploring the role of GDF-15 as a biomarker in IBD could be worthwhile.
Future research should delve into the prognostic role of GDF-15, with a specific focus on its relationship with disease severity. Furthermore, investigating the mechanisms underlying these preliminary results will contribute to a deeper understanding of the role of GDF-15 in IBD.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tu JY, China S-Editor: Chen YL L-Editor: A P-Editor: Yuan YY
| 1. | de Souza HSP. Etiopathogenesis of inflammatory bowel disease: today and tomorrow. Curr Opin Gastroenterol. 2017;33:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Singh S, Singh H, Loftus EV Jr, Pardi DS. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12 382-93.e1:quiz e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 3. | Alicic D, Martinovic D, Rusic D, Zivkovic PM, Tadin Hadjina I, Vilovic M, Kumric M, Tokic D, Supe-Domic D, Lupi-Ferandin S, Bozic J. Urotensin II levels in patients with inflammatory bowel disease. World J Gastroenterol. 2021;27:6142-6153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Zuin M, Rigatelli G, Del Favero G, Andreotti AN, Picariello C, Zuliani G, Carraro M, Galasso MP, Roncon L. Cardiovascular disease in patients with inflammatory bowel disease: An issue in no guidelines land. Int J Cardiol. 2016;222:984-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Brnić D, Martinovic D, Zivkovic PM, Tokic D, Tadin Hadjina I, Rusic D, Vilovic M, Supe-Domic D, Tonkic A, Bozic J. Serum adropin levels are reduced in patients with inflammatory bowel diseases. Sci Rep. 2020;10:9264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Zivkovic PM, Matetic A, Tadin Hadjina I, Rusic D, Vilovic M, Supe-Domic D, Borovac JA, Mudnic I, Tonkic A, Bozic J. Serum Catestatin Levels and Arterial Stiffness Parameters Are Increased in Patients with Inflammatory Bowel Disease. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 7. | Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology. 2021;161:1118-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 462] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 8. | Shah Y, Patel D, Khan N. Iron deficiency anemia in IBD: an overlooked comorbidity. Expert Rev Gastroenterol Hepatol. 2021;15:771-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Tadin Hadjina I, Zivkovic PM, Matetic A, Rusic D, Vilovic M, Bajo D, Puljiz Z, Tonkic A, Bozic J. Impaired neurocognitive and psychomotor performance in patients with inflammatory bowel disease. Sci Rep. 2019;9:13740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Faye AS, Holmer AK, Axelrad JE. Cancer in Inflammatory Bowel Disease. Gastroenterol Clin North Am. 2022;51:649-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 11. | Xiao QA, He Q, Zeng J, Xia X. GDF-15, a future therapeutic target of glucolipid metabolic disorders and cardiovascular disease. Biomed Pharmacother. 2022;146:112582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Kljakovic-Gaspic T, Tokic D, Martinovic D, Kumric M, Supe-Domic D, Stojanovic Stipic S, Delic N, Vrdoljak J, Vilovic M, Ticinovic Kurir T, Bozic J. Prognostic Value of Catestatin in Severe COVID-19: An ICU-Based Study. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Kumric M, Ticinovic Kurir T, Borovac JA, Bozic J. Role of novel biomarkers in diabetic cardiomyopathy. World J Diabetes. 2021;12:685-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 14. | Luo JW, Duan WH, Song L, Yu YQ, Shi DZ. A Meta-Analysis of Growth Differentiation Factor-15 and Prognosis in Chronic Heart Failure. Front Cardiovasc Med. 2021;8:630818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Li C, Wang X, Casal I, Wang J, Li P, Zhang W, Xu E, Lai M, Zhang H. Growth differentiation factor 15 is a promising diagnostic and prognostic biomarker in colorectal cancer. J Cell Mol Med. 2016;20:1420-1426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Breit SN, Johnen H, Cook AD, Tsai VW, Mohammad MG, Kuffner T, Zhang HP, Marquis CP, Jiang L, Lockwood G, Lee-Ng M, Husaini Y, Wu L, Hamilton JA, Brown DA. The TGF-β superfamily cytokine, MIC-1/GDF15: a pleotrophic cytokine with roles in inflammation, cancer and metabolism. Growth Factors. 2011;29:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 17. | He YW, He CS. Association of Growth and Differentiation Factor 15 in Rheumatoid Arthritis. J Inflamm Res. 2022;15:1173-1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Yamamoto H, Takeshima F, Haraguchi M, Akazawa Y, Matsushima K, Kitayama M, Ogihara K, Tabuchi M, Hashiguchi K, Yamaguchi N, Miyaaki H, Kondo H, Nakao K. High serum concentrations of growth differentiation factor-15 and their association with Crohn's disease and a low skeletal muscle index. Sci Rep. 2022;12:6591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Ramasamy J, Jagadish C, Sukumaran A, Varghese J, Mani T, Joseph AJ, Simon EG, Jacob M. Low Serum Hepcidin Levels in Patients with Ulcerative Colitis - Implications for Treatment of Co-existent Iron-Deficiency Anemia. Inflammation. 2023;46:2209-2222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, Calabrese E, Baumgart DC, Bettenworth D, Borralho Nunes P, Burisch J, Castiglione F, Eliakim R, Ellul P, González-Lama Y, Gordon H, Halligan S, Katsanos K, Kopylov U, Kotze PG, Krustinš E, Laghi A, Limdi JK, Rieder F, Rimola J, Taylor SA, Tolan D, van Rheenen P, Verstockt B, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1170] [Article Influence: 195.0] [Reference Citation Analysis (0)] |
| 21. | Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016;6:1393-1407.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 1898] [Article Influence: 210.9] [Reference Citation Analysis (3)] |
| 22. | Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021-3104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7431] [Cited by in RCA: 6375] [Article Influence: 910.7] [Reference Citation Analysis (0)] |
| 23. | Rodrigues BL, Mazzaro MC, Nagasako CK, Ayrizono MLS, Fagundes JJ, Leal RF. Assessment of disease activity in inflammatory bowel diseases: Non-invasive biomarkers and endoscopic scores. World J Gastrointest Endosc. 2020;12:504-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 24. | Sturm A, Maaser C, Calabrese E, Annese V, Fiorino G, Kucharzik T, Vavricka SR, Verstockt B, van Rheenen P, Tolan D, Taylor SA, Rimola J, Rieder F, Limdi JK, Laghi A, Krustiņš E, Kotze PG, Kopylov U, Katsanos K, Halligan S, Gordon H, González Lama Y, Ellul P, Eliakim R, Castiglione F, Burisch J, Borralho Nunes P, Bettenworth D, Baumgart DC, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis. 2019;13:273-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 301] [Article Influence: 50.2] [Reference Citation Analysis (1)] |
| 25. | Yang L, Chang CC, Sun Z, Madsen D, Zhu H, Padkjær SB, Wu X, Huang T, Hultman K, Paulsen SJ, Wang J, Bugge A, Frantzen JB, Nørgaard P, Jeppesen JF, Yang Z, Secher A, Chen H, Li X, John LM, Shan B, He Z, Gao X, Su J, Hansen KT, Yang W, Jørgensen SB. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med. 2017;23:1158-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 478] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 26. | Mullican SE, Lin-Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, Beck SC, South VJ, Dinh TQ, Cash-Mason TD, Cavanaugh CR, Nelson S, Huang C, Hunter MJ, Rangwala SM. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med. 2017;23:1150-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 543] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 27. | Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, Coskun T, Hamang MJ, Sindelar DK, Ballman KK, Foltz LA, Muppidi A, Alsina-Fernandez J, Barnard GC, Tang JX, Liu X, Mao X, Siegel R, Sloan JH, Mitchell PJ, Zhang BB, Gimeno RE, Shan B, Wu X. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med. 2017;23:1215-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 475] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 28. | Patel MS, Lee J, Baz M, Wells CE, Bloch S, Lewis A, Donaldson AV, Garfield BE, Hopkinson NS, Natanek A, Man WD, Wells DJ, Baker EH, Polkey MI, Kemp PR. Growth differentiation factor-15 is associated with muscle mass in chronic obstructive pulmonary disease and promotes muscle wasting in vivo. J Cachexia Sarcopenia Muscle. 2016;7:436-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 29. | Ost M, Igual Gil C, Coleman V, Keipert S, Efstathiou S, Vidic V, Weyers M, Klaus S. Muscle-derived GDF15 drives diurnal anorexia and systemic metabolic remodeling during mitochondrial stress. EMBO Rep. 2020;21:e48804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 30. | Bloch SA, Lee JY, Syburra T, Rosendahl U, Griffiths MJ, Kemp PR, Polkey MI. Increased expression of GDF-15 may mediate ICU-acquired weakness by down-regulating muscle microRNAs. Thorax. 2015;70:219-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | Johann K, Kleinert M, Klaus S. The Role of GDF15 as a Myomitokine. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 32. | Ye D, Liu B, He Z, Huang L, Qian Y, Shao K, Wen C, Mao Y. Assessing the Associations of Growth Differentiation Factor 15 with Rheumatic Diseases Using Genetic Data. Clin Epidemiol. 2021;13:245-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Moschovaki-Filippidou F, Steiger S, Lorenz G, Schmaderer C, Ribeiro A, von Rauchhaupt E, Cohen CD, Anders HJ, Lindenmeyer M, Lech M. Growth Differentiation Factor 15 Ameliorates Anti-Glomerular Basement Membrane Glomerulonephritis in Mice. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Corre J, Hébraud B, Bourin P. Concise review: growth differentiation factor 15 in pathology: a clinical role? Stem Cells Transl Med. 2013;2:946-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 35. | Brown DA, Moore J, Johnen H, Smeets TJ, Bauskin AR, Kuffner T, Weedon H, Milliken ST, Tak PP, Smith MD, Breit SN. Serum macrophage inhibitory cytokine 1 in rheumatoid arthritis: a potential marker of erosive joint destruction. Arthritis Rheum. 2007;56:753-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Song Y, Cui Y, Zhang X, Lin H, Zhang G, Zeng H, Zeng Y. Increased serum levels of MIC1/GDF15 correlated with bone erosion in spondyloarthritis: A pilot study. Medicine (Baltimore). 2018;97:e13733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Qiu M, Sun X, Qi X, Liu X, Zhang Y, Zhang N, Lu F, Liu W, Changjing F, Wang Q, Zhou L. The diagnostic value of GDF-15 for myocardial involvement in idiopathic inflammatory myopathy. Rheumatology (Oxford). 2021;60:2826-2833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015;6:62-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 39. | Jiang F, Yu WJ, Wang XH, Tang YT, Guo L, Jiao XY. Regulation of hepcidin through GDF-15 in cancer-related anemia. Clin Chim Acta. 2014;428:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Mirciov CS, Wilkins SJ, Dunn LA, Anderson GJ, Frazer DM. Characterization of Putative Erythroid Regulators of Hepcidin in Mouse Models of Anemia. PLoS One. 2017;12:e0171054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Corre J, Labat E, Espagnolle N, Hébraud B, Avet-Loiseau H, Roussel M, Huynh A, Gadelorge M, Cordelier P, Klein B, Moreau P, Facon T, Fournié JJ, Attal M, Bourin P. Bioactivity and prognostic significance of growth differentiation factor GDF15 secreted by bone marrow mesenchymal stem cells in multiple myeloma. Cancer Res. 2012;72:1395-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |