Published online Mar 21, 2024. doi: 10.3748/wjg.v30.i11.1488
Peer-review started: January 3, 2024
First decision: January 19, 2024
Revised: January 30, 2024
Accepted: February 28, 2024
Article in press: February 28, 2024
Published online: March 21, 2024
Processing time: 78 Days and 7.1 Hours
The last decade has been notable for increasing high-quality research and dramatic improvement in outcomes with dynamic liver preservation. Robust evidence from numerous randomized controlled trials has been pooled by meta-analyses, providing the highest available evidence on the protective effect of machine perfusion (MP) over static cold storage in liver transplantation (LT). Based on a protective effect with less complications and improved graft survival, the field has seen a paradigm shift in organ preservation. This editorial focuses on the role of MP in LT and how it could become the new “gold standard”. Strong collaborative efforts are needed to explore its effects on long-term outcomes.
Core Tip: Machine perfusion (MP) has garnered the interest of the transplant community given its proven beneficial effects on the clinical outcomes after liver transplantation (LT). Herein, we discuss the historical background of MP in LT and the available clinical evidence. Furthermore, we highlighted the obstacles and the need for future research, in particular with respect to viability assessment and prolonged preservation times.
- Citation: Parente A, Sun K, Dutkowski P, Shapiro AJ, Schlegel A. Routine utilization of machine perfusion in liver transplantation: Ready for prime time? World J Gastroenterol 2024; 30(11): 1488-1493
- URL: https://www.wjgnet.com/1007-9327/full/v30/i11/1488.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i11.1488
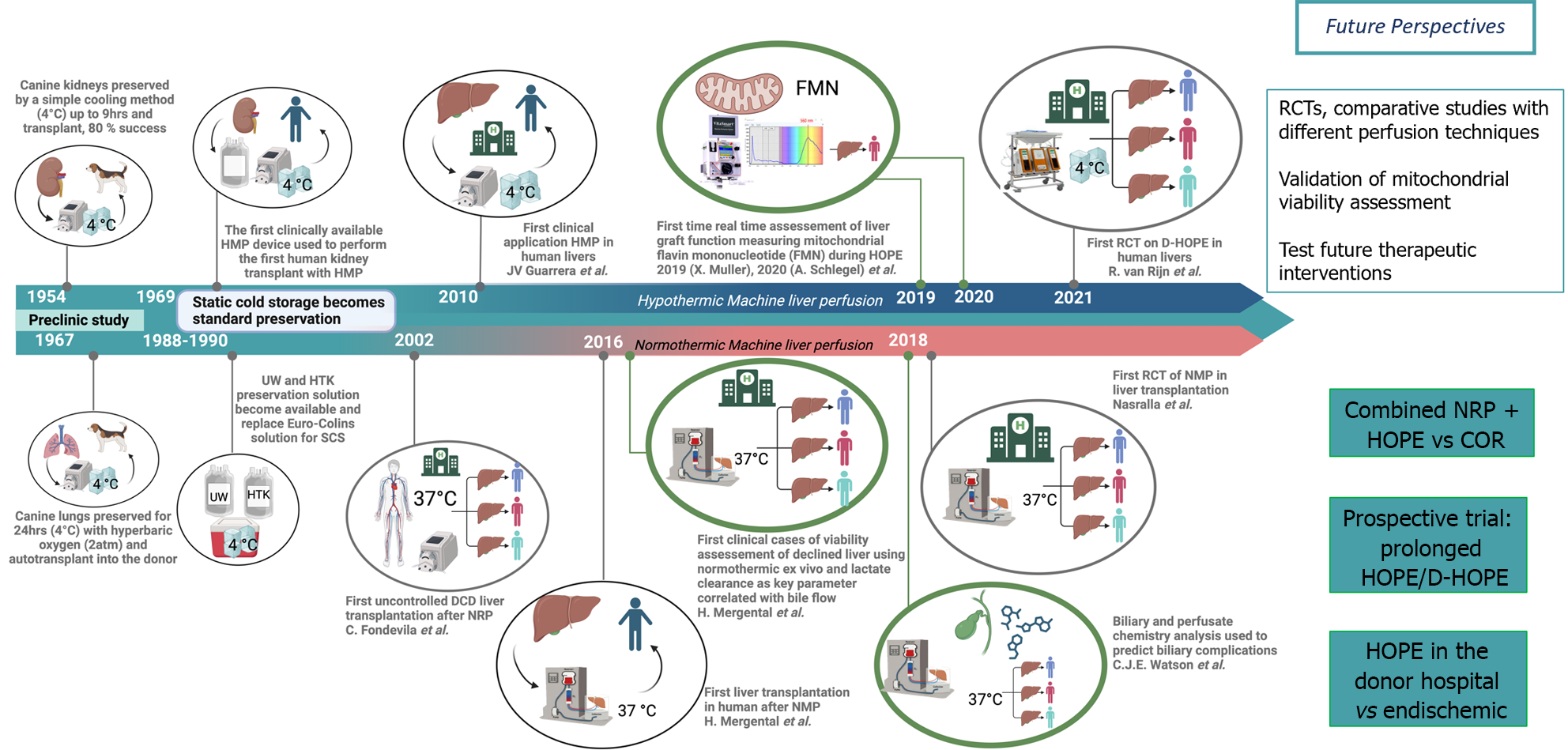
Machine perfusion (MP) technology is an old concept first introduced by Alexis Carrell and Charles Lindberg in the 1920’s, then utilized first in clinical kidney transplantation in 1968[1], however static cold storage (SCS) appeared simpler and more practical, effectively hindering the progress in MP technology development for more than 3 decades. Recently, MP has emerged as one of the most promising approaches to improve post-transplant outcomes after liver transplan
Two main ex-situ liver perfusion and one in-situ donor approach are increasingly used in clinical practice today[2]. The first ex-situ technique is known as hypothermic oxygenated perfusion (HOPE) using a highly oxygenated (pO2: > 60 kPa) artificial solution at hypothermic temperatures (8-12 °C)[2]. The second perfusion technique, normothermic MP (NMP), uses a blood-based perfusates at 37 °C, thus recreating a near-physiologic environment. Both HOPE and NMP are mainly applied after SCS, however NMP-preservation is also started upfront after minimal SCS of 2-3 hrs or as described with “ischemia-free organ transplantation (IFOT)”, as a technique avoiding all SCS[2]. In contrast, during in-situ normothermic regional perfusion (NRP) a veno-arterial extracorporeal membrane oxygenation technique is used to recirculate and oxygenate donor blood immediately after circulatory arrest in donation after circulatory death (DCD) donors[2].
The importance of oxygen was understood early with the use of HOPE in kidney transplants in 1968, however the earlier perfusion concepts in LT were simply hypothermic (HMP). Guarrera et al[3] presented the first clinical study in LT in 2010 with HMP using a homegrown device (Figure 1). NRP was used first in uncontrolled DCD in 2007[4] and NMP started later off in clinics in 2016[5].
The very first randomized controlled trial (RCT) with NMP and the Organox-Metra® device was published in 2018 by Nasralla et al[6]. The authors demonstrated less early allograft dysfunction (EAD) and lower peak aspartate aminotransferase levels during the first week after transplant in the recipient of NMP-treated grafts compared to SCS[6]. Only one year later, similar results in terms of EAD were illustrated by another single center RCT, however with much smaller number of patients[7].
Next, in 2021, another two important RCTs were published. The first was focused on HOPE in DCD livers and demonstrated a four times lower risk of non-anastomotic biliary strictures compared to SCS, together with lower EAD-rates, postreperfusion syndrome and retransplantation[8]. And the second RCT paralleled such results with HOPE and showed less 90-d post-operative complications, shorter intensive care and hospital stay compared to SCS in extended criteria donor livers after brain death (DBD)[9]. The following 2 years, the findings of these studies with HOPE were supported by further RCTs. The Bologna team showed that patients receiving HOPE-treated extended criteria DBD livers had significantly lower EAD-rates, better 1-year graft survival, less post-LT complications and lower hospital re-admission rates compared to SCS[10]. Authors from the United States published the results of another multicenter RCT using the portable OCSTM device provided by Transmedics for upfront NMP[11]. The NMP group in this study had significantly lower EAD-rates and less signs of ischemia-reperfusion injury on histopathology[11].
In the first half of 2023, two RCTs were published: The largest RCT ever conducted with HOPE and the first one with IFOT. In the former, the authors illustrated that, although the number of serious complications between HOPE and SCS was similar, a post hoc analysis revealed that liver-related serious complications occurred less frequently in the HOPE group compared to SCS. Notably, graft failure due to liver-related complications did not occur with HOPE but in 7% of SCS grafts (6 of 85)[12]. Subsequently, a Chinese group reported the results of the first RCT of DBD livers randomly assigned to SCS or IFOT, demonstrating significantly reduced EAD-rates, post-reperfusion syndrome, less non-anastomotic biliary stricture, and less cumulative post-operative complications at 12 months[13]. A group from Poland assessed the role of HOPE in their country. In low-risk DBD donors HOPE had no impact on outcomes, however authors demonstrated significantly lower EAD-rates and post-operative complications in donor livers with higher risk, i.e., with a donor risk index of > 1.7)[14]. Two additional RCTs were recently presented with NMP and HOPE in the United States. NMP with the Organox-Metra® device had a positive effect on the post-reperfusion syndrome[15], and HOPE showed lower primary non function, less EAD rates and reduced biliary strictures[16].
Such RCTs were recently combined with other retrospective studies in several meta-analyses[17], summarizing the beneficial effects of MP over SCS[18,19]. Currently, there are no available RCTs comparing HOPE and NMP, however results of ongoing RCT are awaited (Clinicaltrials.gov: NCT04644744).
While there are no RCT with NRP available yet, this technology appears to be beneficial to reduce biliary complications, improve graft survival and increase organ utilization if combined with limited SCS up to 6-7 hrs[20-22]. It seems unlikely that a RCT would proceed as the clinical outcomes have been so positive to date-in fact it is currently mandated in France and other European countries that DCD donation should not proceed unless NRP is applied. Clinical studies demonstrated however several limitations, especially when donor warm ischemia time before or SCS after NRP are prolonged or when these grafts were used for retransplant candidates[23-26]. Perfusion technologies are not mutually exclusive, and indeed when looking at real-world data have been combined with satisfactory outcomes[27]. In particular, in Italy where the no-touch period following DCD donation is 20 min, combining NRP and HOPE has yielded good outcomes[28].
Despite its clinical benefits, MP is still not routinely used worldwide. There are several reasons behind this which have been identified as lack of funding, clarity as to what is research and what is accepted clinical care delivery, knowledge and availability of healthcare staff trained in the operation of this equipment[29,30]. In addition, it must be noted that the current available evidence is limited at one year follow-up. In fact, all RCTs and meta-analyses that have been published have reported their outcomes at one year. This appears to be one of the main limitations of the current available literature. As life expectancy of LT recipients continues to increase, exceeding 70% at 5 years[31], more evidence is needed to better understand the impact of MP on long-term outcomes after LT. Additionally, many RCTs have reported similar study endpoint outcome measures with however heterogenous definitions often lacking certain endpoints[18]. Future research will need to endeavor in more homogeneous outcome reporting to provide replicable data worldwide.
Perfusing marginal organ with the aim to increase liver utilization rates necessitate reliable viability tests. Although there are several biochemical factors that have been explored both for HOPE and NMP[32,33], there are no clear guidelines regarding a systematic viability assessment during liver perfusion and available parameters often lack sensitivity and acceptable positive predictive value to discriminate between livers of metabolically good quality and others with too high risk for failure in the recipient. Most viability parameters are usually measured in the perfusate, with arbitrary timepoints and cutoffs, reflecting a post ischemia-reperfusion injury downstream of instigating mitochondria in hepatocytes, including perfusate lactate, transaminases, cytokine, and lactate dehydrogenase. Bile chemistry is also applied to assist identification of livers at risk of ischemic cholangiopathy. The early occurring cellular damage during MP was recently assessed with proteomics analysis of bile collected during sequential HOPE, rewarming and subsequent NMP[34]. On the other hand, mitochondrial transition pore opening with danger signal release appears to be a more upfront parameter of liver injury[33,35,36]. There is evidence that MP could be utilized to reprogram mitochondrial metabolism during HOPE before normothermic reperfusion. The slow metabolism of succinate and concomitant ATP reloading are two protective mechanisms described with HOPE. A recent study has demonstrated that the analysis of flavin mononucleotide from complex I, perfusate nicotinamide adenine dinucleotide hydrogen, and mitochondrial CO2 production during HOPE allows a more objective viability assessment of liver quality on a subcellular level which seems to be more reliable when compared to donor derived data, in particular for high-risk organs such as DCD[33].
In the real world, MP is used also for logistic reasons and prolonged liver preservation is increasingly used to enable timely LT with both NMP and HOPE[6,11,15,37]. A human liver graft was preserved for 3 d and successfully tran
Next to a prolonged ex-situ perfusion, super cooling and cryopreservation are potential but as yet untested clinically as additional technique to prolong organ preservation. A recent study has explored the concept of cryopreservation which enabled to preserve experimental kidney grafts for 100 d through vitrification and nanowarming[41]. Despite being compelling novel strategies, such techniques are currently limited to animal models or small studies and their potential future applications remains unclear.
In summary, within the past 5 years, increasing evidence has demonstrated the clinical benefits of MP in the setting of LT (Figure 1). We believe that this progress with MP technology and the clear beneficial effects will soon outpace and replace the standard SCS preservation for the human liver grafts, and now necessitates both a paradigm shift and rapid change in clinical practice to capitalize on these advances. This will pave the way for a new era in organ transplantation, leading to the application of MP routinely in clinical practice, in particular for marginal organs. However, several challenges remain and will need to be addressed in future research.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kalinowski P, Poland S-Editor: Qu XL L-Editor: A P-Editor: Cai YX
| 1. | Belzer FO, Ashby BS, Gulyassy PF, Powell M. Successful seventeen-hour preservation and transplantation of human-cadaver kidney. N Engl J Med. 1968;278:608-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | De Goeij FHC, De Meijer V, Mergental H, Guarrera JV, Asthana S, Ghinolfi D, Boteon YL, Selzner N, Kalisvaart M, Pulitano C, Sonnenday C, Martins PN, Berlakovich G, Schlegel A. Challenges With the Implementation of Machine Perfusion in Clinical Liver Transplantation. Transplantation. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 3. | Guarrera JV, Henry SD, Samstein B, Odeh-Ramadan R, Kinkhabwala M, Goldstein MJ, Ratner LE, Renz JF, Lee HT, Brown RS Jr, Emond JC. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant. 2010;10:372-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 417] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 4. | Fondevila C, Hessheimer AJ, Ruiz A, Calatayud D, Ferrer J, Charco R, Fuster J, Navasa M, Rimola A, Taurá P, Ginés P, Manyalich M, García-Valdecasas JC. Liver transplant using donors after unexpected cardiac death: novel preservation protocol and acceptance criteria. Am J Transplant. 2007;7:1849-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 5. | Ravikumar R, Jassem W, Mergental H, Heaton N, Mirza D, Perera MT, Quaglia A, Holroyd D, Vogel T, Coussios CC, Friend PJ. Liver Transplantation After Ex Vivo Normothermic Machine Preservation: A Phase 1 (First-in-Man) Clinical Trial. Am J Transplant. 2016;16:1779-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 366] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 6. | Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, Chiocchia V, Dutton SJ, García-Valdecasas JC, Heaton N, Imber C, Jassem W, Jochmans I, Karani J, Knight SR, Kocabayoglu P, Malagò M, Mirza D, Morris PJ, Pallan A, Paul A, Pavel M, Perera MTPR, Pirenne J, Ravikumar R, Russell L, Upponi S, Watson CJE, Weissenbacher A, Ploeg RJ, Friend PJ; Consortium for Organ Preservation in Europe. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 851] [Article Influence: 121.6] [Reference Citation Analysis (0)] |
| 7. | Ghinolfi D, Rreka E, De Tata V, Franzini M, Pezzati D, Fierabracci V, Masini M, Cacciatoinsilla A, Bindi ML, Marselli L, Mazzotti V, Morganti R, Marchetti P, Biancofiore G, Campani D, Paolicchi A, De Simone P. Pilot, Open, Randomized, Prospective Trial for Normothermic Machine Perfusion Evaluation in Liver Transplantation From Older Donors. Liver Transpl. 2019;25:436-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 8. | van Rijn R, Schurink IJ, de Vries Y, van den Berg AP, Cortes Cerisuelo M, Darwish Murad S, Erdmann JI, Gilbo N, de Haas RJ, Heaton N, van Hoek B, Huurman VAL, Jochmans I, van Leeuwen OB, de Meijer VE, Monbaliu D, Polak WG, Slangen JJG, Troisi RI, Vanlander A, de Jonge J, Porte RJ; DHOPE-DCD Trial Investigators. Hypothermic Machine Perfusion in Liver Transplantation - A Randomized Trial. N Engl J Med. 2021;384:1391-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 388] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 9. | Czigany Z, Pratschke J, Froněk J, Guba M, Schöning W, Raptis DA, Andrassy J, Kramer M, Strnad P, Tolba RH, Liu W, Keller T, Miller H, Pavicevic S, Uluk D, Kocik M, Lurje I, Trautwein C, Mehrabi A, Popescu I, Vondran FWR, Ju C, Tacke F, Neumann UP, Lurje G. Hypothermic Oxygenated Machine Perfusion Reduces Early Allograft Injury and Improves Post-transplant Outcomes in Extended Criteria Donation Liver Transplantation From Donation After Brain Death: Results From a Multicenter Randomized Controlled Trial (HOPE ECD-DBD). Ann Surg. 2021;274:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 166] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 10. | Ravaioli M, Germinario G, Dajti G, Sessa M, Vasuri F, Siniscalchi A, Morelli MC, Serenari M, Del Gaudio M, Zanfi C, Odaldi F, Bertuzzo VR, Maroni L, Laurenzi A, Cescon M. Hypothermic oxygenated perfusion in extended criteria donor liver transplantation-A randomized clinical trial. Am J Transplant. 2022;22:2401-2408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 11. | Markmann JF, Abouljoud MS, Ghobrial RM, Bhati CS, Pelletier SJ, Lu AD, Ottmann S, Klair T, Eymard C, Roll GR, Magliocca J, Pruett TL, Reyes J, Black SM, Marsh CL, Schnickel G, Kinkhabwala M, Florman SS, Merani S, Demetris AJ, Kimura S, Rizzari M, Saharia A, Levy M, Agarwal A, Cigarroa FG, Eason JD, Syed S, Washburn WK, Parekh J, Moon J, Maskin A, Yeh H, Vagefi PA, MacConmara MP. Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg. 2022;157:189-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 260] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 12. | Schlegel A, Mueller M, Muller X, Eden J, Panconesi R, von Felten S, Steigmiller K, Sousa Da Silva RX, de Rougemont O, Mabrut JY, Lesurtel M, Cerisuelo MC, Heaton ND, Allard MA, Adam R, Monbaliu D, Jochmans I, Haring MPD, Porte RJ, Parente A, Muiesan P, Kron P, Attia M, Kollmann D, Berlakovich G, Rogiers X, Petterson K, Kranich AL, Amberg S, Müllhaupt B, Clavien PA, Dutkowski P. A multicenter randomized-controlled trial of hypothermic oxygenated perfusion (HOPE) for human liver grafts before transplantation. J Hepatol. 2023;78:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 89] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 13. | Guo Z, Zhao Q, Jia Z, Huang C, Wang D, Ju W, Zhang J, Yang L, Huang S, Chen M, Zhu X, Hu A, Ma Y, Wu L, Chen Y, Han M, Tang Y, Wang G, Wang L, Li L, Xiong W, Zhang Z, Shen Y, Tang Z, Zhu C, Chen X, Hu X, Guo Y, Chen H, Zhang T, Zeng P, Lai S, Wang T, Chen Z, Gong J, Yu J, Sun C, Li C, Tan H, Liu Y, Dong Y, Liao B, Ren J, Zhou Z, Andrea S, Björn N, Cai C, Gong F, Rong J, Huang W, Guan X, Clavien PA, Stefan TG, Huang J, He X. A randomized-controlled trial of ischemia-free liver transplantation for end-stage liver disease. J Hepatol. 2023;79:394-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 53] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 14. | Grąt M, Morawski M, Zhylko A, Rykowski P, Krasnodębski M, Wyporski A, Borkowski J, Lewandowski Z, Kobryń K, Stankiewicz R, Stypułkowski J, Hołówko W, Patkowski W, Mielczarek-Puta M, Struga M, Szczepankiewicz B, Górnicka B, Krawczyk M. Routine End-ischemic Hypothermic Oxygenated Machine Perfusion in Liver Transplantation From Donors After Brain Death: A Randomized Controlled Trial. Ann Surg. 2023;278:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Chapman WC, Barbas AS, D'Alessandro AM, Vianna R, Kubal CA, Abt P, Sonnenday C, Barth R, Alvarez-Casas J, Yersiz H, Eckhoff D, Cannon R, Genyk Y, Sher L, Singer A, Feng S, Roll G, Cohen A, Doyle MB, Sudan DL, Al-Adra D, Khan A, Subramanian V, Abraham N, Olthoff K, Tekin A, Berg L, Coussios C, Morris C, Randle L, Friend P, Knechtle SJ. Normothermic Machine Perfusion of Donor Livers for Transplantation in the United States: A Randomized Controlled Trial. Ann Surg. 2023;278:e912-e921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (1)] |
| 16. | Panayotova GG, Lunsford KE, Quillin RC 3rd, Rana A, Agopian VG, Lee-Riddle GS, Markovic D, Paterno F, Griesemer AD, Amin A, Alonso D, Rocca JP, Borja-Cacho D, Hernandez-Alejandro R, Fung JJ, Pelletier SJ, Shah SA, Guarrera JV. Portable hypothermic oxygenated machine perfusion for organ preservation in liver transplantation: A randomized, open-label, clinical trial. Hepatology. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 17. | Parente A, Dondossola D, Dutkowski P, Schlegel A. Current evidence on the beneficial HOPE-effect based on systematic reviews and meta-analyses in liver transplantation. J Hepatol. 2024;80:e116-e119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Parente A, Tirotta F, Pini A, Eden J, Dondossola D, Manzia TM, Dutkowski P, Schlegel A. Machine perfusion techniques for liver transplantation - A meta-analysis of the first seven randomized-controlled trials. J Hepatol. 2023;79:1201-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 19. | Tingle SJ, Dobbins JJ, Thompson ER, Figueiredo RS, Mahendran B, Pandanaboyana S, Wilson C. Machine perfusion in liver transplantation. Cochrane Database Syst Rev. 2023;9:CD014685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Hessheimer AJ, de la Rosa G, Gastaca M, Ruíz P, Otero A, Gómez M, Alconchel F, Ramírez P, Bosca A, López-Andújar R, Atutxa L, Royo-Villanova M, Sánchez B, Santoyo J, Marín LM, Gómez-Bravo MÁ, Mosteiro F, Villegas Herrera MT, Villar Del Moral J, González-Abos C, Vidal B, López-Domínguez J, Lladó L, Roldán J, Justo I, Jiménez C, López-Monclús J, Sánchez-Turrión V, Rodríguez-Laíz G, Velasco Sánchez E, López-Baena JÁ, Caralt M, Charco R, Tomé S, Varo E, Martí-Cruchaga P, Rotellar F, Varona MA, Barrera M, Rodríguez-Sanjuan JC, Briceño J, López D, Blanco G, Nuño J, Pacheco D, Coll E, Domínguez-Gil B, Fondevila C. Abdominal normothermic regional perfusion in controlled donation after circulatory determination of death liver transplantation: Outcomes and risk factors for graft loss. Am J Transplant. 2022;22:1169-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 109] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 21. | Hessheimer AJ, Coll E, Torres F, Ruíz P, Gastaca M, Rivas JI, Gómez M, Sánchez B, Santoyo J, Ramírez P, Parrilla P, Marín LM, Gómez-Bravo MÁ, García-Valdecasas JC, López-Monclús J, Boscá A, López-Andújar R, Fundora-Suárez J, Villar J, García-Sesma Á, Jiménez C, Rodríguez-Laíz G, Lladó L, Rodríguez JC, Barrera M, Charco R, López-Baena JÁ, Briceño J, Pardo F, Blanco G, Pacheco D, Domínguez-Gil B, Sánchez Turrión V, Fondevila C. Normothermic regional perfusion vs. super-rapid recovery in controlled donation after circulatory death liver transplantation. J Hepatol. 2019;70:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 214] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 22. | Oniscu GC, Mehew J, Butler AJ, Sutherland A, Gaurav R, Hogg R, Currie I, Jones M, Watson CJE. Improved Organ Utilization and Better Transplant Outcomes With In Situ Normothermic Regional Perfusion in Controlled Donation After Circulatory Death. Transplantation. 2023;107:438-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 90] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 23. | Richards JA, Gaurav R, Upponi SS, Swift L, Fear C, Webb GJ, Allison MED, Watson CJE, Butler AJ. Outcomes of livers from donation after circulatory death donors with extended agonal phase and the adjunct of normothermic regional perfusion. Br J Surg. 2023;110:1112-1115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 24. | Gaurav R, Butler AJ, Kosmoliaptsis V, Mumford L, Fear C, Swift L, Fedotovs A, Upponi S, Khwaja S, Richards J, Allison M, Watson CJE. Liver Transplantation Outcomes From Controlled Circulatory Death Donors: SCS vs in situ NRP vs ex situ NMP. Ann Surg. 2022;275:1156-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 95] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 25. | Antoine C, Jasseron C, Dondero F, Savier E; French National Steering Committee of Donors After Circulatory Death. Liver Transplantation From Controlled Donors After Circulatory Death Using Normothermic Regional Perfusion: An Initial French Experience. Liver Transpl. 2020;26:1516-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Savier E, Lim C, Rayar M, Orlando F, Boudjema K, Mohkam K, Lesurtel M, Mabrut JY, Pittau G, Begdadi N, Cherqui D, Adam R, Dondero F, Sepulveda A, Soubrane O, Bucur P, Barbier L, Salame E, Jasseron C, Antoine C, Riou B, Scatton O. Favorable Outcomes of Liver Transplantation from Controlled Circulatory Death Donors Using Normothermic Regional Perfusion Compared to Brain Death Donors. Transplantation. 2020;104:1943-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 27. | van Leeuwen OB, Bodewes SB, Lantinga VA, Haring MPD, Thorne AM, Brüggenwirth IMA, van den Berg AP, de Boer MT, de Jong IEM, de Kleine RHJ, Lascaris B, Nijsten MWN, Reyntjens KMEM, de Meijer VE, Porte RJ. Sequential hypothermic and normothermic machine perfusion enables safe transplantation of high-risk donor livers. Am J Transplant. 2022;22:1658-1670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 91] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 28. | De Carlis R, Schlegel A, Frassoni S, Olivieri T, Ravaioli M, Camagni S, Patrono D, Bassi D, Pagano D, Di Sandro S, Lauterio A, Bagnardi V, Gruttadauria S, Cillo U, Romagnoli R, Colledan M, Cescon M, Di Benedetto F, Muiesan P, De Carlis L. How to Preserve Liver Grafts From Circulatory Death With Long Warm Ischemia? A Retrospective Italian Cohort Study With Normothermic Regional Perfusion and Hypothermic Oxygenated Perfusion. Transplantation. 2021;105:2385-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 29. | Parente A, Flores Carvalho M, Panconesi R, Boteon YL, De Carlis R, Dutkowski P, Muiesan P, Dondossola D, Schlegel A. Trends and Obstacles to Implement Dynamic Perfusion Concepts for Clinical Liver Transplantation: Results from a Global Web-Based Survey. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Flores Carvalho M, Boteon YL, Guarrera JV, Modi PR, Lladó L, Lurje G, Kasahara M, Dutkowski P, Schlegel A. Obstacles to implement machine perfusion technology in routine clinical practice of transplantation: Why are we not there yet? Hepatology. 2024;79:713-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. |
OPTN/SRTR 2020 Annual Data Report: Liver.
HRSA.. Available from: |
| 32. | Mergental H, Laing RW, Kirkham AJ, Perera MTPR, Boteon YL, Attard J, Barton D, Curbishley S, Wilkhu M, Neil DAH, Hübscher SG, Muiesan P, Isaac JR, Roberts KJ, Abradelo M, Schlegel A, Ferguson J, Cilliers H, Bion J, Adams DH, Morris C, Friend PJ, Yap C, Afford SC, Mirza DF. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat Commun. 2020;11:2939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 329] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 33. | Eden J, Breuer E, Birrer D, Müller M, Pfister M, Mayr H, Sun K, Widmer J, Huwyler F, Ungethüm U, Humar B, Gupta A, Schiess S, Wendt M, Immer F, Elmer A, Meierhofer D, Schlegel A, Dutkowski P. Screening for mitochondrial function before use-routine liver assessment during hypothermic oxygenated perfusion impacts liver utilization. EBioMedicine. 2023;98:104857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 34. | Thorne AM, Wolters JC, Lascaris B, Bodewes SB, Lantinga VA, van Leeuwen OB, de Jong IEM, Ustyantsev K, Berezikov E, Lisman T, Kuipers F, Porte RJ, de Meijer VE. Bile proteome reveals biliary regeneration during normothermic preservation of human donor livers. Nat Commun. 2023;14:7880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Eden J, Dutkowski P. Prolonging Preservation or Assessment of Organ Quality-What is Key? Transpl Int. 2023;36:12174. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Sampaziotis F, Muraro D, Tysoe OC, Sawiak S, Beach TE, Godfrey EM, Upponi SS, Brevini T, Wesley BT, Garcia-Bernardo J, Mahbubani K, Canu G, Gieseck R 3rd, Berntsen NL, Mulcahy VL, Crick K, Fear C, Robinson S, Swift L, Gambardella L, Bargehr J, Ortmann D, Brown SE, Osnato A, Murphy MP, Corbett G, Gelson WTH, Mells GF, Humphreys P, Davies SE, Amin I, Gibbs P, Sinha S, Teichmann SA, Butler AJ, See TC, Melum E, Watson CJE, Saeb-Parsy K, Vallier L. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science. 2021;371:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 231] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 37. | Brüggenwirth IMA, Mueller M, Lantinga VA, Camagni S, De Carlis R, De Carlis L, Colledan M, Dondossola D, Drefs M, Eden J, Ghinolfi D, Koliogiannis D, Lurje G, Manzia TM, Monbaliu D, Muiesan P, Patrono D, Pratschke J, Romagnoli R, Rayar M, Roma F, Schlegel A, Dutkowski P, Porte RJ, de Meijer VE. Prolonged preservation by hypothermic machine perfusion facilitates logistics in liver transplantation: A European observational cohort study. Am J Transplant. 2022;22:1842-1851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 38. | Clavien PA, Dutkowski P, Mueller M, Eshmuminov D, Bautista Borrego L, Weber A, Muellhaupt B, Sousa Da Silva RX, Burg BR, Rudolf von Rohr P, Schuler MJ, Becker D, Hefti M, Tibbitt MW. Transplantation of a human liver following 3 days of ex situ normothermic preservation. Nat Biotechnol. 2022;40:1610-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 94] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 39. | Lau NS, Ly M, Dennis C, Liu K, Kench J, Crawford M, Pulitano C. Long-term normothermic perfusion of human livers for longer than 12 days. Artif Organs. 2022;46:2504-2510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 40. | Eshmuminov D, Becker D, Bautista Borrego L, Hefti M, Schuler MJ, Hagedorn C, Muller X, Mueller M, Onder C, Graf R, Weber A, Dutkowski P, Rudolf von Rohr P, Clavien PA. An integrated perfusion machine preserves injured human livers for 1 week. Nat Biotechnol. 2020;38:189-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 268] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 41. | Han Z, Rao JS, Gangwar L, Namsrai BE, Pasek-Allen JL, Etheridge ML, Wolf SM, Pruett TL, Bischof JC, Finger EB. Vitrification and nanowarming enable long-term organ cryopreservation and life-sustaining kidney transplantation in a rat model. Nat Commun. 2023;14:3407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 61] [Article Influence: 30.5] [Reference Citation Analysis (0)] |