Published online Dec 15, 1997. doi: 10.3748/wjg.v3.i4.246
Revised: September 9, 1997
Accepted: October 28, 1997
Published online: December 15, 1997
AIM: To study the therapeutic effect of intraperitoneal hyperthermic double-distilled water and cis-diaminodichloro-platinum (DDP) perfusion for cancerous ascites and radical gastrectomy.
METHODS: LACA mice were injected peritoneally with H22 cancer cells (2 × 107 tumor cells). Five days later, the mice received treatments with either intraperitoneal perfusion of 37 °C isotonic fluid (group I), or 43 °C simple hyperthermic double-distilled water (group II), isotonic fluid (group III), DDP (group IV) or a combination of the hyperthermic double-distilled water with DDP (group V). A clinical experiment with intraperitoneal hyperthermic double-distilled water perfusion with DDP was carried out from September 1991 through September 1993 with 32 advanced gastric cancer patients who had undergone radical gastrectomy.
RESULTS: In comparison with the untreated control group of cancer cell-bearing LACA mice, the mice in all treatment groups showed near complete obliteration of cancer cells in the peritoneal cavity, markedly reduced ascites, prolonged survival times, and reduced growth of peritoneal cancerous nodes. In the clinical experiment, all 32 patients with advanced carcinoma had achieved satisfactory results at the 1-year follow-up, but had unsatisfactory results at the 2-year follow-up.
CONCLUSION: The intraperitoneal hyperthermic double-distilled water perfusion with DDP inhibited the occurrence of ascites in LACA mice bearing cancer cells, and prolonged the lifetime of patients with gastric cancer who had undergone radical gastrectomy.
- Citation: Chen ZX, Chen JP, Chen Z, Peng DS, Zhen JX, Tan JS. Treatment of cancerous ascites and radical gastrectomy with intraperitoneal hyperthermic double-distilled water and cis-diaminodichloro-platinum perfusion. World J Gastroenterol 1997; 3(4): 246-248
- URL: https://www.wjgnet.com/1007-9327/full/v3/i4/246.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i4.246
The five-year survival rate of gastric carcinoma after radical gastrectomy is about 30%-40%. Among the various causes of death, peritoneal metastasis recurrence accounts for about 50%. Recent reports from abroad[1-4] have demonstrated the therapeutic efficacy of intraperitoneal hyperthermic isotonic anticarcinogen fluid perfusion, but the long-term outcome has not yet been reported. In this study, we investigated the treatment of intraperitoneal hyperthermic double-distilled water and cis-diaminodichloro-platinum (DDP; the cisplatin chemotherapy drug) perfusion to prevent peritoneal metastasis and treat cancerous ascites in a mouse model and in patients with gastric cancer who had undergone radical gastrectomy.
LACA mice and ascites tumor cells (liver cancer cell ascites, H22) were provided by the Cancer Institute of West-China University of Medical Sciences. Double-distilled water (DDW) and physiological saline were provided by our pharmaceutical department. DDP (2 mL/tube, containing 10 mg DDP) were procured from the manufacturer, Gejiu Biochemical Pharmaceutical Factory (Yunnan, China).
Seventy mice were given an intraperitoneal injection of 2 × 107 tumor cells; the male: female ratio was 35:35 and the range of body weight was 22-24 g. The injected mice were randomly divided into the following 5 groups for treatment at day 5 post-injection: Physiological saline at 1.5 mL[5] (group I, control group); hyperthermic double-distilled water (group II); isotonic fluid (group III); DDP (2.5 mg/kg)[6] (group IV); and hyperthermic double-distilled water combined with DDP (group V). All treatments were delivered as intraperitoneal perfusion, and the various features of the perfusions are presented in Table 1.
| Management | Group I | Group II | Group III | Group IV | Group V |
| Injection of tumors cells | + | + | + | + | + |
| 37 °C | + | - | - | - | - |
| 43 °C | - | + | + | + | + |
| 0.9% normal saline | + | - | + | + | - |
| DDW | - | + | - | - | - |
| DDP | - | - | - | + | + |
Average survival days of mice and quantity of ascites The exact time of death was recorded, after which a small incision was made in the abdominal wall in order to remove the ascitic fluid by suction. The collected fluid was used in subsequent analysis as described below.
Peritoneal cancer nodes examination An autopsy was performed to observe the distribution, number and size of the cancer nodules. All data were recorded for subsequent statistical analysis.
Number of living tumor cells Samples of the collected ascitic fluid from each mouse were diluted with physiological saline and stained with TaiPan blue to distinguish the living tumor cells. The total number were counted and recorded.
Tumor cells under optical and electron microscopic observation A smear was made using the precipitate obtained by centrifugation. After ultra-thin sectioning and staining with hematoxylin-eosin and counterstaining with uranyl, acetate and lead citrate, the sections were observed under both optical and electronic microscopy. F and Q tests were used for statistical comparison.
In the control group, ascites were obvious within 1 wk, while the managed groups (groups II-V) presented a slower appearance of ascites and a reduced amount of ascitic fluid. Four mice in group V developed no ascites. Managed groups II, IV and V, with the exception of group III, showed a significantly low number of tumor cells (P < 0.01 vs control group I). When group V was compared with groups II and IV, the tumor cells were found to be drastically lower in the former and the survival rate to be markedly improved (Table 2).
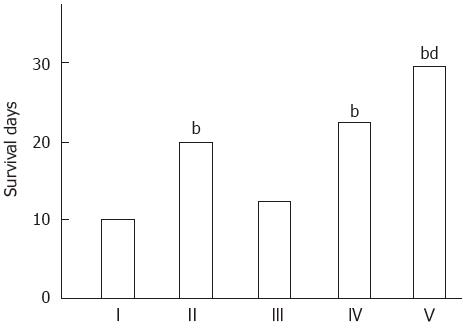
With the exception of group III, the management strategies were associated with significantly improved survival (P < 0.05 vs control group I). When group V was compared with groups II, III and IV, the former showed significantly greater prolonged survival (Figure 1).
The data for effects of the different management strategies on growth of peritoneal cancerous nodes are presented in Table 3.
Under the light microscope, the tumor cells of group I were found to be larger in number, gathered in clots, and presenting with large and deeply-stained nuclei. The tumor cells of group III were found to be reduced in number. The tumor cells of groups II and IV showed a portion with water-like degeneration and necrosis. The tumor cells of group V showed a sharp sharply reduction in number, with the present cells showing necrotic pathological changes, cytoplasm dissolution and nucleus disappearance. Under the electron microscope, the tumor cells of group V showed no complete structures, while those of groups II and IV showed degeneration in various degrees, with distension of mitochondria and nucleoplasm.
The clinical experiment was conducted from September 1991 to September 1993 and involved 60 cases of advanced gastric cancer. The patients, once enrolled, were divided randomly into the following two groups. The first group was a control (28 cases) and was treated with operation only; the second group was experimental (32 cases) and was treated with an intraperitoneal perfusion of 43 °C double-distilled water combined with DDP.
For each patient, the abdominal cavity was opened for perfusion and washing with 200 mL of 37 °C normal saline, which was then sucked out in order to search for the detouched tumor cells. Patients allocated to the second group received the following additional treatment: Before closure of the abdomen, an intraperitoneal perfusion of hyperthermic (44-46 °C) sterile double-distilled water (2500-3000 mL) was delivered by circulating perfusion for 30 min, after which 700-1000 mL of perfusate was allowed to remain in the abdomen, and 300 mg of DDP (200 mg/m2) was added[7]; the abdomen was then closed with a tube in place to facilitate post-operative drainage. After postoperative clamping for 4 h, the tube was opened for drainage. At the same time of the perioperative perfusion, a rapid (20 min) i.v. drip of 12 g of sodium thiosulfate was administered, followed by a gradual (8 h) i.v. drip of 24 g of sodium thiosulfate in 1000 mL of 5% G.S.[8].
In the second group (prevention group), 13 (48.1%) of the 27 patients were positive for detouched tumor cells, and all 13 became negative after treatment. In the first group (control group), 9 patients were positive and 6 remained positive after the operation.
The prevention group had a 1-year survival rate of 96.9% (31/32), which was significantly higher than that of the control group (46.1%, 13/28; P < 0.01), but a 2-year survival rate that was similar to the control group (75.0%, 24/32 vs 42.9%, 12/28; P > 0.05).
The incidence of complications of intraperitoneal perfusion was 65.6% (21/32) and included nausea (43.8%, 14 patients) and vomiting (25.0%, 8 patients). These cases were mild, and easily relieved by paspertin or ondansetron zofran. The levels of serum creatinine and urea nitrogen were higher after perfusion than before the operation, but still within normal limits. Six patients showed a slight decrease in white blood cell count after perfusion, but in all cases the count was above 3.0 × 109. All patients showed normal findings from liver function tests; there were no instances of peritoneal nerve injury, delayed healing of the incision, anastomotic leakage, or intestinal adhesion.
When the serosa of the gastric tumor is invaded, the tumor cell may detach spontaneously or a result of manipulation during operation, thereby entering the free peritoneal cavity or the pelvic fossae[9]. Kaibara[3] reported that the 5-year survival rate in patients free from peritoneal metastasis was 51.4% but only 18.7% for their counterparts with peritoneal metastasis. Thus, it is important to prevent and treat peritoneal metastasis.
Thermochemotherapy is able to eliminate recurrence at the excision site and helps to avoid peritoneal seeding. Moreover, Kaibara[3] reported that it might raise the 5-year survival rate. In our experiment, on day 5 after intraperitoneal injection of H22 cancer cells into the peritoneal cavity of LACA mice, the tumor cells presented with continuous division and proliferation activity, and seeding was present. When the treatment of intraperitoneal perfusion of hyperthermic (43 °C) hypotonic (double-distilled water) fluid combined with DDP was compared with perfusion of normal temperature hypotonic fluid, isotonic fluid and DDP, the former showed the best effect, probably due to the synergic action of the agents when used as a combination therapy.
Original title:
L- Editor: Filipodia E- Editor: Liu WX
| 1. | Fujimoto S, Shrestha RD, Kokubun M, Ohta M, Takahashi M, Kobayashi K, Kiuchi S, Okui K, Miyoshi T, Arimizu N. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann Surg. 1988;208:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 164] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Koga S, Hamazoe R, Maeta M, Shimizu N, Murakami A, Wakatsuki T. Prophylactic therapy for peritoneal recurrence of gastric cancer by continuous hyperthermic peritoneal perfusion with mitomycin C. Cancer. 1988;61:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Kaibara N, Hamazoe R, Iitsuka Y, Maeta M, Koga S. Hyperthermic peritoneal perfusion combined with anticancer chemotherapy as prophylactic treatment of peritoneal recurrence of gastric cancer. Hepatogastroenterology. 1989;36:75-78. [PubMed] |
| 4. | Fujimura T, Yonemura Y, Fushida S, Urade M, Takegawa S, Kamata T, Sugiyama K, Hasegawa H, Katayama K, Miwa K. Continuous hyperthermic peritoneal perfusion for the treatment of peritoneal dissemination in gastric cancers and subsequent second-look operation. Cancer. 1990;65:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Xu SY. Pharmacological experimental methodology, 2nd ed. Beijing: People s Health Publishing House 1991; 1424-1430. |
| 6. | Trave F, Rustum YM, Boransen T. Synergistic antitumor activity of cisplatin (DDP) and 5-fluorouracil (Fura 0 in mice bearing leukemic L1210 cells. Proc Annual Meeting of Association of Cancer Am Res. 1985;26:322. |
| 7. | Xu SY. Clinical guidance of administration drug. 1st ed. Hefei: Anhui Publishing House of Science and Technology 1989; 8-10. |
| 8. | Howell SB, Pfeifle CE, Wung WE, Olshen RA. Intraperitoneal cis-diamminedichloroplatinum with systemic thiosulfate protection. Cancer Res. 1983;43:1426-1431. [PubMed] |
| 9. | Iitsuka Y, Kaneshima S, Tanida O, Takeuchi T, Koga S. Intraperitoneal free cancer cells and their viability in gastric cancer. Cancer. 1979;44:1476-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |