Published online Dec 15, 1997. doi: 10.3748/wjg.v3.i4.213
Revised: May 25, 1997
Accepted: July 11, 1997
Published online: December 15, 1997
AIM: To evaluate the relationship between expression of ras, p53 and bcl-2 gene products and hepatocarcinogenesis since the endotoxemia produced from lipopolysaccharide administration and/or the hypophagocytic state of splenectomy significantly accelerated hepatocarcinogenesis induced by thioacetamide.
METHODS: The hepatocarcinoma model was induced by 6-mo oral intake of 0.03% thioacetamide. During the hepatocarcinoma modeling process, rats were additionally treated with splenectomy and/or lipopolysaccharide administration. The techniques of flow cytometry, immunohistochemistry and immunoelectronmicroscopy were applied for quantitative analysis of the expression of oncogene proteins.
RESULTS: In this model system, overexpression of ras p21 protein mainly occurred in the precancerous cell population or in cells in the early stage of hepatocyte transformation. The levels of ras p21 declined when nuclear DNA aneuploidy increased. Expression of bcl-2 protein slowly and steadily rose, with more hepatocytes staying in S + G2M phases, as the hepatocarcinoma became more malignant. p53 was moderately expressed during hepatocarcinogenesis. There was no statistical correlation between endotoxemia levels and the changes in levels of ras, p53 and bcl-2 gene products.
CONCLUSION: Overexpression of oncogene ras p21 was considered likely to be a precursor of premalignant hepatocytes and possibly as responsible for the initiation of hepatocarcinogenesis. Bcl-2 protein expression is proportional to the severity of malignancy in hepatocarcinogenesis. p53 may be involved in a key pathway underlying the transformation and development processes of hepatocarcinoma. This study confirmed the hypothesis that there are multiple genes and multiple steps involved in hepatocarcinogenesis. Expression of oncogene proteins reflects the properties of the premalignant and malignant cells, but is not directly related to endotoxemia statistically.
- Citation: Yang JM, Han DW, Liang QC, Zhao JL, Hao SY, Ma XH, Zhao YC. Effects of endotoxin on expression of ras, p53 and bcl-2 oncoprotein in hepatocarcinogenesis induced by thioacetamide in rats. World J Gastroenterol 1997; 3(4): 213-217
- URL: https://www.wjgnet.com/1007-9327/full/v3/i4/213.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i4.213
Chemically-induced hepatocarcinogenesis in rats is widely used to assess the carcinogenic risk of chemicals to humans and to study the molecular pathogenesis in order to improve hepatoma prevention and treatment strategies for humans. Based on our previous observations that endotoxin can enhance hepatocarcinogenesis in rats induced by oral intake of thioacetamide (TAA), we hypothesized that endotoxin is responsible for, at least some of, the changes in levels of oncogenes and tumor suppressor genes that occur during the development of TAA-induced hepatocarcinoma.
A multitude of studies have provided evidence that members of the ras oncogene family (Ha-ras, Ki-ras and N-ras, activated by point mutation in codons 12, 13 and 61 respectively) are responsible for cell transformation[1]. NIH-373 cell transfection and immunohistochemical analysis, in particular, have yielded data that demonstrates the presence of a dominant activated N-ras gene in premalignant and malignant cells, which also appears to play a key role in the initiation of hepatocarcinogenesis[2-4].
Mutations in the p53 tumor suppressor gene are frequently detected in many human and animal cancers (approximately 50%), and studies of its consequent misexpression have provided clues to the etiology and molecular pathogenesis of neoplasia[5,6]. In addition to its role in neoplastic transformation, the p53 protein plays an important role in normal cell function and its encoding gene has been found to be highly conserved among vertebrates, allowing extrapolation of data from animal models[7].
The bcl-2 gene was first discovered in non-Hodgkin’s B-cell lymphomas and its presence in tumors is due to a bcl-2 gene translocation from 18q21 to cis-configuration. However, high levels of bcl-2 protein production in a variety of human solid tumors have been observed in the without translocation or alterations in the structure of the bcl-2 gene[8]. It is generally accepted that the bcl-2 protein contributes to the process of neoplastic cell expansion by blocking normal physiological cell death[9]; in addition, gene transfer-mediated elevation in bcl-2 protein levels have been shown to render tumor cells relatively more resistant to induction of apoptosis by chemotherapeutic drugs[10]. Therefore, bcl-2 has been theorized to play a significant role in the origins of cancer and in its therapy.
Considering the important contributions of ras, p53 and bcl-2 genes to carcinogenesis, the aim of this study was to explore the relations between expression of oncoproteins and malignity or incidence of hepatocarcinoma using a TAA-treated model system coupled with splenectomy (ST) and/or endotoxin (lipopolysaccharide, LPS) treatment.
The protocol course of TAA-induced modeling encompassed 4 mo for cirrhosis and 6 mo for liver tumor. Female Wistar rats (provided by the Experimental Animals Center of Shanxi Medical University), weighing 125 ± 9 g, were housed in wire-bottom cages under a 12 h light/dark cycle and fed with a balanced pet diet ad libitum. Animals were randomly assigned to the following five groups: sham group (n = 5), which was the untreated control group that received tap water ad libitum; TAA control group (n = 6), which was given 0.03% w/v TAA (purity > 99%; Shanghai Central Chemical Factory) in drinking water; TAA + ST group (n = 6), which was splenectomized 1 week before commencement of the experiment; for the final two groups, mice first treated as the TAA + LPS group (n = 6) or the TAA + ST + LPS group (n = 6) were given 0.8 mg LPS (Escherichia coli sterotype 055≥B5; Sigma) in drinking water containing 0.03% TAA for the last 2 months of the modeling course. The sham group, without splenectomy, underwent midline laparotomy and spleen manipulation as the sham surgery procedure. All surgeries were carried out under light ether anesthesia and all procedures were performed under sterile conditions. At the end of the 6 mo modeling course, all rats were euthanized by over-anesthetization with ethyl ether and the liver was excised for the following preparations.
Expression of oncogene proteins was quantitatively determined as described by Zuo et al[11]. Briefly, ~2 g of excised liver tissue was immediately minced in 0.05% collagenase (type ; Sigma) and filtered through a 200 mesh stainless steel filter. A single-cell suspension was prepared and fixed in 70% ethanol. After washing with PBS, the cells were resuspended in a solution containing 0.1% Triton X-100 and 5% goat serum, in order to increase the permeability of the cell membrane and to block the non-specific binding sites of IgG. Antibodies, including pan ras (F132, a mouse monoclonal IgG2b antibody), p53 (CM1, a rabbit polyclonal antibody) and bcl-2 (N19, a rabbit polyclonal antibody) were purchased from Santa Cruz Biotechnology Inc., United Kingdom. They were diluted at 1≥100 and incubated with 105 cells respectively at 37 °C for 30 min. After washing twice, the cells were incubated with 100 μL of species-specific secondary-FITC-IgG (Lot 9609; Military Medical Academy) at 37 °C for 30 min. The negative control (omission of the primary antibody) and the positive control (the rat hepatocarcinoma cell line CBRH-7919, provided by Shanghai Institute of Cell Biology) were generated using the same procedure described above. For flow cytometric analysis, 10000 cells from each sample were passed through a 400 mesh filter and then quantitatively examined on the Fluorescence Activated Cell Sorter FACS-420 (Becton Dickinson, United States). Data were analyzed on an IBM PC-compatible computer with HP-300 Consort 30 software. The quantitative expression of oncogene product was calculated according to Morker et al[12], as follows:
The criteria for oncoprotein expression was as follows: FI value > 1, positive; FI ≤ 1, negative.
In addition, liver cells were stained with propidium iodide reagents (50 mg/L propidium iodide, 20 mg/L RNase, and 1% Triton X-100) at 4 °C for 30 min, and the nuclear DNA content was determined by flow cytometry.
Immunohistochemical staining was conducted using the streptavidin/peroxidase kit (SP-TM; Zymed, United States) and the biotin-streptavidin method[13] with slight modification. Briefly, all liver sections were fixed with neutral buffered 10% formalin and embedded in paraffin. The paraffin-embedded sections were deparaffinized through an alcohol series graded with distilled water. The resultant hydrated sections were then incubated in 0.3% hydrogen peroxide for 10 min to quench the endogenous peroxidase activity. Liver sections were then incubated in a salt buffer (pH 6.0) at 92-98 °C for 10 min to restore antigenicity, followed by incubation in 5% normal blocking serum for 20 min to suppress non-specific binding of IgG. The preparations were then incubated sequentially with primary antibodies (diluted 1≥50 in PBS) at 4 °C for overnight, followed by sequential incubation with the biotinylated secondary antibodies (diluted 1≥100 in 1% BSA-PBS) at 37 °C for 10 min and streptavidin horseradish peroxidase (diluted 1≥100) at 37 °C for 20 min. The colored reaction product was developed with diaminobenzidine (DAB). The sections were lightly counterstained with hematoxylin. Immunostaining by replacing primary antibody with PBS was also conducted as a negative control.
Anti-mouse IgG/colloidal gold (10 nm) was obtained from Beijing Zhongshan Biotechnology Co. LTD. The liver ultrathin sections were sequentially incubated with pan ras p21 antibody and the secondary immunogold antibody using the procedures described by Yin et al[14].
All results are expressed as the x ± s. Data were analyzed by Student’s t-test and multiple regression. P values < 0.05 were considered statistically significant.
Expression of oncogene ras p21 was quantitatively detected by flow cytometry. The fluorescence index indicated the potentiality of gene product expression. Table 1 shows that the TAA-treated group with 2-mo administration of LPS presented the highest expression of ras p21 despite their relatively low DNA content, reflecting the malignity of hepatocarcinoma to a great extent. However, the group with the highest DNA content had an average value of fluorescence index that was significantly decreased (P < 0.01) but a percentage of labeled cells that was obviously increased (P < 0.01).
| Group | n | FI, x ± s | % cells labeled, x ± s | Cases with positive ras p21expression | DNA index, x ± s |
| Sham | 5 | 1 | 17 ± 4 | 1 | 1 |
| TAA control | 6 | 1.29 ± 0.085 | 18 ± 5 | 6 | 1.15 ± 0.21 |
| TAA + LPS | 6 | 1.60 ± 0.071a | 24 ± 7a | 6 | 1.24 ± 0.25a |
| TAA + ST | 6 | 1.52 ± 00105a | 15 ± 3b | 6 | 1.44 ± 0015ab |
| TAA + ST + LPS | 6 | 1.32 ± 0.061bc | 49 ± 9abc | 6 | 1.56 ± 0.07abc |
| CBRH-7919 cells | 1 | 1.54 | 42 | 1 | 1.34 |
Similar to the results of ras p21 expression, the flourescence index of the p53 tumor suppressor gene product was highest in the TAA + LPS group (P < 0.01) and relatively higher in the TAA + ST + LPS group (P < 0.05). However, the alteration in expression did not correlate with the hepatocarcinoma rate (Table 2).
Unlike the results of p53 and ras p21, the expression of the bcl-2 protein was consistently correlated with DNA index (r = 0.93, P < 0.01) and the percentage of cells in S plus G-2M phases (r = 0.86, P < 0.05). The rat hepatocarcinoma cell line CBRH-7919 was used as a positive control of expression of oncogene product when the samples of hepatocytes were analyzed by flow cytometry (Table 3).
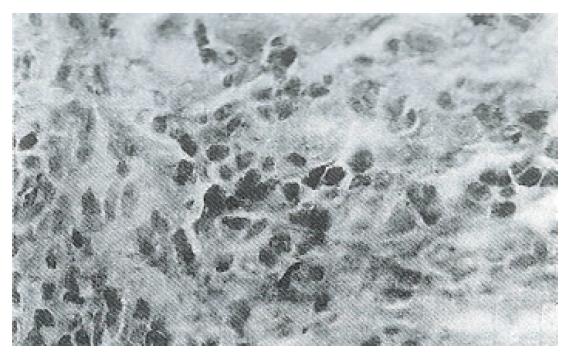
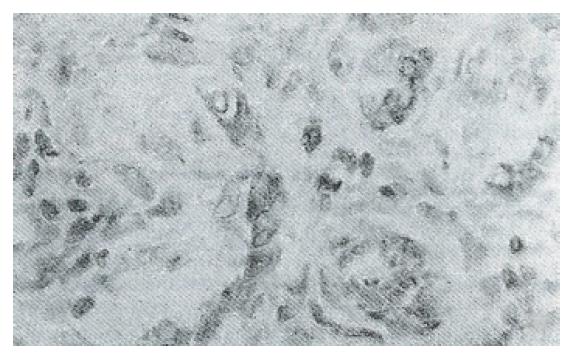
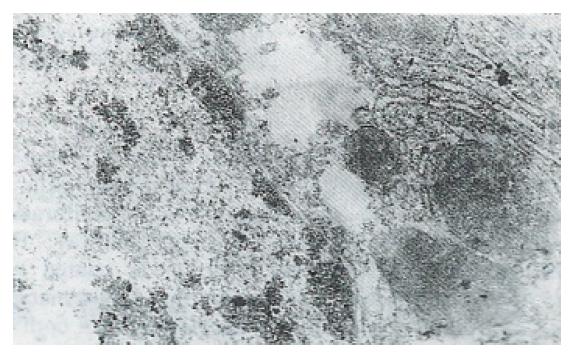
Immunohistochemical analyses showed that immunostaining for the ras p21 oncogene was mainly localized to the cytoplasm in malignant cells and in dysplastic hepatocytes in the hyperplastic nodules. It was noticed that expression of ras p21 was more apparent in hepatocytes adjacent to neoplastic lesions than in hepatic tumor tissues (Figure 1). The hyperexpression of p53 was present in the nuclei of malignant hepatocytes (Figure 2), and bcl-2 protein was moderately expressed in some cytoplasm of neoplastic foci (Figure 3).
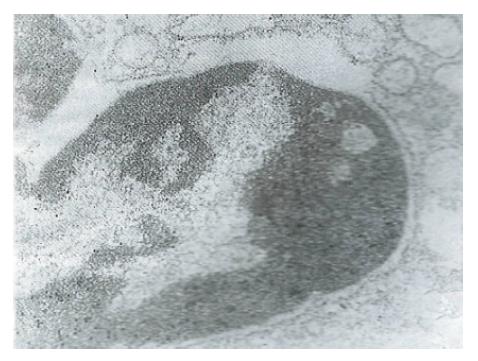
Immunoelectronmicroscopy showed that in contrast to the data presented in Figure 4, overexpression of ras p21 dominantly occurred on the increased nuclear heterochromatin of malignant hepatoma and secondarily on the endoplasmic reticulum adjacent to the nuclear membrane (Figure 5).
The proto-oncogene ras gene family (Ha-ras, Ki-ras and N-ras) commonly exists in normal mammalian cells and its products are involved in cell proliferation, metabolism, differentiation and various signaling pathways. Here, the mutation-activated ras gene was found to be associated with hepatocarcinogenesis. It has been reported that mutation of Ki-ras in codon 13 is present in liver angiosarcoma of humans as well as animals exposed to vinyl chloride[4]. Activated Ha-ras has also been detected in spontaneous hepatoma of the B6C3F1 mouse[15] and 2 amino 3 methyl-imidazo quinolone-induced hepatocarcinoma in rat[16]. However, the mutation-activated N-ras oncogene has been demonstrated frequently in hepatocarcinomas.
The p21 ras gene product plays a key role in cell transformation, as indicated by its strong expression in preneoplastic cells[17]; thus, ras p21 may be a biomarker for tumor diagnosis and prognosis. Our study, along with others[17,18], demonstrate oncogene ras p21 expression as a early event of hepatocarcinogenesis, since its expression levels do not correspond with nuclear DNA content. In contrast, we found that p21 expression declines when the DNA content is increased in the premalignant and malignant liver. Therefore, the inverse correlation between the levels of ras p21 production and the severity of liver cancerous lesions indicates that ras p21 is a potent trigger for transformation of the hepatocyte phenotype and it is not crucial for maintenance of the transformed cell phenotype. It is generally accepted that members of the ras gene family, especially N-ras, are “transforming genes” involved in initiation of carcinogenesis[1,2,17,18]. Therefore, although we observed a high content of p21 protein in the model system that occurred in response to additional administration of LPS purified from E. coli, it is questionable whether LPS itself induces overexpression of ras p21 or the property of preneoplastic hepatocytes. The increased heterochromatin in the malignant hepatocytes showed overexpression of ras p21, which may imply some mechanism of hepatocarcinogenesis.
The 17-y history of research into the p53 tumor suppressor gene has indicated that p53 protein is involved in gene transcription, DNA synthesis and repair, genomic plasticity, and programmed cell death. So it is not surprising that p53 is a functional component of signaling pathways central to human carcinogenesis. Deactivation of p53 causes loss of tumor suppressor function and gain of oncogenic activity, and this dynamic represents one of the explanations for pathogenesis of cell transformation. The genetic mutation of p53 and of some viral oncoproteins binding to the p53 protein can lead to deactivation of p53 functions; for example, aflatoxin B1 induces hepatocellular carcinoma by mutation at codon 249 of p53[19] and HBV X protein binding to the p53 protein mediates p53 inactivation and remains an important possible mechanism of primary hepatocellular carcinoma lacking a p53 mutation[20]. In addition, some small carcinogen-DNA adducts, such as O6 methylguanin, may cause DNA polymerase to misread the base pairing, and bulky DNA adducts may render the bases unreadable, thereby increasing errors during DNA replication[21].
The wild-type p53 protein exists in a very small quantity and has a very short half-life in the cell nucleus, and these two features complicate its detection by routine immunological methods. Missense mutations often increase the half-life and quantity of the p53 protein by 20-fold, so p53 overexpression is a surrogate marker for missense mutation. Previous studies have noted that alteration of the function of the p53 pathway (via mutation or epigenetic inactivation) are associated with aneuploidy and increased proliferative rates in ovarian, colorectal and gastric cancers, features which are also considered precursors for tumor progression and poor prognosis[22]; however, some authors have reported that changes in the levels of p53 protein are not related to tumor differentiation[23]. Our study showed that a high content of p53 protein, as detected by flow cytometry and immunohistochemistry, was present in the model system with LPS treatment or in the hyperplastic nodules and malignant liver lesions, but neither specifically related to endotoxemia levels nor to malignancy. These findings suggested that the overexpression of p53 protein was relatively associated with LPS challenge and to both the early and late events of hepatocarcinogenesis, but the precise mechanism remains unclear.
The bcl-2 proto-oncogene on chromosome 18 may normally exert a regulatory function towards avoidance of cell apoptosis (programmed cell death), mainly in tissues where apoptosis may represent a specific control mechanism of cell turnover. Bcl-2 protein localized in mitochondria, endoplasmic reticulum and nuclear membrane has been shown to contribute to neoplastic cell expansion by blocking programmed cell death[24]. Bcl-2 expression has been detected in hepatocarcinoma and other solid tumors but appears to be absent in normal and dysplastic hepatocytes[25]. In normal liver, the majority of hepatocytes exist in a state of proliferative quiescence (G0 phase), but can enter a cell renewal compartment upon appropriate stimulation[26]. Dysplastic hepatocytes and regenerating hepatocytes in cirrhotic nodules have failed to show bcl-2 protein expression, but some types of dysplasia represent a precancerous lesion[27]. Thus, bcl-2 expression seems to be a relatively late event in hepatocarcinogenesis. In our study, the data from flow cytometry analysis with an antibody specific for bcl-2 protein indicated that expression of bcl-2 protein is significantly correlated with malignity of hepatocarcinoma (r = 0.93, P < 0.01), which is in agreement with findings from other studies[25-27]. On the other hand, the bcl-2 protein was also found to be related to the percentage of hepatocytes in S and G2M phases (r = 0.86, P < 0.05). Therefore, inhibition of apoptosis may be one of the mechanisms of carcinogenesis as little is known about the intracellular mechanisms underlying programmed cell death. Unlike other oncogene proteins described in the literature so far, bcl-2 acts in both S and G2M transitions and the potential impact of bcl-2 functions seem to, in addition to protecting cells from apoptosis, play a more complex role in morphogenesis, differentiation and homeostasis.
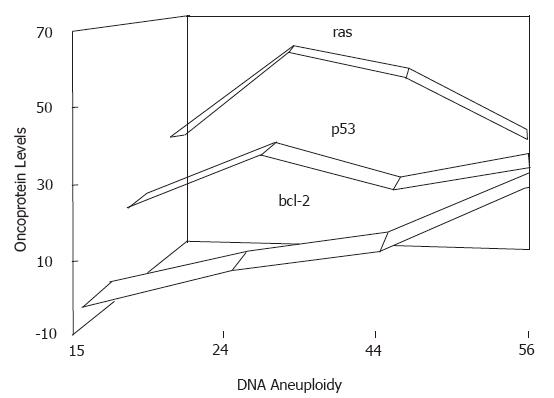
It is generally accepted that there are multiple genes and steps involved in carcinogenesis. The quantitatively analyzed data obtained in the current study showing elevations of ras, p53, and bcl-2 proteins in a mass of normal, premalignant and malignant cells in rat liver, together with the immunohistochemstry data, confirm the hypothesis that ras mainly contributes to neoplastic transformation and bcl-2 to malignant progression, and with p53 contributing to several pathways during hepatocarcinogenesis. Although endotoxemia was found to be significantly correlated with the malignity or incidence of rat hepatocarcinoma, it was not directly related to the overexpression of ras, p53 or bcl-2 proteins (maximum: r = 0.78, P > 0.05). Thus, it is suggested that expression of oncogene proteins may represent some of the properties of the hepatocarcinoma itself (Figure 6).
Original title:
S- Editor: A L- Editor: Filipodia E- Editor: Li RF
| 1. | Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3375] [Cited by in RCA: 3430] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 2. | Notario V. A common mechanism for the malignant activation of ras oncogenes in human neoplasia and in chemically induced animal tumors. Cancer cells. Vol. 2, New York: Cold Spring Harbor Laboratory 1984; . |
| 3. | Jagirdar J, Nonomura A, Patil J, Paronetto F. Activated ras oncogene p21 expression in hepatocellular carcinoma and HBsAg-positive liver cells. Hepatology. 1985;5:1055. |
| 4. | Froment O, Boivin S, Barbin A, Bancel B, Trepo C, Marion MJ. Mutagenesis of ras proto-oncogenes in rat liver tumors induced by vinyl chloride. Cancer Res. 1994;54:5340-5345. [PubMed] |
| 5. | Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2565] [Cited by in RCA: 2682] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 6. | Harris CC. p53: at the crossroads of molecular carcinogenesis and risk assessment. Science. 1993;262:1980-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 304] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Soussi T, Caron de Fromentel C, May P. Structural aspects of the p53 protein in relation to gene evolution. Oncogene. 1990;5:945-952. [PubMed] |
| 8. | Reed JC, Meister L, Tanaka S, Cuddy M, Yum S, Geyer C, Pleasure D. Differential expression of bcl2 protooncogene in neuroblastoma and other human tumor cell lines of neural origin. Cancer Res. 1991;51:6529-6538. [PubMed] |
| 9. | Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1619] [Cited by in RCA: 1686] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 10. | Miyashita T, Reed JC. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993;81:151-157. [PubMed] |
| 11. | Zuo LF, Hu JL, Lin JH, Guo JW, Gao GD. Quantitative Study of oncogene ras p21 expression in carcinomas and non-cancer lesion of the stomach. Prog Biochem Biophys. 1995;22:146-149. |
| 12. | Morker O, Laerum OD. Flow cytometric measurement of quantitative expression of oncogene product. Cytometry. 1991;12:138. |
| 13. | Iwaki T, Miyazono M, Hitosumatsu T, Tateishi J. An immunohistochemical study of tissue transglutaminase in gliomas with reference to their cell dying processes. Am J Pathol. 1994;145:776-781. [PubMed] |
| 14. | Yin GH. Immino-gel-gold technique. Microscopic technology of medical biology and cellular ultrastructure. vol.5. Hong Kong: Modern Press 1992; 114-116. |
| 15. | Reynolds SH, Stowers SJ, Maronpot RR, Anderson MW, Aaronson SA. Detection and identification of activated oncogenes in spontaneously occurring benign and malignant hepatocellular tumors of the B6C3F1 mouse. Proc Natl Acad Sci USA. 1986;83:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Ishikawa F, Takaku F, Nagao M, Ochiai M, Hayashi K, Takayama S, Sugimura T. Activated oncogenes in a rat hepatocellular carcinoma induced by 2-amino-3-methylimidazo[4,5-f]quinoline. Jpn J Cancer Res. 1985;76:425-428. [PubMed] |
| 17. | Wang S, Ying HJ. A study of relationship of p21 expression and DNA ploidy in preneoplastic and neoplastic lesion of stomach. Practical J Cancer. 1996;11:79. |
| 18. | Wang Z, Liao TJ, Yong WC. A study of ras p21 expression and DNA ploidy in bladder tumors. Zhonguo Aizheng Zazhi. 1991;13:245. |
| 19. | Bressac B, Kew M, Wands J, Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991;350:429-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 872] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 20. | Feitelson MA, Zhu M, Duan LX, London WT. Hepatitis B x antigen and p53 are associated in vitro and in liver tissues from patients with primary hepatocellular carcinoma. Oncogene. 1993;8:1109-1117. [PubMed] |
| 21. | Yuspa SH, Poirier MC. Chemical carcinogenesis: from animal models to molecular models in one decade. Adv Cancer Res. 1988;50:25-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 152] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Kihana T, Tsuda H, Teshima S, Okada S, Matsuura S, Hirohashi S. High incidence of p53 gene mutation in human ovarian cancer and its association with nuclear accumulation of p53 protein and tumor DNA aneuploidy. Jpn J Cancer Res. 1992;83:978-984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Campo E, de la Calle-Martin O, Miquel R, Palacin A, Romero M, Fabregat V, Vives J, Cardesa A, Yague J. Loss of heterozygosity of p53 gene and p53 protein expression in human colorectal carcinomas. Cancer Res. 1991;51:4436-4442. [PubMed] |
| 24. | Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1619] [Cited by in RCA: 1686] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 25. | Zhao M, Zhang NX, Economou M, Blaha I, Laissue JA, Zimmermann A. Immunohistochemical detection of bcl-2 protein in liver lesions: bcl-2 protein is expressed in hepatocellular carcinomas but not in liver cell dysplasia. Histopathology. 1994;25:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Ma XH. Hepatocyte regeneration and regulation. Hepatic pathophysiology. Taiyuan: Shanxi United Universities Press 1992; 91-96. |
| 27. | Borzio M, Bruno S, Roncalli M, Mels GC, Ramella G, Borzio F, Leandro G, Podda M. Liver cell dysplasia and risk of hepatocellular carcinoma in cirrhosis: a preliminary report. BMJ. 1991;302:1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |