Published online Dec 15, 1997. doi: 10.3748/wjg.v3.i4.210
Revised: May 21, 1997
Accepted: June 14, 1997
Published online: December 15, 1997
AIM: To isolate and purify the heme oxygenase (HO) isoform in microsomal fractions of Sprague-Dawley rat liver and brain in order to understand the characteristics of the two constitutive forms and the mechanism of the occurrence of hyperbilirubinemia.
METHODS: After induction by hematin and phenylhydrazine, the rat liver and brain microsomal fractions were isolated and purified by DEAE-Sephacel and hydroxyapatite. Activity and the apparent molecular weight of the two isoforms [heme oxygenase 1 (HO-1) and heme oxygenase-2 (HO-2)] were measured. Kunming mice were used to prepare antiserum against purified liver HO-2. Rat liver HO-1 and brain HO-2 preparations were analyzed by the western immunoblotting technique.
RESULTS: Two isoforms were purified and identified in the treated rat liver, and HO-1 was the predominant form with a ratio of 2:1. In the native state, HO-2 activity was detectable but HO-1 activity was increased in response to hematin and phenylhydrazine, while HO-2 activity was fully refractory to these agents. The apparent molecular weights of HO-1 and HO-2 were about Mr 30000 and Mr 36000 under reducing conditions, respectively. In the untreated liver and treated brain, only one peak of HO activity exhibiting an elution profile similar to that of HO-2 of the treated liver was detected. The presence of an activity peak was not found in the elution profile at the region where the inducible isoform of HO (HO-1) was expected. The apparent molecular weight in treated brain preparation was identical to that of the purified liver HO-2. Cross-reactivity of HO-2 in the brain microsomal preparation was established, but a reactivity of HO-1 in the liver was not observed by western immunoblotting analysis when antiserum to liver HO-2 was applied.
CONCLUSION: Two constitutive forms of HO, designated as HO-1 and HO-2, exist in the treated rat liver. HO-1 is an inducible enzyme. In the treated rat brain only HO-2 exists and is a molecular entity similar to that found in liver. The two constitutive forms were different in molecular weight and in inducibility and immunochemical properties.
- Citation: Xia ZW, Li YZ, Chen SN, Shen QX, Ben XM, Yu SC. Analysis of two constitutive forms of microsomal heme oxygenase in different rat tissues. World J Gastroenterol 1997; 3(4): 210-212
- URL: https://www.wjgnet.com/1007-9327/full/v3/i4/210.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i4.210
Oxidative degradation of heme (protoheme, Fe protoporphyrin , heme b) to the open tetrapyrrole, biliverdin, is catalyzed by the microsomal enzymes heme oxygenase (HO) and NADPH-cytochrome C (P-450) reductase. Although the exact mechanism of heme degradation is not yet fully understood, it is generally accepted that the role of HO is to bind the substrate in a specific orientation, such that upon degradation the predominant end product is the -αisomer of biliverdin; the dual function of the reductase is to activate molecular oxygen and to promote the reduction of heme iron to the ferrous state. In mammals, the cytosolic enzyme, biliverdin reductase, catalyzes the conversion of biliverdin to bilirubin[1-6].The purpose of the present study was, therefore, to verify the possibility of the existence of multiple forms of HO in the rat liver and brain, and if existing to compare the properties of these enzymatic isoforms in order to understand their mechanisms of action.
Hematin was purchased from Sigma. Sodium cholate and DTT were purchased from Serva. Sprague-Dawley (S-D) rats (weight range: 180-220 g) and Kunming mice were purchased from the Shanghai Institute of Cell Biology, Academia Sinica.
The S-D rats were given ad libitum access to food and water. Hypoxia was induced in the rats by exposure to a gas mixture of 5%-7% oxygen and 93%-97% nitrogen for 60 min to increase the blood brain barrier permeability, after which injections of 40 μmol/kg hematin (pH 7.4, intraperitoneally) and 100 mg/kg phenylhydrazine (intravenously) were given. The control animals (untreated, hypoxic) received saline injections. The animals were euthanized 20 h after treatment for analysis.
All operations were carried out below 4 °C. The rat tissues were homogenized in 2.0 volumes of 20.0 mmol/L potassium phosphate buffer (pH 7.4) containing 0.1 mmol/L EDTA and 135.0 mmol/L KCl; the resulting homogenate was centrifuged at 10000 ×g for 20 min. The supernatant fractions were collected and subsequently centrifuged at 150000 ×g for 1 h. Washing, resuspension, and solubilization of the microsomes were performed according to Maines’ method[1,4]. The solubilized microsomes were diluted with 1.0 volume of 0.05 mmol/L DTT solution (pH adjusted to 8.0) and loaded onto a DEAE-Sephacel column (2.5 cm × 13.6 cm) that was previously equilibrated with 20.0 mmol/L Tris HCl buffer (pH 7.5) containing 0.05 mmol/L EDTA, 0.5% Triton X-100, 0.1% sodium cholate and 0.05 mmol/L DTT. The column was eluted with concurrent linear gradients of KCl (0-0.4 mol/L) and Triton X-100 (0.5%-0.9%) prepared with equilibration buffer (75 mL). Fractions (3.0-4.0 mL) were collected and analyzed for HO activity.
The pooled DEAE-Sephacel fractions containing the first and second peaks of HO activity were dialyzed against distilled water for 24 h at temperature below 4 °C, and then concentrated and respectively loaded onto a hydroxyapatite column equilibrated with 10.0 mmol/L potassium phosphate buffer (pH 7.5). The column was washed with 10.0 mmol/L potassium phosphate buffer (pH 7.2) containing 0.05 mmol/L EDTA, 0.4% Triton X-100 and 0.1% sodium cholate, followed by the same solution supplemented with 20% glycerol. The column was eluted with a linear gradient of 10.0-260.0 mmol/L potassium phosphate in the above buffer, and the fractions containing HO activity were collected.
Each rat liver sample was homogenized in 2.0 volumes of 0.1 mol/L potassium phosphate buffer (pH 7.4). The resulting homogenate was centrifuged at 9000 ×g for 20 min. The supernatant containing biliverdin reductase was prepared by additional centrifugation of that supernatant fraction at 105000 ×g for 1 h. The protein concentration of the supernatant fraction was adjusted to 10.0 mg/mL. Biliverdin reductase activity was determined by measuring the increase in spectra absorption due to the production of bilirubin.
HO isoform activities were routinely determined according to the previously described methods in[1] and[4]. One unit of HO activity was defined as the amount of enzyme catalyzing the formation of 1 nmol of bilirubin in 1 h.
Electrophoretic procedures were performed according to Laemmli’s method in order to identify HO isoforms.
The purified rat liver HO-2 fraction (69 μg) was mixed with 100 μL of 0.9% NaCl solution and injected intraperitoneally into Kunming mice (10-15 g). These mice were then boosted with antigen (69 μg) every second week, for a total of three times. The mice were bled at 10 days after the final booster and sera were collected and stored at -20 °C.
Western blotting was performed as follows. Protein samples, rat liver HO-1 and rat brain HO-2 fractions, were subjected to SDS-PAGE in 1.5 mm slab gels according to the procedure of Laemmli. The separated polypeptides were then transferred electrophoretically from the gel to a nitrocellulose membrane using a transfer buffer consisting of 48 mmol/L Tris base, 39 mmol/L glycine, 20% (v/v) methanol and 0.037% (w/v) SDS (pH 8.3). Following electroblotting, the nitrocellulose blot was stained with Ponceau S for protein detection. Destaining was performed with distilled water. For immunostaining, the nitrocellulose blot was incubated at 37 °C for 1 h with bovine serum albumin in 20.0 mmol/L Tris-HCl buffer (pH 7.5) containing 0.5 mol/L sodium chloride, in order to saturate all protein binding sites. The nitrocellulose blot was then incubated with antiserum against rat liver HO-2 at 37 °C for 1 h, washed several times with PBS-Tween-20 solution and then incubated for 1 h at 37 °C with peroxidase anti-mouse IgG diluted with PBS solution. The blot was stained with diaminobenzidine (DAB) solution, washed with distilled water, and air-dried.
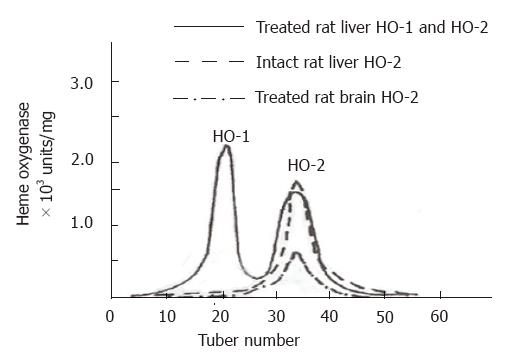
Rat liver microsomal fractions were loaded for DEAE-Sephacel chromatography, and 3.0-4.0 mL fractions were collected and analyzed for HO activity. Two peaks exhibiting HO activity were eluted (Figure 1). These peaks were designated as HO-1 and HO-2 according to the sequence of their elution from the column. The pooled DEAE-Sephacel fractions containing HO-1 and HO-2 activities were loaded onto hydroxyapatite column, and the final purified HO-1 and HO-2 preparations exhibited only single bands, respectively, when visualized by staining with Coomassie blue following SDS-PAGE. The final HO-1 and HO-2 preparations showed specific activities of up to 4300 nmol of bilirubin/mg of protein·h and 2150 nmol of bilirubin/mg of protein·h, respectively (Table 1).
| Purified fractions | Total protein, mg | Total activity, ×103 U | Specific activity, U/mg | Recovery, % | Purification, -fold |
| HO-1 | |||||
| Solubilized microsomes | 82 | 68.6 | 837.5 | 100 | 1.0 |
| (64.0-73.2) | (781.4-893.6) | ||||
| DEAE-Sephacel | 8.7 | 18.3 | 2097.3 | 10.7 | 2.5 |
| (16.6-20.0) | (1902.9-2291.7) | ||||
| Hydroxyapatite | 1.2 | 5.1 | 4301.1 | 1.5 | 5.1 |
| (5.0-5.2) | (3692.8-4909.3) | ||||
| HO.2 | |||||
| Solubilized microsomes | 82 | 68.6 | 837.5 | 100 | 1.0 |
| (64.0-73.2) | (781.4-893.6) | ||||
| DEAE-Sephacel | 4.9 | 7.7 | 1574 | 6.0 | 1.9 |
| (7.3-8.0) | (1504.5-1643.6) | ||||
| Hydroxyapatite | 3.1 | 6.7 | 2146.3 | 3.8 | 2.6 |
| (5.3-8.0) | (1708.5-2584.0) |
The treated rat brain and untreated rat liver microsomal fractions were also solubilized and respectively subjected to ion exchange chromatography of DEAE-Sephacel. The chromatographic elution patterns of HO activity were compared with those of treated rat liver. In the treated rat brain and untreated rat liver, only one peak of HO activity was detected. An activity peak was not present in the eluate corresponding to the region where the inducible isoform of HO, HO-1, was detected (Figure 1). Tables 2 and 3 show the procedures and the activity for purification of treated rat brain HO-2 and untreated liver HO-2, respectively.
| Purified fractions | Total protein,mg | Total activity,×103 U | Specific activity,U/mg | Recovery,% | Purification,-fold |
| HO-2 | |||||
| Solubilized microsomes | 56.0 | 24 | 428.7 | 100 | 1.0 |
| (16.9-31.1) | (302.7-554.7) | ||||
| DEAE-Sephacel | 20.1 | 12.2 | 607.7 | 35.9 | 1.4 |
| (10.3-14.1) | (512.0-703.4) | ||||
| Hydroxyapatite | 1.6 | 10.6 | 6478.1 | 2.9 | 15.1 |
| (10.0-11.2) | (6102.4-6853.8) |
| Purified fractions | Total protein,mg | Total activity,×103 U | Specific activity,U/mg | Recovery,% | Purification,-fold |
| HO-2 | |||||
| Solubilized microsomes | 662.7 | 394.7 | 595.6 | 100 | 1 |
| (351.8-437.6) | (530.9-660.3) | ||||
| DEAE-Sephacel | 10.2 | 16 | 1574.4 | 1.5 | 2.6 |
| (13.8-18.2) | (1358.7-1789.5) | ||||
| Hydroxyapatite | 2.4 | 8.2 | 3381.6 | 0.4 | 5.7 |
| (7.3-9.0) | (3020.1-3743.2) |
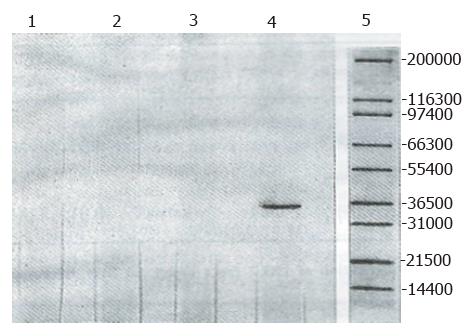
Two forms of HO from the treated rat liver were subjected to electrophoresis and two distinct bands exhibiting HO activity were detected, with the migration patterns of HO-1 and HO-2 being different. This differential separation pattern suggested the presence of distinct molecular properties associated with HO-1 and HO-2. The purified HO-2 after SDS-PAGE treatment displayed a higher monomeric molecular weight. The apparent molecular weights for HO-1 and HO-2 were Mr 30000 and Mr 36000, respectively. The apparent molecular weight in treated brain microsomal preparation was identical to the purified liver HO-2 (Mr 36000). The HO-1 activity was increased in response to hematin and phenylhydrazine, while that of HO-2 was fully refractory to these agents. The blot was treated with anti-rat liver HO-2 serum followed by anti-mouse IgG-peroxidase conjugate and then stained for peroxidase activity. As expected, rat brain HO-2 preparation gave a reddish brown band in the region of molecular weight Mr 36000. However, the rat HO-1 preparation failed to show a stained band (Figure 2).
We have purified and identified two constitutive forms of HO from rat liver and brain, and provided evidence for decidedly different characteristics of the two forms. These two forms are named HO-1 and HO-2. The results indicate that in the liver of treated rats, there exist two different molecular types of HO. It appears that HO-1 and HO-2 substantially differ in their molecular composition and structure as indicated by their chromatographic behavior and electrophoretic migration pattern. Differences in amino acid composition or sequence usually result in such observations. It is rather curious that in the untreated rat liver only the HO-2 isoform exists, and the HO-1 form of the enzyme could not be detected. This finding suggests that the amount of the HO-1 form is too low to be detected. The present findings also show that the activity of HO-1 in the treated rat liver apparently surpasses that of HO-2, and the HO-2 activity is almost refractory, indicating that only the activity of HO-1 is inducible.
With the same procedure, only HO-2 could be clearly detected in the brain, and the HO-1 form was absent, after the brain barrier permeability had been increased by hypoxia in order to induce the brain microsomal HO isoforms with hematin and phenylhydrazine. This finding, however, does not suggest that this isoform is absent in the organ; rather, it may reflect inability of the presently used procedures to detect the exceedingly low level of the isoform and expression of the HO-1 isoform in the brain being suppressed under various conditions. Surprisingly, the brain displayed a higher level of HO-2 activity than the liver, possibly reflecting a major biological adaptation[2]. HO-1 and HO-2 preparations were analyzed by the western immunoblotting technique. As expected, the rat brain HO-2 preparation exhibited immunological reactivity with antibody to rat liver HO-2, but the rat HO-1 preparation did not, indicating that these two HO preparations are antigenically different.
The present studies further suggested that the liver and brain HO-2 are similar protein entities, according to the following criteria: (1) similarity in molecular weight; (2) cross-reactivity with antiserum to rat liver HO-2; and (3) similarity in chromatographic behavior on a DEAE-Sephacel column.
Collectively, this study showed that the two constitutive forms differ in molecular weight and in inducibility and immunochemical properties and that these differences are highly important for understanding the occurrence of hyperbilirubinemia, particularly in the premature newborn.
Original title:
S- Editor: A L- Editor: Filipodia E- Editor: Li RF
| 1. | Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986;261:411-419. [PubMed] |
| 2. | Trakshel GM, Kutty RK, Maines MD. Resolution of the rat brain heme oxygenase activity: absence of a detectable amount of the inducible form (HO-1). Arch Biochem Biophys. 1988;260:732-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Braggins PE, Trakshel GM, Kutty RK, Maines MD. Characterization of two heme oxygenase isoforms in rat spleen: comparison with the hematin-induced and constitutive isoforms of the liver. Biochem Biophys Res Commun. 1986;141:528-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Trakshel GM, Kutty RK, Maines MD. Purification and characterization of the major constitutive form of testicular heme oxygenase. The noninducible isoform. J Biol Chem. 1986;261:11131-11137. [PubMed] |
| 5. | Ishikawa K, Sato M, Yoshida T. Expression of rat heme oxygenase in Escherichia coli as a catalytically active, full-length form that binds to bacterial membranes. Eur J Biochem. 1991;202:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Ishikawa K, Sato M, Ito M, Yoshida T. Importance of histidine residue 25 of rat heme oxygenase for its catalytic activity. Biochem Biophys Res Commun. 1992;182:981-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |