Published online Dec 15, 1997. doi: 10.3748/wjg.v3.i4.201
Revised: March 14, 1997
Accepted: April 6, 1997
Published online: December 15, 1997
- Citation: Huang XQ. Somatostatin: Likely the most widely effective gastrointestinal hormone in the human body. World J Gastroenterol 1997; 3(4): 201-204
- URL: https://www.wjgnet.com/1007-9327/full/v3/i4/201.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i4.201
Somatostatin (SS) was discovered from rat hypothalamus in 1968 and identified as a 14-amino acid peptide (SS-14) in 1973. Research achievements from studies of this fascinating hormone have drawn attention of scientists worldwide, yet it is not yet the time to make a complete conclusion on its broad distribution, physiologic functions, and roles in diagnosis and treatment of diseases. The results reported in the accumulated literature, however, do suggest its potential vast significant influence.
SS-producing cells (D-cells) exist at high densities throughout the central and peripheral nervous systems, in the endocrine pancreas and the gut, as well as in small numbers in the thyroid, adrenals, submandibular glands, kidneys, prostate and placenta (Table 1). In rats, gut-localized SS accounts for about 65% of the total body distribution, followed by brain localization at 25% and the pancreas and the remaining organs at 5% each. The approximate relative amounts of SS in the central nervous system are mainly in the cortex (49%) and spinal cord (30%). The D-cells in pancreatic islets are proximal to the other hormone-producing islet cells (i.e. A (glucagon-producing), B (insulin-producing), and PP (pancreatic polypeptide-producing) cells) and function to mediate function and inhibit hypersecretion of glucagon, insulin and pancreatic polypeptide via paracrine signaling. The D-cells are also located in the mucosal glands located throughout the cardiac region of the stomach to the rectum, and their content of SS accounts for more than 90% of that in the entire gastrointestinal tract.
| Organs | Type of Cells | Location |
| Major sites | ||
| Nervous system | Neurons | Hypothalamus |
| Cerebral cortex | ||
| Limbic system | ||
| Basal ganglia | ||
| Major sensory systems | ||
| Spinal cord | ||
| Dorsal root ganglia | ||
| Autonomic ganglia | ||
| Pancreas | D-cells | Islets |
| Gut | D-cells | Mucosal glands |
| Neurons | Submucous and myenteric plexuses | |
| Minor sites | ||
| Adrenal | Scattered medullary cells | |
| Placenta | Cytotrophoblasts in chorionic villi | |
| Reproductive organs | Testis, epididymis, prostate | |
| Submandibular Gland | D-cells | Scattered ductal cells |
| Thyroid | C-cells | Scattered parafollicular cells, coexisting with calcitonin |
| Urinary system | Scattered cells in renal glomerulus and collecting ducts | |
| Miscellaneous | Thymus, heart, eyes, erythrocytes | |
The SS in neurons populate both the submucosal and myenteric plexuses in all segments of the gastrointestinal tract, accounting for less than 10% of the total. Compared to SS concentration in plasma, that in cerebral spinal fluid is twice as much and in semen is 200-fold as high. Amniotic fluid is rich in SS. It is also present in some tumor tissues, including those of lung cancer, colon cancer and gastroenteropancreatic endocrine tumors. The extensive existence of SS in large amounts in the human body support the notion that it likely plays a significantly influential role in both physiologic and pathologic conditions.
SS exerts inhibitory effects on various organs. The broad array of SS actions includes regulation of neurotransmission, glandular secretion, smooth muscle contractility and cell proliferation. SS has been shown to inhibit virtually every known exocrine and endocrine secreted factor, including growth hormone, prolactin, thyrotropin, corticotropin, insulin, glucagon, vasoactive intestinal polypeptide, pancreatic polypeptide, gastrin, cholecystokin, motilin, neurotensin, thyroxin (T4), triiodothyronine (T3), calcitonin, aldosterone, catecholamine and renin. It has also been shown to inhibit splanchnic blood flow and movement functions (i.e. smooth muscle contractility), such as contractility of stomach, intestine and the biliary system, as well as gastric emptying, contractility of sphincter of Oddi, prolongation of gastrointestinal transit time, secretion such as for gastric acid and bicarbonate from the exocrine pancreas, release of pepsin, trypsin and the hemopoietic intrinsic factor as well as bile flow, absorption of glucose and other carbohydrates, amino acids and triglycerides, activity of the nervous system such as acetylcholine release from the gut mural myenteric plexuses, proliferation of cells and tumor growth, platelet aggregation, and function of activated immune cells. SS is also capable of stimulating water and electrolyte absorption, activating opioid receptors, and appears to excite the central neurons. The above-mentioned extensive inhibitory functions of SS suggest that, the SS is a widespread and powerful inhibitor capable of influencing a number of organs and tissues, and therefore of mediating homeostasis.
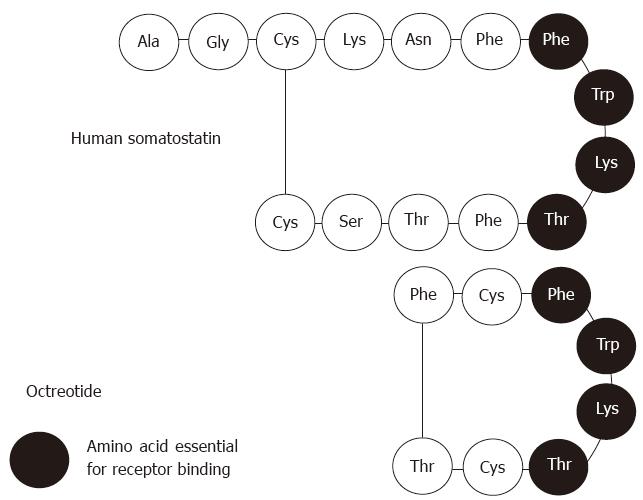
To date, the clinical utility of SS has been limited due to its very short half-life (less than 3 min). Octreotide, the first synthetic SS analogue, first described by Bauer et al in 1982, possesses longer half life (up to 2 h) and could be injected intermittently, and even administered orally. This agent is also able to inhibit the release of growth hormone, glucagon, and insulin by 45-, 11-, and 1.3-times respectively, and more powerfully than SS-14 and without causing rebound hypersecretion of hormones. Octreotide, an 8-amino acid, was modified from SS-14 (Figure 1), with the 7th to 10th amino acids being maintained in common since they are the 4 amino acids essential for receptor binding. Octreotide has similar pharmacological effects as native SS, and its widespread clinical use had illuminated its manifold physiological functions and values in clinical application. The mechanism underlying SS analogues’ clinical utility in diagnosis and treatment is the SS receptors that are present on tissue membranes and which specifically bind to the SS analogues, resulting in cell effects.
There are 5 subtypes of SS receptors (Table 2), which are identical in 42%-60% of their amino acid sequences and belong to a superfamily of receptors. The genes for these subtypes, located on different chromosomes, have suggested their different functions in different organs. As such, a variety of SS analogues might exert pharmacological actions by the specific binding with the various subtypes. The effects of treatment with SS analogues depend on both the ligands and the receptor subtypes.
| Feature | SS receptors | ||||
| Subtype 1 | Subtype 2 | Subtype 3 | Subtype 4 | Subtype 5 | |
| Amino acid sequence | 361 | 369 (A), 346 (B) | 418 | 388 | 364 |
| Chromosomal location | 14 | 17 | 22 20 | 20 22 | 16 |
| G protein coupling | Yes | Yes | Yes | Yes | Yes |
| Effector system | |||||
| Adenylate cyclase activity | Reduced | Reduced | Reduced | Reduced | Reduced |
| Tyrosine phosphatase activity | Increased | Increased | Not investigated | Not investigated | Not investigated |
| Possible binding affinity to receptors | |||||
| SS-14 | ++ | ++ | ++ | ++ | ++ |
| SS-28 | ++ | ++ | ++ | ++ | ++ |
| Octreotide | ± | ++ | + | ± | ++ |
| Vapreotide (RC-100) | ± | ++ | + | + | +++ |
| Lanreotide (BIM-23014) | ± | ++ | + | + | +++ |
| 1Distribution in normal human tissue | Brain, lungs, stomach, jejunum, kidneys, liver, pancreas | Brain, kidneys | Brain, pancreas | Brain, lungs | Brain, heart, adrenal glands, placenta, pituitary, small intestine, skeletal muscle |
Radiolabelled SS analogue, administered intravenously and then binding to the SS receptors on tumor tissues is the resulting formation of radiolabelled aggregation. Scintigraphy using a γ camera allow for visualization of such SS receptor-positive tumors. 111Octreotide is the most commonly used SS analogue because of the high density of SS receptors on neuroendocrine tumors of the gastroenteropancreatic system, with the total positivity rate ranging from 50% to 100%, and in most of the groups investigated it is above 80%. Metastatic liver tumors show lower rates of positivity. Pituitary adenoma may usually be visualized by scintigraphy. Adenocarcinomas originating from the breast, kidney, colon or ovary also express SS receptors. In 111octreotide scintigraphy imaging, the diagnostic reliability and rate of positivity are better than the other current diagnostic imaging techniques. The diagnostic efficiency depends upon the amount of SS receptors expressed on the tumor cells. Octreotide injection therapy does not affect the diagnostic efficiency, but can increase the therapeutic effect.
Octreotide is the most widely used drug among the SS analogues. Its indications and clinical uses are as follows:
These tumors have a large number of SS receptors and are treated with octreotide effectively, especially the growth hormone-secreting pituitary tumors (acromegaly). The agent is usually delivered as a subcutaneous injection of 100 μg three times a day, and may result in rapid control of symptoms and hormone secretion. In about half of the patients treated with octreotide, the size of the tumor is reduced. Some cases require larger doses, up to 300 μg or 600 μg daily, to achieve the maximal benefit, but a dose above 600 μg per day rarely has additional effect. Because of the high sensitivity observed in elderly patients with growth hormone-secreting tumors, octreotide can be recommended as a primary treatment. There have been no reports of loss of the suppressive effect of octreotide on growth hormone secretion in patients with acromegaly, even after 10 years or more of uninterrupted treatment.
Pancreatic islet-cell tumors express SS receptors in more than 80% of cases, and daily injection of 150 μg to 300 μg may improve the clinical outcome in 50% to 85% of cases. This outcome may be mediated by both a direct inhibitory effect on hormone production by the tumor and indirect effects, such as on the resorption of intestinal fluid, the production of gastric acid, or intestinal contractility. Octreotide treatment is often effective initially, but after weeks to months of treatment the symptoms worsen and tumor hormone secretion increases in virtually all the patients; while this effect can be reversed by increasing the dose of octreotide, eventually the drug becomes ineffective in all patients. This resistance phenomenon may be explained by the growth of clones of tumor cells that lack SS receptors. Octreotide is especially valuable in controlling the symptoms associated with vipoma or glucagonoma, and the remaining islet cell tumors often express subtypes of the SS receptor that do not bind octreotide, decreasing the beneficial effect. Reportedly, 70%-90% of metastatic carcinoid tumors respond well to octreotide, with effective control of the symptoms and of the overproduction of serotonin or tachykinin. It may also temporarily inhibit tumor growth, and in about 20% of patients the enlarged lymph nodes and liver metastases shrink. The most important benefits of octreotide are improvements to patient quality of life and patient survival.
In accordance with its various functions, including suppression of endocrine and exocrine secretions of the gastroenteropancreatic system, reduction of splanchnic blood flow, prolongation of gastrointestinal transit time and stimulation of water and electrolytic absorption, octreotide can be used to treat the following diseases: variceal acute bleeding (by 48-h continuous intravenous administration, and which has a similar effect of emergency sclerotherapy); pancreatic fistula; post-elective pancreatic surgery complications when applied perioperatively; and some refractory secretory diarrheas (as in autoimmune disease syndrome, short bowel syndrome, radiation colitis, and chemotherapy conditions).
Octreotide has not been used formally as a clinical oncologic treatment, but several studies have showed its promise in this realm. Octreotide has been shown to inhibit tumor growth in animals and of tumor cell lines in vitro, exerting antiproliferative effects on tumor cells via it effects on the SS receptors. The efficiency of octreotide on tumors depends on both the presence and amount of SS receptors on tumor cells. Tumors of the nervous system have SS receptors, and these tumors account for 50% of solid tumors in children and adolescents, including the neuroblastoma, meningioma, low grade astrocytoma and medulloblastoma.
In Weckbecker’s report, octreotide was applied in combination with the anti-cancer drugs mitomycin, doxorubicin, 5-fluorouracil, or Taxol to a pancreatic tumor cell line and to tumor-bearing nude mice. The results showed that the inhibitory effects of the anti-cancer drugs were enhanced markedly in a synergistic or additive manner, strongly suggesting the clinical utility of octreotide in combination with chemotheraputic agents for tumor therapy. Tamoxifen is an anti-oestrogenic agent used to treat breast cancer, but achievement of long-term effects in metastatic diseases is rare because developing resistance to the drug is common. Studies both in vitro and in vivo showed that the anti-proliferative effects of octreotide can enhance the anti-neoplastic effects of tamoxifen. Compared to tamoxifen mono-treatment, co-administration of octreotide plus tamoxifen yields better results with low toxicity and significantly lowered serum insulin-like growth factor I bioactivity.
A β-emitter-labelled octreotide (90Y-SDZ 413) binds to SS receptors on rat pituitary cells and inhibits growth hormone release. Administration of 90Y-SDZ 413 to a nude mouse model with SS receptor-positive tumors led to SS receptor-specific accumulation of the labelled conjugate (tumor/muscle ratio: 52/1), and a single treatment of 90Y-SDZ 413 led to a decrease of 25% in tumor mass. These results suggest that receptor-targeted radiotherapy by means of a 90Y-labelled octreotide represents a new strategy for the treatment of SS receptor-positive tumors.
The mechanism by which octreotide exerts its therapeutic effects on cancers has not been fully elucidated, but studies have indicated that the effects occur as a result of direct anti-proliferative activities on the tumor cells and direct inhibition of angiogenesis in the tumor mass, and as a result of indirect inhibition of the secretion of growth hormone, insulin, and insulin-like factor . A variety of tumors express different subtypes of the SS receptor, and each of the different kinds of SS analogues may not have high affinity with all subtypes of the SS-receptor. High affinity binding between an SS analogue and its specific subtypes is key for its optimal efficacy on tumors.
In patients with systemic sclerosis, octreotide can resolve the chaotic and non-propagative intestinal motility patterns so that they become well-coordinated-nearly to the extent as in healthy individuals-and can cause symptoms to subside. For patients with irritable bowel syndrome, octreotide can reduce the symptom of rectal pressure that underlies rectal urgency, thereby relieving a key component of the disease manifestation. In patients with dumping syndrome, octreotide can alleviate dumping by slowing gastric emptying, inhibiting insulin release, decreasing enteric peptide secretion, increasing intestinal absorption of water and sodium, slowing monosaccharide absorption, increasing gut transit time and preventing haemodynamic changes. For patients with psoriasis, a 12-wk course of octreotide treatment can ameliorate most symptoms. For patients wit pancreatitis, octreotide can also correct orthostatic hypotension and suppress the inflammatory reaction, the mechanism underlying its therapeutic effect involves inhibiting pancreatic exocrine and endocrine secretions and rectifying perioperative hypotension. Finally, patients with rheumatoid arthritis can benefit from the inflammation-resolving effects of octreotide and the therapeutic effect can be evaluated by scintigraphy of the involved joints.
Octreotide can be delivered via intravenous and subcutaneous routes, but is mostly administered as a subcutaneous injection at present. Dosage ranges from 50 μg to 200 μg, 3 times a day, with the most common being 100 μg, 3 times a day. For advanced tumor cases, 500 μg to 1000 μg or 2000 μg, 3 times a day for 6 months has been used safely, but usage of the larger doses is more rare at present. The most common adverse effects include nausea, diarrhea, flatulence, abdominal cramps, and malabsorption of fat, all of which usually subside spontaneously in 10 to 12 d regardless of continuance of treatment.
Long-term treatment with octreotide (>1 mo) is associated with an increased incidence of cholesterol gallstones, with rates ranging from 20% to 30%. This effect seems to be related to dosage and duration of the octreotide treatment. In most cases, the gallstones remain asymptomatic and the management should be similar to that applied to patients with non-octreotide-induced gallstones. The process of gallstone formation during octreotide therapy probably involves inhibition of gallbladder emptying and reduction of bile flow. Discontinuation of octreotide treatment tends towards gallstone resolution. A smaller dose, such as 100 μg, 3 times a day for less than one month, may present less to no risk of developing this problem. In the case of long-term octreotide treatment, however, intermittent usage may prevent gallbladder formation.
Octreotide has widespread therapeutic effects on various diseases, and hundreds of articles on octreotide treatment are published annually. The reasons why this agent is capable of exerting such a broad range of effects remains unknown and further investigation is necessary. For patients with acromegaly, a long-acting preparation of 20 mg to 30 mg administered intramuscularly is capable of controlling growth hormone hypersecretion for 28 to 42 d as effectively as multiple daily subcutaneous injections. A long-acting intramuscular formulation of lanreotide (another SS analogue) can also maintain therapeutic effects for 2 wk.
Development of novel SS analogues and further development of their diverse preparations may speed up the progression of both basic and clinical research for the wide of array of disease conditions they are related to. Identification and characterization of the various subtypes of SS receptors in different organs and lesions, and of their specific ligands, will improve diagnosis and treatment as well. A current limitation of SS analogues is that they are expensive. When their costs are lowered in the future, they will be used more extensively and their theoretical basis and practical value will become more apparent as well. It can be predicted that octreotide will become a common drug in clinically use when it is able to be administered orally and for lower, more affordable cost.
Original title:
S- Editor: A L- Editor: Filipodia E- Editor: Li RF
| 1. | Patel PC. General aspects of biology and function of somatostatin. Somatostatin. BERLIN: Springer Sandoz 1992; 1-16. |
| 2. | Lamberts SW, van der Lely AJ, de Herder WW, Hofland LJ. Octreotide. N Engl J Med. 1996;334:246-254. [PubMed] |
| 3. | Harris AG. Somatostatin and somatostatin analogues: pharmacokinetics and pharmacodynamic effects. Gut. 1994;35:S1-S4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 205] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Kvols LK, Reubi JC, Horisberger U, Moertel CG, Rubin J, Charboneau JW. The presence of somatostatin receptors in malignant neuroendocrine tumor tissue predicts responsiveness to octreotide. Yale J Biol Med. 1992;65:505-518; discussion 531-536. [PubMed] |
| 5. | Krenning EP, Kooij PP, Pauwels S, Breeman WA, Postema PT, De Herder WW, Valkema R, Kwekkeboom DJ. Somatostatin receptor: scintigraphy and radionuclide therapy. Digestion. 1996;57 Suppl 1:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Faiss S, Scherübl H, Bäder M, Fett U, Koppenhagen K, Wiedenmann B, Riecken EO. [Significance of false-positive findings of somatostatin receptor scintigraphy in diagnosis of neuroendocrine tumors of the gastroenteropancreatic system]. Z Gastroenterol. 1994;32:243-246. [PubMed] |
| 7. | Huang XQ. Octreotide and its clinical applications. Therapeutics of gastrointestinal disease. Tianjin: Tianjin Science and Technology Publication 1996; 60-76. |
| 8. | Eriksson B, Janson ET, Bax ND, Mignon M, Morant R, Opolon P, Rougier P, Oberg KE. The use of new somatostatin analogues, lanreotide and octastatin, in neuroendocrine gastro-intestinal tumours. Digestion. 1996;57 Suppl 1:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Weckbecker G, Raulf F, Tolcsvai L, Bruns C. Potentiation of the anti-proliferative effects of anti-cancer drugs by octreotide in vitro and in vivo. Digestion. 1996;57 Suppl 1:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Pollak M. Enhancement of the anti-neoplastic effects of tamoxifen by somatostatin analogues. Digestion. 1996;57 Suppl 1:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Albers AR, O’Dorisio MS. Clinical use of somatostatin analogues in paediatric oncology. Digestion. 1996;57 Suppl 1:38-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Stolz B, Smith-Jones P, Albert R, Tolcsvai L, Briner U, Ruser G, Mäcke H, Weckbecker G, Bruns C. Somatostatin analogues for somatostatin-receptor-mediated radiotherapy of cancer. Digestion. 1996;57 Suppl 1:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Camilleri M. Effects of somatostatin analogues on human gastrointestinal motility. Digestion. 1996;57 Suppl 1:90-92. [PubMed] |
| 14. | Farthing MJ. The role of somatostatin analogues in the treatment of refractory diarrhoea. Digestion. 1996;57 Suppl 1:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Scarpignato C. The place of octreotide in the medical management of the dumping syndrome. Digestion. 1996;57 Suppl 1:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |