Published online Sep 15, 1997. doi: 10.3748/wjg.v3.i3.182
Revised: January 11, 1997
Accepted: February 18, 1997
Published online: September 15, 1997
AIM: To investigate the effects of Astragalus membranaceus (AM) on intestinal oxygen consumption both in vivo and in vitro.
METHODS: The oxygen consumption of the intestine was measured using an arteriovenous (A-V) O2 difference analyzer after treatment with AM in the intestinal lumen of ten healthy, anesthetized mongrel dogs. The effects of AM on the oxygen consumption of the intestinal mucosa in vitro were observed using constant volume manometers.
RESULTS: The oxygen consumption of the intestine in vivo increased significantly (P < 0.05 or P < 0.01) after treatment with AM compared to the saline control. The oxygen consumption significantly increased after treatment with the 30% AM dilution and the 50% AM dilution compared to that of the 10% AM dilution (P < 0.05). There was no significant difference between the 30% AM dilution and the 50% AM dilution (P > 0.05). The effects of AM on oxygen consumption of the intestinal mucosa in vivo were similar to those in vivo. After treatment with the 5% AM dilution and the 1% AM dilution, the intestinal oxygen consumption increased compared to the control (Krebs Ringer phosphate buffer (KRPB)) (P < 0.05 and P < 0.01, respectively). There was no significant difference between treatment with the 10% AM dilution and the KRPB control (P > 0.05).
CONCLUSION: AM improved the function of intestinal oxidative metabolism.
- Citation: Li SZ, Tan XH. The effects of Astragalus membranaceus on oxygen consumption in the intestine. World J Gastroenterol 1997; 3(3): 182-184
- URL: https://www.wjgnet.com/1007-9327/full/v3/i3/182.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i3.182
It was reported that Astragalus membranaceus (AM) could stabilize the configuration of deoxyhemoglobin, decrease hemoglobin’s affinity for oxygen, shift the oxygen hemoglobin dissociation curve to the right, increase hemoglobin’s capacity for transporting oxygen, and decrease the from an oxygen supply deficiency[1,2]. AM can also decrease the oxygen consumption of cardiac muscle and maintain the balance of oxygen supply in the blood[3]. However, its effects on oxygen consumption in the intestine has not been investigated. This study observed the effects of AM on oxygen consumption in the intestine both in vivo and in vitro.
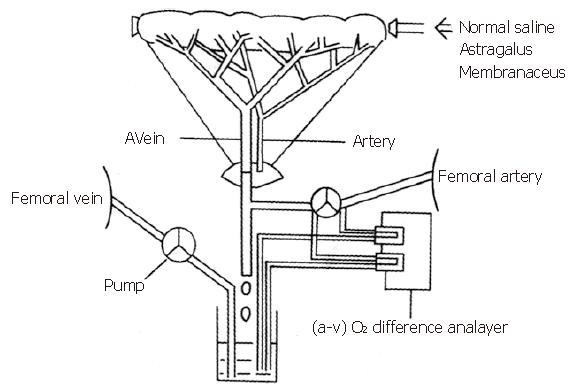
Ten healthy mongrel dogs (15-20 kg) of either sex were fasted for 24 h and anesthetized with pentobarbital sodium (30 mg/kg). All animals were ventilated with a positive pressure respirator (Harvard Apparatus, United States) that was adjusted to achieve normal blood pH, O2 tension, and CO2 tension before each experiment. A midline abdominal incision was made and a segment of jejunum (20-40 g) about 30 cm aboard to the ligament of Treitz was exteriorized. A rubber tube was placed in the lumen of the segment for introduction and withdrawal of the AM solution. After heparin sodium (500 U/kg) was administered intravenously, a vein draining in the jejunal segment and a femoral artery were cannulated for continuous measurement of intestinal oxygen consumption of the observed segment. The measured blood was directed to a reservoir, which was pumped back to the animal via a femoral vein at a rate equal to the total outflow in order to maintain the blood equilibrium. Both ends of the segment were ligated to the adjacent jejunum to exclude collateral flow after the rubber tube was placed into the lumen of the segment (Figure 1). The segment was covered with a plastic sheet and kept at 37 °C with a heat lamp and a thermoregulator (Yellow Spring Instrument, United States)[4,5].
Intestinal oxygen consumption in vivo was measured by an arteriovenous (A-V) O2 difference analyzer (A-Vox. Systems, United States). After the operation was performed, normal saline was used to wash the lumen three times. Ten milliliters of normal saline was placed into the lumen while the intestinal blood flow, motility, and blood pressure were continuously measured for 15 min. This procedure was repeated every 15 min until the blood flow, motility, and blood pressure reached a steady state. Then the effects of different concentrations of AM on oxygen consumption were measured. Each dog was given each AM concentration three times. The intestinal oxygen consumption was recorded from 0-3 min, 4-7 min, 8-11 min, and 12-15 min.
The oxygen consumption of the intestinal mucosa in vitro was measured using constant volume manometers (Warburg Apparatus, United States). Segments of jejunum were harvested from anesthetized mongrel dogs. The tissue was immediately placed in fresh oxygenated ice-cold Krebs Ringer phosphate buffer (KRPB) solution. The container was surrounded by ice in order to keep the temperature low. Blood was removed from the tissue by perfusing KRPB via the local artery after the lumen was washed. The tissue was cut open along the mesenteric border, and intact mucosal sheets were obtained by gently separating the mucosa from the underlying gut wall using a microscope slide. Small pieces of mucosa, 1 mm x 2 mm, were obtained by cutting. These small pieces were transferred to the fresh, oxygenated, cold KRPB. Numerous small pieces were blotted on filter paper, weighed to the nearest 0.15 g, and placed in Warburg flasks. Each flask contained a total volume of 3 mL of incubation media that included oxygenated KRPB with 0.5 mL of one of the concentrations of AM in the experimental groups or without AM in the control group. To measure the absorption of carbon dioxide, the center well of the flask contained 0.2 mL of 10% KOH and 0.1 g of filter paper. The flasks were attached to manometers, placed in a water bath (37 °C), and shaken at 90 cycles per minute. After a 10-15 min equilibration, manometer pressure readings were obtained every 10 min for 1 h. Additional mucosal tissue samples of 5-6 g were weighed, oven dried, and re-weighed to obtain the percentage dry weight, which was used to calculate the dry weight of the experimental samples.
AM was purchased from the Hebei Company of Medicinal Materials. Two hundred milliliters of distilled water was added to a flask containing 50 g of AM. The flask was put on an electric furnace to be decocted until the solution became 100 mL. This was labeled as the 50% concentration of AM. This solution was further diluted to 30%, 10%, 5%, 1% and 0.5% with distilled water. Fresh AM dilutions were prepared on each experimental day.
KRPB solution was prepared with NaCl (7.106 g/L), KCl (0.365 g/L), CaCl2 (0.138 g/L), MgSO4 (0.01 g/L), Na2HPO4 (2.29 g/L), and glucose (0.9 g/L). First, NaCl, KCl, MgSO4 and Na2HPO4 were placed in distilled water and stirred. At the same time, the solution was gassed with a combination of 95% O2 and 5% CO2 for 10 min. CaCl2 dissolved in 100 mL of distilled water was slowly added into the KRPB solution. A fresh solution was prepared on each experimental day.
Analysis of variance (ANOVA) was employed to detect the differences among the four groups. The Q-test (Newman-Keuls test) was used to compare the differences between two groups.
Six different concentrations of AM (50%, 30%, 10%, 5%, 1% and 0.5%) were tested in both in vivo and in vitro experiments to determine the most effective concentration. This study reported the effects of the most effective concentration and the concentration above and below the most effective concentration.
The effects of AM on the intestinal oxygen consumption in vivo are reported in Table 1. After treatment with the AM solution, the intestine increased the oxygen consumption, especially after 4-7 min, compared to the saline control (P < 0.05 or P < 0.01). The intestinal oxygen consumption was different among the different AM dilutions. The oxygen consumption was significantly increased (P < 0.05) after treatment with the 30% and 50% AM dilutions compared to the 10% dilution. There was no significant differences in oxygen consumption between the 30% and 50% AM dilutions (P > 0.05).
| Groups | n | 0-3 min | 4-7 min | 8-11 min | 12-15 min |
| A. Normal saline | 10 | 2.62 ± 0.51 | 2.71 ± 0.54 | 2.68 ± 0.60 | 2.58 ± 0.58 |
| B. 10% concentration | 10 | 2.96 ± 0.64 | 3.48 ± 0.66a | 3.87 ± 0.69b | 4.01 ± 0.72b |
| C. 20% concentration | 10 | 3.18 ± 0.70 | 4.16 ± 0.78bc | 4.82 ± 0.82bc | 4.77 ± 0.83b |
| D. 30% concentration | 10 | 3.23 ± 0.76 | 4.35 ± 0.79bc | 4.74 ± 0.86bc | 4.76 ± 0.84b |
The effects of AM on the oxygen consumption of the intestinal mucosa in vitro is reported in Table 2. There was no significant difference between the 10% AM dilution and the KRPB control (P > 0.05). The intestinal oxygen consumption was increased after treatment with the 5% and 1% AM dilutions. The oxygen consumption significantly increased (P < 0.05) at 50 min and 60 min after treatment with the 1% AM dilution compared to that of the KRPB control. The oxygen consumption significantly increased (P < 0.05 or P < 0.01) at 20 min, 30 min, 40 min, 50 min, and 60 min after treatment with the 5% AM dilution compared to the KRPB control, the 10% AM dilution, and the 1% AM dilution.
| Groups | n | 0-3 min | 4-7 min | 8-11 min | 12-15 min |
| A. Normal saline | 10 | 2.62 ± 0.51 | 2.71 ± 0.54 | 2.68 ± 0.60 | 2.58 ± 0.58 |
| B. 10% concentration | 10 | 2.96 ± 0.64 | 3.48 ± 0.66a | 3.87 ± 0.69b | 4.01 ± 0.72b |
| C. 20% concentration | 10 | 3.18 ± 0.70 | 4.16 ± 0.78bc | 4.82 ± 0.82bc | 4.77 ± 0.83b |
| D. 30% concentration | 10 | 3.23 ± 0.76 | 4.35 ± 0.79bc | 4.74 ± 0.86bc | 4.76 ± 0.84b |
AM has been shown to protect cultured cardiac muscle cells during a glucose and oxygen deficient period. The proposed mechanism was that AM could stabilize the function of cardiac muscle cells, protect the mitochondria and lysosomes, and enhance the resistant capacity of oxygen deficiency[6]. In addition, AM has been shown to lower the oxygen consumption of the heart and liver mitochondria in guinea rats, increase the formation of 2, 3-diphosphoglycerate in healthy human red blood cells (in vitro), and improve hemoglobin’s capacity for transporting oxygen[1]. After a 25% concentrated solution (1 mL/kg) of AM was injected into the empty stomachs of healthy, awakened dogs, the cycle duration of the interdigestive myoelectric complex was changed. Phase I was shortened and phase II were prolonged in the jejunum. The cycle duration was also extended and showed an increase of action potential in phase II. Taken together, this data indicated that AM could strengthen the movement and muscle tone of the intestine[7].
Our previous study also showed that the intestinal blood flow and motility increased after treatment with the AM solution into the canine intestine in vivo. This revealed that AM could improve the physiological function of the intestine. This study also indicated that the pharmaceutical effects of AM on different organs were different.
Guo et al[8] reported that the injection of AM could directly cause peripheral vasodilatation. According to Kvietys and Granger’s[9] analysis of 50 papers about the effects of vasoactive agents on oxygen uptake, vasodilators can increase oxygen uptake. Changes in oxidative metabolism, blood flow, and capillary density appeared to be the major mechanism by which vasoactive agents alter splanchnic oxygen uptake. Under in vitro conditions, any drug-induced changes in tissue oxygen uptake are assumed to reflect only changes in tissue oxidative metabolism. Accordingly, AM probably has the pharmaceutical action of increasing oxygen consumption.
In order to probe the effect of AM on oxygen consumption in this study, the canine intestine was treated with different dilutions of AM. Its effect on the intestinal oxygen consumption was observed. We observed that AM treatment could significantly increase the intestinal oxygen consumption in vivo compared to the saline control. This indicated that AM could improve the physiological function of the intestine. To eliminate the interference involved in in vivo experiments, the effects AM treatment on intestinal oxygen consumption in vitro were also measured. We observed that AM treatment could also significantly increase the intestinal oxygen consumption compared to the KRPB control.
The effects of AM treatment on the intestinal oxygen consumption varied at different dilutions. This might be explained by the use of different experimental methods. In the in vivo experiments, AM was placed into the intestine. This method limited the effect of the AM on the surface of the tissue. While in the in vitro experiments, AM was put in the flasks containing small pieces of intestinal mucosa. In this method, the surface of the tissues was in contact with the AM constantly. Therefore, different pharmaceutical responses were observed among the AM dilutions between the in vivo and in vitro experiments.
We observed that the pharmaceutical function of AM was to increase the intestinal oxygen consumption. It was consistent with the report that AM could improve the intestinal function[7]. Other studies have shown different effects of AM on oxygen consumption in different organs (i.e. decreased oxygen consumption in cardiac muscle cells and liver mitochondria, and increased intestinal oxygen consumption in vivo and in vitro). The mechanism, by which AM affects oxygen consumption, awaits further studies.
The authors are grateful to Dr Adamu Alemayehu and to Wayne Assing, an English teacher from the United States, for their help with the manuscript.
Li Shao Zhi, male, was born on 1953-10-11 in Changsha City, Hunan Province, and graduated from the Hunan College of Traditional Chinese Medicine as a postgraduate in 1984. He is an Associate Professor of Diagnosis, Director of Research Laboratory of Diagnosis, Hunan College of Traditional Chinese Medicine, and has 34 papers and two books published.
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Hu S
| 1. | Zhang XR, Liu CM, Chen WW. Effect of Qixue injection on heart oxygen consumption (in Chinese). Chinese Journal of Integrative Medicine. 1987;7:606-607. |
| 2. | Lei ZY, Wang SR. Effect of Astragalus Membranaceus on cardiovascular system. Chinese Journal of Integrative Medicine (in Chinese). 1993;13:443-446. |
| 3. | Chen JC, Li SY, Miao LJ, Yan A. A study about effect of Astragalus Membranaceus on ultrastructure of cardiac-muscle cells cultured in medium lack of glucose and oxygen (in Chinese). New J TCM. 1990;22:52-53. |
| 4. | Chou CC, Alemayehu A, Mangino MJ. Prostanoids in regulation of postprandial jejunal hyperemia and oxygen uptake. Am J Physiol. 1989;257:G798-G808. [PubMed] |
| 5. | Alemayehu A, Lock KR, Coatney RW, Chou CC. L-NAME, nitric oxide and jejunal motility, blood flow and oxygen uptake in dogs. Br J Pharmacol. 1994;111:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Li SY, Chen JC, Huang X, Yan A, Miao LJ. The study about the mechanism of Qi-boosting function of Astragalus Membranaceus (in Chinese). New J TCM. 1987;19:51-52. |
| 7. | Yang DZ, Bi QH, Din AL, Ying CZ. Effect of Astragalus Membranaceus on Myoelectric activity of small intestine (in Chinese). Chinese Journal of Integrative Medicine. 1993;13:616-617. |
| 8. | Guo ZG, Xu SW, Jia HJ, Zhou HH, Zhang FL, Shen L. The peripheral vasodilation effect of Hunan (Astragalus Membranaceus (fisch) Bunge) and a comparison with that of r-Aminobutyric acid (GABA). J TCM. 1980;21:73-76. |
| 9. | Kvietys PR, Granger DN. Vasoactive agents and splanchnic oxygen uptake. Am J Physiol. 1982;243:G1-G9. [PubMed] |