Published online Sep 15, 1997. doi: 10.3748/wjg.v3.i3.177
Revised: October 9, 1996
Accepted: November 16, 1996
Published online: September 15, 1997
AIM: To investigate the effects of the Yanggan Jieyu (YGJY, nourishing the liver and alleviating mental depression) decoction on the plasma concentrations of fibronectin (FN), fibronectin receptor (FNR), tumor necrosis factor alpha (TNF-α), and the activity of interleukin-1 (IL-1) in patients with cirrhosis.
METHODS: Thirty-four cases of decompensated cirrhosis were divided into the YGJY decoction treatment group and the control group (patients received standard treatment). FN, FNR and TNF-α were measured by ELISA and expressed as mg/L (FN, FNR) and ng/L (TNF-α). IL-1 was measured by mice thymocyte proliferation using a β scintillation counter and was expressed as cpm.
RESULTS: In the YGJY decoction treatment group, FN and TNF-α levels increased significantly (P < 0.01 and P < 0.05, respectively), and FNR and IL-1 levels decreased significantly (P < 0.05 and P < 0.05, respectively). In the control group, FN, FNR, TNF-α, and IL-1 levels did not significantly change.
CONCLUSION: YGJY decoction could prevent hepatic fibrosis by adjusting the plasma levels of FN, FNR, TNF-α and IL-1, which could mediate cirrhosis formation. This data is of clinical significance.
- Citation: Wu H, Gao JS, Fan JZ, Huang J, Deng JW. Analysis of fibronectin, fibronectin receptor and interleukin-1 in patients with cirrhosis treated by the Yanggan Jieyu decoction. World J Gastroenterol 1997; 3(3): 177-179
- URL: https://www.wjgnet.com/1007-9327/full/v3/i3/177.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i3.177
Recent reports have shown that serum levels of some extracellular matrix proteins and cytokines, such as fibronectin and interleukin-1, may be biomarkers of hepatic fibrosis, as well as important molecules for initiating hepatic fibrosis[1-3]. Using the autoimmune hepatitis and cirrhotic model of C57 BL/6 mice, it was suggested that the Yanggan Jieyu (YGJY, nourishing the liver and alleviating mental depression) decoction could prevent liver tissue damage[4]. Clinical studies have confirmed that many cirrhotic patients were in the state of Gan Yu Xue Xu (stagnancy of the Qi and deficiency of blood in the liver). In this study, we evaluated the curative effect of the YGJY decoction in cirrhosis.
Thirty-four patients (30 men and 4 women ranging in ages from 25 years to 68 years) diagnosed with chronic cirrhotic Hepatitis B virus (HBV) were included in this study. Their diagnoses were based on clinical and laboratory evaluations[5]. All of the cases were decompensated cirrhosis and 80% of the cases also had cirrhotic ascites).
The patients were randomly divided into two groups. Twenty patients were treated with the YGJY decoction (35 g per day for 8 wk). The decoction is made of Bupleuri (10 g), Lycium barbarum (10 g), Rapid Angelicae Sinensis (10 g), and Radix Glycyrrhizae (5 g). The remaining 14 patients were treated by the standard methods. Twenty healthy volunteers served as controls.
The FN and FNR levels were assayed by ELISA. FN was detected by using a specific anti-FN rabbit serum (rabbit immunoglobulin to human fibronectin provided by Dakopatts, Denmark)[1,2]. FNR was detected by using an anti-FNR mouse antibody (Takara Biolaboratory, Japan). The results were expressed as mg/L.
IL-1 was produced from human mononuclear cells (macrophages) after stimulation with 40 μg of LPS (Sigma) and 3 μg of indomethacin (Sigma). Thymocyte proliferation was analyzed using the thymocytes from 4 to 8 wk old BALB/C mice. The samples were incubated in the presence or absence of 0.3 mg/L conA (Sigma). Sample dilutions were assayed for IL-1 activity by the incorporation of tritiated thymidine into the cells and counted with a β scintillation counter. The results were expressed as cpm[6].
The serum TNF-α concentrations were determined by the ELISA (Genzyme corporation, Cambridge, MA, United States).
The mean values in the control and cirrhotic patients before and after treatment were compared by the Student’s t test. The correlation between FN and other parameters of hepatic fibrosis were evaluated by a linear regression analysis. Data were expressed as average ± standard deviation.
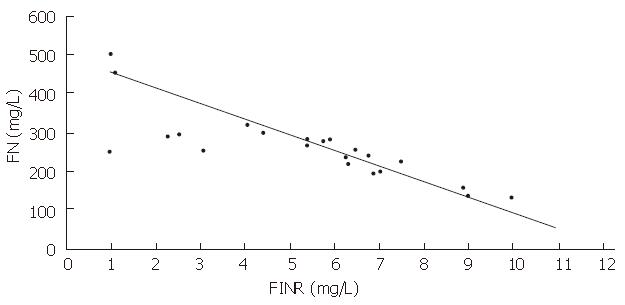
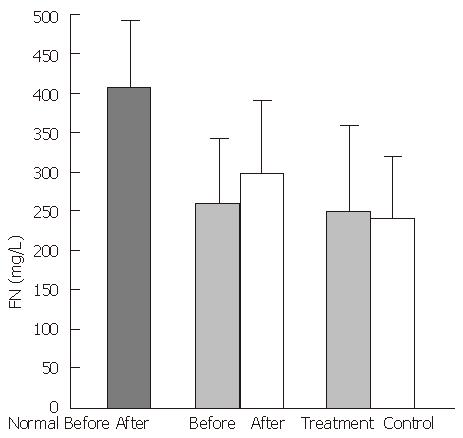
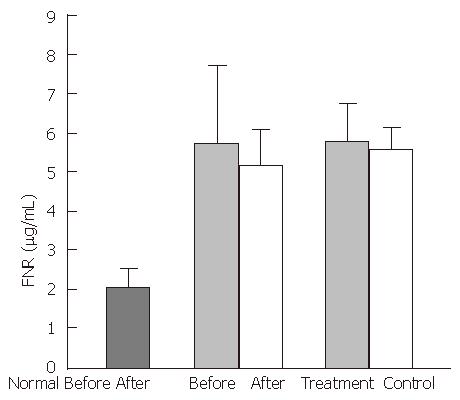
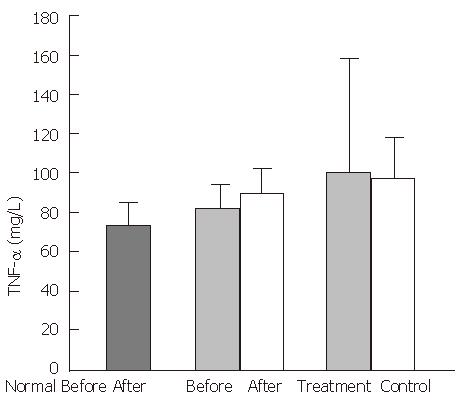
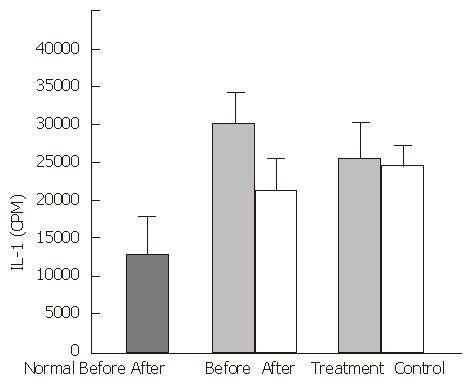
In the healthy control group, mean plasma FN levels were 413.0 ± 72.5 mg/L, FNR levels were 2.3 ± 0.4 mg/L, TNF-α levels were 72.3 ± 8.6 ng/L, and IL-1 activity was 1320.6 ± 419.2 cpm. In the experimental group, cirrhotic patients had significantly decreased FN levels (248 ± 97.0 mg/L, P < 0.01), and significantly increased FNR levels (5.5 ± 2.3 mg/L, P < 0.01), TNF-α levels (97.4 ± 29.4 ng/L), and IL-1 activity, (2760.8 cpm, P < 0.05), compared with the healthy control group. A negative correlation was observed between the serum concentrations of FN and FNR (P < 0.01, r = -0.6534) (Figure 1).
After treatment with the YGJY decoction, the FN levels significantly increased (247.9 ± 97.2 mg/L to 298.3 ± 93.2 mg/L, P < 0.01) (Figure 2). The FNR levels significantly decreased after YGJY treatment (5.6 ± 2.7 mg/L to 4.3 ± 2.3 mg/L, P < 0.01) (Figure 3). The TNF-α levels significantly increased after YGJY treatment (83.9 ± 7.1 ng/L to 93.6 ± 12.0 ng/L, P < 0.05) (Figure 4). The activity of IL-1 after the YGJY treatment decreased significantly (2760.8 ± 813.6 cpm to 1922.3 ± 847.0 cpm, P < 0.01) (Figure 5). In the standard treatment group, the FN levels, FNR levels, TNF-α levels, and the activity of IL-1 were not significantly different (P > 0.05, Figure 2, Figure 3, Figure 4, Figure 5).
In 63.5% of patients with cirrhotic HBV, the serum globulin level was down-regulated to normal levels after treatment with YGJY decoction. However, the serum globulin level returned to normal in only 23.4% of patients treated by standard methods.
Recent studies on cirrhosis have focused on the interactions between cytokines and the extracellular matrix (ECM)[1,8,9]. Several reports have shown that serum levels of some ECM molecules, such as type III procollagen peptide, types I and IV collagen, and fibronectin, were biomarkers of hepatic fibrosis, and the accumulation of ECM molecules played a major role in liver function impairment. The FN and FNR are the main components of the extracellular matrix. We found that in patients with decompensated liver cirrhosis, the plasma FN was significantly lower and FNR was significantly higher, with a strong negative correlation (r = -0.6534). The TNF-α levels and IL-1 activity were also increased as compared with the normal subjects. Hagiwata et al[10] observed that recombinant human IL-1 could increase FN in the liver of rats, and also could directly increase the transcription of type I, III and IV collagen. IL-1 may act synergistically with TNF-α to induce hepatitis[9].
These data show that serum FNR, IL-1 and TNF-α could up-regulate and FN could down-regulate the liver fibrosis process. We found that IL-1 activity and FNR levels, which could indicate increased fibrosis of the liver, were strongly down-regulated by treatment with YGJY decoction. While FN, which could indicate decreased liver fibrosis, was strongly up-regulated by YGJY decoction treatment. These changes showed that YGJY decoction could prevent liver damage and inhibit hepatic fibrosis.
TNF-α is a multifunctional cytokine, which is hypothesized to regulate inflammatory and pathological processes and orchestrate necrosis and regeneration. We detected plasma levels of TNF-α and nitrate in cirrhotic rats by reproduction with CCl4. The TNF-α levels were significantly higher after YGJY treatment than before treatment. It has been shown that TNF-α can positively regulate liver cell regeneration and hepatocyte proliferation in rats induced by the nitrate[11]. Further studies are necessary to clarify the effect and mechanism of YGJY decoction in hepatocyte proliferation and regeneration.
Hong Wu, professor of clinical immunology and integrated traditional Chinese and western medicine, graduated from Hunan Medical College, in 1961, studied in the United States. from 1988-1990.
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Hu S
| 1. | Yamauchi M, Nakajima H, Ohata M, Hirakawa J, Mizuhara Y, Nakahara M, Kimura K, Fujisawa K, Kameda H. Detection of fibronectin receptor in sera: its clinical significance as a parameter of hepatic fibrosis. Hepatology. 1991;14:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Gabrielli GB, Casaril M, Bonazzi L, Baracchino F, Bellisola G, Corrocher R. Plasma fibronectin in liver cirrhosis and its diagnostic value. Clinica Chimica Acta. 1986;160:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Matsuoka M, Pham NT, Tsukamoto H. Differential effects of interleukin-1 alpha, tumor necrosis factor alpha, and transforming growth factor beta 1 on cell proliferation and collagen formation by cultured fat-storing cells. Liver. 1989;9:71-78. [PubMed] |
| 4. | Wu H, Wang ZM, Fan JT, Deng JW, Fen TJ. Effect of six kinds of decoctions on experimental autoimmune liver diseases. Bulletin of Hunan Medical University. 1991;16:263-267. |
| 5. | Chen JZ, Li ZM. Internal medicine. Ed 3. Beijing: The People-s Medical Publishing House 1989; 389-396. |
| 6. | Habicht GS, Beck G, Benach JL, Coleman JL, Leichtling KD. Lyme disease spirochetes induce human and murine interleukin 1 production. J Immunol. 1985;134:3147-3154. [PubMed] |
| 7. | Pizzaro TT, Malinowskak K, Kovacs EJ, Chancy J, Bobinson JA, Piclinini LA. Induction of TNFB and FNF (Lymphotoxin) gene expression during rate Cardige gllogr of regection (Abstract). FASEB J. 1991;5:1708. |
| 8. | Dinaretto CA. Interleukin-1 and Interleukin? antagonism blood. 1991;77:1627-1862. |
| 9. | Gantner F, Leist M, Lohes AW, Germaann RG, Tes G. Concaavalin A induced T cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology. 1995;2:190-198. |
| 10. | Hagiwata T, Suzuid H, Kano C, Kashiwag I, Ykyama Y, Onoiaki K. Regulation of fibronectin synthesis by interleukin-1 and interleukin-6 in rat hepatocytes. Am J Pathol. 1990;136:39-47. |
| 11. | Kubo Y, Yasunaga M, Masuhara M, Terai S, Nakamura T, Okita K. Hepatocyte proliferation induced in rats by lead nitrate is suppressed by several tumor necrosis factor alpha inhibitors. Hepatology. 1996;23:104-114. [PubMed] |