Published online Jun 15, 1997. doi: 10.3748/wjg.v3.i2.84
Revised: November 14, 1996
Accepted: March 1, 1997
Published online: June 15, 1997
AIM: To study gastrin expression in colorectal carcinoma tissues and adjacent mucosa and discuss the function of gastrin in colorectal carcinoma.
METHODS: Gastrin expression in colorectal carcinoma tissues and adjacent mucosa was examined in 58 cases using immunohistochemical and immunoelectron microscopy.
RESULTS: A total of 35.1% of colorectal carcinoma transitional mucosa (TM), 48.3% of nontypical dysplasia mucosa and 60.3% of carcinoma tissue were positive for gastrin expression (P < 0.05). Immunoelectron microscopy revealed that protein A gold (PAG) granules localized to different electron-dense secretory granules in carcinoma cells, the intercellular spaces, and the microvillar membrane surface.
CONCLUSION: Gastrin expression in colorectal carcinoma tissue and adjacent mucosa, and the release of gastrin by carcinoma may be an initiating factor in carcinoma occurrence and development. Positive gastrin expression in colorectal carcinoma tissues can serve as a differentiation marker.
- Citation: Jiang CP, Chen YQ, Zhu JW, Shen HX, Yu X. Immunohistochemical study of gastrin in colorectal carcinoma tissues and adjacent mucosa. World J Gastroenterol 1997; 3(2): 84-86
- URL: https://www.wjgnet.com/1007-9327/full/v3/i2/84.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i2.84
Recent studies have shown that exogenous gastrin can promote human colorectal carcinoma cell strain growth, that colorectal carcinoma cells express gastrin mRNA, and that gastrin-like material can be detected in the culture medium[1-3]. We examined gastrin expression in 58 cases of human colorectal carcinoma tissue and adjacent mucosa by immunohistochemical methods and immunomicroscopy to determine the function of gastrin and clinical significance of its expression in colorectal carcinoma occurrence and development.
Surgically resected specimens were from 58 colorectal cancer cases, including 25 colon cancer cases and 33 rectal cancer cases. Carcinoma tissues and their adjacent mucosal tissues were resected separately, fixed in 10% neutral formalin, embedded in paraffin and sectioned into three slices. Hematoxylin and eosin (HE), high iron diamine-alcian blue (HID-AB), and immunohistochemical staining was performed. (1) HID-AB staining. Sections were dewaxed with dimethylbenzene, dehydrated in alcohol, stained in high iron diamine for 6 h, washed with distilled water, soaked with 1% Alcian blue, 3% acetic aqueous solution for 15 min, dehydrated with anhydrous alcohol, and fixed in xylene. The Filipe and Greaves[4] standard was used for scoring transitional mucosa (TM). (2) Immunohistochemical staining of gastrin by the ABC method. A rabbit anti-human gastrin antibody and all ABC agents were purchased from DAKO Company, and the staining was performed according to the manufacturer’s protocol. Normal gastroantrum mucosa served as a positive control. PBS and normal rabbit serum served as negative controls. The positive judging standard was used as follows: Weak positive (+) tissue had less than 20% of cells that were weakly brown in color with individual brown cells, positive (+++) tissue contained more than 60% of cells stained brown or dark brown with a few weakly stained brown cells, and moderately positive (++) tissue stained tissue in a range between the weak positive and strong positive tissues.
In the five specimens observed by electron microscopy, three of the cases were considered immunohistochemically strong positive (+++) for gastrin expression, one was moderately positive (++) and one was weakly positive (+). According to Ducke′s tumor stages, three cases were stage B and two were stage C. Specimens were acquired from fresh material within one minute of removal and were prefixed in 2.5% glutaraldehyde. Specimens were then fixed with osmic acid, washed with 0.1 mol/L PBS, and embedded in Epon 812. For immuno-gold staining (IGS), the staphylococcus Protein A gold (PAG) method was used as follows: Tissues were incubated in 1% ovalbumin for ten minutes, followed by incubation with a rabbit anti human gastrin antibody (1:100) for two hours. Samples were washed with 0.05 mol/L TBS, and 0.02 mol/L TBS for five minutes each, followed by incubation in 1% ovalbumin for ten minutes, PAG (1:7) for 2 h, 0.02 mol/L-TBS for five minutes, and 0.05 mol/L TBS. Samples were transiently stained with uranium acetate and plumbum citrate, followed by observation on a JEM-100s electron microscope. Normal gastroantrum mucosa served as a positive control. TBS and normal rabbit immunoserum served as negative controls.
A χ2 test and Ridit analysis were performed, with 95%CI, α = 0.050.
We detected non-adenoma and atypical hyperplasia in 29 adjacent mucosa from the 58 colorectal carcinoma cases. Fifty-six out of 58 cases were positive for salivary mucin (blue). The transitional mucosa limits were 0.5-6.7 cm, with a mean of 2.12 cm. We acquired 28 specimens from the distal remnant adjacent to the cancer more than 15 cm away from the neoplasm border. Light microscopic examination was normal, and sulfomucin staining by HID-AB reaction (dark brown) looked comparable to normal mucosa.
Table 1 demonstrates the relationship between the rate of positive gastrin staining in colorectal carcinoma tissue and tumor differentiation, but gastrin expression did not correlate to histological type or Ducke’s tumor stage (P > 0.5). There was no relationship between gastrin expression in the atypical proliferative mucosa and the transitional mucosa, and tumor differentiation, pathological stage or tumor type (P > 0.5). The relationship between gastrin expression in the adjacent transitional mucosa, atypical hyperplasia and colorectal carcinoma tissues was statistically significant (P < 0.05). A total of 28 normal colorectal mucosal cases were negative for gastrin expression.
| Group | Colorectal carcinoma | Atypical dysplasia | Transitional mucosa | |||
| n | Positive (%) | n | Positive (%) | n | Positive (%) | |
| Histological type | ||||||
| Tubular | 47 | 29 (61.5) | 24 | 12 (50.0) | 45 | 16 (35.6) |
| Nipple | 5 | 3 (60.0) | 2 | 1 (50.0) | 5 | 2 (40.0) |
| Mucinous | 6 | 3 (50.0) | 3 | 1 (33.3) | 6 | 2 (33.3) |
| Differentiation | ||||||
| Mild | 12 | 11 (91.7) | 7 | 3 (42.8) | 12 | 5 (41.7) |
| Moderate | 34 | 18 (52.9) | 17 | 9 (52.9) | 32 | 11 (34.4) |
| Low | 6 | 3 (50.0) | 2 | 1 (50.0) | 6 | 2 (33.3) |
| Ducke′s stages | ||||||
| A | 27 | 16 (59.2) | 13 | 6 (46.2) | 26 | 10 (38.5) |
| B | 10 | 6 (60.0) | 4 | 2 (50.0) | 10 | 4 (40.0) |
| C | 21 | 13 (61.9) | 12 | 6 (50.0) | 20 | 6 (30.0) |
| Total | 58 | 35 (60.3) | 29 | 14 (48.3) | 56 | 20 (35.1) |
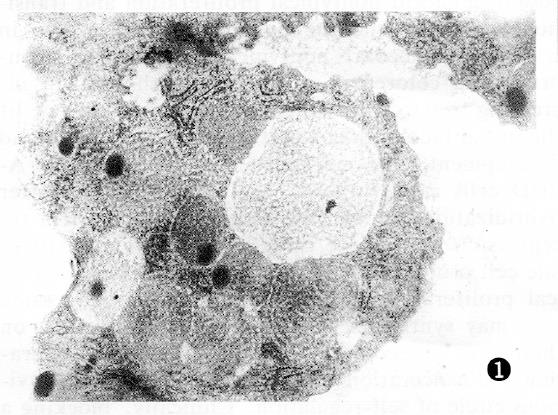
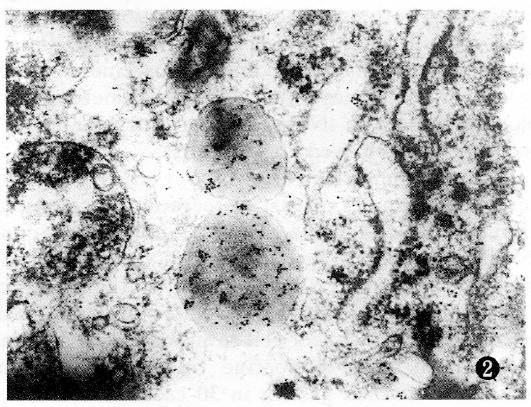
Colorectal cancer cells were polygonal in shape, with unusual variably sized nucleoli concentrated on the edge. The cytoplasm contained many vacuolated mitochondria, and the cells contained secretion granules 400-1500 nm in diameter with a clear membrane border. There were two types of granular appearance: Type A was largest in bulk size, with well distributed low electro density, and the granular core appeared loose; type B was smaller in bulk size, with well distributed high electro density, and the nucleus was usually compact. PAG-positive granules partially localized to secretory granules. PAG-positive granules in highly differentiated cancers primarily localized to type A secretory granules (Figure 1). PAG-positive granules in low differentiated cancer primarily localized to type B secretory granules (Figure 2). PAG granules partially localized to the inside and outside of the cancer cell membrane and microvillus membrane.
Studies have shown that colorectal cancer cells synthesize and secrete gastrin[5,6], and our study further confirmed gastrin was expressed in the mucosa of adjacent atypical proliferative and transitional mucosa, and the rate of gastrin-expressing cells in transitional mucosa, atypical proliferation membrane and colorectal cancer tissues showed an increasing trend, suggesting that the gastrin expression is one of the initial factors regulating colorectal cancer occurrence and development. It has been hypothesized that APUD cells synthesize and secrete gastrin after hybridization, allowing for gastrin receptor activity on the cell surface membrane to stimulate cell proliferation, leading to the development of transitional mucosa, atypical proliferation, and ultimately cancer development. The cancer cells can synthesize and secrete gastrin to activate gastrin receptors and stimulate cell proliferation and cancer formation in a positive feedback loop. Inhibition of the positive feedback loop could inhibit neoplasm growth. This may open a way for the treatment of colorectal carcinoma by using endocrine therapy. Kaneyma et al[7] treated colon carcinoma metastasis to the liver by using gastrin receptor antagonists and achieved good results.
Immunoelectron microscopy revealed that there were gastrin-containing secretory granules in colorectal carcinoma cells, and the gastrin granules were adsorbed into the cancer cell membrane and the inside and outside of the microvillus, and to the carcinoma interstice. These findings provide a morphological basis for colorectal cancer cell production of gastrin, and a theoretical basis for endocrine treatment. The authors examined serum gastrin levels in 30 colorectal cancer cases and found that in some patients, serum gastrin levels were increased, especially in the cases of well-differentiated cancer[8]. However, the mechanism is not yet clear. Our study indicated that gastrin localized to type A granules in highly differentiated adenocarcinoma and to type B granules in poorly differentiated adenocarcinomas. G17-positive granules were of type A, and G34-positive granules were of type B. G17 had higher bioactivity than G34, consistent with the above mentioned mechanism.
Gastrin was expressed in 91.7% of highly differentiated cancers, which was higher than the middle and low differentiated adenocarcinoma cases (P < 0.05). Middle and low differentiated adenocarcinomas have been shown to produce mutated gastrin mRNA, as described by Singh, which leads to insufficient levels of mature gastrin[9]. Thus, the positive expression of gastrin in colorectal carcinoma tissues may serve as a marker of well differentiated cancer. Cases with positive expression in colorectal carcinoma tissues and its adjacent mucosa may be good candidates for treatment with a gastrin antagonist.
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Liu WX
| 1. | Baldwin GS, Zhang QX. Measurement of gastrin and transforming growth factor alpha messenger RNA levels in colonic carcinoma cell lines by quantitative polymerase chain reaction. Cancer Res. 1992;52:2261-2267. [PubMed] |
| 2. | Van Solinge WW, Nielsen FC, Friis-Hansen L, Falkmer UG, Rehfeld JF. Expression but incomplete maturation of progastrin in colorectal carcinomas. Gastroenterology. 1993;104:1099-1107. [PubMed] |
| 3. | Xu Z, Dai B, Dhruva B, Singh P. Gastrin gene expression in human colon cancer cells measured by a simple competitive PCR method. Life Sci. 1994;54:671-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Wang Q, Kao H, Wang Y, Chen YL, He J. The immunohisochemical study of carcinoembryonic antigen in the transitional mucosa adjacent to colorectal cancer. Zhonghua Xiaohua Zazhi. 1990;10:25-27. |
| 5. | Finley GG, Koski RA, Melhem MF, Pipas JM, Meisler AI. Expression of the gastrin gene in the normal human colon and colorectal adenocarcinoma. Cancer Res. 1993;53:2919-2926. [PubMed] |
| 6. | Zhu JW, Chen YQ. Gastrin and neoplasms. Zhongguo Putong Waike Zazhi. 1996;11:7-10. |
| 7. | Kameyama M, Nakamori S, Imaoka S, Yasuda T, Nakano H, Ohigashi H, Hiratsuka M, Sasaki Y, Kabuto T, Ishikawa O. [Adjuvant chemo-endocrine chemotherapy with gastrin antagonist after resection of liver metastasis in colorectal cancer]. Gan To Kagaku Ryoho. 1994;21:2169-2171. [PubMed] |
| 8. | Jiang CP, Shen HX, Chen YQ, Chen RX. The changes of serum gastrin level in 60 cases with gastrointestinal tumors. Jiangsu Yiyao. 1994;20:73-74. |
| 9. | Singh P, Xu Z, Dai B, Rajaraman S, Rubin N, Dhruva B. Incomplete processing of progastrin expressed by human colon cancer cells: role of noncarboxyamidated gastrins. Am J Physiol. 1994;266:G459-G468. [PubMed] |