Published online Mar 7, 2023. doi: 10.3748/wjg.v29.i9.1509
Peer-review started: November 27, 2022
First decision: December 27, 2022
Revised: January 1, 2023
Accepted: February 15, 2023
Article in press: February 15, 2023
Published online: March 7, 2023
Processing time: 100 Days and 15.2 Hours
Small bowel obstruction (SBO) still imposes a substantial burden on the health care system. Traditional evaluation systems for SBO outcomes only focus on a single element. The comprehensive evaluation of outcomes for patients with SBO remains poorly studied. Early intensive clinical care would effectively improve the short-term outcomes for SBO, however, the full spectrum of the potential risk status regarding the high complication-cost burden is undetermined.
We aim to construct a novel system for the evaluation of SBO outcomes and the identification of potential risk status.
Patients who were diagnosed with SBO were enrolled and stratified into the simple SBO (SiBO) group and the strangulated SBO (StBO) group. A principal component (PC) analysis was applied for data simplification and the extraction of patient characteristics, followed by separation of the high PC score group and the low PC score group. We identified independent risk status on admission via a binary logistic regression and then constructed predictive models for worsened management outcomes. Receiver operating characteristic curves were drawn, and the areas under the curve (AUCs) were calculated to assess the effectiveness of the predictive models.
Of the 281 patients, 45 patients (16.0%) were found to have StBO, whereas 236 patients (84.0%) had SiBO. Regarding standardized length of stay (LOS), total hospital cost and the presence of severe adverse events (SAEs), a novel principal component was extracted (PC score = 0.429 × LOS + 0.444 × total hospital cost + 0.291 × SAE). In the multivariate analysis, risk statuses related to poor results for SiBO patients, including a low lymphocyte to monocyte ratio (OR = 0.656), radiological features of a lack of small bowel feces signs (OR = 0.316) and mural thickening (OR = 1.338), were identified as risk factors. For the StBO group, higher BUN levels (OR = 1.478) and lower lymphocytes levels (OR = 0.071) were observed. The AUCs of the predictive models for poor outcomes were 0.715 (95%CI: 0.635-0.795) and 0.874 (95%CI: 0.762-0.986) for SiBO and StBO stratification, respectively.
The novel PC indicator provided a comprehensive scoring system for evaluating SBO outcomes on the foundation of complication-cost burden. According to the relative risk factors, early tailored intervention would improve the short-term outcomes.
Core Tip: A novel outcome indicator based on the standardized length of stay, total hospital cost and the presence of severe adverse events provided a comprehensive system for evaluating small bowel obstruction (SBO) outcomes. Furthermore, risk statuses associated with poor results were identified; specifically, for simple SBO patients, a low lymphocyte to monocyte ratio, as well as radiological features of a lack of small bowel feces signs and mural thickening, should be noticeable. For the strangulated SBO group, higher blood urea nitrogen levels and lower lymphocytes levels were recognized. Accordingly, early clinical intensive care was applicable for outcome improvement.
- Citation: Xu WX, Zhong QH, Cai Y, Zhan CH, Chen S, Wang H, Tu PS, Chen WX, Chen XQ, Zhang JR. Comprehensively evaluate the short outcome of small bowel obstruction: A novel medical-economic score system. World J Gastroenterol 2023; 29(9): 1509-1522
- URL: https://www.wjgnet.com/1007-9327/full/v29/i9/1509.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i9.1509
Small bowel obstructions (SBO) result in over 300000 hospitalizations per year in the United States[1]. With the increasing public health burden, the average cost for SBOs ranges from $30000-$38000 individually, and the total cost for SBOs is estimated to be approximately 9-11.4 billion dollars[2,3]. Recently, the short outcomes of SBO were evaluated by using in-hospital mortality, major complications and the length of hospital stay[3-6]. There is still lack of an integrative medical-economic system to evaluate the overall outcomes for SBO, even though previous studies have confirmed the relationship between worse outcomes and higher hospital costs[7,8]. Furthermore, the question of how to comprehensively evaluate outcomes for patients with SBO remains uncharted.
Principal component analysis (PCA) is commonly used for dimension reduction[9,10], linear correlation resolution and data simplification. By summarizing and maximizing the information encoding a set of outcome variables, a novel principal component for evaluating the clinical and economic effects on SBO is available. For SBO, patients’ statuses on admission, including longer pain duration, acute kidney injury and malnutrition, were found to be closely correlated with severe adverse events (SAEs), based on previous studies[3,5,7,11]. However, the risk factors for the integrative scoring system, including clinical and economic adverse events, have not been extensively evaluated. The method of how to fully evaluate the potential risk status regarding the high complication-cost burden is urgently needed.
As an urgent life-threatening problem, the physical status of strangulated SBO is considerably deteriorating[12-14]. To control this confounding factor[15,16] and to further identify the risk admission status, we divided patients into a simple bowel obstruction group and a strangulated bowel obstruction group for the stratification analysis. We also constructed a novel indicator combining standardized SAEs, length of stay (LOS) and total hospital cost for defining outcomes of SBO. Furthermore, we established a representative model to distinguish high-risk statuses for both the simple small bowel obstruction (SiBO) and strangulated small bowel obstruction (StBO) groups to guide clinical intensive care for SBO.
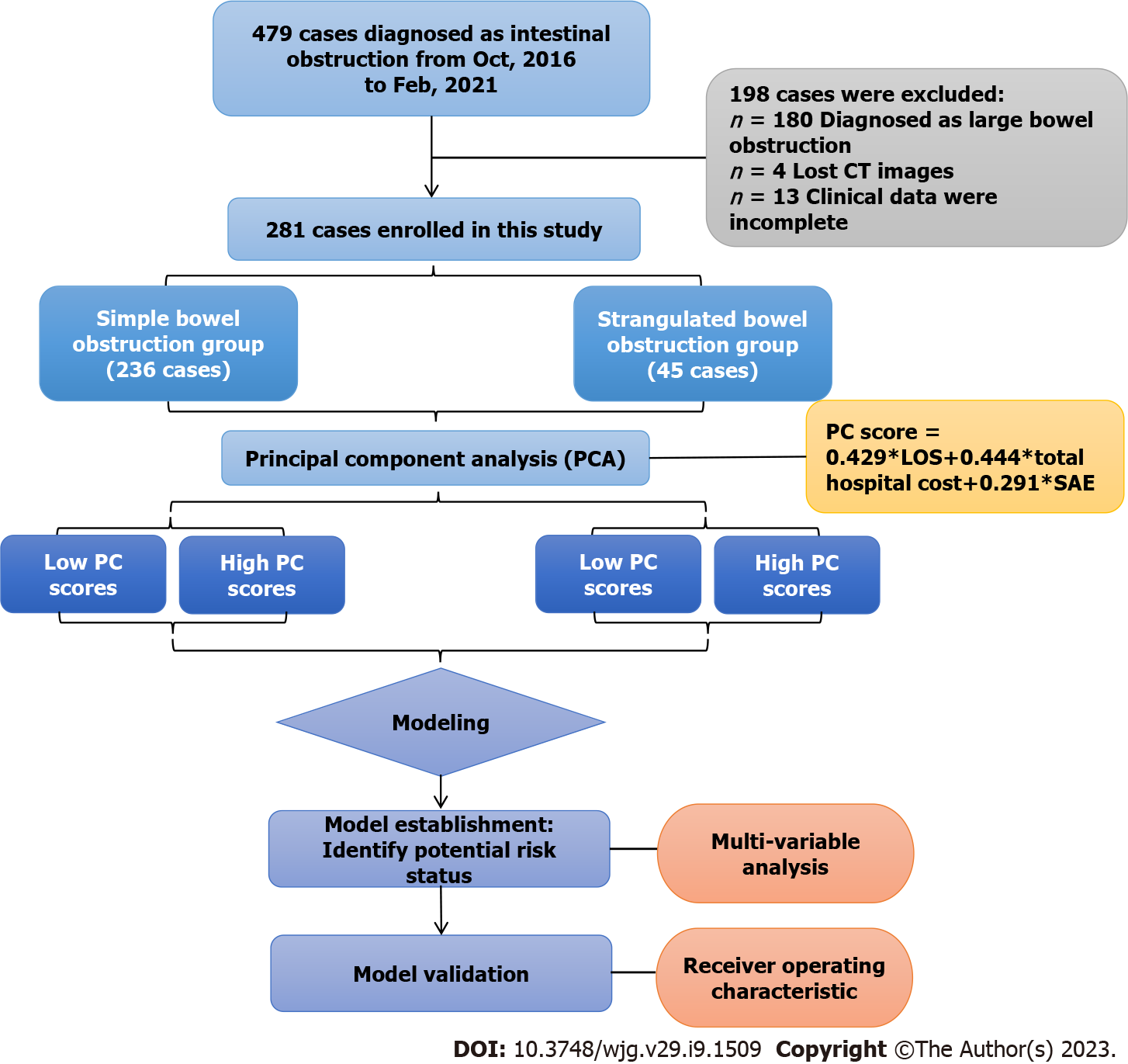
From October 2016 to February 2021, 479 patients diagnosed with intestinal obstructions at Fujian Medical University Union Hospital were included in the study. After excluding 180 cases with large bowel obstructions, 4 cases with missing computed tomography (CT) images and 13 cases with incomplete clinical data, 281 patients were recruited for the final study (shown in Figure 1). The following stratification was made according to the pathological confirmation of intestinal ischemia: A simple bowel obstruction (SiBO, n = 236) group and a strangulated bowel obstruction (StBO, n = 45) group. For patients without acute peritonitis, conservative treatment was applied. Once patients with highly suspect of bowel ischemia or failure to conservative treatment, laparoscopy as well as laparotomy was adopted for SBO patients according to different intrabdominal pressures (shown in Table 1). The study protocol was approved by the Institutional Review Board of Fujian Medical University Union Hospital (Approval No. 2021YF005-02), and all of the patients provided written informed consent for the procedure.
| Characteristics | Simple obstruction (n = 236) | P value | Strangulated obstruction (n = 45) | P value | ||
| Low PC score | High PC score | Low PC score | High PC score | |||
| Baseline data | ||||||
| Gender, n (%) | 1.0001 | 0.4212 | ||||
| Male | 117 (69.2%) | 39 (69.6%) | 18 (52.9%) | 8 (72.7%) | ||
| Female | 52 (30.8%) | 17 (30.4%) | 16 (47.1%) | 3 (27.3%) | ||
| Age (yr) | 60 (47, 69) | 65 (53, 71) | 0.081 | 63 (52.25, 70.00) | (61.0, 71.5) | 0.321 |
| BMI (kg/m2) | 20.70 (18.83, 22.98) | 20.94 (18.21, 22.65) | 0.196 | 20.20 (18.16, 22.00) | 18.75 (17.72, 19.81) | 0.228 |
| Comorbidity, n (%) | 0.2451 | 1.0002 | ||||
| None | 128 (75.7%) | 38 (67.9%) | 27 (79.4%) | 9 (81.8%) | ||
| Yes | 41 (24.3%) | 18 (32.1%) | 7 (20.6%) | 2 (18.2%) | ||
| Pain duration (d) | 2 (1, 5) | 6 (3, 12.5) | < 0.000 | 2.00 (1.00, 3.75) | 2.0 (1.0, 4.0) | 0.989 |
| History of abdominal operation, n (%) | 0.4711 | 0.6032 | ||||
| None | 43 (25.4%) | 17 (30.4%) | 11 (32.4%) | 2 (18.2%) | ||
| Yes | 126 (74.6) | 39 (69.6%) | 23 (67.6%) | 9 (81.8%) | ||
| Temperature (degrees Celsius) | 36.6 (36.5, 36.8) | 36.6 (36.5, 36.8) | 0.401 | 36.6 (36.5, 36.8) | 36.60 (36.50, 36.75) | 0.956 |
| CT characteristics | ||||||
| Mesenteric fluid (%) | 0.430 | 0.9852 | ||||
| None | 32 (18.9%) | 8 (14.3%) | 1 (2.9%) | 1 (9.1%) | ||
| Yes | 137 (81.1%) | 48 (85.7%) | 33 (97.1%) | 10 (90.9%) | ||
| Ascites (%) | 0.849 | 1.0002 | ||||
| None | 58 (34.3%) | 20 (35.7%) | 4 (11.8%) | 1 (9.1%) | ||
| Yes | 111 (65.7%) | 36 (64.3%) | 30 (88.2%) | 10 (90.9%) | ||
| Spiral signs (%) | 0.6122 | 0.4362 | ||||
| None | 151 (89.3%) | 52 (92.9%) | 22 (64.7%) | 5 (45.5%) | ||
| Yes | 18 (10.7%) | 4 (7.1%) | 12 (35.3%) | 6 (54.5%) | ||
| Concentric circle sign (%) | 0.1322 | 0.7452 | ||||
| None | 164 (97.0%) | 51 (91.1%) | 31 (91.2%) | 11 (100%) | ||
| Yes | 5 (3.0%) | 5 (8.9%) | 3 (8.8%) | 0 (0%) | ||
| Small bowel feces sign (%) | 0.006 | 1.0002 | ||||
| None | 70 (41.4%) | 35 (62.5%) | 17 (50.0%) | 5 (45.5%) | ||
| Yes | 99 (58.6%) | 21 (37.5%) | 17 (50.0%) | 5 (54.5%) | ||
| Mural thickness (median) | 3.28 (2.30, 3.75) | 3.63 (2.97, 4.53) | 0.002 | 3.51 (3.16, 4.12) | 3.42 (2.67, 4.07) | 0.634 |
| Laboratory data | ||||||
| WBC (109/L) | 6.770 (4.89, 9.52) | 7.345 (4.87, 11.18) | 0.387 | 8.70 (5.89, 12.23) | 6.83 (10.01, 18.25) | 0.384 |
| NE% | 75.50 (65.9, 83.3) | 77.45 (68.5, 84.03) | 0.422 | 83.60 (69.05, 86.90) | 77.50 (73.30, 90.25) | 0.853 |
| Lymphocyte (109/L) | 1.01 (0.74, 1.42) | 0.94 (0.64, 1.34) | 0.240 | 0.96 (0.65, 1.34) | 0.60 (0.42, 0.76) | 0.020 |
| Monocyte (109/L) | 0.420 (0.30, 0.58) | 0.565 (0.34, 0.73) | 0.011 | 0.570 (0.407, 0.735) | 0.540 (0.33, 0.760) | 0.721 |
| NLR (ratio) | 4.650 (3.03, 8.07) | 6.115 (3.74, 9.30) | 0.159 | 7.750 (4.085, 12.922) | 9.030 (4.990, 15.565) | 0.491 |
| LMR (ratio) | 2.286 (1.67, 3.42) | 1.591 (1.13, 2.84) | 0.002 | 1.681 (2.131, 1.141) | 1.482 (0.957, 1.933) | 0.459 |
| Hb (g/L) | 128.0 (115, 142) | 120.5 (108, 133) | 0.016 | 131.0 (110.0, 137.7) | 129.0 (120.5, 145.0) | 0.587 |
| PLT (109/L) | 205.5 (162.50, 250.75) | 250.5 (180.25, 307.25) | 0.002 | 213 (163, 260) | 180.0 (152.0, 242.5) | 0.256 |
| Albumin (g/L) | 35.9 (32.30, 40.45) | 36.1 (31.80, 39.45) | 0.403 | 34.6 (31.7, 39.6) | 37.1 (28.6, 42.0) | 0.977 |
| ALT (U/L) | 16 (11, 24) | 16 (11, 22) | 0.727 | 15.00 (12.00, 21.75) | 15.00 (13.25, 27.75) | 0.612 |
| AST (U/L) | 20 (16, 26) | 21 (17, 25.5) | 0.619 | 19.50 (17.00, 23.75) | 36.50 (20.75, 45.25) | 0.022 |
| Ca (mmol/L) | 2.19 (2.04,2.32) | 2.15 (2.02,2.26) | 0.152 | 2.19 (2.09, 2.31) | 2.05 (1.95, 2.20) | 0.062 |
| Cl (mmol/L) | 102.30 (100.0, 104.1) | 100.15 (96.85, 104.03) | 0.015 | 100.85 (98.13, 103.85) | 102.00 (101.35, 104.15) | 0.296 |
| K (mmol/L) | 4.035 (3.78, 4.34) | 3.985 (3.74, 4.43) | 0.957 | 4.00 (3.56, 4.31) | 4.36 (3.62, 5.07) | 0.290 |
| Na (mmol/L) | 138.40 (136.68, 140.48) | 138.15 (135.50, 141.23) | 0.533 | 138.05 (134.13, 140.30) | 135.60 (134.75, 137.75) | 0.334 |
| BUN (mmol/L) | 5.5 (4.3, 7.2) | 5.4 (3.68, 8.23) | 0.872 | 6.45 (4.00, 8.57) | 10.6 (7.3, 15.3) | 0.002 |
| Glu (mmol/L) | 6.78 (5.30, 8.67) | 6.59 (5.16, 9.51) | 0.515 | 8.165 (6.963, 9.300) | 8.66 (6.78, 11.04) | 0.428 |
| PT (s) | 13.6 (13.1, 14.3) | 13.6 (13.28, 14.43) | 0.825 | 13.45 (12.90, 13.90) | 15.40 (14.20, 16.65) | 0.004 |
| APTT (s) | 35.6 (33.3, 38.3) | 36.1 (34.0, 40.9) | 0.184 | 35.15 (32.18, 37.10) | 41.6 (36.1, 45.0) | 0.012 |
| DDI (mg/L) | 1.45 (0.71, 2.52) | 1.94 (0.79, 4.67) | 0.151 | 1.64 (0.88, 3.50) | 5.75 (2.39, 6.72) | 0.024 |
| Fib (g/L) | 3.49 (2.92, 4.37) | 3.78 (3.25, 4.59) | 0.150 | 3.69 (2.71, 4.60) | 3.89 (3.17, 4.82) | 0.548 |
| Creatinine (umol/L) | 70.0 (56, 81) | 70.5 (54.75, 88.00) | 0.512 | 67 (57, 76) | 93 (80, 147) | 0.003 |
| Management | < 0.0002 | 0.2152 | ||||
| Conservative treatment | 155 (91.7%) | 17 (30.4%) | 1 (2.9%) | 0 (0%) | ||
| Laparoscopy | 11 (6.5%) | 8 (14.3%) | 8 (23.5%) | 0 (0%) | ||
| Laparotomy | 3 (1.8%) | 31 (55.4%) | 25 (73.5%) | 11 (100%) | ||
| CD, n (%) | < 0.0002 | < 0.0002 | ||||
| Grade I | 141 (83.4%) | 20 (35.7%) | 5 (14.7%) | 0 (0%) | ||
| Grade II | 28 (16.6%) | 32 (57.1%) | 28 (82.4%) | 2 (18.2%) | ||
| Grade III | 0 (0%) | 1 (1.8%) | 1 (2.9%) | 0 (0%) | ||
| Grade IV | 0 (0%) | 3 (5.4%) | 0 (0%) | 9 (81.8%) | ||
| Grade V | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| SAE, n (%) | 0.0182 | < 0.0002 | ||||
| None | 169 (100%) | 53 (94.6%) | 34 (100%) | 2 (18.2%) | ||
| Yes | 0 (0%) | 3 (5.4%) | 0 (0%) | 9 (81.8%) | ||
| Fee (¥) | 12070 (8830, 19935) | 54322 (41370, 74623) | < 0.000 | 51828 (33575, 66954) | 83553.0 (74146.0, 142409.5) | < 0.000 |
| Length of stay (d) | 5 (4, 8) | 16 (13.75, 22.50) | < 0.000 | 14.00 (10.25, 17.00) | 28.0 (18.5, 35.5) | < 0.000 |
All of the patients with suspected SBO underwent CT scans before receiving treatment. The features of the CT scans that were recorded in this study were separated into mesenteric fluid, ascites, spiral signs, concentric circle signs, small bowel feces signs and edema of the bowel wall categories[17-20]. All of the CT scan images were cross-reviewed and evaluated by two senior general surgeons (Chen XQ and Zhang JR, and both surgeons had abundant experience in abdominal emergency surgery. The definitions of CT characteristics are shown in Supplementary Figure 1 and supplied in Supplementary Table 1[21-25].
Baseline demographics consisted of sex, age, body mass index (BMI), comorbidity, temperature, pain duration and history of abdominal pain. Biochemical parameters, including white blood cell count, neutrophil percentage, lymphocyte concentration, monocyte concentration, hemoglobin concentration, platelet concentration, albumin, alanine aminotransferase, aspartate aminotransferase (AST), calcium concentration, chloride concentration, potassium concentration, sodium concentration, blood urea nitrogen (BUN), serum creatinine, glucose, prothrombin time (PT), activated partial thromboplastin time (APTT), D-dimer (DDI) and fibrinogen, were collected within 24 h of admission. Combinations of inflammatory parameters, such as the neutrophil to lymphocyte ratio and lymphocyte to monocyte ratio (LMR), were calculated and recorded accordingly.
Posttreatment outcomes were both clinically and economically evaluated.
Postoperative complications were defined as any deviation from the normal postoperative course during the index admission for SBO treatment, which was guided by the European Perioperative Clinical Outcome definitions[7,26]. The severity of complications was graded according to the Clavien-Dindo (CD) system[27], which is a validated classification system that categorizes complication severity based on the level of required treatment. Grade I was defined as complications without the need for pharmacological treatment or surgical, endoscopic and radiological interventions, as well as only minor interventions such as vomiting; grade II was defined as complications requiring pharmacological or other treatments, such as blood transfusions and total parenteral nutrition; grade III was defined as complications requiring surgical interventions or other interventional treatments; grade IV was defined as life-threatening complications, including central nervous system, cardiac and pulmonary complications, as well as renal failure and those interventions requiring intensive care unit (ICU) management; and grade V was defined as death. CD grade I to grade III were classified as non-SAE, and CD grade IV to grade V were classified as SAE.
The LOS was defined as the number of days from admission to discharge. Total hospital cost was defined as the total expenditure for medical resource utilization during hospitalizations, which included fees for operations (materials and occupancy of the operating room), medications, radiology, laboratory tests, microbiology tests, ward stay, ICU days, feeding and blood products[28].
PCA was used to achieve data simplification by expressing multivariate outcome indicators with fewer dimensions. With standardized LOS, total hospital cost and the presence of SAEs, a novel principal component was extracted: PC score = 0.429 × LOS + 0.444 × total hospital cost + 0.291 × SAE. Furthermore, the patient population was classified in the following manner according to the quartile PC score: The low PC score group (below the 75% quartile) and the high PC score group (in the upper 75% quartile). This analysis was performed in R V.4.1.3 (R Foundation for Statistical Programming, Vienna, Austria) by using the psych packages.
Categorical variables were compared by using the χ2 test or Fisher’s exact test between the two groups. Data are presented as the mean ± SD or median for continuous variables. Independent t tests or Kruskal-Wallis tests were applied according to the characteristics of the variables. The association of admission status with higher PC scores was evaluated by using univariate logistic regression and summarized with an odds ratio (OR) and 95% confidence interval (CI). After setting the variables with a significance level of P < 0.05 and variance inflation factors < 5, a multivariate logistic regression with “binomial” method was performed, and independent risk factors were determined. We extracted the following risk score formulas based on these independent risk factors: Risk score 1 (RS1) = [0.291 × (bowel wall thickness) - 1.150 × (small bowel feces sign) - 0.421 × (LMR)] and RS2 = [-2.632 × (lymphocyte concentration) + 0.391 × (BUN concentration)] for the SiBO group and StBO group, respectively. Receiver operating characteristic curves and the area under the curve were calculated to assess the accuracy of the models. All of the statistical analyses were performed in R Version.4.1.3. The statistical methods of this study were reviewed by Yin YR.
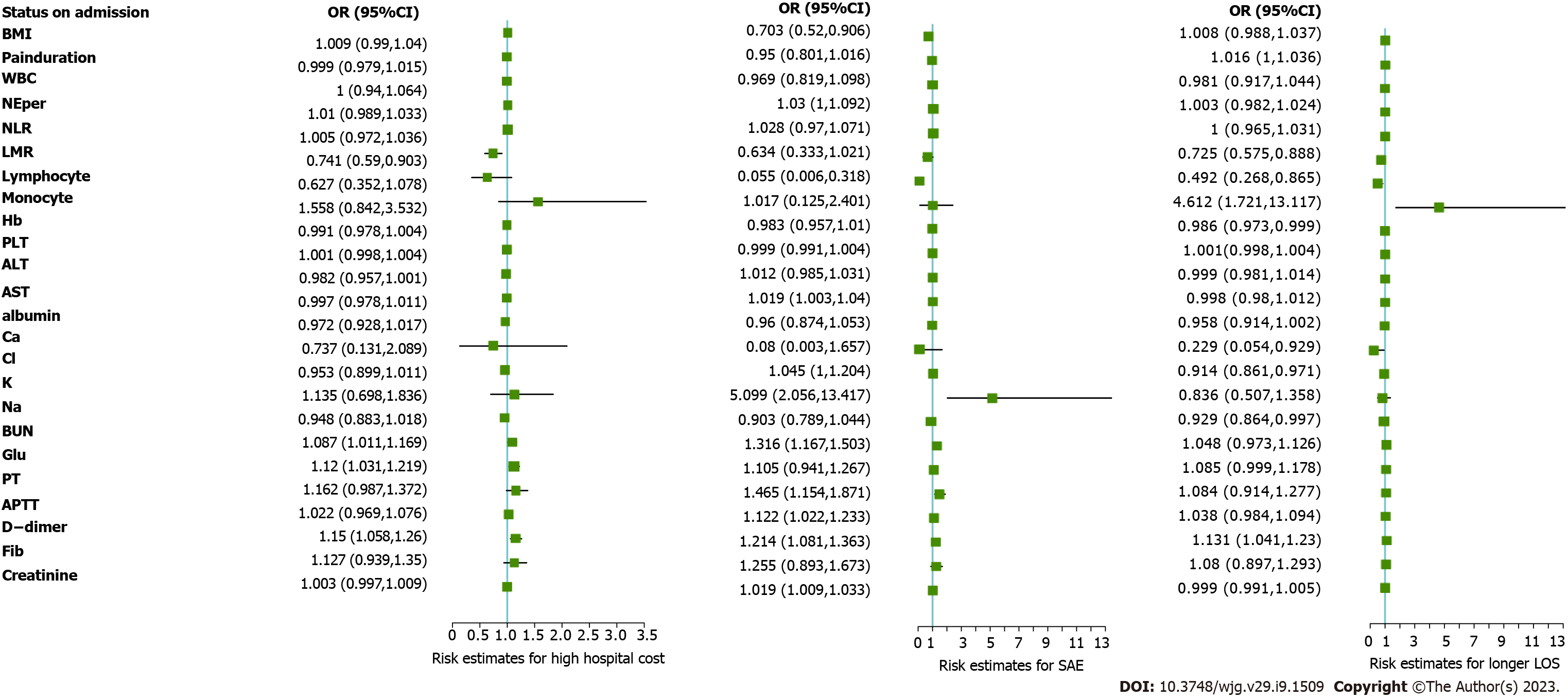
For 281 patients with SBO who were included in this study, posttreatment outcomes were evaluated by LOS, total hospital cost and the presence of SAEs. Via the univariate analysis, admission risk status, including lower LMR (P = 0.005), higher BUN concentration (P = 0.022), higher glucose concentration (P = 0.007) and higher DDI concentration (P = 0.001), was significantly associated with higher hospital costs. Patients with SAE had lower levels of lymphocyte concentration (P = 0.003), higher levels of AST (P = 0.027), higher levels of potassium (P < 0.000), higher levels of BUN (P < 0.000), higher levels of serum creatinine (P < 0.000) and coagulation and fibrinolysis disturbances, including longer PT (P = 0.001), APTT (P = 0.012) and higher levels of DDI (P < 0.000). Furthermore, at admission, lower LMR (P = 0.003), higher monocyte concentration (P = 0.003), lower hemoglobin concentration (P = 0.038), higher level of glucose (P = 0.049), higher level of DDI (P = 0.004) and abnormal electrolyte and metabolic changes, such as lower calcium concentration (P = 0.042), lower chloride concentration (P = 0.003) and lower sodium concentration (P = 0.043), were closely related to a longer LOS (Figure 2 and Supplemen
After maximizing the possible information and variation of the above-mentioned outcome indicators, including total hospital cost, LOS and SAEs, data simplification was performed. Via PCA, one principal component was extracted (Supplementary Figure 2). The PC score was calculated according to weights given to each outcome indicator: PC score = 0.429 × LOS + 0.444 × total hospital cost + 0.291 × SAE (Figure 1).
Of the 281 patients with SBO who were included in this study, 45 patients (16.0%) were found to have StBO, whereas 236 patients (84.0%) were found to have SiBO. The low PC score group (< 75% quartile) and high PC score group (> 75% quartile) were identified according to the quartile PC score. For both the SiBO and StBO groups, no significant difference was observed between the two PC score groups for sex, age, BMI, comorbidity status, temperature or history of abdominal operation (all P values > 0.05, Table 1). For patients with SiBO, a higher PC score was significantly related to longer pain duration (P < 0.000), higher monocyte concentration (P = 0.011), lower LMR (P = 0.002), lower hemoglobin concentration (P = 0.016), lower platelet count (P = 0.002) and low level of chloride (P = 0.015). Through the univariate analysis of radiological characteristics, we determined that a lack of small bowel feces signs and mural thickening were risk factors for a high PC score. In contrast, in the StBO group, low levels of lymphocytes (P = 0.020), high levels of AST (P = 0.022), high levels of BUN (P = 0.002) and coagulation and fibrinolysis disturbances, including abnormal DDI concentrations (P = 0.024), PTs (P = 0.004) and APTTs (P = 0.012), were significantly associated with higher PC scores. None of the risk radiological characteristics were observed in this stratification.
Via the univariate analysis of the admission clinical-laboratory features, we determined potential risk status, including longer pain duration (P = 0.048), higher monocyte concentration (P = 0.003), lower LMR (P = 0.006), lower hemoglobin concentration (P = 0.033), lower platelet count (P = 0.036) and low level of chloride (P = 0.031), as well as radiological characteristics of mural thickening (P = 0.033) and lack of small bowel feces sign (P = 0.006), for high PC scores in the SiBO stratification. Via the multivariate analysis, independent risk factors consisting of radiological findings of small bowel feces sign (OR = 0.316), mural thickening (OR = 1.338) and LMR (OR = 0.656) were identified (all P values < 0.05, Table 2 and Figure 3). For StBO stratification, low levels of lymphocytes (P = 0.038), high levels of AST (P = 0.027), longer PTs (P = 0.015), high levels of BUN (P = 0.004) and creatinine (P = 0.022) seemed to be related to high PC scores. Finally, we found that only lymphocytes (OR = 0.071) and BUN (OR=1.478) were independent risk factors for high PC scores (all P values < 0.05, Table 2 and Figure 3).
| Characteristics | Simple obstruction (n = 236) | Strangulated obstruction (n = 45) | ||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| Pain duration (d) | 1.019 (1.002, 1.041) | 0.0481 | ||||||
| Small bowel feces sign (+)/(-) | 0.424 (0.225, 0.783) | 0.0061 | 0.316 (0.158, 0.612) | < 0.0001 | ||||
| Mural thickening (cm) | 2.119 (1.084, 4.375) | 0.0331 | 1.338 (1.098, 1.664) | 0.0031 | ||||
| Lymphocyte (109/L) | 0.097 (0.007, 0.665) | 0.0381 | 0.071 (0.003, 0.539) | 0.0331 | ||||
| Monocyte (109/L) | 5.472 (1.809, 17.780) | 0.0031 | ||||||
| LMR (ratio) | 0.708 (0.541, 0.891) | 0.0061 | 0.656 (0.496, 0.836) | 0.0011 | ||||
| Hb (g/L) | 0.983 (0.969, 0.998) | 0.0331 | ||||||
| PLT (109/L) | 1.003 (1.001, 1.007) | 0.0361 | ||||||
| AST (U/L) | 1.075 (1.018, 1.156) | 0.0271 | ||||||
| Cl (mmol/L) | 0.931 (0.871, 0.993) | 0.0311 | ||||||
| BUN (mmol/L) | 1.383 (1.133, 1.786) | 0.0041 | 1.478 (1.169, 2.061) | 0.0041 | ||||
| PT (s) | 1.568 (1.141, 2.418) | 0.0151 | ||||||
| APTT (s) | 1.109 (0.999, 1.264) | 0.076 | ||||||
| DDI (mg/L) | 1.196 (1.006, 1.513) | 0.067 | ||||||
| Creatinine (umol/L) | 1.034 (1.011, 1.071) | 0.0221 | ||||||
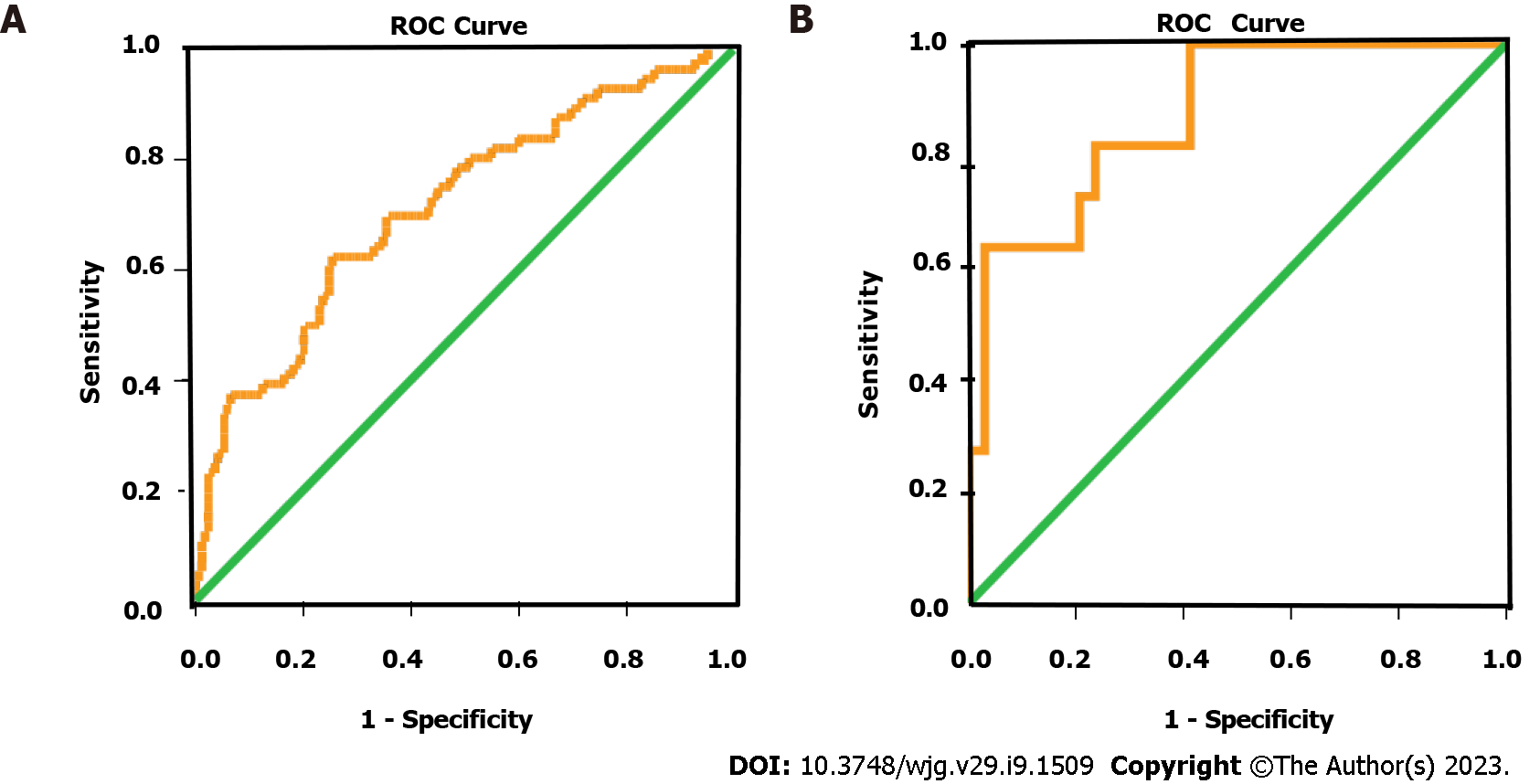
Based on the regression coefficient for each factor, we calculated risk scores and built prediction models for worse outcomes: RS1 = [0.291 × (bowel wall thickness) - 1.150 × (small bowel feces sign) - 0.421 × (LMR)] for the SiBO group and RS2 = [-2.632 × (lymphocyte concentration) + 0.391 × (BUN concentration)] for the StBO group. Furthermore, receiver operating characteristic curves were drawn with areas under the curve of 0.715 (95%CI: 0.635-0.795) and 0.874 (95%CI: 0.762-0.986) for the SiBO and StBO stratifications, respectively (Figure 4).
Given that approximately 9-11.4 billion dollars are the costs per year in the United States, SBO still imposes a substantial burden on the health care system[2]. In contrast to the traditional evaluation systems that only focus on a single element[3-6], in this study, the standardized LOS, total hospital cost and the presence of SAEs were considered as integrative systems to evaluate the clinical-economic outcomes of SBO via PCA[9]. Previous studies have confirmed the close relationship between patients’ statuses on admission (including longer pain duration, acute kidney injury and malnutrition) and adverse outcomes, which provides a potential target for improving outcomes[3,5,7,11]. Commonly, severe statuses, including severe inflammatory reactions, electrolyte disturbances and hemostatic abnormalities, tend to occur in strangulated bowel obstruction[22]. Following the formula that assigned weights to each component, we determined PC score = 0.429 × LOS + 0.444 × total hospital cost + 0.291 × SAE; thus, the posttreatment outcome of SBO could be calculated and precisely evaluated (Figure 1).
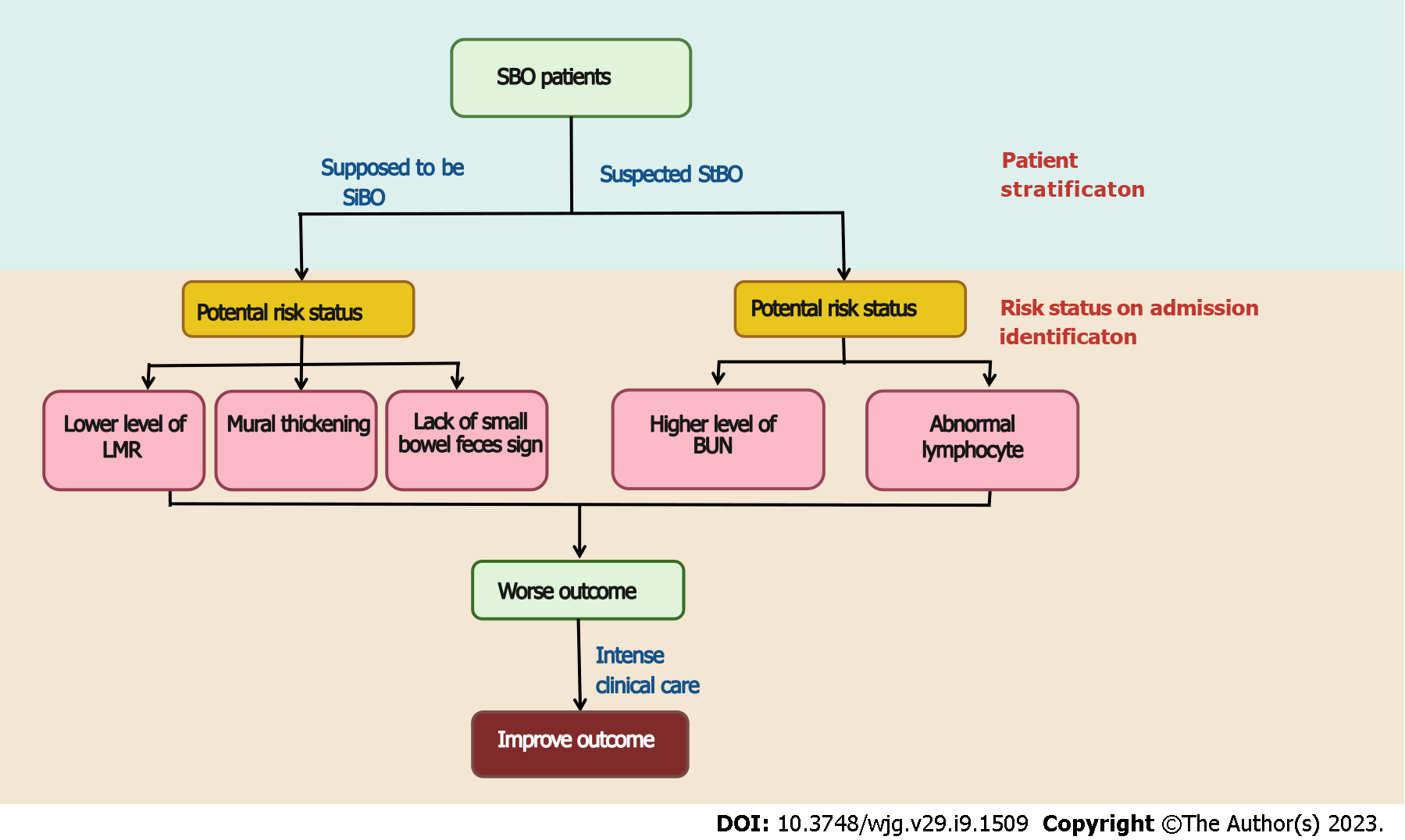
For people with SiBO, only low LMR is observed, as radiological features (such as a lack of small bowel feces signs and mural thickening) were independent risk factors for high PC scores via the multivariate analysis. The area under the curve (AUC) of the predictive model based on the comprehensive scores for SiBO was 0.715 (95%CI: 0.635-0.795). As acute intestinal failure accompanies the obstructive bowel[26], when mechanical obstruction develops, the bowel lumen dilates along with the accumulation of air and intestinal fluid; thus, enteric stasis initiates bacterial proliferation with the intestinal gas produced by the fermentation of ingested food[22]. Conversely, when obstruction is incomplete or mild, the lasting bowel absorptive function can allow for fluid reabsorption across the bowel wall, thus leading to the small bowel feces sign as an independent protective factor for SBO[18,29]. Furthermore, progressive bowel dilation accompanied by compromised venous reflux increases intramural tension, which causes mural edema, secondary intestinal absorptive dysfunction and the loss of mucosal integrity (both functionally and physically)[22,30]. Similarly, as a potential effect on decreasing mural edema, the use of gastrografin challenge has been identified as the standardized management for SBO[31,32]. Moreover, in this study, the LMR was much lower in the high PC group, which may be due to the immune system becoming weakened as a result of the underlying malnutrition, as well as an excessive compensatory anti-inflammatory response[33-37].
Once SiBO deteriorated into StBO, the risk factors were dynamically changed. None of the radiological characteristics were found to be related to the outcomes. In particular, coagulation and fibrinolysis disturbances (including abnormal DDI, PT and APTT), kidney injury (such as increasing BUN and creatinine levels) and relevant lymphocytes were confirmed as being risk factors. Finally, only BUN and lower lymphocyte counts were identified as being independent risk factors for high PC. Partially due to the impaired mucosal barriers[22,38], lactic acid from intestinal anaerobic glycolysis gradually accumulates, which adversely deteriorates renal function with increasing levels of BUN in the peripheral blood[39]. Similarly, it is difficult to correct conventional enteral interventions and intestinal mucosal malnutrition due to the weakened immune status[33,40], which may explain why a lower level of lymphocytes is a risk factor for poorer outcomes. The predictive model for StBO yielded an AUC of 0.874 (95%CI: 0.762-0.986), which provided an excellent differentiating ability.
There were a few limitations to the present study. Primarily, this was a retrospective study conducted in a single center. In addition, the sample size of the initial models was relatively small. However, in both group (SiBO or StBO) the patients evaluated were consecutively enrolled and this could reproduce a real-world situation. Adequately powered and well-designed studies are required to confirm these findings and to establish causality.
The novel PC indicator provided a comprehensive scoring system for evaluating SBO outcomes on the foundation of complication-cost burden. According to the relative risk factors, early tailored intervention would improve the short-term outcomes.
Small bowel obstruction (SBO) still imposes a substantial burden on the health care system. Traditional evaluation systems for SBO outcomes only focus on a single element. There is still lack of an integrative medical-economic system to evaluate the overall outcomes for SBO. Moreover, patients’ statuses on admission, including longer pain duration, acute kidney injury and malnutrition, were found to be closely correlated with severe adverse events (SAEs). However, the risk factors for the integrative scoring system, including clinical and economic adverse events, have not been extensively evaluated.
SBO still imposes a substantial burden on the health care system. Traditional evaluation systems for SBO outcomes only focus on a single element. The comprehensive evaluation of outcomes for patients with SBO remains poorly studied. Early intensive clinical care would effectively improve the short-term outcomes for SBO, however, the full spectrum of the potential risk status regarding the high complication-cost burden is undetermined.
In this study, we aim to construct a novel indicator combining standardized SAEs, length of stay (LOS) and total hospital cost for defining outcomes of SBO. Furthermore, we established a representative model for distinguishing high-risk statuses on admission for the simple SBO (SiBO) or strangulated SBO (StBO) groups. Given that SBO still imposes a substantial burden on the health care system, we believe our findings will provide a new insight for comprehensively evaluation outcomes of SBO as well as a guideline for early intervention.
In this study, we evaluated posttreatment outcomes of SBO both clinically and economically. Principal component analysis (PCA) was used to achieve data simplification by expressing multivariate outcome indicators with fewer dimensions. By summarizing and maximizing the information encoding in standardized LOS, total hospital cost and the presence of SAEs, a novel principal component was extracted: PC score = 0.429 × LOS + 0.444 × total hospital cost + 0.291 × SAE. Furthermore, the patient population was classified in the following manner according to the quartile PC score: The low PC score group (below the 75% quartile) and the high PC score group (in the upper 75% quartile).
In this study, a novel outcome indicator based on the standardized LOS, total hospital cost and the presence of SAEs provided a comprehensive system for evaluating SBO outcomes (PC score = 0.429 × LOS + 0.444 × total hospital cost + 0.291 × SAE). Furthermore, risk statuses associated with poor results were identified; specifically, for SiBO patients, a low LMR, as well as radiological features of a lack of small bowel feces signs and mural thickening, should be noticeable. For the StBO group, higher blood urea nitrogen levels and lower lymphocytes levels were recognized. Accordingly, early clinical intensive care was applicable for outcome improvement. In the future, adequately powered and well-designed studies are required to confirm these findings and to establish causality.
In this study, PCA was innovatively used for dimension reduction, linear correlation resolution and data simplification. Furthermore, a novel comprehensive system for the evaluation of SBO outcomes was constructed and the potential risk status associated with poor results were identified.
Large-scale and prospective studies are going to be designed to confirm these findings and to establish causality.
The authors thank the staff of the Department of General surgery (Emergency surgery) of Fujian Medical University Union Hospital, for their guidance and support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Papazafiropoulou A, Greece; Zharikov YO, Russia S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Sikirica V, Bapat B, Candrilli SD, Davis KL, Wilson M, Johns A. The inpatient burden of abdominal and gynecological adhesiolysis in the US. BMC Surg. 2011;11:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 2. | Bilderback PA, Massman JD 3rd, Smith RK, La Selva D, Helton WS. Small Bowel Obstruction Is a Surgical Disease: Patients with Adhesive Small Bowel Obstruction Requiring Operation Have More Cost-Effective Care When Admitted to a Surgical Service. J Am Coll Surg. 2015;221:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Brandt WS, Wood J, Bhattacharya B, Pei K, Davis KA, Schuster K. Relationship between duration of preoperative symptoms and postoperative ileus for small bowel obstruction. J Surg Res. 2018;225:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Oyasiji T, Angelo S, Kyriakides TC, Helton SW. Small bowel obstruction: outcome and cost implications of admitting service. Am Surg. 2010;76:687-691. [PubMed] |
| 5. | Lee MJ, Sayers AE, Drake TM, Marriott PJ, Anderson ID, Bach SP, Bradburn M, Hind D, Verjee A, Fearnhead NS; NASBO steering group and NASBO collaborators. National prospective cohort study of the burden of acute small bowel obstruction. BJS Open. 2019;3:354-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Byrne J, Saleh F, Ambrosini L, Quereshy F, Jackson TD, Okrainec A. Laparoscopic versus open surgical management of adhesive small bowel obstruction: a comparison of outcomes. Surg Endosc. 2015;29:2525-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Lee DK, Frye A, Louis M, Koshy AN, Tosif S, Yii M, Ma R, Nikfarjam M, Perini MV, Bellomo R, Weinberg L. Postoperative complications and hospital costs following small bowel resection surgery. PLoS One. 2020;15:e0241020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Straatman J, Cuesta MA, de Lange-de Klerk ES, van der Peet DL. Hospital cost-analysis of complications after major abdominal surgery. Dig Surg. 2015;32:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Alavi M, Visentin DC, Thapa DK, Hunt GE, Watson R, Cleary M. Exploratory factor analysis and principal component analysis in clinical studies: Which one should you use? J Adv Nurs. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 10. | Zhang JJ, Cao YY, Tan G, Dong X, Wang BC, Lin J, Yan YQ, Liu GH, Akdis M, Akdis CA, Gao YD. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy. 2021;76:533-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 11. | Soressa U, Mamo A, Hiko D, Fentahun N. Prevalence, causes and management outcome of intestinal obstruction in Adama Hospital, Ethiopia. BMC Surg. 2016;16:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Schwenter F, Poletti PA, Platon A, Perneger T, Morel P, Gervaz P. Clinicoradiological score for predicting the risk of strangulated small bowel obstruction. Br J Surg. 2010;97:1119-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Xu WX, Zhong QH, Cai Y, Zhan CH, Chen S, Wang H, Lin L, Geng YQ, Hou P, Chen XQ, Zhang JR. Prediction and management of strangulated bowel obstruction: a multi-dimensional model analysis. BMC Gastroenterol. 2022;22:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Huang X, Fang G, Lin J, Xu K, Shi H, Zhuang L. A Prediction Model for Recognizing Strangulated Small Bowel Obstruction. Gastroenterol Res Pract. 2018;2018:7164648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Hajibandeh S, Hajibandeh S, Panda N, Khan RMA, Bandyopadhyay SK, Dalmia S, Malik S, Huq Z, Mansour M. Operative versus non-operative management of adhesive small bowel obstruction: A systematic review and meta-analysis. Int J Surg. 2017;45:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Hernandez MC, Finnesgard EJ, Shariq OA, Knight A, Stephens D, Aho JM, Kim BD, Schiller HJ, Zielinski MD. Disease Severity and Cost in Adhesive Small Bowel Obstruction. World J Surg. 2019;43:3027-3034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Chang WC, Ko KH, Lin CS, Hsu HH, Tsai SH, Fan HL, Tung HJ, Huang GS, Chen RC. Features on MDCT that predict surgery in patients with adhesive-related small bowel obstruction. PLoS One. 2014;9:e89804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Zielinski MD, Eiken PW, Bannon MP, Heller SF, Lohse CM, Huebner M, Sarr MG. Small bowel obstruction-who needs an operation? World J Surg. 2010;34:910-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Kim J, Lee Y, Yoon JH, Lee HJ, Lim YJ, Yi J, Jung WB. Non-strangulated adhesive small bowel obstruction: CT findings predicting outcome of conservative treatment. Eur Radiol. 2021;31:1597-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Ofer A, Abadi S, Nitecki S, Karram T, Kogan I, Leiderman M, Shmulevsky P, Israelit S, Engel A. Multidetector CT angiography in the evaluation of acute mesenteric ischemia. Eur Radiol. 2009;19:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Ishikawa E, Kudo M, Minami Y, Ueshima K, Kitai S, Ueda K. Cecal intussusception in an adult with Cronkhite-Canada syndrome relieved by colonoscopy. Intern Med. 2010;49:1123-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Rami Reddy SR, Cappell MS. A Systematic Review of the Clinical Presentation, Diagnosis, and Treatment of Small Bowel Obstruction. Curr Gastroenterol Rep. 2017;19:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 23. | Millet I, Taourel P, Ruyer A, Molinari N. Value of CT findings to predict surgical ischemia in small bowel obstruction: A systematic review and meta-analysis. Eur Radiol. 2015;25:1823-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Scaglione M, Galluzzo M, Santucci D, Trinci M, Messina L, Laccetti E, Faiella E, Beomonte Zobel B. Small bowel obstruction and intestinal ischemia: emphasizing the role of MDCT in the management decision process. Abdom Radiol (NY). 2022;47:1541-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Paulson EK, Thompson WM. Review of small-bowel obstruction: the diagnosis and when to worry. Radiology. 2015;275:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 26. | Jammer I, Wickboldt N, Sander M, Smith A, Schultz MJ, Pelosi P, Leva B, Rhodes A, Hoeft A, Walder B, Chew MS, Pearse RM; European Society of Anaesthesiology (ESA) and the European Society of Intensive Care Medicine (ESICM); European Society of Anaesthesiology; European Society of Intensive Care Medicine. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol. 2015;32:88-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 627] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 27. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24828] [Article Influence: 1182.3] [Reference Citation Analysis (0)] |
| 28. | Krielen P, van den Beukel BA, Stommel MWJ, van Goor H, Strik C, Ten Broek RPG. In-hospital costs of an admission for adhesive small bowel obstruction. World J Emerg Surg. 2016;11:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Yamamoto Y, Miyagawa Y, Kitazawa M, Tanaka H, Kuroiwa M, Hondo N, Koyama M, Nakamura S, Tokumaru S, Muranaka F, Soejima Y. Association of feces sign with prognosis of non-emergency adhesive small bowel obstruction. Asian J Surg. 2021;44:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Grootjans J, Lenaerts K, Buurman WA, Dejong CH, Derikx JP. Life and death at the mucosal-luminal interface: New perspectives on human intestinal ischemia-reperfusion. World J Gastroenterol. 2016;22:2760-2770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 31. | Zielinski MD, Haddad NN, Cullinane DC, Inaba K, Yeh DD, Wydo S, Turay D, Pakula A, Duane TM, Watras J, Widom KA, Cull J, Rodriguez CJ, Toschlog EA, Sams VG, Hazelton JP, Graybill JC, Skinner R, Yune JM; EAST SBO Workgroup: Martin D. Zielinski, MD; Nadeem N. Haddad, MD; Asad J. Choudhry, MBBS; Daniel C. Cullinane, MD; Kenji Inaba, MD; Agustin Escalante; D. Dante Yeh, MD; Salina Wydo, MD; David Turay, MD; Andrea Pakula, MD; Therese M. Duane, MD; Jill Watras, MD; Kenneth A. Widom, MD; John Cull, MD; Carlos J. Rodriguez, DO; Eric A. Toschlog, MD; Valerie G. Sams, MD; Joshua P. Hazelton, DO; John Christopher Graybill, MD, Ruby Skinner, MD, Ji-Ming Yune, MD. Multi-institutional, prospective, observational study comparing the Gastrografin challenge versus standard treatment in adhesive small bowel obstruction. J Trauma Acute Care Surg. 2017;83:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Mahony CR, Traynor MD Jr, Knight AW, Hughes JD, Hernandez MC, Finnesgard EJ, Musa J, Selby SL, Rivera M, Kim BD, Heller SF, Zielinski MD. Small bowel obstruction managed without hospital admission: A safe way to reduce both cost and time in the hospital? Surgery. 2022;171:1665-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 33. | Lee MJ, Sayers AE, Drake TM, Singh P, Bradburn M, Wilson TR, Murugananthan A, Walsh CJ, Fearnhead NS; NASBO Steering Group and NASBO Collaborators. Malnutrition, nutritional interventions and clinical outcomes of patients with acute small bowel obstruction: results from a national, multicentre, prospective audit. BMJ Open. 2019;9:e029235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Vural V, Ozozan OV. The Usefulness of Inflammation-based Prognostic Scores for the Prediction of Postoperative Mortality in Patients Who Underwent Intestinal Resection for Acute Intestinal Ischemia. Cureus. 2019;11:e6372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72:1491-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 552] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 36. | Christou NV, Meakins JL, Gordon J, Yee J, Hassan-Zahraee M, Nohr CW, Shizgal HM, MacLean LD. The delayed hypersensitivity response and host resistance in surgical patients. 20 years later. Ann Surg. 1995;222:534-46; discussion 546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 86] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, López MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG; Inflammation and Host Response to Injury Large-Scale Collaborative Research Program. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581-2590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 777] [Cited by in RCA: 858] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 38. | Wright HK, O'Brien JJ, Tilson MD. Water absorption in experimental closed segment obstruction of the ileum in man. Am J Surg. 1971;121:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Baum N, Dichoso CC, Carlton CE. Blood urea nitrogen and serum creatinine. Physiology and interpretations. Urology. 1975;5:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Clumeck N, George C. Immunological aspects to severe bacterial sepsis. Intensive Care Med. 1981;7:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |