Published online Mar 7, 2023. doi: 10.3748/wjg.v29.i9.1475
Peer-review started: December 5, 2022
First decision: January 2, 2023
Revised: January 11, 2023
Accepted: February 22, 2023
Article in press: February 22, 2023
Published online: March 7, 2023
Processing time: 92 Days and 6.6 Hours
Immunological dysfunction-induced low-grade inflammation is regarded as one of the predominant pathogenetic mechanisms in post-infectious irritable bowel syndrome (PI-IBS). γδ T cells play a crucial role in innate and adaptive immunity. Adenosine receptors expressed on the surface of γδ T cells participate in intestinal inflammation and immunity regulation.
To investigate the role of γδ T cell regulated by adenosine 2A receptor (A2AR) in PI-IBS.
The PI-IBS mouse model has been established with Trichinella spiralis (T. spiralis) infection. The intestinal A2AR and A2AR in γδ T cells were detected by immunohistochemistry, and the inflammatory cytokines were measured by western blot. The role of A2AR on the isolated γδ T cells, including proliferation, apoptosis, and cytokine production, were evaluated in vitro. Their A2AR expression was measured by western blot and reverse transcription polymerase chain reaction (RT-PCR). The animals were administered with A2AR agonist, or A2AR anta
PI-IBS mice exhibited elevated ATP content and A2AR expression (P < 0.05), and suppression of A2AR enhanced PI-IBS clinical characteristics, indicated by the abdominal withdrawal reflex and colon transportation test. PI-IBS was associated with an increase in intestinal T cells, and cytokine levels of interleukin-1 (IL-1), IL-6, IL-17A, and interferon-α (IFN-α). Also, γδ T cells expressed A2AR in vitro and generated IL-1, IL-6, IL-17A, and IFN-α, which can be controlled by A2AR agonist and antagonist. Mechanistic studies demonstrated that the A2AR antagonist improved the function of γδ T cells through the PKA/CREB/NF-κB signaling pathway.
Our results revealed that A2AR contributes to the facilitation of PI-IBS by regulating the function of γδ T cells via the PKA/CREB/NF-κB signaling pathway.
Core Tip: Immunological dysfunction-induced low-grade inflammation is regarded as one of the most important pathogenetic mechanisms in post-infectious irritable bowel syndrome. γδ T cells play a crucial role in innate and adaptive immunity. The adenosine molecule and receptors regulate intestinal inflammation and immunity. Through the PKA/CREB/NF-κB signaling pathway, we showed that adenosine 2A receptor contributes to the facilitation of post-infectious irritable bowel syndrome by T cells.
- Citation: Dong LW, Chen YY, Chen CC, Ma ZC, Fu J, Huang BL, Liu FJ, Liang DC, Sun DM, Lan C. Adenosine 2A receptor contributes to the facilitation of post-infectious irritable bowel syndrome by γδ T cells via the PKA/CREB/NF-κB signaling pathway. World J Gastroenterol 2023; 29(9): 1475-1491
- URL: https://www.wjgnet.com/1007-9327/full/v29/i9/1475.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i9.1475
Irritable bowel syndrome (IBS), a functional gastrointestinal disorder with abnormal bowel habits and/or faecal traits, has been increasing in prevalence in developing countries since 1990[1]. The concept of post-infectious IBS (PI-IBS) was derived from clinical observations of patients with previous acute gastrointestinal infections[2]. Patients often suffer from refractory discomfort, low quality of life, and high healthcare economic burden, but current treatments reap unsatisfactory therapeutic results due to the lack of specific targets[3]. Numerous studies in the literature have shown that 1/3 of IBS patients have a history of gastrointestinal infection, suggesting that a persistent inflammatory response may explain the inconsistency between the intestinal response of PI-IBS patients and the normal gut[4]. Therefore, researchers generally agree that PI-IBS is also essentially a post-inflammatory immune dysfunction[5] and that reducing the intestinal inflammatory response is expected to improve clinical symptoms and life quality. For the potential therapeutic target of PI-IBS, upregulation of EphA2 expression and activation of the NF-κB signaling pathway have been reported to alleviate PI-IBS-related symptoms[6], while miRNA-510 has also been proved to regulate the intestinal inflammatory response in PI-IBS by targeting PRDX1[7].
γδ T cells are a subpopulation of T lymphocytes with a distinct phenotype and function, although they represent only a small proportion of the total T cells. γδ T cells bridge the gap between innate and adaptive immunity, determining the type of immune response and maintaining homeostasis in mucosal tissues. In inflammatory and immune responses, γδ T cells play a complex dual role depending on the context. On the one hand, γδ T cells present antigens to immune cells and trigger the recognition of these antigens by immune cells, leading to an adaptive immune response, promoting the secretion of chemokines and cytokines, and recruiting inflammatory cells[8,9]. On the other hand, γδ T cells inhibit the inflammatory process by conducting regulatory cytokines such as interferon-α (IFN-α), interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α), and IL-17 and eliminating over-activated inflammatory effector cells such as macrophages and polymorphonuclear leukocytes through cytotoxic effects[10]. In animal models of experimental autoimmune encephalomyelitis, rheumatoid arthritis and non-fatty diabetes, γδ T cells are proved to be the causative factor whereas rats with colitis and sepsis and mice with EAU were found to benefit from their presence[11]. According to our previous research, we have demonstrated that γδ T cells could alleviate PI-IBS through promoting Th17 polarization via their HSP70 receptor[12].
One of the many receptors expressed on the surface of γδ T cells is adenosine 2A receptor (A2AR), a classical G protein-coupled receptor with a high affinity for adenosine (ADO)[13]. A2AR is expressed in many immune cells, such as regulatory T cells, cytotoxic T cells, macrophages, etc. Its homologous family members include four isoforms, namely A1R, A2aR, A2bR, and A3R. These ARs are distributed in different tissues. For example, A1R is in the small intestine, A2aR and A2bR are in the cecum and colon, and A3R is in the ileum and colon[14]. A2AR forms the CD39-CD73-A2AR adenosine metabolic pathway with CD39 and CD73: first, CD39 cleaves extracellular ATP into AMP, which then binds to CD73 and is converted to ADO by CD73. ADO then binds to A2AR, which in turn interacts with Gs family proteins to increase intracellular cAMP levels and activate the downstream PKA/CREB-related pathway. Thus, the adenosine-A2AR pathway plays an important role in protecting normal organs and tissues from the autoimmune response of immune cells[15-17]. There are fewer reports on A2AR and intestinal inflammatory injury. Researchers have found that ADO can inhibit intestinal inflammatory injury in chronically septic mice via interacting with A1AR and A2AR[18]. And according to the research from Hou et al[19], electroacupuncture reduced visceral pain in rats with inflammatory bowel disease by interfering with adenosine receptors. However, there are no studies correlating A2AR with the pathogenesis of IBS, especially PI-IBS. Recently, Sun et al[20] reported that A2AR is able to bind to mGluR5 and exhibits a unique role in maintaining the function of the intestinal barrier in enteric glial cells against hypoxia-induced cellular damage. The above findings suggest that ARs, especially the A2AR isoform, may play a key role in the pathogenesis of PI-IBS and could potentially be a novel target for intervention in PI-IBS progression.
Hence, considering that the correlation between A2AR and PI-IBS remains unclear, the function of A2AR on the surface of γδ T cells has not been fully elucidated, and the precise A2AR-related signaling pathway mediating PI-IBS progression in γδ T cells awaits to be explored. It is an urgent issue to evaluate the role of A2AR in regulating γδ T cells on PI-IBS pathogenesis.
Ninety-six female 57BL/6 mice were provided by the Medical Animal Center of the Hainan Medical College. All animals were housed in a sterile, suitable environment and fed ad libitum with food and water that met cleanliness standards. The animals used in the experiments were cared for and handled by the Chinese Guide for Laboratory Animals. The experimental procedures were examined and approved by the Animal Care and Utilization Committee of Hainan.
Animals were randomly divided into 4 groups (n = 24 per group): The control group, the PI-IBS group, the IBS + SCH58261 group, and the IBS + CGS21680 group. A quarter of mice were killed in each group, then intestinal A2AR protein and mRNA levels, cytokine expression and secretion plus signaling pathway activation were investigated, respectively. Another six mice were used for γδ T cell isolation. The remaining mice were used for A2AR lentivirus infection and cell reinfusion assay.
The Trichinella spiralis (T. spiralis) infected the mice as described earlier in Lanzhou Animal Medical Institute[21,22]. Briefly, parasite larvae were isolated from cysts of SD rats, which have been infected with T. spiralis for 60 d and digested with 1.5% pepsin. Mice were then fed with larvae, which were resuspended in 0.2 mL of saline (300 larvae per mouse). At the same time, the control group was only fed with 0.9% saline.
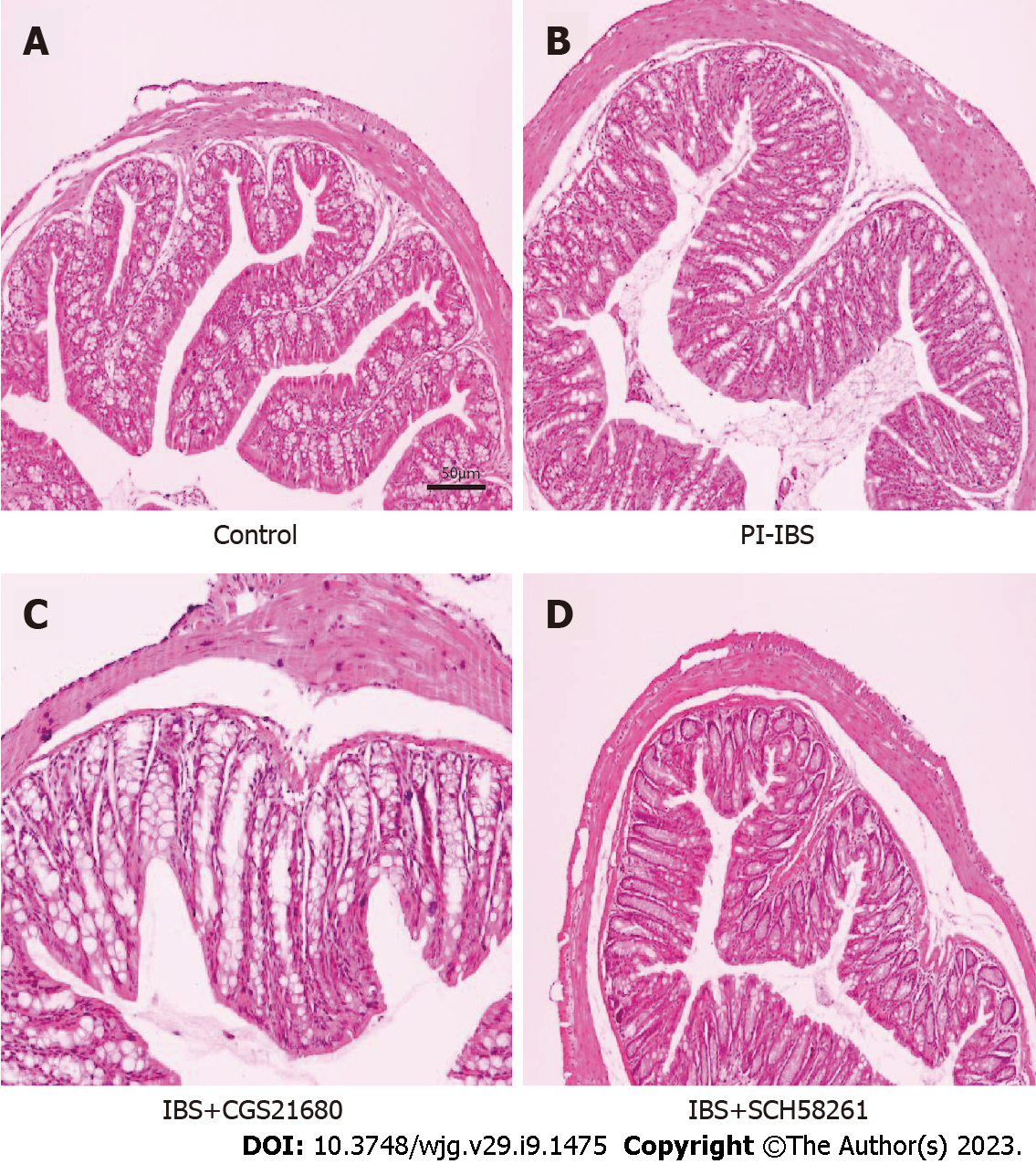
After 8 wk of infection, animals were euthanized, meanwhile, their ileal tissues were fixed with 10% formalin at 4 °C for 10 d, then dried in ethanol, and embedded in paraffin. The 5 micrometers sections were made. After dewaxing and hydration, they were stained with hematoxylin-eosin (HE). In addition, HE staining was used to assess inflammation scores according to a previous scoring system[23].
The abdominal withdrawal reflex (AWR) was administered to assess visceral hypersensitivity[24]. A catheter and air chamber were introduced via the anus of sedated animals. The air chamber was inflated to a capacity of 0.25/0.35/0.5/0.66 mL in 15 min on a number of occasions. The animals were allowed 30 s of rest between each distending period. The AWR criterion for scoring: When stimulated, the animals' mood is stable, 0 points; if they are unstable, occasionally bending their necks, 1 point; slightly contracting their abdomen and back muscles, 2 points; intensively contracting their abdomen muscles and lifting the abdomen from the ground, 3 points; intensively contracting abdomen muscles, bowing abdomen, and lifting abdomen and perineum, 4 points.
The colon transit test (CTT) was used to assess intestinal motility. After ingesting 0.4 mL of active carbon, the timing was documented. The entire feces collected during 8 h were graded using the Bristol stool scale: 1 point for normal-shaped stools; 2 points for stools that are soft or malformed; 3 points for stools that are watery[25].
Immunohistochemistry was performed on the cells as previously described[26]. Briefly, cells were fixed in suspension with 4% PFA for uniform distribution on the slides. After rinsing in phosphate-buffered saline (PBS), slides were incubated with anti-mouse A2AR monoclonal antibody (1:150; Abcam, Cambridge, United Kingdom) overnight at 4 °C and then with HRP secondary antibody (SP-9001, Beijing Sequoia Jinqiao, Beijing, China) for 1 h at room temperature. Reactivity was detected using the DAB reagent (Beijing Sequoia Jinqiao, Beijing, China). The primary antibody was substituted with PBS as a negative control. As the cell membrane was the main localization site of A2AR, the expression of A2AR was evaluated by a semi-quantitative integration technique. A2AR protein expression staining intensity was rated as 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining). The percentage of positively stained cells in the visual field was categorized as 0 (5%), 1 (5%-25%), 2 (26%-50%), 3 (51%-75%), or 4 (> 75%). The evaluation was represented as the product of the positive rate score and staining intensity score, with a score of greater than or equal to 6 indicating a favorable evaluation. The above staining was examined using Nikon DR-Si2 cell counting software and digital image analysis was employed. Moreover, the scoring was done in a double-blind way, which was confirmed by two pathologists who were not involved in this study and unaware of the clinical-pathological data.
Western blot was used to detect altered protein expression. Ileal tissue samples were lysed with RIPA coupled with brief ultrasonic pulverization. After lysis was completed, the homogenate was centrifuged at 4 °C for 30 min, the precipitate was discarded and the supernatant was retained. The protein content of the supernatant was determined by the Bradford method. 40 μg of protein was taken for SDS-PAGE gel electrophoresis, and the protein blot was transferred to the PVDF membrane. Transfer conditions: β-actin, A2AR (200 mA, 90 min); PKA, p-PKA (200 mA, 120 min followed by 300 mA, 30 min); CREB, p-CREB (200 mA, 20 min followed by 300 mA, 15 min); NF-κB (200 mA, 120 min); claudin-1 (200 mA, 70 min), occludin (200 mA, 120 min); ZO-1 (200 mA, 120 min followed by 300 mA, 60 min); IL-1β (200 mA, 50 min); IL-6 (200 mA, 70 min); IL-17A (200 mA, 50 min); IFN-α (200 mA, 60 min). The primary antibodies presented in Table 1 were rabbit anti-mouse multi-clonal.
| Primary antibodies | Dilution ratio |
| β-actin | 1:1000 |
| A2AR | 1:500 |
| NF-κB | 1:2000 |
| PKA | 1:1000 |
| p-PKA | 1:2000 |
| CREB | 1:1000 |
| p-CREB | 1:1000 |
| ZO-1 | 1:1000 |
| Occludin | 1:1000 |
| Claudin-1 | 1:1000 |
| IL-1β | 1:1000 |
| IL-6 | 1:1000 |
| IL-17A | 1:500 |
| IFN-α | 1:1000 |
The diluted primary antibodies were incubated with the PVDF membrane at 4 °C for over 12 h. On the following day, the membrane was rinsed with TBST for three times, and ECL chemiluminescent test was performed. In a dark setting, the grayscale value was identified by improved chemiluminescence. The protein/actin grayscale value represented the relative protein expression level.
mRNA alteration was determined through Real-time quantitative polymerase chain reaction (PCR). Briefly, RNA was extracted from the tissue of the terminal ileum by using Trizol and DNase I. Primers were constructed based on the gene sequences of mice (Table 2). β-Actin served as an internal reference. The RNA was then reverse transcribed to cDNA according to the steps of the TAKARA PrimeScript kit (TAKARA-RR047A), and real-time quantitative PCR was performed according to the following procedure: pre-denaturation at 94 °C for 5 min; denaturation at 94 °C for 1 min; 30 cycles of amplification and qualification at 57 °C and 60 °C; the ultimate step was prolongation at 72 °C for 7 min. Results are expressed as the ratio of target genes to control genes.
| Name | Primer | Sequence | Size |
| β-actin | Forward | 5’-CACGATGGAGGGGCCGGACTCATC-3’ | 240 bp |
| Reverse | 5’-TAAAGACCTCTATGCCAACACAGT-3’ | ||
| NF-κB | Forward | 5’-CACCGGATTGAAGAGAAGCG-3’ | 194 bp |
| Reverse | 5’-AAGTTGATGGTGCTGAGGGA-3’ | ||
| CREB | Forward | 5’-GCTGGCTAACAATGGTACGG-3’ | 230 bp |
| Reverse | 5’-CCATAACAACTCCAGGGGCA-3’ | ||
| PKA | Forward | 5’-GGGCGTGCTGATCTATGAGA -3’ | 169 bp |
| Reverse | 5’-TCGCTTTGTCAGATCCACCT-3’ | ||
| A2AR | Forward | 5’-GCCTCTTCTTCGCCTGCTTTGTCC-3’ | 140 bp |
| Reverse | 5’-GCCCTTCGCCCTCATACCCGTCAC-3’ |
The tissue samples were sonicated and centrifuged at 4 °C for 15 min followed by the addition of double distilled water (1:9). The 10% homogenate was continued to be centrifuged at 3000 rpm for another 10 min. The supernatant was collected for subsequent analysis.
The ATP concentration was determined by the colorimetric method. Specifically, the OD value of the liquid in the colorimetric tube was measured at 636 nm. ATP concentration was calculated as: ATP concentration (mol/g prot) = (measured OD - control OD)/(standard OD - blank OD) × standard concentration (1000 mol/L)/sample concentration (g prot/L).
γδ T cells were extracted from the spleen of animals[27]. Spleens were collected aseptically from 8 to 10-week female mice and stored in 1 × PBS on ice. Splenocytes were then isolated and red blood cells were lysed with a hypotonic solution. γδ TCR cells were selected by depletion of CD11b, B220, CD4 and CD8 cells, negative selection using biotinylated antibodies and EasySep Biotin Positive Selection Kit (StemCell) according to the manufacturer's protocol.
The magnetic separation process was carried out as follows: Biotin Anti-CD11b, Biotin Anti-B220, Biotin Anti-CD4, and Biotin Anti-CD8 (1/100 × dilution, 10 μL/mL) were added respectively, then the whole system was mixed thoroughly and incubated at room temperature for 15 min. After that, 10 mL 1 × PBS + 0.526 mmol/L EDTA + 2% FBS was added and rotated at 1.4 k for 5 min at 4 °C. Discarding the supernatant and then the cells were resuspended at 1 × 108 cells/mL in the mentioned buffer. Then, Biotin Select Cocktail (100 μL/mL) was added and mixed thoroughly, following the incubation at room temperature for 15 min. Next, the beads from the EasySep kit were vortexed for 15 to 30 min and added (50 μL/mL), the new system was mixed thoroughly and incubated at room temperature for 10 min. At room temperature, tubes were placed into silver EasySep magnets, incubated for 5 min, and the supernatant fraction was poured into a new 15 mL tube. Then the cells were counted for further cell sorting.
The antibodies for sorting were as follows: γδTCR Alexa 488 (1/100 × dilution), CD8 PE (1/100 × dilution), CD4 PerCP Cy5.5 (1/100 × dilution), CD11b APC (1/100 × dilution) and B220 PEcy7 (1/100 × dilution). Before sorting, cells were stained at 4 °C for 30 min on ice with the mentioned antibodies. Then, we washed the cells with 1 × PBS and resuspended to 5 × 106 cells/mL for sorting[28].
The proliferation of isolated γδ T cells was detected by CCK8 assay. Cells were resuspended in 100 μL of the medium at the density of 5 × 104 cells per well, then added to 96-well plates and waited for the cells to stick. After that, 100 nmol/L CGS-21680 or 1 μnmol/L SCH58261 was added to different wells of the 96-well plate, and the cells were continued to be cultured at 37 °C with 5% CO2 for more than half an hour. Finally, 20 μL CCK8 was added to each well and the incubation was continued at 37 °C, 5% CO2 for another 2 h, and the OD value at 450 nm was measured by Multiscan Spectrum.
The extracted γδ T cells were uniformly distributed in six-well plates (5 × 105 cells/well). Subsequently, 100 nnmol/L CGS-21680 or 1 μnmol/L SCH58261 were added, respectively, and incubated at 37 °C with 5% CO2 for 0.5 h. After that, the apoptosis rate was detected by fluorescence-activated cell sorting (FACS).
ELISA was performed to determine the levels of IL-1β, IL-6, IL-17A, and IFN-α in cultivated γδ T cells and colonic tissue supernatants. Specifically, the original supernatant was removed and replaced with a new medium (RPMI-1640), and typical culture conditions were re-established for another 24 h. The content of cytokine proteins in the supernatant was quantitatively analyzed by using IL-1, IL-6, IL-17A, and IFN-α high sensitivity (0.25-16 pg/mL sensitivity range) ELISA kits (R&D Systems, Minneapolis, Minnesota, United States) according to the manufacturer's instructions.
Isolated γδ T cells were treated with SCH58261 or CGS21680 at 37 °C, 5% CO2 for 48 h. The cell concentration was then adjusted to 2 × 106 cells/mL with RMPI-1640. Mice were injected separately with γδ T cells at an inoculum of 2 × 106 cells per mouse. Clinical characteristics were observed as described previously.
LV-mA2AR-shRNA was synthesized by Fubio (Suzhou) Biopharmaceutical Technology Co., Ltd. and Suzhou Genepharma Co., Ltd. The sequence is as follows: the sense is 5'-GGAGACAGCUGA
Data are represented as mean ± SD. All data were analyzed using Grubb’s test, followed by one-way analysis of variance (ANOVA) and Levene's test to assess the homogeneity of variance, and finally Ducan's multiple comparison test for multiple comparisons (SPSS 22.0 software). P < 0.05 were considered statistically significant.
First, we chose the T. spiralis infection to construct the PI-IBS mouse model. On day 56 after the T. spiralis infection, the model was evaluated, which demonstrated that there was no obvious inflammation in the animal colon (Figure 1), but the AWR rating was significantly higher than that of the control group, and the results of CTT experiment were significantly abnormal, as shown by the shortened first black stool time and a higher Bristol stool level (Tables 3 and 4). This indicated that a successful PI-IBS mouse model has been established.
After that, we applied the A2AR agonist or antagonist to PI-IBS mice in order to find potential interventions for curing PI-IBS. A further novel finding was that only the injection of A2AR antagonist SCH58261 significantly relieved the severe clinical manifestations of the model animal whereas A2AR agonist CGS21680 could not. At this stage of understanding, these findings suggest that A2AR may have a crucial impact on the development of PI-IBS.
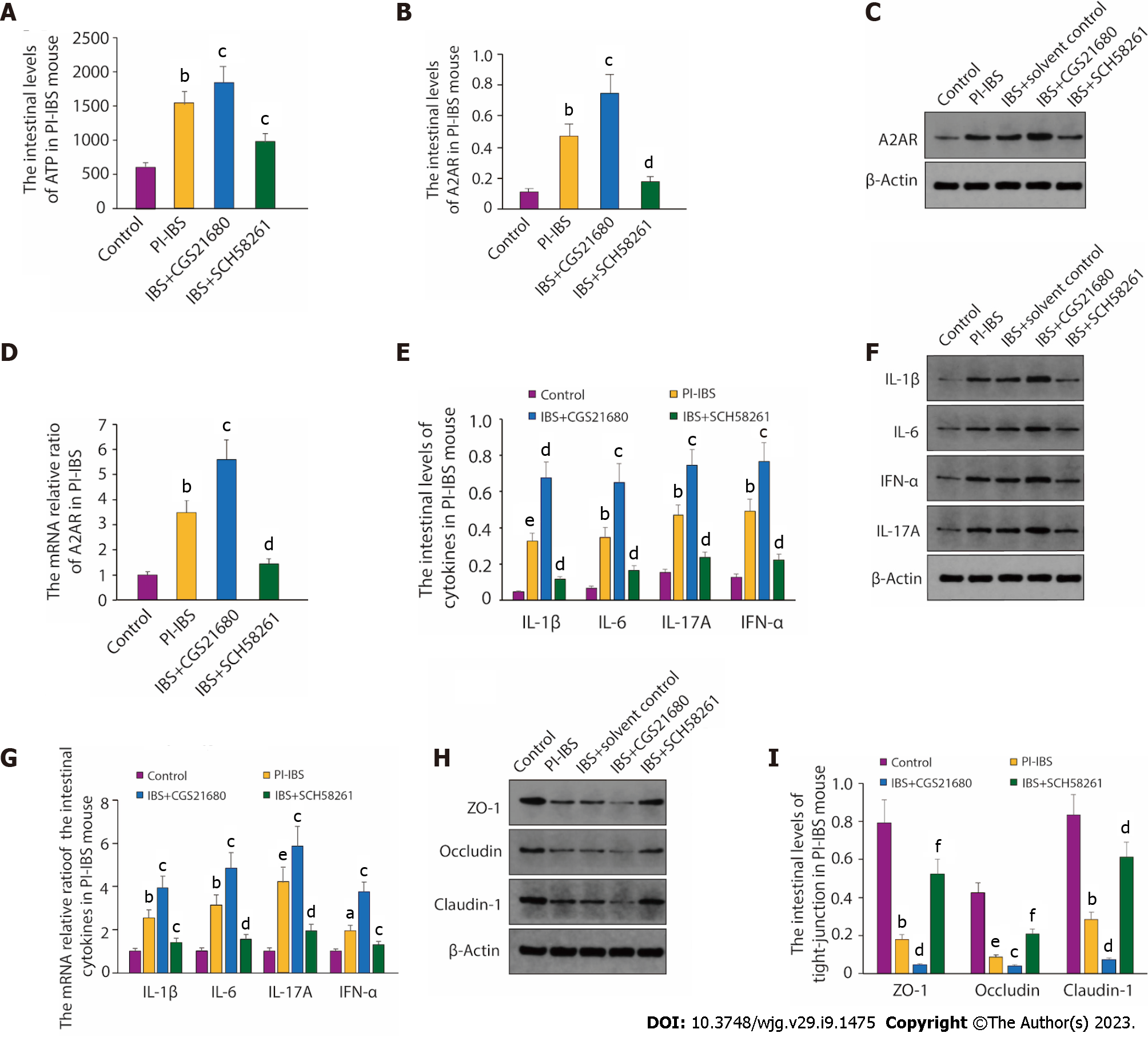
To further assess the relationship between A2AR and PI-IBS, we compared the altered expression of A2AR and ATP content in mice with PI-IBS or in normal mice, as A2AR plays a vital role in the conversion of ATP. From the results, it is clear that compared with the control group, intestinal ATP levels in PI-IBS mice rose considerably (P < 0.01, Figure 2A) as did A2AR protein expression (P < 0.01, Figure 2B-D). Intestinal ATP levels and A2AR expression were further enhanced when the PI-IBS mice were treated with the A2AR agonist CGS21680 (P < 0.05, Figure 2A-D). At the same time, the levels of inflammatory factors such as IL-1, IL-6, IL-17, and IFN-α were also further boosted compared to the PI-IBS model group (Figure 2E-G). Interestingly, when SCH58261, an antagonist of A2AR, was administered in PI-IBS mice, the significant increase in ATP levels and the up-regulation of A2AR expression triggered by PI-IBS was reversed (Figure 2A-D), followed by the drop of inflammatory factors’ expression compared with the PI-IBS group, including IL-1, IL-6, IL-17 and IFN-α (Figure 2E-G).
Intestinal epithelial tight junctions (TJs) proteins, such as ZO-1, Occludin, and Claudin-1, play a vital role in maintaining the epithelial barrier function to restrict the paracellular movement of harmful substances across intestinal mucosa[29]. The disrup- tion of the TJs barrier could increase dysregulated immune reactions, such as the activation of mucosal immune response and the permeation of noxious molecules, and thus inducing gut inflammation[30,31]. Thus, we next tested the change of ZO-1, occludin, and claudin-1 expression. As we can see, the expression of TJ proteins ZO-1, Occludin, and Claudin-1 was sharply reduced in PI-IBS mice compared to the control, while agonism of A2AR further inhibited the expression of the proteins mentioned above. In line with the results in Figure 2A-D, treatment with the antagonism of A2AR SCH58261 also reversed the decreased expression of ZO-1, Occludin, and Claudin-1 in the PI-IBS group (Figure 2H-I). The results of the experiment found clear support for the hypothesis that A2AR is crucial to the pathophysiology of PI-IBS.
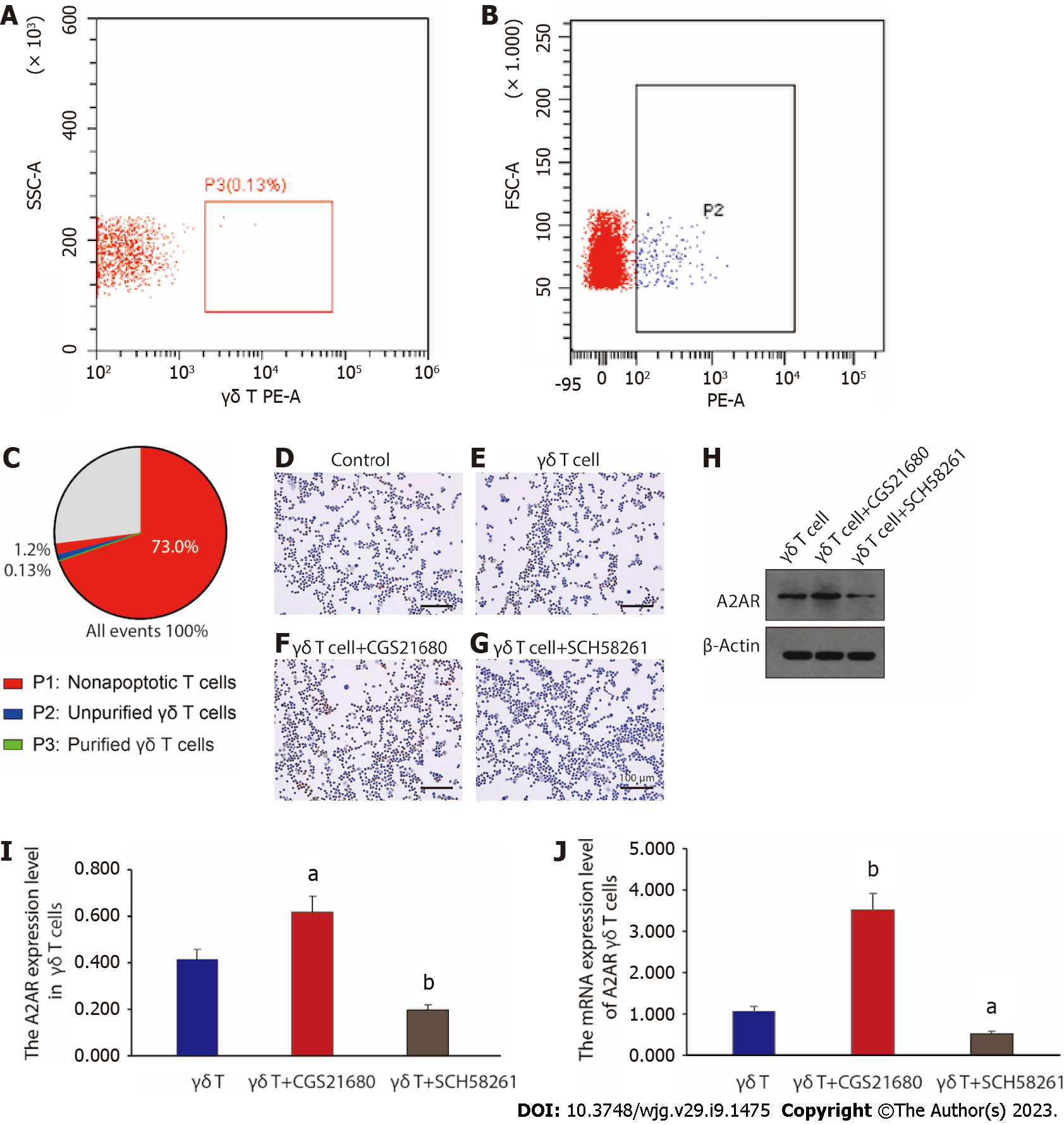
It has been previously reported that the intestinal γδ T cells could exert an important role in a PI-IBS mouse model[32]. However, we wanted to further corroborate whether it is the altered A2AR expression in γδ T cells that directly influences the pathogenesis of PI-IBS. Before starting the exploration, we first purified γδ T cells. Figure 3A-C depicts the extraction and purification of γδ T cells from the spleen of PI-IBS mice for subsequent in vitro functional assessment through FACS sorting. Presently, we examined the A2AR expression in unpurified γδ T cells (the control group) and purified γδ T cells (the γδ T cell group) by immunohistochemical staining, respectively. The results were presented in Figure 3D and E. Notably, the A2AR expression level of γδ T cells was significantly higher than that in the control group, suggesting that A2AR was mainly highly expressed in γδ T cells. Furthermore, we treated γδ T cells with A2AR agonist CGS21680 and A2AR antagonist SCH58261, respectively, and we can visualize through Figure 3F and G that the A2AR expression level was further enhanced in T cells treated with A2AR agonists, while the A2AR expression in T cells was significantly downregulated after the administration of SCH58261 to inhibit A2AR.
Besides, western blot and reverse transcription PCR results further confirmed the immunohistochemical findings: the agonist significantly increased both A2AR protein and mRNA level in γδ T cells, whereas the antagonist did the opposite (Figure 3H-J). Taken together, these results revealed that γδ T cells were the main immune cell subtype mediating the upregulation of A2AR expression in the intestinal immune microenvironment. By interfering with A2AR expression within γδ T cells, the function of γδ T cells might be affected, which could in turn interfere with disease progression throughout PI-IBS.
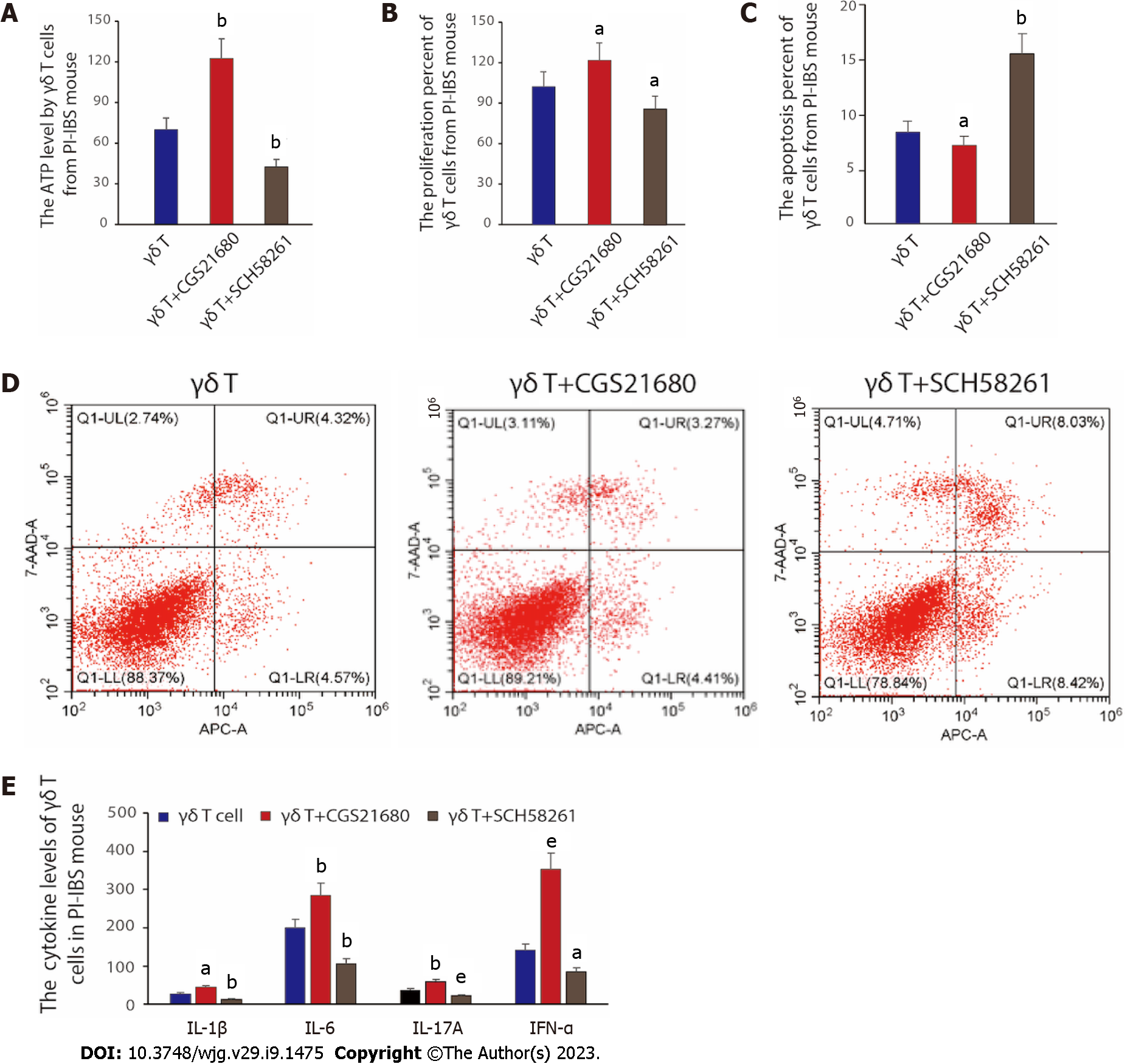
To verify the conjecture of the previous chapter, we would like to further unveil the effect of A2AR on γδ T-cells’ viability and function. First, we assessed the correlation between A2AR and γδ T-cell viability by the measurement of altered ATP content in γδ T cells and the percentage of proliferating γδ T cells. The results are shown in Figure 4A and B. When we treated γδ T cells with the A2AR agonist CGS21680, both the intracellular ATP content and the percentage of proliferating cells increased compared with the control γδ T cells, suggesting that A2AR activation could enhance the cell viability of γδ T cells; whereas the intracellular ATP content and proliferating percentage both decreased when the antagonist of cellular A2AR, SCH58261, was given. Subsequently, we further evaluated whether the apoptosis of γδ T cells was affected by A2AR, and the results demonstrated that inhibition of A2AR promoted the apoptosis of γδ T cells, while activation of A2AR greatly inhibited the apoptosis process (Figure 4C and D). Finally, we examined the function of γδ T cells. To be specific, we reinfused γδ T cells that have been treated differently into PI-IBS mice. A similar pattern of results was obtained in the expression of inflammatory factors, including IL-1β, IL-6, IL-17A and IFN-α in γδ T cells. Virtually, the addition of A2AR agonist CGS21680 promoted the expression of IL-1β, IL-6, IL-17A and IFN-α, while A2AR antagonist SCH58261 inhibited the expression of the above-mentioned inflammatory factors (P < 0.05, Figure 4E). The above results suggest that A2AR has an important effect on the viability and function of γδ T cells, especially on the secretion of inflammatory factors.
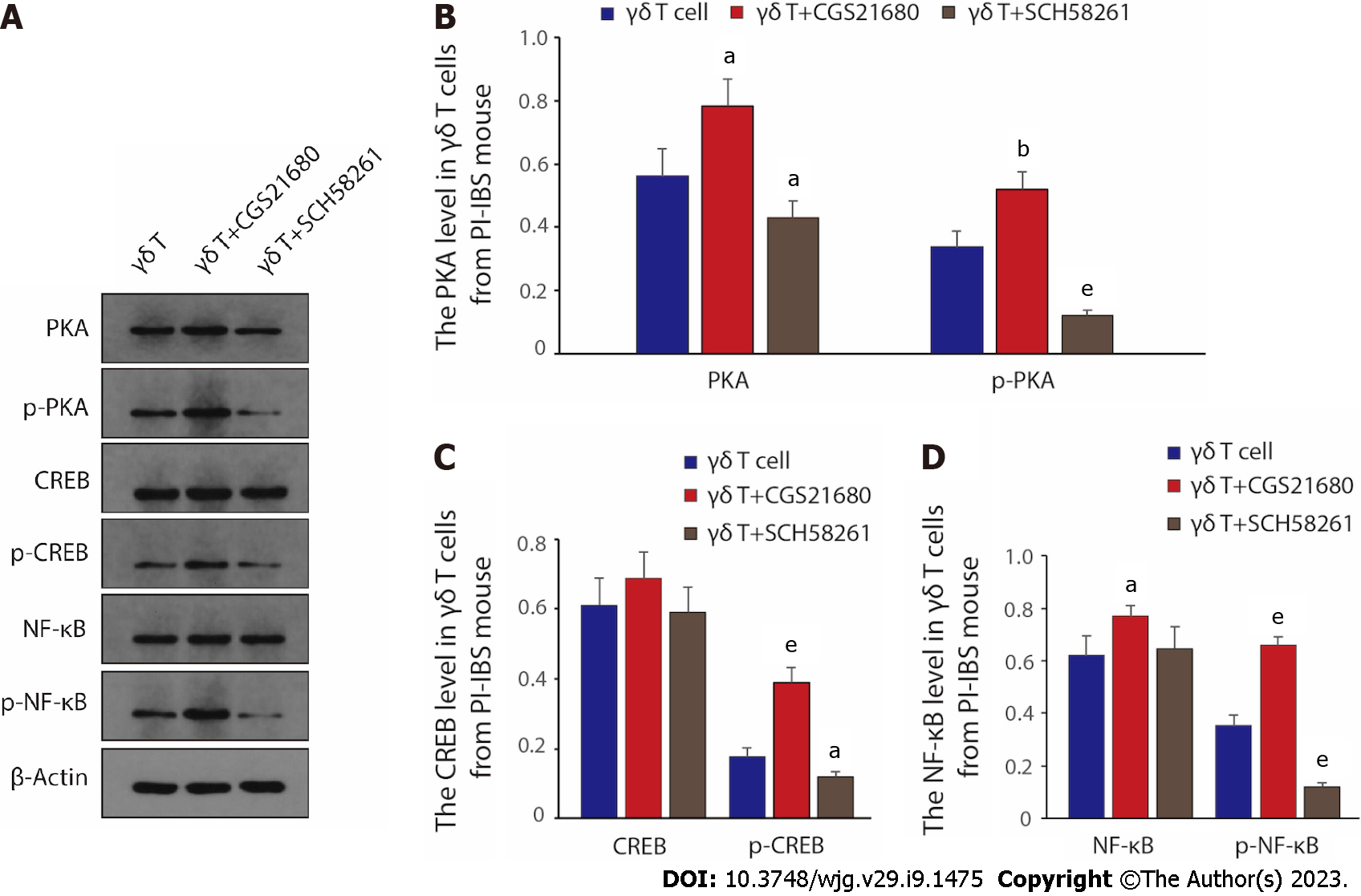
As ADO binds to A2AR, A2AR then interacts with Gs family proteins to increase intracellular cAMP levels and activate downstream PKA/CREB-related pathways[33], we next investigated the activation of signaling pathways in γδ T cells by western blot. The change of PKA/p-PKA, CREB/p-CREB, NF-κB/p-NF-κB protein level, and quantitative results elucidated that compared to control γδ T cells, treatment of γδ T cells with the A2AR antagonist SCH58261 significantly decreased the expression of PKA/CREB/NF-κB and reduced the phosphorylation levels of the above proteins; whereas the introduction of A2AR agonist CGS21680 further promoted the protein expression of PKA/CREB/NF-κB as well as the phosphorylation activation level of the above proteins (Figure 5A-D). Therefore, given the above results, we can safely draw the conclusion that the PKA/CREB/NF-κB pathway is the key downstream signaling pathway for γδ T cells to regulate the relevant clinical manifestations via A2AR during PI-IBS disease progression.
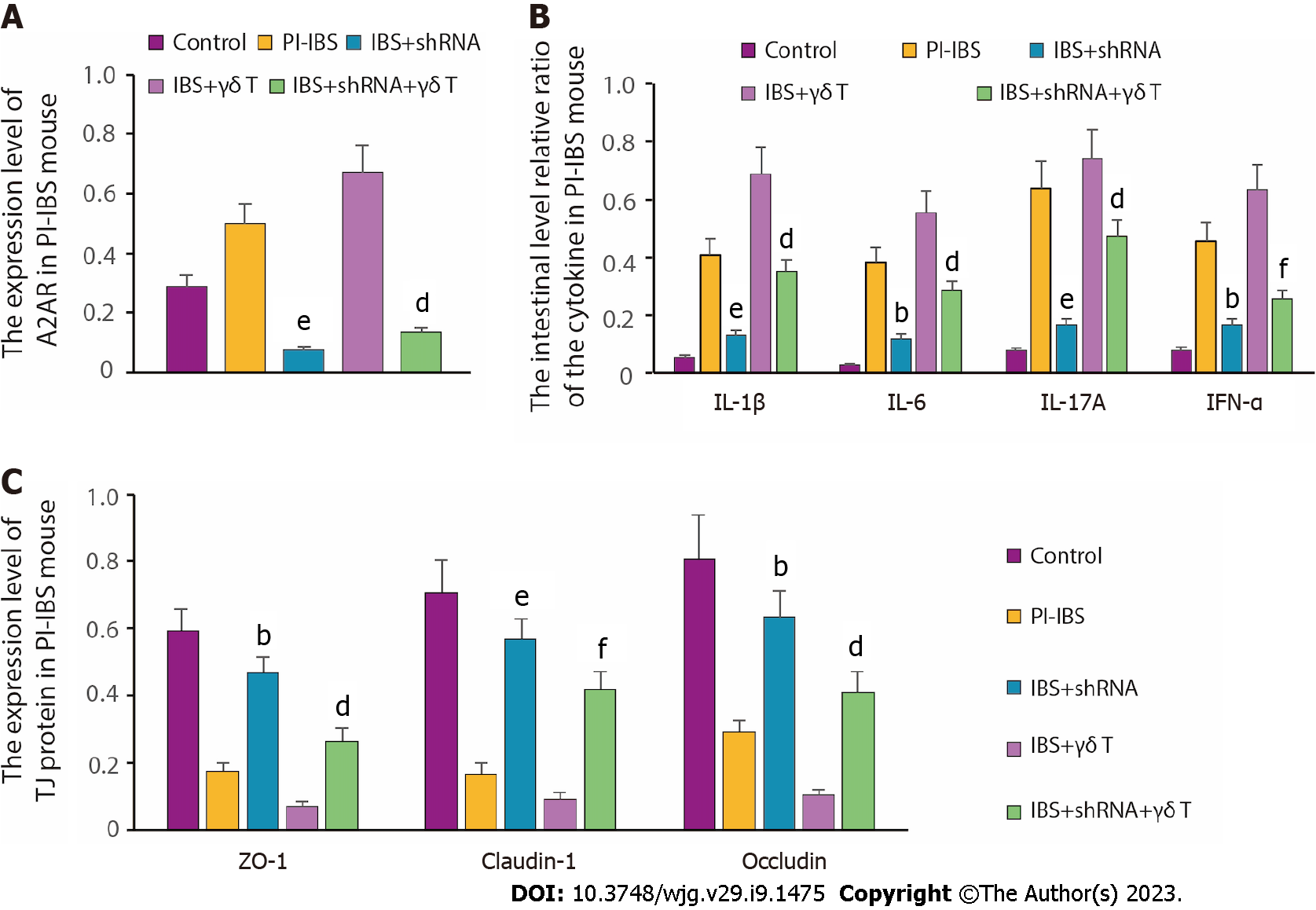
To further clarify the effect of A2AR on the expression of inflammatory factors, we constructed A2AR-shRNA and inhibited the expression of A2AR by plasmid transfection. The results illustrated that A2AR expression was greatly boosted in the PI-IBS group compared with the control group; when transfected with shRNA, A2AR expression was significantly reduced in the IBS + shRNA group. Moreover, when IBS tissue cells were co-incubated with γδ T cells, the A2AR expression level was further increased. Meanwhile, silencing A2AR downregulated A2AR expression in the IBS + shRNA + γδ T cells group compared with the IBS + γδ T cells group (Figure 6A). After clarifying the silencing efficiency, we examined the effect of silencing A2AR on the expression level of inflammatory factors. From the results in Figure 6B, we can clearly see that the inflammatory factor levels, which were originally higher in the PI-IBS model group, were dramatically reduced due to A2AR knockdown. At the same time, given that the reduced expression of TJ proteins leads to increased intestinal permeability, we also evaluated the altered expression levels of TJ proteins and found that the originally suppressed TJ protein expression in PI-IBS mice was significantly reversed after A2AR silencing (Figure 6C). Taken together, the above findings demonstrate that downregulation of A2AR can reduce γδ T cell-induced inflammatory cytokine production and release.
Persistent intestinal low-degree inflammation occurs in IBS due to complex disorders in the immune system, especially in PI-IBS, leading to minor biochemical and pathological changes but continuous clinical symptoms in patients[34]. Nevertheless, the precise immune regulatory mechanism in PI-IBS remains unknown. We have previously reported that γδ T cells could alleviate PI-IBS through promoting Th17 polarization via HSP70 receptor[12]. This study is a follow-up study on the patho
Few literatures have been reported the relationship between adenosine receptor and PI-IBS. According to our research, an increase in intestinal ATP level is followed by the upregulation of intestinal A2AR in PI-IBS, suggesting that ATP and A2AR may be involved in the pathophysiology of PI-IBS. It is unclear if the quantitative alterations of intestinal A2AR originated from PI-IBS could lead to exacerbated PI-IBS. After being administered with the A2AR antagonist, the digestive tissue of the animals remained unchanged, but their clinical symptoms improved. This discovery contradicted earlier notions that A2AR antagonist exhibits a protective function in inflammation[35]. Moreover, an A2AR antagonist may activate an unidentified pathway to control the equilibrium, and an extended persistence of elevated adenosine levels can be deleterious, contributing to the formation of an immunosuppressed niche that is conducive to the initiation and development of neoplasia[36]. These findings also indicate the complexity of PI-IBS.
IL-6, IL-17A, and IFN-α are important pro-inflammatory factors. IL-17A can increase the permeability of cells by binding to specific receptors and can stimulate various types of cells to produce chemokines, IFN-α, and IL-1β to stimulate inflammatory reactions. IFN-α can interrupt the function of intestinal epithelial cells, leading to intestinal epithelial barrier dysfunction[37]. IL-6 is mainly manifested in maintaining regulatory T cells and effector T cells and inhibiting the apoptosis of CD4+ T cells in the inflammatory response[38]. Our study suggested that the levels of pro-inflammatory factors IL-6, IL-17A, and IFN-α were significantly elevated in PI-IBS mice. This indicated that there was a low-grade inflammatory response in the intestinal mucosa, resulting in a defect in the epithelial barrier. The epithelial barrier dysfunction can lead to an increase in intestinal permeability, which further promotes the increase and activation of immune cells[39]. TJ proteins such as ZO-1, Claudin-1, and Occludin form a tight junction structure between adjacent intestinal epithelial cells as the structural basis of the mechanical intestinal barrier, thereby defending against external damaging factors and maintaining intestinal mucosal homeostasis[40,41]. Under the stimulation of severe infection and surgical blows, the tight junction structure between the intestinal epithelium can be destructed, The damaged mechanical barrier and the translocation of intestinal flora into the blood then cause bacteremia and sepsis to stimulate the release of systemic inflammatory factors, leading to systemic inflammatory response syndrome and multiple organ dysfunction[42]. In this study, the expression of ZO-1, Claudin-1, and Occludin was reduced substantially, suggesting that PI-IBS mice had impaired intestinal epithelial barrier function, and were susceptible to stimulation by various etiologies to activate the intestinal epithelial immune system and lead to intestinal inflammatory responses.
The uneven distribution of γδ T cells in normal and inflammatory tissues plays an important role in autoimmunity[43]. In some patients with autoimmune diseases, the proportion of γδ T cells in infiltrating T cells is abnormally increased. γδ T cells are the main source of proinflammatory cytokines IL-17, IL-23, IFN-α, and TGF-β. These inflammatory molecules are responsible for creating an inflammatory environment that enhances disease progression through different pathways[44]. The relationship between different disease phenotypes and γδT cells can be clearly discerned. γδT cells acquire the ability to produce IL-17 during embryonic thymus development, and T cells expressing γδT cell receptors are an important innate source of the pro-inflammatory cytokine IL-17. The potent inflammatory effects of IL-17 are mainly related to its ability to recruit immune cells and synergistic effects with other pro-inflammatory cytokines[45]. Hence, it is no exaggeration to say that γδ T cells play a crucial role in regulating inflammation and the immune response. In our results, we firstly found that the number of γδ T cells markedly increased, accompanied by the increased A2AR expressed on their surfaces. Secondly, the agonist of A2AR boosted the quantity of γδ T cells together with upregulated A2AR expression. Thirdly, the A2AR antagonist reduced γδ T cells’ number and impaired their function. These findings demonstrated that γδ T cells participated in intestinal inflammation mediated by A2AR.
At present, the anti-inflammatory mechanism of A2AR mainly includes: (1) Inhibition of neutrophils and release of peroxidase[46,47]; (2) promotion of the production of IL-10 by monocytes and macrophages; (3) inhibition of the release of IL-12 and TNF-α[48]; and (4) phosphorylation of cAMP response element-binding (CREB) protein by activating the cAMP-PKA pathway, in which phosphory
Furthermore, we decreased the A2AR expression with LV-A2AR-shRNA, which alleviated the symptoms of PI-IBS in model mice. Meanwhile, we then reinfused the γδ T cell to PI-IBS mice, the disease became severer. Interestingly, when the PI-IBS mice reinfused with sh-A2AR γδ T cells, the severity of their clinical symptoms was lessened. Besides, we also observed the alterations in the cytokines and TJ protein expression, and the results were in accordance with the changes in the clinical symptoms. These data suggested that γδ T cells participated in PI-IBS via the A2AR mediated signaling pathway.
Our study included a few drawbacks as well. The type of visceral hypersensitivity was not investigated in PI-IBS mice, and the water content from the feces of mice was not examined. Due to the difficulties of isolating T cells directly from the gut, we utilized spleen-isolated T cells, likely resulting in a greater degree of variety than their intestinal counterparts.
In the present study, we found higher expression of A2AR in PI-IBS mice than normal mice, which initially suggested the relevance of A2AR to PI-IBS disease development. Further mechanistic studies demonstrated that A2AR, which located on the surface of γδ T cells, could regulate the function of γδ T cells. Notably, A2AR regulated T cell viability and increased the secretion of inflammatory factors, mainly through the PKA/CREB/NF-κB signaling pathway, which followed by the progression of PI-IBS. Fortunately, the application of A2AR antagonists could markedly reduce the inflammatory response and improve PI-IBS symptoms by regulating the function of γδ T cells.
In summary, through our study, we identified A2AR, a key protein that promotes disease progression in PI-IBS, and demonstrated the feasibility of antagonizing A2AR to intervene in PI-IBS, thus providing a new therapeutic target for PI-IBS treatment.
Persistent low level of inflammation due to immune dysfunction is regarded as one of the prime pathogenic mechanisms of post-infectious irritable bowel syndrome (PI-IBS). γδ T cells play a key role in innate and adaptive immunity. Adenosine and its receptors expressed on γδ T cells are involved in intestinal inflammation and immune regulation.
To unveil the role of γδ T cells regulated by adenosine 2A receptor (A2AR) in the pathogenesis of PI-IBS.
This study aims to investigate the role of A2AR in γδ T cells and γδ T cells in PI-IBS.
A PI-IBS mouse model was established with Trichinella spiralis (T. spiralis) infection. Intestinal A2AR and A2AR in γδ T cells were detected through immunohistochemistry, and inflammatory cytokines were detected through western blot. The role of A2AR on isolated γδ T cells, including γδ T cell proliferation, apoptosis and γδ T cell-mediated cytokine secretion, was assessed in vitro. A2AR expression in γδ T cells was determined by western blot and reverse transcription polymerase chain reaction (RT-PCR). Mice were injected with A2AR agonist or A2AR antagonist and cultured γδ T cells were also reinfused into the animals, and then the above parameters and clinical features were examined again. In addition, alterations in A2AR-related signaling pathway molecules were detected by western blot and RT-PCR.
The expression levels of ATP and A2AR were increased in PI-IBS mice (P < 0.01), and inhibition of A2AR further enhanced the clinical features of PI-IBS, as reflected by the abdominal withdrawal reflex and colonic transport test results. The development of PI-IBS was associated with an increase in intestinal γδ T cells and cytokines including interleukin-1 (IL-1), IL-6, IL-17A and interferon-α (IFN-α). In addition, γδ T cells obtained by purification in vitro could express A2AR and promote IL-1, IL-6, IL-17A and IFN-α secretion, which is also regulated by A2AR agonists and antagonists. We also found that A2AR antagonists improved γδ T cell function through the PKA/CREB/NF-κB signaling pathway.
Our results suggested that A2AR contributes to the development of PI-IBS after T. spiralis infection via the PKA/CREB/NF-κB signaling pathway by γδ T cells.
Hypo-inflammation caused by immune dysfunction is considered to be one of the main pathogenic mechanisms of PI-IBS. In this study, we discovered that A2AR on the surface of γδ T cells can regulate the function of γδ T cell, thereby increasing inflammatory factor secretion and promoting PI-IBS progression. Luckily, the utilization of A2AR antagonists can improve PI-IBS symptoms by promoting γδ T cells’ function. Through our study, we identified A2AR, a key protein that promotes PI-IBS disease progression, and demonstrated the feasibility of antagonizing A2AR to intervene in PI-IBS, thus providing a novel therapeutic target and an effective intervention strategy for PI-IBS treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee EW, South Korea; Shin CM, South Korea; Yashiro M, Japan S-Editor: Gong ZM L-Editor: A P-Editor: Yuan YY
| 1. | Camilleri M. Diagnosis and Treatment of Irritable Bowel Syndrome: A Review. JAMA. 2021;325:865-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 182] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 2. | Berumen A, Edwinson AL, Grover M. Post-infection Irritable Bowel Syndrome. Gastroenterol Clin North Am. 2021;50:445-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Bonetto S, Fagoonee S, Battaglia E, Grassini M, Saracco GM, Pellicano R. Recent advances in the treatment of irritable bowel syndrome. Pol Arch Intern Med. 2021;131:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Ng QX, Soh AYS, Loke W, Lim DY, Yeo WS. The role of inflammation in irritable bowel syndrome (IBS). J Inflamm Res. 2018;11:345-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 214] [Article Influence: 30.6] [Reference Citation Analysis (2)] |
| 5. | Aguilera-Lizarraga J, Hussein H, Boeckxstaens GE. Immune activation in irritable bowel syndrome: what is the evidence? Nat Rev Immunol. 2022;22:674-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 6. | Zeng L, Li K, Wei H, Hu J, Jiao L, Yu S, Xiong Y. A Novel EphA2 Inhibitor Exerts Beneficial Effects in PI-IBS in Vivo and in Vitro Models via Nrf2 and NF-κB Signaling Pathways. Front Pharmacol. 2018;9:272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, Wu X, Wu J, Li S, Han S, Lin Z, Ding S, Jia X, Gong W. Decreased expression of microRNA-510 in intestinal tissue contributes to post-infectious irritable bowel syndrome via targeting PRDX1. Am J Transl Res. 2019;11:7385-7397. [PubMed] |
| 8. | Kobayashi H, Tanaka Y. γδ T Cell Immunotherapy-A Review. Pharmaceuticals (Basel). 2015;8:40-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Ribot JC, Lopes N, Silva-Santos B. γδ T cells in tissue physiology and surveillance. Nat Rev Immunol. 2021;21:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 313] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 10. | Ramstead AG, Jutila MA. Complex role of γδ T-cell-derived cytokines and growth factors in cancer. J Interferon Cytokine Res. 2012;32:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Su D, Shen M, Li X, Sun L. Roles of γδ T cells in the pathogenesis of autoimmune diseases. Clin Dev Immunol. 2013;2013:985753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | He Z, Sun X, Ma Z, Fu J, Huang B, Liu F, Chen Y, Deng T, Han X, Sun D, Lan C. Heat shock protein 70 protects mouse against post-infection irritable bowel syndrome via up-regulating intestinal γδ T cell's Th17 response. Cell Biosci. 2018;8:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Layland J, Carrick D, Lee M, Oldroyd K, Berry C. Adenosine: physiology, pharmacology, and clinical applications. JACC Cardiovasc Interv. 2014;7:581-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 218] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 14. | Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol Rev. 2018;98:1591-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 539] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 15. | Pelleg A, Porter RS. The pharmacology of adenosine. Pharmacotherapy. 1990;10:157-174. [PubMed] |
| 16. | Kumar V, Sharma A. Adenosine: an endogenous modulator of innate immune system with therapeutic potential. Eur J Pharmacol. 2009;616:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Antonioli L, Fornai M, Colucci R, Ghisu N, Tuccori M, Del Tacca M, Blandizzi C. Regulation of enteric functions by adenosine: pathophysiological and pharmacological implications. Pharmacol Ther. 2008;120:233-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Belikoff B, Hatfield S, Sitkovsky M, Remick DG. Adenosine negative feedback on A2A adenosine receptors mediates hyporesponsiveness in chronically septic mice. Shock. 2011;35:382-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Hou T, Xiang H, Yu L, Su W, Shu Y, Li H, Zhu H, Lin L, Hu X, Liang S, Zhang H, Li M. Electroacupuncture inhibits visceral pain via adenosine receptors in mice with inflammatory bowel disease. Purinergic Signal. 2019;15:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Sun L, Li X, Guan H, Chen S, Fan X, Zhou C, Yang H, Xiao W. A Novel Role of A(2A)R in the Maintenance of Intestinal Barrier Function of Enteric Glia from Hypoxia-Induced Injury by Combining with mGluR5. Front Pharmacol. 2021;12:633403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Long SR, Wang ZQ, Liu RD, Liu LN, Li LG, Jiang P, Zhang X, Zhang ZF, Shi HN, Cui J. Molecular identification of Trichinella spiralis nudix hydrolase and its induced protective immunity against trichinellosis in BALB/c mice. Parasit Vectors. 2014;7:600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Yang B, Zhou X, Lan C. Changes of cytokine levels in a mouse model of post-infectious irritable bowel syndrome. BMC Gastroenterol. 2015;15:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Suzuki R, Kohno H, Sugie S, Tanaka T. Dose-dependent promoting effect of dextran sodium sulfate on mouse colon carcinogenesis initiated with azoxymethane. Histol Histopathol. 2005;20:483-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 24. | Lan C, Sun XN, Zhou XC, Yang B, Huang BL, Deng TZ, He ZT, Han XY. Preinduced intestinal HSP70 improves visceral hypersensitivity and abnormal intestinal motility in PI-IBS mouse model. Asian Pac J Trop Med. 2016;9:302-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Vork L, Wilms E, Penders J, Jonkers DMAE. Stool Consistency: Looking Beyond the Bristol Stool Form Scale. J Neurogastroenterol Motil. 2019;25:625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Wu Z, Yang L, Shi L, Song H, Shi P, Yang T, Fan R, Jiang T, Song J. Prognostic Impact of Adenosine Receptor 2 (A2aR) and Programmed Cell Death Ligand 1 (PD-L1) Expression in Colorectal Cancer. Biomed Res Int. 2019;2019:8014627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 27. | Sauer KA, Scholtes P, Karwot R, Finotto S. Isolation of CD4+ T cells from murine lungs: a method to analyze ongoing immune responses in the lung. Nat Protoc. 2006;1:2870-2875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Williams L, Dery KJ, Lee WH, Li H, Shively JE, Kujawski M. Isolation and expansion of murine γδ T cells from mouse splenocytes. J Immunol Methods. 2022;508:113322. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 2707] [Article Influence: 169.2] [Reference Citation Analysis (0)] |
| 30. | Li B, Lee C, Chuslip S, Lee D, Biouss G, Wu R, Koike Y, Miyake H, Ip W, Gonska T, Pierro A. Intestinal epithelial tight junctions and permeability can be rescued through the regulation of endoplasmic reticulum stress by amniotic fluid stem cells during necrotizing enterocolitis. FASEB J. 2021;35:e21265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70:631-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 959] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 32. | Mangan BA, Dunne MR, O'Reilly VP, Dunne PJ, Exley MA, O'Shea D, Scotet E, Hogan AE, Doherty DG. Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human Vδ3 T cells. J Immunol. 2013;191:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 33. | Mastelic-Gavillet B, Navarro Rodrigo B, Décombaz L, Wang H, Ercolano G, Ahmed R, Lozano LE, Ianaro A, Derré L, Valerio M, Tawadros T, Jichlinski P, Nguyen-Ngoc T, Speiser DE, Verdeil G, Gestermann N, Dormond O, Kandalaft L, Coukos G, Jandus C, Ménétrier-Caux C, Caux C, Ho PC, Romero P, Harari A, Vigano S. Adenosine mediates functional and metabolic suppression of peripheral and tumor-infiltrating CD8(+) T cells. J Immunother Cancer. 2019;7:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 34. | El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Low-grade inflammation in the rectum of patients with sporadic irritable bowel syndrome. Mol Med Rep. 2013;7:1081-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Leone RD, Lo YC, Powell JD. A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J. 2015;13:265-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 36. | D'Antongiovanni V, Fornai M, Pellegrini C, Benvenuti L, Blandizzi C, Antonioli L. The Adenosine System at the Crossroads of Intestinal Inflammation and Neoplasia. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Yang S, Yu M, Sun L, Xiao W, Yang X, Zhang C, Ma Y, Yang H, Liu Y, Lu D, Teitelbaum DH. Interferon-γ-induced intestinal epithelial barrier dysfunction by NF-κB/HIF-1α pathway. J Interferon Cytokine Res. 2014;34:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 285] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 39. | González-Castro AM, Martínez C, Salvo-Romero E, Fortea M, Pardo-Camacho C, Pérez-Berezo T, Alonso-Cotoner C, Santos J, Vicario M. Mucosal pathobiology and molecular signature of epithelial barrier dysfunction in the small intestine in irritable bowel syndrome. J Gastroenterol Hepatol. 2017;32:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Xiao W, Wang W, Chen W, Sun L, Li X, Zhang C, Yang H. GDNF is involved in the barrier-inducing effect of enteric glial cells on intestinal epithelial cells under acute ischemia reperfusion stimulation. Mol Neurobiol. 2014;50:274-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 41. | Xiao W, Feng Y, Holst JJ, Hartmann B, Yang H, Teitelbaum DH. Glutamate prevents intestinal atrophy via luminal nutrient sensing in a mouse model of total parenteral nutrition. FASEB J. 2014;28:2073-2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Lu YZ, Wu CC, Huang YC, Huang CY, Yang CY, Lee TC, Chen CF, Yu LC. Neutrophil priming by hypoxic preconditioning protects against epithelial barrier damage and enteric bacterial translocation in intestinal ischemia/reperfusion. Lab Invest. 2012;92:783-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Paul S, Shilpi, Lal G. Role of gamma-delta (γδ) T cells in autoimmunity. J Leukoc Biol. 2015;97:259-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 44. | Huang Y, Getahun A, Heiser RA, Detanico TO, Aviszus K, Kirchenbaum GA, Casper TL, Huang C, Aydintug MK, Carding SR, Ikuta K, Huang H, Wysocki LJ, Cambier JC, O'Brien RL, Born WK. γδ T Cells Shape Preimmune Peripheral B Cell Populations. J Immunol. 2016;196:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Bao K, Reinhardt RL. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine. 2015;75:25-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 219] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 46. | Gonzales JN, Gorshkov B, Varn MN, Zemskova MA, Zemskov EA, Sridhar S, Lucas R, Verin AD. Protective effect of adenosine receptors against lipopolysaccharide-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;306:L497-L507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 47. | Koizumi S, Odashima M, Otaka M, Jin M, Linden J, Watanabe S, Ohnishi H. Attenuation of gastric mucosal inflammation induced by indomethacin through activation of the A2A adenosine receptor in rats. J Gastroenterol. 2009;44:419-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Nowak M, Lynch L, Yue S, Ohta A, Sitkovsky M, Balk SP, Exley MA. The A2aR adenosine receptor controls cytokine production in iNKT cells. Eur J Immunol. 2010;40:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 49. | Milne GR, Palmer TM. Anti-inflammatory and immunosuppressive effects of the A2A adenosine receptor. ScientificWorldJournal. 2011;11:320-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 50. | Lin G, Field JJ, Yu JC, Ken R, Neuberg D, Nathan DG, Linden J. NF-κB is activated in CD4+ iNKT cells by sickle cell disease and mediates rapid induction of adenosine A2A receptors. PLoS One. 2013;8:e74664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |