Published online Mar 7, 2023. doi: 10.3748/wjg.v29.i9.1446
Peer-review started: October 4, 2022
First decision: October 17, 2022
Revised: October 27, 2022
Accepted: February 27, 2023
Article in press: February 27, 2023
Published online: March 7, 2023
Processing time: 154 Days and 8.9 Hours
Liver fibrosis is a wound-healing response following chronic liver injury caused by hepatitis virus infection, obesity, or excessive alcohol. It is a dynamic and reversible process characterized by the activation of hepatic stellate cells and excess accumulation of extracellular matrix. Advanced fibrosis could lead to cirrhosis and even liver cancer, which has become a significant health burden worldwide. Many studies have revealed that noncoding RNAs (ncRNAs), including microRNAs, long noncoding RNAs and circular RNAs, are involved in the pathogenesis and development of liver fibrosis by regulating signaling pathways including transforming growth factor-β pathway, phosphatidylinositol 3-kinase/protein kinase B pathway, and Wnt/β-catenin pathway. NcRNAs in serum or exosomes have been reported to tentatively applied in the diagnosis and staging of liver fibrosis and combined with elastography to improve the accuracy of diagnosis. NcRNAs mimics, ncRNAs in mesenchymal stem cell-derived exosomes, and lipid nanoparticles-encapsulated ncRNAs have become promising therapeutic approaches for the treatment of liver fibrosis. In this review, we update the latest knowledge on ncRNAs in the pathogenesis and progression of liver fibrosis, and discuss the potentials and challenges to use these ncRNAs for diagnosis, staging and treatment of liver fibrosis. All these will help us to develop a comprehensive understanding of the role of ncRNAs in liver fibrosis.
Core Tip: Liver fibrosis is an inevitable stage in the development of various chronic liver diseases, and manifests as an imbalance between the formation and degradation of extracellular matrix. The key mechanism of liver fibrosis is the activation of hepatic stellate cells, which is coordinately regulated by a variety of cytokines, inflammatory factors and chemokines involved in multiple cells signaling pathways. In this review, we discuss the role of noncoding RNAs (ncRNAs) in regulating the signaling pathways in the formation and regression of liver fibrosis, and the limitations, challenges, and prospects of ncRNAs in the diagnosis and treatment of liver fibrosis.
- Citation: Li QY, Gong T, Huang YK, Kang L, Warner CA, Xie H, Chen LM, Duan XQ. Role of noncoding RNAs in liver fibrosis. World J Gastroenterol 2023; 29(9): 1446-1459
- URL: https://www.wjgnet.com/1007-9327/full/v29/i9/1446.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i9.1446
Liver fibrosis is the result of excessive accumulation of extracellular matrix (ECM) caused by continuous liver injuries that promote wound healing[1]. Liver injuries can be caused by many factors including persistent hepatitis B virus (HBV)/hepatitis C virus (HCV) infections, excessive alcohol consumption, metabolic diseases, drugs, genetic diseases, cholestasis, and autoimmune diseases. Due to an increase in the prevalence of obesity and type 2 diabetes, liver fibrosis caused by nonalcoholic steatohepatitis (NASH) has been increasing annually in recent years[2]. Liver fibrosis can resolve at an early stage if the injuries subside. Progressive fibrosis is associated with architectural changes to hepatic lobules and may lead to cirrhosis, liver failure, portal hypertension, and even hepatocellular carcinoma (HCC).
Hepatic stellate cells (HSCs) play a central role in liver fibrosis. HSCs, also known as perisinusoidal cells, are located in the Disse space under healthy conditions. When injury occurs, HSCs are activated and transdifferentiate into myofibroblast-like cells which are the main source of ECM[3]. Hepatic fibrosis is a dynamic process coordinated by multiple cells in the liver. Acute injury, such as viral infection, induces an inflammatory response, necrosis, and apoptosis in hepatocytes which leads to liver regeneration and limited ECM deposition. However, if the damage persists, the injured hepatocytes attract an infiltration of inflammatory cells such as T lymphocytes and neutrophils, which will in turn activate HSCs by releasing cytokines, chemokines, and reactive oxygen species (ROS). Activated HSCs can maintain the active state by the mediators produced by the autocrine and paracrine system. In addition, platelet-derived growth factor (PDGF) secreted by liver macrophages (Kupffer cells) stimulates the continuous proliferation of HSCs. Therefore, inhibiting the activation and proliferation of HSCs, promoting the apoptosis of activated HSCs, and reducing the expression of fibrogenic factors are considered to be the key measures for the successful treatment of liver fibrosis.
Noncoding RNAs (ncRNAs) refer to RNAs that are transcribed from the genome but do not normally encode proteins, although some of them have recently been reported to encode small proteins[4]. According to their length, ncRNAs can be divided into short ncRNAs and long ncRNAs (lncRNAs). MicroRNAs (miRNAs) are a class of short ncRNAs of approximately 22 nucleotides in length that act as gene repressors by complementary binding to the 3' untranslated region of target mRNA to degrade or prevent it from being translated to protein[5,6]. LncRNAs are defined as ncRNAs longer than 200 bp with 5’-end m7G caps and 3’-end poly(A) tails. LncRNAs can regulate gene expression in cis or trans, change the structure and function of chromatin via interaction with proteins, or act as competitive endogenous RNAs (ce-RNAs) for post-transcriptional regulation[7]. Circular RNAs (circRNAs) are a novel form of ncRNAs with a covalently closed single-stranded structure, which is formed by back-splicing of the 3' and 5' ends of mRNAs[8]. Depending on their subcellular localization, circRNAs have different biological functions: interfering with signal transduction pathways and regulating the transcription and translation of target genes, sponge proteins and miRNAs[9].
In this review, we summarize the latest findings about miRNAs, lncRNAs, and circRNAs in the pathogenesis and progression of liver fibrosis and discuss the potential of ncRNAs as biomarkers for diagnosis and as therapeutic targets for liver fibrosis.
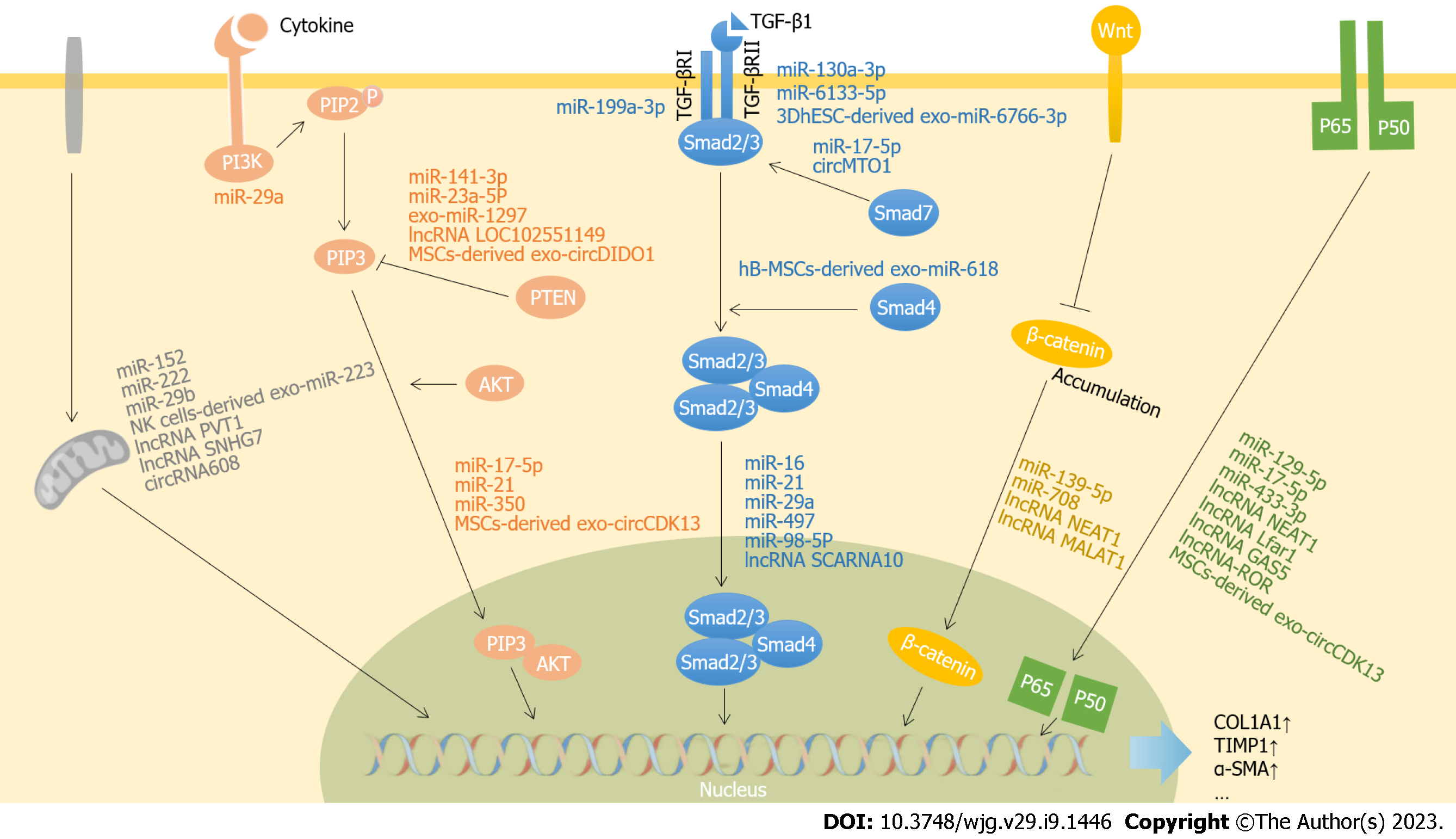
The activation and proliferation of HSCs are essential steps in the development of liver fibrosis. Numerous studies have shown that ncRNAs exert profibrotic effects by regulating genes in the activation and proliferation signaling pathways of HSCs. Signaling pathways closely related to liver fibrosis mainly include transforming growth factor-β (TGF-β)/Smad, phosphatidylinositol 3-kinase (PI3K)/serine/threonine kinase 1 (AKT), Wnt/β-catenin, and nuclear factor κ light chain enhancer of activated B cells (NF-κB) pathways. Although some miRNAs[10] and lncRNAs[11] involved in liver fibrosis have been reviewed elsewhere, we focus on the most recent data published in the past 3 years as summarized in Figure 1.
Transforming growth factor-β1 (TGF-β1) is a well-recognized fibrogenic cytokine that is widely expressed in damaged hepatocytes, Kupffer cells, HSCs, sinusoidal endothelial cells, and platelets. TGF-β1 promotes HSC activation through a canonical (Smad) or noncanonical pathway[12]. In the TGF-β1/Smad signaling pathway, TGF-β1 binds to the TGF-β type II receptor (TGF-βRII) on the cell membrane and then recruits the TGF-β type I receptor (TGF-βRI) to form a heterotetrameric complex. This complex induces the phosphorylation of intracellular Smad2 and Smad3, which then bind with Smad4 and translocate to the nucleus to regulate expression of target genes[13-15]. In addition, TGF-β1 induces the expression of Smad7, which maintains the balance between profibrotic and antifibrosis by negatively regulating TGF-βRI and Smad2[13].
Many miRNAs are involved in the regulation of the TGF-β/Smad signaling pathway and liver fibrosis. These miRNAs include miR-21, miR-497, miR-16, miR-98-5p, miR-199a-3p, miR-29a, and miR-130a-3p. MiR-21 is expressed abundantly in liver, is present in serum, and is positively associated with liver inflammation, fibrosis, and cancer[16]. TGF-β1 induces transcription, processing and maturation of pri-miR-21 through a Smad3-dependent pathway, while mature miR-21 promotes the development of fibrosis by targeting the inhibitory Smad gene-small mothers against decapentaplegic7[15]. Clonorchis sinensis promotes hepatic fibrosis by inducing miR-497 and activating the TGF-β/Smad pathway[17]. Pan et al[18] revealed that miR-16 plays an essential role in the phenotypic remodeling of myofibroblasts. Overexpression of miR-16 restored the phenotype of HSCs and led to fibrotic regression by targeting Smad2 and Wnt3a to interfere with TGF-β and Wnt signaling pathways, respectively[18]. In patients with chronic HBV-induced liver fibrosis, expression of miR-98-5p was significantly downregulated. Further studies indicated that overexpression of miR-98-5p significantly inhibited HSC activation through targeting the TGF-β1/Smad3 signaling pathway[19]. Yang et al[20] demonstrated that expression of miR-199a-3p was upregulated in carbon tetrachloride (CCl4)-induced liver fibrotic rats, and miR-199a-3p activated HSCs by targeting caveolin-2 (CAV2) to increase expression of TGF-βRI. Stimulation with TGF-β resulted in the downregulation of miR-29a, which increased follistatin-like 1 expression and accelerated the progression of fibrosis by enhanced phosphorylation of Smad2[21]. MiR-130a-3p was significantly decreased in liver fibrosis caused by Schistosoma japonicum[22]. It has been shown that miR-130a-3p attenuates fibrosis by inhibiting the activation and proliferation of HSCs and promoting their apoptosis through regulation of mitogen-activated protein kinase 1 and TGF-βRI/II both in vitro and in vivo[22].
In addition to miRNAs, lncRNAs and circRNAs are associated with the TGF-β pathway and liver fibrogenesis. LncRNA small Cajal body-specific RNA 10 (lncRNA SCARNA10) was found to inhibit the expression of polycomb repressive complex 2 to induce hepatocytes apoptosis and HSC activation, thereby stimulating the TGF-β pathway and liver fibrogenesis[23]. CircRNA mitochondrial tRNA translation optimization 1 (circMTO1) was reported to inhibit liver fibrosis through interaction with miR-17-5p and Smad7[24].
The PI3K/AKT pathway is an essential intracellular signaling pathway in the regulation of the cell cycle. The AKT cascade can be activated by cytokine receptors such as receptors of TGF-β and PDGF. PI3K is activated to induce phosphorylation of Phosphatidylinositol-4,5-biophosphate (PIP2) on the cell surface, leading to production of phosphatidylinositol-3,4,5-trisphosphate (PIP3). AKT (also known as protein kinase B, PKB) binds to PIP3 and they are co-translocated to the nucleus, where they regulate target gene expression to stimulate cell proliferation and inhibit apoptosis. Phosphatase and tensin homology deleted on chromosome ten (PTEN) increases the number of activated HSCs by catalyzing dephosphorylation of PIP3 and downregulating the PI3K/AKT signaling pathway. A variety of miRNAs regulate the PI3K/AKT signaling pathway. MiR-21 is significantly upregulated in liver fibrosis induced by cadmium exposure, which leads to the progression of fibrosis by activating the PI3K/AKT pathway[25]. Lipotoxic hepatocyte-derived exosomal miR-1297 promotes HSC proliferation and activation by inhibiting expression of PTEN[26]. MiR-23a-5p activates the PI3K/AKT/mammalian target of rapamycin (mTOR) signaling pathway by inhibiting PTEN and can be targeted by lncRNA LOC102551149 to reduce liver fibrosis[27]. All these results indicate that ncRNAs, especially miRNAs and lncRNAs, play important roles in liver fibrosis through targeting the PI3K/AKT pathway.
Wnt/β-catenin is involved in the development of fibrosis of several tissues, including kidney, lung, skin, and liver. Wnt proteins are cysteine-rich glycoproteins generally secreted to the ECM. β-Catenin is a cytoplasmic protein that can be activated by Wnt and is translocated to the nucleus to activate transcription of target genes, thereby regulating occurrence of fibrosis[28]. Yang et al[29] demonstrated that expression of miR-708 was downregulated in fibrotic liver tissue. The authors further demonstrated that overexpression of miR-708 inhibited activation of HSCs by targeting zinc finger E-box binding homeobox 1 and regulating the Wnt/β-catenin signaling pathway[29]. Different forms of ncRNAs may work together to have a synergistic effect in the pathogenesis and progression of liver fibrosis. For example, lncRNA nuclear enriched abundant transcript1 (lncRNA NEAT1) and miR-139-5p have a synergistic effect that exacerbates the development of liver fibrosis[30]. Another study has revealed that lncRNA metastasis-associated lung adenocarcinoma transcript1 (lncRNA MALAT1) upregulates expression of β-catenin and promotes liver fibrosis through the Wnt/β-catenin pathway[31].
NF-κB is one of the transcription factors that regulates important cellular events, particularly inflammation. NF-κB consists of two subunits p50 and p65, which can be activated by extracellular signals. Activated NF-κB translocate to the nucleus to regulate expression of various cytokines, growth factors, and other target genes. LncRNA NEAT1 plays critical roles in hepatic fibrosis of different etiologies by targeting various miRNAs associated with NF-κB signaling pathways. In NASH-induced liver fibrotic mice, Zhang et al[32] found that lncRNA NEAT1 stimulated expression of paternally expressed gene 3 (PEG3) by inhibiting miR-129-5p, which reduced HSC apoptosis through the NF-κB (p65/p50) signaling pathway. The effect of the lncRNA NEAT1/miR-129-5p axis on liver fibrosis had also been confirmed in alcoholic steatohepatitis mice by targeting suppressor of cytokine signaling 2[33]. In addition, lncRNA NEAT1 also promotes fibrosis via inhibition of miR-148a-3p and miR-22-3p and regulation of cytohesin 3 expression[34]. LncRNA liver fibrosis associated lncRNA1 (lncRNA Lfar1) was demonstrated to promote hepatic fibrosis through activation of HSCs, probably by way of its regulatory effect on macrophages through the NF-κB signaling pathway[35]. Overexpression of lncRNA growth arrest-special transcript 5 (lncRNA GAS5) decreased expression of miR-433-3p, which then intercepted the NF-κB signaling pathway through targeting of toll-like receptor 10[36]. In addition, lncRNA maternally expressed gene 3 (lncRNA MEG3) targeted NLR Family CARD Domain Containing 5 (NLRC5) to reverse liver fibrosis[37]. All of these results indicate that ncRNAs regulating liver fibrosis through targeting the NF-κB pathway are mainly lncRNAs, including lncRNAs NEAT1, Lfar1, GAS5, and MEG3.
Autophagy is a process that regulates self-metabolism and maintains cellular homeostasis by removing cell debris, misfolded proteins and lipid droplets[38]. Activation of autophagy promotes liver fibrosis by increasing the digestion of lipid droplets and activating multiple signaling pathways, which implies that promoting regeneration of lipid droplets and restraining expression of proinflammatory factors inhibits liver fibrosis[38]. In hypoxic conditions, lncRNA plasmacytoma variant translocation 1 (lncRNA PVT1) regulates expression levels of autophagy-related gene (ATG)14 by decreasing miR-152, thereby activating HSCs through the autophagy pathway[39]. LncRNA small nucleolar RNA host gene 7 (lncRNA SNHG7) increased DNA methyltransferase 3 alpha (DNMT3A) expression through binding to miR-29b, which is involved in liver fibrosis and autophagy. Inhibition of lncRNA SNHG7 significantly decreases expression of collagen and autophagy factors, leading to inhibition of liver fibrosis[40]. In addition to lncRNAs, circRNAs are also associated with autophagy and mitophagy. Xu et al[41] illustrated that circRNA608/miR-222 regulates PTEN-induced putative kinase 1-mediated mitophagy and liver fibrosis in NASH-induced fibrotic mice.
Chen et al[42] demonstrated that miR-451 and miR-185 were downregulated in activated HSCs, and they exerted antifibrotic effects synergistically by targeting erythropoietin-producing hepatocellular receptor B2. MiR-451 upregulated expression of miR-185. This occurs at the post-transcriptional level by targeting nuclear export receptor exportin 1 (XPO-1). Zhao et al[43] demonstrated that lncRNA molecule interacting with CasL2 (lncRNA Mical2) upregulated p66 Src homologous-collagen homologue (p66Shc) through sponging miR-203a-3p, which promoted reactive oxygen species (ROS)-mediated epithelial–mesenchymal transition and liver fibrosis. It has been reported that lncRNA X-inactive-specific transcript (lncRNA XIST) damages mitochondrial function and increases ROS production to promote HSC activation by regulating miR-539-3p and ADAM metallopeptidase with thrombospondin type 1 motif 5 (ADAMTS5)[44]. Studies from cholestatic liver injury caused by biliary atresia have indicated that expression of lncRNA H19 is significantly upregulated in exosomes derived from liver and serum. LncRNA H19 deficiency protects mice from liver fibrosis by inhibiting sphingosine-1-phosphate receptor 2/sphingosine kinase 2 activation and by sponging let-7 to upregulate high-mobility group AT-hook 2 expression[45]. It has also been reported that depletion of macrophages significantly reduced lncRNA H19 and inhibited cholestatic liver injury in bile duct ligation mice[46]. LncRNA actin alpha 2-antisense RNA 1 (lncRNA ACTA2-AS1) accelerated liver fibrosis and epigenetic activation by targeting the p300/ETS transcription factor (ELK1) complex in biliary diseases[47]. CircRNA F-box and WD repeat domain containing 4 (circFBXW4) was downregulated significantly in HSCs of mice with liver fibrosis. Overexpression of circFBXW4 inhibited HSC activation by targeting miR-18b-3p to increase FBXW7 expression[48]. Similarly, CircRNA CREB binding protein (circCREBBP) inhibited liver fibrosis by targeting miR-1291 to regulate the expression of left-right determinant cluster 2 (LEFTY2)[49]. Hsa_circ_0071410 inhibited activation of HSCs by binding to miR-9-5p in irradiation-induce liver fibrosis[50]. All these ncRNAs in the pathogenesis and progression of liver fibrosis are summarized in Table 1[51-60].
| ncRNAs | Target genes | Signaling pathways | Ref. |
| miR-199a-3p | CAV2 | TGF-β/Smad | [20] |
| miR-497 | Smad7 | TGF-β/Smad | [17] |
| miR-21 | Smad2/3/7 | TGF-β/Smad | [15] |
| TGF-β | PI3K/AKT | [25] | |
| - | PPARα | [51] | |
| - | PDCD4/AP-1 | [51,52] | |
| - | Smad7/Smad2/3/NOX4, Spry1/ERK/NF-κB | [51,53] | |
| - | HIF-1α/VEGF | [54] | |
| miR-16 | Smad2, Wnt3a | TGF-β/Smad, Wnt | [18] |
| miR-130a-3p | TGF-βRI, TGF-βRII; MAPK1 | TGF-β; MAPK | [22] |
| miR-98-5p | TGF-βRI | TGF-β1/Smad3 | [19] |
| miR-6133-5p | TGF-βRII, FGFRI | TGF-β/Smad2/3, AKT/ERK/JNK | [55] |
| miR-708 | ZEB1 | Wnt/β-catenin | [29] |
| exo-miR-1297 | PTEN | PI3K/AKT | [26] |
| miR-350 | SPRY2 | PI3K/AKT and ERK | [56] |
| miR-34c | ACSL1 | - | [57] |
| miR-200c | HAS2 | - | [58] |
| miR-451, miR-185 | EphB2 | - | [42] |
| miR-20b-5p | STAT3 | STAT3 | [97] |
| lncRNA SNHG7 | miR-29b, DNMT3A | Autophagy pathway | [40] |
| lncRNA PVT1 | miR-152, ATG14 | Autophagy pathway | [39] |
| lncRNA SCARNA10 | PRC2 | TGF-β | [23] |
| lncRNA LOC102551149 | miR-23a-5p, PTEN | PI3K/AKT/mTOR/Snail | [27] |
| lncRNA MALAT1 | - | Wnt/β-catenin | [31] |
| lncRNA NEAT1 | miR-139-5p, β-catenin | β-catenin/SOX9/TGF-β1 | [30] |
| miR-129-5p, PEG3 | NF-κB | [32] | |
| miR-129-5p, SOCS2 | - | [33] | |
| miR-148a-3p, miR-22-3p, Cyth3 | - | [34] | |
| lncRNA Lfar1 | - | NF-κB | [35] |
| lncRNA GAS5 | miR-433-3p, TLR10 | NF-κB | [36] |
| lncRNA-ROR | miR-6499-3p | NF-κB | [99] |
| lncRNA Airn | EZH2 | KLF2-eNOS-sGC | [98] |
| lncRNA MEG3 | NLRC5 | - | [37] |
| lncRNA NORAD | miR-495-3p, S1PR3 | - | [60] |
| lncRNA XIST | miR-539-3p, ADAMTS5 | - | [44] |
| lncRNA Mical2 | miR-203a-3p, p66Shc | - | [43] |
| circRNA608 | miR222, PINK1 | Autophagy pathway | [41] |
| circMTO1 | miR-17-5p, Smad7 | - | [24] |
| circFBXW4 | miR-18b-3p, FBXW7 | - | [48] |
| circCREBBP | miR-1291, LEFTY2 | - | [49] |
| circ_0071410 | miR-9-5p | - | [50] |
| circUbe2k | miR-149-5p, TGF-β2 | - | [59] |
Liver-related mortality increases with the progression of fibrosis. Therefore, it is essential for the early diagnosis of liver fibrosis. At present, the gold standard for the diagnosis of liver fibrosis is still liver biopsy, although it has some limitations such as sampling error, inter- and intra-observer variability[61], invasiveness to patients, and many other complications. Several noninvasive examinations have been introduced in clinical settings, including serum markers, combined indices or scores, and imaging techniques. Hepascore and enhanced liver fibrosis score are based on serum liver fibrosis markers such as tissue metalloproteinases and hyaluronic acid [62]. Elastography, including ultrasound elastography and magnetic resonance elastography, is a method to access liver stiffness quantitatively and it is more accurate than serological markers for diagnosis of advanced liver fibrosis. However, elastography has disadvantages such as unreliable results due to high body mass index (BMI) and high cost, making it unsuitable for population screening[62]. As ncRNAs in the blood are easily accessible for detection, they have potential as novel noninvasive biomarkers for diagnosis of liver fibrosis.
Recent research has shown that stimulation of HSCs with TGF-β and PDGF-BB decreased the intracellular miR-29 expression level but significantly increased miR-29 level in the supernatant vesicles[63]. They verified the results in serum from patients with HCV-related liver fibrosis and mice with CCl4-induced fibrosis[63]. These findings indicate that elevated miR-29 Level in serum may be a promising biomarker for diagnosis of liver fibrosis[63]. Another set of biomarkers (NIS4) consisting of miR-34a-5p, α-2 macroglobulin, YKL-40 and glycated hemoglobin have been developed to successfully identify patients who have a higher risk of disease progression with non-alcoholic fatty liver disease and liver fibrosis. The diagnostic value of the NIS4 algorithm was not affected by age, gender, BMI and transaminase[64]. Similarly, Azar et al[65] constructed a miRNA regulatory network using bioinformatics tools and identified five upregulated miRNAs (miR-21-5p, miR-222-3p, miR-221-3p, miR-181b-5p, and miR-17-5p) that targeted tissue inhibitor of metalloproteinase 3 in activated HSCs, and these results have been verified in a mouse model of liver fibrosis. Zhang et al[66] performed a logistic regression analysis to show that miR-1225-3p, miR-1238, miR-3162-3p, miR-4721, and miR-H7 could distinguish, with high sensitivity and specificity, nonsignificant fibrosis from significant fibrosis in chronic hepatitis B (CHB) patients. Some researchers screened miRNAs in serum from HCV-related liver fibrosis patients and found that miR-484 was significantly downregulated in advanced liver fibrosis compared to early liver fibrosis and liver cancer[67], which indicates that miR-484 may be used as a biomarker for staging liver fibrosis in patients with HCV. Besheer et al[68] performed diffusion-weighted magnetic resonance imaging of livers in patients with liver fibrosis caused by chronic hepatitis C and compared the apparent diffusion coefficient (ADC) with miRNA expression pattern in liver biopsies. They found that ADC was closely associated with expression of miR-200b, miR-21, and miR-29, and the accuracy of ADC combined with miR-200b to distinguish early and late liver fibrosis was 80.2%[68]. In a discovery cohort of 183 patients with non-alcoholic fatty liver disease, scientists identified that plasma miR-193a-5p was consistently maintained at a high level and was closely associated with grade of fibrosis, which was verified in a cohort of 372 additional cases[69]. Results from another study confirmed that miR-103a-3p and miR-425-5p were stably expressed in exosomes of serum derived from mice and humans infected with schistosomiasis[70]. MiR-146a-5p could distinguish mild (grades 0 and I) and severe fibrosis (grades II and III) and could be used for staging liver fibrosis[70].
LncRNAs are useful in the diagnosis of liver fibrosis. A study compared lncRNAs profiles of serum exosomes from patients with liver fibrosis and healthy controls and found that the expression level of lncRNA MALAT1 was significantly increased in the serum of fibrotic patients[31]. Serum lncRNA GAS5 was significantly upregulated in patients with advanced liver fibrosis compared with nonfibrotic patients[71]. Serum lncRNA-p21 had 70% specificity and 100% sensitivity in diagnosing liver fibrosis in patients with CHB[72]. LncRNA SCARNA10 was higher in liver and serum samples in patients with advanced liver fibrosis compared with healthy controls[23].
In addition to miRNAs and lncRNAs, circRNAs have shown differential expression in patients with liver fibrosis. The expression level of circRNA death inducer-obliterator 1 (circDIDO1) was decreased in serous exosomes derived from patients with liver fibrosis[73], while serum circMTO1 was negatively correlated with the degree of liver fibrosis in patients with CHB[24]. All these findings suggest that ncRNAs have potential as novel noninvasive biomarkers for the diagnosis and staging of liver fibrosis with high sensitivity and specificity.
Early liver fibrosis is deemed to be reversible. When the injury is removed, activated HSCs (myofibroblasts) are reduced through deactivation or apoptosis to slow down the fibrotic process and even lead to regression. Studies have shown that patients with chronic hepatitis B or chronic hepatitis C have reduced liver fibrosis after receiving antiviral therapy[74]. In addition, therapies such as antioxidants, renin–angiotensin system inhibitors, and traditional Chinese medicine[75] are also considered promising for treatment of liver fibrosis, although more clinical trials are needed to confirm their safety and efficacy. As extensive cytokines and signaling pathways are involved in the pathogenesis and progression of liver fibrosis, ncRNA-based therapies that target various signaling pathways are being developed based on the outstanding gene silencing effect of miRNAs and the sponging effect of lncRNAs and circRNAs.
With strong inhibitory effects on a variety of fibrotic diseases such as myocardial fibrosis[76], pulmonary fibrosis[77], and renal fibrosis[78], miR-29 families are regarded as a potential therapeutic target for fibrosis. Yang et al[79] reported that miR-29a reduced liver fibrosis and ECM by directly targeting PI3KP85α in cholestatic liver fibrosis, and this supports the potential of miR-29a for the treatment of liver fibrosis. However, recent studies showed that, even though upregulation of miR-29 inhibited fibrosis, it could also lead to type 2 diabetes and insulin resistance[80]. Researchers have assessed the therapeutic effect of a synthetic miR-223 analog in a murine NASH model and have found that miR-223 treatment inhibited HSC activation through the downregulation of transcription of proinflammatory cytokines and chemokines together with NOD-like receptor 3 (NLRP3) inflammasome[81]. In addition, miR-223 was reported to inhibit the activation and proliferation of HSCs by targeting Gliotactin family zinc finger 2 (GLI2) and PDGFRα/β in CCl4-induced liver fibrotic mice[82]. These studies clearly demonstrate the potential of miR-223 as a therapeutic strategy for liver fibrosis, although the underlying mechanisms vary.
Exosomes are small vesicles that are stable in body fluids, low in immunogenicity, can be engulfed by cells, and have been used as delivery vectors for easily degradable molecules such as RNA to treat diseases like liver cancer[83]. Exosomes have also been explored in treating liver fibrosis. Gao et al[84] found that miR-690 produced by Kupffer cells could be delivered to HSCs by exosomes to inhibit fibrosis by targeting nicotinamide adenine dinucleotide kinase. In a murine NASH model, miR-690 mimics decreased liver fibrosis markers and alleviated NASH phenotypes significantly. Exosomal miR-223 derived from natural killer cells has also been shown to target ATG7 in HSCs by inhibiting autophagy, leading to reduced fibrosis[85]. In addition, mesenchymal stem cell (MSC)-derived exosomes have been well studied as a promising treatment option for liver fibrosis[86]. Human bone MSCs (hB-MSCs)-derived exosomal miR-618[87] and human tonsil-derived MSCs (hT-MSCs)-derived exosomal miR-486[88] have been shown to alleviate liver fibrosis by targeting Smad4 and smoothened (Smo) genes, respectively. MiR-6766-3p derived from 3D-cultured human embryonic stem cells were enriched in exosomes and attenuated TGFβ1/SMADs by targeting TGFβRII to inhibit proliferation of HSCs[89]. In another study, adipose-derived stromal cells were transfected with miR-150, and the culture supernatants were collected to treat HSCs or infuse into mice with liver fibrosis. Expression of several fibrosis markers such as Collagen 1A1 and α-smooth muscle actin (α-SMA), as well as the levels of systemic inflammatory cytokines such as interleukin-6 and tumor necrosis factor-α were significantly decreased in miR-150-treated mice compared with the control group[90]. This indicates that the exosomal miR-150 has antifibrotic activity through targeting of the TGF-β pathway. Zhou et al[91] co-cultured HSCs with human umbilical cord MSCs and found that expression of miR-148a-5p in HSCs was significantly upregulated, which decreased liver fibrosis by inhibiting Notch2 in vivo and in vitro.
Bone marrow MSCs have also been shown to reduce liver fibrosis by altering expression of lncRNAs. One such example comes from lncRNA BIHAA1 derived from bone marrow MSC-treated HSCs. Bone marrow MSCs inhibited liver fibrosis by lncRNA BIHAA1 targeting miR-667-5p[92]. Sun et al[93] found that silencing lncRNA SNHG promoted differentiation of bone marrow MSCs into hepatocyte-like cells and reduced cirrhosis through the miR-15a/Smad ubiquitin regulatory factor 1 (SMURF1)/UV radiation resistance associated gene (UVRAG)/ATG5/Wnt5a axis.
CircRNAs have also been investigated to treat liver fibrosis. Ma et al[73] reported that circDIDO1 in exosomes derived from MSCs regulated the PTEN/AKT pathway by sponging miR-141-3p, thereby inhibiting activation of HSCs and reducing expression of α-SMA and Collagen I to alleviate liver fibrosis. Similarly, MSC-derived exosomal circRNA cyclin dependent kinase 13 (circCDK13) inhibited activation of PI3K/AKT and NF-κB signaling pathways to reduce liver fibrosis by regulating miR-17-5p and its target gene K (lysine) acetyltransferase 2B (KAT2B)[94].
Delivery systems are one of the key issues to be resolved in order to protect ncRNAs from being degraded. Lipid nanoparticles (NPs) for ncRNAs delivery have been developed. Hu et al[95] encapsulated miR-30a-5p and an antifibrotic peptide Relaxin into NPs and injected them into fibrotic mice. NPs increased the exosomal miR-30a-5p level, which in turn reversed the activated HSCs into a quiescent state by targeting liver macrophages[95]. Furthermore, NPs encapsulated with miR-29b and Germacrone, a major component of the traditional Chinese medicine Rhizoma curcuma, have been shown to have robust antifibrotic activity in vitro and in vivo[96]. The ncRNAs showed the potential for the treatment of liver fibrosis are collected in Table 2.
| ncRNAs | Target genes | Signaling pathways | Ref. |
| miR-29a | PI3KP85α | PI3K/AKT | [79] |
| Fstl | TGF-β/Smad2, JNK | [21] | |
| miR-223 | NLRP3 inflammasome | NOD signaling pathway | [81] |
| GLI2, PDGFRα/β | Hedgehog, PDGF | [82] | |
| hB-MSCs-derived exo-miR-618 | Smad4 | TGF-β/Smad2 | [87] |
| 3DhESCs-derived exo-miR-6766-3p | TGFβRII | TGF-β/Smad | [89] |
| hT-MSCs-derived exo-miR-486 | Smo | Hedgehog/GlI2 | [88] |
| NK cells-derived exo-miR-223 | ATG7 | Autophagy pathway | [85] |
| KCs-derived exo-miR-690 | NADK | - | [84] |
| lncRNA SNHG | miR-15a, MURF1 | UVRAG/ATG5/Wnt5a | [93] |
| lncRNA BIHAA1 | miR-667-5p | - | [92] |
| MSCs-derived exo-circDIDO1 | miR-141-3p | PTEN/AKT | [73] |
| MSCs-derived exo-circCDK13 | miR-17-5p, KAT2B | PI3K/AKT, NF-κB | [94] |
It is well known that persistent liver fibrosis leads to irreversible fibrosis, decompensated cirrhosis, and even HCC, which emphasizes the importance of treatment during early-stage fibrosis to prevent disease progression. Therefore, it is important to diagnose liver fibrosis before clinical symptoms appear. Although liver biopsy is considered the gold standard for the diagnosis of liver fibrosis, its invasive nature limits its clinical use, especially in early disease stages. Although some ncRNAs are closely associated with the pathogenesis and progression of liver fibrosis, there is still insufficient evidence for diagnosing and staging liver fibrosis using ncRNAs alone. Some studies have suggested combining ncRNAs with other indicators (biomarkers) in blood or with imaging techniques to increase the accuracy of liver fibrosis diagnosis. There is no specific anti-hepatic fibrosis drug in clinical use, although several candidates have already been enrolled in clinical trials. The main strategy for antifibrosis therapy is to treat the etiology and alleviate liver inflammation. NcRNAs are able to target various inflammation-related signaling pathways to reduce liver fibrosis. The latest studies have found that miR-20b-5p[97] and lncRNA Antisense Igf2r RNA (lncRNA Airn)[98] can inhibit HSCs activation to alleviate liver fibrosis process. Salvianolic acid B treatment relieved the activation of HSCs through decreasing the expression of lncRNA regulator of reprogramming (lncRNA-ROR)[99], which providing new targets for the treatment of liver fibrosis. Although most of these findings are based on in vitro studies, and therefore, need validation in vivo. With the rapid progress of techniques such as gene editing, NP-based delivery systems, and synthetic biology, MSC-derived exosomal ncRNAs may become promising treatment options for liver fibrosis in the near future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Agrawal P, United States; Liu Z, China S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 778] [Article Influence: 111.1] [Reference Citation Analysis (0)] |
| 2. | Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70:531-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 1452] [Article Influence: 242.0] [Reference Citation Analysis (1)] |
| 3. | Cai X, Wang J, Zhou Q, Yang B, He Q, Weng Q. Intercellular crosstalk of hepatic stellate cells in liver fibrosis: New insights into therapy. Pharmacol Res. 2020;155:104720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 4. | Wang J, Zhu S, Meng N, He Y, Lu R, Yan GR. ncRNA-Encoded Peptides or Proteins and Cancer. Mol Ther. 2019;27:1718-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 242] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 5. | Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3368] [Cited by in RCA: 4113] [Article Influence: 373.9] [Reference Citation Analysis (1)] |
| 6. | Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20:21-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1523] [Cited by in RCA: 1592] [Article Influence: 265.3] [Reference Citation Analysis (0)] |
| 7. | Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:96-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3257] [Cited by in RCA: 2978] [Article Influence: 744.5] [Reference Citation Analysis (0)] |
| 8. | Xie H, Sun H, Mu R, Li S, Li Y, Yang C, Xu M, Duan X, Chen L. The role of circular RNAs in viral infection and related diseases. Virus Res. 2021;291:198205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Liu CX, Chen LL. Circular RNAs: Characterization, cellular roles, and applications. Cell. 2022;185:2016-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 544] [Article Influence: 181.3] [Reference Citation Analysis (0)] |
| 10. | Tadokoro T, Morishita A, Masaki T. Diagnosis and Therapeutic Management of Liver Fibrosis by MicroRNA. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 11. | He Z, Yang D, Fan X, Zhang M, Li Y, Gu X, Yang M. The Roles and Mechanisms of lncRNAs in Liver Fibrosis. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 12. | Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1221] [Cited by in RCA: 1972] [Article Influence: 246.5] [Reference Citation Analysis (0)] |
| 13. | Xu F, Liu C, Zhou D, Zhang L. TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J Histochem Cytochem. 2016;64:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 566] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 14. | Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, Zhao YY. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 789] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 15. | Noetel A, Kwiecinski M, Elfimova N, Huang J, Odenthal M. microRNA are Central Players in Anti- and Profibrotic Gene Regulation during Liver Fibrosis. Front Physiol. 2012;3:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Wang X, He Y, Mackowiak B, Gao B. MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut. 2021;70:784-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 298] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 17. | Zhou QY, Yang HM, Liu JX, Xu N, Li J, Shen LP, Zhang YZ, Koda S, Zhang BB, Yu Q, Chen JX, Zheng KY, Yan C. MicroRNA-497 induced by Clonorchis sinensis enhances the TGF-β/Smad signaling pathway to promote hepatic fibrosis by targeting Smad7. Parasit Vectors. 2021;14:472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Pan Q, Guo CJ, Xu QY, Wang JZ, Li H, Fang CH. miR-16 integrates signal pathways in myofibroblasts: determinant of cell fate necessary for fibrosis resolution. Cell Death Dis. 2020;11:639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Ma Y, Yuan X, Han M, Xu Y, Han K, Liang P, Liu S, Chen J, Xing H. miR-98-5p as a novel biomarker suppress liver fibrosis by targeting TGFβ receptor 1. Hepatol Int. 2022;16:614-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Yang X, Ma L, Wei R, Ye T, Zhou J, Wen M, Men R, Aqeilan RI, Peng Y, Yang L. Twist1-induced miR-199a-3p promotes liver fibrosis by suppressing caveolin-2 and activating TGF-β pathway. Signal Transduct Target Ther. 2020;5:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 21. | Xu XY, Du Y, Liu X, Ren Y, Dong Y, Xu HY, Shi JS, Jiang D, Xu X, Li L, Xu ZH, Geng Y. Targeting Follistatin like 1 ameliorates liver fibrosis induced by carbon tetrachloride through TGF-β1-miR29a in mice. Cell Commun Signal. 2020;18:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Liu L, Wang P, Wang YS, Zhang YN, Li C, Yang ZY, Liu ZH, Zhan TZ, Xu J, Xia CM. MiR-130a-3p Alleviates Liver Fibrosis by Suppressing HSCs Activation and Skewing Macrophage to Ly6Clo Phenotype. Front Immunol. 2021;12:696069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Zhang K, Han Y, Hu Z, Zhang Z, Shao S, Yao Q, Zheng L, Wang J, Han X, Zhang Y, Chen T, Yao Z, Han T, Hong W. SCARNA10, a nuclear-retained long non-coding RNA, promotes liver fibrosis and serves as a potential biomarker. Theranostics. 2019;9:3622-3638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 24. | Wang W, Dong R, Guo Y, He J, Shao C, Yi P, Yu F, Gu D, Zheng J. CircMTO1 inhibits liver fibrosis via regulation of miR-17-5p and Smad7. J Cell Mol Med. 2019;23:5486-5496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Cui W, Zhou S, Wang Y, Shi X, Liu H. Cadmium exposure activates the PI3K/AKT signaling pathway through miRNA-21, induces an increase in M1 polarization of macrophages, and leads to fibrosis of pig liver tissue. Ecotoxicol Environ Saf. 2021;228:113015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Luo X, Luo SZ, Xu ZX, Zhou C, Li ZH, Zhou XY, Xu MY. Lipotoxic hepatocyte-derived exosomal miR-1297 promotes hepatic stellate cell activation through the PTEN signaling pathway in metabolic-associated fatty liver disease. World J Gastroenterol. 2021;27:1419-1434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 27. | Dong Z, Li S, Si L, Ma R, Bao L, Bo A. Identification lncRNA LOC102551149/miR-23a-5p pathway in hepatic fibrosis. Eur J Clin Invest. 2020;50:e13243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Hu HH, Cao G, Wu XQ, Vaziri ND, Zhao YY. Wnt signaling pathway in aging-related tissue fibrosis and therapies. Ageing Res Rev. 2020;60:101063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 29. | Yang J, Tao Q, Zhou Y, Chen Q, Li L, Hu S, Liu Y, Zhang Y, Shu J, Zhang X, Zhang L. MicroRNA-708 represses hepatic stellate cells activation and proliferation by targeting ZEB1 through Wnt/β-catenin pathway. Eur J Pharmacol. 2020;871:172927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Wang Q, Wei S, Li L, Bu Q, Zhou H, Su W, Liu Z, Wang M, Lu L. miR-139-5p sponged by LncRNA NEAT1 regulates liver fibrosis via targeting β-catenin/SOX9/TGF-β1 pathway. Cell Death Discov. 2021;7:243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Wang T, Zhang C, Meng X, Zhu B, Wang S, Yuan W, Zhang S, Xu J. Long Noncoding RNA Metastasis-Associated Lung Adenocarcinoma Transcript 1 in Extracellular Vesicles Promotes Hepatic Stellate Cell Activation, Liver Fibrosis and β-Catenin Signaling Pathway. Front Physiol. 2022;13:792182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Zhang Z, Wen H, Peng B, Weng J, Zeng F. Downregulated microRNA-129-5p by Long Non-coding RNA NEAT1 Upregulates PEG3 Expression to Aggravate Non-alcoholic Steatohepatitis. Front Genet. 2020;11:563265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Ye J, Lin Y, Yu Y, Sun D. LncRNA NEAT1/microRNA-129-5p/SOCS2 axis regulates liver fibrosis in alcoholic steatohepatitis. J Transl Med. 2020;18:445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 34. | Huang W, Huang F, Zhang R, Luo H. LncRNA Neat1 expedites the progression of liver fibrosis in mice through targeting miR-148a-3p and miR-22-3p to upregulate Cyth3. Cell Cycle. 2021;20:490-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Zhang K, Shi Z, Zhang M, Dong X, Zheng L, Li G, Han X, Yao Z, Han T, Hong W. Silencing lncRNA Lfar1 alleviates the classical activation and pyoptosis of macrophage in hepatic fibrosis. Cell Death Dis. 2020;11:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 36. | Su SB, Tao L, Liang XL, Chen W. Long noncoding RNA GAS5 inhibits LX-2 cells activation by suppressing NF-κB signalling through regulation of the miR-433-3p/TLR10 axis. Dig Liver Dis. 2022;54:1066-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Wu YY, Wu S, Li XF, Luo S, Wang A, Yin SQ, Huang C, Li J. LncRNA MEG3 reverses CCl4-induced liver fibrosis by targeting NLRC5. Eur J Pharmacol. 2021;911:174462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Li Y, Liu R, Wu J, Li X. Self-eating: friend or foe? Theranostics. 2020;10:7993-8017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 39. | Yu F, Dong B, Dong P, He Y, Zheng J, Xu P. Hypoxia induces the activation of hepatic stellate cells through the PVT1-miR-152-ATG14 signaling pathway. Mol Cell Biochem. 2020;465:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Xie Z, Wu Y, Liu S, Lai Y, Tang S. LncRNA-SNHG7/miR-29b/DNMT3A axis affects activation, autophagy and proliferation of hepatic stellate cells in liver fibrosis. Clin Res Hepatol Gastroenterol. 2021;45:101469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Xu ZX, Li JZ, Li Q, Xu MY, Li HY. CircRNA608-microRNA222-PINK1 axis regulates the mitophagy of hepatic stellate cells in NASH related fibrosis. Biochem Biophys Res Commun. 2022;610:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 42. | Chen X, Zhang D, Wang Y, Chen K, Zhao L, Xu Y, Jiang H, Wang S. Synergistic antifibrotic effects of miR-451 with miR-185 partly by co-targeting EphB2 on hepatic stellate cells. Cell Death Dis. 2020;11:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Zhao Y, Wang Z, Zhou J, Feng D, Li Y, Hu Y, Zhang F, Chen Z, Wang G, Ma X, Tian X, Yao J. LncRNA Mical2/miR-203a-3p sponge participates in epithelial-mesenchymal transition by targeting p66Shc in liver fibrosis. Toxicol Appl Pharmacol. 2020;403:115125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Wu XJ, Xie Y, Gu XX, Zhu HY, Huang LX. LncRNA XIST promotes mitochondrial dysfunction of hepatocytes to aggravate hepatic fibrogenesis via miR-539-3p/ADAMTS5 axis. Mol Cell Biochem. 2023;478:291-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Xiao Y, Liu R, Li X, Gurley EC, Hylemon PB, Lu Y, Zhou H, Cai W. Long Noncoding RNA H19 Contributes to Cholangiocyte Proliferation and Cholestatic Liver Fibrosis in Biliary Atresia. Hepatology. 2019;70:1658-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 46. | Tian X, Wang Y, Lu Y, Wang W, Du J, Chen S, Zhou H, Cai W, Xiao Y. Conditional depletion of macrophages ameliorates cholestatic liver injury and fibrosis via lncRNA-H19. Cell Death Dis. 2021;12:646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 47. | Navarro-Corcuera A, Sehrawat TS, Jalan-Sakrikar N, Gibbons HR, Pirius NE, Khanal S, Hamdan FH, Aseem SO, Cao S, Banales JM, Kang N, Faubion WA, LaRusso NF, Shah VH, Huebert RC. Long non-coding RNA ACTA2-AS1 promotes ductular reaction by interacting with the p300/ELK1 complex. J Hepatol. 2022;76:921-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 48. | Chen X, Li HD, Bu FT, Li XF, Chen Y, Zhu S, Wang JN, Chen SY, Sun YY, Pan XY, Yin NN, Xu JJ, Huang C, Li J. Circular RNA circFBXW4 suppresses hepatic fibrosis via targeting the miR-18b-3p/FBXW7 axis. Theranostics. 2020;10:4851-4870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 49. | Yang YR, Hu S, Bu FT, Li H, Huang C, Meng XM, Zhang L, Lv XW, Li J. Circular RNA CREBBP Suppresses Hepatic Fibrosis Via Targeting the hsa-miR-1291/LEFTY2 Axis. Front Pharmacol. 2021;12:741151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Chen Y, Yuan B, Wu Z, Dong Y, Zhang L, Zeng Z. Microarray profiling of circular RNAs and the potential regulatory role of hsa_circ_0071410 in the activated human hepatic stellate cell induced by irradiation. Gene. 2017;629:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 51. | Zhang T, Yang Z, Kusumanchi P, Han S, Liangpunsakul S. Critical Role of microRNA-21 in the Pathogenesis of Liver Diseases. Front Med (Lausanne). 2020;7:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 52. | Zhang Z, Zha Y, Hu W, Huang Z, Gao Z, Zang Y, Chen J, Dong L, Zhang J. The autoregulatory feedback loop of microRNA-21/programmed cell death protein 4/activation protein-1 (MiR-21/PDCD4/AP-1) as a driving force for hepatic fibrosis development. J Biol Chem. 2013;288:37082-37093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 53. | Ning ZW, Luo XY, Wang GZ, Li Y, Pan MX, Yang RQ, Ling XG, Huang S, Ma XX, Jin SY, Wang D, Li X. MicroRNA-21 Mediates Angiotensin II-Induced Liver Fibrosis by Activating NLRP3 Inflammasome/IL-1β Axis via Targeting Smad7 and Spry1. Antioxid Redox Signal. 2017;27:1-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 54. | Sun J, Shi L, Xiao T, Xue J, Li J, Wang P, Wu L, Dai X, Ni X, Liu Q. microRNA-21, via the HIF-1α/VEGF signaling pathway, is involved in arsenite-induced hepatic fibrosis through aberrant cross-talk of hepatocytes and hepatic stellate cells. Chemosphere. 2021;266:129177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 55. | Hamada-Tsutsumi S, Onishi M, Matsuura K, Isogawa M, Kawashima K, Sato Y, Tanaka Y. Inhibitory Effect of a Human MicroRNA, miR-6133-5p, on the Fibrotic Activity of Hepatic Stellate Cells in Culture. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Zhu Y, Pan X, Du N, Li K, Hu Y, Wang L, Zhang J, Liu Y, Zuo L, Meng X, Hu C, Wu X, Jin J, Wu W, Chen X, Wu F, Huang Y. ASIC1a regulates miR-350/SPRY2 by N6 -methyladenosine to promote liver fibrosis. FASEB J. 2020;34:14371-14388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Li B, Liu J, Xin X, Zhang L, Zhou J, Xia C, Zhu W, Yu H. MiR-34c promotes hepatic stellate cell activation and Liver Fibrogenesis by suppressing ACSL1 expression. Int J Med Sci. 2021;18:615-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Kim SM, Song GY, Shim A, Lee JH, Eom CB, Liu C, Yang YM, Seki E. Hyaluronan synthase 2, a target of miR-200c, promotes carbon tetrachloride-induced acute and chronic liver inflammation via regulation of CCL3 and CCL4. Exp Mol Med. 2022;54:739-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 59. | Zhu S, Chen X, Wang JN, Xu JJ, Wang A, Li JJ, Wu S, Wu YY, Li XF, Huang C, Li J. Circular RNA circUbe2k promotes hepatic fibrosis via sponging miR-149-5p/TGF-β2 axis. FASEB J. 2021;35:e21622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 60. | Zou L, Shi C, Wang D, Cheng J, Wang Q, Wang L, Yang G. Long non-coding RNA-non-coding RNA activated by DNA damage inhibition suppresses hepatic stellate cell activation via microRNA-495-3p/sphingosine 1-phosphate receptor 3 axis. Bioengineered. 2022;13:6150-6162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 61. | Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:475-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 370] [Cited by in RCA: 467] [Article Influence: 42.5] [Reference Citation Analysis (2)] |
| 62. | Loomba R, Adams LA. Advances in non-invasive assessment of hepatic fibrosis. Gut. 2020;69:1343-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 229] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 63. | Yu X, Elfimova N, Müller M, Bachurski D, Koitzsch U, Drebber U, Mahabir E, Hansen HP, Friedman SL, Klein S, Dienes HP, Hösel M, Buettner R, Trebicka J, Kondylis V, Mannaerts I, Odenthal M. Autophagy-Related Activation of Hepatic Stellate Cells Reduces Cellular miR-29a by Promoting Its Vesicular Secretion. Cell Mol Gastroenterol Hepatol. 2022;13:1701-1716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 64. | Harrison SA, Ratziu V, Boursier J, Francque S, Bedossa P, Majd Z, Cordonnier G, Sudrik FB, Darteil R, Liebe R, Magnanensi J, Hajji Y, Brozek J, Roudot A, Staels B, Hum DW, Megnien SJ, Hosmane S, Dam N, Chaumat P, Hanf R, Anstee QM, Sanyal AJ. A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:970-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 65. | Azar F, Courtet K, Dekky B, Bonnier D, Dameron O, Colige A, Legagneux V, Théret N. Integration of miRNA-regulatory networks in hepatic stellate cells identifies TIMP3 as a key factor in chronic liver disease. Liver Int. 2020;40:2021-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Zhang Q, Zhang Q, Li B, Qu Y, Li Z, Lu L, Li R, Cai X. The Diagnosis Value of a Novel Model with 5 Circulating miRNAs for Liver Fibrosis in Patients with Chronic Hepatitis B. Mediators Inflamm. 2021;2021:6636947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | El-Maraghy SA, Adel O, Zayed N, Yosry A, El-Nahaas SM, Gibriel AA. Circulatory miRNA-484, 524, 615 and 628 expression profiling in HCV mediated HCC among Egyptian patients; implications for diagnosis and staging of hepatic cirrhosis and fibrosis. J Adv Res. 2020;22:57-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 68. | Besheer T, Elalfy H, Abd El-Maksoud M, Abd El-Razek A, Taman S, Zalata K, Elkashef W, Zaghloul H, Elshahawy H, Raafat D, Elemshaty W, Elsayed E, El-Gilany AH, El-Bendary M. Diffusion-weighted magnetic resonance imaging and micro-RNA in the diagnosis of hepatic fibrosis in chronic hepatitis C virus. World J Gastroenterol. 2019;25:1366-1377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 69. | Johnson K, Leary PJ, Govaere O, Barter MJ, Charlton SH, Cockell SJ, Tiniakos D, Zatorska M, Bedossa P, Brosnan MJ, Cobbold JF, Ekstedt M, Aithal GP, Clément K, Schattenberg JM, Boursier J, Ratziu V, Bugianesi E, Anstee QM, Daly AK; LITMUS Consortium Investigators§; LITMUS Consortium Investigators. Increased serum miR-193a-5p during non-alcoholic fatty liver disease progression: Diagnostic and mechanistic relevance. JHEP Rep. 2022;4:100409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 70. | Cai P, Mu Y, Olveda RM, Ross AG, Olveda DU, McManus DP. Serum Exosomal miRNAs for Grading Hepatic Fibrosis Due to Schistosomiasis. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 71. | Han MH, Lee JH, Kim G, Lee E, Lee YR, Jang SY, Lee HW, Chun JM, Han YS, Yoon JS, Kang MK, Lee WK, Kweon YO, Tak WY, Park SY, Park JG, Hur K. Expression of the Long Noncoding RNA GAS5 Correlates with Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Genes (Basel). 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 72. | Yu F, Zhou G, Huang K, Fan X, Li G, Chen B, Dong P, Zheng J. Serum lincRNA-p21 as a potential biomarker of liver fibrosis in chronic hepatitis B patients. J Viral Hepat. 2017;24:580-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 73. | Ma L, Wei J, Zeng Y, Liu J, Xiao E, Kang Y. Mesenchymal stem cell-originated exosomal circDIDO1 suppresses hepatic stellate cell activation by miR-141-3p/PTEN/AKT pathway in human liver fibrosis. Drug Deliv. 2022;29:440-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 74. | Butt AA, Yan P, Aslam S, Abou-Samra AB, Sherman KE, Shaikh OS. Liver Fibrosis Progression and Mortality in Hepatitis B- and C-Coinfected Persons Treated With Directly Acting Antiviral Agents: Results From ERCHIVES. Clin Infect Dis. 2020;71:664-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Ge C, Tan J, Lou D, Zhu L, Zhong Z, Dai X, Sun Y, Kuang Q, Zhao J, Wang L, Liu J, Wang B, Xu M. Mulberrin confers protection against hepatic fibrosis by Trim31/Nrf2 signaling. Redox Biol. 2022;51:102274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 76. | Liu MN, Luo G, Gao WJ, Yang SJ, Zhou H. miR-29 family: A potential therapeutic target for cardiovascular disease. Pharmacol Res. 2021;166:105510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 77. | Yu Y, Liu X, Zhao Z, Xu Z, Qiao Y, Zhou Y, Qiao H, Zhong J, Dai J, Suo G. The Extracellular Matrix Enriched With Exosomes for the Treatment on Pulmonary Fibrosis in Mice. Front Pharmacol. 2021;12:747223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Shi S, Song L, Yu H, Feng S, He J, Liu Y, He Y. Knockdown of LncRNA-H19 Ameliorates Kidney Fibrosis in Diabetic Mice by Suppressing miR-29a-Mediated EndMT. Front Pharmacol. 2020;11:586895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 79. | Yang YL, Wang FS, Lin HY, Huang YH. Exogenous Therapeutics of Microrna-29a Attenuates Development of Hepatic Fibrosis in Cholestatic Animal Model through Regulation of Phosphoinositide 3-Kinase p85 Alpha. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Dalgaard LT, Sørensen AE, Hardikar AA, Joglekar MV. The microRNA-29 family: role in metabolism and metabolic disease. Am J Physiol Cell Physiol. 2022;323:C367-C377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 81. | Jimenez Calvente C, Del Pilar H, Tameda M, Johnson CD, Feldstein AE. MicroRNA 223 3p Negatively Regulates the NLRP3 Inflammasome in Acute and Chronic Liver Injury. Mol Ther. 2020;28:653-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 82. | Wang X, Seo W, Park SH, Fu Y, Hwang S, Rodrigues RM, Feng D, Gao B, He Y. MicroRNA-223 restricts liver fibrosis by inhibiting the TAZ-IHH-GLI2 and PDGF signaling pathways via the crosstalk of multiple liver cell types. Int J Biol Sci. 2021;17:1153-1167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 83. | Li S, Chen L. Exosomes in Pathogenesis, Diagnosis, and Treatment of Hepatocellular Carcinoma. Front Oncol. 2022;12:793432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 84. | Gao H, Jin Z, Bandyopadhyay G, Cunha E Rocha K, Liu X, Zhao H, Zhang D, Jouihan H, Pourshahian S, Kisseleva T, Brenner DA, Ying W, Olefsky JM. MiR-690 treatment causes decreased fibrosis and steatosis and restores specific Kupffer cell functions in NASH. Cell Metab. 2022;34:978-990.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 80] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 85. | Wang L, Wang Y, Quan J. Exosomal miR-223 derived from natural killer cells inhibits hepatic stellate cell activation by suppressing autophagy. Mol Med. 2020;26:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 86. | Rong X, Liu J, Yao X, Jiang T, Wang Y, Xie F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther. 2019;10:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 239] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 87. | Sun C, Shi C, Duan X, Zhang Y, Wang B. Exosomal microRNA-618 derived from mesenchymal stem cells attenuate the progression of hepatic fibrosis by targeting Smad4. Bioengineered. 2022;13:5915-5927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 88. | Kim J, Lee C, Shin Y, Wang S, Han J, Kim M, Kim JM, Shin SC, Lee BJ, Kim TJ, Jung Y. sEVs from tonsil-derived mesenchymal stromal cells alleviate activation of hepatic stellate cells and liver fibrosis through miR-486-5p. Mol Ther. 2021;29:1471-1486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 89. | Wang N, Li X, Zhong Z, Qiu Y, Liu S, Wu H, Tang X, Chen C, Fu Y, Chen Q, Guo T, Li J, Zhang S, Zern MA, Ma K, Wang B, Ou Y, Gu W, Cao J, Chen H, Duan Y. 3D hESC exosomes enriched with miR-6766-3p ameliorates liver fibrosis by attenuating activated stellate cells through targeting the TGFβRII-SMADS pathway. J Nanobiotechnology. 2021;19:437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 90. | Paik KY, Kim KH, Park JH, Lee JI, Kim OH, Hong HE, Seo H, Choi HJ, Ahn J, Lee TY, Kim SJ. A novel antifibrotic strategy utilizing conditioned media obtained from miR-150-transfected adipose-derived stem cells: validation of an animal model of liver fibrosis. Exp Mol Med. 2020;52:438-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 91. | Zhou Q, Rong C, Gu T, Li H, Wu L, Zhuansun X, Zhao X, Xiao Z, Kuang Y, Xu S, Wang S. Mesenchymal stem cells improve liver fibrosis and protect hepatocytes by promoting microRNA-148a-5p-mediated inhibition of Notch signaling pathway. Stem Cell Res Ther. 2022;13:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 92. | Feng Y, Li Y, Xu M, Meng H, Dai C, Yao Z, Lin N. Bone marrow mesenchymal stem cells inhibit hepatic fibrosis via the AABR07028795.2/rno-miR-667-5p axis. Stem Cell Res Ther. 2022;13:375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 93. | Sun J, Sun X, Hu S, Wang M, Ma N, Chen J, Duan F. Long noncoding RNA SNHG1 silencing accelerates hepatocyte-like cell differentiation of bone marrow-derived mesenchymal stem cells to alleviate cirrhosis via the microRNA-15a/SMURF1/UVRAG axis. Cell Death Discov. 2022;8:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 94. | Ma J, Li Y, Chen M, Wang W, Zhao Q, He B, Zhang M, Jiang Y. hMSCs-derived exosome circCDK13 inhibits liver fibrosis by regulating the expression of MFGE8 through miR-17-5p/KAT2B. Cell Biol Toxicol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 95. | Hu M, Wang Y, Liu Z, Yu Z, Guan K, Liu M, Wang M, Tan J, Huang L. Hepatic macrophages act as a central hub for relaxin-mediated alleviation of liver fibrosis. Nat Nanotechnol. 2021;16:466-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 96. | Ji, Wang Q, Zhao Q, Tong H, Yu M, Wang M, Lu T, Jiang C. Co-delivery of miR-29b and germacrone based on cyclic RGD-modified nanoparticles for liver fibrosis therapy. J Nanobiotechnology. 2020;18:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 97. | Lv L, Wang D, Yin J, Yang T, Huang B, Cao Y, Lu J. Downregulation of miR-20b-5p Contributes to the Progression of Liver Fibrosis via the STAT3 Signaling Pathway In Vivo and In Vitro. Dig Dis Sci. 2023;68:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 98. | Chen T, Shi Z, Zhao Y, Meng X, Zhao S, Zheng L, Han X, Hu Z, Yao Q, Lin H, Du X, Zhang K, Han T, Hong W. LncRNA Airn maintains LSEC differentiation to alleviate liver fibrosis via the KLF2-eNOS-sGC pathway. BMC Med. 2022;20:335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 99. | Wang R, Li S, Chen P, Yue X, Wang S, Gu Y, Yuan Y. Salvianolic acid B suppresses hepatic stellate cell activation and liver fibrosis by inhibiting the NF-κB signaling pathway via miR-6499-3p/LncRNA-ROR. Phytomedicine. 2022;107:154435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |