Published online Feb 28, 2023. doi: 10.3748/wjg.v29.i8.1344
Peer-review started: October 17, 2022
First decision: January 3, 2023
Revised: January 13, 2023
Accepted: February 14, 2023
Article in press: February 14, 2023
Published online: February 28, 2023
Processing time: 133 Days and 1.4 Hours
Cervical cancer is one of the most common gynecological malignant tumors. Radiation enteritis (RE) leads to radiotherapy intolerance or termination of radiotherapy, which negatively impacts the therapeutic effect and seriously affects the quality of life of patients. If the incidence of RE in patients can be predicted in advance, and targeted clinical preventive treatment can be carried out, the side effects of radiotherapy in cervical cancer patients can be significantly reduced. Furthermore, accurate prediction of RE is essential for the selection of individualized radiation dose and the optimization of the radiotherapy plan.
To analyze the relationships between severe acute RE (SARE) of cervical cancer radiotherapy and clinical factors and dose-volume parameters retrospectively.
We included 50 cervical cancer patients who received volumetric modulated arc therapy (VMAT) from September 2017 to June 2018 in the Department of Radiotherapy at The First Affiliated Hospital Soochow University. Clinical and dose-volume histogram factors of patients were collected. Logistic regression analysis was used to evaluate the predictive value of each factor for SARE. A nomogram to predict SARE was developed (SARE scoring system ≥ 3 points) based on the multiple regression coefficients; validity was verified by an internal verification method.
Gastrointestinal and hematological toxicity of cervical cancer VMAT gradually increased with radiotherapy and reached the peak at the end of radiotherapy. The main adverse reactions were diarrhea, abdominal pain, colitis, anal swelling, and blood in the stool. There was no significant difference in the incidence of gastrointestinal toxicity between the radical and postoperative adjuvant radiotherapy groups (P > 0.05). There were significant differences in the small intestine V20, V30, V40, and rectal V40 between adjuvant radiotherapy and radical radiotherapy after surgery (P < 0.05). Univariate and multivariate analyses revealed anal bulge rating (OR: 14.779, 95%CI: 1.281-170.547, P = 0.031) and disease activity index (DAI) score (OR: 53.928, 95%CI: 3.822-760.948, P = 0.003) as independent predictors of SARE.
Anal bulge rating (> 0.500 grade) and DAI score (> 2.165 points) can predict SARE. The nomogram shows potential value in clinical practice.
Core Tip: Radiation enteritis (RE) not only seriously affects the quality of life of patients, but it also leads to radiotherapy intolerance or termination of radiotherapy. The aim of our study was to determine the cumulative incidence of acute RE associated with cervical cancer radiotherapy in patients with RE in organs at risk and changes in dose-volume histogram indices. The nomogram of severe acute RE (SARE) was further developed according to the clinical factors, cumulative incidence of SARE and dosimetric parameters of volumetric modulated arc therapy patients, which may be useful for individualized risk assessment and accurate prediction of SARE to guide clinical treatment strategies.
- Citation: Ma CY, Zhao J, Gan GH, He XL, Xu XT, Qin SB, Wang LL, Li L, Zhou JY. Establishment of a prediction model for severe acute radiation enteritis associated with cervical cancer radiotherapy. World J Gastroenterol 2023; 29(8): 1344-1358
- URL: https://www.wjgnet.com/1007-9327/full/v29/i8/1344.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i8.1344
Cervical cancer is one of the most common gynecological malignant tumors, ranking fourth among malignant tumors in females worldwide[1]. Radiotherapy plays an important role in the postoperative adjuvant treatment of cervical cancer patients and the radical treatment of patients who are ineligible for surgery. Radiation enteritis (RE) is a common and potentially dose-limiting toxic reaction of pelvic radiotherapy. The clinical manifestations include nausea, vomiting, abdominal pain, bloody stool, mucous stool, diarrhea, tenesmus, and urinary incontinence. RE not only seriously affects the quality of life of patients, but it also leads to radiotherapy intolerance or termination of radiotherapy, which negatively impacts the therapeutic effect. If the incidence of RE in patients can be predicted in advance, and targeted clinical preventive treatment can be carried out, the side effects of radiotherapy in cervical cancer patients can be significantly reduced. Furthermore, accurate prediction of RE is essential for the selection of individualized radiation dose and the optimization of the radiotherapy plan.
Many studies have shown that the occurrence and development of RE are related to intestinal radiotherapy technology, total dose, total volume, fraction dose, ratio of dose to volume, and uniformity of radiation dose distribution[2-6]. RE is also influenced by factors such as inflammatory bowel disease[7], collagen vascular disease[8], history of abdominal and pelvic surgery[9], history of pelvic inflammatory disease, diabetes mellitus[10], and acquired immunodeficiency syndrome[11]. However, there is no consensus on the relative importance of these predictors. Only a few studies have explored the dosimetric risk factors for RE in cervical cancer patients[12]. How to predict RE and reduce adverse reactions is the core issue of clinical radiotherapy for cervical cancer. Notably, a single predictor has limited predictive power and cases can show substantial differences in heterogeneity of RE; these issues should be addressed in further research[13]. To specifically and accurately identify RE, there is an urgent need for an accurate predictive model that combines multiple factors.
The initial objective of this study was to determine the cumulative incidence of acute RE associated with intensity modulated radiation therapy (IMRT) for cervical cancer in patients with RE in organs at risk (OAR) and changes in dose-volume histogram indices. The severe acute RE scoring system (SARE-SS) was defined by integrating clinical factors and dosimetric physical parameters. Finally, the nomogram of severe acute RE (SARE) was further developed according to the clinical factors, cumulative incidence of SARE and dosimetric parameters of volumetric modulated arc therapy (VMAT) patients. This nomogram may be useful for individualized risk assessment and accurate prediction of SARE to guide clinical treatment strategies.
Fifty patients with cervical cancer who received pelvic local radiotherapy in the Department of Radiation Oncology of The First Affiliated Hospital of Soochow University from August 2017 to August 2018 were selected. The inclusion criteria were as follows: (1) 18-80 years old; (2) cervical cancer confirmed by pathology; (3) no history of intestinal diseases or metabolic diseases; and (4) good understanding and communication skills. The exclusion criteria were as follows: (1) Severe heart, liver and kidney dysfunction; (2) termination of treatment because of serious complications during and after radiotherapy (such as cardiopulmonary, hepatic and renal insufficiency, severe infection, severe bone marrow suppression, massive hemorrhage, vaginorectal fistula and vaginovesical fistula); and (3) refusal to participate in the study.
This study was approved by the ethics committee of The First Affiliated Hospital of Soochow University Medical Ethics Committee [approval No. 2016(100)]. All enrolled patients provided signed informed consent.
Radiotherapy simulation positioning: On the night before radiotherapy, all patients took laxatives to clean the intestinal track. One hour before positioning, the bladder was emptied and patients drank 800 mL of water to fill the bladder. The patients then took the supine position. A Philips [Brilliance computed tomography (CT) Big Bore] large aperture CT machine was used for simulated CT localization, and enhanced CT scanning was performed with a slice thickness of 5 mm. The scanning range was from the upper edge of T11 vertebral body to 5 cm below the ischial tuberosity. The CT localization images were transferred to the treatment planning system (V5.1.1, Elekta, Monaco, Sweden).
Radiotherapy target delineation: Magnetic resonance imaging (MRI), CT or positron emission tomography-CT (PET-CT) examinations were routinely performed during the pre-radiotherapy evaluation. The target volume and OAR were delineated according to the standards of Radiation Therapy Oncology Group and Japan Clinical Oncology Group.
Radiotherapy plan design: The Monaco treatment planning system was used to design the 7-field reverse dynamic VMAT plan using unified parameters. The X-ray beam energy was 6 MV. At least 95% of the pelvic lymphatic drainage (PTV) is required to reach the prescribed dose, and no hot spot of ≥ 110% dose can occur outside the PTV. The OAR dose limit was uniform (OAR dose limitation: Bladder V40 < 0%, rectum V40 < 40%, colon V40 < 30%, small intestine V40 < 20%, femoral head V45 < 5%).
Prescription dose of the VMAT target volume after operation: We administered 45 Gy/25 fractions for moderate risk included lymph node metastasis, paracytal invasion, and positive margin) PTV1 and 50 Gy/25 fractions for high risk PTV1 50. 4 Gy/28 fractions, the prescription dose of the remaining target volume can be executed according to the radical external irradiation scheme.
Prescription dose of the radical VMAT target volume: We administered the following doses: PTV1 45 Gy/25 fractions, parametrial area (PTV2) 50 Gy/25 fractions, and positive lymph nodes (PGTVnd) 56 Gy/28 fractions. In cases with para-aortic area, the dose for the para-aortic lymphatic drainage area (PTV3) was 36-40 Gy/20 fractions.
Implementation of the VMAT plan: VMAT was performed using the Elekta Synergy linear accelerator. All patients were treated five times a week, with one treatment a day. Cone-beam CT (Elekta) image-guided VMAT was performed at least once a week, and the error of each treatment was kept within 3 mm.
Of the 50 cervical cancer patients included in this study, 18 were treated with concurrent chemoradiotherapy (CCRT) and 32 received sequential chemoradiotherapy (SCRT). All doses and adjustments of the chemotherapy regimen followed the NCCN guidelines[14].
General data collection: Data on age, weight, height, body mass index (BMI)[15], age-adjusted Charlson Comorbidity Index (aCCI), pathological data, HPV, squamous cell carcinoma antigen, risk rating, FIGO 2018 staging information, surgery, radiotherapy and chemotherapy were collected.
Data collection of acute RE: The adverse reactions of all patients were monitored at baseline and 2 wk, 4 wk, and 6 wk after the start of radiotherapy. Adverse reactions included diarrhea, abdominal pain, colitis, anal distension, hematochezia and bone marrow suppression. The diagnosis and classification of RE were determined following the National Institutes of Health Common Adverse Events Evaluation Criteria (CTCAE 5.0).
We established the SARE-SS to reflect the adverse reaction score as follows: SARE-SS = the sum of CTCAE scores of diarrhea + abdominal pain + colitis + anal bulging + hematochezia. A score of ≥ 3 indicated the presence of SARE. The diagnosis of SARE was determined by at least two experienced radiation oncologists on the basis of clinical symptoms and changes in the results of ancillary tests.
Disease activity index determination: The disease activity index (DAI) is based on scores that reflect the weight loss of patients (weight unchanged is 0, 1%-5% is 1 point, 5%-10% is 2 points, 10%-15% is 3 points, more than 15% is 4 points), stool viscosity (normal is 0 points, loose stool is 2 points, diarrhea is 4 points) and stool bleeding (normal is 0 points, occult blood positive is 2 points, dominant bleeding 4 points). The sum of the three scores is divided by 3 to obtain the final DAI score.
Radiotherapy planning parameter collection: Statistics of different OAR dose-volume relationship (percentage of PTV volume receiving prescription dose), recorded as Vx.
Statistical analysis was performed using SPSS 20.0. The quantitative data of normal distribution were expressed as (mean ± SD), and t test was used for comparisons between groups. The data of skew distribution were tested by Mann-Whitney U test. Qualitative data were expressed as number of cases and percentage; Fisher’s exact probability method or χ2test was used to compare unordered categorical data, and Mantel-Haenszel χ2and Mann-Whitney U test were used to compare ordered categorical data. P < 0.05 was considered to indicate statistical significance. Receiver operating characteristic curve (ROC curve) was used to analyze the specificity and sensitivity of OAR dose-volume parameters in predicting RE. Two-factor repeated-measures ANOVA was used to analyze the relationship between DAI scores and time in different groups, and one-way ANOVA was used to compare the differences of DAI scores among four time points in each group.
To establish the prediction model, univariate logistic regression model was used to evaluate the predictive ability of each factor for SARE. Multivariate analysis was performed for significant factors from the univariate analysis. Kendall correlation analysis was used to avoid multicollinearity between factors. Factors with significant predictive value in the multivariate analysis were used to construct a nomogram. The area under the ROC curve (AUC), calibration curve and decision curve analysis (DCA) were used for nomogram validation. Data analysis was performed using R software (software version 4.0.2, R package version rmda 1.6).
This study included 50 patients with cervical cancer. Among the 50 patients, 14 patients received radical radiotherapy, 35 patients received postoperative adjuvant pelvic radiotherapy and 1 patient received pelvic radiotherapy combined with after loading vaginal radiotherapy because the vaginal margin was 2 cm away from the tumor boundary. The specific clinical factors are detailed in Table 1. The mean age of the postoperative adjuvant radiotherapy group was significantly lower than that of radical radiotherapy group (P < 0.05) (Table 2). The BMI of the postoperative adjuvant radiotherapy group was also significantly lower (P < 0.05), but there was no significant difference in weight, pathological type, cumulative incidence of RE and SARE and irradiation of para-aortic extension field between the two groups (P > 0.05).
| Characteristic | n (%) |
| Age, median (IQR) | 51.0 (45.5-62.0) |
| Weight, IQR | 55.0 (50.5-61.8) |
| BMI/kg/m2, IQR | 21.5 (20.7-22.9) |
| aCCI | |
| 2-3/4-5 | 38 (76)/12 (24) |
| Pathological diagnosis | |
| Squamous cell carcinoma | 47 (94) |
| Adenocarcinoma | 1 (2) |
| Adenosquamous carcinoma | 2 (4) |
| T | |
| > 4 cm/≤ 4 cm | 24 (48)/26 (52) |
| N | |
| Negative/positive | 15 (30)/35 (70) |
| Metastatic pelvic lymph nodes | |
| Positive/negative | 14 (28)/36 (72) |
| Metastatic common iliac lymph nodes | |
| Positive/negative | 5 (10)/45 (90) |
| Para-aortic lymph nodes | |
| Positive/negative | 2 (4)/48 (96) |
| FIGO staging | |
| I/II/III/IVA | 19 (38)/21 (42)/5 (10)/5 (10) |
| LVSI | |
| Positive/negative | 16 (32)/34 (68) |
| Degree of differentiation | |
| Low and medium differentiation/high differentiation | 40 (80)/10 (20) |
| Degree of infiltration | |
| Shallow 1/3/Medium 1/3/Deep 1/3 | 10 (20)/16 (32)/24 (48) |
| Incised margin | |
| R1 + R2/R0 | 5 (10)/45 (90) |
| Danger degree | |
| Low/medium/high risk | 15 (30)/12 (24)/23 (46) |
| HPV | |
| Positive/negative | 46 (92)/4 (8) |
| SCC | |
| Abnormally elevated/no abnormality observed | 17 (34)/33 (66) |
| RE | |
| Occurred/not occurred | 42 (84)/8 (16) |
| SARE/Non-SARE | 15 (30)/35 (70) |
| Operation | |
| Radical surgery/no surgery | 36 (72)/14 (28) |
| Chemotherapy | |
| CCRT/SCRT | 18 (36)/32 (64) |
| Pelvic External Radiation Dose, IQR | 45.0 (45.0-48.0) |
| Para-aortic extension field | |
| Radiotherapy/no radiotherapy | 4 (8)/46 (92) |
| Factor | Postoperative adjuvant radiotherapy group | Radical radiotherapy group | T/χ2 values | P value |
| Age/yr | 48.25 ± 8.05 | 61.86 ± 13.17 | -4.450 | < 0.001 |
| Young and middle-aged | 33 (92) | 5 (36) | 17.301 | < 0.001 |
| Old age | 3 (8) | 9 (64) | ||
| Weight | 57.31 ± 8.00 | 53.07 ± 8.74 | 1.638 | 0.108 |
| BMI/kg/m2 | 21.86 ± 2.07 | 20.21 ± 2.79 | 2.284 | 0.027 |
| Tumor type | 0.1861 | |||
| Squamous cell carcinoma | 35 (97) | 12 (86) | ||
| Adenocarcinoma | 0 (0) | 1 (7) | ||
| Adenosquamous carcinoma | 1 (3) | 1 (7) | ||
| CCRT | 0.397 | 0.529 | ||
| Implement | 12 (33) | 6 (43) | ||
| Not implemented | 24 (67) | 8 (57) | ||
| Para-aortic extended field radiotherapy | 0.1861 | |||
| Implement | 1 (3) | 2 (14) | ||
| Not implemented | 35 (97) | 12 (86) | ||
| RE | - | 1.0001 | ||
| Occurred | 30 (83) | 12 (86) | ||
| Did not occur | 6 (17) | 2 (14) | ||
| SARE | 2.286 | 0.131 | ||
| Occurred | 13 (36) | 2 (14) | ||
| Did not occur | 23 (64) | 12 (86) |
Patients in the radical radiotherapy group were treated with total pelvic and parauterine local radiotherapy, and patients in the postoperative adjuvant radiotherapy group were treated with total pelvic prophylactic radiotherapy. The dose and volume of small intestine in the radical radiotherapy group were significantly reduced compared with that in the postoperative adjuvant radiotherapy group, and the decrease of V20, V30 and V40 were statistically significant (P < 0.05) (Table 3). Rectal V30, V35, and V40 in the radical radiotherapy group were significantly higher than those in the postoperative adjuvant radiotherapy group. The increase of V40 was statistically significant (P < 0.05). There was no significant difference in the dose-volume relationship of colon, bladder and femoral head between the two groups (P > 0.05).
| OAR | Group | V20 | V25 | V30 | V35 | V40 |
| Small intestine | Postoperative adjuvant radiotherapy group | 77.41 ± 16.49 | 51.90 ± 10.99 | 39.51 ± 8.24 | 26.40 ± 5.50 | 19.25 ± 4.12 |
| Radical radiotherapy group | 67.75 ± 14.10 | 50.11 ± 9.86 | 32.92 ± 7.91 | 25.18 ± 7.12 | 15.07 ± 4.65 | |
| Z value | -2.377 | -0.864 | -2.701 | -0.367 | -3.003 | |
| P value | 0.017 | 0.387 | 0.007 | 0.713 | 0.003 | |
| Colon | Postoperative adjuvant radiotherapy group | 100.00 ± 0.00 | 99.88 ± 0.73 | 98.26 ± 6.09 | 91.36 ± 11.67 | 75.64 ± 12.59 |
| Radical radiotherapy group | 95.57 ± 16.59 | 95.34 ± 17.44 | 94.21 ± 21.66 | 91.09 ± 21.12 | 84.17 ± 7.94 | |
| Z value | -1.604 | -0.732 | -0.332 | -0.750 | -2.247 | |
| P value | 0.109 | 0.464 | 0.740 | 0.453 | 0.025 | |
| Rectum | Postoperative adjuvant radiotherapy group | 100.00 ± 0.00 | 100.00 ± 0.00 | 99.17 ± 2.90 | 95.63 ± 9.41 | 75.64 ± 12.59 |
| Radical radiotherapy group | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 99.60 ± 1.48 | 84.17 ± 7.94 | |
| Z value | 0.000 | 0.000 | -1.102 | -1.745 | -2.247 | |
| P value | 1.000 | 1.000 | 0.270 | 0.081 | 0.025 | |
| Bladder | Postoperative adjuvant radiotherapy group | 70.87 ± 28.16 | 68.75 ± 24.41 | 47.18 ± 23.63 | 35.58 ± 16.04 | 27.33 ± 12.22 |
| Radical radiotherapy group | 74.08 ± 27.93 | 71.94 ± 25.87 | 52.54 ± 28.93 | 40.40 ± 21.04 | 31.18 ± 16.28 | |
| T-value | -0.354 | -0.523 | -0.519 | -0.605 | -0.627 | |
| P value | 0.723 | 0.601 | 0.604 | 0.545 | 0.531 | |
| Femoral head | Postoperative adjuvant radiotherapy group | 48.55 ± 28.80 | 34.45 ± 22.27 | 24.73 ± 16.00 | 18.12 ± 11.68 | 12.23 ± 8.10 |
| Radical radiotherapy group | 39.60 ± 19.76 | 27.13 ± 14.03 | 19.54 ± 10.07 | 14.42 ± 7.69 | 9.47 ± 5.37 | |
| T-value | -0.951 | -0.929 | -0.994 | -0.929 | -0.983 | |
| P value | 0.342 | 0.353 | 0.320 | 0.353 | 0.326 |
The main radiotherapy-related adverse reactions included diarrhea, abdominal pain, colitis, anal distension, hematochezia and bone marrow suppression. All adverse reactions were grade 1 to 3; no adverse reactions above grade 3 occurred. RE was monitored as follows (Table 4). There was no significant difference in the incidences of adverse reaction between the radical radiotherapy group and postoperative adjuvant radiotherapy group (P > 0.05).
| Adverse reaction | Group | Level 0 | Level 1 | Level 2 | Level 3 | χ2 value | P value |
| Diarrhea | Postoperative adjuvant radiotherapy group | 10 (28) | 19 (53) | 5 (14) | 2 (5) | 2.748 | 0.432 |
| Radical radiotherapy group | 3 (21) | 10 (72) | 0 (0) | 1 (7) | |||
| Abdominal pain | Postoperative adjuvant radiotherapy group | 21 (58) | 12 (33) | 3 (9) | 0 (0) | 1.423 | 0.491 |
| Radical radiotherapy group | 8 (57) | 6 (43) | 0 (0) | 0 (0) | |||
| Colitis | Postoperative adjuvant radiotherapy group | 25 (69) | 11 (31) | 0 (0) | 0 (0) | - | 0.1401 |
| Radical radiotherapy group | 13 (93) | 1 (7) | 0 (0) | 0 (0) | |||
| Anal bulging | Postoperative adjuvant radiotherapy group | 22 (61) | 9 (25) | 5 (14) | 0 (0) | 1.374 | 0.503 |
| Radical radiotherapy group | 11 (79) | 2 (14) | 1 (7) | 0 (0) | |||
| Blood in the stool | Postoperative adjuvant radiotherapy group | 23 (64) | 13 (36) | 0 (0) | 0 (0) | 0.1791 | |
| Radical radiotherapy group | 12 (86) | 2 (14) | 0 (0) | 0 (0) | |||
| Myelosuppression | Postoperative adjuvant radiotherapy group | 12 (33) | 9 (25) | 10 (28) | 5 (14) | 3.691 | 0.297 |
| Radical radiotherapy group | 3 (21) | 4 (29) | 2 (14) | 5 (36) |
ROC curve was used to analyze the specificity and sensitivity of small intestine V20, V30, V40 and rectum V40 in predicting acute/SARE. The AUC was less than 0.60 and P > 0.05. These results suggest that a single OAR dose-volume parameter is not enough to predict the occurrence of acute/SARE.
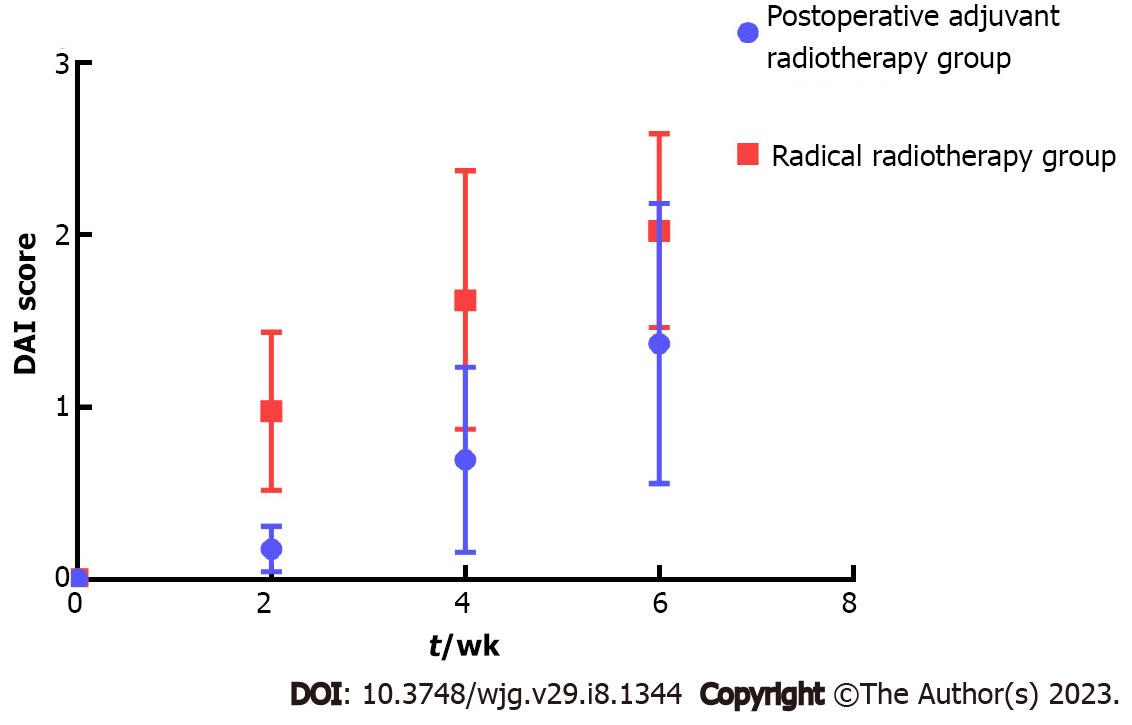
The DAI scores of the radical radiotherapy group were higher than those of the postoperative adjuvant radiotherapy group at the second week, fourth week and the end of radiotherapy (Figure 1). There was an interaction effect between different groups and time (χ2 = 77. 238, P < 0.01). The DAI scores of the two groups showed an increasing trend over time (P < 0.05), and the DAI scores of the radical radiotherapy group increased the most.
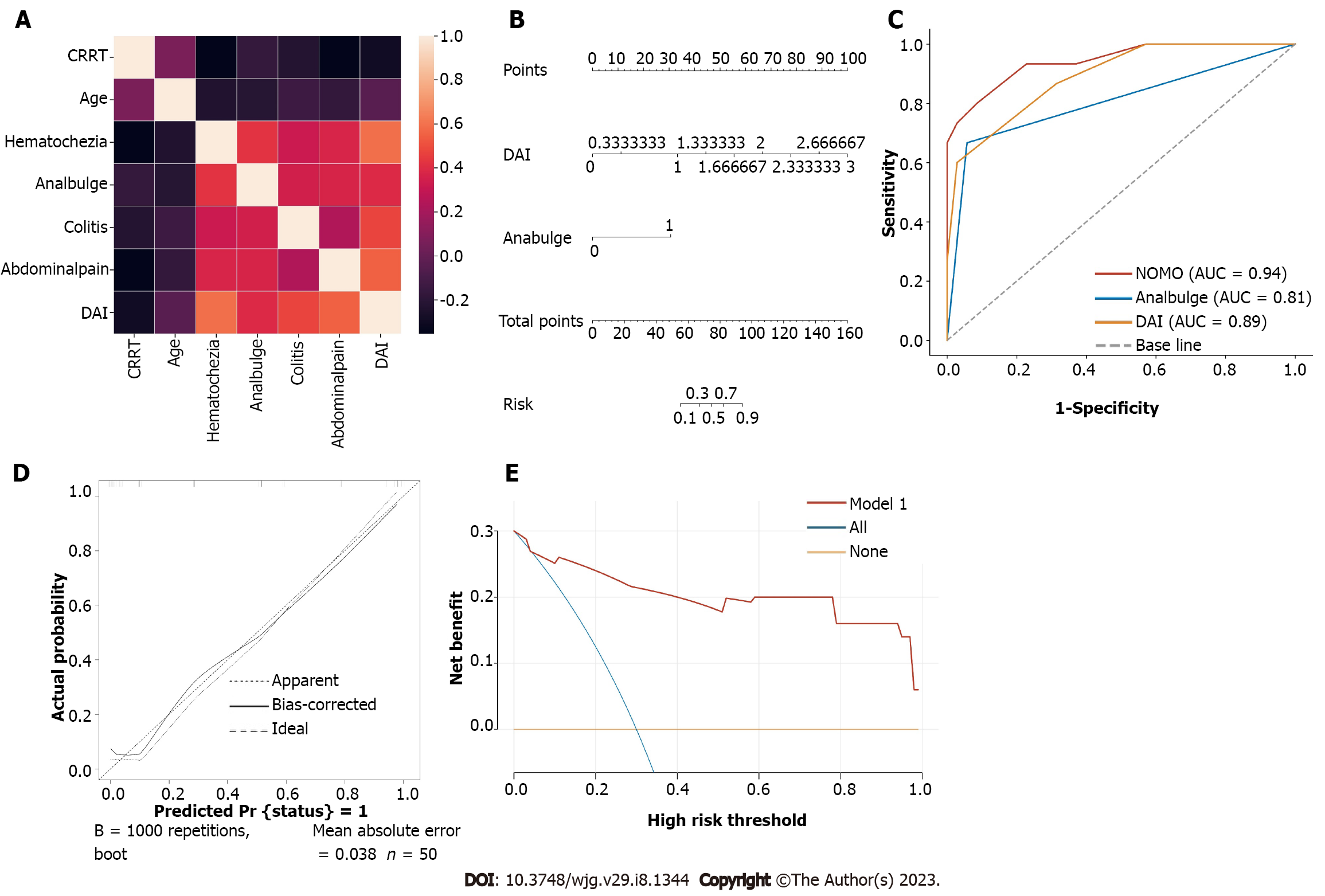
Univariate analysis showed that abdominal pain, colitis, anal bulging, hematochezia, DAI score, age, and CCRT were significantly correlated with the occurrence of severe acute RE (all P < 0.05) (Table 5). Kendall analysis showed a relatively strong correlation between abdominal pain, hematochezia and DAI score (R = 0.715, 0.622, P < 0.001); the correlation between other factors was very weak (Figure 2A). To avoid multicollinearity, abdominal pain, hematochezia, and DAI scores were included in the multivariate analysis. Multivariate logistic regression analysis was conducted to analyze the factors of colitis, anal bulge, DAI score and age. In multivariate analysis, anal bulge rating (OR: 14.779, 95%CI: 1.281-170.547, P = 0.031) and DAI score (OR: 53.928, 95%CI: 3.822-760.948, P = 0.003) were independent predictors of SARE (Table 6). These factors were used to construct the nomogram. The constant in the logistic regression equation was -10.039, and the logistic regression equation was Logit (P) = 3.988 × DAI + 2.693 × anal bulge rating -10.039.
| Factor | SARE (n = X) | No SARE (n = X) | OR (95%CI) | P value |
| Median (IQR) | Median (IQR) | |||
| Clinical factors | ||||
| Elderly (> 60 yr)/young and middle-aged (20-60 yr) | 0.123 (0.015-1.040) | 0.054 | ||
| Age | 47.5 (37.0-54.0) | 53.0 (46.0-63.0) | 0.930 (0.872-0.992) | 0.027 |
| Weight | 53.0 (51.0-60.9) | 56.0 (50.0-61.8) | 0.983 (0.918-1.054) | 0.631 |
| BMI/kg/m2 | 20.5 (19.7-22.2) | 21.9 (20.4-23.0) | 0.936 (0.746-1.174) | 0.566 |
| ACCI (2-3/4-5) | 0.123 (0.015-1.040) | 0.054 | ||
| Tumor type (squamous/non-squamous) | 0.848 (0.139-5.179) | 0.859 | ||
| T (> 4 cm/≤ 4 cm) | 0.510 (0.153-1.695) | 0.272 | ||
| N (positive/negative) | 0.615 (0.165-2.298) | 0.538 | ||
| Metastatic pelvic lymph nodes (positive/negative) | 0.694 (0.185-2.611) | 0.589 | ||
| Common iliac lymph node metastasis (positive/negative) | 0.427 (0.046-3.980) | 0.455 | ||
| Para-aortic lymph nodes (positive/negative) | 1.471 (1.216-1.779) | 0.999 | ||
| FIGO stage (IIB-IVA/IB-IIA) | 1.108 (0.328-3.739) | 0.869 | ||
| LVSI (positive/negative) | 0.548 (0.147-2.034) | 0.368 | ||
| Differentiation degree (low and medium differentiation/high differentiation) | 1.011 (0.225-4.536) | 0.988 | ||
| Infiltration degree (shallow 1/3/medium depth 1/3) | 0.837 (0.191-3.673) | 0.813 | ||
| Incisal margin (R1 + R2/R0) | 0.427 (0.046-3.980) | 0.455 | ||
| Risk (low risk/medium high risk) | 1.227 (0.340-4.242) | 0.754 | ||
| HPV (positive/negative) | 1.324 (0.127-13.785) | 0.815 | ||
| SCC (abnormally elevated/not abnormal) | 0.303 (0.074-1.245) | 0.098 | ||
| Surgery (performed/not performed) | 2.080 (0.497-8.706) | 0.316 | ||
| CCRT (implemented/not implemented) | 6.042 (1.681-21.718) | 0.006 | ||
| Myelosuppression (Grade 1-3/Grade 0) | 0.617 (0.178-2.144) | 0.446 | ||
| Diarrhea (Grade 1-3/Grade 0) | NA | 0.999 | ||
| Abdominal pain (Grade 1-3/Grade 0) | 25.375 (4.750-135.559) | < 0.001 | ||
| Colitis (Grade 1-3/Grade 0) | 38.500 (6.530-226.993) | < 0.001 | ||
| Anal bulge (Grade 1-3/Grade 0) | 5.185 (1.470-18.286) | 0.010 | ||
| Hematochezia (Grade 1-3/Grade 0) | NA | < 0.001 | ||
| DAI score | 2.8 (2.3-3.0) | 1.7 (1.2-2.0) | 152.546 (6.045-3849.771) | 0.002 |
| Physical dose parameters | ||||
| Small intestine | ||||
| V20 (%) | 80.9 (68.6-93.7) | 78.0 (66.8-82.5) | 1.015 (0.975-1.056) | 0.475 |
| V25 (%) | 58.2 (46.0-62.9) | 52.0 (48.0-57.3) | 1.014 (0.955-1.076) | 0.659 |
| V30 (%) | 41.0 (35.1-47.6) | 39.6 (31.1-42.1) | 1.039 (0.961-1.122) | 0.335 |
| V35 (%) | 29.6 (23.5-32.2) | 26.9 (24.4-29.1) | 1.032 (0.927-1.149) | 0.569 |
| V40 (%) | 19.5 (16.8-23.3) | 19.3 (13.3-20.7) | 1.068 (0.928-1.229) | 0.358 |
| Rectum | ||||
| V20 (%) | 80.9 (68.6-93.7) | 78.0 (66.8-82.5) | 1.015 (0.975-1.056) | 0.475 |
| V25 (%) | 58.2 (46.0-62.9) | 52.0 (48.0-57.3) | 1.014 (0.955-1.076) | 0.659 |
| V30 (%) | 41.0 (35.1-47.6) | 39.6 (31.1-42.1) | 1.039 (0.961-1.122) | 0.335 |
| V35 (%) | 29.6 (23.5-32.2) | 26.9 (24.4-29.1) | 1.032 (0.927-1.149) | 0.569 |
| V40 (%) | 19.5 (16.8-23.3) | 19.3 (13.3-20.7) | 1.068 (0.928-1.229) | 0.358 |
| Colon | ||||
| V20 (%) | 100.0 (100.0-100.0) | 100.0 (100.0-100.0) | NA | NA |
| V25 (%) | 100.0 (100.0-100.0) | 100.0 (100.0-100.0) | NA | NA |
| V30 (%) | 100.0 (100.0-100.0) | 100.0 (100.0-100.0) | 0.982 (0.775-1.246) | 0.883 |
| V35 (%) | 100.0 (99.1-100.0) | 100.0 (100.0-100.0) | 0.981 (0.915-1.052) | 0.981 |
| V40 (%) | 80.4 (71.3-89.2) | 79.3 (72.1-87.8) | 1.005 (0.956-1.056) | 0.851 |
| Femoral head | ||||
| V20 (%) | 35.4 (19.7-57.3) | 48.6 (22.9-65.2) | 0.994 (0.971-1.017) | 0.586 |
| V25 (%) | 24.8 (13.7-40.1) | 31.6 (16.0-45.1) | 0.996 (0.967-1.026) | 0.797 |
| V30 (%) | 17.7 (9.8-29.6) | 22.6 (11.4-32.6) | 0.997 (0.957-1.039) | 0.877 |
| V35 (%) | 13.3 (7.4-20.0) | 16.9 (8.6-24.5) | 0.989 (0.935-1.047) | 0.711 |
| V40 (%) | 8.9 (4.9-14.3) | 11.3 (5.7-16.3) | 0.993 (0.916-1.076) | 0.858 |
| Total pelvic lymphatic drainage dose (Gy) | 45.0 (45.0-45.0) | 45.0 (45.0-48.5) | 1.194 (0.877-1.625) | 0.261 |
| Para-aortic extended field radiotherapy (performed/not performed) | 1.485 (1.222-1.804) | 0.999 |
| B | SE | Exp (B) | 95%CI | Wald χ2 | P value | ||
| Lower | Upper | ||||||
| DAI score | 4.106 | 1.371 | 60.705 | 4.134 | 891.506 | 9.463 | 0.003 |
| Anal bulge rating | 2.925 | 1.229 | 18.636 | 1.677 | 207.114 | 7.664 | 0.017 |
Based on the multivariate logistic regression coefficients, the prediction model was visually represented as a nomogram (Figure 2B). The ROC curves for the anal bulge rating, DAI score and nomogram are shown in Figure 2C. ROC analysis showed that the AUC of the prediction model was 0.950 (95%CI: 0.891-1.000), which was much higher than that of each parameter alone (anal bulge rating: 0.805, 95%CI: 0.651-0.959; DAI score: 0.892, 95%CI: 0.873-1.000). The MAL thresholds for anal bulge rating and DAI score were 0.5 and 2.165 points. The nomogram had a high predictive efficiency (sensitivity: 80.0%, specificity: 91.4%). In addition, the calibration curve showed good agreement between the predicted severe acute RE and the actual observations (Figure 2D). In most threshold probabilities, DCA showed a positive net benefit with a satisfactory nomogram, indicating a good potential clinical effect of the model (Figure 2E).
Nearly 80% of patients receiving pelvic radiotherapy for cervical cancer have early acute toxicity and 20% have late toxicity[16]. Acute RE often occurs in the second week after the start of radiotherapy and reaches a peak in the fourth to fifth week. The clinical manifestations are nausea, vomiting, abdominal pain, bloody stool, mucous stool, diarrhea, tenesmus, and incontinence. In gynecological tumor radiotherapy, the clinical application of IMRT shows an increasing trend. This radiotherapy technology can not only obtain satisfactory target coverage dose, but also effectively reduces the radiation dose of OAR[17,18], such as V45 of rectum and small intestine[19,20], and reduces the toxic reaction of bone marrow[21,22]. Although the incidence and severity of RE have decreased under the current radiotherapy technology, these issues cannot be ignored. In addition, mild RE is often underestimated or ignored due to the lack of clinical records and inadequate assessment of toxicity[23].
In this study, we preliminarily demonstrated the close relationship between the anal bulge rating and the DAI score and SARE. More importantly, this study is the first to develop and validate an easy-to-understand visual nomogram prediction model for cervical cancer radiotherapy patients and doctors, providing more personalized and accurate SARE prediction for cervical cancer patients undergoing radiotherapy. Many studies have reported that the most important factor affecting radiation proctitis is the total dose received by the rectum, and it is also affected by the radiotherapy technique, fraction dose, total dose received by the rectum/total volume, dose volume ratio of rectum to radiation dose, and uniformity of radiation dose distribution[4-6]. According to Seppenwoolde et al[24], rectal V40 ≥ 80% is a physical dose factor predicting VMAT-related rectal incontinence and diarrhea in patients with locally advanced cervical cancer. Ballari et al[25] suggested that small bowel V45 has predictive value for acute RE. In addition, individual factors such as IBD, collagen vascular disease, history of abdominal and pelvic surgery, history of pelvic inflammatory disease, diabetes mellitus, and acquired immunodeficiency syndrome affect the determination of radiation dose and volume[7-11]. In patients with cervical cancer, CCRT exerts radiosensitization and increases gastrointestinal toxicity by a factor of 3[18]. However, there is currently no consensus on the relative importance of these relevant predictors. In our study, the dose-volume of the small intestine receiving 20-40 Gy in the radical radiotherapy group of cervical cancer was significantly reduced compared with that in the postoperative adjuvant radiotherapy group, and the reduction of V20, V30, and V40 was statistically significant (P < 0.05), which was considered to be related to the anatomical displacement of organs after hysterectomy. After the removal of the tumor bed and uterus, part of the intestinal loop falls into the pelvic cavity[9], resulting in increased secondary toxicity of radiotherapy. In addition, the rectal V30, V35, and V40 in the radical radiotherapy group were significantly higher than those in the postoperative adjuvant radiotherapy group, and the increase of V40 was statistically significant (P < 0.05). The cervical cancer patients treated with radical radiotherapy in this study were FIGO IIB-IVA. CT or MRI showed obvious parametrial infiltration, and mesorectal invasion occurred in some patients, resulting in a relative increase in the volume of PTV to the left, right and dorsal side. Although we found dose-volume differences in the small intestine and rectum between groups, we were unable to demonstrate that a single physical parameter independently predicted RE or SARE. Therefore, more prospective, well-designed randomized controlled trials with larger sample sizes are needed for validation.
Acute RE is self-limited, and not all acute RE will be classified as chronic RE. The prevalence of chronic RE after 10 years of radiotherapy is 10%-20%[26]. In terms of pathogenesis, inflammation and cell death occur in the intestinal mucosa during the acute phase of RE, and sustained cytokine activation in the submucosa leads to progressive ischemia, fibrosis, and stem cell loss[27]. In contrast, chronic RE is associated with progressive endarteritis obliterans, secondary to capillary fibrosis, leading to chronic ischemia and fibrosis of the affected bowel segments[28]. The outcome of chronic RE often induces intestinal obstruction, which is similar to the Koenig syndrome observed in Crohn’s disease and is considered to be related to ileal stenosis. In view of the similarities between RE and IBD, we innovatively adopted the IBD evaluation index-DAI score. Univariate and multivariate logistic regression analysis confirmed that DAI score was independent of other physical parameters and clinical factors and could be used as a new predictor of SARE. In univariate analysis, abdominal pain, colitis, anal distension, hematochezia, DAI score, age, and CCRT were statistically correlated with the occurrence of severe acute RE (P < 0.05). Kendall analysis showed a relatively strong correlation between abdominal pain, hematochezia, and DAI scores. Therefore, DAI score is a convenient, non-invasive and highly beneficial diagnostic system for SARE.
The clinical manifestations of SARE are complex, and it is difficult for doctors, even with extensive clinical experience, to predict and diagnose the disease early, comprehensively and accurately. If cervical cancer can be identified and treated early before, during or after radiotherapy, the toxic and side effects of pelvic radiotherapy may be improved, and the probability of radiotherapy intolerance or even termination of radiotherapy may be reduced. The multi-factor prediction model is helpful to further improve the prediction of RE. Several prediction models have been described in previous studies, but they are still not used in clinic due to the low recognition ability of RE and the neglect of heterogeneity among different patients[29,30]. In this study, we established a SARE-SS evaluation system and found that anal bulge rating and DAI score were independent predictors of SARE through multivariate statistical analysis. The above predictors were further integrated into the nomogram to allow precise individualized SARE risk assessment for each patient. The AUC of the predictive model was 0.950, which was much higher than the two independent factors of DAI score and anal bulge score. The MAL thresholds for anal bulge rating and DAI score were 0.5 grade and 2.165 points, respectively, indicating an advantage over previous models in predicting RE. We adopted the Bootstrap validation method (including drawing the calibration curve and DCA curve), which showed a statistical advantage in a relatively small sample size statistic[31,32]. In later studies, we will expand the sample size, carry out prospective cohort studies, and use external validation methods to reduce data selection bias and increase test power.
Although our study has many advantages, there are still several limitations to be addressed. First, our sample size is small, so a large cohort is needed to further develop and validate the nomogram for predicting RE. Second, since this was a retrospective study, our study may have had a selection bias. Third, we only integrated the most important and commonly used parameters in clinic. From the perspective of precision medicine, we need to combine biological factors in the future including individual genomics, proteomics, metabolomics, microbiomics, real-time dosimetry and a wider range of clinical parameters to establish a comprehensive prediction model to improve the accuracy of RE prediction. Therefore, we will add biological factors to the analysis of related studies in later studies and need to establish a more comprehensive and specific prediction model of RE.
Our findings showed that during VMAT for cervical cancer, gastrointestinal and hematological system toxicity gradually increased with radiotherapy treatment and reached the peak at the end of radiotherapy. The main adverse reactions were diarrhea, abdominal pain, colitis, anal bulging and hematochezia. There were significant differences in V20, V30, V40 of small intestine and V40 of rectum between postoperative adjuvant radiotherapy and radical radiotherapy, which may be related to intestinal anatomical displacement caused by surgery and primary disease. However, there were no significant differences in the cumulative incidence of adverse reactions between the two groups. Anal bulge rating (> 0.500) and DAI score (> 2.165) were identified as independent predictors of SARE by multivariate analysis. The validity of our established nomogram was verified by the internal verification method. Although further external validation is needed, our results indicate that the nomogram has clinical value for the prediction and evaluation of SARE.
Radiation enteritis (RE) not only seriously affects the quality of life of patients, but it also leads to radiotherapy intolerance or termination of radiotherapy. However, data on the clinical efficacy and side effects of volumetric modulated arc therapy (VMAT) for cervical cancer are limited.
If the incidence of RE in patients can be predicted in advance, and targeted clinical preventive treatment can be carried out, the side effects of radiotherapy in cervical cancer patients can be significantly reduced. Furthermore, accurate prediction of RE is essential for the selection of individualized radiation dose and the optimization of the radiotherapy plan.
To analyze the relationships between severe acute RE (SARE) of cervical cancer radiotherapy and clinical factors and dose-volume parameters retrospectively.
We included 50 cervical cancer patients who received VMAT from September 2017 to June 2018 in the Department of Radiotherapy at The First Affiliated Hospital Soochow University. Clinical and dose-volume histogram factors of patients were collected. Logistic regression analysis was used to evaluate the predictive value of each factor for SARE. A nomogram to predict SARE was developed (SARE scoring system ≥ 3 points) based on the multiple regression coefficients; validity was verified by an internal verification method.
Gastrointestinal and hematological toxicity of cervical cancer VMAT gradually increased with radiotherapy and reached the peak at the end of radiotherapy. The main adverse reactions were diarrhea, abdominal pain, colitis, anal swelling, and blood in the stool. There was no significant difference in the incidence of gastrointestinal toxicity between the radical and postoperative adjuvant radiotherapy groups (P > 0.05). There were significant differences in the small intestine V20, V30, V40, and rectal V40 between adjuvant radiotherapy and radical radiotherapy after surgery (P < 0.05). Univariate and multivariate analysis revealed anal bulge rating (OR: 14.779, 95%CI: 1.281-170.547, P = 0.031) and disease activity index (DAI) score (OR: 53.928, 95%CI: 3.822-760.948, P = 0.003) as independent predictors of SARE.
Anal bulge rating (> 0.500 grade) and DAI score (> 2.165 points) can predict SARE. The nomogram shows potential value in clinical practice.
From the perspective of precision medicine, it will also be necessary to combine biological factors, such as individual genomics, proteomics, metabolomics, microbiomics, real-time dosimetry, and a wider range of clinical parameters to establish comprehensive predictive models. This study is a prospective study with a small sample size. In the later stage, we will expand the sample size, conduct prospective cohort studies, and use external validation methods to reduce data selection bias and increase test efficiency.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Health care sciences and services
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey; Šarenac TM, Serbia S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;70:313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 454] [Article Influence: 90.8] [Reference Citation Analysis (1)] |
| 2. | Hyun Kim T, Choi J, Park SY, Lee SH, Lee KC, Yang DS, Shin KH, Cho KH, Lim HS, Kim JY. Dosimetric parameters that predict late rectal complications after curative radiotherapy in patients with uterine cervical carcinoma. Cancer. 2005;104:1304-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | van de Bunt L, van der Heide UA, Ketelaars M, de Kort GA, Jürgenliemk-Schulz IM. Conventional, conformal, and intensity-modulated radiation therapy treatment planning of external beam radiotherapy for cervical cancer: The impact of tumor regression. Int J Radiat Oncol Biol Phys. 2006;64:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Forrest JL, Ackerman I, Barbera L, Barnes EA, Davidson M, Kiss A, Thomas G. Patient outcome study of concurrent chemoradiation, external beam radiotherapy, and high-dose rate brachytherapy in locally advanced carcinoma of the cervix. Int J Gynecol Cancer. 2010;20:1074-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Kozak MM, Koenig JL, von Eyben R, Kidd EA. Less Than Whole Uterus Irradiation for Locally Advanced Cervical Cancer Maintains Locoregional Control and Decreases Radiation Dose to Bowel. Pract Radiat Oncol. 2019;9:e164-e171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Ray A, Sarkar B. Small bowel toxicity in pelvic radiotherapy for postoperative gynecological cancer: comparison between conformal radiotherapy and intensity modulated radiotherapy. Asia Pac J Clin Oncol. 2013;9:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Willett CG, Ooi CJ, Zietman AL, Menon V, Goldberg S, Sands BE, Podolsky DK. Acute and late toxicity of patients with inflammatory bowel disease undergoing irradiation for abdominal and pelvic neoplasms. Int J Radiat Oncol Biol Phys. 2000;46:995-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Lin A, Abu-Isa E, Griffith KA, Ben-Josef E. Toxicity of radiotherapy in patients with collagen vascular disease. Cancer. 2008;113:648-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 9. | Nuyttens JJ, Robertson JM, Yan D, Martinez A. The influence of small bowel motion on both a conventional three-field and intensity modulated radiation therapy (IMRT) for rectal cancer. Cancer Radiother. 2004;8:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Andreyev HJ, Wotherspoon A, Denham JW, Hauer-Jensen M. "Pelvic radiation disease": new understanding and new solutions for a new disease in the era of cancer survivorship. Scand J Gastroenterol. 2011;46:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Gichangi P, Bwayo J, Estambale B, Rogo K, Njuguna E, Ojwang S, Temmerman M. HIV impact on acute morbidity and pelvic tumor control following radiotherapy for cervical cancer. Gynecol Oncol. 2006;100:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Dröge LH, von Sivers FF, Schirmer MA, Wolff HA. Conventional 3D conformal radiotherapy and volumetric modulated arc therapy for cervical cancer: Comparison of clinical results with special consideration of the influence of patient- and treatment-related parameters. Strahlenther Onkol. 2021;197:520-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Andreyev HJ, Davidson SE, Gillespie C, Allum WH, Swarbrick E; British Society of Gastroenterology; Association of Colo-Proctology of Great Britain and Ireland; Association of Upper Gastrointestinal Surgeons; Faculty of Clinical Oncology Section of the Royal College of Radiologists. Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut. 2012;61:179-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 14. | Abu-Rustum NR, Yashar CM, Bean S, Bradley K, Campos SM, Chon HS, Chu C, Cohn D, Crispens MA, Damast S, Fisher CM, Frederick P, Gaffney DK, Giuntoli R, Han E, Huh WK, Lurain Iii JR, Mariani A, Mutch D, Nagel C, Nekhlyudov L, Fader AN, Remmenga SW, Reynolds RK, Sisodia R, Tillmanns T, Ueda S, Urban R, Wyse E, McMillian NR, Motter AD. NCCN Guidelines Insights: Cervical Cancer, Version 1.2020. J Natl Compr Canc Netw. 2020;18:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 216] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 15. | Eifel PJ, Jhingran A, Bodurka DC, Levenback C, Thames H. Correlation of smoking history and other patient characteristics with major complications of pelvic radiation therapy for cervical cancer. J Clin Oncol. 2002;20:3651-3657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 140] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Shadad AK, Sullivan FJ, Martin JD, Egan LJ. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol. 2013;19:185-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 152] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 17. | Mell LK, Sirák I, Wei L, Tarnawski R, Mahantshetty U, Yashar CM, McHale MT, Xu R, Honerkamp-Smith G, Carmona R, Wright M, Williamson CW, Kasaová L, Li N, Kry S, Michalski J, Bosch W, Straube W, Schwarz J, Lowenstein J, Jiang SB, Saenz CC, Plaxe S, Einck J, Khorprasert C, Koonings P, Harrison T, Shi M, Mundt AJ; INTERTECC Study Group. Bone Marrow-sparing Intensity Modulated Radiation Therapy With Concurrent Cisplatin For Stage IB-IVA Cervical Cancer: An International Multicenter Phase II Clinical Trial (INTERTECC-2). Int J Radiat Oncol Biol Phys. 2017;97:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 18. | Zhang K, Wang H, Wang Z, Li F, Cui Y, Ma S, Chen R, Wang Y, Guo S, Wei Y. Intensity-modulated radiation therapy (IMRT)-based concurrent chemoradiotherapy (CCRT) with Endostar in patients with pelvic locoregional recurrence of cervical cancer: Results from a hospital in the Qinghai-Tibet Plateau. Medicine (Baltimore). 2020;99:e21966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Chino J, Annunziata CM, Beriwal S, Bradfield L, Erickson BA, Fields EC, Fitch K, Harkenrider MM, Holschneider CH, Kamrava M, Leung E, Lin LL, Mayadev JS, Morcos M, Nwachukwu C, Petereit D, Viswanathan AN. Radiation Therapy for Cervical Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract Radiat Oncol. 2020;10:220-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 20. | Yang B, Zhu L, Cheng H, Li Q, Zhang Y, Zhao Y. Dosimetric comparison of intensity modulated radiotherapy and three-dimensional conformal radiotherapy in patients with gynecologic malignancies: a systematic review and meta-analysis. Radiat Oncol. 2012;7:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Contreras J, Srivastava A, Chundury A, Schwarz JK, Markovina S, Thaker PH, Massad LS, Mutch DG, Powell MA, Grigsby PW, Lin AJ. Long-term outcomes of intensity-modulated radiation therapy (IMRT) and high dose rate brachytherapy as adjuvant therapy after radical hysterectomy for cervical cancer. Int J Gynecol Cancer. 2020;30:1157-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Liu Y, Yu J, Qian L, Zhang H, Ma J. Extended field intensity-modulated radiotherapy plus concurrent nedaplatin treatment in cervical cancer. Oncol Lett. 2016;11:3421-3427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Wang W, Zhang F, Hu K, Hou X. Image-guided, intensity-modulated radiation therapy in definitive radiotherapy for 1433 patients with cervical cancer. Gynecol Oncol. 2018;151:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | Seppenwoolde Y, Majercakova K, Buschmann M, Dörr E, Sturdza AE, Schmid MP, Pötter R, Georg D. Early morbidity and dose-volume effects in definitive radiochemotherapy for locally advanced cervical cancer: a prospective cohort study covering modern treatment techniques. Strahlenther Onkol. 2021;197:505-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Ballari N, Rai B, Bahl A, Mittal BR, Ghoshal S. Prospective observational study evaluating acute and delayed treatment related toxicities of prophylactic extended field volumetric modulated arc therapy with concurrent cisplatin in cervical cancer patients with pelvic lymph node metastasis. Tech Innov Patient Support Radiat Oncol. 2021;17:48-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Shen XJ, Liu L, Zhu JY. Radiofrequency ablation in a patient with radiation enteritis: A case report. Medicine (Baltimore). 2018;97:e13328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Denham JW, Hauer-Jensen M. The radiotherapeutic injury--a complex 'wound'. Radiother Oncol. 2002;63:129-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 420] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 28. | Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97:149-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 479] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 29. | Loge L, Florescu C, Alves A, Menahem B. Radiation enteritis: Diagnostic and therapeutic issues. J Visc Surg. 2020;157:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 30. | Hale MF. Radiation enteritis: from diagnosis to management. Curr Opin Gastroenterol. 2020;36:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Halabi S, Li C, Luo S. Developing and Validating Risk Assessment Models of Clinical Outcomes in Modern Oncology. JCO Precis Oncol. 2019;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Steyerberg EW, Harrell FE Jr. Prediction models need appropriate internal, internal-external, and external validation. J Clin Epidemiol. 2016;69:245-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 731] [Article Influence: 73.1] [Reference Citation Analysis (0)] |