Published online Feb 28, 2023. doi: 10.3748/wjg.v29.i8.1261
Peer-review started: October 28, 2022
First decision: November 14, 2022
Revised: December 5, 2022
Accepted: February 16, 2023
Article in press: February 16, 2023
Published online: February 28, 2023
Processing time: 122 Days and 20.5 Hours
Functional constipation (FC) is considered the most common functional gastrointestinal disorder in children with a pooled global prevalence of 14.4% (95% confidence interval: 11.2-17.6) when diagnosed based on the Rome IV criteria. Its pathophysiological mechanisms are thought be multifactorial and complicated, resulting in difficult management. Currently, the most effective medication, when used in parallel with toilet training, is osmotic laxatives. Children’s adherence to medication and parental concern regarding long-term laxative use are the main contributors to treatment failure. Recently, novel therapies with a high safety profile have been developed, such as probiotics, synbiotics, serotonin 5-hydroxytryptamine 4 receptor agonists, chloride channel activators, and herbal and transitional medicines; nonetheless, well-designed research to support the use of these therapies is needed. This review aims to focus on multiple aspects of FC in children, including global prevalence, pathogenesis, diagnostic criteria, tools, as well as conventional and novel treatment options, such as non-pharmacological management, including adequate fiber and fluid intake, physiotherapy, or neuromodulators. We also report that in very difficult cases, surgical intervention may be required.
Core Tip: Functional constipation (FC) is a typical symptom of functional gastrointestinal disorders in children and its prevalence is high worldwide. Since the pathophysiology of FC in children is associated with stool withholding behavior, successful toilet training in combination with osmotic laxatives is crucial for the treatment childhood FC. Additionally, promising and innovative drugs can also aid in treatment success.
- Citation: Tran DL, Sintusek P. Functional constipation in children: What physicians should know. World J Gastroenterol 2023; 29(8): 1261-1288
- URL: https://www.wjgnet.com/1007-9327/full/v29/i8/1261.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i8.1261
Functional constipation (FC) is considered a great disease burden in children that needs early screening and detection. The prognosis of FC is better in children with prompt and proper management. General physicians and pediatricians are usually the first person who take care these children hence understanding the pathophysiology of FC can lead to the proper management and satisfied outcome. In this chapter, the content will be covered all aspect the physician should know about FC for better patient care.
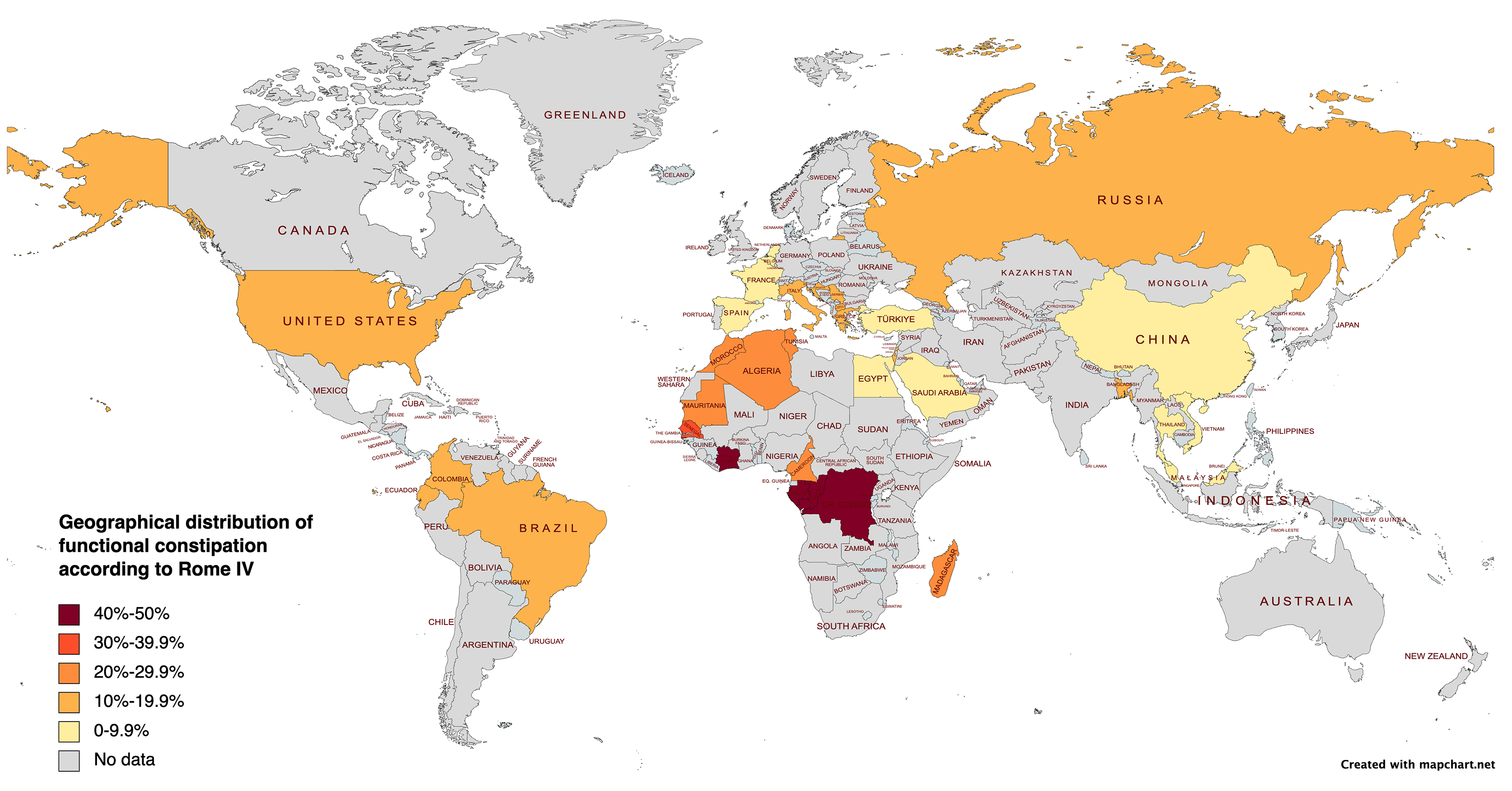
Globally, up to 25% of visits to pediatric gastroenterologists and 3% of all general pediatric outpatient visits are due to FC[1]. It is difficult to determine the true prevalence of FC in children due to the heterogeneity of the studies in terms of target population sampling, diagnostic criteria, participant ethnicity and environment, method of data acquisition, and life style and psychological factors among others[2]. The primary reasons for the global diversity in prevalence among published studied may be due to the lack of agreement on diagnostic standards and cultural differences[3]. A systematic review and meta-analysis published by Koppen et al[4] reported that the worldwide prevalence of FC according to the Rome III criteria was 9.5% [95% confidence interval (CI): 7.5%-12.1%], with significantly more American and European children being affected than Asian ones. Additionally, geographical region, diet, and exposure to traumatic life events were linked to FC in children.
We determined that the pool global prevalence of FC in children was 14.4% (95%CI: 11.2-17.6) using the Rome IV criteria. According to continent, Africa had a highest prevalence of constipation (31.4%), followed by America (12.1%, 95%CI: 9.1%-15.1%), Europe (8.3%, 95%CI: 3.7%-12.9%), and Asia (6.2%, 95%CI: 1.3%-11%). Moreover, the factors significantly associated with FC from these studies are summarized in Figure 1 and Table 1.
| Ref. | Country | Population | Sample size | Age | Method of data collection | Prevalence of FC, n | Factors associated with FC |
| Huang et al[139], 2021 | China | 4 community hospitals in Jinhua and Shanghai | 2604 | 0-4 yr | Guardian interview | 92 (3.5%) | Vaginal delivery (OR = 0.01, 95%CI: 0.00-0.17), forceps delivery (OR: > 999, 95%CI: 154 to > 999) |
| Chew et al[140], 2021 | Malaysia | A well child clinic, University Malaya medical center | 534 | 1-12 mo | Guardian interview | 6 (1.1%) | NA |
| Ibrahim et al[141], 2020 | Egypt | Randomly schools in Cairo | 1082 | 4-18 yr | NA | 91 (8.4%) | NA |
| Khayat et al[142], 2021 | Saudi Arabia | Public survey (random) from Western | 317 | 3-18 yr | Questionnaire by Google form/links share on social apps | 15 (4.7%) | Guardian characteristics, family income, gender, age, development, previous covid ìnfection (P > 0.05) |
| Benzamin et al[29], 2022 | Bangladesh | Schools of Dhaka division | 707 | 5-16 yr | Child interview and examination (face to face) | 134 (19%) | Female (P = 0.003), age (P = 0.001), history of FC in siblings/parents (P = 0.001), fibers intake (P = 0.002), fluid intake (P = 0.001), electronic screen time (P = 0.001) |
| Siajunboriboon et al[143], 2022 | Thailand | 2 high schools | 1700 | 14-18 yr | Child interview | 138 (8.1%) | Guardian characteristics, BMI, history of allergic diseases (P > 0.05) |
| Chia et al[144], 2022 | Vietnam | A government hospital and a government kindergarten | 1511 | 0-48 mo | Guardian interview and examination (face to face) | 46 (3%) | Male (OR = 3.6, 95%CI: 1.5-8.5), bottle feeding (OR = 18.5, 95%CI: 1.5-219.4), low income (OR = 5.8, 95%CI: 1.7-19.3) |
| Asia | 8455 | 522 (6.2%) | |||||
| Zwiener et al[145], 2017 | United States | Online survey | 1075 | 4-18 yr | Guardian interview | 144 (13.4%) | NA |
| Saps et al[146], 2018 | Colombia | 12 schools in 6 cities | 3567 | 8-18 yr | Child interview | 382 (10.7%) | NA |
| Robin et al[147], 2018 | United States | Online survey panels by CINT, United States | 1255 | 0-18 yr | Guardian interview | 186 (14.8%) | NA |
| Játiva-Mariño E et al[148], 2019 | Ecuador | 1 public and 1 private school | 951 | 8-15 yr | Child interview | 137 (14.4%) | NA |
| Saps et al[149], 2020 | Colombia | 6 outpatient clinics | 1334 | 1-48 mo | Guardian interview (face to face) | 281 (15.1%) | NA |
| Velasco-Benitez et al[150], 2020 | Colombia | 4 public schools | 1497 | 10-18 yr | Guardians interview | 194 (13%) | NA |
| Baaleman et al[151], 2021 | Colombia | A public school | 118 | 11-18 yr | Child interview | 16 (13.6%) | NA |
| Velasco-Benítez et al[152], 2021 | Colombia | 5 to 8 grade students in Cali | 465 | 10-18 yr | Children interview | 52 (28.7%) | NA |
| Dos Santos et al[27], 2021 | Brazil | Public parks and school areas | 799 | 5-14 yr | Guardian interview | 163 (20.4%) | Sex, type of school (P > 0.05) |
| de Morais et al[153], 2022 | Brazil | Pediatric private clinics in 5 regions | 4560 | 0-12 mo | Guardian interview | 341 (7.6%) | Age 162-248 d (OR = 1.41, 95%CI: 1.01-1.95), prematurity (OR = 1.44, 95%CI: 1.02-2.02) |
| America | 15621 | 1896 (12.1%) | |||||
| Russo et al[21], 2019 | Italy | General clinics | 214 | 1 mo-17 yr | Guardian/child interview(face to face) | 39 (18.2%) | NA |
| Vladimir et al[154], 2019 | Russia | University clinic | 300 | 0-48 mo | Guardian interview | 45 (15%) | NA |
| Steutel et al[155], 2020 | Belgium, Italy, Netherland | General pediatrics hospital (Belgium, Italy) and well-baby clinic (the Netherlands) | 2751 | 0-48 mo | Guardian interview and examination (face to face) | 151 (5.4%) | NA |
| Campeotto et al[156], 2020 | France | Private out-patient pediatricians and general practitioner | 1570 | 0-12 mo | Guardian interview and examination (face to face) | 141 (9%) | NA |
| Alonso-Bermejo et al[157], 2022 | Spain | A pediatric gastroenterology clinic | 574 | 0-16 yr | Guardian/child interview and examination (face to face) | 41 (7.1%) | NA |
| Beser et al[158], 2021 | Turkey | 9 tertiary Hospital | 2383 | 1-12 mo | Child interview and examination (face to face) | 112 (4.7%) | NA |
| Strisciuglio et al[159], 2022 | 6 Mediterranean countries1 | Nursery schools, primary schools and secondary schools, randomly | 4353 | 4-18 yr | Guardian interview | 475 (10.9%) | NA |
| Europe | 12145 | 1004 (8.3%) | |||||
| Bellaiche et al[160], 2020 | 10 countries in Africa2 | Children with gastrointestinal symptoms | 10458 | 0-12 mo | Guardians interview and examination (face to face) | 3283 (31.4%) | NA |
| Africa | 10458 | 3283 (31.4%) | |||||
| All over the world | 46679 | 6704 (14.4%) |
Frequency of toileting habits in infants and children varies with age. To reduce parental worry and prevent needless testing and treatment, knowing the typical toilet routines for all age groups is important[5]. The frequency of stool passage per day gradually decreases from more than four times per day during the first week of life to three times per day at 4-6 wk of life and one to two times per day by the age of 4 years[6,7]. Healthy infants who are exclusively breastfed will have infrequent stool passage at 1-2 mo of life, with a mean duration of 6 days per stool passage (2-28 d per stool passage) without any abnormalities[8]. This condition will normalize at a mean age of 3.9 mo (range 1-7 mo)[9]. Hence, if the stool is soft, the infrequent stool passage in this age group requires neither intervention nor treatment. From the age of five, the majority of children pass stools daily or every other day without straining or withholding[3]. In early newborns, the average intestinal transit time is around 8.5 h, whereas intestinal transit times after puberty range from 30 to 48 h[5].
FC in adults had been first defined in 1999 according to the Rome II criteria and was mostly based on expert opinion. The diagnostic criteria of FC in children was subsequently established and integrated in Rome III criteria by Rome foundation in 2006. In 2016, the Rome III criteria had been replaced by the Rome IV criteria, with only minor changes being made as shown in Table 2[10]. Children who are not yet toilet trained do not need to include fecal incontinence (FI) and clogged toilet in the diagnostic criteria. In addition, the duration of symptoms in children had been changed from 2 mo in the previous Rome III criteria to 1 mo in the current Rome IV criteria[10,11] to promote early recognition and timely treatment. Although there are some changes of the diagnostic criteria in Rome IV, the prevalence of FC in children was similar using either the Rome IV or Rome III criteria.
| Child’s age | Diagnostic criteria |
| < 4 years old | Two or more criteria for at least 1 mo1: (1) Two or fewer defecations per week; (2) History of excessive stool retention; (3) History of painful or hard bowel movements; (4) History of large diameter stools; (5) Presence of a large fecal mass in the rectum; (6) At least one episode of fecal incontinence per week after the acquisition of toileting skills; and (7) History of large-diameter stools that may obstruct the toilet in toilet trained children |
| ≥ 4 years old | Two or more symptoms for at least 1 mo in children at least 4 yr2: (1) Two or fewer defecations per week; (2) At least one episode of fecal incontinence per week; (3) History of retentive posturing or excessive stool retention; (4) History of painful or hard bowel movements; (5) Presence of a large fecal mass in the rectum; (6) History of large-diameter stool that may obstruct the toilet; and (7) Additional criteria: Without fulfilling irritable bowel syndrome criteria |
FI in children is defined as the involuntary passage of stool into the underwear either as unintentional seepage of small amounts of liquid stools (generally referred to as “soiling” or “leakage”) in a child older than 4 years of age or in a toilet-trained child[12,13]. Irrespective of the amount of stool, this is one of the most unpleasant and embarrassing things for a growing child apart from being an upsetting and mentally distressing issue that has a negative impact on children’s quality of life. FI was divided into 2 types; retentive and nonretentive FI. It is critical to distinguish between retentive and nonretentive FI given their different etiologies and approaches to treatment[14]. Hospital and community studies have shown retentive FI occurs in constipated children with fecal impaction[1,15,16], whereas the nonretentive type could be found in children with psychological problems[3].
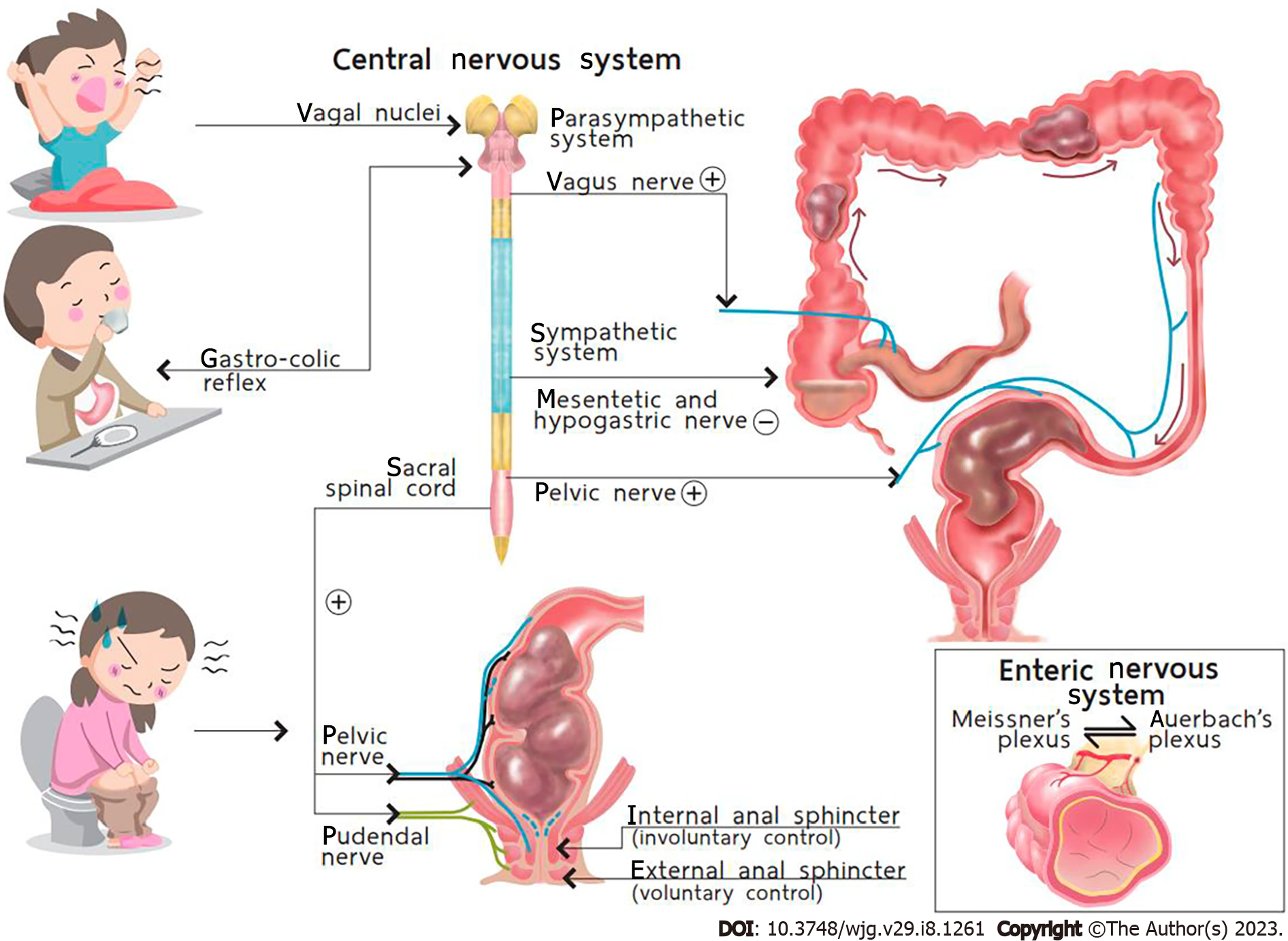
The act of defecation is a process related to the pelvic floor muscles, anal sphincter complex, enteric nervous system, and central nervous system (Figure 2). Normally, children over 18 mo old can initially control defecation through this complicated process, with nearly all of them succeeding in controlling defecation by the age of 4.
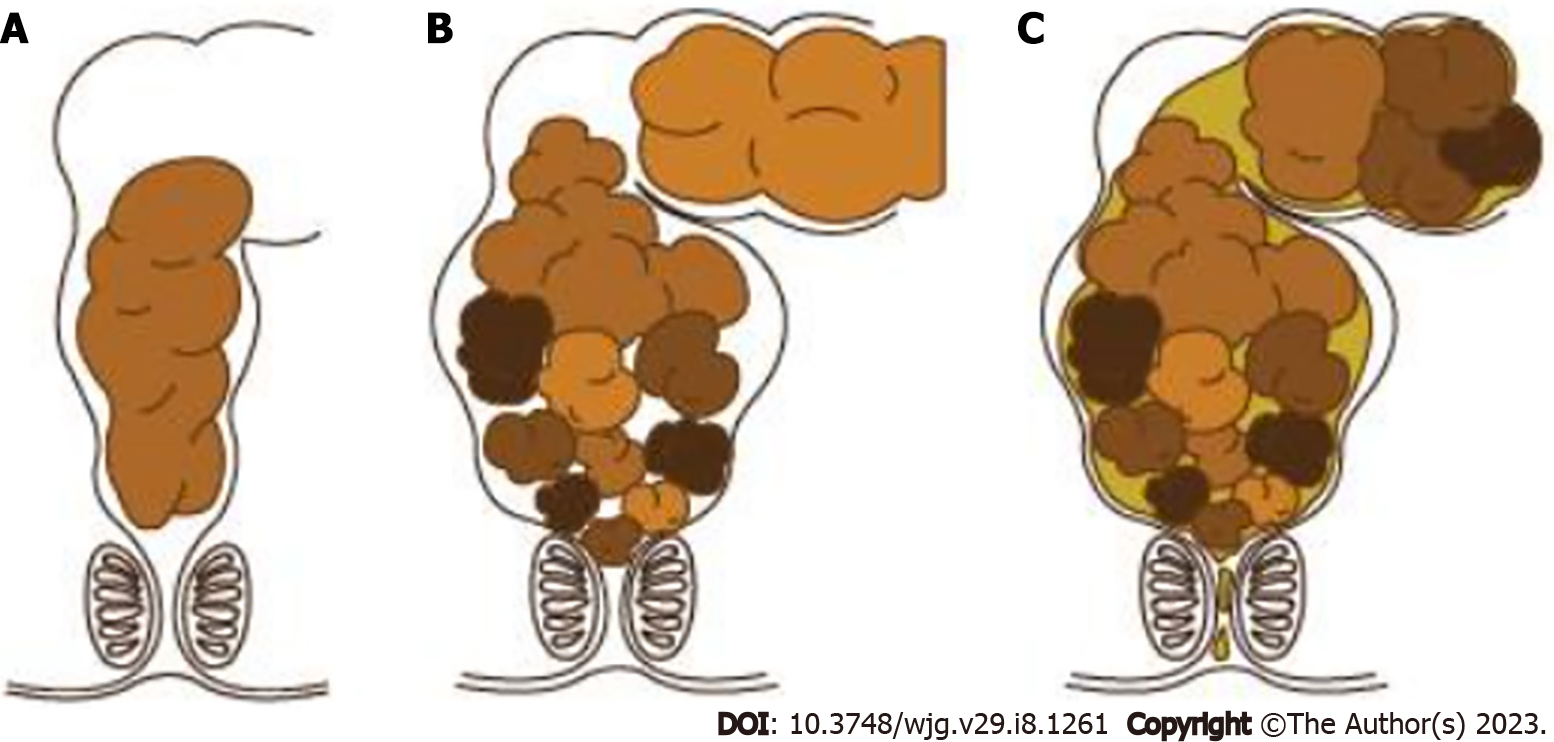
The etiology of constipation can be classified into functional and organic causes, which account for 90% and 10% of the cases, respectively[12]. Regarding FC, the pathophysiological mechanism might be multifactorial, including stool withholding behavior, anorectal dysfunctions, diet, physical activity, genetic predisposition, and psychological issues. Stool withholding behavior is the main pathophysiological mechanism especially in toddlers and young children. Faulty toilet training, painful defecation from hard stool and frequent rectal enema contribute to fear and bad experiences related to defecation, which can cause purposeful or subconscious stool withholding behavior. Instead of relaxing the pelvic floor muscle when feeling the urge to defecate, children will defecate in the standing position and contract the pelvic floor and gluteal muscles, a phenomenon called “retentive posture or defecation in standing position”. This behavior promotes the retention of stool in rectum and causes the stool to become lumpier and harder, making it quite difficult to evacuate, due to water absorption by rectal mucosa. This phenomenon leads to a vicious cycle of difficult defecation. Once large stools are retained in the rectum, the rectal wall stretches and develops into a megarectum[17] with decreased sensation to defecate[12]. Moreover, liquid stool can penetrate the hard stool and leak out of the anus, causing fecal soiling (Figure 3). According to pathophysiology, withholding behavior, palpable fecal mass on abdominal examination, and fecal soiling were reported in 37%-91%, 33%-68%, and 33%-77% of in children with FC, respectively[18-21]. Hence, two of the three characteristics were integrated into the Rome IV criteria.
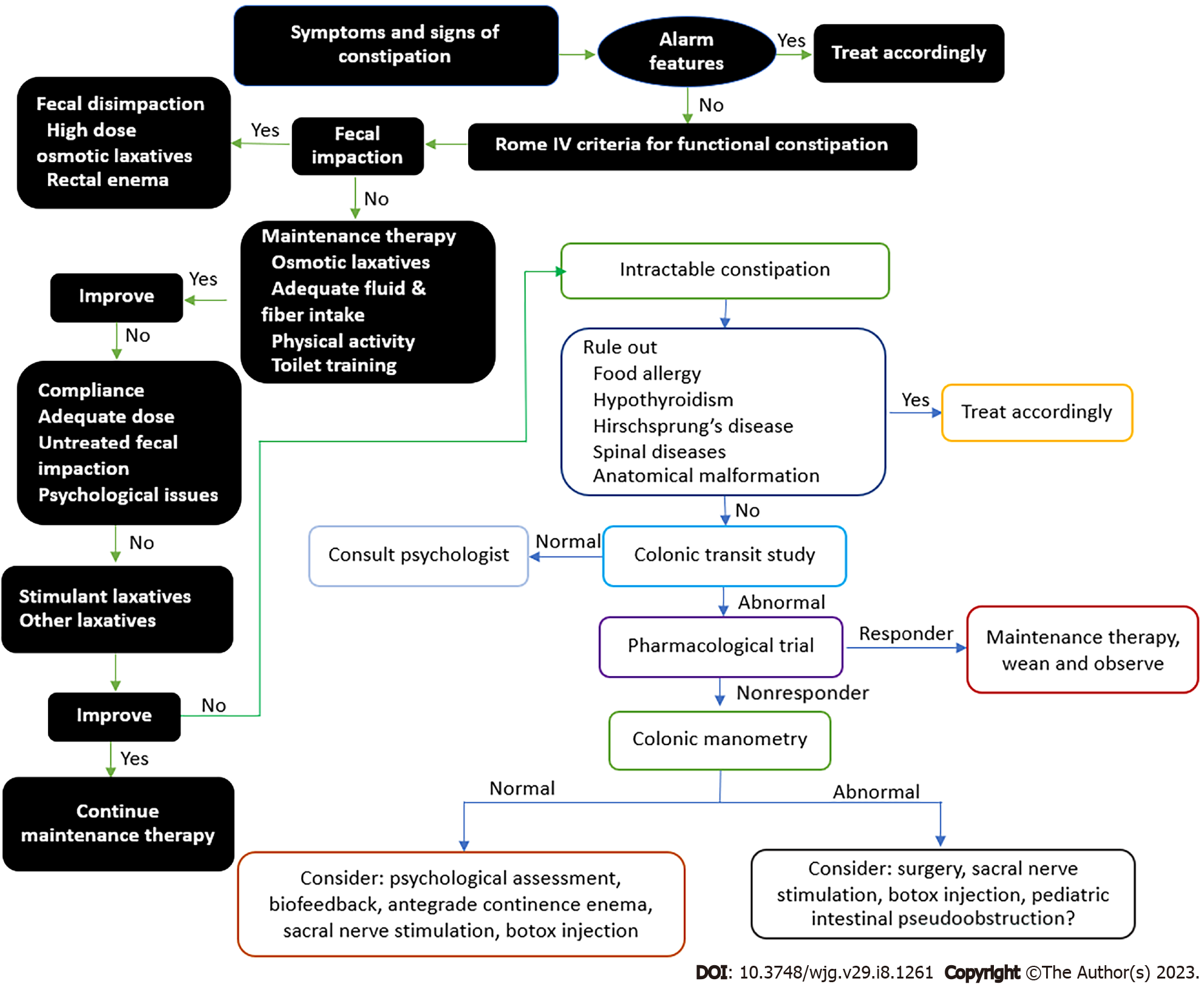
FC is diagnosed based on symptoms detailed in the Rome IV criteria. However, some examinations may help pediatricians in cases with uncertain symptoms and signs[22]. Moreover, in cases that are difficult to treat or have alarm features (Table 3), examinations to exclude organic cause are necessary. Here we will review the clinical manifestations and examinations that can helpful general pediatricians and specialists diagnose functional and organic constipation (Table 4).
| Alarm signs | Symptoms of constipation |
| History | Constipation starting in neonatal period, delay pass meconium (> 48 h of life), family history of Hirschsprung’s disease |
| Stool characteristics | Ribbon stools, blood in the stools in the absence of anal fissures |
| Gastrointestinal features | Bilious vomiting, severe abdominal distension |
| Back | Sacral dimple, tuft of hair on spine, gluteal cleft deviation |
| Anus | Perianal fistula, abnormal position of anus, anal scar, absent anal/cremasteric reflex |
| Neurological features | Decreased lower extremity strength/tone/reflex |
| Others | Abnormal thyroid gland, fever, faltering of growth |
| Organic causes | |
| Abnormalities of colon and rectum | Anal or colonic stenosis. Imperforate anus. Anteriorly displaced or ectopic anus. Cloacal malformations. Chronic intestinal pseudo-obstruction |
| Systemic disorders | Hypothyroidism. Hypercalcemia. Hypocalcemia. Diabetes mellitus. Panhypopituitarism. Cerebral palsy. Myotonia congenita. Scleroderma. Amyloidosis. Mixed connective tissue disease. Myotonic dystrophy. Progressive systemic sclerosis |
| Others | Cystic fibrosis. Celiac disease. Heavy metal ingestion (lead, mercury) |
| Spinal cord abnormalities | Meningomyelocele. Spinal cord tumor. Sacral agenesis. Tethered cord |
| Neuropathic intestinal disorders | Hirschsprung’s disease. Intestinal neuronal dysplasia. Chagas disease. Abnormal muscle of abdomen. Prune belly syndrome. Gastroschisis |
| Drugs | Opiates. Anticholinergics. Antacids. Antihypertensives. Antimotility agents. Cholestyramine. Psychotropics. Diuretics |
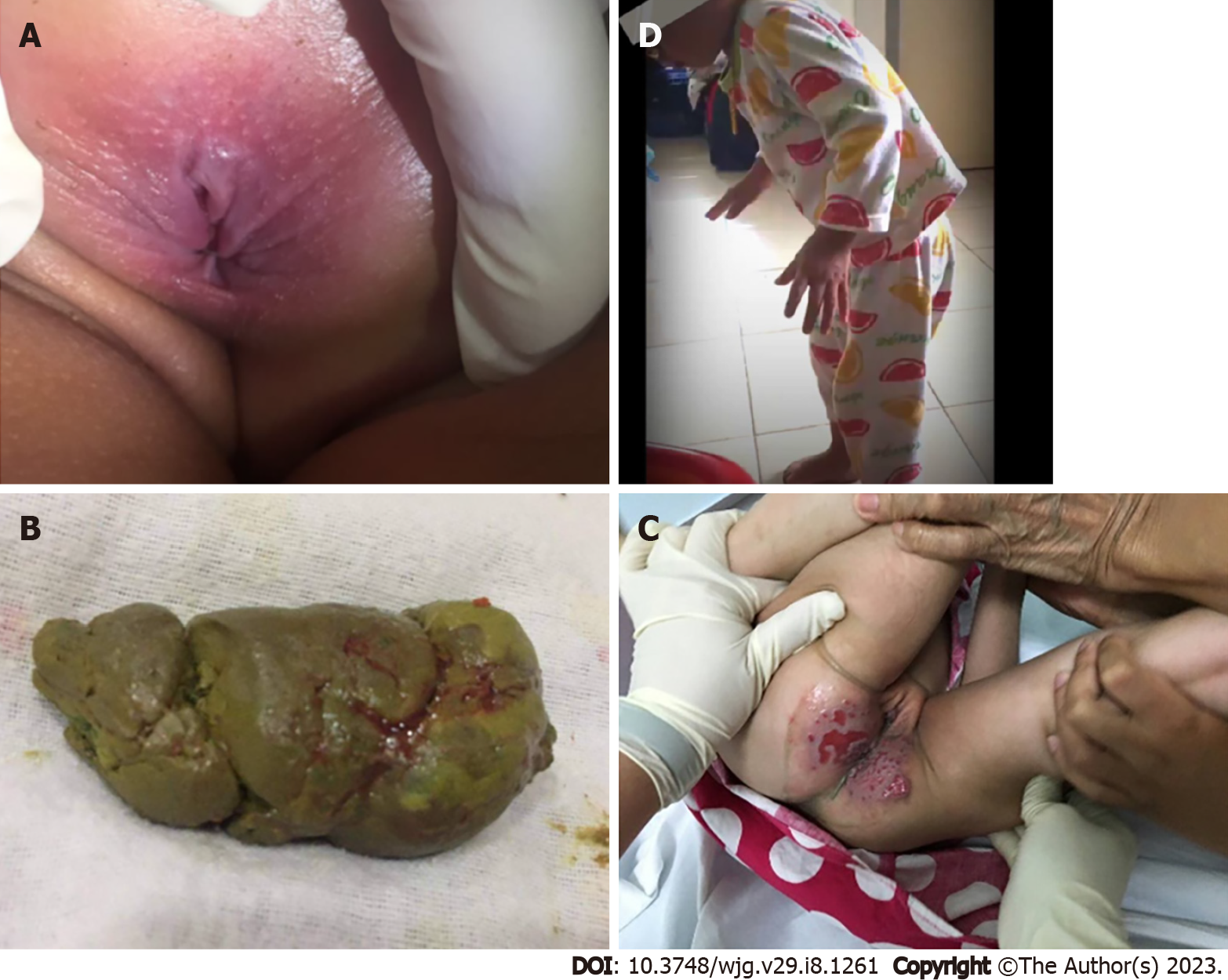
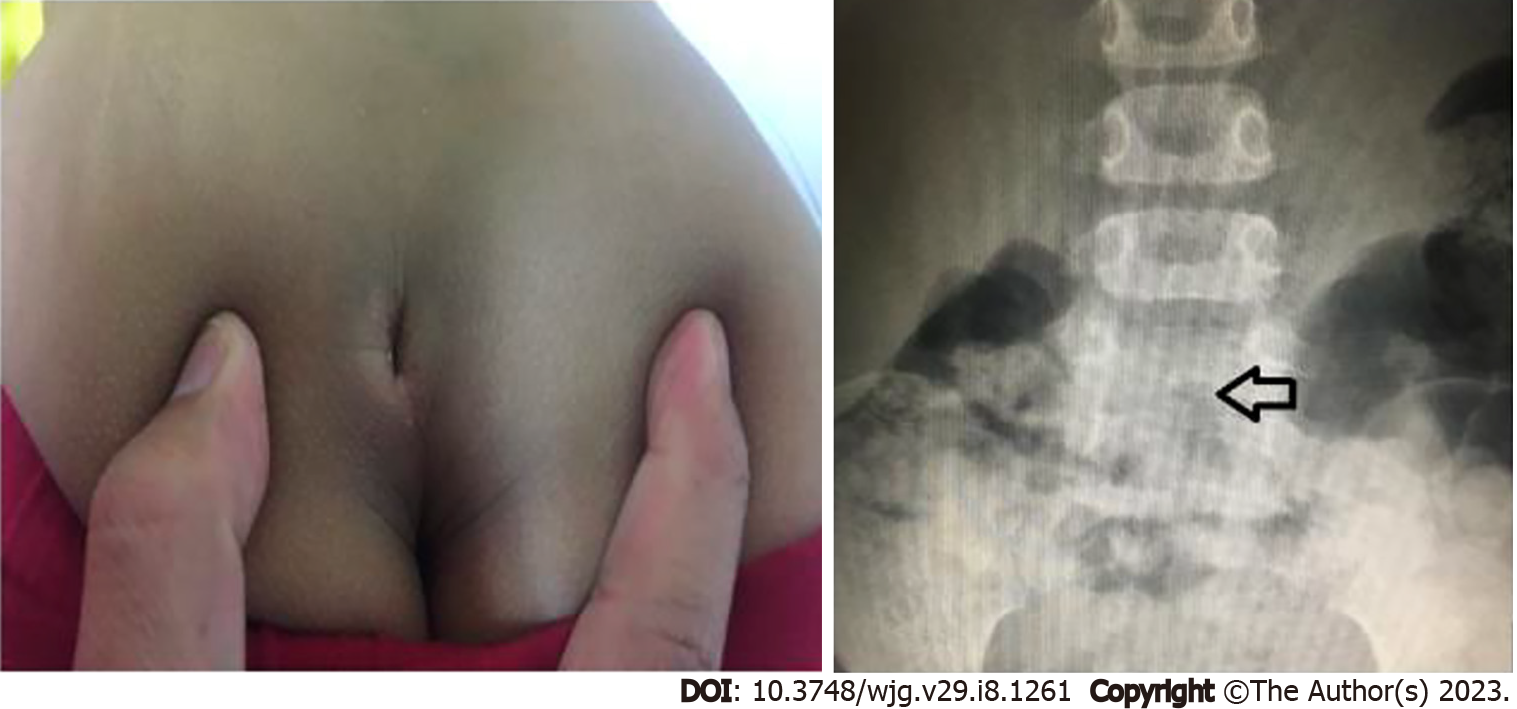
When obtaining the medical history of children, it is important to inquire about when the child had their first bowel movement after delivery. Normally, during the first 24 h of life, more than 90% of term newborns pass meconium[23,24]. This period may be longer in preterm infants due to the delayed maturation of the intestinal motor function[25]. If the passage of meconium is delayed after birth, worrisome diseases, such as Hirschsprung’s disease (HD) and cystic fibrosis, should be excluded[6,26]. Age of onset, frequency, consistency and size of the stool, painful or difficult defecation, and presence of blood coating the stool are all crucial details to note when recording a patient’s history. In addition, frequent clogging of the toilet might reflect a large fecal mass in rectum. Anal fissures should be examined in children with a history of difficult defecation (Figure 4A) and those with blood coating the stool (Figure 4B) or the toilet paper. It is necessary to gather information regarding incontinence or soiling during the day and night. FI can be mistaken for diarrhea by their guardians[1,6] (Figure 4C). Evidence of fecal impaction in children suspected of FI is crucial, and physicians could obtain this information through abdominal palpation of a fecal mass, digital rectal examination, or rarely through plain abdominal radiography in noncooperative children. Importantly, physicians must define how a child defecates. Withholding behaviors are considered the main pathogenesis of FC and have been defined by guardians as defecation in the “standing position”. This can also be described as stiffening up, buttock clenching, walking on tip toes, crossing one leg over the other, bracing against furniture, being in the all-fours position or curling up in a ball, and sitting with legs straight out (Figure 4D)[27,28]. Withholding behaviors also help clinicians determine that the constipation should be of functional etiology without any organic problems. Other factors, such as significant life events like a family member’s death, the birth of a sibling, difficulties in school, sexual abuse, and others, can contribute to retentive behaviors and FC and should be evaluated in detail[1,6].
Although the abdominal pain caused by FC is typically nonspecific and poorly localized, constipation was the cause of acute abdominal pain in 50% of children who presented for a primary care visit and should be considered in this context[1]. Physicians should identify alarm features (Table 4) and other signs and symptoms, including appetite loss, fever, nausea, vomiting, reduced weight gain, issues with neuromuscular development, and behavioral or psychological problems[6,26,29]. Poddar et al[18] reported that children with symptoms such as delayed passage of meconium, growth failure, lack of retentive posturing, and absence of fecal impaction may likely have organic FC. Furthermore, urinary tract infections have been reported in a significant number of children suffering from constipation and FI[30,31]. Dietary and constipation treatment history should be investigated to predict the long-term outcomes of FC in affected children[1,6,32].
Assessing children’s physical development through weight and height measurements should be the first step[6]. Abdominal examination should obtain information on the rectal fecal mass, particularly its height above the pelvic brim, through bimanual palpation on either side of the rectus sheath[7]. With careful abdominal and digital rectal examination, fecal masses can be detected in 30%-75% of children with FC[1,18]. The perineum should be examined given that it can reveal important details regarding the anal position, evidence of FI, skin irritation, eczema, fissures, and signs of possible sexual abuse[6].
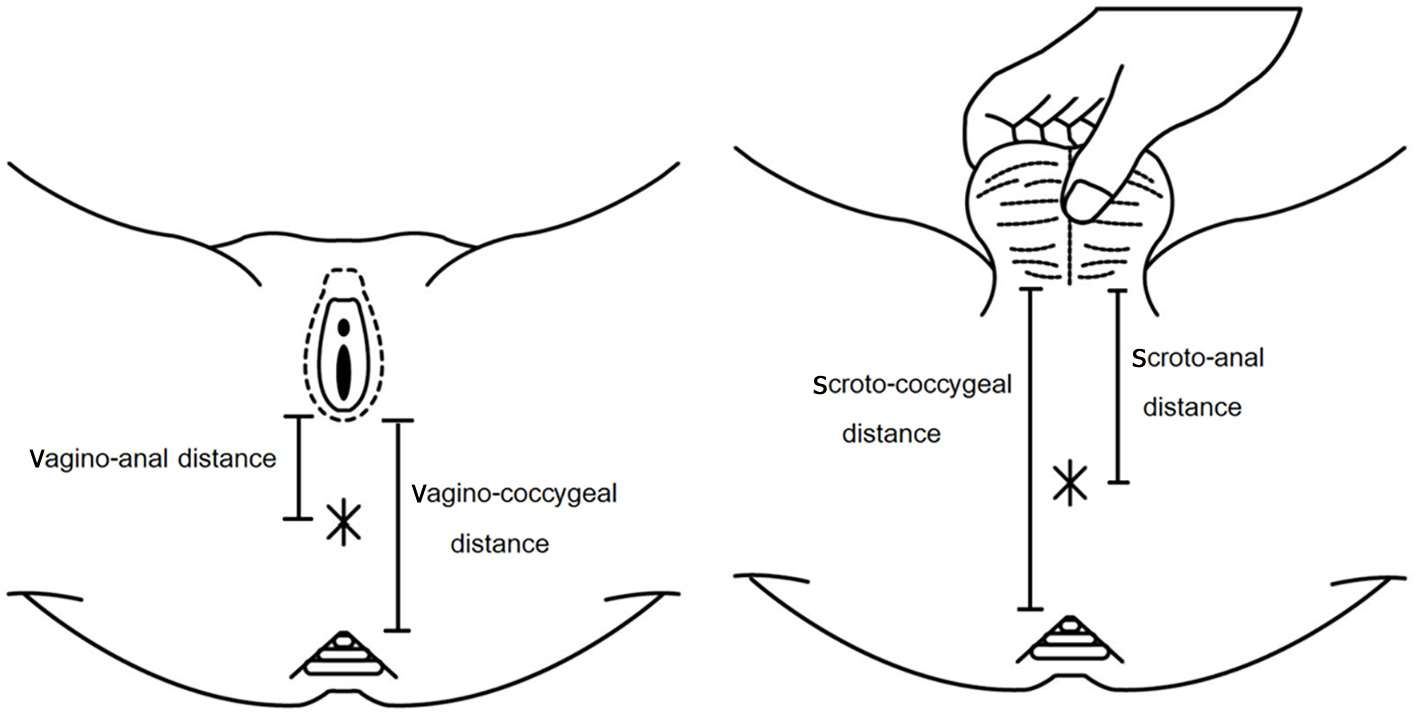
Measuring the anogenital index (Figure 5) is important given that it might be considered a factor associated with FC. The anogenital index can be calculated using the formula presented below[33-36]. The normal anogenital index in males and females is 0.54 ± 0.03 and 0.40 ± 0.04, respectively[35]. Anogenital index = [vagino/scroto to anal distance (cm) ÷ vagino/scroto to coccygeal distance (cm)][35].
When the child’s history suggests the presence of FC, a digital rectal examination may not be necessary[7,37]. Digital rectal examination should be conducted when children present with red flags, a history of delayed meconium passage after birth, intractable constipation, an uncertain diagnosis according to the Rome IV criteria, suspicion of an anatomic problem, and assessment of fecal impaction after disimpaction. Although neurological disease causing organic constipation is very rare, dedicated neurological examination still has merit (Figure 6).
It is necessary to emphasize that FC is a clinical diagnosis based on a detailed medical history and physical examination. The goal of laboratory testing is to determine the presence of a rare organic etiology in children with constipation showing alarm features[37] (Table 3) or confirm the diagnosis of FC in complicated or unclear cases. Hence, further diagnostic interventions are sometimes warranted. Investigations that might be useful for determining organic causes of constipation are described below.
Laboratory testing: Thyroid function and serum calcium tests can be used in children with intractable constipation or chronic constipation who are quite difficult to treat. In countries with a high prevalence of celiac disease and cystic fibrosis, specific tests for these diseases might be considered. Although the prevalence of food allergies in children presenting to tertiary clinics with chronic constipation who are unresponsive to traditional treatment vary from 28% to 78%[38], conflicting data support the use of allergy testing to identify cow’s milk protein allergy (CMPA) in constipated children[39-41]. Therefore, according to the recommendations of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NAPSGHAN), routine allergy testing is not recommended in constipated children suspected for CMPA[37]. Oral food avoidance and rechallenge is the gold standard for diagnosing CMPA that manifests with intractable constipation.
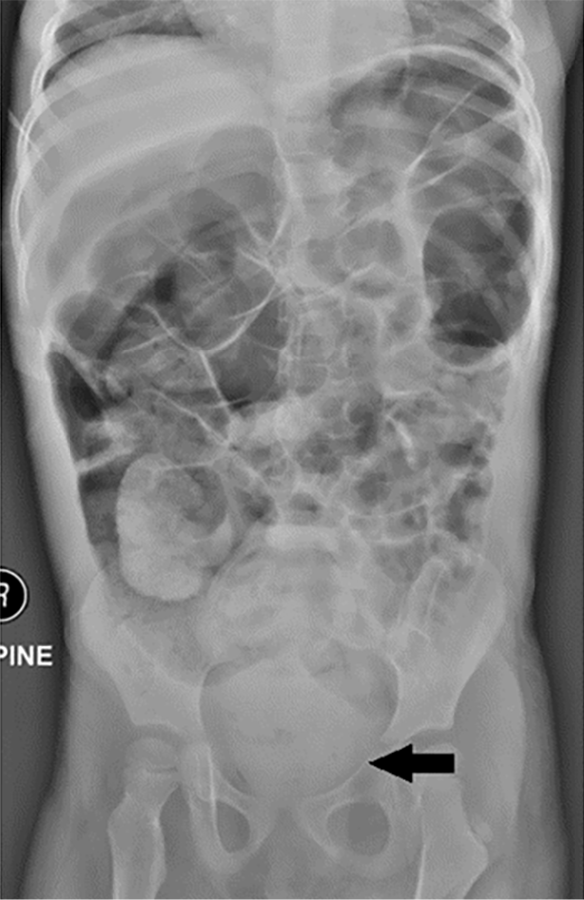
Abdominal radiography: Abdominal radiography can be help pediatricians determine the presence fecal masses in some cases where physical examination is limited (Figure 7), such as obese children, patient refusal, or noncooperation, or exclude some causes of acute abdominal pain.
According to systematic reviews, abdominal radiography can identify constipation with a sensitivity and specificity of 60%-80% and 43%-99%, respectively[42,43]. There was inconsistent evidence to support the diagnostic relationship between constipation symptoms and fecal loading in abdominal radiographs from children; hence, the results should be interpreted with caution. In line with this, three scoring systems have been developed, namely the Barr score[44], Leech score[45], and Blethyn score[46]. However, further validation is needed before they can be widely used in clinical practice.
Abdominal ultrasonography: Abdominal ultrasonography can assess stool retention and estimate the size of the rectum and colon based on the supposition that fecal retention is one of the primary characteristics of constipation in both children and adult[6,47]. Given that ultrasound scanning is noninvasive and radiation-free, it is usually used for assessment in primary and secondary clinical care[1,6]. Rectal diameter measurements were correlated with the results of digital rectal examination and therefore seems to accurately assess fecal impaction[43]. Evidence suggests that digital rectal examination might be replaced by ultrasound scanning given that the latter is less unpleasant[48]. Even though there was a good correlation of transverse rectal diameter with FI and long-term constipation[49], the transverse diameter cannot be used to predict fecal impaction or constipation[37]. Furthermore, the results are largely operator dependent, and patient cooperation is also needed.
Radiopaque marker for colonic transit study: Based on the distribution of markers throughout the colon, the radiopaque marker (ROM) for colonic transit time (CTT) study is one method for distinguishing between different types of colonic function, including normal colonic transit, slow-transit constipation, and obstruction of the rectal outlet. Given its accessibility and strong concordance with scintigraphic methods, the ROM for CTT study has become the most popular method for determining both total and segmental CTT[50]. The sensitivity and specificity of the ROM for CTT study were 71% (95%CI: 57%-83%) and 95% (95%CI: 82%-99%), respectively[51]. The mean transit time in healthy persons has been reported to range from 15.6 to 37.7 h, with a review by Southwell et al[52] revealing that the normal CTT was < 32 h (upper 95th centile: 54 h).
The ESPGHAN and NAPSGHAN recommend that these tests only used to distinguish FC from functional non-retentive fecal incontinence or, where the diagnosis is unclear, provide clarity and allow the selection of alternative diagnostic procedures due to the widespread use of the Rome criteria for the diagnosis of FC and the potential risks caused from repeated radiation exposure from abdominal radiography[37,50].
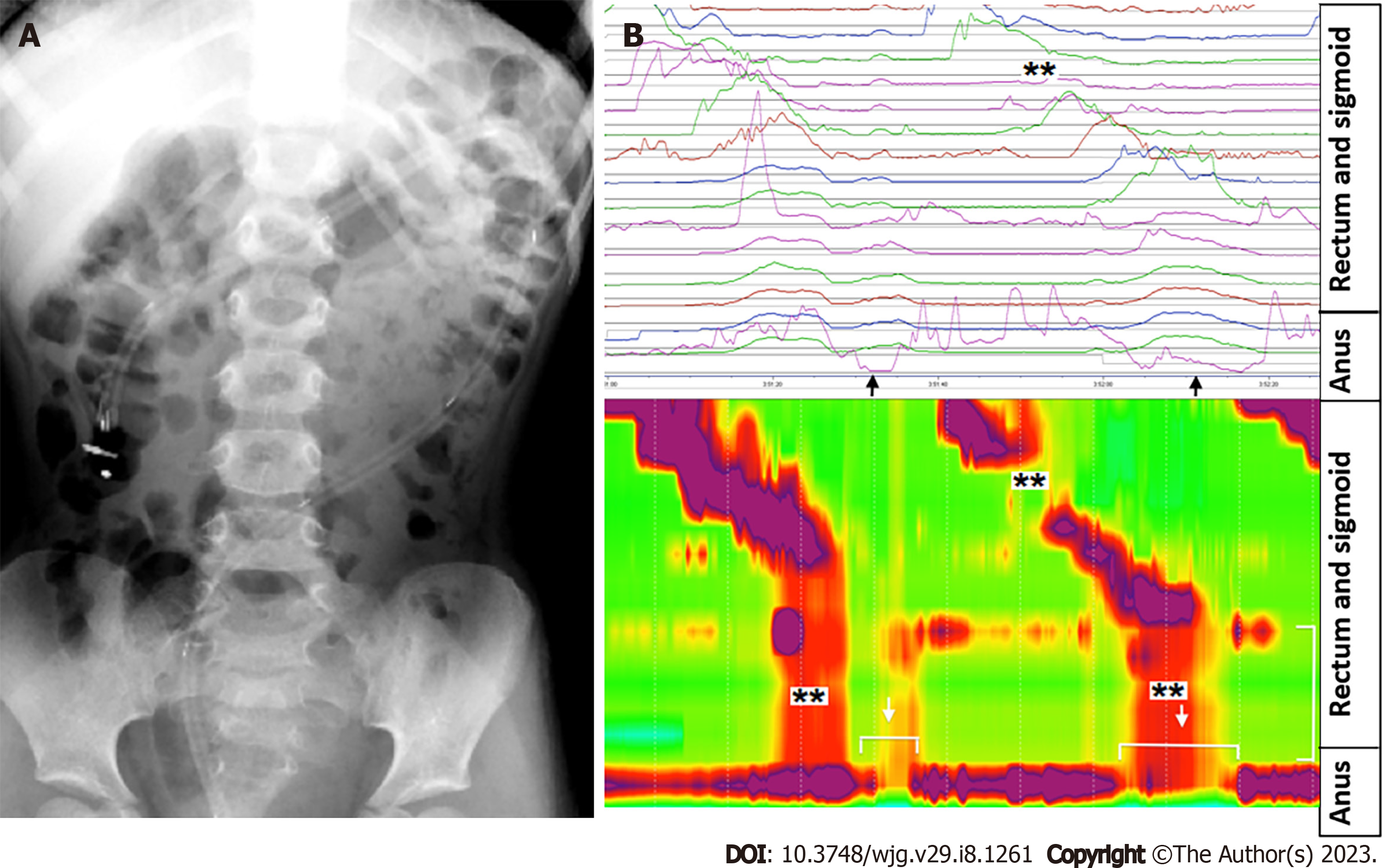
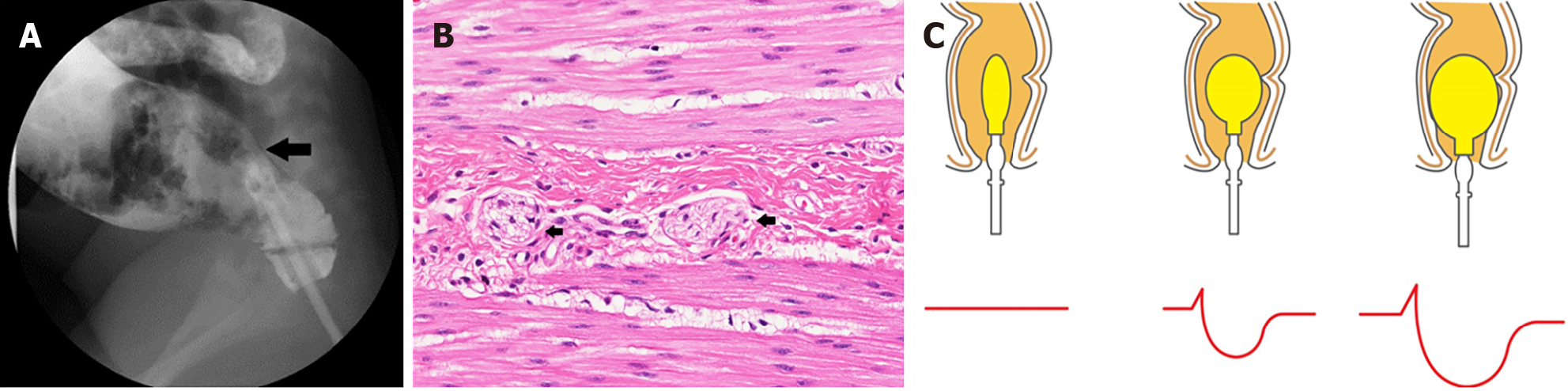
Colonic manometry: To determine the neuromuscular function of the colon in children with intractable constipation, colonic manometry is regarded as the gold standard[50]. Evidence suggests that colonic manometry is most useful in providing subsequent guidance for further therapy, including pharmacological and surgical management, in intractable constipation. According to a review by the ESPGHAN motility group, high amplitude propagating contractions are the most easily recognizable and reliable motor pattern (Figure 8). They are initiated usually in the proximal colon and expected to stop at the recto-sigmoid junction. However, colonic manometry can be difficult for children given its invasive nature and necessity for general anesthesia. Moreover, age can be a potential limiting factor depending on the size of the catheter and endoscope. Additionally, only a few specialist centers globally offer this test.
Wireless motility capsule: The wireless motility capsule (WMC) is a novel, nonradioactive, and minimally invasive tool for the assessment of colonic motor function. Several investigations have reported on the safety and tolerability of WMC. In recent years, an increasing number of studies have used WMC to diagnose children with functional gastrointestinal diseases (FGIDs)[53,54]. This test could provide information on gastrointestinal motility that is similar to information obtained via nuclear medicine gastric emptying time and/or ROM. Moreover, WMC can provide additional information on regional and entire-gut transit[53], which can not only add to our knowledge of colon physiology but also be used as a parameter to tailor treatment[54]. Considering its safety and low invasiveness, the ESPGHAN motility working group recommended that more research be done to assess the effectiveness of the WMC in predicting outcomes among children with intractable constipation[50].
Magnetic resonance imaging: Cine magnetic resonance imaging (cMRI) is a noninvasive tool that uses a high-resolution spatiotemporal approach to facilitate dynamic MRI, which would allow the observation of the gut lumen diameter. The assessment of stomach accommodation and emptying, terminal ileum motility, and the small bowel using cMRI has been documented in the literature[43].
In both adults and children, cMRI can be used to assess colonic motility for various gastrointestinal disorders[55]. However, data on the application of this method in children with intractable FC are currently scarce.
Vriesman et al[56] published the first pediatric study comparing the identification of colonic motility patterns on cMRI with that on colonic manometry, proving potential evidence regarding the feasibility of the technique. cMRI has the advantage of being noninvasive, precluding the need for general anesthesia; however, given that this is still a research-based modality, additional studies are required to establish objective and systematic measurements.
Barium enema and others: A barium enema is used to coat the lining of the colon and rectum in order to create clearer images of the colon (Figure 9A). A contrast enema is often used in the diagnostic workup of HD, in which a transition zone between the aganglionic and ganglionic bowel may be observed in histopathology study[6] (Figure 9B). A 24-h delayed barium enema film could offer comprehensive data on colon transit function in young children, especially those under 4 years old who often cannot undergo CTT study[57]. Moreover, delayed retention of contrast at 48 h provides the strongest negative predictive value to exclude HD. Nonetheless, a limitation of barium enema is that it cannot be used to diagnose ultrashort HD; instead, anorectal manometry is required wherein absence of the anorectal inhibitory reflex is pathognomonic for HD (Figure 9C).
The goals of treatment include establishing regular defecation (ideally once a day, passing soft stools and without difficulties) and preventing relapses[1,6,58]. Oral laxatives and structured toilet training are the main tools of a successful treatment. FC in children is typically managed across four important phases: (1) Education; (2) Fecal disimpaction; (3) Preventing fecal reaccumulation; and (4) Follow-up.
Education is an important initial step of treatment[59] given its association with adherence and the successful of management of constipated children. In fact, studies by Steiner et al[60] and Koppen et al[61] reported that 38% and 37% of patients adhered to therapy, respectively. Inconvenience, dissatisfaction with treatment, and emotional impact of symptoms were linked to low adherence, all of which require attention[61].
Therefore, education should include an explanation of the physiological dynamics of defecation; associated factors of constipation; and the related shame, embarrassment, and social issues to the guardians and their children. In particular, the doctor needs to make it clear to the family that withholding behavior is crucial to the pathophysiology of FC. Furthermore, physicians should create a thorough plan to eliminate the frustration of guardians and children and increase cooperation required for prolonged treatment. Moreover, the timing of a successful treatment is frequently unpredictable, and guardians must understand that there is no quick fix for this problem. Recovery is only feasible with a sufficient, frequent, and prolonged care[1,6,59]. In some complex cases, such as in children with intractable constipation or in those suffering from other comorbidities such as urinary problems, it is necessary to involve a multidisciplinary team that includes pediatric specialist nurses, pediatric research nurses, psychiatrists, urotherapists, and urologists for long-term follow-ups. Hence, such children require customized care.
Fecal disimpaction is crucial before the start of maintenance therapy in order to maximize the success of treatment. If fecal impaction is not eliminated beforehand, maintenance therapy can lead to worsening FI[1,6,59]. Oral drugs, rectal enema, or a combination of both can effectively treat fecal impaction. Two randomized controlled trials (RCTs) showed that polyethylene glycol (PEG) and enemas are equally effective for fecal disimpaction[62,63]. The use of 1-1.5 g/kg/d of PEG with or without electrolytes orally for 3-6 d is recommended as the first-line treatment for constipated children with fecal impaction[37]. However, a RCT study reported that both lactulose and PEG treatment successfully promoted disimpaction and were safe and well tolerated, although PEG achieved disimpaction significantly faster at day 2 (P = 0.001)[64]. Lactulose may be a useful PEG substitution for treating fecal impaction, particularly in areas wherein the availability of PEG is limited.
The oral route is typically less intrusive, better tolerated, and provides children with a better sense of control than rectal enema; however, successful disimpaction can take a few days, with compliance also being an issue. In contrast, the rectal approach (enema) is faster (effect occurs within minutes) but more invasive and traumatic. It may be useful for removing fecal impaction in patients with severe abdominal pain or large fecal mass on abdominal examination. Common side effects of enema include anorectal discomfort and abdominal pain; hence, it should be avoided in scared and resistant children to avoid anal fissures[58]. Recently, a study on the efficacy of olive oil enemas for childhood constipation showed that 79.6% and 66.7% of FC cases in the olive oil and lubricant groups were effectively treated for fecal impaction, respectively[65] (Table 5).
| Agent | Child’s age | Dosage | Side effects |
| Osmotic laxatives | |||
| PEG | Any age | 0.4-0.8g/kg per day for maintenance; 1-1.5g/kg per day for fecal disimpaction | Diarrhea, bloating, flatulence, nausea, vomiting, abdominal cramps |
| Lactulose (70% solution) | Any age | 1 mL/kg once or twice daily (max 120 mL per day) | Bloating, flatulence, abdominal cramps, fecal, incontinence |
| Sorbitol (70% solution) | 1-11 yr | 1 mL/kg once or twice daily (max 30 mL per day) | Bloating, abdominal cramps |
| > 12 yr | 15-30 mL once or twice daily | ||
| Milk of magnesium | > 2 yr | 1-3 mL/kg per day once or twice daily | Abdominal pain, fecal incontinence, hypermagnesaemia, hypocalcaemia, hypophosphataemia (with excess use in children with renal disease) |
| Stimulant laxatives | |||
| Senna (antraquinone) | > 2 yr | 7.5-15 mg/kg per day once daily | Abdominal cramps, idiosyncratic hepatitis, melanosis coli in prolong used, nephropathy, neuropathy, hypertrophic osteoarthropathy |
| Bisacodyl | > 2 yr | 5-10 mg per day once daily | Diarrhoea, abdominal cramps |
| Sodium picosulphate | 4-5 yr | 3 mg per day | Nausea, vomiting, bloating, abdominal cramps, diarrhea, headache, taste impairment |
| > 6 yr | 4-6 mg per day | ||
| Glycerine suppository | < 1 yr | Half for pediatric suppository once daily | Rectal irritation, bloating, abdominal cramps, diarrhea |
| Rectal laxatives/enemas | |||
| Sodium phosphate | > 1 yr | 2.5 mg/kg | Rectal discomfort, diarrhea, abdominal cramps, electrolyte imbalance |
| Bisacodyl | 2-12 yr | 5 mg/dose once daily | Rectal discomfort, diarrhea, abdominal cramps, hypokalemia |
| > 12 yr | 5-10 mg/dose once daily | ||
| Saline enema | Neonate | < 1 kg: 5 mL, > 1 kg: 10 mL | Rectal discomfort, bloating |
| > 1 yr | 6 mL/kg once or twice daily | ||
| Lubricant | |||
| Mineral oil | > 1 yr | 1-2 mL/kg daily (max 90 mL per day) | Rectal discomfort, lipoid pneumonitis |
Following disimpaction, maintenance therapy should be started immediately. The goals of maintenance therapy are to produce soft and painless stools, avoid stool reimpaction, and stop the reemergence of stool withholding behavior. This can be accomplished by combining pharmacological and nonpharmacological interventions.
Osmotic laxatives: Osmotic laxatives, including PEG, lactulose, and milk of magnesium hydroxide (MOM), are a type of osmotically active ions or molecules that are rarely absorbed in the small intestine. As such, they stimulate water retention in the colon, consequently softening stools.
PEG has been demonstrated to be more effective at increasing bowel movement frequency than lactulose, making it the first option for maintenance therapy in constipated children[66-68] or MOM[69]. However, limited evidence of its the utilization and safety has been available in infants, especially for long-term usage[66,70]. Moreover, data from a 10-year survey revealed that 645 children using PEG 3350 between the ages of 0-21 reported 1564 adverse symptoms. Among these adverse symptoms, 58.75% were neurological or neuropsychiatric, such as anxiety, anger, abnormal behaviors, and others[71]. However, the data source from this survey had significant limitations, including sampling bias, lack of verification, inability to seek clarifications, and lack of follow-up data. Comparing the efficacy of lactulose and MOM, one study found a significant difference in the frequency of stool passage per week, favoring MOM over lactulose (MD: 1.51, 95%CI: -2.63 to -0.39, 50 patients). Besides PEG, lactulose and MOM have been used as second-line drugs, with MOM being very cheap and widely available in some Asian countries such as Thailand[72]. However, the main limitation of MOM is its terrible palatability as opposed to lactulose. The concerning adverse effect of MOM is just only awareness of hypermagnesemia, especially with long-term usage in children with chronic renal disease. Conversely, lactulose can used safely even among preterm infants[73], with the only common adverse effect being abdominal distension. Though PEG is the most effective osmotic laxative for the treatment of function constipation in children, in areas or situations where availability is limited, MOM or lactulose might be used as the standard medication for FC instead of PEG[6,37].
Stimulant laxatives: Stimulant laxatives, such as senna and bisacodyl, increase intestinal motility and interfere with water and electrolyte transport across the epithelial layer. Therefore, stimulant laxatives might result in cramping and abdominal discomfort[1,6,74]. When osmotic laxatives alone are ineffective in treating chronic constipation, stimulant laxatives are often considered. Although stimulant laxatives are thought to be safe and beneficial for treating childhood constipation, limited high-quality RCTs have evaluated their use[66,74,75]. Based on expert opinion, the use of stimulant laxatives may be considered as an additional or second-line treatment[37].
Lubricants: The most popular lubricant laxative is mineral oil, often known as liquid paraffin. Mineral oil works by coating and lubricating stools, lowering fecal water absorption in the colon, and making it easier to pass feces. Given that mineral oil has no chemical activity, severe negative impacts are rarely common. The effectiveness of mineral oil and oral laxatives in treating childhood constipation has been compared in a few low-quality trials. Accordingly, mineral oil promoted significantly greater bowel movement frequency compared to lactulose[66,76,77], but no significant difference in treatment response was observed when compared to PEG[78]. Moreover, liquid paraffin was found to induce significantly better defecation frequency and FI episodes compared to senna; however, the evidence was of low quality[79]. Given the risk of aspiration and severe lipoid pneumonitis, mineral oil is not recommended for infants under the age of 1 year[80].
Probiotics/prebiotics: Probiotics are live microorganisms that, when administered in adequate amounts, confer health benefits to the host. Probiotics have been used in the treatment of FC based on the hypothesis that they alter the intestinal microbiota and colonic pH, thereby improving gastrointestinal motility. Studies reported that constipated children have higher amounts of Lactobacillus spp.[81,82] and lower amounts of Bacteroides[83] compared to healthy children, implying that gut dysbiosis in the pathogenesis of constipation. So far, however, strong evidence to support the benefits of probiotics in the treatment or prevention of constipation has been limited[84-87]. However, some studies have demonstrated significantly increased stool frequency or softer stools after receiving probiotics. These findings might imply the significant impact of the pathogenesis of stool withholding in constipated children and that probiotics cannot be expected to overcome withholding behaviors. Therefore, future well-designed studies are needed.
5-HT4 receptor agonists: Serotonin controls gut motility, visceral sensitivity, and intestinal secretion through serotonin 5-HT4 receptors, which are primarily expressed by enteric nervous system interneurons[6].
5-HT4 receptor agonists cisapride and tegaserod, which showed similar benefits for treating childhood constipation, were discontinued due to their increased risk for cardiovascular accidents and prolonged QT interval. Prucalopride is a new generation of selective, high-affinity 5-HT4 receptor agonists that stimulate gastrointestinal motility and act primarily on parts of the lower gastrointestinal tract[6,74,88]. Current research on the benefit of prucalopride for constipated children has been contradictory. In an open-label pilot study, prucalopride had favorable effects on stool frequency, stool consistency, and frequency of FI in children with FC[89]. However, a recent multicenter RCT in 213 constipated children found no significant improvement of symptoms compared to placebo[90]. Common side effects include headache, nausea, abdominal pain, and diarrhea.
Chloride channel activators: Lubiprostone is a prostaglandin E1 derivative that activates the chloride channel, thereby stimulating intestinal fluid secretion without increasing serum electrolyte levels[6,74,88]. A study on 127 children (3-17 years old) with constipation showed that lubiprostone was effective and well tolerated, with only minimal side effects like nausea and vomiting[91]. Another study evaluating the safety and tolerability of oral lubiprostone over the course of 24 wk for the treatment of childhood FC in patients aged 6-17 years old showed that lubiprostone was well tolerated and the frequency of treatment-emergent adverse events was similar to that seen in previous clinical trials and adults[92]. However, a double-blind, placebo-controlled, multicenter study on 606 children aged 6-17 years old (202 placebo; 404 Lubiprostone) with FC who satisfied the Rome IV criteria showed no significant difference in the total spontaneous bowel movements response rate between the lubiprostone and placebo groups. Frequently reported side effects include nausea, vomiting, diarrhea, and stomach pain[93].
Linaclotide and plecanatide bind to and act as an agonist of guanylate cyclase-C receptors, causing an increase in the production of chloride and bicarbonate the intestinal lumen. This increase in intestinal fluid causes an acceleration of the gastrointestinal transit while simultaneously decreasing visceral pain by reducing pain sensation[6,74]. Although no pediatric trials have been conducted, studies in adults showed that plecanatide treatment significantly improved constipation. The use of linaclotide in children with FC (0-18 years old) was only reported in a retrospective study, which showed that 45% patients with FC had a positive clinical response and approximately one-third of children experienced negative side effect, such as diarrhea, abdominal pain nausea, and bloating. Eventually, 27% patients stopped using linaclotid due to adverse events[94].
Herbal and traditional medicine: Stool withholding behavior is the main pathogenesis of FC in children. Given that successful toilet training takes time, normally more than 3-6 mo, osmotic laxatives have been the mainstay of treatment during toilet training. However, the majority of guardians and children wanted to withdraw the medication due to their concerns, sometimes with the taste or the amount of osmotic laxatives, which worsened the constipation. In several countries such as Iran, China, Vietnam, and Thailand, herbal medicine is usually integrated into some parts of the treatment as the main or additional therapy. Furthermore, while developing a new drug is a time-consuming and costly process, traditional drugs can be used to treat FC in children instead.
Several herbal and traditional medicines have been used for managing constipation in children, such as glucomannan[95,96], cocoa husk[97], AFPFF (combined acacia fiber, psyllium and fructose)[98], cassia fistula emulsion[99], inulin[100], black stap molasses[101], XiaojiDaozhi Decoction[102], damask roses[103,104], and other herbal medicines[105]. However, only a small number of herbal remedies for FC have been well supported by RCTs in children (Table 6).
| Ref. | Country | Age (yr) | Study design | No. case (intervention/ control) | Intervention protocol | Probably pharmacological effect of herbal medicine | Duration of treatment/follow-up/end point and outcome measurement | Treatment effect |
| Esmaeilidooki et al[99], 2016 | Iran | 2-15 | Open label, RCT, single center | 109 (52/57) | CFE 1 mL/kg per day in three-divided doses (equivalent to 0.1 g of dried pulp of fruits of Cassia fistula). PEG 0.7-0.8g/kg per day | Phenolic antioxidants such as flavonoids, flavan-3-ol derivatives and anthraquinones: Stimulant laxative | Treatment for 4 wk. Primary outcome: Frequencies of defecation, severity of pain, consistency of stool, fecal incontinence and retentive posturing. Secondary: The safety and compliance of therapy | After 4 wk: 86.5% of children in CFE group and 77.1% in PEG group exited from the criteria of FC (RR = 1.121, CI95%: 0.939-1.338). Frequency of defecation that in CFE group (10.96 ± 5.7 stools per week) was significantly more than PEG group (6.9 ± 3.5 stools per week) (P < 0.001). No serious adverse effects in both groups (25% diarrhea and 3.8% abdominal pain) |
| Cai et al[161], 2018 | China | 1-14 | Double-bline RCT, multicenter | 480 (120/360) | XEBT: (1) 1-3 years old: 2.5 g, 3 times a day; (2) 4-6 years old: 5 g, 2 times a day; and (3) > 7 years old: 5 g, 3 times a day. Placebo | Seven herbs (Houpo contains magnolol, JueMingZi contain anthraquinones, LuHui contains reactive Aloe-emodin, BaiZhu contains Atractylodes japonica, LaiFuZi, XingRen, ZhiQiao): Promote small bowel peristalsis and work against atropine-induced small intestine suppression in mice | Treatment for 14 daysPrimary outcome: Frequency of SBM for 14 d. Secondary outcomes: Effectual time of defecation, mean symptom scores, disappearance rate of symptoms, recurrence rate and safety outcomes | The mean value of SBM for 14 d were 8.89 and 5.63 in the XEBT and placebo group (P < 0.05). The median effectual time of defecation, main symptom score and disappearance rate of symptoms were significant improved in XEBT group without the significant minor adverse effects between groups |
| Dehghani et al[101], 2019 | Iran | 4-12 | Double-blind RCT, single center | 92 (45/47) | BSM (sugarcane extract) 1 mL/kg per day. PEG 1 g/kg per day | The BSM naturally contained polyphenols (960 μg/mL), potassium (12430 μg/mL), iron (10 μg/mL), calcium (3320 μg/mL), zinc (22 μg/mL), sucrose (296000 μg/mL), triterpenoids (11230 μg/mL), phytosterols (7 μg/mL), flavonoids (2 μg/mL and polysaccharides (1250 μg/mL): Polysaccharide act as bulk forming agent, flavonoids/phytosterols and polyphenolic compounds act as natural antioxidants and anti-inflammatory agents | Treatment for 4 wk: Primary outcome: Response rate improvement in frequency of defecations per week, absence of lumpy or hard stools, abdominal pain and retention, soiling and blood-stained stool, sensation of anorectal obstruction/blockage. Secondary outcome: Patients’ body weigh was measured in every visit and serological parameters (count blood cells, BUN, creatinine, calcium, phosphorus, sodium and potassium) | Defecation per week was significantly improved in both groups. Symptoms including volitional stool retention, large diameter stool, painful or hard stool and large fecal mass in the rectum decreased significantly two and four weeks after intervention (P < 0.05). No significant difference between the groups. No adverse effects were observed |
| Qiao et al[102], 2021 | China | 4-14 | Double-blind RCT, multicenter study | 200 (100/100) | Mixture of 12 herbs1 (XiaojiDaozhi Decoction) and placebo (5% drug ingredients and 95% dextrin). All received fiber 20 g per day and toilet training) | 12.5% Raphanus sativus L. (facilitating intestinal motility), 8.33% Areca catechu L. (stimulating gastrointestinal cholinergic receptor), 6.67% Fructus aurantll immaturus (stimulate gastrointestinal smooth muscle), Citrus aurantium L. (stimulate gastrointestinal smooth muscle), 6.67% Crataegus pinnatifida (stimulate gastrointestinal smooth muscle), Magnolia officinalis Rehd (stimulate gastrointestinal smooth muscle), Cannabis sativa L. (moistening the bowel and purging the stools), Atractylodesmacrocephala Koidz. (stimulate gastrointestinal smooth muscle), Semen armeniacae amarum (purgative effect), Paeonia lactiflora Pall, Radix et rhizoma rhei, (purgative stool)0 and honey (moistening the bowel and purgative effect) | Treatment for 8 wk and follow-up for 12 wk. Primary outcome: Complete SBM (≥ 3 per week) and satisfaction with bowel function. Secondary outcome: Safety and adverse effect (blood measurement of liver and kidney function and lead level) | After 8 wk: 56% of CHM group and 25% of placebo satified with bowel movement (P < 0.05). 40% of CHM group and 19% of placebo had complete spontaneous bowel movement (P < 0.05). No serious adverse effects in both groups |
| Tavassoli et al[162], 2021 | Iran | 4-10 | Open label RCT, single center | 133 (66/67) | Viola flower syrups 5 mL, 3 times a day. PEG 4000 1 g/kg per day | Viola flower contains crude methanolic extract, butanolic and aqueous extracts the stimulate gastrointestinal motility | Treatment 4 wk. Primary outcome: Response off treatment (ROME III criteria). Secondary outcome: Stool consistency, defecation frequency, hard stools, painful defecation, fecal retention, and fecal soiling | Both groups demonstrated significant improvement in stool consistency, number off defecation, hard stool, painful defecation, fecal retention and fecal soiling at the end of the study compared to baseline (P < 0.001). No significant difference was observed between the two groups at baseline or at the end of the study (P > 0.05) |
| Nasri et al[163], 2021 | Iran | 2-15 | Open-lable RCT | 120 (60/60) | LaxaPlus Barij Syrup 1 mL/kg divided into 3 doses. PEG 0.7 g/kg | NA | Treatment and follow-up 8 wk. Primary outcome: Stool consistency, number of defecations, intensity of pain, fecal incontinence. Secondary outcome: Satisfaction rate | After 8 wk follow-up: Bowel movements in the intervention group was significantly higher than in the control group (P < 0.05). Pain intensity, and abdominal pain in the group LaxaPlus Barij® decreased significant than control group. No different about satisfaction rate between 2 group |
| Saneian et al[103], 2021 | Iran | 2-15 | Double-blind RCT | 60 (30/30) | Goleghand (including honey and Rosa damascene) 0.5 g/kg in three divided dose. PEG: 0.7 g/kg | NA | Treatment and follow-up 8 wk. Primary outcome: The number and consistency of stools per day, painful defecation, abdominal pain, and fecal incontinence. Secondary outcome: Adverse effects and parental satisfaction | After 8 wk: The number of fecal defecations in Goleghand group was higher than PEG (P < 0.05). The decrease of defecations after following was more significant in the PEG group than in the Goleghand® group (P = 0.001). Parental satisfaction scores did not change in either group (P > 0.05) |
| Imanieh et al[104], 2022 | Iran | 1-18 | Double-blind RCT | 100 (50/50) | Rosa damascena + brown sugar 1-2 mL/kg (1 mL composed of 0.1 g damask rose and 0.85 g brown sugar). PEG 1-2 mL/kg. All received high fiber diet and hydration | Damask rose: Osmotic laxatives and prokinetic effect. Brown sugar: Osmotic laxatives effect. Possible active ingredients might be phenolic compounds and aqueous fraction (terpenes, glucosides, flavonoids, anthocyanins, kaempferol and quercetin) | Treatment and follow-up 4 wk. Primary outcome: the effective of herbs with PEG. Secondary outcome: Adverse effects | After 4 wk: The cure rate was 100% in the R. damascena group and 91.7% in the control group. Adverse effect of intervention group was the taste which was too sweet |
Most studies have reported that herbal and traditional medicine had significant effects on childhood constipation without significant adverse effects. Nonetheless, more high-quality studies are needed. This also suggests the need for studies that identify the active ingredients of herbal and traditional medicine responsible for their beneficial effects against FC in children. With more evidence, herbal or traditional medicine therapies can be integrated into standard treatments for childhood.
Diet (fiber and water): A low dietary fiber intake has been considered a risk factor for the development of FC[1,6,106]. The recommended adequate dietary fiber intake in children older than 2 years of age is equivalent to age (in years) plus 5-10 g/d[1,107]. However, there is still insufficient evidence from RCTs to support the routine use of fiber supplements to reduce constipation in children[108]. While some studies have shown that constipated children have lower fiber intake compared to healthy controls[96,109], other studies do not support this[95,110]. Regarding to fluid intake, lower water intake has been associated with a higher risk for intestinal constipation[111-113]. Hence, adequate water intake may be beneficial for the prevention of FC. The recommended water intake for children is based on the National Institute for Health and Care Excellence Guideline (Table 7).
| Total water intake per day, including water contained in food (mL) | |
| Infants 0-6 mo | 700 (water is assumed to be from breast) milk |
| 7-12 mo | 800 (milk and complementary foods and beverages) |
| 1-3 yr | 1300 |
| 4-8 yr | 1700 |
| Boys 9-13 yr | 2400 |
| Girls 9-13 yr | 2100 |
| Boys 14-18 yr | 3300 |
| Girls 14-18 yr | 2300 |
Physiotherapy: FC in children is thought to be influenced by dysynergic defecation, which refers to pelvic floor dysfunction[88]. Studies have shown the effectiveness of pelvic muscle exercises as part of a combined treatment intervention[59,88]. A multicenter RCT comparing standard medical care (SMC), which includes education, toilet training, and laxatives, with pelvic physiotherapy (PPT) + SMC in constipated children aged 5-16 years old showed that PPT and PPT + SMC were effective in 63% and 92.3% of the children, respectively. Treatment success (based on the global perceived effect) were achieved in 88.5% and 33.3% of subjects receiving PPT + SMC and SMC, respectively (P < 0.001)[114]. A significant study in a primary care environment, however, found no additional benefits of PPT in 134 children aged 4-18[115] and that adding physiotherapy to SMA as a first-line treatment for all children with FC offered no cost benefits compared to SMA alone[116].
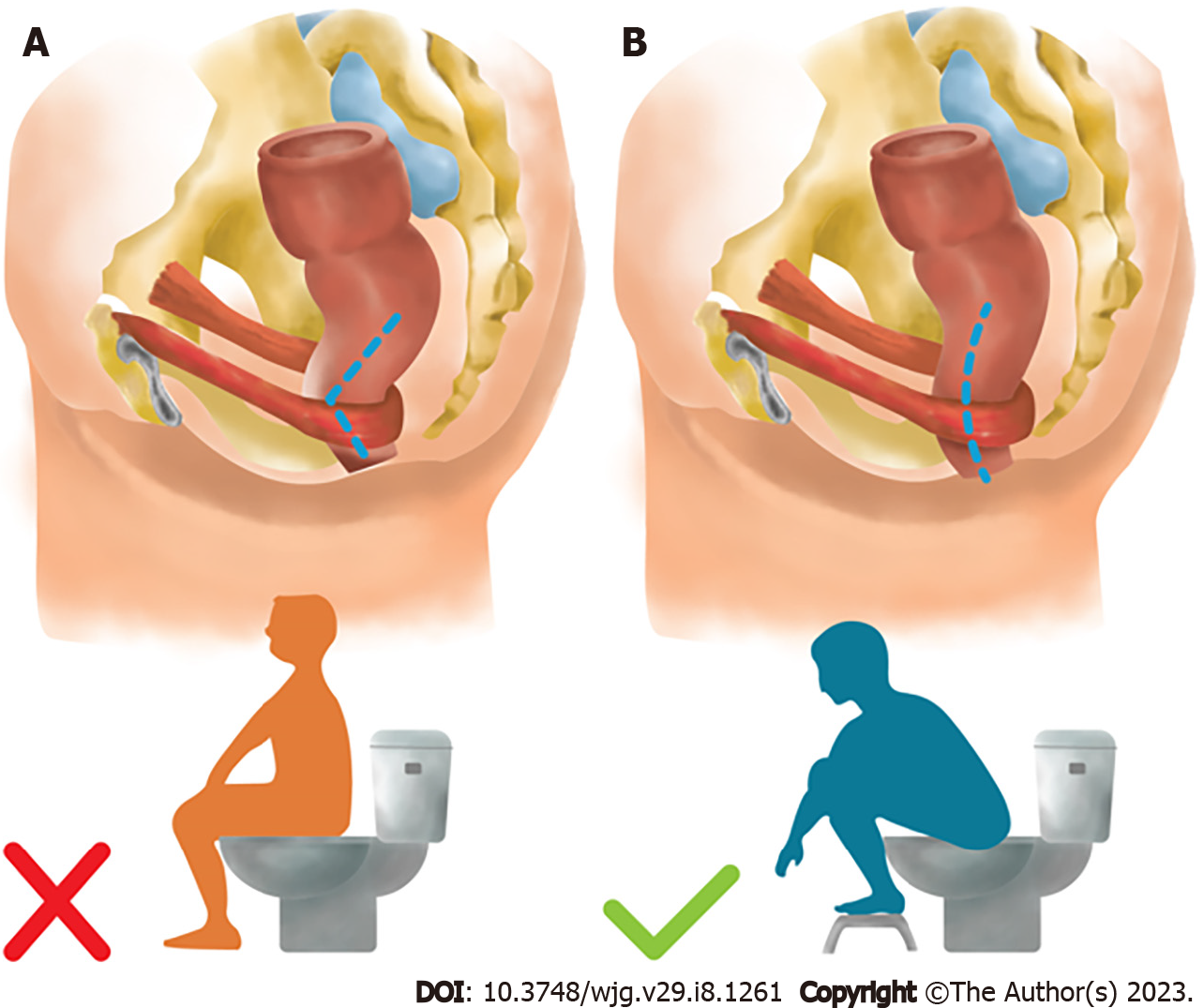
Toilet training: Toilet training aims to reduce symptoms, the child’s defecation anxiety, and toileting avoidance[1,6]. There are four main methods for toilet training, including the child-oriented toilet training method, Azrin and Foxx method, Dr. Spock’s toilet training method, and early elimination toilet training method[117-119]. The most friendly, accepted, and practical methods recommended by the American Academy of Pediatrics and Canadian Pediatric Society is the child-oriented toilet training method, in which the proper age for toilet training is between 18 and 24 mo[120,121]. Parents should be encouraged to be positive and supportive throughout the toilet training. Children should be encouraged to participate in toilet training, which consists of five sequential steps: know, dare, can, will, and do[122]. The child is taught to sit on the toilet for up to 5 min, one to three times a day, following meals to take advantage of the gastrocolic reflex[6]. The position of defecation is necessary to open the anorectal angle (the angle between the longitudinal axis of anal canal and the posterior rectal line, parallel to the longitudinal axis of rectum) and facilitate stool expulsion (Figure 10). To track improvement and compliance, keeping a daily journal of bowel movement, fecal and urine incontinence, and medication is beneficial. Providing stickers or small gifts as positive reinforcement for good behavior might further motivate children[6,122]. Through this process, children gain the ability to perceive their urge to defecate, consequently developing the habit of using the toilet instead of holding it in[59].
Biofeedback: Biofeedback training is a technique for teaching children how to control their perianal muscles for more efficient bowel movements. This technique involves bringing a typically unfamiliar physiological process to the patient’s attention and allowing them to measure it[1,6]. In line with this, a recent meta-analysis incorporated three studies contrasting conventional treatment with add-on biofeedback treatment. Accordingly, two studies showed that treatment success rates were higher in the biofeedback group, whereas one study found no difference. In addition, one study found that the addition of biofeedback training at home offered no benefit in terms of defecation frequency compared to biofeedback at the laboratory[106]. Accordingly, biofeedback therapy is not advised for the regular treatment of children with FC based on the most recent research[59,106].
Abdominal massage: The mechanisms by which abdominal massages reduce constipation are most likely a combination of local stimulation and relaxation, as well as stimulation of the parasympathetic nervous system. Direct pressure over the abdominal wall alternately compresses and releases sections of the digestive tract, briefly distorting the lumen size and activating stretch receptors that can reinforce the gastrocolic reflex and trigger intestinal and rectal contraction[106,123,124]. A meta-analysis including a total of 23 RCTs and 2005 children showed that traditional Chinese medicine (TCM) infant massage had a superior effect on infant FC than drug therapy alone. Moreover, a clinical investigation found that children with FC may defecate more frequently and experience less constipation symptoms when receiving TCM infant massage[123]. There is little evidence to support the idea that using Chinese herbs in combination with other therapies might be beneficial[106,123]. Abdominal massage might be a promising additional therapy to manage FC.
Retrograde enemas: There is insufficient evidence to support the role of retrograde enemas in the maintenance phase of FC in children. Hence, this procedure is reserved for intractable constipation, especially in cases with slow-transit constipation or megarectum. An RCT comparing to the clinical efficacy of supplemental treatment with rectal enemas against conventional treatment alone in 100 children between the ages of 8 and 18 who had symptoms of constipation for at least 2 years revealed that defecation frequency normalized after 1 year of treatment in both groups but was significantly higher in the intervention group compared to controls at 26 and 52 wk (5.6/wk vs 3.9/wk, P = 0.02, and 5.3/wk vs. 3.9/wk, P = 0.02, respectively). Enemas as maintenance therapy for severely constipated children had no substantial side effects compared to oral laxatives alone[125].
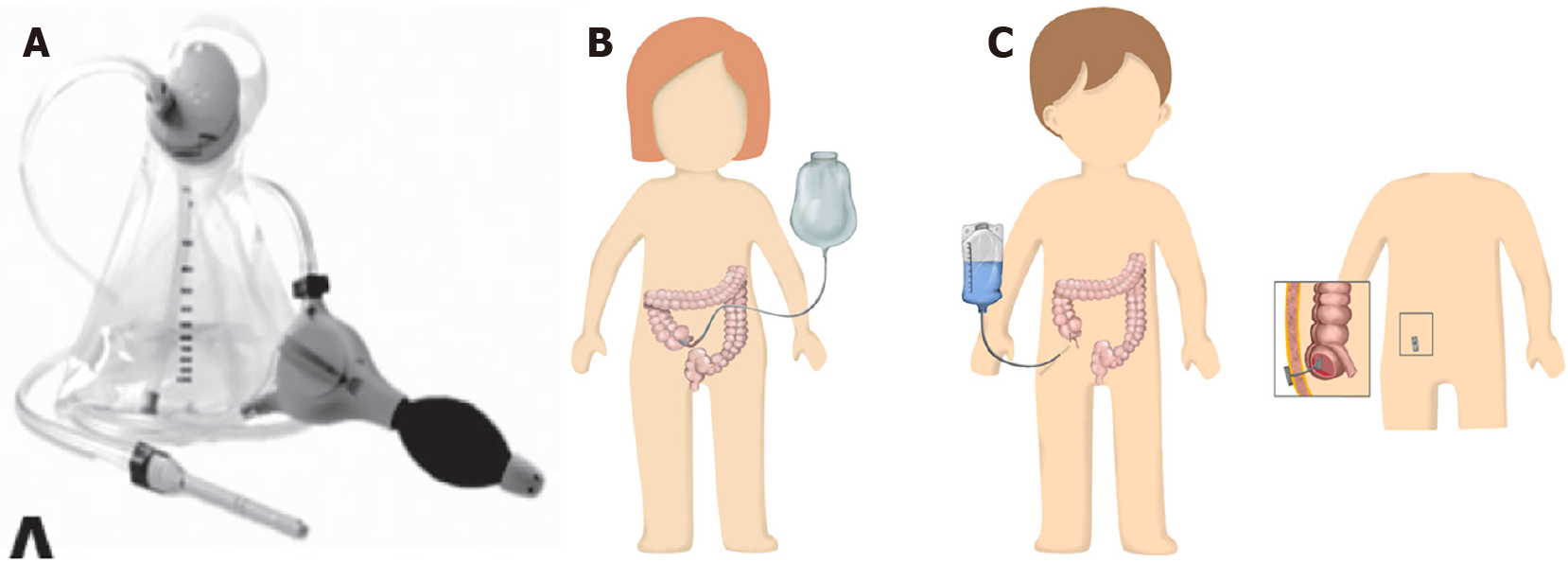
Transanal irrigation: Transanal irrigation (Figure 11A) can be an option for children with FC who do not respond to pharmacological treatment[126,127]. It involves inserting a catheter or cone into the rectum to inject water into the colon, cleaning it completely[126]. This treatment has been previously well established for patients with neurogenic bowel disorders and anorectal malformations[127]. Moreover, evidence has suggested its safety and effectiveness, with an average success rate of 78% for both FI and constipation[127,128]. In addition, 86% of the parents were satisfied with the results of transanal irrigation, and 67% reported that they would continue using transanal irrigation for the treatment of their child’s symptoms[129]. Therefore, transanal irrigation can be considered an effective therapeutic option for severely constipated children with FI who do not respond to conventional therapy[6,127,128].
Botulinum toxin A injection: Botulinum toxin A (Botox) injections into the anal sphincter can be considered in cases suspected of anorectal dysfunction or functional outlet obstruction[59]. Botox injections, which temporarily reduce anal sphincter muscle contraction, serves as both a diagnostic test, indicating whether the obstructive symptoms are being caused by internal anal sphincter hypertonia, and treatment for intractable constipation. Children who exhibit significant withholding behavior or anal sphincter dysfunction may benefit from Botox injections[6,59]. A RCT and systematic review found that Botox injections were as equally effective as internal sphincter myectomy on short-term follow-up[130,131]. However, a retrospective study on 164 children over 7 years old with intractable constipation showed that Botox injections into the internal anal sphincter of children had an overall response rate of 70%. Moreover, anorectal manometry studies in children with normal and abnormal sphincter dynamics observed similar response rates to this therapy[132].
Neuromodulation: Sacral neuromodulation (SNM) is a promising option for the treatment intractable constipation. SNM involves percutaneous placement of an electrode into the third sacral foramen and implantation of a stimulating device under the skin covering the buttocks[6]. The exact working mechanism of SNM remains largely unknown, although evidence has suggested that SNM stimulates anorectal function at a more central level. SNS can affect multiple physiological functions of the pelvis and lower abdomen and supports the propulsive peristalsis of the intestine, which is of special interest in slow-transit constipation[133]. One study in 30 constipated children reported a significant improvement in defecation frequency and abdominal pain after 3 wk of SNM treatment, with the effects being sustained over 22 mo of follow-up in 42% of children. Another study on the treatment of intractable constipation with SNM for over 2 years found that defecation frequency did not change after SNS; however, patients reported that FI decreased from 72% to 20% (P < 0.01) and urinary incontinence decreased from 56% to 28% (P = 0.04). Minor complications include pain after implantation, displacements of the leads, and infection[134,135]. A recent pilot study that assessed noninvasive SNS in 17 constipated children also found it effective in improving symptoms of constipation[136]. Additionally, abdominal transcutaneous electrical stimulation and posterior tibial nerve stimulation, two skin stimulation techniques, have been used to neuromodulate the bowel to treat constipation with promising outcomes[6].
Surgery: Although almost all patients with FC are successfully treated with conventional therapy, a few continue to have intractable symptoms without any organic problems. In such cases, surgical interventions may be beneficial.
Apart from transanal irrigation, there are also surgical treatment options to achieve stool expulsion, such as antegrade colonic irrigation or antegrade continence enemas (ACEs)[59]. When maximal conventional therapy fails, ACEs have been considered a successful therapeutic option for constipated children[1,6]. ACEs allow fluid to be flushed through the entire colon via an external opening into the colonic lumen, which is usually located at the cecum. The most well-established ACE procedures are percutaneous cecostomy and Malone appendicocecostomy (Figures 11B and C). The success rate of ACEs for the management of FC varies from 15% to 100% among studies[130,137,138]. Various enema solutions can be used, including saline and PEG. Complications include skin abrasion, stoma stenosis, granulation tissue, enema fluid leaks, and tube dislodging[137,138]. Regarding other surgical management, there are no clear guidelines on surgical colonic resections and ostomies for children with FC, with such surgical management only reserved for severely constipated children who do not respond to conventional therapy and surgical ACE. These surgical procedures are performed in specialized centers by a multidisciplinary team due to their complexity and potential for problems[6,59].
FC is the most common FGID in children and it affects the quality of life and psychological health of both the child and the family. Stool withholding behavior is the main etiological agent of FC and successful toilet training is the most effective treatment measure as it also prevents FC recurrence in the long term. Nonetheless, osmotic laxatives and lifestyle modifications, along with adequate fiber and fluid intake, are also crucial as first line therapy during toilet training. Even though extensive history taking and physical examination might enable a diagnosis of FC according to Rome IV criteria, children with intractable constipation may require multiple investigations to confirm the diagnosis or to exclude organic causes. Apart from osmotic laxatives, other promising herbal and alternative therapies have been reported to yield satisfactory outcomes in FC with minimal short-term adverse effects; nevertheless, more evidence is needed before these strategies can be adopted worldwide. Children with intractable constipation typically requires a multidisciplinary team approach and the physician should refer the child to a pediatric specialist for re-evaluation and further management (Figure 12).
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bredt LC, Brazil; Patel MV, India; Skogman BH, Sweden S-Editor: Wang JJ L-Editor: A P-Editor: Yu HG
| 1. | Benninga MA, Voskuijl WP, Taminiau JA. Childhood constipation: is there new light in the tunnel? J Pediatr Gastroenterol Nutr. 2004;39:448-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (3)] |
| 2. | Boronat AC, Ferreira-Maia AP, Matijasevich A, Wang YP. Epidemiology of functional gastrointestinal disorders in children and adolescents: A systematic review. World J Gastroenterol. 2017;23:3915-3927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 3. | Vriesman MH, Benninga MA. Functional constipation and fecal incontinence. In: Wyllie R. Pediatric gastrointestinal and liver diseases. Philadelphia: Elsevier, 2021: 106-118. |
| 4. | Koppen IJN, Vriesman MH, Saps M, Rajindrajith S, Shi X, van Etten-Jamaludin FS, Di Lorenzo C, Benninga MA, Tabbers MM. Prevalence of Functional Defecation Disorders in Children: A Systematic Review and Meta-Analysis. J Pediatr. 2018;198:121-130.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 195] [Article Influence: 27.9] [Reference Citation Analysis (4)] |
| 5. | Rial R, Uc A. Functional constipation. In: Kleinman RE, Goulet O-J, Mieli-Vergani G, Sanderson IR, Sherman PM, Shneider BL, editors. Walker’s peadiatric gastrointestinal disease. United States: People’s medical publishing house, 2018: 991-1005. |
| 6. | Weaver LT. Bowel habit from birth to old age. J Pediatr Gastroenterol Nutr. 1988;7:637-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 7. | Osatakul S, Yossuk P, Mo-suwan L. Bowel habits of normal Thai children. J Pediatr Gastroenterol Nutr. 1995;20:339-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 8. | Courdent M, Beghin L, Akré J, Turck D. Infrequent stools in exclusively breastfed infants. Breastfeed Med. 2014;9:442-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Choe YH, Lee JE, Moon KB, Hwang JH, Seo JM. The infrequent bowel movements in young infants who are exclusively breast-fed. Eur J Pediatr. 2004;163:630-1; discussion 632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 10. | Benninga MA, Faure C, Hyman PE, St James Roberts I, Schechter NL, Nurko S. Childhood Functional Gastrointestinal Disorders: Neonate/Toddler. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 364] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 11. | Koppen IJ, Nurko S, Saps M, Di Lorenzo C, Benninga MA. The pediatric Rome IV criteria: what's new? Expert Rev Gastroenterol Hepatol. 2017;11:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (22)] |
| 12. | Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Functional Disorders: Children and Adolescents. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 812] [Article Influence: 90.2] [Reference Citation Analysis (5)] |
| 13. | Rajindrajith S, Devanarayana NM, Thapar N, Benninga MA. Functional Fecal Incontinence in Children: Epidemiology, Pathophysiology, Evaluation, and Management. J Pediatr Gastroenterol Nutr. 2021;72:794-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Rajindrajith S, Devanarayana NM, Benninga MA. Review article: faecal incontinence in children: epidemiology, pathophysiology, clinical evaluation and management. Aliment Pharmacol Ther. 2013;37:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Rajindrajith S, Devanarayana NM, Benninga MA. Constipation-associated and nonretentive fecal incontinence in children and adolescents: an epidemiological survey in Sri Lanka. J Pediatr Gastroenterol Nutr. 2010;51:472-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Voskuijl WP, Heijmans J, Heijmans HS, Taminiau JA, Benninga MA. Use of Rome II criteria in childhood defecation disorders: applicability in clinical and research practice. J Pediatr. 2004;145:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Scott SM, van den Berg MM, Benninga MA. Rectal sensorimotor dysfunction in constipation. Best Pract Res Clin Gastroenterol. 2011;25:103-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Poddar U, Singh S, Pawaria A, Srivastava A, Yachha SK. Aetiological spectrum, clinical differentiation and efficacy of polyethylene glycol over lactulose in children with constipation: Experience of 316 cases. J Paediatr Child Health. 2019;55:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Jang HJ, Chung JY, Seo JH, Moon JS, Choe BH, Shim JO. Nationwide Survey for Application of ROME IV Criteria and Clinical Practice for Functional Constipation in Children. J Korean Med Sci. 2019;34:e183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Dehghani SM, Kulouee N, Honar N, Imanieh MH, Haghighat M, Javaherizadeh H. Clinical Manifestations among Children with Chronic Functional Constipation. Middle East J Dig Dis. 2015;7:31-35. [PubMed] |
| 21. | Russo M, Strisciuglio C, Scarpato E, Bruzzese D, Casertano M, Staiano A. Functional Chronic Constipation: Rome III Criteria Versus Rome IV Criteria. J Neurogastroenterol Motil. 2019;25:123-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 22. | Alshehri DB, Sindi HH, AlMusalami IM, Rozi IH, Shagrani M, Kamal NM, Alahmadi NS, Alfuraikh SS, Vandenplas Y. Saudi Experts Consensus on Diagnosis and Management of Pediatric Functional Constipation. Pediatr Gastroenterol Hepatol Nutr. 2022;25:163-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Ezomike UO, Ugwu EO, Ezomike NE, Eke CB, Ekenze SO. Evaluation of Impact of Perinatal Factors on Time to First Meconium Passage in Nigerian Neonates. Malawi Med J. 2019;31:150-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Tunc VT, Camurdan AD, Ilhan MN, Sahin F, Beyazova U. Factors associated with defecation patterns in 0-24-month-old children. Eur J Pediatr. 2008;167:1357-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 25. | Bekkali N, Hamers SL, Schipperus MR, Reitsma JB, Valerio PG, Van Toledo L, Benninga MA. Duration of meconium passage in preterm and term infants. Arch Dis Child Fetal Neonatal Ed. 2008;93:F376-F379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Xinias I, Mavroudi A. Constipation in Childhood. An update on evaluation and management. Hippokratia. 2015;19:11-19. [PubMed] |
| 27. | Dos Santos IR, de Abreu GE, Dourado ER, Martinelli Braga AAN, Lobo VA, de Carvalho IWB, Bastos Netto JM, Barroso U Jr. Emotional and behavioural problems in children and adolescents: The role of constipation. J Paediatr Child Health. 2021;57:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Singh H, Connor F. Paediatric constipation: An approach and evidence-based treatment regimen. Aust J Gen Pract. 2018;47:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Benzamin M, Karim AB, Rukunuzzaman M, Mazumder MW, Rana M, Alam R, Islam MM, Alam MS, Hossen K, Yasmin A, Fathema K, Khadga M, Aishy AS. Functional constipation in Bangladeshi school aged children: A hidden misty at community. World J Clin Pediatr. 2022;11:160-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Ataee P, Taleshi B, Eskandarifar A, Nuri B, Naghshizadian R, Malekian Taghi A, Eftekhari K. Association between duration of constipation and frequency of urinary tract infection in children. J Comprehensive Pediatrics. 2020;11. [DOI] [Full Text] |
| 31. | Giramonti KM, Kogan BA, Agboola OO, Ribons L, Dangman B. The association of constipation with childhood urinary tract infections. J Pediatr Urol. 2005;1:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Strisciuglio C, Cenni S, Serra MR, Dolce P, Kolacek S, Sila S, Trivic I, Bar Lev MR, Shamir R, Kostovski A, Papadopoulou A, Roma E, Katsagoni C, Jojkic-Pavkov D, Campanozzi A, Scarpato E, Miele E, Staiano A. Diet and Pediatric Functional Gastrointestinal Disorders in Mediterranean Countries. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 33. | Chan WT, Lee HC, Wang WN, Yeung CY, Jiang CB. Determination of the normal position of the anus in Taiwanese infants. Pediatr Neonatol. 2009;50:158-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Merlob P, Reisner SH. Determination of the normal position of the anus (with reference to idiopathic constipation). J Pediatr Gastroenterol Nutr. 1988;7:630. [PubMed] |
| 35. | Núñez-Ramos R, Fabbro MA, González-Velasco M, Núñez Núñez R, Romanato B, Vecchiato L, D'Agostino S, Blesa Sánchez E. Determination of the anal position in newborns and in children with chronic constipation: comparative study in two European healthcare centres. Pediatr Surg Int. 2011;27:1111-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Bar-Maor JA, Eitan A. Determination of the normal position of the anus (with reference to idiopathic constipation). J Pediatr Gastroenterol Nutr. 1987;6:559-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Tabbers MM, DiLorenzo C, Berger MY, Faure C, Langendam MW, Nurko S, Staiano A, Vandenplas Y, Benninga MA; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition; North American Society for Pediatric Gastroenterology. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58:258-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 636] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 38. | Gelsomino M, Vescovo ED, Bersani G, Sopo SM. Functional constipation related to cow's milk allergy in children: A management proposal. Allergol Immunopathol (Madr). 2021;49:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Iacono G, Cavataio F, Montalto G, Florena A, Tumminello M, Soresi M, Notarbartolo A, Carroccio A. Intolerance of cow's milk and chronic constipation in children. N Engl J Med. 1998;339:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 189] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Simeone D, Miele E, Boccia G, Marino A, Troncone R, Staiano A. Prevalence of atopy in children with chronic constipation. Arch Dis Child. 2008;93:1044-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Mohammadi Bourkheili A, Mehrabani S, Esmaeili Dooki M, Haji Ahmadi M, Moslemi L. Effect of Cow's-milk-free diet on chronic constipation in children; A randomized clinical trial. Caspian J Intern Med. 2021;12:91-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 42. | Reuchlin-Vroklage LM, Bierma-Zeinstra S, Benninga MA, Berger MY. Diagnostic value of abdominal radiography in constipated children: a systematic review. Arch Pediatr Adolesc Med. 2005;159:671-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Berger MY, Tabbers MM, Kurver MJ, Boluyt N, Benninga MA. Value of abdominal radiography, colonic transit time, and rectal ultrasound scanning in the diagnosis of idiopathic constipation in children: a systematic review. J Pediatr. 2012;161:44-50.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 44. | Barr RG, Levine MD, Wilkinson RH, Mulvihill D. Chronic and occult stool retention: a clinical tool for its evaluation in school-aged children. Clin Pediatr (Phila). 1979;18:674, 676, 677-679, passim. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Leech SC, McHugh K, Sullivan PB. Evaluation of a method of assessing faecal loading on plain abdominal radiographs in children. Pediatr Radiol. 1999;29:255-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Blethyn AJ, Jenkins HR, Roberts R, Verrier Jones K. Radiological evidence of constipation in urinary tract infection. Arch Dis Child. 1995;73:534-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Matsumoto M, Misawa N, Tsuda M, Manabe N, Kessoku T, Tamai N, Kawamoto A, Sugama J, Tanaka H, Kato M, Haruma K, Sanada H, Nakajima A. Expert Consensus Document: Diagnosis for Chronic Constipation with Faecal Retention in the Rectum Using Ultrasonography. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | Burgers R, de Jong TP, Benninga MA. Rectal examination in children: digital vs transabdominal ultrasound. J Urol. 2013;190:667-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Pop D, Tătar S, Fufezan O, Farcău D. Rectum sizes: Assessment by ultrasonography in children with functional constipation. J Paediatr Child Health. 2021;57:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Rybak A, Martinelli M, Thapar N, Van Wijk MP, Vandenplas Y, Salvatore S, Staiano A, Benninga MA, Borrelli O. Colonic Function Investigations in Children: Review by the ESPGHAN Motility Working Group. J Pediatr Gastroenterol Nutr. 2022;74:681-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 51. | Popescu M, Mutalib M. Bowel transit studies in children: evidence base, role and practicalities. Frontline Gastroenterol. 2022;13:152-159. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 52. | Southwell BR, Clarke MC, Sutcliffe J, Hutson JM. Colonic transit studies: normal values for adults and children with comparison of radiological and scintigraphic methods. Pediatr Surg Int. 2009;25:559-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 53. | Rodriguez L, Heinz N, Colliard K, Amicangelo M, Nurko S. Diagnostic and clinical utility of the wireless motility capsule in children: A study in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. 2021;33:e14032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Fritz T, Hünseler C, Broekaert I. Assessment of whole gut motility in adolescents using the wireless motility capsule test. Eur J Pediatr. 2022;181:1197-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Menys A, Saliakellis E, Borrelli O, Thapar N, Taylor SA, Watson T. The evolution of magnetic resonance enterography in the assessment of motility disorders in children. Eur J Radiol. 2018;107:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 56. | Vriesman MH, de Jonge CS, Kuizenga-Wessel S, Adler B, Menys A, Nederveen AJ, Stoker J, Benninga MA, Di Lorenzo C. Simultaneous assessment of colon motility in children with functional constipation by cine-MRI and colonic manometry: a feasibility study. Eur Radiol Exp. 2021;5:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Yoo HY, Son JS, Park HW, Kwak BO, Kim HS, Bae SH. Value of 24-hour Delayed Film of Barium Enema for Evaluation of Colon Transit Function in Young Children with Constipation. J Neurogastroenterol Motil. 2016;22:483-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Leung AK, Hon KL. Paediatrics: how to manage functional constipation. Drugs Context. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Koppen IJN, Benninga MA. Functional Constipation and Dyssynergic Defecation in Children. Front Pediatr. 2022;10:832877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (2)] |
| 60. | Steiner SA, Torres MR, Penna FJ, Gazzinelli BF, Corradi CG, Costa AS, Ribeiro IG, de Andrade EG, do Carmo Barros de Melo M. Chronic functional constipation in children: adherence and factors associated with drug treatment. J Pediatr Gastroenterol Nutr. 2014;58:598-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Koppen IJN, van Wassenaer EA, Barendsen RW, Brand PL, Benninga MA. Adherence to Polyethylene Glycol Treatment in Children with Functional Constipation Is Associated with Parental Illness Perceptions, Satisfaction with Treatment, and Perceived Treatment Convenience. J Pediatr. 2018;199:132-139.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 62. | Miller MK, Dowd MD, Friesen CA, Walsh-Kelly CM. A randomized trial of enema versus polyethylene glycol 3350 for fecal disimpaction in children presenting to an emergency department. Pediatr Emerg Care. 2012;28:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 63. | Bekkali NL, van den Berg MM, Dijkgraaf MG, van Wijk MP, Bongers ME, Liem O, Benninga MA. Rectal fecal impaction treatment in childhood constipation: enemas versus high doses oral PEG. Pediatrics. 2009;124:e1108-e1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 64. | Shatnawi MS, Alrwalah MM, Ghanma AM, Alqura'an ML, Zreiqat EN, Alzu'bi MM. Lactulose versus polyethylene glycol for disimpaction therapy in constipated children, a randomized controlled study. Sudan J Paediatr. 2019;19:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Yokoi A, Kamata N. The usefulness of olive oil enema in children with severe chronic constipation. J Pediatr Surg. 2021;56:1141-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Gordon M, MacDonald JK, Parker CE, Akobeng AK, Thomas AG. Osmotic and stimulant laxatives for the management of childhood constipation. Cochrane Database Syst Rev. 2016;2016:CD009118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 67. | Jarzebicka D, Sieczkowska-Golub J, Kierkus J, Czubkowski P, Kowalczuk-Kryston M, Pelc M, Lebensztejn D, Korczowski B, Socha P, Oracz G. PEG 3350 Versus Lactulose for Treatment of Functional Constipation in Children: Randomized Study. J Pediatr Gastroenterol Nutr. 2019;68:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 68. | Treepongkaruna S, Simakachorn N, Pienvichit P, Varavithya W, Tongpenyai Y, Garnier P, Mathiex-Fortunet H. A randomised, double-blind study of polyethylene glycol 4000 and lactulose in the treatment of constipation in children. BMC Pediatr. 2014;14:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Loening-Baucke V, Pashankar DS. A randomized, prospective, comparison study of polyethylene glycol 3350 without electrolytes and milk of magnesia for children with constipation and fecal incontinence. Pediatrics. 2006;118:528-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 70. | Roy D, Akriche F, Amlani B, Shakir S. Utilisation and Safety of Polyethylene Glycol 3350 With Electrolytes in Children Under 2 Years: A Retrospective Cohort. J Pediatr Gastroenterol Nutr. 2021;72:683-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 71. | Hussaun SZ, Belkind-Gerson J, Chogle A, Bhuiyan MAN, Hicks T, Misra S. Probable neuropsychiatric toxicity of polyethylene glycol roles of media internet and the caregivers. GastroHep. 2019;1: 118-123. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | Chen SL, Cai SR, Deng L, Zhang XH, Luo TD, Peng JJ, Xu JB, Li WF, Chen CQ, Ma JP, He YL. Efficacy and complications of polyethylene glycols for treatment of constipation in children: a meta-analysis. Medicine (Baltimore). 2014;93:e65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Riskin A, Hochwald O, Bader D, Srugo I, Naftali G, Kugelman A, Cohen E, Mor F, Kaufman B, Shaoul R. The effects of lactulose supplementation to enteral feedings in premature infants: a pilot study. J Pediatr. 2010;156:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 74. | Medina-Centeno R. Medications for constipation in 2020. Curr Opin Pediatr. 2020;32:668-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 75. | Vilanova-Sanchez A, Gasior AC, Toocheck N, Weaver L, Wood RJ, Reck CA, Wagner A, Hoover E, Gagnon R, Jaggers J, Maloof T, Nash O, Williams C, Levitt MA. Are Senna based laxatives safe when used as long term treatment for constipation in children? J Pediatr Surg. 2018;53:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 76. | Urganci N, Akyildiz B, Polat TB. A comparative study: the efficacy of liquid paraffin and lactulose in management of chronic functional constipation. Pediatr Int. 2005;47:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Farahmand F. A randomised trial of liquid paraffin versus lactulose in the treatment of chronic functional constipation in children. Acta Medica Iranica. 2006;45: 183-188. |
| 78. | Rafati M, Karami H, Salehifar E, Karimzadeh A. Clinical efficacy and safety of polyethylene glycol 3350 versus liquid paraffin in the treatment of pediatric functional constipation. Daru. 2011;19:154-158. [PubMed] |
| 79. | Sondheimer JM, Gervaise EP. Lubricant versus laxative in the treatment of chronic functional constipation of children: a comparative study. J Pediatr Gastroenterol Nutr. 1982;1:223-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Sharif F, Crushell E, O'Driscoll K, Bourke B. Liquid paraffin: a reappraisal of its role in the treatment of constipation. Arch Dis Child. 2001;85:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Jomehzadeh N, Javaherizadeh H, Amin M, Rashno M, Teimoori A. Quantification of Intestinal Lactobacillus Species in Children with Functional Constipation by Quantitative Real-Time PCR. Clin Exp Gastroenterol. 2020;13:141-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 82. | de Moraes JG, Motta ME, Beltrão MF, Salviano TL, da Silva GA. Fecal Microbiota and Diet of Children with Chronic Constipation. Int J Pediatr. 2016;2016:6787269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 83. | de Meij TG, de Groot EF, Eck A, Budding AE, Kneepkens CM, Benninga MA, van Bodegraven AA, Savelkoul PH. Characterization of Microbiota in Children with Chronic Functional Constipation. PLoS One. 2016;11:e0164731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 84. | Wojtyniak K, Szajewska H. Systematic review: probiotics for functional constipation in children. Eur J Pediatr. 2017;176:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 85. | Wegner A, Banaszkiewicz A, Kierkus J, Landowski P, Korlatowicz-Bilar A, Wiecek S, Kwiecien J, Gawronska A, Dembinski L, Czaja-Bulsa G, Socha P. The effectiveness of Lactobacillus reuteri DSM 17938 as an adjunct to macrogol in the treatment of functional constipation in children. A randomized, double-blind, placebo-controlled, multicentre trial. Clin Res Hepatol Gastroenterol. 2018;42:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 86. | Jadrešin O, Sila S, Trivić I, Mišak Z, Hojsak I, Kolaček S. Lack of Benefit of Lactobacillus reuteri DSM 17938 as an Addition to the Treatment of Functional Constipation. J Pediatr Gastroenterol Nutr. 2018;67:763-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 87. | Lee KJ, Ryoo E, Lee YM, Yoon JM, Jang HJ, Choi SY, Choi YJ, Kim HJ, Chung JY, Shim JO. Saccharomyces boulardii and Lactulose for Childhood Functional Constipation: A Multicenter Randomized Controlled Trial. J Neurogastroenterol Motil. 2022;28:454-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | van Mill MJ, Koppen IJN, Benninga MA. Controversies in the Management of Functional Constipation in Children. Curr Gastroenterol Rep. 2019;21:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 89. | Winter HS, Di Lorenzo C, Benninga MA, Gilger MA, Kearns GL, Hyman PE, Vandeplassche L, Ausma J, Hoppenbrouwers M. Oral prucalopride in children with functional constipation. J Pediatr Gastroenterol Nutr. 2013;57:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 90. | Mugie SM, Korczowski B, Bodi P, Green A, Kerstens R, Ausma J, Ruth M, Levine A, Benninga MA. Prucalopride is no more effective than placebo for children with functional constipation. Gastroenterology. 2014;147:1285-95.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 91. | Hyman PE, Di Lorenzo C, Prestridge LL, Youssef NN, Ueno R. Lubiprostone for the treatment of functional constipation in children. J Pediatr Gastroenterol Nutr. 2014;58:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 92. | Hussain SZ, Labrum B, Mareya S, Stripling S, Clifford R. Safety of Lubiprostone in Pediatric Patients With Functional Constipation: A Nonrandomized, Open-Label Trial. J Pediatr Gastroenterol Nutr. 2021;73:572-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 93. | Benninga MA, Hussain SZ, Sood MR, Nurko S, Hyman P, Clifford RA, O'Gorman M, Losch-Beridon T, Mareya S, Lichtlen P, Di Lorenzo C. Lubiprostone for Pediatric Functional Constipation: Randomized, Controlled, Double-Blind Study With Long-term Extension. Clin Gastroenterol Hepatol. 2022;20:602-610.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 94. | Baaleman DF, Gupta S, Benninga MA, Bali N, Vaz KH, Yacob D, Di Lorenzo C, Lu PL. The Use of Linaclotide in Children with Functional Constipation or Irritable Bowel Syndrome: A Retrospective Chart Review. Paediatr Drugs. 2021;23:307-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 95. | Chmielewska A, Horvath A, Dziechciarz P, Szajewska H. Glucomannan is not effective for the treatment of functional constipation in children: a double-blind, placebo-controlled, randomized trial. Clin Nutr. 2011;30:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 96. | Loening-Baucke V, Miele E, Staiano A. Fiber (glucomannan) is beneficial in the treatment of childhood constipation. Pediatrics. 2004;113:e259-e264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 97. | Castillejo G, Bulló M, Anguera A, Escribano J, Salas-Salvadó J. A controlled, randomized, double-blind trial to evaluate the effect of a supplement of cocoa husk that is rich in dietary fiber on colonic transit in constipated pediatric patients. Pediatrics. 2006;118:e641-e648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 98. | Quitadamo P, Coccorullo P, Giannetti E, Romano C, Chiaro A, Campanozzi A, Poli E, Cucchiara S, Di Nardo G, Staiano A. A randomized, prospective, comparison study of a mixture of acacia fiber, psyllium fiber, and fructose vs polyethylene glycol 3350 with electrolytes for the treatment of chronic functional constipation in childhood. J Pediatr. 2012;161:710-5.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 99. | Esmaeilidooki MR, Mozaffarpur SA, Mirzapour M, Shirafkan H, Kamalinejad M, Bijani A. Comparison Between the Cassia Fistula`s Emulsion With Polyethylene Glycol (PEG4000) in the Pediatric Functional Constipation: A Randomized Clinical Trial. Iran Red Crescent Med J. 2016;18:e33998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 100. | Closa-Monasterolo R, Ferré N, Castillejo-DeVillasante G, Luque V, Gispert-Llaurado M, Zaragoza-Jordana M, Theis S, Escribano J. The use of inulin-type fructans improves stool consistency in constipated children. A randomised clinical trial: pilot study. Int J Food Sci Nutr. 2017;68:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 101. | Dehghani SM, Bahroloolomifard MS, Yousefi G, Pasdaran A, Hamedi A. A randomized controlled double blinded trial to evaluate efficacy of oral administration of black strap molasses (sugarcane extract) in comparison with polyethylene glycol on pediatric functional constipation. J Ethnopharmacol. 2019;238:111845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 102. | Qiao L, Wang LJ, Wang Y, Chen Y, Zhang HL, Zhang SC. A Randomized, Double-Blind, and Placebo-Controlled Trial of Chinese Herbal Medicine in the Treatment of Childhood Constipation. Clin Transl Gastroenterol. 2021;12:e00345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 103. | Saneian H, Ghaedi S, Famouri F, Khademian M, Ahmadi N, Memarzadeh M, Sadeghi S, Nasri P. Comparing the Effect of a Herbal-based Laxative (Goleghand®) and Polyethylene Glycol on Functional Constipation among Children: A Randomized Controlled Trial. J Res Pharm Pract. 2021;10:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Imanieh MH, Honar N, Mohagheghzadeh A, Haghighat M, Dehghani SM, Mosleh G, Ataollahi M, Safarpour H, Darban B, Karbasian F, Safarpour AR, Hassani AH, Avazpour A. Rosa damascena together with brown sugar mitigate functional constipation in children over 12 months old: A double-blind randomized controlled trial. J Ethnopharmacol. 2022;298:115582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 105. | Paknejad MS, Motaharifard MS, Barimani S, Kabiri P, Karimi M. Traditional, complementary and alternative medicine in children constipation: a systematic review. Daru. 2019;27:811-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 106. | Wegh CAM, Baaleman DF, Tabbers MM, Smidt H, Benninga MA. Nonpharmacologic Treatment for Children with Functional Constipation: A Systematic Review and Meta-analysis. J Pediatr. 2022;240:136-149.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 107. | McClung HJ, Boyne L, Heitlinger L. Constipation and dietary fiber intake in children. Pediatrics. 1995;96:999-1000. [PubMed] |
| 108. | Tabbers MM, Benninga MA. Constipation in children: fibre and probiotics. BMJ Clin Evid. 2015;2015. [PubMed] |
| 109. | Souza DDS, Tahan S, Weber TK, Araujo-Filho HB, de Morais MB. Randomized, Double-Blind, Placebo-Controlled Parallel Clinical Trial Assessing the Effect of Fructooligosaccharides in Infants with Constipation. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 110. | Weber TK, Toporovski MS, Tahan S, Neufeld CB, de Morais MB. Dietary fiber mixture in pediatric patients with controlled chronic constipation. J Pediatr Gastroenterol Nutr. 2014;58:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 111. | Comas Vives A, Polanco Allué I; Grupo de Trabajo Español para el Estudio del Estreñimiento en la Población Infantil. [Case-control study of risk factors associated with constipation. The FREI Study]. An Pediatr (Barc). 2005;62:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 112. | Chien LY, Liou YM, Chang P. Low defaecation frequency in Taiwanese adolescents: association with dietary intake, physical activity and sedentary behaviour. J Paediatr Child Health. 2011;47:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 113. | Park M, Bang YG, Cho KY. Risk Factors for Functional Constipation in Young Children Attending Daycare Centers. J Korean Med Sci. 2016;31:1262-1265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 114. | van Engelenburg-van Lonkhuyzen ML, Bols EM, Benninga MA, Verwijs WA, de Bie RA. Effectiveness of Pelvic Physiotherapy in Children With Functional Constipation Compared With Standard Medical Care. Gastroenterology. 2017;152:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 115. | van Summeren JJGT, Holtman GA, Kollen BJ, Lisman-van Leeuwen Y, van Ulsen-Rust AHC, Tabbers MM, Dekker JH, Berger MY. Physiotherapy for Children with Functional Constipation: A Pragmatic Randomized Controlled Trial in Primary Care. J Pediatr. 2020;216:25-31.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 116. | van Summeren JJGT, Holtman GA, Lisman-van Leeuwen Y, van Ulsen-Rust AHC, Vermeulen KM, Tabbers MM, Kollen BJ, Dekker JH, Berger MY. Cost-effectiveness of physiotherapy in childhood functional constipation: a randomized controlled trial in primary care. Fam Pract. 2022;39:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 117. | Klassen TP, Kiddoo D, Lang ME, Friesen C, Russell K, Spooner C, Vandermeer B. The effectiveness of different methods of toilet training for bowel and bladder control. Evid Rep Technol Assess (Full Rep). 2006;1-57. [PubMed] |
| 118. | Blum NJ, Taubman B, Nemeth N. Relationship between age at initiation of toilet training and duration of training: a prospective study. Pediatrics. 2003;111:810-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 119. | Choby BA, George S. Toilet training. Am Fam Physician. 2008;78:1059-1064. [PubMed] |
| 120. | Stadtler AC, Gorski PA, Brazelton TB. Toilet training methods, clinical interventions, and recommendations. American Academy of Pediatrics. Pediatrics. 1999;103:1359-1368. [PubMed] |
| 121. | Toilet learning: Anticipatory guidance with a child-oriented approach. Paediatr Child Health. 2000;5:333-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 122. | van Dijk M, Benninga MA, Grootenhuis MA, Nieuwenhuizen AM, Last BF. Chronic childhood constipation: a review of the literature and the introduction of a protocolized behavioral intervention program. Patient Educ Couns. 2007;67:63-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 123. | Liu Z, Gang L, Yunwei M, Lin L. Clinical Efficacy of Infantile Massage in the Treatment of Infant Functional Constipation: A Meta-Analysis. Front Public Health. 2021;9:663581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 124. | Sinclair M. The use of abdominal massage to treat chronic constipation. J Bodyw Mov Ther. 2011;15:436-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 125. | Bongers ME, van den Berg MM, Reitsma JB, Voskuijl WP, Benninga MA. A randomized controlled trial of enemas in combination with oral laxative therapy for children with chronic constipation. Clin Gastroenterol Hepatol. 2009;7:1069-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 126. | Vriesman MH, Koppen IJN, Camilleri M, Di Lorenzo C, Benninga MA. Management of functional constipation in children and adults. Nat Rev Gastroenterol Hepatol. 2020;17:21-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 249] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 127. | Mosiello G, Marshall D, Rolle U, Crétolle C, Santacruz BG, Frischer J, Benninga MA. Consensus Review of Best Practice of Transanal Irrigation in Children. J Pediatr Gastroenterol Nutr. 2017;64:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 128. | Patel S, Hopson P, Bornstein J, Safder S. Impact of Transanal Irrigation Device in the Management of Children With Fecal Incontinence and Constipation. J Pediatr Gastroenterol Nutr. 2020;71:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 129. | Koppen IJ, Kuizenga-Wessel S, Voogt HW, Voskeuil ME, Benninga MA. Transanal Irrigation in the Treatment of Children With Intractable Functional Constipation. J Pediatr Gastroenterol Nutr. 2017;64:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 130. | Siminas S, Losty PD. Current Surgical Management of Pediatric Idiopathic Constipation: A Systematic Review of Published Studies. Ann Surg. 2015;262:925-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 131. | Keshtgar AS, Ward HC, Sanei A, Clayden GS. Botulinum toxin, a new treatment modality for chronic idiopathic constipation in children: long-term follow-up of a double-blind randomized trial. J Pediatr Surg. 2007;42:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 132. | Zar-Kessler C, Kuo B, Belkind-Gerson J. Botulinum toxin injection for childhood constipation is safe and can be effective regardless of anal sphincter dynamics. J Pediatr Surg. 2018;53:693-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 133. | Maeda Y, O'Connell PR, Lehur PA, Matzel KE, Laurberg S; European SNS Bowel Study Group. Sacral nerve stimulation for faecal incontinence and constipation: a European consensus statement. Colorectal Dis. 2015;17:O74-O87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 134. | Lu PL, Koppen IJN, Orsagh-Yentis DK, Leonhart K, Ambeba EJ, Deans KJ, Minneci PC, Teich S, Diefenbach KA, Alpert SA, Benninga MA, Yacob D, Di Lorenzo C. Sacral nerve stimulation for constipation and fecal incontinence in children: Long-term outcomes, patient benefit, and parent satisfaction. Neurogastroenterol Motil. 2018;30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 135. | van der Wilt AA, van Wunnik BP, Sturkenboom R, Han-Geurts IJ, Melenhorst J, Benninga MA, Baeten CG, Breukink SO. Sacral neuromodulation in children and adolescents with chronic constipation refractory to conservative treatment. Int J Colorectal Dis. 2016;31:1459-1466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 136. | Besendörfer M, Kohl M, Schellerer V, Carbon R, Diez S. A Pilot Study of Non-invasive Sacral Nerve Stimulation in Treatment of Constipation in Childhood and Adolescence. Front Pediatr. 2020;8:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 137. | Reppucci ML, Nolan MM, Cooper E, Wehrli LA, Schletker J, Ketzer J, Peña A, Bischoff A, De la Torre L. The success rate of antegrade enemas for the management of idiopathic constipation. Pediatr Surg Int. 2022;38:1729-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 138. | Kuizenga-Wessel S, Mousa HM, Benninga MA, Di Lorenzo C. Lack of Agreement on How to Use Antegrade Enemas in Children. J Pediatr Gastroenterol Nutr. 2016;62:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 139. | Huang Y, Tan SY, Parikh P, Buthmanaban V, Rajindrajith S, Benninga MA. Prevalence of functional gastrointestinal disorders in infants and young children in China. BMC Pediatr. 2021;21:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 140. | Chew KS, Em JM, Koay ZL, Jalaludin MY, Ng RT, Lum LCS, Lee WS. Low prevalence of infantile functional gastrointestinal disorders (FGIDs) in a multi-ethnic Asian population. Pediatr Neonatol. 2021;62:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 141. | Ibrahim ATA, Hamdy AM, Elhodhod MA. Prevalence of Functional Gastrointestinal Disorders among School-aged Children and adolescents, A Multicenter Study. QJM. 2020;113. [DOI] [Full Text] |
| 142. | Khayat A, Algethami G, Baik S, Alhajori M, Banjar D. The Effect of Using Rome IV Criteria on the Prevalence of Functional Abdominal Pain Disorders and Functional Constipation among Children of the Western Region of Saudi Arabia. Glob Pediatr Health. 2021;8:2333794X211022265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 143. | Siajunboriboon S, Tanpowpong P, Empremsilapa S, Lertudomphonwanit C, Nuntnarumit P, Treepongkaruna S. Prevalence of functional abdominal pain disorders and functional constipation in adolescents. J Paediatr Child Health. 2022;58:1209-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 144. | Chia LW, Nguyen TVH, Phan VN, Luu TTN, Nguyen GK, Tan SY, Rajindrajith S, Benninga MA. Prevalence and risk factors of functional gastrointestinal disorders in Vietnamese infants and young children. BMC Pediatr. 2022;22:315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 145. | Zwiener R, Keller C, Robin S, Hyman PE, Palsson OS, Saps M, Lorenzo CD, Shulman RJ, Hyams J, Nurko S, Tilburg MALV. Prevalence of Rome IV functional gastrointestinal disorders in children and adolescents in the United States. Gastroenterology. 2017;152:S649. [DOI] [Full Text] |
| 146. | Saps M, Velasco-Benitez CA, Langshaw AH, Ramírez-Hernández CR. Prevalence of Functional Gastrointestinal Disorders in Children and Adolescents: Comparison Between Rome III and Rome IV Criteria. J Pediatr. 2018;199:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 147. | Robin SG, Keller C, Zwiener R, Hyman PE, Nurko S, Saps M, Di Lorenzo C, Shulman RJ, Hyams JS, Palsson O, van Tilburg MAL. Prevalence of Pediatric Functional Gastrointestinal Disorders Utilizing the Rome IV Criteria. J Pediatr. 2018;195:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 148. | Játiva-Mariño E, Rivera-Valenzuela MG, Velasco-Benitez CA, Saps M. The prevalence of functional constipation in children was unchanged after the Rome IV criteria halved the diagnosis period in Rome III. Acta Paediatr. 2019;108:2274-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 149. | Saps M, Velasco-Benitez CA, Fernandez Valdes L, Mejia J, Villamarin E, Moreno J, Ramirez C, González MJ, Vallenilla I, Falcon AC, Axelrod C. The impact of incorporating toilet-training status in the pediatric Rome IV criteria for functional constipation in infant and toddlers. Neurogastroenterol Motil. 2020;32:e13912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 150. | Velasco-Benitez CA, Axelrod CH, Gutierrez S, Saps M. The Relationship Between Prematurity, Method of Delivery, and Functional Gastrointestinal Disorders in Children. J Pediatr Gastroenterol Nutr. 2020;70:e37-e40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 151. | Baaleman DF, Velasco-Benítez CA, Méndez-Guzmán LM, Benninga MA, Saps M. Can We Rely on the Rome IV Questionnaire to Diagnose Children With Functional Gastrointestinal Disorders? J Neurogastroenterol Motil. 2021;27:626-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 152. | Velasco-Benítez CA, Gómez-Oliveros LF, Rubio-Molina LM, Tovar-Cuevas JR, Saps M. Diagnostic Accuracy of the Rome IV Criteria for the Diagnosis of Functional Gastrointestinal Disorders in Children. J Pediatr Gastroenterol Nutr. 2021;72:538-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 153. | de Morais MB, Toporovski MS, Tofoli MHC, Barros KV, Silva LR, Ferreira CHT. Prevalence of Functional Gastrointestinal Disorders in Brazilian Infants Seen in Private Pediatric Practices and Their Associated Factors. J Pediatr Gastroenterol Nutr. 2022;75:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 154. | Vladimir C, Iienkova N. Prevalence of functional gastrointestinal disorders in Russian children [abstract]. Turkey J Gastroenterol. 2019;30:S279. |
| 155. | Steutel NF, Zeevenhooven J, Scarpato E, Vandenplas Y, Tabbers MM, Staiano A, Benninga MA. Prevalence of Functional Gastrointestinal Disorders in European Infants and Toddlers. J Pediatr. 2020;221:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 156. | Campeotto F, Barbaza MO, Hospital V. Functional Gastrointestinal Disorders in Outpatients Aged up to 12 Months: A French Non-Interventional Study. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 157. | Alonso-Bermejo C, Barrio J, Fernández B, García-Ochoa E, Santos A, Herreros M, Pérez C. Functional gastrointestinal disorders frequency by Rome IV criteria. An Pediatr (Engl Ed). 2022;96:441-447. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 158. | Beser OF, Cullu Cokugras F, Dogan G, Akgun O, Elevli M, Yilmazbas P, Ocal M, Bayrak NA, Sezer Yamanel RG, Bozaykut A, Celtik C, Polat E, Gerenli N, Bozlak S, Ayyildiz Civan H, Ozkul Saglam N, Hatipoglu SS, Özgürhan G, Sunnetci Silistre E, Solmaz B, Kutluk G, Genc HS, Onal H, Usta AM, Urganci N, Sahin A, Cam S, Yildirim S, Yildirim A, Vandenplas Y. The frequency of and factors affecting functional gastrointestinal disorders in infants that presented to tertiary care hospitals. Eur J Pediatr. 2021;180:2443-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 159. | Strisciuglio C, Cenni S, Serra MR, Dolce P, Kolacek S, Sila S, Trivic I, Lev MRB, Shamir R, Kostovski A, Papadopoulou A, Roma E, Katsagoni C, Jojkic-Pavkov D, Salvatore S, Pensabene L, Scarpato E, Miele E, Staiano A; Collaborators: Angelo Campanozzi, and Maria Fotoulaki. Functional Gastrointestinal Disorders in Mediterranean Countries According to Rome IV Criteria. J Pediatr Gastroenterol Nutr. 2022;74:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 160. | Bellaiche M, Ategbo S, Krumholz F, Ludwig T, Miqdady M, Abkari A, Vandenplas Y. A large-scale study to describe the prevalence, characteristics and management of functional gastrointestinal disorders in African infants. Acta Paediatr. 2020;109:2366-2373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 161. | Cai QH, Ma R, Hu SY, Li XM, Xiang XX, Ding Y, He P, Han XM, Chang K, Zhang W, Xue Z, Wang XF, Zhong CL, Yang N. A Randomized Controlled Trial of Chinese Patent Medicine Xiao'er Biantong Granules in the Treatment of Functional Constipation in Children. Evid Based Complement Alternat Med. 2018;2018:4941505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 162. | Tavassoli S, Eftekhari K, Karimi M, Ghobadi A, Shati M, Naddaf A, Abbassian A. Effectiveness of Viola Flower Syrup Compared with Polyethylene Glycol in Children with Functional Constipation: A Randomized, Active-Controlled Clinical Trial. Evid Based Complement Alternat Med. 2021;2021:9915289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 163. | Nasri P, Saeidi S, Saneian H, Famouri F, Sadeghi S, Kashani LMT, Khademian M. Comparative Evaluation between the LaxaPlus Barij(®) and Polyethylene Glycol (4000) in the Pediatric Functional Constipation in Children 2-15 Years Old. J Res Pharm Pract. 2021;10:180-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 164. | Constipation in children and young people: diagnosis and management. London: NICE clinical guidelines, 2017. [cited 15 August 2022]. Available from https://www.nice.org.uk/guidance/cg99/resources/constipation-in-children-and-young-people-diagnosis-and-management-pdf-975757753285. |