Published online Feb 7, 2023. doi: 10.3748/wjg.v29.i5.867
Peer-review started: October 29, 2022
First decision: November 30, 2022
Revised: December 7, 2022
Accepted: January 11, 2023
Article in press: January 11, 2023
Published online: February 7, 2023
Processing time: 94 Days and 5.4 Hours
Although the associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) induces more rapid liver regeneration than portal vein embolization, the mechanism remains unclear.
To assess the influence of inflammatory cytokines and endothelial nitric oxide synthase (eNOS) activation on liver regeneration in ALPPS.
The future liver remnant/body weight (FLR/BW) ratio, hepatocyte proliferation, inflammatory cytokine expression, and activation of the Akt-eNOS pathway were evaluated in rat ALPPS and portal vein ligation (PVL) models. Hepatocyte proliferation was assessed based on Ki-67 expression, which was confirmed using immunohistochemistry. The serum concentrations of inflammatory cytokines were measured using enzyme linked immune-solvent assays. The Akt-eNOS pathway was assessed using western blotting. To explore the role of inflammatory cytokines and NO, Kupffer cell inhibitor gadolinium chloride (GdCl3), NOS inhibitor N-nitro-arginine methyl ester (L-NAME), and NO enhancer molsido
The ALPPS group showed significant FLR regeneration (FLR/BW: 1.60% ± 0.08%, P < 0.05) compared with that observed in the PVL group (1.33% ± 0.11%) 48 h after surgery. In the ALPPS group, serum interleukin-6 expression was suppre
Early induction of inflammatory cytokines may not be pivotal for accelerated FLR regeneration after ALPPS, whereas Akt-eNOS pathway activation may contribute to accelerated regeneration of the FLR.
Core Tip: In extended hepatectomy for hepatobiliary tumors, adequate future liver remnant (FLR) is essential to prevent postoperative liver failure. Portal vein embolization (PVE) and associated liver partition and portal vein ligation for staged hepatectomy (ALPPS) are performed to increase the FLR. Although ALPPS induces more rapid liver regeneration than PVE, the mechanism remains unclear. In this study, we compared ALPPS with portal vein ligation (PVL) in a rat model and found that activation of the Akt-endothelial nitric oxide synthase pathway promotes liver regeneration. The combination of PVL and nitric oxide-producing agents may induce liver regeneration comparable to ALPPS in a non-invasive manner.
- Citation: Masuo H, Shimizu A, Motoyama H, Kubota K, Notake T, Yoshizawa T, Hosoda K, Yasukawa K, Kobayashi A, Soejima Y. Impact of endothelial nitric oxide synthase activation on accelerated liver regeneration in a rat ALPPS model. World J Gastroenterol 2023; 29(5): 867-878
- URL: https://www.wjgnet.com/1007-9327/full/v29/i5/867.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i5.867
Hepatectomy is the most curative treatment for hepatobiliary carcinoma[1,2]. Extended hepatectomy is occasionally performed to achieve R0 surgical margins. However, postoperative liver failure may occur in these cases because of an inadequate volume of the future liver remnant (FLR)[3,4]. To resolve this issue, portal vein embolization (PVE) is widely performed before major hepatectomy to obtain a sufficient FLR volume[5,6]. Although PVE results in a 10%-45% increase in FLR, it requires a waiting period of 2-8 wk[6-8]. Hepatectomy cannot be performed in some cases because of tumor progression, inadequate volume increase, or both in the FLR, even after PVE. Therefore, the resection rate after PVE has been reported as only 70%[7,8]. Furthermore, it has been reported that hepatocellular carcinoma (HCC) is nourished by abnormal vessels in the hepatic artery (HA). Thus, PVE may reduce blood flow in the portal vein and increase blood flow in the HA of the liver to be resected, which may result in rapid progression of HCC[9]. As described above, PVE has limited indications and therapeutic effects. Therefore, the development of new surgical or therapeutic methods is desired to promote further liver regeneration in the short term.
As an alternative to PVE, associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) was reported in 2012[6]. This method enables the FLR to increase by 70%-80% within 10 d[6]. ALPPS promotes a much faster increase of FLR than PVE[6,9], but the mechanism of this rapid liver regeneration remains unclear. Although increases in inflammatory cytokines, such as interleukin-6 (IL-6), which is an inducer in the early stage of liver regeneration, have been reported as a cause of rapid liver regeneration[10-13], it remains controversial[14]. However, in previous studies on the mechanism of liver regeneration after liver resection and portal vein ligation (PVL), shear stress caused by blood viscosity, blood flow velocity, and endothelial nitric oxide synthase (eNOS) activation, followed by NO induction, has been reported to promote liver regeneration[15,16]. This study aimed to explore the mechanism of promoting liver regeneration in ALPPS and investigate the involvement of inflammatory cytokines and eNOS activation using PVL and ALPPS rat models.
Eight-week-old male Wistar rats (CLEA Japan, Kanagawa, Japan) weighing 230-300 g were used in this study. The animals were housed in wood-chip-bedded cages in an air-conditioned room (24 ± 1 °C) with a 12 h light/dark cycle under specific pathogen free condition. There were no diet restrictions. Based on national and institutional regulations and guidelines, all procedures for animal experiments were reviewed by the Committee for Animal Experiments and approved by the President of Shinshu University (Approval numbers 270018 and 019067).
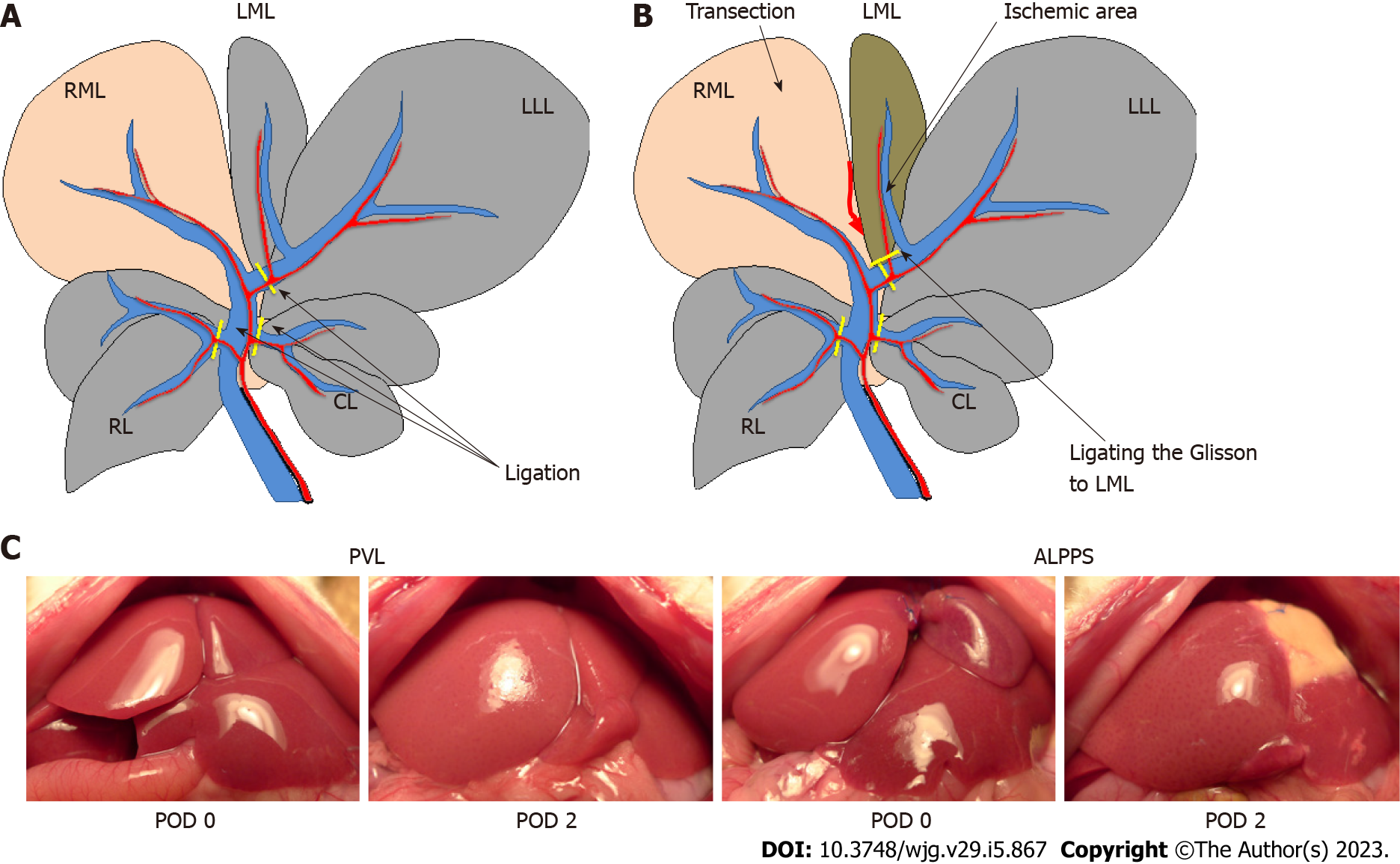
Rats were divided into two groups, PVL and ALPPS, and examined 72 h after surgery. A midline laparotomy was performed under isoflurane-induced anesthesia. In the PVL model, the portal vein branches to the caudate lobe, left lobe, left side of the median lobe, and right lobes were ligated with 7-0 silk (Figure 1A). In the ALPPS model, in addition to PVL, liver parenchymal transection between the right lobe and the left side of the middle lobe was performed based on the gross morphology and demarcation line after PVL. The Glisson flowing into the left side of the median lobe was ligated with 7-0 nylon (Figure 1B). Little bleeding occurred during the liver parenchymal transection because the parenchyma on either side of the dissection line was ligated with 6-0 Prolene before parenchymal transection to control intraoperative bleeding. The abdomen was then closed in layers.
The rats were sacrificed to collect blood samples and liver tissue from the right side of the median lobe (RML) at 1, 4, 6, 24, 48, and 72 h after surgery (n = 5 for each group per time point). Blood samples were collected from the inferior vena cava at the time of liver removal and centrifuged at 2600 × g for 5 min. The serum was stored at -80 °C. Liver tissue samples were frozen in liquid nitrogen and stored at -80 °C. The remaining liver tissue was fixed with 4% paraformaldehyde.
The weight of the FLR, that is, the RML, and body weight (BW) were measured before surgery and at 24, 48, and 72 h after surgery. The BW (FLR/BW) ratio (%) was used as the liver regeneration index. In western blotting analysis and volumetric blood flow analysis, the PVL and ALPPS groups were compared based on the control group, in which only open and closed abdomens were performed.
Serum concentrations of IL-6, tumor necrosis factor-α (TNF-α), and hepatocyte growth factor (HGF) were measured at 1, 4, 6, and 24 h after surgery using ELISA kits (R&D Systems, Minneapolis, MN, United States). IL-6 concentration in the RML tissue was also quantified 1 h after surgery.
The liver tissues were fixed with paraformaldehyde and embedded in paraffin. After deparaffinization, antigen retrieval, and quenching of endogenous peroxidases, the sections were incubated overnight at 4 °C with a mouse monoclonal anti-Ki-67 antibody (1:200 dilution; Dako, Glostrup, Denmark; 1:200 dilution, Abcam, Cambridge, United Kingdom), followed by incubation for 30 min at room temperature with a peroxidase-labeled anti-mouse antibody (Histofine Simplestain Max PO; Nichirei). The sections were immersed in diaminobenzidine solution for visualization and counterstained with hematoxylin. To evaluate hepatocyte proliferation 48 h after surgery, the average percentage of Ki-67-positive cells to total hepatocytes in three random high-power fields was used as the Ki-67 labeling index.
To explore the role of inflammatory cytokines in liver regeneration, the Kupffer cell inhibitor gadolinium chloride (GdCl3; Sigma-Aldrich, St. Louis, MO, United States) was used. Another set of animals was used for the Kupffer cell inhibition experiments. We prepared an ALPPS model for GdCl3 administration (n = 3). GdCl3 (10 mg/kg) was administered intraperitoneally 24 h before surgery. In the control group, physiological saline was administered. All rats were sacrificed 48 h after surgery to obtain liver samples.
To explore the role of NO in liver regeneration, the NOS inhibitor NG-nitro-arginine methyl ester (L-NAME; Sigma-Aldrich) and the NO enhancer molsidomine (Cayman Chemical, MI, United States) were used. Another set of animals was used for the NOS inhibition and NO enhancement experiments. We prepared the ALPPS model for L-NAME administration, the PVL model for molsidomine administration, and the corresponding control PVL and ALPPS models (n = 5 for each group). L-NAME (100 mg/kg) or molsidomine (10 mg/kg) was administered intraperitoneally 24 h before and during surgery. In each control group, physiological saline was administered. All rats were sacrificed 24, 48, and 72 h after surgery to obtain liver samples.
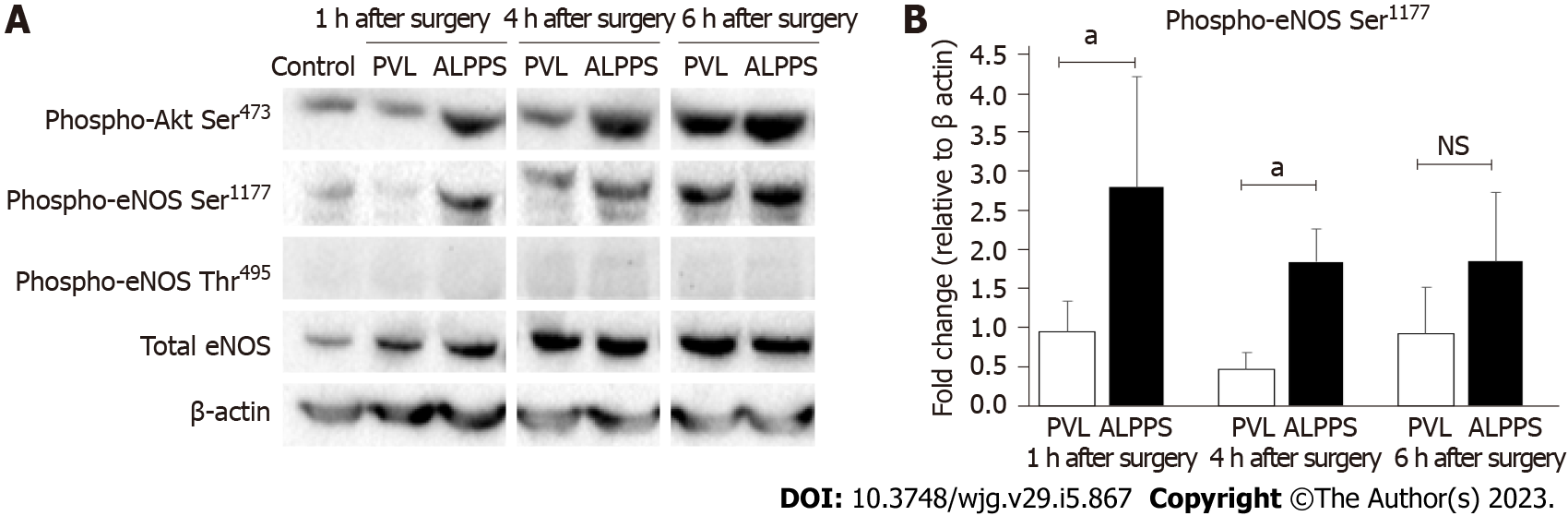
The RML tissue proteins were collected at 1, 4, and 6 h after surgery using radioimmunoprecipitation assay lysis buffer (Santa Cruz Biotechnology, Inc., CA, United States). The protein concentration was measured using the bicinchoninic acid assay method. Samples of 10 μg proteins from FLRs of PVL and ALPPS models were separated on 4%-12% NuPAGE Gels and transferred onto nitrocellulose membranes. After blocking with 5% dry skim milk for 1 h, the membranes were incubated with primary antibodies overnight at 4 °C, followed by incubation with horseradish peroxidase-conjugated secondary antibodies for 1 h. The blots were developed with ECL Select western blotting Detection Reagent (Amersham, GE Healthcare Life Sciences, Chicago, IL, United States) and photographed using a Molecular Imager ChemiDoc XRS device (Bio-Rad Laboratories, Inc., Hercules, CA, United States). The density of the bands in the immunoblots was analyzed using Image Lab Software (Bio-Rad Laboratories, Inc.). The results are expressed as a percentage of the β-actin internal control. The anti-human antibodies used were rabbit monoclonal antibodies against p-Akt (Ser 473) (cat. no. 4060), p-eNOS (Ser1177) (Cat. no. 9570), p-eNOS (Thr495) (Cat. no. 9574), total eNOS (Cat. no. 32027) (Cell Signaling Technology, Inc., Danvers, MA, United States), and mouse monoclonal antibody against β-actin (Cat. no. A5441; Sigma-Aldrich). Anti-β-actin antibody was used at a 1:3000 dilution, and the other antibodies were used at a 1:1000 dilution.
Before the estimation of volumetric blood flow in the HA and PV of the FLR, blood velocity and vascular diameter (r) were measured using ultrasonography (Vevo2100, Primetech, Tokyo, Japan). Volumetric blood flow was estimated from the blood velocity and vascular cross-sectional area (πr2) (volumetric blood flow = blood velocity × πr2) in mm3 per second.
The collected data were evaluated statistically using the JMP software, version 13.2 (SAS Institute, Cary, NC, United States). Data are expressed as mean ± SD. Statistical analysis was performed using an unpaired student’s t-test. Statistical significance was defined as P < 0.05.
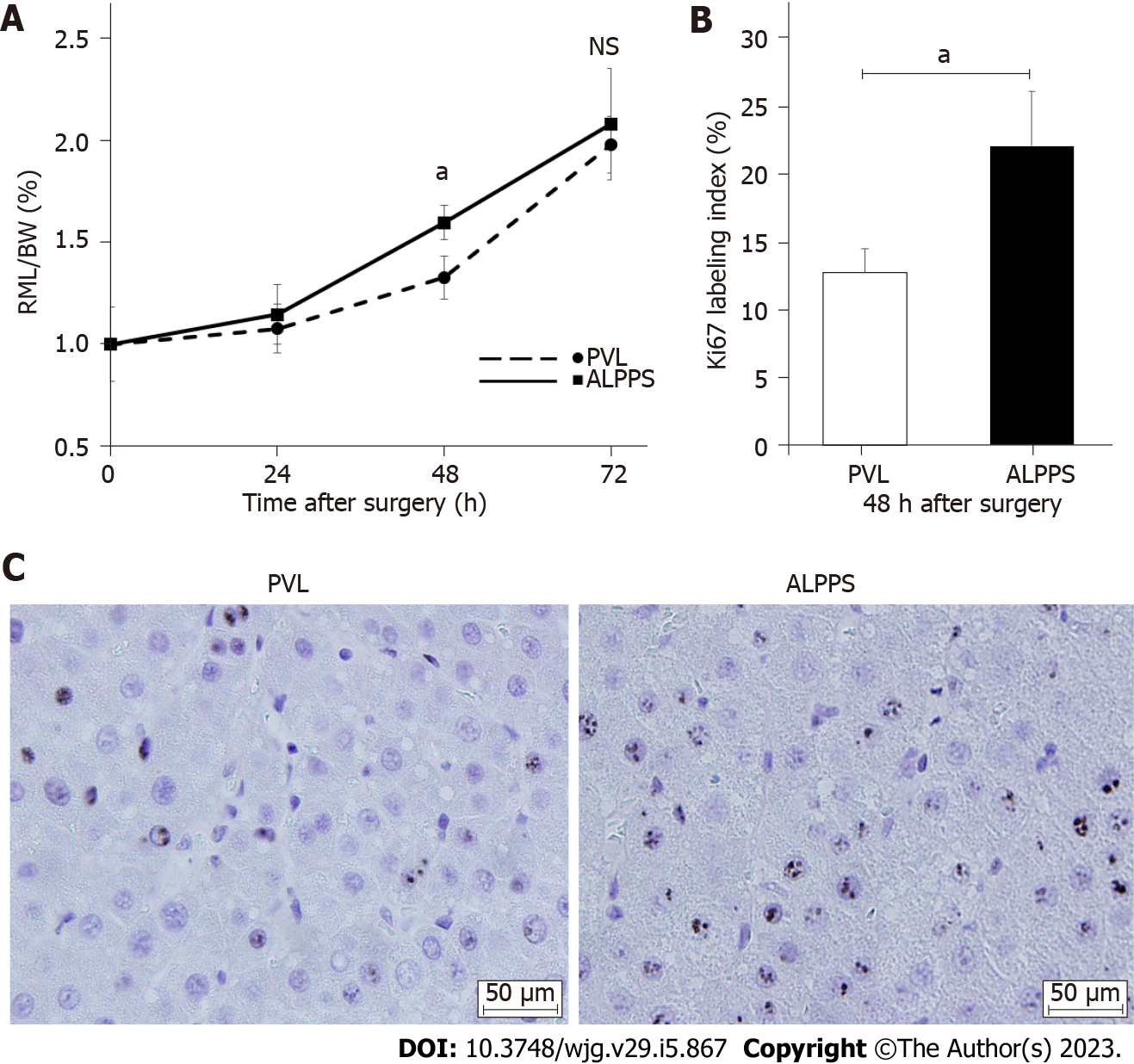
The FLR/BW ratio increased over time in both groups. At 48 h after surgery, the FLR/BW ratio in the ALPPS group was significantly higher (1.60% ± 0.08%, P < 0.05) than that in the PVL group (1.33% ± 0.11%) (Figures 1C and 2A). However, no significant difference was observed between the two groups at 24 and 72 h after surgery. The Ki-67 labeling index of the RML at 48 h after surgery was significantly increased in the ALPPS group (22.1% ± 4.01%, P < 0.05) compared with that in the PVL group (12.8% ± 1.73%), which was consistent with the FLR/BW ratio (Figures 2B and 2C).
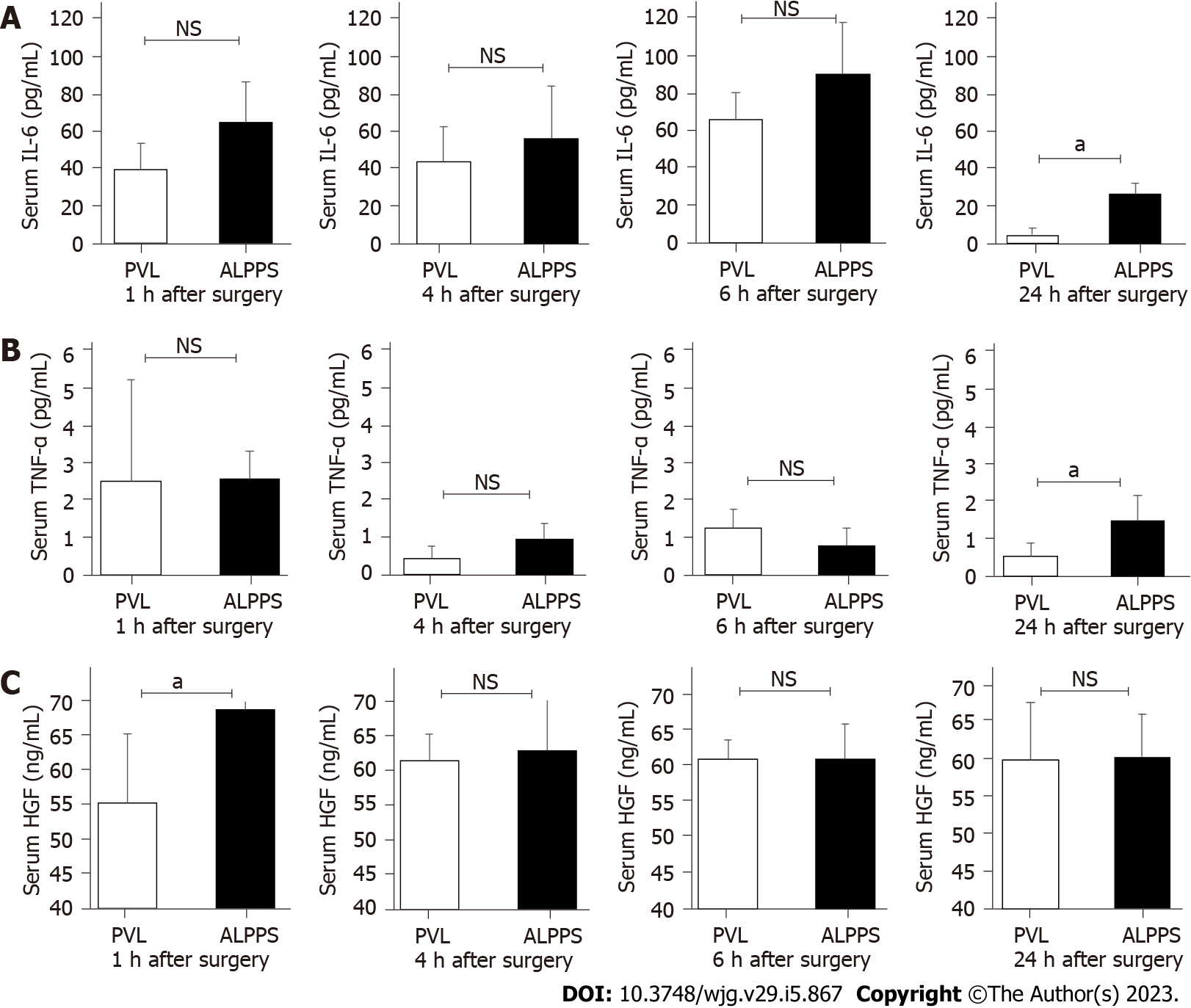
The serum concentrations of IL-6, TNF-α, and HGF in RML were measured at 1, 4, 6, and 24 h after surgery. Serum IL-6 and TNF-α levels increased in both groups after surgery compared with the levels before surgery. However, no difference was found in the two groups at 1, 4, and 6 h after surgery. At 24 h after surgery, IL-6 and TNF-α concentrations were significantly higher in the ALPPS group (25.91 ± 6.05 pg/mL, P < 0.05 and 1.52 ± 0.68 pg/mL, P < 0.05) compared with that in the PVL group (4.11 ± 3.99 pg/mL and 0.54 ± 0.38 pg/mL). Serum HGF concentration at 1 h after surgery was significantly higher in the ALPPS group (68.86 ± 4.89 ng/mL, P < 0.05) compared with that in the PVL group (55.34 ± 9.97 ng/mL). However, no significant difference was observed in serum HGF concentration at 4, 6, and 24 h (Figures 3A-C).
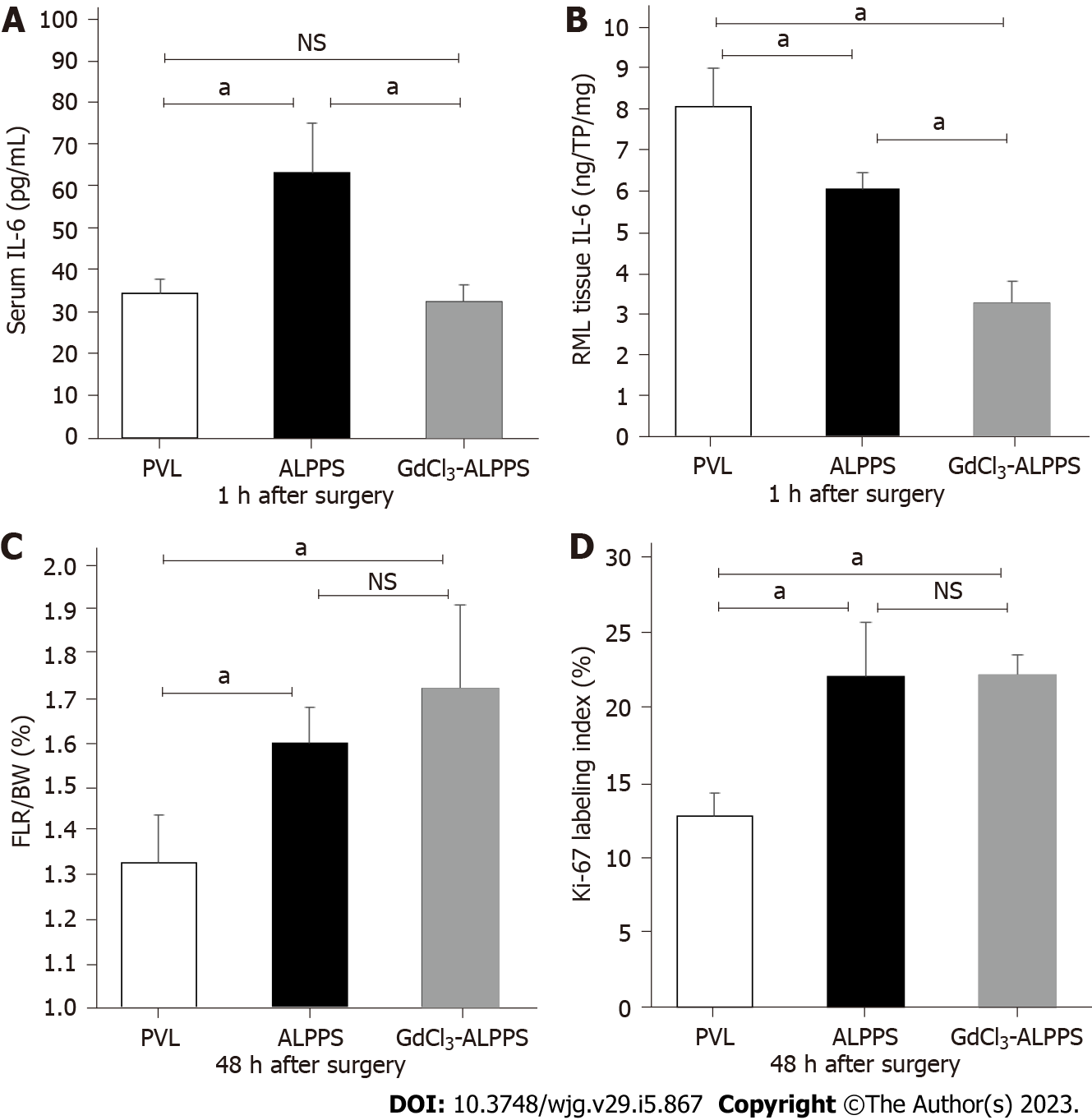
To evaluate the effect of IL-6 on liver regeneration, ALPPS rats were administered GdCl3, which suppressed the activation of Kupffer cells in the liver. In the GdCl3-ALPPS group, the IL-6 concentrations in serum (40.3 ± 11.3 pg/mL, P < 0.05) and the RML tissue (3.27 ± 0.54 ng/TP 1 g, P < 0.05) 1 h after surgery were significantly decreased compared with the concentrations in the corresponding groups without administration of GdCl3 (Figures 4A and 4B). However, there was no significant difference in the FLR/BW ratio or Ki-67 labeling index at 48 h after surgery in the ALPPS group with or without administration of GdCl3 (Figures 4C and 4D).
Phosphorylation of Akt and eNOS in RML tissue at 1, 4, and 6 h after surgery was evaluated using western blotting (Figure 5A). Phospho-Akt Ser473 and phospho-eNOS Ser1177 levels increased in the ALPPS group compared with those in the PVL group. The quantitative measurement revealed that the phosphorylation levels of eNOS Ser1177 in the ALPPS group was significantly higher than that in the PVL group at 1 and 4 h after surgery. However, there was no significant difference at 6 h after surgery (Figure 5B).
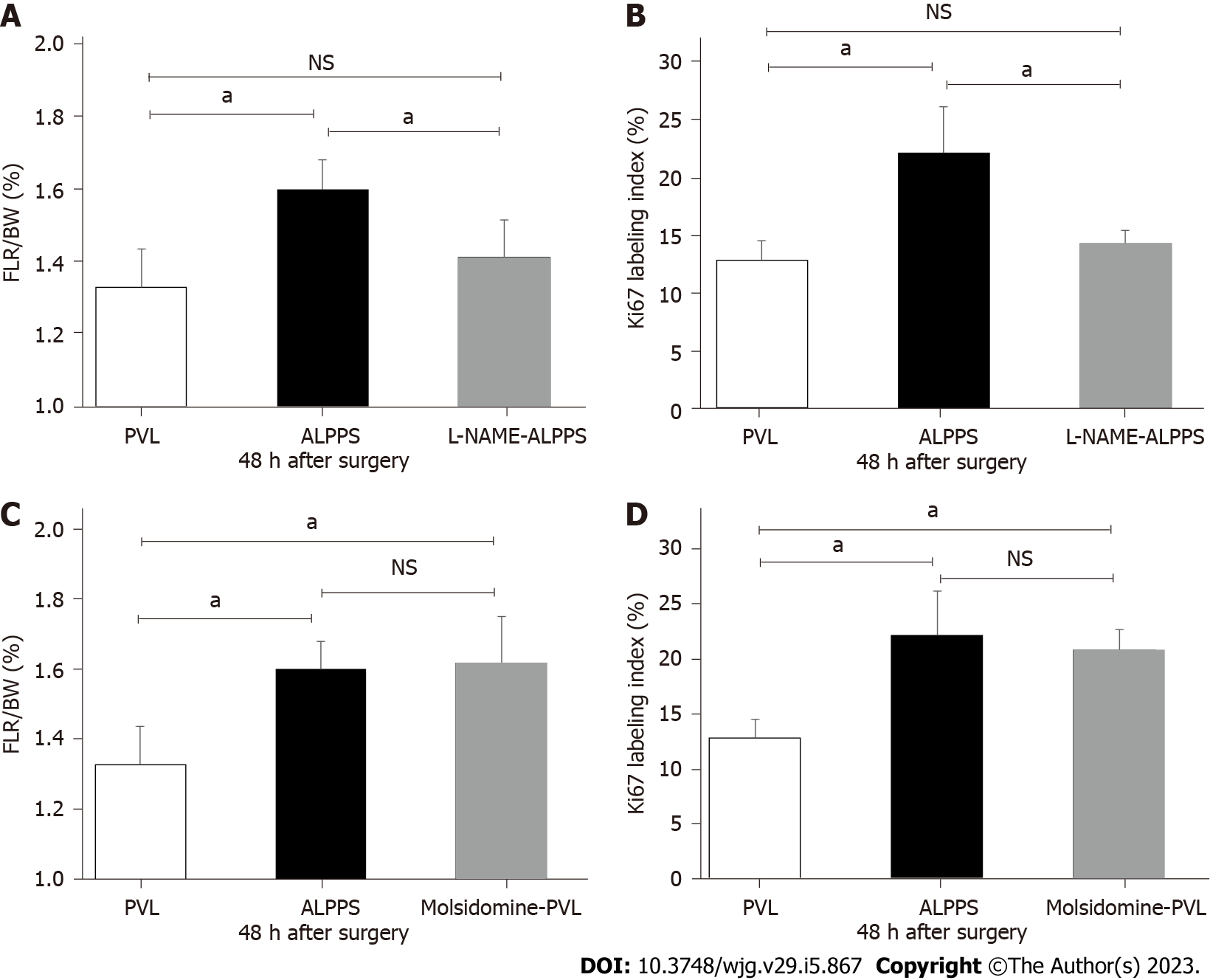
L-NAME, an NOS inhibitor, was administered to rats to examine whether suppression of eNOS affected liver regeneration. The FLR/BW ratio and Ki-67 labeling index at 48 h after surgery in the L-NAME-ALPPS group were significantly lower than those in the ALPPS group without L-NAME administration and were comparable to those in the PVL group (Figures 6A and 6B).
Additionally, molsidomine, which induces eNOS activation, was administered to the rats to examine whether eNOS activation affects liver regeneration. The FLR/BW ratio and Ki-67 labeling index at 48 h after surgery in the molsidomine-administered PVL (molsidomine-PVL) group were significantly higher than those in the PVL group without molsidomine administration and comparable with those in the ALPPS group (Figures 6C and 6D). However, there was no significant difference in the long-term FLR/BW ratio on a postoperative day 7 between the PVL, ALPPS, and molsidomine-administered PVL groups (data not shown).
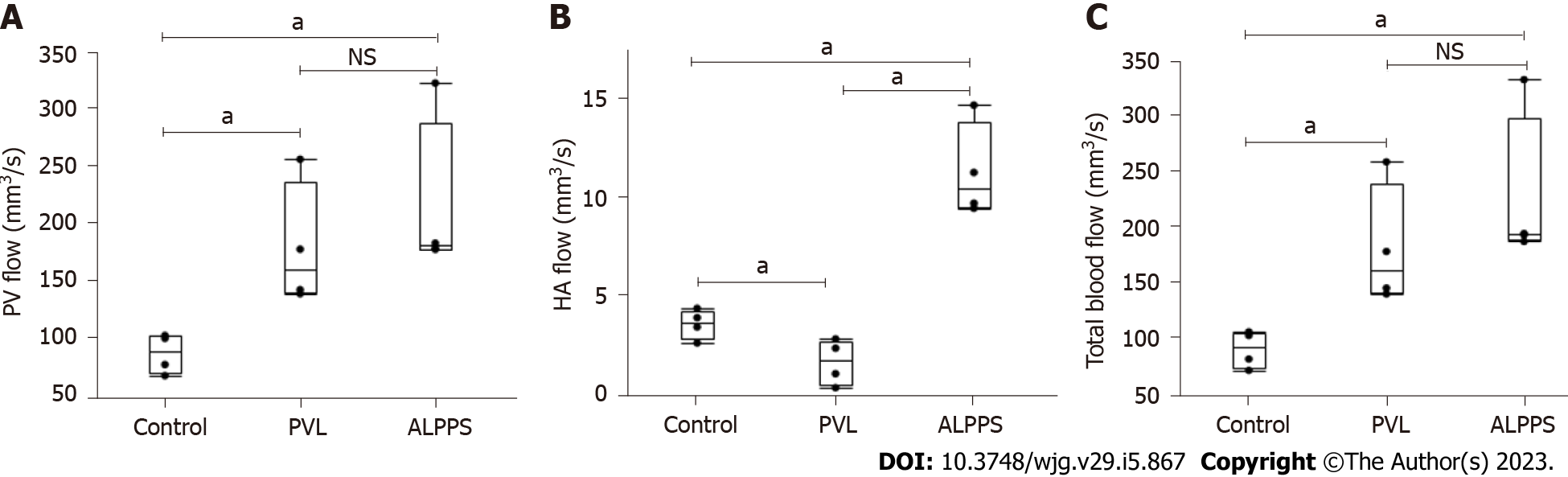
PV flow in the PVL and ALPPS groups was significantly faster than that in the control group; however, there was no significant difference in PV flow between the PVL and ALPPS groups (180.1 ± 54.4, 216.6 ± 71.4 mm3/s) (Figure 7A). HA flow in the PVL group was significantly slower than that in the control group without surgical intervention (1.73 ± 1.14 vs 3.66 ± 0.74 mm3/s, P < 0.05), whereas that in the ALPPS group was significantly faster (11.32 ± 2.40 mm3/s, P < 0.05) than that in control and PVL groups (Figure 7B). The total blood flow, that is, the sum of PV and HA, was not significantly different between the PVL and ALPPS groups (Figure 7C).
Hepatectomy is the most curative treatment for HCC and intraductal cholangiocarcinoma[1,2]. Additionally, major hepatectomy is the standard operative procedure for perihilar cholangiocarcinoma[17,18]. Extended hepatectomy may be required, depending on the location of the cancer. Postoperative liver failure that results from insufficient residual liver volume is a fatal complication of hepatectomy. PVE and ALPPS were developed with the aim of pre-operative liver enlargement to avoid postoperative liver failure[5,6,18]. ALPPS leads to the rapid regeneration of FLR compared with PVE, although high mortality (90-d mortality of 9%) and morbidity (grade IIIb of 40% in the Clavien-Dindo classification) are limitations[19]. Elucidation of the mechanism of rapid liver regeneration after ALPPS may contribute to improving surgical outcomes for patients who undergo extended hepatectomy for hepatobiliary malignancies and to the development of novel alternative treatments that provide effective and safe regeneration of the FLR.
In this study, we obtained two crucial findings regarding the mechanism of liver regeneration in ALPPS. First, the induction of inflammatory cytokines, such as IL-6, might not be pivotal for the rapid regeneration of FLR after ALPPS in the early phase. Second, activation of the Akt-eNOS pathway may be an important factor in promoting liver regeneration after ALPPS.
The mechanism of liver regeneration has been studied in animal models of partial hepatectomy. The regeneration process is distinctive, complex, and well-coordinated and depends on the interactions of several signaling pathways, cytokines, and growth factors. Additionally, endocrine hormones, such as norepinephrine, growth hormone, insulin, and thyroid hormones, have been reported to influence these pathways and factors[20-22]. Since Schnitzbauer et al[6] reported ALPPS in 2012, there have been several reports to elucidate the major factors in liver regeneration of ALPPS, which promote rapid liver regeneration compared with PVL[10-13,23]. Activation of downstream signals, such as c-Jun N-terminal kinase-Indian hedgehog signaling from stellate cells by inflammatory cytokines[24], activation of the Janus kinase 2/signal transducer and activator of transcription 3 pathway via regenerating islet-derived 3α/3β, and hypoxia-induced stabilization of hypoxia-inducible factor-α subunits by hypoxia[14,25,26], have been reported as major factors. However, the mechanism of liver regeneration in ALPPS has not yet been completely elucidated.
Previous studies have reported that the peak of cell proliferation is 48 h after surgery, and inflammatory cytokines and their downstream signal enhancement cause liver regeneration in ALPPS[11,12,23]. Although the peak liver regeneration in this study was consistent with previous studies, the relationship between the early induction of inflammatory cytokines and liver regeneration was not consistent. In this study, serum concentrations of inflammatory cytokines, such as IL-6 and TNF-α, in the short term (1, 4, and 6 h) after surgery did not differ between the ALPPS and PVL groups. However, the ALPPS group showed a greater increase in FLR and a higher Ki-67 labeling index than in the PVL group. Additionally, suppression of inflammatory cytokines using GdCl3 did not suppress liver regeneration. These results suggest that the induction of inflammatory cytokines in the early phase after ALLPS is not necessarily a major factor in accelerating liver regeneration. The reason why no difference was observed in the expression of inflammatory cytokines may be the site of liver resection, setting of FLR, or differences in animal models. The timing of specimen collection may have influenced the results, as specimens collected 24 h after surgery had higher concentrations in the ALPPS group.
Activation of eNOS and NO induction have been reported to be a mechanism of liver regeneration other than inflammatory cytokines[15,16]. In this study, we focused on the effect of eNOS activation on liver regeneration after PVL and ALPPS. Evaluation of eNOS activation in the liver tissue showed that eNOS Ser1177 phosphorylation was significantly increased in the ALPPS model at 1 and 4 h after surgery. Thus, the FLR/BW ratio and Ki-67 labeling index in the ALPPS model were increased compared with those in the PVL model. Furthermore, the activation of Akt, which is upstream of eNOS, was observed, suggesting that the Akt-eNOS pathway contributes to the mechanism of liver regeneration in ALPPS. The administration of L-NAME, which suppresses NO, inhibits liver regeneration. The administration of molsidomine, which activates eNOS, promotes liver regeneration. Molsidomine is a nitrate drug used as a coronary vasodilator for the treatment of angina pectoris; its intermediate metabolite, SIN-1 (ionidamine chlorohydrate) produces NO[27]. When endothelial cells are stimulated by shear stress or vascular endothelial growth factor, phosphoinositide 3-kinase (PI3K) is activated and PIP3 is produced, which activates the PI3K-Akt pathway and activates downstream signals such as eNOS[28,29]. An increase in shear stress, which has been reported to cause NO production[30], is due to hemodynamic changes in the residual liver caused by hepatectomy, which is expected to affect liver regeneration in ALPPS. To evaluate the effect of increased shear stress on liver regeneration, we examined the blood flow exchange after PVL and ALPPS. Contrary to our expectations, there was no difference in PV or total blood flow, which might be associated with shear stress, between the PVL and ALPPS groups; however, HA flow in the ALPPS group was significantly higher than that in the PVL and control groups. Therefore, the difference in oxygenation of the FLR, rather than the shear stress between ALPPS and PVL, might be associated with the difference in liver regeneration. However, Schadde et al[25] reported that hypoxia due to reduced HA flow in the FLR promotes hepatic regeneration in patients who underwent ALPPS and in the rat ALPPS model. However, in their study, HA flow was evaluated only in patients who underwent ALPPS, and this evaluation was not compared with that in patients who underwent PVE. Furthermore, the transition of HA flow before and after ALPPS has not been evaluated in a rat model. In the rat ALPPS model, liver transection between the right and left median lobes with ligation of the Glisson of the left median lobe caused a necrotic change in the left median lobe, which is synonymous with liver resection of the left median lobe considering hemodynamics. These results suggest that both hemodynamic changes and differences in oxygenation of the FLR affect regeneration rates in the ALPPS and PVL models. The increased HA flow to the RML observed in the ALPPS group may have been due to a hepatic arterial buffer response derived from collateral blood flow blockage by hepatectomy. In contrast, the reason for the observed decrease in HA flow to the RML in the PVL group might be the effect of HA influx from the RML to the left median lobe (LML) via collateral circulation after the PV blockade to the LML.
This study had some limitations. First, because we observed short-term changes in rat models, it is unknown whether NO activation promotes clinically meaningful liver regeneration in humans. Second, the mechanism underlying the activation of the Akt-eNOS pathway is unclear and requires further investigation that includes real-time monitoring of oxygenation in the FLR. Despite these shortcomings, we believe that our results are of interest because few reports have focused on the relationship between eNOS activation and liver regeneration after ALPPS.
The activation of the Akt-eNOS pathway in ALPPS may be an important factor in promoting early liver regeneration. If a combination of NO-producing agents and PVL or PVE enables liver regeneration within a short time after surgery, it may be an alternative to ALPPS and is expected to be applied clinically as a less invasive procedure.
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) has already been clinically applied in various countries. Although it has been reported that ALPPS offers faster and larger liver regeneration compared to portal vein embolization (PVE), the mechanism of this phenomenon is still unclear.
The aim of this study was to investigate the underlying mechanism of rapid liver regeneration after ALPPS focusing on inflammatory cytokines and endothelial nitric oxide synthase (eNOS) activation.
Activation of eNOS was considered one of key points on mechanism of rapid liver regeneration after ALPPS.
Liver regeneration was compared between the rat portal vein ligation (PVL) model and the rat ALPPS model. In addition, impact of administration of gadolinium chloride (GdCl3, Kupffer cell inhibitor), NG-nitro-arginine methyl ester (L-NAME, NOS inhibitor), and molsidomine (NO enhancer) on liver regeneration after PVL and/or ALPPS.
Administration of GdCl3 before ALPPS provided no significant negative influence of liver regeneration after ALPPS. Administration of L-NAME before ALPPS suppressed liver regeneration after ALPPS, while administration of molsidomine before PVL accerelated liver regeneration after PVL as well as ALPPS.
ALPPS is an alternative to PVE for reducing posthepatectomy liver failure after major hepatectomy.
Combination of NO-producing agents and less invasive procedure can be an alternative to ALPPS procedure in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: The Japanese Society of Gastroenterology, 048188.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: A JD, China; Kordzaia D, Georgia; Tan W, China; Xiao LK, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Orcutt ST, Anaya DA. Liver Resection and Surgical Strategies for Management of Primary Liver Cancer. Cancer Control. 2018;25:1073274817744621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 216] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 2. | Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014;149:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 594] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 3. | Clavien PA, Oberkofler CE, Raptis DA, Lehmann K, Rickenbacher A, El-Badry AM. What is critical for liver surgery and partial liver transplantation: size or quality? Hepatology. 2010;52:715-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 733] [Article Influence: 40.7] [Reference Citation Analysis (1)] |
| 5. | Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H, Ozaki H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521-527. [PubMed] |
| 6. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, Kroemer A, Loss M, Rümmele P, Scherer MN, Padberg W, Königsrainer A, Lang H, Obed A, Schlitt HJ. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 932] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 7. | Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 473] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 8. | Liu H, Zhu S. Present status and future perspectives of preoperative portal vein embolization. Am J Surg. 2009;197:686-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Knoefel WT, Gabor I, Rehders A, Alexander A, Krausch M, Schulte am Esch J, Fürst G, Topp SA. In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two-stage liver resection. Br J Surg. 2013;100:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Schlegel A, Lesurtel M, Melloul E, Limani P, Tschuor C, Graf R, Humar B, Clavien PA. ALPPS: from human to mice highlighting accelerated and novel mechanisms of liver regeneration. Ann Surg. 2014;260:839-46; discussion 846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 11. | Yao L, Li C, Ge X, Wang H, Xu K, Zhang A, Dong J. Establishment of a rat model of portal vein ligation combined with in situ splitting. PLoS One. 2014;9:e105511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Shi H, Yang G, Zheng T, Wang J, Li L, Liang Y, Xie C, Yin D, Sun B, Sun J, Wang H, Pan S, Jiang H, Lau W, Liu L. A preliminary study of ALPPS procedure in a rat model. Sci Rep. 2015;5:17567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | García-Pérez R, Revilla-Nuin B, Martínez CM, Bernabé-García A, Baroja Mazo A, Parrilla Paricio P. Associated Liver Partition and Portal Vein Ligation (ALPPS) vs Selective Portal Vein Ligation (PVL) for Staged Hepatectomy in a Rat Model. Similar Regenerative Response? PLoS One. 2015;10:e0144096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Otsuka N, Yoshioka M, Abe Y, Nakagawa Y, Uchinami H, Yamamoto Y. Reg3α and Reg3β Expressions Followed by JAK2/STAT3 Activation Play a Pivotal Role in the Acceleration of Liver Hypertrophy in a Rat ALPPS Model. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Schoen JM, Wang HH, Minuk GY, Lautt WW. Shear stress-induced nitric oxide release triggers the liver regeneration cascade. Nitric Oxide. 2001;5:453-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Mei Y, Thevananther S. Endothelial nitric oxide synthase is a key mediator of hepatocyte proliferation in response to partial hepatectomy in mice. Hepatology. 2011;54:1777-1789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Furusawa N, Kobayashi A, Yokoyama T, Shimizu A, Motoyama H, Miyagawa S. Surgical treatment of 144 cases of hilar cholangiocarcinoma without liver-related mortality. World J Surg. 2014;38:1164-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 227] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Schadde E, Raptis DA, Schnitzbauer AA, Ardiles V, Tschuor C, Lesurtel M, Abdalla EK, Hernandez-Alejandro R, Jovine E, Machado M, Malago M, Robles-Campos R, Petrowsky H, Santibanes ED, Clavien PA. Prediction of Mortality After ALPPS Stage-1: An Analysis of 320 Patients From the International ALPPS Registry. Ann Surg. 2015;262:780-5; discussion 785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 20. | Michalopoulos GK. Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology. 2017;65:1384-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 318] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 21. | Abu Rmilah A, Zhou W, Nelson E, Lin L, Amiot B, Nyberg SL. Understanding the marvels behind liver regeneration. Wiley Interdiscip Rev Dev Biol. 2019;8:e340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 22. | Abu Rmilah AA, Zhou W, Nyberg SL. Hormonal Contribution to Liver Regeneration. Mayo Clin Proc Innov Qual Outcomes. 2020;4:315-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Dhar DK, Mohammad GH, Vyas S, Broering DC, Malago M. A novel rat model of liver regeneration: possible role of cytokine induced neutrophil chemoattractant-1 in augmented liver regeneration. Ann Surg Innov Res. 2015;9:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Langiewicz M, Graf R, Humar B, Clavien PA. JNK1 induces hedgehog signaling from stellate cells to accelerate liver regeneration in mice. J Hepatol. 2018;69:666-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Schadde E, Tsatsaris C, Swiderska-Syn M, Breitenstein S, Urner M, Schimmer R, Booy C, Z'graggen BR, Wenger RH, Spahn DR, Hertl M, Knechtle S, Diehl AM, Schläpfer M, Beck-Schimmer B. Hypoxia of the growing liver accelerates regeneration. Surgery. 2017;161:666-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Dirscherl K, Schläpfer M, Roth Z'graggen B, Wenger RH, Booy C, Flury-Frei R, Fatzer R, Aloman C, Bartosch B, Parent R, Kurtcuoglu V, de Zélicourt D, Spahn DR, Beck Schimmer B, Schadde E. Hypoxia sensing by hepatic stellate cells leads to VEGF-dependent angiogenesis and may contribute to accelerated liver regeneration. Sci Rep. 2020;10:4392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Rosenkranz B, Winkelmann BR, Parnham MJ. Clinical pharmacokinetics of molsidomine. Clin Pharmacokinet. 1996;30:372-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2723] [Cited by in RCA: 2749] [Article Influence: 105.7] [Reference Citation Analysis (0)] |
| 29. | Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2015] [Cited by in RCA: 2066] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 30. | Kelm M, Feelisch M, Deussen A, Strauer BE, Schrader J. Release of endothelium derived nitric oxide in relation to pressure and flow. Cardiovasc Res. 1991;25:831-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 65] [Article Influence: 1.9] [Reference Citation Analysis (0)] |