Published online Dec 21, 2023. doi: 10.3748/wjg.v29.i47.6138
Peer-review started: August 4, 2023
First decision: October 25, 2023
Revised: November 7, 2023
Accepted: December 8, 2023
Article in press: December 8, 2023
Published online: December 21, 2023
Processing time: 133 Days and 16.7 Hours
Superficial esophageal squamous cell carcinoma (ESCC) is defined as cancer infiltrating the mucosa and submucosa, regardless of regional lymph node metastasis (LNM). Endoscopic resection of superficial ESCC is suitable for lesions that have no or low risk of LNM. Patients with a high risk of LNM always need further treatment after endoscopic resection. Therefore, accurately assessing the risk of LNM is critical for additional treatment options.
To analyze risk factors for LNM and develop a nomogram to predict LNM risk in superficial ESCC patients.
Clinical and pathological data of superficial ESCC patients undergoing esophagectomy from January 1, 2009 to January 31, 2016 were collected. Logistic regression analysis was used to predict LNM risk factors, and a nomogram was developed based on risk factors derived from multivariate logistic regression analysis. The receiver operating characteristic (ROC) curve was used to obtain the accuracy of the nomogram model.
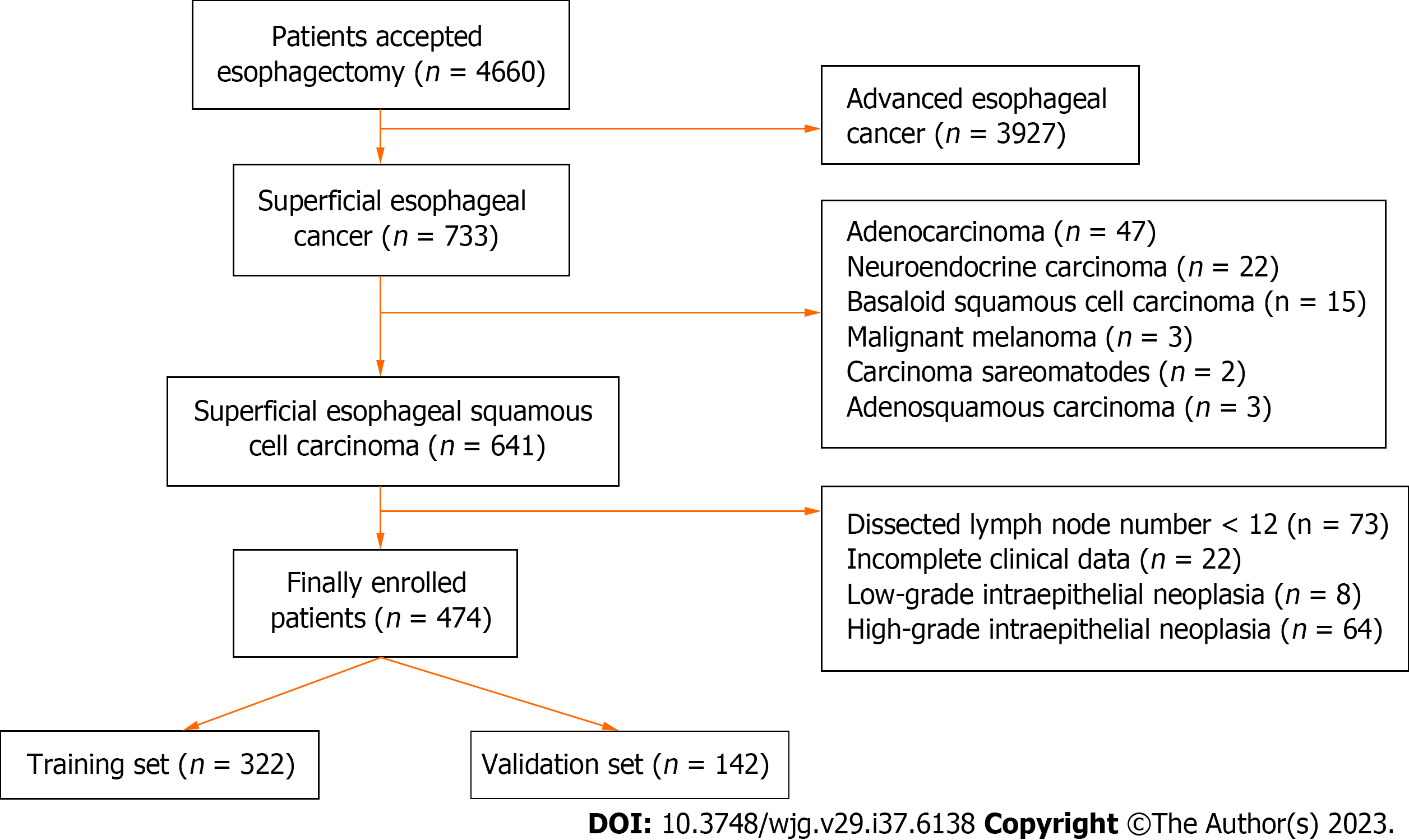
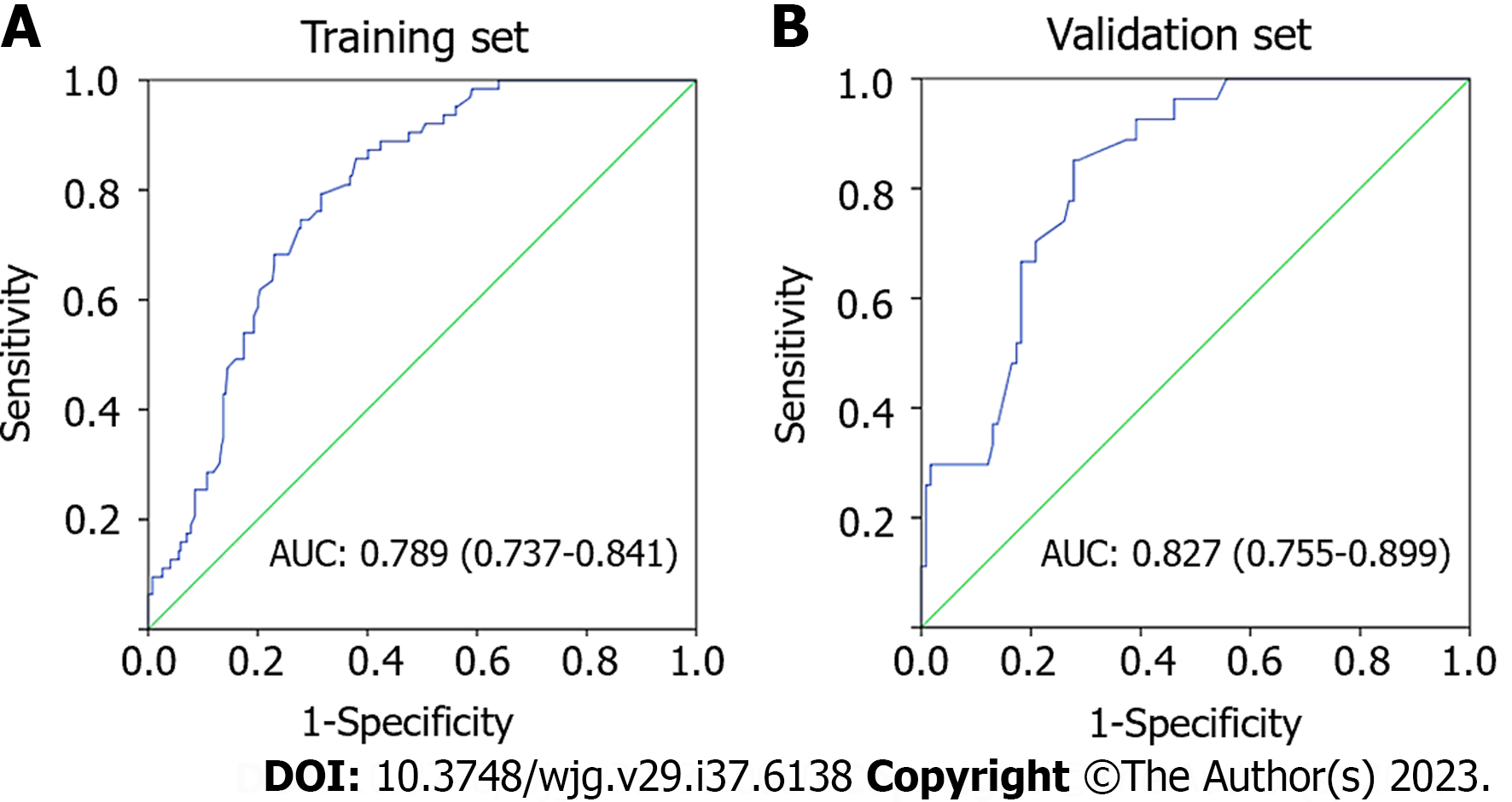
A total of 4660 patients with esophageal cancer underwent esophagectomy. Of these, 474 superficial ESCC patients were enrolled in the final analysis, with 322 patients in the training set and 142 patients in the validation set. The prevalence of LNM was 3.29% (5/152) for intramucosal cancer and increased to 26.40% (85/322) for submucosal cancer. Multivariate logistic analysis showed that tumor size, invasive depth, tumor differentiation, infiltrative growth pattern, tumor budding, and lymphovascular invasion were significantly correlated with LNM. A nomogram using these six variables showed good discrimination with an area under the ROC curve of 0.789 (95%CI: 0.737-0.841) in the training set and 0.827 (95%CI: 0.755-0.899) in the validation set.
We developed a useful nomogram model to predict LNM risk for superficial ESCC patients which will facilitate additional decision-making in treating patients who undergo endoscopic resection.
Core Tip: This is a retrospective study to identify risk factors for lymph node metastasis (LNM) in superficial esophageal squamous cell carcinoma (ESCC) and to develop a nomogram model for predicting LNM. A total of 474 superficial ESCC patients who underwent esophagectomy were enrolled. Multivariate logistic analysis showed that tumor size, invasive depth, tumor differentiation, infiltrative growth pattern, tumor budding, and lymphovascular invasion were significantly correlated with LNM. A predictive nomogram using these six variables showed good performance and will facilitate the treatment choice for superficial ESCC patients.
- Citation: Wang J, Zhang X, Gan T, Rao NN, Deng K, Yang JL. Risk factors and a predictive nomogram for lymph node metastasis in superficial esophageal squamous cell carcinoma. World J Gastroenterol 2023; 29(47): 6138-6147
- URL: https://www.wjgnet.com/1007-9327/full/v29/i47/6138.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i47.6138
Superficial esophageal squamous cell carcinoma (ESCC) is defined as esophageal cancerous lesions infiltrating the mucosa and submucosa, regardless of lymph node metastasis (LNM)[1]. Endoscopic resection has been the first treatment choice for superficial ESCC patients with no or low risk of LNM and has an en bloc rate of more than 90%[2,3]. However, additional treatment is recommended for patients with high risk of LNM, especially for patients with positive lymphovascular invasion (LVI) and positive vertical margins[4]. Assessing pathological characteristics after endoscopic resection and predicting the risk of LNM is critical for additional treatment strategies.
Recent studies have established several predictive models to identify LNM risk for superficial ESCC patients[5-8]. However, some limitations existed in these models. For example, some studies did not incorporate critical factors, such as tumor budding and tumor infiltrative growth (INF) pattern, into LNM risk prediction. In some studies, the submucosa was considered as an entire layer[5,6] and the depth was not classified as submucosa 1 (SM1), SM2 or more[7,8]. Therefore, we aimed to establish a nomogram predictive model based on comprehensive pathological features obtained from esophagectomy to improve the predictive performance of such models.
Patients who underwent esophagectomy at West China Hospital of Sichuan University from January 1, 2009, to January 31, 2016, were enrolled. The inclusion criteria were as follows: (1) Histopathological diagnosis of esophageal cancer; and (2) patients who received esophagectomy. The exclusion criteria were as follows: (1) Not pT1 stage tumor; (2) the histologic type was not squamous cell carcinoma; (3) number of dissected lymph nodes < 12; (4) history of previous malignancies; (5) incomplete clinical data; and (6) lesions with low-grade intraepithelial neoplasia or high-grade intraepithelial neoplasia. This retrospective study was approved by the Institutional Review Board of West China Hospital of Sichuan University (No. 2015-159). Informed consent was signed before the surgery.
General clinical and endoscopic features, such as age, sex, tumor location, and endoscopic type, were retrospectively collected. Tumor location was defined as: (1) Upper: 15 to 24 cm from the incisors; (2) middle: 24 to 32 cm from the incisors; and (3) lower: 32 cm from the incisors to the cardia. Endoscopic types were identified according to the Paris classification criteria for superficial tumors[9]. The pathologic diagnosis was independently confirmed by two experienced pathologists. If the diagnosis is inconsistent, a third expert pathologist will re-examine the specimen, and the final diagnosis will be made when two or more pathologists agree on the diagnosis.
Data regarding pathological characteristics of specimens, such as tumor size, invasion depth, differentiation grade, INF pattern, tumor budding, LVI, and number of dissected lymph nodes, were collected. The vertical invasion depth of submucosal invasion was measured from the muscularis mucosae according to the Japanese guidelines[10]. The invasion depth was classified into four layers: Muscularis mucosae (MM), upper third of submucosa (SM1), middle third of submucosa (SM2) and lower third of submucosa (SM3) according to the Japanese Classification of Esophageal Cancer[10]. The differentiation grade was grouped as well differentiated (G1), moderately differentiated (G2), and poorly differentiated (G3)[11]. The tumor INF pattern was carefully observed and classified into three groups according to previous reports[10,12]: INF-a (expansive type, expansive growth of tumor nests downward continuously from the epithelium as a whole), INF-b (intermediate type between INF-a and INF-c), and INF-c (infiltrative type, tumor infiltrates with a way of single cell or small tumor nests, or trabecular arrangement of tumor cells on the leading edge of the tumor). Tumor budding is defined as a single tumor cell or a small tumor nest consisting of up to 4 cells at the front of the tumor invasion[13]. In this study, tumor budding was assessed on hematoxylin and eosin-stained slides at the front of tumor invasion. Tumor budding was categorized into three types: No budding, low-grade tumor budding (1 to 4 budding foci at a 20 × objective lens) and high-grade tumor budding (≥ 5 budding foci at a 20 × objective lens) according to a previous study[14]. For LVI, two experienced pathologists observed the same specimen to improve diagnostic accuracy.
Continuous variables are presented as mean ± SD and are compared with a t test in the case of a normal distribution. Categorical data are presented as percentages and are compared with the chi-square test or Fisher’s exact test. All variables associated with LNM at a significant level were enrolled in the stepwise multivariate logistic analysis. All data were statistically analyzed by SPSS 22.0 software (IBM SPSS, Chicago, IL, United States). P values < 0.05 were considered statistically significant.
R software (version 4.1.3) with the rms package was used to formulate a predictive nomogram using variables derived from multivariate logistic analysis. The pROC package was used to formulate the receiver operating characteristic (ROC) curve, and the area under the curve (AUC) was used to evaluate the predictive performance of this nomogram as previously reported[15]. The nomogram can convert each regression variable to a scale of 0-100 points based on the regression coefficient. Finally, the predicted probabilities were derived from the total points obtained from each independent variable.
In total, 4660 esophageal cancer patients underwent esophagectomy from January 1, 2009 to January 31, 2016 of which, 474 superficial ESCC patients were enrolled in the final analysis (Figure 1). The clinicopathologic characteristics of enrolled patients are presented in Table 1. Most of the superficial ESCC patients were male (77.4%), the average age was 60 (range, 38-84) years, and the average tumor size was 23 (range, 3-73) mm. Most tumors (63.9%) were located in the middle third of the esophagus. According to the endoscopic appearance, 309 (65.2%) tumors presented as flat lesions. Regarding invasion depth, 152 (32.1%) patients had intramucosal cancer, 80 (16.9%) patients had SM1 cancer, and 242 (51.0%) patients had tumors deeper than SM1. Regarding tumor differentiation, 103 (21.7%) patients, 279 (58.9%) patients, and 92 (19.4%) patients had well differentiated, moderately differentiated, and poorly differentiated tumors, respectively. INF-b was the most common INF pattern, with 232 (48.9%) cases reported as such. The total tumor budding rate was 13.5% (64/474), and the LVI rate was 5.7% (27/474). Overall, 90 of the 474 (16.48%) patients had lymph node metastasis (LNM), and the LNM rate was 3.29% (5/152) in T1a tumors and 26.40% (85/322) in T1b tumors. The average number of dissected lymph nodes was 18.0 (range, 12-53) (Table 1).
| Characteristics | No. of patients, n (%) |
| Gender | |
| Male | 367 (77.4) |
| Female | 107 (22.6) |
| Age (yr), median (range) | 60 (38-84) |
| Tumor size (mm), median (range) | 23 (3-73) |
| Tumor location | |
| Upper third | 28 (5.9) |
| Middle third | 303 (63.9) |
| Lower third | 143 (30.2) |
| Paris classification | |
| 0-I | 95 (20.0) |
| 0-II | 309 (65.2) |
| 0-III | 70 (14.8) |
| Depth of invasion | |
| MM | 152 (32.1) |
| SM1 | 80 (16.9) |
| SM2 | 106 (22.3) |
| SM3 | 136 (28.7) |
| Differentiation | |
| Well | 103 (21.7) |
| Moderate | 279 (58.9) |
| Poor | 92 (19.4) |
| INF pattern | |
| INF-a | 196 (41.4) |
| INF-b | 232 (48.9) |
| INF-c | 46 (9.7) |
| Tumor budding | 64 (13.5) |
| LVI | 27 (5.7) |
| LNM | |
| Yes | 90 (19.0) |
| No | 384 (81.0) |
| Dissected LN, median (range) | 18.0 (12-53) |
The 474 enrolled patients were randomly grouped into a training set and a validation set at a ratio of 7:3. Comparisons of clinicopathological characteristics between the LNM+ and LNM- groups are presented in Table 2. Variables such as tumor size, invasion depth, tumor differentiation, INF pattern, tumor budding and LVI were significantly associated with LNM in both the training set and validation set according to the univariate analysis (Table 2). Furthermore, multivariate logistic regression analysis also showed that tumor size, invasion depth, tumor differentiation, INF pattern, tumor budding, and LVI were independent risk factors for LNM (Table 3).
| Variable | Training set (n = 332) | P value | Validation set (n = 142) | P value | ||
| LNM(-) | LNM(+) | LNM(-) | LNM(+) | |||
| Gender | 0.298 | 0.913 | ||||
| Male | 201 | 51 | 84 | 20 | ||
| Female | 68 | 12 | 31 | 7 | ||
| Age (yr), median (range) | 60 (42-80) | 60 (45-84) | 0.204 | 60 (38-78) | 58 (47-76) | 0.522 |
| Tumor size, (cm), mean ± SD | 2.23 ± 1.21 | 2.65 ± 0.99 | 0.008 | 2.07 ± 0.97 | 2.61 ± 0.86 | 0.008 |
| Tumor location, n (%) | 0.095 | 0.702 | ||||
| Upper third | 14 | 6 | 7 | 1 | ||
| Middle third | 181 | 47 | 62 | 13 | ||
| Lower third | 74 | 10 | 46 | 13 | ||
| Paris classification, n (%) | 0.282 | 0.158 | ||||
| 0-I | 46 | 14 | 26 | 9 | ||
| 0-II | 186 | 37 | 74 | 12 | ||
| 0-III | 37 | 12 | 15 | 6 | ||
| Depth of invasion, n (%) | < 0.001 | < 0.001 | ||||
| MM | 100 | 3 | 47 | 2 | ||
| SM1 | 53 | 6 | 19 | 2 | ||
| > SM1 | 116 | 54 | 49 | 23 | ||
| Differentiation, n (%) | 0.015 | 0.029 | ||||
| Well | 77 | 8 | 15 | 3 | ||
| Moderate | 148 | 38 | 80 | 13 | ||
| Poor | 44 | 17 | 20 | 11 | ||
| INF pattern, n (%) | 0.001 | 0.014 | ||||
| INF-a | 125 | 13 | 53 | 5 | ||
| INF-b | 123 | 41 | 52 | 16 | ||
| INF-c | 21 | 9 | 10 | 6 | ||
| Tumor budding, n (%) | 0.009 | 0.009 | ||||
| No | 241 | 48 | 103 | 18 | ||
| Low | 19 | 8 | 8 | 5 | ||
| High | 9 | 7 | 4 | 4 | ||
| LVI, n (%) | 0.005 | 0.004 | ||||
| Yes | 10 | 8 | 4 | 5 | ||
| No | 259 | 55 | 111 | 22 | ||
| Factors | Training set | Validation set | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Tumor size (cm) | 1.365 | 1.081-1.723 | 0.009 | 1.750 | 1.139-2.688 | 0.011 |
| Invasive depth | ||||||
| MM | Reference | Reference | ||||
| SM1 | 3.774 | 0.907-15.696 | 0.068 | 2.474 | 0.325-18.856 | 0.382 |
| > SM1 | 15.517 | 4.707-51.158 | 0.001 | 11.031 | 2.463-49.401 | 0.002 |
| Tumor differentiation | ||||||
| Well or cis | Reference | Reference | ||||
| Moderate | 2.423 | 0.807-9.451 | 0.072 | 0.715 | 0.179-2.856 | 0.635 |
| Poor | 3.670 | 1.465-9.198 | 0.006 | 4.078 | 0.977-17.026 | 0.054 |
| INF pattern | ||||||
| INF-a | Reference | Reference | ||||
| INF-b | 3.205 | 1.637-6.274 | 0.001 | 3.262 | 1.114-9.552 | 0.031 |
| INF-c | 4.121 | 1.566-10.843 | 0.004 | 6.360 | 1.623-24.922 | 0.008 |
| Tumor budding | ||||||
| Low | 2.114 | 0.875-5.108 | 0.096 | 3.815 | 0.978-14.813 | 0.054 |
| High | 3.905 | 1.387-10.995 | 0.010 | 4.769 | 1.315-17.290 | 0.017 |
| No | Reference | Reference | ||||
| LVI | ||||||
| No | Reference | Reference | ||||
| Yes | 3.767 | 1.422-9.979 | 0.008 | 4.408 | 1.346-14.438 | 0.014 |
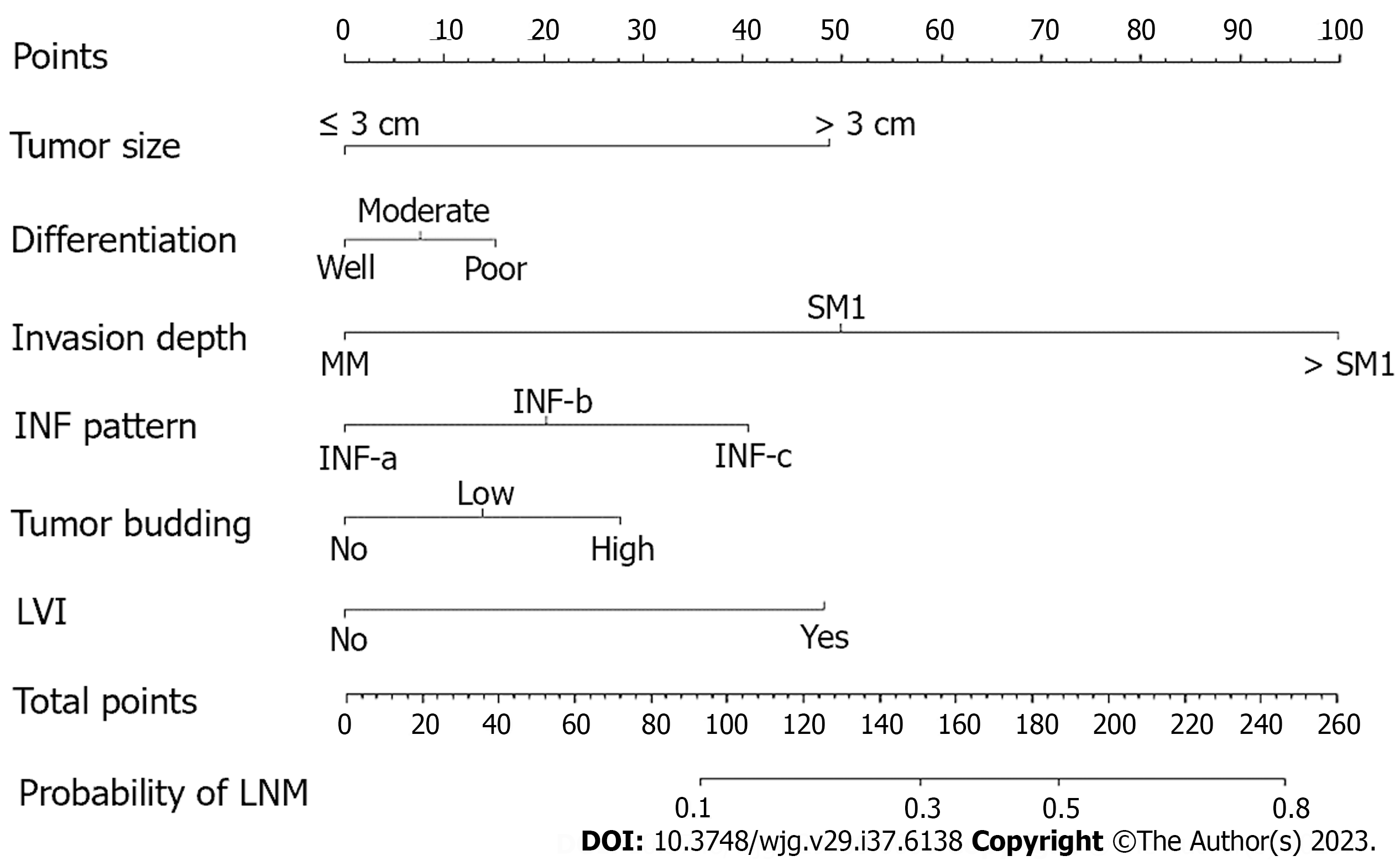
Subsequently, a nomogram was developed based on six independent risk factors derived from the multivariate analysis (Figure 2). The point of each factor was proportional to its own β-coefficient resulted from logistic regression. Finally, the total points of each factor were added and visually corresponded to a predictive value for LNM. The ROC curve showed that this nomogram had good predictive performance both in the training set and in the validation set, with AUCs of 0.789 (95%CI: 0.737-0.841) and 0.827 (95%CI: 0.755-0.899), respectively (Figure 3).
Endoscopic resection has become one of the preferred treatment methods for superficial ESCC. Compared to surgery, it has fewer complications and a shorter recovery time[16]. Guidelines and studies have indicated that endoscopic submucosal dissection (ESD) and endoscopic submucosal tunnel dissection (ESTD) can be used to treat lesions limited to the muscularis mucosa and submucosal lesions with invasion depths ≤ 200 μm, which have no or an extremely low risk of lymph node metastasis[2,17-19]. The en bloc resection rate of ESD for superficial ESCC is 98.2%-100%, and the curative resection rate is 78.2%-96.1%[17-19]. However, patients with a high risk of LNM or noncurative endoscopic resection always need further treatment[4]. Therefore, summarizing post-ESD pathological characteristics and identifying risk factors for predicting LNM are critical to guide post-ESD treatment. In this study, we enrolled superficial ESCC patients who underwent esophagectomy and lymph node dissection and collected detailed pathological information, such as tumor budding and tumor infiltrative growth pattern, to comprehensively analyze and identify the risk factors for LNM, providing favorable evidence for post-ESD treatment decisions.
Our findings in this study indicated that superficial ESCC patients with positive LNM were more likely to have larger tumors, deeper invasion, poorer differentiation, more INF-c infiltrative patterns, more high-grade tumor budding, and more positive LVI both in both the training and validation sets. Some previous studies have likewise indicated that tumor size is positively correlated with LNM risk[5-8]. Ruan et al[8] found that tumor size > 2 cm was an independent risk factor for LNM in superficial ESCC. Our results showed that patients with tumor size > 3 cm had a higher risk of LNM (Figure 2). In another study, it was reported that a 1-cm increase in esophageal tumor length increased the LNM risk by 3.55 times[20]. Therefore, it is necessary to measure the area of cancer cells after the ESD procedure. If possible, pathological recovery should be performed to determine tumor size after the ESD procedure since it is crucial for predicting LNM risk.
In addition, we found that tumor invasion depth is associated with LNM risk. It was reported that the LNM rates of T1a and T1b superficial ESCC were 6.2%-8.0% and 20.0%-29.3%, respectively[21-23]. Similarly, in our study, the LNM rate in T1a tumor was 3.29% (5/152) and increased to 26.40% (85/322) in T1b tumor. Further multivariate analysis showed that an invasion depth deeper than SM1 (OR: 15.517, 95%CI: 4.707-51.158) is an independent risk factor for LNM, which has been confirmed by other studies[5,6]. The esophageal submucosa is an area rich in lymphovascular network, and once intruded into the submucosa, tumor cells are more likely to infiltrate vasculature[24,25]. In our study, LVI positivity (OR: 3.767, 95%CI: 1.422-9.979) was also an independent risk factor for LNM. Therefore, additional treatment, such as surgery or chemoradiotherapy, should be recommended for post-ESD patients with submucosal invasion deeper than 200 μm and positive LVI.
It has been well established that the grade of tumor budding is positively correlated with the rate of LNM in solid cancers, including gastrointestinal cancers[26-28]. We found that high-grade tumor budding (OR: 3.905, 95%CI: 1.387-10.995) was positively correlated with LNM risk in superficial ESCC. Similarly, Li et al[29] checked tumor budding in pT1b ESCC by using Pan-CK immunohistochemical (IHC) staining and found that the tumor budding level is an excellent predictor of LNM and patient survival time. However, there is no gold standard for differentiating the threshold value of tumor budding in superficial ESCC specimens, and a clear and standardized method for distinguishing and reporting tumor budding in superficial ESCC is urgently needed. In addition, tumor budding status in post-ESD specimens should be carefully assessed and reported.
The Japanese guidelines recommend that tumor infiltrative growth patterns should be reported in esophageal cancer[10]. Some studies have identified infiltrative type c (INF-c) as associated with deep tumor invasion, poor tumor differentiation, and a high risk of LNM[12,30,31]. In our study, multivariate logistic regression identified INF-b (OR: 3.205; 95%CI: 1.637-6.274), INF-c (OR: 4.121, 95%CI: 1.566-10.843) and poor differentiation (OR: 3.670, 95%CI: 1.465-9.198) as independent risk factors for LNM. Invasion of surrounding tissue by cancer cells is a key step in tumor progression and metastasis[32]. As the tumor grows, the morphology and behaviour of cancer cells at the tumor front undergo epithelial mesenchymal transition, detaching from the tumor body and infiltrating deep into the submucosa or even deeper[30]. Poorly differentiated tumors are more likely to have LVI and LNM in superficial ESCC, as previously reported[6,8]. Therefore, a detailed assessment of tumor differentiation, INF pattern, tumor budding, and LVI is critical for predicting LNM risk in post-ESD patients.
Overall, we analyzed the risk factors for LNM in superficial ESCC patients by evaluating detailed pathological characteristics and developed a nomogram by incorporating six variables, including tumor size, invasion depth, tumor differentiation, tumor budding, tumor infiltrative growth pattern and LVI. This nomogram showed good predictive performance, with an AUC of 0.789 (95%CI: 0.737-0.841) in the training set and 0.827 (95%CI: 0.755-0.899) in the validation set. Although the data for this nomogram come from surgical specimens, we believe it is also applicable to post-ESD patients, as all six enrolled predictors can be easily obtained from post-ESD specimens. The use of this predictive nomogram will facilitate the assessment of LNM, thus providing references for guiding post-ESD treatment. However, this is a single-center retrospective study. More multicenter studies are needed to further confirm the reliability of the nomogram.
In addition, this study has some limitations. First, this is a retrospective study, and bias in case selection cannot be avoided. Second, differences in surgical procedures and chronological differences in pathological diagnostic criteria may affect the consistency of the results. D2-40 and CD34 IHC staining were not used in LVI diagnoses in this study, which would lead to underestimating the positivity of LVI. Finally, the LNM rate of superficial ESCC in this study cannot accurately represent the overall LNM rate because patients who underwent ESD were excluded because their postoperative LNM rate could not be calculated.
In conclusion, we identified the risk factors for LNM in superficial ESCC patients and developed a useful nomogram for predicting LNM risk by integrating all significant risk factors. This nomogram model will facilitate decision-making regarding additional treatment options in post-ESD patients.
Endoscopic resection of superficial esophageal squamous cell carcinoma (ESCC) is limited to lesions that have no or low risk of lymph node metastasis (LNM). Patients with a high risk of LNM always need further treatment after endoscopic resection.
Accurately assessing the LNM risk is critical for additional treatment choices for superficial ESCC patients who underwent endoscopic resection.
This study aimed to analyze the risk factors for LNM and develop a LNM predictive nomogram for superficial ESCC patients.
Clinical and pathological data from superficial ESCC patients underwent esopha
A total of 474 superficial ESCC patients were enrolled. The prevalence of LNM was 3.29% for intramucosal cancer and increased to 26.40% for submucosal cancer. A nomogram incorporating six variables, including tumor size, invasion depth, tumor differentiation, tumor budding, tumor infiltrative growth pattern, and lymphovascular invasion, was successfully developed.
We developed a useful nomogram model to predict LNM risk for superficial ESCC patients, which will facilitate additional treatment decisions for patients who underwent endoscopic resection.
The nomogram model is a simple and useful tool to facilitate the prediction of LNM risk for superficial ESCC patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bredt LC, Brazil; Guo D, China S-Editor: Yan JP L-Editor: A P-Editor: Cai YX
| 1. | Nishizawa T, Suzuki H. Long-Term Outcomes of Endoscopic Submucosal Dissection for Superficial Esophageal Squamous Cell Carcinoma. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Pimentel-Nunes P, Libânio D, Bastiaansen BAJ, Bhandari P, Bisschops R, Bourke MJ, Esposito G, Lemmers A, Maselli R, Messmann H, Pech O, Pioche M, Vieth M, Weusten BLAM, van Hooft JE, Deprez PH, Dinis-Ribeiro M. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2022. Endoscopy. 2022;54:591-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 355] [Article Influence: 118.3] [Reference Citation Analysis (0)] |
| 3. | Wang J, Zhu XN, Zhu LL, Chen W, Ma YH, Gan T, Yang JL. Efficacy and safety of endoscopic submucosal tunnel dissection for superficial esophageal squamous cell carcinoma and precancerous lesions. World J Gastroenterol. 2018;24:2878-2885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Hatta W, Koike T, Uno K, Asano N, Masamune A. Management of Superficial Esophageal Squamous Cell Carcinoma and Early Gastric Cancer following Non-Curative Endoscopic Resection. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Zhang W, Chen H, Zhang G, Jin G. A nomogram for predicting lymph node metastasis in superficial esophageal squamous cell carcinoma. J Biomed Res. 2021;35:361-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 6. | Min BH, Yang JW, Min YW, Baek SY, Kim S, Kim HK, Choi YS, Shim YM, Choi YL, Zo JI. Nomogram for prediction of lymph node metastasis in patients with superficial esophageal squamous cell carcinoma. J Gastroenterol Hepatol. 2020;35:1009-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Ma DW, Jung DH, Kim JH, Park JJ, Youn YH, Park H. Predicting lymph node metastasis for endoscopic resection of superficial esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. 2019;157:397-402.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Ruan R, Chen S, Tao Y, Yu J, Zhou D, Cui Z, Shen Q, Wang S. Retrospective analysis of predictive factors for lymph node metastasis in superficial esophageal squamous cell carcinoma. Sci Rep. 2021;11:16544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1323] [Article Influence: 60.1] [Reference Citation Analysis (4)] |
| 10. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus. 2017;14:1-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 706] [Article Influence: 88.3] [Reference Citation Analysis (1)] |
| 11. | Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 529] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 12. | Zhao Y, Xu E, Yang X, Zhang Y, Chen H, Wang Y, Jin M. Tumor infiltrative growth pattern correlates with the immune microenvironment and is an independent factor for lymph node metastasis and prognosis in stage T1 esophageal squamous cell carcinoma. Virchows Arch. 2020;477:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Jesinghaus M, Brühl F, Steiger K, Klare P, Reiser M, Scheiter A, Konukiewitz B, Kuhn P, Münch S, Quante M, Schmid RM, Wilhelm D, Feith M, Friess H, Combs SE, Saur D, Boxberg M, Weichert W. Cellular Dissociation Grading Based on the Parameters Tumor Budding and Cell Nest Size in Pretherapeutic Biopsy Specimens Allows for Prognostic Patient Stratification in Esophageal Squamous Cell Carcinoma Independent From Clinical Staging. Am J Surg Pathol. 2019;43:618-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Beer A, Reber A, Paireder M, Schoppmann SF, Heber S, Schiefer AI. Tumor cell budding in preoperative biopsies of esophageal and gastroesophageal junction carcinoma independently predicts survival in a grade-dependent manner. Surgery. 2022;172:567-574. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 2387] [Article Influence: 238.7] [Reference Citation Analysis (0)] |
| 16. | Min YW, Lee H, Song BG, Min BH, Kim HK, Choi YS, Lee JH, Hwang NY, Carriere KC, Rhee PL, Kim JJ, Zo JI, Shim YM. Comparison of endoscopic submucosal dissection and surgery for superficial esophageal squamous cell carcinoma: a propensity score-matched analysis. Gastrointest Endosc. 2018;88:624-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Li P, Ma B, Gong S, Zhang X, Li W. Endoscopic submucosal tunnel dissection for superficial esophageal neoplastic lesions: a meta-analysis. Surg Endosc. 2020;34:1214-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Gong J, Zhou BY, Liang CB, Zhou HJ, Wang HY, Tan YY, Liu DL. Comparison between tunneling and standard endoscopic submucosal dissection for treatment of large esophageal superficial neoplasm. Acta Gastroenterol Belg. 2019;82:469-474. [PubMed] |
| 19. | Huang R, Cai H, Zhao X, Lu X, Liu M, Lv W, Liu Z, Wu K, Han Y. Efficacy and safety of endoscopic submucosal tunnel dissection for superficial esophageal squamous cell carcinoma: a propensity score matching analysis. Gastrointest Endosc. 2017;86:831-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Haisley KR, Hart KD, Fischer LE, Kunio NR, Bakis G, Tieu BH, Schipper PH, Sheppard BC, Hunter JG, Dolan JP. Increasing tumor length is associated with regional lymph node metastases and decreased survival in esophageal cancer. Am J Surg. 2016;211:860-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Dubecz A, Kern M, Solymosi N, Schweigert M, Stein HJ. Predictors of Lymph Node Metastasis in Surgically Resected T1 Esophageal Cancer. Ann Thorac Surg. 2015;99:1879-85; discussion 1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Duan XF, Tang P, Shang XB, Jiang HJ, Yu ZT. The prevalence of lymph node metastasis for pathological T1 esophageal cancer: a retrospective study of 143 cases. Surg Oncol. 2018;27:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Xu W, Liu XB, Li SB, Yang ZH, Tong Q. Prediction of lymph node metastasis in superficial esophageal squamous cell carcinoma in Asia: a systematic review and meta-analysis. Dis Esophagus. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Ruan R, Chen S, Tao Y, Yu J, Zhou D, Cui Z, Shen Q, Wang S. A Nomogram for Predicting Lymphovascular Invasion in Superficial Esophageal Squamous Cell Carcinoma. Front Oncol. 2021;11:663802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Yang J, Lu Z, Li L, Li Y, Tan Y, Zhang D, Wang A. Relationship of lymphovascular invasion with lymph node metastasis and prognosis in superficial esophageal carcinoma: systematic review and meta-analysis. BMC Cancer. 2020;20:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Lugli A, Zlobec I, Berger MD, Kirsch R, Nagtegaal ID. Tumour budding in solid cancers. Nat Rev Clin Oncol. 2021;18:101-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 220] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 27. | Yao G, Fang Y, Fu Y, Xu J, Song H, Zhu H, Gu M, Ding X. Tumor budding as an indicator for lymph node metastasis and prognosis of early gastric cancer. J Cancer Res Clin Oncol. 2023;149:5603-5616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Archilla I, Díaz-Mercedes S, Aguirre JJ, Tarragona J, Machado I, Rodrigo MT, Lopez-Prades S, Gorostiaga I, Landolfi S, Alén BO, Balaguer F, Castells A, Camps J, Cuatrecasas M. Lymph Node Tumor Burden Correlates With Tumor Budding and Poorly Differentiated Clusters: A New Prognostic Factor in Colorectal Carcinoma? Clin Transl Gastroenterol. 2021;12:e00303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Li Z, Liu L, Wang B, Ying J, He J, Xue L. Tumor budding and tumor-infiltrating lymphocytes can predict prognosis in pT1b esophageal squamous cell carcinoma. Thorac Cancer. 2023;14:2608-2617. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Anciaux M, Demetter P, De Wind R, Gomez Galdon M, Vande Velde S, Lens G, Craciun L, Deleruelle A, Larsimont D, Lenaerts T, Sclafani F, Deleporte A, Donckier V, Hendlisz A, Vandeputte C. Infiltrative tumour growth pattern correlates with poor outcome in oesophageal cancer. BMJ Open Gastroenterol. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 31. | Ebisumoto K, Okami K, Ogura G, Sakai A, Sugimoto R, Saito K, Kaneda S, Hanakita T, Nakamura N, Iida M. The predictive role of infiltrative growth pattern in early pharyngeal cancers. Acta Otolaryngol. 2015;135:1172-1177. [PubMed] |
| 32. | de Visser KE, Joyce JA. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41:374-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1541] [Reference Citation Analysis (0)] |