Published online Dec 7, 2023. doi: 10.3748/wjg.v29.i45.5974
Peer-review started: September 17, 2023
First decision: October 8, 2023
Revised: October 19, 2023
Accepted: November 17, 2023
Article in press: November 17, 2023
Published online: December 7, 2023
Processing time: 74 Days and 14.9 Hours
Trastuzumab constitutes the fundamental component of initial therapy for patients with advanced human epidermal growth factor receptor 2 (HER-2)-positive gastric cancer (GC). However, the efficacy of this treatment is hindered by substantial challenges associated with both primary and acquired drug resistance. While S-phase kinase associated protein 2 (Skp2) overexpression has been implicated in the malignant progression of GC, its role in regulating trastuzumab resistance in this context remains uncertain. Despite the numerous studies investigating Skp2 inhibitors among small molecule compounds and natural products, there has been a lack of successful commercialization of drugs specifically targeting Skp2.
To discover a Skp2 blocker among currently available medications and develop a therapeutic strategy for HER2-positive GC patients who have experienced progression following trastuzumab-based treatment.
Skp2 exogenous overexpression plasmids and small interfering RNA vectors were utilized to investigate the correlation between Skp2 expression and trastuzumab resistance in GC cells. Q-PCR, western blot, and immunohistochemical analyses were conducted to evaluate the regulatory effect of thioridazine on Skp2 expression. A cell counting kit-8 assay, flow cytometry, a amplex red glucose/glucose oxidase assay kit, and a lactate assay kit were utilized to measure the proliferation, apoptosis, and glycolytic activity of GC cells in vitro. A xenograft model established with human GC in nude mice was used to assess thioridazine's effectiveness in vivo.
The expression of Skp2 exhibited a negative correlation with the sensitivity of HER2-positive GC cells to trastuzumab. Thioridazine demonstrated the ability to directly bind to Skp2, resulting in a reduction in Skp2 expression at both the transcriptional and translational levels. Moreover, thioridazine effectively inhibited cell proliferation, exhibited antiapoptotic properties, and decreased the glucose uptake rate and lactate production by suppressing Skp2/protein kinase B/mammalian target of rapamycin/glucose transporter type 1 signaling pathways. The combination of thioridazine with either trastuzumab or lapatinib exhibited a more pronounced anticancer effect in vivo, surpassing the efficacy of either monotherapy.
Thioridazine demonstrates promising outcomes in preclinical GC models and offers a novel therapeutic approach for addressing trastuzumab resistance, particularly when used in conjunction with lapatinib. This compound has potential benefits for patients with Skp2-proficient tumors.
Core Tip: S-phase kinase-interacting protein 2 (Skp2) has been shown to be a reliable prognostic indicator of unfavorable outcomes for gastric cancer (GC). However, no agents specifically targeting Skp2 have been successfully developed. In this study, we proved that thioridazine restores the sensitivity of GC cells to trastuzumab both in vivo and in vitro by inhibiting Skp2-mediated glycolysis. Furthermore, the combination of thioridazine and lapatinib exhibits enhanced inhibitory effects compared with either monotherapy on the growth and survival of trastuzumab-resistant GC cells. Overall, this study suggests the potential of a thioridazine-based therapy to overcome trastuzumab resistance in human epidermal growth factor receptor 2-positive GC by targeting Skp2.
- Citation: Yang ZY, Zhao YW, Xue JR, Guo R, Zhao Z, Liu HD, Ren ZG, Shi M. Thioridazine reverses trastuzumab resistance in gastric cancer by inhibiting S-phase kinase associated protein 2-mediated aerobic glycolysis. World J Gastroenterol 2023; 29(45): 5974-5987
- URL: https://www.wjgnet.com/1007-9327/full/v29/i45/5974.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i45.5974
Gastric cancer (GC) is the fifth most common cancer worldwide, with more than 1 million new cases diagnosed in 2020, and it is the fourth leading cause of cancer-related death[1]. Approximately 7.3%-20.2% of GCs are positive for human epidermal growth factor (EGF) receptor 2 (HER2) /neu, c-ERBB2[2]. Positive expression of HER2 was proven to be associated with many tumorigenic processes and poor prognosis in patients with GC[3,4]. Trastuzumab is an effective anti-HER2 therapeutic agent that showed a survival benefit in the ToGA trial[5]. Trastuzumab in combination with chemotherapy was previously the first-line treatment for HER2-positive metastatic GC. However, due to primary or acquired drug resistance, only 12.8% of patients with HER2-positive GC respond to trastuzumab[6,7]. To overcome trastuzumab resistance, many new agents and combination therapies, such as pertuzumab, margetuximab, lapatinib, tucatinib, trastuzumab emtansine, and pembrolizumab, have emerged. However, the application of most of these drugs in the treatment of trastuzumab-resistant HER-2-positive GC is still in the investigative stage. Thus, the development of new drugs or combination therapies to increase trastuzumab sensitivity is a critical need.
Cancer cells exhibit high levels of glucose uptake and glycolysis, which allow the production of high levels of ATP to facilitate cell proliferation and survival, a phenomenon called the “Warburg effect”[8]. It has been reported that the GATA6 binding protein 6 protein contributes to resistance to trastuzumab in GC by regulating metabolic reprogram
S-phase kinase associated protein 2 (Skp2) is a constituent of the F-box protein family and functions as a substrate recognition component within the Skp2-SCF complex, which plays a crucial role in the regulation of ubiquitination, cell cycle progression, cell proliferation, and apoptosis[13]. Extensive evidence has demonstrated that Skp2 acts as an oncogene[14], exhibiting elevated expression levels in breast cancer[15], GC[16], prostate cancer[17], and various other malignant tumors, thereby exhibiting a strong association with poor outcomes in affected individuals. Recent studies have shown that Skp2 regulates glycolysis, trastuzumab sensitivity, and tumorigenesis in breast cancer[18]. However, whether Skp2 regulates trastuzumab sensitivity in GC is unknown.
Several investigations have been conducted on small structure-based inhibitors of Skp2. For instance, the compounds SZL-P1-41[19], SKPin C1[20,21], and DT204[22] were identified as Skp2 inhibitors that could suppress tumor growth. However, treatment with these chemical inhibitors is accompanied by adverse effects. Several natural compounds, such as diosmetin[23], safranal[24], dioscin[25], gartanin[26], betulinic acid[27], linichlorin A[28], and gentian violet[29], have been identified to function as potential antitumor agents through Skp2 inhibition. However, these studies are still in the preliminary stages of preclinical development.
This study proposes that the antipsychotic drug thioridazine can specifically decrease the expression of Skp2, thereby increasing the responsiveness of HER-2-positive GC cells to trastuzumab through the attenuation of glycolysis.
The human GC cell lines HGC-27, SGC-7901, MGC-803, MKN-45, and NCI-N87 were purchased from the American Type Culture Collection (Manassas, United States). HGC-27, NCI-N87, and MGC-803 cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS); Biological Industries, Israel). SGC-7901 and MKN-45 cells were grown in RPMI 1640 medium containing 10% FBS. Trastuzumab-resistant HGC-27 and SGC-7901 cells were established by culturing cells with increasing concentrations of trastuzumab (Roche, Switzerland) over half a year and were designated HGC-27-R and SGC-7901-R cells, respectively. All cells were cultured in incubators at 37 ℃ with 5% CO2. Thioridazine and lapatinib were obtained from Selleck Chemicals (United States).
Quantitative real-time reverse transcription polymerase chain reaction analysis using a Real-Time PCR Detection System (Agilent Technologies) was performed to validate the effect of thioridazine on SKP2 gene expression. The sequences of the primers used were as follows: 5’-ATGCCCCAATCTTGTCCATCT-3’ and 5’-CACCGACTGAGTGATAGGTGT-3’ for SKP2; 5’-GTGGGGCGCCCCAGGCACCA-3’ and 5’-CTTCCTTAATGTCACGCACGATTTC-3’ for β-actin.
The pcDNA3.1-3 × Flag-C plasmid carrying the Skp2 coding sequence was constructed. The sense primer sequence was 5'-CCGGAATTCCGGAGGATGCACAGGAAGCACCTCCAGGAG-3', and the antisense primer sequence was 5'-CCGCTCGAGTAGACAACTGGGCTTTTGCAGTGT-3'. The two recombinant plasmids confirmed to contain the correct sequence were named OX-SKP2-1 and OX-SKP2-2. Small interfering RNA (siRNA) for SKP2 and the negative control (NC) oligonucleotide sequence were synthesized by Sangon Biotech (Guangdong, China). The siRNA duplexes were transfected into HGC27-R cells using Lipofectamine 2000 (Invitrogen, United States).
A cell counting kit-8 (CCK-8), Japan, was used to assess cell proliferation. In brief, cell suspensions (5 × 103 cells/well) were seeded in 96-well plates in triplicate, and the plates were incubated for 48 h. Each well was filled with 10 µL of CCK-8 assay solution and incubated for 4 h. A microplate reader was used to measure the optical density at 450 nm.
Immunoblotting was performed using antibodies against the following proteins: Poly ADP-ribose polymerase (PARP); 9352, glucose transporter type 1 (Glut1); 73015, Skp2 (2652), p-signal transducer and activator of transcription 3 (9134p), p-AKT (4060), p-mammalian target of rapamycin (mTOR) (5336), and GAPDH (5174) (all obtained from Cell Signaling Technology, United States).
The 3D structure of thioridazine was obtained from the PubChem Substance database (https://www.ncbi.nlm.nih.gov/) by minimizing structural energy using the ChemBioDraw 3D module. The crystal structure of Skp2 was retrieved from the RCSB Protein Data Bank (PDB ID: 1fs2) and subsequently modified (dehydration and hydrogenation) using AutoDockTools 1.5.6 21 before being exported in pdbqt format. Following definition of the grid on the active site of the receptor protein, the docking procedure was executed using AutoDock Vina 1.1.2, and the output score was displayed in kcal/mol. PyMOL 2.3.0 and BIOVIA Discovery Studio were utilized in this process.
For apoptosis assays, the following steps were performed according to the instructions of the annexin V-FITC Apoptosis Detection Kit (Solarbio, China). Cells in each sample were washed, suspended in 100 µL of 1 × binding buffer and stained with 5 µL of FITC-labeled annexin V and 5 µL of PI for 5 min. Apoptotic cells were detected by a flow cytometer (BD, United States) at wavelengths of 488 nm and 630 nm.
HGC-27-R cells were exposed to dimethyl sulfoxide or thioridazine for 24 h, collected, washed with PBS containing protease inhibitors, aliquoted into PCR tubes, and heated in a thermal cycler (Bio-Rad, T100) at the indicated temperature for 3 min to denature proteins. The cells were then resuspended in NP40 buffer, subjected to three freeze-thaw cycles with liquid nitrogen, and centrifuged at 20000 × g for 20 min at 4 ℃. The supernatant was boiled in loading buffer for western blotting.
Cells were seeded in 12-well plates at 5 × 105 cells/well. After the cells were treated with different reagents for 48 h, the supernatant was collected. An amplex red glucose/glucose oxidase assay kit (Molecular Probes, Carlsbad, CA, United States) was used for glucose uptake measurements. A lactate assay kit (BioVision, Mountain View, CA, United States) was used to detect the production of lactate in the medium.
The animal procedures were approved by the Henan University Institutional Experimental Animal Care and Use Committee (ID: HUSOM2022-439). All experiments were designed and conducted in accordance with the Animal Research: Reporting of In Vivo Experiments guidelines; the United Kingdom Animals (Scientific Procedures) Act 1986 and associated guidelines; and the European Union (EU) Directive 2010/63/EU for animal experiments. Five-week-old male BALB/c athymic nude mice (weighing 16-18 g, SPF grade) were purchased from Peking Vital River Laboratory Animal Technology Company. Prior to use, all cages, bedding, and drinking water were sterilized. The cages, feed, and drinking water were replaced biweekly. The breeding environment adhered to the following specifications: temperature range from 20 to 26 ℃, humidity range from 40% to 70%, and light cycle consisting of 12 h of illumination followed by 12 h of darkness (lights activated from 8 am to 8 pm). A total of 3 × 106 HGC27-R or 1 × 106 SGC7901-R cells were injected subcutaneously (s.c.) into each mouse. The mice were randomly grouped into four groups with five mice in each group. The mice received either vehicle control, thioridazine (25 mg/kg), trastuzumab (5 mg/kg), lapatinib (70 mg/kg), and thioridazine (25 mg/kg) plus trastuzumab (5 mg/kg) or lapatinib (70 mg/kg) by intraperitoneal injection daily. After two weeks of drug administration, the mice were sacrificed, and tumor weights were determined.
Data were expressed as mean ± SD. Comparisons between two groups were performed using a t test. One-way ANOVA with the Bonferroni correction was used to analyze differences among three or more groups. Statistical significance was defined as a value of P < 0.05.
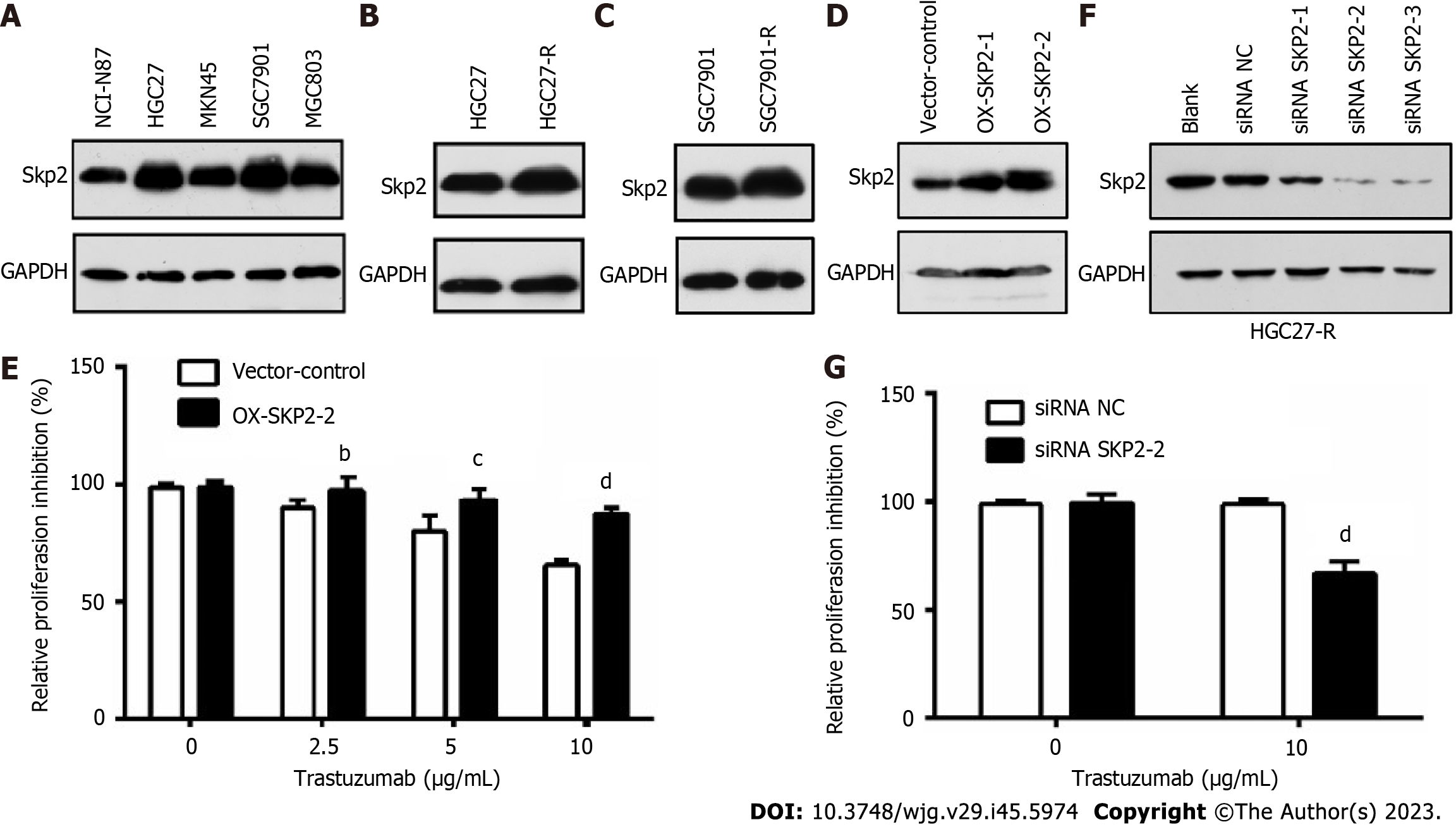
To ascertain the correlation between Skp2 expression and trastuzumab sensitivity in GC, Skp2 expression was analyzed by immunoblotting in a panel of human GC cell lines with HER2-positive status. Subsequently, the HGC27 and SGC7901 cell lines, exhibiting Skp2 overexpression, were selected for further examination (Figure 1A). Following chronic treatment with 10 μg/mL trastuzumab, two cell lines, HGC27-R and SGC7901-R, were identified as being more resistant to trastuzumab than their parental counterparts. Notably, the Skp2 level was higher in HGC27-R and SGC7901-R cells than in the corresponding parental HGC27 and SGC7901 cells (Figure 1B and C). HGC27 cells with exogenous Skp2 overexpression were employed to investigate the potential decrease in antiproliferative activity associated with upregulated Skp2 expression. The results indicated a decline in trastuzumab activity in isogenic stable Skp2 transfectants (HGC27-OX-SKP2-2 cells) (Figure 1D and E). Additionally, transfection of the SKP2-targeted siRNA effectively downregulated Skp2 expression and significantly enhanced trastuzumab activity in HGC27-R cells (Figure 1F and G). These findings suggest a significant relationship between Skp2 expression and trastuzumab insensitivity, highlighting the importance of Skp2 as a potential therapeutic target in GC.
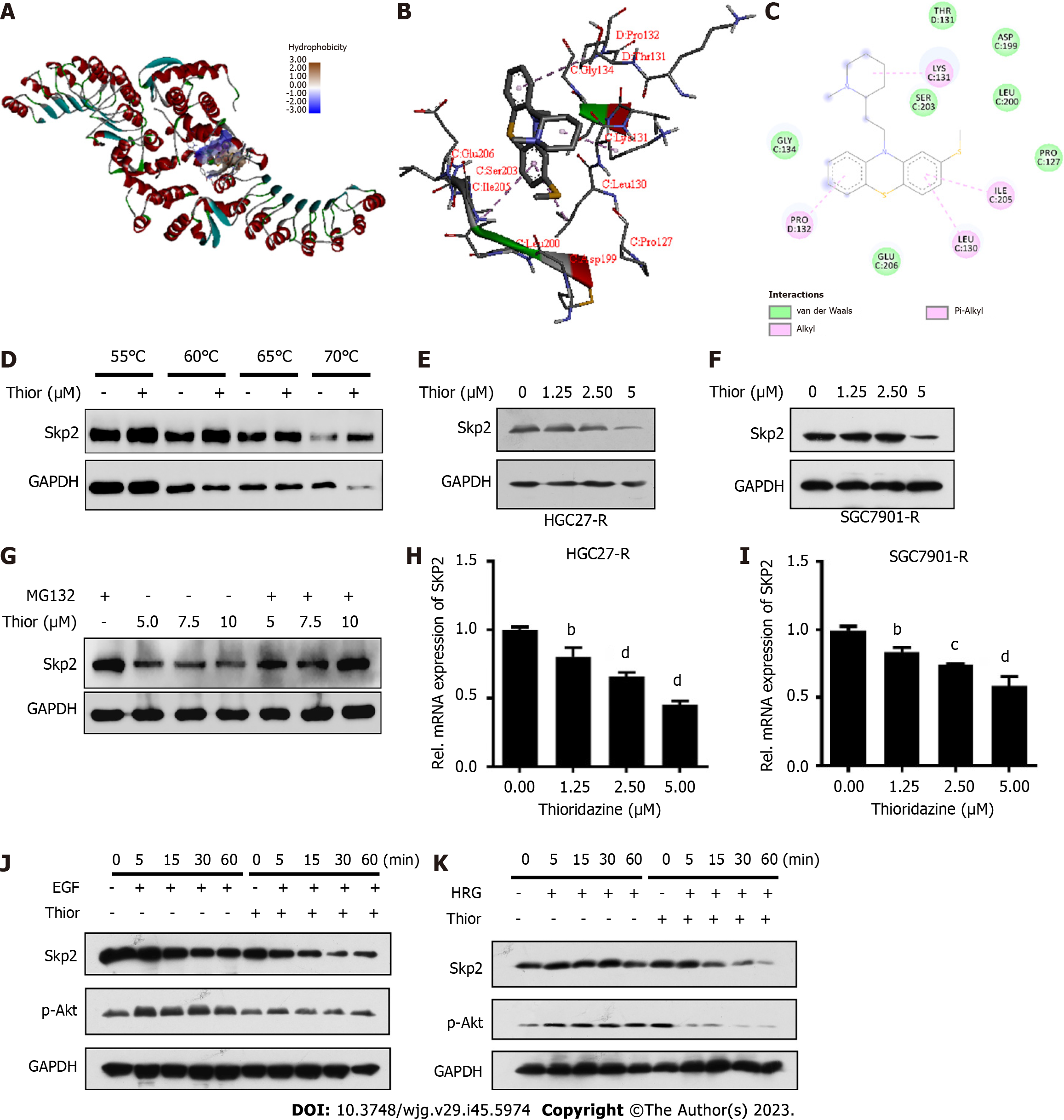
A docking analysis conducted with the drug repurposing compound library revealed that thioridazine exhibits favorable binding potential with Skp2. Thioridazine exhibits a high binding potential (affinity score: -7.0 kcal/mol) for the active pocket of Skp2, as shown in Figure 2A. Specifically, thioridazine engages in van der Waals, pi-alkyl and alkyl interactions with the branched-chain amino acids Leu130, Lys131, Pro132 and Ile205 in Skp2, resulting in a robust interaction between the ligand and the Skp2 protein (Figure 2B and C). Cellular thermal shift assay was performed to evaluate the binding affinity of thioridazine for the Skp2 protein. Administration of thioridazine increased the thermal stability of Skp2 but not of GAPDH (Figure 2D), implying a direct binding interaction between thioridazine and the Skp2 protein.
To explore the effect of thioridazine on Skp2 expression, HGC27-R and SGC7901-R cells were treated with varying concentrations of thioridazine (0, 1.25, 2.5, and 5 µM). Western blot analysis revealed a notable decrease in the expression level of Skp2 protein after treatment with thioridazine (Figure 2E and F). MG132 reversed the decrease in Skp2 protein expression induced by thioridazine, thereby indicating that thioridazine may increase the degradation of Skp2 via the ubiquitin–proteasome pathway (Figure 2G). Additionally, the mRNA expression level of SKP2 exhibited a dose-dependent decline as the concentration of thioridazine increased, as shown in Figure 2H and I. It has been established that Skp2 plays a role in governing the phosphorylation and activation of Akt in response to ErbB receptor signaling[18], and we found that the phosphorylation of Akt, which is stimulated by EGF and facilitated by Skp2, was abolished after thioridazine treatment (Figure 2J). In a similar vein, our observations also revealed that Skp2 expression and Akt phosphorylation induced by heregulin was effectively inhibited through the administration of thioridazine (Figure 2K). These results suggest that thioridazine can impede the Skp2-mediated Akt phosphorylation and activation induced by ligands of the ErbB2 family.
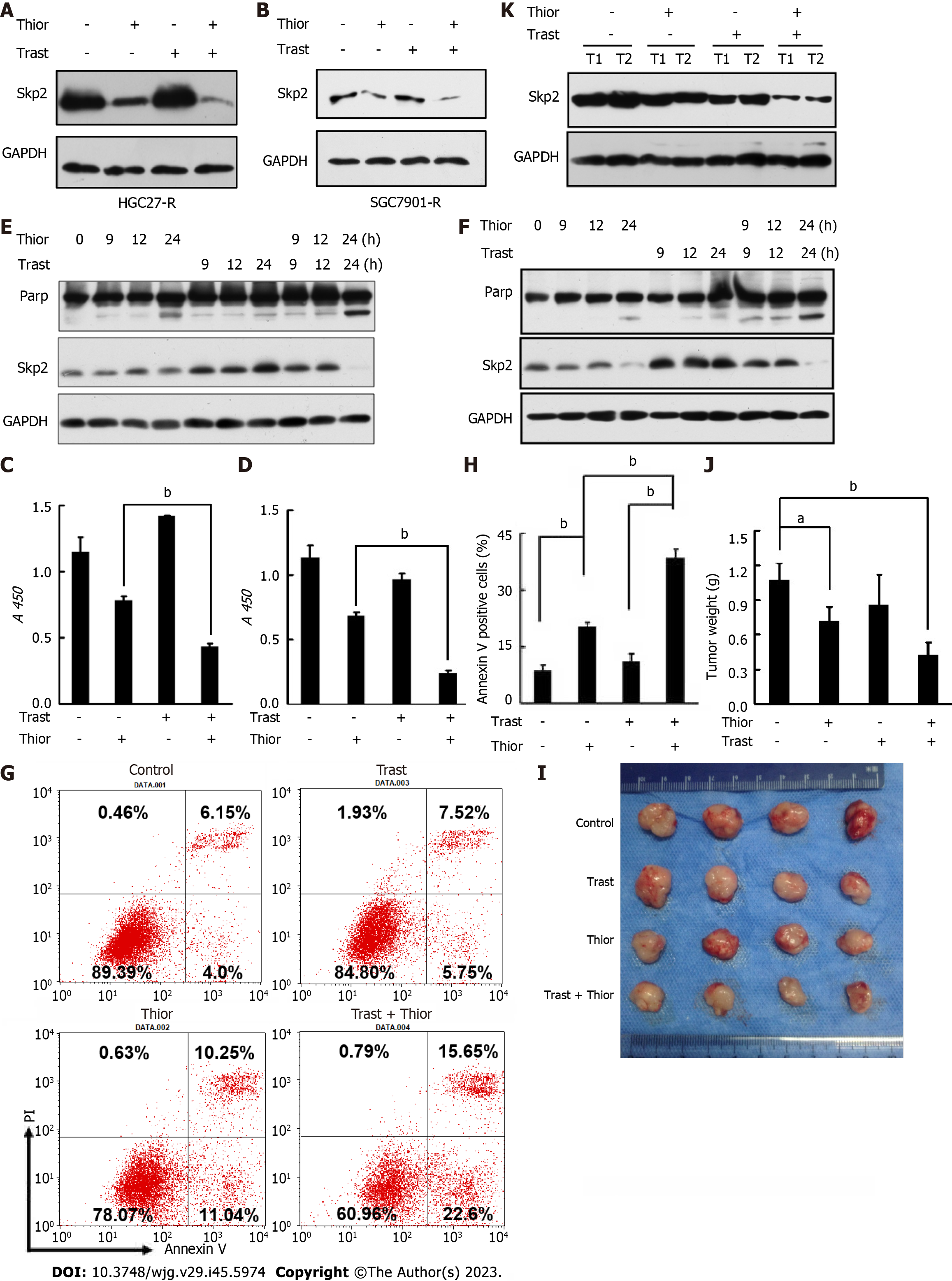
Furthermore, we verified that cotreatment with thioridazine and trastuzumab resulted in a further decrease in Skp2 expression (Figure 3A and B). We next assessed the effect of thioridazine on the proliferation and survival of HGC27-R and SGC7901-R cells. As shown in Figure 3C and D, thioridazine significantly decreased the viability and restored the trastuzumab sensitivity of HGC27-R and SGC7901-R cells. To determine whether thioridazine promotes apoptosis in trastuzumab-resistant cells, we treated HGC27-R and SGC7901-R cells with thioridazine and trastuzumab alone and in combination. PARP is a substrate of caspases. PARP splicing is a key indicator of apoptosis. As shown in Figure 3E and F, trastuzumab induced increased expression of Skp2 but not splicing of PARP. In contrast, combined treatment with thioridazine and trastuzumab increased PARP splicing. The rate of early apoptotic HGC27-R cells treated with thioridazine was 2-fold that of HGC27-R cells treated with trastuzumab, and the difference even increased to 4-fold thioridazine was combined with trastuzumab (Figure 3G and H). These data suggest that thioridazine and trastuzumab synergistically suppress the proliferation and decrease the survival of GC cells.
To investigate the efficacy of trastuzumab and thioridazine in vivo, we treated mice bearing HGC27-R xenografts with trastuzumab and thioridazine alone or in combination. As monotherapies, trastuzumab and thioridazine showed a limited effect on tumor growth, whereas combined administration of trastuzumab and thioridazine resulted in greater reductions in tumor volume and tumor weight (Figure 3I and J). However, lower expression of Skp2 was found in xenograft tissues of the combined administration group (Figure 3K). These data demonstrate that thioridazine enhances the antitumor activity of trastuzumab in vitro as well as in vivo.
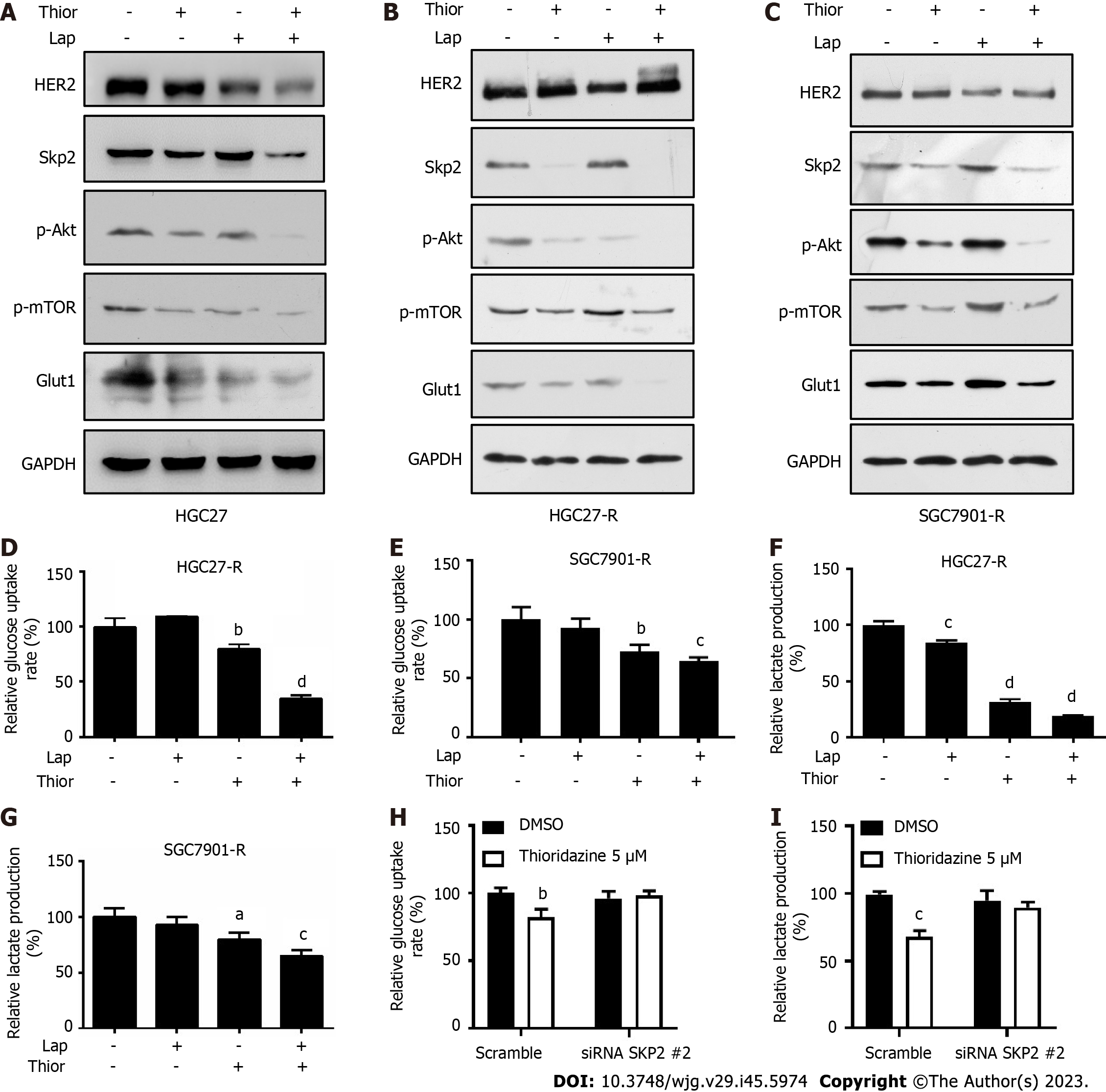
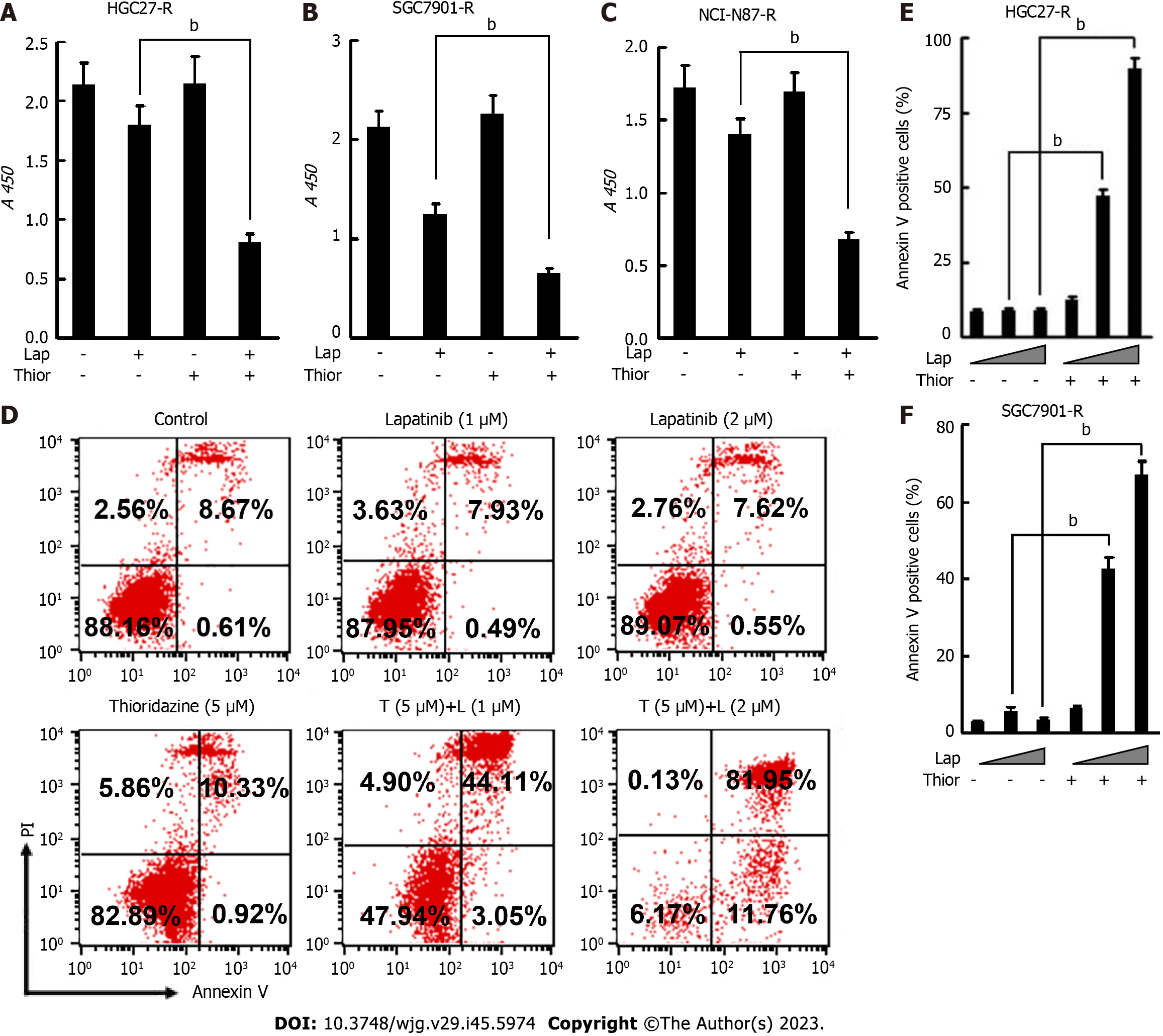
Trastuzumab-resistant advanced breast cancer responds to lapatinib, a dual tyrosine kinase inhibitor (TKI), according to the results of the phase III trial EGF104900 and the HER2CLIMB Randomized Clinical Trial[30,31]. However, the addition of lapatinib to a regimen of capecitabine and oxaliplatin did not improve overall survival (OS) in patients with HER2-amplified gastroesophageal adenocarcinoma[32]. In addition, lapatinib plus paclitaxel demonstrated activity in the second-line treatment of patients with HER2 FISH-positive IHC3+ advanced GC but did not significantly improve OS in the intent-to-treat population[33]. Here, we explored the effect of combination treatment with lapatinib and thioridazine on the glycolytic phenotype in GC cells. The combination of thioridazine and lapatinib completely abolished Skp2 expression (Figure 4A-C). It has been reported that Skp2 regulates glycolysis by inducing Glut1 expression and Akt/mTOR pathway activation[34,35]. The decreased protein levels of p-Akt, p-mTOR, and Glut1 were consistent with the downregulation of Skp2 (Figure 4A-C). Furthermore, both glucose uptake and lactate production were decreased more apparently in HGC27-R and SGC7901-R cells treated with thioridazine and lapatinib together than in those treated with either alone (Figure 4D-G). Compared to siRNA-NC-transfected cells, HGC27 cells transfected with SKP2-2 siRNA did not show inhibitory effects of thioridazine on glucose uptake or lactate production (Figure 4H and I). These results indicate that in combination, thioridazine and lapatinib can markedly suppress glycolysis by downregulating Skp2 in trastuzumab-resistant GC cells.
We further examined the anticancer effect of thioridazine combined with lapatinib. As shown in Figure 5A-C, thioridazine significantly enhanced the anti-proliferative activity of lapatinib in GC cells (HGC27-R, SGC-7901, and NCI-N87-R). Thioridazine (5 µM) in combination with lapatinib (1 or 2 µM) markedly increased apoptosis in HGC27-R and SGC7901-R cells (Figure 5D-F).
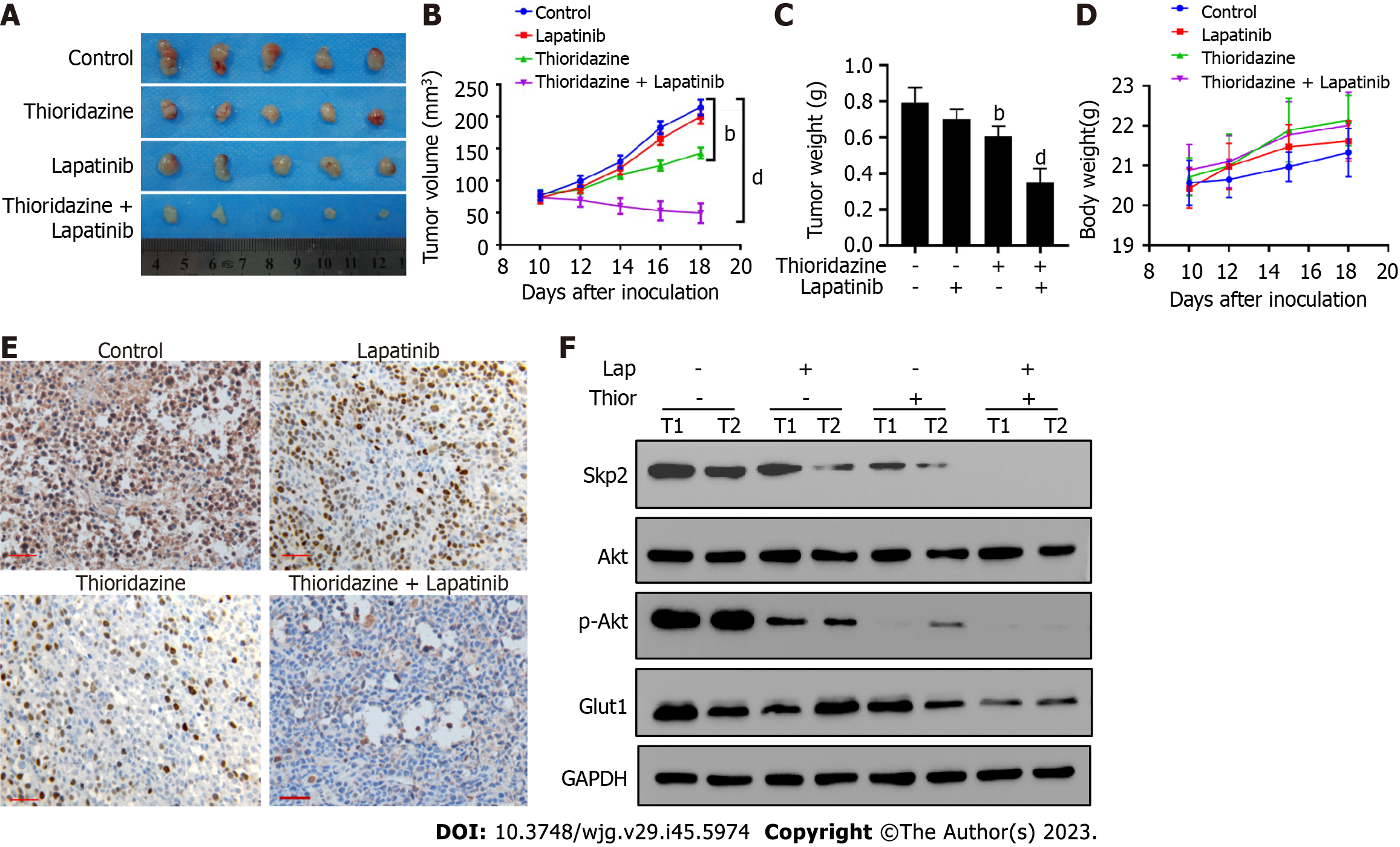
Moreover, Figure 6A-C shows that combined administration of thioridazine and lapatinib strongly decreased the growth, volume, and weight of tumors in mice bearing SGC7901-R xenografts. No toxic or side effects were observed during the administration period, and the weight of the mice did not significantly decrease (Figure 6D). Lower expression of Skp2 was found in the combined thioridazine and lapatinib treatment group than in the other groups (Figure 6E). The protein levels of Skp2, p-Akt, and Glut1 were greatly decreased in xenograft tissues from mice treated with both thioridazine and lapatinib (Figure 6F). These data demonstrate that thioridazine enhances the antitumor activity of lapatinib by inhibiting Skp2 expression in vitro and in vivo.
Our study demonstrates that thioridazine can overcome trastuzumab resistance by blocking glycolysis, growth, and apoptosis resistance by downregulating the expression of Skp2 in GC cells.
It has been confirmed that HER-2 has the most outstanding clinical significance in advanced GC. However, targeting HER-2 in advanced GC remains challenging due to the high heterogeneity and subsequent resistance caused by prolonged therapy. Despite the development of numerous HER2-targeted drugs, including antibody-drug conjugates, TKIs, bispecific antibodies, vaccines, and immune checkpoint inhibitors, to combat trastuzumab resistance in HER2-positive breast cancer, the efficacy of these treatments in HER2-positive GC remains uncertain.
Trastuzumab combined with palbociclib, a CDK4/6 inhibitor, was demonstrated to yield favorable survival outcomes in patients with advanced breast cancer[36,37]. Multiple studies have demonstrated that the simultaneous administration of supplementary inhibitors, including figitumumab (an insulin-like growth factor 1 receptor inhibitor), ipatasertib (an AKT inhibitor), and MK2206 (another AKT inhibitor), in individuals with HER2-overexpressing tumors increases the efficacy of trastuzumab[38-40]. However, additional extensive research is needed for clinical incorporation of these agents. In contrast to the aforementioned drugs currently under development, the expedited introduction of approved drugs for new therapeutic applications could be facilitated in the clinical market. For example, the potential of combining trastuzumab with metformin as an innovative adjuvant therapy for HER-2-positive breast cancer is being investigated in an ongoing phase II clinical trial[41]. Our findings in the current study highlight the ability of thioridazine to augment the effect of trastuzumab in GC.
The antipsychotic drug thioridazine was first discovered as a phenothiazine-type piperidine drug. In 2013, Sachlos et al[42] first discovered that thioridazine can induce the differentiation of acute myeloid leukemia cells and breast cancer stem cells and increase sensitivity to doxorubicin without affecting the function of normal hematopoietic stem cells. It has been shown that thioridazine can inhibit the expression of a multidrug resistance protein (P-gp) and increase sensitivity to chemotherapy drugs in glioblastoma[43]. In addition to inducing reactive oxygen species accumulation and DNA damage, thioridazine can increase autophagy and apoptosis in ovarian cancer cells[44]. It reduces ovarian cancer angiogenesis by inhibiting vascular endothelial growth factor receptor-2, PI3K, and mTOR signaling[45]. Recent studies have demonstrated that thiolidazine exhibits the potential to augment the susceptibility of glioblastoma cells towards temozolomide through the inhibition of autophagy[46]. Furthermore, the concurrent administration of thiazidine and oxaliplatin has been found to stimulate immunogenic cell death in colon cancer by inducing endoplasmic reticulum stress[47]. In this study, we first revealed that thioridazine can inhibit glycolysis in GC by downregulating Skp2 expression at both the transcriptional and translational levels. However, further evidence is needed to determine whether the main regulatory mechanism by which thioridazine regulates the expression and function of Skp2 is mediated through transcriptional inhibition, posttranslational inhibition, or blockade by protein interactions. The effectiveness of combining thioridazine with other HER-2- or non-HER-2-targeted drugs, such as neratinib, tucatinib, and apatinib, in treating trastuzumab-resistant GC remains uncertain.
Based on our findings, Skp2 may serve as a predictor of the trastuzumab response in patients with HER-2-positive GC, thereby aiding in identifying the patient subgroups most likely to benefit from HER-2-targeted therapies. Additionally, our study underscores the advantageous impacts of thioridazine when used in combination with synergistic drugs for the management of GC. These findings offer valuable support for future clinical investigations aimed at exploring the potential of thioridazine as a viable treatment option for GC. Notably, HER-2-targeted drugs possess dosage-independent potential for cardiac toxicity. In addition, thioridazine has been reported to be associated with arrhythmias in a minority of schizophrenia patients. Consequently, when considering the administration of these drugs individually or in combination, it becomes imperative to exclude individuals with preexisting heart conditions and implement vigilant cardiac surveillance.
In conclusion, our data demonstrate that thioridazine combined with lapatinib exhibits synergistic effects in impeding cell proliferation, inducing apoptosis, and suppressing tumor growth in GC. This study provides an experimental foundation for the potential utilization of thioridazine in overcoming trastuzumab resistance in GC. The objective of this study is to offer potential drug selection and administration strategies for patients with advanced GC who exhibit resistance to trastuzumab. Furthermore, these findings offer novel insights for the future investigation of Skp2 inhibitors.
The drug resistance observed in patients with human epidermal growth factor receptor 2 (HER-2)-positive advanced gastric cancer (GC) treated with trastuzumab is a significant concern, as no established targeted therapy regimen for use after the development of drug resistance is available. S-phase kinase associated protein 2 (Skp2) has been identified as a crucial target for GC treatment; however, the development of new drugs targeting Skp2 remains a considerable challenge.
To investigate potential pharmacological interventions targeting Skp2 to increase the efficacy of subsequent therapies for patients with HER-2-positive GC who have developed resistance to trastuzumab.
This study aims to elucidate the inhibitory effect of thioridazine on Skp2 expression and to preliminarily assess the potential of thioridazine in reversing the resistance of HER2-positive GC cells to trastuzumab through both in vivo and in vitro experiments.
The impact of altering the Skp2 protein expression level through overexpression or knockdown on the sensitivity of HER2-positive GC cells to trastuzumab was assessed using a cell counting kit-8 assay. The influence of thioridazine on Skp2 protein expression was demonstrated through computational docking analysis and Cellular Thermal Shift Assay. Flow cytometry, a glucose uptake assay, a lactate production assay, and xenograft experiments in nude mice were employed to evaluate the effects of thioridazine alone or in combination with trastuzumab and lapatinib on the cell cycle, apoptosis, glucose metabolism, and tumor growth.
Trastuzumab sensitivity can be increased in HER-2-positive GC cells through negative modulation of Skp2 expression. Thioridazine can selectively inhibit Skp2 expression and the protein kinase B/mammalian target of rapamycin signaling pathway. Thioridazine combined with lapatinib effectively reverses trastuzumab resistance in GC cells by diminishing glycolysis.
Combining thioridazine with lapatinib is a potential strategy to reverse trastuzumab resistance in GC by suppressing Skp2 expression.
Further investigation into the optimal combination ratio, initial dosage, and dose-response correlation between thioridazine and lapatinib in GC xenograft models will contribute to the development of more precise drug reference protocols for subsequent clinical trials. A new therapeutic strategy for the management of GC by simultaneous targeting of Skp2 and HER-2 could be introduced.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Melo FF, Brazil; Kotelevets SM, Russia S-Editor: Qu XL L-Editor: A P-Editor: Zhao S
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55774] [Article Influence: 7967.7] [Reference Citation Analysis (132)] |
| 2. | Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: An update. World J Gastroenterol. 2016;22:4619-4625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 155] [Cited by in RCA: 207] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 3. | Kurokawa Y, Matsuura N, Kimura Y, Adachi S, Fujita J, Imamura H, Kobayashi K, Yokoyama Y, Shaker MN, Takiguchi S, Mori M, Doki Y. Multicenter large-scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer. Gastric Cancer. 2015;18:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Liang JW, Zhang JJ, Zhang T, Zheng ZC. Clinicopathological and prognostic significance of HER2 overexpression in gastric cancer: a meta-analysis of the literature. Tumour Biol. 2014;35:4849-4858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5309] [Article Influence: 353.9] [Reference Citation Analysis (3)] |
| 6. | Saeki H, Oki E, Kashiwada T, Arigami T, Makiyama A, Iwatsuki M, Narita Y, Satake H, Matsuda Y, Sonoda H, Shimokawa M, Maehara Y; Kyushu Study Group of Clinical Cancer (KSCC). Re-evaluation of HER2 status in patients with HER2-positive advanced or recurrent gastric cancer refractory to trastuzumab (KSCC1604). Eur J Cancer. 2018;105:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Gomez-Martín C, Lopez-Rios F, Aparicio J, Barriuso J, García-Carbonero R, Pazo R, Rivera F, Salgado M, Salud A, Vázquez-Sequeiros E, Lordick F. A critical review of HER2-positive gastric cancer evaluation and treatment: from trastuzumab, and beyond. Cancer Lett. 2014;351:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Warburg O. On the origin of cancer cells. Science. 1956;123:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9117] [Cited by in RCA: 9914] [Article Influence: 143.7] [Reference Citation Analysis (0)] |
| 9. | Chang J, Wang Q, Bhetuwal A, Liu W. Metabolic pathways underlying GATA6 regulating Trastuzumab resistance in Gastric Cancer cells based on untargeted metabolomics. Int J Med Sci. 2020;17:3146-3164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Liu J, Pan C, Guo L, Wu M, Guo J, Peng S, Wu Q, Zuo Q. A new mechanism of trastuzumab resistance in gastric cancer: MACC1 promotes the Warburg effect via activation of the PI3K/AKT signaling pathway. J Hematol Oncol. 2016;9:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 11. | Wang J, Huang Q, Hu X, Zhang S, Jiang Y, Yao G, Hu K, Xu X, Liang B, Wu Q, Ma Z, Wang Y, Wang C, Wu Z, Rong X, Liao W, Shi M. Disrupting Circadian Rhythm via the PER1-HK2 Axis Reverses Trastuzumab Resistance in Gastric Cancer. Cancer Res. 2022;82:1503-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 12. | Fan T, Sun G, Sun X, Zhao L, Zhong R, Peng Y. Tumor Energy Metabolism and Potential of 3-Bromopyruvate as an Inhibitor of Aerobic Glycolysis: Implications in Tumor Treatment. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 13. | Asmamaw MD, Liu Y, Zheng YC, Shi XJ, Liu HM. Skp2 in the ubiquitin-proteasome system: A comprehensive review. Med Res Rev. 2020;40:1920-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 14. | Wang H, Cui J, Bauzon F, Zhu L. A comparison between Skp2 and FOXO1 for their cytoplasmic localization by Akt1. Cell Cycle. 2010;9:1021-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Radke S, Pirkmaier A, Germain D. Differential expression of the F-box proteins Skp2 and Skp2B in breast cancer. Oncogene. 2005;24:3448-3458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Masuda TA, Inoue H, Sonoda H, Mine S, Yoshikawa Y, Nakayama K, Mori M. Clinical and biological significance of S-phase kinase-associated protein 2 (Skp2) gene expression in gastric carcinoma: modulation of malignant phenotype by Skp2 overexpression, possibly via p27 proteolysis. Cancer Res. 2002;62:3819-3825. [PubMed] |
| 17. | Wang Z, Gao D, Fukushima H, Inuzuka H, Liu P, Wan L, Sarkar FH, Wei W. Skp2: a novel potential therapeutic target for prostate cancer. Biochim Biophys Acta. 2012;1825:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Chan CH, Li CF, Yang WL, Gao Y, Lee SW, Feng Z, Huang HY, Tsai KK, Flores LG, Shao Y, Hazle JD, Yu D, Wei W, Sarbassov D, Hung MC, Nakayama KI, Lin HK. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell. 2012;149:1098-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 322] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 19. | Chan CH, Morrow JK, Li CF, Gao Y, Jin G, Moten A, Stagg LJ, Ladbury JE, Cai Z, Xu D, Logothetis CJ, Hung MC, Zhang S, Lin HK. Pharmacological inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem cell traits and cancer progression. Cell. 2013;154:556-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 323] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 20. | Zhao H, Iqbal NJ, Sukrithan V, Nicholas C, Xue Y, Yu C, Locker J, Zou J, Schwartz EL, Zhu L. Targeted Inhibition of the E3 Ligase SCF(Skp2/Cks1) Has Antitumor Activity in RB1-Deficient Human and Mouse Small-Cell Lung Cancer. Cancer Res. 2020;80:2355-2367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Zhao H, Pan H, Wang H, Chai P, Ge S, Jia R, Fan X. SKP2 targeted inhibition suppresses human uveal melanoma progression by blocking ubiquitylation of p27. Onco Targets Ther. 2019;12:4297-4308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Malek E, Abdel-Malek MA, Jagannathan S, Vad N, Karns R, Jegga AG, Broyl A, van Duin M, Sonneveld P, Cottini F, Anderson KC, Driscoll JJ. Pharmacogenomics and chemical library screens reveal a novel SCF(SKP2) inhibitor that overcomes Bortezomib resistance in multiple myeloma. Leukemia. 2017;31:645-653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Liu Y, Zhou Z, Yan J, Wu X, Xu G. Diosgenin Exerts Antitumor Activity via Downregulation of Skp2 in Breast Cancer Cells. Biomed Res Int. 2020;2020:8072639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Jiang X, Li Y, Feng JL, Nik Nabil WN, Wu R, Lu Y, Liu H, Xi ZC, Xu HX. Safrana l Prevents Prostate Cancer Recurrence by Blocking the Re-activation of Quiescent Cancer Cells via Downregulation of S-Phase Kinase-Associated Protein 2. Front Cell Dev Biol. 2020;8:598620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Zhou L, Yu X, Li M, Gong G, Liu W, Li T, Zuo H, Li W, Gao F, Liu H. Cdh1-mediated Skp2 degradation by dioscin reprogrammes aerobic glycolysis and inhibits colorectal cancer cells growth. EBioMedicine. 2020;51:102570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Pham V, Rendon R, Le VX, Tippin M, Fu DJ, Le TH, Miller M, Agredano E, Cedano J, Zi X. Gartanin is a novel NEDDylation inhibitor for induction of Skp2 degradation, FBXW2 expression, and autophagy. Mol Carcinog. 2020;59:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | He DH, Chen YF, Zhou YL, Zhang SB, Hong M, Yu X, Wei SF, Fan XZ, Li SY, Wang Q, Lu Y, Liu YQ. Phytochemical library screening reveals betulinic acid as a novel Skp2-SCF E3 ligase inhibitor in non-small cell lung cancer. Cancer Sci. 2021;112:3218-3232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Estévez-Sarmiento F, Saavedra E, Ruiz-Estévez M, León F, Quintana J, Brouard I, Estévez F. Chlorinated Guaiane-Type Sesquiterpene Lactones as Cytotoxic Agents against Human Tumor Cells. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Ooi LC, Watanabe N, Futamura Y, Sulaiman SF, Darah I, Osada H. Identification of small molecule inhibitors of p27(Kip1) ubiquitination by high-throughput screening. Cancer Sci. 2013;104:1461-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J, Baselga J, O'Shaughnessy J. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 706] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 31. | Blackwell KL, Burstein HJ, Sledge GW. Updated survival analysis of a randomized study of lapatinib alone or in combination with trastuzumab in women with HER2-positive metastatic breast cancer progressing on trastuzumab therapy. Cancer Res. 2009;69 Suppl 24:61. [DOI] [Full Text] |
| 32. | Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero A, Salman P, Li J, Protsenko SA, Wainberg ZA, Buyse M, Afenjar K, Houé V, Garcia A, Kaneko T, Huang Y, Khan-Wasti S, Santillana S, Press MF, Slamon D. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC--A Randomized Phase III Trial. J Clin Oncol. 2016;34:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 476] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 33. | Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, Tsuji A, Omuro Y, Li J, Wang JW, Miwa H, Qin SK, Chung IJ, Yeh KH, Feng JF, Mukaiyama A, Kobayashi M, Ohtsu A, Bang YJ. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol. 2014;32:2039-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 488] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 34. | Barthel A, Okino ST, Liao J, Nakatani K, Li J, Whitlock JP Jr, Roth RA. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J Biol Chem. 1999;274:20281-20286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 255] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 460] [Cited by in RCA: 456] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 36. | Gianni L, Bisagni G, Colleoni M, Del Mastro L, Zamagni C, Mansutti M, Zambetti M, Frassoldati A, De Fato R, Valagussa P, Viale G. Neoadjuvant treatment with trastuzumab and pertuzumab plus palbociclib and fulvestrant in HER2-positive, ER-positive breast cancer (NA-PHER2): an exploratory, open-label, phase 2 study. Lancet Oncol. 2018;19:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 37. | Ciruelos E, Villagrasa P, Pascual T, Oliveira M, Pernas S, Paré L, Escrivá-de-Romaní S, Manso L, Adamo B, Martínez E, Cortés J, Vazquez S, Perelló A, Garau I, Melé M, Martínez N, Montaño A, Bermejo B, Morales S, Echarri MJ, Vega E, González-Farré B, Martínez D, Galván P, Canes J, Nuciforo P, Gonzalez X, Prat A. Palbociclib and Trastuzumab in HER2-Positive Advanced Breast Cancer: Results from the Phase II SOLTI-1303 PATRICIA Trial. Clin Cancer Res. 2020;26:5820-5829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 38. | Chakraborty AK, Zerillo C, DiGiovanna MP. In vitro and in vivo studies of the combination of IGF1R inhibitor figitumumab (CP-751,871) with HER2 inhibitors trastuzumab and neratinib. Breast Cancer Res Treat. 2015;152:533-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Laterza MM, Ciaramella V, Facchini BA, Franzese E, Liguori C, De Falco S, Coppola P, Pompella L, Tirino G, Berretta M, Montella L, Facchini G, Ciardiello F, de Vita F. Enhanced Antitumor Effect of Trastuzumab and Duligotuzumab or Ipatasertib Combination in HER-2 Positive Gastric Cancer Cells. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Hudis C, Swanton C, Janjigian YY, Lee R, Sutherland S, Lehman R, Chandarlapaty S, Hamilton N, Gajria D, Knowles J, Shah J, Shannon K, Tetteh E, Sullivan DM, Moreno C, Yan L, Han HS. A phase 1 study evaluating the combination of an allosteric AKT inhibitor (MK-2206) and trastuzumab in patients with HER2-positive solid tumors. Breast Cancer Res. 2013;15:R110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Rocca A, Cortesi P, Cortesi L, Gianni L, Matteucci F, Fantini L, Maestri A, Giunchi DC, Cavanna L, Ciani R, Falcini F, Bagni A, Meldoli E, Dall'Agata M, Volpi R, Andreis D, Nanni O, Curcio A, Lucchi L, Amadori D, Fedeli A. Phase II study of liposomal doxorubicin, docetaxel and trastuzumab in combination with metformin as neoadjuvant therapy for HER2-positive breast cancer. Ther Adv Med Oncol. 2021;13:1758835920985632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Sachlos E, Risueño RM, Laronde S, Shapovalova Z, Lee JH, Russell J, Malig M, McNicol JD, Fiebig-Comyn A, Graham M, Levadoux-Martin M, Lee JB, Giacomelli AO, Hassell JA, Fischer-Russell D, Trus MR, Foley R, Leber B, Xenocostas A, Brown ED, Collins TJ, Bhatia M. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149:1284-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 381] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 43. | Choi AR, Kim JH, Yoon S. Thioridazine specifically sensitizes drug-resistant cancer cells through highly increase in apoptosis and P-gp inhibition. Tumour Biol. 2014;35:9831-9838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Yong M, Yu T, Tian S, Liu S, Xu J, Hu J, Hu L. DR2 blocker thioridazine: A promising drug for ovarian cancer therapy. Oncol Lett. 2017;14:8171-8177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Park MS, Dong SM, Kim BR, Seo SH, Kang S, Lee EJ, Lee SH, Rho SB. Thioridazine inhibits angiogenesis and tumor growth by targeting the VEGFR-2/PI3K/mTOR pathway in ovarian cancer xenografts. Oncotarget. 2014;5:4929-4934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 46. | Johannessen TC, Hasan-Olive MM, Zhu H, Denisova O, Grudic A, Latif MA, Saed H, Varughese JK, Røsland GV, Yang N, Sundstrøm T, Nordal A, Tronstad KJ, Wang J, Lund-Johansen M, Simonsen A, Janji B, Westermarck J, Bjerkvig R, Prestegarden L. Thioridazine inhibits autophagy and sensitizes glioblastoma cells to temozolomide. Int J Cancer. 2019;144:1735-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 47. | Tran TH, Kao M, Liu HS, Hong YR, Su Y, Huang CF. Repurposing thioridazine for inducing immunogenic cell death in colorectal cancer via eIF2α/ATF4/CHOP and secretory autophagy pathways. Cell Commun Signal. 2023;21:184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |