Published online Nov 28, 2023. doi: 10.3748/wjg.v29.i44.5935
Peer-review started: September 19, 2023
First decision: October 8, 2023
Revised: October 15, 2023
Accepted: November 14, 2023
Article in press: November 14, 2023
Published online: November 28, 2023
Processing time: 68 Days and 5.5 Hours
Esophageal carcinoma is a highly aggressive digestive cancer responsible for a notable proportion of cancer-related deaths worldwide. Its elevated metastatic rate contributes to a poor prognosis in affected patients. In this case review, we aim to summarize the metastatic characteristics of intramural gastric metastasis (IGM) in mucosal esophageal squamous carcinoma.
A 56-year-old man was admitted to our hospital because of a dry cough with an esophageal sensation for one year. Endoscopic examination revealed a 2.0 cm 1.0 cm, superficial esophageal squamous cell carcinoma, and the patient underwent endoscopic submucosal dissection (ESD). Fifteen months after ESD, positron emission tomography/computed tomography revealed that the metabolism of the stomach cardia wall had increased slightly. However, the mucosa of the gastric cardia was smooth under gastroendoscopy. Two years after ESD, endoscopic examination revealed a giant gastric cardia carcinoma, while the esophageal mucosa was smooth, and no advanced cancer was found. A biopsy of the gastric cardia indicated squamous-cell carcinoma. The patient received immunochemotherapy and radiotherapy for esophageal cancer for 8 mo and is currently under follow-up.
Early-stage esophageal carcinoma with IGM is rare. Despite the ESD of the primary lesion, IGM may still occur and should be closely monitored after ESD.
Core Tip: This study presents a rare case of mucosal esophageal squamous cell carcinoma (ESCC) post endoscopic submucosal dissection with giant gastric cardia metastasis. intramural gastric metastasis has also been reported to plays a role in distant metastasis, particularly in the liver. Preoperative and postoperative endoscopic follow-up of patients with ESCC of any stage, although the depth of an esophageal cancer may be T1.
- Citation: Yang MQ, Sun MJ, Zhang HJ. Mucosal esophageal carcinoma following endoscopic submucosal dissection with giant gastric metastasis: A case report and review of literature. World J Gastroenterol 2023; 29(44): 5935-5944
- URL: https://www.wjgnet.com/1007-9327/full/v29/i44/5935.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i44.5935
Globally, esophageal squamous cell carcinoma (SCC) (ESCC) is the sixth leading cause of cancer-related deaths. In Asian countries, SCC is the most common pathological type of esophageal cancer. The prognosis of ESCC is poor owing to its increased tendencyfor direct spread and metastasis. The 5-year survival rate of ESCC patients with metastatic disease is less than 5%[1]. ESCC often metastasizes to the lymph nodes and distant organs, and intramural metastasis (IM) occasionally occurs, which is seen in approximately 10% of esophageal carcinomas[2,3]. IM is a form that tumor can occur as a skip lesion in the other parts of esophagus or to the stomach. IM incidence in ESCC in the head and neck is 25%, compared with 11% in stage I ESCC[4]. However, the reported intramural gastric metastasis (IGM) is seldom, accounting for only 1%–4.58% of ESCC through surgical treatment[5-9]. A higher incidence of IGM has been reported in the advanced ESCC, whereas only a few cases of IGM have been reported in the early-stage ESCC[10-13]. Herein, we report a case of mucosal esophageal carcinoma with multi-pathway and multi-organ metastases (including IGM, lymphatic metastasis of celiac lymph nodes, and hematogenous metastasis of the liver and right adrenal gland) that occurred in a metachronous manner, while the IGM progressed rapidly over 1 year. We hope that this case will guideclinicians in identifying unique metastasis of ESCC in some cases when making a treatment plan.
In August 2020, a 56-year-old man was admitted to our hospital for a dry cough with an esophageal sensation.
The symptoms started 1 year before presentation.
The patient had no relevant past illness.
The patient had a smoking history for 20 years, with 20 cigarettes daily and drinking approximately 4000 mL of beer 3–4 times a week for 20 years.
There was no abnormal in physical examination.
All blood test results, including tumor markers, were within normal limits.
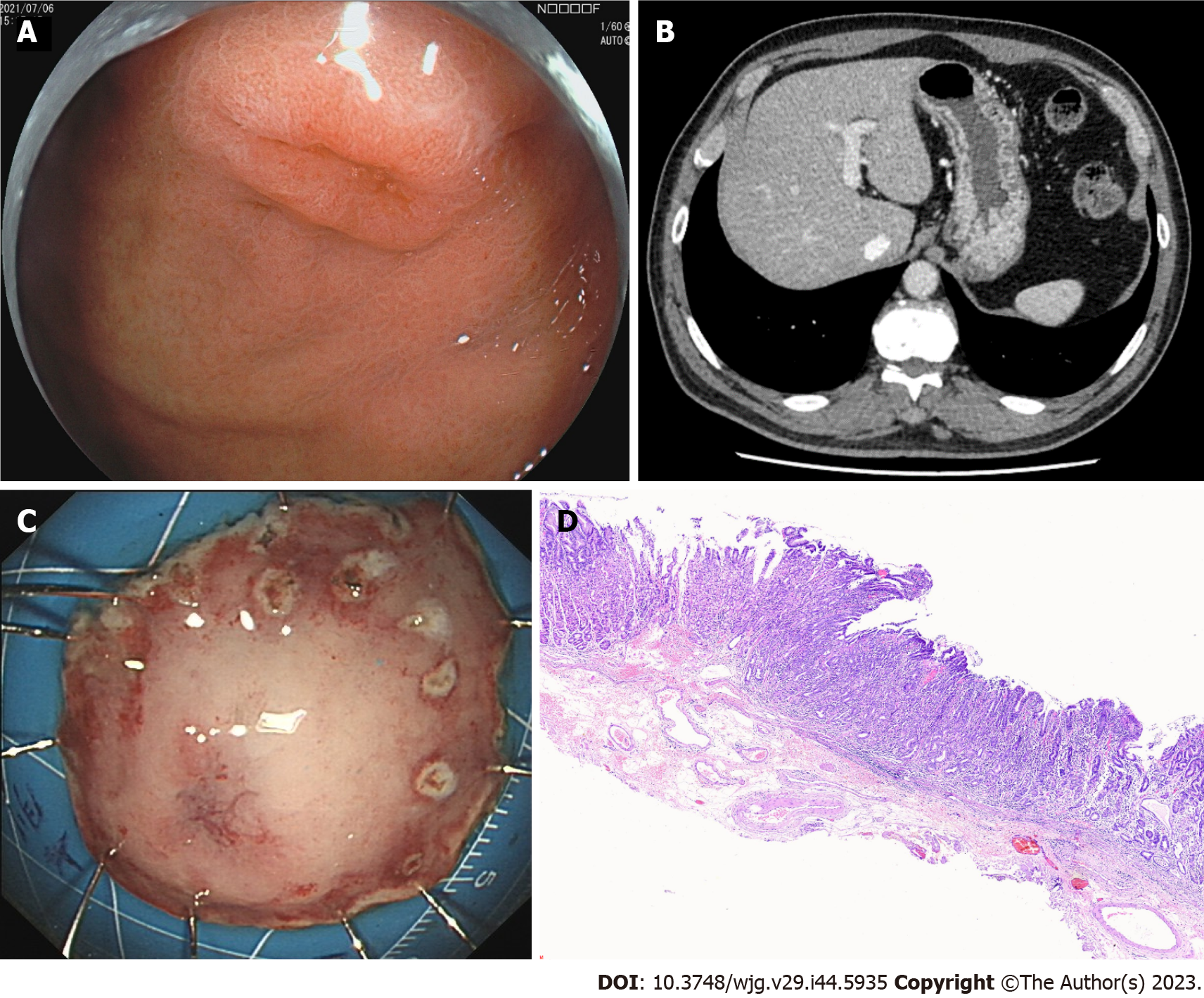
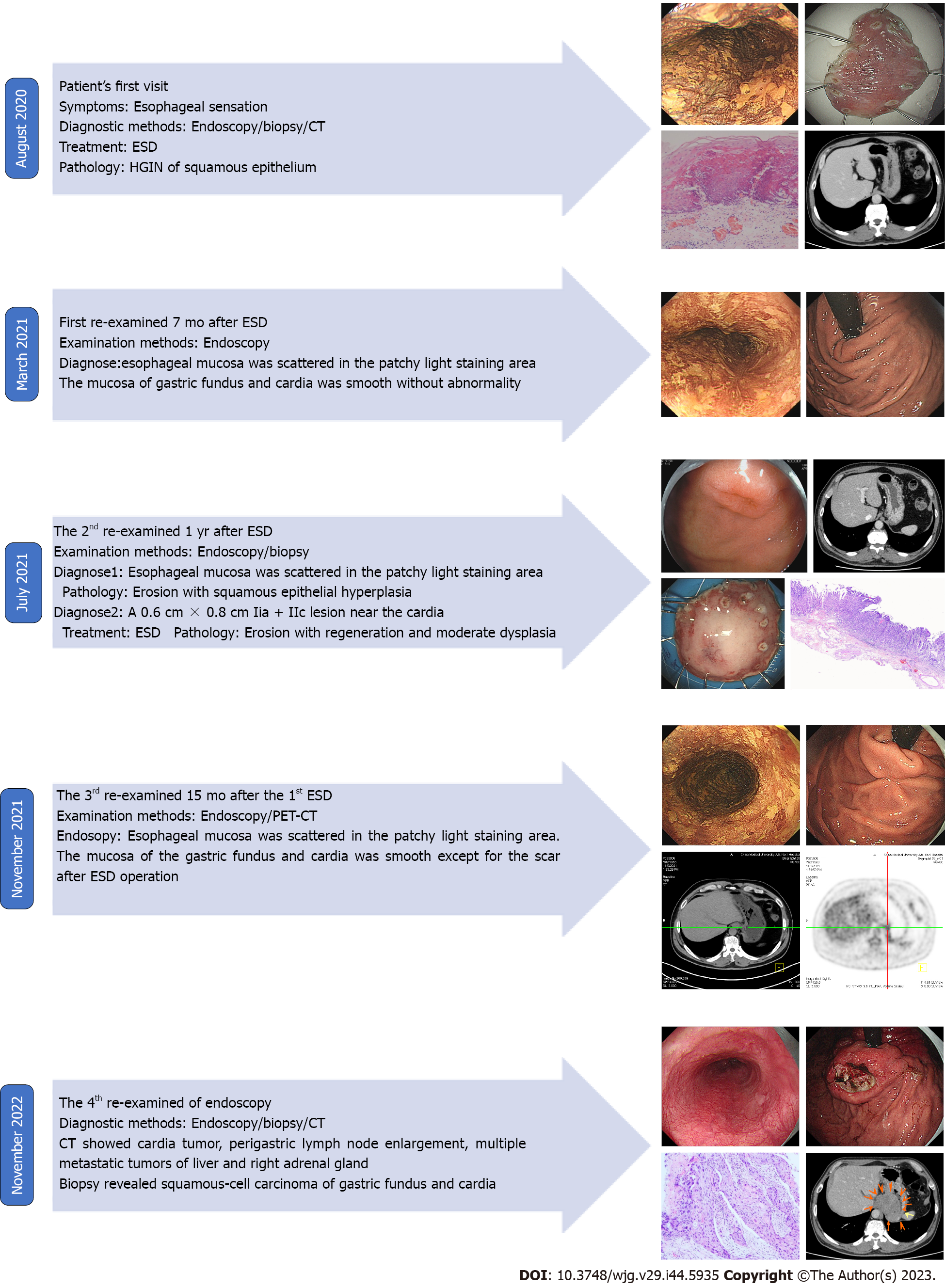
Gastroendoscopy showed a 2.0 cm × 1.0 cm, superficial, flat, slightly rough, and red esophageal lesion 30 cm from the incisor teeth. Magnifying endoscopy with narrow-band imaging observations showed that the background mucus was brown, and the intrapapillary capillary loop was Type B1 (JES). Iodine staining showed no stain but showed its apparent margins more, and the pink sign was positive in the central part. The remaining esophageal mucosa was scattered in patchy light-stained areas, ranging from 0.2–0.4 cm (Figure 1A). Computed tomography (CT) revealed a small cyst in the liver; however, no abnormal thickening of the gastrointestinal wall was observed (Figure 1B). Endoscopic submucosal dissection (ESD) was performed 1 wk after the endoscopy (Figure 1C), and postoperative pathology revealed mucosal ESCC (Figure 1D).
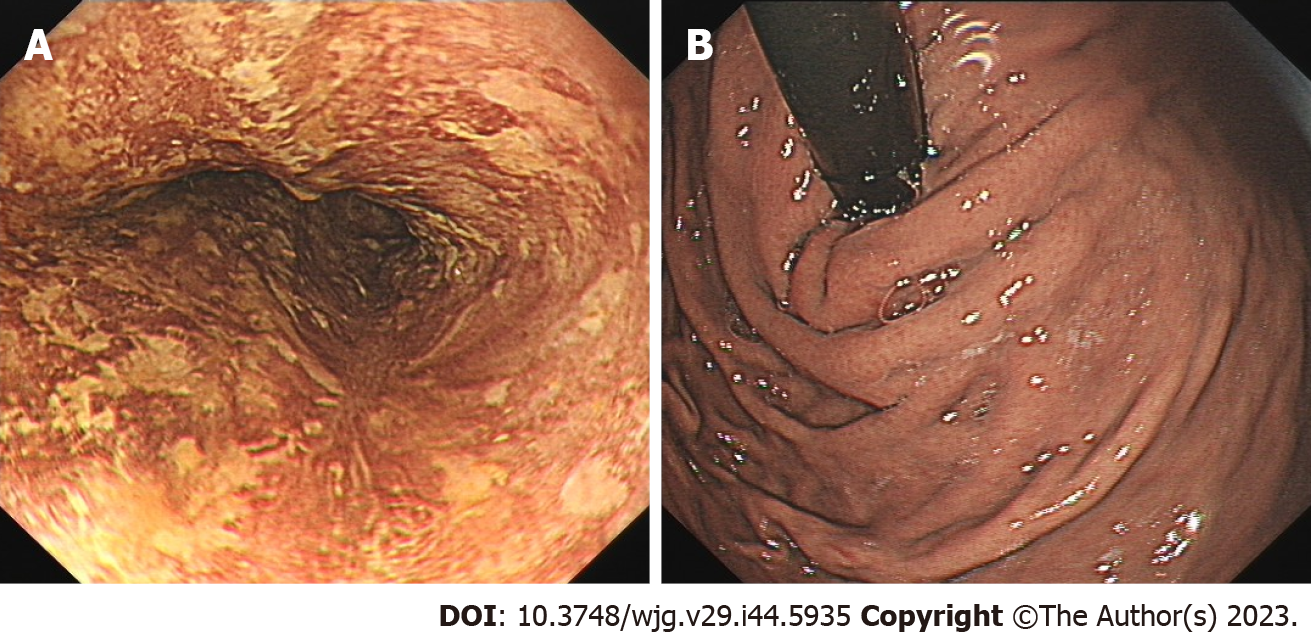
In March 2021, the patient was re-examined 7 mo after ESD. Endoscopic examination showed a 1.0 cm × 2.0 cm scar on the right posterior wall of the esophagus 30 cm from the incisor teeth. Iodine staining showed that the esophageal mucosa was scattered in a patchy light-stained area, with a pink sign (-) (Figure 2A). The mucosa of the gastric fundus and cardia were smooth without any abnormalities (Figure 2B).
In July 2021, the patient was examined again 1 year after ESD. The esophageal mucosa was the same as that at the final endoscopic examination. While gastroendoscopy also showed a 0.6 cm × 0.8 cm IIa + IIc lesion near the anterior wall of the gastric fundus near the cardia, biopsy of this lesion revealed erosion with regeneration and moderate dysplasia locally. The other mucosa of the gastric fundus and cardia were smooth without any abnormalities (Figure 3A). CT also revealed a small cyst in the liver; however, no abnormal thickening was observed in the gastrointestinal wall (Figure 3B). The patient underwent ESD of the fundus lesion (Figure 3C), and postoperative pathology revealed moderate-to-severe dysplasia of the gastric mucosa (Figure 3D).
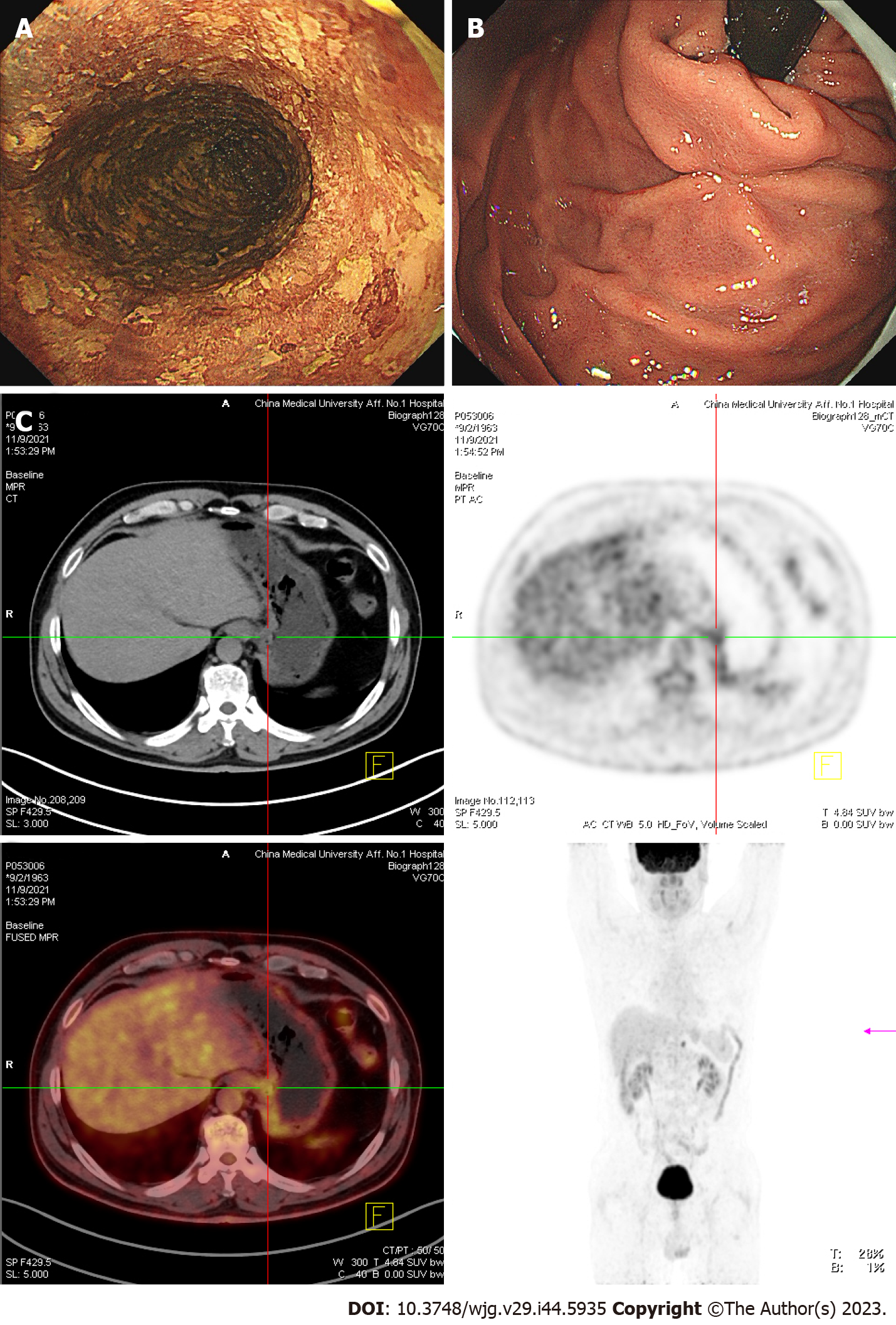
In November 2021, the patient received a 3rd examination 15 mo after the 1st ESD. Gastroendoscopy revealed patchy light-stained areas throughout the esophagus after iodine staining (Figure 4A). The mucosa of the gastric fundus and cardia was smooth, except for the scar after ESD, and no abnormalities were found on a gastroendoscopy (Figure 4B). CT showed no abnormal thickening of the gastrointestinal wall. However, positron emission tomography (PET)-CT revealed that the metabolism of the lower esophagus increased slightly, the metabolism of the stomach cardia wall increased slightly, and lymph nodes in the retroperitoneal area had high-density shadows and increased metabolism (Figure 4C).
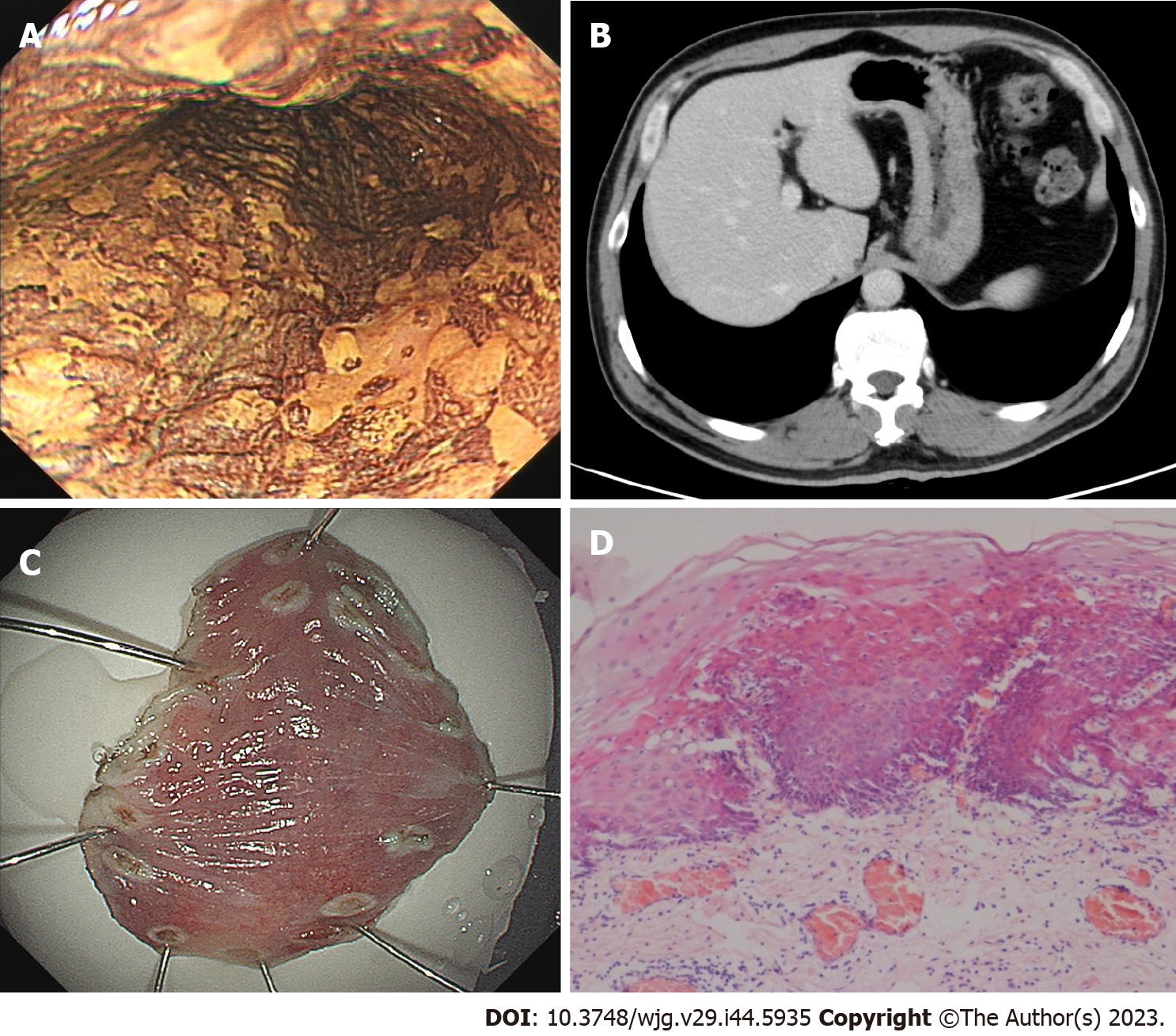
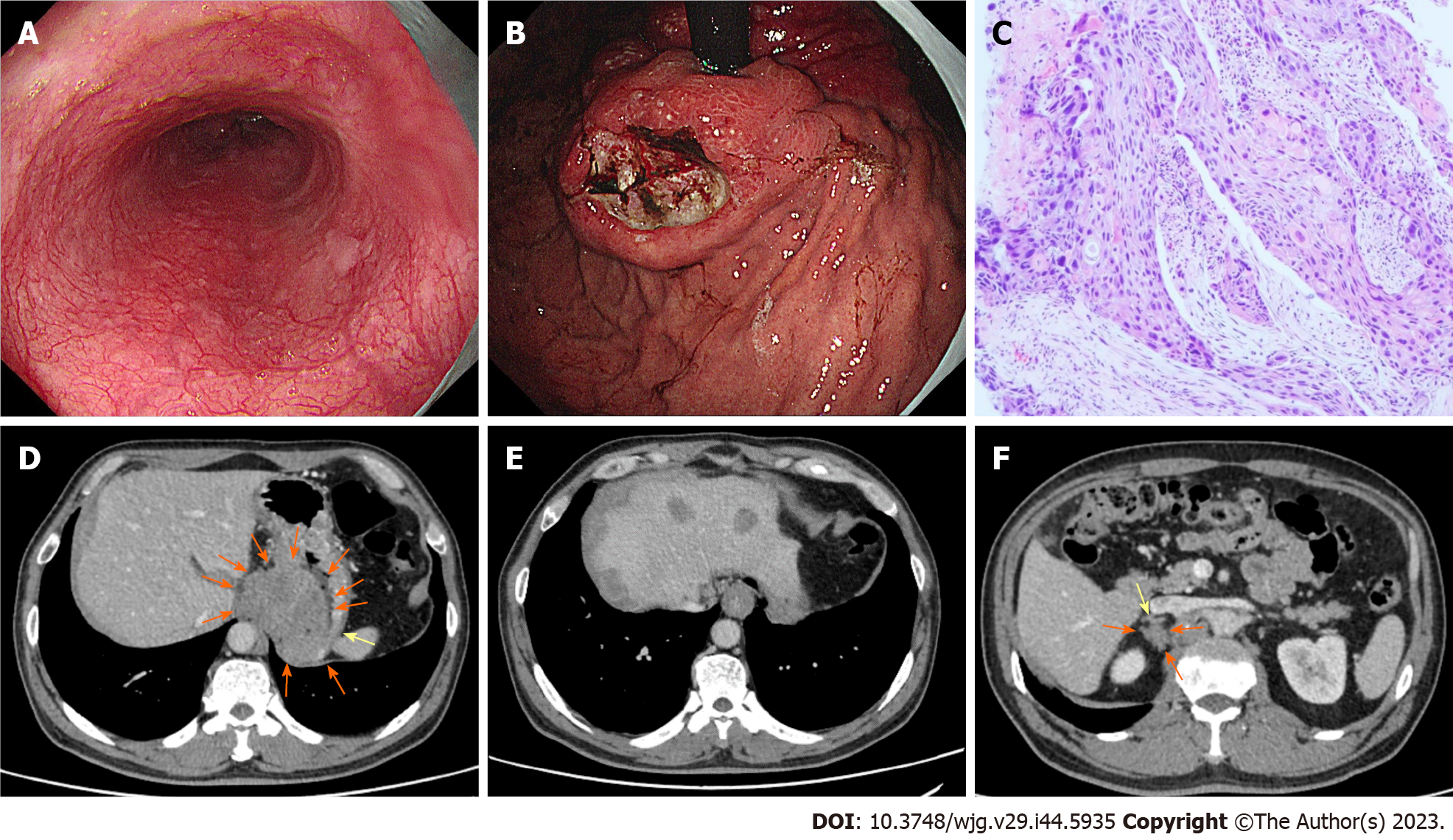
In November 2022, the patient received a 4th endoscopy examination. The esophageal mucosa was smooth, and no advanced cancer was found (Figure 5A). Circumferential submucosal tumor (SMT)-like swelling was observed in the cardia and stomach body; local ulceration was observed, and multiple ulcers were formed. The mucosa surrounding the ulcer was regular and tough, the mucosa surrounding the lesion was clustered, and the lower edge of the lesion involved a minor curvature in the middle of the stomach body (Figure 5B). A biopsy of the cardia revealed SCC (Figure 5C). CT revealed a cardia tumor (Figure 5D), perigastric lymph node enlargement, and multiple metastatic tumors of the liver (Figure 5E) and right adrenal gland (Figure 5F).
The patient was diagnosed as early-stage esophageal carcinoma with giant gastric cardia metastasis.
To alleviate the observed multi-organ metastasis, the patient was administered immunochemotherapy (carrelizumab 200 mg D1, albumin paclitaxel 400 mg D1, and carboplatin 450 mg D1) and radiotherapy (30 courses) for esophageal cancer. Six immunochemotherapy and 30 radiotherapy courses were completed.
The development and treatment of the disease in the present case are summarized in Figure 6.
The immunochemotherapy and radiotherapy was completed and the patient was still alive. The follow-up is ongoing.
ESCC is an extremely aggressive cancer and has a poor prognosis owing to its higher propensity for lymph nodes and distant metastases. Historically, cancer-distal metastases are considered as the most important barrier to achieving significant advances in cancer management; moreover, it is responsible for 90% of cancer-related deaths[14,15]. The incidence of distant metastases in newly diagnosed ESCC is approximately 20%–30%. However, this proportion raised to approximately 40%–50% in recurrent cases after radical esophagectomy[16] or concurrent radiochemotherapy[17] in ESCC. IM is another metastasis pathway in esophageal cancer. Based on previous endoscopic findings, clinical IM, including gastric metastasis from ESCC, had mainly three characters: (1) Metastatic tumor often separated distantly from the primary tumor; (2) The metastatic lesion presented in the esophagus or stomach wall; and (3) the SMT lacked of an intraepithelial component, occasionally with an erosive change[6]. Accompanied by these findings, the ESCC strongly suggests an IM tumor[18].
Longitudinal lymphatic vessels contained in the mucosa lamina propria and submucosa of the esophagus are the anatomic basis for IM[19]. The mucosal lymphatic channels in esophagus are not directly connected to those in stomach; however, submucosal channels may have a direct connection with the submucosal gastric lymphatics[20]. Therefore, tumor cells go through the submucosal lymphatic system to the stomach wall and form metastatic loci[20]. Watson and Goodner[21] first reported there existed an interflow of lymphatic channels between the esophagus and stomach through virtue of neo-lymphangiogenesis in the carcinomatous lesion and explained IM as an extension of submucous lymphatics. Kato et al[6] documented that the location of the IM toward the primary tumor did not indicate a predilection in the direction of the lymphogenic spread of carcinoma of the esophagus. However, another study[5] showed that among 1259 patients, although the depth of esophageal cancer may be T1, the possibility of IGM still exists. The incidence of IGM is higher in advanced-stage ESCC[5,22], as only a few cases of IGM in the early-stage ESCC have been reported. From mucosal ESCC, to our best knowledge, only 6 cases (including the present one) have been documented[10-13,23]. Therefore, although the probability of lymphatic metastasis is very low, it can still occur even if the tumor is confined to the mucosa.
IGM from esophageal cancer is rare[5,9]; nonetheless, it is considered to be one of the most significant poor prognostic factors due to its higher rate of lymphatic invasion[24,25] and distant metastasis. The correlation between metastatic tumors and lymph node metastasis can be attributed to the fact that gastric metastases occur via the lymphatic system. In the middle and lower parts of the esophagus, submucosal lymphatic drainage is regarded to be connected to gastric from the cardia and fundus. At times, the primary esophageal lesion could appear to be small, compared with the gastric metastatic lesion, which is exophytic in morphology because of the submucosal site of implantation[22]. In this report, the primary tumor was in the middle-thoracic esophagus, and the IGM was found in the gastric cardia.
In addition, IGM plays a role in distant metastasis, particularly in the liver[6]. IGM of ESCC is categorized as distant metastasis, described as M1b stage IVB, according to the Union for International Cancer Control for International Cancer Control Classification[26]. However, other studies have reported metastases in the reconstructed stomach. IGM develops principally in the upper third of the stomach wall; however, it can also appear in the gastric body[5,9]. These mean IGM happen without direct involvement of the lymphatic system[27]. In this report, the tumor cells giving rise to IGM could not have spread via the lymphatic system because the primary tumor was confined to the mucosa with no lymphatic connection to the submucosa. Thus, IGM sometimes develops via routes other than the lymphatic system, including vascular routes. In addition, we found multiple distant metastases to the liver and right adrenal gland, which indicated that there were hematogenous pathways of metastasis in our case.
In our case, what really surprised us was that from November 2021 (PET-CT suggested abnormal cardiac metabolism) to November 2022 (gastroendoscopy revealed a Borrmann type II lesion in the cardiac area), metastases to the cardia grew rapidly and were so large that no significant primary lesions were found in the esophagus. The cardiac area is rich in blood supply, which is conducive to the root proliferation of squamous cells, and the cardiac area is significantly large[23,24]. Primary gastric adenocarcinoma sometimes has a similar gross appearance but a higher incidence in the lower part of the stomach. Gastrointestinal stromal tumors also appear as exophytic lesions. The detection rate of IM of ESCC ranges 5.5% between 16.6%[6,28]. General endoscopy has certain limitations when the metastases are confined below the mucosa without breaking through the surface. The radiological imaging, especially fluorodeoxyglucose-PET, can detect out early metastatic lesions in time[29-31]. Therefore, preoperative and postoperative radiographic follow-up is conducive to indicating the presence of IM[20,32]. The five-year disease-free survival rate of patients with ESCC with IM is significantly lower than that of patients without IM[6,33]. Therefore, multimodal treatments with strong antitumor effects should be administered.
In conclusion, preoperative and postoperative endoscopic follow-up of patients with ESCC of any stage, although the depth of an esophageal cancer may be T1, in addition to paying attention to esophageal lesions, the stomach should also be carefully examined, especially for the submucosal eminence in the upper part of the stomach, to consider the possibility of IGM, and the nature of the submucosal eminence can be clarified by combining endoscopic ultrasonography and ultrasonic puncture biopsy. Therefore, enhancing the preoperative and postoperative imaging and endoscopic ultrasonography are beneficial for indicating the existence of IM.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lu P, United States; Mori F, Italy S-Editor: Lin C L-Editor: A P-Editor: Lin C
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 12986] [Article Influence: 1442.9] [Reference Citation Analysis (2)] |
| 2. | Takubo K, Sasajima K, Yamashita K, Tanaka Y, Fujita K. Prognostic significance of intramural metastasis in patients with esophageal carcinoma. Cancer. 1990;65:1816-1819. [PubMed] [DOI] [Full Text] |
| 3. | Seki M. [Clinicopathological study on the intramural metastasis of the esophageal cancer]. Nihon Geka Gakkai Zasshi. 1991;92:1426-1435. [PubMed] |
| 4. | Shaheen O, Ghibour A, Alsaid B. Esophageal Cancer Metastases to Unexpected Sites: A Systematic Review. Gastroenterol Res Pract. 2017;2017:1657310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Ebihara Y, Hosokawa M, Kondo S, Katoh H. Thirteen cases with intramural metastasis to the stomach in 1259 patients with oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg. 2004;26:1223-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Kato H, Tachimori Y, Watanabe H, Itabashi M, Hirota T, Yamaguchi H, Ishikawa T. Intramural metastasis of thoracic esophageal carcinoma. Int J Cancer. 1992;50:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Kuwano H, Baba K, Ikebe M, Kitamura K, Adachi Y, Mori M, Sugimachi K. Gastric involvement of oesophageal squamous cell carcinoma. Br J Surg. 1992;79:328-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Oda, Kondo H, Yamao T, Saito D, Ono H, Gotoda T, Yamaguchi H, Yoshida S, Shimoda T. Metastatic tumors to the stomach: analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy. 2001;33:507-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 165] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Saito T, Iizuka T, Kato H, Watanabe H. Esophageal carcinoma metastatic to the stomach. A clinicopathologic study of 35 cases. Cancer. 1985;56:2235-2241. [PubMed] [DOI] [Full Text] |
| 10. | Ebihara Y, KT, Kobayashi M, Hosokawa M, Kato H. A case of esophageal carcinoma with giant intramural gastric metastasis. Jpn J Surg Assoc. 2002;63:8. |
| 11. | Yoshida K, Ide H, Murata Y, Kobayashi A, Hanyuu H, Yamada A. [A case of mucosal esophageal carcinoma with a large intramural metastasis in the stomach]. Nihon Kyobu Geka Gakkai Zasshi. 1989;37:1430-1435. [PubMed] |
| 12. | Nishimura T, TT, Shibuya Y, Nakamura T, Goto M, Fukui Y. A case of mucosal esophageal carcinoma with metastasis to the gastric wall. Jpn J Surg Assoc. 2008;69:5. |
| 13. | Nabeki B, O. H., Omoto I, Matsumoto M, Uchikado Y, Setoyama T et al, A case of upper thoracic superficial esophageal carcinoma with intramural metastasis to the stomach and lymph node metastasis along the common hepatic artery. Jpn J Gastroenterol Surg. 2012;45:7. |
| 14. | Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3059] [Cited by in RCA: 3615] [Article Influence: 258.2] [Reference Citation Analysis (0)] |
| 15. | Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2932] [Cited by in RCA: 3206] [Article Influence: 168.7] [Reference Citation Analysis (0)] |
| 16. | Sudo K, Kato K, Kuwabara H, Sasaki Y, Takahashi N, Shoji H, Iwasa S, Honma Y, Okita NT, Takashima A, Hamaguchi T, Yamada Y, Ito Y, Itami J, Fukuda T, Tobinai K, Boku N. Patterns of Relapse after Definitive Chemoradiotherapy in Stage II/III (Non-T4) Esophageal Squamous Cell Carcinoma. Oncology. 2018;94:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Yamashita K, Watanabe M, Mine S, Kurogochi T, Okamura A, Hayami M, Imamura Y. Patterns and Outcomes of Recurrent Esophageal Cancer After Curative Esophagectomy. World J Surg. 2017;41:2337-2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Okamura A, Yoshimizu S, Kanamori J, Imamura Y, Asari T, Nakayama I, Ogura M, Ishiyama A, Yoshio T, Chin K, Fujisaki J, Watanabe M. Treatment Strategy for Esophageal Squamous Cell Carcinoma With Endoscopic Intramural Metastasis. Cureus. 2022;14:e23028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Wang Y, Zhu L, Xia W, Wang F. Anatomy of lymphatic drainage of the esophagus and lymph node metastasis of thoracic esophageal cancer. Cancer Manag Res. 2018;10:6295-6303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Riquet M, Saab M, Le Pimpec Barthes F, Hidden G. Lymphatic drainage of the esophagus in the adult. Surg Radiol Anat. 1993;15:209-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | WATSON WL, GOODNER JT. Carcinoma of the esophagus. J Int Coll Surg. 1957;28:715-723. [PubMed] |
| 22. | Roul PK, Saran S, Poonia DR, Paul P. Intramural Gastric Metastasis: A Rare Presentation of Esophageal Squamous Cell Carcinoma. J Med Ultrasound. 2022;30:47-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 23. | Nakazawa N, Fukuchi M, Sakurai S, Naitoh H, Kiriyama S, Fukasawa T, Tabe Y, Yamauchi H, Suzuki M, Yoshida T, Kuwano H. Mucosal esophageal squamous cell carcinoma with intramural gastric metastasis invading liver and pancreas: a case report. Int Surg. 2014;99:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Sun K, Lv H, Chen B, Nie C, Zhao J, Wang S, Wang J, Xu W, Chen X. Dawning precision treatment for gastric cancer: The latest biomarkers. J Transl Int Med. 2021;9:228-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology. 2018;154:360-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1154] [Article Influence: 164.9] [Reference Citation Analysis (1)] |
| 26. | Okumura T, Shimada Y, Hojo S, Sekine S, Hirano K, Moriyama M, Miwa S, Nagata T, Tsukada K. Perforation of intramural gastric metastasis during preoperative chemotherapy in a patient with thoracic esophageal squamous cell carcinoma. Int J Surg Case Rep. 2015;17:23-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Matsutani T, SK, Yoshida H, Hosone M, Katayama H, Uchida EA. A case of intramural gastric tube metastasis from esophageal squamous cell carcinoma. Esophagus. 2012;9:239-242. |
| 28. | Nishimaki T, Suzuki T, Tanaka Y, Aizawa K, Hatakeyama K, Muto T. Intramural metastases from thoracic esophageal cancer: local indicators of advanced disease. World J Surg. 1996;20:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Nishida K, Okuyama C, Kubota T, Matsushima S, Oda M, Akazawa K, Nishimura T. Intramural metastasis of esophageal carcinoma to the reconstructed gastric tube detected by FDG PET/CT. Clin Nucl Med. 2009;34:523-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Liu P, Zhu H, Zhang X, Feng A, Zhu X, Sun Y. Predicting Survival for Hepatic Arterial Infusion Chemotherapy of Unresectable Colorectal Liver Metastases: Radiomics Analysis of Pretreatment Computed Tomography. J Transl Int Med. 2022;10:56-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | Lee GD, Kim YH, Kim JB, Choi SH, Kim HR, Kim DK, Park SI. Esophageal Cancer Associated with Multiple Primary Cancers: Surgical Approaches and Long-term Survival. Ann Surg Oncol. 2013;20:4260-4266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Szántó I, Vörös A, Nagy P, Gonda G, Gamal EM, Altorjay A, Banai J, Kiss J. Esophageal intramural metastasis from adenocarcinoma of the gastroesophageal junction. Endoscopy. 2002;34:418-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Watanabe M, Kuwano H, Araki K, Kawaguchi H, Saeki H, Kitamura K, Ohno S, Sugimachi K. Prognostic factors in patients with submucosal carcinoma of the oesophagus. Br J Cancer. 2000;83:609-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |