Published online Nov 21, 2023. doi: 10.3748/wjg.v29.i43.5834
Peer-review started: August 19, 2023
First decision: September 28, 2023
Revised: October 10, 2023
Accepted: October 29, 2023
Article in press: October 29, 2023
Published online: November 21, 2023
Processing time: 92 Days and 16 Hours
14C urea breath test (14C UBT) and immunohistochemical staining (IHC) are widely used for detection Helicobacter pylori (H. pylori) infection with different sensitivity, and there is a difference in H. pylori infection rate in Uyghur and Han ethnic groups. Both need large cohort studies to evaluate the differences more accura
To analyze the difference between 14C UBT and IHC for H. pylori detection in Xinjiang Uyghur Autonomous Region and the difference between Uyghur and Han populations.
There were 3944 cases of H. pylori infection detected by both IHC and 14C UBT at the same time (interval < 1 wk, with sampling site including gastric antrum, selected from 5747 patients). We compared the sensitivity of
The sensitivity was 94.9% for 14C UBT and 65.1% for IHC, which was a significant difference (n = 3944, P < 0.001). However, among those cases negative for H. pylori by 14C UBT (detection value ≤ 100), 4.8% were positive by IHC. Combining both methods, the overall H. pylori infection rate was 48.6% (n = 5747), and differences in gender, age group, ethnicity and region of residence significantly affected the H. pylori positive rates. According to age group (Han/Uyghur), the positive rates were ≤ 30 years (62.2%/100.0%), 31-40 years (45.2%/85.7%), 41-50 years (47.2%/79.2%), 51-60 years (44.6%/76.1%), 61-70 years (40.9%/68.2%), 71-80 years (41.7%/54.1%) and ≥ 81 years (42.9%/NA). The H. pylori infection rates of Han/Uyghur paired cases were 41.4% and 73.3%, which was a significant difference (P < 0.001) (555 pairs). H. pylori positivity was significantly related to moderate-severe grade 2-3 chronic/active gastritis and intestinal metaplasia (all P < 0.05).
The sensitivity of 14C UBT was significantly higher, but combined application can still increase the accuracy. The prevention H. pylori should be emphasized for Uygur and young people.
Core Tip: The sensitivity of 14C urea breath test (14C UBT) for detecting Helicobacter pylori (H. pylori) is significantly higher than that of immunohistochemistry (IHC) with endoscopy specimens. Combination of 14C UBT and IHC is necessary to improve detection accuracy, and increasing the number of biopsy specimens (≥ 2) can improve the positive rate significantly. The overall rate of H. pylori infection in Xinjiang Uyghur Autonomous Region was higher than in previous studies. H. pylori infection was more prevalent in the Uyghur population and the infection rate decreased as age increased. Therefore, the prevention and intervention of H. pylori infection in Xinjiang Uyghur Autonomous Region should emphasize Uyghur and young people.
- Citation: Peng YH, Feng X, Zhou Z, Yang L, Shi YF. Helicobacter pylori infection in Xinjiang Uyghur Autonomous Region: Prevalence and analysis of related factors. World J Gastroenterol 2023; 29(43): 5834-5847
- URL: https://www.wjgnet.com/1007-9327/full/v29/i43/5834.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i43.5834
Helicobacter pylori (H. pylori) is a Gram-negative bacterium that mainly parasitizes the oral cavity, stomach, and duodenum after infection[1]. It was first isolated and cultured from patients with gastritis by Warren and Marshall[2] in Australia. It was extensively studied and reported that the vast majority of gastric and duodenal ulcers are associated with H. pylori infection, which is also associated with gastric cancer. H. pylori has been classified by World Health Organization as a class I carcinogen[3]. Diseases related to H. pylori are infectious as emphasized by the Kyoto Consensus[4]. It is known that transmission pathway of H. pylori includes fecal-oral and oral-oral transmission[1,5].
The Xinjiang Uyghur Autonomous Region of China has a vast territory and is inhabited by multiple ethnic groups; the main two being Han and Uyghur. Due to the differences in climate, lifestyle, dietary habits, and medical and health conditions, there are differences in H. pylori infection status. Although there are some previous reports from Chinese journals, the studies involved small series or their detection methods were not sufficiently sensitive[6-9].
The most important way to more accurately investigate the infection rate of H. pylori is to use a more accurate method of detection. Clinical H. pylori testing is divided into noninvasive and invasive types[10-12]. The noninvasive tests are mainly carried out in the endoscopy rooms or laboratories of various medical institutions, and the highly recommended method is the 14C urea breath test (14C UBT) test. For the invasive tests, detection of H. pylori is through gastroscopic biopsy of pathological tissue. Previous studies have shown that immunohistochemical staining to detect H. pylori infection has the highest sensitivity and specificity among all histopathological methods for endoscopic biopsies[13-15]. At present, the consensus is that both immunohistochemistry (IHC) and 14C UBT detection of H. pylori infection can be used for diagnosing H. pylori infection[16-18]. However, there is controversy in the literature about the sensitivity of IHC and 14C UBT. One study (n = 150) reported that UBT was more sensitive[19] but another study (n = 239) reported that IHC had higher sensitivity[20]. Larger studies are needed to compare the differences between 14C UBT and IHC for detection of H. pylori infection.
Thus, we retrospectively studied a large series of patients who underwent both 14C UBT and IHC and on histopathological diagnosed gastroscopy specimens between January 2019 and February 2021 and through gastroscopy at our hospital in Urumqi. We aimed to investigate the differences between the two detection methods; evaluate H. pylori infection rate in Xinjiang Uyghur Autonomous Region; and establish whether there were differences in H. pylori infection due to factors such as age, gender, region, and especially, ethnic group (Uyghur and Han). It is hoped that our study might provide more accurate data about the difference in the two widely used H. pylori detection methods, and would be useful for the detection, prevention and intervention of H. pylori infection in Xinjiang Uyghur Autonomous Region.
A retrospective control study was conducted among patients who went to the Traditional Chinese Medical Hospital of Xinjiang Medical University between March 2019 and February 2021 due to gastrointestinal discomfort for which they underwent pathological examination of gastric biopsy tissue. The screening criteria were as follows: (1) All enrolled patients also underwent 14C UBT; (2) No history of medication (such as antibiotics, proton pump inhibitors, and bismuth-based agents) that affected H. pylori activity, or a positive detection rate within 1 mo before the examination; and (3) Complete clinical information. This study met the ethical requirements of the Ethics Committee of Traditional Chinese Medical Hospital of Xinjiang Medical University.
H. pylori infection was detected using hematoxylin and eosin (H&E) staining, IHC staining, and 14C UBT.
All specimens were fixed with 10% neutral formaldehyde solution, routinely dehydrated, embedded, and cut into 4-5 μm sections. We used an automated staining machine (LEICA Company, AUTOSTAINER) for H&E staining.
IHC staining was performed using a BENCHMARK-XT automatic staining machine (Roche) with H. pylori antibody working solution (MX104 mouse antihuman monoclonal antibody; Fuzhou Maixin Company), and the positive signal was stained with DAB. Both positive and negative controls were established. The positive control was confirmed to be H. pylori positive gastric biopsy tissue with plenty of H. pylori (staining > 100 bacterial bodies/H. pylori, F). The negative control used a blank antibody solution instead of the first antibody working solution.
H&E staining: Pathological diagnosis and gastritis grading (including grading of chronic gastritis, active gastritis, atrophy, and intestinal metaplasia). Classification: Grade 0 (none), grade 1 (mild), grade 2 (moderate), and grade 3 (severe), and statistical analysis was conducted in sequence.
Positive/negative results were based on the presence or absence of H. pylori bacteria under the microscope. All sections were independently reviewed by two senior pathologists, and for those cases with inconsistency, a third senior pathologist provided the final interpretation.
Patients were tested on an empty stomach or fasting for > 2 h, and the test was positive if ≥ 100 dpm/mmol, and negative if 0-100 dpm/mmol. The 14C urea capsule and related reagents, as well as the H. pylori detector, were all provided by Shenzhen Zhonghe Headway Bio-Sci & Tech Co. Ltd.
At least one positive result from 14C UBT and IHC is considered as a positive H. pylori infection. In the literature, both are the preferred methods for noninvasive and invasive detection; therefore, a positive result with either one can directly diagnose H. pylori infection. Both methods have high specificity (default close to 100%). Comparison of the two detection methods in this study focused on the sensitivity aspect. Sensitivity was calculated as: sensitivity = number of true positive cases/(number of true positive cases + number of false negative cases) × 100%.
In order to accurately compare the results of 14C UBT and IHC, it is required that: (1) Gastroscopic pathological examination (H&E and IHC), 14C UBT and the interval between pathological examinations should be < 1 wk; (2) The sampling sites should include the gastric antrum; and (3) The number of samples taken by clinicians should be recorded as it may affect the detection results. A total of 3944 eligible cases were selected from all cases undergoing both 14C UBT and IHC.
We speculated that ethnic differences would be the main factor affecting H. pylori infection. We used pathological IHC and 14C UBT, with Uyghur as the case group and Han as the control group, and matched them 1:1. The matching variables included gender and age. A total of 555 fully matched Uyghur and Han cases were obtained, including 249 males and 306 females (male to female ratio of approximately 1:1.23), aged 24-80 years (average 54.1 years, median 48 years), to further investigate the exact impact of ethnic differences on H. pylori infection. SPSS version 23.0 was used for statistical analysis and comparison (using χ2 tests), and P < 0.05 represented a statistically significant difference.
A total of 5747 cases were included. Overall, the male to female ratio was 1:1.15 (2672/3075). Age ranged from 19 to 92 years, with a median of 56 years. There were 4925 cases of Han and 822 cases of Uyghur. Divided by the Tianshan Mountains, there were 4919 cases residing in Northern Xinjiang, 508 in Southern Xinjiang, 265 in the Xinjiang Production and Construction Corps (XPCC), and 55 in areas outside Xinjiang Uyghur Autonomous Region. The case information is detailed in Table 1.
| Characteristics | Total | Han | Uyghur | |
| Gender (cases) | Male | 2672 (46.5%) | 2303 (46.8%) | 369 (44.9%) |
| Female | 3075 (35.5%) | 2622 (53.2%) | 453 (55.1%) | |
| Age (yr) | Range | 19-92 | 19-92 | 22-80 |
| Average | 56.3 | 56.7 | 53.4 | |
| Median | 56.0 | 56.0 | 54.0 | |
| Residing region (cases) | Northern Xinjiang | 4919 (85.6%) | 4405 (89.4%) | 514 (62.5%) |
| Southern Xinjiang | 508 (8.8%) | 207 (4.2%) | 301 (36.6%) | |
| Xinjiang Production and Construction Corps | 265 (4.6%) | 263 (5.3%) | 2 (0.2%) | |
| Extra Xinjiang Uyghur Autonomous Region | 55 (1.0%) | 50 (1.0%) | 5 (0.6%) | |
| Total (cases) | 5747 | 4925 (85.7%) | 822 (14.3%) | |
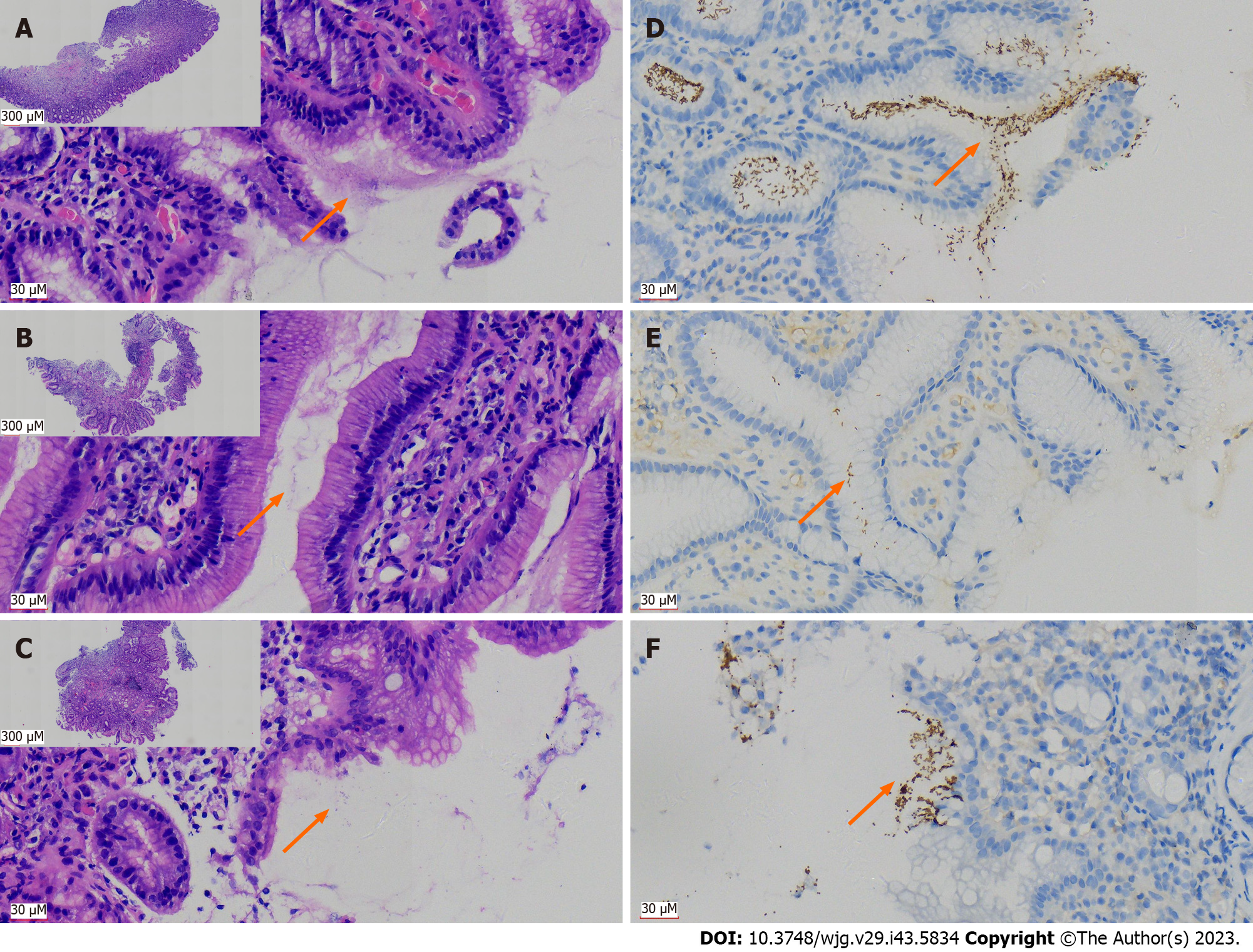
For the typical H. pylori-positive cases, which were definitively confirmed in the H&E sections (Figure 1A), spiral-shaped or curved bacteria were seen in the superficial mucus secreted from the gastric mucosa, or in the gastric glandular cavity. However, when the number of H. pylori was limited or their morphology was atypical (such as coccoid shapes), it was difficult to recognize them (Figures 1B and C). Using H. pylori antibodies and IHC can specifically label H. pylori. For typical positive cases, H. pylori uniformly appeared like small brown rods (Figure 1D). For those cases with low bacterial count, they can also be more easily identified and confirmed, which helps to prevent missed diagnosis or false negative result (Figures 1E and F).
Among the 3944 cases who underwent both IHC and 14C UBT during gastroscopy (interval < 1 wk, including gastric antrum), the overall positive rate of H. pylori infection was 49.7% (1962/3944): 29.8% (1176/3944) of the cases were positive by both 14C UBT and IHC; 2.6% (101/3944) were solely positive for IHC; 17.4% (685/3944) were solely positive for 14C UBT; and 50.3% (1982/3944) were negative. According to the previously established standards, the sensitivity of
When the H. pylori positive rates detected by IHC were compared with the value ranges detected by 14C UBT, it would be 4.2%,11.1%, 25.6%, 52.6%, 66.8% and 73.9% in the value ranges 0-49, 50-100, 101-199, 200-499, 500-999 and ≥ 1000, respectively. For cases negative by 14C UBT, the H. pylori positive rate by IHC was 4.8%, while for cases defined as positive by 14C UBT, the H. pylori positive rate by IHC was 63.2% (Tables 2 and 3).
| H. pylori positive rate (%) | ||||||||
| Positive1 | Negative1 | P value1 | Positive2 | Negative2 | P value (single vs multiple biopsy)2 | |||
| 14C UBT (total, n = 3944) | Positive | 1176 (29.8%) | 685 (17.4%) | P < 0.001 | 1962 (49.7%) | 1982 (50.3%) | - | |
| Negative | 101 (2.6%) | 1982 (50.3%) | ||||||
| Subgroup | Single biopsy (1 piece, n = 3476) | Positive | 987 (28.4%) | 608 (17.5%) | P < 0.001 | 1068 (30.7%) | 2408 (69.3%) | P < 0.001 |
| Negative | 81 (2.3%) | 1800 (51.8%) | ||||||
| Multiple biopsies (≥ 2 pieces, n = 468) | Positive | 189 (40.4%) | 77 (16.5%) | P < 0.001 | 209 (44.7%) | 259 (55.3%) | ||
| Negative | 20 (4.3%) | 182 (38.9%) | ||||||
To clarify the impact of the difference in the number of biopsies taken during gastric endoscopy on the positive rate by IHC, we divided those cases into two groups: Single biopsy (only 1 specimen) and multiple biopsies (≥ 2 specimens). Pearson’s test confirmed that the positive rate for immunohistochemical detection in the single biopsy group was 28.4% (987/3476), and the positive rate in the multiple biopsy group was 40.4% (189/468). The two groups were significantly different (P < 0.001) by Pearson χ2 test, and the H. pylori-positive rate in the multiple biopsy group was significantly increased (Table 2).
According to the H. pylori positive criteria, the overall positive rate of H. pylori infection was 48.6% (2794/5747). The infection rate in male and female patients was 51.0% (1362/2672) and 46.6% (1432/3075), respectively, with a significant difference by χ2 test (P = 0.001). The H. pylori infection rates in the Han and Uyghur ethnic groups were 44.2% (2175/4925) and 75.3% (619/822), respectively, with a significant difference (P < 0.001). Further statistical analysis showed that the H. pylori infection rate was 47.4% (1091/2303) in Han Chinese males and 41.3% (1084/2622) in Han females, which was a significant difference by χ2 test (P < 0.001). The infection rate was 73.4% (271/369) in Uighur men and 76.8% (348/453) in Uighur women, which was not a significant difference by χ2 test (P = 0.291).
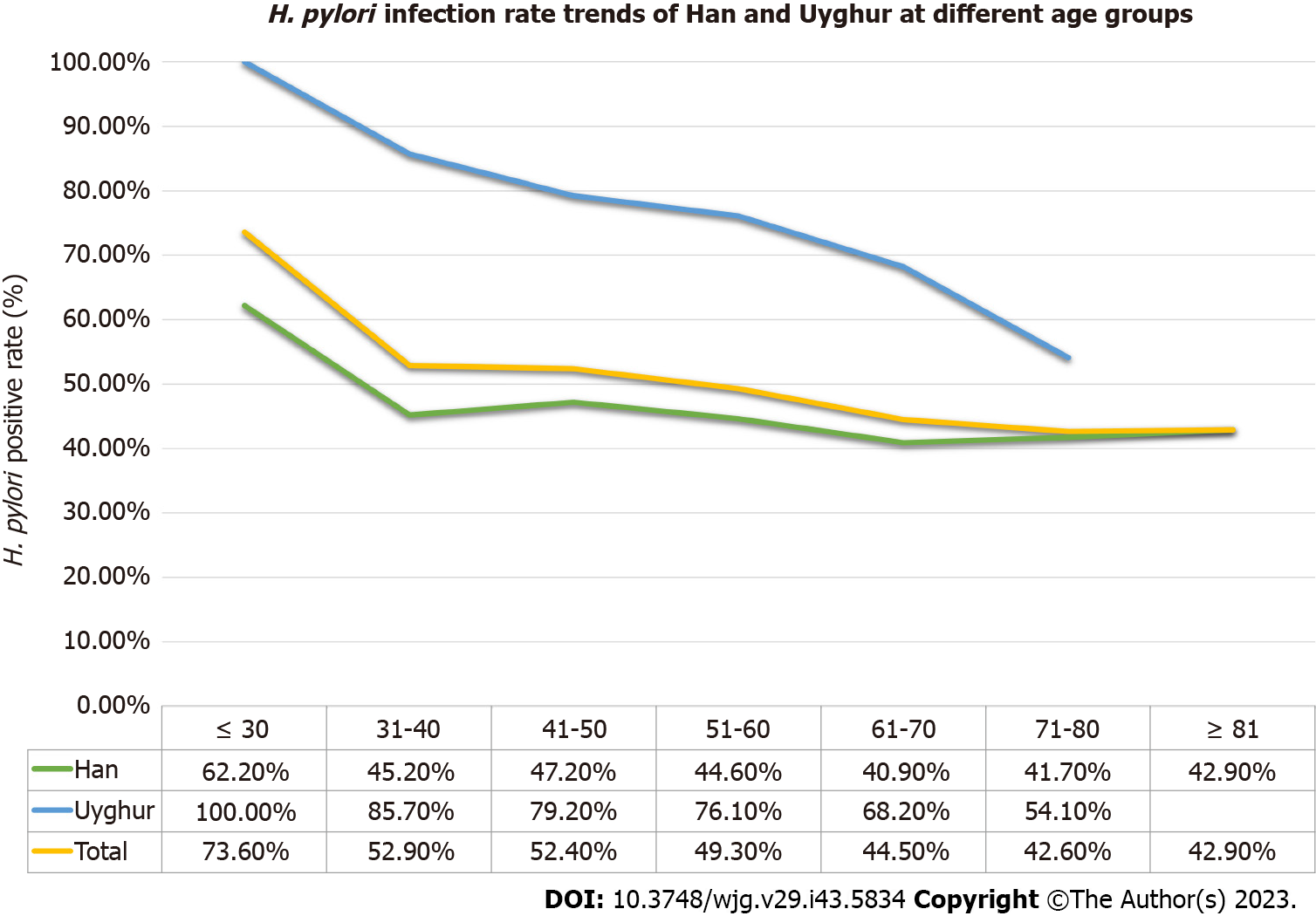
When grouped according to age, the H. pylori infection rates in the Han ethnic group were 62.2% (23/37) in the ≤ 30 years group, 45.2% (109/241) in the 31-40 years group, 47.2% (523/1108) in the 41-50 years group, 44.6% (814/1825) in the 51-60 years group, 40.9% (488/1193) in the 61-70 years group, 41.7% (194/465) in the 71-80 years group, and 42.9% (24/56) in the ≥ 81 years group. The H. pylori infection rates in the Uyghur ethnic group were 100.0% (16/16) in the ≤ 30 years group, 85.7% (48/56) in the 31-40 years group, 79.2% (171/216) in the 41-50 years group, 76.1% (242/318) in the 51-60 years group, 68.2% (122/179) in the 61-70 years group, 54.1% (20/77) in the 71-80 years group, and data for the ≥ 81 years group were missing. The trend in H. pylori-positive rate with age group can be seen in Figure 2.
According to region of residence, the H. pylori infection rate was 47.4% (2333/4919) in northern Xinjiang, 66.3% (337/508) in southern Xinjiang, 37.0% (98/265) in the XPCC, and 47.3% (26/55) outside Xinjiang Uyghur Autonomous Region. There was a significant difference in H. pylori infection rate among the regions, and the rate in southern Xinjiang was the highest (P < 0.001). The H. pylori-positive rates of the Han and Uyghur ethnic groups in northern Xinjiang region were 44.5% (1959/4405) and 72.8% (374/514), respectively, and 47.8% (99/207) and 79.1% (238/301) in southern Xinjiang. The infection rates in the Han and Uyghur ethnic groups in the XPCC region were 36.5% (96/263) and 100.0% (2/2), and 42.0% (96/263) and 100.0% (5/5), respectively.
For the 555 matched cases of Uyghur and Han groups, the positive rate of H. pylori infection in the Uyghur group was 73.3%, while the positive rate in the matched Han cases was 41.4%. The positive rates of H. pylori infection in the Uyghur group were higher than those in the Han group by using IHC (53.2% and 27.7%) or 14C UBT (69.5% and 38.2%). These differences were significant by χ2 test (all P < 0.001). In the matched cases, the infection rate of H. pylori in Han men was 47.4%, which was higher than that in Han women (36.6%), which was a significant difference by χ2 test (P = 0.012). The infection rate in Uyghur men was 72.3%, and that of Uyghur women was 74.2%, but there was no significant difference by χ2 test (P = 0.630), Table 4.
| Method | H. pylori positive rate | ||||||||
| Male (n = 249)1 | Female (n = 306)1 | P value (male/female, Pearson)1 | Subtotal (n = 555)1 | Male (n = 249)2 | Female (n = 306)2 | P value (male/female, Pearson)2 | Subtotal (n = 555)2 | P value (total, Han and Uyghur), Pearson | |
| IHC | 89 (35.7%) | 65 (21.2%) | P < 0.001 | 154 (27.7%) | 134 (53.8%) | 161 (52.6%) | P = 0.778 | 295 (53.2%) | P < 0.001 |
| 14C UBT | 111 (44.6%) | 101 (33.0%) | P = 0.005 | 212 (38.2%) | 170 (68.3%) | 216 (70.0%) | P = 0.556 | 386 (69.5%) | P < 0.001 |
| IHC & 14C UBT | 118 (47.4%) | 112 (36.6%) | P = 0.012 | 230 (41.4%) | 180 (72.3%) | 227 (74.2%) | P = 0.630 | 407 (73.3%) | P < 0.001 |
The pathological changes in the H&E-stained slides of gastric mucosa were classified as follows: (1) Benign lesions: 4609 cases of gastritis; 4 with ulcer diagnosed definitively; 440 cases of gastric fundic gland polyps; 502 cases of hyperplastic (regenerative) polyps; three cases of xanthoma; and 36 cases of duodenal ectopic gastric mucosa; (2) Precancerous lesions: Nine cases of LGIN; seven cases of gastric HGIN; two cases of esophageal LGIN; two cases of esophageal HGIN; 92 cases of BE; and one case of duodenal LGIN; and (3) Tumors: 34 cases of gastric adenocarcinoma; four cases of lymphoma; one case of neuroendocrine tumor; and one case of stromal tumor.
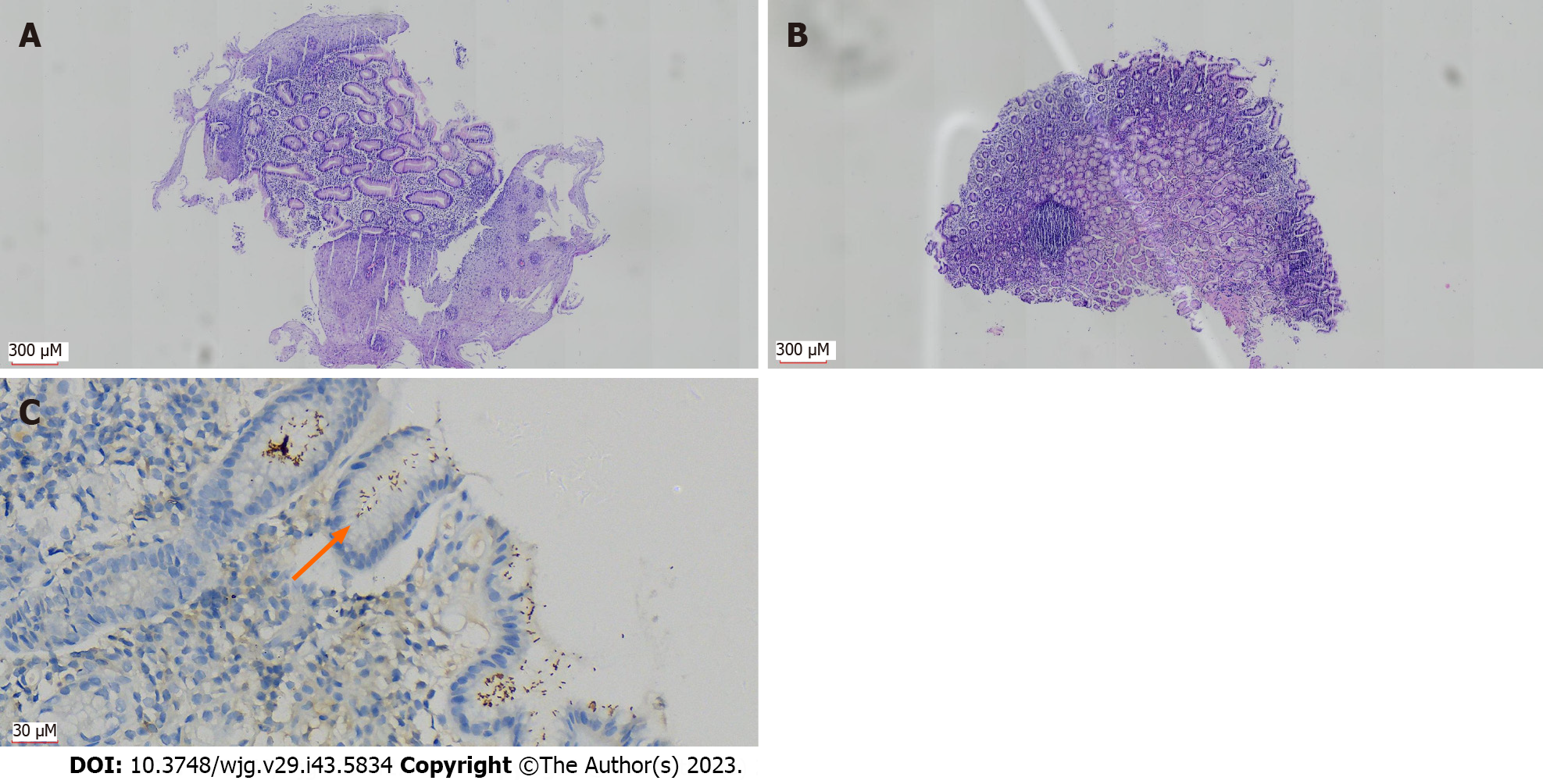
To ensure the accuracy of H. pylori positive rates, we only show disease types with more than 20 samples here, and the other types are included in Table 5. The H. pylori positive rates obtained solely by IHC were as follows: (1) Benign lesions: Gastritis 35.8%, duodenal ectopic gastric mucosa 19.4%, gastric fundic gland polyps 17.3%, and hyperplastic (regenerative) polyps 7.4%; (2) Precancerous lesions: BE 41.3%; and (3) Malignant tumors: 41.2% of gastric adenocarcinoma. If the combination of two detection methods was used, the H. pylori infection rate in patients with various types of diseases diagnosed by gastroscopy was higher: (1) Benign lesions: Gastritis 53.8%, gastric fundic gland polyps 27.5%, duodenal ectopic gastric mucosa 22.2%, and hyperplastic (regenerative) polyps 17.5%; (2) Precancerous lesions: H. pylori-positive rate of BE was 58.7% (based on gastric samples taken simultaneously, Figure 3); and (3) Malignant tumors: 67.6% gastric adenocarcinoma, Table 5.
| Classification of disease | Pathological diagnosis | H. pylori positive rate | |
| IHC | IHC & 14C UBT combined | ||
| Benign lesions | Gastritis | 35.8% (1652/4609) | 53.8% (2478/4609) |
| Duodenal ectopic gastric mucosa | 19.4% (7/36) | 22.2% (8/36) | |
| Gastric fundic gland polyps | 17.3% (76/440) | 27.5% (121/440) | |
| Hyperplastic (regenerative) polyps | 7.4% (37/502) | 17.5% (88/502) | |
| Ulcer | 50.0% (2/4) | 100% (4/4) | |
| Xanthoma | 66.7% (2/3) | 100% (3/3) | |
| Pre-malignant lesions | Barrett’s esophagus | 41.3% (38/92) | 58.7% (54/92) |
| Duodenal LGIN | 100% (1/1) | 100% (1/1) | |
| Gastric LGIN | 55.6% (5/9) | 55.6% (5/9) | |
| Esophageal HGIN | 50.0% (1/2) | 100% (2/2) | |
| Gastric HGIN | 28.6% (2/7) | 57.1% (4/7) | |
| Esophageal HGIN | 0% (0/2) | 0% (0/2) | |
| Malignant tumors | Gastric adenocarcinoma | 41.2% (14/34) | 67.6% (23/34) |
| Lymphoma | 75.0% (3/4) | 75% (3/4) | |
| Neuroendocrine tumor | 0% (0/1) | 0% (0/1) | |
| GIST | 0% (0/1) | 0% (0/1) | |
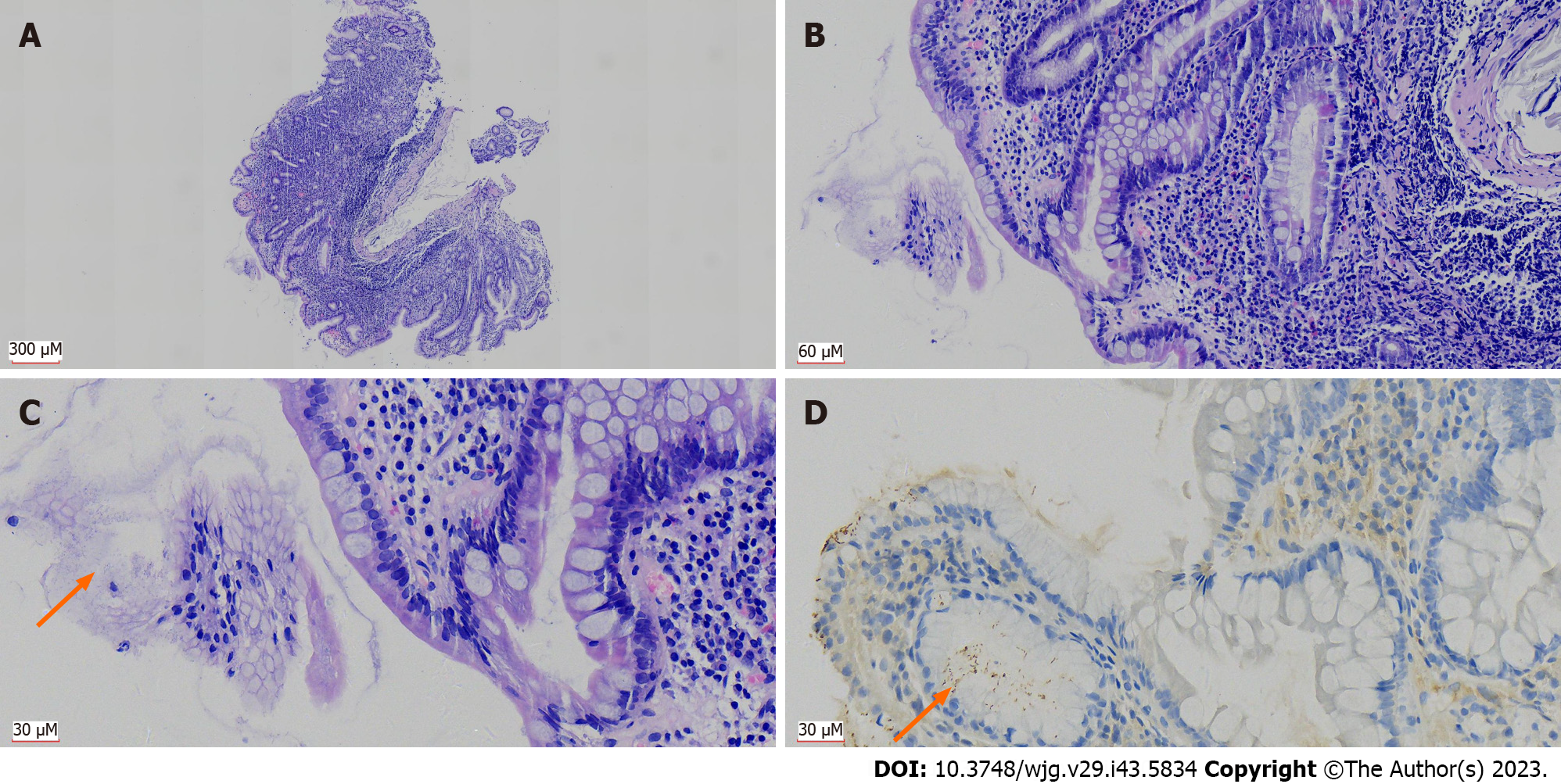
For gastritis, 91.6% (4222/4609) of the cases showed intact structure of the mucosal lamina propria, which was evaluated and graded according to chronic gastritis, active gastritis, atrophy and intestinal metaplasia. The positive rates for H. pylori were as follows: (1) Chronic gastritis: H. pylori positive rates of grades 1-3 were 29.9%, 85.2% and 95.6%, respectively; (2) Active gastritis: The positive rates of H. pylori in grades 0-3 were 27.6%, 74.9%, 95.6% and 100%, respectively; (3) Atrophy: The positive rates of H. pylori in grades 0-3 were 54.4%, 57.3%, 65.5% and 80%, respectively; and (4) Intestinal metaplasia: The positive rates of H. pylori in grades 0-3 were 55.6%, 48.6%, 63.5% and 66.7%, respectively. The H. pylori infection rates in patients with different grades of chronic gastritis and active gastritis differed significantly (both P < 0.001). There was a significant difference in the H. pylori infection rate among patients with different grades of intestinal metaplasia, by χ2 test (P = 0.032), indicating that H. pylori infection was associated with higher grade of chronic/active gastritis and intestinal metaplasia (Figure 4). However, there was no significant difference (P = 0.084 by χ2 test) in the H. pylori infection rates between patients with different grades of atrophy (Table 6).
| Characteristics | Grading | H. pylori positive rate | ||
| IHC | IHC & 14C UBT combined1 | P value | ||
| Chronic | G1 | 13.3% (312/2348) | 29.9% (703/2348) | P < 0.0012 |
| G2 | 65.6% (1110/1692) | 85.2% (1441/1692) | ||
| G3 | 81.3% (148/182) | 95.6% (174/182) | ||
| Active | G0 | 10.5% (229/2174) | 27.6% (599/2174) | P < 0.0012 |
| G1 | 54.1% (626/1157) | 74.9% (867/1157) | ||
| G2 | 80.3% (705/878) | 95.6% (839/878) | ||
| G3 | 76.9% (10/13) | 100% (13/13) | ||
| Atrophy | G0 | 37.0% (1367/3697) | 54.4% (2010/3697) | P = 0.0841 |
| G1 | 37.2% (170/457) | 57.3% (262/457) | ||
| G2 | 46.6% (27/58) | 65.5% (38/58) | ||
| G3 | 60.0% (6/10) | 80.0% (8/10) | ||
| Intestinal metaplasia | G0 | 38.8% (1235/3183) | 55.6% (1771/3183) | P = 0.0321 |
| G1 | 30.9% (289/934) | 48.6% (480/934) | ||
| G2 | 43.8% (42/96) | 63.5% (61/96) | ||
| G3 | 44.4% (4/9) | 66.7% (6/9) | ||
For diagnosis of H. pylori infection, both invasive and noninvasive tests are used. Histology with special stains, rapid urease tests (RUTs), bacterial culture, and polymerase chain reaction (PCR) are examples of invasive tests, which require endoscopy and biopsy. Although PCR-based methods were proposed as gold standard tests[10], expensive, complex, and time-consuming methods, they were not widely used for screening, and were preferred for cases when an etiological role of H. pylori was clinically suggested but histopathological confirmation was not possible[21]. To increase the specificity of the histological test, special stains such as modified Giemsa stain, Warthin-Starry Silver stain, and immunohistochemical stain can be used, and IHC is considered to be the most reasonable histological method[21-23]. Serology, stool antigen testing, and UBT are examples of noninvasive tests[24]. UBT is often considered as the gold standard diagnostic test for H. pylori infection[10], and consistently produces better results in comparison to many other available tests. IHC and 14C UBT are most widely used in our hospital for routine screening of H. pylori. It was not clear whether we needed to perform the two methods simultaneously for H. pylori detection, and there were controversial reports about the sensitivity of IHC as being either superior[20] or inferior[19] to 14C UBT. The number of cases of the two studies mentioned above is relatively small, thus the conclusion might have coincidence or selection bias.
The novelty of our study was that it strongly proved that the sensitivity of 14C UBT was significantly higher than that of IHC. We also found about 20% inconsistency between the results of 14C UBT and IHC, although most were negative for IHC and positive for 14C UBT (> 17%). It is speculated that there is a significant focal distribution pattern of H. pylori infection in these cases[25,26], and the site and size of biopsy specimen obtained by endoscopic sampling are limited, causing false-negative results[24]. Our research showed that increasing the number of biopsy specimens (n ≥ 2) significantly improved the positive rate of H. pylori detection in pathological IHC methods as expected, and the greater the number of biopsy specimens, the fewer false-negative results from sampling errors[24]. However, it might be unacceptable to do multiple biopsies for almost-healthy patients. 14C UBT can overcome this drawback and reflect the overall H. pylori infection status in the upper gastrointestinal tract[18].
We should point out that 14C UBT might be unreliable when the reported value is around the cut-off value, and these cases might be infected with a lower density of H. pylori[10,27]. That could explain why a small number of cases (< 3%) were negative for 14C UBT but positive for IHC in our study. IHC is superior for tracing H. pylori infections in such circumstances, relying on both morphological review and the specificity of the H. pylori antibodies used for IHC[15]. The specificity of the primary antibody used in IHC prevents mistaking other resident or contaminating bacteria for H. pylori[13,15].
We showed that although IHC gave direct evidence of H. pylori infection, its sensitivity was lower and there was a higher risk of false-negative results. 14C UBT is still the preferred method for detecting H. pylori infection clinically. However, to improve the positive rate of H. pylori detection, we recommend performing 14C UBT and IHC simultaneously, especially for patients unexplained gastritis, previously treated with low organism density, and with results around the cut-off value[15]. Our statistical analysis supports that the H. pylori positive rate can increase significantly as multiple endoscopic biopsies are submitted. One limitation of our was is that we did not perform a PCR-based method as the gold standard. However, potential pitfalls still exist with PCR methods, and more studies need to be conducted on diagnostic tests for H. pylori to find a reliable gold standard. Positive findings with either IHC or 14C UBT are highly specific[14,28], and are widely recognized to establish diagnosis of H. pylori infection[18,29,30]; thus, we focused on their sensitivity.
H. pylori infection is found worldwide, but the rate varies significantly among different countries (11%-91%)[29]. China has a high incidence of H. pylori infection, and it has decreased to 40%-60% after economic development and changes in lifestyle[30,31]. Our study showed that the overall infection rate of H. pylori in a single center in Xinjiang Uyghur Autonomous Region (Urumqi) was 48.6%, which was close to the national infection rate[30,31]. However, the infection rate was significantly higher than previously reported (36.5%) in Shihezi (located adjacent to Urumqi) in the XPCC[7]. We speculate that H. pylori infection was detected by morphological examination without special stains, and was lower in sensitivity. The majority of the population in Shihezi area is of Han nationality. The overall infection rate of H. pylori in this center was also higher than the 43.6% overall positive infection rate reported by the First Affiliated Hospital of Xinjiang Medical University in the same region of Urumqi in 2012[6], which used a combination of RUT and 14C UBT. Thus, the higher H. pylori positivity rate in our center was likely related to the higher sensitivity of our immunohistochemical assay compared to their RUT assay. Our overall infection rate was lower than that reported in Yili region[9], because the majority of cases in the other study were from multiple ethnic groups, whose H. pylori infection rate were all higher than in the Han group.
Xinjiang Uyghur Autonomous Region is a vast region with multiple ethnic groups living together. Previous reports have shown that there are differences in H. pylori infection rates between different genders, ages, ethnic groups, and regions[6-9]. Our single center data also showed that the overall infection rate in males was significantly higher than in females, which is consistent with reports in the Shihezi area[7]. However, these were inconsistent with the reports in the Ili area[9], with no significant gender difference. We believe that both our cases and the cases in Shihezi area were randomly enrolled with a large sample size, and the majority were Han ethnicity. The infection rate among Uyghur women in our study was also higher than in men, and the proportion of ethnic minorities in the study from Ili region[9] is larger than in our cases from Urumqi.
From a perspective of regional difference, patients from southern Xinjiang had the highest positive rate of H. pylori infection, followed by patients from northern Xinjiang, and patients from the XPCC had the lowest H. pylori infection rate. We speculate that it may be related to the fact that southern Xinjiang is mainly inhabited by Uyghur ethnic groups, while northern Xinjiang is mainly inhabited by Han ethnic groups, as is the XPCC[7].
These results mentioned above suggest that ethnic difference is the most important cause of difference in H. pylori infection rate, which was confirmed by our matched comparative analysis. The differences in ethnicity might be one of the most important factors affecting the epidemic characteristics of H. pylori infection. The positive rate for H. pylori obtained by combining the two most sensitive methods in Uyghur was 75.3%, which was higher than previously reported (59.8%-62.38%)[6,8,9], indicating that the prevention and control for H. pylori infection in Uyghur people might be more challenging.
In addition, regardless of the Uyghur or Han ethnic groups, the H. pylori infection rate calculated by age group showed a decreasing trend as age increased. In the Han ethnic group, there was an increase after the age of 70 years, which is consistent with previous reports[6,32,33], indicating that we might need to eradicate H. pylori infection in young people as early as possible.
Since our hospital is a general hospital focused on traditional Chinese medicine, the majority of patients have mild stomach discomfort. Overall, benign inflammatory lesions were the main cause (> 90%) in this continuous randomized study. Therefore, there were few cases of malignant lesions such as gastric adenocarcinoma included in this study. Malignant tumors have a closer correlation with H. pylori infection, with over two-thirds of gastric adenocarcinoma cases and 75% of lymphoma cases having H. pylori infection. According to the literature, eradicating H. pylori in the first-degree relatives of gastric cancer patients is an important factor in reducing the incidence of gastric cancer[34]. In addition, H. pylori-related gastric lymphoma patients can achieve complete remission by eradicating H. pylori[35,36]. BE is a precancerous lesion and some studies have shown that H. pylori infection and BE are inversely related[37,38]. The H. pylori infection rate (58.7%) was higher than the overall positive level, and BE may be related to H. pylori infection; however, the positive association between H. pylori infection and BE seemed to be a paradox. Well-designed prospective cohort studies with a powered sample size are required[39].
For gastritis, the H. pylori infection rate significantly increased with grade of chronic/active gastritis, and intestinal metaplasia grading. Infection with H. pylori may be an important factor leading to gastritis and intestinal metaplasia[40]. Eradicating H. pylori can improve the inflammatory response of the gastric mucosa, prevent or delay development of atrophy and intestinal metaplasia[40,41], block the Correa mode of intestinal gastric cancer evolution, and eliminate the risk of (intestinal) gastric cancer[18,29,33,34]. By combining the IHC and 14C UBT results, we can obtain a better linear correlation between H. pylori infection and severity of gastritis, thus it is important to use two diagnostic tests at the same time.
Our results show that the sensitivity of 14C UBT for detecting H. pylori is significantly higher than that of IHC with pathological specimens obtained by gastroscopy. The combination of 14C UBT and IHC is necessary to improve the detection rate of H. pylori infection, and increasing the number of biopsy specimen (≥ 2) can significantly improve the positive rate of H. pylori detection in pathological immunohistochemical methods. The overall H. pylori infection rate in Xinjiang Uyghur Autonomous Region was higher than previously reported, and ethnicity was the most important factor for detection by combination of the two methods. Uyghur people have a higher H. pylori infection rate. The overall H. pylori infection rate calculated by age group showed a decreasing trend as age increased. Therefore, the key populations for the prevention and control of H. pylori infection in Xinjiang Uyghur Autonomous Region are the Uyghur and young people.
There are differences in Helicobacter pylori (H. pylori) infection rate in Uyghur and Han ethnic groups. 14C urea breath test (14C UBT) and immunohistochemistry (IHC) with tissue from gastroscopic biopsy are widely used detection methods, but both lack large cohort studies to accurately evaluate their performance.
To compare the difference between 14C UBT and IHC for accurate testing for H. pylori infection, and to study the difference in infection positive rate between Uyghur and Han ethnic groups.
We included 5747 cases with H. pylori infection detected by both IHC and 14C UBT. We detected 3944 by simultaneous IHC and 14C UBT and 555 pairs of Han/Uyghur were compared for their H. pylori infection rate.
IHC and 14C UBT were performed at the same time (interval < 1 wk, with sampling site including gastric antrum), and 3944 cases were screened out. The overall H. pylori infection positive rate was calculated by combining IHC and 14C UBT results (n = 5747). Correlation between H. pylori infection and patients’ clinical parameters (gender, age, ethnicity and region) was analyzed. 555 pairs of Han/Uyghur cases (completely matched for gender and age) were compared for their H. pylori infection rates. The H. pylori infection rate and pathological diagnosis, including gastritis (chronic/active inflammation, atrophy, and intestinal metaplasia), were analyzed.
Among the 3944 cases for which 14C UBT and IHC were performed at the same time, the sensitivity was 94.9% for 14C UBT and 65.1% for IHC, which was a significant difference (P < 0.001). Among those positive by 14C UBT (detection value > 100), the H. pylori positive rate with IHC was 63.2%, and among those negative for 14C UBT (detection value ≤ 100), the IHC positive rate was 4.8%. In combination with both detection methods, the total rate of H. pylori infection in all 5747 patients was 48.6%, and there were significant differences for gender, age, ethnicity, and region (P values were 0.001, < 0.001, < 0.001 and < 0.001). The H. pylori infection rates for the 555 Chinese/Uyghur paired cases (completely matched for gender and age) were 41.4% and 73.3%, which was a significant difference (P < 0.001). For benign gastric lesions, the combined H. pylori infection rate was 53.8% for inflammation, 27.5% for fundus gland polyps, 22.2% for duodenal ectopic gastric mucosa, 17.5% for hyperplastic polyps, 58.7% for BE, and 67.6% for gastric adenocarcinoma. Positivity for H. pylori infection was significantly related to moderate-severe (grade 2-3) chronic inflammation, moderate-severe active inflammation and moderate-severe (grade 2-3) intestinal metaplasia (P < 0.001, < 0.001 and 0.032 in order).
The sensitivity of 14C UBT was significantly higher than that of IHC when detecting H. pylori infection, but there were still H. pylori positive cases missed that were detected by IHC. Combination of both methods can increase the detection accuracy of H. pylori infection, and the overall infection rate of H. pylori in our study was higher than previously reported in Xinjiang Uyghur Autonomous Region. Ethnic difference was the most important factor affecting the H. pylori infection rate, and the Uyghur people had more H. pylori infection. The H. pylori infection rate decreased with age, and was more correlated with precancerous lesions and malignant tumors, and increased with severity of inflammation.
Our study highlights the importance of using IHC and 14C UBT together for H. pylori infection, and the prevention and intervention of H. pylori infection in Xinjiang Uyghur Autonomous Region and emphasizes that the Uyghur and young people should be examined as early as possible.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bordin DS, Russia; Keikha M, Iran S-Editor: Wang JJ L-Editor: A P-Editor: Yuan YY
| 1. | Duan M, Li Y, Liu J, Zhang W, Dong Y, Han Z, Wan M, Lin M, Lin B, Kong Q, Ding Y, Yang X, Zuo X. Transmission routes and patterns of helicobacter pylori. Helicobacter. 2023;28:e12945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 2. | Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 454] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Møller H, Heseltine E, Vainio H. Working group report on schistosomes, liver flukes and Helicobacter pylori. Int J Cancer. 1995;60:587-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1322] [Cited by in RCA: 1180] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 5. | Epidemiology of, and risk factors for, Helicobacter pylori infection among 3194 asymptomatic subjects in 17 populations. The EUROGAST Study Group. Gut. 1993;34:1672-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 293] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Aisikaier A, Maimaitituersun M, Xie ZH. Helicobacterpylori Infection and Associated Diseases and Risk Factors Analysis among Xinjiang Uyghur, Han, Kazak Ethnic People. Xinjiang Med J. 2012;42:4-9. |
| 7. | Qi XY, Li R, Chen WG, Tian SX, Liu F, Han YZ, Ruan KX, Zheng Y, Shang GC. Analysis of Helicobacter pylori infection about 25699 cases by gastroscopy examination in Shihezi region, Xinjiang. Shandong Med J. 2012;32-34. |
| 8. | Freedland SJ, Aronson WJ. Commentary on "Integrative clinical genomics of advanced prostate cancer". Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, Beltran H, Abida W, Bradley RK, Vinson J, Cao X, Vats P, Kunju LP, Hussain M, Feng FY, Tomlins SA, Cooney KA, Smith DC, Brennan C, Siddiqui J, Mehra R, Chen Y, Rathkopf DE, Morris MJ, Solomon SB, Durack JC, Reuter VE, Gopalan A, Gao J, Loda M, Lis RT, Bowden M, Balk SP, Gaviola G, Sougnez C, Gupta M, Yu EY, Mostaghel EA, Cheng HH, Mulcahy H, True LD, Plymate SR, Dvinge H, Ferraldeschi R, Flohr P, Miranda S, Zafeiriou Z, Tunariu N, Mateo J, Perez-Lopez R, Demichelis F, Robinson BD, Schiffman M, Nanus DM, Tagawa ST, Sigaras A, Eng KW, Elemento O, Sboner A, Heath EI, Scher HI, Pienta KJ, Kantoff P, de Bono JS, Rubin MA, Nelson PS, Garraway LA, Sawyers CL, Chinnaiyan AM. Cell. 21 May 2015; 161(5): 1215-1228. Urol Oncol. 2017;35:535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 284] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 9. | Huo XL, Qin J, Zhang W, Zhang WZ, Zhu XL, Dou YQ, Ye NN. Factors related to rate of Helicobacter pylori infection in patients with upper gastrointestinal tract diseases in Yili. World Chinese J Digestol. 2013;21:1568-1572. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Patel SK, Pratap CB, Jain AK, Gulati AK, Nath G. Diagnosis of Helicobacter pylori: what should be the gold standard? World J Gastroenterol. 2014;20:12847-12859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 146] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (3)] |
| 11. | Wang YK, Kuo FC, Liu CJ, Wu MC, Shih HY, Wang SS, Wu JY, Kuo CH, Huang YK, Wu DC. Diagnosis of Helicobacter pylori infection: Current options and developments. World J Gastroenterol. 2015;21:11221-11235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 218] [Cited by in RCA: 264] [Article Influence: 26.4] [Reference Citation Analysis (8)] |
| 12. | Kalali B, Formichella L, Gerhard M. Diagnosis of Helicobacter pylori: Changes towards the Future. Diseases. 2015;3:122-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Benoit A, Hoyeau N, Fléjou JF. Diagnosis of Helicobacter pylori infection on gastric biopsies: Standard stain, special stain or immunohistochemistry? Ann Pathol. 2018;38:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Kocsmár É, Szirtes I, Kramer Z, Szijártó A, Bene L, Buzás GM, Kenessey I, Bronsert P, Csanadi A, Lutz L, Werner M, Wellner UF, Kiss A, Schaff Z, Lotz G. Sensitivity of Helicobacter pylori detection by Giemsa staining is poor in comparison with immunohistochemistry and fluorescent in situ hybridization and strongly depends on inflammatory activity. Helicobacter. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Wang XI, Zhang S, Abreo F, Thomas J. The role of routine immunohistochemistry for Helicobacter pylori in gastric biopsy. Ann Diagn Pathol. 2010;14:256-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Helicobacter pylori Study Group; Chinese Society of Gastroenterology; Chinese Medical Association. Sixth Chinese national consensus report on the management of Helicobacter pylori infection (treatment excluded). Chinese J Digest. 2022;. |
| 17. | Godbole G, Mégraud F, Bessède E. Review: Diagnosis of Helicobacter pylori infection. Helicobacter. 2020;25 Suppl 1:e12735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Shakir SM, Shakir FA, Couturier MR. Updates to the Diagnosis and Clinical Management of Helicobacter pylori Infections. Clin Chem. 2023;69:869-880. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Dechant FX, Dechant R, Kandulski A, Selgrad M, Weber F, Reischl U, Wilczek W, Mueller M, Weigand K. Accuracy of Different Rapid Urease Tests in Comparison with Histopathology in Patients with Endoscopic Signs of Gastritis. Digestion. 2020;101:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Wan WS, Wang L, Hu YC, Liu YF, Zheng DP, Hu QL. Comparison of immunohistochemical stain and 14C urea breath test in the diagnosis of helicobacter pylori associated gastritis. Chinese J Clin Experiment Pathol. 2019;35:47-50. [DOI] [Full Text] |
| 21. | Daugule I, Megraud F, Leja M. Reply to Kocsmár, É.; Lotz, G. Comment on "Skrebinska et al. Who Could Be Blamed in the Case of Discrepant Histology and Serology Results for Helicobacter pylori Detection? Diagnostics 2022, 12, 133". Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Wan W, Wang L, Liu Y, Hu Y. Improving the detection of Helicobacter pylori in biopsies of chronic gastritis: a comparative analysis of H&E, methylene blue, Warthin-Starry, immunohistochemistry, and quantum dots immunohistochemistry. Front Oncol. 2023;13:1229871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 23. | Urgessa NA, Geethakumari P, Kampa P, Parchuri R, Bhandari R, Alnasser AR, Akram A, Kar S, Osman F, Mashat GD, Tran HH, Arcia Franchini AP. A Comparison Between Histology and Rapid Urease Test in the Diagnosis of Helicobacter Pylori in Gastric Biopsies: A Systematic Review. Cureus. 2023;15:e39360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Lee JH, Park YS, Choi KS, Kim DH, Choi KD, Song HJ, Lee GH, Jang SJ, Jung HY, Kim JH. Optimal biopsy site for Helicobacter pylori detection during endoscopic mucosectomy in patients with extensive gastric atrophy. Helicobacter. 2012;17:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Lee JY, Kim N. Diagnosis of Helicobacter pylori by invasive test: histology. Ann Transl Med. 2015;3:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 26. | Kobayashi D, Eishi Y, Ohkusa T, Ishige I, Suzuki T, Minami J, Yamada T, Takizawa T, Koike M. Gastric mucosal density of Helicobacter pylori estimated by real-time PCR compared with results of urea breath test and histological grading. J Med Microbiol. 2002;51:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Perri F. Diagnosis of Helicobacter pylori infection: which is the best test? The urea breath test. Dig Liver Dis. 2000;32 Suppl 3:S196-S198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Ji YH, Shi YM, Hei QW, Sun JM, Yang XF, Wu T, Sun DL, Qi YX. Evaluation of guidelines for diagnosis and treatment of Helicobacter pylori infection. Helicobacter. 2023;28:e12937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 29. | Cho JH, Jin SY. Current guidelines for Helicobacter pylori treatment in East Asia 2022: Differences among China, Japan, and South Korea. World J Clin Cases. 2022;10:6349-6359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (8)] |
| 30. | Xie C, Lu NH. Review: clinical management of Helicobacter pylori infection in China. Helicobacter. 2015;20:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Mitchell HM, Li YY, Hu PJ, Liu Q, Chen M, Du GG, Wang ZJ, Lee A, Hazell SL. Epidemiology of Helicobacter pylori in southern China: identification of early childhood as the critical period for acquisition. J Infect Dis. 1992;166:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 248] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Marginean CM, Cioboata R, Olteanu M, Vasile CM, Popescu M, Popescu AIS, Bondari S, Pirscoveanu D, Marginean IC, Iacob GA, Popescu MD, Stanciu M, Mitrut P. The Importance of Accurate Early Diagnosis and Eradication in Helicobacter pylori Infection: Pictorial Summary Review in Children and Adults. Antibiotics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 33. | Suzuki H, Mori H. World trends for H. pylori eradication therapy and gastric cancer prevention strategy by H. pylori test-and-treat. J Gastroenterol. 2018;53:354-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 34. | Cavanna L, Pagani R, Seghini P, Zangrandi A, Paties C. High grade B-cell gastric lymphoma with complete pathologic remission after eradication of Helicobacter pylori infection: report of a case and review of the literature. World J Surg Oncol. 2008;6:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Hu Q, Zhang Y, Zhang X, Fu K. Gastric mucosa-associated lymphoid tissue lymphoma and Helicobacter pylori infection: a review of current diagnosis and management. Biomark Res. 2016;4:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Wang C, Yuan Y, Hunt RH. Helicobacter pylori infection and Barrett's esophagus: a systematic review and meta-analysis. Am J Gastroenterol. 2009;104:492-500; quiz 491, 501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Fujita M, Nakamura Y, Kasashima S, Furukawa M, Misaka R, Nagahara H. Risk factors associated with Barrett's epithelial dysplasia. World J Gastroenterol. 2014;20:4353-4361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Polyzos SA, Zeglinas C, Artemaki F, Doulberis M, Kazakos E, Katsinelos P, Kountouras J. Helicobacter pylori infection and esophageal adenocarcinoma: a review and a personal view. Ann Gastroenterol. 2018;31:8-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Toyokawa T, Suwaki K, Miyake Y, Nakatsu M, Ando M. Eradication of Helicobacter pylori infection improved gastric mucosal atrophy and prevented progression of intestinal metaplasia, especially in the elderly population: a long-term prospective cohort study. J Gastroenterol Hepatol. 2010;25:544-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 40. | Vázquez Romero M, Boixeda de Miquel D, Valer López-Fando MP, Albéniz Arbizu E, González Alonso R, Bermejo San José F. Intestinal metaplasia: evolution after Helicobacter pylori eradication and influence in the success of eradicating therapy. Rev Esp Enferm Dig. 2003;95:781-784, 777. [PubMed] |
| 41. | Crafa P, Russo M, Miraglia C, Barchi A, Moccia F, Nouvenne A, Leandro G, Meschi T, De' Angelis GL, Di Mario F. From Sidney to OLGA: an overview of atrophic gastritis. Acta Biomed. 2018;89:93-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |