Published online Oct 28, 2023. doi: 10.3748/wjg.v29.i40.5593
Peer-review started: August 28, 2023
First decision: September 11, 2023
Revised: September 25, 2023
Accepted: October 17, 2023
Article in press: October 17, 2023
Published online: October 28, 2023
Processing time: 60 Days and 0.5 Hours
Gastric cancer (GC) is the sixth most common cancer and third leading cause of cancer-related deaths worldwide. Current treatments mainly rely on surgery- and chemotherapy-based systemic; however, the prognosis remains poor for advanced disease. Recent studies have suggested that immunotherapy has significant potential in cancer therapy; thus, GC immunotherapy may improve quality of life and survival for patients with this disease.
To provide a comprehensive overview of the knowledge structure and research hotspots of GC immunotherapy.
We conducted a bibliometric analysis of publications on immunotherapy related to GC in the Web of Science Core Collection database. We analyzed 2013 pub-lications from 1999 to February 1, 2023, using the VOSviewer and CiteSpace software. We assessed publication and citation distributions using the WoS platform and explored research countries, institutions, journals, authors, references, and keywords (co-occurrence, timeline view, and burst analysis). In addition, we examined 228 trials on immunotherapy, 137 on adoptive cell therapy, 274 on immune checkpoint inhibitors (ICIs), and 23 on vaccines from ClinicalTrials.gov and the International Clinical Trials Registry Platform. The Impact Index Per Article for the top ten high-cited papers collected from Reference Citation Analysis (RCA) are presented.
Our bibliometric analysis revealed that the study of immunotherapy in GC has developed rapidly in recent years. China accounted for almost half the publications, followed by the United States. The number of publications in recent years has been growing continuously, and most institutions and authors with the most publications are from China. The main keywords or clusters identified were “tumor microenvironment”, “adoptive immunotherapy”, “dendritic therapy”, and “microsatellite instability”.
Our analysis of 2013 publications indicated that immunotherapy for GC has led to several new developments in recent years. Considerable progress has been made in vaccinations, immune checkpoint therapy, and adoptive cellular therapy. In particular, ICIs and chimeric antigen receptor T-cells are novel options for the treatment of GC. We suggest that the combination of ICIs, chemotherapy, targeted therapy, and other immunotherapies should be the primary research direction in the future.
Core Tip: In this study, we systematically analyzed studies related to immunotherapy for gastric cancer (GC). We used scientometrics to explore research hotspots in the field and summarize the current developmental status of GC immunotherapy, as well as the advantages and disadvantages of different immunotherapy modalities. We also compiled information on ongoing clinical trials and predicted future developmental trends in this field based on the direction and stage of these trials. This research can help advance our understanding of the latest progress and future development trends in the field, as well as provide scientific research recommendations.
- Citation: Li YN, Xie B, Zhang Y, He MH, Xing Y, Mu DM, Wang H, Guo R. Advances and key focus areas in gastric cancer immunotherapy: A comprehensive scientometric and clinical trial review (1999-2023). World J Gastroenterol 2023; 29(40): 5593-5617
- URL: https://www.wjgnet.com/1007-9327/full/v29/i40/5593.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i40.5593
Gastric cancer (GC) is the sixth most prevalent cancer and the third leading cause of cancer-related fatalities worldwide, thus presenting a significant global health concern[1]. The incidence rates of GC are highest in Eastern Europe and Eastern Asia, and it remains the most prevalent cancer and the leading cause of cancer-related deaths in specific regions of South and Central Asia[2]. Despite a trend toward decreasing morbidity and mortality rates in several countries and regions, GC continues to be a substantial health burden[3]. Numerous factors have been established to contribute to the pathogenesis of GC, including family history, dietary habits, smoking, and Helicobacter pylori or Epstein-Barr virus (EBV) infection[4]. GC can be categorized into four distinct molecular subgroups based on the patterns of molecular alterations, with each subgroup corresponding to a different disease progression and prognosis: (1) Microsatellite stable/epithelial-mesenchymal transition; (2) Microsatellite instability (MSI); (3) Tumor protein 53 (TP53)-active; and (4) TP53-inactive types[5].
Owing to its predominantly asymptomatic nature, early detection of GC poses a significant challenge, with > 50% of patients being diagnosed after the cancer has already metastasized[6,7]. The prognosis of advanced GC remains dismal; the five-year overall survival (OS) rate is < 5% and the median OS is approximately 8 mo[8-10]. Although surgery remains the primary curative approach, chemotherapy forms the foundation of treatment for metastatic GC, with multimodal therapy employed to enhance survival outcomes[9]. However, the efficacy of conventional treatments, including surgery, radiotherapy, chemotherapy, and anti-human epidermal growth factor receptor-2 (HER2) therapy, in combating this lethal disease[11].
Surgery, chemotherapy, radiation therapy, and targeted therapy have long been considered the four pillars of GC management; however, immunotherapy has recently emerged as a promising “fifth pillar”, and its use is rapidly expanding[12]. For cases of unresectable locally advanced, recurrent, or metastatic GC, a combination therapy of anti-HER2, chemotherapy, and optional pembrolizumab is preferred for HER2-positive diseases, with the inclusion of pembrolizumab demonstrating a high objective response rate (ORR)[13]. Irrespective of HER2 status, nivolumab [for programmed death-ligand 1 (PD-L1) CPS ≥ 5] is recommended as part of systemic treatment regimens[14]. Currently, there are four principal strategies for tumor immunotherapy: Immune checkpoint inhibitors (ICIs), tumor vaccines, adoptive immunotherapy, and nonspecific immunomodulators. With advancements in the understanding of the tumor microenvironment (TME), immunotherapy for advanced GC has evolved rapidly, demonstrating superior efficacy and tolerable toxicity compared to traditional therapies, leading to the rapidly increasing use of ICIs[7]. As most GCs are relatively resistant to ICI monotherapy, patients may benefit from combination therapy to achieve enhanced therapeutic effects[15]. Multiple studies have demonstrated that immunotherapy combined with conventional therapy provides superior efficacy compared with monotherapy[16]. Although immunotherapy holds promise for the treatment of GC, the complexity of the immune microenvironment and the heterogeneity of immunogenicity present significant challenges that require further investigation[17].
Chemotherapies for patients with locally advanced or metastatic GC include S-1 + oxaliplatin, docetaxel + oxaliplatin + fluorouracil, docetaxel + oxaliplatin + S-1 (DOS), capecitabine + oxaliplatin (XELOX), and folinic acid/5-fluorouracil/oxaliplatin chemotherapy (FOLFOX). Trastuzumab or pembrolizumab should be added to first-line chemotherapy for patients with HER2 overexpression-positive GC[18,19]. There are two commonly used combinations: Trastuzumab combined with a fluoropyrimidine and a platinum agent and a combination of trastuzumab, pembrolizumab, and XELOX/PF. Regimens for HER2-negative disease include nivolumab, cindilimab, and tislelizumab combined with first-line chemotherapy[20]. Docetaxel, cisplatin, 5-fluorouracil (DCF), modified DCF, and POF also exhibit promising activity. The selection of regimens for second-line or subsequent therapy depends on prior therapy and performance status. Ramucirumab combined with paclitaxel is the preferred second-line or subsequent therapy[19]. Single-agent docetaxel, paclitaxel, irinotecan, albumin-paclitaxel, pembrolizumab, nivolumab, vedicitumab, and apatinib mesylate have also been used as second-line or subsequent therapies. Although there is currently a relatively complete treatment plan, the OS of patients remains short. Therefore, more effective treatment options are required. Based on traditional treatment, explore combination therapy with immunotherapy, subdivide the population, and improve curative effects.
Recent phase I/II trials focusing on the perioperative use of ICIs in combination with chemotherapy for resectable locally advanced gastric/gastroesophageal junction cancer (GC/GEJC) have yielded positive results, thus expanding the potential applications of ICIs in GC management[21]. The addition of sintilimab to chemotherapy has demonstrated encouraging pathological complete response (pCR) and major pathological response rates as a perioperative treatment for resectable locally advanced GC/GEJC with manageable safety profiles. In a phase II study, durvalumab combined with DOS as neoadjuvant chemotherapy reached its primary efficacy endpoint, confirming a pCR in 29.0% of the patients (9 of 31) with acceptable toxicity (safety endpoint < 20%)[22]. In a separate multicenter, single-arm, multi-cohort, phase II trial of tremelimumab and durvalumab as neoadjuvant treatments in patients with MSI-high (MSI-H) resectable gastric adenocarcinoma/gastroesophageal junction adenocarcinoma (GAC/GEJAC; NCT04817826), a pCR rate of 60% (9 of 15) and a major-complete pathological response rate of 18% (< 10% viable cells) were achieved[23].
Bibliometrics is a methodological approach that encompasses the analysis and summary of data produced within a specific timeframe. It offers invaluable insights into scientific productivity, behavior, and advancements within the research domain[24]. The objective of this study was to employ bibliometric analysis to elucidate the current status and emergent trends in the field of GC immunotherapy research.
This analysis was conducted on February 1, 2023, and searched the Web of Science Core Collection (WoSCC) and Science Citation Index Expanded. The retrieval terms in the topic: (“gastric cancer” OR “gastric adenocarcinoma” OR “gastric neoplasm” OR “gastric tumor” OR “stomach cancer” OR “stomach adenocarcinoma” OR “stomach neoplasm” OR “stomach tumor” OR “gastric cancers” OR “gastric adenocarcinoma” OR “gastric neoplasms” OR “gastric tumors” OR “stomach cancers” OR “stomach adenocarcinoma” OR “stomach neoplasms” OR “stomach tumors” OR “tumor of stomach”) AND (“immunotherapeutic” OR “immunotherapy” OR “immunotherapies” OR “immunotherapeutics”). Types of documents: Article and review. Finally, the information for a total of 2013 documents was downloaded as Plain Text Files and Tab Delimited Files, and full records and cited references were included. All duplicate records were removed. All documents published in 2013 were included in the analysis.
We searched ClinicalTrials. gov and the International Clinical Trials Registry Platform (ICTRP) (clinical). The retrieval terms: (“gastric cancer” OR “gastric adenocarcinoma” OR “gastric neoplasm” OR “gastric tumor” OR “stomach cancer” OR “stomach adenocarcinoma” OR “stomach neoplasm” OR “stomach tumor”) AND (“immunotherapy”). There were 228 registered clinical trials, 25 of which were completed, and 113 were recruiting or not yet recruiting. We searched for some of the main immunotherapies in clinical trials using these two platforms. The research strategy: (“dendritic cells” OR “DNA vaccine” OR “RNA vaccine”) AND “gastric cancer” for vaccine clinical trials; (ACT OR TIL OR TCR-T OR CAR-T OR TCR T OR CAR T OR NK OR CIK) AND “gastric cancer” for ACT clinical trials; (ICI OR PD-1 OR PD-L1 OR CTLA-4) AND “gastric cancer” for ICI clinical trials. In total, 274, 137, and 23 clinical trials were incorporated, respectively. These retrievals were conducted on February 19, 2023.
We used CiteSpace (6.1.6) and VOSviewer (1.6.18) to analyze the data. Plain Text Files were analyzed using CiteSpace, and Tab Delimited Files were analyzed using the VOSviewer. CiteSpace made time slicings for the original files from January 1999 to December 2023 with 1 year per slice, and qualified records from 2012 were analyzed. The literature search and screening processes are illustrated in Figure 1. The Citation Report of WoS on February 1, 2023, provided the number of publications and citations annually. The figures used in this study were mapped using CiteSpace, VOSviewer, and Excel.
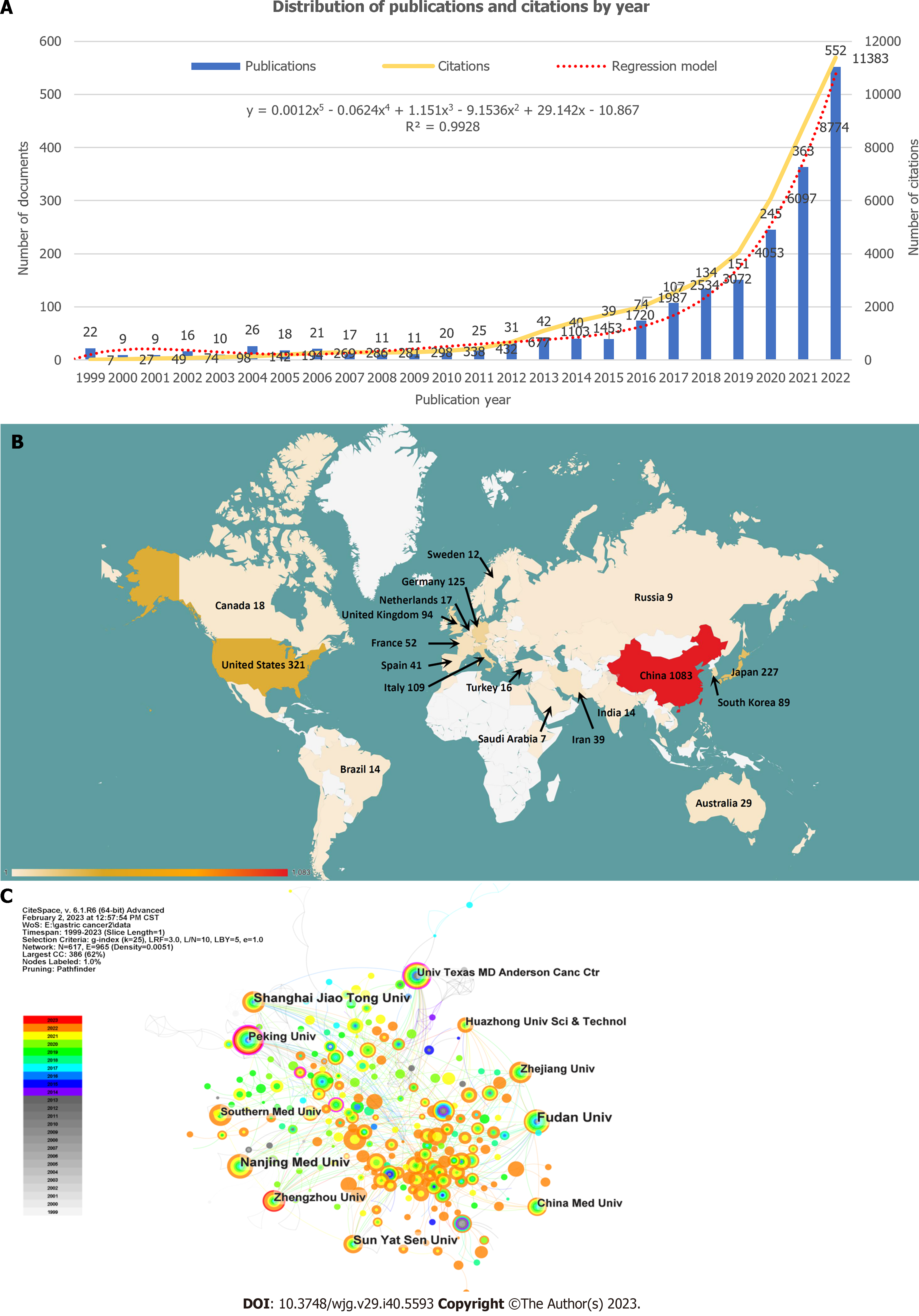
Annual distribution of publications and citations: The spatial-temporal distribution of scholarly publications has been demonstrating a noteworthy ascending trajectory. As per the WoSCC, the 2013 documents collectively garnered 45700 citations, averaging 22.7 citations per document until February 1, 2023. The H-index was 86, indicating that 86 documents each received over 86 citations. Figure 2A illustrates the annual distribution of publications and citations. Only 22 publications were released in 1999, with the number fluctuating between 9 and 42 from 1999 to 2015. However, starting in 2016, the number of publications experienced steady and significant growth, reaching 552 in 2022. Although there were only seven citations in 1999, this figure escalated to 11382 in 2022, marking 23 years of consistent growth. We adopted the regression model y = 0.0012x5 - 0.0624x4 + 1.151x3 - 9.1536x2 + 29.142x - 10.867 (R² = 0.9928) to show how the number of publications in this field changed over time and forecast it in the following year.
Related countries and institutions: The CiteSpace analysis revealed that contributions to publications in this field originated from 72 countries and 617 institutions, as depicted in Figures 2B and C. Of these, 26 countries and 58 institutions contributed ten or more publications. China had the highest number of publications (n = 1070), representing 53.2% of the total, which was significantly higher than that of other countries. The United States was second with 321 publications (16.0 %), followed by Japan (n = 227, 11.3%), Germany (n = 125, 6.2%), and Italy (n = 109, 5.4%). The top ten institutions with the highest number of publications were located in China, five of which were Fudan University (n = 85), Nanjing Medical University (n = 64), Shanghai Jiao Tong University (n = 64), Sun Yat-sen University (n = 52), and Zhengzhou University (n = 43).
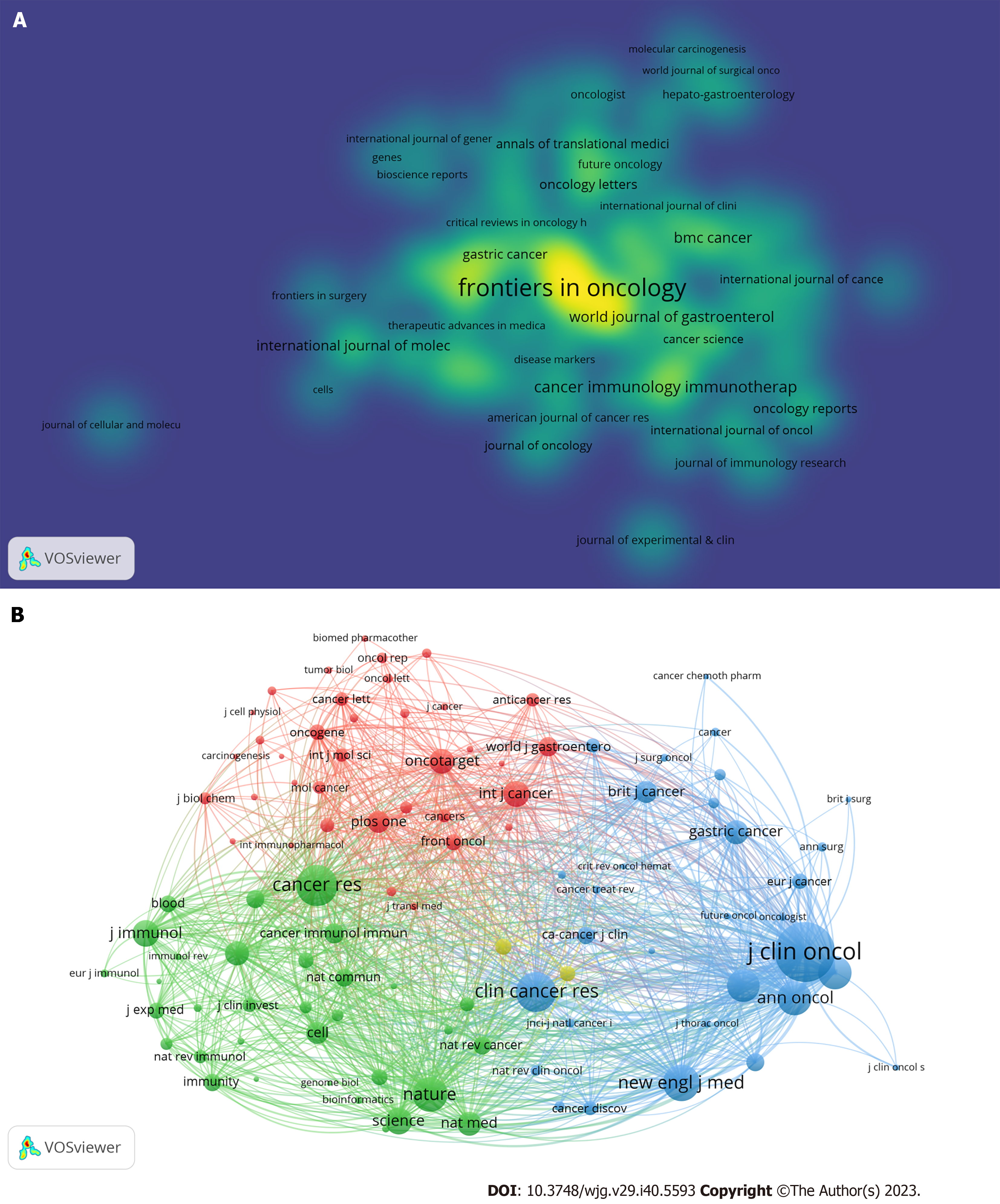
Journals: The 2013 analyzed documents were disseminated across 532 journals, with those comprising at least five documents incorporated into Figure 3A using the VOSviewer. The nodes exhibiting high brightness in this figure denote a higher frequency of occurrence. Frontiers in Oncology led the list with 104 documents, followed by Frontiers in Immunotherapy (n = 78) and Cancers (n = 69). Additionally, we conducted a co-citation analysis of the cited journals. These articles cited 5445 journals, and we analyzed 102 journals that received at least 200 citations (Figure 3B). The Journal of Clinical Oncology garnered the highest number of citations (n = 5044), followed by Cancer Research, with 3018 citations.
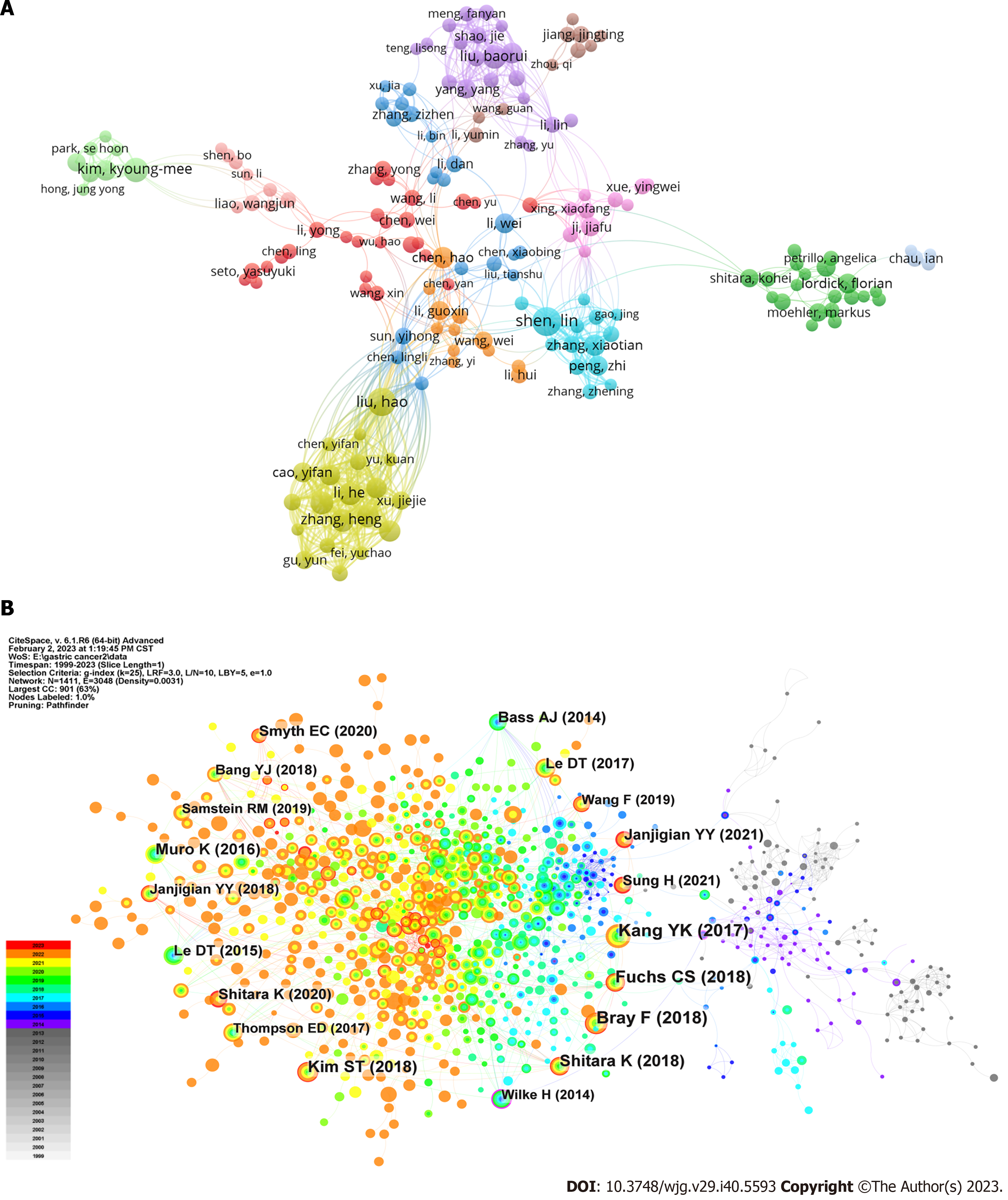
Authors: After excluding documents coauthored by more than 25 authors, we identified 11730 authors across the documents. Only authors with at least five publications were considered, resulting in an analysis of 212 authors who fulfilled the criteria (Figure 4A). In the VOSviewer analysis, Lin Shen had the highest number of publications (525 citations across 25 documents), followed by Hao Liu coming with the second highest number (444 citations across 24 documents). Regarding citations, Sakamoto Junichi had the most citations (810 citations across 6 documents), followed by Xin Wang (705 citations across seven documents). Thirty authors had at least 10 publications in this field, and 17 authors garnered over 400 citations.
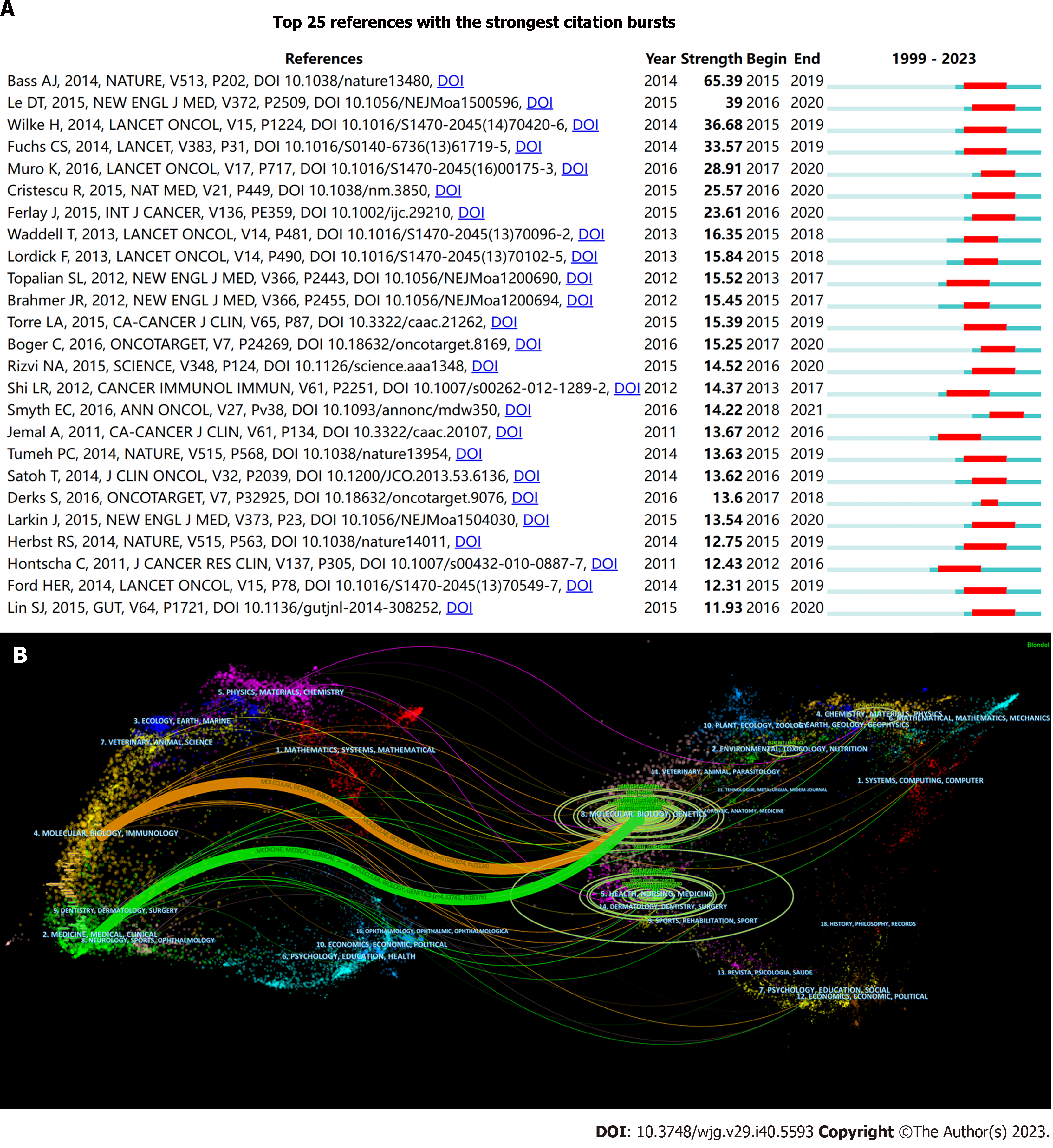
References: Table 1 shows the ten most frequently co-cited articles based on citation frequency data retrieved from WoS as of February 1, 2023. Seven of these articles were disseminated in the past seven years, and the majority of the highly cited documents had recent publication dates, suggesting a swift progression in this field (Figure 4B). The clinical trial spearheaded by Brahmer et al[25], illustrating the induction of tumor regression through an antibody-mediated PD-L1 blockade, amassed 5555 citations, which is significantly more than that of all other articles. This trial, published in the New England Journal of Medicine in 2012, experienced a citation surge from 2014 to 2017, exhibiting a strength of 15.45 (Figure 5A). Two additional articles focused on programmed cell death protein 1 (PD-1) or PD-L1, a topic that continues to garner significant attention[26,27]. The second most cited article, also a clinical trial, validated the efficacy of pembrolizumab in patients with advanced triple-negative breast cancer by evaluating its safety and antitumor activity[28]. The remaining articles in this table pertained to the TME, adjuvant chemotherapy, or review articles. The impact index per article of the top ten articles ranged from 30.8 to 524.5, which is also shown in Table 1.
| | Title | Year | Type | First author | Journal | IF (2021) | JCR | Co-citation | DOI | Impact index per article |
| 1 | Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer | 2012 | Clinical trial | Brahmer JR | N Engl J Med | 176.08 | Q1 | 5555 | 10.1056/NEJMoa1200694 | 524.5 |
| 2 | Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study | 2016 | Clinical trial | Nanda R | J Clin Oncol | 50.72 | Q1 | 1297 | 10.1200/JCO.2015.64.8931 | 133.7 |
| 3 | Benefit of Adjuvant Chemotherapy for Resectable Gastric Cancer A Meta-analysis | 2010 | Review | Paoletti X | JAMA | 157.34 | Q1 | 604 | 10.1001/jama.2010.534 | 47.9 |
| 4 | Progress in the treatment of advanced gastric cancer | 2017 | Review | Song ZY | Tumour Biol | 3.65 (2016) | Q2 | 490 | 10.1177/1010428317714626 | 75.7 |
| 5 | m(6)A regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer | 2020 | Article | Zhang B | Mol Cancer | 41.44 | Q1 | 442 | 10.1186/s12943-020-01170-0 | 134.0 |
| 6 | PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer | 2019 | Article | Kamada T | Proc Natl Acad Sci U S A | 12.78 | Q1 | 428 | 10.1073/pnas.1822001116 | 106.8 |
| 7 | Tumor Microenvironment Characterization in Gastric Cancer Identifies Prognostic and Immunotherapeutically Relevant Gene Signatures | 2019 | Article | Zeng DQ | Cancer Immunol Res | 12.02 | Q1 | 418 | 10.1158/2326-6066.CIR-18-0436 | 93.7 |
| 8 | PD-L1 expression in human cancers and its association with clinical outcomes | 2016 | Review | Wang X | Onco Targets Ther | 4.35 | Q2 | 412 | 10.2147/OTT.S105862 | 59.0 |
| 9 | Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer | 2019 | Article | Kather JN | Nat Med | 87.24 | Q1 | 400 | 10.1038/s41591-019-0462-y | 114.0 |
| 10 | The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial | 2010 | Clinical trial | Heiss MM | Int J Cancer | 7.32 | Q1 | 349 | 10.1002/ijc.25423 | 30.8 |
Figure 5B presents an overlay map of journals, illustrating aspects such as the distribution, citation trajectory, and shift in the center of gravity of papers across each discipline. The label on the left represents the discipline of the cited journal, whereas the label on the right indicates the discipline of the journal in which the cited paper was published. In the figure on the left, the vertical axis of the ellipse extends as the number of papers published by a journal increases, whereas the horizontal axis increases as the number of authors increases. Our analysis revealed that most publications appeared in journals associated with molecular biology, genetics, health, nursing, and medicine. Furthermore, most publications have been cited in journals pertaining to molecular biology, immunology, and medicine. The orange and green citation trajectories suggest that research journals in the molecular/biology/genetics domain garner frequent citations in molecular/biology/immunology and medicine/medical/clinical journals.
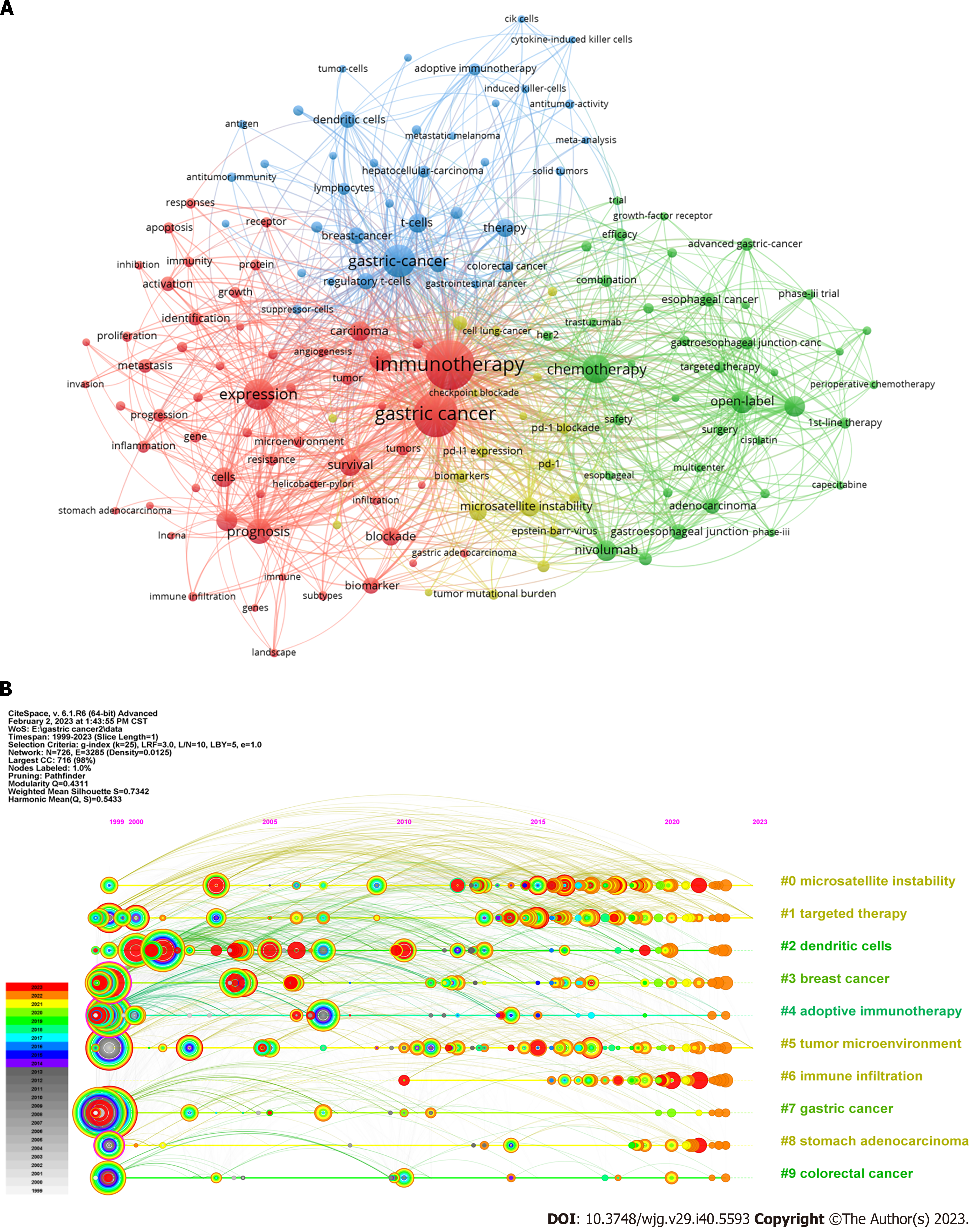
Co-occurrence analysis of keywords: Keywords play a pivotal role in encapsulating scientific research trends. To identify the most prevalent keywords, we used the VOSviewer tool for keyword co-occurrence analysis. From the 6337 keywords, we focused on 152 keywords that appeared 25 times or more (Figure 6A). “Gastric cancer” emerged as the most recurrent keyword, appearing 1257 times. The top ten most frequently occurring keywords included GC (n = 1257), immunotherapy (n = 988), expression (n = 394), chemotherapy (n = 320), prognosis (n = 253), open label (n = 206), survival (n = 194), cancer (n = 190), double-blind (n = 177), and TME (n = 174).
Timeline view of keywords: Following cluster analysis, the keywords were segregated into ten clusters and subsequently subjected to a timeline view analysis in CiteSpace (Figure 6B): #0 MSI, #1 targeted therapy, #2 dendritic cells (DCs), #3 breast cancer, #4 adoptive immunotherapy, #5 TME, #6 immune infiltration, #7 GC, #8 stomach adenocarcinoma, and #9 colorectal cancer. Each cluster comprised multiple closely associated words with smaller ranks or cluster numbers that contained more keywords. The premier cluster (#0) had the largest number of keywords, showing minimal past activity with two minor bursts but became more active in the recent decade. Cluster #1 followed a trend akin to that of cluster #0, signifying that MSI and targeted therapy have drawn significant interest. Clusters #2, #3, and #4 were notably active around 2000 and have undergone minor bursts in recent years. The term “tumor microenvironment” first appeared in 2010 in cluster #5 and has maintained activity in recent years.
Burst analysis of keywords: Following the burst analysis, we selected 25 keywords from the burst keywords based on burst strength and initiation time (Figures 7A and B). The keywords exhibiting the highest burst strengths included “Adoptive immunotherapy” (strength = 17.37), “dendritic cells” (strength = 16.79), and “carcinoma” (strength = 12.14). Three keywords that initiated bursting in the past 5 years and were identified as hotspots encompassed “mismatch repair deficiency” (initiated in 2018), “tumors” (initiated in 2019), and “plus chemotherapy” (initiated in 2021). The emergence of new burst keywords and prevalence of strong bursts in recent years have led to rapid developments in this field.
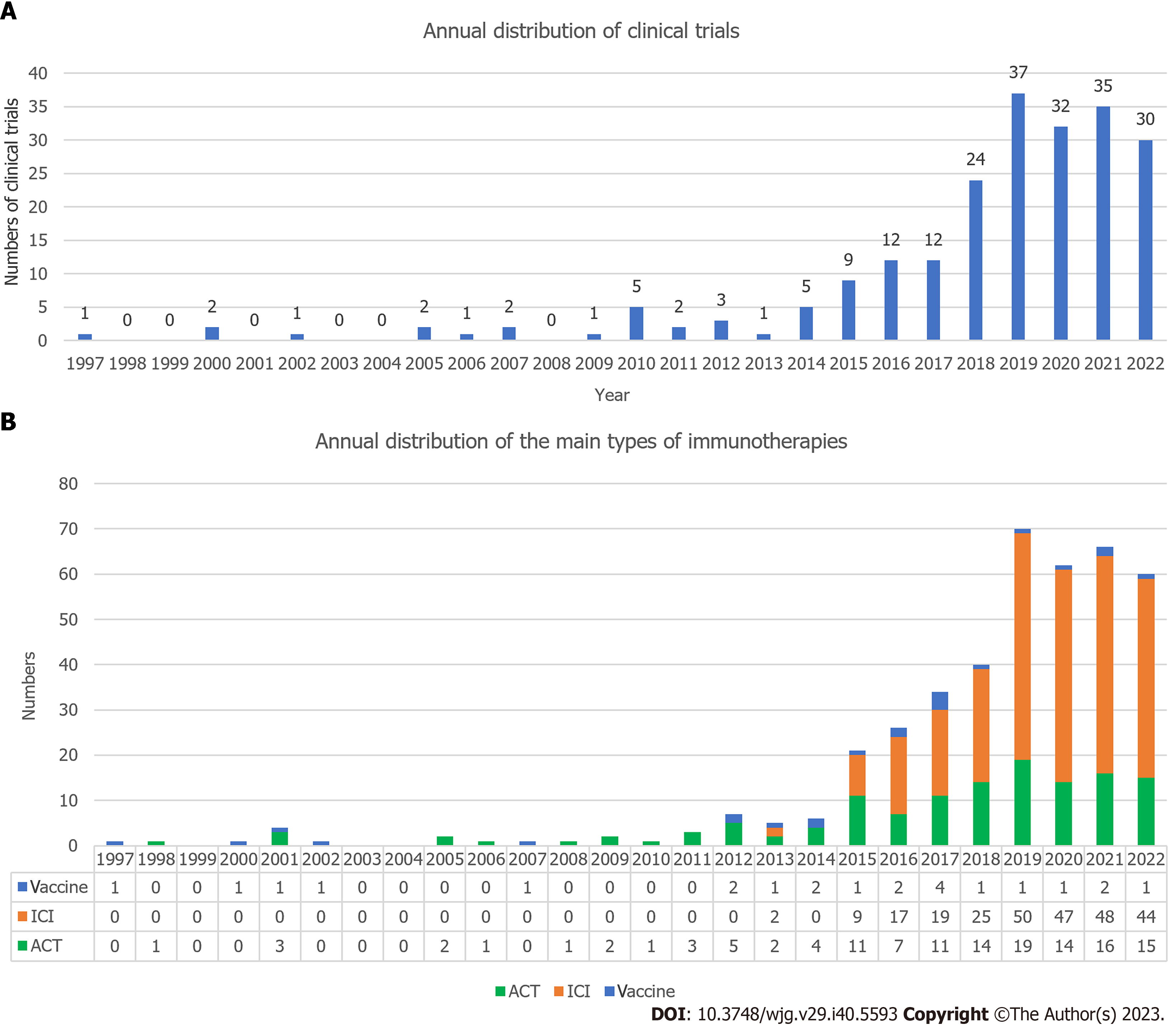
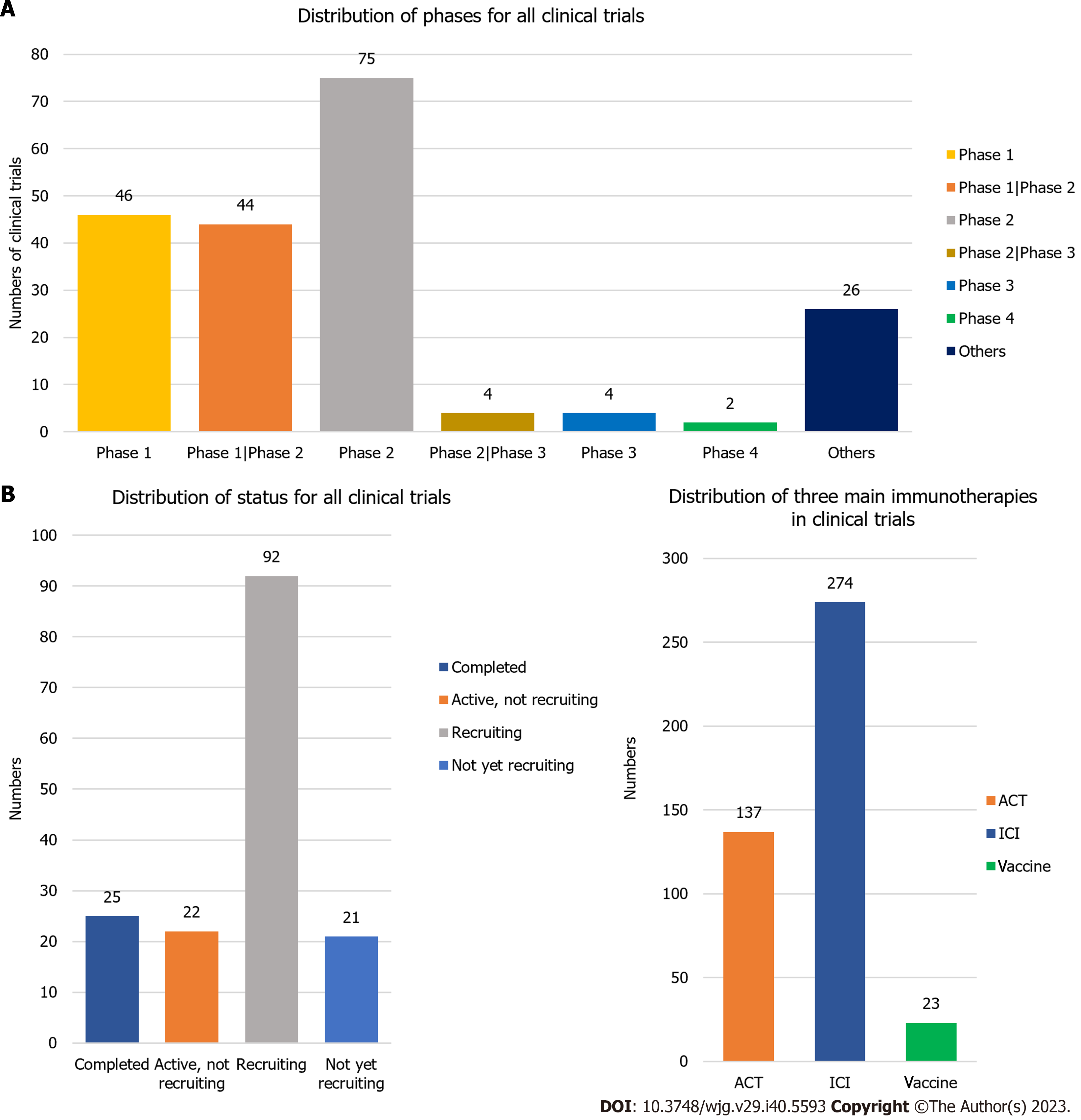
The clinical trial with the earliest enrolment commenced in 1987, and was a randomized controlled trial predicated on preoperative serum glycoproteins assessing the efficacy of immunotherapy in GC (JPRN-UMIN000037472). The annual distribution of clinical trials (Figure 8A) indicated a sharp recent progress in the study of GC immunotherapy. The peak in the number of clinical trials occurred in 2019 (n = 37), with high numbers observed in the subsequent three years (2020 n = 32, 2021 n = 35, 2022 n = 30). The trials were predominantly in phase I (n = 46), phase I/II (n = 44), or phase II (n = 75) (Figure 9A). We have consolidated the clinical trials of phase II/III (n = 4), III (n = 4), and IV (n = 2) in Table 2, offering scholars an update on the latest advancements in this field. The status of the clinical trials is shown in Figure 9B, with a significant number of trials either recruiting (n = 92) or not yet recruiting (n = 21). The three primary immunotherapies for GC, namely adoptive cell therapy (ACT), ICI, and vaccination, were subjected to separate clinical trial searches (Supplementary material, Figures 8B and 9C).
| No. | Trial ID | Status | Phases | Start date | Title |
| 1 | NCT00503321 | Terminated | Phase 2/3 | October 1, 2006 | Phase II Study of TS-1 Therapy and TS-1+PSK Therapy Against Advanced Gastric Carcinoma |
| 2 | EUCTR2017-004896-30-IE | Not recruiting | Phase 3 | July 6, 2018 | A Randomized, Active-Controlled, Blinded, Phase III Clinical Trial of BMS- 986213 (Fixed Dose Combination of Relatlimab [anti-LAG-3] and Nivolumab) in Combination with Chemotherapy versus Placebo in Combination with Chemotherapy as First-Line Treatment in Participants with Unresectable, Locally Advanced or Metastatic LAG-3 Positive Gastric or Gastroesophageal Junction Adenocarcinoma |
| 3 | NCT04078152 | Active, not recruiting | Phase 4 | September 5, 2019 | Durvalumab Long-Term Safety and Efficacy Study |
| 4 | ChiCTR2000039110 | Recruiting | Phase 4 | October 14, 2020 | Effect of immunotherapy combined with chemotherapy on gastric cancer |
| 5 | NCT05002686 | Recruiting | Phase 2/3 | August 7, 2021 | Safety and Efficacy of Sintilimab in Combination With Chemoradiothrapy Followed by D2 Surgical Resection in Patients With Advanced Gastric Cancer With Retroperitoneal Lymph Node Metastasis |
| 6 | NCT05152147 | Recruiting | Phase 3 | December 2, 2021 | A Study of Zanidatamab in Combination With Chemotherapy Plus or Minus Tislelizumab in Patients With HER2-positive Advanced or Metastatic Gastric and Esophageal Cancers |
| 7 | NCT05270824 | Not yet recruiting | Phase 3 | March 1, 2022 | Study Evaluating Neoadjuvant Immunotherapy Increasing CD8+ Cell Infiltration in Advance Gastric Adenocarcinoma |
| 8 | NCT05325528 | Recruiting | Phase 2/3 | April 4, 2022 | Study of Tislelizumab in Combination With SOX for the Treatment of Gastric Cancer With Liver Metastases |
| 9 | NCT05677490 | Not yet recruiting | Phase 3 | January 6, 2023 | mFOLFIRINOX Versus mFOLFOX With or Without Nivolumab for the Treatment of Advanced, Unresectable, or Metastatic HER2 Negative Esophageal, Gastroesophageal Junction, and Gastric Adenocarcinoma |
| 10 | NCT05699655 | Not yet recruiting | Phase 2/3 | March 1, 2023 | Tislelizumab Combined With Apatinib and Oxaliplatin Plus S1 Vs Oxaliplatin Plus S1 as Neoadjuvant Therapy for Borrmann IV, Large Borrmann III Type and Bulky N Positive Advanced Gastric Cancer |
In recent years, there has been a surge in the bibliometric analysis of articles across various fields. Tools such as CiteSpace and the VOSviewer enable raw data visualization, offering comprehensive and intuitive data representation. Cancer is a persistent medical challenge that has caused researchers to work globally toward enhancing treatments to improve progression-free survival (PFS) and OS. GC is one of the most prevalent cancers and requires more effective and targeted treatments. Bibliometric analysis of documents pertaining to GC immunotherapy elucidated the current research hotspots, thereby providing researchers with insights and directions. This analysis incorporated every related article from the WoSCC database until December 31, 2022, offering a visual and systematic overview. It summarizes and analyzes the countries, institutions, authors, keywords, and references. Clinical trials included every related trial from ClinicalTrials.gov and ICTRP until February 18, 2023.
With regard to the volume of documents produced by each country, China held a dominant position, contributing 1070 documents, accounting for 53.2% of the total. The ten institutions with the highest publication counts were all located in China. The substantial population base of China facilitates a larger number of researchers and institutions in related fields, thereby leading to a higher volume of literature production. Furthermore, China’s substantial investment in GC immunotherapy research underscores the promising future of this field. In addition to the patient count correlating with the large population base, the incidence of GC in China significantly surpasses that in the United States and United Kingdom[29]. Elevated rates of smoking and Helicobacter pylori infection in China could contribute to a higher incidence of GC. The extent of early cancer screening in China remains unclear.
The United States holds the second position with regard to the volume of documents produced, contributing 16.0% of the publications, and is potentially linked to factors such as obesity, alcohol consumption, and high-fat diets. Japan ranked third (11.3%). The high incidence of GC in Japan mirrors that in China, likely because of Asian dietary habits, including high salt intake and excessive nitrite consumption, which are associated with a high incidence of digestive system tumors.
Co-citation analysis revealed that Sakamoto Junichi had the highest citation count (810 citations across six documents). His research has primarily focused on immunochemotherapy or adjuvant chemotherapy, with a meta-analysis of the benefits of adjuvant chemotherapy for resectable GC with over 600 citations. Oba et al[30] also highlighted the therapeutic effects of polysaccharide K, lentinan, and OK-432 in GC[31,32]. This highlights the pivotal role of chemotherapy in GC treatment and ongoing advancements in research on the integration of immunotherapy and chemotherapy. Xin Wang, with 705 citations across seven documents, held the second position. His contributions include the development of clinical guidelines for GC diagnosis and treatment and a review of PD-L1 expression in human cancers and its correlation with clinical outcomes, which has received over 400 citations. PD-L1-related research has emerged as a significant focus in recent years, offering new prospects for GC immunotherapy, which will be discussed in subsequent sections.
Keyword analysis, excluding less-specific terms, revealed several significant keywords and clusters. Notably, clusters #0 (MSI), #2 (DCs), #4 (adoptive immunotherapy), and #5 (TME) stood out; these were also keywords with notable bursts. Moreover, keyword “mismatch repair deficiencies” have garnered considerable attention. We delved into these categories in detail. Because of the substantial progress and potential of ICIs reported in recent studies, we discuss them separately.
TME (#5 TME): Among the top ten most frequently occurring keywords in this study, three only surfaced in recent years: “open label” (first appearance in 2015, 206 occurrences), “double-blind” (first appearance in 2015, 177 occurrences), and “tumor microenvironment” (first appearance in 2017, 174 occurrences). Open-label and double-blind denote two contrasting types of experiments. In open-label trials, both investigators and participants are aware of the treatment status, whereas in double-blind trials, neither party knows the treatment status. The frequent appearance of these two keywords likely reflects the growing number of immunotherapeutic drugs entering clinical trials in recent years. Pembrolizumab and nivolumab have shown efficacy against certain types of GC[33,34]. Toripalimab, currently under evaluation for its safety and efficacy in treating advanced GC resistant to chemotherapy, is also part of an ongoing phase III randomized trial assessing the combined therapy of toripalimab and XELOX[33,35].
The sixth keyword cluster, represented by “tumor microenvironment”, gained prominence in 1999 and has received consistent attention since 2010. The TME refers to non-tumor cells and their metabolites and secretions within the tumor, including immune cells such as myeloid suppressor cells, tumor-infiltrating lymphocytes (TILs), macrophages, stromal fibroblasts, endothelial cells, extracellular matrix components, growth factors, and cytokines[36]. Five highly cited articles in this study featured “tumor microenvironment” as a keyword, focusing on tumor-associated macrophages (TAMs) or pyroptosis. Macrophages are primarily categorized into two phenotypes, classically activated (M1) and alternatively activated (M2). M1 macrophages exhibit antitumor effects; however, M2 macrophages promote angiogenesis and tumor progression. TAMs, comprising M2 and a fraction of M1 cells, foster pro-angiogenic and immunosuppressive signals in gastric tumors, thereby presenting potential therapeutic targets[37,38]. Another study highlighted the significant role of RNA N6-methyladenosine modifications in shaping the diversity and complexity of the TME[39]. Pyroptosis aids cytotoxic lymphocytes in eliminating tumor cells and reprograms the TME toward an immunostimulatory state[40]. A recent study has suggested that the GC microenvironment of patients may provide a more accurate prediction of chemotherapy sensitivity, thereby enabling improved staging and prognostic assessment of patients[41]. The role of TME in GC continues to garner interest.
MSI (#0 MSI) and defective mismatch repair (mismatch repair deficiency): MSI arises from the inability to repair replication errors in microsatellite sequences owing to defective mismatch repair (dMMR). When these errors accumulate significantly, they result in MSI-H, a subtype of GAC constituting up to 22% of the cases[42]. The keyword “mismatch repair deficiency” experienced a burst from 2018 to 2020, with a burst strength of 10.82, ranking eighth (Figure 7A). As depicted in Figure 6B, MSI ranks first in the cluster, signifying its high association and occurrence. The MSI-driven cancer pathway prompts tumor cells to produce abnormal and potentially immunogenic neoantigens, suggesting high antigenic potential in MSI GC[43,44]. Studies have established the independent prognostic significance of dMMR (P = 0.0001), with patients with metastatic GC exhibiting defective MMR systems demonstrating improved prognoses[44]. MSI cancer, characterized by extensive expression of immune checkpoint ligands and robust immunogenicity, exhibits heightened sensitivity to immunotherapy, albeit with potential resistance to chemotherapy[45,46]. The significance of the MSI-H subtype is well established, with numerous recent studies focusing on this subtype. Both nivolumab as a standalone treatment and in combination with ipilimumab have demonstrated antitumor activity with a tolerable toxicity profile in chemotherapy-resistant gastroesophageal adenocarcinomas. Moreover, pembrolizumab has received regulatory approval as a therapeutic alternative for MSI-H tumors[47,48]. Emerging evidence indicates a potential correlation between MSI-H and PD-L1 expression in various cancers, suggesting that MMR defects may serve as predictors of ICI therapy[45].
DC (#2 DCs): “Dendritic cells” constituted the third cluster in Figure 6B, exhibiting a significant burst from 2001 to 2013, as indicated by the second highest burst strength (Figure 7A, strength = 16.79). DCs are antigen-presenting cells essential for the induction and regulation of adaptive immune responses. DCs can phagocytose and process antigens and present these antigens to naive T-cells, resulting in the activation, differentiation, and polarization of T-cells toward specific effector functions. In GC immunotherapy, DCs play a key role in the activation and regulation of antitumor immune responses[49]. DCs can be used as adjuvants or vaccine adjuvants to enhance antitumor immunity by presenting tumor-specific antigens to naïve T-cells[49,50].
DC-based vaccination and ACT are the two main types of cellular immunotherapies used in GC[50]. Our analysis revealed that 23 clinical trials have focused on GC vaccines, with new trials initiated annually since 2012, as depicted in Figures 8B and 9C. Vaccines have surfaced as a pivotal modality in cancer immunotherapy, with DC-based vaccination being the predominant approach, constituting 20 out of 23 clinical trials. In 1990, Inaba et al[51] demonstrated that injection of in vitro antigens from DCs could sensitize normal mice to protein antigens. A decade later, researchers discovered that DCs can elicit antitumor responses[52]. A study in 2015 illustrated that DCs, when loaded with tumor RNA, can stimulate lymphocytes to differentiate into effector cells that respond to tumors and are capable of eliminating tumor cells[53]. The fusion of GC cells and DCs significantly enhances their ability to stimulate anti-tumor immune responses and prevents their proliferation into newly implanted tumors in vivo, highlighting the potential of DCs as a safe and effective anti-tumor vaccine[54]. Recombinant adenovirus-bearing secondary lymphoid tissue chemokine-modified DCs could serve as adjuvants to induce a robust immune response against GC[55]. DCs derived from cord blood in combination with cytokine-induced killer (CIK) cells have also been clinically utilized for the treatment of GC, showing significant disease-free survival (DFS, P = 0.0448) and OS (P = 0.0646) rates[56]. As of February 6, 2023, we identified 18 trials on ClinicalTrials.gov using the keywords “gastric cancer” and “dendritic cells”, with seven completed, nine with unknown status (indicating that the study had passed its completion date and its status had not been verified for over two years), one active, and one recruiting.
ACT (#4 adoptive immunotherapy): Adoptive immunotherapy was identified as the fifth cluster (Figure 6B, #4 adoptive immunotherapy) and experienced a surge in research from 1999 to 2016, as evidenced by its high strength (Figures 7A and B, strength = 17.37). A total of 137 clinical trials pertaining to ACT were initiated during this period, with relevant trials conducted almost annually for the past two decades (Figures 8B and 9C). ACTs involve the transfer of immune cells, such as lymphocytes or DCs, to a patient to enhance the antitumor immune response. In GC immunotherapy, ACTs generate effector T-cells that specifically target tumor antigens and induce long-term antitumor immunity[57]. ACTs can also enhance the function of regulatory T-cells and improve patient outcomes[50,57].
ACT has a rich history demonstrating its importance and progression in the field of immunotherapy. Currently, four types of ACTs have made significant contributions to international research: (1) TIL therapy; (2) Engineered T-cell receptor (TCR) therapy; (3) Chimeric antigen receptor T-cell (CAR-T) therapy; and (4) Natural killer (NK) cell therapy. CIK cell therapy, another form of ACT, is considered a promising approach for next-generation tumor-adaptive cell immunotherapies, with CIK cells often referred to as precision-guided missiles that target tumor cells[58].
First, we investigated TIL therapy. Unlike several types of cell immunotherapies that use blood-derived cells, TIL therapy leverages immune cells extracted directly from tumor tissues, thereby enhancing the capacity of these cells for tumor recognition. TILs play a critical role in curbing tumor growth and adjusting therapeutic response in cancer patients and could potentially act as predictive markers, providing insights into patient response to treatment and prognosticating survival outcomes[59,60]. GC can be stratified into four distinct TME subtypes based on the assessment of PD-L1 expression and TILs: (1) PD-L1+/TIL+, associated with the best PFS [Hazards ratio (HR = 2.044)] and OS (HR = 1.993); (2) PD-L1-/TIL-, linked with the poorest survival outcomes; (3) PD-L1+/TIL-; and (4) PD-L1+/TIL+[61]. Increased counts of T-bet+ TILs were found to be correlated with non-invasion of the muscle layer (P = 0.0138), smaller tumor size (P = 0.0202), and early Union for International Cancer Control stage (P = 0.0196), and studies have demonstrated that higher numbers of T-bet+ TILs are associated with longer median PFS (41 vs 26 mo, P = 0.0481) and median survival time (MST) (55 vs 32 mo, P = 0.0455)[62].
Second, we examined engineered TCR therapies. TCR-engineered T-cells require the presentation of antigens by specific major histocompatibility complex (MHC) molecules for activation, which presents certain constraints on their clinical applications[63]. Initial trials with TCR therapy showcased cancer regression; however, the associated toxicities were severe, underscoring the need for caution when implementing high-avidity TCRs[10,64]. Later clinical trials achieved efficacy without substantial toxicity by using T-cells redirected against cancer testis antigens[63,65,66]. A 2022 study profiled the hypervariable complementarity determining region 3 of the TCR beta chain in the peripheral blood of GC patients to identify new biomarkers[67].
Thirdly, we turn our attention to CAR-T therapy. Emerging over the past 30 years, CAR-T-cell therapy is a relatively novel approach, evolving from prior clinical applications of ACT, including TILs[11]. CAR-T-cells, a subset of genetically engineered T-cells, can identify specific antigens, and their cytotoxic effects operate independently of MHC interactions[50]. The first-generation CAR, constructed in 1993, comprises an extracellular antigen recognition domain-commonly a single-chain Fragment variant derived from an antibody-a transmembrane domain, and the intracellular T-cell activation domain of CD3+[68,69]. The second-generation CAR introduces a costimulatory domain, enhancing antitumor effects and promoting the establishment of immunological memory in patients with hematological tumors[69,70]. Third-generation CAR integrate two tandem costimulatory molecules[11]. The fourth-generation CAR adopts a more intricate design that aims to mitigate off-target toxicity and immunosuppression and boost the anti-tumor transport activity targeted by solid tumors[11]. Various potential targets for GC therapy, including claudin 18.2, mesothelin, ANTXR1 (TEM8), and MUC3A, have been identified, and claudine18.2-specific CAR-T-cell therapy is currently being explored in clinical trials[71,72]. As of February 6, 2023, a search of ClinicalTrials.gov using the keywords “gastric cancer” and “CAR T” yielded 37 trials, 23 of which were recruiting or not yet recruiting. Numerous related experiments will be conducted in the coming years, likely catalyzing rapid advancements in CAR-T therapy.
NK cell therapy was also considered. Patients with digestive cancers frequently demonstrate elevated PD-1 expression in both peripheral and tumor-infiltrating NK cells, and this heightened expression could potentially signify an unfavorable prognosis[73]. A decade ago, a study revealed that among patients with GAC, those with high concentrations of NK cells exhibited higher survival rates than those with low concentrations (P = 0.0027, HR = 0.343)[74]. As NK cell therapy continues to evolve, researchers have begun to integrate NK cells with other forms of immunotherapy to investigate the potential improvements in patient outcomes. A recent animal study employed a combination of interleukin (IL)-2-activated NK cells and anti-PD-1 therapy, demonstrating that this synergistic approach could hinder gastric tumor progression and promote tumor immune cell infiltration[75]. Moreover, various strategies involving CAR NK cell therapy have demonstrated efficacy in the treatment of certain tumor types[76-78].
Finally, CIK cell therapy is discussed. As illustrated in Figure 7A, the term “cytokine-induced killer cells” experienced a research publication surge from 2013 to 2017, with a burst strength of 11.39, whereas “cik cells” followed a similar trend from 2012 to 2017, with a burst strength of 10.75. CIK cells have several attributes that make them a promising therapeutic avenue for cancer treatment, including the potent antitumor capabilities of T lymphocytes and the non-MHC-restricted tumoricidal activity characteristic of NK cells. The earliest research document we obtained featuring the terms “cytokine-induced killer cells” or “CIK” dates back to a 2006 study, which indicated that the total remission rate (CR + PR + MR) in the group treated with a combination of chemotherapy and CIK cells was higher than in the group treated with chemotherapy alone (56.3% vs 48.0%)[79]. A clinical trial in 2010 showed that GC patients treated with CIK cell adoptive immunotherapy combined with chemotherapy had a significantly longer survival time than those treated with chemotherapy alone (MST: 49 m vs 27 m, P < 0.05; 2- and 5-year survival rates: 73.5% vs 52.6%, 40.4% vs 23.9%, P < 0.05). Another report evaluated 11 studies proving that adjuvant immunotherapy with CIK cells may prevent recurrence and improve the quality of life and PFS rate in patients with cancer[80,81]. However, a recent meta-analysis revealed that the incorporation of cellular immunotherapy into chemotherapy resulted in a statistically significant improvement in OS and DFS only in patients with stage III GC[82].
Despite the significant anti-tumor effects demonstrated by CAR-T therapy across various iterations, numerous challenges remain in its widespread application. Future research in this area will likely concentrate on the development of more potent CAR-T-cells with enhanced anti-tumor activity and mitigated toxicity[83]. Despite the significant anti-tumor effects demonstrated by CAR-T therapy across various iterations, there are still numerous challenges to its widespread application. Future research in this area will likely concentrate on the development of more potent CAR-T-cells with enhanced anti-tumor activity and mitigated toxicity[84]. NK cells display high anti-tumor activity and antibody-dependent cytotoxicity. However, their therapeutic application is limited by the challenge of generating large quantities of highly pure and functional NK cells[7,85]. In contrast, immunotherapies involving CIK cells and TILs have a significant potential for cancer treatment. Our analysis shows that CIK cells exhibit potent anti-tumor activity, and that combination therapies incorporating CIK cells demonstrate improved efficacy in patients with GC. TILs immunotherapy has also been extensively used in advanced GC treatment[7]. TILs offer several key advantages, including lower off-target toxicity and increased specificity, making them superior in addressing tumor heterogeneity in comparison to other ACT therapies; they can also regulate the immune response by releasing cytokines, which subsequently bolster anti-tumor immunity[86]. Moreover, TIL levels are prognostic indicators[87].
ICIs: Clinical research on ICIs is the most advanced and widespread cancer immunotherapy. These inhibitors have shown notable efficacy in the treatment of various solid and hematological cancers, often leading to durable, long-lasting responses that are typically well tolerated by patients[88].
The interaction of PD-1 (also referred to as CD279) with PD-L1 (also denoted as CD274 or B7-H1) can induce T-cell dysfunction, exhaustion, and tolerance, thereby inhibiting the PD-1/PD-L1 and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4, also known as CD152) signaling pathways, T-cell function can be rejuvenated, leading to an increase in the destruction of tumor cells[17,89]. Studies over the past 20 years have indicated that inhibiting PD-L1 can boost anti-tumor immunity[90-92]. In fact, it has been demonstrated that most patients with GAC express CTLA-4 on their tumor cells (CTLA-4 86.6%, PD-L1 44.9%) and those who tested negative for CTLA-4 or PD-L1 had a significantly better mean OS than those who tested positive (CTLA-4: 62.0 vs 44.4 mo, P = 0.018; PD-L1: 54.2 vs 39.1 mo, P = 0.011)[93]. Contrary to a previous study, another study demonstrated that the expression of PD-L1 was associated with improved DFS and OS in GC (PD-L1+ vs PD-L1- tumors: 5-year DFS rate, 82.6% vs 66.9%; 5-year OS rate, 83.0% vs 69.1%, P < 0.05)[94]. These contrasting findings could be due to differences in the ethnic backgrounds of the patients (Caucasians in the first study and Asians in the second) or variations in the antibodies used in each study, indicating the need for larger studies to clarify the roles of PD-L1 and CTLA-4 in GC.
As of February 18, 2023, 274 clinical trials were registered at ClinicalTrials.gov and ICTRP, focusing on ICIs, which is a considerably larger number than those related to ACT and vaccine therapies (Figure 9C). The first clinical trials centered on ICIs began to emerge in 2013, and their counts saw a steady increase from 2015 to 2019, maintaining a high level over the last four years (Figure 8B). ICIs have received growing academic attention and have shown robust potential in GC immunotherapy. Some PD-1 inhibitors with relatively advanced research include avelumab, pembrolizumab, nivolumab, tislelizumab, camrelizumab, atezolizumab, and ipilimumab, function in combination with CTLA-4.
Published studies indicate that therapy using ICIs may present less toxicity, better tolerance than chemotherapy, and potentially superior outcomes. In trials with GC patients, avelumab did not outperform chemotherapy in terms of OS or RFS, but it did showcase a more manageable safety profile [grade ≥ 3 treatment-related adverse events (AEs): 9.2% vs 31.6% and 12.8% vs 32.8%, NCT02625623 and NCT02625610][95,96]. Pembrolizumab, which has undergone phase III clinical trials (NCT02370498 and NCT02494583), showed promising results; compared to chemotherapy, it demonstrated a trend toward improved OS and ORR while reducing the occurrence of treatment-related AEs[97,98].
In recent years, notable advances have been made in the combination of ICIs and chemotherapy. In patients with advanced GAC, the combination of tislelizumab and chemotherapy results in sustained responses and manageable side effects[99]. Similarly, a combination of Camrelizumab and CAPOX has demonstrated encouraging anti-tumor effects and manageable toxicity in patients with metastatic or advanced GAC, having successfully completed phase II clinical trials (NCT03469557 and NCT03472365)[100]. The effectiveness of ipilimumab in combination with chemotherapy has been assessed in both phase II and III clinical trials (NCT03241173 and NCT03126110, respectively).
The HER2, also known as Neu or ErbB2, is part of the epidermal growth factor receptors family, which encodes the transmembrane glycoprotein p185. It was initially discovered to have high expression in GC in 1986 and has since become a significant focus in translational cancer research due to its role in the HER2 signaling pathway[101,102]. For HER2-positive GCs, the preferred treatment regimen often includes a combination of pembrolizumab, trastuzumab, and chemotherapy[13]. A recent clinical trial (NCT03409848) demonstrated that combination therapy with trastuzumab, nivolumab, and FOLFOX was more effective than previous treatment protocols in treating HER2-positive esophagogastric adenocarcinoma[103].
We screened 228 clinical trials on GC immunotherapy identified in a preliminary search and further analyzed 174 eligible clinical trials. There were 38 phase I clinical trials, 40 phase I/phase II clinical trials, 71 phase II clinical trials, three phase II/phase III clinical trials, four phase III clinical trials, and two phase IV clinical trials. For status, 25 clinical trials were completed: 20 were active but not recruited, 72 were recruited, 17 were not yet recruited, and the others were unknown or difficult to classify.
Completed trials: There are 25 completed clinical trials were completed, all of which were in phase I or II. After some immunotherapeutic drugs have proven effective, researchers have begun to explore the potential of multiple immunotherapy combinations.
Monoclonal antibodies (mAbs) are the predominant form of immunotherapy used to manage GC. The anticipated synergies arise primarily from the combination of anti-PD-1 and anti-CTLA-4 antibodies, a therapeutic pairing that has been extensively explored by numerous researchers, as indicated by associated clinical trials (NCT02340975, NCT02983045). Moreover, the inclusion of anti-HER2 antibodies in this treatment paradigm has also been investigated (NCT03409848). Beyond ICIs, other emergent forms of immunotherapy, such as ACT, have also been initiated in clinical trials. The completed trials included an array of treatment strategies. These include the integration of autologous DC-CIK cell immunotherapy with chemotherapy (NCT01783951), implementation of CAR-T immunotherapy (NCT02850536), and combination of NK cell therapy with ICIs (NCT03319459, NCT03841110). A phase II clinical trial (ChiCTR-OCH-12002610) investigating postoperative immunotherapies involving tumor lysate-loaded DCs, in vitro DC-activated T-cells, and activated T-cells in conjunction with chemotherapy demonstrated significantly improved mean survival rates in patients with operable colorectal cancer (59.74 ± 3.21 years vs 49.99 ± 2.54 years, P = 0.034), though its applicability to GC has not been substantiated with published results[104].
Trials active, not recruiting: Eighteen active clinical trials that completed recruitment primarily employed a therapeutic approach involving the synergistic use of multiple ICIs alongside chemotherapy, with notable targets, including PD-1/PD-L1, CTLA-4, HER2, and LAG3 (NCT03662659, NCT03647969, NCT03443856, NCT04062656). A phase I/II clinical trial (NCT03093688) preliminarily corroborated the tolerability of an immunotherapeutic approach based on invariant NK T cells and PD-1+CD8+ T-cells for lung adenocarcinoma, suggesting their potential applicability to other solid tumors[105].
Trails in recruiting: With 66 clinical trials currently recruiting, the discovery of novel cellular immunotherapeutic approaches continues to facilitate the development of increasingly effective immune cells, and consequently, the integration of more cellular immunotherapies into clinical trials.
Four clinical trials focused on peptide vaccines (NCT03784040, KCT0005481, NCT05269381, and NCT05311176), all of which implemented treatment regimens that incorporated a combination of vaccines and ICIs or the addition of chemotherapy. Notably, a phase II clinical trial was initiated to explore the potential of IMU-131, a B-cell peptide vaccine, in combination with anti-vascular endothelial growth factor receptor (VEGFR) and anti-PD-1 antibodies (NCT05311176).
Current research indicates that, although the combination of DC-CIK with S-1 does not yield a statistically significant advantage over S-1 in combination with cisplatin (P = 0.892), DC-CIK exhibit good tolerability, suggesting their potential as an alternative in cases where multi-agent chemotherapy is not tolerated[106]. Therefore, researchers continue to explore more efficacious cellular immunotherapies. T-cells stimulated by Claudin18.2 peptide demonstrate potent anti-tumor activity and are emerging as a beacon of hope and a focal point of interest in the landscape of GC cellular immunotherapy, with corresponding clinical trials already underway (NCT04683939)[107].
Zanidatamab (ZW25) is an asymmetrically structured antibody that binds to two non-overlapping HER2 epitopes. A phase III clinical trial aims to examine the efficacy of ZW25 in combination with chemotherapy, both with and without tislelizumab, as a first-line treatment for patients with advanced or metastatic HER2-positive gastroesophageal adenocarcinomas (NCT05152147). Investigations involving ZW25 as a stand-alone treatment or in conjunction with chemotherapy or ICIs are ongoing. On May 29, 2019, the United States Food and Drug Administration (FDA) approved ZW25 as a first-line treatment in conjunction with standard-of-care chemotherapy for patients with HER2-overexpressing gastroesophageal adenocarcinoma[102]. ZW49, an antibody-drug conjugate (ADC) derivative of ZW25, utilizes the proprietary cytotoxic and cleavage adapters of the Zymework. A Phase I clinical trial (NCT03821233) is currently underway to explore the potential of ZW49 in patients with HER2-positive malignancies.
IL-2 is known for its ability to foster the growth of diverse immune cell populations, modulate cellular differentiation, and depending on the cellular milieu, either promote survival or induce apoptosis. Being the first cytokine to gain FDA approval for cancer treatment, IL-2’s role in strategies that synergistically enhance immune responses is garnering increasing interest. Several clinical trials have investigated the synergistic use of interleukins with other treatments, including IL-2 combined with an anti-PD-1 antibody (NCT05086692), and AU-007 (interleukin antibodies) in conjunction with aldesleukin (NCT05267626). IL-15, which is similar to IL-2, is currently under investigation in a phase II clinical trial (NCT04847466) that examines the combination of N803 (an IL-15 agonist), pembrolizumab, and PD-L1 CAR NK cells. Additionally, an ongoing investigation involves a combination of KK-LC-1 TCR-T cells and aldesleukin (NCT05483491). However, no associated literature has been published on these trials. The future utility and progress of interleukins in cancer therapy warrants further investigation.
Trails not yet recruiting: Sixteen trials have not yet started recruitment, and most of them were phase II trials, with some phase I and III trials being planned. One or more ICIs combined with chemotherapy continue to be the most frequently studied, but some new treatment strategies are also being explored.
Research has demonstrated that the addition of anti-PD-1 antibodies to anti-HER2 antibodies and chemotherapy substantially diminishes tumor size and significantly enhances the ORR to 74.4%, as opposed to 51.9% without anti-PD-1 antibodies in HER2-positive GC[13]. In recent years, simultaneous blockade of PD-1 and HER2 has emerged as a significant area of research. Studies on VEGFR continue to progress. The anti-VEGFR2 antibody, ramucirumab, has demonstrated clinical efficacy in GC, both as a standalone therapy and in combination with chemotherapy[15]. Furthermore, clinical trials have been initiated to investigate the combination of anti-VEGFR and anti-PD-1 antibodies with and without chemotherapy (NCT05585580 and NCT05721651).
Olaparib, a PARP inhibitor approved for the treatment of ovarian cancer, is currently being explored for its potential clinical utility in GC. A phase I/II clinical trial (NCT04592211) has investigated the combination of olaparib, pembrolizumab, and paclitaxel in patients with GC. Over the past decades, 5-fluorouracil has remained a mainstay in the realm of chemotherapy. Recently, researchers have introduced NUC-3373, which has demonstrated a more favorable safety profile in patients[108]. A phase I/II clinical trial is currently centered on the use of NUC-3373 in conjunction with other agents in patients with advanced solid tumors, including GC (NCT05714553). IRAK4 can induce T cell dysfunction, making it a promising and novel target for immunotherapy[109]. A phase I clinical trial (NCT05187182) is currently investigating the use of CA-4948 (an IRAK4 inhibitor) combined with chemotherapy and ICIs for untreated unresectable gastric and esophageal cancer. A phase II clinical trial (NCT05671822) focusing on SHR-A1811 (an ADC drug targeting HER2 and TOP1) combined with SHR-1701 (a PD-L1 and TGFBR2 inhibitor) and chemotherapy in advanced HER2-positive GC is being conducted, which we believe will be significant.
The trends of clinical trials: (1) Discovering novel biomarkers to subclassify patients and exploring more specific treatment options. NCT05593419, ChiCTR2100052367, NCT02757391, NCT03158571; (2) Integration of immunotherapy with surgery, radiotherapy, and chemotherapy. NCT04688801, chemotherapy ± immunotherapy ± radiotherapy after surgery; (3) Transition from single-agent to multi-agent therapy. NCT05152147, trastuzumab (anti-HER2)/ZW25 (anti-HER2) ± tislelizumab (anti-PD-1) + chemotherapy; (4) Combination therapy involving various immunotherapies such as ICI, ADC, and ACT. NCT05269381, vaccine + pembrolizumab (anti-PD-1, ICI) + chemotherapy; NCT05671822, SHR-A1811 (HER2, ADC) + SHR-1701 (PD-L1 and transforming growth factor-β double antibody) + capecitabine + oxaliplatin; NCT05313906, RC48 (HER2, ADC) + AK105 (anti-PD-1) + cisplatin; (5) Discovery of new ICIs. NCT05187182, CA-4948(IRAK4/FLT3 inhibitor) + FOLFOX + PD-1 inhibitor ± trastuzumab (anti-HER2); NCT05714553, NUC-3373 (thymine synthase inhibitors) + leucovorin+ pembrolizumab/docetaxel.
Through this study, we have understood that: (1) Immunotherapy has become an important treatment modality for GC, representing the greatest advancement since chemotherapy and anti-HER2 therapy; (2) Immunotherapy has evolved from a sole focus on replacing chemotherapy to a concept of combined therapy; (3) Immunotherapy has developed from a simple pursuit of efficacy to one that balances efficacy and toxicity; and (4) Immunotherapy has progressed from the use of ICIs to the exploration of CAR-T therapy. For future research, we suggest: (1) Continued exploration of new targets; (2) Investigation into new prognostic and predictive biomarkers for immunotherapy, enabling individualized precision treatment; (3) Exploration of the optimal treatment modalities for immunotherapy in combination with ADC therapy; (4) Investigation into the application scenarios for immune therapy bispecific antibodies; and (5) Further development of CAR-T therapy targets and reduction of CAR-T therapy-related toxicities.
Currently, there are many limitations and challenges in GC immunotherapy research that need to be overcome in future studies. Firstly, GC is highly heterogeneous, both inter- and intratumorally. This variability affects the response to immunotherapeutic agents and poses challenges for identifying universal targets. Comprehensive genomic and transcriptomic analyses could identify reliable biomarkers and offer a more individualized treatment approach. Secondly, current biomarkers like PD-L1 expression, and MSI are not wholly predictive of the treatment response. So the development and validation of new biomarkers or a set of biomarkers are essential for better patient stratification and response prediction. Thirdly, immunotherapies have shown limited efficacy in the late stages of GC thus far. Combining immunotherapy with other treatment modalities such as chemotherapy or targeted therapy could potentially synergize to improve outcomes. Fourthly, the use of ICIs can lead to autoimmunity and other side effects. Therefore, developing methods for early identification and management of AEs is crucial, or discovering new lower toxic agents. Fifthly, the lack of clinically relevant animal models (e.g., TIL, ACT, TME/TIME) for GC hampers the pre-clinical evaluation of immunotherapeutic strategies. The development of patient-derived xenograft models and organoids could enhance the translational potential of pre-clinical findings.
Trends in publications and significant breakthroughs: As presented in Figure 2A, discernible surges in both publications and citations characterize the years 2016-2017 and 2020, with a pronounced resurgence beginning in 2020. These periods align with seminal studies and landmark events in the field. For instance, a 2015 meta-analysis reported that PD-L1 overexpression was correlated with adverse prognoses in solid tumors[110]. Furthermore, in 2016, a distinguished trial (NCT02447003) examining the antitumor efficacy and safety of pembrolizumab amassed 1298 citations, culminating in the FDA’s subsequent approval for HER2-positive GC treatment on May 5, 2021[28,48]. Post-2020, propelled by technological advancements, breakthroughs such as the use of single-cell RNA sequencing in GC cells and transcriptome analysis of peritoneal cancer samples from patients with GAC have emerged, enriching our understanding of tumor biology and uncovering novel immunotherapy targets[111,112]. Notably, EBV-positive status has been identified as a potential biomarker for EBV-associated GC, paving the way for future studies[113].
Evolving clinical trial landscape: With only ten phase III or IV clinical trials and 113 in either the recruiting stage or yet to commence (Figures 9A and B), immunotherapy application in GC is rapidly evolving and requires for further investigation. Analysis of ongoing trials portends emerging directions in the field, emphasizing the continuance of surgery, chemotherapy, and radiotherapy as foundational modalities, supplemented by significant advancements in targeted therapy and immunotherapy[12,114].
Monotherapy and combination therapies: The current therapeutic armamentarium includes monotherapies with ICIs and combination regimens that demonstrate efficacy against solid tumors, including GC. Specifically, ICIs have become prevalent in the treatment of advanced GC, with all analyzed phase III and IV trials incorporating anti-PD-1 antibodies, highlighting this avenue as a focal research point[114].
Anticipated future developments: Concurrently, advancements in anti-HER2 and anti-VEGFR therapies are steadily progressing, with research anticipated to encompass a wide spectrum, including ICI monotherapies, various ICI combinations, and integration with ADCs. ADCs, which bridge the specificity of mAbs with the potency of cytotoxic agents, hold immense potential as therapeutic modalities for GC, with ongoing trials investigating their synergistic application with chemotherapy. These collective developments indicate a well-defined direction for future research, affirming a promising era for immunotherapy in the field of GC.
In conclusion, this study delineates a clear trajectory in GC therapy, from the present use of monotherapies and chemo/target therapy to future integrative and combinatorial approaches, with CAR-T technologies at the forefront. It encapsulates the potential for enhanced therapeutic paradigms and heralds a new era of precision medicine for GC treatment. The findings presented here highlight substantial progress as well as pinpoint the direction for continued research and clinical innovation, aligning with the rigorous academic standards of premier oncological journals.
Strengths: This study offers systematic and specialized insights into the treatment and current status of GC by merging published literature with clinical research. By comprehensively articulating GC immunotherapy, readers can decode this complex subject into multiple dimensions. This inclusive approach synthesizes the current understanding and offers a valuable reference for future investigations, thereby enriching the collective knowledge of this field.
Limitations: Literature selection: Although extensive literature related to immunotherapy for GC was included, enabling comprehensive and objective conclusions, our search was restricted to articles and reviews within the WoSCC, using limited terms. Although this strategy emphasizes high-impact data, potentially enhancing the accuracy of our analysis, it may also lead to incomplete literature retrieval. Time constraints: Conducted in early 2023, our literature review spans from 1999 to the present, imposing time limitations that may create delays in reflecting the most recent advancements. Citation bias: Newly published critical research findings may lack substantial citation numbers, thereby obscuring their value in our data analysis.
In summary, the strengths of this study lie in its systematic and specialized approach, providing a multidimensional understanding of GC immunotherapy, whereas the limitations primarily pertain to literature selection, temporal boundaries, and potential citation bias. These factors must be considered when interpreting the findings and their implications for future research and clinical practice.
Gastric cancer (GC) ranks sixth in incidence among all cancers, and is the third leading cause of cancer-related deaths worldwide. However, there are significant limitations to the treatment of GC, and more effective and less toxic treatment options are required to prolong the survival of patients with GC. Immunotherapy has recently shown rapid development for the treatment of GC and has great potential.
In recent years, scientometrics has been applied to analyze literature related to certain fields to identify hotspots and predict future trends. However, to the best of our knowledge, there have been no previous studies on scientometric analyses in the field of GC immunotherapy.
To present a comprehensive review of the knowledge framework and research hotspots in the field of GC immunotherapy, we aimed to assist scholars in promptly understanding the current research status and latest developments in this field and provide new ideas and directions for future studies.
Publications related to GC immunotherapy between 1999 and 2023 were retrieved from the Web of Science Core Collection database on February 1, 2023. An analysis of the 2013 relevant articles retrieved using CiteSpace and VOSviewer was conducted. We get Impact Index Per Article from Reference Citation Analysis (RCA). Additionally, we searched for clinical trials on ClinicalTrials.gov and the International Clinical Trials Registry Platform, and analyzed ongoing clinical trials in this field to predict future developmental trends.
Through a literature search, we included 2013 relevant articles in this study and used the scientometric software CiteSpace and VOSviewer to analyze 11730 authors, 617 institutions, 71 countries, 726 keywords, citations, and the emergence and timeline of certain information. We have provided specific explanations for the main keywords and analyzed the significance of these research hotspots, including “tumor microenvironment”, “microsatellite instability”, “mismatch repair deficiency”, “dendritic cells”, and “adoptive immunotherapy”. Because immune checkpoint inhibitors (ICIs) play an important role in GC immunotherapy, we have also provided a separate discussion on them. Additionally, we classified the retrieved clinical trials based on the type of immunotherapy and stage of disease and further analyzed the research hotspots and trends in this field in the coming years. The integration of literature and clinical trials has enabled the production of comprehensive and objective research findings.
Through the use of novel research methods, ideas, and software, we gained innovative and comprehensive insights into the current status and future trends in GC immunotherapy. Our research findings summarize the current state of development in this field and facilitate understanding and learning among scholars. Additionally, this article identifies future developmental directions and trends in this field and provides scientific research ideas for researchers to promote the development of GC immunotherapy.
Burst keywords and clusters, including tumor microenvironment, microsatellite instability, mismatch repair deficiency, dendritic cells, and adoptive immunotherapy represent the current frontiers of research on immunological factors in cirrhosis. ICIs have also been studied. The combination of multiple drugs and immunotherapeutic methods has received increasing attention.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fu L, China; Nishida T, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Chen YX
| 1. | Chhikara BS, Parang K. Global Cancer Statistics 2022: the trends projection analysis. Chem Biol Lett. 2023;10:451. |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 63856] [Article Influence: 15964.0] [Reference Citation Analysis (174)] |
| 3. | Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 502] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 4. | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 834] [Article Influence: 166.8] [Reference Citation Analysis (0)] |
| 5. | Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, Ye XS, Do IG, Liu S, Gong L, Fu J, Jin JG, Choi MG, Sohn TS, Lee JH, Bae JM, Kim ST, Park SH, Sohn I, Jung SH, Tan P, Chen R, Hardwick J, Kang WK, Ayers M, Hongyue D, Reinhard C, Loboda A, Kim S, Aggarwal A. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1071] [Cited by in RCA: 1565] [Article Influence: 156.5] [Reference Citation Analysis (0)] |
| 6. | Högner A, Moehler M. Immunotherapy in Gastric Cancer. Curr Oncol. 2022;29:1559-1574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 111] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 7. | Jin X, Liu Z, Yang D, Yin K, Chang X. Recent Progress and Future Perspectives of Immunotherapy in Advanced Gastric Cancer. Front Immunol. 2022;13:948647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (17)] |
| 8. | Li K, Zhang A, Li X, Zhang H, Zhao L. Advances in clinical immunotherapy for gastric cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 222] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 9. | Charalampakis N, Economopoulou P, Kotsantis I, Tolia M, Schizas D, Liakakos T, Elimova E, Ajani JA, Psyrri A. Medical management of gastric cancer: a 2017 update. Cancer Med. 2018;7:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 10. | Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry RM, Phan GQ, Hughes MS, Kammula US, Miller AD, Hessman CJ, Stewart AA, Restifo NP, Quezado MM, Alimchandani M, Rosenberg AZ, Nath A, Wang T, Bielekova B, Wuest SC, Akula N, McMahon FJ, Wilde S, Mosetter B, Schendel DJ, Laurencot CM, Rosenberg SA. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36:133-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 871] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 11. | Long B, Qin L, Zhang B, Li Q, Wang L, Jiang X, Ye H, Zhang G, Yu Z, Jiao Z. CAR Tcell therapy for gastric cancer: Potential and perspective (Review). Int J Oncol. 2020;56:889-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Kumar AR, Devan AR, Nair B, Vinod BS, Nath LR. Harnessing the immune system against cancer: current immunotherapy approaches and therapeutic targets. Mol Biol Rep. 2021;48:8075-8095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 13. | Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, Wyrwicz LS, Xu J, Shitara K, Qin S, Van Cutsem E, Tabernero J, Li L, Shah S, Bhagia P, Chung HC. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 471] [Article Influence: 117.8] [Reference Citation Analysis (1)] |
| 14. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1833] [Article Influence: 458.3] [Reference Citation Analysis (1)] |
| 15. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2792] [Article Influence: 558.4] [Reference Citation Analysis (5)] |
| 16. | Sato H, Okonogi N, Nakano T. Rationale of combination of anti-PD-1/PD-L1 antibody therapy and radiotherapy for cancer treatment. Int J Clin Oncol. 2020;25:801-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 17. | Zeng Z, Yang B, Liao Z. Progress and prospects of immune checkpoint inhibitors in advanced gastric cancer. Future Oncol. 2021;17:1553-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5285] [Article Influence: 352.3] [Reference Citation Analysis (3)] |
| 19. | Shah MA, Kennedy EB, Alarcon-Rozas AE, Alcindor T, Bartley AN, Malowany AB, Bhadkamkar NA, Deighton DC, Janjigian Y, Karippot A, Khan U, King DA, Klute K, Lacy J, Lee JJ, Mehta R, Mukherjee S, Nagarajan A, Park H, Saeed A, Semrad TJ, Shitara K, Smyth E, Uboha NV, Vincelli M, Wainberg Z, Rajdev L. Immunotherapy and Targeted Therapy for Advanced Gastroesophageal Cancer: ASCO Guideline. J Clin Oncol. 2023;41:1470-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 103] [Article Influence: 51.5] [Reference Citation Analysis (35)] |
| 20. | Sato Y, Okamoto K, Kida Y, Mitsui Y, Kawano Y, Sogabe M, Miyamoto H, Takayama T. Overview of Chemotherapy for Gastric Cancer. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 21. | Ding X, Wang X, Li B, Wang L, Guo H, Shang L, Huang W, Wu L, Ke B, Liu Y, Liu N, Wang B, Cai M, Deng J, Zhang R, Zhang J, Chai J, Zhao Q, Li L, Liang H. PERSIST: A multicenter, randomized phase II trial of perioperative oxaliplatin and S-1 (SOX) with or without sintilimab in resectable locally advanced gastric/gastroesophageal junction cancer (GC/GEJC). J Clin Oncol. 2023;41:364-364. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Kim HD, Ryu MH, Park YS, Lee JS, Kang YK. A phase 2 study of durvalumab plus DOS (docetaxel, oxaliplatin, S-1) neoadjuvant chemotherapy followed by surgery and adjuvant durvalumab plus S-1 chemotherapy in patients with resectable locally advanced gastric cancer: An interim efficacy report. J Clin Oncol. 2023;41:408-408. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Pietrantonio F, Raimondi A, Lonardi S, Murgioni S, Cardellino GG, Tamberi S, Strippoli A, Palermo F, Prisciandaro M, Randon G, Corti F, Bergamo F, Nappo F, Leone AG, Leoncini G, Sabella G, Kaneva K, Sposito C, Bartolomeo MD, Mazzaferro V. INFINITY: A multicentre, single-arm, multi-cohort, phase II trial of tremelimumab and durvalumab as neoadjuvant treatment of patients with microsatellite instability-high (MSI) resectable gastric or gastroesophageal junction adenocarcinoma (GAC/GEJAC). J Clin Oncol. 2023;41:358-358. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Sur D, Lungulescu C, Puscariu II, Volovat SR, Preda M, Mateianu EA, Lungulescu CV. Immunotherapy-Related Publications in Colorectal Cancer: A Bibliometric Analysis. Healthcare (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5599] [Cited by in RCA: 6265] [Article Influence: 481.9] [Reference Citation Analysis (0)] |
| 26. | Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. 2016;9:5023-5039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 594] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 27. | Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, Sato E, Fukuoka S, Tada Y, Tanaka A, Morikawa H, Kawazoe A, Kinoshita T, Shitara K, Sakaguchi S, Nishikawa H. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A. 2019;116:9999-10008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 401] [Cited by in RCA: 743] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 28. | Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, Karantza V, Buisseret L. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol. 2016;34:2460-2467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 1144] [Article Influence: 127.1] [Reference Citation Analysis (0)] |
| 29. | Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). 2021;41:1037-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 567] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 30. | Oba K, Kobayashi M, Matsui T, Kodera Y, Sakamoto J. Individual patient based meta-analysis of lentinan for unresectable/recurrent gastric cancer. Anticancer Res. 2009;29:2739-2745. [PubMed] |
| 31. | Oba K, Teramukai S, Kobayashi M, Matsui T, Kodera Y, Sakamoto J. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curative resections of gastric cancer. Cancer Immunol Immunother. 2007;56:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Oba MS, Teramukai S, Ohashi Y, Ogawa K, Maehara Y, Sakamoto J. The efficacy of adjuvant immunochemotherapy with OK-432 after curative resection of gastric cancer: an individual patient data meta-analysis of randomized controlled trials. Gastric Cancer. 2016;19:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Qiu H. Safety and efficacy of toripalimab in advanced gastric cancer: A new clinical trial bringing hope for immunotherapy in gastric cancer. Cancer Commun (Lond). 2020;40:194-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1413] [Cited by in RCA: 1452] [Article Influence: 207.4] [Reference Citation Analysis (0)] |
| 35. | Wang F, Wei XL, Wang FH, Xu N, Shen L, Dai GH, Yuan XL, Chen Y, Yang SJ, Shi JH, Hu XC, Lin XY, Zhang QY, Feng JF, Ba Y, Liu YP, Li W, Shu YQ, Jiang Y, Li Q, Wang JW, Wu H, Feng H, Yao S, Xu RH. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol. 2019;30:1479-1486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 371] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 36. | Liu Y, Li C, Lu Y, Liu C, Yang W. Tumor microenvironment-mediated immune tolerance in development and treatment of gastric cancer. Front Immunol. 2022;13:1016817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 86] [Reference Citation Analysis (0)] |
| 37. | Gambardella V, Castillo J, Tarazona N, Gimeno-Valiente F, Martínez-Ciarpaglini C, Cabeza-Segura M, Roselló S, Roda D, Huerta M, Cervantes A, Fleitas T. The role of tumor-associated macrophages in gastric cancer development and their potential as a therapeutic target. Cancer Treat Rev. 2020;86:102015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 38. | Zhou J, Tang Z, Gao S, Li C, Feng Y, Zhou X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front Oncol. 2020;10:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 437] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 39. | Zhang B, Wu Q, Li B, Wang D, Wang L, Zhou YL. m(6)A regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer. Mol Cancer. 2020;19:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 731] [Article Influence: 146.2] [Reference Citation Analysis (0)] |
| 40. | Tsuchiya K. Switching from Apoptosis to Pyroptosis: Gasdermin-Elicited Inflammation and Antitumor Immunity. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 177] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 41. | Jiang Y, Xie J, Huang W, Chen H, Xi S, Han Z, Huang L, Lin T, Zhao LY, Hu YF, Yu J, Cai SR, Li T, Li G. Tumor Immune Microenvironment and Chemosensitivity Signature for Predicting Response to Chemotherapy in Gastric Cancer. Cancer Immunol Res. 2019;7:2065-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 42. | Farmkiss L, Hopkins I, Jones M. Idylla microsatellite instability assay versus mismatch repair immunohistochemistry: a retrospective comparison in gastric adenocarcinoma. J Clin Pathol. 2021;74:604-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Colle R, Cohen R, Cochereau D, Duval A, Lascols O, Lopez-Trabada D, Afchain P, Trouilloud I, Parc Y, Lefevre JH, Fléjou JF, Svrcek M, André T. Immunotherapy and patients treated for cancer with microsatellite instability. Bull Cancer. 2017;104:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 44. | Giampieri R, Maccaroni E, Mandolesi A, Del Prete M, Andrikou K, Faloppi L, Bittoni A, Bianconi M, Scarpelli M, Bracci R, Scartozzi M, Cascinu S. Mismatch repair deficiency may affect clinical outcome through immune response activation in metastatic gastric cancer patients receiving first-line chemotherapy. Gastric Cancer. 2017;20:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 45. | Ratti M, Lampis A, Hahne JC, Passalacqua R, Valeri N. Microsatellite instability in gastric cancer: molecular bases, clinical perspectives, and new treatment approaches. Cell Mol Life Sci. 2018;75:4151-4162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 46. | Janjigian YY, Sanchez-Vega F, Jonsson P, Chatila WK, Hechtman JF, Ku GY, Riches JC, Tuvy Y, Kundra R, Bouvier N, Vakiani E, Gao J, Heins ZJ, Gross BE, Kelsen DP, Zhang L, Strong VE, Schattner M, Gerdes H, Coit DG, Bains M, Stadler ZK, Rusch VW, Jones DR, Molena D, Shia J, Robson ME, Capanu M, Middha S, Zehir A, Hyman DM, Scaltriti M, Ladanyi M, Rosen N, Ilson DH, Berger MF, Tang L, Taylor BS, Solit DB, Schultz N. Genetic Predictors of Response to Systemic Therapy in Esophagogastric Cancer. Cancer Discov. 2018;8:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 297] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 47. | Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, Ott PA, Peltola K, Jaeger D, Evans J, de Braud F, Chau I, Harbison CT, Dorange C, Tschaika M, Le DT. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J Clin Oncol. 2018;36:2836-2844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 556] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 48. | Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 1058] [Article Influence: 264.5] [Reference Citation Analysis (0)] |
| 49. | Guo Z, Yuan Y, Chen C, Lin J, Ma Q, Liu G, Gao Y, Huang Y, Chen L, Chen LZ, Huang YF, Wang H, Li B, Chen Y, Zhang X. Durable complete response to neoantigen-loaded dendritic-cell vaccine following anti-PD-1 therapy in metastatic gastric cancer. NPJ Precis Oncol. 2022;6:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (37)] |
| 50. | Faghfuri E, Shadbad MA, Faghfouri AH, Soozangar N. Cellular immunotherapy in gastric cancer: adoptive cell therapy and dendritic cell-based vaccination. Immunotherapy. 2022;14:475-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (36)] |
| 51. | Inaba K, Metlay JP, Crowley MT, Steinman RM. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172:631-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 453] [Cited by in RCA: 498] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 52. | Yu Z, Restifo NP. Cancer vaccines: progress reveals new complexities. J Clin Invest. 2002;110:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 53. | Ohshita A, Yamaguchi Y, Minami K, Okita R, Toge T. Generation of tumor-reactive effector lymphocytes using tumor RNA-introduced dendritic cells in gastric cancer patients. Int J Oncol. 2006;28:1163-1171. [PubMed] |
| 54. | Zhang K, Gao PF, Yu PW, Rao Y, Zhou LX. Study on biological characters of SGC7901 gastric cancer cell-dendritic cell fusion vaccines. World J Gastroenterol. 2006;12:3438-3441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Xue G, Cheng Y, Ran F, Li X, Huang T, Yang Y, Zhang Y. SLC gene-modified dendritic cells mediate T cell-dependent anti-gastric cancer immune responses in vitro. Oncol Rep. 2013;29:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Mu Y, Wang WH, Xie JP, Zhang YX, Yang YP, Zhou CH. Efficacy and safety of cord blood-derived dendritic cells plus cytokine-induced killer cells combined with chemotherapy in the treatment of patients with advanced gastric cancer: a randomized Phase II study. Onco Targets Ther. 2016;9:4617-4627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Park R, Williamson S, Kasi A, Saeed A. Immune Therapeutics in the Treatment of Advanced Gastric and Esophageal Cancer. Anticancer Res. 2018;38:5569-5580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 58. | Fucikova J, Hensler M, Kasikova L, Lanickova T, Pasulka J, Rakova J, Drozenova J, Fredriksen T, Hraska M, Hrnciarova T, Sochorova K, Rozkova D, Sojka L, Dundr P, Laco J, Brtnicky T, Praznovec I, Halaska MJ, Rob L, Ryska A, Coosemans A, Vergote I, Cibula D, Bartunkova J, Galon J, Galluzzi L, Spisek R. An Autologous Dendritic Cell Vaccine Promotes Anticancer Immunity in Patients with Ovarian Cancer with Low Mutational Burden and Cold Tumors. Clin Cancer Res. 2022;28:3053-3065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 59. | Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3108] [Cited by in RCA: 3593] [Article Influence: 276.4] [Reference Citation Analysis (0)] |
| 60. | Yu W, Wang S, Rong Q, Ajayi OE, Hu K, Wu Q. Profiling the Tumor-Infiltrating Lymphocytes in Gastric Cancer Reveals Its Implication in the Prognosis. Genes (Basel). 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Cho J, Chang YH, Heo YJ, Kim S, Kim NK, Park JO, Kang WK, Lee J, Kim KM. Four distinct immune microenvironment subtypes in gastric adenocarcinoma with special reference to microsatellite instability. ESMO Open. 2018;3:e000326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 62. | Chen LJ, Zheng X, Shen YP, Zhu YB, Li Q, Chen J, Xia R, Zhou SM, Wu CP, Zhang XG, Lu BF, Jiang JT. Higher numbers of T-bet(+) intratumoral lymphoid cells correlate with better survival in gastric cancer. Cancer Immunol Immunother. 2013;62:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 63. | Mandriani B, Pelle' E, Pezzicoli G, Strosberg J, Abate-Daga D, Guarini A, Cives M, Porta C. Adoptive T-cell immunotherapy in digestive tract malignancies: Current challenges and future perspectives. Cancer Treat Rev. 2021;100:102288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 806] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 65. | D'Angelo SP, Melchiori L, Merchant MS, Bernstein D, Glod J, Kaplan R, Grupp S, Tap WD, Chagin K, Binder GK, Basu S, Lowther DE, Wang R, Bath N, Tipping A, Betts G, Ramachandran I, Navenot JM, Zhang H, Wells DK, Van Winkle E, Kari G, Trivedi T, Holdich T, Pandite L, Amado R, Mackall CL. Antitumor Activity Associated with Prolonged Persistence of Adoptively Transferred NY-ESO-1 (c259)T Cells in Synovial Sarcoma. Cancer Discov. 2018;8:944-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 336] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 66. | Lu YC, Parker LL, Lu T, Zheng Z, Toomey MA, White DE, Yao X, Li YF, Robbins PF, Feldman SA, van der Bruggen P, Klebanoff CA, Goff SL, Sherry RM, Kammula US, Yang JC, Rosenberg SA. Treatment of Patients With Metastatic Cancer Using a Major Histocompatibility Complex Class II-Restricted T-Cell Receptor Targeting the Cancer Germline Antigen MAGE-A3. J Clin Oncol. 2017;35:3322-3329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 67. | Wang M, Gao P, Ren L, Duan J, Yang S, Wang H, Sun J, Gao X, Li B, Li S, Su W. Profiling the peripheral blood T cell receptor repertoires of gastric cancer patients. Front Immunol. 2022;13:848113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 68. | Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 1166] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 69. | Jaspers JE, Brentjens RJ. Development of CAR T cells designed to improve antitumor efficacy and safety. Pharmacol Ther. 2017;178:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 70. | Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1684] [Cited by in RCA: 1877] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 71. | Sotoudeh M, Shirvani SI, Merat S, Ahmadbeigi N, Naderi M. MSLN (Mesothelin), ANTXR1 (TEM8), and MUC3A are the potent antigenic targets for CAR T cell therapy of gastric adenocarcinoma. J Cell Biochem. 2019;120:5010-5017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 72. | Bębnowska D, Grywalska E, Niedźwiedzka-Rystwej P, Sosnowska-Pasiarska B, Smok-Kalwat J, Pasiarski M, Góźdź S, Roliński J, Polkowski W. CAR-T Cell Therapy-An Overview of Targets in Gastric Cancer. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 73. | Liu Y, Cheng Y, Xu Y, Wang Z, Du X, Li C, Peng J, Gao L, Liang X, Ma C. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene. 2017;36:6143-6153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 282] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 74. | Rosso D, Rigueiro MP, Kassab P, Ilias EJ, Castro OA, Novo NF, Lourenço LG. [Correlation of natural killer cells with the prognosis of gastric adenocarcinoma]. Arq Bras Cir Dig. 2012;25:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 75. | Abdolahi S, Ghazvinian Z, Muhammadnejad S, Ahmadvand M, Aghdaei HA, Ebrahimi-Barough S, Ai J, Zali MR, Verdi J, Baghaei K. Adaptive NK Cell Therapy Modulated by Anti-PD-1 Antibody in Gastric Cancer Model. Front Pharmacol. 2021;12:733075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 76. | Klapdor R, Wang S, Hacker U, Büning H, Morgan M, Dörk T, Hillemanns P, Schambach A. Improved Killing of Ovarian Cancer Stem Cells by Combining a Novel Chimeric Antigen Receptor-Based Immunotherapy and Chemotherapy. Hum Gene Ther. 2017;28:886-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 77. | Zhang Q, Zhang H, Ding J, Liu H, Li H, Lu M, Miao Y, Li L, Zheng J. Combination Therapy with EpCAM-CAR-NK-92 Cells and Regorafenib against Human Colorectal Cancer Models. J Immunol Res. 2018;2018:4263520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 78. | Klapdor R, Wang S, Morgan M, Dörk T, Hacker U, Hillemanns P, Büning H, Schambach A. Characterization of a Novel Third-Generation Anti-CD24-CAR against Ovarian Cancer. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 79. | Jiang J, Xu N, Wu C, Deng H, Lu M, Li M, Xu B, Wu J, Wang R, Xu J, Nilsson-Ehle P. Treatment of advanced gastric cancer by chemotherapy combined with autologous cytokine-induced killer cells. Anticancer Res. 2006;26:2237-2242. [PubMed] |
| 80. | Jiang JT, Shen YP, Wu CP, Zhu YB, Wei WX, Chen LJ, Zheng X, Sun J, Lu BF, Zhang XG. Increasing the frequency of CIK cells adoptive immunotherapy may decrease risk of death in gastric cancer patients. World J Gastroenterol. 2010;16:6155-6162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 81. | Hontscha C, Borck Y, Zhou H, Messmer D, Schmidt-Wolf IG. Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC). J Cancer Res Clin Oncol. 2011;137:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 82. | Hu G, Zhong K, Wang S, Ding Q, Xu F, Chen W, Cheng P, Huang L. Cellular immunotherapy plus chemotherapy ameliorates survival in gastric cancer patients: a meta-analysis. Int J Clin Oncol. 2020;25:1747-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 1492] [Article Influence: 373.0] [Reference Citation Analysis (0)] |
| 84. | Zhao Q, Jiang Y, Xiang S, Kaboli PJ, Shen J, Zhao Y, Wu X, Du F, Li M, Cho CH, Li J, Wen Q, Liu T, Yi T, Xiao Z. Engineered TCR-T Cell Immunotherapy in Anticancer Precision Medicine: Pros and Cons. Front Immunol. 2021;12:658753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 85. | Ishikawa T, Okayama T, Sakamoto N, Ideno M, Oka K, Enoki T, Mineno J, Yoshida N, Katada K, Kamada K, Uchiyama K, Handa O, Takagi T, Konishi H, Kokura S, Uno K, Naito Y, Itoh Y. Phase I clinical trial of adoptive transfer of expanded natural killer cells in combination with IgG1 antibody in patients with gastric or colorectal cancer. Int J Cancer. 2018;142:2599-2609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (41)] |
| 86. | Wang S, Sun J, Chen K, Ma P, Lei Q, Xing S, Cao Z, Sun S, Yu Z, Liu Y, Li N. Perspectives of tumor-infiltrating lymphocyte treatment in solid tumors. BMC Med. 2021;19:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 87. | Zhang D, He W, Wu C, Tan Y, He Y, Xu B, Chen L, Li Q, Jiang J. Scoring System for Tumor-Infiltrating Lymphocytes and Its Prognostic Value for Gastric Cancer. Front Immunol. 2019;10:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 88. | Mazloom A, Ghalehsari N, Gazivoda V, Nimkar N, Paul S, Gregos P, Rateshwar J, Khan U. Role of Immune Checkpoint Inhibitors in Gastrointestinal Malignancies. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 89. | Xu-Monette ZY, Zhang M, Li J, Young KH. PD-1/PD-L1 Blockade: Have We Found the Key to Unleash the Antitumor Immune Response? Front Immunol. 2017;8:1597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 217] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 90. | Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, Kasperbauer JL, Ballman KV, Chen L. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501-6505. [PubMed] |
| 91. | Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 1004] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 92. | Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 610] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 93. | Schlößer HA, Drebber U, Kloth M, Thelen M, Rothschild SI, Haase S, Garcia-Marquez M, Wennhold K, Berlth F, Urbanski A, Alakus H, Schauss A, Shimabukuro-Vornhagen A, Theurich S, Warnecke-Ebertz U, Stippel DL, Zippelius A, Büttner R, Hallek M, Hölscher AH, Zander T, Mönig SP, von Bergwelt-Baildon M. Immune checkpoints programmed death 1 ligand 1 and cytotoxic T lymphocyte associated molecule 4 in gastric adenocarcinoma. Oncoimmunology. 2016;5:e1100789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 94. | Kim JW, Nam KH, Ahn SH, Park DJ, Kim HH, Kim SH, Chang H, Lee JO, Kim YJ, Lee HS, Kim JH, Bang SM, Lee JS, Lee KW. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer. 2016;19:42-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 95. | Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, Alsina M, Ryu MH, Chung HC, Evesque L, Al-Batran SE, Park SH, Lichinitser M, Boku N, Moehler MH, Hong J, Xiong H, Hallwachs R, Conti I, Taieb J. Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29:2052-2060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 410] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 96. | Moehler M, Dvorkin M, Boku N, Özgüroğlu M, Ryu MH, Muntean AS, Lonardi S, Nechaeva M, Bragagnoli AC, Coşkun HS, Cubillo Gracian A, Takano T, Wong R, Safran H, Vaccaro GM, Wainberg ZA, Silver MR, Xiong H, Hong J, Taieb J, Bang YJ. Phase III Trial of Avelumab Maintenance After First-Line Induction Chemotherapy Versus Continuation of Chemotherapy in Patients With Gastric Cancers: Results From JAVELIN Gastric 100. J Clin Oncol. 2021;39:966-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 178] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 97. | Fuchs CS, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, Fornaro L, Olesinski T, Caglevic C, Chung HC, Muro K, Van Cutsem E, Elme A, Thuss-Patience P, Chau I, Ohtsu A, Bhagia P, Wang A, Shih CS, Shitara K. Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial. Gastric Cancer. 2022;25:197-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 98. | Chao J, Fuchs CS, Shitara K, Tabernero J, Muro K, Van Cutsem E, Bang YJ, De Vita F, Landers G, Yen CJ, Chau I, Elme A, Lee J, Özgüroglu M, Catenacci D, Yoon HH, Chen E, Adelberg D, Shih CS, Shah S, Bhagia P, Wainberg ZA. Assessment of Pembrolizumab Therapy for the Treatment of Microsatellite Instability-High Gastric or Gastroesophageal Junction Cancer Among Patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol. 2021;7:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 284] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 99. | Xu J, Bai Y, Xu N, Li E, Wang B, Wang J, Li X, Wang X, Yuan X. Tislelizumab Plus Chemotherapy as First-line Treatment for Advanced Esophageal Squamous Cell Carcinoma and Gastric/Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res. 2020;26:4542-4550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 100. | Peng Z, Wei J, Wang F, Ying J, Deng Y, Gu K, Cheng Y, Yuan X, Xiao J, Tai Y, Wang L, Zou J, Zhang Y, Shen L. Camrelizumab Combined with Chemotherapy Followed by Camrelizumab plus Apatinib as First-line Therapy for Advanced Gastric or Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res. 2021;27:3069-3078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 101. | Sakai K, Mori S, Kawamoto T, Taniguchi S, Kobori O, Morioka Y, Kuroki T, Kano K. Expression of epidermal growth factor receptors on normal human gastric epithelia and gastric carcinomas. J Natl Cancer Inst. 1986;77:1047-1052. [PubMed] |
| 102. | Zhu Y, Zhu X, Wei X, Tang C, Zhang W. HER2-targeted therapies in gastric cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 103. | Stein A, Paschold L, Tintelnot J, Goekkurt E, Henkes SS, Simnica D, Schultheiss C, Willscher E, Bauer M, Wickenhauser C, Thuss-Patience P, Lorenzen S, Ettrich T, Riera-Knorrenschild J, Jacobasch L, Kretzschmar A, Kubicka S, Al-Batran SE, Reinacher-Schick A, Pink D, Sinn M, Lindig U, Hiegl W, Hinke A, Hegewisch-Becker S, Binder M. Efficacy of Ipilimumab vs FOLFOX in Combination With Nivolumab and Trastuzumab in Patients With Previously Untreated ERBB2-Positive Esophagogastric Adenocarcinoma: The AIO INTEGA Randomized Clinical Trial. JAMA Oncol. 2022;8:1150-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 104. | Du XH, Liu HL, Li L, Xia SY, Ning N, Zou ZY, Teng D, Xiao CH, Li R, Xu YX. Clinical significance of immunotherapy with combined three kinds of cells for operable colorectal cancer. Tumour Biol. 2015;36:5679-5685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 105. | Cheng X, Wang J, Qiu C, Jin Y, Xia B, Qin R, Hu H, Yan J, Zhang X, Xu J. Feasibility of iNKT cell and PD-1+CD8+ T cell-based immunotherapy in patients with lung adenocarcinoma: Preliminary results of a phase I/II clinical trial. Clin Immunol. 2022;238:108992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 106. | Qiao G, Wang X, Zhou L, Zhou X, Song Y, Wang S, Zhao L, Morse MA, Hobeika A, Song J, Yi X, Xia X, Ren J, Lyerly HK. Autologous Dendritic Cell-Cytokine Induced Killer Cell Immunotherapy Combined with S-1 Plus Cisplatin in Patients with Advanced Gastric Cancer: A Prospective Study. Clin Cancer Res. 2019;25:1494-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 107. | Xu B, Chen F, Zhang X, Wang Z, Che K, Wu N, Yu L, Fan X, Liu B, Wei J. Antigen-Specific T Cell Immunotherapy Targeting Claudin18.2 in Gastric Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 108. | Bré J, Dickson AL, Read OJ, Zhang Y, McKissock FG, Mullen P, Tang P, Zickuhr GM, Czekster CM, Harrison DJ. The novel anti-cancer fluoropyrimidine NUC-3373 is a potent inhibitor of thymidylate synthase and an effective DNA-damaging agent. Cancer Chemother Pharmacol. 2023;91:401-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 109. | Somani VK, Zhang D, Dodhiawala PB, Lander VE, Liu X, Kang LI, Chen HP, Knolhoff BL, Li L, Grierson PM, Ruzinova MB, DeNardo DG, Lim KH. IRAK4 Signaling Drives Resistance to Checkpoint Immunotherapy in Pancreatic Ductal Adenocarcinoma. Gastroenterology. 2022;162:2047-2062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 110. | Wu P, Wu D, Li L, Chai Y, Huang J. PD-L1 and Survival in Solid Tumors: A Meta-Analysis. PLoS One. 2015;10:e0131403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 285] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 111. | Wang R, Dang M, Harada K, Han G, Wang F, Pool Pizzi M, Zhao M, Tatlonghari G, Zhang S, Hao D, Lu Y, Zhao S, Badgwell BD, Blum Murphy M, Shanbhag N, Estrella JS, Roy-Chowdhuri S, Abdelhakeem AAF, Wang Y, Peng G, Hanash S, Calin GA, Song X, Chu Y, Zhang J, Li M, Chen K, Lazar AJ, Futreal A, Song S, Ajani JA, Wang L. Single-cell dissection of intratumoral heterogeneity and lineage diversity in metastatic gastric adenocarcinoma. Nat Med. 2021;27:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 175] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 112. | Sathe A, Grimes SM, Lau BT, Chen J, Suarez C, Huang RJ, Poultsides G, Ji HP. Single-Cell Genomic Characterization Reveals the Cellular Reprogramming of the Gastric Tumor Microenvironment. Clin Cancer Res. 2020;26:2640-2653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 236] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 113. | Salnikov M, Prusinkiewicz MA, Lin S, Ghasemi F, Cecchini MJ, Mymryk JS. Tumor-Infiltrating T Cells in EBV-Associated Gastric Carcinomas Exhibit High Levels of Multiple Markers of Activation, Effector Gene Expression, and Exhaustion. Viruses. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 114. | Guan WL, He Y, Xu RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. 2023;16:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 377] [Reference Citation Analysis (5)] |