Published online Jan 28, 2023. doi: 10.3748/wjg.v29.i4.706
Peer-review started: September 18, 2022
First decision: November 15, 2022
Revised: November 28, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 28, 2023
Processing time: 124 Days and 4.2 Hours
The diagnostic and economic value of carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9) and CA72-4 for gastrointestinal malignant tumors lacked evaluation in a larger scale.
To reassess the diagnostic and economic value of the three tumor biomarkers.
A retrospective analysis of all 32857 subjects who underwent CEA, CA19-9, CA72-4, gastroscopy and colonoscopy from October 2006 to May 2018 was conducted. Then, we assessed the discrimination and clinical usefulness. Total cost, cost per capita and cost-effectiveness ratios were used to evaluate the economic value of two schemes (gastrointestinal endoscopy for all people without blood tests vs both gastroscopy and colonoscopy when blood tests were positive).
The analysis of 32857 subjects showed that CEA was a qualified biomarker for colorectal cancer (CRC), while the diagnostic efficiencies of CA72-4 were catastrophic for all gastrointestinal cancers (GICs). Regarding early diagnosis, only CEA could be used for early CRC. The combination of biomarkers didn’t greatly increase the area under the curve. The economic indicators of CEA were superior to those of CA19-9, CA72-4 and any combination. At the threshold of 1.8 μg/L to 10.4 μg/L, all four indicators of CEA were lower than those in the scheme that conducted gas-trointestinal endoscopy only. Subgroup analysis implied that the health checkup of CEA for people above 65 years old was economically valuable.
CEA had qualified diagnostic value for CRC and superior economic value for GICs, especially for elderly health checkup subjects. CA72-4 was not suitable as a diagnostic biomarker.
Core Tip: This is a retrospective study to reassess the diagnostic and economic value of traditional tumor biomarkers carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9) and CA72-4 for gastrointestinal malignant tumors in a large sample with novel indicators. Instead of increasing the diagnostic value, CA72-4 should be removed from the list of the health checkup items to avoid the waste of social medical resources for CEA were superior to those of CA19-9, CA72-4 or any other combinations in which it could be applied for early colorectal cancer and a health checkup of CEA for people above 65 years old was economically valuable.
- Citation: Liu HN, Yao C, Wang XF, Zhang NP, Chen YJ, Pan D, Zhao GP, Shen XZ, Wu H, Liu TT. Diagnostic and economic value of carcinoembryonic antigen, carbohydrate antigen 19-9, and carbohydrate antigen 72-4 in gastrointestinal cancers. World J Gastroenterol 2023; 29(4): 706-730
- URL: https://www.wjgnet.com/1007-9327/full/v29/i4/706.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i4.706
Blood carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) are widely used as classic diagnostic markers for malignant tumors, and they are recommended by several clinical guidelines for gastrointestinal cancer (GIC) screening[1-3]. Following the introduction of the CEA and CA19-9 assessment, in 1990, blood CA72-4 was proposed as a diagnostic biomarker for gastric cancer (GC)[4]. Subsequent studies showed that CA72-4 could be used to diagnose GC and colorectal cancer (CRC)[5,6]. These studies reported sensitivities of 19%-47% in GC and 25%-43% in CRC at the cut-off value of 6 kU/L[7-13]. The clinical guidelines published by the European Group on Tumor Markers (EGTM) in 2003 suggested that CA72-4 could be a potential biomarker for CRC[14].
Based on these previous studies, blood CA72-4 began to be widely used as a tumor biomarker since 2010 in China. Nevertheless, after large-scale clinical application, we noticed, empirically, an extremely high false positive rate of CA72-4 for diagnosis. A positive result would lead the subject to undergo further examinations, including gastroscopy, colonoscopy, chest computed tomography (CT), abdominal CT, and even positron emission tomography-CT (PET-CT). The blood test with a low positive predictive value (PPV) not only brings unnecessary anxiety, invasive examinations, and extra costs to the subjects but also leads to the waste of medical resources and increases the social medical burden.
The massive data and real-world diagnostic cohorts make it possible to further explore the diagnostic and economic value of biomarkers. Through a real-world diagnostic cohort, we comprehensively analyzed the differences in the levels of CEA, CA19-9, and CA72-4 and their diagnostic and economic value in gastrointestinal tumors. Four indicators were used to comprehensively evaluate the economic value, namely, the total cost and the average cost per person for each positive patient diagnosed and their corresponding cost-effectiveness ratios. We evaluated whether age and health checkup could help us make useful recommendations for thresholds of tumor biomarkers and medical insurance policies.
We retrospectively analyzed all patients from October 2006 to May 2018. The inclusion criteria included: (1) Patients from the medical examination center, outpatient department or inpatient department of Zhongshan Hospital of Fudan University; and (2) patients had completed all five examinations, namely, CEA, CA19-9, CA72-4, gastroscopy and colonoscopy, within half a year. The exclusion criteria were as follows: (1) Duplicate patients; and (2) patients who had accepted anti-tumor therapies such as radiotherapy, chemotherapy or surgery.
All data were abstracted from our hospital information system (HIS). They included general information (e.g., age, sex, medical record number, whether health checkup, past history), the concentrations of each of the three tumor biomarkers, reports of auxiliary examinations (e.g., endoscopy, pathology, ultrasonography, CT, magnetic resonance, PET-CT, electrocardiogram), and the medical records of outpatients and inpatients. The generation time of these data was also provided.
The concentrations of serum CEA, CA19-9, and CA72-4 were measured with an electrochemiluminescence immunoassay (Elecsys2010, Roche Diagnostics, indianapolis, IN, United States). The traditional cut-off values for CEA, CA19-9, and CA72-4 were 5 μg/L, 37 kU/L, and 6 kU/L, respectively.
According to the regular practice of our hospital, pathological biopsy was taken when gastroscopy was performed, while colon biopsy was not necessary taken unless some lesions were found by colonoscopy. The diagnosis of GIC depends on the gold standard of pathology, and other gas-trointestinal diseases are diagnosed by endoscopy and pathology. Other malignant tumors were comprehensively judged based on the medical history, pathology and imaging exams that we could collect. TNM staging of cancers was based on the American Joint Committee on Cancer Staging or case data at that time.
According to the type of test and the order of endoscopy procedures, we assumed six schemes (Table 1). Four economic indicators combined with the proportion of endoscopies and the missed diagnosis rate was used to evaluate the economic value of tumor biomarkers. The four economic indicators were the total cost and cost per capita of correctly diagnosing one case of GIC and the cost-effectiveness ratio of the above two indicators. The cost-effectiveness ratio was the total cost or cost per capita divided by sensitivity. We assumed that the missed diagnosis rate and misdiagnosis of endoscopy plus necessary pathological examination for gastrointestinal malignancies were all 0.
| Item | Description |
| Schemes | Scheme 1 Gastrointestinal endoscopy for all people without blood tests |
| Scheme 2 Both of gastroscopy and colonoscopy when blood tests were positive | |
| Scheme 3 Gastroscopy first when blood tests were positive, and then colonoscopy when the result of gastroscopy was negative | |
| Scheme 4 Colonoscopy first when blood tests were positive, and then gastroscopy when the result of colonoscopy was negative | |
| Scheme 5 Only gastroscopy when blood tests were positive | |
| Scheme 6 Only colonoscopy when blood tests were positive | |
| Examination prices | CEA, $4.64; CA19-9, $7.25; CA72-4, $7.25 |
| Gastroscopy & biopsy, $87.99 | |
| Colonoscopy, $57.98; biopsy after colonoscopy, $32.62 |
The costs of blood tests, endoscopy and pathological examination were the cost of these procedures at Zhongshan Hospital in 2019 (Table 1). All costs were converted to United States dollars.
Considering the preliminary results, further analyses were performed on Scheme 1 (gastrointestinal endoscopy for all people without blood tests) and Scheme 2 (both gastroscopy and colonoscopy when blood tests were positive). We also calculated 9 conditions when CEA and CA19-9 were combined. They were parallel (any positive was considered positive), serial (all positive was considered positive), and the formula under the traditional cut-off value (the coefficients of CEA and CA19-9 were calculated according to the logistic regression), the minimum total cost, and the minimum total cost-effectiveness ratio.
Subgroup analysis (age, health checkup/active consultation) was utilized to analyze the economic value of the three biomarkers under the traditional threshold, with a view to drawing some medical insurance recommendations.
The statistical analyses were performed using R software 3.3.5 (R Foundation for Statistical Computing, Vienna, Austria). The level of significance was set at P < 0.05. All tests were two-sided.
Student’s t-test or Wilcoxon test was used to assess the differences in continuous variables, as appropriate. The chi-square test was used for counting variables. Correlations between two variables were calculated by Pearson correlation analysis or Spearman correlation analysis. The influences of age and sex on the biomarker levels were analyzed with the regression coefficient of linear regression. Categorical regression analysis was utilized to calculate the regression coefficient quantification of each stage of GC and CRC.
The diagnostic value was evaluated by means of the area under the curve (AUC) values of the receiver operating characteristics (ROC) curve, as well as the diagnostic odds ratio (DOR), sensitivity, specificity, Youden index (sensitivity + specificity-1), accuracy, predicted value and likelihood ratio on the traditional and best cut-off values. The best cut-off value referred to the threshold when the Youden index was the largest. When multiple diagnostic biomarkers were combined, logistic regression was used to calculate the formula coefficients. We used Delong’s test to compare AUC.
Decision curve analysis (DCA) was performed to determine the clinical usefulness of the radiomics nomogram by quantifying the net benefits at different threshold probabilities. The clinical net benefit was defined as the true positive rate (sensitivity) minus the false positive rate (misdiagnosis rate) and was then weighted by the relative damage of the positive rate and the negative rate.
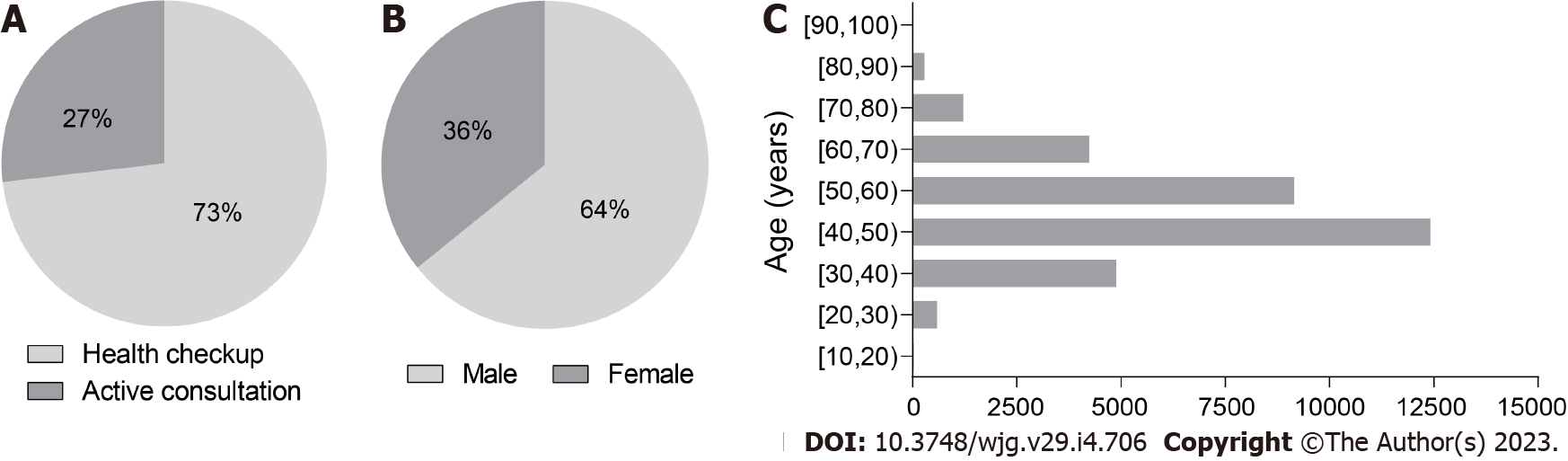
According to the inclusion criteria, we screened a total of 32857 subjects aged 15 to 97 years in the HIS, including 21099 males and 11758 females. There were 24045 subjects who underwent health checkup and 8812 subjects with an active consultation (Figure 1). The ages and sexes of the subjects with GC, CRC, and GIC were significantly different from those of the subjects without the disease (Table 2).
| Age median (quartile) | P value | Male, n (%) | Female, n (%) | P value | |
| Gastric cancer | 61 (51, 68) | < 0.001 | 268 (68.4) | 124 (31.6) | 0.084 |
| Non-gastric cancer | 48 (42, 56) | 20831 (64.2) | 11634 (35.8) | ||
| Colorectal cancer | 62 (55, 70) | < 0.001 | 522 (58.5) | 370 (41.5) | < 0.001 |
| Non-colorectal cancer | 48 (42, 55) | 20577 (64.4) | 11388 (35.6) | ||
| Gastrointestinal cancer | 62 (53, 69) | < 0.001 | 816 (62.4) | 491 (37.6) | 0.170 |
| Non-gastrointestinal cancer | 48 (42, 55) | 20283 (64.3) | 11267 (35.7) |
The constituent ratios of the diseases detected by gastroscopy, colonoscopy and pathological examination are displayed in Table 3. Among them, there were 392 GC cases, 892 CRC cases and 1307 GIC cases.
| Examination | Disease | No. | % |
| Gastroscope (without pathological examination) | Esophagitis | 2887 | 8.8% |
| Esophageal erosion | 88 | 0.3% | |
| Esophageal ulcer | 30 | 0.1% | |
| Esophageal protuberant lesion | 491 | 1.5% | |
| Esophageal non-protuberant lesion | 77 | 0.2% | |
| Barrett’s esophagus | 66 | 0.2% | |
| Bile reflux | 1344 | 4.1% | |
| Gastric atrophy | 45 | 0.1% | |
| Gastric erosion | 12457 | 37.9% | |
| Gastric hemorrhage | 1117 | 3.4% | |
| Gastric ulcer | 1088 | 3.3% | |
| Gastric protuberant lesion | 3329 | 10.1% | |
| Gastric non-protuberant lesion | 225 | 0.7% | |
| Duodenitis | 1473 | 4.5% | |
| Duodenal erosion | 26 | 0.1% | |
| Duodenal ulcer | 1548 | 4.7% | |
| Duodenal protuberant lesion | 666 | 2.0% | |
| Duodenal non-protuberant lesion | 31 | 0.1% | |
| Colonoscopy (without pathological examination) | Colorectitis | 653 | 2.0% |
| Colorectal erosion | 29 | 0.1% | |
| Colorectal ulcer | 99 | 0.3% | |
| Colorectal protuberant lesion | 7312 | 22.3% | |
| Colorectal non-protuberant lesion | 36 | 0.1% | |
| Pathological examination | Esophageal mucositis | 398 | 1.2% |
| Esophageal dysplasia | 44 | 0.1% | |
| Esophageal adenoma | 1 | < 0.1% | |
| Esophageal hyperplastic polyp | 2 | < 0.1% | |
| Esophageal glandular hyperplasia | 4 | < 0.1% | |
| Chronic atrophic gastritis | 1809 | 5.5% | |
| Gastric dysplasia | 308 | 0.9% | |
| Gastric adenoma | 13 | < 0.1% | |
| Gastric hyperplastic polyps | 117 | 0.4% | |
| Gastric glandular hyperplasia | 761 | 2.3% | |
| Gastric juvenile polyps | 1 | < 0.1% | |
| Duodenal mucositis | 276 | 0.8% | |
| Duodenal dysplasia | 24 | 0.1% | |
| Duodenal adenoma | 12 | < 0.1% | |
| Duodenal hyperplastic polyps | 10 | < 0.1% | |
| Duodenal gland hyperplasia | 31 | 0.1% | |
| Colorectal mucositis | 2206 | 6.7% | |
| Colorectal high-grade intraepithelial neoplasia | 330 | 1.0% | |
| Colorectal low-grade intraepithelial neoplasia | 3364 | 10.2% | |
| Colorectal adenoma | 3707 | 11.3% | |
| Colorectal hyperplastic polyps | 1037 | 3.2% | |
| Colorectal inflammatory polyps | 13 | < 0.1% | |
| Colorectal gland hyperplasia | 567 | 1.7% | |
| Colorectal juvenile polyps | 3 | < 0.1% | |
| Peutz-Jeghers polyps | 5 | < 0.1% | |
| Familial polyposis coli | 3 | < 0.1% | |
| Esophageal cancer | 57 | 0.2% | |
| Gastric cancer | 392 | 1.2% | |
| Duodenal cancer | 25 | 0.1% | |
| Small intestine cancer | 4 | < 0.1% | |
| Colorectal cancer | 892 | 2.7% | |
| Liver cancer | 127 | 0.4% | |
| Pancreatic cancer | 47 | 0.1% | |
| Gallbladder cancer | 20 | 0.1% | |
| Bile duct cancer & ampulla cancer | 10 | < 0.1% | |
| Lung cancer | 129 | 0.4% | |
| Breast cancer | 55 | 0.2% | |
| Ovarian cancer | 57 | 0.2% | |
| Uterine malignancy | 28 | 0.1% | |
| Kidney cancer | 37 | 0.1% | |
| Prostate cancer | 28 | 0.1% | |
| Bladder Cancer | 17 | 0.1% | |
| Leukemia | 1 | < 0.1% | |
| Lymphoma | 29 | 0.1% | |
| Other malignant tumors | 15 | < 0.1% |
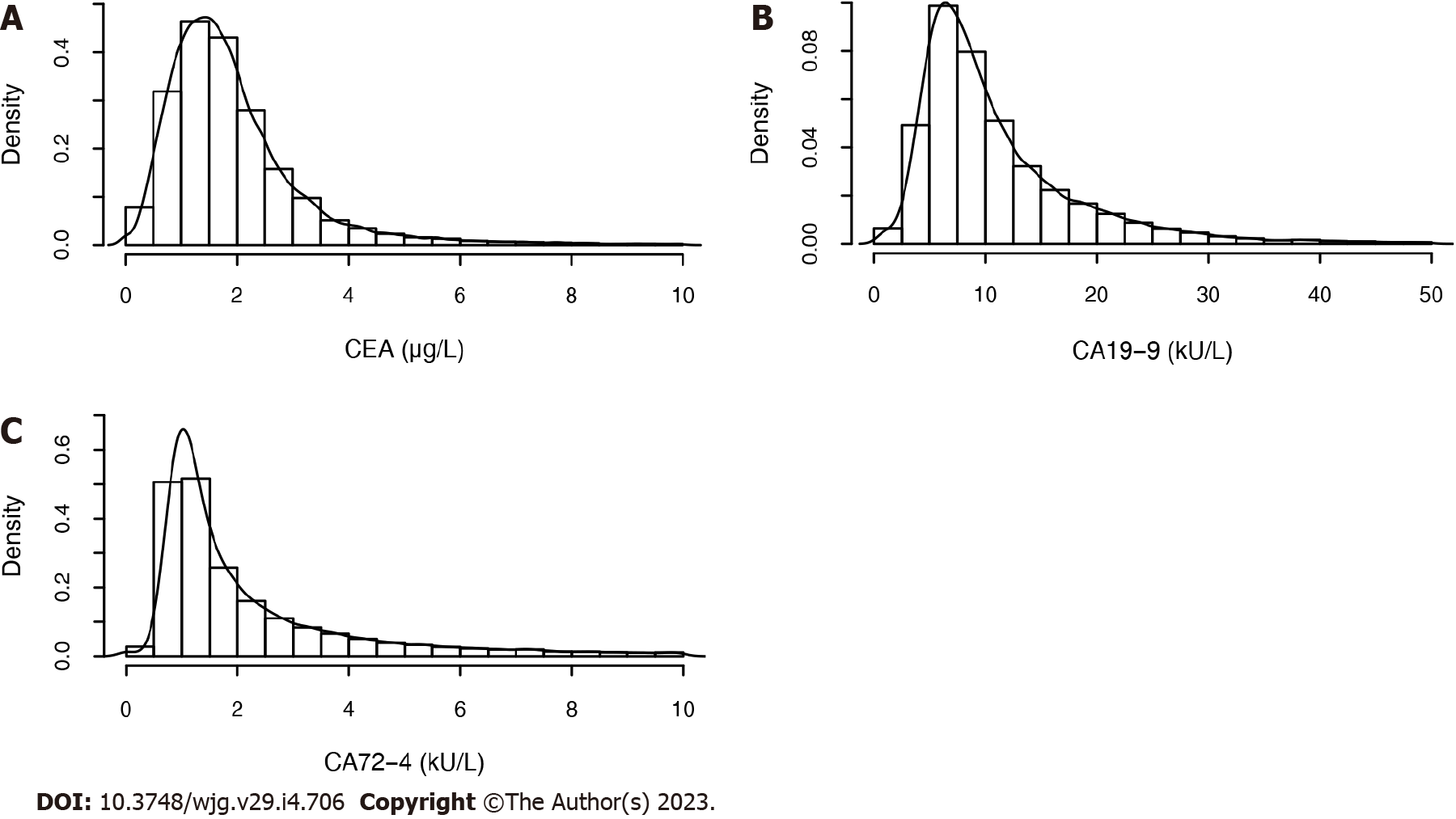
The concentrations of the three biomarkers were skewed (Figure 2). The correlations between the pairwise biomarkers are shown in Table 4. We found that there were significant correlations between CEA and CA19-9 in all subjects and various GICs, and the correlation coefficients were all exceed 0.245.
| Correlation coefficient | P value | |||||
| CEA and CA19-9 | CEA and CA72-4 | CA19-9 and CA724-4 | CEA and CA19-9 | CEA and CA72-4 | CA19-9 and CA724-4 | |
| Whole | 0.245 | -0.005 | -0.046 | < 0.001 | 0.359 | < 0.001 |
| Gastric cancer | 0.291 | 0.048 | 0.022 | < 0.001 | 0.342 | 0.657 |
| Colorectal cancer | 0.385 | 0.2 | 0.169 | < 0.001 | < 0.001 | < 0.001 |
| Gastrointestinal cancer | 0.354 | 0.164 | 0.134 | < 0.001 | < 0.001 | < 0.001 |
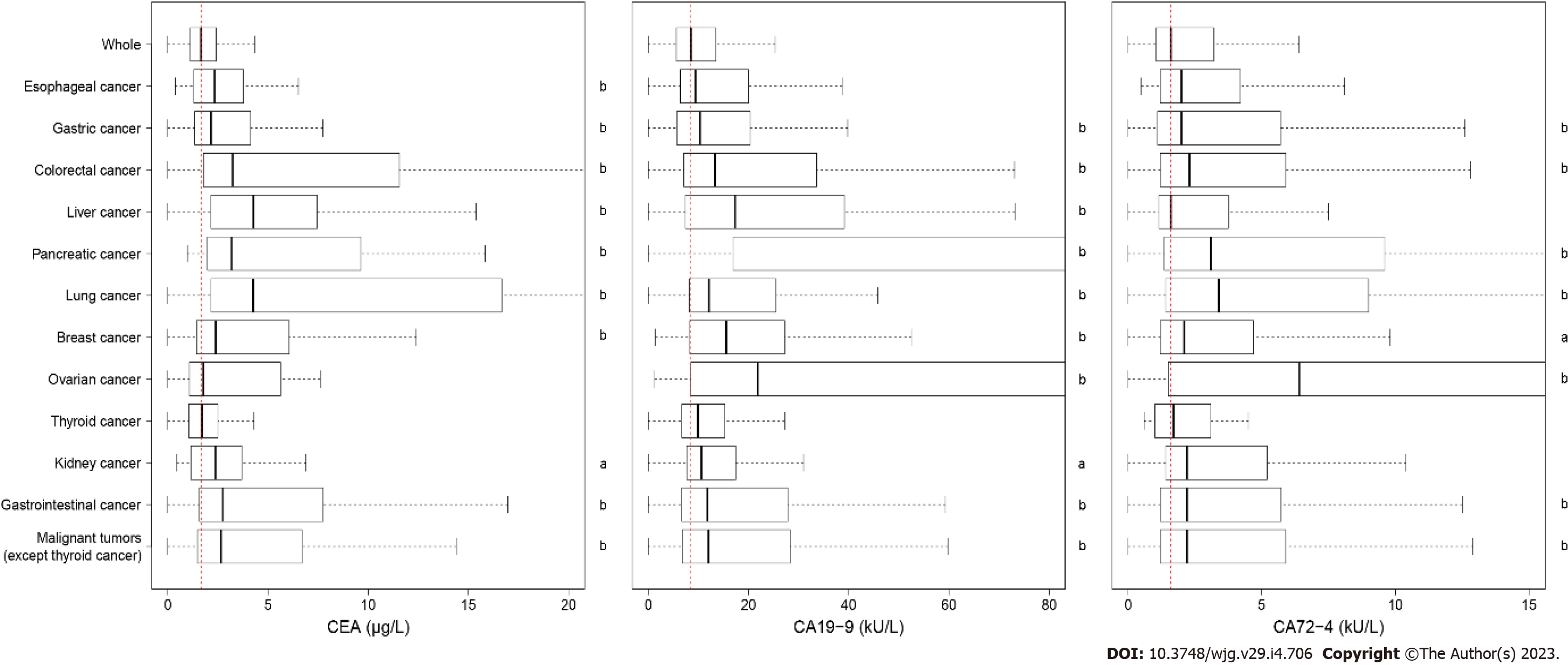
The median values for CEA, CA19-9, and CA72-4 Level were 1.67 μg/L, 8.50 kU/L and 1.60 kU/L, respectively. The expression levels of the biomarkers for the diseases that had more than 30 cases are shown in Table 5. The concentrations of the three biomarkers in patients with several malignant tumors were significantly different from those without malignant tumors (Figure 3). The CEA level increased but did not exceed 0.3 μg/L in esophageal erosion, gastric erosion, gastric ulcer, chronic atrophic gastritis, and colorectal adenoma.
| Disease | No. | CEA | CA19-9 | CA72-4 | ||||||
| Median (quartile) (μg/L) | P value | AUC | Median (quartile) (kU/L) | P value | AUC | Median (quartile) (kU/L) | P value | AUC | ||
| Whole | 32857 | 1.67 (1.13, 2.41) | - | - | 8.50 (5.60, 13.50) | - | - | 1.60 (1.05, 3.20) | - | - |
| Esophagitis | 3137 | 1.88 (1.29, 2.72) | < 0.001 | 0.566 | 8.60 (5.70, 13.50) | 0.439 | 0.504 | 1.60 (1.10, 3.40) | 0.001 | 0.518 |
| Esophageal erosion | 109 | 1.84 (1.40, 2.90) | 0.007 | 0.575 | 8.30 (5.00, 15.00) | 0.938 | 0.498 | 1.50 (1.10, 3.20) | 0.644 | 0.487 |
| Esophageal ulcer | 30 | 1.73 (0.90, 2.29) | 0.818 | 0.488 | 7.90 (5.73, 10.38) | 0.166 | 0.573 | 1.59 (1.10, 3.95) | 0.572 | 0.470 |
| Barrett’s esophagus | 66 | 1.91 (1.21, 3.01) | 0.095 | 0.559 | 8.95 (6.25, 12.70) | 0.565 | 0.520 | 1.95 (1.10, 3.98) | 0.306 | 0.536 |
| Bile reflux | 1344 | 1.65 (1.07, 2.43) | 0.435 | 0.506 | 8.90 (5.60, 14.73) | 0.121 | 0.512 | 1.60 (1.10, 3.30) | 0.130 | 0.512 |
| Gastric erosion | 13094 | 1.78 (1.22, 2.57) | < 0.001 | 0.555 | 8.60 (5.70, 13.70) | 0.006 | 0.509 | 1.60 (1.00, 3.20) | 0.748 | 0.499 |
| Gastric ulcer | 1091 | 1.98 (1.41, 2.98) | < 0.001 | 0.598 | 8.40 (5.50, 14.40) | 0.646 | 0.496 | 1.60 (1.00, 3.20) | 0.789 | 0.498 |
| Gastric hemorrhage | 1125 | 1.68 (1.12, 2.46) | 0.492 | 0.506 | 8.70 (5.80, 14.50) | 0.092 | 0.515 | 1.60 (1.10, 3.40) | 0.331 | 0.509 |
| Chronic atrophic gastritis | 1839 | 1.90 (1.32, 2.89) | < 0.001 | 0.578 | 9.20 (6.00, 14.90) | < 0.001 | 0.535 | 1.70 (1.10, 3.40) | < 0.001 | 0.528 |
| Gastric xanthoma | 100 | 1.65 (1.13, 2.41) | 0.935 | 0.498 | 10.35 (6.45, 15.18) | 0.072 | 0.552 | 1.80 (1.20, 3.43) | 0.111 | 0.546 |
| Gastrointestinal stromal tumor | 48 | 1.56 (1.09, 2.69) | 0.903 | 0.505 | 7.80 (5.78, 13.41) | 0.614 | 0.521 | 1.50 (1.10, 2.23) | 0.683 | 0.517 |
| Gastric hyperplastic polyps | 117 | 1.71 (1.14, 2.89) | 0.282 | 0.529 | 11.20 (7.00, 22.72) | < 0.001 | 0.612 | 1.70 (1.10, 2.40) | 0.964 | 0.501 |
| Gastric glandular hyperplasia | 761 | 1.57 (1.09, 2.29) | 0.050 | 0.521 | 9.50 (6.00, 15.00) | < 0.001 | 0.543 | 1.70 (1.10, 3.80) | 0.010 | 0.527 |
| Colorectitis | 2592 | 1.83 (1.25, 2.70) | < 0.001 | 0.552 | 8.70 (5.70, 14.30) | 0.014 | 0.515 | 1.60 (1.10, 3.40) | 0.034 | 0.513 |
| Colorectal erosion | 167 | 1.72 (1.17, 2.73) | 0.198 | 0.529 | 8.80 (5.95, 13.90) | 0.495 | 0.515 | 1.60 (1.00, 3.10) | 0.788 | 0.494 |
| Colorectal ulcer | 107 | 1.54 (1.03, 2.61) | 0.658 | 0.512 | 9.10 (6.30, 16.90) | 0.069 | 0.551 | 1.70 (1.09, 2.80) | 0.859 | 0.495 |
| Colorectal hemorrhage | 36 | 1.57 (1.12, 3.14) | 0.799 | 0.488 | 9.05 (5.73, 13.75) | 0.550 | 0.529 | 1.25 (0.90, 1.95) | 0.061 | 0.590 |
| Colorectal cyst | 41 | 1.53 (0.93, 2.70) | 0.455 | 0.534 | 9.20 (6.50, 13.70) | 0.321 | 0.545 | 1.40 (1.10, 2.40) | 0.353 | 0.542 |
| Colorectal adenoma | 3707 | 1.91 (1.29, 2.84) | < 0.001 | 0.578 | 9.04 (6.00, 14.70) | < 0.001 | 0.532 | 1.60 (1.10, 3.30) | 0.010 | 0.513 |
| Colorectal hyperplastic polyps | 1037 | 1.88 (1.31, 2.75) | < 0.001 | 0.565 | 8.70 (5.90, 13.70) | 0.065 | 0.517 | 1.60 (1.00, 3.20) | 0.379 | 0.492 |
| Colorectal gland hyperplasia | 567 | 1.87 (1.35, 2.62) | < 0.001 | 0.560 | 8.30 (5.45, 13.45) | 0.308 | 0.512 | 1.50 (1.10, 3.30) | 0.687 | 0.505 |
| Esophageal cancer | 57 | 2.34 (1.30, 3.78) | < 0.001 | 0.645 | 9.40 (6.40, 20.00) | 0.114 | 0.560 | 2.00 (1.20, 4.20) | 0.073 | 0.568 |
| Gastric cancer | 392 | 2.15 (1.35, 4.13) | < 0.001 | 0.625 | 10.30 (5.70, 20.23) | < 0.001 | 0.577 | 2.00 (1.10, 5.70) | < 0.001 | 0.570 |
| Colorectal cancer | 892 | 3.25 (1.78, 11.55) | < 0.001 | 0.736 | 13.30 (7.10, 33.45) | < 0.001 | 0.649 | 2.30 (1.20, 5.90) | < 0.001 | 0.598 |
| Liver cancer | 127 | 4.27 (2.15, 7.46) | < 0.001 | 0.786 | 17.30 (7.40, 39.15) | < 0.001 | 0.674 | 1.60 (1.15, 3.75) | 0.070 | 0.547 |
| Pancreatic cancer | 47 | 3.20 (1.98, 9.63) | < 0.001 | 0.771 | 99.60 (16.95, 307.15) | < 0.001 | 0.830 | 3.10 (1.35, 9.60) | < 0.001 | 0.680 |
| Lung cancer | 129 | 4.25 (2.15, 16.67) | < 0.001 | 0.787 | 12.10 (8.20, 25.50) | < 0.001 | 0.668 | 3.40 (1.40, 9.00) | < 0.001 | 0.660 |
| Breast cancer | 55 | 2.39 (1.44, 6.06) | < 0.001 | 0.654 | 15.60 (8.30, 27.20) | < 0.001 | 0.693 | 2.10 (1.20, 4.70) | 0.022 | 0.589 |
| Ovarian cancer | 57 | 1.78 (1.08, 5.63) | 0.228 | 0.546 | 21.85 (8.50, 130.50) | < 0.001 | 0.718 | 6.40 (1.50, 17.70) | < 0.001 | 0.698 |
| Thyroid cancer | 74 | 1.71 (1.06, 2.48) | 0.936 | 0.497 | 9.90 (6.80, 15.28) | 0.067 | 0.562 | 1.70 (1.03, 3.08) | 0.874 | 0.495 |
| Kidney cancer | 37 | 2.37 (1.16, 3.70) | 0.015 | 0.615 | 10.60 (7.70, 17.46) | 0.039 | 0.598 | 2.20 (1.40, 5.20) | 0.050 | 0.593 |
| Malignant tumors (except thyroid cancer) | 1955 | 2.65 (1.49, 6.70) | < 0.001 | 0.692 | 12.00 (6.80, 28.30) | < 0.001 | 0.636 | 2.20 (1.20, 5.90) | < 0.001 | 0.589 |
The influences of age and sex on the biomarker levels are presented in Table 6. Due to the fact that the patients with malignant tumors were elder, the age baselines of the patients with and without tumors were no equal. Moreover, the sex baseline of the CRC patients was not the same. The correlation coefficients of age and sex were both less than 0.25, indicating small influences. The regression coefficients were used to calculate the effect of age on the biomarker levels. CEA, CA19-9, and CA72-4 increased by 0.41, 2.69, and 0.69, respectively, for the subjects without malignant tumors for every 10-year increase.
| Age | Gender | ||||||
| P value | Correlation coefficient | Regression coefficient | P value | Correlation coefficient | Regression coefficient | ||
| CEA | Whole | < 0.001 | 0.227 | 0.176 | < 0.001 | 0.236 | 0.004 |
| With malignant tumors | < 0.001 | 0.231 | 0.263 | < 0.001 | 0.144 | -2.965 | |
| Without malignant tumors | < 0.001 | 0.195 | 0.041 | < 0.001 | 0.248 | 0.472 | |
| CA19-9 | Whole | < 0.001 | 0.135 | 1.076 | < 0.001 | -0.070 | -1.400 |
| With malignant tumors | < 0.001 | 0.111 | 1.898 | 0.356 | -0.021 | - | |
| Without malignant tumors | < 0.001 | 0.113 | 0.269 | < 0.001 | -0.071 | -2.482 | |
| CA72-4 | Whole | < 0.001 | 0.084 | 0.076 | < 0.001 | -0.043 | -0.927 |
| With malignant tumors | 0.064 | 0.042 | - | 0.814 | -0.005 | - | |
| Without malignant tumors | < 0.001 | 0.069 | 0.052 | < 0.001 | -0.044 | -0.773 | |
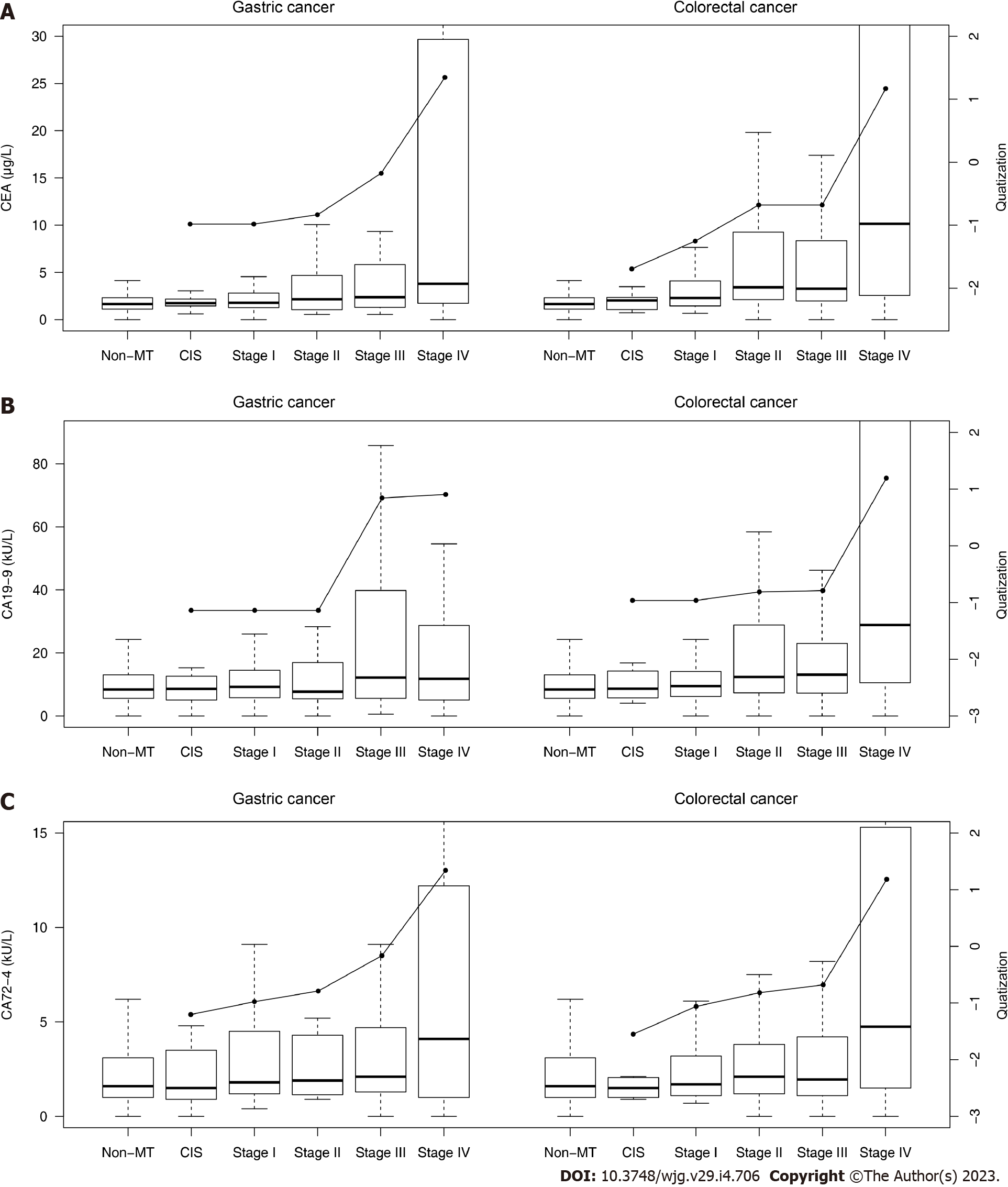
The biomarker levels in different malignant tumor stages are shown in Table 7 and Figure 4.
| Gastric cancer | Colorectal cancer | ||||
| Median (quartile) | Quatization | Median (quartile) | Quatization | ||
| CEA (μg/L) | CIS | 1.74 (1.45, 2.18) | -0.983 | 2.04 (1.17, 2.32) | -1.695 |
| Stage I | 1.78 (1.29, 2.79) | -0.983 | 2.30 (1.45, 4.10) | -1.252 | |
| Stage II | 2.16 (1.07, 4.03) | -0.835 | 3.42 (2.13, 9.26) | -0.680 | |
| Stage III | 2.37 (1.31, 5.78) | -0.176 | 3.28 (2.00, 8.21) | -0.680 | |
| Stage IV | 3.79 (1.76, 29.1) | 1.346 | 10.1 (2.57, 57.4) | 1.168 | |
| CA19-9 (kU/L) | CIS | 8.60 (5.05, 12.7) | -1.138 | 8.66 (6.18, 13.2) | -0.963 |
| Stage I | 9.25 (5.88, 14.4) | -1.138 | 9.50 (6.20, 14.1) | -0.963 | |
| Stage II | 7.73 (5.53, 16.8) | -1.138 | 12.4 (7.38, 28.8) | -0.812 | |
| Stage III | 12.2 (5.63, 37.7) | 0.842 | 13.1 (7.33, 23.0) | -0.790 | |
| Stage IV | 11.8 (5.08, 28.7) | 0.903 | 28.9 (10.5, 216.4) | 1.192 | |
| CA72-4 (kU/L) | CIS | 1.50 (0.90, 3.50) | -1.203 | 1.50 (1.00, 2.03) | -1.550 |
| Stage I | 1.80 (1.20, 4.33) | -0.977 | 1.70 (1.10, 3.20) | -1.060 | |
| Stage II | 1.90 (1.18, 4.30) | -0.789 | 2.10 (1.20, 3.81) | -0.818 | |
| Stage III | 2.10 (1.30, 4.63) | -0.164 | 1.95 (1.10, 4.21) | -0.679 | |
| Stage IV | 4.10 (1.00, 12.0) | 1.340 | 4.75 (1.50, 15.2) | 1.182 | |
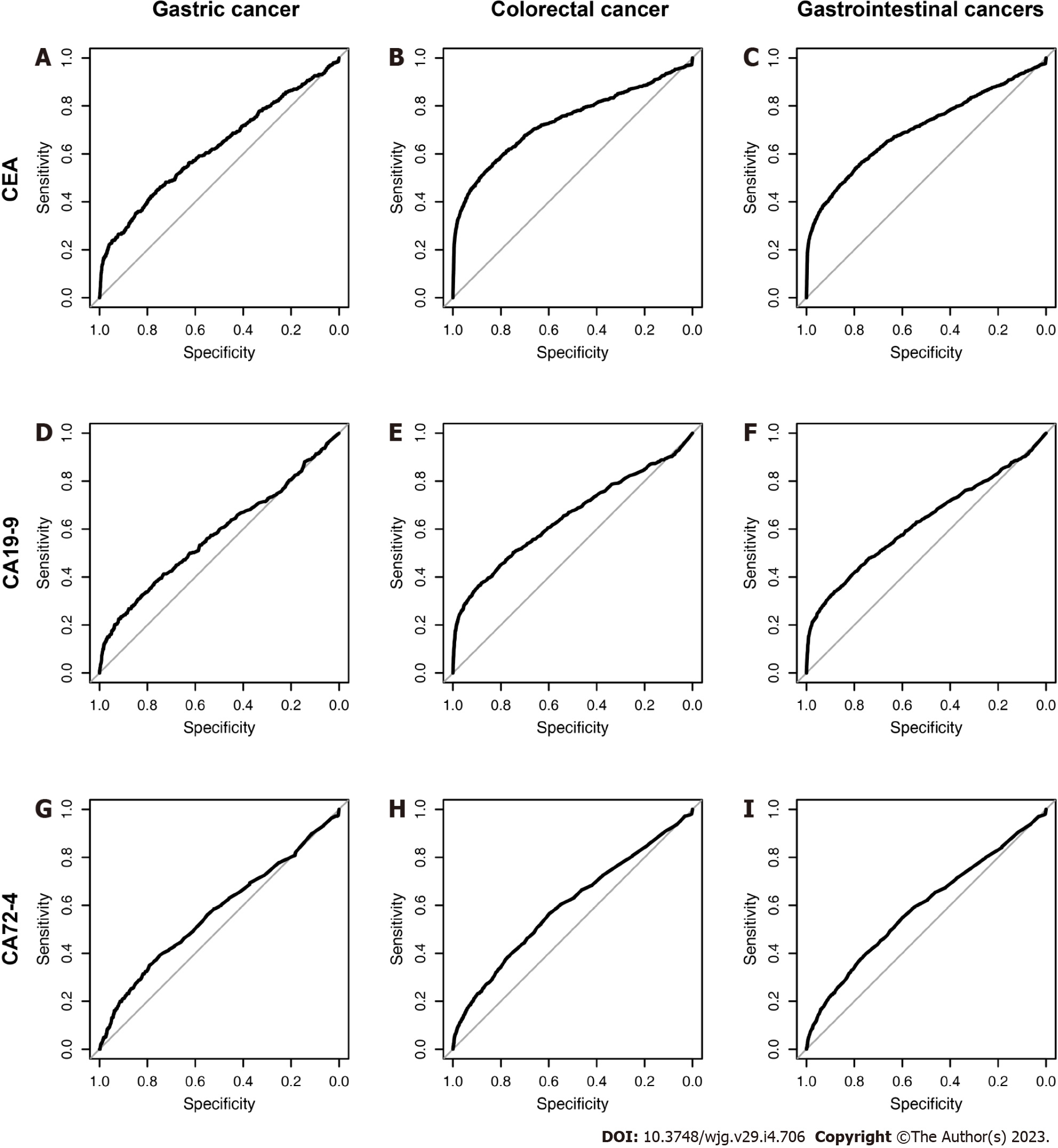
The AUCs of the three biomarkers in various benign and malignant diseases are displayed in Table 5, and the ROC curves are shown in Figure 5. An AUC above 0.7 was of moderate diagnostic value, and an AUC above 0.9 was of high diagnostic value. We found that even though the biomarker levels of several diseases were significantly different, the diagnostic values of these biomarkers were not high enough. The AUC of the CEA level reached 0.7 for CRC, liver cancer, pancreatic cancer and lung cancer, while those of the CA19-9 Level reached 0.830 for pancreatic cancer and 0.7 for ovarian cancer. There was no disease in which the AUC of CA72-4 reached 0.7.
We show the diagnostic value of GC, CRC and gastrointestinal malignant tumors (the DOR, sensitivity, specificity, Youden index, accuracy, predictive value, likelihood ratio under the traditional and the best threshold) in Table 8. Furthermore, we provide several criteria for evaluating their diagnostic efficiencies as the qualified standards: positive likelihood ratio, negative likelihood ratio and DOR should be > 5.0, < 0.2 and > 10.0, respectively. Generally, there is no ideal biomarker for GC. In this study, CEA was better than CA19-9 and CA72-4. The positive likelihood ratio and DOR of CEA and CA19-9 were qualified for CRC and GIC, while those of CA72-4 were not qualified for GC, CRC or GIC.
| Gastric cancer | Colorectal cancer | Gastrointestinal cancers | ||||||||
| CEA | CA19-9 | CA72-4 | CEA | CA19-9 | CA72-4 | CEA | CA19-9 | CA72-4 | ||
| AUC | 0.625 | 0.577 | 0.570 | 0.736 | 0.649 | 0.598 | 0.705 | 0.627 | 0.590 | |
| Traditional cut-off value | Cut-off value | 5.0 | 37.0 | 6.0 | 5.0 | 37.0 | 6.0 | 5.0 | 37.0 | 6.0 |
| DOR | 6.083 | 5.089 | 2.220 | 14.854 | 11.895 | 2.376 | 12.459 | 10.367 | 2.337 | |
| Sensitivity | 0.227 | 0.140 | 0.240 | 0.377 | 0.241 | 0.249 | 0.323 | 0.210 | 0.243 | |
| Specificity | 0.954 | 0.969 | 0.876 | 0.961 | 0.974 | 0.878 | 0.963 | 0.975 | 0.879 | |
| Youden index | 0.181 | 0.109 | 0.115 | 0.338 | 0.215 | 0.127 | 0.286 | 0.185 | 0.122 | |
| Accuracy | 0.945 | 0.959 | 0.868 | 0.945 | 0.954 | 0.861 | 0.938 | 0.945 | 0.854 | |
| PPV | 0.056 | 0.052 | 0.023 | 0.212 | 0.204 | 0.054 | 0.266 | 0.261 | 0.077 | |
| NPV | 0.990 | 0.989 | 0.990 | 0.982 | 0.979 | 0.977 | 0.972 | 0.968 | 0.966 | |
| PLR | 4.927 | 4.516 | 1.927 | 9.640 | 9.269 | 2.034 | 8.759 | 8.400 | 2.012 | |
| NLR | 0.810 | 0.888 | 0.868 | 0.649 | 0.779 | 0.856 | 0.703 | 0.810 | 0.861 | |
| Best cut-off value | Cut-off value | 2.6 | 16.3 | 3.8 | 2.8 | 20.7 | 2.0 | 2.5 | 19.6 | 3.4 |
| DOR | 2.687 | 2.233 | 2.038 | 6.345 | 4.825 | 1.933 | 4.419 | 3.872 | 2.068 | |
| Sensitivity | 0.423 | 0.324 | 0.349 | 0.558 | 0.361 | 0.566 | 0.556 | 0.339 | 0.375 | |
| Specificity | 0.785 | 0.823 | 0.791 | 0.834 | 0.895 | 0.597 | 0.779 | 0.883 | 0.775 | |
| Youden index | 0.209 | 0.147 | 0.141 | 0.392 | 0.256 | 0.163 | 0.335 | 0.222 | 0.150 | |
| Accuracy | 0.781 | 0.817 | 0.786 | 0.827 | 0.881 | 0.596 | 0.770 | 0.861 | 0.759 | |
| PPV | 0.023 | 0.022 | 0.020 | 0.086 | 0.088 | 0.038 | 0.094 | 0.107 | 0.065 | |
| NPV | 0.991 | 0.990 | 0.990 | 0.985 | 0.980 | 0.980 | 0.977 | 0.970 | 0.968 | |
| PLR | 1.972 | 1.833 | 1.675 | 3.363 | 3.445 | 1.405 | 2.519 | 2.900 | 1.667 | |
| NLR | 0.734 | 0.821 | 0.822 | 0.530 | 0.714 | 0.727 | 0.570 | 0.749 | 0.806 | |
The AUCs of diverse subgroups, including age, health checkup/active consultation and malignant tumor stage, are shown in Table 9. We defined an AUC greater than 0.7 as the qualified line. Then, the AUCs of CEA, CA19-9, and CA72-4 in the health checkup population were all unqualified. If we looked at the stages alone, CEA for stage-IV GC, CA19-9 for stage-IV CRC and CEA for stage-II-IV CRC were qualified. However, neither CEA nor CA199 can diagnose early GICs.
| Gastric cancer | Colorectal cancer | Gastrointestinal cancer | |||||||
| CEA | CA19-9 | CA72-4 | CEA | CA19-9 | CA72-4 | CEA | CA19-9 | CA72-4 | |
| Whole | 0.625 | 0.577 | 0.570 | 0.736 | 0.649 | 0.598 | 0.705 | 0.627 | 0.590 |
| ≥ 60 years | 0.585 | 0.521 | 0.544 | 0.701 | 0.614 | 0.577 | 0.675 | 0.592 | 0.572 |
| < 60 years | 0.578 | 0.571 | 0.570 | 0.683 | 0.616 | 0.593 | 0.648 | 0.598 | 0.583 |
| HC | 0.570 | 0.570 | 0.525 | 0.584 | 0.539 | 0.514 | 0.584 | 0.554 | 0.526 |
| AC | 0.595 | 0.544 | 0.547 | 0.724 | 0.637 | 0.577 | 0.696 | 0.615 | 0.571 |
| CIS | 0.551 | 0.478 | 0.542 | 0.540 | 0.536 | 0.526 | - | - | - |
| Stage I | 0.554 | 0.525 | 0.565 | 0.675 | 0.546 | 0.512 | - | - | - |
| Stage II | 0.603 | 0.489 | 0.591 | 0.781 | 0.657 | 0.578 | - | - | - |
| Stage III | 0.658 | 0.645 | 0.603 | 0.770 | 0.642 | 0.565 | - | - | - |
| Stage IV | 0.739 | 0.614 | 0.634 | 0.810 | 0.778 | 0.698 | - | - | - |
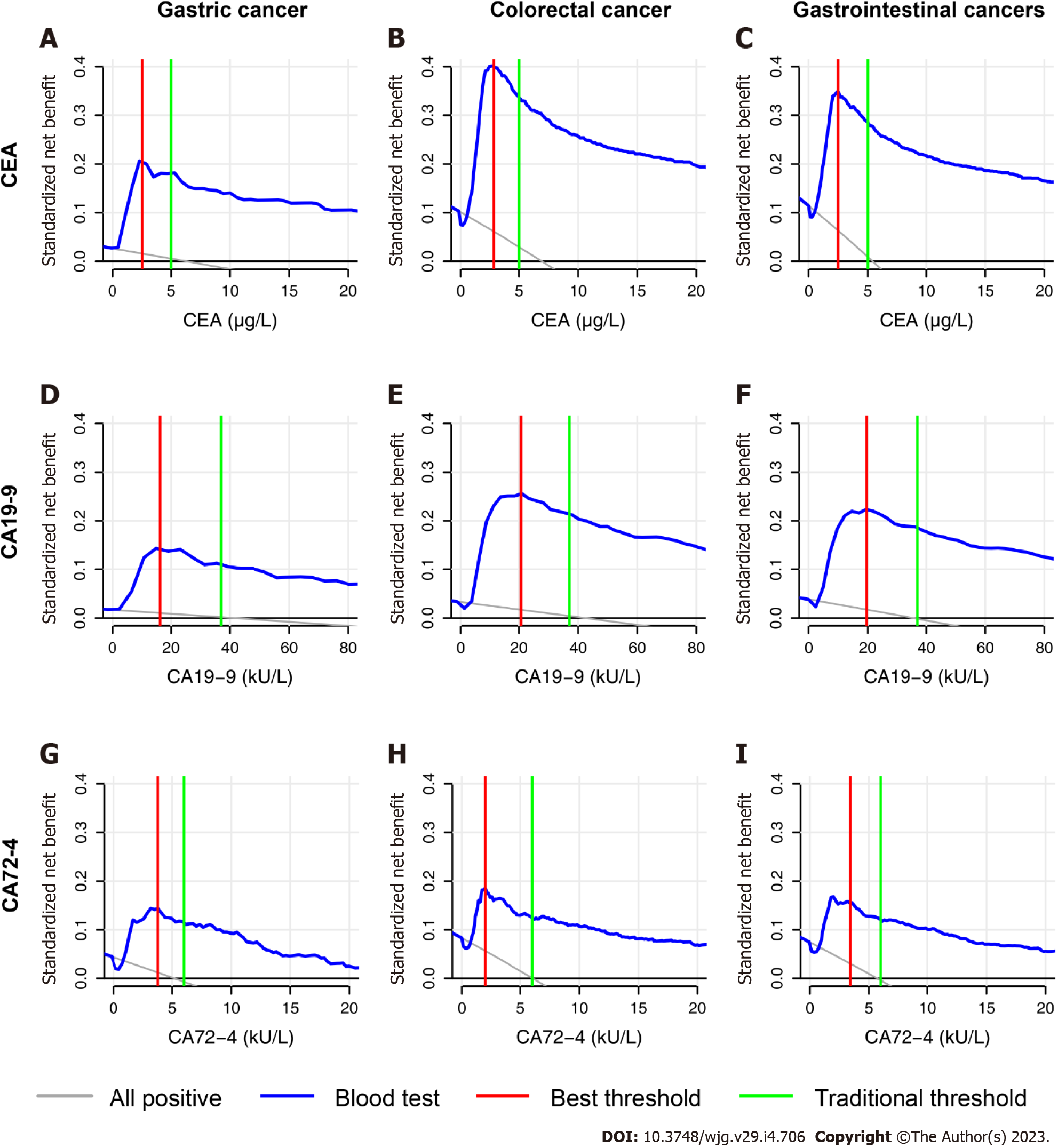
The DCA curves of the three biomarkers are presented in Figure 6. The DCA curve showed that under the traditional threshold and the best threshold, the clinical benefits of CEA were higher than those of CA19-9, while the clinical benefits of CA72-4 were the lowest.
Four panels were conducted with the combination of the three biomarkers. We selected the panel with the highest AUC and compared it with the single biomarker with the highest AUC (Table 10). The combination of biomarkers in the CRC and gastrointestinal malignant tumors significantly increased the AUC (Delong’s test, P < 0.05) by less than 0.3, while that in GC did not. Therefore, the combination of the three biomarkers could not greatly improve the diagnostic value.
| Best combination | Best single biomarker | P value | |||
| Biomarkers | AUC | Biomarker | AUC | ||
| Gastric cancer | CEA + CA19-9 + CA72-4 | 0.653 | CEA | 0.625 | 0.067 |
| Colorectal cancer | CEA + CA19-9 | 0.761 | CEA | 0.736 | < 0.001 |
| Gastrointestinal cancers | CEA + CA19-9 | 0.727 | CEA | 0.705 | < 0.001 |
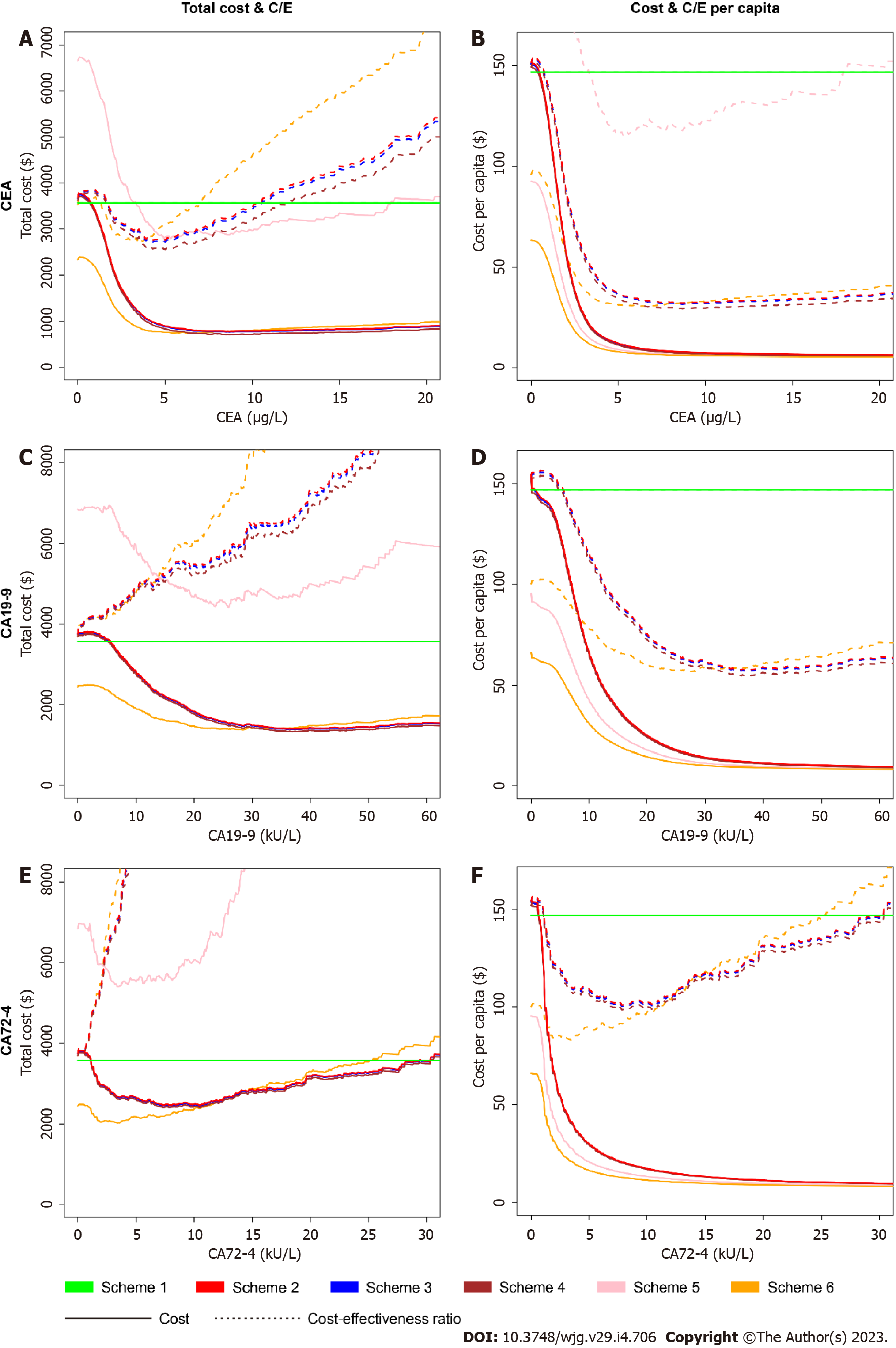
We analyzed the four economic indicators of the six schemes with changes in the serum levels of the three biomarkers, as shown in Figure 7. For gastroscopy only, the total cost and cost-effectiveness ratio of correctly diagnosing one case of GIC were unacceptably high. For colonoscopy only, various cost indicators were reduced within a certain range of biomarker levels. The four economic indicators of CEA in Scheme 6 (only colonoscopy conducted when blood tests were positive) were lower than those in other schemes because the diagnostic efficiencies of CEA for CRC were high, and the prevalence rate of CRC was higher than that of GC in this study. If both gastroscopy and colonoscopy were conducted, the influence of the order of gastroscopy on the four economic indicators was small. Therefore, in the follow-up study, we only calculated the economic indicators in Scheme 2 (both gastroscopy and colonoscopy when blood tests were positive) compared to those in Scheme 1 (both gastroscopy and colonoscopy for all people without blood tests).
In terms of threshold selection, we found that the traditional threshold of CEA (5 μg/L) was exactly between the CEA level under the minimum total cost-effectiveness ratio (4.3 μg/L) and that under the minimum total cost (equal to cost-effectiveness ratio per capita, 8.7 μg/L). If we decrease the cut-off value, the four indicators grew rapidly. If we increase the cut-off value, then the total cost-effectiveness ratio rose sharply, while the other three indicators had fewer changes. One can use 5 μg/L for CEA as an economic cut-off value. For CA19-9, we found that a similarly high economic cut-off value was approximately 30 kU/L, not the traditional threshold of 37 kU/L. Compared with that at the threshold of 30 kU/L, the total cost-effectiveness ratio at the threshold of 37 kU/L was greatly increased because of the lower sensitivity of the marker. We evaluated the economic efficiencies as the qualified standard: all four indicators in Scheme 2 were lower than those in Scheme 1. CEA met the standards at the threshold of 1.8 μg/L to 10.4 μg/L. CA19-9 and CA72-4 failed at the whole threshold, caused by the high total cost-effectiveness ratio in Scheme 2.
Compared with CEA, the combination of the three biomarkers in pairs or altogether caused the cost and cost-effectiveness ratio to be higher (Table 11). From an economic perspective, the combination of biomarkers is not superior to the single biomarker, CEA.
| Cut-off value | Proportion of endoscopy | Missed diagnosis rate | Total cost ($) | Cost per capita ($) | Total C/E ($) | C/E per capita ($) | Remarks | |
| Non-blood test | - | 1.000 | 0.000 | 3574.3 | 146.9 | 3574.3 | 146.9 | Gold standard |
| CEA (μg/L) | 0.0 | 1.000 | 0.000 | 3691.9 | 151.7 | 3691.9 | 151.7 | Lowest cut-off value |
| 2.5 | 0.230 | 0.451 | 1718.1 | 38.7 | 3130.1 | 70.6 | Highest youden index | |
| 4.3 | 0.065 | 0.642 | 990.0 | 14.6 | 2767.1 | 40.7 | Lowest total cost-effectiveness ratio | |
| 5.0 | 0.049 | 0.674 | 903.1 | 12.1 | 2770.9 | 37.1 | Traditional diagnostic cut-off value | |
| 8.7 | 0.020 | 0.759 | 783.9 | 7.8 | 3246.2 | 32.2 | Lowest total cost & lowest cost-effectiveness ratio per capita | |
| CA19-9 (kU/L) | 0.0 | 1.000 | 0.000 | 3755.4 | 154.3 | 3755.4 | 154.3 | Lowest cut-off value & lowest total cost-effectiveness ratio |
| 20.0 | 0.121 | 0.665 | 1836.7 | 25.3 | 5485.7 | 75.5 | Highest youden index | |
| 36.9 | 0.033 | 0.787 | 1398.5 | 12.2 | 6578.5 | 57.5 | Lowest total cost & lowest cost-effectiveness ratio per capita | |
| 37.0 | 0.032 | 0.789 | 1405.2 | 12.2 | 6656.1 | 57.7 | Traditional diagnostic cut-off value | |
| CA72-4 (kU/L) | 0.0 | 1.000 | 0.000 | 3755.4 | 154.3 | 3755.4 | 154.3 | Lowest cut-off value & lowest total cost-effectiveness ratio |
| 3.4 | 0.231 | 0.623 | 2670.7 | 41.4 | 7083.5 | 109.7 | Highest youden index | |
| 6.0 | 0.126 | 0.756 | 2584.5 | 25.9 | 10605.1 | 106.2 | Traditional diagnostic cut-off value | |
| 10.5 | 0.064 | 0.833 | 2451.6 | 16.8 | 14709.6 | 100.7 | Lowest total cost & lowest cost-effectiveness ratio per capita | |
| CEA | 5.0 | 0.069 | 0.601 | 1365.1 | 22.4 | 3419.2 | 56.1 | Traditional diagnostic cut-off value in parallel |
| CA19-9 | 37.0 | |||||||
| CEA | 6.9 | 0.036 | 0.676 | 1309.0 | 17.4 | 4034.6 | 53.8 | Lowest cut-off value & lowest total cost-effectiveness ratio in parallel |
| CA19-9 | 69.2 | |||||||
| CEA | 3.9 | 0.098 | 0.554 | 1455.6 | 26.7 | 3264.3 | 59.8 | Lowest total cost-effectiveness ratio in parallel |
| CA19-9 | 38.1 | |||||||
| CEA | 5.0 | 0.012 | 0.862 | 2433.3 | 13.8 | 17661.0 | 100.0 | Traditional diagnostic cut-off value in serial |
| CA19-9 | 37.0 | |||||||
| CEA | 5.4 | 0.042 | 0.689 | 1437.7 | 18.4 | 4621.3 | 59.1 | Lowest cut-off value & lowest total cost-effectiveness ratio in serial |
| CA19-9 | 0.0 | |||||||
| CEA | 2.1 | 0.335 | 0.361 | 2339.4 | 61.4 | 3659.5 | 96.1 | Lowest total cost-effectiveness ratio in serial |
| CA19-9 | 0.0 | |||||||
| CEA | 5.0 | 0.044 | 0.666 | 1362.9 | 18.7 | 4079.6 | 56.0 | Traditional diagnostic cut-off value in the logistic model |
| CA19-9 | 37.0 | |||||||
| CEA | 4.9 | 0.052 | 0.641 | 1341.0 | 19.8 | 3732.7 | 55.1 | Lowest cut-off value & lowest total cost-effectiveness ratio in the logistic model |
| CA19-9 | 23.2 | |||||||
| CEA | 2.0 | 0.213 | 0.436 | 1874.8 | 43.5 | 3321.5 | 77.0 | Lowest total cost-effectiveness ratio in the logistic model |
| CA19-9 | 33.5 |
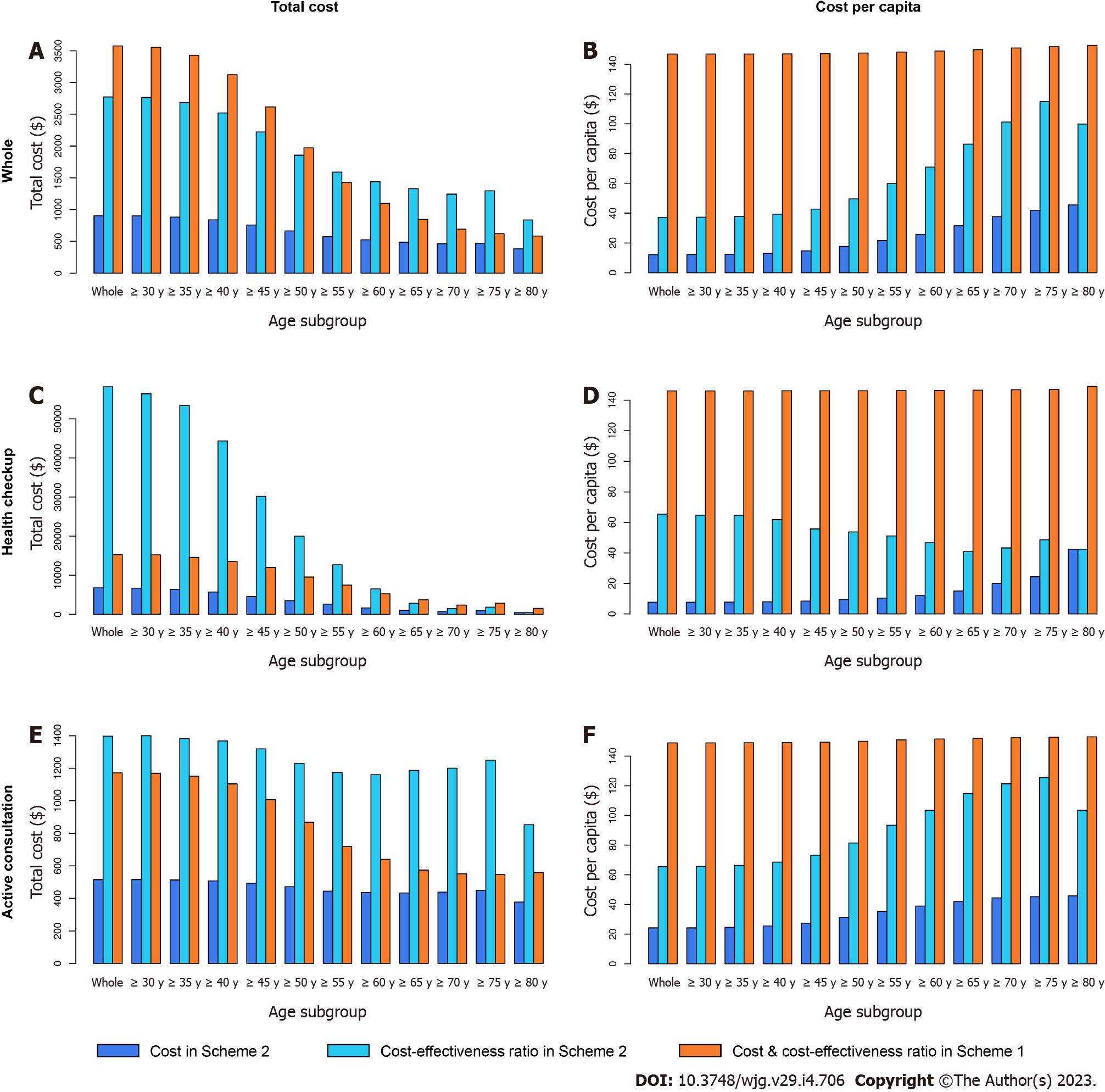
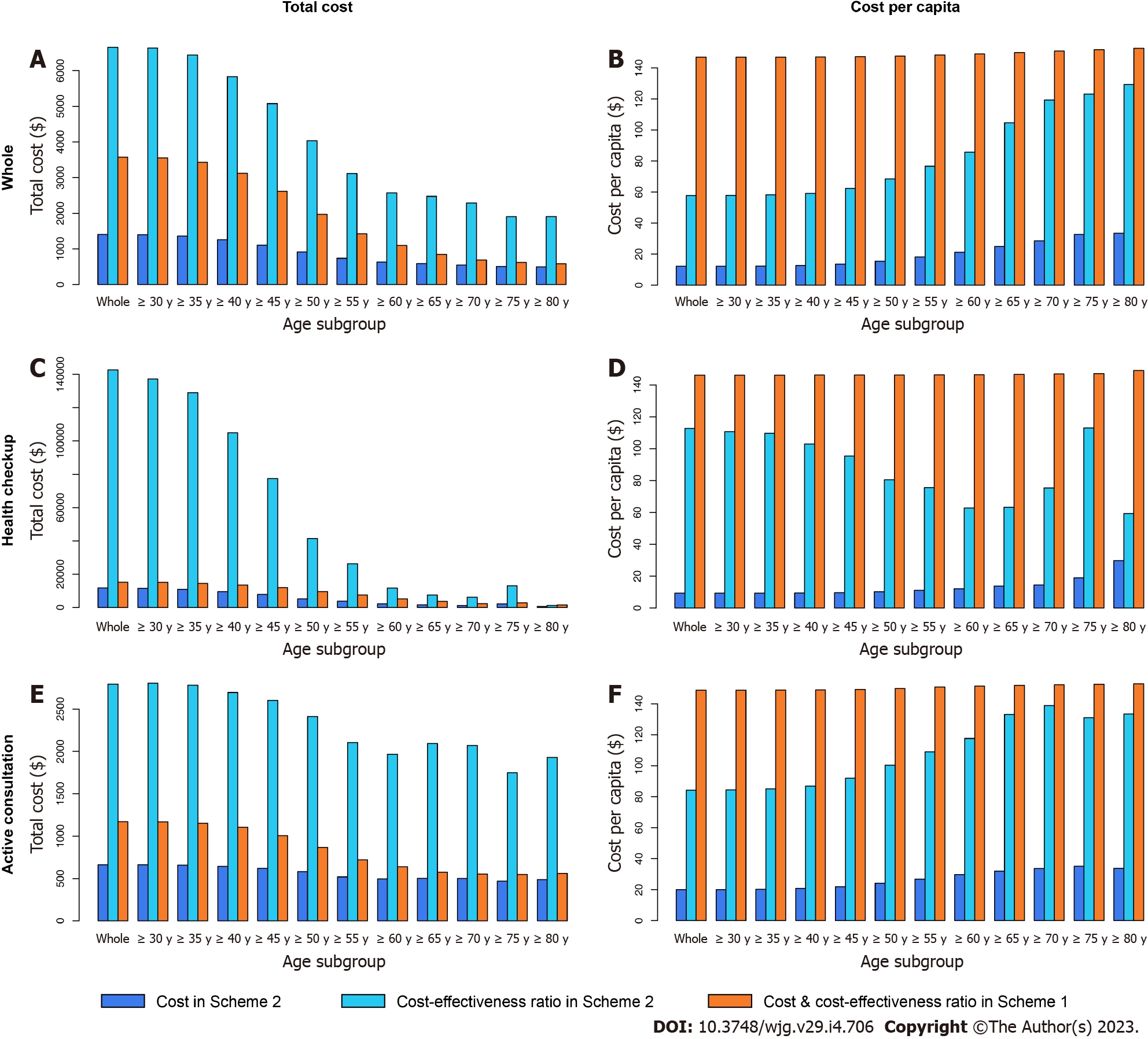
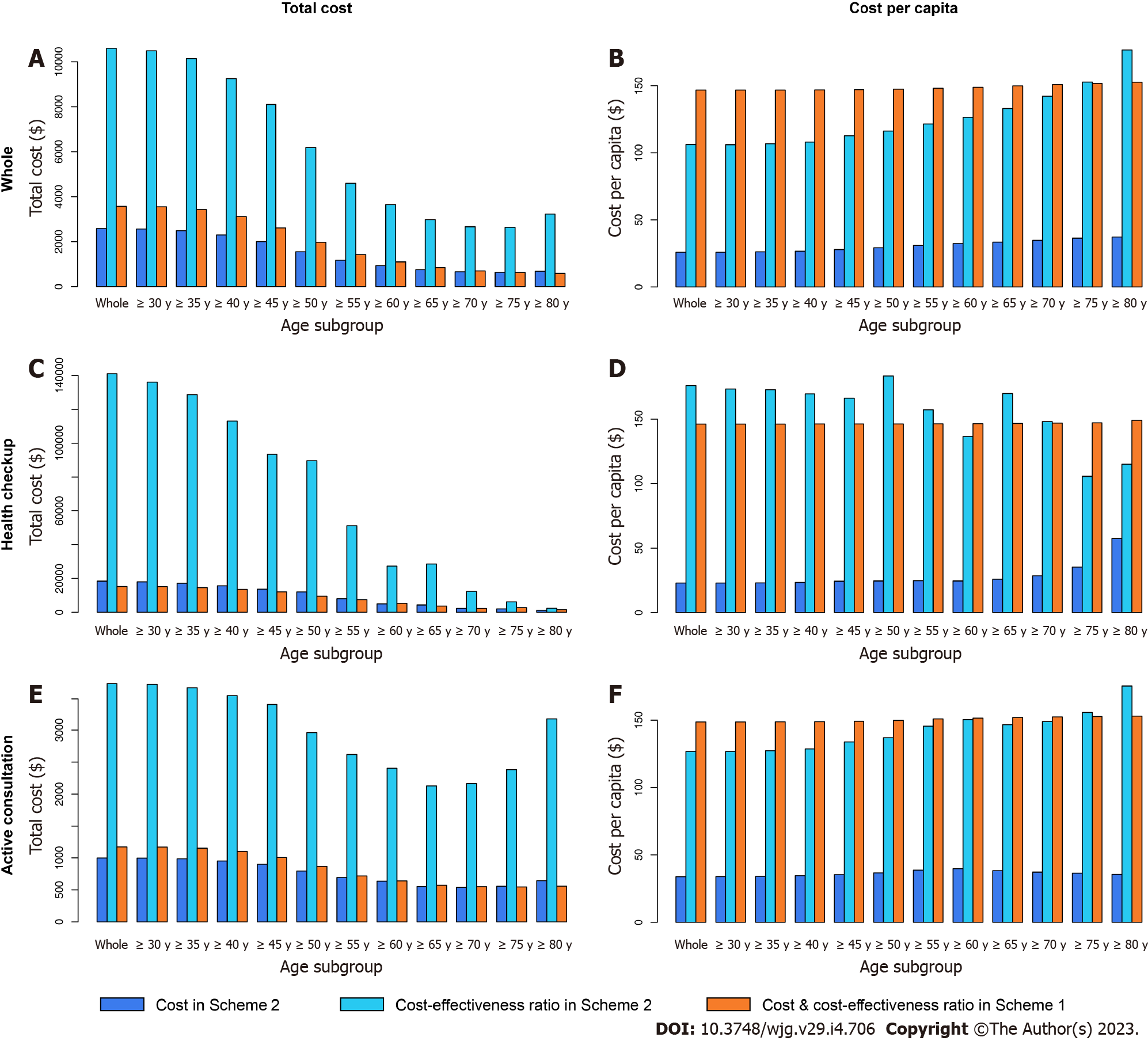
The subgroup analysis under the traditional threshold is displayed in Table 12, Figures 8-10.
| Scheme 2 | Scheme 1 | ||||||||||
| Cut-off value | Proportion of endoscopy | Missed diagnosis rate | Total cost ($) | Cost per capita ($) | Total C/E ($) | C/E per capita ($) | Total cost & C/E ($) | Cost & C/E per capita ($) | |||
| CEA | Whole | Whole | 5.0 | 0.049 | 0.674 | 903.1 | 12.1 | 2770.9 | 37.1 | 3574.3 | 146.9 |
| ≥ 80 yr | 5.0 | 0.258 | 0.543 | 381.8 | 45.6 | 835.9 | 99.8 | 584.4 | 152.7 | ||
| ≥ 75 yr | 5.0 | 0.240 | 0.635 | 472.1 | 41.9 | 1294.7 | 115.0 | 623.2 | 151.8 | ||
| ≥ 70 yr | 5.0 | 0.214 | 0.627 | 463.5 | 37.7 | 1242.1 | 101.2 | 691.5 | 150.9 | ||
| ≥ 65 yr | 5.0 | 0.174 | 0.634 | 486.4 | 31.6 | 1329.1 | 86.3 | 844.6 | 149.9 | ||
| ≥ 60 yr | 5.0 | 0.137 | 0.636 | 523.6 | 25.8 | 1438.6 | 71.0 | 1099.1 | 149.0 | ||
| ≥ 55 yr | 5.0 | 0.110 | 0.639 | 574.1 | 21.6 | 1588.8 | 59.8 | 1424.3 | 148.3 | ||
| ≥ 50 yr | 5.0 | 0.085 | 0.643 | 662.6 | 17.7 | 1854.8 | 49.6 | 1972.8 | 147.6 | ||
| ≥ 45 yr | 5.0 | 0.065 | 0.658 | 758.5 | 14.6 | 2220.9 | 42.7 | 2614.6 | 147.2 | ||
| ≥ 40 yr | 5.0 | 0.055 | 0.668 | 837.4 | 13.1 | 2520.1 | 39.4 | 3121.4 | 147.0 | ||
| ≥ 35 yr | 5.0 | 0.051 | 0.671 | 882.1 | 12.4 | 2684.9 | 37.8 | 3427.4 | 146.9 | ||
| ≥ 30 yr | 5.0 | 0.049 | 0.674 | 902.2 | 12.2 | 2764.8 | 37.3 | 3552.0 | 146.9 | ||
| HC | Whole | 5.0 | 0.021 | 0.883 | 6834.3 | 7.7 | 58218.5 | 65.4 | 15277.9 | 146.1 | |
| ≥ 80 yr | 5.0 | 0.238 | 0.000 | 446.3 | 42.5 | 446.3 | 42.5 | 1565.3 | 149.1 | ||
| ≥ 75 yr | 5.0 | 0.129 | 0.500 | 941.8 | 24.4 | 1883.7 | 48.7 | 2843.9 | 147.1 | ||
| ≥ 70 yr | 5.0 | 0.101 | 0.538 | 693.8 | 20.0 | 1503.3 | 43.3 | 2356.2 | 146.9 | ||
| ≥ 65 yr | 5.0 | 0.070 | 0.630 | 1046.6 | 15.1 | 2832.1 | 40.9 | 3755.2 | 146.6 | ||
| ≥ 60 yr | 5.0 | 0.049 | 0.744 | 1677.6 | 12.0 | 6542.7 | 46.7 | 5254.5 | 146.4 | ||
| ≥ 55 yr | 5.0 | 0.040 | 0.794 | 2611.7 | 10.5 | 12685.3 | 51.1 | 7474.5 | 146.3 | ||
| ≥ 50 yr | 5.0 | 0.033 | 0.824 | 3519.3 | 9.5 | 19989.5 | 53.8 | 9558.2 | 146.2 | ||
| ≥ 45 yr | 5.0 | 0.026 | 0.848 | 4577.8 | 8.5 | 30179.8 | 55.7 | 12010.0 | 146.2 | ||
| ≥ 40 yr | 5.0 | 0.023 | 0.871 | 5726.3 | 8.0 | 44326.1 | 61.8 | 13537.7 | 146.2 | ||
| ≥ 35 yr | 5.0 | 0.021 | 0.879 | 6443.2 | 7.8 | 53455.1 | 64.6 | 14572.3 | 146.1 | ||
| ≥ 30 yr | 5.0 | 0.021 | 0.881 | 6737.6 | 7.7 | 56396.5 | 64.7 | 15225.1 | 146.1 | ||
| AC | Whole | 5.0 | 0.126 | 0.631 | 515.4 | 24.2 | 1397.6 | 65.5 | 1170.9 | 148.8 | |
| ≥ 80 yr | 5.0 | 0.260 | 0.557 | 378.1 | 45.8 | 853.5 | 103.4 | 559.6 | 153.0 | ||
| ≥ 75 yr | 5.0 | 0.260 | 0.640 | 449.7 | 45.2 | 1249.2 | 125.5 | 547.0 | 152.7 | ||
| ≥ 70 yr | 5.0 | 0.256 | 0.634 | 439.0 | 44.4 | 1200.5 | 121.3 | 551.5 | 152.4 | ||
| ≥ 65 yr | 5.0 | 0.240 | 0.634 | 433.8 | 42.0 | 1186.4 | 114.8 | 574.1 | 152.0 | ||
| ≥ 60 yr | 5.0 | 0.219 | 0.624 | 436.5 | 38.9 | 1161.3 | 103.4 | 639.4 | 151.5 | ||
| ≥ 55 yr | 5.0 | 0.197 | 0.621 | 445.2 | 35.4 | 1173.3 | 93.4 | 719.0 | 150.9 | ||
| ≥ 50 yr | 5.0 | 0.171 | 0.616 | 471.6 | 31.2 | 1229.4 | 81.5 | 868.1 | 149.9 | ||
| ≥ 45 yr | 5.0 | 0.146 | 0.626 | 493.5 | 27.4 | 1319.3 | 73.2 | 1006.5 | 149.3 | ||
| ≥ 40 yr | 5.0 | 0.134 | 0.628 | 508.3 | 25.5 | 1367.6 | 68.6 | 1103.8 | 149.0 | ||
| ≥ 35 yr | 5.0 | 0.129 | 0.629 | 513.2 | 24.6 | 1383.2 | 66.3 | 1151.7 | 148.9 | ||
| ≥ 30 yr | 5.0 | 0.126 | 0.631 | 516.1 | 24.2 | 1400.2 | 65.7 | 1168.9 | 148.8 | ||
| CA19-9 | Whole | Whole | 37.0 | 0.032 | 0.789 | 1405.2 | 12.2 | 6656.1 | 57.7 | 3574.3 | 146.9 |
| ≥ 80 yr | 37.0 | 0.168 | 0.741 | 494.9 | 33.5 | 1908.7 | 129.3 | 584.4 | 152.7 | ||
| ≥ 75 yr | 37.0 | 0.164 | 0.735 | 505.6 | 32.7 | 1906.7 | 123.2 | 623.2 | 151.8 | ||
| ≥ 70 yr | 37.0 | 0.137 | 0.761 | 546.5 | 28.5 | 2288.5 | 119.3 | 691.5 | 150.9 | ||
| ≥ 65 yr | 37.0 | 0.115 | 0.762 | 589.9 | 25.0 | 2474.1 | 104.7 | 844.6 | 149.9 | ||
| ≥ 60 yr | 37.0 | 0.090 | 0.754 | 633.2 | 21.2 | 2568.7 | 85.8 | 1099.1 | 149.0 | ||
| ≥ 55 yr | 37.0 | 0.070 | 0.764 | 736.3 | 18.1 | 3114.0 | 76.7 | 1424.3 | 148.3 | ||
| ≥ 50 yr | 37.0 | 0.054 | 0.774 | 913.9 | 15.5 | 4035.0 | 68.4 | 1972.8 | 147.6 | ||
| ≥ 45 yr | 37.0 | 0.042 | 0.782 | 1108.4 | 13.6 | 5075.1 | 62.4 | 2614.6 | 147.2 | ||
| ≥ 40 yr | 37.0 | 0.036 | 0.785 | 1254.6 | 12.7 | 5833.6 | 59.1 | 3121.4 | 147.0 | ||
| ≥ 35 yr | 37.0 | 0.033 | 0.789 | 1359.0 | 12.3 | 6434.6 | 58.2 | 3427.4 | 146.9 | ||
| ≥ 30 yr | 37.0 | 0.032 | 0.789 | 1398.6 | 12.2 | 6634.7 | 57.8 | 3552.0 | 146.9 | ||
| HC | Whole | 37.0 | 0.014 | 0.917 | 11789.9 | 9.3 | 142719.5 | 112.8 | 15277.9 | 146.1 | |
| ≥ 80 yr | 37.0 | 0.143 | 0.500 | 622.7 | 29.7 | 1245.5 | 59.3 | 1565.3 | 149.1 | ||
| ≥ 75 yr | 37.0 | 0.078 | 0.833 | 2187.1 | 18.9 | 13122.9 | 113.1 | 2843.9 | 147.1 | ||
| ≥ 70 yr | 37.0 | 0.048 | 0.808 | 1207.9 | 14.5 | 6281.3 | 75.3 | 2356.2 | 146.9 | ||
| ≥ 65 yr | 37.0 | 0.043 | 0.783 | 1621.1 | 13.8 | 7457.1 | 63.3 | 3755.2 | 146.6 | ||
| ≥ 60 yr | 37.0 | 0.032 | 0.808 | 2252.2 | 12.1 | 11711.6 | 62.8 | 5254.5 | 146.4 | ||
| ≥ 55 yr | 37.0 | 0.026 | 0.853 | 3855.6 | 11.1 | 26218.2 | 75.5 | 7474.5 | 146.3 | ||
| ≥ 50 yr | 37.0 | 0.020 | 0.873 | 5259.2 | 10.2 | 41488.9 | 80.5 | 9558.2 | 146.2 | ||
| ≥ 45 yr | 37.0 | 0.016 | 0.899 | 7832.3 | 9.6 | 77452.6 | 95.3 | 12010.0 | 146.2 | ||
| ≥ 40 yr | 37.0 | 0.014 | 0.909 | 9540.9 | 9.4 | 104950.4 | 103.0 | 13537.7 | 146.2 | ||
| ≥ 35 yr | 37.0 | 0.014 | 0.915 | 10937.8 | 9.3 | 128950.9 | 109.7 | 14572.3 | 146.1 | ||
| ≥ 30 yr | 37.0 | 0.014 | 0.916 | 11530.0 | 9.3 | 137146.2 | 110.7 | 15225.1 | 146.1 | ||
| AC | Whole | 37.0 | 0.082 | 0.763 | 663.4 | 20.0 | 2793.3 | 84.3 | 1170.9 | 148.8 | |
| ≥ 80 yr | 37.0 | 0.170 | 0.747 | 488.5 | 33.8 | 1929.4 | 133.5 | 559.6 | 153.0 | ||
| ≥ 75 yr | 37.0 | 0.180 | 0.731 | 469.9 | 35.2 | 1749.5 | 131.1 | 547.0 | 152.7 | ||
| ≥ 70 yr | 37.0 | 0.171 | 0.757 | 502.4 | 33.7 | 2069.9 | 138.9 | 551.5 | 152.4 | ||
| ≥ 65 yr | 37.0 | 0.159 | 0.760 | 503.3 | 32.0 | 2093.5 | 133.2 | 574.1 | 152.0 | ||
| ≥ 60 yr | 37.0 | 0.144 | 0.748 | 496.7 | 29.7 | 1967.4 | 117.7 | 639.4 | 151.5 | ||
| ≥ 55 yr | 37.0 | 0.126 | 0.753 | 519.6 | 26.9 | 2105.0 | 109.0 | 719.0 | 150.9 | ||
| ≥ 50 yr | 37.0 | 0.109 | 0.759 | 581.1 | 24.2 | 2411.0 | 100.4 | 868.1 | 149.9 | ||
| ≥ 45 yr | 37.0 | 0.095 | 0.762 | 620.3 | 21.9 | 2601.4 | 92.0 | 1006.5 | 149.3 | ||
| ≥ 40 yr | 37.0 | 0.087 | 0.761 | 644.3 | 20.8 | 2694.8 | 87.0 | 1103.8 | 149.0 | ||
| ≥ 35 yr | 37.0 | 0.083 | 0.763 | 659.0 | 20.2 | 2780.6 | 85.2 | 1151.7 | 148.9 | ||
| ≥ 30 yr | 37.0 | 0.082 | 0.763 | 663.9 | 20.0 | 2805.1 | 84.5 | 1168.9 | 148.8 | ||
| CA72-4 | Whole | Whole | 6.0 | 0.126 | 0.756 | 2584.5 | 25.9 | 10605.1 | 106.2 | 3574.3 | 146.9 |
| ≥ 80 yr | 6.0 | 0.194 | 0.790 | 676.1 | 37.1 | 3221.6 | 176.7 | 584.4 | 152.7 | ||
| ≥ 75 yr | 6.0 | 0.190 | 0.762 | 627.4 | 36.3 | 2641.0 | 152.8 | 623.2 | 151.8 | ||
| ≥ 70 yr | 6.0 | 0.180 | 0.755 | 651.5 | 34.8 | 2661.4 | 142.2 | 691.5 | 150.9 | ||
| ≥ 65 yr | 6.0 | 0.173 | 0.749 | 749.3 | 33.4 | 2980.7 | 133.0 | 844.6 | 149.9 | ||
| ≥ 60 yr | 6.0 | 0.166 | 0.745 | 933.2 | 32.3 | 3653.5 | 126.5 | 1099.1 | 149.0 | ||
| ≥ 55 yr | 6.0 | 0.158 | 0.746 | 1167.6 | 30.9 | 4599.8 | 121.6 | 1424.3 | 148.3 | ||
| ≥ 50 yr | 6.0 | 0.147 | 0.749 | 1552.7 | 29.1 | 6194.2 | 116.2 | 1972.8 | 147.6 | ||
| ≥ 45 yr | 6.0 | 0.139 | 0.753 | 2003.3 | 27.9 | 8106.4 | 112.8 | 2614.6 | 147.2 | ||
| ≥ 40 yr | 6.0 | 0.132 | 0.752 | 2293.2 | 26.7 | 9259.2 | 108.0 | 3121.4 | 147.0 | ||
| ≥ 35 yr | 6.0 | 0.128 | 0.755 | 2488.4 | 26.2 | 10145.7 | 106.7 | 3427.4 | 146.9 | ||
| ≥ 30 yr | 6.0 | 0.127 | 0.755 | 2565.2 | 25.9 | 10489.2 | 106.1 | 3552.0 | 146.9 | ||
| HC | Whole | 6.0 | 0.108 | 0.870 | 18401.4 | 23.0 | 141077.7 | 176.0 | 15277.9 | 146.1 | |
| ≥ 80 yr | 6.0 | 0.333 | 0.500 | 1206.6 | 57.5 | 2413.3 | 114.9 | 1565.3 | 149.1 | ||
| ≥ 75 yr | 6.0 | 0.190 | 0.667 | 2042.4 | 35.2 | 6127.2 | 105.6 | 2843.9 | 147.1 | ||
| ≥ 70 yr | 6.0 | 0.144 | 0.808 | 2375.7 | 28.5 | 12353.8 | 148.1 | 2356.2 | 146.9 | ||
| ≥ 65 yr | 6.0 | 0.126 | 0.848 | 4345.5 | 25.8 | 28556.3 | 169.7 | 3755.2 | 146.6 | ||
| ≥ 60 yr | 6.0 | 0.118 | 0.821 | 4898.1 | 24.5 | 27289.4 | 136.5 | 5254.5 | 146.4 | ||
| ≥ 55 yr | 6.0 | 0.119 | 0.843 | 8033.4 | 24.7 | 51212.7 | 157.2 | 7474.5 | 146.3 | ||
| ≥ 50 yr | 6.0 | 0.118 | 0.866 | 11985.7 | 24.5 | 89577.1 | 183.4 | 9558.2 | 146.2 | ||
| ≥ 45 yr | 6.0 | 0.116 | 0.854 | 13653.0 | 24.3 | 93470.7 | 166.2 | 12010.0 | 146.2 | ||
| ≥ 40 yr | 6.0 | 0.111 | 0.861 | 15690.0 | 23.5 | 113076.5 | 169.4 | 13537.7 | 146.2 | ||
| ≥ 35 yr | 6.0 | 0.109 | 0.866 | 17224.4 | 23.1 | 128608.7 | 172.7 | 14572.3 | 146.1 | ||
| ≥ 30 yr | 6.0 | 0.108 | 0.867 | 18051.9 | 23.0 | 135991.3 | 173.3 | 15225.1 | 146.1 | ||
| AC | Whole | 6.0 | 0.177 | 0.733 | 997.5 | 33.8 | 3736.5 | 126.8 | 1170.9 | 148.8 | |
| ≥ 80 yr | 6.0 | 0.183 | 0.797 | 643.0 | 35.6 | 3174.8 | 175.8 | 559.6 | 153.0 | ||
| ≥ 75 yr | 6.0 | 0.190 | 0.766 | 558.4 | 36.5 | 2383.4 | 155.8 | 547.0 | 152.7 | ||
| ≥ 70 yr | 6.0 | 0.194 | 0.751 | 539.5 | 37.2 | 2165.0 | 149.1 | 551.5 | 152.4 | ||
| ≥ 65 yr | 6.0 | 0.202 | 0.739 | 554.2 | 38.2 | 2126.5 | 146.7 | 574.1 | 152.0 | ||
| ≥ 60 yr | 6.0 | 0.212 | 0.736 | 634.8 | 39.7 | 2405.9 | 150.4 | 639.4 | 151.5 | ||
| ≥ 55 yr | 6.0 | 0.206 | 0.735 | 694.1 | 38.6 | 2617.8 | 145.6 | 719.0 | 150.9 | ||
| ≥ 50 yr | 6.0 | 0.194 | 0.732 | 793.2 | 36.7 | 2963.2 | 137.0 | 868.1 | 149.9 | ||
| ≥ 45 yr | 6.0 | 0.186 | 0.736 | 901.9 | 35.4 | 3410.7 | 133.8 | 1006.5 | 149.3 | ||
| ≥ 40 yr | 6.0 | 0.182 | 0.731 | 953.6 | 34.6 | 3547.9 | 128.7 | 1103.8 | 149.0 | ||
| ≥ 35 yr | 6.0 | 0.179 | 0.732 | 984.8 | 34.1 | 3674.4 | 127.3 | 1151.7 | 148.9 | ||
| ≥ 30 yr | 6.0 | 0.177 | 0.733 | 995.6 | 33.9 | 3723.6 | 126.8 | 1168.9 | 148.8 | ||
As we expected, for all ages, the four economic indicators of CEA in the health checkup subgroup were much higher than those in the active consultation subgroup. In the subgroup of health checkup subjects above 65 years old, all four indicators of CEA in Scheme 2 were lower than those in Scheme 1, while the total cost-effectiveness ratio in Scheme 2 was higher than that in Scheme 1 in the subgroup of health checkup subjects under 60 years. This highlights that conducting CEA testing in the health checkup for people over 65 years old is economically valuable, especially the lower cost per capita ($40.9 in Scheme 2 vs $146.6 in Scheme 1).
In the active consultation subgroup, the total cost-effectiveness ratio in Scheme 2 was higher than that in Scheme 1 for all ages. CA19-9 and CA72-4 had higher total cost-effectiveness ratios in almost all subgroups, different from CEA (Figures 9 and 10). This also indicates that blood tests for the active consultation group are not enough and that the necessary gastrointestinal endoscopy procedure is more important.
This study included more than 32000 subjects who received CEA, CA19-9, CA72-4, gastroscopy and colonoscopy assessments. In our study, CEA and CA19-9 again have been proved to be ideal serum biomarkers for screening GICs. The specificity of CEA and CA19-9 was approximately 95.0%-97.5% at the traditional cut-off value, which was highly consistent with previous studies[5,6]. While for the diagnostic value of CA72-4, there is a discrepancy between the results of previous literatures and our clinical practice. In our study, the specificity of CA72-4 was less than 90%, indicating that the cut-off value could be higher, which made the sensitivity even lower. If the cut-off value of CA72-4 was 10, the sensitivity and specificity of GC were 0.163 and 0.933, respectively, and the sensitivity and specificity of CRC were 0.177 and 0.935, respectively.
Besides the sensitivity and specificity, another important indicator is the PPV. Even for the best performing CEA, the PPV for GC was as low as 5.6% and that for CRC was only 21.2%. At the traditional cut-off value, the PPV of CA72-4 for GC was 2.3%, which meant that 97.7% of CA72-4-positive patients were false positive. The PPV also explained why there was no evidence of malignant disease in a large number of CA72-4-positive patients after a full set of auxiliary examinations. Of course, in view of the fact that the PPV is greatly affected by the prevalence, the real-world PPV would be lower. Therefore, our data on the predictive value is mainly used for comparison among the three biomarkers.
Several novel indicators are proposed to evaluate the economic value of blood markers for GICs. To calculate the economic value of a blood biomarker, it is inadequate to focus on the biomarker itself. A blood test is used as a screening test, and its significance also lies in the following gold standard test. By combining blood tests and endoscopy, the total cost and cost per capita of correctly diagnosing one case of GIC are excellent indicators, which are related to the cost, prevalence rate and sensitivity of blood tests. However, these two indicators are not sufficient. If the prevalence of a disease increases, the cost per capita would also increase owing to more endoscopy examinations. It seemed that the cost increased, but the effect had actually improved even more. Therefore, it was necessary to calculate the cost-effectiveness ratio. What is the ‘effect’? As a screening test, the sensitivity is its effect. The cost-effectiveness ratio is cost divided by sensitivity, which means the total cost for correctly diagnosing all subjects, including missed patients. We found that the total cost and the cost-effectiveness ratio per capita are positively correlated and change synchronously. Through our economic research, we have discovered the impacts of the order of gastrointestinal endoscopy and diagnostic thresholds on economic benefits. It is also clear that the economic value of combined blood biomarkers is not as good as that of the single CEA. Subgroup analysis shows that CEA had qualified diagnostic value for health checkup subjects above 65 years old.
In this study, only the subjects who received CEA, CA19-9, CA72-4, gastroscopy and colonoscopy were included. These inclusion criteria avoided or reduced several biases, such as workup bias, spectrum bias and measurement bias. For example, all of the included cases were examined by the gold standard test, so there was no situation in which the subject with negative blood test results was not examined with the gold standard test. But on the other hand, the inclusion criteria led to an inevitable selection bias because the subjects undergoing gastrointestinal endoscopy are those with a high risk of digestive diseases, and the incidences of GC and CRC in this study were higher than that in the real world[15]. Many people undergo only blood tests but not gastrointestinal endoscopy when receiving a health checkup. As a result, some early GIC patients with normal CEA, CA19-9, CA72-4 Levels were not included. If these patients were included, the number of false negative subjects might have increased, and the sensitivity would have further decreased.
The advantages of this study are its continuous inclusion of subjects, use of the cohort study inclusion method (not case-control study), large sample size, inclusion of multiple tumors and use of multiple indicators. Especially for CA72-4 test, our sample size exceeded the sum of all previous reported studies. The comparison among multiple indicators highlighted the shortcomings of the diagnostic and economic value of CA72-4. In particular, the results of the classic markers CEA and CA19-9 were consistent with previous studies. We also proposed a new evaluation method for the economic efficiencies of tumor biomarkers for GIC and provided a reference for medical insurance policies.
CEA had qualified diagnostic value for CRC and superior economic value for GICs, especially for health checkup subjects above 65 years old. CA72-4 was not suitable as a diagnostic biomarker.
Studies showed that blood carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9) could be used to diagnose gastric cancer (GC) and colorectal cancer (CRC). Blood CA72-4 could be a potential biomarker to diagnose GC and CRC. A positive result in blood test would lead the subject to undergo further examinations.
Large-scale clinical application showed an extremely high false positive rate of CA72-4 for diagnosis, which leads to the waste of medical resources and heave social medical burden. The massive data and real-world diagnostic cohorts make it possible to further explore the diagnostic and economic value of biomarkers.
Through a real-world diagnostic cohort, we aimed to reassess the diagnostic and economic value of CEA, CA19-9, and CA72-4 for gastrointestinal malignant tumors in a large sample.
Data from patients the medical examination center, outpatient department or inpatient department of Zhongshan Hospital of Fudan University from October 2006 to May 2018 were retrospectively evaluated. Four economic indicators were used to evaluate the economic value of tumor biomarkers. The diagnostic value of the three biomarkers was further evaluated.
The clinical benefits of CEA were higher than those of CA19-9, while the clinical benefits of CA72-4 were the lowest. The combination of biomarkers in the CRC and gastrointestinal malignant tumors significantly increased the AUC by less than 0.3, while that in GC did not. Compared to the economic indicators of the single biomarker CEA, the combination of biomarkers is not superior. At the threshold of 1.8 μg/L to 10.4 μg/L, all four indicators of CEA were lower than those in the scheme that conducted gastrointestinal endoscopy only. Subgroup analysis implied that the health checkup of CEA for people above 65 years old was economically valuable.
CEA had qualified diagnostic value for CRC and superior economic value for gastrointestinal cancers, especially for health checkup subjects above 65 years old while CA72-4 was not suitable as a diagnostic biomarker.
In real world, many people undergo only blood tests but not gastrointestinal endoscopy when receiving a health checkup. Those undergone gastrointestinal endoscopy were at a higher risk of digestive diseases, which leads to an inevitable selection bias. Future researches may emphasize on the involvement of patients with normal CEA, CA19-9, CA72-4 Levels to decrease the number of false negative subjects.
The authors would like to thank the members of Professor Xi-Zhong Shen’s laboratory for helpful discussions and critical reading of the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dauyey K, Kazakhstan; Grossi U, Italy S-Editor: Chen YL L-Editor: A P-Editor: Yuan YY
| 1. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1335] [Article Influence: 333.8] [Reference Citation Analysis (2)] |
| 2. | Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC Jr; ASCO. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313-5327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1111] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 3. | Duffy MJ, Lamerz R, Haglund C, Nicolini A, Kalousová M, Holubec L, Sturgeon C. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. Int J Cancer. 2014;134:2513-2522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 248] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 4. | Byrne DJ, Browning MC, Cuschieri A. CA72-4: a new tumour marker for gastric cancer. Br J Surg. 1990;77:1010-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 367] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 6. | Hundt S, Haug U, Brenner H. Blood markers for early detection of colorectal cancer: a systematic review. Cancer Epidemiol Biomarkers Prev. 2007;16:1935-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Guadagni F, Roselli M, Amato T, Cosimelli M, Perri P, Casale V, Carlini M, Santoro E, Cavaliere R, Greiner JW. CA 72-4 measurement of tumor-associated glycoprotein 72 (TAG-72) as a serum marker in the management of gastric carcinoma. Cancer Res. 1992;52:1222-1227. [PubMed] |
| 8. | Filella X, Fuster J, Molina R, Grau JJ, García-Valdecasas JC, Grande L, Estapé J, Ballesta AM. TAG-72, CA 19.9 and CEA as tumor markers in gastric cancer. Acta Oncol. 1994;33:747-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Fernández-Fernández L, Tejero E, Tieso A, Rabadán L, Munoz M, Santos I. Receiver operating characteristic (ROC) curve analysis of the tumor markers CEA, CA 19-9 and CA 72-4 in gastric cancer. Int Surg. 1996;81:400-402. [PubMed] |
| 10. | Tocchi A, Costa G, Lepre L, Liotta G, Mazzoni G, Cianetti A, Vannini P. The role of serum and gastric juice levels of carcinoembryonic antigen, CA19.9 and CA72.4 in patients with gastric cancer. J Cancer Res Clin Oncol. 1998;124:450-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Guadagni F, Roselli M, Cosimelli M, Mannella E, Tedesco M, Cavaliere F, Grassi A, Abbolito MR, Greiner JW, Schlom J. TAG-72 (CA 72-4 assay) as a complementary serum tumor antigen to carcinoembryonic antigen in monitoring patients with colorectal cancer. Cancer. 1993;72:2098-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Carpelan-Holmström M, Louhimo J, Stenman UH, Alfthan H, Haglund C. CEA, CA 19-9 and CA 72-4 improve the diagnostic accuracy in gastrointestinal cancers. Anticancer Res. 2002;22:2311-2316. [PubMed] |
| 13. | Carpelan-Holmström M, Louhimo J, Stenman UH, Alfthan H, Järvinen H, Haglund C. Estimating the probability of cancer with several tumor markers in patients with colorectal disease. Oncology. 2004;66:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Duffy MJ, van Dalen A, Haglund C, Hansson L, Klapdor R, Lamerz R, Nilsson O, Sturgeon C, Topolcan O. Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines. Eur J Cancer. 2003;39:718-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 251] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |