Published online Jan 28, 2023. doi: 10.3748/wjg.v29.i4.670
Peer-review started: September 28, 2022
First decision: November 18, 2022
Revised: November 26, 2022
Accepted: January 9, 2023
Article in press: January 9, 2023
Published online: January 28, 2023
Processing time: 114 Days and 8.8 Hours
Colon cancer has attracted much attention due to its annually increasing incidence. Conventional chemotherapeutic drugs are unsatisfactory in clinical application because of their lack of targeting and severe toxic side effects. In the past decade, nanomedicines with multimodal therapeutic strategies have shown potential for colon cancer because of their enhanced permeability and retention, high accumulation at tumor sites, co-loading with different drugs, and comb-ination of various therapies. This review summarizes the advances in research on various nanomedicine-based therapeutic strategies including chemotherapy, radiotherapy, phototherapy (photothermal therapy and photodynamic therapy), chemodynamic therapy, gas therapy, and immunotherapy. Additionally, the therapeutic mechanisms, limitations, improvements, and future of the above therapies are discussed.
Core Tip: Nanomedicine has exhibited great potential in the colon cancer therapy over the past decades. In this review, we summarize the advances in research on various nanomedicine-based therapeutic strategies including chemotherapy, radiotherapy, phototherapy (photothermal therapy and photodynamic therapy), chemodynamic therapy, gas therapy, and immunotherapy. Additionally, the therapeutic mechanism, limitations, and improvement in these therapies are also introduced. The challenges and future prospect of the nanomedicine-based multimodal therapies for colon cancer are discussed.
- Citation: He YC, Hao ZN, Li Z, Gao DW. Nanomedicine-based multimodal therapies: Recent progress and perspectives in colon cancer. World J Gastroenterol 2023; 29(4): 670-681
- URL: https://www.wjgnet.com/1007-9327/full/v29/i4/670.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i4.670
Colon cancer is one of the most intractable gastrointestinal diseases with increasing incidence worldwide[1,2]. For the past few years, human lifestyles and diets have changed markedly with the rapid development of the global economy, which further increases the risk of colon cancer. According to the global cancer statistics, the incidence and mortality of colon cancer were 6.1% and 5.8% in 2018[3], which ranked fourth and fifth among all cancers, respectively. The characteristics of colon cancer are mainly reflected in rapid energy metabolism and proliferation that enhance tumor invasion and metastasis. Therefore, colon cancer has become one of the major unresolved problems in medicine[4,5]. Conventional small molecule chemotherapeutic drugs (such as paclitaxel, doxorubicin, and camptothecin) are unsatisfactory because of their lack of targeting and solubility, and severe toxic side effects. Thus, there is an urgent need to develop novel and efficient therapeutic strategies for colon cancer. In the past decade, the emergence of nanomedicine has shown potential in cancer therapy. Compared with traditional chemotherapeutic drugs, nanomedicine has better tumor targeting because the vascular gaps in tumor tissue are wider than those of normal tissue, so that nanomedicine can penetrate tumor tissue through these vascular gaps but not into normal tissue. Because of the lack of lymphatic reflux in the tumor region, nanomedicine can remain in the tumor tissue, and this mechanism of nanomedicine-based tumor targeting is called the enhanced permeability and retention (EPR) effect[6]. Additionally, various nanoscale drug delivery systems can load the chemotherapeutic drugs to enhance their solubility, which improves their utilization. Finally, nanomedicine is able to combine multimodal therapies to enhance the antitumor effect. Above all, nanomedicine has shown numerous advantages and potential for multimodal therapy of colon cancer.
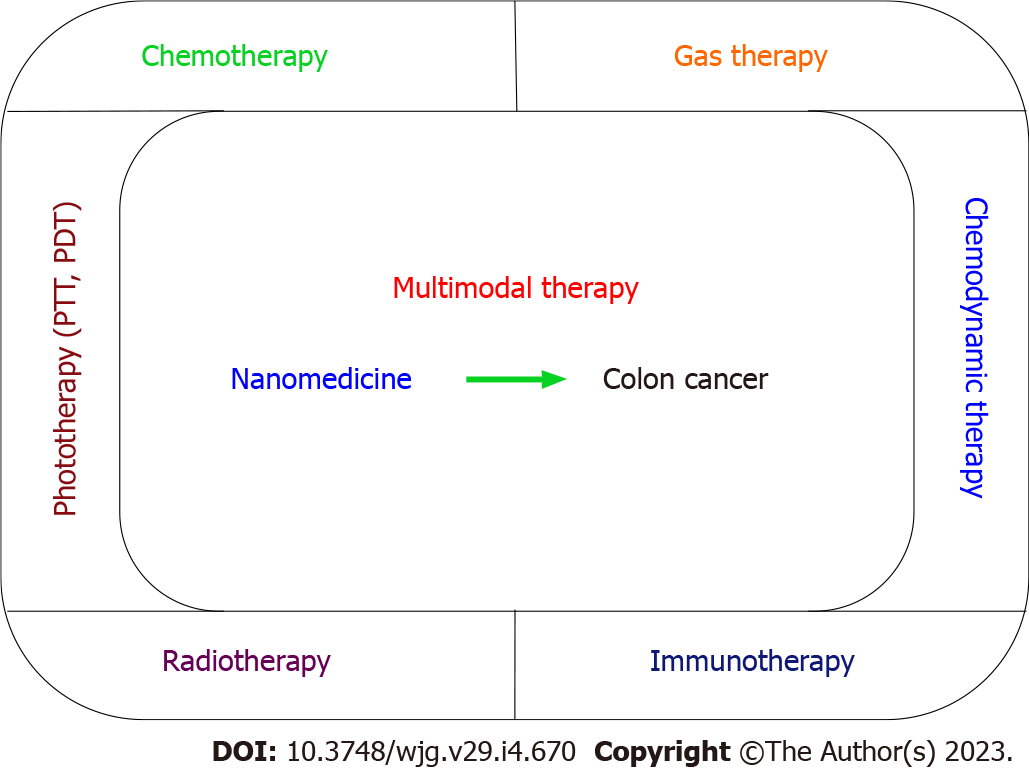
In this review, we summarize recent progress of nanomedicine-based multimodal colon cancer therapy. First, we introduce all types of organic and inorganic nanomedicine and explore their drug loading, drug release, and tumor targeting. Moreover, the biosafety of nanomedicine is also discussed. Then, we introduce various therapeutic strategies for colon cancer including chemotherapy, phototherapy [photothermal therapy (PTT) and photodynamic therapy (PDT)], radiotherapy, gas therapy, chemodynamic therapy (CDT), and immunotherapy (Figure 1). The therapeutic mechanisms of these approaches are also discussed. Among them, nano drug delivery systems (NDDSs) are widely used to improve the therapeutic effect due to their characteristics of improving the water solubility of chemotherapy drugs, prolonging the blood circulation time, targeted drug delivery, few side effects, and reversing multi-drug resistance. PDT is a new treatment for colon cancer that uses specific wavelengths of light to excite photosensitizers. In the excited state, the photosensitizers transfer energy or electrons to the surrounding oxygen, thus producing singlet oxygen and killing cancer cells. Radiation therapy can cause DNA strand break of tumor cells under X-ray irradiation, and produce high cytotoxic free radicals to damage colon tumor cells. Compared with other reactive oxygen species (ROS) therapies, CDT has stronger in situ catalytic ROS generation, higher tumor specificity, and deeper tissue penetration, and does not require additional stimulation, providing a new idea for the future treatment of colon cancer. Gas therapy can enhance drug release, and when used with chemotherapy and synergistic therapy with other therapies, it can improve therapeutic effects, but its application in colon cancer requires extensive studies. Immunotherapy has been widely used in the treatment of colon cancer. The immunogenicity of tumor cells is activated by means of photothermal and ROS, and immunoadjuvant is used to reduce the immunosuppression in the tumor microenvironment and enhance the immune effect. These strategies provide new insights into the clinical treatment of colon cancer. Finally, the main limitations and challenges in the development of nanomedicine for colon cancer are addressed, and future research directions proposed. It is believed that nanomedicine-based multimodal therapy will play an important role in colon cancer.
Chemotherapy is the core method in current cancer treatment, and various drugs such as 5-fluorouracil (5-FU), platinum drugs, irinotecan, and epirubicin, are widely used[7-11]. However, there are still some problems in conventional chemotherapy: (1) Free small-molecule drugs have a limited half-life in vivo and lack of tumor targeting, leading to severe side effect; (2) Poor aqueous solubility of drugs limits their clinical effect; (3) Dense solid tumor tissue hinders drug delivery, resulting in insufficient drug dose in tumor tissue; and (4) Tumor microenvironment, such as hypoxia, low pH, and high H2O2 concentration, leads to multidrug resistance. To improve the therapeutic effect of chemotherapy, NDDSs have received extensive attention because of their properties such as improving the aqueous solubility of drugs, prolonging the blood circulation time, achieving targeted delivery to tumors, and few side effects. Various NDDSs have been designed to enhance tumor targeting and aqueous solubility of drugs, leading to improved therapeutic effect[12-15].
Most drugs exhibit poor aqueous solubility and low bioavailability. To solve this problem, Chen et al[16] adopted a cucurbituril-based supramolecular chemical strategy to improve the aqueous solubility and long-term circulation of the drugs for enhancing the therapeutic effect of oxaliplatin on colon cancer. Chen et al[17] prepared fisetin micelles using monomethyl poly(ethylene glycol)-poly(ε-caprolactone) copolymers. Compared with free fisetin, the micelles exhibited excellent aqueous solubility and cytotoxicity. Additionally, Xiao et al[18] used the intermolecular noncovalent interaction of curcumin and irinotecan to self-assemble into nanoparticles, which enhanced the aqueous solubility of curcumin, reduced the side effects of irinotecan, and showed better targeting and therapeutic effect. To prolong the blood circulation of drugs, Jiang et al[19] designed OxPt/SN38 nanoparticles to hitchhike on low-density lipoprotein (LDL) particles and accumulate at the tumor site through LDL-receptor-mediated endocytosis, which showed excellent antitumor efficacy in murine tumor models. Liu et al[20] developed an active targeting strategy to specifically combine glucose-regulated protein 78 overexpressed on the surface of colon cancer cells with PEGylated WL8 peptide, which enhanced the enrichment of doxorubicin in the tumor region.
Inflammation is an important reason for promoting tumor proliferation, invasion, metastasis, and drug resistance. Therefore, anti-inflammatory drugs such as aspirin and dexamethasone can improve the therapeutic effect of antitumor drugs[21,22]. Natural products such as curcumin and fisetin, which show good anti-inflammatory and antitumor properties, have also been widely used as chemotherapeutic drugs[23-26]. Wang et al[27] found that the anti-inflammatory drug dexamethasone significantly enhanced the antitumor activity of carboplatin and gemcitabine and increased their accumulation in tumors, providing a basis for dexamethasone as a chemosensitizer. Ma et al[28] developed a pH- and redox-responsive peptide-dexamethasone conjugate (L-SS-DEX) that reduces inflammation and modulates the tumor microenvironment for an effective antitumor effect.
Multidrug resistance is another reason for the failure of chemotherapy. The multidrug-resistance-related proteins such as P-glycoprotein (P-gp) of tumor cells result in significant drug excretion[29,30]. Currently, some NDDSs have been designed to co-deliver P-gp inhibitors or microRNAs to suppress multidrug resistance and enhance the drug sensitivity of tumor cells[31,32]. Sivak et al[33] overcame multidrug resistance by simultaneously delivering doxorubicin and the P-gp inhibitor (reversin 121) into cancer cells. The neurokinin-1 receptor antagonists inhibited expression of P-gp to enhance the chemotherapy effect[34].
Studies have shown that the development of colon cancer is closely related to the gut microbiota, which is involved in regulating the sensitivity of tumor cells to chemotherapy. As a Gram-negative anaerobic bacterium, Fusobacterium nucleatum (F. nucleatum) is enriched in colon cancer patients, adheres to the intestinal mucosa, and invades epithelial cells to induce carcinogenesis. It can combine with E-cadherin on the surface of colon cancer cells to form a tumor immunosuppressive microenvironment, promote tumor proliferation, and enhance drug resistance of colon cancer cells[35-38]. Therefore, inhibiting the activity of F. nucleatum is important for enhancing the efficacy of colon cancer chemotherapy. Lauric acid has a specific inhibitory effect on F. nucleatum. Yan et al[39] used poly-glycidyl ether as a nanodrug carrier, introduced the antibacterial agent lauric acid and oxaliplatin through esterification, selectively inhibited the biological activity of F. nucleatum, and improved the resistance of colon cancer cells to oxaliplatin. The antibiotic metronidazole and the chemotherapy drug 5-FU were mixed into the metal polyphenol network coated mesoporous silica nanoparticles (MSNs), and then added with carboxymethyl cellulose to obtain anti-colorectal cancer gel to eliminate F. nucleatum in colon cancer and inhibit the drug resistance, and proliferation and metastasis of colon cancer cells[40].
Phototherapy is an emerging strategy to kill tumor cells by stimulating photosensitizers under light irradiation. In recent years, phototherapy, as a noninvasive treatment, has attracted widespread attention because of its specificity, low toxicity for normal tissues, and excellent antitumor effect. PTT and PDT are two common methods in colon cancer treatment[41-44]. PTT utilizes photosensitizer accumulated in tumor tissue to convert light energy into heat for killing tumor cells under light irradiation (generally near-infrared, NIR), which shows spatiotemporal controllability, high selectivity, and low cost. Recently, NDDSs have been designed to delivery photothermal agents for enhancing tumor targeting. For example, Ren et al[45] designed CT26 cell membrane-coated Bi nanoparticles, which had good long-term circulation and tumor homologous targeting ability in vivo compared with Bi nanoparticles. In addition, it is reported that epidermal growth factor receptor (EGFR) is abundantly expressed on the surface of some colorectal cancer cells. Shih et al[46] combined cetuximab (EGFR inhibitor) with the organic NIR dye IR780 to target colon cancer cells with high EGFR expression for PTT. Excessive H2S (0.3-3.4 mmol/L) produced by colon cancer cells can promote the proliferation of colon cancer cells and angiogenesis in the tumor area[47,48]. Biocompatible iron oxide nanospindles have been developed, which can efficiently remove endogenous H2S gas in colon tumor tissues and inhibit tumor growth, and generate FeS in situ for magnetic resonance imaging (MRI) and PTT under NIR irradiation[49-51].
PDT is a new method for colon cancer therapy that utilizes light of a specific wavelength to excite a photosensitizer, and the photosensitizer in the excited state transfers energy or electrons to the surrounding oxygen, thereby producing singlet oxygen to kill cancer cells[52]. Various NDDSs have been designed to deliver PDT-based photosensitizers to colon tumors. By adjusting the size of the NDDSs and modifying with hydrophilic groups, the photosensitizers can be passively targeted to the tumor area through the EPR effect. Besides the EPR effect, biomimetic membrane or tumor-specific affinity ligands-modified NDDSs have also been extensively studied for tumor targeting. Xie et al[53] designed a translocator protein (TSPO)-targeted photosensitizer (IR700DX-6T) for tumor targeting of photosensitizers via combination with overexpressed TSPO in colon cancer cells. Additionally, because of the high expression of EGFR in colon cancer cells, EGFR antibody has been used to target delivery of the photosensitizer IR700, which effectively eradicated colon cancer cells[54]. Traditional pho-tosensitizers have high fluorescence quantum yields in dilute solutions, which leads to weaker fluorescence in the aggregated state. Aggregation of photosensitizers during delivery can lead to reduced ROS yields, so it is crucial to develop novel nanocarriers that efficiently load photosensitizers and prevent their aggregation. Covalent organic frameworks as a class of organic polymers, have attracted much attention because of their excellent biocompatibility and biodegradability. Gan et al[55] showed enhanced phototherapeutic effects by adsorbing the NIR dye indocyanine green (ICG) onto the covalent organic framework via π–π interaction to prevent its aggregation. In addition to this, aggregation-induced emission luminescence agents have been used to enhance PDT because the agents exhibit enhanced fluorescence emission in the aggregated state[56]. Hypoxia is one of the main reasons for the poor effect of PDT. Thus, researchers have developed a variety of oxygen generators such as hemoglobin, MnO2, and perfluorocarbon, to increase oxygen in the tumor to enhance the effect of PDT[57-59]. For example, He et al[60] designed gold nanocages coated with MnO2 and hyaluronic acid (HA) for tumor targeting, and MnO2 was designed to react with the overproduced H2O2 in the tumor to relieve tumor hypoxia and enhance the effect of gold nanocage-based PDT.
Radiotherapy is a local cancer treatment that is widely applied in clinical therapy. The mechanism of action of radiotherapy is to cause DNA strand breaks in tumor cells and generate highly cytotoxic free radicals under X-ray irradiation to damage tumor cells[61-65]. Radiosensitizers are usually used to boost the effect of radiotherapy against colon cancer[66]. 7-Dehydrocholesterol is utilized as a radiosensitizer, which can react with ROS to promote lipid peroxidation, double-strand breaks, and mitochondrial damage in cancer cells, enhancing the radiotherapeutic effect[67]. As we know from the mechanism of action of radiotherapy, tumor hypoxia limits the efficacy of radiotherapy; thus, relief of hypoxia by nanomedicine can improve the therapeutic effect. MnO2 can react with excess H2O2 in the tumor to generate oxygen, which can relieve the hypoxic microenvironment, eliminate tumor resistance to radiotherapy, and reshape the immunosuppressive microenvironment. Zhang et al[68] designed bovine-serum-albumin-coated MnO2 as a radiosensitizer. MnO2 can decompose excess H2O2 in the tumor into oxygen to relieve tumor hypoxia and convert tumor-promoting M2 tumor-associated macrophages into antitumor M1-type macrophages to reshape the immunosuppressive microenvironment and eliminate tumor resistance to radiotherapy. In addition, perfluorocarbon is a good oxygen carrier that can be used to delivery oxygen to tumors and reverse hypoxia, leading to enhancement of radiotherapy[69].
CDT is a promising therapeutic strategy that utilizes endogenously overexpressed H2O2 in tumors to generate toxic hydroxyl radicals (·OH) through Fenton/Fenton-like reactions catalyzed by metals (Fe2+, Cu+, Mn2+, Mo4+, W4+, Ti3+, etc.)[70-73]. Compared with other ROS therapies, CDT has the advantages of stronger in situ catalytic ROS generation, tumor specificity, and deep tissue penetration, which does not require additional stimulation. However, the effect of CDT is still limited by its high dependence on tumor endogenous H2O2 concentration (10-100 μM) and slow ion release from inorganic nanoparticles[74,75]. The problem of low levels of H2O2 in tumor tissue can be solved by directly loading H2O2 or encapsulating H2O2-producing drugs such as glucose oxidase and calcium peroxide. However, nanocarriers directly encapsulating exogenous H2O2 have the risk of leakage causing damage to normal tissues. Therefore, new strategies are urgently needed to address the challenges associated with CDT. Su et al[76] used a microfluidic method to prepare a nanogel (DOX@Mn-Alg) composed of alginate (Alg), Mn2+, and doxorubicin as an ideal CDT/chemotherapy synergistic therapeutic nanoplatform, because doxorubicin can activate NADP oxidases to convert oxygen to ·O2– and then superoxide dismutase further catalyzes ·O2– to generate endogenous H2O2 via a disproportionation reaction. Subsequently, the elevated H2O2 can be converted into a sufficient amount of ·OH through a Mn2+-mediated Fenton-like reaction. Ultimately, DOX@Mn-Alg can rationally combine doxorubicin chemotherapy with Mn2+-mediated CDT and immunotherapy for synergistic cancer treatment. Chen et al[77] selected Pd nanoparticles as a CDT reagent, and showed that the ultra-small Pd nanozyme as the core had high catalytic activity and pH selectivity. Under acidic conditions, it exhibited peroxidase activity to produce OH and 1O2, while under neutral conditions, it promoted the decomposition of H2O2 to produce O2 through catalase activity. In terms of biological activity, the bidirectional anisotropic nanocluster not only directly inhibited tumor cells through ROS production, but also induced H2O2 production in CT26 cells, which enhanced the therapeutic effect. The nanoparticles inhibited tumor growth in CT26 mice, and improved tumor hypoxia and enhanced the therapeutic effect.
The intracellular glutathione in tumor cells can eliminate the oxidative activity of ·OH through powerful reducing activity. Lin et al[78] devised a strategy to enhance CDT by inhibiting expression of glutathione in tumors and remodeling the reductive state of the tumor microenvironment, indicating that inhibition of glutathione can improve the effect of CDT. Wang et al[79] reported a degradable MnSiO3 nanosystem for CDT/chemical synergistic therapy. First, MnSiO3 nanoparticles were synthesized, and then the surface-initiated living radical polymerization of monomer of SN38 and oligo(ethylene glycol) methacrylate was conducted to obtain the product of CAMNSN@PSN38. Nanoparticles delivered to tumor tissues were gradually biodegraded by glutathione over time, during which SN38 and Mn2+ were gradually released. The released SN38 showed a favorable chemotherapeutic effect and increased accumulation of H2O2. The interaction of CAMNSN@PSN38 with glutathione depleted glutathione in tumor tissues and led to Mn2+ release for CDT and MRI-guided therapy. CAMNSN@PSN38 had a good inhibitory effect on colon tumor growth and assisted MRI-guided imaging through ROS accumulation in vivo. Unlike other tumor types, colon tumor shows high expression of H2S (0.3-3.4 mmol/L), whose reductive activity is stronger than that of glutathione[80,81]. Therefore, in the treatment of colon cancer, the effect of CDT is also limited by endogenous H2S. Liu et al[82] constructed CuFe2O4 nanoparticles to explore the potential of endogenous H2S depletion to enhance CDT for colon cancer. CuFe2O4 nanoparticles remodel endogenous H2S in colon cancer and enhance the Fenton or Fenton-like reaction of Cu(I) and Fe(II) by a photothermal effect to generate more ·OH. The results suggest that CuFe2O4 nanoparticles effectively enhance the effect of CDT by depleting H2S. In addition, H2S-responsive therapeutic nanoplatforms have been designed. Xiao et al[18] synthesized a copper-based metal-organic framework named HKUST-1 as a smart therapeutic platform. PTT and CDT were activated in the presence of H2S in colon cancer cells. H2S-triggered nanosystems can minimize side effects on surrounding normal tissues and precisely inhibit colon cancer growth. Above all, CDT shows potential for colon cancer treatment.
As an emerging treatment method, gas therapy has attracted research interest in recent years[83-86]. Gas therapy refers to use of H2S[87], NO[88], CO, etc. to kill tumor cells[89]. Liu et al[90] designed a nanoplatform (PEG/SCNPs@DMSN-SNO-g-C3N4) to release NO under X-ray irradiation, and then NO reacted with superoxide anions to generate ONOO– toxic free radicals, leading to apoptosis through mitochondrial damage. NO has been proven to activate innate and adaptive responses of the immune system against tumors. Previous in vivo results showed that all NO-treated colon tumor-bearing (CT26 model) mice were resistant to secondary CT26 cell inoculation. Nonsteroidal anti-inflammatory drugs (NSAIDs) are prototypical anticancer agents. NO and H2S are gaseous mediators with physiological relevance and NSAIDs that possess an H2S- and NO-releasing moiety have shown beneficial effects. Chattopadhyay et al[91] synthesized and characterized a new class of anti-inflammatory NO- and H2S -releasing compounds. This induced apoptosis, inhibited cell proliferation, and reduced colon tumor growth in a mouse xenograft model. Zhang et al[92] designed gas-generating MSNs, which can load ammonium bicarbonate and doxorubicin in the pores, and ICG coated on a polydopamine layer and modified with RGD peptides on the outer surface [M(ABC)-DOX@PDA-ICG-PEG-RGD] for triggering drug release and targeted chemotherapeutic photothermal combination treatment. At high temperature and low pH, the encapsulated ammonium bicarbonate can effectively generate CO2. The CO2 can damage the polydopamine layer and accelerate the release of doxorubicin. The results proved the excellent antitumor effect of gas therapy and chemotherapy, as well as good biosafety. Therefore, the gas therapy showed potential for colon cancer therapy.
Immunotherapy exhibits potential against colon cancer because it relies on the autoimmune system to attack malignant tumors. Immunotherapy for colon cancer is mainly divided into the following categories: (1) Activation of tumor immunogenicity; (2) Relief of tumor microenvironment immunosuppression; (3) Design of antitumor neoantigen vaccines and novel immune adjuvants; and (4) Design of therapeutic strategies using macrophages as target cells. However, only a subset of cancer patients responds to current immunotherapies because of the low immunogenicity of tumor cells and the immunosuppressive tumor microenvironment. Therefore, new strategies are needed to activate tumor immunogenicity and relieve the immunosuppression of the tumor microenvironment to improve the effect of immunotherapy. Fan et al[93] reported pH-responsive core-shell nanoparticles (HCLO NPs) for co-delivery of oxaliplatin intermediate and cytosine-guanine-containing oligodeoxynucleotide (CpG) for colon cancer treatment, and the oxaliplatin intermediate intratumoral injection induced in situ antigen production via immunogenic cell death. Subsequently, CpG enhanced antigen presentation and promoted production of cytotoxic T lymphocytes (CTLs). The results indicated that the HCLO NPs enhanced the toxicity of oxaliplatin intermediate for CT26 cells and upregulated expression of calreticulin, which exhibited significant immunity and antitumor effect. Hu et al[94] integrated HA, pheophorbide A heterodimer, and NLG919 into a supramolecular nanocomposite, which generated ROS under NIR laser irradiation to kill tumor cells, stimulated antitumor immunogenicity, and enhanced intratumoral infiltration of CTLs. The immunosuppressive tumor microenvironment was reversed by NLG919-mediated inhibition of indoleamine 2,3-dioxygenase 1. The results showed that this strategy could effectively kill CT26 colon tumors. Ding et al[95] designed liposome-encapsulating phosphatidylinositol 3-kinase γ inhibitor IPI-549 and photosensitizer Ce6 for immunotherapy of colon cancer. When the liposomes were internalized into CT26 cells, ROS were generated under laser irradiation, causing immunogenic tumor cell death. IPI-549 transported by liposomes promoted apoptosis of myeloid-derived suppressor cells and reduced the immunosuppressive activity of CD8+ T cells to inhibit growth of CT26 tumors. Checkpoint inhibitors, such as antibodies that block the programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) pathway, are among the most promising immunotherapies for metastatic cancer. However, the responses rates remain low. To solve this problem, Yu et al[96] developed nanoparticles with PD-L1 blocking ability, which integrated PTT, antitumor immunity, and PD-1/PD-L1 blockade to enhance antitumor efficacy. In the mouse CT26 bilateral tumor model, intravenously injected nanoparticles accumulated at the tumor site and mediated a strong photothermal effect, eliminated the primary tumor by inducing immunogenic cell death, and elicited strong antitumor immunity. Growth of untreated distant tumors was inhibited by the synergistic effect of systemic antitumor immune activation and PD-L1 blockade. This strategy provided a promising approach for the treatment of metastatic cancer.
The reported immunoadjuvants have many limitations, such as poor cellular uptake and biocompatibility, excessive particle size, single function, and unsatisfactory therapeutic effect. Ding et al[97] prepared mesoporous silica-coated upconversion nanoparticles (UCMSs) and used them as a novel immune adjuvant. UCMSs had significant loading of the photosensitizer merocyanine 540, chicken ovalbumin, and tumor cell fragments. The UCMSs exhibited the best synergistic immune enhancement under 980 nm NIR irradiation, with the strongest Th1 and Th2 immune responses, and the highest frequencies of CD4+, CD8+, and effector memory T cells. In addition, nanovaccine UCMSs inhibited tumor growth more effectively and improved survival of tumor-bearing mice compared with PDT or immunotherapy alone, indicating that UCMSs have higher immunotherapeutic efficacy and clinical potential. As a new tumor vaccine based on zymosan shell particles[98], GP-Neoantigen can stimulate the body to generate a strong antigen-specific CD8+ T cell immune response and an immune response to a variety of neoantigen peptides, and thereby be used for effective tumor treatment. The vaccine induced strong specific CD8+ T cell immune responses and humoral immune responses in vivo, which also showed strong tumor growth inhibitory activity in the CT26 colon cancer model. Binding to Toll-like receptor agonists PolyI:C and CpG 2395 enhanced the antitumor effect and achieved complete tumor clearance. These results provide broad possibilities for further clinical promotion and personalized vaccine therapy.
M2 macrophages are polarized by stimulatory factors in the tumor microenvironment and promote tumor growth. They are involved in limiting T cell function, tumor angiogenesis, and tumor invasion and metastasis. Increasing the ratio of M1/M2 macrophages in the tumor microenvironment is a promising cancer immunotherapy strategy. An erythrocyte membrane nanoparticle encapsulating Porphyromonas gingivalis can modulate the ratio of M1/M2 macrophages for cancer immunotherapy[99], and such nanoparticles inhibited the growth of primary and secondary tumors of CT26 colon cancer under the action of laser and anti-PD-1. Immunotherapy based on nanomedicine has been widely used in cell and animal models, and has shown good anti-tumor efficacy. It is expected to become one of the most potential therapeutic means in cancer treatment.
Several advanced nanomedicine applications have been developed for colon cancer therapy, which overcome the poor tumor targeting and efficacy of conventional drugs. This review presents various organic- and inorganic-based nanomedicines applied in colon cancer therapy using CT26 cells as the tumor model. We have introduced the mechanism of nanomedicine-based therapeutic strategies including chemotherapy, phototherapy (PTT and PDT), radiotherapy, gas therapy, CDT, and immunotherapy. These multimodal therapeutic strategies based on nanomedicine against colon cancer have shown excellent antitumor effect and potential.
Although the nanomedicine-based multimodal therapies have shown a superior effect against colon cancer, several limitations need to be overcome in future development. The first limitation is the unsatisfactory tumor penetration of nanomedicine. Drug delivery in vivo includes circulation, accumulation, penetration, internalization, and release. Poor tumor penetration has become a long-standing problem for the development of nanomedicine, which leads to the survival of tumor stem cells in deep tumor sites. The reason is the serious hinders of dense extracellular matrix and elevated tumor interstitial pressure. Thus, there is an urgent need to develop novel strategies to enhance tumor penetration of nanomedicine. The second limitation is obstruction of various therapies by the tumor microenvironment. For example, tumor hypoxia limits oxygen-dependent therapy such as PDT and radiotherapy. Additionally, M2 tumor-associated macrophages construct the tumor immunosuppression environment, which limits the effect of immunotherapy. Not only that, the immune checkpoint protein on the tumor cell inhibits the recognition and combination of cytotoxic T cells. Therefore, reversing the adverse effects of the tumor microenvironment is the key to improving the therapeutic effect of nanomedicine. It is expected that nanomedicine-based multimodal therapeutic strategies will have potential for clinical translation into colon cancer therapy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nakaji K, Japan; Wang YP, Taiwan S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2907] [Article Influence: 363.4] [Reference Citation Analysis (3)] |
| 2. | Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2952] [Cited by in RCA: 3032] [Article Influence: 159.6] [Reference Citation Analysis (0)] |
| 3. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55686] [Article Influence: 7955.1] [Reference Citation Analysis (132)] |
| 4. | Peng L, Xing X, Li W, Qu L, Meng L, Lian S, Jiang B, Wu J, Shou C. PRL-3 promotes the motility, invasion, and metastasis of LoVo colon cancer cells through PRL-3-integrin beta1-ERK1/2 and-MMP2 signaling. Mol Cancer. 2009;8:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Ogasawara M, Matsubara T, Suzuki H. Inhibitory effects of evodiamine on in vitro invasion and experimental lung metastasis of murine colon cancer cells. Biol Pharm Bull. 2001;24:917-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Greish K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol Biol. 2010;624:25-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 544] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 7. | Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1561] [Cited by in RCA: 2009] [Article Influence: 143.5] [Reference Citation Analysis (0)] |
| 8. | Rocha CRR, Silva MM, Quinet A, Cabral-Neto JB, Menck CFM. DNA repair pathways and cisplatin resistance: an intimate relationship. Clinics (Sao Paulo). 2018;73:e478s. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 285] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 9. | Therizols G, Bash-Imam Z, Panthu B, Machon C, Vincent A, Ripoll J, Nait-Slimane S, Chalabi-Dchar M, Gaucherot A, Garcia M, Laforêts F, Marcel V, Boubaker-Vitre J, Monet MA, Bouclier C, Vanbelle C, Souahlia G, Berthel E, Albaret MA, Mertani HC, Prudhomme M, Bertrand M, David A, Saurin JC, Bouvet P, Rivals E, Ohlmann T, Guitton J, Dalla Venezia N, Pannequin J, Catez F, Diaz JJ. Alteration of ribosome function upon 5-fluorouracil treatment favors cancer cell drug-tolerance. Nat Commun. 2022;13:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Wulaningsih W, Wardhana A, Watkins J, Yoshuantari N, Repana D, Van Hemelrijck M. Irinotecan chemotherapy combined with fluoropyrimidines versus irinotecan alone for overall survival and progression-free survival in patients with advanced and/or metastatic colorectal cancer. Cochrane Database Syst Rev. 2016;2:CD008593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Huang L, Zhang S, Zhou J, Li X. Effect of resveratrol on drug resistance in colon cancer chemotherapy. RSC Adv. 2019;9:2572-2580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Luan X, Guan YY, Lovell JF, Zhao M, Lu Q, Liu YR, Liu HJ, Gao YG, Dong X, Yang SC, Zheng L, Sun P, Fang C, Chen HZ. Tumor priming using metronomic chemotherapy with neovasculature-targeted, nanoparticulate paclitaxel. Biomaterials. 2016;95:60-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Pan P, Dong X, Chen Y, Ye JJ, Sun YX, Zhang XZ. A heterogenic membrane-based biomimetic hybrid nanoplatform for combining radiotherapy and immunotherapy against breast cancer. Biomaterials. 2022;289:121810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 14. | Yao Y, Chen H, Tan N. Cancer-cell-biomimetic nanoparticles systemically eliminate hypoxia tumors by synergistic chemotherapy and checkpoint blockade immunotherapy. Acta Pharm Sin B. 2022;12:2103-2119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Xue C, Hu S, Gao ZH, Wang L, Luo MX, Yu X, Li BF, Shen Z, Wu ZS. Programmably tiling rigidified DNA brick on gold nanoparticle as multi-functional shell for cancer-targeted delivery of siRNAs. Nat Commun. 2021;12:2928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 16. | Chen H, Chen Y, Wu H, Xu JF, Sun Z, Zhang X. Supramolecular polymeric chemotherapy based on cucurbit[7]uril-PEG copolymer. Biomaterials. 2018;178:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Chen Y, Wu Q, Song L, He T, Li Y, Li L, Su W, Liu L, Qian Z, Gong C. Polymeric micelles encapsulating fisetin improve the therapeutic effect in colon cancer. ACS Appl Mater Interfaces. 2015;7:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Xiao H, Guo Y, Liu H, Liu Y, Wang Y, Li C, Císař J, Škoda D, Kuřitka I, Guo L, Sedlařík V. Structure-based design of charge-conversional drug self-delivery systems for better targeted cancer therapy. Biomaterials. 2020;232:119701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Jiang X, Han W, Liu J, Mao J, Lee MJ, Rodriguez M, Li Y, Luo T, Xu Z, Yang K, Bissonnette M, Weichselbaum RR, Lin W. Tumor-Activatable Nanoparticles Target Low-Density Lipoprotein Receptor to Enhance Drug Delivery and Antitumor Efficacy. Adv Sci (Weinh). 2022;9:e2201614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Liu HJ, Luan X, Feng HY, Dong X, Yang SC, Chen ZJ, Cai QY, Lu Q, Zhang Y, Sun P, Zhao M, Chen HZ, Lovell JF, Fang C. Integrated Combination Treatment Using a “Smart” Chemotherapy and MicroRNA Delivery System Improves Outcomes in an Orthotopic Colorectal Cancer Model. Adv Funct Mater. 2018;28:1801118. [DOI] [Full Text] |
| 21. | Kumar D, Rahman H, Tyagi E, Liu T, Li C, Lu R, Lum D, Holmen SL, Maschek JA, Cox JE, VanBrocklin MW, Grossman D. Aspirin Suppresses PGE(2) and Activates AMP Kinase to Inhibit Melanoma Cell Motility, Pigmentation, and Selective Tumor Growth In Vivo. Cancer Prev Res (Phila). 2018;11:629-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Martin JD, Panagi M, Wang C, Khan TT, Martin MR, Voutouri C, Toh K, Papageorgis P, Mpekris F, Polydorou C, Ishii G, Takahashi S, Gotohda N, Suzuki T, Wilhelm ME, Melo VA, Quader S, Norimatsu J, Lanning RM, Kojima M, Stuber MD, Stylianopoulos T, Kataoka K, Cabral H. Dexamethasone Increases Cisplatin-Loaded Nanocarrier Delivery and Efficacy in Metastatic Breast Cancer by Normalizing the Tumor Microenvironment. ACS Nano. 2019;13:6396-6408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 23. | Chen J, Xue F, Du W, Yu H, Yang Z, Du Q, Chen H. An Endogenous H(2)S-Activated Nanoplatform for Triple Synergistic Therapy of Colorectal Cancer. Nano Lett. 2022;22:6156-6165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 24. | Li Y, Su Y, Pan H, Deng W, Wang J, Liu D, Pan W. Nanodiamond-based multifunctional platform for oral chemo-photothermal combinational therapy of orthotopic colon cancer. Pharmacol Res. 2022;176:106080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Park HH, Lee S, Oh JM, Lee MS, Yoon KH, Park BH, Kim JW, Song H, Kim SH. Anti-inflammatory activity of fisetin in human mast cells (HMC-1). Pharmacol Res. 2007;55:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Alonso S, Saltz L. The Landmark Series: Chemotherapy for Non-Metastatic Colon Cancer. Ann Surg Oncol. 2021;28:995-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Wang H, Li M, Rinehart JJ, Zhang R. Pretreatment with dexamethasone increases antitumor activity of carboplatin and gemcitabine in mice bearing human cancer xenografts: in vivo activity, pharmacokinetics, and clinical implications for cancer chemotherapy. Clin Cancer Res. 2004;10:1633-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Ma S, Song W, Xu Y, Si X, Zhang D, Lv S, Yang C, Ma L, Tang Z, Chen X. Neutralizing tumor-promoting inflammation with polypeptide-dexamethasone conjugate for microenvironment modulation and colorectal cancer therapy. Biomaterials. 2020;232:119676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 29. | Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4043] [Cited by in RCA: 4140] [Article Influence: 180.0] [Reference Citation Analysis (0)] |
| 30. | Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 966] [Cited by in RCA: 1127] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 31. | Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey DJ, Choi W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820-5828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 711] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 32. | Juang V, Chang CH, Wang CS, Wang HE, Lo YL. pH-Responsive PEG-Shedding and Targeting Peptide-Modified Nanoparticles for Dual-Delivery of Irinotecan and microRNA to Enhance Tumor-Specific Therapy. Small. 2019;15:e1903296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 33. | Sivak L, Subr V, Tomala J, Rihova B, Strohalm J, Etrych T, Kovar M. Overcoming multidrug resistance via simultaneous delivery of cytostatic drug and P-glycoprotein inhibitor to cancer cells by HPMA copolymer conjugate. Biomaterials. 2017;115:65-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Shi Y, Wang X, Meng Y, Ma J, Zhang Q, Shao G, Wang L, Cheng X, Hong X, Wang Y, Yan Z, Cao Y, Kang J, Fu C. A Novel Mechanism of Endoplasmic Reticulum Stress- and c-Myc-Degradation-Mediated Therapeutic Benefits of Antineurokinin-1 Receptor Drugs in Colorectal Cancer. Adv Sci (Weinh). 2021;8:e2101936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Chen S, Su T, Zhang Y, Lee A, He J, Ge Q, Wang L, Si J, Zhuo W. Fusobacterium nucleatum promotes colorectal cancer metastasis by modulating KRT7-AS/KRT7. Gut Microbes. 2020;11:511-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 36. | Komiya Y, Shimomura Y, Higurashi T, Sugi Y, Arimoto J, Umezawa S, Uchiyama S, Matsumoto M, Nakajima A. Patients with colorectal cancer have identical strains of Fusobacterium nucleatum in their colorectal cancer and oral cavity. Gut. 2019;68:1335-1337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 205] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 37. | Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Bérard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1834] [Cited by in RCA: 2526] [Article Influence: 252.6] [Reference Citation Analysis (0)] |
| 38. | Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N, Chen Y, Chen H, Hong J, Zou W, Fang JY. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell. 2017;170:548-563.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 1455] [Article Influence: 181.9] [Reference Citation Analysis (0)] |
| 39. | Yan X, Ma F, Chen Q, Gou X, Li X, Zhang L, Gao H. Construction of size-transformable supramolecular nano-platform against drug-resistant colorectal cancer caused by Fusobacterium nucleatum. Chem Eng J. 2022;450:137605. [DOI] [Full Text] |
| 40. | Chen ZX, Li JL, Pan P, Bao P, Zeng X, Zhang XZ. Combination gut microbiota modulation and chemotherapy for orthotopic colorectal cancer therapy. Nano Today. 2021;41:101329. [DOI] [Full Text] |
| 41. | Yan J, Wang C, Jiang X, Wei Y, Wang Q, Cui K, Xu X, Wang F, Zhang L. Application of phototherapeutic-based nanoparticles in colorectal cancer. Int J Biol Sci. 2021;17:1361-1381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 42. | He J, Wei Q, Wang S, Hua S, Zhou M. Bioinspired protein corona strategy enhanced biocompatibility of Ag-Hybrid hollow Au nanoshells for surface-enhanced Raman scattering imaging and on-demand activation tumor-phototherapy. Biomaterials. 2021;271:120734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 43. | Lee DY, Kim JY, Lee Y, Lee S, Miao W, Kim HS, Min JJ, Jon S. Black Pigment Gallstone Inspired Platinum-Chelated Bilirubin Nanoparticles for Combined Photoacoustic Imaging and Photothermal Therapy of Cancers. Angew Chem Int Ed Engl. 2017;56:13684-13688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 44. | Wang W, Wang L, Li Y, Liu S, Xie Z, Jing X. Nanoscale Polymer Metal-Organic Framework Hybrids for Effective Photothermal Therapy of Colon Cancers. Adv Mater. 2016;28:9320-9325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 45. | Ren X, Yang S, Yu N, Sharjeel A, Jiang Q, Macharia DK, Yan H, Lu C, Geng P, Chen Z. Cell membrane camouflaged bismuth nanoparticles for targeted photothermal therapy of homotypic tumors. J Colloid Interface Sci. 2021;591:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 46. | Shih YH, Luo TY, Chiang PF, Yao CJ, Lin WJ, Peng CL, Shieh MJ. EGFR-targeted micelles containing near-infrared dye for enhanced photothermal therapy in colorectal cancer. J Control Release. 2017;258:196-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 47. | An L, Wang X, Rui X, Lin J, Yang H, Tian Q, Tao C, Yang S. The In Situ Sulfidation of Cu(2) O by Endogenous H(2) S for Colon Cancer Theranostics. Angew Chem Int Ed Engl. 2018;57:15782-15786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 48. | Tao C, An L, Lin J, Tian Q, Yang S. Surface Plasmon Resonance-Enhanced Photoacoustic Imaging and Photothermal Therapy of Endogenous H(2) S-Triggered Au@Cu(2) O. Small. 2019;15:e1903473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 49. | Li Y, Chen W, Qi Y, Wang S, Li L, Li W, Xie T, Zhu H, Tang Z, Zhou M. H(2) S-Scavenged and Activated Iron Oxide-Hydroxide Nanospindles for MRI-Guided Photothermal Therapy and Ferroptosis in Colon Cancer. Small. 2020;16:e2001356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 50. | Feng J, Ren WX, Kong F, Dong YB. A covalent organic framework-based nanoagent for H(2)S-activable phototherapy against colon cancer. Chem Commun (Camb). 2021;57:7240-7243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 51. | Zhu Y, Chen C, Wu Q, Yang G, Liu Z, Hao E, Cao H, Gao Y, Zhang W. Single-wavelength phototheranostics for colon cancer via the thiolytic reaction. Nanoscale. 2020;12:12165-12171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Gunduz H, Bilici K, Cetin S, Muti A, Sennaroglu A, Yagci Acar H, Kolemen S. Dual laser activatable brominated hemicyanine as a highly efficient and photostable multimodal phototherapy agent. J Photochem Photobiol B. 2021;217:112171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Xie Q, Li Z, Liu Y, Zhang D, Su M, Niitsu H, Lu Y, Coffey RJ, Bai M. Translocator protein-targeted photodynamic therapy for direct and abscopal immunogenic cell death in colorectal cancer. Acta Biomater. 2021;134:716-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 54. | She T, Shi Q, Li Z, Feng Y, Yang H, Tao Z, Li H, Chen J, Wang S, Liang Y, Cheng J, Lu X. Combination of long-acting TRAIL and tumor cell-targeted photodynamic therapy as a novel strategy to overcome chemotherapeutic multidrug resistance and TRAIL resistance of colorectal cancer. Theranostics. 2021;11:4281-4297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 55. | Gan S, Tong X, Zhang Y, Wu J, Hu Y, Yuan A. Covalent Organic Framework‐Supported Molecularly Dispersed Near‐Infrared Dyes Boost Immunogenic Phototherapy against Tumors. Adv Funct Mater. 2019;29:1902757. [DOI] [Full Text] |
| 56. | Huang C, Zhang T, Li Y, Lyu M, Suo M, Xia L, Liu L, Tang B, Zhang Q. Type-I AIE photosensitizer triggered cascade catalysis system for tumor targeted therapy and postoperative recurrence suppression. Chem Eng J. 2022;446:136381. [DOI] [Full Text] |
| 57. | Chen Z, Liu L, Liang R, Luo Z, He H, Wu Z, Tian H, Zheng M, Ma Y, Cai L. Bioinspired Hybrid Protein Oxygen Nanocarrier Amplified Photodynamic Therapy for Eliciting Anti-tumor Immunity and Abscopal Effect. ACS Nano. 2018;12:8633-8645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 263] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 58. | Cheng Y, Cheng H, Jiang C, Qiu X, Wang K, Huan W, Yuan A, Wu J, Hu Y. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat Commun. 2015;6:8785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 698] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 59. | Zhu H, Li J, Qi X, Chen P, Pu K. Oxygenic Hybrid Semiconducting Nanoparticles for Enhanced Photodynamic Therapy. Nano Lett. 2018;18:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 241] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 60. | He H, Liu L, Liang R, Zhou H, Pan H, Zhang S, Cai L. Tumor-targeted nanoplatform for in situ oxygenation-boosted immunogenic phototherapy of colorectal cancer. Acta Biomater. 2020;104:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 61. | Wang C, Dong Z, Hao Y, Zhu Y, Ni J, Li Q, Liu B, Han Y, Yang Z, Wan J, Yang K, Liu Z, Feng L. Coordination Polymer-Coated CaCO(3) Reinforces Radiotherapy by Reprogramming the Immunosuppressive Metabolic Microenvironment. Adv Mater. 2022;34:e2106520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 62. | Pierini S, Mishra A, Perales-Linares R, Uribe-Herranz M, Beghi S, Giglio A, Pustylnikov S, Costabile F, Rafail S, Amici A, Facciponte JG, Koumenis C, Facciabene A. Combination of vasculature targeting, hypofractionated radiotherapy, and immune checkpoint inhibitor elicits potent antitumor immune response and blocks tumor progression. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 63. | Walker JM, Rolig AS, Charych DH, Hoch U, Kasiewicz MJ, Rose DC, McNamara MJ, Hilgart-Martiszus IF, Redmond WL. NKTR-214 immunotherapy synergizes with radiotherapy to stimulate systemic CD8(+) T cell responses capable of curing multi-focal cancer. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 64. | Chen J, Liang C, Song X, Yi X, Yang K, Feng L, Liu Z. Hybrid Protein Nano-Reactors Enable Simultaneous Increments of Tumor Oxygenation and Iodine-131 Delivery for Enhanced Radionuclide Therapy. Small. 2019;15:e1903628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 65. | Gong L, Zhang Y, Zhao J, Tu K, Jiao L, Xu Q, Zhang M, Han S. All-In-One Biomimetic Nanoplatform Based on Hollow Polydopamine Nanoparticles for Synergistically Enhanced Radiotherapy of Colon Cancer. Small. 2022;18:e2107656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 66. | Nag D, Bhanja P, Riha R, Sanchez-Guerrero G, Kimler BF, Tsue TT, Lominska C, Saha S. Auranofin Protects Intestine against Radiation Injury by Modulating p53/p21 Pathway and Radiosensitizes Human Colon Tumor. Clin Cancer Res. 2019;25:4791-4807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 67. | Delahunty I, Li J, Jiang W, Lee C, Yang X, Kumar A, Liu Z, Zhang W, Xie J. 7-Dehydrocholesterol Encapsulated Polymeric Nanoparticles As a Radiation-Responsive Sensitizer for Enhancing Radiation Therapy. Small. 2022;18:e2200710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Zhang J, Yang M, Fan X, Zhu M, Yin Y, Li H, Chen J, Qin S, Zhang H, Zhang K, Yu F. Biomimetic radiosensitizers unlock radiogenetics for local interstitial radiotherapy to activate systematic immune responses and resist tumor metastasis. J Nanobiotechnology. 2022;20:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 69. | Zhou Z, Zhang B, Wang H, Yuan A, Hu Y, Wu J. Two-stage oxygen delivery for enhanced radiotherapy by perfluorocarbon nanoparticles. Theranostics. 2018;8:4898-4911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 70. | Wang C, Xue F, Wang M, An L, Wu D, Tian Q. 2D Cu-Bipyridine MOF Nanosheet as an Agent for Colon Cancer Therapy: A Three-in-One Approach for Enhancing Chemodynamic Therapy. ACS Appl Mater Interfaces. 2022;14:38604-38616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 71. | Wang W, Jin Y, Xu Z, Liu X, Bajwa SZ, Khan WS, Yu H. Stimuli-activatable nanomedicines for chemodynamic therapy of cancer. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12:e1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 72. | Li SL, Jiang P, Jiang FL, Liu Y. Recent Advances in Nanomaterial-Based Nanoplatforms for Chemodynamic Cancer Therapy. Adv Funct Mater. 2021;31:2100243. [DOI] [Full Text] |
| 73. | Zhang L, Li CX, Wan SS, Zhang XZ. Nanocatalyst-Mediated Chemodynamic Tumor Therapy. Adv Healthc Mater. 2022;11:e2101971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 135] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 74. | Li S, Shang L, Xu B, Wang S, Gu K, Wu Q, Sun Y, Zhang Q, Yang H, Zhang F, Gu L, Zhang T, Liu H. A Nanozyme with Photo-Enhanced Dual Enzyme-Like Activities for Deep Pancreatic Cancer Therapy. Angew Chem Int Ed Engl. 2019;58:12624-12631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 235] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 75. | Zhai S, Hu X, Hu Y, Wu B, Xing D. Visible light-induced crosslinking and physiological stabilization of diselenide-rich nanoparticles for redox-responsive drug release and combination chemotherapy. Biomaterials. 2017;121:41-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 76. | Su M, Zhu Y, Chen J, Zhang B, Sun C, Chen M, Yang X. Microfluidic synthesis of manganese-alginate nanogels with self-supplying H2O2 capability for synergistic chemo/chemodynamic therapy and boosting anticancer immunity. Chem Eng J. 2022;435:134926. [DOI] [Full Text] |
| 77. | Chen X, Jia Z, Wen Y, Huang Y, Yuan X, Chen Y, Liu Y, Liu J. Bidirectional anisotropic palladium nanozymes reprogram macrophages to enhance collaborative chemodynamic therapy of colorectal cancer. Acta Biomater. 2022;151:537-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 78. | Lin LS, Song J, Song L, Ke K, Liu Y, Zhou Z, Shen Z, Li J, Yang Z, Tang W, Niu G, Yang HH, Chen X. Simultaneous Fenton-like Ion Delivery and Glutathione Depletion by MnO(2) -Based Nanoagent to Enhance Chemodynamic Therapy. Angew Chem Int Ed Engl. 2018;57:4902-4906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 952] [Article Influence: 136.0] [Reference Citation Analysis (0)] |
| 79. | Wang L, Xia J, Fan H, Hou M, Wang H, Wang X, Zhang K, Cao L, Liu X, Ling J, Yu H, Wu X, Sun J. A tumor microenvironment responsive nanosystem for chemodynamic/chemical synergistic theranostics of colorectal cancer. Theranostics. 2021;11:8909-8925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | He R, Hu B, Zhong H, Jin F, Fan J, Hu YH, Jing Z. Reduction of CO(2) with H(2)S in a simulated deep-sea hydrothermal vent system. Chem Commun (Camb). 2019;55:1056-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Wu YC, Wang XJ, Yu L, Chan FK, Cheng AS, Yu J, Sung JJ, Wu WK, Cho CH. Hydrogen sulfide lowers proliferation and induces protective autophagy in colon epithelial cells. PLoS One. 2012;7:e37572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 82. | Liu D, Liu M, Wan Y, Zhou X, Yang S, An L, Huang G, Tian Q. Remodeling endogenous H2S microenvironment in colon cancer to enhance chemodynamic therapy. Chem Eng J. 2021;422:130098. [DOI] [Full Text] |
| 83. | He Q. Precision gas therapy using intelligent nanomedicine. Biomater Sci. 2017;5:2226-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 84. | Jing YZ, Li SJ, Sun ZJ. Gas and gas-generating nanoplatforms in cancer therapy. J Mater Chem B. 2021;9:8541-8557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 85. | Liu R, Peng Y, Lu L, Peng S, Chen T, Zhan M. Near-infrared light-triggered nano-prodrug for cancer gas therapy. J Nanobiotechnology. 2021;19:443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 86. | Wu CG, Liang JL, Wang X, Zhou X, Cai X, Xu J, Wang M, Wang WB, Ma D, Xue W. Light-activated nitric-oxide overproduction theranostic nanoplatform based on long-circulating biomimetic nanoerythrocyte for enhanced cancer gas therapy. Sci China Chem. 2021;64:1796-1810. [RCA] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 87. | Kang J, Li Z, Organ CL, Park CM, Yang CT, Pacheco A, Wang D, Lefer DJ, Xian M. pH-Controlled Hydrogen Sulfide Release for Myocardial Ischemia-Reperfusion Injury. J Am Chem Soc. 2016;138:6336-6339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 88. | Zhou HF, Yan H, Hu Y, Springer LE, Yang X, Wickline SA, Pan D, Lanza GM, Pham CT. Fumagillin prodrug nanotherapy suppresses macrophage inflammatory response via endothelial nitric oxide. ACS Nano. 2014;8:7305-7317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 89. | He Q, Kiesewetter DO, Qu Y, Fu X, Fan J, Huang P, Liu Y, Zhu G, Qian Z, Chen X. NIR-Responsive On-Demand Release of CO from Metal Carbonyl-Caged Graphene Oxide Nanomedicine. Adv Mater. 2015;27:6741-6746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 90. | Liu S, Li W, Zhang Y, Zhou J, Du Y, Dong S, Tian B, Fang L, Ding H, Gai S, Yang P. Tailoring Silica-Based Nanoscintillators for Peroxynitrite-Potentiated Nitrosative Stress in Postoperative Radiotherapy of Colon Cancer. Nano Lett. 2022;22:6409-6417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 91. | Chattopadhyay M, Kodela R, Olson KR, Kashfi K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid is a potent inhibitor of colon cancer cell growth in vitro and in a xenograft mouse model. Biochem Biophys Res Commun. 2012;419:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 92. | Zhang Z, Zhang L, Huang C, Guo Q, Zuo Y, Wang N, Jin X, Zhu D. Gas-generating mesoporous silica nanoparticles with rapid localized drug release for enhanced chemophotothermal tumor therapy. Biomater Sci. 2020;8:6754-6763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Fan T, Ye W, Zhao P, Zhou W, Chen Y, He C, Zhang X, Yan R, Chen C, Luo J, Yang T, Ma X, Xiang G, Lu Y. pH-responsive core-shell nanogels induce in situ antigen production for cancer treatment. Chem Eng J. 2021;426:130839. [DOI] [Full Text] |
| 94. | Hu X, Hou B, Xu Z, Saeed M, Sun F, Gao Z, Lai Y, Zhu T, Zhang F, Zhang W, Yu H. Supramolecular Prodrug Nanovectors for Active Tumor Targeting and Combination Immunotherapy of Colorectal Cancer. Adv Sci (Weinh). 2020;7:1903332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 95. | Ding D, Zhong H, Liang R, Lan T, Zhu X, Huang S, Wang Y, Shao J, Shuai X, Wei B. Multifunctional Nanodrug Mediates Synergistic Photodynamic Therapy and MDSCs-Targeting Immunotherapy of Colon Cancer. Adv Sci (Weinh). 2021;8:e2100712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 96. | Yu Y, Li J, Song B, Ma Z, Zhang Y, Sun H, Wei X, Bai Y, Lu X, Zhang P, Zhang X. Polymeric PD-L1 blockade nanoparticles for cancer photothermal-immunotherapy. Biomaterials. 2022;280:121312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 97. | Ding B, Shao S, Yu C, Teng B, Wang M, Cheng Z, Wong KL, Ma P, Lin J. Large-Pore Mesoporous-Silica-Coated Upconversion Nanoparticles as Multifunctional Immunoadjuvants with Ultrahigh Photosensitizer and Antigen Loading Efficiency for Improved Cancer Photodynamic Immunotherapy. Adv Mater. 2018;30:e1802479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 98. | Jing Z, Wang S, Xu K, Tang Q, Li W, Zheng W, Shi H, Su K, Liu Y, Hong Z. A Potent Micron Neoantigen Tumor Vaccine GP-Neoantigen Induces Robust Antitumor Activity in Multiple Tumor Models. Adv Sci (Weinh). 2022;9:e2201496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 99. | Chen Q, Liu C, Zhong D, Hua S, He J, Wang K, Zhou M. Wrapping Porphyromonas gingivalis for tumor microenvironment immunomodulation and photothermal immunotherapy. Nano Today. 2021;41:101311. [DOI] [Full Text] |