Published online Jan 28, 2023. doi: 10.3748/wjg.v29.i4.616
Peer-review started: October 2, 2022
First decision: October 29, 2022
Revised: November 3, 2022
Accepted: December 30, 2022
Article in press: December 30, 2022
Published online: January 28, 2023
Processing time: 110 Days and 1.8 Hours
It was clearly realized more than 50 years ago that iron deposition in the liver may be a critical factor in the development and progression of liver disease. The recent clarification of ferroptosis as a specific form of regulated hepatocyte death different from apoptosis and the description of ferritinophagy as a specific variation of autophagy prompted detailed investigations on the association of iron and the liver. In this review, we will present a brief discussion of iron absorption and handling by the liver with emphasis on the role of liver macrophages and the significance of the iron regulators hepcidin, transferrin, and ferritin in iron homeostasis. The regulation of ferroptosis by endogenous and exogenous mod-ulators will be examined. Furthermore, the involvement of iron and ferroptosis in various liver diseases including alcoholic and non-alcoholic liver disease, chronic hepatitis B and C, liver fibrosis, and hepatocellular carcinoma (HCC) will be analyzed. Finally, experimental and clinical results following interventions to reduce iron deposition and the promising manipulation of ferroptosis will be presented. Most liver diseases will be benefited by ferroptosis inhibition using exogenous inhibitors with the notable exception of HCC, where induction of ferroptosis is the desired effect. Current evidence mostly stems from in vitro and in vivo experimental studies and the need for well-designed future clinical trials is warranted.
Core Tip: Iron overload may damage the liver in a variety of liver diseases such as cirrhosis and hepatocellular carcinoma affecting patient survival. In this review, we present the evidence, both experimental and clinical, of the detrimental effects of iron deposition in hepatocytes and other liver sinusoidal cells. Moreover, we examine the mechanism and implications of the recently described ferroptosis in the evolution of liver disease. Ferroptosis is a form of regulated hepatocyte death caused by excess iron and lipid peroxidation. Inhibition or induction of ferroptosis may profoundly improve the course of many liver diseases as demonstrated by a large number of experimental studies as well as a small number of clinical trials.
- Citation: Kouroumalis E, Tsomidis I, Voumvouraki A. Iron as a therapeutic target in chronic liver disease. World J Gastroenterol 2023; 29(4): 616-655
- URL: https://www.wjgnet.com/1007-9327/full/v29/i4/616.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i4.616
The major suppliers of plasma iron are duodenal enterocytes and iron-recycling macrophages[1-3]. Duodenal cytochrome B reductase reduces inorganic trivalent iron reaching the duodenum to form divalent iron (Fe2+), and surface divalent metal transporter 1 (DMT1) imports Fe2+ into the cytoplasm. The gene SLC11A2 encoding DMT1 is activated in cases of iron deficiency or hypoxia as it interacts with the hypoxia-inducible factors (HIF1α and HIF2α) overexpressed in these situations[4-7]. The cytoplasmic iron sensor iron-responsive element (IRE) and iron regulatory proteins (IRP1 and IRP2) also participate in iron absorption control as they stabilize the SLC11A2 transcript in iron deficiency or dissociate and degrade in iron overload[8]. Then, the cytoplasmic divalent iron is bound to ferroportin, the only known iron exporter protein, and exported to the portal vein blood. Transportation is mediated by the chaperone protein poly (rC)-binding protein 2 encoded by the SLC40A gene[9]. The main regulator of ferroportin is hepcidin[10], but the IRP/IRE proteins and microRNAs are also involved[11]. Once in the portal vein, the divalent iron is oxidized back to trivalent by the ferroxidases hephestin and ceruloplasmin and then carried in different cells bound to transferrin. Peripheral cells import iron by the internalization of transferrin after it binds to its receptor TFR1[12] and is sorted into endosomes where iron is removed in the acidic environment, reduced again to Fe2+ by the ferrireductase STEAP3, and released into the cytosol by DMT1[1,3]. Iron is then either exported by ferroportin or stored in ferritin or in the labile iron pool (LIP). On the other hand, heme oxygenases (Hos) localized mainly in iron-recycling macrophages of liver and spleen, degrade heme to recover Fe2+[2,13].
The regulation of hepcidin is critical in iron metabolism as binding of hepcidin to ferroportin in hepatocytes, macrophages, or enterocytes leads to internalization and degradation of ferroportin, thus limiting iron export to the blood[2,3,10]. A decrease in hepcidin when iron is needed leads to enhancement of ferroportin expression and increased iron absorption from the duodenum. In iron overload, ferroportin is downregulated and iron absorption is decreased[14]. In addition to iron deficiency, inflammatory molecules like interleukin (IL)-6 also upregulate hepcidin expression[15]. The HAMP gene encodes hepcidin, and its promoter is activated by the complex of bone morphogenic proteins (BMP2, BMP4, BMP6) and their receptor. This complex phosphorylates the SMAD pathway, which in turn activates HAMP expression[16,17]. Hemojuvelin (HJV) is a necessary co-factor for BMP-BMP receptor complex function[18]. BMP6 is mainly expressed in liver sinusoidal cells and induces hepcidin upregulation via paracrine signaling during iron overload[19-21].
The second receptor of transferrin (TFR2), a low-affinity receptor found in hepatocytes and erythroid precursors, is also an important inducer of hepcidin through the BMP/SMAD pathway[22-24] after forming a complex with HFE (the protein involved in hereditary hemochromatosis)[25]. Anomalies of either of the genes encoding these proteins will lead to hepcidin downregulation[26-28].
In contrast to iron overload, hypoxia, anemia, and erythropoiesis reduce hepcidin expression[29,30]. The main inhibitor of hepcidin expression is erythroferrone (ERFE)[31], which is produced by erythroid cells in response to erythropoietic stimuli. ERFE downregulates hepcidin, interfering with the BMP/SMAD pathway in hepatocytes[32-34]. Three other hepcidin inhibitors have been described. PIEZO1 and the immunophilin FKBP reduce HAMP expression by inhibiting the BMP/SMAD pathway[35,36]. The third hepcidin inhibitor is the ferritinophagy axis operating in both the enterocyte and the macrophage. Ferritinophagy is a specialized form of autophagy resulting in the lysosomal breakdown of ferritin and subsequent iron release to increase the LIP. It is controlled by the nuclear receptor coactivator 4 (NCOA4)[37,38] during transport of absorbed iron to ferritin. In increased iron demand, NCOA4 functions as a cargo receptor for lysosomal degradation of ferritin. Excess iron leads to lipid peroxidation-mediated ferroptosis[38]. NCOA4 is similarly involved in macrophage ferritinophagy and iron release for erythropoiesis[39].
Iron ions are dangerous to cells. In iron overload, redox-active iron increases and oxidative stress is induced through the formation of reactive oxygen species (ROS). Non-transferrin bound iron is mainly responsible for the redox-active iron when the capacity of iron binding proteins is not able to accommodate for the increased iron load. An additional dangerous form is the transit iron pool, which comprises iron that is not bound to ferritin or other chelating proteins. This iron may also induce the formation of ROS[40]. Iron is a double-edged sword[41], which even under normal conditions may cause pathological damage. Iron induces hydroxyl radical production through the Fenton reaction[42]. The Fenton-Haber-Weiss reaction is caused by the free donation and acceptance of electrons during the transition between Fe2+ and Fe3+ states. Iron-catalyzed generation of hydroxide ions and hydroperoxyl and hydroxyl radicals is the result of this exchange. Under normal conditions, free-radicals are quenched by cellular antioxidant mechanisms[43]. However, when overproduced, these free radicals promote the formation of other ROS such as thiyl and peroxyl radicals and a vicious circle is initiated leading to oxidation of lipids, proteins and nucleic acids[44]. Thus, in iron-loaded animals the products of lipid peroxidation such as malondialdehyde (MDA), isoprostanes, and 4-hydroxynonenal (4-HNE) can be detected in the liver[45]. MDA and 4-HNE form mutagenic adducts, reacting with amino groups and DNA bases[46,47] that target the p53 tumor suppressor gene initiating apoptotic resistance to the cells[48]. Levels of 4-HNE correlate well with hepatic iron levels[49]. Iron metabolism was recently reviewed in detail[50-53].
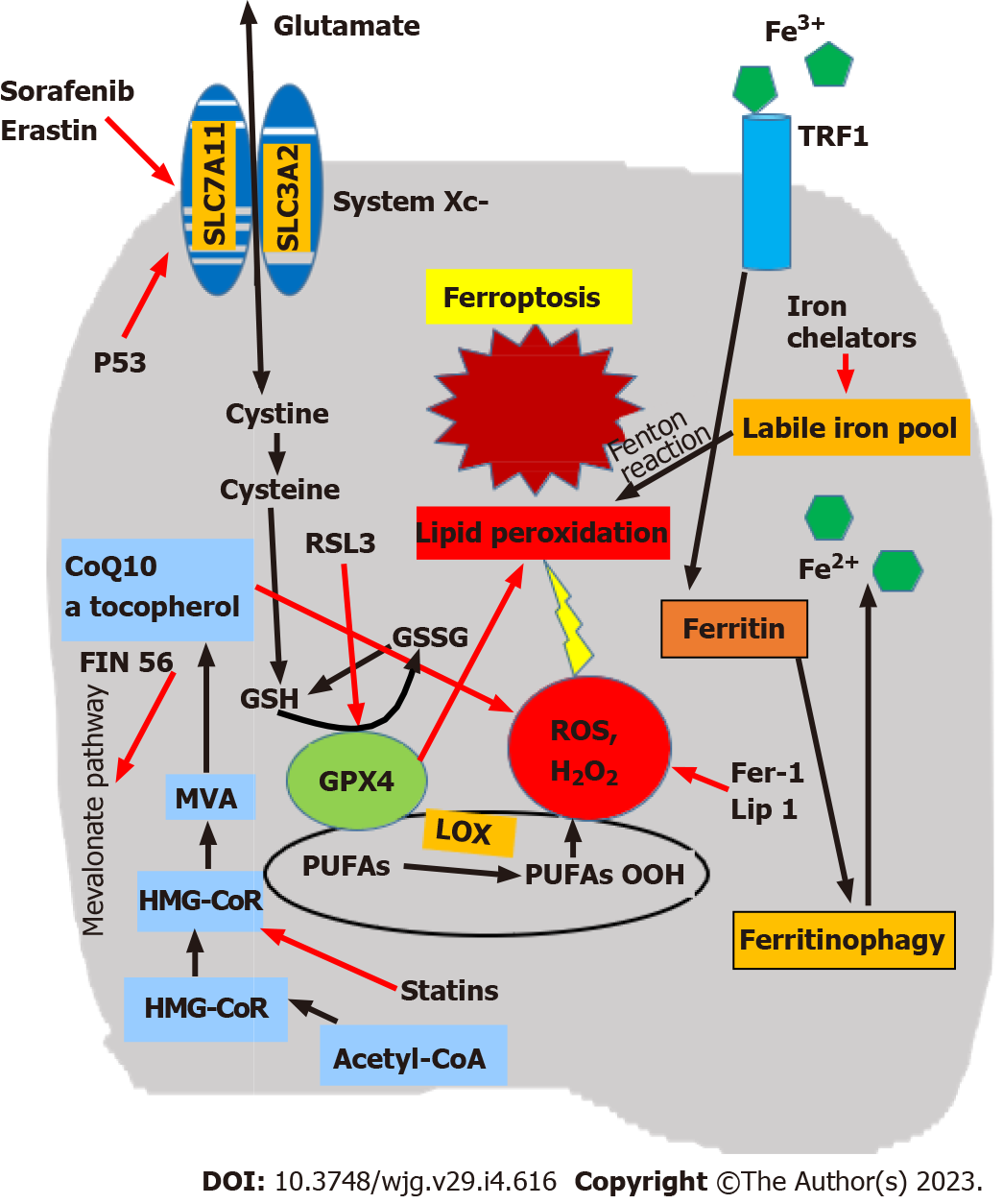
The most important mechanism of iron-induced liver damage is the recently described ferroptosis, a name derived from the Greek word “ptosis,” meaning a fall, and the Latin “ferrum” or iron[54]. It is an iron-dependent regulated cell death characterized by iron accumulation, lipid peroxidation, and the production of ROS that depends on the activity of NADPH oxidases[55,56]. The mitochondrial respiratory chain initiates lipid peroxidation by lipoxygenase (LOX) or cytochrome P450 reductase. The enzyme glutathione peroxidase 4 (GPX4), the antioxidant glutathione (GSH), the coenzyme Q10 (CoQ10), and the tetrahydrobiopterin (BH4) system are the defense mechanisms of the cell. They are further regulated by the nuclear factor erythroid 2-related factor (Nrf2)[57-59]. The process is controlled by multiple genes associated with iron uptake[60,61], lipotoxicity[62,63], and antioxidation responses[64,65].
Ferroptosis is regulated by several metabolic events such as lipogenesis and ferritinophagy. The mitochondrial tricarboxylic acid cycle fueled by glutaminolysis may promote ferroptosis induction. Phospholipid peroxidation is the critical event in ferroptosis. Production of ROS, iron, and phospholipids containing polyunsaturated fatty acids (PUFA-PLs) are the necessary requirements. The executioner of ferroptosis is phospholipid hydroperoxide (PLOOH) synthesized from its precursor, PUFA[66].
Both non-enzymatic/exogenous and enzymatic/endogenous pathways are implicated in lipid peroxidation. For the latter, LOXs and/or cytochrome P450 oxidoreductase mediate the induction of lipid peroxidation by the dioxygenation of lipids. Exogenous transporter mediated signaling pathways include the E cadherin-NF2-Hippo-YAP pathway, the glucose-regulated AMPK signaling pathway, and the p53 tumor suppressor pathway[67].
Mechanisms inhibiting ferroptosis are provided by three main biological pathways (Figure 1)[68,69]. The first is the GSH/GPX4 pathway, implicating the system Xc-, which is a membrane cystine/ glutamate exchanger that imports cystine and exports glutamate. A critical mediator in this system is the cystine/glutamate antiporter SLC7A11, and GPX4 is the major protective system against lipid peroxidation[70]. In addition, ferroptosis suppressor protein 1 acts mainly on the plasma membrane, and dihydroorotate dehydrogenase is an important defense molecule in mitochondria[71-75]. The second comprises iron metabolism pathways, particularly the p62-Kelch-like ECH-associated protein 1 (Keap1)-Nrf2 regulatory pathway[54]. Inhibition of ferritinophagy increases mitochondrial ferritin and protects from ferroptosis as evidenced in hypoxic macrophages. This is regulated by a hypoxia-induced decrease of NCOA4 transcription, in combination with a microRNA 6862-5p-dependent degradation of NCOA4 mRNA[76]. Nrf2 is a transcription factor that protects cells against oxidative and toxic damage and plays a significant role in regulating ferroptosis[77-79]. In hepatocellular carcinoma (HCC) and other tumors, activation of the p62-Keap1-Nrf2 pathway leads to reduced Nrf2 degradation, the protection of tumor cells against ferroptosis, and resistance to anticancer drugs[80]. The third includes lipid metabolism pathways implicating p53 and various enzymes[54,66]. p53 is a tumor suppressor transcription factor that may prevent cancer by controlling the cell cycle, cellular senescence, and apoptosis. Ferroptosis is one of its antitumor mechanisms; p53 increases cell sensitivity to ferroptosis through repression of SLC7A11. The ferroptosis inhibitor fer-1 reverses this effect and induces SLC7A11 overexpression[62,81-83]. Additional biological factors inhibiting ferroptosis were also recently identified: (1) GTP Cyclohydrolase-1 (the rate-limiting enzyme for biosynthesis of tetrahydrobiopterin (BH4), which counteracts ferroptosis[84]); (2) Transferrin and its cell surface TFR1 receptor[12]; and (3) CDGSH (iron sulfur domain 1, which negatively regulates ferroptosis protecting against lipid peroxidation in mitochondria)[85].
Exogenous ferroptosis modulators discussed here are summarized in Table 1. Ferroptosis inhibitors are divided into two major groups: (1) Class I inhibitors, such as Deferoxamine (DFO) mesylate[86], which suppresses iron accumulation; and (2) Class II inhibitors, including ferrostatin-1, liproxstatin-1 and vitamin E, which react with chain free radicals and can inhibit lipid peroxidation[87-91]. The activity of drugs in the first generation of ferrostatin class specifically reduce the accumulation of lipid ROS. The second generation (SRS 11-92) and the third generation (SRS 16-86) ferrostatin drugs function through conferring increased metabolic stability[90].
| Inducers | Mechanisms | Compounds |
| Class 1 | Inhibition of system Xc- | Erastin, sorafenib, sulfasalazin |
| Prevention of cystine import | Glutamate | |
| Class 2 | Inhibition of GPX4 | RLS3, DPIs (DPI7, DPI10) |
| Class 3 | Degradation of GPX4 | FIN56 |
| Depletion of CoQ10 | ||
| Class 4 | Initiation of lipid peroxidation | FINO2, PUFAs |
| Indirect reduction of GPX4 activity | ||
| Inhibitors | ||
| Class 1 | Suppression of iron accumulation | Deferoxamine |
| Class 2 | Inhibition of lipid peroxidation | Ferrostatin-1, liproxstatin-1, vitamin E |
| Unclassified | Dynasore, probucol, selenium, nitroxide XJB-5-131 | |
| Bicyclol, rosiglitazone |
The recently described inhibitor dynasore has characteristics of both classes, and prevents both iron accumulation and lipid peroxidation[92]. Other inhibitors of ferroptosis have been identified. For example, the cholesterol-reducing drug probucol was found to suppress ferroptosis[93]. The RIPK1 inhibitor necrostatin-1, which suppresses necroptosis, also has the additional effect of suppressing ferroptosis. Selenium administration has also been seen to suppress ferroptosis during stroke[94], and the nitroxide XJB-5-131 targets mitochondria and suppresses both apoptosis and ferroptosis[95]. However, it should be noted that these inhibitors have not been tested in the liver.
Interestingly, a recent experimental finding showed that the mechanism of action of bicyclol, a common hepatoprotectant in China, is via the prevention of ferroptosis. Furthermore, bicyclol attenuates cellular damage and lipid peroxidation induced by erastin. Additionally, Nrf2 inhibition and the subsequent reduction of GPX4 levels impedes the effects of bicyclol[96]. Finally, the anti-diabetic drug rosiglitazone inhibits ferroptosis and reduces hepatocyte death, acting as an ACSL4 inhibitor[97,98].
Class I inducers such as erastin, sorafenib, sulfasalazine and glutamate, deplete cellular cysteine by inhibiting system Xc- and the biosynthesis of GSH, resulting in the loss of GPX4 activity[81,99-102]. The low water solubility and metabolic instability of erastin has limited its clinical application[103], but a metabolically stable erastin derivative has been tested[104].
Class II inducers, including RSL3 and DPI compounds, act by directly inhibiting GPX4[88,105-107], leading to the accumulation of lipid peroxides and eventual cell death. BSO and cisplatin also deplete GSH inducing ferroptosis. Cisplatin and erastin have a significant synergistic effect[108]. Interestingly, erastin promotes ferritinophagy and increased the free iron, lipid peroxidation, while RSL3 does not interfere with ferritinophagy, suggesting that RSL3 induction of ferroptosis is not dependent on ferritin degradation[109].
Class III inducers such as FIN56 act by both direct degradation of GPX4 and indirect inactivation of GPX4 via the squalene synthase-mevalonate pathway of the mitochondrial electron transport chain[103,110]. FIN56 also acts by depleting GPX4 and CoQ10. It seems that the cellular lethality of FIN56 is increased when cells are co-treated with statins and FIN56[110]. In addition, statins, such as simvastatin, enhance ferroptosis by inhibiting HMG-CoA reductase[110].
In class IV inducers, ferroptosis is induced by excess iron, omega-3 PUFAs, or peroxides, such as FINO2, that initiate lipid peroxidation and indirectly reduce GPX4 activity[111,112]. FINO2 is the only class IV ferroptosis inducer tested so far, but several other have been synthesized[103]. PUFAs show anticancer activity[113], but shortcomings such as reduced bioavailability, limited resistance to oxidative degradation, and lack of uptake specificity impede their use. However, the application of nanotechnology improves their therapeutic use[114]. Low density lipoproteins (LDLs) are taken up by LDL receptor expressed in tumor cells. LDL-based nanoparticles with docosahexaenoic acid (LDL-DHA NPs) were found to maintain their stability and specificity[115,116].
Experimental evidence suggests that there are additional biological inducers of ferroptosis, but their significance in human disease is still unknown. As mentioned above, ferritinophagy is a special recycling process of autophagy for the autophagic degradation of ferritin in lysosomes. It is mediated by the autophagic cargo receptor NCOA4, and leads to the initiation of ferroptosis[117]. Augmented ferritinophagy mediated by an increase of NCOA4 leads to induction of ferroptosis[64] (Table 1).
Reduction of iron-response element binding protein 2 significantly reduces erastin induced ferroptosis[55]. Increased activity of HO-1, the enzyme responsible for degradation of heme into ferrous iron, carbon monoxide, and biliverdin, increases LIP and initiated ferroptosis[118,119]. Artesunate (a derivative of artemisinin) is used in severe malaria[120] and induces hematopoietic stem cell (HSC) ferroptosis. However, the malaria drug chloroquine (a ferritinophagy inhibitor) reverses this effect, implying that artesunate induces HSC ferroptosis by activating ferritinophagy[121].
Finally, magnesium isoglycyrrhizinate (MgIG) is a natural product with anticancer activity[122] that has been shown to promote HSC ferroptosis. Inhibition of HO-1 reduces MgIG-induced HSC ferroptosis, suggesting that the promotion of HSC ferroptosis is mediated through upregulation of this enzyme[123].
Kupffer cells and other liver and spleen macrophages take up heme from damaged or senescent erythrocytes and either export the extracted Fe2+ using ferroportin or store it in ferritin in the cytoplasm[124]. It has been shown that intracellular iron regulates the differentiation of macrophages into M1 (pro-inflammatory) and M2 (anti-inflammatory) subtypes[125,126]. M1 macrophages have an iron storage capability with higher HAMP but lower FPN and IRP1/2 compared to M2 subtype[127]. M1 polarization is regulated by iron overload[128], but also by ROS production and p53 acetylation induced by iron overload[129]. Recently, experiments with cultured macrophages demonstrated that chronic iron overload may in fact downregulate M1 markers and show signs of M2 differentiation[130].
During infection, hepcidin blocks macrophage differentiation to reduce iron export that could increase the growth of pathogens[131], which is reversed in the case of intracellular pathogens. This is possibly achieved by an increased production of nitric oxide[132] and the expression of the pha-golysosomal protein NRAMP1 both leading to induction of ferroportin and intracellular iron reduction[133].
Kupffer cells exhibit phagocytic dysfunction and impair iron homeostasis during the development of non-alcoholic fatty liver disease (NAFLD)[134-136]. In addition, they participate in the clearance of lipids in nonalcoholic steatohepatitis (NASH) through M1 differentiation with the help of invariant natural killer T cells[137-139]. This composite role indicates that Kupffer cells can influence the development of ferroptosis, providing a new target for therapy in NAFLD.
Moreover, acute iron deprivation led to changes in metabolic and immunoregulatory genes in human macrophages resulting in impaired cell proliferation and reduced inflammation[140]. This is in contrast to the pro-inflammatory production of leukotrienes by the enzyme 5-LOX mediated by ferric iron in human macrophages[141]. As expected, ferroptosis has been the subject of several detailed reviews[69,142-145], which include descriptions of ferroptosis regulators[146,147], ferroptosis in viral disease[148], and the role of macrophages in ferroptosis[149].
Patients with chronic liver disease may exhibit hepatic and splenic iron loading, usually inside Kupffer cells and splenic and bone marrow macrophages[150]. Sometimes, this is accompanied by low hemoglobin levels and other hemolysis indices, indicating that hemolysis may have a role in the development of secondary iron overload[151]. However, a recent review emphasized the role of low levels of hepcidin in various liver diseases as implicated in both iron deposition in hepatocytes and participation in stellate cell activation and liver fibrosis[152].
Excess free iron exerts a toxic effect on the liver, favoring the progression of liver disease[58,153], and indeed abnormalities of iron regulation are reported in various liver diseases apart from inherited hemochromatosis[154]. Hyperferritinemia has been the main manifestation of disturbed iron homeostasis in chronic liver disease[155,156].
Opposing views have also been expressed in the literature. Data from cell culture experiments and animal models suggest that iron overload is only a weak fibrosis inducer and rarely causes serious liver damage not supporting the concept that iron overload is an important cause of liver toxicity. Iron may co-exist with other causes of inflammation, and the resulting hepatocyte necrosis is the real driving force leading to fibrosis[157].
The role of iron overload and the significance of ferroptosis have been investigated in the context of several liver diseases. The most common liver diseases will be discussed as well as the common end points of all, namely cirrhosis sometimes followed by the development of HCC. The rather limited available information on other liver diseases will be presented.
The role of iron in liver damage has been extensively researched in the case of NAFLD. A new term was introduced, the dysmetabolic or insulin-resistance hepatic iron overload syndrome (DIOS or IR-HIO), which is characterized by high serum ferritin levels, unexplained iron overload, and is associated with metabolic abnormalities[158-161]. IR-HIO is detected in one third to half of patients with NAFLD[155,158,162,163]. The reason for the observed iron overload in NAFLD is still uncertain. A proposed mechanism is the redistribution of transferrin receptors (TfRs) to the cell surface, a process induced by insulin[158,163,164]. Tfr1 is upregulated in mice on a high fat diet, which may enhance hepatocellular iron uptake in NAFLD despite already increased hepatocellular iron[165]. The increase in serum ferritin may be due to increased iron stores, oxidative stress caused by lipid abnormalities, systemic inflammation, and genetics[166,167]. The implication of the presence of the Cys282Tyr HFE gene variant of hereditary hemochromatosis was also examined. A heterozygous mutation is associated with bridging fibrosis or cirrhosis in Caucasians[168-170]. By contrast, in knock out mouse models of hemochromatosis no progression to steatohepatitis or liver fibrosis was noted with a high-fat diet[171].
In addition, certain variants of ceruloplasmin are associated with increased liver iron stores and high ferritin in patients with NAFLD and advanced liver fibrosis[172,173]. Ceruloplasmin mutations have been associated with iron deposition in the liver of other chronic liver diseases as well[174]. Excess dietary iron causes hepatic oxidative stress, inflammation and hepatocellular ballooning injury leading to NASH[175,176]. Oxidative stress interferes with mitochondrial function, impairing fatty acid oxidation and producing different pro-inflammatory factors such as tumor necrosis factor (TNF)-α, IL-6, IL-8, MDA and nitric oxide[177-180], leading to NASH. Moreover, liver iron deposition increases cholesterol synthesis, lipid accumulation, and impairs cellular stress responses, which further exacerbate NAFLD[181-184].
The pattern of hepatic iron deposition is important in NAFLD patients, as iron deposition in macrophages is associated with more advanced disease[185]. An important observation was recently reported emphasizing the role of liver macrophages in the pathogenesis of NASH. A histological structure, the crown-like structure, has been described in NASH: Iron-rich Kupffer cells surround dead hepatocytes, take up debris, and induce inflammation and fibrosis. They have proinflammatory and profibrotic phenotypes, driving liver fibrosis[186]. Hepatic iron was significantly higher in patients with HCC associated with NASH and it was mostly localized in Kupffer cells[187]. Evidence suggests that iron may contribute to NAFLD pathogenesis and fuel the progression to NASH[178-180].
Red blood cell fragility and erythrophagocytosis may also explain iron deposition in NAFLD. It could be the result of insulin resistance and membrane lipid abnormalities[188]. Recently, aristolochic acid-associated drugs (atypical antipsychotic medications) were reported to induce NAFLD and link insulin resistance with iron metabolism dysregulation irrespective of drug-associated weight gain[189]. Regardless, whatever the etiology of the iron deposition in NAFLD and NASH, the clinical con-sequences are well documented.
Hyperferritinemia is also frequent in patients with NAFLD. Sometimes, it is the first laboratory abnormality leading to further clinical investigation[190]. In a large prospective population-based study from South Korea, serum ferritin was a strong early predictor of future development of steatosis, indicating that the ferritin association with NAFLD is not a simple consequence of the disease itself[191]. Patients with high ferritin have more severe steatosis[192,193], inflammation[194], advanced fibrosis[195], and increased mortality[196,197]. It has been suggested that serum ferritin could be used as a marker to identify NAFLD patients likely to have NASH and fibrosis[166]. However, a clear association between serum ferritin and fibrosis could not be verified in other studies that reported that ferritin could not accurately predict advanced fibrosis in NAFLD[198,199,200]. This discrepancy may be explained by the findings of a recent investigation in which hyperferritinemia was found in a quarter of NAFLD patients. In this study, stainable iron was present in hepatocytes, Kupffer cells, or more frequently, in both. Importantly, serum ferritin was not related to the presence of NASH, but it increased with worsening of fibrosis and decreased in the cirrhotic stage of the disease[201].
Iron measurement by magnetic resonance imaging (MRI) demonstrates that liver iron is the most important determinant of serum ferritin in NAFLD[202]. An important association of serum ferritin with the gut microbiome was also recently reported. In this study, ferritin levels were associated with differences in gut microbial composition. Both negative and positive associations with particular microbial species were found, and ferritin-related bacterial species correlated with hepatic iron-related genes. Moreover, the iron-associated microbiome was also linked to liver fat load. Fecal transplantation from high-ferritin mice to normal mice confirmed the human results and demonstrated an interplay among iron load, liver fat, and gut microbiome that could be exploited in future treatments[203].
As in other liver diseases, extensive research has been conducted on the possible role of hepcidin in NAFLD. Investigations have tried to identify if the reported hepcidin abnormalities were the cause or the result of the iron overload observed in many cases of NAFLD. In various studies, hepcidin has been demonstrated to be either increased or decreased in NAFLD. In obese individuals, adipose tissue expression of hepcidin was upregulated, irrespective of steatosis and NASH. The contribution of adipose tissue hepcidin to the serum hepcidin is not well studied, but it may potentially explain the increased serum hepcidin in NAFLD[182,204-207].
Furthermore, leptin was found to correlate with hepcidin levels in obese children. Leptin also upregulates hepcidin expression in hepatocyte cultures, indicating that an increase in hepcidin may correlate to the leptin abnormalities in NAFLD[208,209]. Hepcidin downregulation, on the other hand, may be a consequence of oxidative stress secondary to iron overload[158-160,208,210]. Experimental evidence has demonstrated that hepcidin downregulation is a secondary phenomenon occurring after deposition of iron in the liver and the concomitant increase in oxidative stress[211]. Furthermore, an investigation on the relationship between iron stores and cardiovascular damage in patients with NAFLD showed that ferritin was associated with the components of the metabolic syndrome but not with liver inflammation and damage. In this study, hepcidin was increased due to the increased iron load[198], and fat in the liver of mice increased the expression of BMP-binding endothelial regulator, which was produced in sinusoidal endothelial cells and inhibited the BMP-SMAD pathway leading to a secondary inhibition of hepcidin. This is an additional explanation for the iron deposition in NAFLD[212].
Clinical data also indicate that hepcidin abnormalities are not the primary cause of the excess iron in the liver observed in NAFLD. HJV levels were low and hepcidin levels were high in iron-overloaded NAFLD patients. These findings support the suggestion that iron accumulation may be the primary inciting event in this disease[213].
Individuals with the metabolic syndrome preserve the iron regulatory control of hepcidin, and hepcidin progressively increases in response to the increase of iron stores[205,214-216]. In addition, serum hepcidin and HAMP mRNA in the liver correlate to body iron stores irrespective of the degree of iron deposition. Thus, the dysmetabolic iron overload syndrome (DIOS) syndrome seen in NAFLD is not related to altered hepcidin synthesis[217].
However, despite the elevated serum hepcidin, duodenal iron absorption is increased because DMT1 is upregulated by IRP1 activation, likely due to unidentified humoral factors in the sera of NASH patients[218]. It seems, therefore, that elevated hepcidin in NAFLD is either a reflection of hepatocellular inflammation in NASH, or that increased iron and the associated induction of hepcidin appears before the development of NAFLD or NASH[219].
So far, data suggest that the interplay between iron and lipid metabolism is multifaceted in NAFLD. Moreover, it could be suggested that iron is directly implicated in NAFLD pathogenesis. Reports that increased dietary iron from red meat may predispose individuals to type II diabetes and insulin resistance are supportive evidence for such an idea[220-222].
Contrasting results have also been reported. For example, inadequate hepcidin production in response to a given level of iron load in NAFLD patients compared to controls was found in one study[159]. An impairment in the ability of hepcidin to inhibit iron absorption was also demonstrated in DIOS, suggesting hepcidin resistance in this condition[223]. The recent description of ferroptosis has prompted new investigations on the effects of liver iron load in NAFLD, although its exact role in this disease process has not been fully clarified.
Ferroptosis was recently related to the induction of inflammation in the early stages of NASH, making it a possible the “first hit” in its pathogenesis[98]. Further studies indicated that ferroptosis plays a critical role in the progression of NASH, making it a promising treatment target[224,225]. The enzyme arachidonate 12-LOX is known to promote the progression of NASH[226,227], and arachidonic acid metabolism has been shown to trigger ferroptosis in a diet-induced NASH mouse model[224]. Furthermore, the levels of the central regulator of ferroptosis ACSL4 were increased in a rat NASH model, and inhibition of the Mfn2/IRE1αACSL4 pathway was found to prevent incidence and development of NASH[228]. However, the connection between NAFLD and ferroptosis is still debatable, and many reviews of iron and NAFLD pathogenesis have been presented[229-231].
Early reports showed that stainable iron is present in the livers of alcoholics[232,233]. Hepatocyte iron deposition is considered an important feature of alcoholic liver disease (ALD), although stainable iron in Kupffer cells is more prominent, particularly in the advanced stages of disease[234]. Ethanol con-sumption triggers iron overload[235]; it has been shown in patients with ALD that ethanol increases iron uptake from circulating de-sialylated transferrin by hepatocytes[236].
Almost half of patients with ALD have hepatic iron overload (HIO)[237] with high values of plasma ferritin and transferrin saturation[238,239]. Drinkers, from an early age, have increased iron markers[208,240]. High liver iron was found to be predictive of HCC development or death in patients with alcoholic cirrhosis[241,242], often acting synergistically with diabetes mellitus and viral hepatitis[243,244]. Ethanol is metabolized into acetaldehyde, forming DNA and protein adducts that predispose individuals to HCC[103]. Iron is directly implicated in HCC development, since it accumulates in lysosomes through ferritinophagy and reaches the cytoplasm as free iron[245]. The resultant production of free radicals through the Fenton reaction initially activates Kupffer and stellate cells, ultimately leading to ferroptosis[246]. Additional significant production of ROS is mediated by cytochrome P450 2E1 (CYP2E1), which is directly induced by alcohol[247]. Alcohol consumption results in up to 20 folds increase of CYP2E1[248]. Additional mechanisms of alcohol-induced HCC have also been reviewed[249].
The question of increased iron load in the liver of patients with ALD has prompted research on hepcidin regulation in ALD. Suppression of hepcidin expression by ethanol has been reported in cell culture and experimental animal models, possibly via the inhibition of CCAAT enhancer binding protein-α (C/EBP-α)[250-253]. Iron induces activation of C/EBP-α, but ethanol inhibits this action and leads to inadequate hepcidin expression[254]. Suppression of the BMP6/SMAD pathway by alcohol has also been reported[255]. Hepcidin downregulation is also mediated by the induction of oxidative stress caused by either the effects of ethanol itself or free iron. As such, antioxidant treatment attenuates hepcidin downregulation. Ethanol may also increase hepatocyte iron uptake by upregulating the expression of TfR[246], even in habitual drinkers[256]. Additionally, ethanol may reduce hepcidin through proteins involved in liver regeneration; however, this requires further investigation[257].
Ethanol exposure simultaneously increases the expression of DMT1 and FPN in the duodenum[254], which has been linked to liver fibrosis[254,258]. Iron absorption is increased two-fold in chronic alcoholics[259], and ethanol administration in a mouse model overexpressing adipose tissue lipin-1 accelerated iron accumulation followed by lipid peroxidation, reduction of GSH, and induction of ferroptotic liver damage[260].
The effect of ethanol on hepcidin seems to be more complex than previously thought[261]. Ethanol has been shown to increase transforming growth factor (TGF)-β expression and phosphorylation of SMAD2[262]. Increased activation of SMAD2/3 can abrogate the TGF-β-induced hepcidin upregulation[263]. Hepcidin is also suppressed by ethanol through the toll-like receptor 4 (TLR4) pathway, and ethanol does not suppress hepcidin in TLR4 receptor mutant mice[264]. Interestingly, TLR4 deficiency has been shown to protect animals from liver fibrosis[265,266]. Further evidence suggests that ethanol action on TLR4 involves HSCs, as TLR4 on Kupffer cells or mature hepatocytes are unlikely targets of the effects of ethanol[267,268].
Both serum transferrin and serum hepcidin have been used as prognostic markers in ALD. To this end, low transferrin levels[269,270] have been associated with worse prognosis[197,270-272]. Importantly, the prognostic value of serum transferrin is similar to other traditional prognostic scores, such as the model of end-stage liver disease (MELD) and the Glasgow alcoholic hepatitis scores[273].
The recent identification of ferroptosis has allowed for a better understanding of the connection between lipid and iron abnormalities observed in ALD[274]. Ferroptosis is downregulated during the repair of ethanol-induced liver damage, while ferroptosis inhibition or activation of the Nfr2 pathway reversed ROS accumulation and lipid peroxidation induced by ethanol[275,276]. Excessive ethanol activates genes like frataxin that promote liver injury[277]. More importantly, ferroptosis provides a strong link for the recently demonstrated crosstalk between the liver and the gut[278]. Lack of intestinal sirtuin 1 has been shown to limit ferroptosis, normalize iron overload, and ameliorate ethanol-induced liver damage[279]. Ferroptosis is also implicated in adipose-liver axis abnormalities observed in alcoholic steatohepatitis[260]. Moreover, the overexpression of adipose-specific lipin-1 aggravates alcoholic steatohepatitis and iron deposition, increasing hepatic MDA levels[153,260].
Finally, an additional mechanism of ethanol-induced liver damage has been identified in severe alcoholic hepatitis patients. Iron overload triggers activation of the metallopeptidase ADAM17, which leads to the increase of TNF-α and soluble CD163, resulting in macrophage activation and promotion of hepatic inflammation[280]. Detailed reviews on iron and ALD were recently published[143,281,282].
The effect of iron on the activity and infection cycle of the hepatitis C virus (HCV) has been controversial. Inhibition of viral replication by iron due to the suppression of the nonstructural protein 5B has been reported[283], but enhancement of viral replication has also been observed[284]. HCV alters the expression of hepcidin and therefore cellular iron metabolism[285,286]. Experimental evidence in early HCV infection has demonstrated increased hepcidin expression followed by enhanced viral translation and replication. In this study, iron loading of macrophages accompanied hepcidin upregulation and resulted in increased viral transmission to naïve cells[287]. Other experimental studies, however, have shown that hepcidin levels are low in HCV-infected cell lines[288,289].
Inhibition of hepcidin expression has been attributed to HCV-induced oxidative stress[290,291]. Experiments in chimpanzees on high iron diets have demonstrated that liver damage is observed only in animals infected with HCV, indicating a harmful effect of iron in HCV infection[292]. In chronic infection, HCV interferes with the expression of the iron uptake receptor TfR1, a known mediator of HCV internalization[293,294]. The observed downregulation of hepcidin despite hepatic inflammation in chronic HCV[295] may be related to impairment of the BMP6/HJV pathway by TNF-α, which would suppress the transcription of HJV[296].
Clinical studies have verified that HCV infection downregulates hepcidin[297-299], and serum hepcidin has been correlated with severity of liver disease[300]. More than 40% of patients have iron overload associated with a high rate of liver damage and inflammatory activity, as well as an increased risk of hepatocarcinogenesis[301-303]. Hepatic iron and HCV proteins in combination produce a toxic hydroxyl radical (·OH) that forms mutagenic bases such as 8-hydroxy-2-deoxyguanosine (8-oxodG)[304,305]. HCV patients have been shown to have an approximately 10-fold increase of 8-oxodG in liver tissue compared to non-HCV control patients[306].
The Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis trial has convincingly demonstrated that iron in hepatocytes and portal tract cells predicts progression to decompensated cirrhosis, HCC, and death[307]. Almost all liver tissue from HCV patients had some lysosomal iron deposits detected by electron microscopy and X-ray microanalysis, despite negative results with classical Prussian Blue staining[308]. Moreover, increased serum aminotransferases were found only in HCV patients with stainable iron in Kupffer cells but not in those with hepatocellular iron[309].
Even minor increases in iron load in heterozygous carriers of C282Y or H63D gene mutations for hemochromatosis were found to induce more fibrosis in chronic HCV infection[310,311]. Genotype 3-infected patients have more frequently elevated liver iron, which has been associated with hepatic steatosis in this type of HCV infection[312]. Evidence from thalassemia patients further indicates that iron adversely affects the disease course of HCV, increasing morbidity and mortality due to more severe liver disease[313].
Liver iron also adversely affects the response to interferon (IFN)-based treatments[314]. In studies involving IFN treatment, ferritin levels increased regardless of sustained virologic response (SVR) and decreased at about 3 years post-treatment. This is not the case with direct-acting antivirals (DAAs)[315,316], where SVR is achieved irrespective of iron status[317-320]. A recent study demonstrated that pre-treatment elevated serum ferritin and ERFE levels were restored after treatment with DAAs and correlated with changes in LDL cholesterol levels, but only in men[321].
Plasma ferritin, liver iron, and transferrin saturation are also increased in HCV infection[322] and elevated serum ferritin has been related to liver fibrosis[323]. An additional reason for increased liver iron in HCV patients is the reported increased hemolysis particularly in advanced stages of the disease[151]. Despite the evidence presented above, different results in relation to the role of HCV-induced iron overload have been presented[324,325]. In several studies, elevated serum ferritin and hepatic iron played no significant role in the progression of liver damage[326,327]. Moreover, the significance of hemochromatosis mutations has been questioned as a risk factor in the progression of HCV-related disease[328].
Recently, it has been suggested that ferroptosis may be implicated in the natural course of HCV[58]. Importantly, HCV replication is inhibited by an iron-dependent mechanism like ferroptosis, which is mediated by the desaturation of oleate to highly unsaturated fatty acids by the enzyme fatty acid desaturase 2 (FADS2). This is a key determinant of cellular sensitivity to ferroptosis; FADS2 suppression significantly enhances HCV replication, whereas the ferroptosis inducer erastin sensitizes HCV to DAAs, altering the conformation of HCV replicase[329].
Iron favors hepatitis B virus (HBV) mRNA expression in HepG2 cells[330]. Increased serum and cellular iron uptake and decreased hepcidin expression have been reported in HBV infection[297,331]. Hepatitis B-infected patients frequently show iron deposition in hepatocytes and elevated liver iron concentration (LIC) leading to increased disease severity[332,333]. Serum ferritin levels are also increased in patients with chronic HBV[332].
Levels of hepcidin are increased in early stages of HBV and reduced in the cirrhotic stage[334,335]. Co-infection with hepatitis D increases the iron load[332]. However, results of studies regarding serum hepcidin in HBV infection are not uniform. Reduced serum hepcidin has been reported in HBV patients with or without cirrhosis[336], while another report found that hepcidin is slightly increased in HBV patients without cirrhosis and in those with HCC[335]. The reason for this discrepancy is not clear. Nonetheless, decreased hepcidin levels and elevated transferrin saturation and ferritin levels have been associated with fibrosis severity in patients with chronic HBV[337].
It should be noted that iron deposition in the liver has been considered a secondary phenomenon. Damaged hepatocytes in viral hepatitis undergo necrosis and the released iron is scavenged by Kupffer cells[240,338]. However, this mechanism cannot entirely account for the deposition of iron in hepatocytes. The implication of HBV in iron deposition is exemplified in a case report in which a female patient with symptoms of iron overload had highly increased serum ferritin and transferrin saturation. In this case, all of the patient’s symptoms resolved and her iron abnormalities normalized after HBV antiviral monotherapy[339].
Nearly 6 decades ago, it was shown that iron on liver biopsy is associated with manifestations of advanced disease compared to that in non-iron overloaded cirrhotic patients[340]. Cirrhotic patients with hemosiderosis are more likely to be classified as Child Pugh class B or C with higher MELD scores than those without stainable iron[341,342].
As mentioned before, hyperferritinemia and high liver iron predict the risk of advanced liver fibrosis in NAFLD[166,179,343]. A recent study of a large number of NAFLD patients with a long follow-up (mean 8.4 years) emphasized the fact that it is the non-parenchymal iron deposition that leads to serious liver disease[344].
Fibrosis is increased by the presence of iron through increased HSC proliferation and selectively increased collagen synthesis without interference by non-collagen proteins[345,346]. Experiments with cultured HSCs have shown that incubation with either ferritin or transferrin increases nuclear factor kappa-B translocation and HSC activation[347,348], and enhances α-smooth muscle actin, collagen, and vimentin synthesis[349]. Isoprostanes, products of arachidonic acid peroxidation produced during iron-induced oxidative stress, increase HSC-collagen-production and TGF-β release from Kupffer cells[350]. Furthermore, 4-HNE upregulates the expression of collagen and the TIMP-1 inhibitor of metalloproteases in HSCs[351].
Elastin, another component of the extracellular matrix, is also affected by iron. Elastogenesis is modulated in cultured human skin fibroblasts by iron, as evidenced by the levels of both elastin protein and elastin mRNA are increasing 3-fold[194]. Liver iron load also induces both TGF-β[352] and BMP-6[353,354]. The connection between fibrosis and hepcidin pathways and the significance of SMAD4 as their common link has been demonstrated[353]. Other signaling pathways related to fibrosis are also modulated by iron. For example, iron deficiency stimulates Notch signaling[355], and recently, iron-loading revealed a protective role of β-catenin (a component of the cadherin complex that stimulates Wnt signaling) against liver fibrosis[356]. Hepcidin also has a protective role in liver fibrosis by suppressing HSC activation[357]. BMP6, the main hepcidin inducer, has a similar protective role in fibrosis inhibiting HSCs activation[358]. Evidence regarding the role of ferroportin in liver fibrosis is limited. However, ferroportin has been shown to be increased in activated HSCs and the anti-fibrotic action of hepcidin in HSCs mentioned above may be mediated by degradation of ferroportin[357].
Clinical evidence confirms the importance of iron metabolism in the development of fibrosis. For example, ferritin levels have been associated with decompensation and increased mortality in cirrhosis[359]. However, ferritin concentration has poor sensitivity as a marker of liver fibrosis, since it also increases as a result of inflammation[360]. Transferrin also has clinical significance in HCV- and HBV-related cirrhosis; it has been associated with advanced fibrosis and is a predictor of survival in cirrhotic patients[269,301,338]. Additionally, low hepcidin levels can cause iron overload and increased oxidative stress in the liver[361], which in combination with other factors such as genetic variables, viral infections, and alcohol use, can eventually lead to liver fibrosis[362].
Low hepcidin has also been demonstrated as a predictor of mortality and development of HCC in alcoholic cirrhosis[258,363]. Similarly, in HBV cirrhosis, hepcidin is low compared to patients without cirrhosis[335,364], where values are similar to healthy controls[335,365]. In HCV-related cirrhosis and alcoholic cirrhosis, hepcidin is significantly lower than in HBV cirrhosis[365-367].
Hepcidin levels are not reduced in the early stages of NAFLD, but eventually drop in advanced fibrosis, similar to what has been observed in other liver diseases[368]. Unlike ferritin[369], serum hepcidin is a reliable marker of severity of fibrosis in NAFLD[368,370]. A low hepcidin/ferritin ratio can differentiate between cirrhosis and non-cirrhosis in patients with HBV, HCV and NAFLD[367], but not in ALD patients, possibly because ethanol directly inhibits hepcidin expression as mentioned above.
The role of ferroptosis in liver fibrosis was recently investigated. Its role is debatable as both induction and attenuation of liver fibrosis by ferroptosis has been reported. Ferroptosis increased susceptibility to fibrosis in mice on a high-iron diet, an effect reversed by a ferroptosis inhibitor[12]. However, other studies have shown that ferroptosis attenuates HSC activation and reduces liver fibrosis. Moreover, the ferroptosis inducers erastin and sorafenib reduce liver fibrosis increasing ferritinophagy[371], and MgIG increases ferroptosis, leading to reversion of fibrosis[123]. The anti-malarial agent artemether increases the p53-dependent ferroptosis and inhibits HSC activation[372] and artesunate, a derivative of artemisinin with immunomodulating properties, induced ferroptosis of activated HSCs possibly triggering ferritinophagy[121]. The role of iron in liver fibrosis has been recently reviewed[152,373].
Hepatic iron overload has long been linked to HCC tumorigenesis and tumor growth[147,374-376]. Iron incubation of an HCC cell line has been shown to increase mesenchymal and metastatic markers, representing a fundamental defect in cancer development[377]. Patients with hereditary hemochromatosis show a 20-200-fold increase risk of HCC development[378,379]. Additionally, iron score has been demonstrated to be significantly higher in HCC-NASH patients than in NASH controls[343]. In HCC patients, iron localization is mainly sinusoidal[187], and iron deposition in the portal tract has been associated with poor survival after tumor resection[380]. Similar findings have been reported in prospective studies of HCC in alcoholic cirrhosis[241] and in HCV-associated cirrhosis[381].
Several studies suggest an association between HCC and dietary iron overload from beer fermented in steel drums in black Africans[382-385]. Furthermore, experimental evidence has identified several mechanisms of iron involvement in HCC development. Namely, HCC cells, like many other cancer cells, upregulate iron uptake and intracellular iron accumulation since they are dependent on iron[386,387]. The generation of ROS by this iron favors carcinogenesis through promotion of genomic instability and generation of DNA repair defects[388,389]; in other words,, this generation of ROS maintains the oncogenic phenotype of cancer cells[390,391]. The direct hepatocarcinogenic effect of free iron in the pathogenesis of HCC has also been demonstrated in an animal model of iron-rich diet where the tumor developed without fibrosis or cirrhosis[392,393]. Additionally, iron deposition directly decreases p53 protein level and its activity in the liver, facilitating the development of HCC[394]. An important mediator of intracellular iron is the protein leucine-rich repeat protein 5 (FBXL5); exposure of FBXL5 knockout animals to chemical or viral carcinogens has been shown to result in increased liver tumor formation. More importantly, low levels of FBXL5 in HCC patients are associated with a poor prognosis[395]. Ferritin heavy chain (FTH) acts as a protector of HCC cells, increasing their cellular resistance to ferroptosis, thereby acting as an oncogene in the pathogenesis and progression of HCC[396].
HCC patients in contrast to those with other cancers have low hepcidin levels[397-399]. Many mechanisms lead to the final decrease of hepcidin in HCC, including downregulation of inducers such as HAMP, TfR and HJV, and upregulation of suppressors such as matriptase 2 and GDF15[400]. Hepcidin downregulation increases cellular proliferation and HCC risk via reduction of the hepcidin protection against HSC activation. The downregulation of hepcidin in HCC has been attributed to the effects of cirrhosis rather than to HCC itself. Cirrhotic patients also show decreased hepcidin expression irrespective of disease etiology[152,336,399], while the hepcidin:ferritin ratio has been reported to decrease with fibrosis progression[373].
Ferroptosis and its inducers have been extensively investigated in HCC as it is considered an effective tumor suppression mechanism[81,401-403]. On the other hand, genes negatively regulating ferroptosis increase HCC drug resistance[404]. Sorafenib, a drug used for treatment of advanced HCC, is one example. This drug can induce the expression of metallothionein-1G (MT-1G), and upregulation of MT-1G has been demonstrated to serve as a negative regulator of ferroptosis, conferring resistance to sorafenib[405]. Some studies have also found that haloperidol can facilitate the cascade of ferroptosis induced by sorafenib in HCC[406].
In contrast to the negative regulators of ferroptosis, ACSL4 can positively regulate ferroptosis in HCC[97]. Inhibition of ACSL4 protects sorafenib-induced ferroptosis in HCC cells. A human study demonstrated an upregulation of the ACSL4 protein in HCC tissue from surgical specimens with a good response to sorafenib as a postsurgical adjunct treatment[407]. ACSL4 may therefore serve as a prognostic factor for survival and disease-free survival time[407,408].
Natural omega-3 PUFAs are the main peroxide substrates in ferroptosis and have anti-tumor activity[409], a fact that has been therapeutically exploited[410]. PUFAs consumed in the form of fish can reduce the risk of HCC development[411]. Ceruloplasmin has also been shown to inhibit ferroptosis in HCC cells, interfering with iron metabolism. Moreover, inhibition of ceruloplasmin increases the accumulation of iron and ROS production, facilitating erastin-induced ferroptosis in HCC cells[412].
Additional regulators of ferroptosis in HCC are the long non coding RNA molecules (lncRNAs), but their role has not been fully elucidated[413]. Erastin-induced ferroptosis upregulates the lncRNA GABPB1-AS1 in HepG2 cells, silencing the gene encoding peroxiredoxin-5 peroxidase and eventually leading to a reduction in cellular antioxidant capacity[414]. The predictive value of lncRNAs associated with ferroptosis in HCC has been recently addressed. Nine and five ferroptosis signature models have been established, which identified two groups of patients; the high-risk group in this study was shown to have enhanced tumorigenesis and worse prognosis[415,416].
Equally, the non-coding circular RNAs (circRNAs) seem to play a role in the development of HCC through ferroptosis. The circ0097009 endogenous RNA regulates the expression of SLC7A11, a key regulator of cancer cell ferroptosis in HCC. Circ0097009 therefore may be used as a potential target for HCC treatment[417].
Ferroptosis-related genes (FRGs) have also been identified and found to be upregulated in HCC tissue. In one study, three clusters have been determined, and a high expression of cluster 3 has been associated with worse prognosis and a higher histological stage[418]. Another approach regarding the use of ferroptosis as a prognostic marker in HCC has also recently been presented in which a novel ferroptosis-related 10-gene signature stratified HCC patients into two risk groups[419]. Those in the high-risk group have significantly reduced survival. The role of ferroptosis in HCC generation and progress has been recently reviewed[420].
Hepcidin is significantly lower in patients with primary biliary cholangitis and primary sclerosing cholangitis compared to patients with other chronic viral and metabolic liver diseases. In one study, low hepcidin was maintained even after two years of treatment[421]. The reason for low hepcidin may be the suppression of STAT3 phosphorylation by accumulated bile acids. Furthermore, hepcidin remains lower in cholestatic cirrhosis compared to non-cholestatic cirrhosis, suggesting the critical role of cholestasis in maintaining low values of hepcidin[422].
There is experimental evidence suggesting that iron is implicated in autoimmune hepatitis (AIH) through ferroptosis involvement. The classical AIH-inducer Concanavalin A (ConA) has been linked to an overproduction of reactive nitrogen species (RNS) such as nitric oxide and peroxynitrite in a mouse model of AIH. This effect is attenuated by Fer-1, indicating that ConA induces ferroptosis in the liver. Moreover, gadolinium chloride (a Kupffer cell depleting agent) inhibits RNS and hepatocyte ferroptosis[423]. Indoleamine 2,3-dioxygenase 1 (IDO1) is an intracellular heme enzyme involved in autoimmune diseases[424]. Upregulation of IDO1 has also been shown to be involved in ConA-induced hepatocyte ferroptosis through RNS accumulation and hepatocyte ferroptosis. An IDO1 inhibitor and an IDO1 knockout were shown to induce this effect, indicating that IDO1 promotes hepatocyte ferroptosis by triggering nitrative stress[425].
Clinical evidence also supports the detrimental effect of iron in AIH. Ferritin and iron are increased in serum of 65% and 58% of naïve patients with AIH respectively, which is resolved after successful treatment[426]. Increased serum ferritin has been independently associated with advanced fibrosis in patients with untreated AIH[427]. Moreover, serum hepcidin is low in patients with liver autoimmune disease[367,421]. Interestingly, in AIH, low serum hepcidin levels remain after 2 years of treatment, a finding similar to observations in autoimmune cholestasis. A plausible explanation could be that hepcidin is involved in hepatic autoimmune processes[428].
Although ischemia-reperfusion injury (IRI) is not strictly a liver disease as it also occurs with other organ transplantations, iron is clearly involved in the pathogenesis of IRI-related hepatic abnormalities. Ferroptosis is implicated in the pathogenesis of IRI through GPX4 inactivation[59,429]. Iron overload and upregulation of the ferroptosis indicator PTGS2 are prominent characteristics of IRI in the liver[59]. An analysis of 202 live-donor liver transplantation patients showed a high serum ferritin level indicating iron overload[430]. In this study, use of ferroptosis inhibitors such as Fer-1, α-tocopherol, and DFO prevented hepatic IRI.
Ferroptosis is also involved in the development of acute liver failure (ALF). In sepsis-induced ALF, analysis of the liver infiltrate has shown that FRGs may be responsible for the development of liver failure through the activities of B cells and natural killer cells[431]. The most common reason for ALF, however, is acetaminophen (APAP) toxicity in which lipid peroxidation leads to hepatocyte ferroptosis[432].
GSH is important for the inactivation of the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) responsible for APAP toxicity. GSH reduction and GPX4 inhibition are common in APAP-induced cell death[433]. The viability of mouse hepatocytes in the presence of APAP is improved by fer-1 without restoring the cellular GSH level, suggesting that suppression of the conversion of APAP to NAPQI is not the reason for the protective effect of fer-1[434]. Consistently, other experiments have confirmed the role of ferroptosis in APAP-induced hepatocyte cell death[432,435-437]. An additional mechanism of APAP-induced ferroptosis is the significant hepcidin reduction, likely via activation of HIF1α[434,438-440].
However, the role of ferroptosis in APAP toxicity and other drug-induced liver injury is disputed. An earlier report showed that α-tocopherol does not improve APAP-induced liver injury and that lipid peroxidation is not involved in APAP hepatotoxicity[441]. A recent review suggests that APAP-induced hepatotoxicity should be identified as programmed necrosis and not ferroptosis or other types of cell death[442]. Therefore, more research is required before ferroptosis inhibitors are recommended as treatments for APAP toxicity.
Sickle cell liver disease (SCD) is an inherited disease caused by the presence of hemoglobin S. Under hypoxic conditions, red blood cells are dehydrated and form the characteristic sickle cells[443,444]. The formation of hemoglobin S is due to a single substitution of an amino (glutamic acid to valine) in the beta globin chain[444]. Viral hepatitis and iron overload are two major reasons for the development of liver disease in SCD, both of which are typically related to patients receiving multiple blood transfusions[445]. Sources of hepatic iron in SCD include these multiple blood transfusions and chronic intravascular hemolysis[446]. Liver iron deposition occurs mainly in Kupffer cells[447]. Liver iron deposition can also occur in non-transfusion dependent patients[448], and there is a single case described in a patient who never received any blood transfusion[449]. Hemosiderosis in SCD may lead to fibrosis and overt cirrhosis[445,448,449].
There is considerable evidence to suggest an association between ferroptosis and coronavirus disease 2019. Cytokines produced during the infection have been shown to upregulate hepcidin expression, which leads to ferroportin suppression and iron accumulation. In addition, severe acute respiratory disease coronavirus 2 downregulates the expression of GPX4, contributing further to the initiation of the Fenton reaction and production of massive amounts of ROS and associated ferroptosis[450].
There have been many attempts to reduce iron overload, which is uniformly considered detrimental in liver disease irrespective of etiology. However, it should be remembered that iron loading is not always similar between patients and between stages of various diseases[152]. Dietary iron restriction has been shown to be effective in reducing liver fibrosis and steatosis in diet-induced NAFLD animal models[451,452].
Phlebotomy is the traditional treatment in hereditary hemochromatosis, as it increases erythropoiesis, partially reverses liver fibrosis, and increases life expectancy[453,454]. Phlebotomy has been used to treat NASH patients, but the clinical benefit is unclear[455]. Phlebotomy improves liver enzymes, insulin resistance, and liver histology in the majority of NAFLD patients, but it is not fully successful in DIOS insulin resistant patients with slight ferritin increase[182,456-458]. Insulin sensitivity is improved by phlebotomy in type II diabetics with a high serum ferritin[459]. Moreover, in patients with the metabolic syndrome, phlebotomy improves metabolic parameters, including glycosylated hemoglobin A1c and LDL/high-density lipoprotein ratio[460]. In a meta-analysis of four interventional studies with more than 400 patients, phlebotomy was shown to improve liver enzymes, insulin resistance, and lipid abnormalities[461].
In contrast, no effect was reported in two prospective randomized controlled trials. The first, which is the largest series so far, was conducted in NAFLD patients[462], and the second in DIOS patients with insulin resistance[463]. To this end, the benefit of phlebotomy in patients with NASH remains unclear until more extensive studies are available[464].
Phlebotomy reduces the marker of oxidative stress 8-hydroxy-2’-deoxyguanosine in HCV patients who have failed IFN therapy. Fibrosis and inflammation are also reduced, but HCV titers are unaffected. None of the patients in these studies were shown to develop HCC at the six year follow-up point[306,465]. Reduction of HCC development in HCV patients after phlebotomy has been verified in additional studies[466,467]. Phlebotomy has also been reported to improve the response to IFN in chronic HCV[468].
Iron chelation is an additional intervention to reduce liver iron. DFO has been successfully used to control fibrosis in hemochromatosis[469]. Studies in several animal models have revealed that iron chelation decreases the stability of procollagen mRNA[470] and reduces elastin mRNA[194]. DFO has also been shown to reverse HSC activation and induces apoptosis of activated murine HSCs[471]. More recently, a study of the combination of DFO with pegylated IFN-α showed a synergistic anti-fibrotic effect in rats[472]. ROS degrade the apolipoprotein B100 (apoB100) component of VLDL, thereby enhancing hepatocyte steatosis in rodents. In another study, DFO restored apoB100 and increased VLDL secretion[473]. No firm conclusions can be drawn, however, without the results of clinical trials. It should be noted that inhibition of hemoxygenase-1 decreases hepatic iron deposition and attenuates liver fibrosis in rats[474].
Interestingly, commonly used drugs like the calcium channel blockers have been found to induce HSC apoptosis and reduce DMT1 expression, hepatic iron deposition, and liver collagen in mouse and cellular experiments[475]. Hepcidin may be a promising agent for the treatment of liver iron overload, as hepcidin administration has been shown to attenuate iron deposition in mouse models of hemochromatosis[476-478], while its overexpression ameliorates fibrosis severity. This is due to the inhibition by hepcidin of the TGFβ1-induced SMAD3 phosphorylation in HSCs, a pathway that requires the presence of ferroportin in stellate cells[357]. Similar reduction of liver fibrosis has been observed with BMP6 overexpression in murine and human NAFLD[358].
Hepcidin responds to iron conditions in HCV patients, but the response is impaired. Thus, correction of hepcidin regulation may improve the clinical progress in iron-overloaded HCV patients[479]. Hepcidin manipulation may be beneficial in the management of HCC as well. The iron chelator deferasirox induces apoptosis in hepatoma cells lines and decreases liver tumor development in mice, increasing HAMP mRNA expression. However, toxicity and the lack of response in some patients may be a problem in human trials[480]. Additionally, some HCC patients have increased hepcidin expression and downregulation of hepcidin may be required. In a murine HCC model with high liver hepcidin, the traditional Chinese medicinal herb dandelion polysaccharide has been shown to reduce hepcidin expression, arrest the cell cycle, and suppress the HCC proliferation[481]. Hepcidin, therefore, is a logical candidate target for clinical trials in HCC. Indeed, both hepcidin agonists and inhibitors have been tested in vitro and in laboratory animals[482]. It should be noted that synthetic mini-hepcidins have also been tested in Hamp -/- mice; in one study, serum iron was reduced after chronic administration of the drug[476].
Ferroptosis is the current therapeutic target in the treatment of iron overload diseases. It should be stressed, however, that the effects of ferroptosis in chronic liver disease depends on the cell type and the specific environment. In liver fibrosis, for example, ferroptosis has different effects on hepatocytes and HSCs as will be detailed later[483]. A future challenge is to develop drug delivery systems targeting ferroptosis in specific cell types. In ALD and in NAFLD, ferroptosis is implicated in liver damage, and ferroptosis inhibition would theoretically be beneficial[225,276,432]. For example, ferroptosis-induced liver injury could be reversed by sestrin 2, an antioxidant protein increased by ferroptosis inducers[484].
In contrast to other liver diseases where ferroptosis is detrimental and therapies are directed towards inhibition of ferroptosis, HCC is benefited by enhancement of ferroptosis. Thus, ferroptosis inducers are used in advanced HCC. Sorafenib, a multi-kinase inhibitor, is the most extensively studied ferroptosis inducer[103,485]. In HCC, this drug acts by inhibiting cellular proliferation and neo-angiogenesis. Additionally, it induces ferroptosis in HCC cells[486]. It has been reported that sorafenib decreases the uptake of cystine in the Xc- system and starts the chain of events leading to ferroptosis induction through the accumulation of ROS, which is the result of GSH depletion and loss of GXP4 activity[487]. Excessive ROS production also results in the inhibition of the retinoblastoma protein Rb, an important negative regulator of cell proliferation[488].
Prolonged administration increases the resistance of HCC cells to sorafenib. ABCC5, a recently described regulator of ferroptosis, increases the generation of GSH and reduces the production of ROS through stabilization of SLCA11 and subsequent inhibition of ferroptosis. Accordingly, downregulation of ACCC5 reduces resistance to sorafenib[489]. Other proteins reducing the sorafenib-induced ferroptosis through stabilization of SLCA11 have also been recently described[490,491].
Haloperidol has also been shown to promote erastin- and sorafenib-induced ferroptosis, suggesting that it could be used in combination with sorafenib to achieve either dosage or resistance reduction[404,406,492]. An upregulation of Nrf2 through activation of the p62-Keap1-Nrf2 pathway inhibits sorafenib-induced ferroptosis in HCC cell lines[63,493]. Interestingly, trigonelline, the active ingredient of the traditional Chinese medicine fenugreek, increases ferroptosis by acting on Nrf2, therefore reducing sorafenib resistance[494]. Overexpression of the leukemia inhibitory factor receptor (LIFR) has also been shown to increase sorafenib-induced ferroptosis of HCC cell lines, whereas reduced LIFR expression increases resistance to ferroptosis[495].
A recent study reported an another target for HCC treatment. In this study, lactate-rich hepatoma cells were shown to exhibit increased resistance to the ferroptosis generated by common ferroptosis inducers. Moreover, lactate uptake was shown to be mediated by monocarboxylate transporter 1 (MCT1), which enhances the production of monounsaturated fatty acids, blocking ferroptosis. Inhibition of MCT1-mediated lactate uptake enhances ferroptosis[496]. In contrast to the presented evidence, a recent report indicated that sorafenib may not be an inducer of ferroptosis at least in many cancer cell lines[497]. Other drugs that could be used in the treatment of HCC based on increased ferroptosis have also been recently described[498,499]. Heteronemin, a marine terpenoid, induces ferroptosis in HCC cells by reducing GPX4[500]. IFN-γ has also been confirmed to inhibit system Xc- activity and increase ferroptosis[501]. Lenvatinib, another kinase inhibitor used in advanced HCC treatment, also acts through the inhibition of the system Xc-. Fibroblast growth factor receptor-4 (FGFR4) increases the activity of the system Xc- and lenvatinib inhibited FGFR4 increasing ferroptosis. Interestingly, patients with HCC positive for FGFR4 have a longer progression-free survival compared to those with FGFR4-negative HCC. Nrf2 upregulation has also been shown to decrease the sensitivity of HCC to lenvatinib[502]. Moreover, low-density lipoprotein nanoparticles (LDL-DHA NPs), selectively induce HCC cell death in mouse models, and LDL-DHA NPs enhance lipid peroxidation due to both GSH depletion (leading to GPX4 inactivation) and direct degradation[410].
Ferroptosis can be used for stratification of HCC patients to predict both prognosis and suitability for immunotherapy. For that purpose, a ferroptosis-related prognosis risk score model has been developed to stratify patients into two subgroups based on six FRGs (FRGs)[503].
Ferroptosis inhibitors are promising drugs in the treatment of various liver diseases, although evidence is mainly based on laboratory data. NAFLD and NASH progress is worsened by induction of ferroptosis[98,224,504]. Alleviation of NASH can be achieved by ferroptosis inhibitors, such as liproxstatin-1 or ferrostatin-1[225,505]. In one study, administration of the ferroptosis inducer RSL3 aggravated hepatic steatosis and inflammation in diet-induced NASH mice, while administration of liproxstatin-1 ameliorated NASH severity and rescued animals from cell death[225].
Other drugs, such as Ginkgolide B and dehydroabietic acid, alleviate NASH severity by inhibiting ferroptosis via upregulation of the p62-Keap1-Nrf2 pathway[506-508]. Thymosin β4 (Tβ4) improves liver lipid metabolism markers in NAFLD rat models and inhibits the palmitic acid-induced hepatocyte death in the LO2 cell line. Ferrostatin-1 increases the effect of Tβ4, which is attenuated by erastin, indicating that the protection of hepatocytes is mediated by ferroptosis reduction[509]. The enzyme enoyl coenzyme A hydratase 1 (ECH1) is an important component of mitochondrial fatty acid β-oxidation. ECH1 knockdown aggravates liver inflammation and fibrosis in mouse NAFLD models while fer-1 administration alleviates liver damage, again suggesting that the beneficial effect of ECH1 may be due to inhibition of ferroptosis[505].
Liver fibrosis is another disease that may be treated by ferroptosis regulators[510]. Inhibition of ferroptosis by ferrostatin 1 reverses liver fibrosis induced by a high-iron diet or and carbon tetrachloride[511], while induction of ferroptosis by liver iron overload aggravates APAP-induced fibrosis in mice[483]. However, ferroptosis is a double-edged sword in liver fibrosis. When ferroptosis is targeting activated HSCs, the induction of ferroptosis is beneficial. The cystine/glutamate antiporter SLCA11 has been shown to increase ferroptosis as mentioned before[55]. Inhibition of SLC7A11 enhances ferroptosis in HSCs and attenuates liver fibrosis[512]. Likewise, erastin and sorafenib induce ferroptosis in HSCs, and reduced liver fibrosis in mice[371,513].
There is growing evidence that natural products may effectively be used in the treatment of liver fibrosis. Artesunate can attenuate liver fibrosis by triggering ferritinophagy-mediated ferroptosis in HSCs[121]. Artemether can also induce ferroptosis in HSCs by increasing iron and ROS in HSCs[514] and promoting p53-dependent ferroptosis[372]. MgIG can also induce ferroptosis in HSCs by increasing the activity of the enzyme HO-1[123].
Chrysophanol isolated from the rhizome of rhubarb can inhibit the HBV x protein-induced activation of HSCs through ferroptosis and alleviate HBV-related fibrosis[515]. Additionally, wild bitter melon extracts can downregulate GPX4 and SLC7A11 in activated HSCs by inducing ferroptosis[516]. Two other proteins regulating ferroptosis in HSCs could be the future targets in the treatment of liver fibrosis: ZFP36/TTP and ELAVL1/HuR. These are critical regulators of HSCs ferroptosis[371,513]; ZFP36 protects against ferroptosis and ELAVL1 contributes to ferroptotic cell death.
Three more diseases may be benefited from ferroptosis inhibitors. Fer-1 improves I/R-mediated liver disease[59,224,276]. ALF is also a candidate for similar treatment based on experimental data. Glycyrrhizin, an active constituent of the licorice root, reduces ferroptosis during ALF, inhibiting oxidative stress through the Nrf2/HO-1/high mobility group box 1 pathway[517]. Finally, reduction of liver iron load will most certainly benefit ALD. Phlebotomy, however, is not recommended in patients with ALD.
An interesting approach to reduce iron load in ALD is the stabilization of erythrocytes and associated reduction in hemolysis. Administration of N-acetylcysteine or protective heme carriers like haptoglobin and hemopexin has been tested. Erythrocyte stabilizers include vitamins such as B12 or folate[281]. Ferrostatin-1 can also reduce alcoholic liver damage[276], indicating participation of ferroptosis in ALD progression. Dimethylfumarate reduces lipid peroxidation and alleviates liver cell ferroptosis leading to ALD improvement in a murine model[275]. Currently, no effective treatment can be recommended for ethanol-induced iron overload. Modulation of ferroptosis for the treatment of chronic liver diseases has been recently reviewed[282].
Retinoid signaling is decreased in the livers of humans and mice with NAFLD[518,519], and is epigenetically silenced in HCC[520]. Administration of the synthetic retinoid tamibarotene improved oxidative stress and iron deposition in iron-fed mice. Retinoids downregulate the hepatic expression of HJV, leading to liver hepcidin downregulation and ferroportin upregulation[521,522]. Retinoids also attenuate insulin resistance and hepatic steatosis in a murine model of NAFLD[523,524]. Attenuation of hyperinsulinemia may prevent the development of HCC in NAFLD[525].
A very large observational study with more than 8000 participants demonstrated that dietary vitamin C supplementation decreases plasma ferritin levels[526], indicating that vitamin C limits iron deposition and thereby increases iron mobilization. In a murine model of ALD, vitamin C administration was shown to restore hepatic hepcidin and downregulate intestinal ferroportin, leading to HIO amelioration[527]. Therefore, it is reasonable to supplement vitamin C in ALD and chronic HCV patients with hepatic iron deposition.
Evidence from patients with thalassemia major and hereditary hemochromatosis indicates that iron overload suppresses vitamin D, as there is a negative correlation between liver iron and 25-hydroxyvitamin D levels[528-530]. In hereditary hemochromatosis, levels of vitamin D are partially restored after phlebotomy[531]. Moreover, vitamin D depletion exacerbates HIO in HJV knockout mice, an effect that is corrected by the administration of the calcium channel blocker verapamil but not by vitamin D supplementation[475,532]. These results indicate a link between iron and calcium and justify the use of calcium channel blockers as a treatment modality for iron deposition in patients with decreased levels of vitamin D, as is frequently observed in ALD, NAFLD, and chronic HCV[533-536].
Zinc-deficient diet has been shown to lead to increased plasma ferritin and development of HIO in rats, while zinc supplementation returns liver iron to normal[537]. Clinical studies also indicate that iron deficiency anemia is frequently associated with zinc deficiency[538,539], implying a physiological crosstalk between iron and zinc. For example, zinc plus iron administration in rats has been demonstrated to ameliorate anemia more efficiently than iron alone[540]. The therapeutic effects of zinc on chronic liver diseases have been reviewed[541].
The Solute Carrier Family 46 Member 1 (SLC46A1) is the major importer of hemeiron in the duodenum, and it is also present in the liver. In murine liver-specific SLC46A1 knockdowns, its role in liver iron deposition has been investigated. In these studies, SLC46A1 was found to take up heme in the liver and contribute to hepatic iron deposition. Interestingly, heme inhibited folate uptake after downregulation of SLC46A1 expression, but folate supplementation had no effect in heme uptake and SLC46A1 expression indicating that folate deficiency was the result of secondary liver heme uptake excess[542]. Accordingly, the combined administration of iron and folate in rats also significantly reduced liver iron compared with iron alone[543].
Contrary to the agonistic use of the previously discussed nutrients in the treatment of liver iron deposition, riboflavin antagonists, such as galactoflavin, may be used in HIO[544]. This is because riboflavin deficiency leads to a decrease in iron absorption[544,545]. A detailed discussion on the role of nutrients in chronic liver diseases was recently published[546].
It is well documented, that iron in the liver is a double-edged sword. It is a necessary element in many metabolic pathways, but it is equally harmful if either the amount or its cellular localization are unbalanced. Increased iron deposition negatively affects most chronic liver diseases. The interplay with lipid metabolism prompted an extensive investigation for the role of iron in NAFLD/NASH. Moreover, the description of ferroptosis as a discrete form of regulated hepatocyte death opened the way for the therapeutic modulation of iron overload in many diseases. Interestingly, most liver diseases are benefited by ferroptosis inhibition. A notable exception is HCC, where the therapeutic target is ferroptosis induction.
The current evidence involves the integration of information from experimental models and less so, from patient findings. Further experimental in vitro and in vivo investigations are warranted to find more suitable molecules with wider availability and better specificity that could regulate ferroptosis. In this context, it is interesting to note that many natural products may influence iron metabolism and ferroptosis. Furthermore, it should be stressed that clinical trials involving ferroptosis regulation are scarce and sometimes inconclusive. Therefore, to draw valid conclusions, further well-designed randomized trials in humans are urgently required.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen LJ, China; Liang Y, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Dev S, Babitt JL. Overview of iron metabolism in health and disease. Hemodial Int. 2017;21 Suppl 1:S6-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 286] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 2. | Muckenthaler MU, Rivella S, Hentze MW, Galy B. A Red Carpet for Iron Metabolism. Cell. 2017;168:344-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 915] [Article Influence: 114.4] [Reference Citation Analysis (0)] |
| 3. | Wang CY, Babitt JL. Liver iron sensing and body iron homeostasis. Blood. 2019;133:18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 223] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 4. | Wang D, Wang LH, Zhao Y, Lu YP, Zhu L. Hypoxia regulates the ferrous iron uptake and reactive oxygen species level via divalent metal transporter 1 (DMT1) Exon1B by hypoxia-inducible factor-1. IUBMB Life. 2010;62:629-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Taylor M, Qu A, Anderson ER, Matsubara T, Martin A, Gonzalez FJ, Shah YM. Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology. 2011;140:2044-2055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 6. | Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9:152-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 7. | Yeh KY, Yeh M, Polk P, Glass J. Hypoxia-inducible factor-2α and iron absorptive gene expression in Belgrade rat intestine. Am J Physiol Gastrointest Liver Physiol. 2011;301:G82-G90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Anderson CP, Shen M, Eisenstein RS, Leibold EA. Mammalian iron metabolism and its control by iron regulatory proteins. Biochim Biophys Acta. 2012;1823:1468-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 367] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 9. | Yanatori I, Yasui Y, Tabuchi M, Kishi F. Chaperone protein involved in transmembrane transport of iron. Biochem J. 2014;462:25-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3535] [Article Influence: 168.3] [Reference Citation Analysis (0)] |
| 11. | Sangokoya C, Doss JF, Chi JT. Iron-responsive miR-485-3p regulates cellular iron homeostasis by targeting ferroportin. PLoS Genet. 2013;9:e1003408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Yu Y, Jiang L, Wang H, Shen Z, Cheng Q, Zhang P, Wang J, Wu Q, Fang X, Duan L, Wang S, Wang K, An P, Shao T, Chung RT, Zheng S, Min J, Wang F. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood. 2020;136:726-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 13. | Kwon MY, Park E, Lee SJ, Chung SW. Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget. 2015;6:24393-24403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 475] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 14. | Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823:1434-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 878] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 15. | Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 995] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 16. | Truksa J, Peng H, Lee P, Beutler E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc Natl Acad Sci U S A. 2006;103:10289-10293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 250] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 17. | Andriopoulos B Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, Babitt JL. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 633] [Cited by in RCA: 592] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 18. | Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, Andrews NC, Lin HY. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 780] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 19. | Canali S, Zumbrennen-Bullough KB, Core AB, Wang CY, Nairz M, Bouley R, Swirski FK, Babitt JL. Endothelial cells produce bone morphogenetic protein 6 required for iron homeostasis in mice. Blood. 2017;129:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 20. | Kautz L, Besson-Fournier C, Meynard D, Latour C, Roth MP, Coppin H. Iron overload induces BMP6 expression in the liver but not in the duodenum. Haematologica. 2011;96:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Rausa M, Pagani A, Nai A, Campanella A, Gilberti ME, Apostoli P, Camaschella C, Silvestri L. Bmp6 expression in murine liver non parenchymal cells: a mechanism to control their high iron exporter activity and protect hepatocytes from iron overload? PLoS One. 2015;10:e0122696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105:260-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 416] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 23. | Nai A, Lidonnici MR, Rausa M, Mandelli G, Pagani A, Silvestri L, Ferrari G, Camaschella C. The second transferrin receptor regulates red blood cell production in mice. Blood. 2015;125:1170-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Kawabata H, Yang R, Hirama T, Vuong PT, Kawano S, Gombart AF, Koeffler HP. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem. 1999;274:20826-20832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 451] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 25. | Goswami T, Andrews NC. Hereditary hemochromatosis protein, HFE, interaction with transferrin receptor 2 suggests a molecular mechanism for mammalian iron sensing. J Biol Chem. 2006;281:28494-28498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 247] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 26. | Kawabata H, Fleming RE, Gui D, Moon SY, Saitoh T, O'Kelly J, Umehara Y, Wano Y, Said JW, Koeffler HP. Expression of hepcidin is down-regulated in TfR2 mutant mice manifesting a phenotype of hereditary hemochromatosis. Blood. 2005;105:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 159] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Bridle KR, Frazer DM, Wilkins SJ, Dixon JL, Purdie DM, Crawford DH, Subramaniam VN, Powell LW, Anderson GJ, Ramm GA. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet. 2003;361:669-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 442] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 28. | Muckenthaler M, Roy CN, Custodio AO, Miñana B, deGraaf J, Montross LK, Andrews NC, Hentze MW. Regulatory defects in liver and intestine implicate abnormal hepcidin and Cybrd1 expression in mouse hemochromatosis. Nat Genet. 2003;34:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 213] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 29. | Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730-3735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 374] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 30. | Silvestri L, Nai A. Iron and erythropoiesis: A mutual alliance. Semin Hematol. 2021;58:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46:678-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 817] [Cited by in RCA: 823] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 32. | Arezes J, Foy N, McHugh K, Sawant A, Quinkert D, Terraube V, Brinth A, Tam M, LaVallie ER, Taylor S, Armitage AE, Pasricha SR, Cunningham O, Lambert M, Draper SJ, Jasuja R, Drakesmith H. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood. 2018;132:1473-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 206] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 33. | Andolfo I, Rosato BE, Marra R, De Rosa G, Manna F, Gambale A, Iolascon A, Russo R. The BMP-SMAD pathway mediates the impaired hepatic iron metabolism associated with the ERFE-A260S variant. Am J Hematol. 2019;94:1227-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Coffey R, Jung G, Olivera JD, Karin G, Pereira RC, Nemeth E, Ganz T. Erythroid overproduction of erythroferrone causes iron overload and developmental abnormalities in mice. Blood. 2022;139:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Andolfo I, Rosato BE, Manna F, De Rosa G, Marra R, Gambale A, Girelli D, Russo R, Iolascon A. Gain-of-function mutations in PIEZO1 directly impair hepatic iron metabolism via the inhibition of the BMP/SMADs pathway. Am J Hematol. 2020;95:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 36. | Colucci S, Pagani A, Pettinato M, Artuso I, Nai A, Camaschella C, Silvestri L. The immunophilin FKBP12 inhibits hepcidin expression by binding the BMP type I receptor ALK2 in hepatocytes. Blood. 2017;130:2111-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Bellelli R, Federico G, Matte' A, Colecchia D, Iolascon A, Chiariello M, Santoro M, De Franceschi L, Carlomagno F. NCOA4 Deficiency Impairs Systemic Iron Homeostasis. Cell Rep. 2016;14:411-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 184] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 38. | Kotla NK, Dutta P, Parimi S, Das NK. The Role of Ferritin in Health and Disease: Recent Advances and Understandings. Metabolites. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 39. | Nai A, Lidonnici MR, Federico G, Pettinato M, Olivari V, Carrillo F, Geninatti Crich S, Ferrari G, Camaschella C, Silvestri L, Carlomagno F. NCOA4-mediated ferritinophagy in macrophages is crucial to sustain erythropoiesis in mice. Haematologica. 2021;106:795-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 40. | Kawabata T. Iron-Induced Oxidative Stress in Human Diseases. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 41. | Galaris D, Barbouti A, Pantopoulos K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim Biophys Acta Mol Cell Res. 2019;1866:118535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 536] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 42. | Fenton HJH. LXXIII.—Oxidation of tartaric acid in presence of iron. J Chem Soc Trans. 1984;65:899-910. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2257] [Cited by in RCA: 2250] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 43. | Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res Commun Mol Pathol Pharmacol. 1997;95:179-189. [PubMed] |
| 44. | Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013;11:371-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 1468] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 45. | Houglum K, Filip M, Witztum JL, Chojkier M. Malondialdehyde and 4-hydroxynonenal protein adducts in plasma and liver of rats with iron overload. J Clin Invest. 1990;86:1991-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 175] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 46. | Uchida K, Szweda LI, Chae HZ, Stadtman ER. Immunochemical detection of 4-hydroxynonenal protein adducts in oxidized hepatocytes. Proc Natl Acad Sci U S A. 1993;90:8742-8746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 259] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 47. | Bartsch H, Nair J. Oxidative stress and lipid peroxidation-derived DNA-lesions in inflammation driven carcinogenesis. Cancer Detect Prev. 2004;28:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 48. | Hu W, Feng Z, Eveleigh J, Iyer G, Pan J, Amin S, Chung FL, Tang MS. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis. 2002;23:1781-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 203] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 49. | Kohgo Y, Ohtake T, Ikuta K, Suzuki Y, Hosoki Y, Saito H, Kato J. Iron accumulation in alcoholic liver diseases. Alcohol Clin Exp Res. 2005;29:189S-193S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Kawabata H. The mechanisms of systemic iron homeostasis and etiology, diagnosis, and treatment of hereditary hemochromatosis. Int J Hematol. 2018;107:31-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 51. | Cronin SJF, Woolf CJ, Weiss G, Penninger JM. The Role of Iron Regulation in Immunometabolism and Immune-Related Disease. Front Mol Biosci. 2019;6:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 52. | Xiao X, Alfaro-Magallanes VM, Babitt JL. Bone morphogenic proteins in iron homeostasis. Bone. 2020;138:115495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 53. | Quatredeniers M, Mendes-Ferreira P, Santos-Ribeiro D, Nakhleh MK, Ghigna MR, Cohen-Kaminsky S, Perros F. Iron Deficiency in Pulmonary Arterial Hypertension: A Deep Dive into the Mechanisms. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 54. | Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 639] [Cited by in RCA: 2519] [Article Influence: 503.8] [Reference Citation Analysis (0)] |
| 55. | Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4711] [Cited by in RCA: 11690] [Article Influence: 899.2] [Reference Citation Analysis (1)] |
| 56. | Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D'Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, García-Sáez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jäättelä M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, López-Otín C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Muñoz-Pinedo C, Nagata S, Nuñez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3672] [Cited by in RCA: 4507] [Article Influence: 643.9] [Reference Citation Analysis (0)] |
| 57. | Kuang F, Liu J, Tang D, Kang R. Oxidative Damage and Antioxidant Defense in Ferroptosis. Front Cell Dev Biol. 2020;8:586578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 365] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 58. | Macías-Rodríguez RU, Inzaugarat ME, Ruiz-Margáin A, Nelson LJ, Trautwein C, Cubero FJ. Reclassifying Hepatic Cell Death during Liver Damage: Ferroptosis-A Novel Form of Non-Apoptotic Cell Death? Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 59. | Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Rådmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Förster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O'Donnell VB, Kagan VE, Schick JA, Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 2640] [Article Influence: 240.0] [Reference Citation Analysis (0)] |
| 60. | Wang Y, Yang L, Zhang X, Cui W, Liu Y, Sun QR, He Q, Zhao S, Zhang GA, Wang Y, Chen S. Epigenetic regulation of ferroptosis by H2B monoubiquitination and p53. EMBO Rep. 2019;20:e47563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 61. | Ou Y, Wang SJ, Li D, Chu B, Gu W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci U S A. 2016;113:E6806-E6812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 591] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 62. | Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J, Zhong M, Yuan H, Zhang L, Billiar TR, Lotze MT, Zeh HJ 3rd, Kang R, Kroemer G, Tang D. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017;20:1692-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 687] [Article Influence: 98.1] [Reference Citation Analysis (0)] |
| 63. | Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 1488] [Article Influence: 165.3] [Reference Citation Analysis (0)] |
| 64. | Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd, Kang R, Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1649] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 65. | Lin CC, Mabe NW, Lin YT, Yang WH, Tang X, Hong L, Sun T, Force J, Marks JR, Yao TP, Alvarez JV, Chi JT. RIPK3 upregulation confers robust proliferation and collateral cystine-dependence on breast cancer recurrence. Cell Death Differ. 2020;27:2234-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 66. | Zheng J, Conrad M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020;32:920-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 852] [Article Influence: 170.4] [Reference Citation Analysis (0)] |
| 67. | Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 4202] [Article Influence: 1050.5] [Reference Citation Analysis (0)] |
| 68. | Sassetti E, Clausen MH, Laraia L. Small-Molecule Inhibitors of Reactive Oxygen Species Production. J Med Chem. 2021;64:5252-5275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 69. | Bekric D, Ocker M, Mayr C, Stintzing S, Ritter M, Kiesslich T, Neureiter D. Ferroptosis in Hepatocellular Carcinoma: Mechanisms, Drug Targets and Approaches to Clinical Translation. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 70. | Carlson BA, Tobe R, Yefremova E, Tsuji PA, Hoffmann VJ, Schweizer U, Gladyshev VN, Hatfield DL, Conrad M. Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biol. 2016;9:22-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 71. | Zhang J, Bi J, Ren Y, Du Z, Li T, Wang T, Zhang L, Wang M, Wei S, Lv Y, Wu R. Involvement of GPX4 in irisin's protection against ischemia reperfusion-induced acute kidney injury. J Cell Physiol. 2021;236:931-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 72. | Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, Bassik MC, Nomura DK, Dixon SJ, Olzmann JA. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1498] [Cited by in RCA: 2416] [Article Influence: 402.7] [Reference Citation Analysis (0)] |
| 73. | Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius E, Scheel CH, Mourão A, Buday K, Sato M, Wanninger J, Vignane T, Mohana V, Rehberg M, Flatley A, Schepers A, Kurz A, White D, Sauer M, Sattler M, Tate EW, Schmitz W, Schulze A, O'Donnell V, Proneth B, Popowicz GM, Pratt DA, Angeli JPF, Conrad M. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1492] [Cited by in RCA: 2125] [Article Influence: 354.2] [Reference Citation Analysis (0)] |
| 74. | Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, Koppula P, Wu S, Zhuang L, Fang B, Poyurovsky MV, Olszewski K, Gan B. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 675] [Cited by in RCA: 1167] [Article Influence: 291.8] [Reference Citation Analysis (0)] |
| 75. | Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12:599-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 1451] [Article Influence: 290.2] [Reference Citation Analysis (0)] |
| 76. | Fuhrmann DC, Mondorf A, Beifuß J, Jung M, Brüne B. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 2020;36:101670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 268] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 77. | Dodson M, de la Vega MR, Cholanians AB, Schmidlin CJ, Chapman E, Zhang DD. Modulating NRF2 in Disease: Timing Is Everything. Annu Rev Pharmacol Toxicol. 2019;59:555-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 369] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 78. | Qiu YB, Wan BB, Liu G, Wu YX, Chen D, Lu MD, Chen JL, Yu RQ, Chen DZ, Pang QF. Nrf2 protects against seawater drowning-induced acute lung injury via inhibiting ferroptosis. Respir Res. 2020;21:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 79. | Song X, Long D. Nrf2 and Ferroptosis: A New Research Direction for Neurodegenerative Diseases. Front Neurosci. 2020;14:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 401] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 80. | Liu Q, Wang K. The induction of ferroptosis by impairing STAT3/Nrf2/GPx4 signaling enhances the sensitivity of osteosarcoma cells to cisplatin. Cell Biol Int. 2019;43:1245-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 81. | Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS, Stockwell BR. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1349] [Cited by in RCA: 1551] [Article Influence: 141.0] [Reference Citation Analysis (0)] |
| 82. | Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 2397] [Article Influence: 239.7] [Reference Citation Analysis (0)] |
| 83. | Tarangelo A, Magtanong L, Bieging-Rolett KT, Li Y, Ye J, Attardi LD, Dixon SJ. p53 Suppresses Metabolic Stress-Induced Ferroptosis in Cancer Cells. Cell Rep. 2018;22:569-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 431] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 84. | Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X, Anastasov N, Kössl J, Brandner S, Daniels JD, Schmitt-Kopplin P, Hauck SM, Stockwell BR, Hadian K, Schick JA. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent Sci. 2020;6:41-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 837] [Article Influence: 167.4] [Reference Citation Analysis (0)] |
| 85. | Yuan H, Li X, Zhang X, Kang R, Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun. 2016;478:838-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 366] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 86. | Angeli JPF, Shah R, Pratt DA, Conrad M. Ferroptosis Inhibition: Mechanisms and Opportunities. Trends Pharmacol Sci. 2017;38:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 424] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 87. | Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A, Stockwell BR. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136:4551-4556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 502] [Cited by in RCA: 831] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 88. | Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2388] [Cited by in RCA: 5173] [Article Influence: 470.3] [Reference Citation Analysis (0)] |
| 89. | Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113:E4966-E4975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 1615] [Article Influence: 179.4] [Reference Citation Analysis (0)] |
| 90. | Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D. Ferroptosis: process and function. Cell Death Differ. 2016;23:369-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1553] [Cited by in RCA: 2617] [Article Influence: 290.8] [Reference Citation Analysis (0)] |
| 91. | Imai H, Matsuoka M, Kumagai T, Sakamoto T, Koumura T. Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis. Curr Top Microbiol Immunol. 2017;403:143-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 257] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 92. | Clemente LP, Rabenau M, Tang S, Stanka J, Cors E, Stroh J, Culmsee C, von Karstedt S. Dynasore Blocks Ferroptosis through Combined Modulation of Iron Uptake and Inhibition of Mitochondrial Respiration. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 93. | Bueno DC, Canto RFS, de Souza V, Andreguetti RR, Barbosa FAR, Naime AA, Dey PN, Wüllner V, Lopes MW, Braga AL, Methner A, Farina M. New Probucol Analogues Inhibit Ferroptosis, Improve Mitochondrial Parameters, and Induce Glutathione Peroxidase in HT22 Cells. Mol Neurobiol. 2020;57:3273-3290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 94. | Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E, Seravalli J, Ai Y, Sansing LH, Ste Marie EJ, Hondal RJ, Mukherjee S, Cave JW, Sagdullaev BT, Karuppagounder SS, Ratan RR. Selenium Drives a Transcriptional Adaptive Program to Block Ferroptosis and Treat Stroke. Cell. 2019;177:1262-1279.e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 715] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 95. | Krainz T, Gaschler MM, Lim C, Sacher JR, Stockwell BR, Wipf P. A Mitochondrial-Targeted Nitroxide Is a Potent Inhibitor of Ferroptosis. ACS Cent Sci. 2016;2:653-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 189] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 96. | Zhao T, Yu Z, Zhou L, Wang X, Hui Y, Mao L, Fan X, Wang B, Zhao X, Sun C. Regulating Nrf2-GPx4 axis by bicyclol can prevent ferroptosis in carbon tetrachloride-induced acute liver injury in mice. Cell Death Discov. 2022;8:380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 97. | Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trümbach D, Mao G, Qu F, Bayir H, Füllekrug J, Scheel CH, Wurst W, Schick JA, Kagan VE, Angeli JP, Conrad M. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 2702] [Article Influence: 300.2] [Reference Citation Analysis (0)] |
| 98. | Tsurusaki S, Tsuchiya Y, Koumura T, Nakasone M, Sakamoto T, Matsuoka M, Imai H, Yuet-Yin Kok C, Okochi H, Nakano H, Miyajima A, Tanaka M. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. 2019;10:449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 367] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 99. | Stockwell BR. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185:2401-2421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1082] [Cited by in RCA: 1605] [Article Influence: 535.0] [Reference Citation Analysis (0)] |
| 100. | Feng H, Stockwell BR. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? PLoS Biol. 2018;16:e2006203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 563] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 101. | Capelletti MM, Manceau H, Puy H, Peoc'h K. Ferroptosis in Liver Diseases: An Overview. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 240] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 102. | Su Y, Zhao B, Zhou L, Zhang Z, Shen Y, Lv H, AlQudsy LHH, Shang P. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020;483:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 390] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 103. | Liang C, Zhang X, Yang M, Dong X. Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv Mater. 2019;31:e1904197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 1045] [Article Influence: 174.2] [Reference Citation Analysis (0)] |
| 104. | Zhang Y, Tan H, Daniels JD, Zandkarimi F, Liu H, Brown LM, Uchida K, O'Connor OA, Stockwell BR. Imidazole Ketone Erastin Induces Ferroptosis and Slows Tumor Growth in a Mouse Lymphoma Model. Cell Chem Biol. 2019;26:623-633.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 500] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 105. | Yang WS, Stockwell BR. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016;26:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 2099] [Article Influence: 209.9] [Reference Citation Analysis (0)] |
| 106. | Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med. 2019;133:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 1005] [Article Influence: 167.5] [Reference Citation Analysis (0)] |
| 107. | Weïwer M, Bittker JA, Lewis TA, Shimada K, Yang WS, MacPherson L, Dandapani S, Palmer M, Stockwell BR, Schreiber SL, Munoz B. Development of small-molecule probes that selectively kill cells induced to express mutant RAS. Bioorg Med Chem Lett. 2012;22:1822-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 108. | Guo J, Xu B, Han Q, Zhou H, Xia Y, Gong C, Dai X, Li Z, Wu G. Ferroptosis: A Novel Anti-tumor Action for Cisplatin. Cancer Res Treat. 2018;50:445-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 551] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 109. | Gryzik M, Asperti M, Denardo A, Arosio P, Poli M. NCOA4-mediated ferritinophagy promotes ferroptosis induced by erastin, but not by RSL3 in HeLa cells. Biochim Biophys Acta Mol Cell Res. 2021;1868:118913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 110. | Shimada K, Skouta R, Kaplan A, Yang WS, Hayano M, Dixon SJ, Brown LM, Valenzuela CA, Wolpaw AJ, Stockwell BR. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12:497-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 786] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 111. | Abrams RP, Carroll WL, Woerpel KA. Five-Membered Ring Peroxide Selectively Initiates Ferroptosis in Cancer Cells. ACS Chem Biol. 2016;11:1305-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 112. | Gaschler MM, Andia AA, Liu H, Csuka JM, Hurlocker B, Vaiana CA, Heindel DW, Zuckerman DS, Bos PH, Reznik E, Ye LF, Tyurina YY, Lin AJ, Shchepinov MS, Chan AY, Peguero-Pereira E, Fomich MA, Daniels JD, Bekish AV, Shmanai VV, Kagan VE, Mahal LK, Woerpel KA, Stockwell BR. FINO(2) initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol. 2018;14:507-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 549] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 113. | Giordano C, Plastina P, Barone I, Catalano S, Bonofiglio D. n-3 Polyunsaturated Fatty Acid Amides: New Avenues in the Prevention and Treatment of Breast Cancer. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 114. | Ahmad MZ, Ahmad J, Zafar S, Warsi MH, Abdel-Wahab BA, Akhter S, Alam MA. Omega-3 fatty acids as adjunctive therapeutics: prospective of nanoparticles in its formulation development. Ther Deliv. 2020;11:851-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 115. | Serini S, Cassano R, Trombino S, Calviello G. Nanomedicine-based formulations containing ω-3 polyunsaturated fatty acids: potential application in cardiovascular and neoplastic diseases. Int J Nanomedicine. 2019;14:2809-2828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 116. | Reynolds L, Mulik RS, Wen X, Dilip A, Corbin IR. Low-density lipoprotein-mediated delivery of docosahexaenoic acid selectively kills murine liver cancer cells. Nanomedicine (Lond). 2014;9:2123-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 117. | Fang Y, Chen X, Tan Q, Zhou H, Xu J, Gu Q. Inhibiting Ferroptosis through Disrupting the NCOA4-FTH1 Interaction: A New Mechanism of Action. ACS Cent Sci. 2021;7:980-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 308] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 118. | Chang LC, Chiang SK, Chen SE, Yu YL, Chou RH, Chang WC. Heme oxygenase-1 mediates BAY 11-7085 induced ferroptosis. Cancer Lett. 2018;416:124-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 404] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 119. | Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell. 2019;35:830-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 1620] [Article Influence: 270.0] [Reference Citation Analysis (0)] |
| 120. | Gugliandolo E, D'Amico R, Cordaro M, Fusco R, Siracusa R, Crupi R, Impellizzeri D, Cuzzocrea S, Di Paola R. Neuroprotective Effect of Artesunate in Experimental Model of Traumatic Brain Injury. Front Neurol. 2018;9:590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 121. | Kong Z, Liu R, Cheng Y. Artesunate alleviates liver fibrosis by regulating ferroptosis signaling pathway. Biomed Pharmacother. 2019;109:2043-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 268] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 122. | Li P, Li S, Gu H, Lu Q, Jiang W, Pei X, Sun Y, Xu H, Wang G, Hao K. The exposure-effect-toxicity correlation of docetaxel and magnesium isoglycyrrhizinate in non-small cell lung tumor-bearing mice. Biomed Pharmacother. 2018;97:1000-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 123. | Sui M, Jiang X, Chen J, Yang H, Zhu Y. Magnesium isoglycyrrhizinate ameliorates liver fibrosis and hepatic stellate cell activation by regulating ferroptosis signaling pathway. Biomed Pharmacother. 2018;106:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 124. | Theurl I, Hilgendorf I, Nairz M, Tymoszuk P, Haschka D, Asshoff M, He S, Gerhardt LM, Holderried TA, Seifert M, Sopper S, Fenn AM, Anzai A, Rattik S, McAlpine C, Theurl M, Wieghofer P, Iwamoto Y, Weber GF, Harder NK, Chousterman BG, Arvedson TL, McKee M, Wang F, Lutz OM, Rezoagli E, Babitt JL, Berra L, Prinz M, Nahrendorf M, Weiss G, Weissleder R, Lin HY, Swirski FK. On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nat Med. 2016;22:945-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 314] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 125. | Recalcati S, Locati M, Gammella E, Invernizzi P, Cairo G. Iron levels in polarized macrophages: regulation of immunity and autoimmunity. Autoimmun Rev. 2012;11:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 126. | Haldar M, Kohyama M, So AY, Kc W, Wu X, Briseño CG, Satpathy AT, Kretzer NM, Arase H, Rajasekaran NS, Wang L, Egawa T, Igarashi K, Baltimore D, Murphy TL, Murphy KM. Heme-mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell. 2014;156:1223-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 327] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 127. | Marques L, Negre-Salvayre A, Costa L, Canonne-Hergaux F. Iron gene expression profile in atherogenic Mox macrophages. Biochim Biophys Acta. 2016;1862:1137-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 128. | Handa P, Thomas S, Morgan-Stevenson V, Maliken BD, Gochanour E, Boukhar S, Yeh MM, Kowdley KV. Iron alters macrophage polarization status and leads to steatohepatitis and fibrogenesis. J Leukoc Biol. 2019;105:1015-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 129. | Zhou Y, Que KT, Zhang Z, Yi ZJ, Zhao PX, You Y, Gong JP, Liu ZJ. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer Med. 2018;7:4012-4022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 237] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 130. | Kao JK, Wang SC, Ho LW, Huang SW, Lee CH, Lee MS, Yang RC, Shieh JJ. M2-like polarization of THP-1 monocyte-derived macrophages under chronic iron overload. Ann Hematol. 2020;99:431-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 131. | Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol. 2015;15:500-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 565] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 132. | Nairz M, Schleicher U, Schroll A, Sonnweber T, Theurl I, Ludwiczek S, Talasz H, Brandacher G, Moser PL, Muckenthaler MU, Fang FC, Bogdan C, Weiss G. Nitric oxide-mediated regulation of ferroportin-1 controls macrophage iron homeostasis and immune function in Salmonella infection. J Exp Med. 2013;210:855-873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 133. | Lim D, Kim KS, Jeong JH, Marques O, Kim HJ, Song M, Lee TH, Kim JI, Choi HS, Min JJ, Bumann D, Muckenthaler MU, Choy HE. The hepcidin-ferroportin axis controls the iron content of Salmonella-containing vacuoles in macrophages. Nat Commun. 2018;9:2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 134. | Bennett H, Troutman TD, Sakai M, Glass CK. Epigenetic Regulation of Kupffer Cell Function in Health and Disease. Front Immunol. 2020;11:609618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 135. | Asanuma T, Ono M, Kubota K, Hirose A, Hayashi Y, Saibara T, Inanami O, Ogawa Y, Enzan H, Onishi S, Kuwabara M, Oben JA. Super paramagnetic iron oxide MRI shows defective Kupffer cell uptake function in non-alcoholic fatty liver disease. Gut. 2010;59:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 136. | Cheong H, Lee SS, Lee JS, Kim J, Kim SW, Lee WJ. Phagocytic function of Kupffer cells in mouse nonalcoholic fatty liver disease models: Evaluation with superparamagnetic iron oxide. J Magn Reson Imaging. 2015;41:1218-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 137. | Wang H, Li L, Li Y, Sha Y, Wen S, You Q, Liu L, Shi M, Zhou H. Intravital imaging of interactions between iNKT and kupffer cells to clear free lipids during steatohepatitis. Theranostics. 2021;11:2149-2169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 138. | Yin Y, Wang Q, Qi M, Zhang C, Li Z, Zhang W. Ghrelin ameliorates nonalcoholic steatohepatitis induced by chronic low-grade inflammation via blockade of Kupffer cell M1 polarization. J Cell Physiol. 2021;236:5121-5133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 139. | Tran S, Baba I, Poupel L, Dussaud S, Moreau M, Gélineau A, Marcelin G, Magréau-Davy E, Ouhachi M, Lesnik P, Boissonnas A, Le Goff W, Clausen BE, Yvan-Charvet L, Sennlaub F, Huby T, Gautier EL. Impaired Kupffer Cell Self-Renewal Alters the Liver Response to Lipid Overload during Non-alcoholic Steatohepatitis. Immunity. 2020;53:627-640.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 252] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 140. | Pereira M, Chen TD, Buang N, Olona A, Ko JH, Prendecki M, Costa ASH, Nikitopoulou E, Tronci L, Pusey CD, Cook HT, McAdoo SP, Frezza C, Behmoaras J. Acute Iron Deprivation Reprograms Human Macrophage Metabolism and Reduces Inflammation In Vivo. Cell Rep. 2019;28:498-511.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 141. | Dufrusine B, Di Francesco A, Oddi S, Scipioni L, Angelucci CB, D'Addario C, Serafini M, Häfner AK, Steinhilber D, Maccarrone M, Dainese E. Iron-Dependent Trafficking of 5-Lipoxygenase and Impact on Human Macrophage Activation. Front Immunol. 2019;10:1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 142. | Wu J, Wang Y, Jiang R, Xue R, Yin X, Wu M, Meng Q. Ferroptosis in liver disease: new insights into disease mechanisms. Cell Death Discov. 2021;7:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 169] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 143. | Zhou X, Fu Y, Liu W, Mu Y, Zhang H, Chen J, Liu P. Ferroptosis in Chronic Liver Diseases: Opportunities and Challenges. Front Mol Biosci. 2022;9:928321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 144. | Chen S, Zhu JY, Zang X, Zhai YZ. The Emerging Role of Ferroptosis in Liver Diseases. Front Cell Dev Biol. 2021;9:801365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 145. | Chen J, Li X, Ge C, Min J, Wang F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 2022;29:467-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 399] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 146. | Ma S, Adzavon YM, Wen X, Zhao P, Xie F, Liu M, Ma X. Novel Insights in the Regulatory Mechanisms of Ferroptosis in Hepatocellular Carcinoma. Front Cell Dev Biol. 2022;10:873029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 147. | Liao H, Shi J, Wen K, Lin J, Liu Q, Shi B, Yan Y, Xiao Z. Molecular Targets of Ferroptosis in Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:985-996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 148. | Wang MP, Joshua B, Jin NY, Du SW, Li C. Ferroptosis in viral infection: the unexplored possibility. Acta Pharmacol Sin. 2022;43:1905-1915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 149. | Yang Y, Wang Y, Guo L, Gao W, Tang TL, Yan M. Interaction between macrophages and ferroptosis. Cell Death Dis. 2022;13:355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 198] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 150. | França M, Martí-Bonmatí L, Porto G, Silva S, Guimarães S, Alberich-Bayarri Á, Vizcaíno JR, Pessegueiro Miranda H. Tissue iron quantification in chronic liver diseases using MRI shows a relationship between iron accumulation in liver, spleen, and bone marrow. Clin Radiol. 2018;73:215.e1-215.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 151. | Fierro-Fine A, Guerin L, Hicsasmaz H, Brown KE. Clinical Factors Associated with Hepatocellular Iron Deposition in End-stage Liver Disease. J Clin Transl Hepatol. 2020;8:231-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 152. | Vela D. Low hepcidin in liver fibrosis and cirrhosis; a tale of progressive disorder and a case for a new biochemical marker. Mol Med. 2018;24:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 153. | Gautheron J, Gores GJ, Rodrigues CMP. Lytic cell death in metabolic liver disease. J Hepatol. 2020;73:394-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 154. | Siddique A, Kowdley KV. Review article: the iron overload syndromes. Aliment Pharmacol Ther. 2012;35:876-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 155. | Datz C, Felder TK, Niederseer D, Aigner E. Iron homeostasis in the metabolic syndrome. Eur J Clin Invest. 2013;43:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 156. | Alla V, Bonkovsky HL. Iron in nonhemochromatotic liver disorders. Semin Liver Dis. 2005;25:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 157. | Bloomer SA, Brown KE. Iron-Induced Liver Injury: A Critical Reappraisal. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 158. | Dongiovanni P, Fracanzani AL, Fargion S, Valenti L. Iron in fatty liver and in the metabolic syndrome: a promising therapeutic target. J Hepatol. 2011;55:920-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 267] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 159. | Barisani D, Pelucchi S, Mariani R, Galimberti S, Trombini P, Fumagalli D, Meneveri R, Nemeth E, Ganz T, Piperno A. Hepcidin and iron-related gene expression in subjects with Dysmetabolic Hepatic Iron Overload. J Hepatol. 2008;49:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 160. | Trombini P, Paolini V, Pelucchi S, Mariani R, Nemeth E, Ganz T, Piperno A. Hepcidin response to acute iron intake and chronic iron loading in dysmetabolic iron overload syndrome. Liver Int. 2011;31:994-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 161. | Riva A, Trombini P, Mariani R, Salvioni A, Coletti S, Bonfadini S, Paolini V, Pozzi M, Facchetti R, Bovo G, Piperno A. Revaluation of clinical and histological criteria for diagnosis of dysmetabolic iron overload syndrome. World J Gastroenterol. 2008;14:4745-4752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 162. | Nelson JE, Klintworth H, Kowdley KV. Iron metabolism in Nonalcoholic Fatty Liver Disease. Curr Gastroenterol Rep. 2012;14:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 163. | Aigner E, Weiss G, Datz C. Dysregulation of iron and copper homeostasis in nonalcoholic fatty liver. World J Hepatol. 2015;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 164. | Manco M, Alisi A, Real JF, Equitani F, DeVito R, Valenti L, Nobili V. Early interplay of intra-hepatic iron and insulin resistance in children with non-alcoholic fatty liver disease. J Hepatol. 2011;55:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 165. | Dongiovanni P, Lanti C, Gatti S, Rametta R, Recalcati S, Maggioni M, Fracanzani AL, Riso P, Cairo G, Fargion S, Valenti L. High fat diet subverts hepatocellular iron uptake determining dysmetabolic iron overload. PLoS One. 2015;10:e0116855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 166. | Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, Sanyal AJ, Nelson JE; NASH Clinical Research Network. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 401] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 167. | Valenti L, Dongiovanni P, Fargion S. Diagnostic and therapeutic implications of the association between ferritin level and severity of nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18:3782-3786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 168. | Nelson JE, Bhattacharya R, Lindor KD, Chalasani N, Raaka S, Heathcote EJ, Miskovsky E, Shaffer E, Rulyak SJ, Kowdley KV. HFE C282Y mutations are associated with advanced hepatic fibrosis in Caucasians with nonalcoholic steatohepatitis. Hepatology. 2007;46:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 169. | Valenti L. Uncovering the genetics of cirrhosis: New plots for the usual suspects. Liver Int. 2020;40:281-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 170. | Chen VL, Chen Y, Du X, Handelman SK, Speliotes EK. Genetic variants that associate with cirrhosis have pleiotropic effects on human traits. Liver Int. 2020;40:405-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 171. | Wagner J, Fillebeen C, Haliotis T, Charlebois E, Katsarou A, Mui J, Vali H, Pantopoulos K. Mouse models of hereditary hemochromatosis do not develop early liver fibrosis in response to a high fat diet. PLoS One. 2019;14:e0221455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 172. | Corradini E, Buzzetti E, Dongiovanni P, Scarlini S, Caleffi A, Pelusi S, Bernardis I, Ventura P, Rametta R, Tenedini E, Tagliafico E, Fracanzani AL, Fargion S, Pietrangelo A, Valenti LV. Ceruloplasmin gene variants are associated with hyperferritinemia and increased liver iron in patients with NAFLD. J Hepatol. 2021;75:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 173. | Corradini E, Valenti LV. Reply to: "Ceruloplasmin variants might have different effects in different iron overload disorders". J Hepatol. 2021;75:1004-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 174. | Viveiros A, Schaefer B, Panzer M, Henninger B, Plaikner M, Kremser C, Franke A, Franzenburg S, Hoeppner MP, Stauder R, Janecke A, Tilg H, Zoller H. MRI-Based Iron Phenotyping and Patient Selection for Next-Generation Sequencing of Non-Homeostatic Iron Regulator Hemochromatosis Genes. Hepatology. 2021;74:2424-2435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 175. | Handa P, Morgan-Stevenson V, Maliken BD, Nelson JE, Washington S, Westerman M, Yeh MM, Kowdley KV. Iron overload results in hepatic oxidative stress, immune cell activation, and hepatocellular ballooning injury, leading to nonalcoholic steatohepatitis in genetically obese mice. Am J Physiol Gastrointest Liver Physiol. 2016;310:G117-G127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 176. | Masarone M, Rosato V, Dallio M, Gravina AG, Aglitti A, Loguercio C, Federico A, Persico M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid Med Cell Longev. 2018;2018:9547613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 482] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 177. | Uysal S, Armutcu F, Aydogan T, Akin K, Ikizek M, Yigitoglu MR. Some inflammatory cytokine levels, iron metabolism and oxidan stress markers in subjects with nonalcoholic steatohepatitis. Clin Biochem. 2011;44:1375-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 178. | Valenti L, Dongiovanni P, Fracanzani AL, Santorelli G, Fatta E, Bertelli C, Taioli E, Fiorelli G, Fargion S. Increased susceptibility to nonalcoholic fatty liver disease in heterozygotes for the mutation responsible for hereditary hemochromatosis. Dig Liver Dis. 2003;35:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 179. | Valenti L, Fracanzani AL, Bugianesi E, Dongiovanni P, Galmozzi E, Vanni E, Canavesi E, Lattuada E, Roviaro G, Marchesini G, Fargion S. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2010;138:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 180. | Bonkovsky HL, Jawaid Q, Tortorelli K, LeClair P, Cobb J, Lambrecht RW, Banner BF. Non-alcoholic steatohepatitis and iron: increased prevalence of mutations of the HFE gene in non-alcoholic steatohepatitis. J Hepatol. 1999;31:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 236] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 181. | Graham RM, Chua AC, Carter KW, Delima RD, Johnstone D, Herbison CE, Firth MJ, O'Leary R, Milward EA, Olynyk JK, Trinder D. Hepatic iron loading in mice increases cholesterol biosynthesis. Hepatology. 2010;52:462-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 182. | Britton LJ, Subramaniam VN, Crawford DH. Iron and non-alcoholic fatty liver disease. World J Gastroenterol. 2016;22:8112-8122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (4)] |
| 183. | O'Brien J, Powell LW. Non-alcoholic fatty liver disease: is iron relevant? Hepatol Int. 2012;6:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 184. | Tan TC, Crawford DH, Jaskowski LA, Subramaniam VN, Clouston AD, Crane DI, Bridle KR, Anderson GJ, Fletcher LM. Excess iron modulates endoplasmic reticulum stress-associated pathways in a mouse model of alcohol and high-fat diet-induced liver injury. Lab Invest. 2013;93:1295-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 185. | Nelson JE, Wilson L, Brunt EM, Yeh MM, Kleiner DE, Unalp-Arida A, Kowdley KV; Nonalcoholic Steatohepatitis Clinical Research Network. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology. 2011;53:448-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 186. | Kanamori Y, Tanaka M, Itoh M, Ochi K, Ito A, Hidaka I, Sakaida I, Ogawa Y, Suganami T. Iron-rich Kupffer cells exhibit phenotypic changes during the development of liver fibrosis in NASH. iScience. 2021;24:102032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 187. | Sorrentino P, D'Angelo S, Ferbo U, Micheli P, Bracigliano A, Vecchione R. Liver iron excess in patients with hepatocellular carcinoma developed on non-alcoholic steato-hepatitis. J Hepatol. 2009;50:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 188. | Otogawa K, Kinoshita K, Fujii H, Sakabe M, Shiga R, Nakatani K, Ikeda K, Nakajima Y, Ikura Y, Ueda M, Arakawa T, Hato F, Kawada N. Erythrophagocytosis by liver macrophages (Kupffer cells) promotes oxidative stress, inflammation, and fibrosis in a rabbit model of steatohepatitis: implications for the pathogenesis of human nonalcoholic steatohepatitis. Am J Pathol. 2007;170:967-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 189. | May M, Barlow D, Ibrahim R, Houseknecht KL. Mechanisms Underlying Antipsychotic-Induced NAFLD and Iron Dysregulation: A Multi-Omic Approach. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 190. | Du SX, Lu LL, Geng N, Victor DW, Chen LZ, Wang C, Yue HY, Xin YN, Xuan SY, Jin WW. Association of serum ferritin with non-alcoholic fatty liver disease: a meta-analysis. Lipids Health Dis. 2017;16:228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 191. | Kim CW, Chang Y, Sung E, Shin H, Ryu S. Serum ferritin levels predict incident non-alcoholic fatty liver disease in healthy Korean men. Metabolism. 2012;61:1182-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 192. | Manousou P, Kalambokis G, Grillo F, Watkins J, Xirouchakis E, Pleguezuelo M, Leandro G, Arvaniti V, Germani G, Patch D, Calvaruso V, Mikhailidis DP, Dhillon AP, Burroughs AK. Serum ferritin is a discriminant marker for both fibrosis and inflammation in histologically proven non-alcoholic fatty liver disease patients. Liver Int. 2011;31:730-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 193. | Jung JY, Shim JJ, Park SK, Ryoo JH, Choi JM, Oh IH, Jung KW, Cho H, Ki M, Won YJ, Oh CM. Serum ferritin level is associated with liver steatosis and fibrosis in Korean general population. Hepatol Int. 2019;13:222-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 194. | Bunda S, Kaviani N, Hinek A. Fluctuations of intracellular iron modulate elastin production. J Biol Chem. 2005;280:2341-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 195. | Hagström H, Nasr P, Bottai M, Ekstedt M, Kechagias S, Hultcrantz R, Stål P. Elevated serum ferritin is associated with increased mortality in non-alcoholic fatty liver disease after 16 years of follow-up. Liver Int. 2016;36:1688-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 196. | Ghamarchehreh ME, Jonaidi-Jafari N, Bigdeli M, Khedmat H, Saburi A. Iron Status and Metabolic Syndrome in Patients with Non-Alcoholic Fatty Liver Disease. Middle East J Dig Dis. 2016;8:31-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 197. | Maiwall R, Kumar S, Chaudhary AK, Maras J, Wani Z, Kumar C, Rastogi A, Bihari C, Vashisht C, Sarin SK. Serum ferritin predicts early mortality in patients with decompensated cirrhosis. J Hepatol. 2014;61:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 198. | Valenti L, Swinkels DW, Burdick L, Dongiovanni P, Tjalsma H, Motta BM, Bertelli C, Fatta E, Bignamini D, Rametta R, Fargion S, Fracanzani AL. Serum ferritin levels are associated with vascular damage in patients with nonalcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2011;21:568-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 199. | Chitturi S, Weltman M, Farrell GC, McDonald D, Kench J, Liddle C, Samarasinghe D, Lin R, Abeygunasekera S, George J. HFE mutations, hepatic iron, and fibrosis: ethnic-specific association of NASH with C282Y but not with fibrotic severity. Hepatology. 2002;36:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 200. | Chandok N, Minuk G, Wengiel M, Uhanova J. Serum ferritin levels do not predict the stage of underlying non-alcoholic fatty liver disease. J Gastrointestin Liver Dis. 2012;21:53-58. [PubMed] |
| 201. | Buzzetti E, Petta S, Manuguerra R, Luong TV, Cabibi D, Corradini E, Craxì A, Pinzani M, Tsochatzis E, Pietrangelo A. Evaluating the association of serum ferritin and hepatic iron with disease severity in non-alcoholic fatty liver disease. Liver Int. 2019;39:1325-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 202. | Ryan JD, Armitage AE, Cobbold JF, Banerjee R, Borsani O, Dongiovanni P, Neubauer S, Morovat R, Wang LM, Pasricha SR, Fargion S, Collier J, Barnes E, Drakesmith H, Valenti L, Pavlides M. Hepatic iron is the major determinant of serum ferritin in NAFLD patients. Liver Int. 2018;38:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 203. | Mayneris-Perxachs J, Cardellini M, Hoyles L, Latorre J, Davato F, Moreno-Navarrete JM, Arnoriaga-Rodríguez M, Serino M, Abbott J, Barton RH, Puig J, Fernández-Real X, Ricart W, Tomlinson C, Woodbridge M, Gentileschi P, Butcher SA, Holmes E, Nicholson JK, Pérez-Brocal V, Moya A, Clain DM, Burcelin R, Dumas ME, Federici M, Fernández-Real JM. Iron status influences non-alcoholic fatty liver disease in obesity through the gut microbiome. Microbiome. 2021;9:104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 204. | Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Iannelli A, Staccini-Myx A, Casanova D, Ben Amor I, Saint-Paul MC, Huet PM, Sadoul JL, Gugenheim J, Srai SK, Tran A, Le Marchand-Brustel Y. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 349] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 205. | Coimbra S, Catarino C, Santos-Silva A. The role of adipocytes in the modulation of iron metabolism in obesity. Obes Rev. 2013;14:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 206. | Simcox JA, McClain DA. Iron and diabetes risk. Cell Metab. 2013;17:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 384] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 207. | Smith BW, Adams LA. Nonalcoholic fatty liver disease and diabetes mellitus: pathogenesis and treatment. Nat Rev Endocrinol. 2011;7:456-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 208. | Corradini E, Pietrangelo A. Iron and steatohepatitis. J Gastroenterol Hepatol. 2012;27 Suppl 2:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 209. | Chung B, Matak P, McKie AT, Sharp P. Leptin increases the expression of the iron regulatory hormone hepcidin in HuH7 human hepatoma cells. J Nutr. 2007;137:2366-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 210. | Zumerle S, Mathieu JR, Delga S, Heinis M, Viatte L, Vaulont S, Peyssonnaux C. Targeted disruption of hepcidin in the liver recapitulates the hemochromatotic phenotype. Blood. 2014;123:3646-3650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 211. | Tsuchiya H, Ebata Y, Sakabe T, Hama S, Kogure K, Shiota G. High-fat, high-fructose diet induces hepatic iron overload via a hepcidin-independent mechanism prior to the onset of liver steatosis and insulin resistance in mice. Metabolism. 2013;62:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 212. | Hasebe T, Tanaka H, Sawada K, Nakajima S, Ohtake T, Fujiya M, Kohgo Y. Bone morphogenetic protein-binding endothelial regulator of liver sinusoidal endothelial cells induces iron overload in a fatty liver mouse model. J Gastroenterol. 2017;52:341-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 213. | Boga S, Alkim H, Alkim C, Koksal AR, Bayram M, Yilmaz Ozguven MB, Tekin Neijmann S. The Relationship of Serum Hemojuvelin and Hepcidin Levels with Iron Overload in Nonalcoholic Fatty Liver Disease. J Gastrointestin Liver Dis. 2015;24:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 214. | Senates E, Yilmaz Y, Colak Y, Ozturk O, Altunoz ME, Kurt R, Ozkara S, Aksaray S, Tuncer I, Ovunc AO. Serum levels of hepcidin in patients with biopsy-proven nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2011;9:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 215. | Martinelli N, Traglia M, Campostrini N, Biino G, Corbella M, Sala C, Busti F, Masciullo C, Manna D, Previtali S, Castagna A, Pistis G, Olivieri O, Toniolo D, Camaschella C, Girelli D. Increased serum hepcidin levels in subjects with the metabolic syndrome: a population study. PLoS One. 2012;7:e48250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 216. | Handa P, Vemulakonda AL, Maliken BD, Morgan-Stevenson V, Nelson JE, Dhillon BK, Hennessey KA, Gupta R, Yeh MM, Kowdley KV. Differences in hepatic expression of iron, inflammation and stress-related genes in patients with nonalcoholic steatohepatitis. Ann Hepatol. 2017;16:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 217. | Marmur J, Beshara S, Eggertsen G, Onelöv L, Albiin N, Danielsson O, Hultcrantz R, Stål P. Hepcidin levels correlate to liver iron content, but not steatohepatitis, in non-alcoholic fatty liver disease. BMC Gastroenterol. 2018;18:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 218. | Hoki T, Miyanishi K, Tanaka S, Takada K, Kawano Y, Sakurada A, Sato M, Kubo T, Sato T, Sato Y, Takimoto R, Kobune M, Kato J. Increased duodenal iron absorption through up-regulation of divalent metal transporter 1 from enhancement of iron regulatory protein 1 activity in patients with nonalcoholic steatohepatitis. Hepatology. 2015;62:751-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 219. | Wang H, Li H, Jiang X, Shi W, Shen Z, Li M. Hepcidin is directly regulated by insulin and plays an important role in iron overload in streptozotocin-induced diabetic rats. Diabetes. 2014;63:1506-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 220. | Fleming DJ, Tucker KL, Jacques PF, Dallal GE, Wilson PW, Wood RJ. Dietary factors associated with the risk of high iron stores in the elderly Framingham Heart Study cohort. Am J Clin Nutr. 2002;76:1375-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 221. | Bowers K, Yeung E, Williams MA, Qi L, Tobias DK, Hu FB, Zhang C. A prospective study of prepregnancy dietary iron intake and risk for gestational diabetes mellitus. Diabetes Care. 2011;34:1557-1563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 222. | Qiu C, Zhang C, Gelaye B, Enquobahrie DA, Frederick IO, Williams MA. Gestational diabetes mellitus in relation to maternal dietary heme iron and nonheme iron intake. Diabetes Care. 2011;34:1564-1569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 223. | Rametta R, Dongiovanni P, Pelusi S, Francione P, Iuculano F, Borroni V, Fatta E, Castagna A, Girelli D, Fargion S, Valenti L. Hepcidin resistance in dysmetabolic iron overload. Liver Int. 2016;36:1540-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 224. | Li X, Wang TX, Huang X, Li Y, Sun T, Zang S, Guan KL, Xiong Y, Liu J, Yuan HX. Targeting ferroptosis alleviates methionine-choline deficient (MCD)-diet induced NASH by suppressing liver lipotoxicity. Liver Int. 2020;40:1378-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 225. | Qi J, Kim JW, Zhou Z, Lim CW, Kim B. Ferroptosis Affects the Progression of Nonalcoholic Steatohepatitis via the Modulation of Lipid Peroxidation-Mediated Cell Death in Mice. Am J Pathol. 2020;190:68-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 236] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 226. | Zhang XJ, She ZG, Wang J, Sun D, Shen LJ, Xiang H, Cheng X, Ji YX, Huang YP, Li PL, Yang X, Cheng Y, Ma JP, Wang HP, Hu Y, Hu F, Tian S, Tian H, Zhang P, Zhao GN, Wang L, Hu ML, Yang Q, Zhu LH, Cai J, Yang J, Zhang X, Ma X, Xu Q, Touyz RM, Liu PP, Loomba R, Wang Y, Li H. Multiple omics study identifies an interspecies conserved driver for nonalcoholic steatohepatitis. Sci Transl Med. 2021;13:eabg8117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 227. | Zhang XJ, Ji YX, Cheng X, Cheng Y, Yang H, Wang J, Zhao LP, Huang YP, Sun D, Xiang H, Shen LJ, Li PL, Ma JP, Tian RF, Yang J, Yao X, Xu H, Liao R, Xiao L, Zhang P, Zhang X, Zhao GN, Wang X, Hu ML, Tian S, Wan J, Cai J, Ma X, Xu Q, Wang Y, Touyz RM, Liu PP, Loomba R, She ZG, Li H. A small molecule targeting ALOX12-ACC1 ameliorates nonalcoholic steatohepatitis in mice and macaques. Sci Transl Med. 2021;13:eabg8116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 228. | Wei S, Qiu T, Wang N, Yao X, Jiang L, Jia X, Tao Y, Zhang J, Zhu Y, Yang G, Liu X, Liu S, Sun X. Ferroptosis mediated by the interaction between Mfn2 and IREα promotes arsenic-induced nonalcoholic steatohepatitis. Environ Res. 2020;188:109824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 229. | Shao M, Ye Z, Qin Y, Wu T. Abnormal metabolic processes involved in the pathogenesis of non-alcoholic fatty liver disease (Review). Exp Ther Med. 2020;20:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 230. | Xu L, Liu W, Bai F, Xu Y, Liang X, Ma C, Gao L. Hepatic Macrophage as a Key Player in Fatty Liver Disease. Front Immunol. 2021;12:708978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 231. | Paganoni R, Lechel A, Vujic Spasic M. Iron at the Interface of Hepatocellular Carcinoma. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 232. | Lundvall O, Weinfeld A, Lundin P. Iron stores in alcohol abusers. I. Liver iron. Acta Med Scand. 1969;185:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 233. | Jakobovits AW, Morgan MY, Sherlock S. Hepatic siderosis in alcoholics. Dig Dis Sci. 1979;24:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 234. | Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Body iron metabolism and pathophysiology of iron overload. Int J Hematol. 2008;88:7-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 391] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 235. | Ioannou GN, Dominitz JA, Weiss NS, Heagerty PJ, Kowdley KV. The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemia. Gastroenterology. 2004;126:1293-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 236. | Chapman RW, Morgan MY, Boss AM, Sherlock S. Acute and chronic effects of alcohol on iron absorption. Dig Dis Sci. 1983;28:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 237. | Mueller S, Rausch V. The role of iron in alcohol-mediated hepatocarcinogenesis. Adv Exp Med Biol. 2015;815:89-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 238. | Bell H, Skinningsrud A, Raknerud N, Try K. Serum ferritin and transferrin saturation in patients with chronic alcoholic and non-alcoholic liver diseases. J Intern Med. 1994;236:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 239. | Ford C, Wells FE, Rogers JN. Assessment of iron status in association with excess alcohol consumption. Ann Clin Biochem. 1995;32 ( Pt 6):527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 240. | Milic S, Mikolasevic I, Orlic L, Devcic E, Starcevic-Cizmarevic N, Stimac D, Kapovic M, Ristic S. The Role of Iron and Iron Overload in Chronic Liver Disease. Med Sci Monit. 2016;22:2144-2151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 241. | Nahon P, Sutton A, Rufat P, Ziol M, Thabut G, Schischmanoff PO, Vidaud D, Charnaux N, Couvert P, Ganne-Carrie N, Trinchet JC, Gattegno L, Beaugrand M. Liver iron, HFE gene mutations, and hepatocellular carcinoma occurrence in patients with cirrhosis. Gastroenterology. 2008;134:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 242. | Ganne-Carrié N, Christidis C, Chastang C, Ziol M, Chapel F, Imbert-Bismut F, Trinchet JC, Guettier C, Beaugrand M. Liver iron is predictive of death in alcoholic cirrhosis: a multivariate study of 229 consecutive patients with alcoholic and/or hepatitis C virus cirrhosis: a prospective follow up study. Gut. 2000;46:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 243. | Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 534] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 244. | Grewal P, Viswanathen VA. Liver cancer and alcohol. Clin Liver Dis. 2012;16:839-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 245. | Tirnitz-Parker JE, Glanfield A, Olynyk JK, Ramm GA. Iron and hepatic carcinogenesis. Crit Rev Oncog. 2013;18:391-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 246. | Li LX, Guo FF, Liu H, Zeng T. Iron overload in alcoholic liver disease: underlying mechanisms, detrimental effects, and potential therapeutic targets. Cell Mol Life Sci. 2022;79:201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 247. | Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 766] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 248. | Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem. 2006;387:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 249. | Ganne-Carrié N, Nahon P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J Hepatol. 2019;70:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 250] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 250. | Harrison-Findik DD, Schafer D, Klein E, Timchenko NA, Kulaksiz H, Clemens D, Fein E, Andriopoulos B, Pantopoulos K, Gollan J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J Biol Chem. 2006;281:22974-22982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 222] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 251. | Bridle K, Cheung TK, Murphy T, Walters M, Anderson G, Crawford DG, Fletcher LM. Hepcidin is down-regulated in alcoholic liver injury: implications for the pathogenesis of alcoholic liver disease. Alcohol Clin Exp Res. 2006;30:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 252. | Costa-Matos L, Batista P, Monteiro N, Simões M, Egas C, Pereira J, Pinho H, Santos N, Ribeiro J, Cipriano MA, Henriques P, Girão F, Rodrigues A, Carvalho A. Liver hepcidin mRNA expression is inappropriately low in alcoholic patients compared with healthy controls. Eur J Gastroenterol Hepatol. 2012;24:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 253. | Ohtake T, Saito H, Hosoki Y, Inoue M, Miyoshi S, Suzuki Y, Fujimoto Y, Kohgo Y. Hepcidin is down-regulated in alcohol loading. Alcohol Clin Exp Res. 2007;31:S2-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 254. | Harrison-Findik DD, Klein E, Crist C, Evans J, Timchenko N, Gollan J. Iron-mediated regulation of liver hepcidin expression in rats and mice is abolished by alcohol. Hepatology. 2007;46:1979-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 255. | Tang Y, Li Y, Yu H, Gao C, Liu L, Chen S, Xing M, Yao P. Quercetin prevents ethanol-induced iron overload by regulating hepcidin through the BMP6/SMAD4 signaling pathway. J Nutr Biochem. 2014;25:675-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 256. | Suzuki Y, Saito H, Suzuki M, Hosoki Y, Sakurai S, Fujimoto Y, Kohgo Y. Up-regulation of transferrin receptor expression in hepatocytes by habitual alcohol drinking is implicated in hepatic iron overload in alcoholic liver disease. Alcohol Clin Exp Res. 2002;26:26S-31S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 257. | Kumar S, Wang J, Rani R, Gandhi CR. Hepatic Deficiency of Augmenter of Liver Regeneration Exacerbates Alcohol-Induced Liver Injury and Promotes Fibrosis in Mice. PLoS One. 2016;11:e0147864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 258. | Dostalikova-Cimburova M, Balusikova K, Kratka K, Chmelikova J, Hejda V, Hnanicek J, Neubauerova J, Vranova J, Kovar J, Horak J. Role of duodenal iron transporters and hepcidin in patients with alcoholic liver disease. J Cell Mol Med. 2014;18:1840-1850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 259. | Duane P, Raja KB, Simpson RJ, Peters TJ. Intestinal iron absorption in chronic alcoholics. Alcohol Alcohol. 1992;27:539-544. [PubMed] |
| 260. | Zhou Z, Ye TJ, Bonavita G, Daniels M, Kainrad N, Jogasuria A, You M. Adipose-Specific Lipin-1 Overexpression Renders Hepatic Ferroptosis and Exacerbates Alcoholic Steatohepatitis in Mice. Hepatol Commun. 2019;3:656-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 261. | Harrison-Findik DD, Lu S. The effect of alcohol and hydrogen peroxide on liver hepcidin gene expression in mice lacking antioxidant enzymes, glutathione peroxidase-1 or catalase. Biomolecules. 2015;5:793-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 262. | Gerjevic LN, Liu N, Lu S, Harrison-Findik DD. Alcohol Activates TGF-Beta but Inhibits BMP Receptor-Mediated Smad Signaling and Smad4 Binding to Hepcidin Promoter in the Liver. Int J Hepatol. 2012;2012:459278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 263. | Chen S, Feng T, Vujić Spasić M, Altamura S, Breitkopf-Heinlein K, Altenöder J, Weiss TS, Dooley S, Muckenthaler MU. Transforming Growth Factor β1 (TGF-β1) Activates Hepcidin mRNA Expression in Hepatocytes. J Biol Chem. 2016;291:13160-13174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 264. | Zmijewski E, Lu S, Harrison-Findik DD. TLR4 signaling and the inhibition of liver hepcidin expression by alcohol. World J Gastroenterol. 2014;20:12161-12170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 265. | Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 1555] [Article Influence: 86.4] [Reference Citation Analysis (1)] |
| 266. | Weber SN, Bohner A, Dapito DH, Schwabe RF, Lammert F. TLR4 Deficiency Protects against Hepatic Fibrosis and Diethylnitrosamine-Induced Pre-Carcinogenic Liver Injury in Fibrotic Liver. PLoS One. 2016;11:e0158819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 267. | Harrison-Findik DD, Klein E, Evans J, Gollan J. Regulation of liver hepcidin expression by alcohol in vivo does not involve Kupffer cell activation or TNF-alpha signaling. Am J Physiol Gastrointest Liver Physiol. 2009;296:G112-G118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 268. | Yang L, Seki E. Toll-like receptors in liver fibrosis: cellular crosstalk and mechanisms. Front Physiol. 2012;3:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 269. | Viveiros A, Finkenstedt A, Schaefer B, Mandorfer M, Scheiner B, Lehner K, Tobiasch M, Reiberger T, Tilg H, Edlinger M, Zoller H. Transferrin as a predictor of survival in cirrhosis. Liver Transpl. 2018;24:343-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 270. | Maras JS, Maiwall R, Harsha HC, Das S, Hussain MS, Kumar C, Bihari C, Rastogi A, Kumar M, Trehanpati N, Sharma S, Pandey A, Sarin SK. Dysregulated iron homeostasis is strongly associated with multiorgan failure and early mortality in acute-on-chronic liver failure. Hepatology. 2015;61:1306-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 271. | Anastasiou OE, Kälsch J, Hakmouni M, Kucukoglu O, Heider D, Korth J, Manka P, Sowa JP, Bechmann L, Saner FH, Paul A, Gerken G, Baba HA, Canbay A. Low transferrin and high ferritin concentrations are associated with worse outcome in acute liver failure. Liver Int. 2017;37:1032-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 272. | Bruns T, Nuraldeen R, Mai M, Stengel S, Zimmermann HW, Yagmur E, Trautwein C, Stallmach A, Strnad P. Low serum transferrin correlates with acute-on-chronic organ failure and indicates short-term mortality in decompensated cirrhosis. Liver Int. 2017;37:232-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 273. | Atkinson SR, Hamesch K, Spivak I, Guldiken N, Cabezas J, Argemi J, Theurl I, Zoller H, Cao S, Mathurin P, Shah VH, Trautwein C, Bataller R, Thursz MR, Strnad P. Serum Transferrin Is an Independent Predictor of Mortality in Severe Alcoholic Hepatitis. Am J Gastroenterol. 2020;115:398-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 274. | Wu J, Meng QH. Current understanding of the metabolism of micronutrients in chronic alcoholic liver disease. World J Gastroenterol. 2020;26:4567-4578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 275. | Zhang Y, Zhao S, Fu Y, Yan L, Feng Y, Chen Y, Wu Y, Deng Y, Zhang G, Chen Z, Liu T. Computational repositioning of dimethyl fumarate for treating alcoholic liver disease. Cell Death Dis. 2020;11:641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 276. | Liu CY, Wang M, Yu HM, Han FX, Wu QS, Cai XJ, Kurihara H, Chen YX, Li YF, He RR. Ferroptosis is involved in alcohol-induced cell death in vivo and in vitro. Biosci Biotechnol Biochem. 2020;84:1621-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 277. | Liu J, He H, Wang J, Guo X, Lin H, Chen H, Jiang C, Chen L, Yao P, Tang Y. Oxidative stress-dependent frataxin inhibition mediated alcoholic hepatocytotoxicity through ferroptosis. Toxicology. 2020;445:152584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 278. | Poole LG, Dolin CE, Arteel GE. Organ-Organ Crosstalk and Alcoholic Liver Disease. Biomolecules. 2017;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 279. | Zhou Z, Ye TJ, DeCaro E, Buehler B, Stahl Z, Bonavita G, Daniels M, You M. Intestinal SIRT1 Deficiency Protects Mice from Ethanol-Induced Liver Injury by Mitigating Ferroptosis. Am J Pathol. 2020;190:82-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 280. | Maras JS, Das S, Sharma S, Sukriti S, Kumar J, Vyas AK, Kumar D, Bhat A, Yadav G, Choudhary MC, Kumar G, Bihari C, Trehanpati N, Maiwall R, Sarin SK. Iron-Overload triggers ADAM-17 mediated inflammation in Severe Alcoholic Hepatitis. Sci Rep. 2018;8:10264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 281. | Mueller S, Chen C, Mueller J, Wang S. Novel Insights into Alcoholic Liver Disease: Iron Overload, Iron Sensing and Hemolysis. J Transl Int Med. 2022;10:92-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (1)] |
| 282. | Jia M, Zhang H, Qin Q, Hou Y, Zhang X, Chen D, Chen Y. Ferroptosis as a new therapeutic opportunity for nonviral liver disease. Eur J Pharmacol. 2021;908:174319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 283. | Fillebeen C, Pantopoulos K. Iron inhibits replication of infectious hepatitis C virus in permissive Huh7.5.1 cells. J Hepatol. 2010;53:995-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 284. | Theurl I, Zoller H, Obrist P, Datz C, Bachmann F, Elliott RM, Weiss G. Iron regulates hepatitis C virus translation via stimulation of expression of translation initiation factor 3. J Infect Dis. 2004;190:819-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 285. | Mancinelli R, Rosa L, Cutone A, Lepanto MS, Franchitto A, Onori P, Gaudio E, Valenti P. Viral Hepatitis and Iron Dysregulation: Molecular Pathways and the Role of Lactoferrin. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 286. | Zou DM, Sun WL. Relationship between Hepatitis C Virus Infection and Iron Overload. Chin Med J (Engl). 2017;130:866-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 287. | Foka P, Dimitriadis A, Karamichali E, Kyratzopoulou E, Giannimaras D, Koskinas J, Varaklioti A, Mamalaki A, Georgopoulou U. Alterations in the iron homeostasis network: A driving force for macrophage-mediated hepatitis C virus persistency. Virulence. 2016;7:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 288. | Miura K, Taura K, Kodama Y, Schnabl B, Brenner DA. Hepatitis C virus-induced oxidative stress suppresses hepcidin expression through increased histone deacetylase activity. Hepatology. 2008;48:1420-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 210] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 289. | Bartolomei G, Cevik RE, Marcello A. Modulation of hepatitis C virus replication by iron and hepcidin in Huh7 hepatocytes. J Gen Virol. 2011;92:2072-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 290. | Hino K, Nishina S, Sasaki K, Hara Y. Mitochondrial damage and iron metabolic dysregulation in hepatitis C virus infection. Free Radic Biol Med. 2019;133:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 291. | Nishina S, Hino K, Korenaga M, Vecchi C, Pietrangelo A, Mizukami Y, Furutani T, Sakai A, Okuda M, Hidaka I, Okita K, Sakaida I. Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology. 2008;134:226-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 292. | Bassett SE, Di Bisceglie AM, Bacon BR, Sharp RM, Govindarajan S, Hubbard GB, Brasky KM, Lanford RE. Effects of iron loading on pathogenicity in hepatitis C virus-infected chimpanzees. Hepatology. 1999;29:1884-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 293. | Martin DN, Uprichard SL. Identification of transferrin receptor 1 as a hepatitis C virus entry factor. Proc Natl Acad Sci U S A. 2013;110:10777-10782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 294. | Wessling-Resnick M. Crossing the Iron Gate: Why and How Transferrin Receptors Mediate Viral Entry. Annu Rev Nutr. 2018;38:431-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 295. | Eddowes LA, Al-Hourani K, Ramamurthy N, Frankish J, Baddock HT, Sandor C, Ryan JD, Fusco DN, Arezes J, Giannoulatou E, Boninsegna S, Chevaliez S, Owens BMJ, Sun CC, Fabris P, Giordani MT, Martines D, Vukicevic S, Crowe J, Lin HY, Rehwinkel J, McHugh PJ, Binder M, Babitt JL, Chung RT, Lawless MW, Armitage AE, Webber C, Klenerman P, Drakesmith H. Antiviral activity of bone morphogenetic proteins and activins. Nat Microbiol. 2019;4:339-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 296. | Salama MF, Bayele HK, Srai SS. Tumour necrosis factor alpha downregulates human hemojuvelin expression via a novel response element within its promoter. J Biomed Sci. 2012;19:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 297. | Armitage AE, Stacey AR, Giannoulatou E, Marshall E, Sturges P, Chatha K, Smith NM, Huang X, Xu X, Pasricha SR, Li N, Wu H, Webster C, Prentice AM, Pellegrino P, Williams I, Norris PJ, Drakesmith H, Borrow P. Distinct patterns of hepcidin and iron regulation during HIV-1, HBV, and HCV infections. Proc Natl Acad Sci U S A. 2014;111:12187-12192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 298. | Girelli D, Pasino M, Goodnough JB, Nemeth E, Guido M, Castagna A, Busti F, Campostrini N, Martinelli N, Vantini I, Corrocher R, Ganz T, Fattovich G. Reduced serum hepcidin levels in patients with chronic hepatitis C. J Hepatol. 2009;51:845-852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 299. | Fujita N, Sugimoto R, Takeo M, Urawa N, Mifuji R, Tanaka H, Kobayashi Y, Iwasa M, Watanabe S, Adachi Y, Kaito M. Hepcidin expression in the liver: relatively low level in patients with chronic hepatitis C. Mol Med. 2007;13:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 300. | Tsochatzis E, Papatheodoridis GV, Koliaraki V, Hadziyannis E, Kafiri G, Manesis EK, Mamalaki A, Archimandritis AJ. Serum hepcidin levels are related to the severity of liver histological lesions in chronic hepatitis C. J Viral Hepat. 2010;17:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 301. | Metwally MA, Zein CO, Zein NN. Clinical significance of hepatic iron deposition and serum iron values in patients with chronic hepatitis C infection. Am J Gastroenterol. 2004;99:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 302. | Shan Y, Lambrecht RW, Bonkovsky HL. Association of hepatitis C virus infection with serum iron status: analysis of data from the third National Health and Nutrition Examination Survey. Clin Infect Dis. 2005;40:834-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 303. | Fujita N, Sugimoto R, Urawa N, Araki J, Mifuji R, Yamamoto M, Horiike S, Tanaka H, Iwasa M, Kobayashi Y, Adachi Y, Kaito M. Hepatic iron accumulation is associated with disease progression and resistance to interferon/ribavirin combination therapy in chronic hepatitis C. J Gastroenterol Hepatol. 2007;22:1886-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 304. | Fujita N, Horiike S, Sugimoto R, Tanaka H, Iwasa M, Kobayashi Y, Hasegawa K, Ma N, Kawanishi S, Adachi Y, Kaito M. Hepatic oxidative DNA damage correlates with iron overload in chronic hepatitis C patients. Free Radic Biol Med. 2007;42:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 305. | Miyanishi K, Tanaka S, Sakamoto H, Kato J. The role of iron in hepatic inflammation and hepatocellular carcinoma. Free Radic Biol Med. 2019;133:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 306. | Kato J, Kobune M, Nakamura T, Kuroiwa G, Takada K, Takimoto R, Sato Y, Fujikawa K, Takahashi M, Takayama T, Ikeda T, Niitsu Y. Normalization of elevated hepatic 8-hydroxy-2'-deoxyguanosine levels in chronic hepatitis C patients by phlebotomy and low iron diet. Cancer Res. 2001;61:8697-8702. [PubMed] |
| 307. | Lambrecht RW, Sterling RK, Naishadham D, Stoddard AM, Rogers T, Morishima C, Morgan TR, Bonkovsky HL; HALT-C Trial Group. Iron levels in hepatocytes and portal tract cells predict progression and outcomes of patients with advanced chronic hepatitis C. Gastroenterology. 2011;140:1490-500.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 308. | Isomura T, Yano M, Hayashi H, Sakamoto N. Excess iron in the liver of patients with chronic hepatitis C. J Clin Electron Microsc. 1992;25:231-237. [DOI] [Full Text] |
| 309. | Mitsuyoshi H, Yasui K, Yamaguchi K, Minami M, Okanoue T, Itoh Y. Pathogenic Role of Iron Deposition in Reticuloendothelial Cells during the Development of Chronic Hepatitis C. Int J Hepatol. 2013;2013:686420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 310. | Geier A, Reugels M, Weiskirchen R, Wasmuth HE, Dietrich CG, Siewert E, Gartung C, Lorenzen J, Bosserhoff AK, Brügmann M, Gressner AM, Matern S, Lammert F. Common heterozygous hemochromatosis gene mutations are risk factors for inflammation and fibrosis in chronic hepatitis C. Liver Int. 2004;24:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 311. | Smith BC, Gorve J, Guzail MA, Day CP, Daly AK, Burt AD, Bassendine MF. Heterozygosity for hereditary hemochromatosis is associated with more fibrosis in chronic hepatitis C. Hepatology. 1998;27:1695-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 144] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 312. | Sebastiani G, Vario A, Ferrari A, Pistis R, Noventa F, Alberti A. Hepatic iron, liver steatosis and viral genotypes in patients with chronic hepatitis C. J Viral Hepat. 2006;13:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 313. | Bou Daher H, Sharara AI. Treatment of Chronic HCV Infection in Patients With Thalassemia. Clin Liver Dis (Hoboken). 2019;14:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 314. | Di Bisceglie AM, Bonkovsky HL, Chopra S, Flamm S, Reddy RK, Grace N, Killenberg P, Hunt C, Tamburro C, Tavill AS, Ferguson R, Krawitt E, Banner B, Bacon BR. Iron reduction as an adjuvant to interferon therapy in patients with chronic hepatitis C who have previously not responded to interferon: a multicenter, prospective, randomized, controlled trial. Hepatology. 2000;32:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 315. | Carvalho JR, Velosa J, Serejo F. Lipids, glucose and iron metabolic alterations in chronic hepatitis C after viral eradication - comparison of the new direct-acting antiviral agents with the old regimens. Scand J Gastroenterol. 2018;53:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 316. | Chang ML, Hu JH, Yen CH, Chen KH, Kuo CJ, Lin MS, Lee CH, Chen SC, Chien RN. Evolution of ferritin levels in hepatitis C patients treated with antivirals. Sci Rep. 2020;10:19744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 317. | Mangia A, Sarli R, Gamberini R, Piga A, Cenderello G, Piazzolla V, Santoro R, Caruso V, Quarta A, Ganga R, Copetti M, Forni G. Randomised clinical trial: sofosbuvir and ledipasvir in patients with transfusion-dependent thalassaemia and HCV genotype 1 or 4 infection. Aliment Pharmacol Ther. 2017;46:424-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 318. | Nagral A, Jhaveri A, Sawant S, Parikh NS, Nagral N, Merchant R, Gandhi M. Treatment of Chronic Hepatitis C Infection with Direct Acting Antivirals in Adolescents with Thalassemia Major. Indian J Pediatr. 2019;86:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 319. | Sharara AI, Rustom LBO, Marrache M, Rimmani HH, Bou Daher H, Koussa S, Taher A. Sofosbuvir/velpatasvir for chronic hepatitis C infection in patients with transfusion-dependent thalassemia. Am J Hematol. 2019;94:E43-E45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 320. | Sinakos E, Kountouras D, Koskinas J, Zachou K, Karatapanis S, Triantos C, Vassiliadis T, Goulis I, Kourakli A, Vlachaki E, Toli B, Tampaki M, Arvaniti P, Tsiaoussis G, Bellou A, Kattamis A, Maragkos K, Petropoulou F, Dalekos GN, Akriviadis E, Papatheodoridis GV. Treatment of chronic hepatitis C with direct-acting antivirals in patients with β-thalassaemia major and advanced liver disease. Br J Haematol. 2017;178:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 321. | Inomata S, Morihara D, Anan A, Yamauchi E, Yamauchi R, Takata K, Tanaka T, Yokoyama K, Takeyama Y, Irie M, Shakado S, Sohda T, Sakisaka S, Hirai F. Male-specific Association between Iron and Lipid Metabolism Changes and Erythroferrone after Hepatitis C Virus Eradication. Intern Med. 2022;61:461-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 322. | Fujita N, Takei Y. Iron, hepatitis C virus, and hepatocellular carcinoma: iron reduction preaches the gospel for chronic hepatitis C. J Gastroenterol. 2007;42:923-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 323. | Batsaikhan B, Gantumur G, Huang CI, Yeh ML, Huang CF, Lin ZY, Chen SC, Huang JF, Yu ML, Chuang WL, Lee JC, Dai CY. Elevated serum ferritin level associated with hepatic steatosis and fibrosis in hepatitis C virus-infected patients. J Chin Med Assoc. 2019;82:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 324. | Sikorska K, Stalke P, Izycka-Swieszewska E, Romanowski T, Bielawski KP. The role of iron overload and HFE gene mutations in the era of pegylated interferon and ribavirin treatment of chronic hepatitis C. Med Sci Monit. 2010;16:CR137-CR143. [PubMed] |
| 325. | Sikorska K. The iron homeostasis network and hepatitis C virus - a new challenge in the era of directly acting antivirals. Virulence. 2016;7:620-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 326. | D'Souza RF, Feakins R, Mears L, Sabin CA, Foster GR. Relationship between serum ferritin, hepatic iron staining, diabetes mellitus and fibrosis progression in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2005;21:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 327. | Silva IS, Perez RM, Oliveira PV, Cantagalo MI, Dantas E, Sisti C, Figueiredo-Mendes C, Lanzoni VP, Silva A, Ferraz ML. Iron overload in patients with chronic hepatitis C virus infection: clinical and histological study. J Gastroenterol Hepatol. 2005;20:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 328. | Gattoni A, Parlato A, Vangieri B, Bresciani M, Derna R, Baldassarre R. Role of hemochromatosis genes in chronic hepatitis C. Clin Ter. 2006;157:61-68. [PubMed] |
| 329. | Yamane D, Hayashi Y, Matsumoto M, Nakanishi H, Imagawa H, Kohara M, Lemon SM, Ichi I. FADS2-dependent fatty acid desaturation dictates cellular sensitivity to ferroptosis and permissiveness for hepatitis C virus replication. Cell Chem Biol. 2022;29:799-810.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 330. | Park SO, Kumar M, Gupta S. TGF-β and iron differently alter HBV replication in human hepatocytes through TGF-β/BMP signaling and cellular microRNA expression. PLoS One. 2012;7:e39276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 331. | Felton C, Lustbader ED, Merten C, Blumberg BS. Serum iron levels and response to hepatitis B virus. Proc Natl Acad Sci U S A. 1979;76:2438-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 332. | Sebastiani G, Tempesta D, Alberti A. Hepatic iron overload is common in chronic hepatitis B and is more severe in patients coinfected with hepatitis D virus. J Viral Hepat. 2012;19:e170-e176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 333. | Sikorska K, Romanowski T, Stalke P, Izycka Swieszewska E, Bielawski KP. Association of hepcidin mRNA expression with hepatocyte iron accumulation and effects of antiviral therapy in chronic hepatitis C infection. Hepat Mon. 2014;14:e21184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 334. | Lin D, Ding J, Liu JY, He YF, Dai Z, Chen CZ, Cheng WZ, Zhou J, Wang X. Decreased serum hepcidin concentration correlates with brain iron deposition in patients with HBV-related cirrhosis. PLoS One. 2013;8:e65551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 335. | Wang J, Dong A, Liu G, Anderson GJ, Hu TY, Shi J, Hu Y, Nie G. Correlation of serum hepcidin levels with disease progression in hepatitis B virus-related disease assessed by nanopore film based assay. Sci Rep. 2016;6:34252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 336. | Gao YH, Wang JY, Liu PY, Sun J, Wang XM, Wu RH, He XT, Tu ZK, Wang CG, Xu HQ, Niu JQ. Iron metabolism disorders in patients with hepatitis B-related liver diseases. World J Clin Cases. 2018;6:600-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 337. | Çam H, Yılmaz N. Serum hepcidin levels are related to serum markers for iron metabolism and fibrosis stage in patients with chronic hepatitis B: A cross-sectional study. Arab J Gastroenterol. 2020;21:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 338. | Mao W, Hu Y, Lou Y, Chen Y, Zhang J. Abnormal serum iron markers in chronic hepatitis B virus infection may be because of liver injury. Eur J Gastroenterol Hepatol. 2015;27:130-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 339. | Tey TT, Yiu R, Leow WQ. Hepatitis B-Associated Symptomatic Iron Overload, with Complete Resolution after Nucleoside Analogue Treatment. Case Rep Gastrointest Med. 2021;2021:8407257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 340. | Zimmerman HJ, Chomet B, Kulesh MH, McWhorter CA. Hepatic hemosiderin deposits. Incidence in 558 biopsies from patients with and without intrinsic hepatic disease. Arch Intern Med. 1961;107:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 341. | Kayali Z, Ranguelov R, Mitros F, Shufelt C, Elmi F, Rayhill SC, Schmidt WN, Brown KE. Hemosiderosis is associated with accelerated decompensation and decreased survival in patients with cirrhosis. Liver Int. 2005;25:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 342. | Philippe MA, Ruddell RG, Ramm GA. Role of iron in hepatic fibrosis: one piece in the puzzle. World J Gastroenterol. 2007;13:4746-4754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 343. | Yip TC, Lee HW, Chan WK, Wong GL, Wong VW. Asian perspective on NAFLD-associated HCC. J Hepatol. 2022;76:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 92] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 344. | Eder SK, Feldman A, Strebinger G, Kemnitz J, Zandanell S, Niederseer D, Strasser M, Haufe H, Sotlar K, Stickel F, Paulweber B, Datz C, Aigner E. Mesenchymal iron deposition is associated with adverse long-term outcome in non-alcoholic fatty liver disease. Liver Int. 2020;40:1872-1882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 345. | Bridle KR, Crawford DH, Ramm GA. Identification and characterization of the hepatic stellate cell transferrin receptor. Am J Pathol. 2003;162:1661-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 346. | Gardi C, Arezzini B, Fortino V, Comporti M. Effect of free iron on collagen synthesis, cell proliferation and MMP-2 expression in rat hepatic stellate cells. Biochem Pharmacol. 2002;64:1139-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 347. | Mao Q, Xie Z, Wang X, Chen W, Ren M, Shang M, Lei H, Tian Y, Li S, Liang P, Chen T, Liang C, Xu J, Li X, Huang Y, Yu X. Clonorchis sinensis ferritin heavy chain triggers free radicals and mediates inflammation signaling in human hepatic stellate cells. Parasitol Res. 2015;114:659-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 348. | Ruddell RG, Hoang-Le D, Barwood JM, Rutherford PS, Piva TJ, Watters DJ, Santambrogio P, Arosio P, Ramm GA. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology. 2009;49:887-900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 229] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 349. | Mehta KJ, Coombes JD, Briones-Orta M, Manka PP, Williams R, Patel VB, Syn WK. Iron Enhances Hepatic Fibrogenesis and Activates Transforming Growth Factor-β Signaling in Murine Hepatic Stellate Cells. Am J Med Sci. 2018;355:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 350. | Comporti M, Arezzini B, Signorini C, Vecchio D, Gardi C. Oxidative stress, isoprostanes and hepatic fibrosis. Histol Histopathol. 2009;24:893-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 351. | Zamara E, Novo E, Marra F, Gentilini A, Romanelli RG, Caligiuri A, Robino G, Tamagno E, Aragno M, Danni O, Autelli R, Colombatto S, Dianzani MU, Pinzani M, Parola M. 4-Hydroxynonenal as a selective pro-fibrogenic stimulus for activated human hepatic stellate cells. J Hepatol. 2004;40:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 352. | Houglum K, Ramm GA, Crawford DH, Witztum JL, Powell LW, Chojkier M. Excess iron induces hepatic oxidative stress and transforming growth factor beta1 in genetic hemochromatosis. Hepatology. 1997;26:605-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 353. | Corradini E, Meynard D, Wu Q, Chen S, Ventura P, Pietrangelo A, Babitt JL. Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice. Hepatology. 2011;54:273-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 354. | Kautz L, Meynard D, Monnier A, Darnaud V, Bouvet R, Wang RH, Deng C, Vaulont S, Mosser J, Coppin H, Roth MP. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112:1503-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 353] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 355. | Jian J, Yang Q, Shao Y, Axelrod D, Smith J, Singh B, Krauter S, Chiriboga L, Yang Z, Li J, Huang X. A link between premenopausal iron deficiency and breast cancer malignancy. BMC Cancer. 2013;13:307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 356. | Preziosi ME, Singh S, Valore EV, Jung G, Popovic B, Poddar M, Nagarajan S, Ganz T, Monga SP. Mice lacking liver-specific β-catenin develop steatohepatitis and fibrosis after iron overload. J Hepatol. 2017;67:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 357. | Han CY, Koo JH, Kim SH, Gardenghi S, Rivella S, Strnad P, Hwang SJ, Kim SG. Hepcidin inhibits Smad3 phosphorylation in hepatic stellate cells by impeding ferroportin-mediated regulation of Akt. Nat Commun. 2016;7:13817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 358. | Arndt S, Wacker E, Dorn C, Koch A, Saugspier M, Thasler WE, Hartmann A, Bosserhoff AK, Hellerbrand C. Enhanced expression of BMP6 inhibits hepatic fibrosis in non-alcoholic fatty liver disease. Gut. 2015;64:973-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 359. | Tornai D, Antal-Szalmas P, Tornai T, Papp M, Tornai I, Sipeki N, Janka T, Balogh B, Vitalis Z. Abnormal ferritin levels predict development of poor outcomes in cirrhotic outpatients: a cohort study. BMC Gastroenterol. 2021;21:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 360. | Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014;6:748-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 423] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 361. | Puntarulo S. Iron, oxidative stress and human health. Mol Aspects Med. 2005;26:299-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 362. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4118] [Article Influence: 205.9] [Reference Citation Analysis (3)] |
| 363. | Nahon P, Nuraldeen R, Rufat P, Sutton A, Trautwein C, Strnad P. In alcoholic cirrhosis, low-serum hepcidin levels associate with poor long-term survival. Liver Int. 2016;36:185-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 364. | Yonal O, Akyuz F, Demir K, Ciftci S, Keskin F, Pinarbasi B, Uyanikoglu A, Issever H, Ozdil S, Boztas G, Besisik F, Kaymakoglu S, Cakaloglu Y, Mungan Z, Okten A. Decreased prohepcidin levels in patients with HBV-related liver disease: relation with ferritin levels. Dig Dis Sci. 2010;55:3548-3551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 365. | Jaroszewicz J, Rogalska M, Flisiak R. Serum prohepcidin reflects the degree of liver function impairment in liver cirrhosis. Biomarkers. 2008;13:478-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 366. | Nagashima M, Kudo M, Chung H, Ishikawa E, Hagiwara S, Nakatani T, Dote K. Regulatory failure of serum prohepcidin levels in patients with hepatitis C. Hepatol Res. 2006;36:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 367. | Tan TC, Crawford DH, Franklin ME, Jaskowski LA, Macdonald GA, Jonsson JR, Watson MJ, Taylor PJ, Fletcher LM. The serum hepcidin:ferritin ratio is a potential biomarker for cirrhosis. Liver Int. 2012;32:1391-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 368. | Jamali R, Razavizade M, Arj A, Aarabi MH. Serum adipokines might predict liver histology findings in non-alcoholic fatty liver disease. World J Gastroenterol. 2016;22:5096-5103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 369. | Angulo P, George J, Day CP, Vanni E, Russell L, De la Cruz AC, Liaquat H, Mezzabotta L, Lee E, Bugianesi E. Serum ferritin levels lack diagnostic accuracy for liver fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12:1163-1169.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 370. | Cakir M, Erduran E, Turkmen ES, Aliyazicioglu Y, Reis GP, Cobanoglu U, Demir S. Hepcidin levels in children with chronic liver disease. Saudi J Gastroenterol. 2015;21:300-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 371. | Zhang Z, Guo M, Li Y, Shen M, Kong D, Shao J, Ding H, Tan S, Chen A, Zhang F, Zheng S. RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy. 2020;16:1482-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 333] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 372. | Wang L, Zhang Z, Li M, Wang F, Jia Y, Zhang F, Shao J, Chen A, Zheng S. P53-dependent induction of ferroptosis is required for artemether to alleviate carbon tetrachloride-induced liver fibrosis and hepatic stellate cell activation. IUBMB Life. 2019;71:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 373. | Mehta KJ, Farnaud SJ, Sharp PA. Iron and liver fibrosis: Mechanistic and clinical aspects. World J Gastroenterol. 2019;25:521-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 132] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (7)] |
| 374. | Kew MC. Hepatic iron overload and hepatocellular carcinoma. Liver Cancer. 2014;3:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 375. | Recalcati S, Correnti M, Gammella E, Raggi C, Invernizzi P, Cairo G. Iron Metabolism in Liver Cancer Stem Cells. Front Oncol. 2019;9:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 376. | Torti SV, Manz DH, Paul BT, Blanchette-Farra N, Torti FM. Iron and Cancer. Annu Rev Nutr. 2018;38:97-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 307] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 377. | Mehta KJ, Sharp PA. Iron elevates mesenchymal and metastatic biomarkers in HepG2 cells. Sci Rep. 2020;10:21926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 378. | Fracanzani AL, Conte D, Fraquelli M, Taioli E, Mattioli M, Losco A, Fargion S. Increased cancer risk in a cohort of 230 patients with hereditary hemochromatosis in comparison to matched control patients with non-iron-related chronic liver disease. Hepatology. 2001;33:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 166] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 379. | Kowdley KV. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology. 2004;127:S79-S86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 278] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 380. | Chung JW, Shin E, Kim H, Han HS, Cho JY, Choi YR, Hong S, Jang ES, Kim JW, Jeong SH. Hepatic iron overload in the portal tract predicts poor survival in hepatocellular carcinoma after curative resection. Liver Int. 2018;38:903-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 381. | Chapoutot C, Esslimani M, Joomaye Z, Ramos J, Perney P, Laurent C, Fabbro-Peray P, Larrey D, Domergue J, Blanc F. Liver iron excess in patients with hepatocellular carcinoma developed on viral C cirrhosis. Gut. 2000;46:711-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 382. | Bothwell TH, Seftel H, Jacobs P, Torrance JD, Baumslag N. Iron overload in bantu subjects; studies on the availability of iron in bantu beer. Am J Clin Nutr. 1964;14:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 81] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 383. | Gordeuk VR, McLaren CE, MacPhail AP, Deichsel G, Bothwell TH. Associations of iron overload in Africa with hepatocellular carcinoma and tuberculosis: Strachan's 1929 thesis revisited. Blood. 1996;87:3470-3476. [PubMed] |
| 384. | Mandishona E, MacPhail AP, Gordeuk VR, Kedda MA, Paterson AC, Rouault TA, Kew MC. Dietary iron overload as a risk factor for hepatocellular carcinoma in Black Africans. Hepatology. 1998;27:1563-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 112] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 385. | Moyo VM, Makunike R, Gangaidzo IT, Gordeuk VR, McLaren CE, Khumalo H, Saungweme T, Rouault T, Kiire CF. African iron overload and hepatocellular carcinoma (HA-7-0-080). Eur J Haematol. 1998;60:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 386. | Adachi M, Kai K, Yamaji K, Ide T, Noshiro H, Kawaguchi A, Aishima S. Transferrin receptor 1 overexpression is associated with tumour de-differentiation and acts as a potential prognostic indicator of hepatocellular carcinoma. Histopathology. 2019;75:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 387. | Hsu MY, Mina E, Roetto A, Porporato PE. Iron: An Essential Element of Cancer Metabolism. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 388. | Paul VD, Lill R. Biogenesis of cytosolic and nuclear iron-sulfur proteins and their role in genome stability. Biochim Biophys Acta. 2015;1853:1528-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 389. | Zhang C. Essential functions of iron-requiring proteins in DNA replication, repair and cell cycle control. Protein Cell. 2014;5:750-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 390. | Huang X. Does iron have a role in breast cancer? Lancet Oncol. 2008;9:803-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 391. | Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 666] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 392. | Asare GA, Mossanda KS, Kew MC, Paterson AC, Kahler-Venter CP, Siziba K. Hepatocellular carcinoma caused by iron overload: a possible mechanism of direct hepatocarcinogenicity. Toxicology. 2006;219:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 393. | Asare GA, Paterson AC, Kew MC, Khan S, Mossanda KS. Iron-free neoplastic nodules and hepatocellular carcinoma without cirrhosis in Wistar rats fed a diet high in iron. J Pathol. 2006;208:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 394. | Shen J, Sheng X, Chang Z, Wu Q, Wang S, Xuan Z, Li D, Wu Y, Shang Y, Kong X, Yu L, Li L, Ruan K, Hu H, Huang Y, Hui L, Xie D, Wang F, Hu R. Iron metabolism regulates p53 signaling through direct heme-p53 interaction and modulation of p53 localization, stability, and function. Cell Rep. 2014;7:180-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 175] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 395. | Muto Y, Moroishi T, Ichihara K, Nishiyama M, Shimizu H, Eguchi H, Moriya K, Koike K, Mimori K, Mori M, Katayama Y, Nakayama KI. Disruption of FBXL5-mediated cellular iron homeostasis promotes liver carcinogenesis. J Exp Med. 2019;216:950-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 396. | Hu W, Zhou C, Jing Q, Li Y, Yang J, Yang C, Wang L, Hu J, Li H, Wang H, Yuan C, Zhou Y, Ren X, Tong X, Du J, Wang Y. FTH promotes the proliferation and renders the HCC cells specifically resist to ferroptosis by maintaining iron homeostasis. Cancer Cell Int. 2021;21:709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 397. | Kijima H, Sawada T, Tomosugi N, Kubota K. Expression of hepcidin mRNA is uniformly suppressed in hepatocellular carcinoma. BMC Cancer. 2008;8:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 398. | Maegdefrau U, Arndt S, Kivorski G, Hellerbrand C, Bosserhoff AK. Downregulation of hemojuvelin prevents inhibitory effects of bone morphogenetic proteins on iron metabolism in hepatocellular carcinoma. Lab Invest. 2011;91:1615-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 399. | Udali S, Castagna A, Corbella M, Ruzzenente A, Moruzzi S, Mazzi F, Campagnaro T, De Santis D, Franceschi A, Pattini P, Gottardo R, Olivieri O, Perbellini L, Guglielmi A, Choi SW, Girelli D, Friso S. Hepcidin and DNA promoter methylation in hepatocellular carcinoma. Eur J Clin Invest. 2018;48:e12870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 400. | Joachim JH, Mehta KJ. Hepcidin in hepatocellular carcinoma. Br J Cancer. 2022;127:185-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 401. | Carbone M, Melino G. Stearoyl CoA Desaturase Regulates Ferroptosis in Ovarian Cancer Offering New Therapeutic Perspectives. Cancer Res. 2019;79:5149-5150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 402. | Shin D, Kim EH, Lee J, Roh JL. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic Biol Med. 2018;129:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 384] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 403. | Sui X, Zhang R, Liu S, Duan T, Zhai L, Zhang M, Han X, Xiang Y, Huang X, Lin H, Xie T. RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front Pharmacol. 2018;9:1371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 489] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 404. | Nie J, Lin B, Zhou M, Wu L, Zheng T. Role of ferroptosis in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2018;144:2329-2337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 405. | Sun X, Niu X, Chen R, He W, Chen D, Kang R, Tang D. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. 2016;64:488-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 527] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 406. | Bai T, Wang S, Zhao Y, Zhu R, Wang W, Sun Y. Haloperidol, a sigma receptor 1 antagonist, promotes ferroptosis in hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2017;491:919-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 407. | Feng J, Lu PZ, Zhu GZ, Hooi SC, Wu Y, Huang XW, Dai HQ, Chen PH, Li ZJ, Su WJ, Han CY, Ye XP, Peng T, Zhou J, Lu GD. ACSL4 is a predictive biomarker of sorafenib sensitivity in hepatocellular carcinoma. Acta Pharmacol Sin. 2021;42:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 408. | Sun XJ, Xu GL. Overexpression of Acyl-CoA Ligase 4 (ACSL4) in Patients with Hepatocellular Carcinoma and its Prognosis. Med Sci Monit. 2017;23:4343-4350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 409. | Han YM, Jeong M, Park JM, Kim MY, Go EJ, Cha JY, Kim KJ, Hahm KB. The ω-3 polyunsaturated fatty acids prevented colitis-associated carcinogenesis through blocking dissociation of β-catenin complex, inhibiting COX-2 through repressing NF-κB, and inducing 15-prostaglandin dehydrogenase. Oncotarget. 2016;7:63583-63595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 410. | Ou W, Mulik RS, Anwar A, McDonald JG, He X, Corbin IR. Low-density lipoprotein docosahexaenoic acid nanoparticles induce ferroptotic cell death in hepatocellular carcinoma. Free Radic Biol Med. 2017;112:597-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 411. | Weylandt KH, Krause LF, Gomolka B, Chiu CY, Bilal S, Nadolny A, Waechter SF, Fischer A, Rothe M, Kang JX. Suppressed liver tumorigenesis in fat-1 mice with elevated omega-3 fatty acids is associated with increased omega-3 derived lipid mediators and reduced TNF-α. Carcinogenesis. 2011;32:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 412. | Shang Y, Luo M, Yao F, Wang S, Yuan Z, Yang Y. Ceruloplasmin suppresses ferroptosis by regulating iron homeostasis in hepatocellular carcinoma cells. Cell Signal. 2020;72:109633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 413. | Lim LJ, Wong SYS, Huang F, Lim S, Chong SS, Ooi LL, Kon OL, Lee CG. Roles and Regulation of Long Noncoding RNAs in Hepatocellular Carcinoma. Cancer Res. 2019;79:5131-5139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 414. | Qi W, Li Z, Xia L, Dai J, Zhang Q, Wu C, Xu S. LncRNA GABPB1-AS1 and GABPB1 regulate oxidative stress during erastin-induced ferroptosis in HepG2 hepatocellular carcinoma cells. Sci Rep. 2019;9:16185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 415. | Xiong Y, Ouyang Y, Fang K, Sun G, Tu S, Xin W, Wei Y, Xiao W. Prediction of Prognosis and Molecular Mechanism of Ferroptosis in Hepatocellular Carcinoma Based on Bioinformatics Methods. Comput Math Methods Med. 2022;2022:4558782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Reference Citation Analysis (0)] |
| 416. | Xu Z, Peng B, Liang Q, Chen X, Cai Y, Zeng S, Gao K, Wang X, Yi Q, Gong Z, Yan Y. Construction of a Ferroptosis-Related Nine-lncRNA Signature for Predicting Prognosis and Immune Response in Hepatocellular Carcinoma. Front Immunol. 2021;12:719175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 417. | Lyu N, Zeng Y, Kong Y, Chen Q, Deng H, Ou S, Bai Y, Tang H, Wang X, Zhao M. Ferroptosis is involved in the progression of hepatocellular carcinoma through the circ0097009/miR-1261/SLC7A11 axis. Ann Transl Med. 2021;9:675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 418. | Zhang B, Zhao J, Liu B, Shang Y, Chen F, Zhang S, He J, Fan Y, Tan K. Development and Validation of a Novel Ferroptosis-Related Gene Signature for Prognosis and Immunotherapy in Hepatocellular Carcinoma. Front Mol Biosci. 2022;9:940575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 419. | Liang JY, Wang DS, Lin HC, Chen XX, Yang H, Zheng Y, Li YH. A Novel Ferroptosis-related Gene Signature for Overall Survival Prediction in Patients with Hepatocellular Carcinoma. Int J Biol Sci. 2020;16:2430-2441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 412] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 420. | Pan F, Lin X, Hao L, Wang T, Song H, Wang R. The Critical Role of Ferroptosis in Hepatocellular Carcinoma. Front Cell Dev Biol. 2022;10:882571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 421. | Lyberopoulou A, Chachami G, Gatselis NK, Kyratzopoulou E, Saitis A, Gabeta S, Eliades P, Paraskeva E, Zachou K, Koukoulis GK, Mamalaki A, Dalekos GN, Simos G. Low Serum Hepcidin in Patients with Autoimmune Liver Diseases. PLoS One. 2015;10:e0135486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 422. | Huang YH, Chuang JH, Yang YL, Huang CC, Wu CL, Chen CL. Cholestasis downregulate hepcidin expression through inhibiting IL-6-induced phosphorylation of signal transducer and activator of transcription 3 signaling. Lab Invest. 2009;89:1128-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 423. | Deng G, Li Y, Ma S, Gao Z, Zeng T, Chen L, Ye H, Yang M, Shi H, Yao X, Zeng Z, Chen Y, Song Y, Liu B, Gao L. Caveolin-1 dictates ferroptosis in the execution of acute immune-mediated hepatic damage by attenuating nitrogen stress. Free Radic Biol Med. 2020;148:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 424. | El-Zaatari M, Bass AJ, Bowlby R, Zhang M, Syu LJ, Yang Y, Grasberger H, Shreiner A, Tan B, Bishu S, Leung WK, Todisco A, Kamada N, Cascalho M, Dlugosz AA, Kao JY. Indoleamine 2,3-Dioxygenase 1, Increased in Human Gastric Pre-Neoplasia, Promotes Inflammation and Metaplasia in Mice and Is Associated With Type II Hypersensitivity/Autoimmunity. Gastroenterology. 2018;154:140-153.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 425. | Zeng T, Deng G, Zhong W, Gao Z, Ma S, Mo C, Li Y, Huang S, Zhou C, Lai Y, Xie S, Xie Z, Chen Y, He S, Lv Z, Gao L. Indoleamine 2, 3-dioxygenase 1enhanceshepatocytes ferroptosis in acute immune hepatitis associated with excess nitrative stress. Free Radic Biol Med. 2020;152:668-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 426. | Taubert R, Hardtke-Wolenski M, Noyan F, Lalanne C, Jonigk D, Schlue J, Krech T, Lichtinghagen R, Falk CS, Schlaphoff V, Bantel H, Muratori L, Manns MP, Jaeckel E. Hyperferritinemia and hypergammaglobulinemia predict the treatment response to standard therapy in autoimmune hepatitis. PLoS One. 2017;12:e0179074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 427. | Chen Q, Gao M, Yang H, Mei L, Zhong R, Han P, Liu P, Zhao L, Wang J, Li J. Serum ferritin levels are associated with advanced liver fibrosis in treatment-naive autoimmune hepatitis. BMC Gastroenterol. 2022;22:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 428. | Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, Ho LP, Townsend AR, Drakesmith H. Hepcidin regulation by innate immune and infectious stimuli. Blood. 2011;118:4129-4139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 429. | Mao L, Zhao T, Song Y, Lin L, Fan X, Cui B, Feng H, Wang X, Yu Q, Zhang J, Jiang K, Wang B, Sun C. The emerging role of ferroptosis in non-cancer liver diseases: hype or increasing hope? Cell Death Dis. 2020;11:518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 430. | Yamada N, Karasawa T, Wakiya T, Sadatomo A, Ito H, Kamata R, Watanabe S, Komada T, Kimura H, Sanada Y, Sakuma Y, Mizuta K, Ohno N, Sata N, Takahashi M. Iron overload as a risk factor for hepatic ischemia-reperfusion injury in liver transplantation: Potential role of ferroptosis. Am J Transplant. 2020;20:1606-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 194] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 431. | Chen Q, Liu L, Ni S. Screening of ferroptosis-related genes in sepsis-induced liver failure and analysis of immune correlation. PeerJ. 2022;10:e13757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 432. | Yamada N, Karasawa T, Kimura H, Watanabe S, Komada T, Kamata R, Sampilvanjil A, Ito J, Nakagawa K, Kuwata H, Hara S, Mizuta K, Sakuma Y, Sata N, Takahashi M. Ferroptosis driven by radical oxidation of n-6 polyunsaturated fatty acids mediates acetaminophen-induced acute liver failure. Cell Death Dis. 2020;11:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 237] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 433. | Du K, Ramachandran A, Jaeschke H. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. 2016;10:148-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 395] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 434. | Lőrincz T, Jemnitz K, Kardon T, Mandl J, Szarka A. Ferroptosis is Involved in Acetaminophen Induced Cell Death. Pathol Oncol Res. 2015;21:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 435. | Jaeschke H, Ramachandran A. Response to the opinion letter entitled Role of Ferroptosis in Acetaminophen Hepatotoxicity by Yamada et al. Arch Toxicol. 2020;94:1771-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 436. | Niu B, Lei X, Xu Q, Ju Y, Xu D, Mao L, Li J, Zheng Y, Sun N, Zhang X, Mao Y, Li X. Protecting mitochondria via inhibiting VDAC1 oligomerization alleviates ferroptosis in acetaminophen-induced acute liver injury. Cell Biol Toxicol. 2022;38:505-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 437. | Wang Z, Hao W, Hu J, Mi X, Han Y, Ren S, Jiang S, Wang Y, Li X, Li W. Maltol Improves APAP-Induced Hepatotoxicity by Inhibiting Oxidative Stress and Inflammation Response via NF-κB and PI3K/Akt Signal Pathways. Antioxidants (Basel). 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 438. | He Y, Liu CY, He CC, Zhao J, Sun YH, Xu HS, Cai XQ, Li YF, Kurihara Hiroshi, He RR. [Protective effect of Fuzheng Yanggan Mixture on drug-induced liver injury]. Zhongguo Zhong Yao Za Zhi. 2018;43:4685-4691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 439. | Moon MS, Richie JP, Isom HC. Iron potentiates acetaminophen-induced oxidative stress and mitochondrial dysfunction in cultured mouse hepatocytes. Toxicol Sci. 2010;118:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 440. | van Swelm RP, Laarakkers CM, Blous L, Peters JG, Blaney Davidson EN, van der Kraan PM, Swinkels DW, Masereeuw R, Russel FG. Acute acetaminophen intoxication leads to hepatic iron loading by decreased hepcidin synthesis. Toxicol Sci. 2012;129:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 441. | Knight TR, Fariss MW, Farhood A, Jaeschke H. Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicol Sci. 2003;76:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 442. | Jaeschke H, Ramachandran A, Chao X, Ding WX. Emerging and established modes of cell death during acetaminophen-induced liver injury. Arch Toxicol. 2019;93:3491-3502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 443. | Praharaj DL, Anand AC. Sickle Hepatopathy. J Clin Exp Hepatol. 2021;11:82-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 444. | Theocharidou E, Suddle AR. The Liver in Sickle Cell Disease. Clin Liver Dis. 2019;23:177-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 445. | Olivieri NF. Progression of iron overload in sickle cell disease. Semin Hematol. 2001;38:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 446. | Yassin M, Soliman A, De Sanctis V, Nashwan A, Abusamaan S, Moustafa A, Kohla S, Soliman D. Liver Iron Content (LIC) in Adults with Sickle Cell Disease (SCD): Correlation with Serum Ferritin and Liver Enzymes Concentrations in Trasfusion Dependent (TD-SCD) and Non-Transfusion Dependent (NT-SCD) Patients. Mediterr J Hematol Infect Dis. 2017;9:e2017037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 447. | Hankins JS, Smeltzer MP, McCarville MB, Aygun B, Hillenbrand CM, Ware RE, Onciu M. Patterns of liver iron accumulation in patients with sickle cell disease and thalassemia with iron overload. Eur J Haematol. 2010;85:51-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 448. | Feld JJ, Kato GJ, Koh C, Shields T, Hildesheim M, Kleiner DE, Taylor JG 6th, Sandler NG, Douek D, Haynes-Williams V, Nichols JS, Hoofnagle JH, Jake Liang T, Gladwin MT, Heller T. Liver injury is associated with mortality in sickle cell disease. Aliment Pharmacol Ther. 2015;42:912-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 449. | Demosthenous C, Rizos G, Vlachaki E, Tzatzagou G, Gavra M. Hemosiderosis causing liver cirrhosis in a patient with Hb S/beta thalassemia and no other known causes of hepatic disease. Hippokratia. 2017;21:43-45. [PubMed] |
| 450. | Chen Y, Xu Y, Zhang K, Shen L, Deng M. Ferroptosis in COVID-19-related liver injury: A potential mechanism and therapeutic target. Front Cell Infect Microbiol. 2022;12:922511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 451. | Abe N, Tsuchida T, Yasuda SI, Oka K. Dietary iron restriction leads to a reduction in hepatic fibrosis in a rat model of non-alcoholic steatohepatitis. Biol Open. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 452. | Crawford DHG, Ross DGF, Jaskowski LA, Burke LJ, Britton LJ, Musgrave N, Briskey D, Rishi G, Bridle KR, Subramaniam VN. Iron depletion attenuates steatosis in a mouse model of non-alcoholic fatty liver disease: Role of iron-dependent pathways. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 453. | Falize L, Guillygomarc'h A, Perrin M, Lainé F, Guyader D, Brissot P, Turlin B, Deugnier Y. Reversibility of hepatic fibrosis in treated genetic hemochromatosis: a study of 36 cases. Hepatology. 2006;44:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 454. | Powell LW, Dixon JL, Ramm GA, Purdie DM, Lincoln DJ, Anderson GJ, Subramaniam VN, Hewett DG, Searle JW, Fletcher LM, Crawford DH, Rodgers H, Allen KJ, Cavanaugh JA, Bassett ML. Screening for hemochromatosis in asymptomatic subjects with or without a family history. Arch Intern Med. 2006;166:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 455. | Murali AR, Gupta A, Brown K. Systematic review and meta-analysis to determine the impact of iron depletion in dysmetabolic iron overload syndrome and non-alcoholic fatty liver disease. Hepatol Res. 2018;48:E30-E41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 456. | Facchini FS, Hua NW, Stoohs RA. Effect of iron depletion in carbohydrate-intolerant patients with clinical evidence of nonalcoholic fatty liver disease. Gastroenterology. 2002;122:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 457. | Valenti L, Fracanzani AL, Dongiovanni P, Rovida S, Rametta R, Fatta E, Pulixi EA, Maggioni M, Fargion S. A randomized trial of iron depletion in patients with nonalcoholic fatty liver disease and hyperferritinemia. World J Gastroenterol. 2014;20:3002-3010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 458. | Valenti L, Fracanzani AL, Fargion S. Effect of iron depletion in patients with nonalcoholic fatty liver disease without carbohydrate intolerance. Gastroenterology. 2003;124:866; author reply 866-866; author reply 867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 459. | Fernández-Real JM, Peñarroja G, Castro A, García-Bragado F, Hernández-Aguado I, Ricart W. Blood letting in high-ferritin type 2 diabetes: effects on insulin sensitivity and beta-cell function. Diabetes. 2002;51:1000-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 460. | Houschyar KS, Lüdtke R, Dobos GJ, Kalus U, Broecker-Preuss M, Rampp T, Brinkhaus B, Michalsen A. Effects of phlebotomy-induced reduction of body iron stores on metabolic syndrome: results from a randomized clinical trial. BMC Med. 2012;10:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 461. | Jaruvongvanich V, Riangwiwat T, Sanguankeo A, Upala S. Outcome of phlebotomy for treating nonalcoholic fatty liver disease: A systematic review and meta-analysis. Saudi J Gastroenterol. 2016;22:407-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 462. | Adams LA, Crawford DH, Stuart K, House MJ, St Pierre TG, Webb M, Ching HL, Kava J, Bynevelt M, MacQuillan GC, Garas G, Ayonrinde OT, Mori TA, Croft KD, Niu X, Jeffrey GP, Olynyk JK. The impact of phlebotomy in nonalcoholic fatty liver disease: A prospective, randomized, controlled trial. Hepatology. 2015;61:1555-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 463. | Lainé F, Ruivard M, Loustaud-Ratti V, Bonnet F, Calès P, Bardou-Jacquet E, Sacher-Huvelin S, Causse X, Beusnel C, Renault A, Bellissant E, Deugnier Y; Study Group. Metabolic and hepatic effects of bloodletting in dysmetabolic iron overload syndrome: A randomized controlled study in 274 patients. Hepatology. 2017;65:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 464. | Adams PC. The (II)logic of iron reduction therapy for steatohepatitis. Hepatology. 2015;62:668-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 465. | Kato J, Miyanishi K, Kobune M, Nakamura T, Takada K, Takimoto R, Kawano Y, Takahashi S, Takahashi M, Sato Y, Takayama T, Niitsu Y. Long-term phlebotomy with low-iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis C. J Gastroenterol. 2007;42:830-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 466. | Nirei K, Matsuoka S, Nakamura H, Matsumura H, Moriyama M. Incidence of hepatocellular carcinoma reduced by phlebotomy treatment in patients with chronic hepatitis C. Intern Med. 2015;54:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 467. | Yano M, Hayashi H, Wakusawa S, Sanae F, Takikawa T, Shiono Y, Arao M, Ukai K, Ito H, Watanabe K, Yoshioka K. Long term effects of phlebotomy on biochemical and histological parameters of chronic hepatitis C. Am J Gastroenterol. 2002;97:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 468. | Desai TK, Jamil LH, Balasubramaniam M, Koff R, Bonkovsky HL. Phlebotomy improves therapeutic response to interferon in patients with chronic hepatitis C: a meta-analysis of six prospective randomized controlled trials. Dig Dis Sci. 2008;53:815-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 469. | Nielsen P, Fischer R, Buggisch P, Janka-Schaub G. Effective treatment of hereditary haemochromatosis with desferrioxamine in selected cases. Br J Haematol. 2003;123:952-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 470. | Ikeda H, Wu GY, Wu CH. Evidence that an iron chelator regulates collagen synthesis by decreasing the stability of procollagen mRNA. Hepatology. 1992;15:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 471. | Jin H, Terai S, Sakaida I. The iron chelator deferoxamine causes activated hepatic stellate cells to become quiescent and to undergo apoptosis. J Gastroenterol. 2007;42:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 472. | Darwish SF, El-Bakly WM, El-Naga RN, Awad AS, El-Demerdash E. Antifibrotic mechanism of deferoxamine in concanavalin A induced-liver fibrosis: Impact on interferon therapy. Biochem Pharmacol. 2015;98:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 473. | Pan M, Cederbaum AI, Zhang YL, Ginsberg HN, Williams KJ, Fisher EA. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J Clin Invest. 2004;113:1277-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 198] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 474. | Wang QM, Du JL, Duan ZJ, Guo SB, Sun XY, Liu Z. Inhibiting heme oxygenase-1 attenuates rat liver fibrosis by removing iron accumulation. World J Gastroenterol. 2013;19:2921-2934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 475. | Zhang Y, Zhao X, Chang Y, Zhang Y, Chu X, Zhang X, Liu Z, Guo H, Wang N, Gao Y, Zhang J, Chu L. Calcium channel blockers ameliorate iron overload-associated hepatic fibrosis by altering iron transport and stellate cell apoptosis. Toxicol Appl Pharmacol. 2016;301:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 476. | Preza GC, Ruchala P, Pinon R, Ramos E, Qiao B, Peralta MA, Sharma S, Waring A, Ganz T, Nemeth E. Minihepcidins are rationally designed small peptides that mimic hepcidin activity in mice and may be useful for the treatment of iron overload. J Clin Invest. 2011;121:4880-4888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 477. | Ramos E, Ruchala P, Goodnough JB, Kautz L, Preza GC, Nemeth E, Ganz T. Minihepcidins prevent iron overload in a hepcidin-deficient mouse model of severe hemochromatosis. Blood. 2012;120:3829-3836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 478. | Schmidt PJ, Racie T, Westerman M, Fitzgerald K, Butler JS, Fleming MD. Combination therapy with a Tmprss6 RNAi-therapeutic and the oral iron chelator deferiprone additively diminishes secondary iron overload in a mouse model of β-thalassemia intermedia. Am J Hematol. 2015;90:310-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 479. | Sugimoto R, Fujita N, Tomosugi N, Hara N, Miyachi H, Tanaka H, Takeo M, Nakagawa N, Iwasa M, Kobayashi Y, Kaito M, Takei Y. Impaired regulation of serum hepcidin during phlebotomy in patients with chronic hepatitis C. Hepatol Res. 2009;39:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 480. | Saeki I, Yamamoto N, Yamasaki T, Takami T, Maeda M, Fujisawa K, Iwamoto T, Matsumoto T, Hidaka I, Ishikawa T, Uchida K, Tani K, Sakaida I. Effects of an oral iron chelator, deferasirox, on advanced hepatocellular carcinoma. World J Gastroenterol. 2016;22:8967-8977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (2)] |
| 481. | Ren F, Yang Y, Wu K, Zhao T, Shi Y, Song M, Li J. The Effects of Dandelion Polysaccharides on Iron Metabolism by Regulating Hepcidin via JAK/STAT Signaling Pathway. Oxid Med Cell Longev. 2021;2021:7184760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 482. | Liu J, Sun B, Yin H, Liu S. Hepcidin: A Promising Therapeutic Target for Iron Disorders: A Systematic Review. Medicine (Baltimore). 2016;95:e3150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 483. | Aldrovandi M, Conrad M. Ferroptosis: the Good, the Bad and the Ugly. Cell Res. 2020;30:1061-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 484. | Park SJ, Cho SS, Kim KM, Yang JH, Kim JH, Jeong EH, Yang JW, Han CY, Ku SK, Cho IJ, Ki SH. Protective effect of sestrin2 against iron overload and ferroptosis-induced liver injury. Toxicol Appl Pharmacol. 2019;379:114665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 485. | Louandre C, Ezzoukhry Z, Godin C, Barbare JC, Mazière JC, Chauffert B, Galmiche A. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer. 2013;133:1732-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 442] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 486. | Kong FH, Ye QF, Miao XY, Liu X, Huang SQ, Xiong L, Wen Y, Zhang ZJ. Current status of sorafenib nanoparticle delivery systems in the treatment of hepatocellular carcinoma. Theranostics. 2021;11:5464-5490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 127] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 487. | Galmiche A, Chauffert B, Barbare JC. New biological perspectives for the improvement of the efficacy of sorafenib in hepatocellular carcinoma. Cancer Lett. 2014;346:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 488. | Louandre C, Marcq I, Bouhlal H, Lachaier E, Godin C, Saidak Z, François C, Chatelain D, Debuysscher V, Barbare JC, Chauffert B, Galmiche A. The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Lett. 2015;356:971-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 298] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 489. | Huang W, Chen K, Lu Y, Zhang D, Cheng Y, Li L, Huang W, He G, Liao H, Cai L, Tang Y, Zhao L, Pan M. ABCC5 facilitates the acquired resistance of sorafenib through the inhibition of SLC7A11-induced ferroptosis in hepatocellular carcinoma. Neoplasia. 2021;23:1227-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 490. | Gao R, Kalathur RKR, Coto-Llerena M, Ercan C, Buechel D, Shuang S, Piscuoglio S, Dill MT, Camargo FD, Christofori G, Tang F. YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol Med. 2021;13:e14351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 325] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 491. | Wang Q, Guo Y, Wang W, Liu B, Yang G, Xu Z, Li J, Liu Z. RNA binding protein DAZAP1 promotes HCC progression and regulates ferroptosis by interacting with SLC7A11 mRNA. Exp Cell Res. 2021;399:112453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 492. | Bai T, Lei P, Zhou H, Liang R, Zhu R, Wang W, Zhou L, Sun Y. Sigma-1 receptor protects against ferroptosis in hepatocellular carcinoma cells. J Cell Mol Med. 2019;23:7349-7359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 493. | Houessinon A, François C, Sauzay C, Louandre C, Mongelard G, Godin C, Bodeau S, Takahashi S, Saidak Z, Gutierrez L, Régimbeau JM, Barget N, Barbare JC, Ganne N, Chauffert B, Coriat R, Galmiche A. Metallothionein-1 as a biomarker of altered redox metabolism in hepatocellular carcinoma cells exposed to sorafenib. Mol Cancer. 2016;15:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 494. | Wang Q, Bin C, Xue Q, Gao Q, Huang A, Wang K, Tang N. GSTZ1 sensitizes hepatocellular carcinoma cells to sorafenib-induced ferroptosis via inhibition of NRF2/GPX4 axis. Cell Death Dis. 2021;12:426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 225] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 495. | Yao F, Deng Y, Zhao Y, Mei Y, Zhang Y, Liu X, Martinez C, Su X, Rosato RR, Teng H, Hang Q, Yap S, Chen D, Wang Y, Chen MM, Zhang M, Liang H, Xie D, Chen X, Zhu H, Chang JC, You MJ, Sun Y, Gan B, Ma L. A targetable LIFR-NF-κB-LCN2 axis controls liver tumorigenesis and vulnerability to ferroptosis. Nat Commun. 2021;12:7333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 194] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 496. | Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z, Cai K, Zhao Y, Luo Z. HCAR1/MCT1 Regulates Tumor Ferroptosis through the Lactate-Mediated AMPK-SCD1 Activity and Its Therapeutic Implications. Cell Rep. 2020;33:108487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 254] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 497. | Zheng J, Sato M, Mishima E, Sato H, Proneth B, Conrad M. Sorafenib fails to trigger ferroptosis across a wide range of cancer cell lines. Cell Death Dis. 2021;12:698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 498. | Liu X, Zhu X, Qi X, Meng X, Xu K. Co-Administration of iRGD with Sorafenib-Loaded Iron-Based Metal-Organic Framework as a Targeted Ferroptosis Agent for Liver Cancer Therapy. Int J Nanomedicine. 2021;16:1037-1050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 499. | Tang H, Chen D, Li C, Zheng C, Wu X, Zhang Y, Song Q, Fei W. Dual GSH-exhausting sorafenib loaded manganese-silica nanodrugs for inducing the ferroptosis of hepatocellular carcinoma cells. Int J Pharm. 2019;572:118782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 500. | Chang WT, Bow YD, Fu PJ, Li CY, Wu CY, Chang YH, Teng YN, Li RN, Lu MC, Liu YC, Chiu CC. A Marine Terpenoid, Heteronemin, Induces Both the Apoptosis and Ferroptosis of Hepatocellular Carcinoma Cells and Involves the ROS and MAPK Pathways. Oxid Med Cell Longev. 2021;2021:7689045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 501. | Kong R, Wang N, Han W, Bao W, Lu J. IFNγ-mediated repression of system xc(-) drives vulnerability to induced ferroptosis in hepatocellular carcinoma cells. J Leukoc Biol. 2021;110:301-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 502. | Iseda N, Itoh S, Toshida K, Tomiyama T, Morinaga A, Shimokawa M, Shimagaki T, Wang H, Kurihara T, Toshima T, Nagao Y, Harada N, Yoshizumi T, Mori M. Ferroptosis is induced by lenvatinib through fibroblast growth factor receptor-4 inhibition in hepatocellular carcinoma. Cancer Sci. 2022;113:2272-2287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 503. | Li J, Tao H, Wang W, Li J, Zhang E. The Detection and Verification of Two Heterogeneous Subgroups and a Risk Model Based on Ferroptosis-Related Genes in Hepatocellular Carcinoma. J Oncol. 2022;2022:1182383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 504. | Lu D, Xia Q, Yang Z, Gao S, Sun S, Luo X, Li Z, Zhang X, Han S, Li X, Cao M. ENO3 promoted the progression of NASH by negatively regulating ferroptosis via elevation of GPX4 expression and lipid accumulation. Ann Transl Med. 2021;9:661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 505. | Liu B, Yi W, Mao X, Yang L, Rao C. Enoyl coenzyme A hydratase 1 alleviates nonalcoholic steatohepatitis in mice by suppressing hepatic ferroptosis. Am J Physiol Endocrinol Metab. 2021;320:E925-E937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 506. | Gao G, Xie Z, Li EW, Yuan Y, Fu Y, Wang P, Zhang X, Qiao Y, Xu J, Hölscher C, Wang H, Zhang Z. Dehydroabietic acid improves nonalcoholic fatty liver disease through activating the Keap1/Nrf2-ARE signaling pathway to reduce ferroptosis. J Nat Med. 2021;75:540-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 507. | Slocum SL, Skoko JJ, Wakabayashi N, Aja S, Yamamoto M, Kensler TW, Chartoumpekis DV. Keap1/Nrf2 pathway activation leads to a repressed hepatic gluconeogenic and lipogenic program in mice on a high-fat diet. Arch Biochem Biophys. 2016;591:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 508. | Yang Y, Chen J, Gao Q, Shan X, Wang J, Lv Z. Study on the attenuated effect of Ginkgolide B on ferroptosis in high fat diet induced nonalcoholic fatty liver disease. Toxicology. 2020;445:152599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 509. | Zhu Z, Zhang Y, Huang X, Can L, Zhao X, Wang Y, Xue J, Cheng M, Zhu L. Thymosin beta 4 alleviates non-alcoholic fatty liver by inhibiting ferroptosis via up-regulation of GPX4. Eur J Pharmacol. 2021;908:174351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 510. | Pan Q, Luo Y, Xia Q, He K. Ferroptosis and Liver Fibrosis. Int J Med Sci. 2021;18:3361-3366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 511. | Daher R, Manceau H, Karim Z. Iron metabolism and the role of the iron-regulating hormone hepcidin in health and disease. Presse Med. 2017;46:e272-e278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 512. | Du K, Oh SH, Dutta RK, Sun T, Yang WH, Chi JT, Diehl AM. Inhibiting xCT/SLC7A11 induces ferroptosis of myofibroblastic hepatic stellate cells but exacerbates chronic liver injury. Liver Int. 2021;41:2214-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 513. | Zhang Z, Yao Z, Wang L, Ding H, Shao J, Chen A, Zhang F, Zheng S. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy. 2018;14:2083-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 362] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 514. | Li Y, Jin C, Shen M, Wang Z, Tan S, Chen A, Wang S, Shao J, Zhang F, Zhang Z, Zheng S. Iron regulatory protein 2 is required for artemether -mediated anti-hepatic fibrosis through ferroptosis pathway. Free Radic Biol Med. 2020;160:845-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 515. | Kuo CY, Chiu V, Hsieh PC, Huang CY, Huang SJ, Tzeng IS, Tsai FM, Chen ML, Liu CT, Chen YR. Chrysophanol attenuates hepatitis B virus X protein-induced hepatic stellate cell fibrosis by regulating endoplasmic reticulum stress and ferroptosis. J Pharmacol Sci. 2020;144:172-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 516. | Ho CH, Huang JH, Sun MS, Tzeng IS, Hsu YC, Kuo CY. Wild Bitter Melon Extract Regulates LPS-Induced Hepatic Stellate Cell Activation, Inflammation, Endoplasmic Reticulum Stress, and Ferroptosis. Evid Based Complement Alternat Med. 2021;2021:6671129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 517. | Wang Y, Chen Q, Shi C, Jiao F, Gong Z. Mechanism of glycyrrhizin on ferroptosis during acute liver failure by inhibiting oxidative stress. Mol Med Rep. 2019;20:4081-4090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 518. | Ashla AA, Hoshikawa Y, Tsuchiya H, Hashiguchi K, Enjoji M, Nakamuta M, Taketomi A, Maehara Y, Shomori K, Kurimasa A, Hisatome I, Ito H, Shiota G. Genetic analysis of expression profile involved in retinoid metabolism in non-alcoholic fatty liver disease. Hepatol Res. 2010;40:594-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 519. | Saeed A, Bartuzi P, Heegsma J, Dekker D, Kloosterhuis N, de Bruin A, Jonker JW, van de Sluis B, Faber KN. Impaired Hepatic Vitamin A Metabolism in NAFLD Mice Leading to Vitamin A Accumulation in Hepatocytes. Cell Mol Gastroenterol Hepatol. 2021;11:309-325.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 520. | Kim S, Bolatkan A, Kaneko S, Ikawa N, Asada K, Komatsu M, Hayami S, Ojima H, Abe N, Yamaue H, Hamamoto R. Deregulation of the Histone Lysine-Specific Demethylase 1 Is Involved in Human Hepatocellular Carcinoma. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 521. | Tsuchiya H, Akechi Y, Ikeda R, Nishio R, Sakabe T, Terabayashi K, Matsumi Y, Ashla AA, Hoshikawa Y, Kurimasa A, Suzuki T, Ishibashi N, Yanagida S, Shiota G. Suppressive effects of retinoids on iron-induced oxidative stress in the liver. Gastroenterology. 2009;136:341-350.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 522. | Yoshikawa O, Ebata Y, Tsuchiya H, Kawahara A, Kojima C, Ikeda Y, Hama S, Kogure K, Shudo K, Shiota G. A retinoic acid receptor agonist tamibarotene suppresses iron accumulation in the liver. Obesity (Silver Spring). 2013;21:E22-E25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 523. | Ebata Y, Takino J, Tsuchiya H, Sakabe T, Ikeda Y, Hama S, Kogure K, Takeuchi M, Shiota G. Presence of glyceraldehyde-derived advanced glycation end-products in the liver of insulin-resistant mice. Int J Vitam Nutr Res. 2013;83:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 524. | Tsuchiya H, Ikeda Y, Ebata Y, Kojima C, Katsuma R, Tsuruyama T, Sakabe T, Shomori K, Komeda N, Oshiro S, Okamoto H, Takubo K, Hama S, Shudo K, Kogure K, Shiota G. Retinoids ameliorate insulin resistance in a leptin-dependent manner in mice. Hepatology. 2012;56:1319-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 525. | Plaz Torres MC, Jaffe A, Perry R, Marabotto E, Strazzabosco M, Giannini EG. Diabetes medications and risk of HCC. Hepatology. 2022;76:1880-1897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 526. | Luo X, Zhang W, He Z, Yang H, Gao J, Wu P, Ma ZF. Dietary Vitamin C Intake Is Associated With Improved Liver Function and Glucose Metabolism in Chinese Adults. Front Nutr. 2021;8:779912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 527. | Guo Xiaoqiang, Li Wenjie, Xin Qiliang, Ding Hui, Zhang Caiyun, Chang Yanzhong, Duan Xianglin. Vitamin C protective role for alcoholic liver disease in mice through regulating iron metabolism. Toxicol Ind Health. 2011;27:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 528. | Bajoria R, Rekhi E, Almusawy M, Chatterjee R. Hepatic Hemosiderosis Contributes to Abnormal Vitamin D-PTH Axis in Thalassemia Major. J Pediatr Hematol Oncol. 2019;41:e83-e89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 529. | Wood JC, Claster S, Carson S, Menteer JD, Hofstra T, Khanna R, Coates T. Vitamin D deficiency, cardiac iron and cardiac function in thalassaemia major. Br J Haematol. 2008;141:891-894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 530. | Yu U, Chen L, Wang X, Zhang X, Li Y, Wen F, Liu S. Evaluation of the vitamin D and biomedical statuses of young children with β-thalassemia major at a single center in southern China. BMC Pediatr. 2019;19:375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 531. | Chow LH, Frei JV, Hodsman AB, Valberg LS. Low serum 25-hydroxyvitamin D in hereditary hemochromatosis: relation to iron status. Gastroenterology. 1985;88:865-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 532. | Otto-Duessel M, Brewer C, Wood JC. Interdependence of cardiac iron and calcium in a murine model of iron overload. Transl Res. 2011;157:92-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 533. | Anty R, Canivet CM, Patouraux S, Ferrari-Panaia P, Saint-Paul MC, Huet PM, Lebeaupin C, Iannelli A, Gual P, Tran A. Severe Vitamin D Deficiency May be an Additional Cofactor for the Occurrence of Alcoholic Steatohepatitis. Alcohol Clin Exp Res. 2015;39:1027-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 534. | Bjelakovic M, Nikolova D, Bjelakovic G, Gluud C. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2021;8:CD011564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 535. | Gabr SA, Alghadir AH. Handgrip Strength and Vitamin D as Predictors of Liver Fibrosis and Malnutrition in Chronic Hepatitis C Patients. Dis Markers. 2021;2021:6665893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 536. | Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, Arcaro G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 321] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 537. | Kondaiah P, Palika R, Mashurabad P, Singh Yaduvanshi P, Sharp P, Pullakhandam R. Effect of zinc depletion/repletion on intestinal iron absorption and iron status in rats. J Nutr Biochem. 2021;97:108800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 538. | Ergul AB, Turanoglu C, Karakukcu C, Karaman S, Torun YA. Increased Iron Deficiency and Iron Deficiency Anemia in Children with Zinc Deficiency. Eurasian J Med. 2018;50:34-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 539. | Houghton LA, Parnell WR, Thomson CD, Green TJ, Gibson RS. Serum Zinc Is a Major Predictor of Anemia and Mediates the Effect of Selenium on Hemoglobin in School-Aged Children in a Nationally Representative Survey in New Zealand. J Nutr. 2016;146:1670-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 540. | Chen YH, Feng HL, Jeng SS. Zinc Supplementation Stimulates Red Blood Cell Formation in Rats. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 541. | Himoto T, Masaki T. Associations between Zinc Deficiency and Metabolic Abnormalities in Patients with Chronic Liver Disease. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 542. | Li H, Wang D, Wu H, Shen H, Lv D, Zhang Y, Lu H, Yang J, Tang Y, Li M. SLC46A1 contributes to hepatic iron metabolism by importing heme in hepatocytes. Metabolism. 2020;110:154306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 543. | Suliburska J, Skrypnik K, Chmurzyńska A. Folic Acid Affects Iron Status in Female Rats with Deficiency of These Micronutrients. Biol Trace Elem Res. 2020;195:551-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 544. | Lane M, Alfrey CP, Mengel CE, Doherty MA, Doherty J. The rapid induction of human riboflavin deficiency with galactoflavin. J Clin Invest. 1964;43:357-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 545. | Chen H, Kimura M, Itokawa Y. Changes in iron, calcium, magnesium, copper, and zinc levels in different tissues of riboflavin-deficient rats. Biol Trace Elem Res. 1997;56:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 546. | Tsuchiya H. Iron-Induced Hepatocarcinogenesis-Preventive Effects of Nutrients. Front Oncol. 2022;12:940552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |