Published online Jan 28, 2023. doi: 10.3748/wjg.v29.i4.597
Peer-review started: September 19, 2022
First decision: October 22, 2022
Revised: October 28, 2022
Accepted: November 7, 2022
Article in press: November 7, 2022
Published online: January 28, 2023
Processing time: 122 Days and 17.3 Hours
In recent years, there has been a steady growth of interest in non-alcoholic fatty liver disease (NAFLD), which is associated with negative epidemiological data on the prevalence of the disease and its clinical significance. NAFLD is closely related to the metabolic syndrome and these relationships are the subject of active research. A growing body of evidence shows cross-linkages between metabolic abnormalities and the innate immune system in the development and progression of NAFLD. These links are bidirectional and largely still unclear, but a better understanding of them will improve the quality of diagnosis and management of patients. In addition, lipid metabolic disorders and the innate immune system link NAFLD with other diseases, such as atherosclerosis, which is of great clinical importance.
Core Tip: Non-alcoholic fatty liver disease (NAFLD) is an important medical and social problem. The development of NAFLD is closely related to the metabolic syndrome, which further increases attention to the problem. The pathogenesis of NAFLD is complex and involves closely intertwined metabolic and immune mechanisms, a better understanding of which will improve the effectiveness of measures to prevent and treat the disease. Lipid metabolism has multiple connections with the innate immune system, in which various liver cells are involved.
- Citation: Kotlyarov S. Immune and metabolic cross-links in the pathogenesis of comorbid non-alcoholic fatty liver disease. World J Gastroenterol 2023; 29(4): 597-615
- URL: https://www.wjgnet.com/1007-9327/full/v29/i4/597.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i4.597
Interest in non-alcoholic fatty liver disease (NAFLD) has increased significantly in recent years, due to an increasing number of reports on its high prevalence and clinical significance[1]. Epidemiologic data show that the prevalence of NAFLD in the adult population ranges from 17% to 46%, but the data vary by region and depend on age, sex, and several other characteristics[2]. These negative epidemiologic findings are thought to be related to the high prevalence of metabolic diseases, such as obesity and diabetes mellitus, which is due to the effects of low physical activity and poor diet[3]. The links of NAFLD with the metabolic syndrome are attracting increasing attention from clinicians. Dyslipidemia, obesity, insulin resistance, and diabetes are important features of the metabolic syndrome and are closely related to NAFLD[4-6]. Indeed, the prevalence of NAFLD among obese adults is 80%-90%, approximately 30%-50% in patients with diabetes, and up to 90% in patients with hyperlipidemia[7].
Another problem associated with NAFLD is that the disease is often not diagnosed in a timely manner, as patients do not seek medical care for a long time. Most patients are asymptomatic or the symptoms are nonspecific, and patients may not pay enough attention to them. In addition, these patients often have comorbidities, the clinical picture of which may be more pronounced and of greater concern to patients. Atherosclerotic cardiovascular diseases are common in these patients, significantly affecting quality of life and prognosis[8-10]. It is important to note that accurate diagnosis of NAFLD is currently associated with a number of difficulties, primarily, the limited availability of modern diagnostic tools in the primary care setting. Thus, NAFLD is currently a growing burden on patients and healthcare systems.
NAFLD includes two morphological forms, non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH)[11,12]. At the same time, the diagnosis of NAFLD assumes the exclusion of secondary causes and significant alcohol consumption.
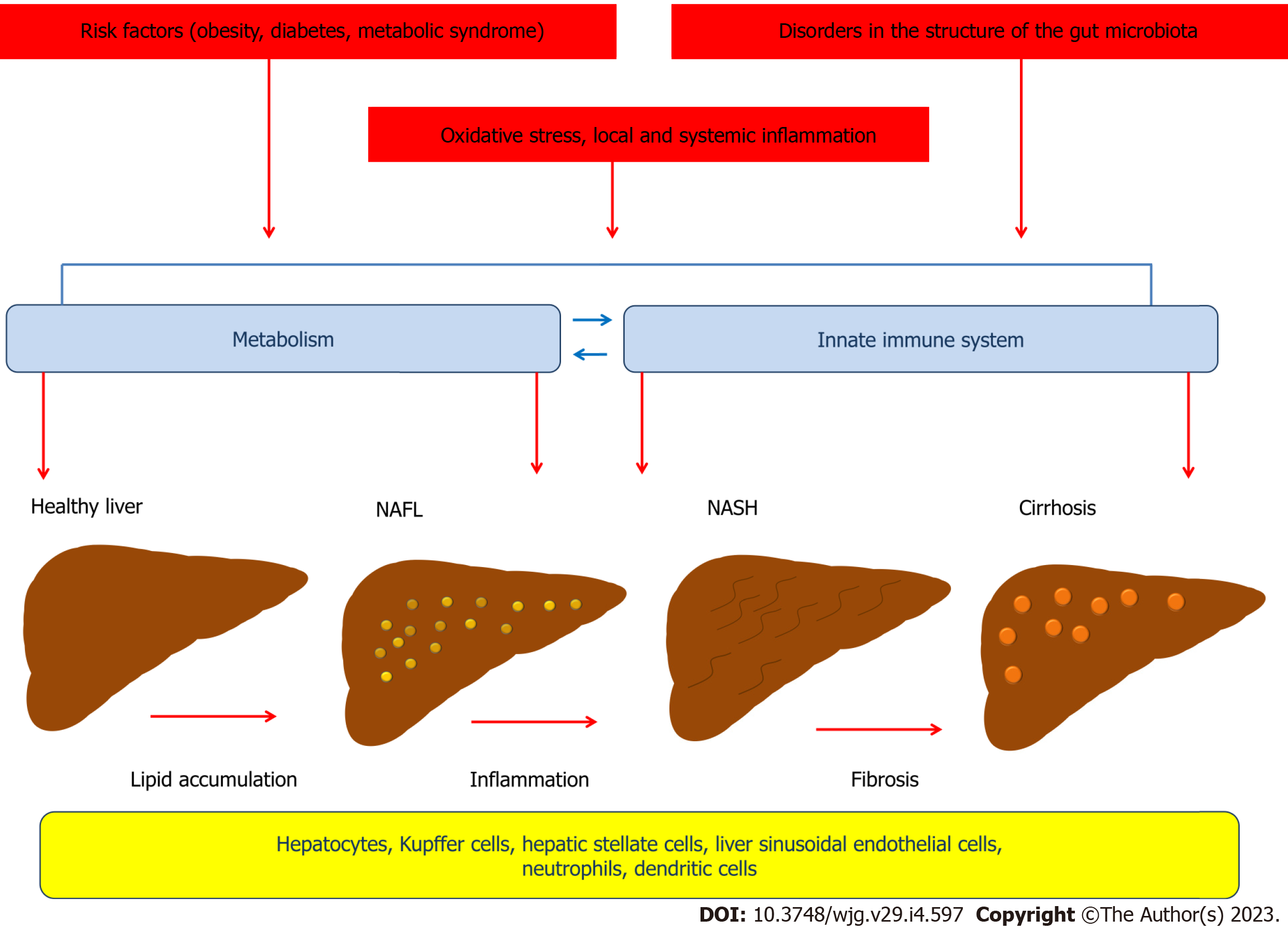
NAFLD is characterized by excessive fat accumulation in the liver, but the pathophysiology of this disorder involves complex mechanisms. According to the "two-hit hypothesis" model proposed in 1998 by Day et al[13], the "first hit" involves lipid accumulation in hepatocytes and development of steatosis, which is associated with the negative impact of obesity, type 2 diabetes, dyslipidemia and other metabolic risk factors on the liver[13-15]. The "second hit" leads to damage to the hepatocellular system and liver inflammation and is associated with the effects of oxidative stress and proinflammatory cytokines[13]. A growing body of evidence suggests that NAFLD develops as a result of a complex chain of events, many of whose links are cross-linked, consistent with the newly proposed "multiple parallel-hit" concept. Thus, insulin resistance, de novo lipogenesis, local and systemic inflammation, disorders in the structure of the gut microbiota, and oxidative stress play an important role in the pathophysiology of NAFLD and have crosslinks that involve different cells (Figure 1)[16]. Recent advances in the study of the mechanisms that contribute to the development and progression of NAFLD have led to a better understanding of the complex interplay between environmental factors, the gut microbiota, metabolism, and the innate immune system, which include both intrahepatic and extrahepatic events[17].
The results of studies suggest that NAFLD exhibits a close bidirectional relationship with the metabolic syndrome[18]. The development of the metabolic syndrome may precede NAFLD or be a consequence of it[19,20]. NAFLD significantly increases the risk of metabolic syndrome and may also be considered an independent risk factor for some cardiovascular diseases[21-23]. Given that NAFLD is often combined with metabolic diseases such as obesity, type 2 diabetes, hyperlipidemia, and hypertension, it may have negative prognostic implications[24,25]. Thus, an overweight person is a typical NAFLD patient phenotype[26,27]. Moreover, body mass index and NAFLD show a strong correlation[27,28]. Interestingly, NAFLD also occurs in non-obese individuals, with the majority of these findings occurring in Asian countries, although they have been described worldwide[29-32]. Despite the phenotypic differences, NAFLD patients who were not obese had similar severity of histologic liver damage[33]. At the same time, NAFLD patients without obesity had a higher degree of fibrosis[34-37].
A key histological characteristic of NAFLD is the cellular accumulation of triglyceride (triacylglycerides, TAGs) containing lipid droplets[38-40]. TAG biosynthesis is carried out using fatty acids, which may enter the cells from the blood or be formed by de novo lipogenesis and endocytotic recycling of lipoprotein remnants[40,41]. In most cases, the main source of fatty acids used for TAG formation is absorption from the blood[41,42]. Interestingly, some data suggest that TAG accumulation per se is not harmful to hepatocytes and can even be considered as a certain protective mechanism against lipotoxicity induced by free fatty acids[43]. This is supported by the data that an excess of free fatty acids in nonfat cells can lead to their dysfunction and apoptotic death[44]. Moreover, levels of free fatty acids in the blood are related to the severity of NAFLD, with saturated fatty acids being more hepatotoxic than unsaturated fatty acids[45]. Thus, free fatty acids are important mediators of excessive lipid accumulation in the liver.
Studies have shown that monounsaturated fatty acids such as oleic or palmitoleic acids are less toxic than saturated fatty acids such as palmitic or stearic acids[46,47]. Long-chain saturated palmitate induces apoptosis in Chinese hamster ovary cells through a mechanism involving reactive oxygen species (ROS) and ceramide formation, which can enhance palmitate-induced apoptosis signals[43]. In turn, unsaturated fatty acids prevent palmitate-induced apoptosis by directing palmitate to triglyceride pools and removing them from pathways leading to apoptosis[43]. In doing so, reducing the ability of cells to synthesize triglycerides contributes to lipotoxicity[43]. The mechanism of this action may be related to the fact that palmitate is poorly incorporated into cellular triglyceride pools in the absence of additional signals, but the presence of unsaturated fatty acids can help direct palmitate toward triglyceride storage, thereby excluding palmitate from apoptotic pathways. Moreover, unsaturated fatty acids, which come both as additives to the medium, such as the addition of oleate, and as a result of the action of desaturase (e.g., stearoyl-CoA desaturase), demonstrate this action[43]. Stearoyl-CoA desaturase-1 (SCD), known as fatty acid desaturase, is an enzyme that is expressed in the liver and is involved in the biosynthesis of monounsaturated fatty acids, primarily oleate and palmitoleate from corresponding saturated fatty acids. Decreased expression and activity of SCD1, leads to the intake of excessive amounts of saturated fatty acids, increasing their lipotoxic effects and the development of steatohepatitis and fibrosis[48,49]. Indeed, oleic acid has been shown to be more steatogenic but has less apoptotic effects than palmitic acid in hepatocyte cell cultures[50].
Increased fat in the liver correlates directly with changes in plasma saturated fatty acids and inversely with polyunsaturated fatty acids (PUFAs)[51]. Saturated fatty acids markedly induce fat deposition in the liver and serum ceramides, whereas dietary PUFAs prevent fat accumulation in the liver and reduce ceramides and hyperlipidemia with excess energy intake in overweight people[51]. Higher concentrations of total ω-6 PUFAs and serum linoleic acid have been shown to be associated with lower odds of developing NAFLD in the future[52]. Meanwhile, ω-3 PUFAs such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) may have a protective effect on the liver by reducing insulin resistance, reducing inflammation, and inhibiting apoptosis of hepatocytes[53].
These and other data allow us to expand our views on the features of metabolic processes in NAFLD, as well as NAFLD comorbid relationships. It has been shown that in NAFLD, regardless of the presence or absence of obesity, there is a high risk of coronary atherosclerosis, which contributes to the clinical picture[54]. It is widely known that NAFLD is associated with the development of atherosclerosis[55-57]. Moreover, NAFLD is associated with an increased risk of cardiovascular disease beyond that due to established risk factors[57]. Moreover, cardiovascular disease is the main cause of death in NAFLD patients[55].
NAFLD patients often have dyslipidemia along with other features of the metabolic syndrome. NAFLD patients have significantly elevated levels of oxidized low-density lipoprotein (LDL), and a significant association has been shown between LDL levels and the prevalence of NAFLD[58,59]. Elevated LDL levels within the normal range were associated with an increased risk of NAFLD[59]. In addition, there are important differences in LDL and high-density lipoprotein (HDL) subfractions in NAFLD patients. Liver fat has been shown to correlate more strongly with circulating HDL2 cholesterol and the ratio of HDL2 to HDL3 cholesterol than with total HDL cholesterol[60]. Patients with NASH had an increased number of small, dense LDL3 and LDL4 particles[61]. These changes may contribute to the increased risk of atherosclerosis and cardiovascular disease in these patients.
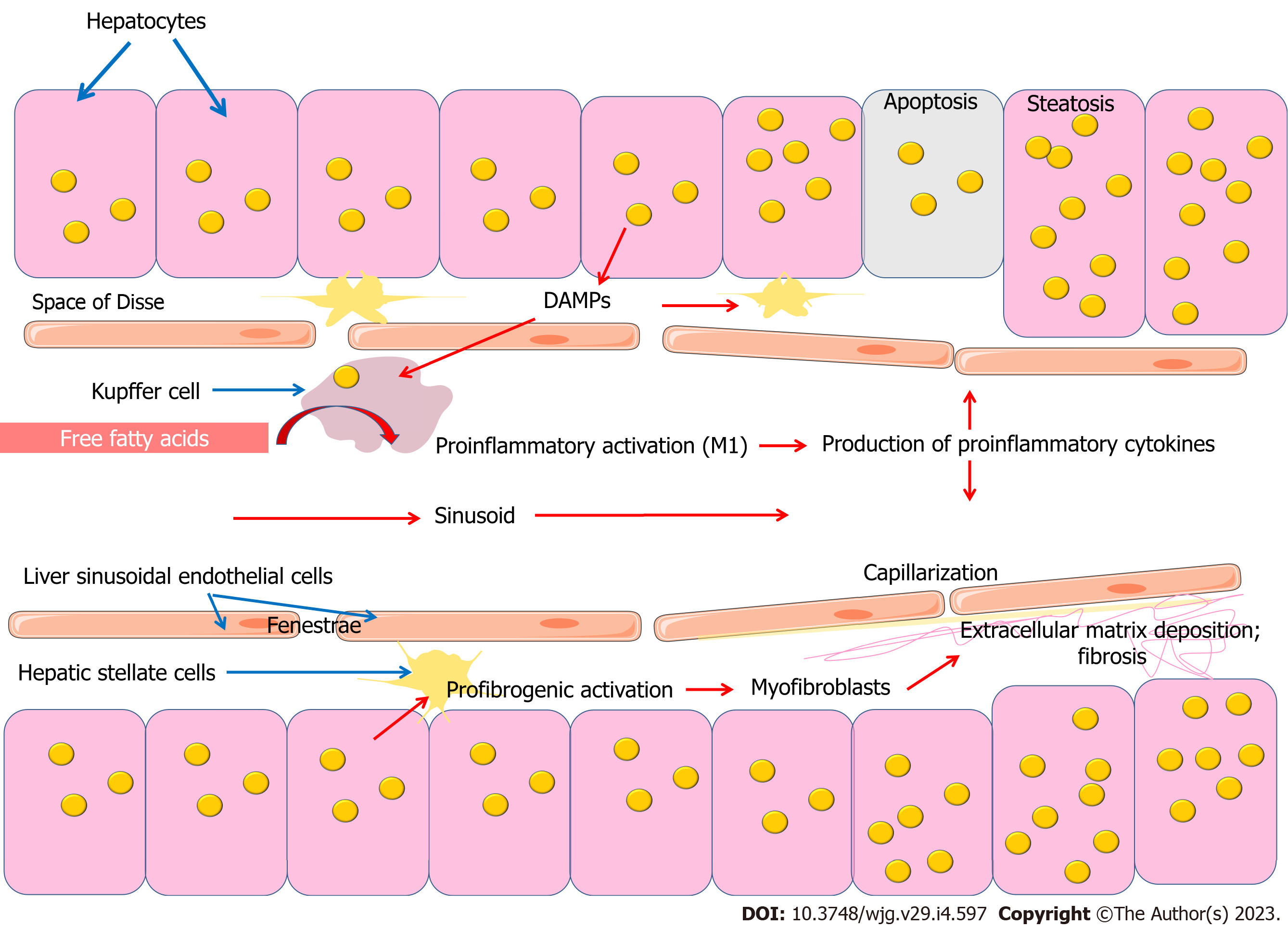
A growing body of evidence is increasing the understanding of the importance of the innate immune system in the development of NAFLD (Figure 2). The innate immune cells, which include Kupffer cells, neutrophils, dendritic cells (DCs), and natural killer (NK) cells, play an important role in the pathogenesis of NAFLD. Kupffer cells, which constitute 80% to 90% of the total macrophage population, are under physiological conditions a long-lived and self-renewing population[62]. Due to their location, they are central to innate immunity and are responsible for the rapid removal of exogenous particles such as lipopolysaccharide (LPS)[63-65]. Like other macrophages, Kupffer cells are also capable of detecting endogenous molecular signals resulting from homeostasis disruption[62].
Steatohepatitis is characterized by marked enlargement and aggregation of Kupffer cells in perivenular regions, with scattered large fat vacuoles found within Kupffer cells[66]. The contribution of macrophages originating from blood monocytes to this cell pool is not entirely clear, as there is currently no marker to distinguish them from resident macrophages[67].
Kupffer cells, which are resident macrophages of the liver, uptake large amounts of free fatty acids, which contributes to their proinflammatory activation. During inflammatory activation, Kupffer cells produce proinflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, which are important participants in the progression of inflammation and development of NASH[68]. Thus, free fatty acids mediate the link between lipid metabolism and the innate immune system[69-71].
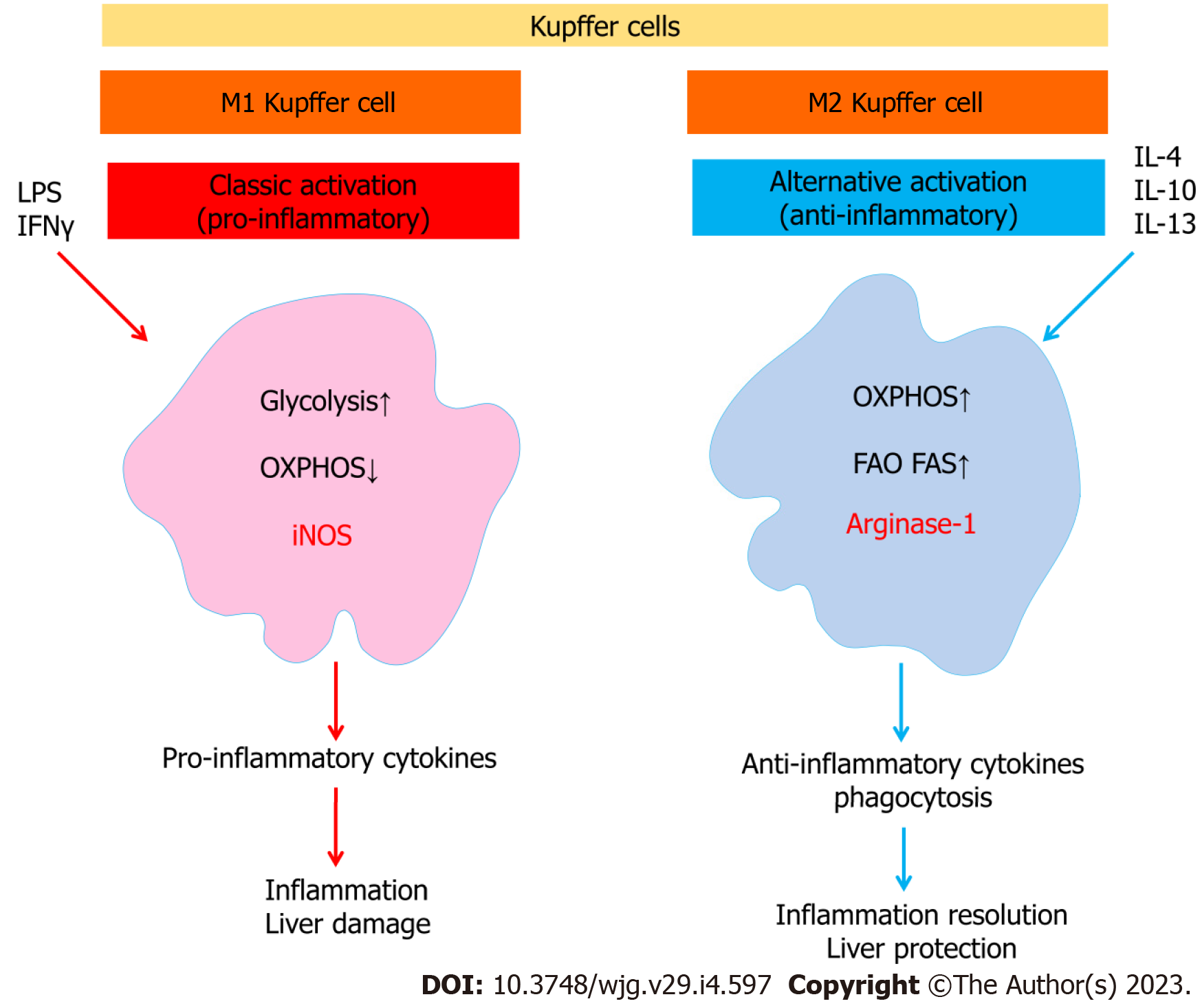
It is important to note that Kupffer cells, like other macrophages, have complex immunometabolic regulation (Figure 3). It has been shown that a prolonged high-fat diet increased the number of Kupffer cells with a proinflammatory M1 phenotype producing proinflammatory cytokines. Saturated fatty acids promoted M1 polarization of Kupffer cells, whereas ω-3 PUFAs polarized Kupffer cells to the M2 phenotype, which was associated with activation of the NF-κB and PPAR-γ signaling pathways, respectively[72]. The proinflammatory M1 phenotype of macrophages is characterized by enhanced glycolysis and fatty acid synthesis, whereas the anti-inflammatory M2 macrophages use fatty acid oxidation[73].
It has been suggested that polarization of M2 Kupffer cells may protect against fatty liver disease. M2 macrophages have been shown to be predominant in individuals with limited liver lesions, corresponding to little hepatocyte apoptosis compared with patients with more severe lesions[74]. Interestingly, M2-induced apoptosis of M1 macrophages is one of the mechanisms regulating the balance between M1 and M2 macrophages[74].
It has been suggested that elevated levels of free fatty acids, resulting from their excessive intake with food or by release from adipose tissue during starvation, may be the main cause of TNF release from Kupffer cells, leading to hepatocyte steatosis. Toll-like receptor 4 (TLR4) is able to detect free fatty acids on Kupffer cells to detect excess and overload of fatty acids in the liver[75]. It is known that saturated fatty acids can participate in the activation of TLR4, a receptor of the innate immune system[76-78]. This action can be associated with both direct stimulation of the receptor, confirming the evolutionary connection with the structure of LPS, which is the receptor aimed at detecting. In addition, fatty acids can be incorporated into the phospholipids of the plasma membrane and thus influence their biophysical properties and function[77,79]. The saturation and length of the alkyl chain are important. By influencing the biophysical properties of the plasma membrane and the stability of lipid rafts in this way, the function of some membrane proteins can be regulated. It is suggested that unsaturated fatty acids contribute to a decrease in lipid ordering and the stability of lipid rafts, which may lead to anti-inflammatory effects, given the role of lipid rafts as platforms for the assembly and function of many signaling pathways. Thus, unlike saturated fatty acids, unsaturated fatty acids do not have the ability to activate TLR4. In addition, their effect on the biophysical properties of plasma membranes is opposite[77].
Unsaturated fatty acids can participate in the regulation of inflammation not only due to their biophysical properties. They are also precursors for the formation of many lipid mediators associated with inflammation. The family of lipid mediators called "specialized pro-resolving mediators" includes lipoxins, resolvins, protectins and maresins. They are formed enzymatically from ω-3 and ω-6 PUFAs such as arachidonic acid, EPA and DHA. Lipoxins are formed from arachidonic acid, E-series resolvins from EPA, and D-series resolvins, protectins and maresins from DHA[80].
Circulating maresin-1 (MaR1) levels were shown to be decreased in NAFLD patients, and a negative correlation between NAFLD and serum MaR1 concentrations was found[81]. MaR1 is mainly synthesized in M2-macrophages and plays an important anti-inflammatory role. It improves insulin sensitivity and eliminates adipose tissue inflammation[82]. In addition, MaR1 improves hepatic steatosis by inhibiting endoplasmic reticulum stress and lipogenic enzymes, and inducing autophagy via the AMP-activated protein kinase (AMPK) pathway[81,83,84]. Activation of Kupffer cells leads to M1 polarization and a decrease in the M2 phenotype, which corresponds to a decrease in maresin production and a decrease in their anti-inflammatory effect. Resolvin D1 (RvD1), which is an endogenous mediator produced from ω-3 DHA, reduced macrophage accumulation in adipose tissue and improved insulin sensitivity in obese and diabetic mice[85]. RvD1 shifted macrophages from an M1-to-M2-like anti-inflammatory phenotype, triggering the resolution process initiated by caloric restriction in obesity-induced steatohepatitis[86]. Protectin DX, derived from DHA, showed suppressive effects on inflammation and insulin resistance and improved hepatic steatosis by suppressing endoplasmic reticulum stress through AMPK-induced ORP150 expression[87].
On the other hand, the development of NAFLD correlates with an increase in serum eicosanoids. Moreover, profiling of plasma eicosanoids and other PUFA metabolites can differentiate NAFLD from NASH[88]. 11,12-dihydroxy-eicosatrienoic acid (11,12-diHETrE) was used as a biomarker to differentiate NAFLD from NASH[88]. In another study, patients with NASH had significantly elevated levels of 9- and 13-HODE and 9- and 13-oxoODE, products of linoleic acid oxidation, compared with patients with steatosis[89]. Interestingly, patients with stage I NAFLD had lower plasma levels of 5-HETE, whereas patients with stage II steatosis had higher concentrations of 9-HODE[90].
Thus, lipid metabolites derived from fatty acids are involved in the development of NAFLD, which is an interesting topic for further research (Figure 2).
Hepatocellular accumulation of lipids can modulate the biological activity of Kupffer cells through a number of mechanisms. On the one hand, fat-saturated hepatocyte swelling changes the architecture of the sinusoidal network, reducing intrasinusoidal volume and microvascular blood flow. Disruption of microvascular blood flow also contributes to the involvement of sinusoidal endothelial cells, Kupffer cells, stellate cells and involvement of inflammatory cells and platelets[91]. Later developing fibrosing steatohepatitis with capillarization of the sinusoids, increases narrowing and distortion of the sinusoidal lumen, further limiting microvascular blood flow. In addition, leukocytes entering the narrowed sinusoids may adhere to the endothelium as a result of activation of the hepatic microvascular inflammatory response[91]. On the other hand, fat overload of hepatocytes causes lipotoxicity and the release of damage-associated molecular patterns (DAMPs), which can activate Kupffer cells and hepatic stellate cells (HSCs), promoting inflammation and fibrosis[92]. Lipid accumulation in hepatocytes has been shown to induce the release of factors that accelerate the activation and proliferation of HSCs and increase their resistance to apoptosis[93]. Conditioned medium from steatotic hepatocytes induced expression of the profibrogenic genes transforming growth factor (TGF)-beta, tissue inhibitor of metalloproteinase-1 (TIMP-1), TIMP-2 and matrix metalloproteinase-2, and expression of the NF-κB-dependent monocyte chemotactic protein-1 (MCP-1) in HSCs[93]. Thus, quiescent HSCs participate in the maintenance of liver architecture by maintaining the balance of extracellular matrix, while disruption of this balance, for example, due to metabolic disorders, leads to HSCs activation and fibrosis[94,95].
Hepatocytes exposed to apoptosis form apoptotic bodies, which are phagocytosed by HSCs and Kupffer cells, triggering a profibrogenic response due to transdifferentiation of HSCs into collagen-producing myofibroblasts[96]. Apoptotic cell uptake has been shown to stimulate Kupffer cell production of death ligands, including Fas ligand and TNF-alpha, which promotes inflammation and fibrogenesis[97].
An important pathogenetic mechanism involved in the pathogenesis of NAFLD is the role of the intestinal microbiota and a defect in the intestinal barrier caused by liver damage. Impaired gut barrier function is thought to accelerate translocation of enteric LPS, which activates proinflammatory signaling pathways and the release of related inflammatory factors in the liver[98]. Intestinal bacterial microflora and TLR4 have been shown to be involved in liver fibrogenesis[99]. Escherichia coli LPS can enhance liver damage in NAFLD by inducing macrophage and platelet activation through the TLR4 pathway[100]. Plasma endotoxin levels and inflammatory markers have been shown to be significantly higher in NAFLD compared with controls and to increase with the severity of hepatic steatosis[101]. Proinflammatory activity and immune imbalance associated with the pathophysiology of NAFLD may be related to gut dysbiosis[102]. For example, decreased Bacteroidetes and increased Firmicutes were observed in obese individuals[102]. Changes in gut microflora ratios may also increase endogenous ethanol production, which generally increases gut permeability, and contributes to translocation of endotoxins from the gut lumen into the portal bloodstream[102,103].
Another immunometabolic link between the gut microbiota and NAFLD, related to short-chain fatty acids (SCFAs), should also be noted[104,105]. SCFAs are formed by the gut microbiota during the fermentation of non-digestible fibers such as resistant starch, cellulose, and pectin[106]. SCFAs are used by colonic mucosal epithelial cells as an energy substrate, are involved in the regulation of a number of processes in the intestinal wall or enter the portal bloodstream, and may be involved in the formation of immunometabolic connections with other organs[107].
A growing body of evidence strengthens the understanding of the importance of SCFAs in inflammation. SCFAs act via receptors associated with the G-protein GPR43 and GPR41, also known as free fatty acid receptor (FFA)2 and FFA3, respectively[108-111]. In addition, SCFAs realize their action through inhibition of histone deacetylase (HDAC)[112,113].
Butyrate is well known for its anti-inflammatory properties and is of great clinical interest[107,114,115]. Through HDAC3 inhibition, butyrate can induce a metabolic switch of macrophages toward an anti-inflammatory M2 phenotype[112,113].
SCFAs are also known to affect the differentiation, recruitment and activation of neutrophils, DCs, macrophages and monocytes as well as T cells[116,117]. Butyrate is involved in the regulation of DC differentiation derived from human monocytes, keeping DCs in the immature stage[118].
In addition to their involvement in inflammation, SCFAs regulate lipid metabolism in the liver. Butyrate levels have been shown to decrease in NAFLD patients and mice with decreased estrogen levels, with butyrate administration attenuating liver steatosis[119]. Studies in rats fed a high-fat diet (HFD) have shown that butyrate increases β-oxidation of fatty acids, inhibits lipid synthesis and suppresses nuclear factor-kappa B and inflammation[120,121]. The addition of sodium butyrate protects mice from developing NASH. It is important to note that the metabolic role of SCFAs in liver function is rather complex[122]. In addition to attenuating hepatic steatosis, acetate, another SCFA derived from the microbiota, may conversely promote hepatic lipogenesis after excessive fructose intake[123,124].
A growing body of evidence strengthens the understanding that lipoproteins are part of an important transport mechanism that is utilized by the innate immune system. The mechanism of LPS elimination involves LPS disaggregation and binding to circulating lipoproteins, uptake of lipoprotein-associated LPS by the liver, and excretion of LPS with the bile[125,126]. This pathway, known as reverse LPS transport, involves lipoproteins as the main carriers of LPS in the plasma and includes the proteins LBP, BPI, phospholipid-transfer protein (PLTP), and cholesteryl ester transfer protein (CETP), which belong to the lipid transfer/LPS binding gene family (LT/LBP) and play different roles in LPS metabolism[126]. In addition, reverse cholesterol transport is at the beginning of the cross-talk between cholesterol metabolism and the innate immune system[126]. ABCA1, a key participant in reverse cholesterol transport also contributes to the efflux of LPS from macrophages[127]. HDL and other plasma lip-oproteins have been shown to contribute to the release of LPS from the cell surface of monocytes[128].
Lipid transfer proteins (lecithin-cholesterol acyltransferase (LCAT), CETP, and PLTP) as well as hepatic and endothelial lipases remodel HDL in the bloodstream. CETP is part of a family of proteins including LPS-binding protein (LBP) and bactericidal permeability increasing protein (BPI) and may participate in the transport of LPS between lipoproteins for further utilization in the liver. CETP transports cholesterol esters from HDL to apoB-containing LDL and very low density lipoproteins (VLDLs).
Kupffer cells take up most of the LPS and can inactivate it by deacylation with acyloxyacyl hydrolase. Kupffer cells express high levels of class A scavenger receptors (SR-A), which bind oxidized low-density lipoproteins (LDL) and are also involved in LPS uptake[64,129]. SR-A expression is increased by oxidized LDL[130,131]. Importantly, in the liver, SR-A is also important for cell adhesion, suggesting a role for SR-A in the recruitment and retention of cells in various organs or in sites of pathological conditions, such as foci of inflammation or areas of atherosclerotic lesions[64]. In addition to Kupffer cells, SR-A types I and II are expressed in the liver on endothelial cells, which are less able to bind LPS[132].
Interestingly, plasma CETP predominantly originates from Kupffer cells, and plasma CETP levels predict the content of Kupffer cells in the liver in humans[133]. In addition, activation of Kupffer cells by LPS strongly decreases CETP expression[134]. LPS has been shown to activate resting Kupffer cells, resulting in decreased hepatic CETP expression and decreased CETP in plasma and increased HDL cholesterol levels[135]. Importantly, CETP inhibition improves HDL function but leads to liver obesity and insulin resistance in CETP-expressing transgenic mice on a HFD[136]. Information obtained in recent years has improved the understanding of the role of CETP in inflammation. Experimental evidence suggests that CETP in macrophages as well as in the liver prevents LPS interaction with TLR4, thereby reducing the inflammatory response[137]. Compared with wild-type mice, CETP mice showed a higher survival rate after polymicrobial sepsis. CETP mice had lower plasma IL-6 concentrations and decreased levels of hepatic TLR4 and acyloxyacyl hydrolase protein[137]. Species-specific differences in CETP expression should be noted[138]. In mice and rats, in contrast to humans, as well as primates, rabbits, and hamsters, CETP is absent in plasma. Consequently, wild-type mice, have naturally low LDL and high HDL levels, in which up to 90% of cholesterol is transported and have low susceptibility to developing atherosclerosis. Transgenic mice expressing human CETP have increased reverse cholesterol transport, which is associated with increased clearance of apoB lipoproteins in the liver. They also show increased postprandial triglyceridemia, increased liver uptake of LPS, and increased survival in endotoxemia[139,140]. Transgenic expression of CETP in mice also reduces liver fat accumulation and improves insulin sensitivity in diet-induced obesity[141,142]. CETP has been shown to reduce liver TAG content in female mice through enhanced β-oxidation and to promote the synthesis and assembly of VLDL[142]. CETP inhibition in transgenic CETP-expressing mice disrupted TAG metabolic pathways, leading to liver TG accumulation and insulin resistance in diet-induced obese mice[136]. In addition, CETP inhibition by anacetrapib increased systemic and hepatic inflammation to a greater extent in obese mice[136].
Despite its weaker ability to bind LPS compared to LBP or BPI, CETP is associated with resistance to sepsis. Experiments with human CETP transgenic mice showed lower mortality after LPS administration compared to wild-type mice. The pathway involving CETP is of interest because it represents a cross-talk mechanism of reverse cholesterol transport and the innate immune system, in which LPS and cholesterol share common transport and utilization pathways[140].
Neutrophils, other important participants in the innate immune system, are also involved in the pathogenesis of NAFLD[143]. Given that inflammation is a key event that contributes to the progression of fatty liver dystrophy to NAFLD, these patients show significant neutrophil infiltration into the liver, often accompanied by increased expression of chemokines that promote neutrophil chemotaxis[144].
Neutrophils exhibit cross-links with HSCs. On the one hand, neutrophils activate HSCs through the production of ROS[145-147]. On the other hand, activated HSCs have been shown to support neutrophil survival by producing granulocyte-macrophage colony-stimulating factor and IL-15. This may serve as a positive direct loop contributor to liver damage and fibrosis under a HFD[147].
Interestingly, it has been shown that neutrophils in blood in patients with NASH had increased expression of receptors reflecting the preparation of neutrophils to migrate into tissue. In addition to preparation for migration, blood neutrophils in NASH were also functionally activated[148]. They were characterized by increased IL-8 production and had more than double the spontaneous oxidative burst. In analyzing these data, it was noted that neutrophils can not only move from the vascular lumen into extravascular tissues but can also move back into the bloodstream, through a process known as reverse transendothelial migration. Reverse transendothelial migration is of interest due to its possible interaction with the immune system[149]. However, its possible role in NAFLD has yet to be studied.
Thus, neutrophils play an important role in the development of inflammation and liver fibrosis[150]. On the other hand, neutrophils contribute to the spontaneous resolution of inflammation and liver fibrosis. Acting via miR-223, neutrophils act as resolving effector cells that induce the transition of proinflammatory macrophages to a restorative phenotype by suppressing NLRP3 inflammasome expression[151]. Another study in a diet-induced NASH mouse model also showed a phase-dependent contrasting role of neutrophils as triggers and pro-resolutive mediators of liver injury and fibrosis[150]. In addition to these findings, miR-223 was shown to be elevated in hepatocytes from HFD-treated mice and patients with NASH, which may be due to the fact that miR-223 can be transferred from neutrophils via the exosome. Moreover, miR-223 in hepatocytes acts as an anti-inflammatory molecule, directly affecting several inflammatory genes[152].
Thus, neutrophils play a complex multifaceted role in the pathogenesis of NAFLD, which is a promising topic for further research.
Liver DCs are a heterogeneous population of hepatic sinusoidal antigen-presenting cells[153,154]. DCs exist in mature or immature states and undergo maturation when exposed to immune or inflammatory signals such as microbial products and proinflammatory cytokines. DCs are involved in maintaining immune homeostasis and liver tolerance by promoting CD8+ T-cell elimination, as well as secreting anti-inflammatory cytokines that maintain the quiescent HSC state and promote TLR4 refractoriness to LPS. In addition, DCs regulate the number and activity of cells involved in the development of fibrosis and may play a role in the regression of liver fibrosis[155]. Dendritic cells can contribute to liver fibrosis regression by activating metalloproteinases and contribute to the homeostasis of NK cells, which are mainly antifibrogenic[154].
Natural killer cells are a heterogeneous multifunctional population of lymphoid cells located inside the sinusoidal space, where they can attach to endothelium and Kupffer cells[156]. A key factor determining the activity of these cells in NASH is their metabolic reprogramming.
Liver NK cells are part of the innate immune system and may play an important role in NAFLD. However, the regulation and function of NK cells in NAFLD remains controversial due to their different involvement at different stages of the disease. On the one hand, NK cells are active and may be useful in the early stages of fibrosis, when they contribute to TRAIL-mediated HSC death. On the other hand, NK cell involvement becomes detrimental when they lose their antitumor capacity, which may contribute to disease progression in later stages[156]. Indeed, metabolic reprogramming of NK cells in obesity limits the antitumor response, which is known as "metabolic paralysis"[157]. Overload of NK cells with lipids absorbed from the environment in obesity leads to metabolic defects that cause inhibition of the cytotoxic mechanism, resulting in loss of antitumor functions[157]. Overall, the available data suggest a possible therapeutic potential for the regulation of NK cell function, which is a promising topic for further research.
The innate immune system relies on a large number of pattern recognition receptors (PRRs) to recognize both DAMPs and pathogen-associated molecular patterns. Toll-like receptors (TLRs) are the most well characterized representatives of PRRs. They are expressed in a variety of liver cells, including Kupffer cells, HSCs, hepatocytes, sinusoidal endothelial cells, and biliary epithelial cells[158-160]. A growing body of evidence reinforces the importance of TLRs in the pathogenesis of NAFLD[161]. TLR4 is of particular interest in connection with liver inflammation and fibrogenesis[158,162,163]. TLR4 is a receptor that detects the LPS of Gram-negative bacteria and is widely known for its role in various diseases.
TLR4 is expressed on all types of liver cells, including Kupffer cells, HSCs, and hepatocytes. Under normal conditions, hepatic cells express minimal TLRs, indicating a high tolerance of the liver to TLR ligands[164]. At the same time, receptor expression in the liver is associated with inflammation and fibrosis[164]. TLR4 plays a central role in Kupffer cell activation by responding to LPS. LPS is considered a potent inducer of hepatic inflammation. It promotes the production of TNF-α in Kupffer cells, which is a mediator of inflammation in the pathogenesis of NAFLD[165]. In addition, LPS can activate HSCs, and Kupffer cells can enhance this process by producing TGF-β and making HSCs more sensitive to TGF-β[164]. Despite the fact that Kupffer cells are the main targets for LPS in the liver, it is HSCs that contribute to TLR4-dependent fibrosis[99]. In addition, modulation of TGF-β signaling along the TLR4-MyD88-NF-κB axis provides a link between proinflammatory and profibrogenic signals[99]. Numerous data support the involvement of HSCs as central mediators of hepatic fibrosis. Activation of TLR4 in quiescent HSCs enhances chemokine secretion and induces Kupffer cell chemotaxis and inhibits the TGF-β pseudoreceptor Bambi, which increases HSCs sensitivity to signals induced by TGF-β and enables unrestricted activation by Kupffer cells[99]. A significantly reduced expression of the Bambi gene in HSCs was seen when incubated with the TLR4 LPS ligand[166].
It was found that a diet high in cholesterol leads to the accumulation of free cholesterol in HSCs, which promotes TLR4 signaling by increasing TLR4 levels in the membrane and can suppress Bambi gene expression. As a consequence, TGF-β signaling in HSCs was enhanced, leading to HSCs activation and progression of liver fibrosis[166].
Endothelial cells, which form the inner membrane of blood vessels, play an important role in the functioning of the barrier between blood and tissues. Endothelium is characterized by heterogeneity and plasticity due to phenotypic specialization of different tissue types. This endothelial specialization can provide dense connections necessary for functioning of histo-tissue barriers, or on the contrary can promote infiltration and extravasation of molecules and particles circulating in the bloodstream due to fenestrated endothelium in the liver and kidneys[167]. Given that the liver is a highly vascularized organ (accounting for 20% of cardiac output), hepatic sinusoidal endothelial cells constitute a significant proportion of the total number of liver cells[168]. Liver sinusoidal endothelial cells (LSECs) have a unique morphological phenotype characterized by a combination of numerous fenestrae and lack of a basement membrane, which provides open access for dissolved substances between the sinusoidal blood and the Disse space (Figure 2). LSECs are involved in regulation of the liver microenvironment and act as the liver's first protective barrier. An important functional phenotypic feature of hepatic sinusoidal endothelial cells is their high endocytic capacity[169]. These cells are capable of absorbing and removing soluble macromolecules from the portal venous blood in addition to Kupffer cells located on the lumen side of the endothelium[168].
Disruption of the LSECs phenotype is a critical step in the liver fibrosis process (Figure 2). Capillarization, in which there is a lack of fenestration of hepatic sinusoidal endothelial cells and formation of an organized basal membrane, precedes fibrosis and contributes to HSC activation[169]. Vascular endothelial growth factor (VEGF) produced by hepatocytes and HSCs has been shown to be a key regulator of the LSEC phenotype[169-173]. The maintenance of the fenestrated LSEC phenotype is provided by the action of VEGF through a nitric oxide (NO)-dependent and NO-independent pathway[169-171]. In this case, VEGF, which is produced by hepatocytes or stellate cells, promotes NO formation from LSECs via endothelial nitric oxide synthase (eNOS)[171].
A growing body of evidence supports the important role of the endothelium in vascular biology. Endothelial cells can detect changes in blood flow and are involved in the regulation of hemodynamics and inflammation through the production of several bioactive substances. Endothelial production of NO is the best known way to regulate vascular hemodynamics. Nitric oxide is an important signaling molecule that is at the crossroads between the regulation of vascular hemodynamics and innate immunity[174]. Importantly, NO demonstrates active involvement in the regulation of inflammation in the vascular wall, which is important in the development of atherosclerosis. Endothelial NO actively regulates the innate immune response involved in atherogenesis by regulating macrophage and lymphocyte uptake and vessel wall migration via adhesion molecules[174].
Nitric oxide synthesis in the endothelium is carried out by a specific constitutive eNOS isoform. Mechanical stimulation of endothelial cells by blood flow triggers a complex chain of events involving numerous cellular mechanosensors and enzymes, leading to activation of eNOS[175]. eNOS is expressed in LSECs and produces small amounts of NO, which maintain intrahepatic sinusoidal vascular tone and hemodynamics in the liver. Another isoform of nitric oxide synthase, inducible NOS (iNOS) is expressed in various liver cells, including LSECs, hepatocytes, Kupffer cells, HSCs and other immune cells[176-178]. LPS induces iNOS expression and NO production and increases caveolin-1 and decreases eNOS phosphorylation[179].
It should be noted that while the NO produced by eNOS has a hepatoprotective effect by inhibiting inflammatory activation of Kupffer cells, the NO produced by iNOS, in contrast, promotes NAFLD[180]. iNOS produces significantly more NO than eNOS, which can have negative effects. This is due to the cytotoxicity of NO in high concentrations. In particular, peroxynitrite (ONOO-) can damage a wide range of cellular molecules[181]. Interestingly, peroxynitrite can affect cyclooxygenase (COX)-1 and COX-2 activity depending on the concentration[182,183]. It has been suggested that NO can interact directly with COX, for example via S-nitrosylation, causing an increase in its enzymatic activity[184,185]. Thus, NO production has closely overlapping connections with innate immunity. These and other data suggested a role for COX enzymes as important endogenous receptor targets for NO functions[186]. COX-2-mediated inflammation is important for insulin resistance associated with obesity and fatty liver dystrophy. Daily aspirin intake was associated with less severe histologic signs of NAFLD and NASH and a reduced risk of fibrosis progression over time[187].
Importantly, eNOS activity is decreased in pathological conditions, whereas iNOS activity is increased. Decreased NO production in LSECs causes endothelial cell capillarization and HSCs activation. This leads to deposition of extracellular matrix, proliferation of HSCs, increased intrahepatic resistance and impaired sinusoidal blood flow[180].
Thus, the function of NO is related to the maintenance of liver cell function. NO derived from eNOS protects against liver disease, whereas NO derived from iNOS has a proinflammatory effect[180]. When mice were fed a HFD, a decrease in liver NO was shown to precede the onset of liver inflammation through the NF-κB pathway as well as impaired insulin signaling at the IRS-1 and phospho-Akt levels. Thus, an important physiological role of endothelial NO has been shown to limit obesity-associated inflammation and impaired insulin signaling in hepatocytes and Kupffer cells via the NO/cGMP-dependent protein kinase (PKG)/ vasodilator-stimulated phosphoprotein (VASP) pathway as part of a cross-talk mechanism with metabolic disturbances associated with obesity[168].
LSECs exhibit a proinflammatory phenotype during the progression of NAFLD to NASH. It is characterized by surface overexpression of adhesion molecules such as ICAM-1, VCAM-1, and VAP-1 (AOC3) and production of proinflammatory molecules such as TNF-α, IL-6, IL-1, and MCP1 (CCL2)[188,189]. Interestingly, LSECs and HSCs are involved in maintaining each other's differential phenotype. On the one hand, VEGF-A production by either HSCs or hepatocytes supports LSECs differentiation[170]; on the other hand, fenestrated LSECs prevent HSCs activation and promote the conversion of activated HSCs to a dormant state. However, LSECs lose this effect when they are undifferentiated or have a capillarized phenotype[171,190].
Thus, LSECs play an important role in liver immunology and the development of NAFLD. In contrast to hepatocytes, free fatty acids such as palmitic acid and oleic acid inhibit LPS-induced production of proinflammatory chemokines in LSECs and inhibit inflammatory cell recruitment. These findings suggest a potentially protective role for LSECs in the liver with excess free fatty acids, as in NAFLD[191].
A growing body of evidence suggests that the role of lipid metabolism in endothelial cell function is not only as a structural or energetic substrate, but also as a participant in cell mechanobiology. In doing so, lipids are at the intersection of chemo- and mechanobiological signaling pathways.
NAFLD is a widespread disease whose clinical and pathophysiological links are only beginning to be understood. TAG accumulation in hepatocytes in NAFLD results from a complex chain of events and is complicated in nature, involving many exogenous and endogenous factors. Obesity and impaired lipid metabolism are considered to be the key links in the development of NAFLD. Moreover, impaired fatty acid metabolism is one of the central events in the pathogenesis of NAFLD due to their involvement not only as an energy substrate or their structural function in cells, but also due to their connection with the innate immune system. Lipid metabolism has multiple cross-links with the innate immune system, and these links are important in the pathogenesis of NAFLD.
Analysis of the data allows us to emphasize the need for a better study of the multifaceted role of lipid metabolism and its disorders as a link in the complex chain of processes underlying the development of NAFLD.
The pathogenesis of NAFLD is an important target for further research, among which immunometabolic cross-linkages can be considered as one of the promising directions. Immunometabolic regulation of cells and intercellular connections at different stages of liver disease development can be a significant target for therapeutic intervention. In addition, the immune and metabolic axes that link the liver to other organs are also of research and clinical interest. There is a growing understanding that the gut microbiota is an important participant in immune and metabolic processes not only in the gut, but also in other organs. There is also interest in information on the cross-linkages of lipid-transport function and innate immunity, which have evolutionarily conservative roots and link a number of diseases that mutually influence their natural history.
In summary, NAFLD is a complex multifaceted disease whose keys are still unknown to clinicians and researchers, but a better understanding of metabolic and immune cross-linkages will improve patient diagnosis and treatment approaches.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He F, China; Zhou T, China S-Editor: Gong ZM L-Editor: Webster JR P-Editor: Gong ZM
| 1. | Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 1184] [Article Influence: 394.7] [Reference Citation Analysis (1)] |
| 2. | Mitra S, De A, Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol. 2020;5:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 336] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 3. | Zelber-Sagi S, Ratziu V, Oren R. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World J Gastroenterol. 2011;17:3377-3389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 210] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 4. | Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2402] [Cited by in RCA: 2653] [Article Influence: 189.5] [Reference Citation Analysis (0)] |
| 5. | Dietrich P, Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28:637-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 317] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 6. | Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1520] [Cited by in RCA: 1712] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 7. | Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 653] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 8. | Lonardo A, Ballestri S, Guaraldi G, Nascimbeni F, Romagnoli D, Zona S, Targher G. Fatty liver is associated with an increased risk of diabetes and cardiovascular disease - Evidence from three different disease models: NAFLD, HCV and HIV. World J Gastroenterol. 2016;22:9674-9693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Fleischman MW, Budoff M, Zeb I, Li D, Foster T. NAFLD prevalence differs among hispanic subgroups: the Multi-Ethnic Study of Atherosclerosis. World J Gastroenterol. 2014;20:4987-4993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Treeprasertsuk S, Björnsson E, Enders F, Suwanwalaikorn S, Lindor KD. NAFLD fibrosis score: a prognostic predictor for mortality and liver complications among NAFLD patients. World J Gastroenterol. 2013;19:1219-1229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Vvedenskaya O, Rose TD, Knittelfelder O, Palladini A, Wodke JAH, Schuhmann K, Ackerman JM, Wang Y, Has C, Brosch M, Thangapandi VR, Buch S, Züllig T, Hartler J, Köfeler HC, Röcken C, Coskun Ü, Klipp E, von Schoenfels W, Gross J, Schafmayer C, Hampe J, Pauling JK, Shevchenko A. Nonalcoholic fatty liver disease stratification by liver lipidomics. J Lipid Res. 2021;62:100104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3179] [Article Influence: 353.2] [Reference Citation Analysis (4)] |
| 13. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3129] [Article Influence: 115.9] [Reference Citation Analysis (36)] |
| 14. | Schreuder TC, Verwer BJ, van Nieuwkerk CM, Mulder CJ. Nonalcoholic fatty liver disease: an overview of current insights in pathogenesis, diagnosis and treatment. World J Gastroenterol. 2008;14:2474-2486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 121] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Duvnjak M, Lerotić I, Barsić N, Tomasić V, Virović Jukić L, Velagić V. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J Gastroenterol. 2007;13:4539-4550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 16. | Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, Nieuwdorp M, Clément K. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17:279-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 691] [Article Influence: 138.2] [Reference Citation Analysis (0)] |
| 17. | Kotlyarov S, Bulgakov A. Lipid Metabolism Disorders in the Comorbid Course of Nonalcoholic Fatty Liver Disease and Chronic Obstructive Pulmonary Disease. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Glass LM, Hunt CM, Fuchs M, Su GL. Comorbidities and Nonalcoholic Fatty Liver Disease: The Chicken, the Egg, or Both? Fed Pract. 2019;36:64-71. [PubMed] |
| 19. | Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, Roverato A, Guaraldi G, Lonardo A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:936-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 541] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 20. | Ballestri S, Nascimbeni F, Romagnoli D, Lonardo A. The independent predictors of non-alcoholic steatohepatitis and its individual histological features.: Insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol Res. 2016;46:1074-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 21. | Tana C, Ballestri S, Ricci F, Di Vincenzo A, Ticinesi A, Gallina S, Giamberardino MA, Cipollone F, Sutton R, Vettor R, Fedorowski A, Meschi T. Cardiovascular Risk in Non-Alcoholic Fatty Liver Disease: Mechanisms and Therapeutic Implications. Int J Environ Res Public Health. 2019;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 22. | Misra VL, Khashab M, Chalasani N. Nonalcoholic fatty liver disease and cardiovascular risk. Curr Gastroenterol Rep. 2009;11:50-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, Steffen HM. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2021;110:921-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 383] [Article Influence: 76.6] [Reference Citation Analysis (1)] |
| 24. | Bang KB, Cho YK. Comorbidities and Metabolic Derangement of NAFLD. J Lifestyle Med. 2015;5:7-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Rosato V, Masarone M, Dallio M, Federico A, Aglitti A, Persico M. NAFLD and Extra-Hepatic Comorbidities: Current Evidence on a Multi-Organ Metabolic Syndrome. Int J Environ Res Public Health. 2019;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 26. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2209] [Article Influence: 441.8] [Reference Citation Analysis (1)] |
| 27. | Loomis AK, Kabadi S, Preiss D, Hyde C, Bonato V, St Louis M, Desai J, Gill JM, Welsh P, Waterworth D, Sattar N. Body Mass Index and Risk of Nonalcoholic Fatty Liver Disease: Two Electronic Health Record Prospective Studies. J Clin Endocrinol Metab. 2016;101:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 28. | Fan R, Wang J, Du J. Association between body mass index and fatty liver risk: A dose-response analysis. Sci Rep. 2018;8:15273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 29. | Molina-Molina E, Krawczyk M, Stachowska E, Lammert F, Portincasa P. Non-Alcoholic Fatty Liver Disease in Non-Obese Individuals: Prevalence, Pathogenesis and Treatment. Clin Res Hepatol Gastroenterol. 2019;43:638-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Rahman MM, Kibria MG, Begum H, Haque M, Sultana N, Akhter M, Rowshon AHM, Ahmed F, Hasan M. Prevalence, risk factors and metabolic profile of the non-obese and obese non-alcoholic fatty liver disease in a rural community of South Asia. BMJ Open Gastroenterol. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Tobari M, Hashimoto E, Taniai M, Ikarashi Y, Kodama K, Kogiso T, Tokushige K, Takayoshi N, Hashimoto N. Characteristics of non-alcoholic steatohepatitis among lean patients in Japan: Not uncommon and not always benign. J Gastroenterol Hepatol. 2019;34:1404-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Adams LC, Lübbe F, Bressem K, Wagner M, Hamm B, Makowski MR. Non-alcoholic fatty liver disease in underweight patients with inflammatory bowel disease: A case-control study. PLoS One. 2018;13:e0206450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Kim D, Kim W, Joo SK, Kim JH, Harrison SA, Younossi ZM, Ahmed A. Predictors of nonalcoholic steatohepatitis and significant fibrosis in non-obese nonalcoholic fatty liver disease. Liver Int. 2019;39:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 34. | Conus F, Rabasa-Lhoret R, Péronnet F. Characteristics of metabolically obese normal-weight (MONW) subjects. Appl Physiol Nutr Metab. 2007;32:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 35. | Fracanzani AL, Valenti L, Bugianesi E, Vanni E, Grieco A, Miele L, Consonni D, Fatta E, Lombardi R, Marchesini G, Fargion S. Risk of nonalcoholic steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease and low visceral adiposity. J Hepatol. 2011;54:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Leung JC, Loong TC, Wei JL, Wong GL, Chan AW, Choi PC, Shu SS, Chim AM, Chan HL, Wong VW. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017;65:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 275] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 37. | Golabi P, Paik J, Fukui N, Locklear CT, de Avilla L, Younossi ZM. Patients With Lean Nonalcoholic Fatty Liver Disease Are Metabolically Abnormal and Have a Higher Risk for Mortality. Clin Diabetes. 2019;37:65-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 38. | Poss AM, Summers SA. Too Much of a Good Thing? Front Endocrinol (Lausanne). 2020;11:505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Minehira K. Role of Lipid Droplet Proteins in the Development of NAFLD and Hepatic Insulin Resistance: IntechOpen, 2018. |
| 40. | Tamura S, Shimomura I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1139-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 41. | Mashek DG. Hepatic lipid droplets: A balancing act between energy storage and metabolic dysfunction in NAFLD. Mol Metab. 2021;50:101115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 42. | Barrows BR, Parks EJ. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J Clin Endocrinol Metab. 2006;91:1446-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 43. | Listenberger LL, Han X, Lewis SE, Cases S, Farese RV Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077-3082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1538] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 44. | Rada P, González-Rodríguez Á, García-Monzón C, Valverde ÁM. Understanding lipotoxicity in NAFLD pathogenesis: is CD36 a key driver? Cell Death Dis. 2020;11:802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 335] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 45. | Zhang J, Zhao Y, Xu C, Hong Y, Lu H, Wu J, Chen Y. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: a cross-sectional study. Sci Rep. 2014;4:5832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 46. | Pardo V, González-Rodríguez Á, Muntané J, Kozma SC, Valverde ÁM. Role of hepatocyte S6K1 in palmitic acid-induced endoplasmic reticulum stress, lipotoxicity, insulin resistance and in oleic acid-induced protection. Food Chem Toxicol. 2015;80:298-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 47. | Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, Charlton MR, Gores GJ. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol. 2010;52:586-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 48. | Fernández Gianotti T, Burgueño A, Gonzales Mansilla N, Pirola CJ, Sookoian S. Fatty liver is associated with transcriptional downregulation of stearoyl-CoA desaturase and impaired protein dimerization. PLoS One. 2013;8:e76912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Silbernagel G, Kovarova M, Cegan A, Machann J, Schick F, Lehmann R, Häring HU, Stefan N, Schleicher E, Fritsche A, Peter A. High hepatic SCD1 activity is associated with low liver fat content in healthy subjects under a lipogenic diet. J Clin Endocrinol Metab. 2012;97:E2288-E2292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 50. | Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, Fantoni LI, Marra F, Bertolotti M, Banni S, Lonardo A, Carulli N, Loria P. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24:830-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 476] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 51. | Rosqvist F, Iggman D, Kullberg J, Cedernaes J, Johansson HE, Larsson A, Johansson L, Ahlström H, Arner P, Dahlman I, Risérus U. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63:2356-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 323] [Article Influence: 29.4] [Reference Citation Analysis (3)] |
| 52. | Mäkelä TNK, Tuomainen TP, Hantunen S, Virtanen JK. Associations of serum n-3 and n-6 polyunsaturated fatty acids with prevalence and incidence of nonalcoholic fatty liver disease. Am J Clin Nutr. 2022;116:759-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 53. | Maciejewska D, Drozd A, Ossowski P, Ryterska K, Jamioł-Milc D, Banaszczak M, Raszeja-Wyszomirska J, Kaczorowska M, Sabinicz A, Stachowska E. Fatty acid changes help to better understand regression of nonalcoholic fatty liver disease. World J Gastroenterol. 2015;21:301-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Chang Y, Ryu S, Sung KC, Cho YK, Sung E, Kim HN, Jung HS, Yun KE, Ahn J, Shin H, Wild SH, Byrne CD. Alcoholic and non-alcoholic fatty liver disease and associations with coronary artery calcification: evidence from the Kangbuk Samsung Health Study. Gut. 2019;68:1667-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 55. | Chatrath H, Vuppalanchi R, Chalasani N. Dyslipidemia in patients with nonalcoholic fatty liver disease. Semin Liver Dis. 2012;32:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 56. | Bonci E, Chiesa C, Versacci P, Anania C, Silvestri L, Pacifico L. Association of Nonalcoholic Fatty Liver Disease with Subclinical Cardiovascular Changes: A Systematic Review and Meta-Analysis. Biomed Res Int. 2015;2015:213737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 57. | Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1487] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 58. | Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1497-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 260] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 59. | Sun DQ, Liu WY, Wu SJ, Zhu GQ, Braddock M, Zhang DC, Shi KQ, Song D, Zheng MH. Increased levels of low-density lipoprotein cholesterol within the normal range as a risk factor for nonalcoholic fatty liver disease. Oncotarget. 2016;7:5728-5737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 60. | Kantartzis K, Rittig K, Cegan A, Machann J, Schick F, Balletshofer B, Fritsche A, Schleicher E, Häring HU, Stefan N. Fatty liver is independently associated with alterations in circulating HDL2 and HDL3 subfractions. Diabetes Care. 2008;31:366-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Sonmez A, Nikolic D, Dogru T, Ercin CN, Genc H, Cesur M, Tapan S, Karslioğlu Y, Montalto G, Banach M, Toth PP, Bagci S, Rizzo M. Low- and high-density lipoprotein subclasses in subjects with nonalcoholic fatty liver disease. J Clin Lipidol. 2015;9:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 62. | Bouwens L, Baekeland M, De Zanger R, Wisse E. Quantitation, tissue distribution and proliferation kinetics of Kupffer cells in normal rat liver. Hepatology. 1986;6:718-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 268] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 63. | Smedsrød B, De Bleser PJ, Braet F, Lovisetti P, Vanderkerken K, Wisse E, Geerts A. Cell biology of liver endothelial and Kupffer cells. Gut. 1994;35:1509-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 134] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 64. | Terpstra V, van Amersfoort ES, van Velzen AG, Kuiper J, van Berkel TJ. Hepatic and extrahepatic scavenger receptors: function in relation to disease. Arterioscler Thromb Vasc Biol. 2000;20:1860-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 65. | Praaning-van Dalen DP, Brouwer A, Knook DL. Clearance capacity of rat liver Kupffer, Endothelial, and parenchymal cells. Gastroenterology. 1981;81:1036-1044. [PubMed] |
| 66. | Lefkowitch JH, Haythe JH, Regent N. Kupffer cell aggregation and perivenular distribution in steatohepatitis. Mod Pathol. 2002;15:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 67. | Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 374] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 68. | Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate Immunity and Inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61:1294-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 383] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 69. | Lancaster GI, Langley KG, Berglund NA, Kammoun HL, Reibe S, Estevez E, Weir J, Mellett NA, Pernes G, Conway JRW, Lee MKS, Timpson P, Murphy AJ, Masters SL, Gerondakis S, Bartonicek N, Kaczorowski DC, Dinger ME, Meikle PJ, Bond PJ, Febbraio MA. Evidence that TLR4 Is Not a Receptor for Saturated Fatty Acids but Mediates Lipid-Induced Inflammation by Reprogramming Macrophage Metabolism. Cell Metab. 2018;27:1096-1110.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 340] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 70. | Rogero MM, Calder PC. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 473] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 71. | Boden G. Obesity and free fatty acids. Endocrinol Metab Clin North Am. 2008;37:635-646, viii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 653] [Cited by in RCA: 624] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 72. | Luo W, Xu Q, Wang Q, Wu H, Hua J. Effect of modulation of PPAR-γ activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci Rep. 2017;7:44612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 73. | Qian X, Yang Z, Mao E, Chen E. Regulation of fatty acid synthesis in immune cells. Scand J Immunol. 2018;88:e12713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 74. | Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, Pecker F, Tran A, Gual P, Mallat A, Lotersztajn S, Pavoine C. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59:130-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 438] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 75. | Diehl KL, Vorac J, Hofmann K, Meiser P, Unterweger I, Kuerschner L, Weighardt H, Förster I, Thiele C. Kupffer Cells Sense Free Fatty Acids and Regulate Hepatic Lipid Metabolism in High-Fat Diet and Inflammation. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 76. | Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2565] [Cited by in RCA: 2744] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 77. | Hwang DH, Kim JA, Lee JY. Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur J Pharmacol. 2016;785:24-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 78. | Korbecki J, Bajdak-Rusinek K. The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm Res. 2019;68:915-932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 312] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 79. | Ibarguren M, López DJ, Escribá PV. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim Biophys Acta. 2014;1838:1518-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 80. | Kotlyarov S, Kotlyarova A. Involvement of Fatty Acids and Their Metabolites in the Development of Inflammation in Atherosclerosis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 81. | Fang X, Wang H, Ye T, Fu X, Tan X, Zeng Y, Fan J, Xu Y. Low serum Maresin-1 levels are associated with non-alcoholic fatty liver disease: a cross-sectional study. Lipids Health Dis. 2021;20:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 82. | Martínez-Fernández L, González-Muniesa P, Laiglesia LM, Sáinz N, Prieto-Hontoria PL, Escoté X, Odriozola L, Corrales FJ, Arbones-Mainar JM, Martínez JA, Moreno-Aliaga MJ. Maresin 1 improves insulin sensitivity and attenuates adipose tissue inflammation in ob/ob and diet-induced obese mice. FASEB J. 2017;31:2135-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 83. | Laiglesia LM, Lorente-Cebrián S, Martínez-Fernández L, Sáinz N, Prieto-Hontoria PL, Burrell MA, Rodríguez-Ortigosa CM, Martínez JA, Moreno-Aliaga MJ. Maresin 1 mitigates liver steatosis in ob/ob and diet-induced obese mice. Int J Obes (Lond). 2018;42:572-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 84. | Jung TW, Kim HC, Abd El-Aty AM, Jeong JH. Maresin 1 attenuates NAFLD by suppression of endoplasmic reticulum stress via AMPK-SERCA2b pathway. J Biol Chem. 2018;293:3981-3988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 85. | Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 2011;25:2399-2407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 86. | Rius B, Titos E, Morán-Salvador E, López-Vicario C, García-Alonso V, González-Périz A, Arroyo V, Clària J. Resolvin D1 primes the resolution process initiated by calorie restriction in obesity-induced steatohepatitis. FASEB J. 2014;28:836-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 87. | Jung TW, Kyung EJ, Kim HC, Shin YK, Lee SH, Park ES, Hacımüftüoğlu A, Abd El-Aty AM, Jeong JH. Protectin DX Ameliorates Hepatic Steatosis by Suppression of Endoplasmic Reticulum Stress via AMPK-Induced ORP150 Expression. J Pharmacol Exp Ther. 2018;365:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 88. | Loomba R, Quehenberger O, Armando A, Dennis EA. Polyunsaturated fatty acid metabolites as novel lipidomic biomarkers for noninvasive diagnosis of nonalcoholic steatohepatitis. J Lipid Res. 2015;56:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 163] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 89. | Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M, Zhang R, McIntyre TM, Hazen SL. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res. 2010;51:3046-3054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 224] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 90. | Maciejewska D, Ossowski P, Drozd A, Ryterska K, Jamioł-Milc D, Banaszczak M, Kaczorowska M, Sabinicz A, Raszeja-Wyszomirska J, Stachowska E. Metabolites of arachidonic acid and linoleic acid in early stages of non-alcoholic fatty liver disease--A pilot study. Prostaglandins Other Lipid Mediat. 2015;121:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 91. | Farrell GC, Teoh NC, McCuskey RS. Hepatic microcirculation in fatty liver disease. Anat Rec (Hoboken). 2008;291:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 92. | Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Kupffer cells in the liver. Compr Physiol. 2013;3:785-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 461] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 93. | Wobser H, Dorn C, Weiss TS, Amann T, Bollheimer C, Büttner R, Schölmerich J, Hellerbrand C. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res. 2009;19:996-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 94. | Winau F, Quack C, Darmoise A, Kaufmann SH. Starring stellate cells in liver immunology. Curr Opin Immunol. 2008;20:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 95. | Schwabe RF, Tabas I, Pajvani UB. Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis. Gastroenterology. 2020;158:1913-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 444] [Article Influence: 88.8] [Reference Citation Analysis (1)] |
| 96. | Heyens LJM, Busschots D, Koek GH, Robaeys G, Francque S. Liver Fibrosis in Non-alcoholic Fatty Liver Disease: From Liver Biopsy to Non-invasive Biomarkers in Diagnosis and Treatment. Front Med (Lausanne). 2021;8:615978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 97. | Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, Gores GJ. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 352] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 98. | Fukunishi S, Sujishi T, Takeshita A, Ohama H, Tsuchimoto Y, Asai A, Tsuda Y, Higuchi K. Lipopolysaccharides accelerate hepatic steatosis in the development of nonalcoholic fatty liver disease in Zucker rats. J Clin Biochem Nutr. 2014;54:39-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 99. | Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 1556] [Article Influence: 86.4] [Reference Citation Analysis (1)] |
| 100. | Carpino G, Del Ben M, Pastori D, Carnevale R, Baratta F, Overi D, Francis H, Cardinale V, Onori P, Safarikia S, Cammisotto V, Alvaro D, Svegliati-Baroni G, Angelico F, Gaudio E, Violi F. Increased Liver Localization of Lipopolysaccharides in Human and Experimental NAFLD. Hepatology. 2020;72:470-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 263] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 101. | Nier A, Huber Y, Labenz C, Michel M, Bergheim I, Schattenberg JM. Adipokines and Endotoxemia Correlate with Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 102. | Saltzman ET, Palacios T, Thomsen M, Vitetta L. Intestinal Microbiome Shifts, Dysbiosis, Inflammation, and Non-alcoholic Fatty Liver Disease. Front Microbiol. 2018;9:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 103. | An L, Wirth U, Koch D, Schirren M, Drefs M, Koliogiannis D, Nieß H, Andrassy J, Guba M, Bazhin AV, Werner J, Kühn F. The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. J Gastrointest Surg. 2022;26:671-683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 119] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 104. | Li P, Ma C, Li J, You S, Dang L, Wu J, Hao Z, Zhi Y, Chen L, Sun S. Proteomic characterization of four subtypes of M2 macrophages derived from human THP-1 cells. J Zhejiang Univ Sci B. 2022;23:407-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 105. | Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, Perlemuter G, Cassard-Doulcier AM, Gérard P. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 715] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 106. | Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019;10:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 970] [Cited by in RCA: 2181] [Article Influence: 363.5] [Reference Citation Analysis (0)] |
| 107. | Ding Y, Yanagi K, Cheng C, Alaniz RC, Lee K, Jayaraman A. Interactions between gut microbiota and non-alcoholic liver disease: The role of microbiota-derived metabolites. Pharmacol Res. 2019;141:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 108. | Sturm EM, Knuplez E, Marsche G. Role of Short Chain Fatty Acids and Apolipoproteins in the Regulation of Eosinophilia-Associated Diseases. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 109. | Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, Parmentier M, Detheux M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481-25489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1107] [Cited by in RCA: 1233] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 110. | Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun. 2003;303:1047-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 420] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 111. | Ulven T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front Endocrinol (Lausanne). 2012;3:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 112. | Zhang Z, Tang H, Chen P, Xie H, Tao Y. Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome. Signal Transduct Target Ther. 2019;4:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 113. | Jardou M, Lawson R. Supportive therapy during COVID-19: The proposed mechanism of short-chain fatty acids to prevent cytokine storm and multi-organ failure. Med Hypotheses. 2021;154:110661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 114. | Kotlyarov S. Role of Short-Chain Fatty Acids Produced by Gut Microbiota in Innate Lung Immunity and Pathogenesis of the Heterogeneous Course of Chronic Obstructive Pulmonary Disease. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 115. | Vinolo MA, Rodrigues HG, Festuccia WT, Crisma AR, Alves VS, Martins AR, Amaral CL, Fiamoncini J, Hirabara SM, Sato FT, Fock RA, Malheiros G, dos Santos MF, Curi R. Tributyrin attenuates obesity-associated inflammation and insulin resistance in high-fat-fed mice. Am J Physiol Endocrinol Metab. 2012;303:E272-E282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 116. | Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology. 2016;5:e73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 596] [Cited by in RCA: 885] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 117. | Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 1852] [Article Influence: 308.7] [Reference Citation Analysis (1)] |
| 118. | Liu L, Li L, Min J, Wang J, Wu H, Zeng Y, Chen S, Chu Z. Butyrate interferes with the differentiation and function of human monocyte-derived dendritic cells. Cell Immunol. 2012;277:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 119. | Liu L, Fu Q, Li T, Shao K, Zhu X, Cong Y, Zhao X. Gut microbiota and butyrate contribute to nonalcoholic fatty liver disease in premenopause due to estrogen deficiency. PLoS One. 2022;17:e0262855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 120. | Sun B, Jia Y, Hong J, Sun Q, Gao S, Hu Y, Zhao N, Zhao R. Sodium Butyrate Ameliorates High-Fat-Diet-Induced Non-alcoholic Fatty Liver Disease through Peroxisome Proliferator-Activated Receptor α-Mediated Activation of β Oxidation and Suppression of Inflammation. J Agric Food Chem. 2018;66:7633-7642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 121. | Zhou D, Chen YW, Zhao ZH, Yang RX, Xin FZ, Liu XL, Pan Q, Zhou H, Fan JG. Sodium butyrate reduces high-fat diet-induced non-alcoholic steatohepatitis through upregulation of hepatic GLP-1R expression. Exp Mol Med. 2018;50:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 122. | Jin CJ, Sellmann C, Engstler AJ, Ziegenhardt D, Bergheim I. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH). Br J Nutr. 2015;114:1745-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 123. | Zhang S, Zhao J, Xie F, He H, Johnston LJ, Dai X, Wu C, Ma X. Dietary fiber-derived short-chain fatty acids: A potential therapeutic target to alleviate obesity-related nonalcoholic fatty liver disease. Obes Rev. 2021;22:e13316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 124. | Zhao S, Jang C, Liu J, Uehara K, Gilbert M, Izzo L, Zeng X, Trefely S, Fernandez S, Carrer A, Miller KD, Schug ZT, Snyder NW, Gade TP, Titchenell PM, Rabinowitz JD, Wellen KE. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature. 2020;579:586-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 366] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 125. | Gautier T, Lagrost L. Plasma PLTP (phospholipid-transfer protein): an emerging role in 'reverse lipopolysaccharide transport' and innate immunity. Biochem Soc Trans. 2011;39:984-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 126. | Dusuel A, Deckert V, Pais de Barros JP, van Dongen K, Choubley H, Charron É, Le Guern N, Labbé J, Mandard S, Grober J, Lagrost L, Gautier T. Human cholesteryl ester transfer protein lacks lipopolysaccharide transfer activity, but worsens inflammation and sepsis outcomes in mice. J Lipid Res. 2021;62:100011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 127. | Thompson PA, Gauthier KC, Varley AW, Kitchens RL. ABCA1 promotes the efflux of bacterial LPS from macrophages and accelerates recovery from LPS-induced tolerance. J Lipid Res. 2010;51:2672-2685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 128. | Kitchens RL, Wolfbauer G, Albers JJ, Munford RS. Plasma lipoproteins promote the release of bacterial lipopolysaccharide from the monocyte cell surface. J Biol Chem. 1999;274:34116-34122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 129. | Ono K, Nishitani C, Mitsuzawa H, Shimizu T, Sano H, Suzuki H, Kodama T, Fujii N, Fukase K, Hirata K, Kuroki Y. Mannose-binding lectin augments the uptake of lipid A, Staphylococcus aureus, and Escherichia coli by Kupffer cells through increased cell surface expression of scavenger receptor A. J Immunol. 2006;177:5517-5523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 130. | Han J, Nicholson AC. Lipoproteins modulate expression of the macrophage scavenger receptor. Am J Pathol. 1998;152:1647-1654. [PubMed] |
| 131. | Yoshida H, Quehenberger O, Kondratenko N, Green S, Steinberg D. Minimally oxidized low-density lipoprotein increases expression of scavenger receptor A, CD36, and macrosialin in resident mouse peritoneal macrophages. Arterioscler Thromb Vasc Biol. 1998;18:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 132. | van Oosten M, van de Bilt E, van Berkel TJ, Kuiper J. New scavenger receptor-like receptors for the binding of lipopolysaccharide to liver endothelial and Kupffer cells. Infect Immun. 1998;66:5107-5112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 133. | Wang Y, van der Tuin S, Tjeerdema N, van Dam AD, Rensen SS, Hendrikx T, Berbée JF, Atanasovska B, Fu J, Hoekstra M, Bekkering S, Riksen NP, Buurman WA, Greve JW, Hofker MH, Shiri-Sverdlov R, Meijer OC, Smit JW, Havekes LM, van Dijk KW, Rensen PC. Plasma cholesteryl ester transfer protein is predominantly derived from Kupffer cells. Hepatology. 2015;62:1710-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 134. | Blauw LL, Li Z, Rensen SS, Greve JWM, Verhoeven A, Derks RJ, Giera M, Wang Y, Rensen PCN. Metabolic liver inflammation in obesity does not robustly decrease hepatic and circulating CETP. Atherosclerosis. 2018;275:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 135. | van der Tuin SJL, Li Z, Berbée JFP, Verkouter I, Ringnalda LE, Neele AE, van Klinken JB, Rensen SS, Fu J, de Winther MPJ, Groen AK, Rensen PCN, Willems van Dijk K, Wang Y. Lipopolysaccharide Lowers Cholesteryl Ester Transfer Protein by Activating F4/80+Clec4f+Vsig4+Ly6C- Kupffer Cell Subsets. J Am Heart Assoc. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 136. | Zhu L, Luu T, Emfinger CH, Parks BA, Shi J, Trefts E, Zeng F, Kuklenyik Z, Harris RC, Wasserman DH, Fazio S, Stafford JM. CETP Inhibition Improves HDL Function but Leads to Fatty Liver and Insulin Resistance in CETP-Expressing Transgenic Mice on a High-Fat Diet. Diabetes. 2018;67:2494-2506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 137. | Venancio TM, Machado RM, Castoldi A, Amano MT, Nunes VS, Quintao EC, Camara NO, Soriano FG, Cazita PM. CETP Lowers TLR4 Expression Which Attenuates the Inflammatory Response Induced by LPS and Polymicrobial Sepsis. Mediators Inflamm. 2016;2016:1784014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 138. | Kotlyarov SN, Kotlyarova AA. Role of lipid metabolism and systemic inflammation in the development of atherosclerosis in animal models. IP Pavlov Russian Medical Biological Herald. 2021;29:134-146. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 139. | Cazita PM, Barbeiro DF, Moretti AI, Quintão EC, Soriano FG. Human cholesteryl ester transfer protein expression enhances the mouse survival rate in an experimental systemic inflammation model: a novel role for CETP. Shock. 2008;30:590-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 140. | Azzam KM, Fessler MB. Crosstalk between reverse cholesterol transport and innate immunity. Trends Endocrinol Metab. 2012;23:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 141. | Cappel DA, Palmisano BT, Emfinger CH, Martinez MN, McGuinness OP, Stafford JM. Cholesteryl ester transfer protein protects against insulin resistance in obese female mice. Mol Metab. 2013;2:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 142. | Palmisano BT, Le TD, Zhu L, Lee YK, Stafford JM. Cholesteryl ester transfer protein alters liver and plasma triglyceride metabolism through two liver networks in female mice. J Lipid Res. 2016;57:1541-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 143. | Liu K, Wang FS, Xu R. Neutrophils in liver diseases: pathogenesis and therapeutic targets. Cell Mol Immunol. 2021;18:38-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 144. | Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, Schuppan D, Grønbæk H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 670] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 145. | Svegliati-Baroni G, Saccomanno S, van Goor H, Jansen P, Benedetti A, Moshage H. Involvement of reactive oxygen species and nitric oxide radicals in activation and proliferation of rat hepatic stellate cells. Liver. 2001;21:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 146. | Casini A, Ceni E, Salzano R, Biondi P, Parola M, Galli A, Foschi M, Caligiuri A, Pinzani M, Surrenti C. Neutrophil-derived superoxide anion induces lipid peroxidation and stimulates collagen synthesis in human hepatic stellate cells: role of nitric oxide. Hepatology. 1997;25:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 147. | Zhou Z, Xu MJ, Cai Y, Wang W, Jiang JX, Varga ZV, Feng D, Pacher P, Kunos G, Torok NJ, Gao B. Neutrophil-Hepatic Stellate Cell Interactions Promote Fibrosis in Experimental Steatohepatitis. Cell Mol Gastroenterol Hepatol. 2018;5:399-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 148. | Lauszus JS, Eriksen PL, Hansen MM, Eriksen LL, Shawcross DL, Vilstrup H, Thomsen KL, Stoy S. Activation and Functional Priming of Blood Neutrophils in Non-Alcoholic Fatty Liver Disease Increases in Non-Alcoholic Steatohepatitis. Clin Exp Gastroenterol. 2021;14:441-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 149. | Hirano Y, Aziz M, Wang P. Role of reverse transendothelial migration of neutrophils in inflammation. Biol Chem. 2016;397:497-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 150. | Kim AD, Kim SE, Leszczynska A, Kaufmann B, Reca A, Kim DJ, Feldstein AE. Dual role of neutrophils in modulating liver injury and fibrosis during development and resolution of diet-induced murine steatohepatitis. Sci Rep. 2021;11:24194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 151. | Calvente CJ, Tameda M, Johnson CD, Del Pilar H, Lin YC, Adronikou N, De Mollerat Du Jeu X, Llorente C, Boyer J, Feldstein AE. Neutrophils contribute to spontaneous resolution of liver inflammation and fibrosis via microRNA-223. J Clin Invest. 2019;129:4091-4109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 152. | He Y, Hwang S, Cai Y, Kim SJ, Xu M, Yang D, Guillot A, Feng D, Seo W, Hou X, Gao B. MicroRNA-223 Ameliorates Nonalcoholic Steatohepatitis and Cancer by Targeting Multiple Inflammatory and Oncogenic Genes in Hepatocytes. Hepatology. 2019;70:1150-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 153. | Adema GJ. Dendritic cells from bench to bedside and back. Immunol Lett. 2009;122:128-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 154. | Méndez-Sánchez N, Córdova-Gallardo J, Barranco-Fragoso B, Eslam M. Hepatic Dendritic Cells in the Development and Progression of Metabolic Steatohepatitis. Front Immunol. 2021;12:641240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 155. | Almeda-Valdes P, Aguilar Olivos NE, Barranco-Fragoso B, Uribe M, Méndez-Sánchez N. The Role of Dendritic Cells in Fibrosis Progression in Nonalcoholic Fatty Liver Disease. Biomed Res Int. 2015;2015:768071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 156. | Martínez-Chantar ML, Delgado TC, Beraza N. Revisiting the Role of Natural Killer Cells in Non-Alcoholic Fatty Liver Disease. Front Immunol. 2021;12:640869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 157. | Michelet X, Dyck L, Hogan A, Loftus RM, Duquette D, Wei K, Beyaz S, Tavakkoli A, Foley C, Donnelly R, O'Farrelly C, Raverdeau M, Vernon A, Pettee W, O'Shea D, Nikolajczyk BS, Mills KHG, Brenner MB, Finlay D, Lynch L. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol. 2018;19:1330-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 434] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 158. | Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 552] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 159. | Kesar V, Odin JA. Toll-like receptors and liver disease. Liver Int. 2014;34:184-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 160. | Petrasek J, Csak T, Szabo G. Toll-like receptors in liver disease. Adv Clin Chem. 2013;59:155-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 161. | Miura K, Ohnishi H. Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:7381-7391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 252] [Cited by in RCA: 284] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 162. | Pradere JP, Troeger JS, Dapito DH, Mencin AA, Schwabe RF. Toll-like receptor 4 and hepatic fibrogenesis. Semin Liver Dis. 2010;30:232-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 163. | Bieghs V, Trautwein C. Innate immune signaling and gut-liver interactions in non-alcoholic fatty liver disease. Hepatobiliary Surg Nutr. 2014;3:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 164. | Vespasiani-Gentilucci U, Carotti S, Perrone G, Mazzarelli C, Galati G, Onetti-Muda A, Picardi A, Morini S. Hepatic toll-like receptor 4 expression is associated with portal inflammation and fibrosis in patients with NAFLD. Liver Int. 2015;35:569-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 165. | Kawaratani H, Tsujimoto T, Douhara A, Takaya H, Moriya K, Namisaki T, Noguchi R, Yoshiji H, Fujimoto M, Fukui H. The effect of inflammatory cytokines in alcoholic liver disease. Mediators Inflamm. 2013;2013:495156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 166. | Teratani T, Tomita K, Suzuki T, Oshikawa T, Yokoyama H, Shimamura K, Tominaga S, Hiroi S, Irie R, Okada Y, Kurihara C, Ebinuma H, Saito H, Hokari R, Sugiyama K, Kanai T, Miura S, Hibi T. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology. 2012;142:152-164.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 167. | Dejana E, Hirschi KK, Simons M. The molecular basis of endothelial cell plasticity. Nat Commun. 2017;8:14361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 328] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 168. | Tateya S, Rizzo NO, Handa P, Cheng AM, Morgan-Stevenson V, Daum G, Clowes AW, Morton GJ, Schwartz MW, Kim F. Endothelial NO/cGMP/VASP signaling attenuates Kupffer cell activation and hepatic insulin resistance induced by high-fat feeding. Diabetes. 2011;60:2792-2801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 169. | DeLeve LD. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology. 2015;61:1740-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 345] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 170. | DeLeve LD, Wang X, Hu L, McCuskey MK, McCuskey RS. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G757-G763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 207] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 171. | Xie G, Wang X, Wang L, Atkinson RD, Kanel GC, Gaarde WA, Deleve LD. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology. 2012;142:918-927.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 298] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 172. | May D, Djonov V, Zamir G, Bala M, Safadi R, Sklair-Levy M, Keshet E. A transgenic model for conditional induction and rescue of portal hypertension reveals a role of VEGF-mediated regulation of sinusoidal fenestrations. PLoS One. 2011;6:e21478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 173. | Yamane A, Seetharam L, Yamaguchi S, Gotoh N, Takahashi T, Neufeld G, Shibuya M. A new communication system between hepatocytes and sinusoidal endothelial cells in liver through vascular endothelial growth factor and Flt tyrosine kinase receptor family (Flt-1 and KDR/Flk-1). Oncogene. 1994;9:2683-2690. [PubMed] |
| 174. | Kotlyarov S. Immune Function of Endothelial Cells: Evolutionary Aspects, Molecular Biology and Role in Atherogenesis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 175. | Sriram K, Laughlin JG, Rangamani P, Tartakovsky DM. Shear-Induced Nitric Oxide Production by Endothelial Cells. Biophys J. 2016;111:208-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 176. | Carnovale CE, Ronco MT. Role of nitric oxide in liver regeneration. Ann Hepatol. 2012;11:636-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 177. | Abu-Amara M, Yang SY, Seifalian A, Davidson B, Fuller B. The nitric oxide pathway--evidence and mechanisms for protection against liver ischaemia reperfusion injury. Liver Int. 2012;32:531-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 178. | Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase up-regulates Notch-1 in mouse cholangiocytes: implications for carcinogenesis. Gastroenterology. 2005;128:1354-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 179. | La Mura V, Pasarín M, Rodriguez-Vilarrupla A, García-Pagán JC, Bosch J, Abraldes JG. Liver sinusoidal endothelial dysfunction after LPS administration: a role for inducible-nitric oxide synthase. J Hepatol. 2014;61:1321-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 180. | Iwakiri Y, Kim MY. Nitric oxide in liver diseases. Trends Pharmacol Sci. 2015;36:524-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 204] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 181. | Abdelmegeed MA, Song BJ. Functional roles of protein nitration in acute and chronic liver diseases. Oxid Med Cell Longev. 2014;2014:149627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 182. | Fujimoto Y, Uno E, Sakuma S. Effects of reactive oxygen and nitrogen species on cyclooxygenase-1 and -2 activities. Prostaglandins Leukot Essent Fatty Acids. 2004;71:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 183. | Bachschmid M, Schildknecht S, Ullrich V. Redox regulation of vascular prostanoid synthesis by the nitric oxide-superoxide system. Biochem Biophys Res Commun. 2005;338:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 184. | Kim SF. The role of nitric oxide in prostaglandin biology; update. Nitric Oxide. 2011;25:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 185. | Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993;90:7240-7244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 978] [Cited by in RCA: 964] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 186. | Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev. 2005;57:217-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 253] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 187. | Simon TG, Henson J, Osganian S, Masia R, Chan AT, Chung RT, Corey KE. Daily Aspirin Use Associated With Reduced Risk For Fibrosis Progression In Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2019;17:2776-2784.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 188. | Novo E, Cannito S, Zamara E, Valfrè di Bonzo L, Caligiuri A, Cravanzola C, Compagnone A, Colombatto S, Marra F, Pinzani M, Parola M. Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. Am J Pathol. 2007;170:1942-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 189. | Miyachi Y, Tsuchiya K, Komiya C, Shiba K, Shimazu N, Yamaguchi S, Deushi M, Osaka M, Inoue K, Sato Y, Matsumoto S, Kikuta J, Wake K, Yoshida M, Ishii M, Ogawa Y. Roles for Cell-Cell Adhesion and Contact in Obesity-Induced Hepatic Myeloid Cell Accumulation and Glucose Intolerance. Cell Rep. 2017;18:2766-2779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 190. | Deleve LD, Wang X, Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology. 2008;48:920-930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 295] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 191. | McMahan RH, Porsche CE, Edwards MG, Rosen HR. Free Fatty Acids Differentially Downregulate Chemokines in Liver Sinusoidal Endothelial Cells: Insights into Non-Alcoholic Fatty Liver Disease. PLoS One. 2016;11:e0159217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |