Published online Oct 21, 2023. doi: 10.3748/wjg.v29.i39.5494
Peer-review started: June 5, 2023
First decision: August 8, 2023
Revised: August 18, 2023
Accepted: September 28, 2023
Article in press: September 28, 2023
Published online: October 21, 2023
Processing time: 135 Days and 23 Hours
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic inflammatory disease of the digestive tract with inc
To estimate the proportion of VTE among IBD patients and assess genetic risk factors (monogenic and polygenic) for VTE.
Incident VTE was followed for 8465 IBD patients in the UK Biobank (UKB). The associations of VTE with F5 factor V leiden (FVL) mutation, F2 G20210A prothrombin gene mutation (PGM), and polygenic score (PGS003332) were tested using Cox hazards regression analysis, adjusting for age at IBD diagnosis, gender, and genetic background (top 10 principal components). The performance of genetic risk factors for discriminating VTE diagnosis was estimated using the area under the receiver operating characteristic curve (AUC).
The overall proportion of incident VTE was 4.70% in IBD patients and was similar for CD (4.46%), UC (4.49%), and unclassified (6.42%), and comparable to that of cancer patients (4.66%) who are well-known at increased risk for VTE. Mutation carriers of F5/F2 had a significantly increased risk for VTE compared to non-mutation carriers, hazard ratio (HR) was 1.94, 95% confidence interval (CI): 1.42-2.65. In contrast, patients with the top PGS decile had a considerably higher risk for VTE compared to those with intermediate scores (middle 8 deciles), HR was 2.06 (95%CI: 1.57-2.71). The AUC for differentiating VTE diagnosis was 0.64 (95%CI: 0.61-0.67), 0.68 (95%CI: 0.66-0.71), and 0.69 (95%CI: 0.66-0.71), respectively, for F5/F2 mutation carriers, PGS, and combined.
Similar to cancer patients, VTE complications are common in IBD patients. PGS provides more informative risk information than F5/F2 mutations (FVL and PGM) for personalized thromboprophylaxis.
Core Tip: Based on 8475 inflammatory bowel disease (IBD) patients from a population-based biobank, we showed they have an elevated risk for venous thromboembolism (VTE), with the overall proportion of incident VTE at 4.70%, similar to 4.66% observed in cancer patients. Polygenic score (PGS) is a significant predictor for VTE events, stronger than the well-known F5 factor V leiden mutation and F2 G20210A prothrombin gene mutation. The overall proportion of incident VTE is 8.53% in patients at the top 10 PGS percentile. These findings highlight the importance of VTE complications in IBD patients and provide genetic tools for personalized thromboprophylaxis.
- Citation: Rifkin AS, Shi Z, Wei J, Zheng SL, Helfand BT, Cordova JS, Biank VF, Tafur AJ, Khan O, Xu J. Risk assessment of venous thromboembolism in inflammatory bowel disease by inherited risk in a population-based incident cohort. World J Gastroenterol 2023; 29(39): 5494-5502
- URL: https://www.wjgnet.com/1007-9327/full/v29/i39/5494.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i39.5494
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is a disease characterized by chronic inflammation of the digestive tract. Its prevalence was estimated at 1.3% (3 million adults) in the United States in 2015 and is expected to increase globally[1,2]. IBD is associated with several adverse complications, including venous thromboembolism (VTE). IBD patients have a 2 to 3-fold higher risk of developing a VTE as compared to the general population[3]. Although the pathophysiology underlying this observation remains largely unknown, it may involve changes in the coagulation system, increased platelet count and reactivity, and altered fibrinolysis[4]. The importance of elevated VTE risk in IBD patients is emphasized in a recent international consensus on the prevention of venous and arterial thrombotic events in patients with IBD, which encourages screening for VTE risk factors. Statement 2 of the consensus states that “Patients with IBD should be screened for VTE risk factors” (consensus reached 100%)[4].
Several inherited factors are associated with risk for VTE, including relatively common gain of function mutations in genes for coagulation factors: Factor V leiden (FVL) mutation in the F5 gene and the G20210A prothrombin gene mutation (PGM) in the F2 gene[5]. Recent advances in genome-wide association studies (GWAS) have also revealed multiple common single nucleotide polymorphisms (SNPs) that are associated with increased VTE risk[6]. Polygenic scores (PGS) based on these SNPs have been shown to be effective to stratify VTE risk in the general population. While the consensus recognizes several genetic factors such as F5 and F2 for VTE risk among IBD patients, it does not mention PGS[4].
The objectives of this study are to utilize a large population-based cohort to: (1) Estimate the proportion of VTE among IBD patients and compare it to that in cancer patients who are well-known to have an increased risk for VTE; (2) test the association of inherited risk factors (F5/F2 mutations and PGS) and VTE among IBD patients; and (3) assess the performance of PGS for predicting VTE among IBD patients, alone or in combination with F5/F2 mutations. Results from this study may provide needed evidence for PGS to be included in the updated international consensus for VTE prevention among IBD patients.
Subjects in this study were patients who had a diagnosis of IBD in the UK Biobank (UKB), a population-based study of 500000 volunteers from the United Kingdom[7]. IBD diagnoses were obtained based on International Classification of Diseases-10 (ICD-10) codes (K50 for CD and K51 for UC) of primary care, death register, inpatient diagnosis, and self-report. Incident VTE after IBD diagnosis were identified based on the inclusion criteria described by Klarin et al[8] Briefly, subjects were defined as a VTE case based on at least one of the following criteria: (1) VTE (deep vein thrombosis and pulmonary embolism) ascertained at baseline by self-report; (2) Hospitalization for ICD-10 Code I80.1, I80.2, I82.2, I26.0, or I26.9; and (3) Hospitalization for Office of Population and Censuses and Survey-4 Procedures Codes L79.1 or L90.2. Only incident VTE, i.e., those that occurred after a diagnosis of IBD were included in the analysis. As a comparison, VTE events after a cancer diagnosis were also estimated among cancer patients (ICD-10: C00-C96) in the UKB[9].
Genotypes for the F5 FVL mutation (c.1601G>A, rs6025) and the F2 PGM (c.*97G>A, rs1799963) as well as genome-wide SNPs were obtained from the UKB Axiom SNP genotype array (genotyped or imputed). A published pan-ancestry PGS for VTE (PGS003332) was selected from the PGS catalog for the study because it was developed from the largest GWAS of VTE with 81190 cases and 1419671 controls sampled from six cohorts[6,10]. Based on the scoring file of the PGS, raw PGS was first calculated by taking the product of the count of risk alleles and the risk allele weight at each locus in the PGS (1092045 SNPs) and then summing across available risk loci. Ancestry-adjusted PGS was calculated based on the first four principal components using a previously described method[11]. To remove the contribution of SNPs from the F5 and F2 genes to the score, a modified PGS (PGSnonF5/F2) was also calculated by removing 1515 SNPs in these two genes (chr1: 168519049-170519049 for F5 and chr11: 45761055-47761055 for F2).
The proportion of incident VTE was calculated as (number of patients with incident VTE)/(number of patients with IBD). The difference in VTE proportion among groups of patients was tested using a χ2 test. Time to VTE diagnosis from the time of IBD diagnosis was estimated using Kaplan-Meier survival analysis and its difference among various groups of IBD patients was tested using and log-rank test. Association of genetic risk factors (PGS and F5/F2 mutations) and other known risk factors with VTE among IBD patients were tested using the Cox proportional hazards regression analysis, adjusting for gender, body mass index at study recruitment, and genetic background (top 10 principal components). In addition, the performance of genetic risk factors for discriminating VTE was estimated using the area under the receiver operating characteristic curve (AUC). All statistical analyses were performed using R-package.
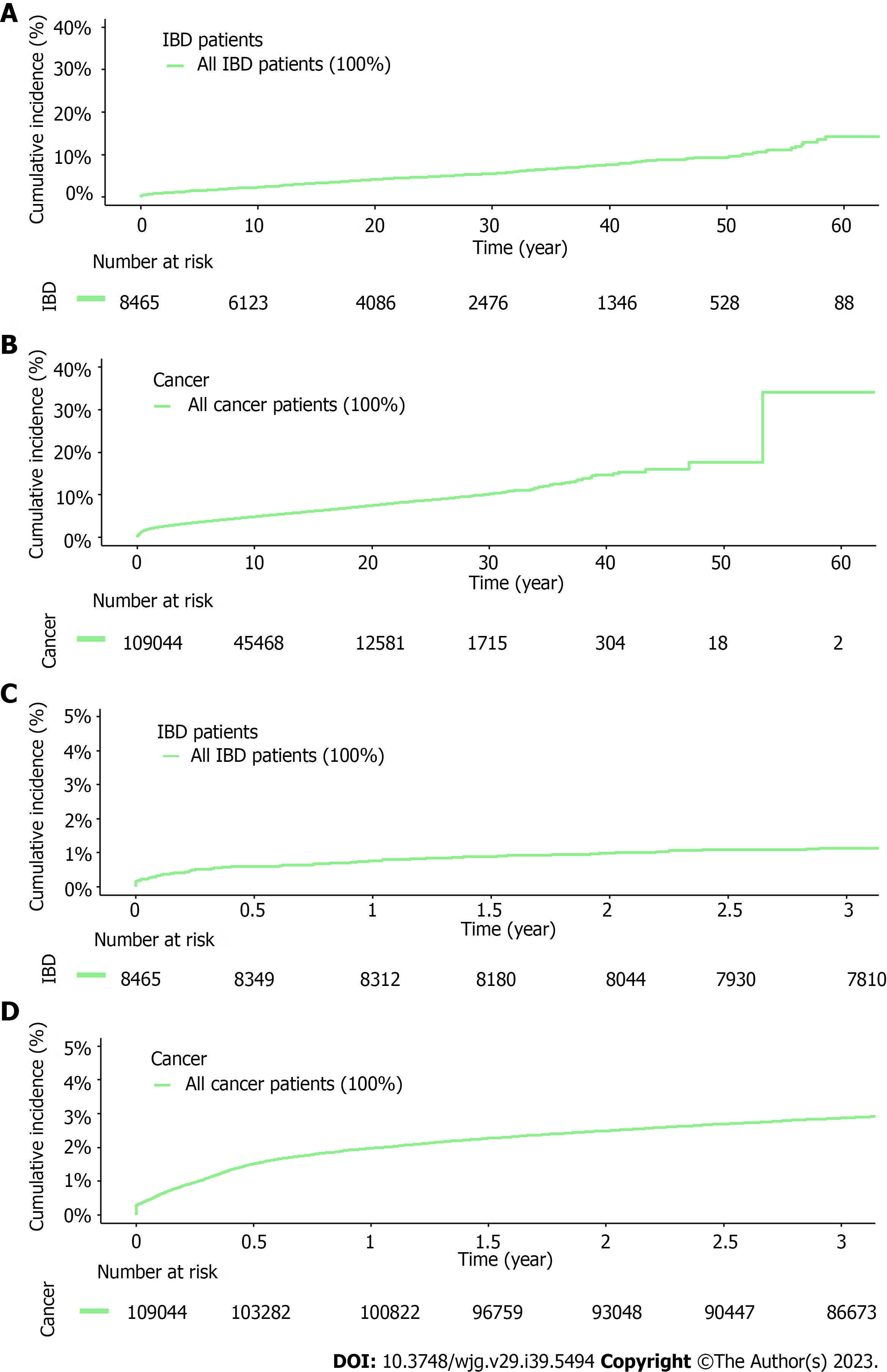
As of the last UKB accession date (October 7, 2022), 8465 (1.68%) IBD patients were identified from 501095 subjects in the UKB, including 2267 (0.45%), 5233 (1.05%), and 965 (0.19%) patients with CD only, UC only, and IBD unclassified (IBD-U) (diagnosed with both CD and UC), respectively (Table 1). The median age at diagnosis was 49.60, 51.34, and 44.09 years for CD only, UC only, and IBD-U, respectively. Among these IBD patients, 398 developed VTE events after IBD diagnosis, with the overall proportion of incident VTE of 4.70%. The proportion of VTE was statistically different among patients with CD only (4.46%), UC only (4.49%), and IBD-U colitis (6.42%), χ2 = 7.22, degree of freedom = 2, P = 0.03. It is noted that the overall proportion of incident VTE in these IBD patients was similar to that of 107520 cancer patients (4.66%) in the UKB who are well-known to have an increased risk for VTE. However, a different pattern of VTE events during the follow-up was noticed; VTE events occurred continuously throughout the follow-up period for IBD patients where a disproportionally higher number of VTE events occurred in the first several years in cancer patients (Figure 1).
| Subjects, n (%) | Age dx, median (IQR), yr | Incident VTE, n (%) | |
| IBD, all | 8465 (1.68) | 50.08 (35.24-61.76) | 398 (4.7) |
| Crohn's disease | 2267 (0.45) | 49.68 (32.48-62.37) | 101 (4.46) |
| Ulcerative colitis | 5233 (1.05) | 51.38 (36.87-62.50) | 235 (4.49) |
| Indeterminate colitis | 965 (0.19) | 44.26 (31.56-54.92) | 62 (6.42) |
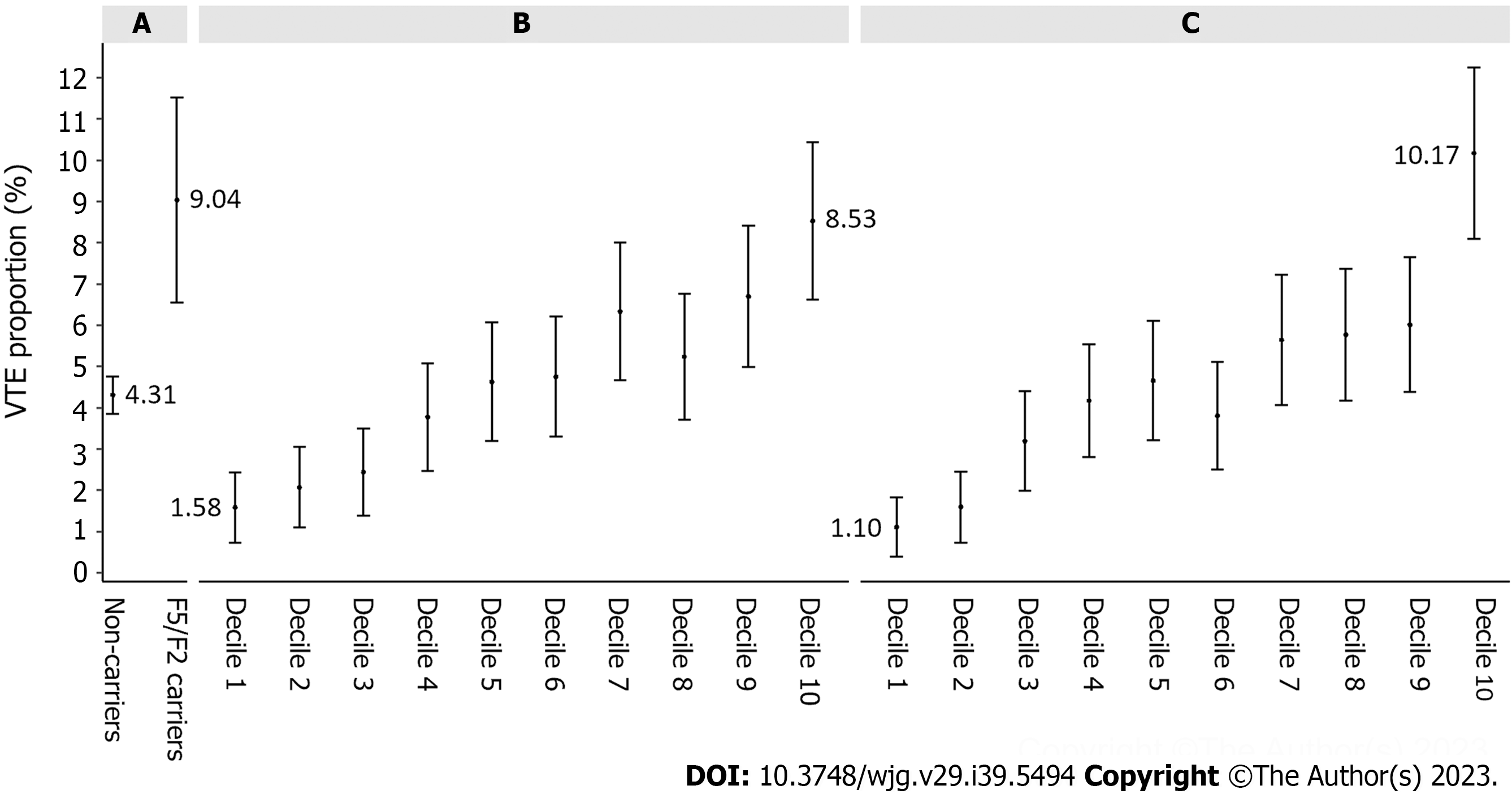
Among the 8210 IBD patients with genetic data, 509 (6.20%) were carriers of F5/F2 mutations, including 351 (4.28%) carriers of F5 FVL and 163 (1.99%) carriers of F2 PGM (Table 2). The overall proportion of incident VTE among mutation carriers was 9.04%, higher than that of non-mutation carriers (4.31%) (Table 2 and Figure 2A). In comparison, PGS was more informative in differentiating VTE events and the proportion of incident VTE increased with increasing decile of PGSnonF5/F2, Ptrend = 1.60E-18 (Figure 2B). For example, the proportion of incident VTE was 1.58% for IBD patients at the bottom of PGSnonF5/F2 decile, which was considerably lower than that of non-mutation carriers of F5/F2. On the other hand, the proportion of incident VTE was 8.53% for IBD patients at the top of PGSnonF5/F2 decile, which was higher than that of mutation carriers of F5/F2. As expected, original PGS (including SNPs in the F5/F2 regions) performed well in stratifying VTE risk and is slightly better than that of PGSnonF5/F2 (Figure 2C). For example, the proportion of incident VTE was 1.10% and 10.17% for IBD patients at the bottom and top decile, respectively.
| IBD patients, n (%) | Incident VTE, n (%) | HR (95%CI)1 | P value | |
| FVL/F2 carrier status | ||||
| Non-carriers | 7701/8210 (93.8) | 332/7701 (4.31) | Ref. | |
| FVL carriers | 351/8210 (4.28) | 37/351 (10.54) | 2.27 (1.61-3.21) | 2.84E-06 |
| F2 carriers | 163/8210 (1.99) | 10/163 (6.13) | 1.29 (0.69-2.43) | 0.43 |
| Any FVL/F2 carriers | 509/8210 (6.2) | 46/509 (9.04) | 1.94 (1.42-2.65) | 3.27E-05 |
| PGSnon-F5/F2 decile | ||||
| Bottom | 821/8210 (10) | 13/821 (1.58) | 0.36 (0.21-0.63) | 3.12E-04 |
| Intermediate | 6568/8210 (80) | 295/6568 (4.49) | Ref. | |
| Top | 821/8210 (10) | 70/821 (8.53) | 1.98 (1.52-2.57) | 3.26E-07 |
Compared to non-mutation carriers of F5/F2, mutation carriers were at a marginally increased risk for VTE, with a hazard ratio (HR) of 1.94, 95% confidence interval (CI): 1.42-2.65 (Table 2). For PGSnonF5/F2, patients at the top decile had a considerably higher risk for VTE compared to IBD patients with intermediate scores (middle 8 deciles), HR was 1.98 (95%CI: 1.52-2.57). Furthermore, PGSnonF5/F2 can also identify patients at lower risk for VTE, HR was 0.36 (95%CI: 0.21-0.63) for patients at the bottom decile. In a multivariable analysis where both F5/F2 mutation carrier status and PGSnonF5/F2 (continuous variable) were included, both were significantly associated with VTE risk, P < 0.001.
PGSnonF5/F2 also had a significantly better discriminative performance for VTE diagnosis than F5/F2 mutations, with the AUC estimated at 0.68 (95%CI: 0.65-0.71) and 0.64 (95%CI: 0.61-0.67), respectively, P = 0.05 (Table 3). Combining these two risk factors increased the AUC to 0.69 (95%CI: 0.66-0.71), which was also significantly higher than that of F5/F2 mutations, P = 0.01. Interestingly, the original PGS for VTE, which includes SNPs in the F5/F2 gene regions, had a similar AUC (0.69, 95%CI: 0.67-0.72) to that of combined PGSnonF5/F2 and F5/F2 mutations.
By following incident VTE events among 8465 IBD patients from a population-based biobank, three major findings that have potentially translational implications were obtained from our study. First, VTE was a common complication among IBD patients, with a proportion similar to diseases commonly considered at elevated risk for VTE such as cancer. This elevated VTE risk among IBD patients was also reported in previously published studies[12,13]. In a cohort of 2811 IBD patients recruited from 14 referral centers in Austria, a VTE prevalence of 5.6% was reported, similar to that in our study[12]. In another large cohort study of hospitalized and ambulatory IBD patients (n = 13756), as well as matched controls (n = 71672) from the General Practice Research Database in the United Kingdom, a higher VTE rate compared to the general population was also reported[13]. For hospitalized patients with IBD, HR for VTE was 3.2, 95%CI: 1.7-6.3. Similarly, for ambulatory patients with IBD, the HR was 8.4, 95%CI: 5.5-12.8. In addition, several studies have also indicated that there is a high risk of recurrent VTE following hospital discharge[13,14]. Interestingly, this increased VTE risk seems to be unique to IBD, setting it apart from other autoimmune diseases like rheumatoid arthritis or celiac disease where this association is not commonly observed[15]. Despite the consistent research observation of an association between IBD and VTE, VTE complication in IBD patients is commonly overlooked in clinical practice. Adherence to VTE prophylaxis among IBD patients is low and inconsistent, with only 68% of hospitalized UC patients prescribed prophylaxis[16]. Even when prescribed, one-third of the doses were not actually administered. Factors contributing to low adherence could include patient noncompliance, physician unawareness, fragmented care, and bleeding risk concerns.
Second, results from our study suggest the VTE risk conferred by F5/F2 mutations (FVL and PGM) in IBD patients was modest and considerably lower than previously reported. For F5 FVL, two meta-analyses published in 2011 reported a combined OR of 4.0 and 5.3, respectively, for VTE in IBD patients[17,18]. These ORs, however, were likely over-estimated due to a combination of several factors, including small sample sizes, study designs (the vast majority was case-control studies), genetic heterogeneity (ancestry and genetic background were not accounted for), and publication bias (positive finding were more likely to be published). For example, in the meta-analysis performed by Zhong et al[17], the total sample size of IBD patients from 10 individual studies published before 2008 was 938, including 124 (13%) with VTE. In particular, 9 of the 10 studies had fewer than 80 IBD patients. The only relatively large study (477 IBD patients, including 14 (2.9%) with VTE) failed to observe a significant association between FVL and VTE, OR = 1.28, 95%CI: 0.16-10.17[19]. For F2 PGM, its association with VTE in IBD patients was inconclusive from two small studies published prior to 2007, with fewer than 100 IBD patients in each study[20,21]. In comparison, the association results and OR estimates from our study were more reliable because the study was based on a large cohort of 8300 IBD patients whose VTE diagnosis was uniformly followed. Furthermore, our association test was performed adjusted for genetic background, therefore, reduced the confounder of genetic heterogeneity. However, additional large cohorts of IBD patients with follow-up information for VTE are still needed to validate our findings.
Third, compared to F5/F2 mutations, we showed new PGS was more informative for stratifying VTE risk and performs better in discriminating VTE diagnosis after IBD. The better performance of PGS is consistent with the strong genetic basis for VTE where about 60% of the variance in VTE incidence is attributable to genetic effects[22]. Our finding is also consistent with a previous polygenic risk score study in 792 IBD patients where the score was based on 265 established VTE risk-associated SNPs[23]. However, the PGS used in the current study differs from the published study in that it includes many more SNPs (more than a million) and captures both established risk-associated SNPs implicated in GWAS and many more SNPs in the genome that did not reach the GWAS significance level individually (due to either modest-effect or low allele frequency)[6,23,24]. Consequently, the performance of PGS for stratifying VTE risk is improved. Additionally, our PGS not only identified patients at high-risk for VTE, but also identified patients at considerably low risk for VTE. This information is important for considering the need, dosage and duration for anticoagulant treatment to balance its potential benefits and harms. Importantly, it is worth noting that the PGS evaluated in this study was developed and validated in large external study populations with over a million subjects[6]. Our study simply assessed its performance in IBD patients, therefore, is not susceptible to issues related to model development such as overfitting and multiple testing. Furthermore, the risk estimate for PGS is not susceptible to potential observer bias because neither physicians nor patients are aware of their PGS for VTE.
Considering the similar AUC performance for the combined PGSnonF5/F2 and F5/F2 mutations and the original PGS, either approach may be used for VTE risk assessment in IBD patients in the clinic. While the first approach has the advantage of integrating well-known F5/F2 mutations with new PGS, the latter approach is simpler to use (single scoring file) and easier to interpret (risk estimate derived from a large number of subjects is more reliable with a narrow CI). Furthermore, as a pan-ancestry PGS, PGS is applicable to patients of various ancestry populations. The added value of PGS over F5/F2 mutations is more relevant in non-European ancestries because of their relatively low F5/F2 mutation carrier rates (carrier rate of F5/F2 mutations was 6.31% and 4.16% in European and non-European IBD patients, respectively). However, considering this PGS was developed and validated primarily in subjects of European ancestry, its performance in other ancestries is less certain. As shown in Supplementary Table 1 for the performance of PGS in 409 non-European IBD patients, compared to patients with intermediate scores, those at the top decile had HR of 1.48 (95%CI: 0.17-13.11) for VTE. Additional data for non-European IBD patients are urgently needed.
Our findings have potential clinical utilities. Genetic risk assessment using PGSnonF5/F2 and F5/F2 mutations may be integrated into clinical presentations to develop personalized strategies for thromboprophylaxis. For example, IBD patients with high genetic risk may consider higher dosage and longer duration of anticoagulation therapy. On the other hand, patients with low PGS and without F5/F2 mutations may consider lower dosages and shorter duration to minimize risk for bleeding events.
Several limitations of our study are noted. Considering possible under-diagnosis of VTE (especially deep vein thrombosis) in subjects without IBD or other major diseases requiring hospitalization and intensive clinical care, we did not compare the VTE proportion between IBD and non-IBD subjects. Furthermore, due to the difficulty in obtaining detailed clinical variables related to the clinical characteristics of IBD and treatment, we were unable to include key clinical variables to assess the independent and added value of PGS in existing clinical risk assessment models. Another major limitation of our study is a lack of ancestral diversity in study subjects (96% of IBD patients in our study are of European ancestry), therefore it is critical to validate our findings in other diverse ancestry groups in future studies. Lastly, we did not include other known major genes for VTE (SERPINC1, PROC, and PROS1) in this study. Mutations in these genes are rare and can only be detected by whole exome sequencing (WES) which is currently available in only 40% of subjects in the UKB[7]. Among 3227 IBD patients with WES data, only 6 (0.19%), 2 (0.06%), and 2 (0.06%) carriers of loss of function mutations were identified, 2 (20%) of whom developed VTE. Larger studies with WES data are required to test these genetic risk factors.
In conclusion, we demonstrated that VTE complications are common in IBD patients with the proportion similar to diseases known at increased VTE risk. Furthermore, we showed SNP-based PGS is more informative and superior to F5/F2 mutations in identifying IBD patients at high risk for VTE. These results may have potential implications for developing personalized anticoagulant treatment.
Venous thromboembolism (VTE) is a major complication in patients with inflammatory bowel disease (IBD). However, it is often underappreciated among physicians and patients. Furthermore, limited VTE risk stratification tools are available for personalized thromboprophylaxis.
A large IBD patient cohort is available from the UK Biobank (UKB). Its long-term clinical follow-up data and genome-wide genetic data provide a rare and efficient opportunity to address these two major challenges.
To estimate the prevalence of VTE complications among IBD patients and assess the performance of known and novel genetic predictors of VTE risk stratification in these patients.
We retrospectively followed the incident VTE complication among 8465 IBD patients in the UKB. The associations of VTE with factor V leiden (FVL) mutation in the F5 gene, G20210A prothrombin gene mutation (PGM) in the F2 gene, and polygenic score (PGS) were tested using Cox hazards regression analysis, adjusting for age at IBD diagnosis, gender, and genetic background (top 10 principal components). The performance of genetic risk factors for discriminating VTE complications was estimated using the area under the receiver operating characteristic curve (AUC).
The overall prevalence of VTE complication after an IBD diagnosis was 4.70%. This prevalence was comparable to that of cancer patients (4.66%) who are well-known at increased risk for VTE. A novel genetic predictor (PGS) was significantly associated with VTE risk and was independent of known genetic predictors (FVL/PGM). The AUC of differentiating VTE complication was significantly higher for PGS [0.68, 95% confidence interval (CI): 0.66-0.71] than that of FVL/PGM (0.64, 95%CI: 0.61-0.67) and was highest by combining these two genetic predictors (0.69, 95%CI: 0.66-0.71).
VTE complication is common in IBD patients and is similar to that of cancer patients. Newly developed PGS provides a more informative VTE risk stratification tool than known mutations (FVL/PGM).
Findings from this large study of IBD patients have potential clinical utilities. It not only highlights the significance of VTE complications in IBD patients, but also provides an informative VTE risk assessment tool for developing personalized thromboprophylaxis strategies.
We are grateful to the Ellrodt-Schweighauser family for establishing Endowed Chair of Cancer Genomic Research (Xu), and Chez and Melman families for establishing Endowed Chairs of Personalized Prostate Cancer Care (Helfand).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang JG, Singapore; Yasuda H, Japan S-Editor: Fan JR L-Editor: A P-Editor: Cai YX
| 1. | Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of Inflammatory Bowel Disease Among Adults Aged ≥18 Years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1166-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 477] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 2. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4109] [Article Influence: 513.6] [Reference Citation Analysis (110)] |
| 3. | Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375:657-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 556] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 4. | Olivera PA, Zuily S, Kotze PG, Regnault V, Al Awadhi S, Bossuyt P, Gearry RB, Ghosh S, Kobayashi T, Lacolley P, Louis E, Magro F, Ng SC, Papa A, Raine T, Teixeira FV, Rubin DT, Danese S, Peyrin-Biroulet L. International consensus on the prevention of venous and arterial thrombotic events in patients with inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18:857-873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 5. | Rosendaal FR, Reitsma PH. Genetics of venous thrombosis. J Thromb Haemost. 2009;7 Suppl 1:301-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 6. | Ghouse J, Tragante V, Ahlberg G, Rand SA, Jespersen JB, Leinøe EB, Vissing CR, Trudsø L, Jonsdottir I, Banasik K, Brunak S, Ostrowski SR, Pedersen OB, Sørensen E, Erikstrup C, Bruun MT, Nielsen KR, Køber L, Christensen AH, Iversen K, Jones D, Knowlton KU, Nadauld L, Halldorsson GH, Ferkingstad E, Olafsson I, Gretarsdottir S, Onundarson PT, Sulem P, Thorsteinsdottir U, Thorgeirsson G, Gudbjartsson DF, Stefansson K, Holm H, Olesen MS, Bundgaard H. Genome-wide meta-analysis identifies 93 risk loci and enables risk prediction equivalent to monogenic forms of venous thromboembolism. Nat Genet. 2023;55:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 84] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 7. | Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O'Connell J, Cortes A, Welsh S, Young A, Effingham M, McVean G, Leslie S, Allen N, Donnelly P, Marchini J. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4971] [Cited by in RCA: 5281] [Article Influence: 754.4] [Reference Citation Analysis (0)] |
| 8. | Klarin D, Emdin CA, Natarajan P, Conrad MF; INVENT Consortium, Kathiresan S. Genetic Analysis of Venous Thromboembolism in UK Biobank Identifies the ZFPM2 Locus and Implicates Obesity as a Causal Risk Factor. Circ Cardiovasc Genet. 2017;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Shi Z, Wei J, Rifkin AS, Wang CH, Billings LK, Woo JSH, Talamonti MS, Vogel TJ, Moore E, Brockstein BE, Khandekar JD, Dunnenberger HM, Hulick PJ, Duggan D, Zheng SL, Lee CJ, Helfand BT, Tafur AJ, Xu J. Cancer-associated thrombosis by cancer sites and inherited factors in a prospective population-based cohort. Thromb Res. 2023;229:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Lambert SA, Gil L, Jupp S, Ritchie SC, Xu Y, Buniello A, McMahon A, Abraham G, Chapman M, Parkinson H, Danesh J, MacArthur JAL, Inouye M. The Polygenic Score Catalog as an open database for reproducibility and systematic evaluation. Nat Genet. 2021;53:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 381] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 11. | Khera AV, Chaffin M, Zekavat SM, Collins RL, Roselli C, Natarajan P, Lichtman JH, D'Onofrio G, Mattera J, Dreyer R, Spertus JA, Taylor KD, Psaty BM, Rich SS, Post W, Gupta N, Gabriel S, Lander E, Ida Chen YD, Talkowski ME, Rotter JI, Krumholz HM, Kathiresan S. Whole-Genome Sequencing to Characterize Monogenic and Polygenic Contributions in Patients Hospitalized With Early-Onset Myocardial Infarction. Circulation. 2019;139:1593-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 12. | Papay P, Miehsler W, Tilg H, Petritsch W, Reinisch W, Mayer A, Haas T, Kaser A, Feichtenschlager T, Fuchssteiner H, Knoflach P, Vogelsang H, Platzer R, Tillinger W, Jaritz B, Schmid A, Blaha B, Dejaco C, Sobala A, Weltermann A, Eichinger S, Novacek G. Clinical presentation of venous thromboembolism in inflammatory bowel disease. J Crohns Colitis. 2013;7:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | McCurdy JD, Israel A, Hasan M, Weng R, Mallick R, Ramsay T, Carrier M. A clinical predictive model for post-hospitalisation venous thromboembolism in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49:1493-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Faye AS, Hung KW, Cheng K, Blackett JW, Mckenney AS, Pont AR, Li J, Lawlor G, Lebwohl B, Freedberg DE. Minor Hematochezia Decreases Use of Venous Thromboembolism Prophylaxis in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2020;26:1394-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Miehsler W, Reinisch W, Valic E, Osterode W, Tillinger W, Feichtenschlager T, Grisar J, Machold K, Scholz S, Vogelsang H, Novacek G. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut. 2004;53:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 343] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 16. | Tinsley A, Naymagon S, Enomoto LM, Hollenbeak CS, Sands BE, Ullman TA. Rates of pharmacologic venous thromboembolism prophylaxis in hospitalized patients with active ulcerative colitis: results from a tertiary care center. J Crohns Colitis. 2013;7:e635-e640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Zhong M, Dong XW, Zheng Q, Tong JL, Ran ZH. Factor V Leiden and thrombosis in patients with inflammatory bowel disease (IBD): a meta-analysis. Thromb Res. 2011;128:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Liang J, Wu S, Feng B, Lei S, Luo G, Wang J, Li K, Li X, Xie H, Zhang D, Wang X, Wu K, Miao D, Fan D. Factor V Leiden and inflammatory bowel disease: a systematic review and meta-analysis. J Gastroenterol. 2011;46:1158-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Bernstein CN, Sargent M, Vos HL, Rosendaal FR. Mutations in clotting factors and inflammatory bowel disease. Am J Gastroenterol. 2007;102:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Guédon C, Le Cam-Duchez V, Lalaude O, Ménard JF, Lerebours E, Borg JY. Prothrombotic inherited abnormalities other than factor V Leiden mutation do not play a role in venous thrombosis in inflammatory bowel disease. Am J Gastroenterol. 2001;96:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Koutroubakis IE, Sfiridaki A, Tsiolakidou G, Theodoropoulou A, Livadiotaki A, Paspatis G, Kouroumalis EA. Genetic risk factors in patients with inflammatory bowel disease and vascular complications: case-control study. Inflamm Bowel Dis. 2007;13:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Heit JA, Phelps MA, Ward SA, Slusser JP, Petterson TM, De Andrade M. Familial segregation of venous thromboembolism. J Thromb Haemost. 2004;2:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Naito T, Botwin GJ, Haritunians T, Li D, Yang S, Khrom M, Braun J; NIDDK IBD Genetics Consortium, Abbou L, Mengesha E, Stevens C, Masamune A, Daly M, McGovern DPB. Prevalence and Effect of Genetic Risk of Thromboembolic Disease in Inflammatory Bowel Disease. Gastroenterology. 2021;160:771-780.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Klarin D, Busenkell E, Judy R, Lynch J, Levin M, Haessler J, Aragam K, Chaffin M, Haas M, Lindström S, Assimes TL, Huang J, Min Lee K, Shao Q, Huffman JE, Kabrhel C, Huang Y, Sun YV, Vujkovic M, Saleheen D, Miller DR, Reaven P, DuVall S, Boden WE, Pyarajan S, Reiner AP, Trégouët DA, Henke P, Kooperberg C, Gaziano JM, Concato J, Rader DJ, Cho K, Chang KM, Wilson PWF, Smith NL, O'Donnell CJ, Tsao PS, Kathiresan S, Obi A, Damrauer SM, Natarajan P; INVENT Consortium; Veterans Affairs’ Million Veteran Program. Genome-wide association analysis of venous thromboembolism identifies new risk loci and genetic overlap with arterial vascular disease. Nat Genet. 2019;51:1574-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |