Published online Oct 14, 2023. doi: 10.3748/wjg.v29.i38.5374
Peer-review started: July 27, 2023
First decision: August 8, 2023
Revised: September 16, 2023
Accepted: September 26, 2023
Article in press: September 26, 2023
Published online: October 14, 2023
Processing time: 77 Days and 7.2 Hours
Many studies have shown that interstitial Cajal-like cell (ICLC) abnormalities are closely related to a variety of dynamic gastrointestinal disorders. ICLCs are pacemaker cells for gastrointestinal movement and are involved in the trans
To elucidate the expression profile and significance of cholecystokinin-A (CCK-A) receptors in ICLCs in the common bile duct (CBD), as well as the role of CCK in regulating CBD motility through CCK-A receptors on CBD ICLCs.
The levels of tyrosine kinase receptor (c-kit) and CCK-A receptors in CBD tissues and isolated CBD cells were quantified using the double immunofluorescence labeling technique. The CCK-mediated enhancement of the movement of CBD muscle strips through CBD ICLCs was observed by a muscle strip contraction test.
Immunofluorescence showed co-expression of c-kit and CCK-A receptors in the CBD muscularis layer. Observations of isolated CBD cells showed that c-kit was expressed on the surface of ICLCs, the cell body and synapse were colored and polygonal, and some cells presented protrusions and formed networks adjacent to the CBD while others formed filaments at the synaptic terminals of local cells. CCK-A receptors were also expressed on CBD ICLCs. At concentrations ranging from 10-6 mol/L to 10-10 mol/L, CCK promoted CBD smooth muscle contractility in a dose-dependent manner. In contrast, after ICLC removal, the contractility mediated by CCK in CBD smooth muscle decreased.
CCK-A receptors are highly expressed on CBD ICLCs, and CCK may regulate CBD motility through the CCK-A receptors on ICLCs.
Core Tip: Interstitial Cajal-like cells (ICLCs) are pacemaker cells for gastrointestinal movement and are involved in the transmission of nerve impulses. This projects intends to elucidate the expression profiling and significance of cholecystokinin-A (CCK-A) receptors in ICLCs of common bile duct (CBD), as well as the role of CCK in regulating CBD motility through CCK-A receptors on CBD ICLCs.
- Citation: Xu D, Ma SL, Huang ML, Zhang H. Expression and functional study of cholecystokinin-A receptors on the interstitial Cajal-like cells of the guinea pig common bile duct. World J Gastroenterol 2023; 29(38): 5374-5382
- URL: https://www.wjgnet.com/1007-9327/full/v29/i38/5374.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i38.5374
Cholecystokinin (CCK) was first found in the gastrointestinal tract (GIT) and was named for its function of stimulating gallbladder contraction. Endothelial cells in the proximal small intestine release CCK after food intake[1]. CCK was thought to directly stimulate gallbladder smooth muscle cells (SMCs) to cause gallbladder contraction[2]. In recent years, the existence of CCK receptors has been confirmed by isolating gallbladder SMCs and radioimmunoassays[3]. CCK, classified into either the CCK-A subtype or CCK-B subtype, can bind to receptors and participate in modulating a series of physiological functions, such as gallbladder contraction, pancreatic enzyme secretion and gastric acid secretion[4]. CCKs in the blood circulation are present as four peptides with chains of different lengths, namely, CCK octapeptide (CCK-8), CCK-33, CCK-39 and CCK-58, among which CCK-8 has the greatest physiological effect on gallbladder contraction[2]. CCK has been reported to regulate guinea pig ileal contraction via CCK-A and CCK-B receptors[5], but CCK modulates guinea pig gallbladder contraction through only CCK-A receptors[6].
CCK regulates biliary system motility in two ways: By neural mechanisms and through receptor binding. Abundant intrinsic and extrinsic nerve plexuses are distributed around the biliary system, with cholinergic and adrenergic fibers and a large number of peptidergic fibers containing various neuropeptides, such as CCK peptidergic fibers. The neural mechanism by which CCK is involved in gallbladder contraction is its direct action on presynaptic neurons and promotion of the release of the neurotransmitter acetylcholine, thus enhancing gallbladder smooth muscle contraction. In addition, CCK directly boosts gallbladder smooth muscle contractility by binding to specific CCK-A receptors on gallbladder smooth muscle[7].
Interstitial Cajal cells (ICCs) are interstitial cells that exist in the GIT and are related to the basic electric rhythm (BER). The tyrosine kinase receptor (c-kit) expressed by ICCs can activate a tyrosine kinase, trigger intracellular signal transduction and maintain the ICC phenotype[7], making c-kit a specific marker for gastrointestinal ICCs. Essentially, ICCs are a kind of BER pacemaker cell, and the network formed by their processes can assist in spreading BER[8]. In recent years, interstitial Cajal-like cells (ICLCs) or ICCs outside the GIT have become a research hotspot[9], with evidence indicating the presence of ICLCs in mice[7], guinea pigs[10] and human gallbladder[11]. ICLCs have also been reported in the common bile duct (CBD) of guinea pigs[12].
From these research directions, this study aims to preliminarily investigate the pathogenesis of biliary tract diseases to deepen our understanding of biliary tract dynamics and provide novel ideas for the pathogenesis and clinical treatment of related dynamic diseases by determining the following: (1) The distribution, expression, morphology and ultra
The experimental animals were adult guinea pigs of both sexes weighing 250-300 g and were supplied by the Center for Animal Experiment of Wuhan University. All protocols were approved by the Institutional Animal Care and Use Committee of The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology.
Expression of c-kit and CCK-A receptors in CBD sections: Preparation of CBD tissue. The guinea pigs were fasted overnight and euthanized via CO2 inhalation[13] in the morning (10 a.m.) of the following day. The CBD tissue was collected from the guinea pigs and immediately placed in a Ca2+-free physiological saline solution (PSS) petri dish, which was continuously oxygenated with 95% O2: 5% CO2. The CBD blood vessels were then peeled off under a microscope, and the CBD tissue was washed with Ca2+-free PSS (20 mL). All experimental procedures conformed to the guidelines from the Committee on the Ethics of Animal Experiments of the Central Hospital of Wuhan.
Immunohistochemistry: The collected tissues were immersed in 4% paraformaldehyde fixative overnight (4 °C, 16-18 h). After the samples were dehydrated and made transparent using different concentration gradients of ethanol and xylene according to routine procedures followed by embedding, slicing and conventional dehydration. The tissue was then cultivated with 3% hydrogen peroxide for 10 min before rinsing with distilled water, followed by washing with phosphate buffered saline (PBS) (3 min each). Diluted normal goat serum was added dropwise to block the samples at room temperature for 10 min. Then, the following diluted primary antibodies were added dropwise: Anti-c-kit monoclonal (1:100) and anti-CCK-A receptor (1:8000), and the sample was placed in a wet box sealed and kept at 4 °C overnight. Subsequently, FITC-labeled rabbit anti-rat IgG (1:50) and Cy3-labeled goat anti-rabbit antibody (1:50) were added for 1 h of incubation at room temperature away from light, followed by washing with PBS and mounting with 50% glycerol. The CBD tissue was finally observed under a laser confocal microscope and photographed. Confocal fluorescence images were captured using an electron multiplying charge-coupled device camera (EMCCD, iXon Ultra, Andor, Tokyo, Japan) mounted onto an inverted microscope (IX83, Olympus), and the fluorescence intensity was quantified using capture software (iQ3, Andor).
Preparation of the CBD ICLC single-cell suspension: Guinea pig CBD tissue was collected in the same way as described above. Then, the CBD tissue was fixed for longitudinal and horizontal dissection, and the mucous layer was stripped. Next, the muscle strips were cut into pieces, and 1-2 mm3 pieces were placed into a beaker to which 5 mL of type II collagenase digestive solution was added with slight oscillation and digestion at 37 °C for 23 min. Then, the strips were rinsed five times with Ca2+-free PSS. After that, the tissue blocks were mixed with a wide-mouth fire-polished pipette to produce a cell suspension, which was then added to an equal volume of Ficoll 400 gradient solution for 10 min of centrifugation (30 g). The cells at the interface of the liquid surface were precipitated and plated.
Cell inoculation: First, cells were suspended in M199 culture medium to adjust the concentration to 1 × 106/mL, followed by seeding the cell suspension into the wells of a 6-well culture plate, in which a rat tail collagen-coated (2 ng/cm2) cover glass was placed in advance. The plate was then put into an incubator to culture under 5% CO2 at 37 °C.
Expression of c-kit and CCK-A receptors on ICLCs determined by immunofluorescence. The culture medium in the petri dish was removed, and the nonadherent cells were washed away after 24 h of cell culture. The remaining cells were rinsed with PBS three times, fixed at room temperature for 10 min with acetone, washed again with PBS, and sheep serum was added for 30 min of blocking at ambient temperature. Then, anti-c-kit monoclonal antibody (1:100) and anti-CCK-A receptor polyclonal antibody (1:8000) were added for incubation overnight at 4 °C. Finally, FITC-labeled rabbit anti-rat IgG (1:50) and Cy3-labeled goat anti-rabbit IgG (1:50) were added for 1 h of incubation at room temperature away from light, followed by washing with PBS and mounting with 50% glycerol. The cells were observed under a laser confocal microscope and photographs were taken.
Preparation of guinea pig CBD muscle strips: Muscle strips (8 mm × 2 mm) were used to prepare two types of smooth muscle strips: (1) CBD muscle strips with ICLCs; and (2) Muscle strips with damaged CBD ICLCs.
Damaged CBD ICLCs: ICLCs were selectively damaged by 50 μmol of methylene blue (MB) + 50 mW/cm light irradiation. Using an adjustable 540 nm spotlight, the tissue sample was placed 315 cm away from the light source and irradiated on the tissue surface for 5 min. A digital photometer was used to measure light intensity. This approach damaged only the ICLC structure and not the CBD intermuscular neural network or SMCs[14].
Contractile response of CCK-8 to CBD muscle strips: Four prepared CBD muscle strips were placed in tissue chambers with one strip per chamber. One end of the CBD muscle strip was fixed on the hook at the bottom of the tissue chamber, and the other end was fixed on the tension sensor. The input signal was input into the physiological recorder to record the spontaneous contractive activity of the CBD muscle strip. Each tissue chamber contained 5 mL of Krebs solution at 37 °C and was continuously filled with mixed gas composed of 5% CO2 and 95% O2. The muscle strips were incubated for approximately 1 h under a 1 g preload, and after the spontaneous contraction of the muscle strips was observed to be smooth, atropine (10-6 mol/L) and tetrodotoxin (TTX) (3 × 10-7 mol/L) were added to observe the contractile activity of the CBD muscle strips. Subsequently, CCK-8 at concentrations ranging from 10-8 to 10-6 mol/L was added to observe the reaction after ICLC removal. The test procedure was performed as described above.
Identification of damaged CBD ICLCs: After the muscle strip experiment, a 1 mm3 section of the CBD tissue was put into buffered glutaraldehyde fixative (2.5%, 2 h), rinsed with PBS, and refixed with 1% OsO4 (1 h). Then, the samples were dehydrated with a gradient series of alcohol and acetone and embedded in Epon 812 resin. Following ultrathin sectioning, the tissue was double-stained with uranyl acetate and lead citrate to be observed and photographed using an FEI Tecnai G2 12 transmission electron microscope.
Experiments were repeated at least three times, and the measured data were averaged.
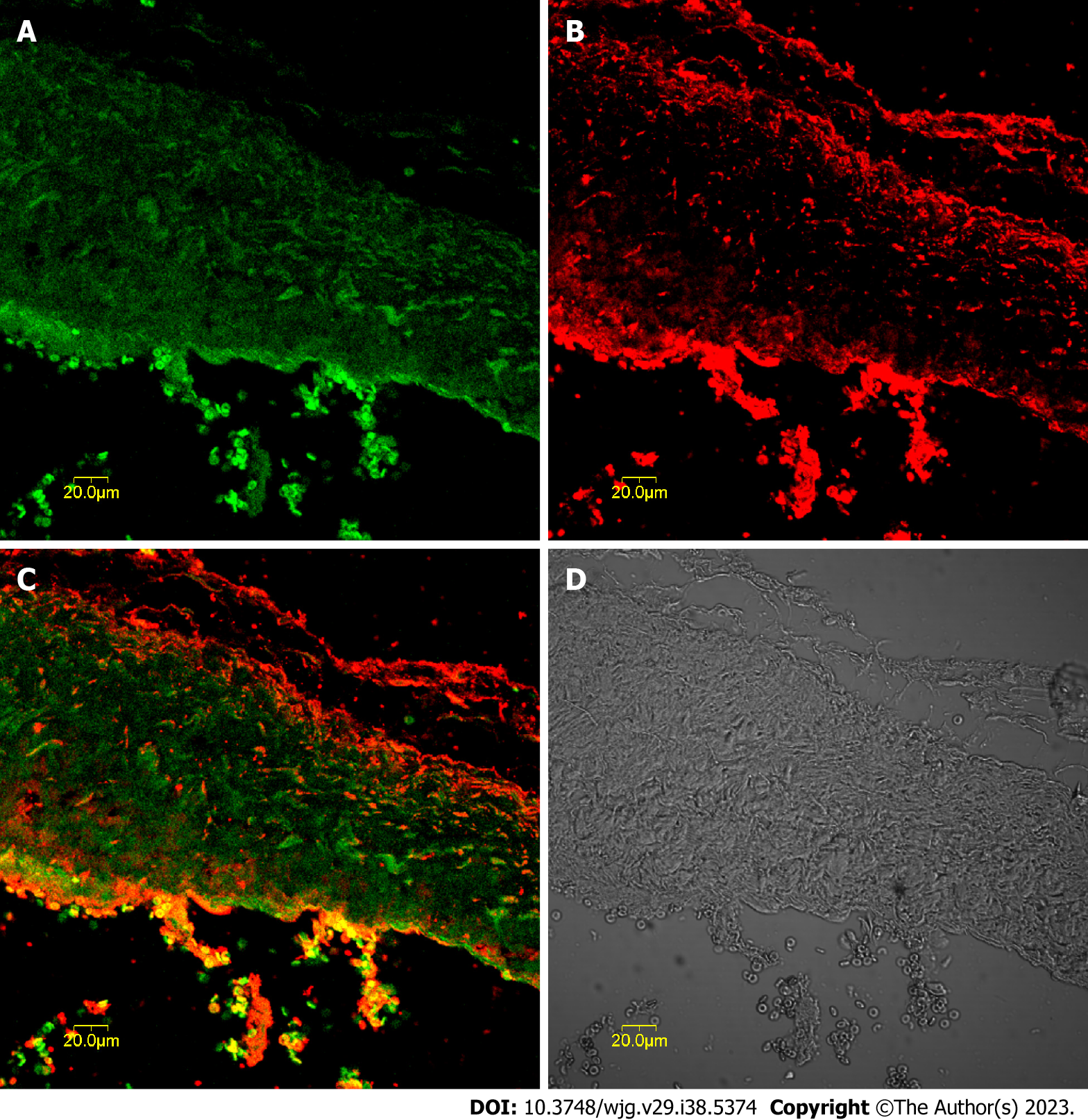
c-kit-positive cells were mainly distributed in the muscularis layer of the CBD co-expressing CCK-A receptors. These cells aggregated in a network and were distributed parallel to the circular muscle, as shown in Figure 1. The optimal concentration of CCK-A receptor primary antibody was 1:8000; below this concentration, the CCK-A receptors were hardly expressed in CBD SMCs, but once the antibody concentration was above 1:8000, CCK-A receptor immunopositivity was observed in CBD SMCs (Figure 1).
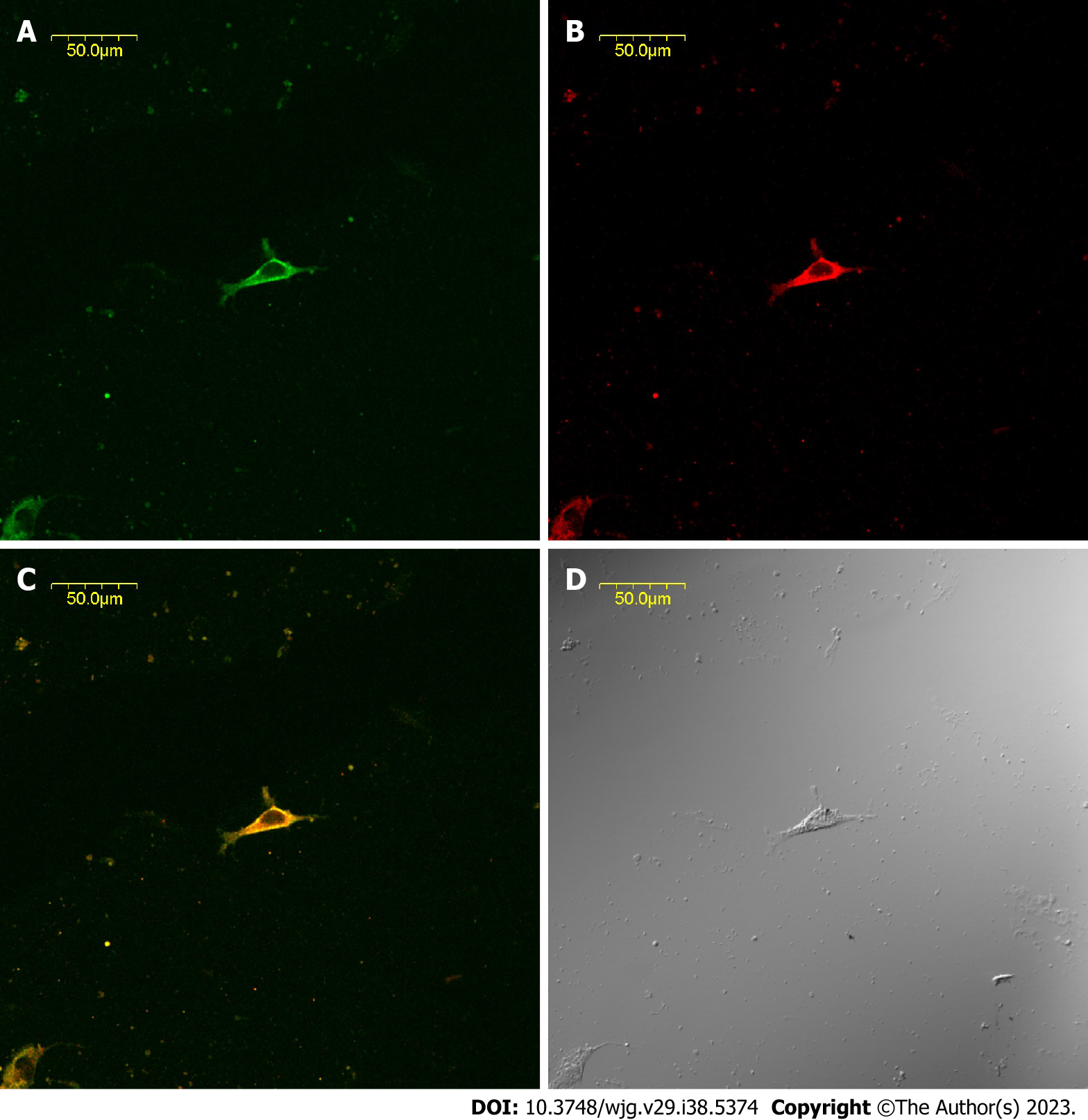
The CBD ICLCs of guinea pigs after immunofluorescence staining were observed under a laser confocal microscope. It was found that the ICLCs were polygonal and c-kit positive with well-stained cell bodies and synapses. Some cells presented long and thin protrusions, while others had more slender synaptic terminals that formed filaments. There were few CBD ICLCs, and they were distributed roughly parallel to the circular muscle and clustered in a network. Moreover, CCK-A receptors were highly expressed in CBD ICLCs. In ICLCs with multiple synapses, the synapses extended into adjacent SMCs, with very large hyperchromatic nuclei in most of the cells, scattered chromatin, and relatively little cytoplasm. However, the organelles were abundant, including well-developed perinuclear endoplasmic reticulum and rough endoplasmic reticulum, which were closely arranged in the cytoplasm. Mitochondria and free ribosomes were also abundant, and the cells had many filaments and intermediate filaments but no myofilaments, distinguishing them from peripheral SMCs. The characteristic structure that distinguished ICLCs from fibrocytes, glial cells and macrophages was caveolae. ICLCs were surrounded by collagen fibers and formed cellular junctions with surrounding SMCs (Figure 2).
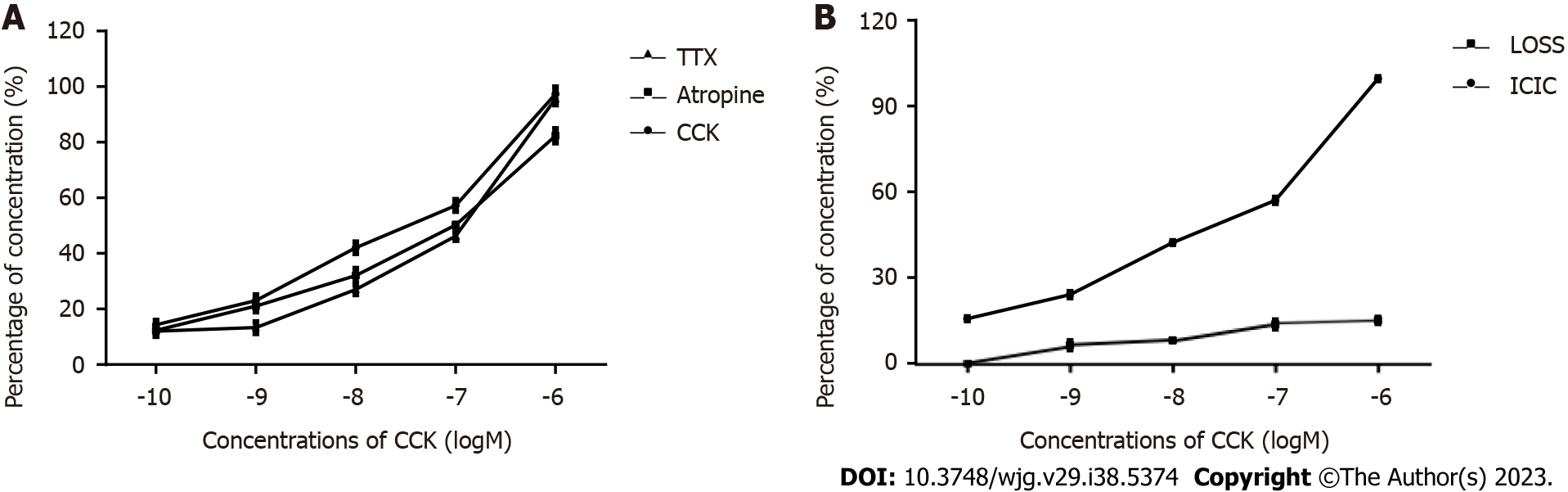
The response of CBD muscle strips with ICLCs to CCK: Figure 3A shows the contractile response curve of guinea pig CBD muscle strips treated with CCK-8 at concentrations ranging from 10-10 to 10-6 mol/L. CCK-8 dose dependently enhanced the contractile amplitude of the CBD muscle strips. Pre-addition of atropine (10-6 mol/L) and TTX (3 × 10-7 mol/L) was performed to eliminate nervous system contraction of the CBD strips in guinea pigs and did not affect contraction of the CBD muscle strips by CCK-8 in guinea pigs.
Response of ICLC-removed CBD muscle strips to CCK-8: Figure 3B shows the contractile response curve of guinea pig CBD after ICLC removal to CCK-8 (concentration range: 10-6 to 10-10 mol/L), which showed a rightward shift.
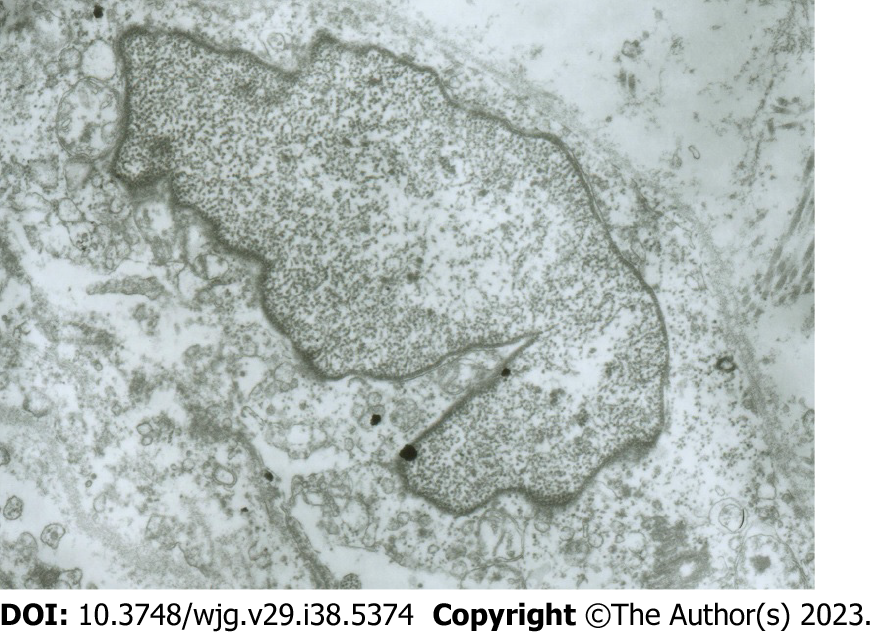
Electron microscopy images of the damaged CBD ICLCs: The following features were observed in CBD ICLCs damaged by MB plus light irradiation under an electron microscope: Incomplete cell membrane, nuclear swelling, chromatin homogenization, partial expansion of the endoplasmic reticulum, unrecognizable mitochondrial damage, and a reduction in or even the disappearance of the characteristic caveolae. However, and no damage was observed to CBD SMCs after treatment with MB plus light (Figure 4).
Biliary tract motility includes the movements of the gallbladder and bile duct[15]. The motility of the CBD is regulated by autonomic nerves as well as joint regulation by gastrointestinal hormones (CCK, motilin, etc.) and neuromediators. Together with nerve regulation, these substances coordinate the contraction and relaxation of the CBD[16]. In contrast, biliary system dysfunction causes cholestasis, which is an important precursor of choledocholithiasis. There are few intrahepatic bile duct SMCs. The distribution of extrahepatic bile duct SMCs also varies, with SMC detection rates in the common hepatic duct, upper duodenum of the CBD, and pancreatic segment being 24%, 53%, and 87%, respectively. There are only a few longitudinal or circular SMCs in the upper segment of the CBD, which form the CBD sphincter (the CBD and pancreatic duct end and ampullary around the ring of the sphincter, collectively known as the Oddi sphincter[17]) at the ampulla[7]. In the bile duct wall, no obvious intramural nerve plexus formed, although there were many nerve cells. CBD motility can be divided into active lengthening and shortening movements for bile transportation[18].
ICCs, which are involved in transmitting nerve impulses to mediate gastrointestinal motility, are the pacemakers of gastrointestinal motility. Extra-gastrointestinal ICLCs share similar functions with gastrointestinal pacemaker cells. These ICLCs lack contractile activity but have morphological characteristics similar to ICCs, including elongated and abundant protrusions, intermediate filaments, mitochondria, and characteristic caveolae that can form network structures. ICLCs also function similarly to ICCs in the GIT. Studies have shown that there are special pacemaker cells in the rabbit urethra; such ICLCs are similar to rabbit intestinal ICCs and generate slow waves[19]. ICLCs are also present in the CBD sphincter[20].
In this study, it was found that guinea pig CBD ICLCs had abundant mitochondria, which were active and provided energy for slow-wave pacemaker cells. The CBD ICLCs contained a large amount of endoplasmic reticulum, especially rough endoplasmic reticulum, which explains their active synthesis and secretion functions. In addition, the cellular junctions formed between ICLCs and SMCs may act as an intermediary regulating nervous system-mediated control of the movement of CBD SMCs.
Cholecystic contractile function is enhanced as the CCK level increases; a decrease in the CCK level and its concomitant gallbladder contraction dysfunction are important pathological mechanisms leading to the formation of gallstones[21]. CCK-A receptors are found in gallbladder smooth muscle, and CCK binding to CCK-A receptors plays a vital role in gallbladder motility. The conventional view is that CCK regulates gallbladder contraction through a neural mechanism that acts on presynaptic neurons to increase the release of acetylcholine, which affects smooth muscle contraction. CCK can also directly contract gallbladder smooth muscle, mainly through specific binding with CCK-A receptors on gallbladder smooth muscle, activating non-G protein-mediated signaling pathways and G protein-coupled signaling pathways to promote signal transduction, thus causing relevant physiological effects[22].
The expression of CCK-A receptors on isolated cultured ICLC slides was confirmed in guinea pig CBD ICLCs by choledochal histology and immunofluorescence. In the choledochal tissue section, clear immunofluorescence was observed, and CCK-A receptors were distributed in the choledochal smooth muscle layer. A CCK-A receptor antibody concentration higher than 1:8000 was initially used in the immunofluorescence assay of CBD tissue sections and revealed no difference in fluorescence intensity between CBD smooth muscle and ICLCs. When the antibody concentration was 1:8000, the SMCs in the CBD tissue sections were not detected, while the ICLC fluorescence intensity was strongly positive. Therefore, CCK-A receptors were expressed in both SMCs and ICLCs of the guinea pig CBD, with a higher concentration in the former.
CCK-A was also found to be expressed on CBD ICLCs in this study. Therefore, how does CCK regulate CBD motility? Is it through CCK-A receptors on ICLCs in addition to directly binding with CCK-A receptors to promote CBD motility? We found that guinea pig CBD ICLCs strongly expressed CCK-A receptors, suggesting the important role of ICCs in CCK-induced CBD smooth muscle contraction. Thus, CCK may regulate biliary system pressure through CCK-A receptors on CBD ICLCs.
In this experiment, CCK-8 notably and dose-dependently increased the contraction of CBD muscle strips. Following the removal of ICLCs, however, we observed a significant rightward shift in the contractile dose-response curve of the CBD smooth muscle strips induced by CCK-8. After light + MB treatment, the guinea pig ICLCs were clearly altered. The electron microscopy images of CBD ICLCs showed swollen cell bodies, loose cytoplasm, notably fewer mitochondria, disappearance of the characteristic caveolae, and destroyed cell membranes. Light + MB treatment specifically damages only the ICLCs but not the SMCs that are closely connected to them[17]. These findings suggest that CBD ICLCs are crucial in initiating SMC contraction. After the ICLCs were removed, CCK caused a significant decrease in the contractility of the CBD muscle strips, indicating that CCK may partially induce the contraction of guinea pig CBD smooth muscle through the CCK-A receptors on the CBD ICLCs.
This research demonstrates the dose-dependent contraction of smooth muscle by CCK at concentrations ranging from 10-10 to 10-6 mol/L and reduced responses of ICLC-removed CBD smooth muscle to CCK. These data suggest that ICLCs not only regulate CBD smooth muscle motility through the slow wave potentials caused by the known pacemaker current pathway but also mediate CCK-induced CBD smooth muscle contraction through chemical excitation. In addition to acting on CCK-A receptors on CBD SMCs, CCK may also regulate CBD contraction by binding to CCK-A receptors on ICLCs. CBD ICLCs not only excite smooth muscle slow wave potentials and modulate enteric neurotransmitters but also regulate CBD contraction by regulating hormones such as CCK in blood circulation.
Interstitial Cajal cells (ICCs) are a kind of interstitial cells existing in the gastrointestinal tract, which are related to basic electric rhythm. Many studies have shown that Interstitial Cajal-like cells (ICLCs) abnormalities are closely related to a variety of gastrointestinal dynamic disorders. ICLCs are pacemaker cells for gastrointestinal movement and are involved in the transmission of nerve impulses.
This study aims to preliminarily investigate the pathogenesis of biliary tract diseases to deepen our understanding of biliary tract dynamics and provide novel ideas for the pathogenesis and clinical treatment of related dynamic diseases.
Figure out the pathogenesis of biliary tract dynamics and related dynamic diseases such as choledocholithiasis. As well as the expression of cholecystokinin-A (CCK-A) receptors on common bile duct (CBD) ICLCs. Investigate how CCK regulates biliary tract dynamics through CBD ICLCs
Of 250-300g adult guinea pigs of either sex were used to obtain CBD tissue. Then, the levels of tyrosine kinase receptor and CCK-A receptors in CBD tissues and CBD isolated cells were quantified using the double immunofluorescence labeling technique. The enhancement effect of CCK on the movement of CBD muscle strips through CBD ICLCs was observed by muscle strip contraction test.
The guinea pig CBD ICLCs had abundant mitochondria, which were active in function and provided energy for slow-wave pacemaker cells. The expression of CCK-A receptors was confirmed on guinea pig CBD ICLCs by choledochal histology and immunofluorescence method of isolated ICLC-cultured slides.
This research demonstrates a dose-dependent contraction of smooth muscle by CCK with a concentration ranging 10-10 to 10-6 mol/L, and reduced responses of ICLC-removed CBD smooth muscle to CCK, suggesting that ICLCs not only regulate CBD smooth muscle motility by slow wave potentials caused by the known pacemaker current pathway, but also mediate CCK-induced CBD smooth muscle contraction through chemical excitation.
Investigate the distribution, expression, morphology and ultrastructure of CBD ICLCs. Also, the expression and function of CCK-A receptors on CBD ICLCs.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Haas RJ, Netherlands; Venkat V, United States S-Editor: Wang JJ L-Editor: A P-Editor: Yuan YY
| 1. | Liu Y, Fan Y, Wu S. Developments in research on interstitial Cajal-like cells in the biliary tract. Expert Rev Gastroenterol Hepatol. 2021;15:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Nadella S, Ciofoaia V, Cao H, Kallakury B, Tucker RD, Smith JP. Cholecystokinin Receptor Antagonist Therapy Decreases Inflammation and Fibrosis in Chronic Pancreatitis. Dig Dis Sci. 2020;65:1376-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Wang HH, Portincasa P, Liu M, Tso P, Wang DQ. An Update on the Lithogenic Mechanisms of Cholecystokinin a Receptor (CCKAR), an Important Gallstone Gene for Lith13. Genes (Basel). 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Zeng Q, Ou L, Wang W, Guo DY. Gastrin, Cholecystokinin, Signaling, and Biological Activities in Cellular Processes. Front Endocrinol (Lausanne). 2020;11:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 5. | Wang DQ. Emerging Trends in Deciphering the Pathogenesis of Human Diseases through Genetic Analysis. Genes (Basel). 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Tang C, Biemond I, Lamers CB. Cholecystokinin receptors in human pancreas and gallbladder muscle: a comparative study. Gastroenterology. 1996;111:1621-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Sun X, Yu B, Xu L, Dong W, Luo H. Interstitial cells of Cajal in the murine gallbladder. Scand J Gastroenterol. 2006;41:1218-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Chen L, Yu B. Telocytes and interstitial cells of Cajal in the biliary system. J Cell Mol Med. 2018;22:3323-3329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Huizinga JD, Faussone-Pellegrini MS. About the presence of interstitial cells of Cajal outside the musculature of the gastrointestinal tract. J Cell Mol Med. 2005;9:468-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Lavoie B, Balemba OB, Nelson MT, Ward SM, Mawe GM. Morphological and physiological evidence for interstitial cell of Cajal-like cells in the guinea pig gallbladder. J Physiol. 2007;579:487-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Hinescu ME, Ardeleanu C, Gherghiceanu M, Popescu LM. Interstitial Cajal-like cells in human gallbladder. J Mol Histol. 2007;38:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Xu D, Liu J, Huang M, Zhang H, Ma S. Kit-positive cells in the murine common bile duct. AQCH. 2021;. |
| 13. | Greulich S, de Wiza DH, Preilowski S, Ding Z, Mueller H, Langin D, Jaquet K, Ouwens DM, Eckel J. Secretory products of guinea pig epicardial fat induce insulin resistance and impair primary adult rat cardiomyocyte function. J Cell Mol Med. 2011;15:2399-2410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Liu LW, Thuneberg L, Huizinga JD. Selective lesioning of interstitial cells of Cajal by methylene blue and light leads to loss of slow waves. Am J Physiol. 1994;266:G485-G496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Feng H, Wang F, Wang C. C-Kit expression in the gallbladder of guinea pig with chronic calculous cholecystitis and the effect of Artemisia capillaris Thunb on interstitial cells of Cajal. Iran J Basic Med Sci. 2016;19:720-725. [PubMed] |
| 16. | Zhou M, Guo YT, Chang YB, Zhong ZH. Effects of anisodamine and gabexate on biliary dynamics in patients after biliary operation. World Chin J Dig. 2009;17:2748-2751. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Woods CM, Mawe GM, Toouli J, Saccone GT. The sphincter of Oddi: understanding its control and function. Neurogastroenterol Motil. 2005;17 Suppl 1:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Fratantoni ME, Giuffrida P, Di Menno J, Ardiles V, de Santibañes M, Clariá RS, Palavecino M, de Santibañes E, Pekolj J, Mazza O. Prevalence of Persistent Common Bile Duct Stones in Acute Biliary Pancreatitis Remains Stable Within the First Week of Symptoms. J Gastrointest Surg. 2021;25:3178-3187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pacemaking cells in the rabbit urethra. J Physiol. 2000;526 Pt 2:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 194] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Park CG, Wu MJ, Hong C, Jo JY, Jiao HY, Park H, Jun JY, Choi S. Regulation of Intracellular Calcium by Endoplasmic Reticulum Proteins in Small Intestinal Interstitial Cells of Cajal. J Neurogastroenterol Motil. 2018;24:128-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Fu BB, Zhao JN, Wu SD, Fan Y. Cholesterol gallstones: Focusing on the role of interstitial Cajal-like cells. World J Clin Cases. 2021;9:3498-3505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Sato N, Miyasaka K, Suzuki S, Kanai S, Ohta M, Kawanami T, Yoshida Y, Takiguchi S, Noda T, Takata Y, Funakoshi A. Lack of cholecystokinin-A receptor enhanced gallstone formation: a study in CCK-A receptor gene knockout mice. Dig Dis Sci. 2003;48:1944-1947. [PubMed] [DOI] [Full Text] |