INTRODUCTION
Primary biliary cholangitis (PBC), previously named primary biliary cirrhosis[1], was probably already described in the middle of the nineteenth century. In fact, important similarities with PBC were reported in a clinical case observed at Guy’s Hospital in 1851[2]. Almost one century later, a description of the clinical course of the disease was reported in a group of patients, mainly achieving diagnosis after a surgical approach[3]. The etiology of PBC remains elusive today, even if the pathogenesis is related to the onset of an autoimmune response characterized by the presence of anti-mitochondrial antibodies (AMA) in the sera of affected patients[4]. The target of the immune response is represented by the intrahepatic biliary tract, thereby determining progressive chronic cholestasis, fibrosis, and a possible evolution toward liver cirrhosis[5]. Women are prevalently affected by PBC, with an age of onset that usually falls within the fourth or fifth decade[5]. The prevalence can change among different countries, accounting in general for 20-30/100.000 inhabitants in United States and Europe[5,6]. Liver biopsy is generally not required for diagnosis since AMA positivity, coupled with increased cholestasis indexes in the absence of biliary obstruction, represents a specific and characteristic presentation[7]. Ursodeoxycholic acid (UDCA), 13-15 mg/kg daily is the main and mandatory treatment to slow the progression in patients affected by PBC. The use of obeticholic acid (OCA) should help increase the response to UDCA or in subjects UDCA intolerant[8]. Despite the identification of effective medical treatments for PBC, a third of patients have a scarce response, thus evolving toward liver cirrhosis that may also require liver transplantation[9]. This problem and uncertainty about the mechanisms of onset and progression of the disease suggest that further research is needed to decipher the natural/molecular history of PBC. In this review, which also describes possible predisposing factors, we examine current knowledge on sequential molecular and cellular mechanisms in the onset of PBC. Reversion of these chain processes may possibly prevent the most severe sequelae of this chronic cholestatic disorder. The manuscript was prepared on the base of a literature search (PubMed, Scopus, Web of Science) using several key words (alone or in combination) such as: PBC, primary biliary cirrhosis, cholangiocyte, bile duct cells, secretion, proliferation, cholestasis, molecular mechanism, risk factors, pathology, antibody, immunology, and others.
Before PBC (predisposing factors)
Some generic risk factors, such as female sex and age, have been identified in patients with PBC. More interestingly, and suggesting a genetic predisposition, studies examine the prevalence of autoimmune disorders in patients with PBC and their relatives[10]. The family history of PBC seems, in fact, to be the strongest predisposing factor for the disease with an odds ratio of nearly 10 compared to controls. Furthermore, this familiar occurrence also increases the possibility of developing other autoimmune disorders such as polymyositis, systemic lupus erythematosus, and others[10]. These observations claim a possible similar genetic background between PBC and other autoimmune disorders, as also suggested by a clinical study focusing on this category of diseases[11]. However, we must emphasize that in PBC, such as other complex disorders, genetics represents only a permissive trait, which requires exposure to possible environmental/external factors for the development of injury[12]. Human leukocyte antigens (HLA) have long been investigated for their possible relationship with the onset of immune diseases[13]. Similarly, early data suggested an association between PBC and HLA DRB1*08[12]. This finding was later expanded, demonstrating an opposite protective effect of DRB1*11 and DRB1*13 and not only with regard to PBC, but also extended to other hepatic diseases such as viral B and C hepatitis[14]. The approach of genome-wide association studies has more recently allowed to identify other genetic changes in PBC, including several non-HLA variants[15]. These may affect the interleukin-12/JAK-STAT pathway, the B cell response, and other steps of inflammatory signalling. However, the trigger factors that may cause disease in the presence of a favourable genetic background are still not clearly defined. Smoking or drinking habits have been associated with the development of PBC, as well as recurrent urinary infections, hair dye, and hormonal replacement therapy in women[10]. The large diffusion of these conditions, together with the limited prevalence of PBC, allow us to foresee the scarce utility of possible preventive strategies for this disease, at present.
The beginning of injury: the antimitochondrial antibody
In parallel with what has been observed in other autoimmune disorders, the presence of self-directed antibodies (AMA) is the basis of the physio-pathological process in PBC. The main target of AMA is represented by the mitochondrial E2 subunits of the pyruvate dehydrogenase complexes (PDC-E2)[4]. Since E2 is a highly preserved portion in other species and bacteria, the possible origin of AMA by molecular mimicry (cross-reaction with self-antigen after a previous exposure to exogenous pathogens with similar moieties) has long been suggested[16]. Several agents have been proposed in the past to induce AMA formation, including microorganisms or environmental substances[17]. Among bacteria, Escherichia coli (E. Coli) has long been suggested as a possible important actor in eliciting the immune response in PBC[18]. The contribution of E. Coli may also explain the frequent association between the onset of PBC and recurrent urinary tract infection, as this microorganism is an important causative agent of this latter disorder. More recently, exposure to Novosphingobium aromaticivorans (N. Aromaticivorans), a ubiquitous gram-negative bacteria capable of metabolizing xenobiotics, has been suggested as a possible trigger factor in PBC. In fact, the immune reactivity of sera from patients with PBC is 100 times stronger for N. Aromaticivorans compared to E. Coli and the ubiquity of this bacteria is demonstrated by its presence in 25% of fecal samples from both PBC patients or control[19]. Regarding the mechanism linking bacteria to PBC progression, also a role for intestinal dysbiosis has been claimed[20]. This may increase intestinal permeability and lipopolysaccharide flux toward the liver, thus resulting in an enhanced immune/inflammatory response. Interestingly and supporting this view, UDCA treatment was reported to attenuate the difference in intestinal microbioma between PBC patients and normal controls[21]. However, to underscore that studies on gut microbial composition are particularly complex, affected by individual, environmental and dietary factors as well as by sampling procedure, so that a conclusive picture on this issue is not available at present. The development of an AMA titer in blood has been considered in the clinic to play an important causal role in the following biliary damage. However, in this regard, while some authors suggest that the onset of this antibody is an important predictive factor for the development of PBC[22], others underscore the higher prevalence (1/1000) of AMA compared to PBC (0.4/1000), therefore suggesting that some subjects may be healthy for life despite displaying these autoantibodies in blood[4]. With this regard, some data show that AMA does not seem pathogenic by itself, and its complex with the corresponding antigen is needed to prompt immunity. However, why the formation of immune complexes occurs in PBC patients is a question that remains unanswered at present[23]. The AMA target within PDC-E2 is represented by the lipoyl domain, and the different degradations of this epitope seem related to the reason that the antibodies mainly target bile duct cells (i.e., cholangiocytes). In fact, PDC-E2 is ubiquitously present in cells; however, it appears that after the apoptotic death of cholangiocytes, differently from other epithelial cells, the epitope is released in its intact form, thus maintaining the immune response and lymphocyte homing[17]. This pattern appears to be also followed by the salivary and lacrimal glands, explaining the frequent association between PBC and Sjögren syndrome[24], being the latter a disease that presents characteristically with dryness in the mouth and eyes and also recognizing an autoimmune origin[25]. Finally, from a clinical point of view, the AMA titer does not have a significant relationship with the prognosis or extent of liver damage in PBC[26] while some patients with this disease exhibit an antinuclear antibody (ANA) instead of AMA (AMA negative PBC)[27].
Onset of chronic inflammation
Biliary cell apoptosis is a critical event in maintaining the injury since the early stages of PBC. This process allows exposure to specific epitopes that support the autoimmune response[28]. The possible contribution of innate immunity to the inflammatory process of PBC remains an argument for debate. Destruction of biliary cells has been described after toll-like receptor 4 stimulation of natural killer cells (NK) in the presence of interferon alpha[29]. Interestingly, biliary damage is proportional to the degree of NK infiltration of the biliary tract[30]. In fact, when the ratio of NK/bile duct cells is low, no biliary damage occurs. On the contrary, high NK infiltration is associated with extensive cholangiocyte damage and epitope exposure. These are targeted by T cells, which in turn maintain chronic injury realizing the adaptive immune response[30]. In this perspective, the activity of NK cells would be the main determinant of the evolution from an innate to an adaptive immunological response, during the onset of PBC. Anti-PDC E2163-176 specific T cells then expand and are preferentially located in the lymph nodes and liver. These cells are recovered only in patients with PBC, and their specific location in the liver supports their pathogenic role in chronic injury involving this organ[31]. Once the inflammatory process is established, changes that occur in the activities of bile duct cells further contribute to injury, as reported in the following paragraphs.
Changes in biliary physiology
Several changes occur in the function of cholangiocytes when the PBC inflammatory damage is established. These involve the secretory and proliferative processes of the biliary epithelium and other aspects. These issues are discussed in the following paragraphs, including their contribution to perpetuating and worsening the damage.
Impairment of secretive processes
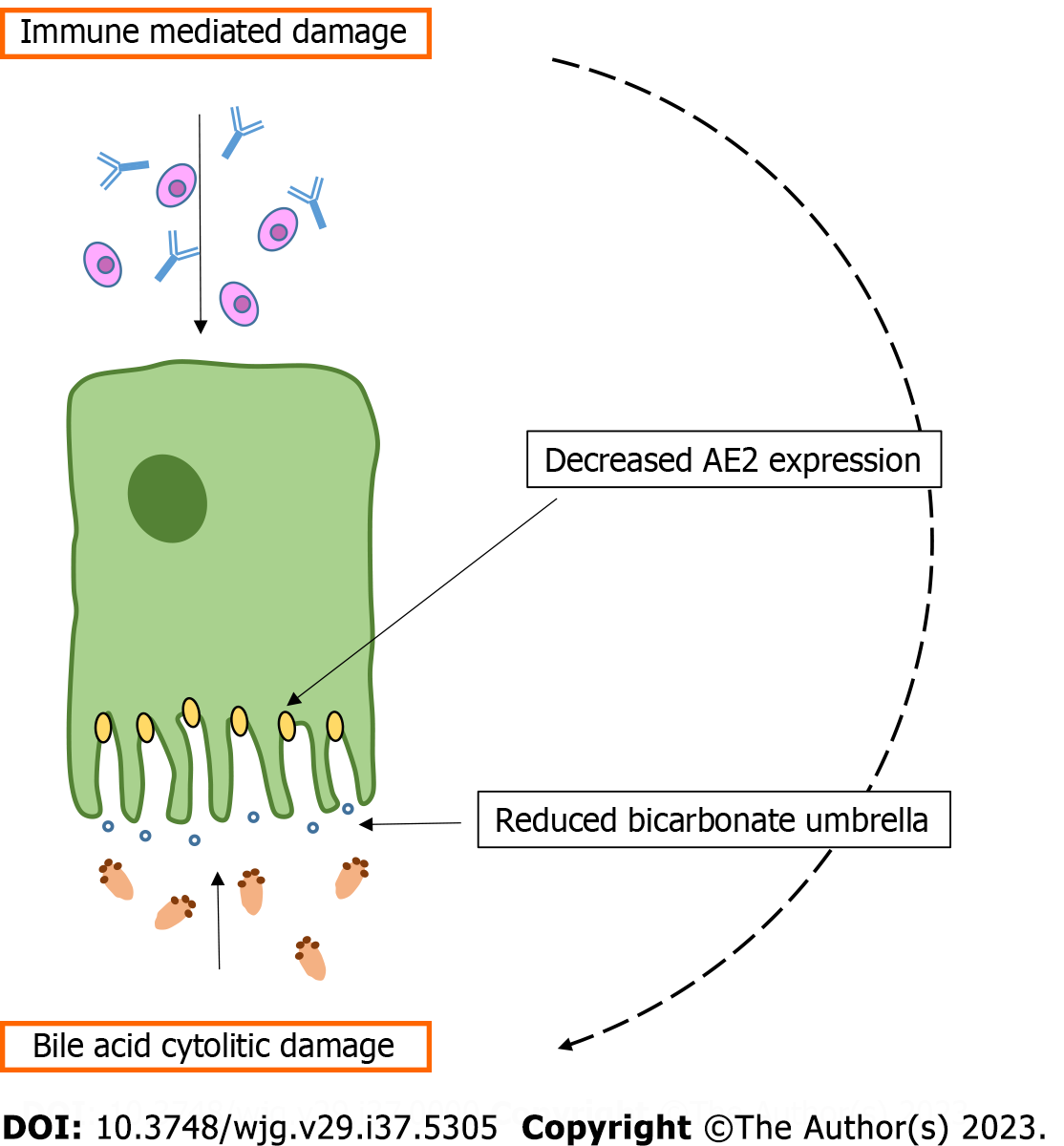
Several studies in recent decades have elucidated the paramount importance of biliary epithelia in bile secretion and the physiopathological changes that occur in this system in cholestatic disorders (when the bile does not reach the intestine) such as PBC[32]. Differently from hepatocytes, in which bile secretion is constant and is driven by canalicular bile acids (BA) transfer, the cholangiocyte bile output can change significantly according to exposure to different gastro-intestinal hormones or peptides[32]. Moreover, endogenous (inflammatory) or exogenous stimuli (bacterial, toxic, drug-induced, and others) altering the ductal microenvironment can change cholangiocyte secretory response[33]. The classical molecular process that stimulates biliary bile secretion recognizes as a first step the stimulation by secretin (Sec) of a specific sec receptor (SR) expressed only in cholangiocytes within the liver[34]. Sec/SR binding activates a cascade of molecular events involving, in turn: (1) Increased intracellular cyclic adenosine 3',5'-monophosphate (cAMP); (2) phosphorylation of protein kinase A (PKA); (3) Cl- extrusion in canalicular space by cystic fibrosis transmembrane regulator; and (4) reabsorption of Cl- and its replacement with bicarbonate operated by the chloride-bicarbonate exchanger (AE2)[34]. This determines the realization of a BA-independent bicarbonate-enriched choleresis that may contribute, under some conditions, to more than 50% of total bile flow. Several human and experimental evidences demonstrated impaired AE2 activity in PBC. In 1993, a decrease in AE2 mRNA was first described in the liver of PBC patients[35]. In a subsequent human study, a reduction in AE2 was also demonstrated with respect to protein synthesis, employing the immune staining technique[36]. Finally, the same group demonstrated with positron emission tomography reduced (both basal and Sec-stimulated) bicarbonate biliary secretion in patients with PBC[37]. Further evidence supporting the impairment of AE2 in PBC came from the Ae2 knockout mouse model, which resembles several features of this human disease[38]. The mechanism at the base of the down-regulation of AE2, in the course of PBC, is not clear at present. However, research demonstrated epigenetic changes in the AE2 promoter region (both in liver and peripheral blood mononuclear cells of PBC patients) characterized by enhanced methylation, thus determining reduced mRNA transcription[39]. Despite the uncertainty with respect to the chain of events that leads to decreased AE2 activity, the outcome of this occurrence is well evidenced by the impairment of bicarbonate-enriched choleresis after Sec stimulation[37]. The fall in biliary bile flow and, in particular, of bicarbonate secretion, opens the door to BA-induced cytolitic damage, according to the theory of bicarbonate umbrella[40]. In agreement with this view, given the decreased AE2 activity, the delicate bicarbonate film (bicarbonate umbrella), laying on the luminal side of the cholangiocyte, would be altered, allowing cellular damage by hydrophobic BA monomers. The main determinants of this process are shown in Figure 1. In this perspective, two pathological components would contribute to chronic biliary damage during PBC: (1) The autoimmune inflammatory process; and (2) the cytotoxic BA-related injury. The role of hydrophobic BA accumulation, in perpetuating PBC damage, is also indirectly supported by the beneficial results obtained when the BA pool is supplemented with less detergent and more hydrophilic BA such as UDCA or when their synthesis is inhibited with OCA.
Figure 1 From immune mediated damage to bile acids injury.
In primary biliary cholangitis the inflammatory process induces a decrease in chloride-bicarbonate exchangerexpression. This in turn determines a reduction of bicarbonate film (bicarbonate umbrella) on the canalicular portion of bile duct cells, exposing them to hydrophobic bile acids injury. AE2: Chloride-bicarbonate exchanger.
Changes in proliferative processes
Bile duct proliferation is a complex process, characterized by important changes during pathological conditions. As observed with respect to the secretive process, intracellular cAMP levels also play a key role in modulating biliary growth[41]. After enhanced cAMP formation, the PKA/Sc/MEK/ERK1/2 axis is activated in order to stimulate cellular proliferation[42]. Several gastro-intestinal hormones and neuropeptides may enhance cholangiocyte proliferation using the cAMP route (such as Sec, acetylcholine, serotonin, histamine, etc.), while others (such as gastrin and somatostatin) may negatively regulate this pathway, obtaining the opposite effect[43]. Finally, BA are also important key molecules for biliary growth[44], interacting with specific cholangiocyte receptors. For example, the interaction of BA with the cholangiocyte Takeda G protein-coupled receptor 5 modulates proliferation, with an opposite effect in ciliated and nonciliated cells[45]. From a morphological point of view, three different proliferative pictures can be recognized in the biliary tree[46]. Type I 'typical' proliferation is observed in humans after high-grade acute biliary obstruction or in the early phase of chronic cholestatic disease, including PBC. In Type I, biliary proliferation is restricted to portal spaces and characterized by ducts with a preserved architecture and orientation. In Type 2 'atypical' proliferation, the extension of truncated, poorly organized, and possibly nonfunctional ducts, with an indefinite lumen, is observed in the liver parenchima, usually coupled with enhanced inflammatory processes. Type II proliferation is generally observed in the more advanced stage of PBC and may represent the introductive step toward the progressive loss of bile ducts (ductopenia) found in the late phase of the disease. Finally, Type III 'oval cell' proliferation is also reported. This irregular proliferation (distorted duct with architectural changes in the liver parenchima) is observed in the early phase of experimental carcinogenesis and may have a relationship with the onset of cholangiocarcinoma, as it originates from multipotent (oval) cells[44,46]. As mentioned above and according to this morphological classification, the repairing process during PBC: (1) Would stimulate biliary growth that; (2) may evolve from a typical phase to an atypical phase, and possibly; and (3) may lead to a ductopenic end stage. This complex cellular/molecular process is morphologically recapitulated in the so-called 'ductular reaction (DR)', which probably promotes the evolution towards liver fibrosis and duct loss, both characteristic features of end-stage PBC.
Mixing biliary proliferation, fibrosis, and cellular senescence(CS): The DR
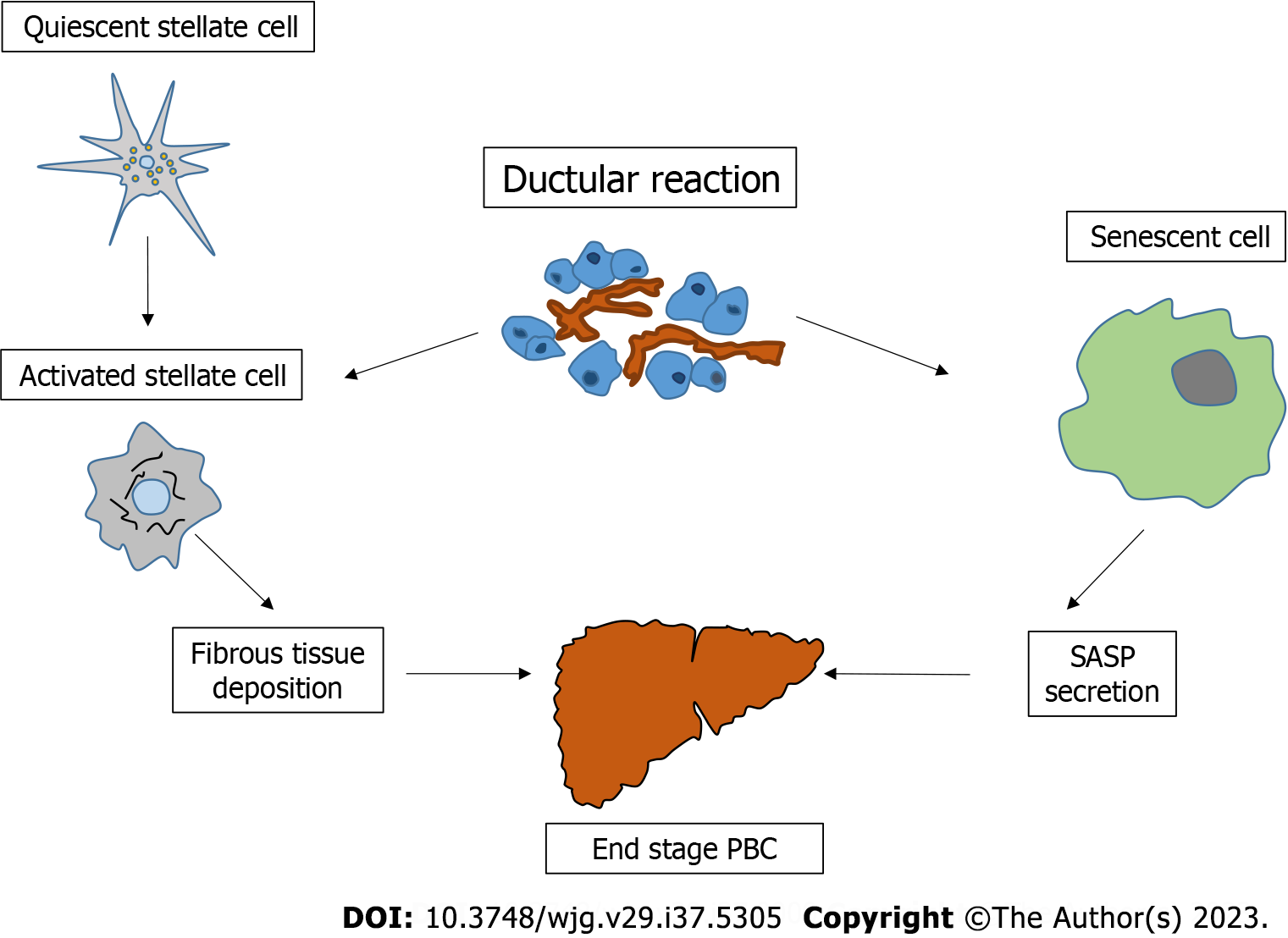
The term DR was coined and is generally preferred to proliferation in liver pathology, since it implies a reactive expansion of the ductular phenotype that may or may not derive from a ductal origin[47]. This suggested definition comes from evidence reporting the presence of inflammatory cells, as well as cells with an intermediate progenitor phenotype, in the DR microenvironment. Although DR may be detected in various liver disorders, it seems particularly frequent and widespread in chronic cholestatic diseases, compared to the normal liver[48,49]. The pathogenetic role of DR in the course of chronic cholestatic diseases has not yet been fully elucidated. However, some experimental data suggest that inhibition of biliary growth in bile duct ligated mice also negatively regulates hepatic stellate cells (HSC) activation and fibrous tissue deposition, suggesting a close link between DR and the onset of liver fibrosis[50]. On the other hand, DR has also been associated with CS[51]. CS refers to a cell that shows permanent G1 phase growth arrest. This cell acquires a so-called senescence-associated secretory phenotype that has been implicated in the pathogenesis of cholangiopathies[52]. With regard to PBC, CS is largely observed within biliary cells and its occurrence (since the possible replacement of vital cells with senescent ones) has been related to the evolution toward ductopenia[53]. In agreement with this view, CS has generally been observed in the late stage of PBC. On the other hand, autophagy, likely supported by the impairment of the bicarbonate umbrella[54], is more frequent in the early phase of the disease, suggesting that this last cellular event may be a possible trigger for CS[55].
As described above, it is clear how the PBC liver represents a pathology compendium in which several cellular/molecular determinants (inflammation, changes in biliary secretion, HSC activation, CS, autophagy, etc.) combine to support the injury. The common field in which all these events are recapitulated or prompted could possibly be represented by DR. In this perspective, we emphasize that DR began to attract the attention of pathologists more than 60 years ago, due to its characteristic features and its relationship with human liver injury[56]. In PBC, a recent clinical study again demonstrated DR as the most relevant pathological characteristic in predicting the stage of the disease, fibrosis progression, and response to UDCA therapy[57]. Unfortunately, most of the research focusing on DR and its relationship with other liver pathological features (such as fibrosis or cell senescence) is mainly descriptive. Mechanistic studies are needed to reveal the molecular pathways that trigger the sequential cascade of pathological events in PBC. In Figure 2 the relationship between DR and other pathological processes during PBC, is depicted.
Figure 2 Central role of ductular reaction in the progression of primary biliary cholangitis.
Ductular reaction contributes to the activation of hepatic stellate cells and simultaneously favours a cell senescent phenotype. These events support the ductopenic/cirrhotic evolution of liver tissue during this cholestatic disease. PBC: Primary biliary cholangitis; SASP: Senescence-associated secretory phenotype.
CONCLUSION
Several sequential molecular/cellular events may occur to develop end-stage PBC. While the presence of the principal predisposing factors (family inheritance or gender) should not be modified, a deep knowledge of the mechanisms involved in the onset and perpetuation of the disease can reveal possible therapeutic targets. However, to decipher the progression of injury during PBC, research cannot be restricted to the study of the immune-inflammatory process, since important physiological and pathological changes in cholangiocytes also contribute to liver damage. To support this view, clinical studies approaching PBC with immunosuppressive or anti-inflammatory drugs, which are usually effective in other autoimmune disorders, gave limited results in these patients[8]. In this perspective and as observed in other liver diseases (such as alcoholic and nonalcoholic steato-hepatitis)[58], multiple hits are likely needed in PBC to develop severe liver damage. Therefore, in an attempt to design a possible pathological route leading to this disorder, a series of events linking together should be considered. As reported in Figure 1 and described in this minireview, the autoimmune process not only promotes inflammation, but also radically changes the secretive and proliferative activities of bile duct cells. Regarding biliary secretion, decreased expression of AE2 negatively regulates biliary flow and bicarbonate secretion. This in turn allows BA cytolitic damage from the impairment of the bicarbonate umbrella. Parallel to this, biliary growth is enhanced in an attempt to balance cellular loss. The chronic enhancement of proliferative activities is recapitulated in the pathological finding of the DR. This represents a microenvironment in which CS and profibrotic processes originate, leading to ductopenic/fibrotic end-stage liver injury (Figure 2). While previous reviews consist in a conventional broad description of PBC or consider a specific aspect of the disorder (genetic, immunology, diagnosis, therapy, etc.), in this manuscript we aimed: (1) To design with clarity the sequential steps allowing the PBC evolution from liver inflammation to end-stage fibrosis; and (2 )to examine and define in detail the key consecutive molecular/cellular events supporting this path. We believe that deciphering the natural history of PBC as a step by step route, may facilitate the identification of important therapeutic targets. It is clear that several pieces are still lacking to complete the pathogenetic puzzle of this disease. In this sense, studies that examine in detail the molecular pathways that lead to different changes/responses of cholangiocytes may improve our knowledge of PBC and should be helpful in developing new pharmacological treatments.