Published online Sep 21, 2023. doi: 10.3748/wjg.v29.i35.5166
Peer-review started: July 2, 2023
First decision: July 14, 2023
Revised: July 22, 2023
Accepted: August 31, 2023
Article in press: August 31, 2023
Published online: September 21, 2023
Processing time: 74 Days and 4.6 Hours
The clinical and histological features of chronic hepatitis B (CHB) patients who fall into the "grey zone (GZ)" and do not fit into conventional natural phases are unclear.
To explore the impact of varying the threshold of alanine aminotransferase (ALT) levels in identifying significant liver injury among GZ patients.
This retrospective analysis involved a cohort of 1617 adult patients diagnosed with CHB who underwent liver biopsy. The clinical phases of CHB patients were determined based on the European Association for the Study of the Liver 2017 Clinical Practice Guidelines. GZ CHB patients were classified into four groups: GZ-A (HBeAg positive, normal ALT levels, and HBV DNA ≤ 107 IU/mL), GZ-B (HBeAg positive, elevated ALT levels, and HBV DNA < 104 or > 107 IU/mL), GZ-C (HBeAg negative, normal ALT levels, and HBV DNA ≥ 2000 IU/mL), and GZ-D (HBeAg negative, elevated ALT levels, and HBV DNA ≤ 2000 IU/mL). Significant hepatic injury (SHI) was defined as the presence of notable liver inflammation (≥ G2) and/or significant fibrosis (≥ S2).
The results showed that 50.22% of patients were classified as GZ, and 63.7% of GZ patients developed SHI. The study also found that lowering the ALT treatment thresholds to the American Association for the Study of Liver Diseases 2018 treatment criteria (35 U/L for men and 25 U/L for women) can more accurately identify patients with significant liver damage in the GZ phases. In total, the proportion of patients with ALT ≤ 40 U/L who required antiviral therapy was 64.86% [(221 + 294)/794]. When we lowered the ALT treatment threshold to the new criteria (30 U/L for men and 19 U/L for women), the same outcome was revealed, and the proportion of patients with ALT ≤ 40 U/L who required antiviral therapy was 75.44% [(401 + 198)/794]. Additionally, the proportion of SHI was 49.1% in patients under 30 years old and increased to 55.3% in patients over 30 years old (P = 0.136).
These findings suggest the importance of redefining the natural phases of CHB and using new ALT treatment thresholds for better diagnosis and management of CHB patients in the GZ phases.
Core Tip: In clinical practice, 27.8%-55% of chronic hepatitis B patients fall into the “grey zone” or “indeterminate phase” that does not meet the diagnostic criteria of the traditional stages. Additionally, there is still debate regarding how best to treat these grey zone (GZ) patients and the advantages of antiviral therapy. Hence, we evaluated the clinical and histological characteristics, and additionally explored the impact of adjusting the threshold of alanine aminotransferase (ALT) in identifying significant liver injury among GZ patients. Based on these data, lowering ALT thresholds can more accurately identify patients with significant hepatic injury at an earlier stage and reduce the need for unnecessary liver biopsies.
- Citation: Yu HS, Jiang H, Li MK, Yang BL, Smayi A, Chen JN, Wu B, Yang YD. Lowering the threshold of alanine aminotransferase for enhanced identification of significant hepatic injury in chronic hepatitis B patients. World J Gastroenterol 2023; 29(35): 5166-5177
- URL: https://www.wjgnet.com/1007-9327/full/v29/i35/5166.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i35.5166
Hepatitis B poses a significant global public health challenge, as evidenced by the estimated 316 million individuals worldwide who were afflicted with the hepatitis B virus (HBV) in 2019. The impact of HBV-related diseases is substantial, as they result in approximately 555000 fatalities globally, which constitutes 48.8% of all hepatitis-related deaths. Notably, hepatitis B stands as the primary cause of mortality in cases of liver cancer and ranks as the third leading cause of death in cirrhosis cases[1]. The progression of chronic hepatitis B (CHB) is a multifaceted interplay involving viral, host, and environmental factors. HBV interacts with the immune system of the host, and the infection status undergoes continuous changes as the disease progresses[2,3]. According to the European Association for the Study of the Liver (EASL) 2017 Clinical Practice Guidelines, CHB can be categorized into five phases: HBeAg-positive chronic HBV infection, HBeAg-positive CHB, HBeAg-negative chronic HBV infection, HBeAg-negative CHB and HBsAg-negative phase[4]. Antiviral treatment is recommended for patients with HBeAg-positive and HBeAg-negative CHB, while regular monitoring is suggested in HBeAg-positive and HBeAg-negative chronic HBV infection phases[4-6].
Nevertheless, in clinical practice, a considerable proportion of CHB patients (27.8%-55%) fall into the “grey zone (GZ)” or “indeterminate phase” that does not meet the diagnostic criteria for the five stages previously indicated[7-9]. Additionally, there is still debate regarding how best to treat these GZ patients and the advantages of antiviral therapy. A study from the United States showed the incidence of hepatocellular carcinoma (HCC) is higher in GZ patients than in HBeAg-negative chronic HBV infection patients (2.67% vs 0.61%)[7]. Furthermore, Huang et al[10] demonstrated a significant decrease in the risk of HCC among GZ patients who underwent antiviral therapy[10]. In contrast, another study found that none of the patients progressed to advanced fibrosis or cirrhosis, and only a small proportion (6.3%) of GZ patients transitioned to HBeAg-negative CHB, necessitating the use of antiviral therapy[11].
There is a crucial indication for receiving antiviral therapy when patients with CHB have significant liver fibrosis and/or inflammation, which are risk factors for HCC and liver-related mortality[4-6]. However, few studies have explored liver histological injury in the GZ phase[12]. Therefore, assessing the clinical and histological features may provide useful recommendations for managing the GZ phase. Moreover, an issue that complicates the management of CHB is the disagreement regarding the appropriate treatment threshold for alanine aminotransferase (ALT) levels. The American Association for the Study of Liver Diseases (AASLD) guidelines suggest that the upper normal limit (ULN) for ALT should be 35 U/L for men and 25 U/L for women, while the EASL guidelines consider 40 U/L as the ULN[4,5]. In China, a recent publication titled "Expert opinion on expanding anti-HBV treatment for chronic hepatitis B" proposed a lower ALT threshold (30 U/L for males and 19 U/L for females) as initiating antiviral therapy in CHB patients[13]. However, the efficacy of reducing ALT thresholds in accurately identifying patients with significant liver damage at an earlier stage and mitigating the necessity for superfluous liver biopsy remains uncertain.
Therefore, using a retrospective cohort of treatment-naive CHB patients who underwent liver biopsy, we evaluated the clinical and histological characteristics, and additionally explored the impact of adjusting the threshold of ALT in identifying significant liver injury among GZ patients.
Patient selection: Between January 2008 and December 2020, a total of 1617 consecutive adult patients (age, ≥ 18 years) diagnosed with CHB (hepatitis B surface antigen positive > 6 mo) who had undergone liver biopsy at the Third Affiliated Hospital of Sun Yat-Sen University were included in this retrospective analysis. The exclusion criteria were as follows: (1) Viral coinfection (hepatitis C virus, hepatitis D virus, or HIV); (2) Alcohol abuse (≥ 30 g of alcohol per day for men, ≥ 20 g of alcohol per day for women), nonalcoholic fatty liver disease (diagnosed by liver biopsy) and autoimmune liver disease; (3) Decompensated cirrhosis, HCC, and nonliver cancer; (4) Liver transplantation; and (5) Prior or current antiviral treatment (Supplementary Figure 1). The study was approved by the Ethics Committee of The Third Affiliated Hospital of Sun Yat-sen University. Written informed consent was obtained from each participant prior to the liver biopsy.
Definitions: The clinical phases of patients with CHB were determined in accordance with the EASL 2017 clinical practice guidelines, taking into consideration the highest HBV-DNA levels and ALT levels observed in at least two determinations within the 12 mo preceding enrolment (Supplementary Table 1)[4]. Patients who did not meet the criteria for any of the five phases were classified as GZ, with subcategories including GZ-A (HBeAg positive, normal ALT levels, and HBV DNA ≤ 107 IU/mL), GZ-B (HBeAg positive, elevated ALT levels, and HBV DNA < 104 or > 107 IU/mL), GZ-C (HBeAg negative, normal ALT levels, and HBV DNA ≥ 2000 IU/mL), and GZ-D (HBeAg negative, elevated ALT levels, and HBV DNA ≤ 2000 IU/mL)[9,12]. We used ALT and gamma-glutamyl transpeptidase (GGT) levels of 40 U/L and 60 U/L as the ULN, respectively[4]. The calculations were formulated as follows: Aspartate aminotransferase (AST)-to-platelet ratio index (APRI) = [AST (U/L)/ULN]/[PLT (109/L)] × 100; fibrosis score based on four factors (FIB-4) = [age (years) × AST (U/L)]/[PLT (109/L) × √ALT (U/L)]; GGT-to-platelet ratio (GPR) = [GGT (U/L)/ULN]/[PLT (109/L)] × 100.
Histological assessment: Ultrasonography-guided percutaneous liver biopsy was conducted using a 16-gauge disposable needle. The minimum sample length required was 15 mm, with a minimum inclusion of 6 portal tracts. Inflammation grade (G0-G4) and fibrosis stage (S0-S4) were estimated according to Scheuer's classification[14]. In accordance with the pathological staging system, significant liver inflammation and significant fibrosis were defined as ≥ G2 and ≥ S2, respectively. Significant hepatic injury (SHI) was defined as ≥ G2 and/or ≥ S2[12,15]. The biopsy samples were subjected to blind and independent observation and interpretation by two proficient pathologists. In cases where discordance arose between the two pathologists, a third pathologist, Jianning Chen, was consulted for additional evaluation, leading to a consensus being achieved through subsequent discussion.
SPSS version 25.0 software (SPSS Inc., Chicago, IL, United States) was used for statistical analyses. Continuous variables are expressed as the median and interquartile range, and categorical data are expressed as counts and percentages. Kruskal-Wallis tests and Pearson’s chi-squared tests were applied to compare variables that were significantly different between groups. The independent predictors of SHI were determined by univariate and multiple logistic regression analyses. Areas under the receiver operating characteristic curve (AUROC) were calculated to investigate the diagnostic performance of the noninvasive scores and were compared by the Delong test. All P values were two-sided, and P < 0.05 was deemed statistically significant.
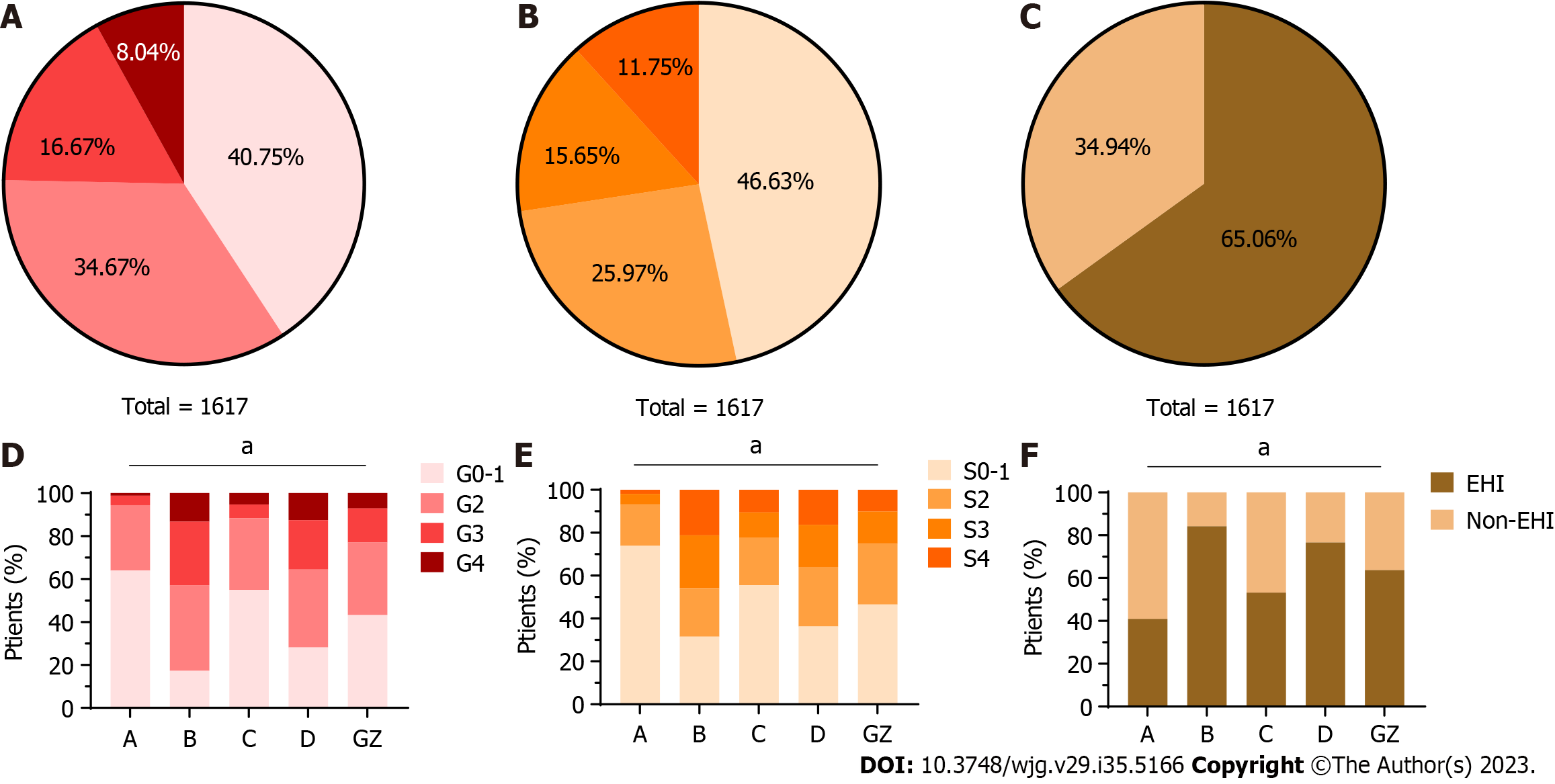
The baseline characteristics of the 1617 treatment-naive patients are presented in Table 1. Based on the defined criteria, 161 (9.96%) patients were classified as having HBeAg-positive chronic HBV infection, 203 (12.55%) patients were categorized as HBeAg-positive CHB, 171 (10.58%) patients were identified as having HBeAg-negative chronic HBV infection, and 270 (16.70%) patients were classified as HBeAg-negative CHB. Interestingly, 812 (50.22%) patients did not meet the criteria for any of the aforementioned phases and were therefore designated as GZ. Notably, there were significant variations in clinical characteristics across the different phases. The average age of patients with GZ was 36.0 years, with 74.1% being male. These patients had a mean HBV DNA level of 5.24 Log10 IU/mL and an intermediate ALT level of 37.0 U/L.
| Clinical characteristics | HBeAg-positive chronic infection (n = 161) | HBeAg-positive chronic hepatitis (n = 203) | HBeAg-negative chronic infection (n = 171) | HBeAg-negative chronic hepatitis (n = 270) | Grey zone (n = 812) | P value |
| Age (year) | 31.0 (26.0-36.0) | 33.0 (27.0-39.0) | 37.0 (32.0-42.0) | 39.0 (33.0-45.0) | 36.0 (30.0-42.0) | < 0.001 |
| Male (%) | 97 (60.2) | 168 (82.8) | 133 (77.8) | 221 (81.9) | 602 (74.1) | < 0.001 |
| BMI (kg/m2) | 21.3 (19.1-23.0) | 22.5 (20.3-24.9) | 22.6 (20.3-24.5) | 22.8 (20.4-25.1) | 22.0 (20.0-24.4) | < 0.001 |
| Diabetes (%) | 0 (0) | 3 (1.5) | 4 (2.4) | 10 (3.7) | 14 (1.7) | 0.071 |
| HBV DNA (log10 IU/mL) | 8.12 (7.60-8.23) | 6.17 (5.38-6.61) | 2.52 (2.00-2.89) | 5.47 (4.50-6.31) | 5.24 (3.92-7.50) | < 0.001 |
| PLT (109/L) | 211.0 (179.5-238.0) | 185.0 (144.0-217.0) | 191.0 (156.0-230.0) | 185.0 (154.8-219.3) | 199.0 (160.0-234.0) | < 0.001 |
| ALT (U/L) | 27.0 (21.0-33.0) | 69.0 (51.0-111.0) | 25.0 (19.0-31.0) | 61.5 (50.0-100.3) | 37.0 (27.0-59.0) | < 0.001 |
| AST (U/L) | 24.0 (21.0-31.0) | 48.0 (36.0-73.0) | 24.0 (20.0-29.0) | 44.0 (33.0-66.0) | 31.0 (24.0-45.0) | < 0.001 |
| GGT (U/L) | 18.0 (14.0-30.0) | 51.0 (30.0-93.0) | 25.0 (17.0-36.0) | 40.0 (27.8-80.5) | 29.0 (20.0-50.0) | < 0.001 |
| Tbil (μmol/L) | 12.7 (9.3-17.6) | 14.1 (11.0-19.5) | 12.6 (9.9-16.5) | 14.4 (10.3-21.6) | 13.0 (9.6-18.0) | < 0.001 |
| ALB (g/L) | 45.2 (43.3-47.6) | 44.1 (41.0-46.2) | 45.8 (43.7-48.0) | 44.5 (41.2-46.9) | 44.9 (42.3-47.0) | < 0.001 |
| AFP (ng/mL) | 2.4 (1.7-3.5) | 5.1 (3.1-14.6) | 2.3 (1.6-3.8) | 3.8 (2.5-8.0) | 3.1 (2.1-5.3) | < 0.001 |
| PT (s) | 13.4 (13.0-13.8) | 13.4 (12.8-14.1) | 13.4 (12.9-14.0) | 13.5 (12.9-14.1) | 13.4 (12.9-14.0) | 0.073 |
| APRI | 0.29 (0.24-0.39) | 0.69 (0.47-1.12) | 0.32 (0.24-0.43) | 0.63 (0.42-0.99) | 0.40 (0.28-1.27) | < 0.001 |
| FIB-4 | 0.72- (0.55-1.08) | 1.05 (0.71-1.55) | 0.92 (0.71-1.33) | 1.18 (0.77-1.81) | 0.93 (0.67-1.40) | < 0.001 |
| GPR | 0.23 (0.16-0.30) | 0.71 (0.39-1.44) | 0.32 (0.22-0.52) | 0.56 (0.34-1.23) | 0.37 (0.23-0.68) | < 0.001 |
| Inflammation | < 0.001 | |||||
| G0-1 | 103 (64.0%) | 35 (17.2%) | 94 (55.0%) | 76 (28.1%) | 351 (43.2%) | |
| ≥ G2 | 58 (36.0%) | 168 (82.8%) | 77 (45.0%) | 194 (71.9%) | 461 (56.8%) | |
| Fibrosis | < 0.001 | |||||
| S0-1 | 119 (73.9%) | 64 (31.5%) | 95 (55.6%) | 98 (36.3%) | 378 (46.6%) | |
| ≥ S2 | 42 (26.1%) | 139 (68.5%) | 76 (44.4%) | 172 (63.7%) | 434 (53.4%) | |
| SHI | < 0.001 | |||||
| No | 95 (59.0%) | 32 (15.8%) | 80 (46.8%) | 63 (23.3%) | 295 (36.3%) | |
| Yes | 66 (41.0%) | 171 (84.2%) | 91 (53.2%) | 207 (76.7%) | 517 (63.7%) |
The clinical and liver histological characteristics are presented in Table 1 and Figure 1. In the GZ group, the proportion of significant liver inflammation (≥ G2) was 56.8%, and the proportion of significant fibrosis (≥ S2) was 53.4%. Among GZ patients, 63.7% had SHI. Higher proportions of SHI were observed in HBeAg-positive (84.2%) and HBeAg-negative (76.7%) CHB patients. However, HBeAg-positive (41.0%) and HBeAg-negative chronic HBV infection (53.2%) had lower but still relatively high proportions of SHI.
The clinical characteristics of 812 GZ patients are shown in Supplementary Table 2. Among these patients, the proportion of GZ-C was the highest (41.1%), followed by GZ-B (34.6%), GZ-A (15.8%), and GZ-D (8.5%). Notably, patients in the GZ-A (35.0 years) and GZ-B (31.0 years) subgroups were younger than those in the GZ-C (40.0 years) and GZ-D (36.0 years) subgroups. Furthermore, higher HBV DNA levels were observed in GZ-B (7.86 Log10 IU/mL), followed by GZ-A (5.39 Log10 IU/mL), GZ-C (4.40 Log10 IU/mL), and GZ-D (2.40 Log10 IU/mL).
As shown in Supplementary Figure 2, HBeAg-positive GZ patients exhibited a significantly higher rate of significant liver inflammation (≥ G2) (66.1% vs 47.4%, P < 0.001) and SHI (70.4% vs 56.8%, P < 0.001) than HBeAg-negative GZ patients. However, there was no statistically significant difference in fibrosis stages between HBeAg-positive and HBeAg-negative GZ patients (55.8% vs 51.2%, P = 0.186). The distributions of liver inflammation grades, fibrosis stages, and SHI are shown in Supplementary Table 2 and Supplementary Figure 2. The highest prevalence of significant liver inflammation (≥ G2) was observed in GZ-B (66.5%), while GZ-D (62.3%) had the highest proportion of significant fibrosis (≥ S2). In terms of SHI, the highest proportion was found in patients from GZ-B (70.5%), followed by GZ-A (70.3%), GA-D (69.6%), and GZ-C (54.2%).
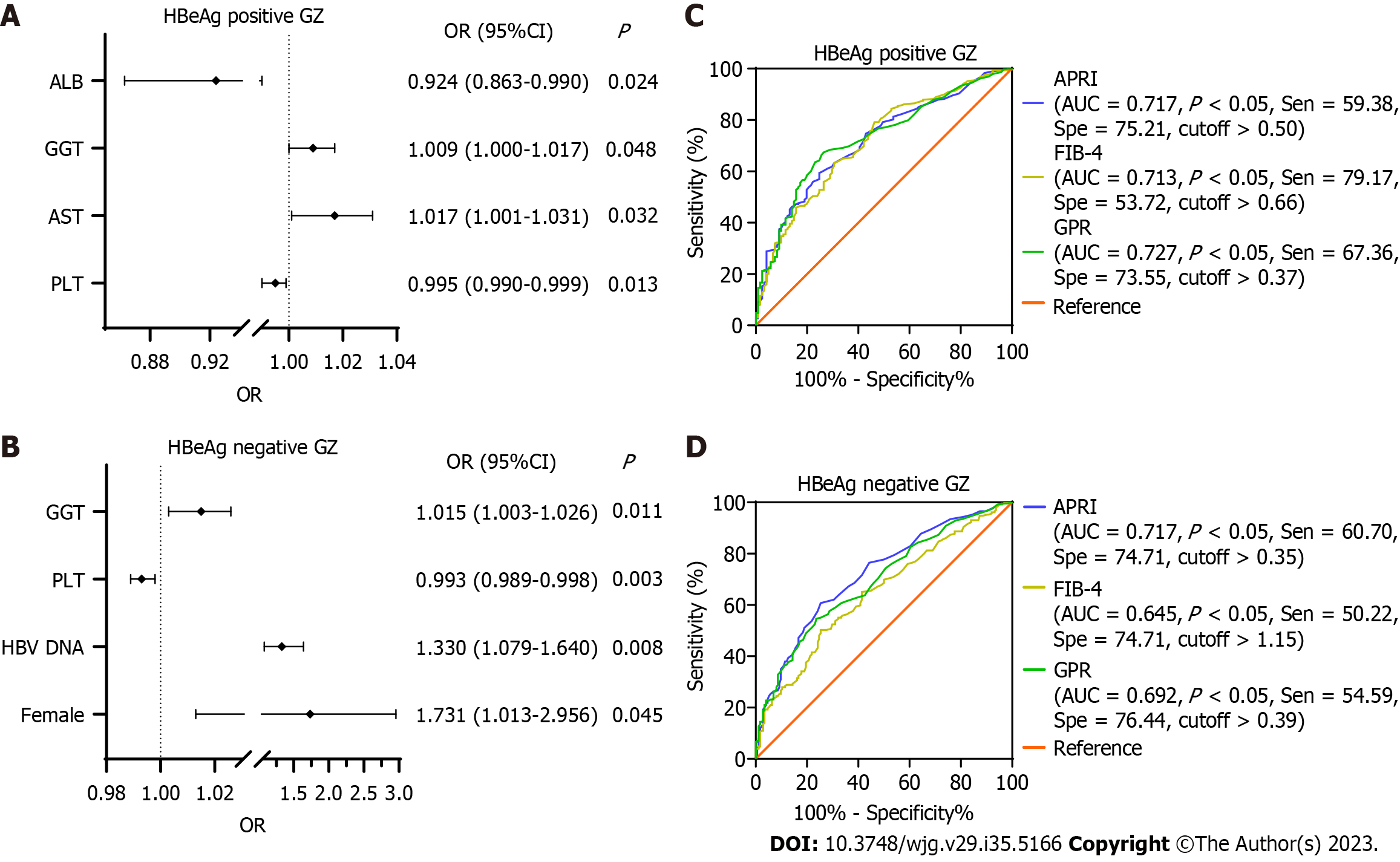
SHI was observed in 517 (63.7%) GZ patients. Of those, 288 were HBeAg-positive GZ patients, and 229 were HBeAg-negative GZ patients. In the HBeAg-positive cohort, univariate analysis indicated that PLT, ALT, AST, GGT, total bilirubin (Tbil), albumin (ALB), and prothrombin time(PT) were associated with SHI. However, multiple logistic regression analysis indicated that only PLT, AST, GGT, and ALB remained significantly associated with SHI. For the HBeAg-negative cohort, female sex, HBV-DNA, ALT, AST, GGT, Tbil, and PT were associated with higher SHI, whereas PLT and ALB were negatively associated with this event by univariate analysis. By multiple logistic regression analysis, female sex, HBV-DNA, GGT, and PLT were associated with SHI (Supplementary Table 3 and Figure 2).
Further investigation was conducted to assess the diagnostic efficacy of established scoring systems such as APRI, FIB-4, and GPR in predicting SHI (Figure 2). The AUROCs for these three tests showed no significant difference in the HBeAg-positive GZ (P > 0.05). However, in HBeAg-negative GZ, APRI demonstrated superior performance compared to FIB-4 (P < 0.001) and was comparable to GPR (P = 0.30).
To examine the significance of lowering the treatment threshold for ALT, a total of 794 patients with normal ALT levels (ULN ≤ 40 U/L) were chosen for further investigation. The distribution of their immune states was as follows: 161 (20.28%) patients had HBeAg-positive chronic HBV infection, 128 (16.12%) patients fell within GZ-A, 171 (21.54%) patients had HBeAg-negative chronic HBV infection, and 334 (42.07%) patients were categorized under GZ-C.
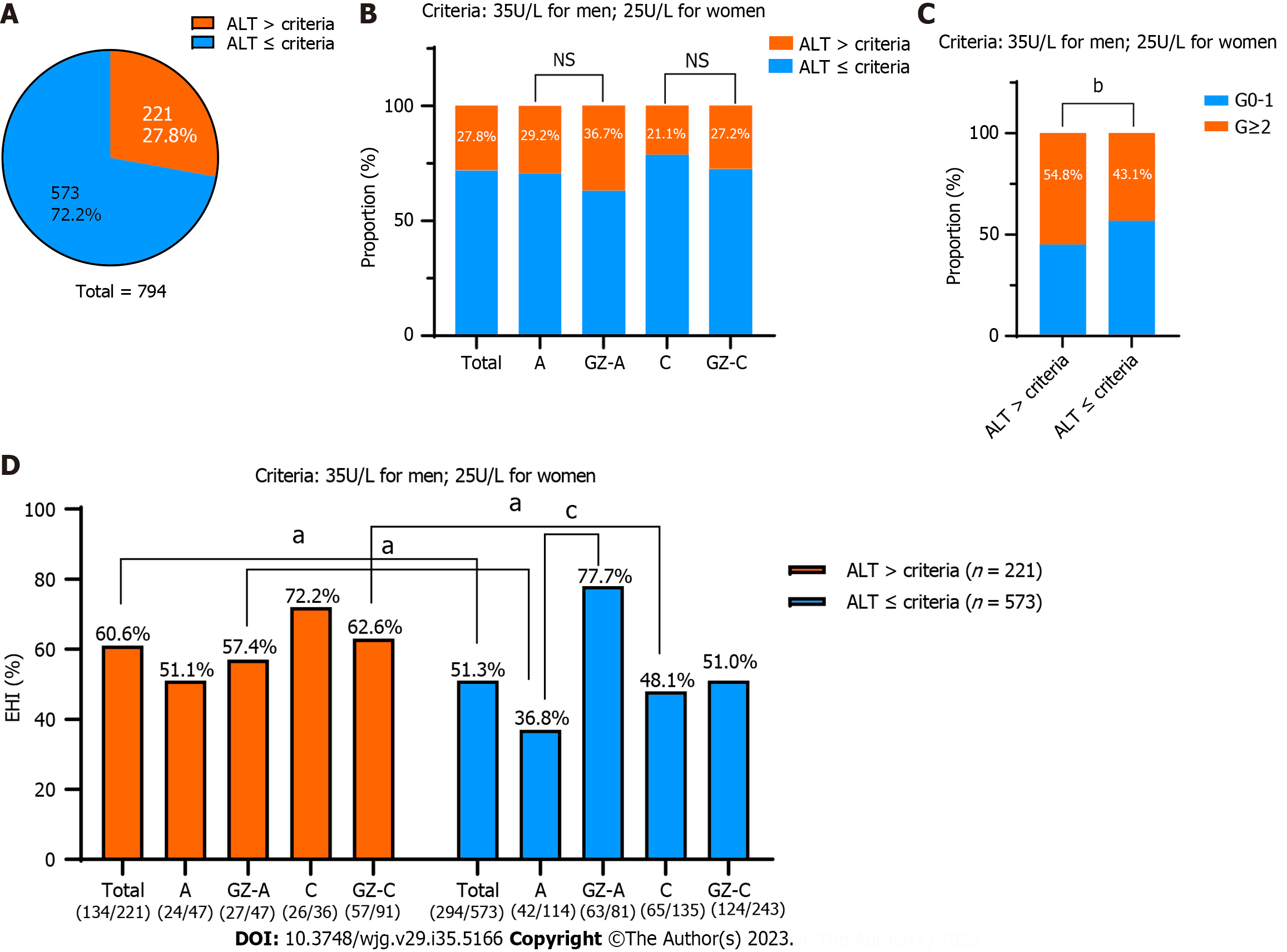
Based on the AASLD 2018 criteria of the ALT antiviral treatment threshold (35 U/L for males and 25 U/L for females), we subsequently evaluated the ratio of SHI in different groups. Among the 794 chronic HBV infections, more than one-quarter of them (221/794, 27.8%) were above the AASLD criteria (Figure 3A). Of these 221 individuals, 29.2% (47/161) with HBeAg-positive chronic HBV infection, 36.7% (47/128) with GZ-A, 21.1% (36/171) with HBeAg-negative chronic HBV infection, and 27.2% (91/334) with GZ-C were above this ALT threshold (Figure 3B). It is worth noting that 54.8% (121/221) of patients had significant liver inflammation (≥ G2), which was significantly higher than that of patients below the ALT threshold (43.1%, 247/573) (P = 0.003) (Figure 3C). In addition, the proportion of SHI in the high ALT group was significantly higher than that in the low ALT group (60.6% vs 51.3%, P = 0.018). In total, the proportion of patients with ALT ≤ 40 U/L who required antiviral therapy was 64.86% [(221 + 294)/794] according to the AASLD 2018 Clinical Practice Guidelines.
Obviously, the SHI value in patients with HBeAg-positive chronic HBV infection below the new ALT threshold was substantially lower than that of GZ-A patients above the ALT threshold (36.8% vs 57.4%, P = 0.016). The former was in the “truly” HBeAg-positive chronic HBV infection group due to high HBV DNA levels and low ALT levels. Similarly, GZ-C patients had a significantly higher SHI ratio than the “truly” HBeAg-negative chronic HBV infection patients (62.6% vs 48.1%, P = 0.032) (Figure 3D).
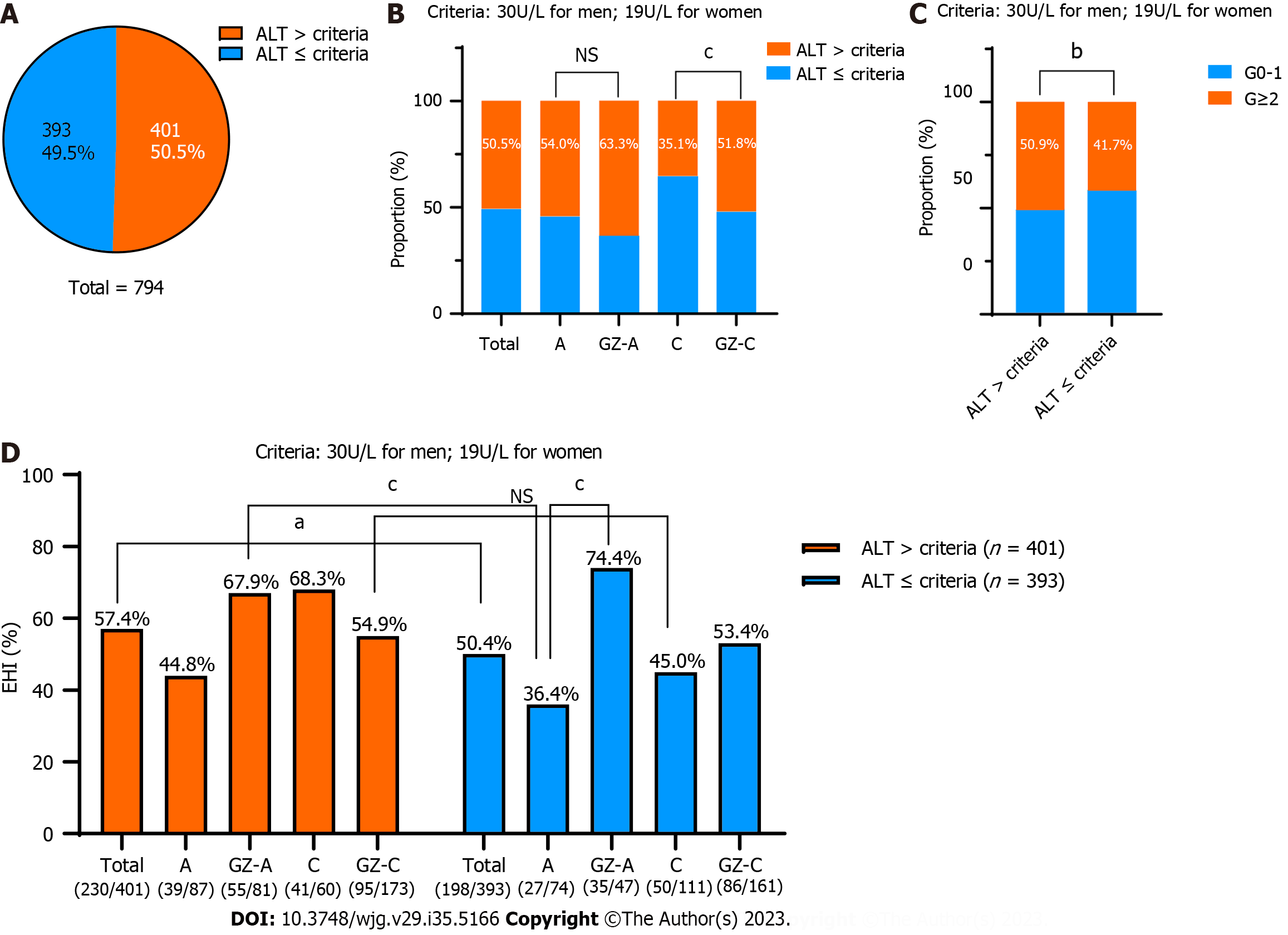
According to the new recommendations for the treatment threshold of ALT (30 U/L for males and 19 U/L for females), we investigated the rate of SHI in different groups separately. Among the 794 chronic HBV infections, nearly half of them (393/794, 49.5%) were below the new criteria (Figure 4A). Among these 393 patients, 46.0% (74/161) with HBeAg-positive chronic HBV infection, 36.7% (47/128) with GZ-A, 64.9% (111/171) with HBeAg-negative chronic HBV infection and 48.2% (161/334) with GZ-C were below this ALT threshold (Figure 4B). Notably, the proportions of significant liver inflammation (≥ G2) and SHI in patients above the ALT threshold were significantly higher than those in patients below the ALT threshold (50.9% vs 41.7%, P = 0.01; 57.4% vs 50.4%, P = 0.049) (Figure 4C). In total, the proportion of patients with ALT ≤ 40 U/L who required antiviral therapy was 75.44% [(401 + 198)/794] according to the “expert opinion on expanding anti-HBV treatment for chronic hepatitis B” in China.
The SHI values in patients with HBeAg-positive chronic HBV infection below the new ALT threshold was 36.4%. However, higher SHI values of 67.9% were seen in the GZ-A patients above the new ALT threshold, and the difference was statistically significant (P < 0.001). However, there was no significant difference in the proportion of SHI between patients with HBeAg-negative chronic HBV infection below the new ALT threshold and GZ-C patients above the new ALT threshold (45.0% vs 54.9%, P = 0.105) (Figure 4D).
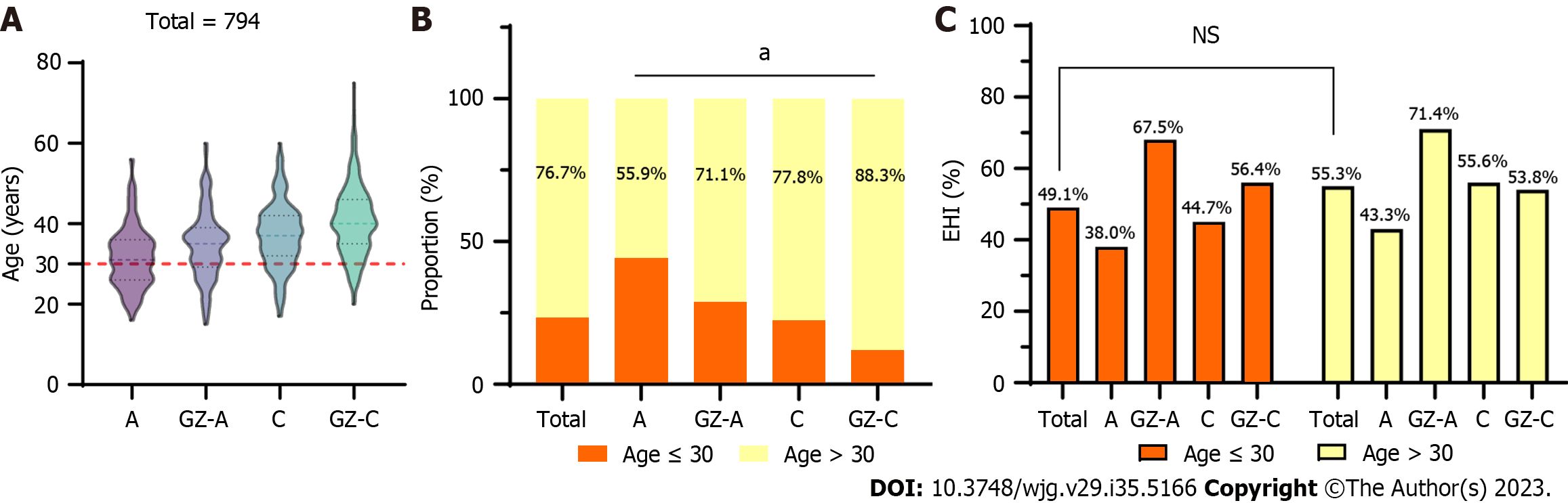
The median age was 31, 35, 37, and 40 years for HBeAg-positive chronic HBV infection, GZ-A, HBeAg-negative chronic HBV infection, and GZ-C patients, respectively. There was an increasing trend of age in these states (Figure 5A). Among 794 patients, 76.7% (609/794) were > 30 years old, and almost 70% were HBeAg-negative patients, including 77.8% with HBeAg-negative chronic HBV infection and 88.3% with GZ-C (Figure 5B). The ratio of SHI in patients ≤ 30 years old was 49.1%, and it increased to 55.3% for those patients > 30 years old. However, there was no significant difference (P = 0.136). A similar result was observed in all states regardless of whether they were older or younger than 30 years old (P > 0.05) (Figure 5C).
This retrospective cohort study examined a group of CHB patients who underwent liver biopsy at the Third Affiliated Hospital of Sun Yat-Sen University. The study showed that 50.22% of the patients with HBV infection fell into the GZ category, with 56.8% and 53.4% having significant liver inflammation (≥ G2) and fibrosis (≥ S2), respectively. More than half of the patients (63.7%) in the GZ category exhibited SHI, which was less than the proportion observed in HBeAg-positive and HBeAg-negative chronic hepatitis patients but more severe than those in the HBeAg-positive and HBeAg-negative chronic infection categories. While current guidelines do not require urgent antiviral therapy for GZ patients[4-6], the study findings indicated that HBeAg-positive and HBeAg-negative chronic HBV infections had relatively high proportions of SHI. The proportions were higher than those of a meta-analysis, which indicated the prevalence of significant fibrosis for chronic HBV infection as 16.9% (95%CI: 7.8-26.1) for HBeAg-positive and 24.8% (95%CI: 4.5-45.1) for HBeAg-negative chronic HBV infection[16]. This may be because the population included in the study is Asian and the genotypes are mainly B and C. Therefore, noninvasive methods, including liver biopsy, should be considered to evaluate liver inflammation and fibrosis in these individuals[17-19].
To better direct clinical diagnosis and treatment strategies, we analysed the risk factors for SHI in GZ patients. In the HBeAg-positive cohort, multiple logistic regression analysis indicated that PLT, AST, GGT, and ALB were associated with SHI. For the HBeAg-negative cohort, female sex, HBV-DNA, GGT, and PLT were associated with SHI by multiple logistic regression analysis. Based on these risk factors, we compared the diagnostic performance of APRI, FIB-4, and GPR in predicting SHI. The AUROCs of APRI, FIB-4, and GPR were 0.717, 0.713, and 0.727, respectively, in the HBeAg-positive GZ phases and 0.717, 0.645, and 0.692, respectively, in the HBeAg-negative GZ phases. Previous studies have shown that GPR provided a significantly higher AUROC than APRI and FIB-4, implying the superiority of GPR in predicting significant liver fibrosis and cirrhosis. For diagnosing significant fibrosis, the AUROCs of GPR were 0.66-0.86 and the cut-off was 0.32-0.43[20-22]. Thus, for simplicity of use in clinical practice, we advised utilizing a GPR cut-off of 0.37 as the optimal cut-off for predicting SHI in GZ patients. Treatment should be individualized for GZ patients, especially those who are over 40 years of old, HBeAg positive, and exhibit high ALT and HBV DNA levels. The state of the GZ is not constant but should be dynamic. Periodic monitoring is particularly important.
The main purpose of this study was to investigate the effect of lowering the treatment threshold of ALT according to different clinical guidelines in identifying SHI patients with CHB virus infection in the GZ. A total of 794 patients with normal ALT levels (ULN ≤ 40 U/L) were selected for further investigation; of these patients, 53.90% (428/794) necessitated antiviral therapy. The proportion of patients with ALT ≤ 40 U/L who required antiviral therapy was 64.86% [(221 + 294)/794] according to the AASLD 2018 Clinical Practice Guidelines. Furthermore, the proportion of patients with ALT ≤ 40 U/L who required antiviral therapy was 75.44% [(401 + 198)/794] according to the “expert opinion on expanding anti-HBV treatment for chronic hepatitis B” in China.
The current criteria for determining "normal" ALT levels were established based on populations that encompassed individuals with subclinical liver disease. Prati et al[23] propose that it is prudent to reconsider the established thresholds for ALT levels in patients diagnosed with chronic HCV infection or nonalcoholic fatty liver disease[23]. Previous studies have shown that even if the ALT level is within the normal range, the ALT level correlates with the degree of liver inflammation and fibrosis. Sonneveld et al[24] showed that 52% of 168 patients without liver fibrosis and 82% of 66 patients with significant liver fibrosis with normal ALT levels had mild and moderate inflammation[24]. More importantly, even if the ALT level is within the normal range, higher ALT levels have a higher incidence of decompensated cirrhosis and HCC. Compared to patients with ALT levels < 0.5 × ULN (53 U/L and 31 U/L for males and females, respectively), patients with ALT levels of 0.5-1 × ULN had an increased risk for the development of complications including ascites, spontaneous bacterial peritonitis, oesophageal varices, encephalopathy and HCC[25]. Similarly, REVEAL-HBV research demonstrated that compared to ALT < 15 U/L, patients with ALT 15-44 U/L had an increased risk of cirrhosis (aHR = 1.97, 95%CI: 1.56-2.48) and HCC (aHR = 2.45, 95%CI: 1.74-2.48)[26]. Therefore, lowering the ALT threshold in CHB patients is conducive to early initiation of antiviral therapy, which in turn reduces the incidence of cirrhosis and HCC, especially in the GZ phases.
Current studies and guidelines recommend that age > 30 years old is an independent risk factor for disease progression and can be an indication for initiating antiviral therapy. A linear correlation between age and the mortality risk of primary liver cancer, chronic liver disease and cirrhosis, and viral hepatitis was found in those whose ages ranged from 15 years to 74 years[27]. Among 794 individuals in our study with normal ALT levels (ULN ≤ 40 U/L), 23.3% of the patients were under 30 years old. The ratio of SHI in patients ≤ 30 years old was 49.1%, and it increased to 55.3% for those patients > 30 years old. However, the difference was not significant. Huang et al[7] showed that among patients who remained indeterminate, an age ≥ 40 years (aHR = 9.06) and ≥ 45 years (aHR = 18.40) were independently associated with HCC development[7]. This suggested that setting 30 years old as a threshold is not suitable for GZ patients. We noticed that a large proportion of CHB patients ≤ 30 years old with normal ALT levels still had inflammation and fibrosis. This finding was consistent with a previous study that noted that among 432 CHB patients with normal or mildly elevated ALT who underwent liver biopsy, the inflammation and fibrosis scores increased with age. Of these patients < 30 years old, G ≥ 2 accounted for approximately 50%, and S ≥ 2 accounted for approximately 40%[28]. Hence, age may not be a limitation for initiating antiviral therapy in patients with CHB who have normal ALT levels. Instead, more individualized attention should be given to the patient's liver inflammation and fibrosis, with the aim of reducing misdiagnosis and underdiagnosis. Additionally, patients with hepatitis B who need treatment and are at risk of disease progression should be placed on antiviral therapy in a timely manner.
Nevertheless, our study has several limitations. First, selection bias could not be ruled out because this was a retrospective and cross-sectional study. Second, the proportion of patients with SHI in this cohort may be higher than the natural population because the patients were sourced from tertiary care hospitals rather than the community, and there may be a bias in the enrolled patients. Third, because the follow-up of patients after liver biopsy was insufficient, the phase transition, benefits of antiviral therapy, and prognosis of GZ patients could not be assessed. Fourth, the study was unable to obtain information on the genotypes of all hepatitis B patients, and the limited data suggest a predominance of genotype B (65%) and genotype C (33%).
In conclusion, this study showed that 50.22% of CHB patients were in the GZ, and over half of GZ patients (63.7%) had SHI. Lowering ALT thresholds can more accurately identify patients with significant liver damage at an earlier stage and reduce the need for some unnecessary liver biopsies. Furthermore, age may not be a limitation for initiating antiviral therapy in patients with CHB who have normal ALT levels. This may have significance for refining the natural history of CHB and providing supporting evidence of lowering the antiviral therapy threshold for ALT.
In clinical practice, a considerable proportion of chronic hepatitis B (CHB) patients (27.8%-55%) fall into the “grey zone (GZ)” or “indeterminate phase”. Additionally, there is still debate regarding how best to treat these GZ patients and the advantages of antiviral therapy. Moreover, an issue that complicates the management of CHB is the disagreement regarding the appropriate treatment threshold for alanine aminotransferase (ALT) levels.
To explore the impact of varying the threshold of ALT levels in identifying significant hepatic injury (SHI) among GZ patients.
Our research evaluated the clinical and histological characteristics and additionally explored the impact of adjusting the threshold of ALT in identifying significant liver injury among GZ patients.
This retrospective analysis involved a cohort of 1617 adult patients diagnosed with CHB who underwent liver biopsy. Significant hepatic injury was defined as the presence of notable liver inflammation (≥ G2) and/or significant fibrosis (≥ S2). Kruskal-Wallis tests and Pearson’s chi-squared tests were applied to compare variables that were significantly different between groups.
The study showed that 50.22% of the patients with HBV infection fell into the GZ category, and more than half of the patients (63.7%) in the GZ category exhibited SHI. The areas under the receiver operating characteristic curves of Aspartate aminotransferase-to-platelet ratio index, fibrosis score based on four factors, and gamma-glutamyl transpeptidase-to-platelet ratio in predicting SHI were 0.717, 0.713, and 0.727, respectively, in the HBeAg-positive GZ phases and 0.717, 0.645, and 0.692, respectively, in the HBeAg-negative GZ phases. Lowering the ALT treatment thresholds to the American Association for the Study of Liver Diseases 2018 treatment criteria can more accurately identify patients with significant liver damage in the GZ phases. When we lowered the ALT treatment threshold to the new criteria, the same outcome was revealed.
This study showed that 50.22% of CHB patients were in the GZ, and over half of GZ patients (63.7%) had SHI. Lowering ALT thresholds can more accurately identify patients with significant liver damage at an earlier stage and reduce the need for some unnecessary liver biopsies. Furthermore, age may not be a limitation for initiating antiviral therapy in patients with CHB who have normal ALT levels.
Further investigation is needed to determine the assessment and treatment strategy for CHB patients in the GZ phases.
We would like to thank all the participants in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Marchesini G, Italy; Sirli RLD, Romania S-Editor: Fan JR L-Editor: A P-Editor: Zhao S
| 1. | GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:796-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 416] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 2. | Croagh CM, Lubel JS. Natural history of chronic hepatitis B: phases in a complex relationship. World J Gastroenterol. 2014;20:10395-10404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 3. | Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 967] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 4. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3802] [Article Influence: 475.3] [Reference Citation Analysis (1)] |
| 5. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2846] [Article Influence: 406.6] [Reference Citation Analysis (0)] |
| 6. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1961] [Article Influence: 217.9] [Reference Citation Analysis (0)] |
| 7. | Huang DQ, Li X, Le MH, Le AK, Yeo YH, Trinh HN, Zhang J, Li J, Wong C, Cheung RC, Yang HI, Nguyen MH. Natural History and Hepatocellular Carcinoma Risk in Untreated Chronic Hepatitis B Patients With Indeterminate Phase. Clin Gastroenterol Hepatol. 2022;20:1803-1812.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (1)] |
| 8. | Spradling PR, Xing J, Rupp LB, Moorman AC, Gordon SC, Teshale ET, Lu M, Boscarino JA, Schmidt MA, Trinacty CM, Holmberg SD; Chronic Hepatitis Cohort Study Investigators. Distribution of disease phase, treatment prescription and severe liver disease among 1598 patients with chronic hepatitis B in the Chronic Hepatitis Cohort Study, 2006-2013. Aliment Pharmacol Ther. 2016;44:1080-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 9. | Yao K, Liu J, Wang J, Yan X, Xia J, Yang Y, Wu W, Liu Y, Chen Y, Zhang Z, Li J, Huang R, Wu C. Distribution and clinical characteristics of patients with chronic hepatitis B virus infection in the grey zone. J Viral Hepat. 2021;28:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Huang DQ, Tran A, Yeh ML, Yasuda S, Tsai PC, Huang CF, Dai CY, Ogawa E, Ishigami M, Ito T, Kozuka R, Enomoto M, Suzuki T, Yoshimaru Y, Preda CM, Marin RI, Sandra I, Tran S, Quek SXZ, Khine HHTW, Itokawa N, Atsukawa M, Uojima H, Watanabe T, Takahashi H, Inoue K, Maeda M, Hoang JK, Trinh L, Barnett S, Cheung R, Lim SG, Trinh HN, Chuang WL, Tanaka Y, Toyoda H, Yu ML, Nguyen MH. Antiviral therapy substantially reduces HCC risk in patients with chronic hepatitis B infection in the indeterminate phase. Hepatology. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 11. | Bonacci M, Lens S, Mariño Z, Londoño MC, Rodríguez-Tajes S, Mas A, García-López M, Pérez-Del-Pulgar S, Sánchez-Tapias JM, Forns X. Anti-viral therapy can be delayed or avoided in a significant proportion of HBeAg-negative Caucasian patients in the Grey Zone. Aliment Pharmacol Ther. 2018;47:1397-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Wang J, Yan X, Zhu L, Liu J, Qiu Y, Li Y, Liu Y, Xue R, Zhan J, Jiang S, Geng Y, Wan Y, Li M, Mao M, Gao D, Yin S, Tong X, Xia J, Ding W, Chen Y, Li J, Zhu C, Huang R, Wu C. Significant histological disease of patients with chronic hepatitis B virus infection in the grey zone. Aliment Pharmacol Ther. 2023;57:464-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 13. | Chinese Society of Hepatology; Chinese Medical Association. [Expert opinion on expanding anti-HBV treatment for chronic hepatitis B]. Zhonghua Gan Zang Bing Za Zhi. 2022;30:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Desmet VJ. Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis [Hepatology 1981;1:431-435]. J Hepatol. 2003;38:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Ren S, Wang W, Lu J, Wang K, Ma L, Zheng Y, Zheng S, Chen X. Effect of the change in antiviral therapy indication on identifying significant liver injury among chronic hepatitis B virus infections in the grey zone. Front Immunol. 2022;13:1035923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Lin MH, Li HQ, Zhu L, Su HY, Peng LS, Wang CY, He CP, Liang XE, Wang Y. Liver Fibrosis in the Natural Course of Chronic Hepatitis B Viral Infection: A Systematic Review with Meta-Analysis. Dig Dis Sci. 2022;67:2608-2626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Lee HW, Chan HL. Unresolved issues of immune tolerance in chronic hepatitis B. J Gastroenterol. 2020;55:383-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Howell J, Chan HLY, Feld JJ, Hellard ME, Thompson AJ. Closing the Stable Door After the Horse Has Bolted: Should We Be Treating People With Immune-Tolerant Chronic Hepatitis B to Prevent Hepatocellular Carcinoma? Gastroenterology. 2020;158:2028-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Kawanaka M, Nishino K, Kawamoto H, Haruma K. Hepatitis B: Who should be treated?-managing patients with chronic hepatitis B during the immune-tolerant and immunoactive phases. World J Gastroenterol. 2021;27:7497-7508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 20. | Lemoine M, Shimakawa Y, Nayagam S, Khalil M, Suso P, Lloyd J, Goldin R, Njai HF, Ndow G, Taal M, Cooke G, D'Alessandro U, Vray M, Mbaye PS, Njie R, Mallet V, Thursz M. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (1)] |
| 21. | Li Q, Li W, Huang Y, Chen L. The gamma-glutamyl transpeptidase-to-platelet ratio predicts liver fibrosis and cirrhosis in HBeAg-positive chronic HBV infection patients with high HBV DNA and normal or mildly elevated alanine transaminase levels in China. J Viral Hepat. 2016;23:912-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Liu X, Li H, Wei L, Tang Q, Hu P. Optimized cutoffs of gamma-glutamyl transpeptidase-to-platelet ratio, aspartate aminotransferase-to-platelet ratio index, and fibrosis-4 scoring systems for exclusion of cirrhosis in patients with chronic hepatitis B. Hepatol Commun. 2022;6:1664-1672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S, Conte D, Colombo M, Sirchia G. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1025] [Cited by in RCA: 1049] [Article Influence: 45.6] [Reference Citation Analysis (4)] |
| 24. | Sonneveld MJ, Brouwer WP, Hansen BE, Chan HL, Piratvisuth T, Jia JD, Zeuzem S, Chien RN, Choi H, de Knegt RJ, Wat C, Pavlovic V, Gaggar A, Xie Q, Buti M, de Man RA, Janssen HLA; SONIC-B Study Group. Very low probability of significant liver inflammation in chronic hepatitis B patients with low ALT levels in the absence of liver fibrosis. Aliment Pharmacol Ther. 2020;52:1399-1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 25. | Yuen MF, Yuan HJ, Wong DK, Yuen JC, Wong WM, Chan AO, Wong BC, Lai KC, Lai CL. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut. 2005;54:1610-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 292] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 26. | Lee MH, Yang HI, Liu J, Batrla-Utermann R, Jen CL, Iloeje UH, Lu SN, You SL, Wang LY, Chen CJ; R. E.V.E.A.L.-HBV Study Group. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: risk scores integrating host and virus profiles. Hepatology. 2013;58:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 27. | Sun Y, Chang J, Liu X, Liu C. Mortality trends of liver diseases in mainland China over three decades: an age-period-cohort analysis. BMJ Open. 2019;9:e029793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Zhao Q, Liu K, Zhu X, Yan L, Ding Y, Xu Y, Lou S, Zhao G, Xie Q, Gao Y, Bao S, Wang H. Anti-viral effect in chronic hepatitis B patients with normal or mildly elevated alanine aminotransferase. Antiviral Res. 2020;184:104953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |