Published online Aug 28, 2023. doi: 10.3748/wjg.v29.i32.4851
Peer-review started: May 28, 2023
First decision: July 8, 2023
Revised: July 18, 2023
Accepted: August 2, 2023
Article in press: August 2, 2023
Published online: August 28, 2023
Processing time: 88 Days and 16 Hours
Marginal zone lymphomas rank as the third most prevalent form of non-Hodgkin B-cell lymphoma, trailing behind diffuse large B-cell lymphoma and follicular lymphoma. Gastric mucosa-associated lymphoid tissue lymphoma (GML) is a low-grade B-cell neoplasia frequently correlated with Helicobacter pylori (H. pylori)-induced chronic gastritis. On the other hand, a specific subset of individuals diagnosed with GML does not exhibit H. pylori infection. In contrast to its H. pylori-positive counterpart, it was previously believed that H. pylori-negative GML was less likely to respond to antimicrobial therapy. Despite this, surprisingly, in-creasing evidence supports that a considerable proportion of patients with H. pylori-negative GML show complete histopathological remission after bacterial eradication therapy. Nonetheless, the precise mechanisms underlying this treatment responsiveness are not yet fully comprehended. In recent years, there has been growing interest in investigating the role of non-H. pylori gastric helicobacters (NHPHs) in the pathogenesis of H. pylori-negative GML. However, additional research is required to establish the causal relationship between NHPHs and GML. In this minireview, we examined the current understanding and proposed prospects on the involvement of NHPHs in H. pylori-negative GML, as well as their potential response to bacterial eradication therapy.
Core Tip: Gastric mucosa-associated lymphoid tissue lymphoma (GML) is a type of non-hodgkin lymphoma that arises in the stomach. It has been well-established that Helicobacter pylori (H. pylori) infection plays a crucial role in the development of GML. However, a subset of patients diagnosed with GML are negative for H. pylori. In recent years, there has been growing interest in investigating the role of non-H. pylori gastric helicobacters (NHPHs) in the pathogenesis of H. pylori-negative GML. This minireview aims to explore the current understanding of the involvement of NHPHs in the development of GML and its potential responsiveness to bacterial eradication therapy.
- Citation: Lemos FFB, Silva Luz M, Rocha Pinheiro SL, Teixeira KN, Freire de Melo F. Role of non-Helicobacter pylori gastric Helicobacters in helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma. World J Gastroenterol 2023; 29(32): 4851-4859
- URL: https://www.wjgnet.com/1007-9327/full/v29/i32/4851.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i32.4851
Marginal zone lymphomas (MZLs) rank as the third most prevalent form of non-hodgkin B-cell lymphoma, trailing behind diffuse large B-cell lymphoma and follicular lymphoma[1]. The 5th edition of the World Health Organization Classification of Hematolymphoid Tumors-Lymphoid Neoplasms further categorizes MZL into four subtypes: Extranodal MZL of mucosa-associated lymphoid tissue (MALT), primary cutaneous MZL, nodal MZL, and pediatric MZL[2].
Gastric MALT lymphoma (GML) is a low-grade B-cell neoplasia often correlated with Helicobacter pylori (H. pylori)-induced chronic gastritis[3]. Although the normal gastric mucosa lacks lymphoid follicles, chronic inflammation can lead to the formation of MALT. Continuous antigenic stimulation fosters the clonal expansion of B cells within the MALT, supported by specific T helper cells, which may lead to malignant transformation[4,5]. As GML progresses, genetic and epigenetic alterations occur in both oncogenes and tumour suppressor genes, resulting in dysregulated cell growth and survival. Common genetic alterations seen in MALT lymphoma include chromosomal translocations involving the API2-MALT1 gene fusion and mutations in genes such as TP53 and MYD88[5-7].
The current clinical guidelines advocate for the use of H. pylori eradication therapy as the primary treatment approach for localized GML[8-10]. In a recent systematic review conducted by our group, including meta-analyses, it was highlighted that bacterial eradication treatment resulted in the disappearance of lymphoma in over 75% of patients with low-grade, H. pylori-positive GML[11]. Hence, our results ratified that bacterial eradication is effective as the sole initial therapy for early-stage GML.
On the other hand, a specific subset of individuals diagnosed with GML does not exhibit H. pylori infection[12-15]. Consequently, it was assumed that these patients might not respond favorably to bacterial eradication therapy. However, another meta-analysis conducted by Jung et al[16] showed that 29.3% (95% confidence interval: 22.2%-37.4%, I2 = 41.5%) of H. pylori-negative GML patients experienced complete histopathological remission after eradication therapy. Nonetheless, the underlying mechanisms for this responsiveness remain unclear[16].
There has been a growing interest in exploring the involvement of species of non-H. pylori gastric helicobacters (NHPHs) in the development of H. pylori-negative GML and its responsiveness to bacterial eradication therapy[17-20]. NHPHs represent a group of bacterial species that colonize the stomach but differ genetically and phenotypically from H. pylori[21-24]. These differences include variances in flagella, urease activity, and other virulence factors[25,26]. While NHPHs have been detected in some patients with gastritis and peptic ulcers, their precise role and contribution to disease progression are not yet fully understood[27].
Some studies have indeed suggested an association between specific NHPH species and the development of GML, particularly in H. pylori-negative cases[28]. However, further research is required to establish a definitive causal relationship between NHPHs and GML. This article aims to explore the current understanding and propose prospects on the role of NHPHS in H. pylori-negative GML and its potential responsiveness to bacterial eradication therapy.
H. pylori-negative GML accounts for around 10% of all GML cases[29-31]. The cause of H. pylori-negative GML is not fully understood, and ongoing research aims to uncover the underlying factors contributing to its development. Symptoms of this type of lymphoma, such as abdominal pain, indigestion, bloating, nausea, vomiting, and weight loss, are similar to other gastric lymphomas but are nonspecific and can be caused by various conditions[32]. Diagnosis is made based on morphologic, immunophenotypic, and genetic analysis of biopsy material. Once the diagnosis is confirmed, a staging procedure to evaluate the extent of lymphoma dissemination is imperative[33].
In contrast to its H. pylori-positive counterpart, H. pylori-negative GML was previously believed to have a reduced likelihood of responding to antimicrobial therapy. In this context, treatment options may involve watchful waiting, radiation therapy (RT), chemotherapy (ChT), and immunotherapy[9]. Watchful waiting is suitable for slow-growing lymphomas without significant symptoms and with regular monitoring[33]. However, RT is the preferred treatment for localized disease in the management of H. pylori-negative GML. Several series have reported excellent disease control using RT alone, highlighting the efficacy of moderate-dose involved-field RT. Typically, a dose of 24-30 Gy is delivered to the stomach and perigastric nodes throughout 3-4 wk. To achieve optimal outcomes in gastric extranodal MZL[34,35]. Systemic treatment with ChT, immunotherapy, or a combination of both (chemoimmunotherapy) is recommended for patients with symptomatic systemic disease, contraindications to RT, treatment failure following antibiotic therapy or local treatments (such as RT or surgery), and those with histological transformation[36].
Despite this, surprisingly, increasing evidence supports that a considerable proportion of patients with H. pylori-negative GML show complete histopathological remission after bacterial eradication therapy[16,28,37]. Nonetheless, the precise mechanisms underlying this treatment responsiveness are not yet fully comprehended. Initially, it was attributed to false-negative tests for H. pylori[8,37]. However, more recently, the infection with other Helicobacter species (NHPHs) is acknowledged as a potential explanation for this phenomenon.
The Helicobacter genus includes gram-negative, microaerophilic, spiral, helical, curved, or fusiform rod-shaped bacteria that inhabit the gastrointestinal tract of several animals, such as humans, cats, dogs, pigs, and mice[38,39]. Currently, 53 species with validly published names comprise this genus[40], with H. pylori being the most prevalent in humans and well-known to be related to the development of chronic gastritis, peptic ulcer, and gastric cancer[41-43]. However, emerging evidence has highlighted the potential role of NHPHs in the progression of these diseases, including GML[24,44-46].
Among the NHPHs, H. suis, H. heilmannii, H. felis, H. salomonis and H. bizzozeronii are the most common species associated with human infection[47,48]. According to Yakoob et al[49], the prevalence of H. heilmannii and H. felis among patients with dyspepsia was 6% and 4%, respectively. On the other hand, Øverby et al[48] revealed a prevalence of gastric NHPH in Japanese patients of 6.1% and within this group, H. suis was the most prevalent, followed by H. heilmannii. This latter finding agrees with Nakamura et al[50], who found a prevalence of NHPHs of 20.8% in gastric mucosal samples of H. pylori-negative gastric disease patients, with H. suis and H. heilmannii also as the most prevalent species. However, it is important to note that the current diagnostic methods available, such as polymerase chain reaction (PCR) and immunohistochemistry, have limited accuracy in detecting NHPHs infections[26]. In a specific study, researchers faced difficulties in identifying the species associated with the infection in approximately 50% of the cases[50]. This challenge can be attributed to several factors, including the high genetic similarity between different NHPH species, significant genetic variation within a single species, limitations imposed by identification methods, and the concurrent presence of multiple NHPH species[51-53]. As a result, there is a concern that the actual prevalence of NHPHs infections among patients with dyspepsia may be underestimated.
Regarding the association of NHPHs with GML, some studies have evaluated the prevalence of infections by these species and its correlation with the complete remission of H. pylori-negative GML through eradication therapy. In this regard, Takigawa et al[54] report that the rate of complete remission in NHPH positive group of patients was significantly higher (75%) when compared to the negative cases (23%) of H. pylori-negative GML, which suggests a potential role for NHPHs in the pathogenesis of GML and the treatment effectiveness of H. pylori-negative GML. Such data are corroborated by Morgner et al[17], which advocate that H. heilmannii infection might be a causative factor in GML and that the current eradication therapy employed for H. pylori (standard antibiotics combined with proton pump inhibitors) is effective and results in complete remission of the lymphoma[17]. Nevertheless, upon confirming the presence of NHPH infection, it is strongly advised to implement a therapeutic approach that is tailored to the susceptibility profile of the individual bacterium.
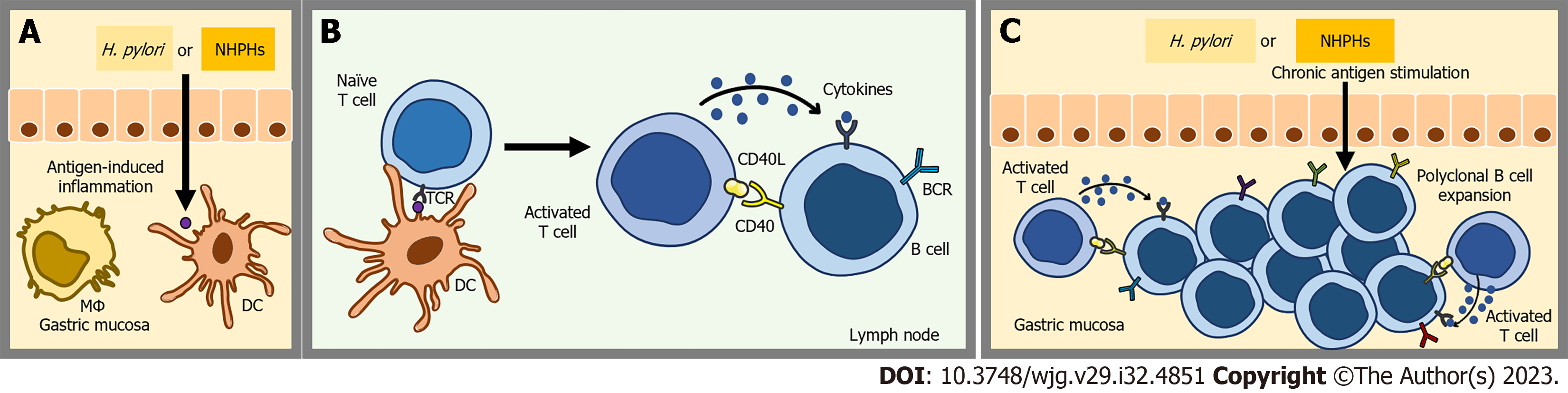
The pathogenesis of GML is a complex event that involves antigen-induced transformation of normal marginal-zone B-cells into malignant cells[55]. In contrast to MALT lymphomas observed in various locations, GML is distinguished by its association with specific microbial species: Primarily, H. pylori, and to a lesser extent, Helicobacter heilmannii[17,54,56]. Under normal physiological conditions, the stomach does not possess MALT. However, in the presence of chronic antigenic stimulation, gastric mucosal cells produce proinflammatory cytokines (such as lymphotoxin beta) and B-cell homing factors (e.g., bicinchoninic acid-1), leading to the infiltration of lymphoid cells into the gastric tissue. This cascade of events leads to the development of MALT[32,57,58] (Figure 1).
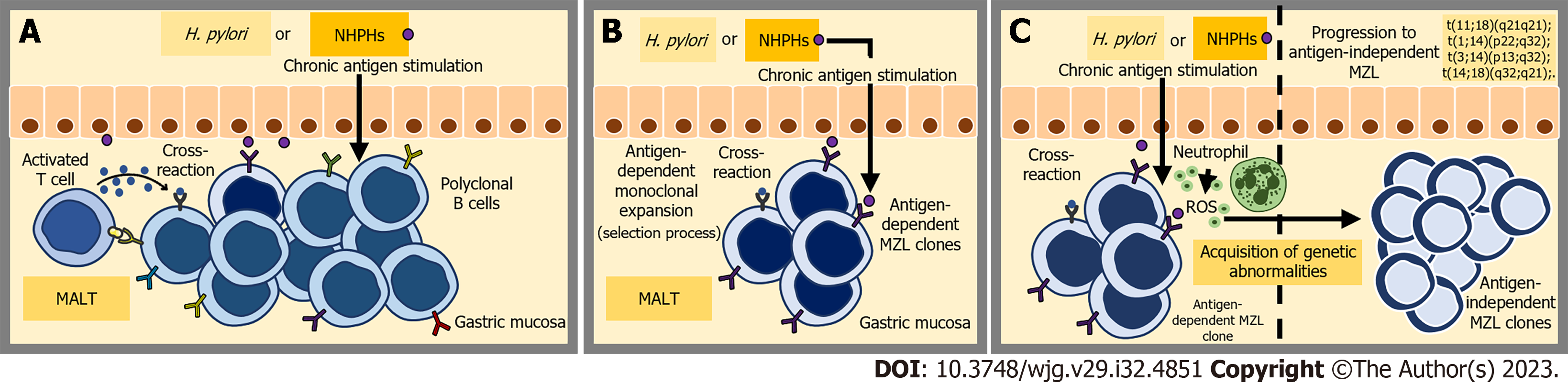
Regarding H. pylori infection, it is well-established that certain T helper cells target specific epitopes of the bacterium and support polyclonal B cells[59,60]. These B cells possess receptors that are able recognize autoantigens found in the gastric mucosa due to cross-reactivity. Consequently, the polyclonal B cell population undergoes expansion and a selection process, resulting in the emergence of an antigen-dependent MZL clone[61,62].
Sustained antigenic exposure not only stimulates the proliferation of a diverse array of B cells but also attracts neutrophils to the site of inflammation. The inflammatory process initiates the release of reactive oxygen species, leading to the occurrence of various genetic abnormalities[55,63,64]. Furthermore, the persistent proliferation of B cells during chronic inflammation increases the risk of double-stranded DNA breaks and translocations[5] (Figure 2).
Likewise, the involvement of NHPHs in H. pylori-negative GML could also be attributed to the induction of chronic inflammation, resulting in the local aggregation and proliferation of antigen-dependent B cells and T cells. Indeed, the infection of mice with NHPHs species, including H. felis, H. suis and H. heilmannii, also leads to a similar process of chronic gastritis and GML development with similarities to the human disease[65-69]. Possibly, the inflammatory microenvironment associated with NHPH-induced gastritis also facilitates the acquisition of genetic abnormalities by B cell clones. Nevertheless, further studies are required to construct a more comprehensive pathogenesis model.
Irrespective of etiology, progression towards antigen-independent MZL is associated with genetic events, while the role of direct antigenic stimulation gradually decreases in the development of GML[5,70] (Figure 2). Four recurrent chromosomal translocations have been found in MZL: t (1; 14) (p22; q32), t (11; 18) (q21; q21), t (14; 18) (q32; q21), and t (3; 14) (p14.1; q32)[71-73]. In GML, the translocation t (11; 18) (q21; q21) is the prominent structural chromosomal abnormality, occurring in approximately 10%-50% of cases[74-76]. This translocation results in the activation of NF-kappaB, which is a downstream target of B-cell receptor (BCR) signaling, independent of BCR signaling itself. The activation is mediated by the disruption of a signalosome complex involving CARD11, BCL10, and MALT1. Within this context, the presence of the MALT1 fusion protein is notably linked to more advanced stages of MALT lymphoma[77-80].
Indeed, numerous studies have demonstrated that GMLs harboring the t (11; 18) (q21; q21) translocation are frequently resistant to H. pylori eradication treatment compared to tumors that do not possess this specific translocation[11,81,82]. The decrease in the rate of complete histopathological remission following eradication therapy was also observed in H. pylori-negative GML cases; however, its influence on the treatment of NHPH-positive GML is still unclear[16].
Given the limited regression observed in H. pylori-negative GML after antibiotic treatment, clinical guidelines previously advised prompt initiation of targeted anti-lymphoma treatments[8,83]. Currently, the European Society for Medical Oncology Guidelines Committee suggests that a trial of anti-Helicobacter therapy may be worthwhile in H. pylori-negative early-stage GML (stages I and II1)[9]. This recommendation presents new opportunities for research in this field. Specifically, future studies could focus on investigating the mechanisms underlying the response to this therapy and further exploring the involvement of other Helicobacter species (NHPHs) in the development of H. pylori-negative GML. Additionally, it is crucial to investigate the long-term outcomes and assess the effects of early intervention with targeted anti-lymphoma treatments on patient prognosis.
In this context, accurate detection of NHPHs is vital for precise clinical diagnosis and targeted treatment strategies. However, current diagnostic methods primarily focus on H. pylori, leaving a gap in the detection of NHPHs infections. Goji et al[26] conducted a review of 26 articles and determined that the sensitivities of diagnostic methods for H. pylori infection, such as the rapid urease test, urea breath test, blood antibody analysis, immunohistochemical analysis, and stool antigen analysis, were low for NHPHs. The calculated sensitivities were only 40.0%, 14.8%, 23.1%, 40.0%, and 0%, respectively[26]. Therefore, at present, the most effective diagnostic tools for identifying NHPH infections are histological techniques and genetic diagnosis based on PCR, which hinders the clinical diagnosis of NHPHs infection, both due to the inflated cost and the dependence on laboratory apparatus. To address this, the development of tests that possess sensitivity, specificity, and the ability to detect different strains of NHPHs is crucial. The availability of reliable diagnostic methods for NHPHs will not only enable timely diagnosis and treatment for H. pylori-negative GML, but also contribute to a better understanding of their epidemiology and impact on human health.
When it comes to comprehending the pathogenesis of NHPH-positive GML, the significance of molecular and immunological studies cannot be overstated. These investigations should encompass the analysis of gene expression profiles in affected tissues, identification of pertinent genetic mutations, and study of cellular signaling pathways involved in the development and progression of the lymphoma. Additionally, it would also be interesting to analyze cytokine profiles, characterize immune cells infiltrating the affected gastric tissue, and conduct studies on the interaction between NHPHs and the host immune system. This deeper understanding might open doors to the development of targeted therapeutic strategies and hold promise for improved clinical outcomes in patients with NHPH-positive GML.
While H. pylori remains the primary pathogenic factor in the development of GML, the role of NHPHs in H. pylori-negative cases is an emerging area of research. It is crucial to identify these alternative pathogens and understand their mechanisms of pathogenesis to improve diagnostic accuracy and guide appropriate treatment strategies for patients with H. pylori-negative GML. Further research is warranted to elucidate the complex interplay between these bacteria, the host immune system, and the gastric microenvironment, which may lead to the development of novel therapeutic interventions and personalized approaches for this subset of patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu Q, China; Watanabe T, Japan; Yuan Y, China S-Editor: Qu XL L-Editor: A P-Editor: Chen YX
| 1. | Smith A, Crouch S, Lax S, Li J, Painter D, Howell D, Patmore R, Jack A, Roman E. Lymphoma incidence, survival and prevalence 2004-2014: sub-type analyses from the UK's Haematological Malignancy Research Network. Br J Cancer. 2015;112:1575-1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 308] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 2. | Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D, Calaminici M, Chadburn A, Chan JKC, Cheuk W, Chng WJ, Choi JK, Chuang SS, Coupland SE, Czader M, Dave SS, de Jong D, Du MQ, Elenitoba-Johnson KS, Ferry J, Geyer J, Gratzinger D, Guitart J, Gujral S, Harris M, Harrison CJ, Hartmann S, Hochhaus A, Jansen PM, Karube K, Kempf W, Khoury J, Kimura H, Klapper W, Kovach AE, Kumar S, Lazar AJ, Lazzi S, Leoncini L, Leung N, Leventaki V, Li XQ, Lim MS, Liu WP, Louissaint A Jr, Marcogliese A, Medeiros LJ, Michal M, Miranda RN, Mitteldorf C, Montes-Moreno S, Morice W, Nardi V, Naresh KN, Natkunam Y, Ng SB, Oschlies I, Ott G, Parrens M, Pulitzer M, Rajkumar SV, Rawstron AC, Rech K, Rosenwald A, Said J, Sarkozy C, Sayed S, Saygin C, Schuh A, Sewell W, Siebert R, Sohani AR, Tooze R, Traverse-Glehen A, Vega F, Vergier B, Wechalekar AD, Wood B, Xerri L, Xiao W. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36:1720-1748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 1923] [Article Influence: 641.0] [Reference Citation Analysis (0)] |
| 3. | Diaconu S, Predescu A, Moldoveanu A, Pop CS, Fierbințeanu-Braticevici C. Helicobacter pylori infection: old and new. J Med Life. 2017;10:112-117. [PubMed] |
| 4. | Rossi D, Bertoni F, Zucca E. Marginal-Zone Lymphomas. N Engl J Med. 2022;386:568-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 5. | Troppan K, Wenzl K, Neumeister P, Deutsch A. Molecular Pathogenesis of MALT Lymphoma. Gastroenterol Res Pract. 2015;2015:102656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Liu H, Ye H, Dogan A, Ranaldi R, Hamoudi RA, Bearzi I, Isaacson PG, Du MQ. T(11;18)(q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10. Blood. 2001;98:1182-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Suzuki H, Saito Y, Hibi T. Helicobacter pylori and Gastric Mucosa-associated Lymphoid Tissue (MALT) Lymphoma: Updated Review of Clinical Outcomes and the Molecular Pathogenesis. Gut Liver. 2009;3:81-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, Boot H, Du MQ, Megraud F, Montalban C, Raderer M, Savio A, Wotherspoon A; EGILS group. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 2011;60:747-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 9. | Zucca E, Arcaini L, Buske C, Johnson PW, Ponzoni M, Raderer M, Ricardi U, Salar A, Stamatopoulos K, Thieblemont C, Wotherspoon A, Ladetto M; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Marginal zone lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:17-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 218] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 10. | Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, Gasbarrini A, Hunt RH, Leja M, O'Morain C, Rugge M, Suerbaum S, Tilg H, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study group. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 657] [Article Influence: 219.0] [Reference Citation Analysis (0)] |
| 11. | Lemos FFB, de Castro CT, Calmon MS, Silva Luz M, Pinheiro SLR, Faria Souza Mendes Dos Santos C, Correa Santos GL, Marques HS, Delgado HA, Teixeira KN, Souza CL, Oliveira MV, Freire de Melo F. Effectiveness of Helicobacter pylori eradication in the treatment of early-stage gastric mucosa-associated lymphoid tissue lymphoma: An up-to-date meta-analysis. World J Gastroenterol. 2023;29:2202-2221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Asano N, Iijima K, Koike T, Imatani A, Shimosegawa T. Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphomas: A review. World J Gastroenterol. 2015;21:8014-8020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 13. | Matysiak-Budnik T, Jamet P, Ruskoné-Fourmestraux A, de Mascarel A, Velten M, Maynadié M, Woronoff AS, Trétarre B, Marrer E, Delafosse P, Ligier K, Lapôtre Ledoux B, Daubisse L, Bouzid L, Orazio S, Cowppli-Bony A, Monnereau A. Gastric MALT lymphoma in a population-based study in France: clinical features, treatments and survival. Aliment Pharmacol Ther. 2019;50:654-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Strati P, Lee ST, Teegavarupu P, Karri A, Anireddy S, Hagemeister FB, Romaguera J, Fayad LE, Rodriguez MA, Samaniego F, Fowler N, Westin J, Wang M, Lee HJ, Pinnix C, Gunther JR, Dabaja B, Feng L, Davis RE, Neelapu SS. Frontline antibiotic therapy for early-stage Helicobacter pylori-negative gastric MALT lymphoma. Am J Hematol. 2019;94:E150-E153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Kuo SH, Yeh KH, Wu MS, Lin CW, Wei MF, Liou JM, Wang HP, Chen LT, Cheng AL. First-line antibiotic therapy in Helicobacter pylori-negative low-grade gastric mucosa-associated lymphoid tissue lymphoma. Sci Rep. 2017;7:14333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Jung K, Kim DH, Seo HI, Gong EJ, Bang CS. Efficacy of eradication therapy in Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma: A meta-analysis. Helicobacter. 2021;26:e12774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 17. | Morgner A, Lehn N, Andersen LP, Thiede C, Bennedsen M, Trebesius K, Neubauer B, Neubauer A, Stolte M, Bayerdörffer E. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterology. 2000;118:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 184] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Okamura T, Iwaya Y, Yokosawa S, Suga T, Arakura N, Matsumoto T, Ogiwara N, Higuchi K, Ota H, Tanaka E. A case of Helicobacter heilmannii-associated primary gastric mucosa-associated lymphoid tissue lymphoma achieving complete remission after eradication. Clin J Gastroenterol. 2013;6:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | O'Rourke JL, Dixon MF, Jack A, Enno A, Lee A. Gastric B-cell mucosa-associated lymphoid tissue (MALT) lymphoma in an animal model of 'Helicobacter heilmannii' infection. J Pathol. 2004;203:896-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Nakagawa S, Shimoyama T, Nakamura M, Chiba D, Kikuchi H, Sawaya M, Chinda D, Mikami T, Fukuda S. The Resolution of Helicobacter suis-associated Gastric Lesions after Eradication Therapy. Intern Med. 2018;57:203-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Yasuda T, Lee HS, Nam SY, Katoh H, Ishibashi Y, Yamagata Murayama S, Matsui H, Masuda H, Rimbara E, Sakurazawa N, Suzuki H, Yoshida H, Seto Y, Ishikawa S, Jeon SW, Nakamura M, Nomura S. Non-Helicobacter pylori Helicobacter (NHPH) positive gastric cancer. Sci Rep. 2022;12:4811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Shafaie S, Kaboosi H, Peyravii Ghadikolaii F. Prevalence of non Helicobacter pylori gastric Helicobacters in Iranian dyspeptic patients. BMC Gastroenterol. 2020;20:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 23. | Bento-Miranda M, Figueiredo C. Helicobacter heilmannii sensu lato: an overview of the infection in humans. World J Gastroenterol. 2014;20:17779-17787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (2)] |
| 24. | Rimbara E, Suzuki M, Matsui H, Nakamura M, Morimoto M, Sasakawa C, Masuda H, Nomura S, Osaki T, Nagata N, Shibayama K, Tokunaga K. Isolation and characterization of Helicobacter suis from human stomach. Proc Natl Acad Sci U S A. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Joosten M, Lindén S, Rossi M, Tay AC, Skoog E, Padra M, Peters F, Perkins T, Vandamme P, Van Nieuwerburgh F, D'Herde K, Van den Broeck W, Flahou B, Deforce D, Ducatelle R, Marshall B, Haesebrouck F, Smet A. Divergence between the Highly Virulent Zoonotic Pathogen Helicobacter heilmannii and Its Closest Relative, the Low-Virulence "Helicobacter ailurogastricus" sp. nov. Infect Immun. 2016;84:293-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Goji S, Tamura Y, Sasaki M, Nakamura M, Matsui H, Murayama SY, Ebi M, Ogasawara N, Funaki Y, Kasugai K. Helicobacter suis-Infected Nodular Gastritis and a Review of Diagnostic Sensitivity for Helicobacter heilmannii-Like Organisms. Case Rep Gastroenterol. 2015;9:179-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Keikha M, Karbalaei M. Clinical aspects of Helicobacter heilmannii-associated gastritis in patients with dyspepsia: A systematic review and meta-analysis. Microb Pathog. 2022;166:105518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Xie YL, He CY, Wei SQ, Guan WJ, Jiang Z. Clinical efficacy of the modified Helicobacter pylori eradication therapy for Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma: a meta analysis. Chin Med J (Engl). 2020;133:1337-1346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Nakamura S, Matsumoto T. Helicobacter pylori and gastric mucosa-associated lymphoid tissue lymphoma: recent progress in pathogenesis and management. World J Gastroenterol. 2013;19:8181-8187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Zullo A, Hassan C, Andriani A, Cristofari F, De Francesco V, Ierardi E, Tomao S, Morini S, Vaira D. Eradication therapy for Helicobacter pylori in patients with gastric MALT lymphoma: a pooled data analysis. Am J Gastroenterol. 2009;104:1932-7; quiz 1938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Nakamura S, Sugiyama T, Matsumoto T, Iijima K, Ono S, Tajika M, Tari A, Kitadai Y, Matsumoto H, Nagaya T, Kamoshida T, Watanabe N, Chiba T, Origasa H, Asaka M; JAPAN GAST Study Group. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut. 2012;61:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 32. | Violeta Filip P, Cuciureanu D, Sorina Diaconu L, Maria Vladareanu A, Silvia Pop C. MALT lymphoma: epidemiology, clinical diagnosis and treatment. J Med Life. 2018;11:187-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 33. | Nakamura S, Hojo M. Diagnosis and Treatment for Gastric Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma. J Clin Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 34. | Wirth A, Gospodarowicz M, Aleman BM, Bressel M, Ng A, Chao M, Hoppe RT, Thieblemont C, Tsang R, Moser L, Specht L, Szpytma T, Lennard A, Seymour JF, Zucca E. Long-term outcome for gastric marginal zone lymphoma treated with radiotherapy: a retrospective, multi-centre, International Extranodal Lymphoma Study Group study. Ann Oncol. 2013;24:1344-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Goda JS, Gospodarowicz M, Pintilie M, Wells W, Hodgson DC, Sun A, Crump M, Tsang RW. Long-term outcome in localized extranodal mucosa-associated lymphoid tissue lymphomas treated with radiotherapy. Cancer. 2010;116:3815-3824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 36. | Kuo SH, Yeh KH, Lin CW, Liou JM, Wu MS, Chen LT, Cheng AL. Current Status of the Spectrum and Therapeutics of Helicobacter pylori-Negative Mucosa-Associated Lymphoid Tissue Lymphoma. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Zullo A, Hassan C, Ridola L, De Francesco V, Rossi L, Tomao S, Vaira D, Genta RM. Eradication therapy in Helicobacter pylori-negative, gastric low-grade mucosa-associated lymphoid tissue lymphoma patients: a systematic review. J Clin Gastroenterol. 2013;47:824-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | On SLW, Miller WG, Houf K, Fox JG, Vandamme P. Minimal standards for describing new species belonging to the families Campylobacteraceae and Helicobacteraceae: Campylobacter, Arcobacter, Helicobacter and Wolinella spp. Int J Syst Evol Microbiol. 2017;67:5296-5311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 39. | Ochoa S, Collado L. Enterohepatic Helicobacter species - clinical importance, host range, and zoonotic potential. Crit Rev Microbiol. 2021;47:728-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 40. | Parte AC, Sardà Carbasse J, Meier-Kolthoff JP, Reimer LC, Göker M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int J Syst Evol Microbiol. 2020;70:5607-5612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 1170] [Article Influence: 234.0] [Reference Citation Analysis (0)] |
| 41. | Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273-1275. [PubMed] |
| 42. | Liu J, He C, Chen M, Wang Z, Xing C, Yuan Y. Association of presence/absence and on/off patterns of Helicobacter pylori oipA gene with peptic ulcer disease and gastric cancer risks: a meta-analysis. BMC Infect Dis. 2013;13:555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Alipour M. Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancer. J Gastrointest Cancer. 2021;52:23-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 150] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 44. | Joo M, Kwak JE, Chang SH, Kim H, Chi JG, Kim KA, Yang JH, Lee JS, Moon YS, Kim KM. Helicobacter heilmannii-associated gastritis: clinicopathologic findings and comparison with Helicobacter pylori-associated gastritis. J Korean Med Sci. 2007;22:63-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Stolte M, Kroher G, Meining A, Morgner A, Bayerdörffer E, Bethke B. A comparison of Helicobacter pylori and H. heilmannii gastritis. A matched control study involving 404 patients. Scand J Gastroenterol. 1997;32:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 118] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Takigawa H, Masaki S, Naito T, Yuge R, Urabe Y, Tanaka S, Sentani K, Matsuo T, Matsuo K, Chayama K, Kitadai Y. Helicobacter suis infection is associated with nodular gastritis-like appearance of gastric mucosa-associated lymphoid tissue lymphoma. Cancer Med. 2019;8:4370-4379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Matos R, Sousa HS, Nogueiro J, Magalhães A, Reis CA, Carneiro F, Amorim I, Haesebrouck F, Gärtner F. Helicobacter species binding to the human gastric mucosa. Helicobacter. 2022;27:e12867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Øverby A, Murayama SY, Michimae H, Suzuki H, Suzuki M, Serizawa H, Tamura R, Nakamura S, Takahashi S, Nakamura M. Prevalence of Gastric Non-Helicobacter pylori-Helicobacters in Japanese Patients with Gastric Disease. Digestion. 2017;95:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Yakoob J, Abbas Z, Khan R, Naz S, Ahmad Z, Islam M, Awan S, Jafri F, Jafri W. Prevalence of non Helicobacter pylori species in patients presenting with dyspepsia. BMC Gastroenterol. 2012;12:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Nakamura M, Øverby A, Michimae H, Matsui H, Takahashi S, Mabe K, Shimoyama T, Sasaki M, Terao S, Kamada T, Yanaka A, Iwamoto J, Tanabe S, Tari A, Nasu S, Suzuki H, Yamagata Murayama S. PCR analysis and specific immunohistochemistry revealing a high prevalence of non-Helicobacter pylori Helicobacters in Helicobacter pylori-negative gastric disease patients in Japan: High susceptibility to an Hp eradication regimen. Helicobacter. 2020;25:e12700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 51. | Smet A, Yahara K, Rossi M, Tay A, Backert S, Armin E, Fox JG, Flahou B, Ducatelle R, Haesebrouck F, Corander J. Macroevolution of gastric Helicobacter species unveils interspecies admixture and time of divergence. ISME J. 2018;12:2518-2531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Mannion A, Shen Z, Fox JG. Comparative genomics analysis to differentiate metabolic and virulence gene potential in gastric versus enterohepatic Helicobacter species. BMC Genomics. 2018;19:830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Liu J, He L, Haesebrouck F, Gong Y, Flahou B, Cao Q, Zhang J. Prevalence of Coinfection with Gastric Non-Helicobacter pylori Helicobacter (NHPH) Species in Helicobacter pylori-infected Patients Suffering from Gastric Disease in Beijing, China. Helicobacter. 2015;20:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 54. | Takigawa H, Yuge R, Masaki S, Otani R, Kadota H, Naito T, Hayashi R, Urabe Y, Oka S, Tanaka S, Chayama K, Kitadai Y. Involvement of non-Helicobacter pylori helicobacter infections in Helicobacter pylori-negative gastric MALT lymphoma pathogenesis and efficacy of eradication therapy. Gastric Cancer. 2021;24:937-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 55. | Sagaert X, Van Cutsem E, De Hertogh G, Geboes K, Tousseyn T. Gastric MALT lymphoma: a model of chronic inflammation-induced tumor development. Nat Rev Gastroenterol Hepatol. 2010;7:336-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Di Rocco A, Petrucci L, Assanto GM, Martelli M, Pulsoni A. Extranodal Marginal Zone Lymphoma: Pathogenesis, Diagnosis and Treatment. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 57. | Lenze D, Berg E, Volkmer-Engert R, Weiser AA, Greiner A, Knörr-Wittmann C, Anagnostopoulos I, Stein H, Hummel M. Influence of antigen on the development of MALT lymphoma. Blood. 2006;107:1141-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Mazzucchelli L, Blaser A, Kappeler A, Schärli P, Laissue JA, Baggiolini M, Uguccioni M. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J Clin Invest. 1999;104:R49-R54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 230] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 59. | Guindi M. Role of activated host T cells in the promotion of MALT lymphoma growth. Semin Cancer Biol. 2000;10:341-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 60. | Suarez F, Lortholary O, Hermine O, Lecuit M. Infection-associated lymphomas derived from marginal zone B cells: a model of antigen-driven lymphoproliferation. Blood. 2006;107:3034-3044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 302] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 61. | Nakamura S, Aoyagi K, Furuse M, Suekane H, Matsumoto T, Yao T, Sakai Y, Fuchigami T, Yamamoto I, Tsuneyoshi M, Fujishima M. B-cell monoclonality precedes the development of gastric MALT lymphoma in Helicobacter pylori-associated chronic gastritis. Am J Pathol. 1998;152:1271-1279. [PubMed] |
| 62. | Du MQ, Isaccson PG. Gastric MALT lymphoma: from aetiology to treatment. Lancet Oncol. 2002;3:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 179] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 63. | Ziech D, Franco R, Pappa A, Panayiotidis MI. Reactive oxygen species (ROS)--induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res. 2011;711:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 391] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 64. | Rodríguez-Sevilla JJ, Salar A. Recent Advances in the Genetic of MALT Lymphomas. Cancers (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 65. | Lee A, O'Rourke J, Enno A. Gastric mucosa-associated lymphoid tissue lymphoma: implications of animal models on pathogenic and therapeutic considerations--mouse models of gastric lymphoma. Recent Results Cancer Res. 2000;156:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 66. | Zhao Y, Lu F, Ye J, Ji M, Pang Y, Wang Y, Wang L, Li G, Sun T, Li J, Ma D, Ji C. Myeloid-Derived Suppressor Cells and γδT17 Cells Contribute to the Development of Gastric MALT Lymphoma in H. felis-Infected Mice. Front Immunol. 2019;10:3104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 67. | Yamamoto K, Tanaka H, Nishitani Y, Nishiumi S, Miki I, Takenaka M, Nobutani K, Mimura T, Ben Suleiman Y, Mizuno S, Kawai M, Uchiyama I, Yoshida M, Azuma T. Helicobacter suis KB1 derived from pig gastric lymphoid follicles induces the formation of gastric lymphoid follicles in mice through the activation of B cells and CD4 positive cells. Microbes Infect. 2011;13:697-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 68. | Mimura T, Yoshida M, Nishiumi S, Tanaka H, Nobutani K, Takenaka M, Suleiman YB, Yamamoto K, Ota H, Takahashi S, Matsui H, Nakamura M, Miki I, Azuma T. IFN-γ plays an essential role in the pathogenesis of gastric lymphoid follicles formation caused by Helicobacter suis infection. FEMS Immunol Med Microbiol. 2011;63:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 69. | Nobutani K, Yoshida M, Nishiumi S, Nishitani Y, Takagawa T, Tanaka H, Yamamoto K, Mimura T, Bensuleiman Y, Ota H, Takahashi S, Matsui H, Nakamura M, Azuma T. Helicobacter heilmannii can induce gastric lymphoid follicles in mice via a Peyer's patch-independent pathway. FEMS Immunol Med Microbiol. 2010;60:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Zucca E, Bertoni F, Vannata B, Cavalli F. Emerging role of infectious etiologies in the pathogenesis of marginal zone B-cell lymphomas. Clin Cancer Res. 2014;20:5207-5216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 71. | Willis TG, Jadayel DM, Du MQ, Peng H, Perry AR, Abdul-Rauf M, Price H, Karran L, Majekodunmi O, Wlodarska I, Pan L, Crook T, Hamoudi R, Isaacson PG, Dyer MJ. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell. 1999;96:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 491] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 72. | Streubel B, Lamprecht A, Dierlamm J, Cerroni L, Stolte M, Ott G, Raderer M, Chott A. T(14;18)(q32;q21) involving IGH and MALT1 is a frequent chromosomal aberration in MALT lymphoma. Blood. 2003;101:2335-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 302] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 73. | Streubel B, Vinatzer U, Lamprecht A, Raderer M, Chott A. T(3;14)(p14.1;q32) involving IGH and FOXP1 is a novel recurrent chromosomal aberration in MALT lymphoma. Leukemia. 2005;19:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 74. | Lima KS, Albuquerque W, Arantes VN, Drummond-Lage AP, Coelho LG. Helicobacter pylori and t(11;18)(q21;q21) translocation in gastric malt lymphoma. Arq Gastroenterol. 2014;51:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Ye H, Liu H, Attygalle A, Wotherspoon AC, Nicholson AG, Charlotte F, Leblond V, Speight P, Goodlad J, Lavergne-Slove A, Martin-Subero JI, Siebert R, Dogan A, Isaacson PG, Du MQ. Variable frequencies of t(11;18)(q21;q21) in MALT lymphomas of different sites: significant association with CagA strains of H pylori in gastric MALT lymphoma. Blood. 2003;102:1012-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 216] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 76. | Streubel B, Simonitsch-Klupp I, Müllauer L, Lamprecht A, Huber D, Siebert R, Stolte M, Trautinger F, Lukas J, Püspök A, Formanek M, Assanasen T, Müller-Hermelink HK, Cerroni L, Raderer M, Chott A. Variable frequencies of MALT lymphoma-associated genetic aberrations in MALT lymphomas of different sites. Leukemia. 2004;18:1722-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 267] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 77. | Sagaert X, Laurent M, Baens M, Wlodarska I, De Wolf-Peeters C. MALT1 and BCL10 aberrations in MALT lymphomas and their effect on the expression of BCL10 in the tumour cells. Mod Pathol. 2006;19:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Knies N, Alankus B, Weilemann A, Tzankov A, Brunner K, Ruff T, Kremer M, Keller UB, Lenz G, Ruland J. Lymphomagenic CARD11/BCL10/MALT1 signaling drives malignant B-cell proliferation via cooperative NF-κB and JNK activation. Proc Natl Acad Sci U S A. 2015;112:E7230-E7238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 79. | Ye H, Gong L, Liu H, Ruskone-Fourmestraux A, de Jong D, Pileri S, Thiede C, Lavergne A, Boot H, Caletti G, Wündisch T, Molina T, Taal BG, Elena S, Neubauer A, Maclennan KA, Siebert R, Remstein ED, Dogan A, Du MQ. Strong BCL10 nuclear expression identifies gastric MALT lymphomas that do not respond to H pylori eradication. Gut. 2006;55:137-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Rosebeck S, Rehman AO, Lucas PC, McAllister-Lucas LM. From MALT lymphoma to the CBM signalosome: three decades of discovery. Cell Cycle. 2011;10:2485-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 81. | Zullo A, Hassan C, Cristofari F, Andriani A, De Francesco V, Ierardi E, Tomao S, Stolte M, Morini S, Vaira D. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol. 2010;8:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 82. | Liu H, Ye H, Ruskone-Fourmestraux A, De Jong D, Pileri S, Thiede C, Lavergne A, Boot H, Caletti G, Wündisch T, Molina T, Taal BG, Elena S, Thomas T, Zinzani PL, Neubauer A, Stolte M, Hamoudi RA, Dogan A, Isaacson PG, Du MQ. T(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology. 2002;122:1286-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 272] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 83. | Zucca E, Copie-Bergman C, Ricardi U, Thieblemont C, Raderer M, Ladetto M; ESMO Guidelines Working Group. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi144-vi148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |