Published online Jul 28, 2023. doi: 10.3748/wjg.v29.i28.4466
Peer-review started: February 10, 2023
First decision: May 16, 2023
Revised: May 30, 2023
Accepted: June 14, 2023
Article in press: June 14, 2023
Published online: July 28, 2023
Processing time: 165 Days and 18.1 Hours
Hemodynamic instability and shock are associated with untoward outcomes in gastrointestinal bleeding. However, there are no studies in the existing literature on the proportion of patients who developed these outcomes after gastrointestinal bleeding.
To determine the pooled event rates in the available literature and specify them based on the bleeding source.
The protocol was registered on PROSPERO in advance (CRD42021283258). A systematic search was performed in three databases (PubMed, EMBASE, and CENTRAL) on 14th October 2021. Pooled proportions with 95%CI were calculated with a random-effects model. A subgroup analysis was carried out based on the time of assessment (on admission or during hospital stay). Heterogeneity was assessed by Higgins and Thompson’s I2 statistics. The Joanna Briggs Institute Prevalence Critical Appraisal Tool was used for the risk of bias assessment. The Reference Citation Analysis (https://www.referencecitationanalysis.com/) tool was applied to obtain the latest highlight articles.
We identified 11589 records, of which 220 studies were eligible for data extraction. The overall proportion of shock and hemodynamic instability in general gastrointestinal bleeding patients was 0.25 (95%CI: 0.17-0.36, I2 = 100%). In non-variceal bleeding, the proportion was 0.22 (95%CI: 0.14-0.31, I2 = 100%), whereas it was 0.25 (95%CI: 0.19-0.32, I2 = 100%) in variceal bleeding. The proportion of patients with colonic diverticular bleeding who developed shock or hemodynamic instability was 0.12 (95%CI: 0.06-0.22, I2 = 90%). The risk of bias was low, and heterogeneity was high in all analyses.
One in five, one in four, and one in eight patients develops shock or hemodynamic instability on admission or during hospitalization in the case of non-variceal, variceal, and colonic diverticular bleeding, respectively.
Core Tip: Gastrointestinal bleeding is one of the most common gastrointestinal emergencies with estimated mortality up to 10%. It is associated with significant morbidity, additional burden, and health care costs. It is documented that hemodynamic instability and shock are highly associated with untoward outcomes; they lead to a higher mortality rate, rebleeding risk, prehospital transfusion, and sedation complications. Our study provides clear evidence that hemodynamic instability and shock are common presentations and complications in gastrointestinal bleeding and gives insight into some possible predictor factors.
- Citation: Obeidat M, Teutsch B, Rancz A, Tari E, Márta K, Veres DS, Hosszúfalusi N, Mihály E, Hegyi P, Erőss B. One in four patients with gastrointestinal bleeding develops shock or hemodynamic instability: A systematic review and meta-analysis. World J Gastroenterol 2023; 29(28): 4466-4480
- URL: https://www.wjgnet.com/1007-9327/full/v29/i28/4466.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i28.4466
The annual incidence of gastrointestinal bleeding (GIB) is 100 per 100000 population, and it is one of the most common gastroenterological emergencies with an estimated mortality rate in the range of 2%-10%, primarily due to complications related to the admission state and individual patient factors[1-3]. It is associated with significant morbidity, additional burden, and health care costs[4,5]. The mortality rate of upper GIB has not considerably decreased over the past decades, despite the improvement in the diagnosis and endoscopic treatment[6]. We contemplate that pre-endoscopic assessment and post-endoscopic care may contribute effectively to better outcomes.
Several studies showed that hemodynamic instability (HI) and shock in GIB are highly associated with untoward outcomes; they can lead to higher mortality rates, prehospital transfusion, rebleeding risk, and endoscopic sedation might be complicated with unfavorable hemodynamics if the patient presents with massive bleeding[7-9]. Furthermore, the hospital mortality rate of bleeding with shock can be 10 times higher than without shock[10].
Early intensive resuscitation of HI decreases complications in patients with upper GIB[11]. However, there are not enough details in the guidelines regarding the management of hemodynamically unstable patients; there are still some uncertainties about the optimal fluid rate and the ideal type of fluid to be used in treating those patients[12-15].
At the time of our systematic search, there were no published systematic reviews assessing the proportion of hemodynamically unstable and shocked patients in GIB. There are large variations in the proportions of these outcomes. Some studies in variceal and non-variceal bleeding resulted in proportions of 10% or lower[16-19], whereas others exceeded 60%[20-22]. Therefore, we aimed to highlight the importance of recognizing those patients by quantifying the pooled event rates based on the bleeding source. Additionally, we did a subgroup analysis based on the assessment time of these outcomes (on admission or during hospital stay).
Our systematic review and meta-analysis was conducted following the recommendation of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guideline[23]. The recommendations of the Cochrane Handbook were also followed[24]. The study protocol was registered on PROSPERO (CRD42021283258), and we fully adhered to it[25]. In addition, we applied the Reference Citation Analysis (RCA) tool, which is based on artificial intelligence technology. This tool allowed us to access a comprehensive database of citations across multiple disciplines, aiding us in identifying the most recent and significant articles for our research.
We applied the CoCoPop (condition, context, and population) framework to establish the eligibility criteria[26]; the condition was hemodynamic instability and/or shock, gastrointestinal bleeding as a context, and our population was adult patients. All definitions of hemodynamic instability and shock were accepted.
Randomized Controlled Trials (RCTs), cohorts, and case-control studies were included. Cross-sectional studies were included only if the hemodynamic parameters were assessed on admission. We included studies only if the primary cause of hospital admission was gastrointestinal bleeding and excluded articles that assessed our investigated outcomes after specific interventions. Articles that could not be found were sought for retrieval by contacting the journals and the authors. In the case of studies with overlapping populations, we kept the ones with larger sample sizes.
Our systematic search was conducted in three main databases: MEDLINE (via PubMed), EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL) from the inception to 14th October 2021. No language or other restrictions were applied.
Our search key contained two main concepts: All types of bleeding sources and hemodynamic instability or shock. For the detailed search strategy, see Supplementary Table 1.
Following the systematic search, the yielded articles were imported into a reference management program (EndNote 20.1). Duplicate articles were eliminated automatically and manually with overlapping publication years, authors, and titles. The screening and selection were performed by two independent reviewers (Obeidat M and Tari E) first by title and abstract, and then by full text (considering the eligibility criteria). Cohen’s kappa coefficient (κ) was calculated at both levels of selection to measure the inter-reviewer reliability. In case of any disagreement, a consensus was reached after a discussion with the corresponding author (Erőss B).
The relevant data from the eligible studies were extracted independently by two authors (Obeidat M and Rancz A). Disagreements were resolved by involving the corresponding author (Erőss B). All data were manually collected and introduced into an Excel spreadsheet (Office 365, Microsoft, Redmond, WA, United States) for analysis. The following data were extracted: First author, the year of publication, Digital Object Identifier, geographical location, study period and design, number of centers, basic demographics, source of bleeding, the total number of GIB patients and those who developed HI or shock, definitions of the investigated outcomes, and the time of detection (on admission or during hospital stay).
Two independent authors (Obeidat M and Tari E) performed the risk of bias assessment using the ‘Joanna Briggs Institute Prevalence Critical Appraisal Tool’[26]. A third reviewer resolved potential disagreements (Rancz A). The tool contains nine items regarding the target population and study settings. Each item was rated as ‘yes’, ‘no’, ‘unclear’, or ‘not applicable’ according to information provided in each study, with a maximum score of nine points. The higher the score, the lower the risk of bias.
We followed the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach[27] to evaluate the quality of evidence of our results, and the GRADEpro tool (software) was used. Study design, risk of bias, inconsistency, indirectness, and imprecision were the determinant factors.
The statistical analysis of the data was conducted by the R programming language using the meta package. We used forest plots to summarize the findings of the studies and show the pooled result. Pooled event rates were calculated with 95%CIs. The random-effect model was anticipated as applied in all analyses as considerable between-study heterogeneity. The random intercept logistic regression model method was used for pooling method as recommended by Schwarzer et al[28]. To estimate the heterogeneity variance measure τ2, the maximum likelihood method was used. For the outcomes where the study number was at least five, a Hartung-Knapp adjustment was used[29,30]. Below five studies, we applied the adjustment if it was more conservative than without the adjustment. Statistical heterogeneity was assessed by Higgins and Thompson’s I²[31].
Egger’s test with the Peter’s modification and funnel plots were applied to report and visualize publication bias if at least 10 studies were involved in the analysis[32]; P < 0.1 indicates potential publication bias. We also performed an influential sensitivity analysis with leave-one-out method to evaluate whether a single study could have a marked influence on the overall proportional rate or heterogeneity.
A subgroup analysis was carried out based on the time of assessment (on admission or during hospitalization) of HI or shock. Studies where there were no data about the time when the patients were assessed, were considered (during hospitalization). We used a fixed-effects “plural” model. We assumed that subgroups had different τ2 values as we anticipated differences in the between-study heterogeneity in the subgroups, although a common τ2 assumption was used for practical reasons if the subgroup size was maximum five. To assess the difference between the subgroups, a Cochrane Q test was used between subgroups[33]. We did not calculate the overall effect and heterogeneity for subgroups where less than three studies were included. We calculated the prediction intervals for our outcomes to assess the probability that future studies would have the same result in a similar setting[34]. The statistical methods of this study were reviewed by Veres DS who is a verified biostatistician from the Centre for Translational Medicine, Semmelweis University.
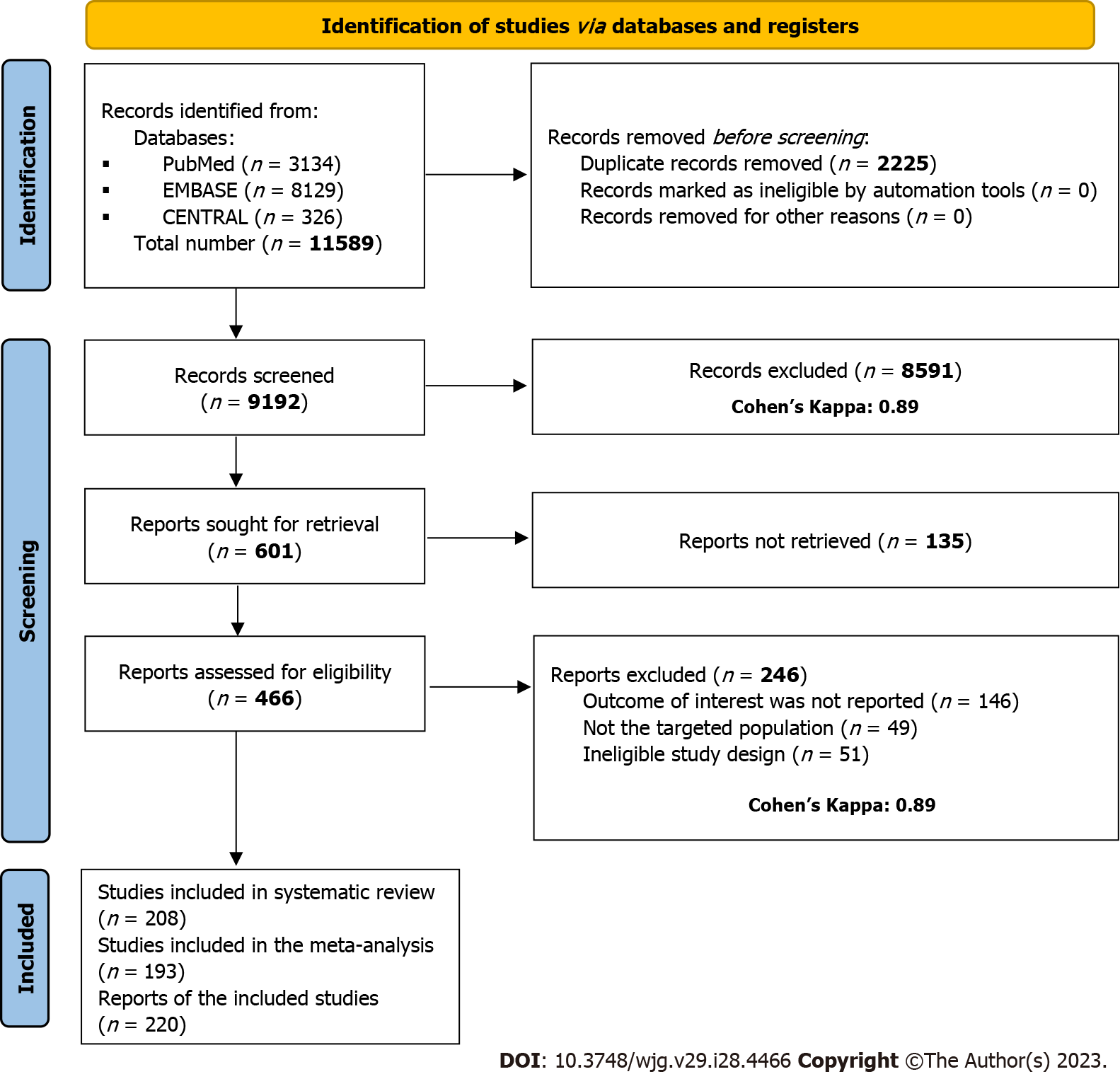
Altogether, 11589 studies were identified by our search key through three main databases, 8129 in EMBASE, 3134 in Medline (via PubMed), and 326 in CENTRAL. Of them, 9192 records remained for title and abstract selection after duplicate removal. A total of 601 studies were sought for full-text selection, out of which 164 records were not found. We managed to retrieve 29, but 135 records were still inaccessible. In total, 466 studies were assessed for full-text eligibility, of which 246 were excluded (Supplementary Table 2). Eleven studies were removed for overlapping populations (SupplementaryTable 3). Details of search and selection are illustrated in the PRISMA 2020 flow chart (Figure 1).
Most of the included studies were cohort studies. We also included 28 RCTs, 6 case-control, and 4 cross-sectional studies. Eighty records were from Asia, 66 from Europe, 25 from North America, and 13 from Africa. In total, more than six million patients were included in the analysis. However, the study with the largest sample size included 6411838 patients with different bleeding sources from a 12-year national analysis in the United States[10]. The main characteristics of the enrolled studies are detailed in Supplementary Table 4.
We included all studies with unspecified bleeding sources[8,10,35-50]. HI was assessed on admission and during hospital stay with pooled event rates of 0.29 (95%CI: 0.12-0.56, I2 = 87%) and 0.34 (95%CI: 0.11-0.68, I2 = 93%), respectively. Shock on admission was 0.27 (95%CI: 0.08-0.60, I2 = 92%), whereas during hospital stay it was 0.15 (95%CI: 0.05-0.36, I2 = 99%). One in four patients with GIB developed HI or shock; 0.25 (95%CI: 0.17-0.36, I2 = 100%) (Figure 2).
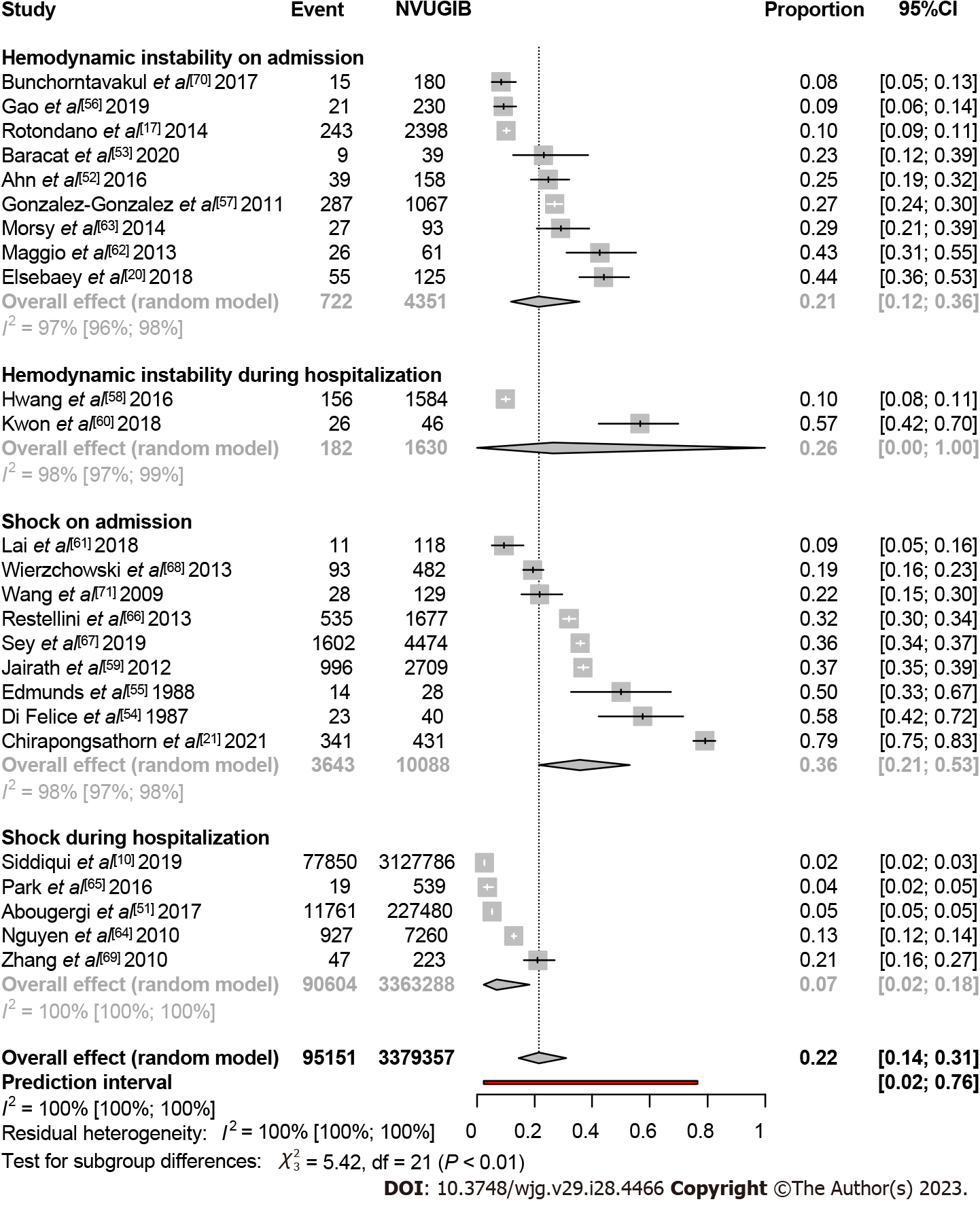
In the case of non-variceal bleeding, more than three million patients were included in the analysis from 25 studies[10,17,20,21,51-71]. The proportion of hemodynamically unstable patients on admission was 0.21 (95%CI: 0.12-0.36, I2 = 97%). Two studies assessed HI during hospitalization, Hwang et al[58] and Kwon et al[60] where the event rate was 0.10 (95%CI: 0.08-0.11) and 0.57 (95%CI: 0.42-0.70), respectively. Moreover, shock on admission was the highest at 0.36 (95%CI: 0.21-0.53, I2 = 98%), with a noticeable difference from those who developed shock during hospitalization with a rate of 0.07 (95%CI: 0.02-0.18, I2 = 100%). Altogether, 0.22 (95%CI: 0.14-0.31, I2 = 100%) of non-variceal bleeders developed shock or HI on admission or during the hospital stay (Figure 3).
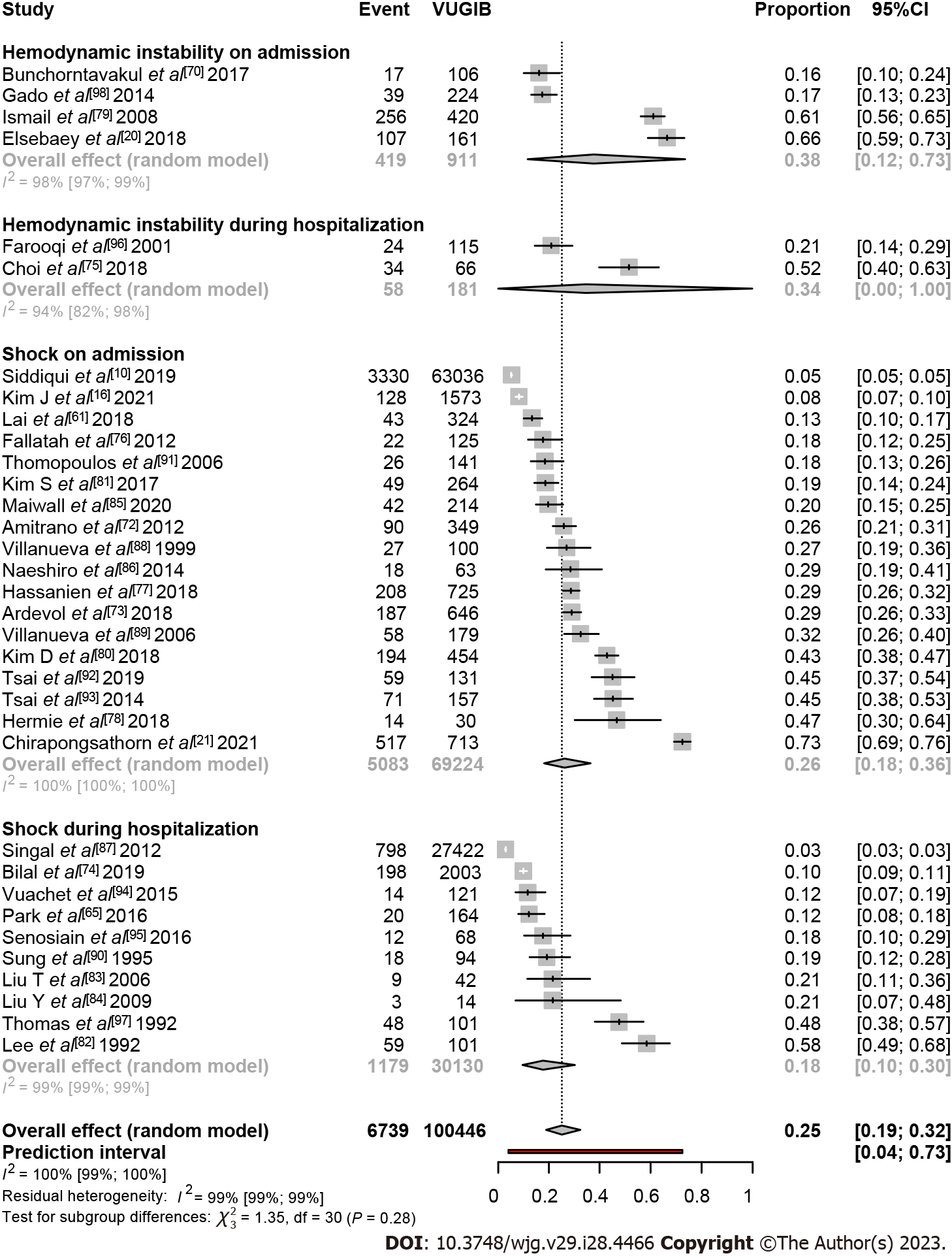
In total, 34 studies were included in this analysis[10,16,20,21,61,65,70,72-98]. The rate of patients with variceal bleeding who presented with HI on admission was 0.38 (95%CI: 0.12-0.73, I2 = 98%). Two studies assessed HI during hospitalization, Farooqi and Farooqi[96] and Choi et al[75] where the event rate was 0.21 (95%CI: 0.14-0.29) and 0.52 (95%CI: 0.40-0.63), respectively. The shock rate on admission was 0.26 (95%CI: 0.18-0.36, I2 = 100%), whereas it was 0.18 (95%CI: 0.10-0.30, I2 = 99%) during the hospital stay. In total, one in four patients with variceal bleeding developed shock or HI at presentation or during hospital stay 0.25 (95%CI: 0.19-0.32, I2 = 100%) (Figure 4).
Peptic ulcer bleeding (PUB) was the most reported source of bleeding among the included studies. Sixty-seven studies were involved in the subgroups. On admission, 0.22 (95%CI: 0.09-0.44, I2 = 96%) of the patients were hemodynamically unstable, whereas during the hospital stay, it was 0.41 (95%CI: 0.12-0.78, I2 = 89%). The rate of shock on admission was 0.25 (95%CI: 0.19-0.32, I2 = 98%), whereas 0.24 (95%CI: 0.17-0.33, I2 = 97%) developed shock during hospitalization. As an overall effect, one in four PUB patients was affected by HI or shock on admission or during hospital stay; 0.25 (95%CI: 0.21-0.30, I2 = 98%) (SupplementaryFigure 1).
The studies included in this plot contain various upper GIB sources. All the studies that reported HI were assessed on admission, with a rate of 0.33 (95%CI: 0.21-0.48, I2 = 97%). Seventeen studies were included in the shock on admission subgroup with a rate of 0.15 (95%CI: 0.09-0.25, I2 = 99%), whereas 18 studies evaluated shock during hospitalization with a rate of 0.20 (95%CI: 0.12-0.32, I2 = 100%). In total, one in five patients with upper GIB developed shock or HI; 0.20 (95%CI: 0.15-0.27, I2 = 100%) (Supplementary Figure 2).
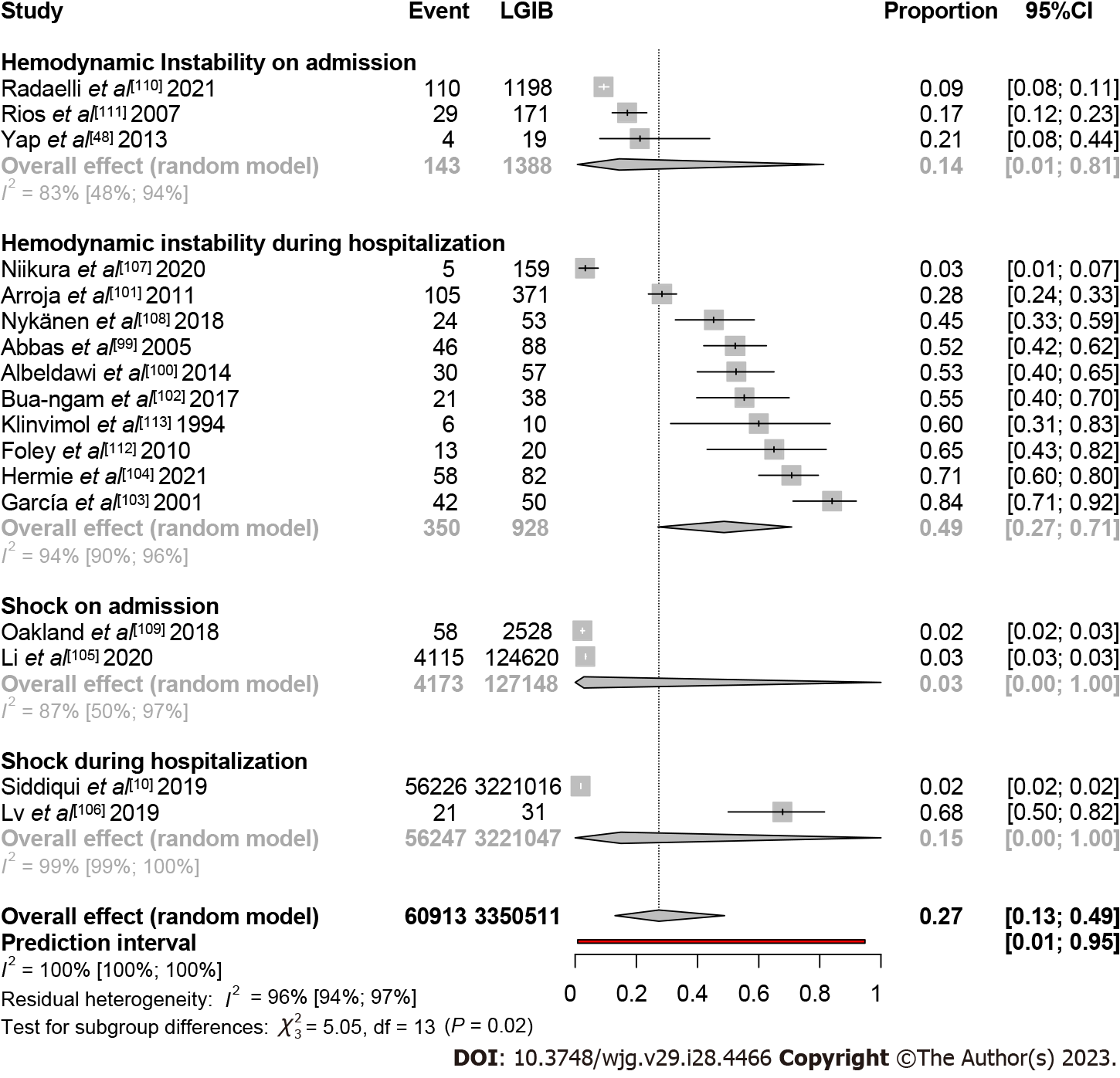
In total, 17 studies were included in this analysis[10,48,99-113]. Thirteen studies evaluated HI in lower GIB population: Three studies on admission with a rate of 0.14 (95%CI: 0.01-0.81, I2 = 83%), and 10 studies during hospitalization with a rate of 0.49 (95%CI: 0.27-0.71, I2 = 94%). Two studies assessed shock on admission, Oakland et al[109] and Li et al[105] where the pooled event rates were 0.02 (95%CI: 0.02-0.03) and 0.03 (95%CI: 0.03-0.03), respectively. Another two studies assessed shock during hospital stay. In the study by Siddiqui et al[10] the shock rate was 0.02 (95%CI: 0.02-0.02). The study by Lv and Gu[106], which involved patients with life-threatening bleeding, resulted in the highest pooled event rate of shock with a rate of 0.68 (95%CI: 0.50-0.82). In total, of the general lower GIB population, 0.27 (95%CI: 0.13-0-49, I2 = 100%) developed shock or HI (Figure 5).
All studies assessed the investigated outcomes on admission only. Six studies evaluated shock in colonic diverticular bleeding (CDB) with a rate of 0.12 (95%CI: 0.05-0.26, I2 = 91%). Only two studies reported HI, that of Gilshtein et al[114] reported a rate of 0.05 (95%CI: 0.02-0.11), and Ichiba et al[115] a rate of 0.21 (95%CI: 0.17-0.26). As an overall effect, the proportion of shock and HI in CDB was 0.12 (95%CI: 0.06-0.22, I2 = 90%) (Supplementary Figure 3).
Most of the studies received a score of 6 or higher, indicating a moderate to low risk of bias. Only 10 studies were rated with a score less than six. The sample size was not adequate in 33 studies. The results of the risk of bias assessment are presented in Supplementary Table 5.
Serious heterogeneity (with more than 80%) was observed in all our analyses. The large number of included studies with heterogeneous populations regarding age and sex could explain this. The definitions of HI and shock in the studies were not the same resulting in considerable heterogeneity, too.
All of our meta-analytical calculations that included 10 or more studies were investigated for publication bias. CDB was an exception where only eight studies were included. We found potential publication bias in all of our analyses except for non-variceal bleeding based on Egger’s test. This result could be explained by the very large heterogeneity of the study estimates. Additionally, a highly influential large study by Siddiqui et al[10] led to a false positive result for Egger’s test.
Leave-one-out sensitivity analysis showed some variability for some potential outliers. The proportion of our outcomes changed from 0.25 (95%CI: 017-0.36, I2 = 100%) to 0.29 (95%CI: 0.22-0.37, I2 = 90%) if Siddiqui et al[10] study was eliminated from the GIB analysis. This study did not only include a large sample size compared to other studies but also used the National Inpatient Sample database using International Classification of Diseases (ICD-9) codes to analyze patient data, which might have failed to identify some affected patients. Results of Egger’s test, funnel plots, and leave-one-out analysis are found in Supplementary Figures 4-16.
Based on the results and the careful evaluation of the evidence level, the certainty levels were low or very low for each outcome. The very high heterogeneity in almost all analyses was the main reason for that. In addition, all the included studies were considered observational studies, which contributes to the low level of evidence. (SupplementaryTables 6-12).
Our study found that HI and shock are common complications of GIB. Either shock or HI affects one in every four patients; even the lowest proportion, one in eight colonic diverticular bleeders, is still a significant portion of patients.
Variceal bleeding resulted in the highest HI on admission, with a rate of (38%) among various bleeding sources. In contrast, the highest HI rates during hospitalization were observed in PUB (41%) and LGIB (49%). The rate of shock on admission was generally the highest among different non-variceal bleeding sources (36%), whereas PUB specifically led to the highest rate of shock during hospitalization (24%).
Our results about unspecified GIB sources, non-variceal, and PUB showed higher rates of HI during hospitalization than on admission and higher rates of shock on admission than during hospitalization. In contrast, variceal bleeding showed higher rates of HI and shock on admission than during hospitalization. Lower GIB, on the other hand, showed higher rates of these outcomes during hospitalization than on admission.
Blood loss leads to HI characterized by a decrease in systolic blood pressure (BP) and an increase in heart rate (HR). Eventually, it can lead to a more severe state of shock, which is caused by a rapid reduction of intravascular blood volume resulting in decreasing hemoglobin levels, thereby decreasing the oxygen delivery capacity of the heart. HI is not just a sign; it is the starting point of a chain of events leading to hypoxemia and hypoperfusion. If it is not appropriately treated as soon as possible, it will lead to multiple organ failures. Therefore, health care providers must emphasize continuous monitoring and efficient stabilization for those patients[11].
Serious heterogeneity was observed in all our analyses. The reason for this lies in the large number of included articles. The population had different geographical locations, ethnicities, several comorbidities, age ranges, and access to different qualities of health care systems. Thus, there was even a variation in the definitions; most of the included studies defined HI as a decrease of systolic BP < 100 mmHg and/or an increase in HR > 100 bpm[6]. However, some definitions included syncope, orthostatic changes[115], or signs of organ hypoperfusion[52]. All these factors contributed noticeably, resulting in a very serious heterogeneity. All definitions of HI and shock can be found in Supplementary Tables 13 and 14, respectively.
Possible predictors were observed that resulted in higher rates of our investigated outcomes. We observed some outliers in different sources of bleeding; in variceal bleeding, intensive care unit admission[79,82,97], elderly population[20], and severe uncontrolled bleeding[75] were possible predictors for higher rates of shock and HI. In non-variceal bleeding, elderly patients > 60 years[20] and those who underwent embolization[60] accounted for the highest rate of HI on admission and during hospitalization, respectively. As for upper GIB in general, the study by Chirapongsathorn et al[21] included variceal and non-variceal bleeders, where they defined shock as mean arterial pressure lower than 50 mmHg, which results in a very high rate of shock (75%).
Lower GIB is three times less common than upper GIB and has not been the focus of much attention yet. Mortality rises to 20%-40% in the case of massive lower GIB complicated by unstable hemodynamics[116]. Super-selective patients who underwent arterial embolization[104], angiography[112], or were diagnosed with acute severe bleeding[103] showed higher rates of the investigated outcomes.
This is the first comprehensive overview to assess the proportion of patients affected by HI and shock in GIB and specify it according to the bleeding source. Our study included many studies with an extensive sample size. Additionally, subgroup analysis, which was based on the time of assessment, whether on admission or during hospital stay, provided a more precise overview. This study also gives an insight into some of the possible predictors that result in higher rates of our investigated outcomes.
Considering the limitations of this work, the definitions of HI and shock were different among the included studies or even missing. Different characteristics of the included population led to high heterogeneity in almost all analyses. The presence of low certainty of evidence in some domains is another limitation.
Based on our results, we suggest standardizing the definition of HI and shock, and establishing a protocol to proactively screen and monitor the affected patients in routine management. Physicians involved in the treatment of the affected patients should focus more on early and rapid correction of hemodynamics because it significantly decreases mortality[11]. Therefore, a careful pre-endoscopic assessment and strong adherence to risk stratification scores need to be highlighted. Furthermore, cautious care and continuous monitoring of the affected patients should be emphasized, especially for high-risk patients.
Our study has provided clear evidence that hemodynamic instability and shock are common presentations and complications of GIB. On the basis of our findings, a high majority of patients are affected; one in five, one in four and one in eight patients develops shock or hemodynamic instability on admission or during the hospital stay in the case of non-variceal, variceal, and colonic diverticular bleeding, respectively. Patients need a more proactive treatment strategy and require continuous monitoring to prevent untoward outcomes.
Hemodynamic instability (HI) and shock are associated with unfavorable outcomes in gastrointestinal bleeding (GIB). Understanding the proportion of these outcomes is essential for several reasons. Firstly, it provides valuable insight into the severity and potential risks associated with the condition. Knowing the proportion of patients who develop shock or HI helps healthcare providers anticipate the need for immediate interventions and allocate appropriate resources accordingly.
At the time of our systematic search, there was no data in the current literature describing these proportions in GIB based on the bleeding source. Additionally, monitoring changes in these patients over time can serve as an indicator of the effectiveness of medical interventions and guide future treatment strategies to improve patient outcomes.
Our aim is to quantify the pooled event rates of HI and shock in GIB. This will help in risk stratification and determining the overall severity of the condition. By understanding how frequently these outcomes occur, healthcare providers can identify high-risk patients who require immediate and intensive management.
We conducted a systematic review with meta-analysis to determine the proportions of HI and shock in different GIB sources. The R programming language, using the meta package, was employed to perform statistical analysis on the data. Forest plots were utilized to summarize the study findings and present the results. Pooled event rates with 95%CIs, were computed to provide a measure of the overall outcomes.
The overall proportion of HI and shock was found to be 25% across all sources of GIB, 22% in non-variceal bleeding, 25% in variceal bleeding, and 12% in colonic diverticular bleeding. However, our findings also revealed a high degree of heterogeneity, highlighting the significance of our study. This heterogeneity suggests a lack of consensus in the guidelines in this field, as evidenced by the varied definitions of our included outcomes.
Our study provides compelling evidence that HI and shock are frequently observed complications and presentations in GIB. One in four patients with GIB develops shock or HI on admission or during the hospital stay.
Given our findings, we recommend the establishment of a standardized definition for HI and shock in GIB. Additionally, implementing a protocol for proactive screening and continuous monitoring of affected patients should be considered as part of routine management. Emphasizing a thorough pre-endoscopic assessment and strict adherence to risk stratification scores is crucial. Furthermore, rigorous care and attentive monitoring should be emphasized, particularly for high-risk patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Hungarian Society of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Hungary
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kotlyarov S, Russia; Sánchez JIA, Colombia S-Editor: Li L L-Editor: A P-Editor: Yuan YY
| 1. | Oakland K. Changing epidemiology and etiology of upper and lower gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2019;42-43:101610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 2. | Marmo R, Koch M, Cipolletta L, Capurso L, Pera A, Bianco MA, Rocca R, Dezi A, Fasoli R, Brunati S, Lorenzini I, Germani U, Di Matteo G, Giorgio P, Imperiali G, Minoli G, Barberani F, Boschetto S, Martorano M, Gatto G, Amuso M, Pastorelli A, Torre ES, Triossi O, Buzzi A, Cestari R, Della Casa D, Proietti M, Tanzilli A, Aragona G, Giangregorio F, Allegretta L, Tronci S, Michetti P, Romagnoli P, Nucci A, Rogai F, Piubello W, Tebaldi M, Bonfante F, Casadei A, Cortini C, Chiozzini G, Girardi L, Leoci C, Bagnalasta G, Segato S, Chianese G, Salvagnini M, Rotondano G. Predictive factors of mortality from nonvariceal upper gastrointestinal hemorrhage: a multicenter study. Am J Gastroenterol. 2008;103:1639-47; quiz 1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Zheng NS, Tsay C, Laine L, Shung DL. Trends in characteristics, management, and outcomes of patients presenting with gastrointestinal bleeding to emergency departments in the United States from 2006 to 2019. Aliment Pharmacol Ther. 2022;56:1543-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Abougergi MS, Travis AC, Saltzman JR. The in-hospital mortality rate for upper GI hemorrhage has decreased over 2 decades in the United States: a nationwide analysis. Gastrointest Endosc. 2015;81:882-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 5. | Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, Jensen ET, Shaheen NJ, Barritt AS, Lieber SR, Kochar B, Barnes EL, Fan YC, Pate V, Galanko J, Baron TH, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156:254-272.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1079] [Article Influence: 179.8] [Reference Citation Analysis (1)] |
| 6. | Moledina SM, Komba E. Risk factors for mortality among patients admitted with upper gastrointestinal bleeding at a tertiary hospital: a prospective cohort study. BMC Gastroenterol. 2017;17:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Laursen SB, Leontiadis GI, Stanley AJ, Møller MH, Hansen JM, Schaffalitzky de Muckadell OB. Relationship between timing of endoscopy and mortality in patients with peptic ulcer bleeding: a nationwide cohort study. Gastrointest Endosc. 2017;85:936-944.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 8. | Parker ME, Khasawneh MA, Thiels CA, Berns KS, Stubbs JR, Jenkins DH, Zietlow SP, Zielinski MD. Prehospital Transfusion for Gastrointestinal Bleeding. Air Med J. 2017;36:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Edelson JC, Edelson CV, Rockey DC, Chung KK, Robles MJ, Subramanian SR, Aden JK, Gancayco JG. Improving haemodynamics in acute gastrointestinal bleeding: Ketamine for endoscopic sedation in active gastrointestinal bleeding in critically Ill patients. Gastro Hep. 2020;2:288-294. [DOI] [Full Text] |
| 10. | Siddiqui NS, Paul S, Khan Z, Javaid T, Hasan SS, Saleh J, Federman DJ, Khuder S, Nawras A. Rising Events and Improved Outcomes of Gastrointestinal Bleed With Shock in USA: A 12-year National Analysis. J Clin Gastroenterol. 2019;53:e194-e201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Baradarian R, Ramdhaney S, Chapalamadugu R, Skoczylas L, Wang K, Rivilis S, Remus K, Mayer I, Iswara K, Tenner S. Early intensive resuscitation of patients with upper gastrointestinal bleeding decreases mortality. Am J Gastroenterol. 2004;99:619-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Gralnek IM, Stanley AJ, Morris AJ, Camus M, Lau J, Lanas A, Laursen SB, Radaelli F, Papanikolaou IS, Cúrdia Gonçalves T, Dinis-Ribeiro M, Awadie H, Braun G, de Groot N, Udd M, Sanchez-Yague A, Neeman Z, van Hooft JE. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2021. Endoscopy. 2021;53:300-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 266] [Article Influence: 66.5] [Reference Citation Analysis (1)] |
| 13. | Gralnek IM, Camus Duboc M, Garcia-Pagan JC, Fuccio L, Karstensen JG, Hucl T, Jovanovic I, Awadie H, Hernandez-Gea V, Tantau M, Ebigbo A, Ibrahim M, Vlachogiannakos J, Burgmans MC, Rosasco R, Triantafyllou K. Endoscopic diagnosis and management of esophagogastric variceal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:1094-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 14. | Laine L, Barkun AN, Saltzman JR, Martel M, Leontiadis GI. ACG Clinical Guideline: Upper Gastrointestinal and Ulcer Bleeding. Am J Gastroenterol. 2021;116:899-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 282] [Article Influence: 70.5] [Reference Citation Analysis (36)] |
| 15. | Sengupta N, Feuerstein JD, Jairath V, Shergill AK, Strate LL, Wong RJ, Wan D. Management of Patients With Acute Lower Gastrointestinal Bleeding: An Updated ACG Guideline. Am J Gastroenterol. 2023;118:208-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 81] [Reference Citation Analysis (33)] |
| 16. | Kim JH, Park SW, Jung JH, Park DH, Bang CS, Park CH, Park JW, Park JG. Bedside risk-scoring model for predicting 6-week mortality in cirrhotic patients undergoing endoscopic band ligation for acute variceal bleeding. J Gastroenterol Hepatol. 2021;36:1935-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Rotondano G, Cipolletta L, Koch M, Bianco MA, Grossi E, Marmo R; PNED (Progetto Nazionale Emorragie Digestive) Investigators. Predictors of favourable outcome in non-variceal upper gastrointestinal bleeding: implications for early discharge? Dig Liver Dis. 2014;46:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Lanas A, Polo-Tomas M, García-Rodríguez LA, García S, Arroyo-Villarino MT, Ponce J, Bujanda L, Calleja JL, Calvet X, Feu F, Perez-Aisa A, Sung JJ. Effect of proton pump inhibitors on the outcomes of peptic ulcer bleeding: comparison of event rates in routine clinical practice and a clinical trial. Scand J Gastroenterol. 2013;48:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Tsoi KK, Chiu PW, Chan FK, Ching JY, Lau JY, Sung JJ. The risk of peptic ulcer bleeding mortality in relation to hospital admission on holidays: a cohort study on 8,222 cases of peptic ulcer bleeding. Am J Gastroenterol. 2012;107:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Elsebaey MA, Elashry H, Elbedewy TA, Elhadidy AA, Esheba NE, Ezat S, Negm MS, Abo-Amer YE, Abgeegy ME, Elsergany HF, Mansour L, Abd-Elsalam S. Predictors of in-hospital mortality in a cohort of elderly Egyptian patients with acute upper gastrointestinal bleeding. Medicine (Baltimore). 2018;97:e0403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Chirapongsathorn S, Akkarachinores K, Chaiprasert A. Development and validation of prognostic model to predict mortality among cirrhotic patients with acute variceal bleeding: A retrospective study. JGH Open. 2021;5:658-663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Garrido A, Giráldez A, Trigo C, Leo E, Guil A, Márquez JL. Intravenous proton-pump inhibitor for acute peptic ulcer bleeding--is profound acid suppression beneficial to reduce the risk of rebleeding? Rev Esp Enferm Dig. 2008;100:466-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40519] [Article Influence: 10129.8] [Reference Citation Analysis (2)] |
| 24. | Chandler J, Hopewell S. Cochrane methods--twenty years experience in developing systematic review methods. Syst Rev. 2013;2:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, Stewart L. PROSPERO at one year: an evaluation of its utility. Syst Rev. 2013;2:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 1780] [Article Influence: 178.0] [Reference Citation Analysis (0)] |
| 27. | Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4813] [Cited by in RCA: 7110] [Article Influence: 474.0] [Reference Citation Analysis (0)] |
| 28. | Schwarzer G, Chemaitelly H, Abu-Raddad LJ, Rücker G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods. 2019;10:476-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 389] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 29. | IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 670] [Cited by in RCA: 1283] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 30. | Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693-2710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 928] [Cited by in RCA: 1223] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 31. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25801] [Article Influence: 1121.8] [Reference Citation Analysis (0)] |
| 32. | Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3796] [Cited by in RCA: 4932] [Article Influence: 352.3] [Reference Citation Analysis (0)] |
| 33. | Harrer M, Cuijpers P, Furukawa T, Ebert D. Doing Meta-Analysis With R: A Hands-On Guide. 1st ed. New York: Chapman and Hall/CRC Press, 2021. [DOI] [Full Text] |
| 34. | IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6:e010247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 688] [Cited by in RCA: 1230] [Article Influence: 136.7] [Reference Citation Analysis (0)] |
| 35. | Ballester-Clau R, Torres Vicente G, Voltà-Pardo T, López-Barroso L, Cucala-Ramos M, Reñé-Espinet JM, Planella de Rubinat M. Clinical experience with ferric carboxymaltose in the management of anemia in acute gastrointestinal bleeding. Eur J Gastroenterol Hepatol. 2019;31:116-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Cangemi DJ, Krill T, Weideman R, Cipher DJ, Spechler SJ, Feagins LA. A Comparison of the Rate of Gastrointestinal Bleeding in Patients Taking Non-Vitamin K Antagonist Oral Anticoagulants or Warfarin. Am J Gastroenterol. 2017;112:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Catano J, Sacleux SC, Gornet JM, Camus M, Bigé N, Saliba F, Azoulay E, Dumas G, Zafrani L. Gastrointestinal bleeding in critically ill immunocompromised patients. Ann Intensive Care. 2021;11:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 38. | Hampers MJ, Surgenor SD, Spanjian K, Clerico T, Corwin HL. ICU care for patients with gastrointestinal bleeding: Impact on cost and outcome. Clin Intensive Care. 2002;13:109-113. [DOI] [Full Text] |
| 39. | Konecki D, Grabowska-Derlatka L, Pacho R, Rowiński O. Correlation Between Findings of Multislice Helical Computed Tomography (CT), Endoscopic Examinations, Endovascular Procedures, and Surgery in Patients with Symptoms of Acute Gastrointestinal Bleeding. Pol J Radiol. 2017;82:676-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Lee L, Iqbal S, Najmeh S, Fata P, Razek T, Khwaja K. Mesenteric angiography for acute gastrointestinal bleed: predictors of active extravasation and outcomes. Can J Surg. 2012;55:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Mehta A, Kim S, Ahmed O, Zangan S, Ha TV, Navuluri R, Funaki B. Outcomes of Patients with Left Ventricular Assist Devices Undergoing Mesenteric Angiography for Gastrointestinal Bleeding. J Vasc Interv Radiol. 2015;26:1710-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Mohan P, Manov J, Diaz-Bode A, Venkat S, Langston M, Naidu A, Howse R, Narayanan G. Clinical predictors of arterial extravasation, rebleeding and mortality following angiographic interventions in gastrointestinal bleeding. J Gastrointestin Liver Dis. 2018;27:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Nagata N, Sakurai T, Moriyasu S, Shimbo T, Okubo H, Watanabe K, Yokoi C, Yanase M, Akiyama J, Uemura N. Impact of INR monitoring, reversal agent use, heparin bridging, and anticoagulant interruption on rebleeding and thromboembolism in acute gastrointestinal bleeding. PLoS One. 2017;12:e0183423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Nishida K, Nojiri I, Kato M, Higashijima M, Takagi K, Akashi R. Upper gastrointestinal bleeding in the elderly. Nihon Ronen Igakkai Zasshi. 1992;29:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 45. | Oprita R, Ilie M, Sandru V, Berceanu D, Constantinescu G. Gastrointestinal bleeding in patients admitted to the intensive care unit. Arch Balk Med Union. 2018;53:544-550. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 46. | Robert R, Gissot V, Pierrot M, Laksiri L, Mercier E, Prat G, Villers D, Vincent JF, Hira M, Vignon P, Charlot P, Burucoa C. Helicobacter pylori infection is not associated with an increased hemorrhagic risk in patients in the intensive care unit. Crit Care. 2006;10:R77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Sàbat M, Kolle L, Soriano G, Ortiz J, Pamplona J, Novella MT, Villanueva C, Sainz S, Torras J, Balanzó J, Guarner C. Parenteral antibiotic prophylaxis of bacterial infections does not improve cost-efficacy of oral norfloxacin in cirrhotic patients with gastrointestinal bleeding. Am J Gastroenterol. 1998;93:2457-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Yap FY, Omene BO, Patel MN, Yohannan T, Minocha J, Knuttinen MG, Owens CA, Bui JT, Gaba RC. Transcatheter embolotherapy for gastrointestinal bleeding: a single center review of safety, efficacy, and clinical outcomes. Dig Dis Sci. 2013;58:1976-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, Giovo I, Uschner FE, Jansen C, Jimenez C, Mookerjee R, Gustot T, Albillos A, Bañares R, Jarcuska P, Steib C, Reiberger T, Acevedo J, Gatti P, Shawcross DL, Zeuzem S, Zipprich A, Piano S, Berg T, Bruns T, Danielsen KV, Coenraad M, Merli M, Stauber R, Zoller H, Ramos JP, Solé C, Soriano G, de Gottardi A, Gronbaek H, Saliba F, Trautwein C, Kani HT, Francque S, Ryder S, Nahon P, Romero-Gomez M, Van Vlierberghe H, Francoz C, Manns M, Garcia-Lopez E, Tufoni M, Amoros A, Pavesi M, Sanchez C, Praktiknjo M, Curto A, Pitarch C, Putignano A, Moreno E, Bernal W, Aguilar F, Clària J, Ponzo P, Vitalis Z, Zaccherini G, Balogh B, Gerbes A, Vargas V, Alessandria C, Bernardi M, Ginès P, Moreau R, Angeli P, Jalan R, Arroyo V; PREDICT STUDY group of the EASL-CLIF CONSORTIUM. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021;74:1097-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 50. | Van Weyenberg SJ, Van Turenhout ST, Jacobs MA, Bouma G, Mulder CJ. Video capsule endoscopy for previous overt obscure gastrointestinal bleeding in patients using anti-thrombotic drugs. Dig Endosc. 2012;24:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Abougergi MS, Peluso H, Mrad C, Saltzman JR. The Impact of Obesity on Mortality and Other Outcomes in Patients With Nonvariceal Upper Gastrointestinal Hemorrhage in the United States. J Clin Gastroenterol. 2019;53:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Ahn DW, Park YS, Lee SH, Shin CM, Hwang JH, Kim JW, Jeong SH, Kim N, Lee DH. Clinical outcome of acute nonvariceal upper gastrointestinal bleeding after hours: the role of urgent endoscopy. Korean J Intern Med. 2016;31:470-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Baracat FI, de Moura DTH, Brunaldi VO, Tranquillini CV, Baracat R, Sakai P, de Moura EGH. Randomized controlled trial of hemostatic powder versus endoscopic clipping for non-variceal upper gastrointestinal bleeding. Surg Endosc. 2020;34:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 54. | Di Felice G. Endoscopic injection treatment in patients with shock and gastrointestinal bleeding or stigmata of recent hemorrhage. Endoscopy. 1987;19:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 55. | Edmunds SEJ, Laurence BH. Endoscopic ethanol sclerotherapy in non-variceal gastrointestinal bleeding. J Gastroen Hepatol. 1988;3:355-360. [DOI] [Full Text] |
| 56. | Gao F, Chen X, Zhang J. Treatment of Acute Nonvariceal Upper Gastrointestinal Bleeding in Chinese Patients on Antithrombotic Therapy. Gastroenterol Res Pract. 2019;2019:9190367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | González-González JA, Vázquez-Elizondo G, García-Compeán D, Gaytán-Torres JO, Flores-Rendón ÁR, Jáquez-Quintana JO, Garza-Galindo AA, Cárdenas-Sandoval MG, Maldonado-Garza HJ. Predictors of in-hospital mortality in patients with non-variceal upper gastrointestinal bleeding. Rev Esp Enferm Dig. 2011;103:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Hwang S, Jeon SW, Kwon JG, Lee DW, Ha CY, Cho KB, Jang B, Park JB, Park YS; Daegu-Gyengbuk Gastrointestinal Study Group (DGSG). The Novel Scoring System for 30-Day Mortality in Patients with Non-variceal Upper Gastrointestinal Bleeding. Dig Dis Sci. 2016;61:2002-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Jairath V, Kahan BC, Stanworth SJ, Logan RF, Hearnshaw SA, Travis SP, Palmer KR, Murphy MF. Prevalence, management, and outcomes of patients with coagulopathy after acute nonvariceal upper gastrointestinal bleeding in the United Kingdom. Transfusion. 2013;53:1069-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 60. | Kwon JH, Han YH. Efficacy and safety of superselective trans-catheter arterial embolization of upper and lower gastrointestinal bleeding using N-butyl-2-cyanoacrylate. Emerg Radiol. 2018;25:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Lai YC, Hung MS, Chen YH, Chen YC. Comparing AIMS65 Score With MEWS, qSOFA Score, Glasgow-Blatchford Score, and Rockall Score for Predicting Clinical Outcomes in Cirrhotic Patients With Upper Gastrointestinal Bleeding. J Acute Med. 2018;8:154-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 62. | Maggio D, Barkun AN, Martel M, Elouali S, Gralnek IM; Reason Investigators. Predictors of early rebleeding after endoscopic therapy in patients with nonvariceal upper gastrointestinal bleeding secondary to high-risk lesions. Can J Gastroenterol. 2013;27:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | Morsy KH, Ghaliony MA, Mohammed HS. Outcomes and predictors of in-hospital mortality among cirrhotic patients with non-variceal upper gastrointestinal bleeding in upper Egypt. Turk J Gastroenterol. 2014;25:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Nguyen GC, Dinani AM, Pivovarov K. Endoscopic management and outcomes of pregnant women hospitalized for nonvariceal upper GI bleeding: a nationwide analysis. Gastrointest Endosc. 2010;72:954-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Park CH, Han DS, Jeong JY, Eun CS, Yoo KS, Jeon YC, Sohn JH. Outcomes of Propofol Sedation During Emergency Endoscopy Performed for Upper Gastrointestinal Bleeding. Dig Dis Sci. 2016;61:825-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Restellini S, Kherad O, Jairath V, Martel M, Barkun AN. Red blood cell transfusion is associated with increased rebleeding in patients with nonvariceal upper gastrointestinal bleeding. Aliment Pharmacol Ther. 2013;37:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Sey MSL, Mohammed SB, Brahmania M, Singh S, Kahan BC, Jairath V. Comparative outcomes in patients with ulcer- vs non-ulcer-related acute upper gastrointestinal bleeding in the United Kingdom: a nationwide cohort of 4474 patients. Aliment Pharmacol Ther. 2019;49:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Wierzchowski P, Dabrowiecki S, Szczesny W, Szmytkowski J. Nonvariceal upper gastrointestinal tract bleeding - risk factors and the value of emergency endoscopy. Arch Med Sci. 2013;9:843-848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Zhang JY, Wang Y, Zhang J, Ding SG, Zhou LY, Lin SR. Risk factors associated with failure from endoscopic therapy in acute non-variceal upper gastrointestinal bleeding. Beijing Daxue Xuebao Yixueban. 2010;42:703-707. [PubMed] |
| 70. | Bunchorntavakul C, Yodket Y, Singhasena N. Clinical Characteristics, Treatment Outcomes and Risk Assessment of Patients with Acute Upper Gastrointestinal Bleeding in Rajavithi Hospital, Thailand. J Med Assoc Thai. 2017;100 Suppl 1:S104-S115. [PubMed] |
| 71. | Wang HM, Hsu PI, Lo GH, Chen TA, Cheng LC, Chen WC, Lin CK, Yu HC, Chan HH, Tsai WL, Wang EM, Lai KH. Comparison of hemostatic efficacy for argon plasma coagulation and distilled water injection in treating high-risk bleeding ulcers. J Clin Gastroenterol. 2009;43:941-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Amitrano L, Guardascione MA, Martino R, Manguso F, Menchise A, Balzano A. Hypoxic hepatitis occurring in cirrhosis after variceal bleeding: still a lethal disease. J Clin Gastroenterol. 2012;46:608-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Ardevol A, Ibañez-Sanz G, Profitos J, Aracil C, Castellvi JM, Alvarado E, Cachero A, Horta D, Miñana J, Gomez-Pastrana B, Pavel O, Dueñas E, Casas M, Planella M, Castellote J, Villanueva C. Survival of patients with cirrhosis and acute peptic ulcer bleeding compared with variceal bleeding using current first-line therapies. Hepatology. 2018;67:1458-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 74. | Bilal M, Abougergi MS, Tayyem O, Parupudi S, Rockey DC. Thirty-Day Readmission After Esophageal Variceal Hemorrhage and its Impact on Outcomes in the United States. J Clin Gastroenterol. 2020;54:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Choi JY, Jo YW, Lee SS, Kim WS, Oh HW, Kim CY, Yun EY, Kim JJ, Lee JM, Kim HJ, Kim TH, Jung WT, Lee OJ, Kim RB. Outcomes of patients treated with Sengstaken-Blakemore tube for uncontrolled variceal hemorrhage. Korean J Intern Med. 2018;33:696-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Fallatah HI, Al Nahdi H, Al Khatabi M, Akbar HO, Qari YA, Sibiani AR, Bazaraa S. Variceal hemorrhage: Saudi tertiary center experience of clinical presentations, complications and mortality. World J Hepatol. 2012;4:268-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Hassanien M, El-Ghannam M, El-Talkawy MD, Abdelrahman Y, Attar GE, Taleb HA. Risk scoring systems to predict in-hospital mortality in patients with acute variceal bleeding due to HCV-induced liver cirrhosis. Gastroenterology Insights. 2018;9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 78. | Hermie L, Dhondt E, Vanlangenhove P, Hoste E, Geerts A, Defreyne L. Model for end-stage liver disease score and hemodynamic instability as a predictor of poor outcome in early transjugular intrahepatic portosystemic shunt treatment for acute variceal hemorrhage. Eur J Gastroenterol Hepatol. 2018;30:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Ismail FW, Shah HA, Hamid S, Abbas Z, Abid S, Mumtaz K, Jafri W. Noninvasive predictors of large varices in patients hospitalized with gastroesophageal variceal hemorrhage. Hepatol Int. 2008;2:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 80. | Kim DH, Cho E, Jun CH, Son DJ, Lee MJ, Park CH, Cho SB, Park SY, Kim HS, Choi SK, Rew JS. Risk Factors and On-site Rescue Treatments for Endoscopic Variceal Ligation Failure. Korean J Gastroenterol. 2018;72:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 81. | Kim SE, Jung DM, Park JW, Ju Y, Lee B, Kim HS, Suk KT, Jang MK, Park SH, Kang JG, Soh JS, Lim H, Kang HS, Moon SH, Kim C, Lee S, Kim JH, Lee MS, Kim DJ, Ihm SH, Park C. Baseline Renal Function Predicts Hyponatremia in Liver Cirrhosis Patients Treated with Terlipressin for Variceal Bleeding. Gastroenterol Res Pract. 2017;2017:7610374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Lee H, Hawker FH, Selby W, McWilliam DB, Herkes RG. Intensive care treatment of patients with bleeding esophageal varices: results, predictors of mortality, and predictors of the adult respiratory distress syndrome. Crit Care Med. 1992;20:1555-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 83. | Liu TT, Wong WJ, Hou MC, Lin HC, Chang FY, Lee SD. Hemorheology in patients with liver cirrhosis: special emphasis on its relation to severity of esophageal variceal bleeding. J Gastroenterol Hepatol. 2006;21:908-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 84. | Liu Y, Yang J, Wang J, Chai G, Sun G, Wang Z, Yang Y. Clinical characteristics and endoscopic treatment with cyanoacrylate injection in patients with duodenal varices. Scand J Gastroenterol. 2009;44:1012-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 85. | Maiwall R, Kumar A, Bhadoria AS, Jindal A, Kumar G, Bhardwaj A, Maras JS, Sharma MK, Sharma BC, Sarin SK. Utility of N-acetylcysteine in ischemic hepatitis in cirrhotics with acute variceal bleed: a randomized controlled trial. Hepatol Int. 2020;14:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Naeshiro N, Aikata H, Kakizawa H, Hyogo H, Kan H, Fujino H, Kobayashi T, Fukuhara T, Honda Y, Ohno A, Miyaki D, Kawaoka T, Tsuge M, Hiraga N, Hiramatsu A, Imamura M, Kawakami Y, Takahashi S, Awai K, Chayama K. Long-term outcome of patients with gastric varices treated by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol. 2014;29:1035-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 87. | Singal AK, Jampana SC, Singal V, Kuo YF. Hepatocellular carcinoma predicts in-hospital mortality from acute variceal hemorrhage among patients with cirrhosis. J Clin Gastroenterol. 2012;46:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 88. | Villanueva C, Ortiz J, Sàbat M, Gallego A, Torras X, Soriano G, Sáinz S, Boadas J, Cussó X, Guarner C, Balanzó J. Somatostatin alone or combined with emergency sclerotherapy in the treatment of acute esophageal variceal bleeding: a prospective randomized trial. Hepatology. 1999;30:384-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 89. | Villanueva C, Piqueras M, Aracil C, Gómez C, López-Balaguer JM, Gonzalez B, Gallego A, Torras X, Soriano G, Sáinz S, Benito S, Balanzó J. A randomized controlled trial comparing ligation and sclerotherapy as emergency endoscopic treatment added to somatostatin in acute variceal bleeding. J Hepatol. 2006;45:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 90. | Sung JJ, Chung SC, Yung MY, Lai CW, Lau JY, Lee YT, Leung VK, Li MK, Li AK. Prospective randomised study of effect of octreotide on rebleeding from oesophageal varices after endoscopic ligation. Lancet. 1995;346:1666-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 134] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 91. | Thomopoulos K, Theocharis G, Mimidis K, Lampropoulou-Karatza Ch, Alexandridis E, Nikolopoulou V. Improved survival of patients presenting with acute variceal bleeding. Prognostic indicators of short- and long-term mortality. Dig Liver Dis. 2006;38:899-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 92. | Tsai MH, Huang HC, Peng YS, Chen YC, Tian YC, Yang CW, Lien JM, Fang JT, Hou MC, Shen CH, Huang CC, Wu CS, Lee FY. Nutrition Risk Assessment Using the Modified NUTRIC Score in Cirrhotic Patients with Acute Gastroesophageal Variceal Bleeding: Prevalence of High Nutrition Risk and its Independent Prognostic Value. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Tsai MH, Huang HC, Peng YS, Chen YC, Tian YC, Yang CW, Lien JM, Fang JT, Wu CS, Lee FY. Critical illness-related corticosteroid insufficiency in cirrhotic patients with acute gastroesophageal variceal bleeding: risk factors and association with outcome*. Crit Care Med. 2014;42:2546-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 94. | Vuachet D, Cervoni JP, Vuitton L, Weil D, Dritsas S, Dussaucy A, Koch S, Di Martino V, Thevenot T. Improved survival of cirrhotic patients with variceal bleeding over the decade 2000-2010. Clin Res Hepatol Gastroenterol. 2015;39:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 95. | Senosiain Lalastra C, Arribas Anta J, Moreira Vicente V, Martínez González J, Maroto Castellanos M, García Sánchez MC, Zaera de la Fuente C, López Durán S, Cañete Ruiz Á, Albillos Martínez A. Acute liver ischaemia after gastro-oesophageal variceal bleeding. Gastroenterol Hepatol. 2016;39:590-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 96. | Farooqi JI, Farooqi RJ. Predictors of the outcome after the first episode of acute variceal bleeding in liver cirrhosis patients. J Coll Physicians Surg Pak. 2001;11:379-382. |
| 97. | Thomas GA, Sugawa C, Joseph AL, Nakamura R, Saihara T, Inoue Y. Upper GI bleeding in an emergency hospital: Etiology, prognosis and improved survival by endoscopic hemostasis. Dig Endosc. 1992;4:199-208. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 98. | Gado A, Ebeid B, Abdelmohsen A, Axon A. Predictors of mortality in patients with acute upper gastrointestinal hemorrhage who underwent endoscopy and confirmed to have variceal hemorrhage. Alexandria journal of medicine. 2015;51:295-304. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 99. | Abbas SM, Bissett IP, Holden A, Woodfield JC, Parry BR, Duncan D. Clinical variables associated with positive angiographic localization of lower gastrointestinal bleeding. ANZ J Surg. 2005;75:953-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Albeldawi M, Ha D, Mehta P, Lopez R, Jang S, Sanaka MR, Vargo JJ. Utility of urgent colonoscopy in acute lower gastro-intestinal bleeding: a single-center experience. Gastroenterol Rep (Oxf). 2014;2:300-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 101. | Arroja B, Cremers I, Ramos R, Cardoso C, Rego AC, Caldeira A, Eliseu L, Silva JD, Glória L, Rosa I, Pedrosa J. Acute lower gastrointestinal bleeding management in Portugal: a multicentric prospective 1-year survey. Eur J Gastroenterol Hepatol. 2011;23:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 102. | Bua-Ngam C, Norasetsingh J, Treesit T, Wedsart B, Chansanti O, Tapaneeyakorn J, Panpikoon T, Vallibhakara SA. Efficacy of emergency transarterial embolization in acute lower gastrointestinal bleeding: A single-center experience. Diagn Interv Imaging. 2017;98:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 103. | García Sánchez M, González Galilea A, López Vallejos P, Gálvez Calderón C, Naranjo Rodríguez A, de Dios Vega J, Miño Fugarolas G. Role of early colonoscopy in severe acute lower gastrointestinal bleeding. Gastroenterol Hepatol. 2001;24:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Hermie L, Dhondt E, Vanlangenhove P, De Waele J, Degroote H, Defreyne L. Empiric cone-beam CT-guided embolization in acute lower gastrointestinal bleeding. Eur Radiol. 2021;31:2161-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 105. | Li B, Stein DJ, Schwartz J, Lipscey M, Feuerstein JD. Outcomes in lower GI bleeding comparing weekend with weekday admission. Gastrointest Endosc. 2020;92:675-680.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 106. | Lv LS, Gu JT. Super-selective arterial embolization in the control of acute lower gastrointestinal hemorrhage. World J Clin Cases. 2019;7:3728-3733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 107. | Niikura R, Nagata N, Yamada A, Honda T, Hasatani K, Ishii N, Shiratori Y, Doyama H, Nishida T, Sumiyoshi T, Fujita T, Kiyotoki S, Yada T, Yamamoto K, Shinozaki T, Takata M, Mikami T, Mabe K, Hara K, Fujishiro M, Koike K. Efficacy and Safety of Early vs Elective Colonoscopy for Acute Lower Gastrointestinal Bleeding. Gastroenterology. 2020;158:168-175.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 108. | Nykänen T, Peltola E, Kylänpää L, Udd M. Transcatheter Arterial Embolization in Lower Gastrointestinal Bleeding: Ischemia Remains a Concern Even with a Superselective Approach. J Gastrointest Surg. 2018;22:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 109. | Oakland K, Guy R, Uberoi R, Hogg R, Mortensen N, Murphy MF, Jairath V; UK Lower GI Bleeding Collaborative. Acute lower GI bleeding in the UK: patient characteristics, interventions and outcomes in the first nationwide audit. Gut. 2018;67:654-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 110. | Radaelli F, Frazzoni L, Repici A, Rondonotti E, Mussetto A, Feletti V, Spada C, Manes G, Segato S, Grassi E, Musso A, Di Giulio E, Coluccio C, Manno M, De Nucci G, Festa V, Di Leo A, Marini M, Ferraris L, Feliziani M, Amato A, Soriani P, Del Bono C, Paggi S, Hassan C, Fuccio L. Clinical management and patient outcomes of acute lower gastrointestinal bleeding. A multicenter, prospective, cohort study. Dig Liver Dis. 2021;53:1141-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 111. | Ríos A, Montoya MJ, Rodríguez JM, Serrano A, Molina J, Ramírez P, Parrilla P. Severe acute lower gastrointestinal bleeding: risk factors for morbidity and mortality. Langenbecks Arch Surg. 2007;392:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 112. | Foley PT, Ganeshan A, Anthony S, Uberoi R. Multi-detector CT angiography for lower gastrointestinal bleeding: Can it select patients for endovascular intervention? J Med Imaging Radiat Oncol. 2010;54:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 113. | Klinvimol T, Ho YH, Parry BR, Goh HS. Small bowel causes of per rectum haemorrhage. Ann Acad Med Singap. 1994;23:866-868. [PubMed] |
| 114. | Gilshtein H, Kluger Y, Khoury A, Issa N, Khoury W. Massive and recurrent diverticular hemorrhage, risk factors and treatment. Int J Surg. 2016;33 Pt A:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 115. | Ichiba T, Hara M, Miyahara K, Urashima M, Shintani A, Naitou H, Higashi R. Impact of Computed Tomography Evaluation Before Colonoscopy for the Management of Colonic Diverticular Hemorrhage. J Clin Gastroenterol. 2019;53:e75-e83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 116. | Miyakuni Y, Nakajima M, Ohbe H, Sasabuchi Y, Kaszynski RH, Ishimaru M, Matsui H, Fushimi K, Yamaguchi Y, Yasunaga H. Angiography versus colonoscopy in patients with severe lower gastrointestinal bleeding: a nation-wide observational study. Acute Med Surg. 2020;7:e533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |