Published online Jul 14, 2023. doi: 10.3748/wjg.v29.i26.4200
Peer-review started: April 24, 2023
First decision: May 16, 2023
Revised: May 20, 2023
Accepted: June 2, 2023
Article in press: June 2, 2023
Published online: July 14, 2023
Processing time: 77 Days and 1 Hours
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract. Tyrosine kinase inhibitors, such as imatinib, have been used as first-line therapy for the treatment of GISTs. Although these drugs have achieved considerable efficacy in some patients, reports of resistance and recurrence have emerged. Extracellular signal-regulated kinase 1/2 (ERK1/2) protein, as a member of the mitogen-activated protein kinase (MAPK) family, is a core molecule of this signaling pathway. Nowadays, research reports on the important clinical and prognostic value of phosphorylated-ERK (P-ERK) and phosphorylated-MAPK/ERK kinase (P-MEK) proteins closely related to raf kinase inhibitor protein (RKIP) have gradually emerged in digestive tract tumors such as gastric cancer, colon cancer, and pancreatic cancer. However, literature on the expression of these downstream proteins combined with RKIP in GIST is scarce. This study will focus on this aspect and search for answers to the problem.
To detect the expression of RKIP, P-ERK, and P-MEK protein in GIST and to analyze their relationship with clinicopathological characteristics and prognosis of this disease. Try to establish a new prognosis evaluation model using RKIP and P-ERK in combination with analysis and its prognosis evaluation efficacy.
The research object of our experiment was 66 pathologically diagnosed GIST patients with complete clinical and follow-up information. These patients received surgical treatment at China Medical University Affiliated Hospital from January 2015 to January 2020. Immunohistochemical method was used to detect the expression of RKIP, P-ERK, and P-MEK proteins in GIST tissue samples from these patients. Kaplan-Meier method was used to calculate the survival rate of 63 patients with complete follow-up data. A Nomogram was used to represent the new prognostic evaluation model. The Cox multivariate regression analysis was conducted separately for each set of risk evaluation factors, based on two risk classification systems [the new risk grade model vs the modified National Institutes of Health (NIH) 2008 risk classification system]. Receiver operating characteristic (ROC) curves were used for evaluating the accuracy and efficiency of the two prognostic evaluation systems.
In GIST tissues, RKIP protein showed positive expression in the cytoplasm and cell membrane, appearing as brownish-yellow or brown granules. The expression of RKIP was related to GIST tumor size, NIH grade, and mucosal invasion. P-ERK protein exhibited heterogeneous distribution in GIST cells, mainly in the cytoplasm, with occasional presence in the nucleus, and appeared as brownish-yellow granules, and the expression of P-ERK protein was associated with GIST tumor size, mitotic count, mucosal invasion, and NIH grade. Meanwhile, RKIP protein expression was negatively correlated with P-ERK expression. The results in COX multivariate regression analysis showed that RKIP protein expression was not an independent risk factor for tumor prognosis. However, RKIP combined with P-ERK protein expression were identified as independent risk factors for prognosis with statistical significance. Furthermore, we establish a new prognosis evaluation model using RKIP and P-ERK in combination and obtained the nomogram of the new prognosis evaluation model. ROC curve analysis also showed that the new evaluation model had better prognostic performance than the modified NIH 2008 risk classification system.
Our experimental results showed that the expression of RKIP and P-ERK proteins in GIST was associated with tumor size, NIH 2008 staging, and tumor invasion, and P-ERK expression was also related to mitotic count. The expression of the two proteins had a certain negative correlation. The combined expression of RKIP and P-ERK proteins can serve as an independent risk factor for predicting the prognosis of GIST patients. The new risk assessment model incorporating RKIP and P-ERK has superior evaluation efficacy and is worth further practical application to validate.
Core Tip: Nowadays, research reports on the expression of downstream proteins of the extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase pathway combined with raf kinase inhibitor protein (RKIP) in gastrointestinal stromal tumor (GIST) is scarce. This study will focus on this aspect and use immunohistochemistry methods, large sample survival analysis data, and the latest bioinformatics analysis techniques to answer the problem. Our experimental results showed that the expression of the two proteins had a certain negative correlation. The combined expression of RKIP and phosphorylated-ERK proteins can serve as an independent risk factor for predicting the prognosis of GIST patients. Furthermore, the new risk assessment model incorporating RKIP and phosphorylated-ERK has superior evaluation efficacy.
- Citation: Qu WZ, Wang L, Chen JJ, Wang Y. Raf kinase inhibitor protein combined with phosphorylated extracellular signal-regulated kinase offers valuable prognosis in gastrointestinal stromal tumor. World J Gastroenterol 2023; 29(26): 4200-4213
- URL: https://www.wjgnet.com/1007-9327/full/v29/i26/4200.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i26.4200
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract. It is believed that GISTs originate from Cajal cells, and mutations in the kit and platelet-derived growth factor receptor-alpha (PDGFR-α) genes are considered to be the cause of most cases of GISTs. Based on this discovery, specific tyrosine kinase inhibitors, such as imatinib, have been used as first-line therapy for the treatment of GISTs. Although these drugs have achieved considerable efficacy in some patients, reports of resistance and recurrence have emerged[1]. Even second-line target therapy drugs such as sunitinib, protein kinase C (PKC) 412, or BMS-354825 are not effective for all patients[2].
To overcome tumor resistance, research on GISTs has focused on the search and validation of new targeted therapy sites and regulatory genes. The Raf-MEK-ERK pathway, which is closely related to GIST, is one of the well-studied pathways. Extracellular signal-regulated kinase 1/2 (ERK1/2) protein, as a member of the mitogen-activated protein kinase (MAPK) family, is a core molecule of this signaling pathway. It works with upstream activating molecules and downstream effector molecules to form an efficient and accurate signal transduction system. The MAPK/ERK Kinase 1/2 (MEK 1/2) protein is a MAPKK (MAPK kinase) that can activate the Thr and Tyr sites on the ERK1/2 protein, thereby phosphorylating and activating it to produce the activated form of P-ERK1/2. Raf-1 protein is an upstream MAPKKK (MAPKK Kinase) that can activate the entire pathway by phosphorylating MEK1/2 to obtain P-MEK1/2. The pathway is regulated by the cell cycle and extracellular stimuli, thus regulating downstream protein kinases, phospholipases, and transcription factors to play important biological functions[3-5].
The RKIP protein is a structurally complex protein with a "multidirectional switch" regulatory role in multiple signaling pathways. First, it can regulate multiple signaling pathways and has an inhibitory effect on the Raf-MEK-ERK pathway, affecting cell invasion and proliferation[6]. Additionally, the RKIP protein is also a phosphorylation target, such as its binding to G protein-coupled receptor kinase 2 (GRK2) in the G protein-coupled pathway[7]. Recently, the study of RKIP protein's tumor regulatory function has gradually become a hot spot, and previous studies have reported that RKIP protein is related to the size of GIST tumors, National Institutes of Health (NIH) staging, and whether the tumor invades the mucosa, but RKIP cannot be used as an independent factor for GIST prognosis evaluation[8].
Nowadays, research reports on the important clinical and prognostic value of phosphorylated (P)-ERK and P-MEK proteins closely related to RKIP have gradually emerged in digestive tract tumors such as gastric cancer, colon cancer, and pancreatic cancer. However, literature on the expression of downstream proteins of the ERK/MAPK pathway combined with RKIP in GIST is scarce. This study will focus on this aspect and use immunohistochemistry methods, large sample survival analysis data, and the latest bioinformatics analysis techniques to search for answers to the problem. We aim to find new tumor treatment targets for GIST and develop more reliable and efficient diagnostic and prognostic evaluation methods.
The research object of the immunohistochemical experiment was 66 paraffin-embedded specimens that were surgically resected and pathologically diagnosed at China Medical University Affiliated Hospital from January 2015 to January 2020, with complete clinical and follow-up information. There were 36 male cases and 30 female cases, with an age range of 21-83 years and a mean age of 56.2 years. The tumor occurrence sites were: Stomach in 37 cases (56.1%), duodenum in 12 cases (18.2%), jejunum in 15 cases (22.7%), and colon in 2 cases (3.0%) (Table 1).
| Characteristic | n | RKIP | P-ERK | P-MEK | ||||||
| Negative | Positive | P valuea | Negative | Positive | P valuea | Negative | Positive | P valuea | ||
| Sex | ||||||||||
| Male | 36 | 16 | 20 | 0.652 | 16 | 20 | 0.716 | 22 | 14 | 0.365 |
| Female | 30 | 15 | 15 | 12 | 18 | 15 | 15 | |||
| Age in yr | ||||||||||
| > 56 | 33 | 17 | 16 | 0.459 | 13 | 20 | 0.618 | 15 | 18 | 0.083 |
| ≤ 56 | 33 | 14 | 19 | 15 | 18 | 22 | 11 | |||
| Tumor location | ||||||||||
| Stomach | 37 | 20 | 17 | 0.585 | 16 | 21 | 0.902 | 21 | 16 | 0.224 |
| Duodenum | 12 | 4 | 8 | 4 | 8 | 4 | 8 | |||
| Jejunoileum | 15 | 6 | 9 | 7 | 8 | 11 | 4 | |||
| Colon | 2 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Tumor size in cm | ||||||||||
| < 2 | 12 | 1 | 11 | < 0.001 | 8 | 4 | 0.009 | 7 | 5 | 0.353 |
| 2-5 | 20 | 5 | 15 | 12 | 8 | 14 | 6 | |||
| 6-10 | 19 | 14 | 5 | 6 | 13 | 10 | 9 | |||
| > 10 | 15 | 11 | 4 | 2 | 13 | 6 | 9 | |||
| NIH risk grade | ||||||||||
| Very low | 10 | 2 | 8 | < 0.001 | 7 | 3 | 0.004 | 7 | 3 | 0.319 |
| Low | 20 | 3 | 17 | 13 | 7 | 11 | 9 | |||
| Moderate | 11 | 8 | 3 | 3 | 8 | 8 | 3 | |||
| High | 25 | 18 | 7 | 5 | 20 | 11 | 14 | |||
| Mitotic figures as /50 HPFs | ||||||||||
| 0 | 11 | 3 | 8 | 0.074 | 5 | 6 | 0.015 | 5 | 6 | 0.604 |
| 1-4 | 37 | 15 | 22 | 21 | 16 | 22 | 15 | |||
| 5-10 | 7 | 5 | 2 | 1 | 6 | 5 | 2 | |||
| > 10 | 11 | 8 | 3 | 1 | 10 | 5 | 6 | |||
| Mucosal invasion | ||||||||||
| Yes | 34 | 21 | 13 | 0.013 | 10 | 24 | 0.027 | 18 | 16 | 0.599 |
| No | 32 | 10 | 22 | 18 | 14 | 19 | 13 | |||
The diagnostic criteria for GISTs were histopathological features consistent with GISTs and immunohistochemical positivity for CD117, immunohistochemical negativity for CD117 but positivity for CD34, or immunohistochemical negativity for CD117 and CD34 as well as smooth muscle actin, desmin, and S-100 (to exclude smooth muscle tumors and neurogenic tumors). Among them, 59 cases (90.8%) were CD117-positive, and 50 cases (76.9%) were CD34-positive.
The risk grade criteria for GIST used the modified NIH 2008 risk classification system, which combines three assessment factors: Tumor location (gastric vs non-gastric: Small intestine and colon, etc.), tumor diameter (< 2 cm, 2-5 cm, 5-10 cm, > 10 cm), and mitotic count [< 5/50 high-power fields (HPFs), 5-10/50 HPFs, > 10/50 HPFs]. GISTs were classified as very low risk (I), low risk (II), moderate risk (III), and high risk (IV).
Among the 66 cases of GIST in this study, except for 2 cases who died due to other reasons and 1 case who was lost to follow-up, the follow-up information of the remaining 63 cases was complete. The follow-up time ranged from 5 to 61 mo, with a mean of 48 mo.
The GIST specimens were fixed with formalin, embedded in paraffin, and sectioned into 4 μm-thick slices. The sections underwent routine dewaxing in water, followed by H2O2 treatment at room temperature to inactivate endogenous enzymes. Subsequently, they were washed three times with distilled water. Antigen retrieval was performed, followed by incubation with a blocking solution containing 5% BSA at room temperature for 20 min. Primary antibodies (RKIP/P-ERK/P-MEK antibodies, rabbit IgG) were then added at an appropriate dilution and incubated at room temperature for 12 h. Next, the sections were treated with a secondary antibody (goat anti-rabbit IgG) and incubated at 25 °C for 20 min. SABC-AP reagent was applied to the sections, followed by incubation at 25 °C for 20 min. For color development, the BCIP/NBT substrate was prepared by diluting it in TBS, thoroughly mixed, and added to the slides. The sections were incubated at 25 °C for 20 min, with the reaction time monitored under a microscope. After washing with distilled water, the sections were lightly counterstained with nuclear red, rinsed, dried, and mounted with a water-soluble mounting medium for microscopic observation.
The positive staining of RKIP, P-ERK, and P-MEK proteins was mainly located in the cytoplasm and cell membrane, while P-ERK and P-MEK were occasionally observed in the nucleus. The semi-quantitative dual scoring method was used to evaluate the protein expression of proteins. Under high-power microscopy, scoring was performed based on the staining intensity and the proportion of positive cells. Ten random high-power fields were selected on each slide, and the number of positive cells was counted among 100 cells in each field. The average value was calculated and expressed as a percentage, representing the positivity index of proteins. The detailed scoring criteria were as follows:
(1) Staining intensity scoring criteria: no staining, 0 points; yellow, 1 point; light brown, 2 points; dark brown, 3 points;
(2) Scoring criteria for the proportion of positive cells: < 25% positive cells, 0 points; 25%-50%, 1 point; 51%-75%, 2 points; > 75%, 3 points;
And (3) The overall score was the product of the staining intensity and the proportion of positive cells, and graded as negative (0-2), mildly positive (+, 3), moderately positive (++, 4-6), or strongly positive (+++, 9). RKIP/P-ERK/P-MEK expression was judged to be either negative (0-2) or positive (3-9).
Telephone follow-up was the primary method, with outpatient review and correspondence as secondary measures. Survival time was calculated as the time from the date of surgery to the date of the last follow-up for surviving patients, the date of death for deceased patients, and the date of the last follow-up for lost patients. Among them, 63 cases had complete follow-up data, 2 cases were excluded due to death from other diseases or accidents, and 1 case was excluded due to loss to follow-up. The follow-up period was from January 2020 to December 2022.
SPSS 22.0 software (IBM Corp., Armonk, NY, United States) was used for statistical analysis. Chi-square test was used for comparing percentages. Spearman analysis was used for correlation analysis of two groups of ordinal data. Kaplan-Meier method was used to calculate survival rate, and log-rank test was used. Cox univariate analysis was used for single-factor prognosis analysis, and Cox multivariate regression was used for multi-factor prognosis analysis. A Nomogram was used to represent the new prognostic evaluation model. The Cox multivariate regression analysis was conducted separately for each set of risk evaluation factors, based on two risk classification systems (the new risk grade model vs the modified NIH 2008 risk classification system). Receiver operating characteristic (ROC) curves were used for evaluating the accuracy and efficiency of the two prognostic evaluation systems, and statistical significance was set at P < 0.05.
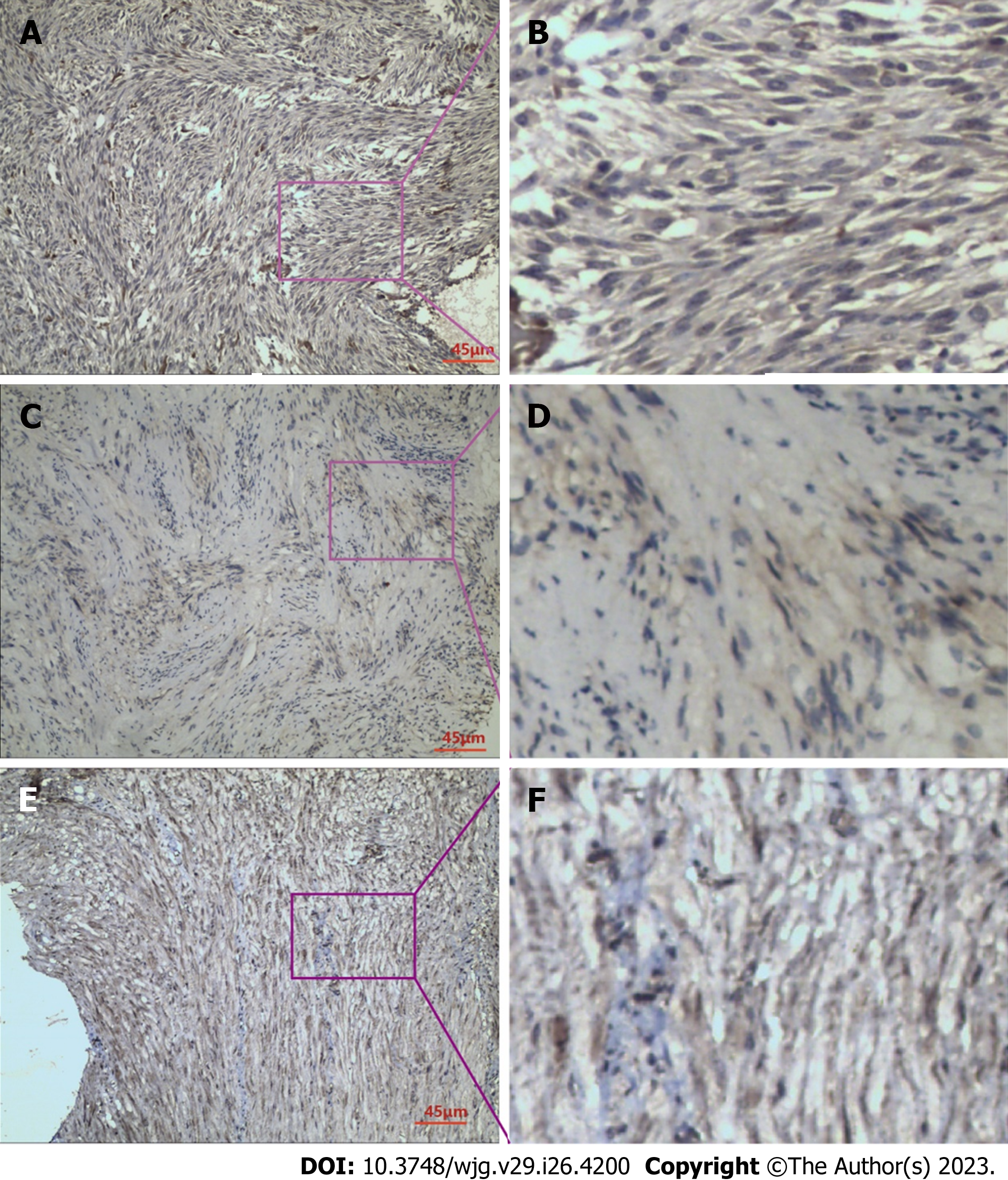
In GIST tissues, RKIP protein showed positive expression in the cytoplasm and cell membrane, appearing as brownish-yellow or brown granules. The immunohistochemical staining result is shown in Figure 1A and B under light microscopy. Among the 66 specimens tested, RKIP protein was expressed positively in 35 cases (53%) and negatively in 31 cases (47%). Statistical analysis revealed that the expression of RKIP was related to GIST tumor size, NIH grade, and mucosal invasion (P < 0.05) (Table 1).
P-ERK protein exhibited heterogeneous distribution in GIST cells, mainly in the cytoplasm, with occasional presence in the nucleus, and appeared as brownish-yellow granules, as shown in Figure 1C and D. Positive expression of P-ERK protein was detected in 38 cases (57.6%), while negative expression was detected in 28 cases (42.4%). Clinical pathology analysis indicated that the expression of P-ERK protein was associated with GIST tumor size, mitotic count, mucosal invasion, and NIH grade (P < 0.05) (Table 1).
P-MEK protein expression in GIST cells was observed as brownish-yellow granules, mainly distributed in the cytoplasm, with some in the nucleus, and showed a relatively uniform distribution, as shown in Figure 1E and F. Among the 66 cases, positive expression of P-MEK protein was found in 29 cases (43.9%), while negative expression was detected in 37 cases (56.1%). However, no significant correlation was observed between P-MEK protein expression and any independent clinical-pathological factors of GIST (Table 1).
Spearman's correlation test was used to analyze the correlation between RKIP protein expression and P-ERK/P-MEK expression. The results showed that in GIST cases, RKIP protein expression was negatively correlated with P-ERK expression (correlation coefficient = -0.575, P < 0.001); RKIP protein expression also had a certain negative correlation with P-MEK protein expression (correlation coefficient = -0.323, P < 0.001) (Table 2).
Univariate regression analysis was performed on complete follow-up data from 63 cases, which indicated that tumor location, tumor size, mitotic count, RKIP protein expression, P-ERK protein expression, RKIP combined with P-ERK protein co-expression, and NIH grading were all correlated with patient prognosis (P < 0.05).
These univariate factors were further included in COX multivariate regression analysis. The results showed that RKIP protein expression was not an independent risk factor for tumor prognosis (P = 0.061). However, tumor size (P = 0.037), NIH grading (P = 0.014), P-ERK protein expression (P = 0.041), and RKIP combined with P-ERK protein expression (P = 0.044) were identified as independent risk factors for prognosis with statistical significance. Among them, the impact of RKIP combined with P-ERK protein expression on survival time was the highest with a weight factor of Exp (B) at 11.320, followed by NIH grading, P-ERK expression, and tumor size (Table 3).
| Characteristic | n | Univariatea | Multivariate1 | ||||
| HR | 95%CI | P value | B | Exp (B) | P value | ||
| Sex | |||||||
| Male | 34 | 0.87 | 0.32-2.34 | 0.783 | 0.004 | 1.004 | 0.994 |
| Female | 29 | ||||||
| Age in yr | |||||||
| > 56 | 30 | 0.99 | 0.96-1.03 | 0.751 | -0.011 | 0.989 | 0.609 |
| ≤ 56 | 33 | ||||||
| Tumor location | |||||||
| Stomach | 36 | 3.86 | 1.23-12.18 | 0.021 | 0.669 | 1.953 | 0.057 |
| Duodenum | 12 | ||||||
| Jejunoileum | 15 | ||||||
| Tumor size in cm | |||||||
| < 2 | 11 | 2.32 | 1.32-4.07 | 0.003 | 0.734 | 2.083 | 0.037 |
| 2-5 | 19 | ||||||
| 6-10 | 18 | ||||||
| > 10 | 14 | ||||||
| NIH risk grade | |||||||
| Very low | 10 | 2.32 | 1.32-4.07 | 0.002 | 1.393 | 4.025 | 0.014 |
| Low | 19 | ||||||
| Moderate | 10 | ||||||
| High | 24 | ||||||
| Mitotic figures as /50 HPFs | |||||||
| 0 | 11 | 1.7 | 1.06-2.70 | 0.026 | 0.179 | 1.196 | 0.505 |
| 1-4 | 36 | ||||||
| 5-10 | 5 | ||||||
| > 10 | 11 | ||||||
| RKIP expression | |||||||
| Positive | 35 | 0.14 | 0.04-0.50 | 0.002 | -2.064 | 0.127 | 0.061 |
| Negative | 28 | ||||||
| P-ERK expression | |||||||
| Positive | 36 | 3.63 | 1.03-12.73 | 0.044 | 1.761 | 5.821 | 0.041 |
| Negative | 27 | ||||||
| P-MEK expression | |||||||
| Positive | 28 | 1.78 | 0.66-4.79 | 0.252 | |||
| Negative | 35 | ||||||
| RKIP and P-ERK co-expression2 | |||||||
| Positive | 25 | 0.34 | 012-0.94 | 0.037 | 2.427 | 11.32 | 0.044 |
| Negative | 38 | ||||||
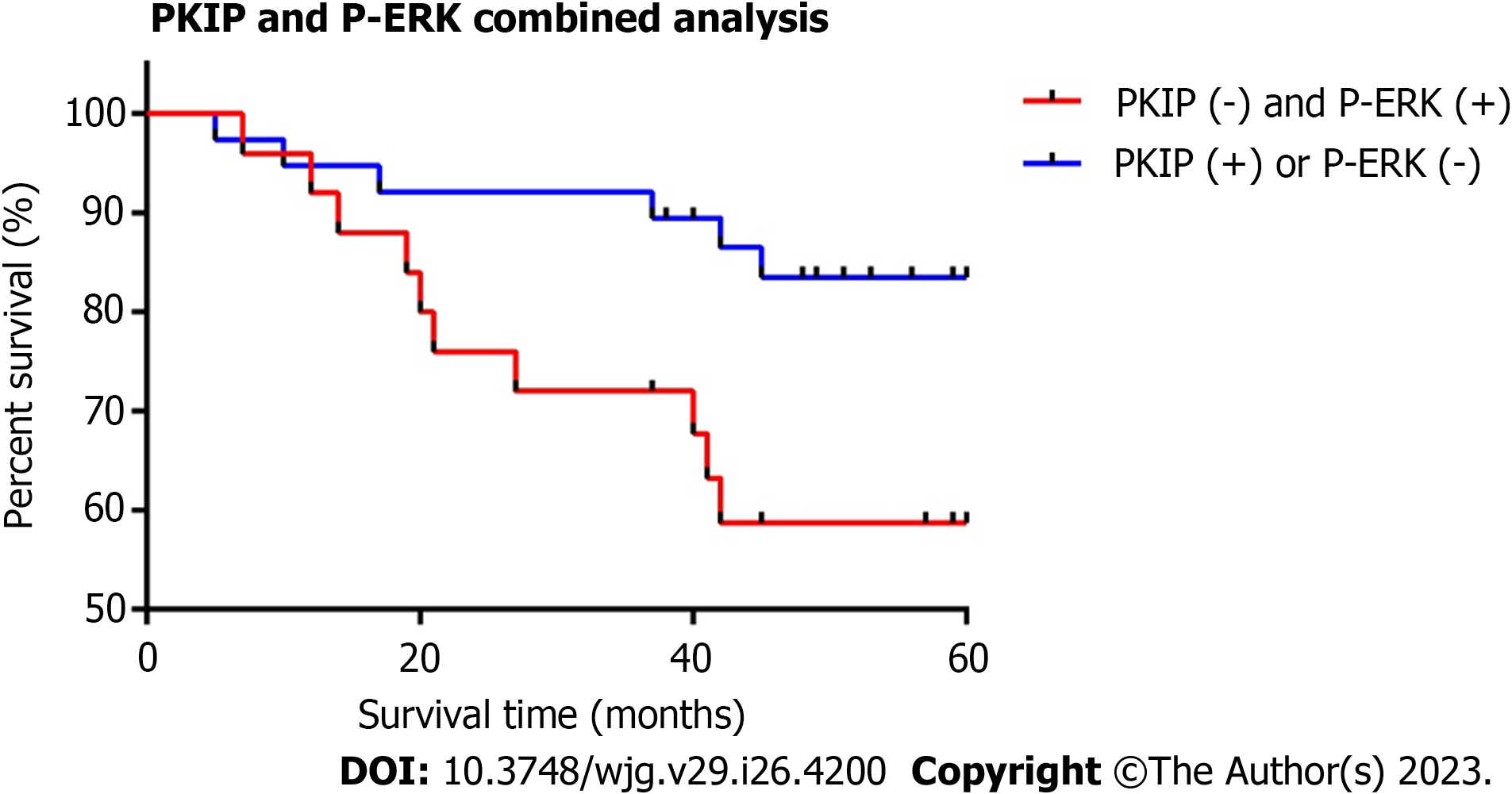
Based on the expression of RKIP and P-ERK proteins in GIST specimens, we divided the cases into two groups: 1. RKIP
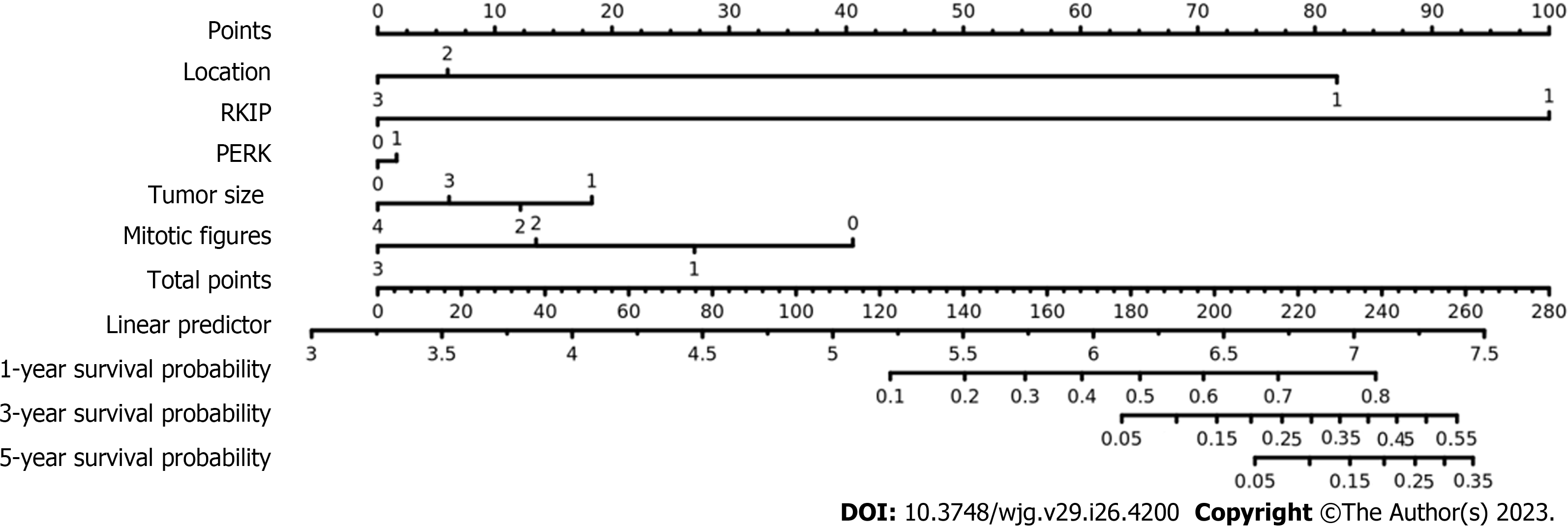
We included "RKIP expression, P-ERK protein expression, tumor size, tumor location, and mitotic count" as five risk assessment factors (Table 4: Multivariate 2) and used multivariate Cox regression analysis to draw a new nomogram for this new evaluation model. We also conducted ROC analysis to evaluate its prognostic performance. As a control, we used the three conventional risk factors (tumor location, tumor size, and mitotic count) in the modified NIH 2008 risk classification system as evaluation factors (Table 4: Multivariate 1) and performed the same statistical analysis.
| Characteristic | n | Multivariate1 | Multivariate2 | ||||
| HR | 95%CI | P valuea | HR | 95%CI | P valuea | ||
| Tumor location | |||||||
| Stomach | 36 | 2.59 | 0.69-9.69 | 0.156 | 4.13 | 1.07-15.98 | 0.04 |
| Duodenum | 12 | ||||||
| Jejunoileum | 15 | ||||||
| Tumor size in cm | |||||||
| < 2 | 11 | 1.92 | 0.93-3.96 | 0.079 | 1.17 | 0.52-2.62 | 0.71 |
| 2-5 | 19 | ||||||
| 6-10 | 18 | ||||||
| > 10 | 14 | ||||||
| Mitotic figures as /50 HPFs | |||||||
| 0 | 11 | 1.22 | 0.71-2.10 | 0.472 | 1.28 | 0.75-2.18 | 0.361 |
| 1-4 | 36 | ||||||
| 5-10 | 5 | ||||||
| > 10 | 11 | ||||||
| RKIP expression | |||||||
| Positive | 35 | 0.13 | 0.03-0.66 | 0.014 | |||
| Negative | 28 | ||||||
| P-ERK expression | |||||||
| Positive | 36 | 0.91 | 0.19-4.32 | 0.909 | |||
| Negative | 27 | ||||||
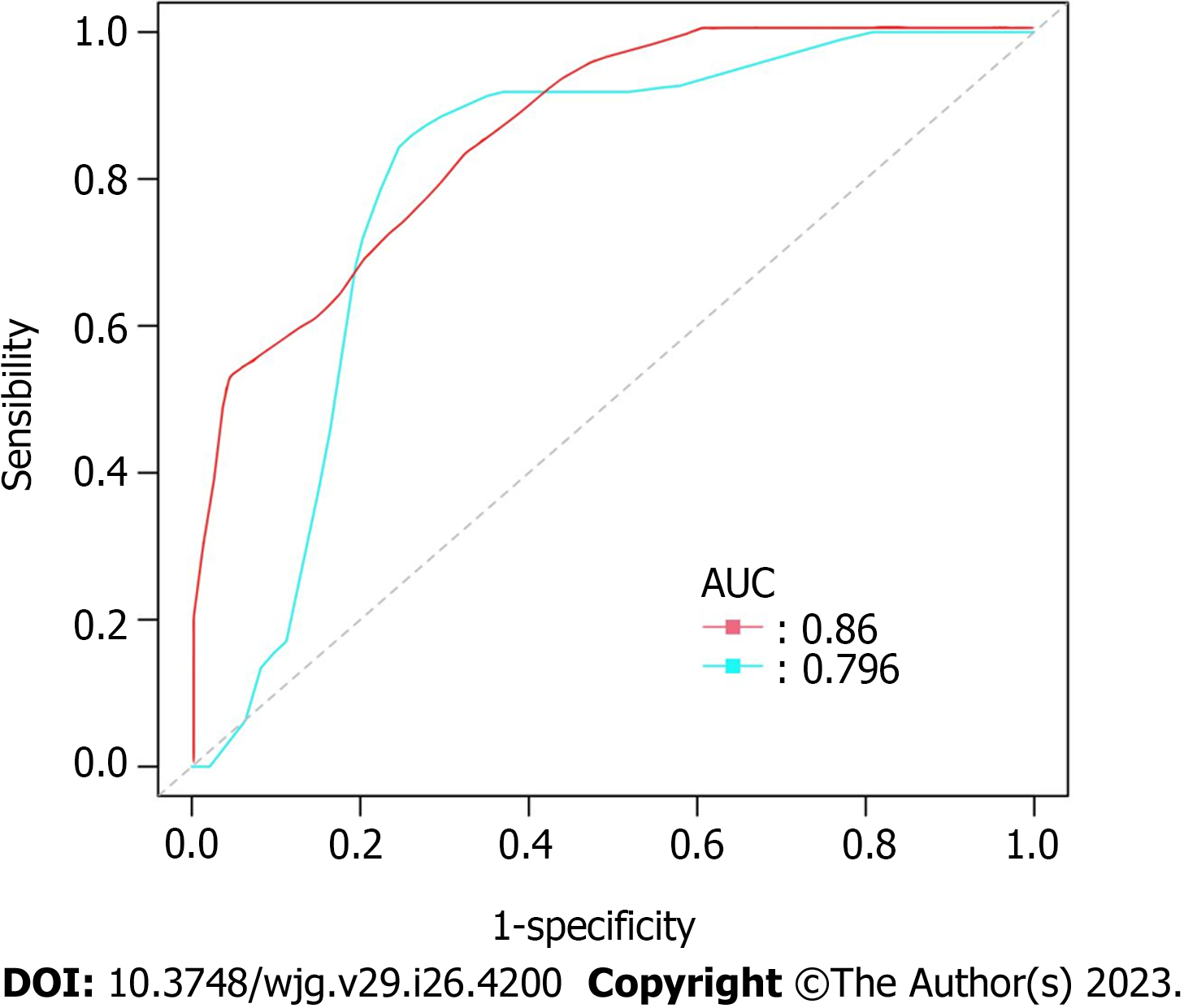
After analyzing the results of the above analysis (Table 4), we obtained the nomogram of the new prognosis evaluation model (Figure 3). ROC curve analysis also showed that the new evaluation model (red curve, AUC = 0.860) had better prognostic performance than the modified NIH 2008 risk classification system (blue curve, AUC = 0.796) (P = 0.0312) (Figure 4).
GIST is the most common mesenchymal tumor in the gastrointestinal tract, accounting for approximately 60%-70% of all cases. Currently, effective treatment for GIST involves surgery combined with appropriate targeted therapy[9]. However, resistance to targeted therapy often occurs, and prognosis assessment is still being explored and modified. Therefore, there is an urgent need to find better therapeutic targets and a more efficient risk grading system.
ERK and MEK proteins are two core proteins in the Raf1-MEK-ERK pathway, which are downstream targets activated by abnormal C-kit and PDGFR-α genes closely related to GIST. ERK protein is associated with various diseases[10] such as tumors, heart failure, developmental disorders, and autoimmune diseases[11-13]. RKIP protein is a natural inhibitor of Raf-1 protein and is widely present in living organisms. It has an inhibitory effect on the metastasis and proliferation of various tumors.
In our preliminary experiments, we found that high expression of RKIP protein was related to GIST tumor size, invasion of the mucosa, and NIH grading. However, the single expression of RKIP protein alone is not sufficient as a single risk factor for evaluating GIST prognosis. Recent reports have suggested that RKIP protein regulates the expression of the MEK/ERK pathway in pancreatic cancer[14,15], thereby affecting tumor resistance and invasion. Additionally, it has been found that the combination of RKIP and P-ERK proteins in gastric cancer has a predictive significance for tumor prognosis[16]. These findings inspired us to focus on the combined detection of RKIP, P-ERK, and P-MEK proteins in GIST, in hopes of gaining further insights.
In this experiment, we analyzed the expression of RKIP, P-ERK, and P-MEK proteins in GIST and found that RKIP expression is related to GIST tumor size and NIH staging. This result is consistent with the research findings of Yuan et al[17], who found that knocking down the RKIP gene promotes cell growth in nasopharyngeal carcinoma cell lines[17].
In addition to its correlation with tumor size and NIH staging, P-ERK protein is also related to the mitotic count in GIST cells. Zhang et al[18] found that high expression of RKIP protein leads to low expression of P-ERK protein in the human choriocarcinoma cell line JEG-3. Furthermore, transfection with low P-ERK level tumor cells significantly reduced their growth activity compared to the control group, indicating that P-ERK protein is related to tumor cell proliferation[18].
The mechanism may be that low RKIP expression in GIST tumor cells leads to more phosphorylation of downstream ERK1/2 protein, forming P-ERK protein, which can control important structures in cell division such as centrosomes, spindle fibers, and centromeres[19,20], accelerating the tumor cell cycle and mitosis[21]. In addition, low RKIP expression causes dysregulation of its ligand Raf-1 protein, which upregulates the cell mitotic cycle[22,23], accelerating tumor growth. Moreover, ERK protein can be rapidly transported into the nucleus, further phosphorylating and activating proliferation-related transcription factors such as AP-1, ELK-1, and Serum Response Factor Accessory Protein (SAP), promoting cell proliferation and causing abnormal nuclear division[24]. Thus, low RKIP expression in GIST upregulates P-ERK, leading to tumor enlargement and increased nuclear division.
The NIH 2008 risk classification system is a recognized postoperative prognostic assessment system for GIST, which is based on three independent risk factors: Tumor location, tumor size, and mitotic index. RKIP and P-ERK proteins are associated with two of these factors, and it is easy to understand that protein expression is also related to NIH 2008 risk classification results. Schoppmann et al's report suggests that loss of RKIP expression tends to increase the risk level of GIST cases in Fletcher's risk classification and a similar conclusion is drawn in Miettinen's risk classification[25]. Wang et al[26] also found that RKIP protein expression is related to TNM staging in non-small cell lung cancer[26].
We also found that both RKIP protein and P-ERK protein are related to the mucosal invasion status of GIST tumors. Martinho et al[27] found in experiments that low expression of RKIP protein often indicates a tendency of GIST tumors to invade and metastasize[27]. A similar correlation is found in gastric cancer between RKIP protein expression and tumor invasion depth, lymph node metastasis, and distant metastasis. The mechanism may be related to the interaction between RKIP protein and Snail protein, which regulates tumor Epithelial-Mesenchymal Transition (EMT)[28]: Snail protein can bind and downregulate E-cadherin protein, thereby regulating EMT; at the same time, it binds to the RKIP protein promoter E-box region to downregulate RKIP protein transcription. Thus, when Snail upregulates EMT, RKIP is often reduced[29].
In the analysis of clinical and pathological factors, no statistically significant results were found for P-MEK protein expression. However, there is a certain negative correlation between RKIP and P-MEK expression, which is statistically significant. This may indicate that RKIP has a certain regulatory effect on P-MEK. Schoppmann et al[25] found in their immunohistochemical study of GIST specimens that P-MEK1/2 expression is related to whether the tumor can be completely resected, but has no significant relationship with other factors. The conclusions of this study are mutually corroborated.
In this experiment, it was found that the expression of RKIP and P-ERK in GIST was negatively correlated (P < 0.05). Yang et al[14] found that inhibiting ERK phosphorylation would upregulate RKIP expression in pancreatic cancer cell lines; while Yuan et al[17] reported that high expression of RKIP in nasopharyngeal carcinoma cells can also inhibit ERK protein activation. Therefore, RKIP protein and P-ERK protein have mutual regulatory functions. Recently, the novel and revolutionary "Phospho-Theft" hypothesis about RKIP provides a perfect explanation for its possible mechanism[30]. RKIP protein has two completely different functions: When not phosphorylated, RKIP protein inhibits the activation and signal transmission of Raf-1 protein, which can reduce the activation of downstream MEK/ERK; after RKIP is phosphorylated by PKC protein at S153, P-RKIP substrate transfers to GRK2[31], which can enhance the activation of the β-AR pathway and activate downstream substrate ERK[32]. In addition, RKIP is regulated by feedback of KRAS-ERK pathway. At the same time, P-ERK can undergo self-phosphorylation, thereby activating downstream substrates such as Elk1, mitogen-and stress-activated protein Kinase, c-myc, etc[33].
Combining univariate and multivariate COX regression analysis, it was found that RKIP protein expression could not serve as an independent risk factor for predicting tumor prognosis (P = 0.061). Tumor size (P = 0.037), NIH grading (P = 0.014), P-ERK protein expression (P = 0.041), and RKIP combined with P-ERK protein expression (P = 0.044) could serve as independent prognostic factors with statistical significance. Among them, the combined expression of RKIP and P-ERK had the highest impact on survival, with a weight factor of Exp (B) at 11.320, followed by NIH grading, P-ERK expression, and tumor size. Furthermore, we plotted survival curves for GIST cases based on their combined RKIP and P-ERK expression, revealing a significant difference in survival rates between groups. Therefore, the combined expression of RKIP and P-ERK has significant prognostic value for evaluating the prognosis of GIST patients and carries greater weight. This is consistent with findings in a study on nasopharyngeal carcinoma where RKIP was found to be an independent risk factor for predicting prognosis. In gastric cancer, the combined expression of P-ERK and RKIP was found to be associated with a 5-year relapse-free survival after surgery.
Standardized treatment for GIST includes timely surgery and rational targeted therapy. The indication for targeted therapy is currently based on the NIH 2008 risk classification system applied to postoperative pathology results, with Imatinib (a representative TKI drug) for the intermediate and high-risk population[34]. Meanwhile, the Armed Forces Institute of Pathology and World Health Organization 2013 risk classifications are also used[35]. However, there are differences in the results among various risk classification systems, and there are difficulties in using and interpreting them for the public. As mentioned in this article, we found that the combination of RKIP and P-ERK protein expression is of great significance for the prognostic evaluation of GIST patients. Therefore, we incorporated RKIP and P-ERK protein expression, as well as tumor size, tumor location, and mitotic count, into a new GIST risk assessment model and plotted a survival curve. We analyzed its evaluation efficiency using the ROC curve, and the results showed that it is superior to the NIH 2008 risk classification system (Area Under Curve 0.860 vs 0.796).
In recent years, GIST-related research has been devoted to identifying new biomarkers and finding new therapeutic targets[36]. As one of the hotspots, our study found that the combination of RKIP and P-ERK proteins can effectively indicate the prognosis of GIST and can be used to guide GIST targeted therapy. More researchers have focused on the application and exploration of RKIP and P-ERK proteins in tumor diagnosis and treatment, such as the recent successful case of using urine RKIP monitoring to evaluate the prognosis of clear cell renal cell carcinoma (ccRCC)[37]. Serum RKIP levels have also been used to monitor the efficacy of treatment for multiple sclerosis[38]. In terms of treatment, ERK inhibitors combined with chloroquine can improve the adjuvant therapy for pancreatic cancer with K-Ras mutations[39], and imatinib combined with chloroquine (to inhibit autophagy) has also been attempted in GIST drug-resistant experimental studies[40].
Our experiment fills the gap in the research on the relevance of RKIP and P-ERK in GIST, further confirming the significance of these proteins in tumor physiology and clinical treatment. However, the experiment still has some limitations. Firstly, due to the lower incidence of GIST comparing to other gastrointestinal tumors, the sample size was limited. We will continue to recruit more samples and track their follow-up, using more abundant data for further validation and research. Secondly, this experiment used immunohistochemical methods for protein level research. In the future, we will supplement molecular level experiments to further elucidate the mechanism of action of relevant sites on the RKIP and ERK/MAPK pathways, and better guide targeted therapy. The above is also the focus of our project and ongoing work. We are confident in making improvements and will strive to promote a new chapter in the RKIP-related diagnosis and treatment of GIST.
Our experimental results showed that the expression of RKIP and p-ERK proteins in GIST was associated with tumor size, NIH 2008 staging, tumor invasion, and p-ERK expression was also related to mitotic count. The expression of the two proteins had a certain negative correlation. The combined expression of RKIP and p-ERK proteins can serve as an independent risk factor for predicting the prognosis of GIST patients. The new risk assessment model incorporating RKIP and p-ERK has superior evaluation efficacy and is worth further practical application to help validate results.
Nowadays, research reports on the important clinical and prognostic value of phosphorylated-extracellular signal-regulated kinase (P-ERK) and phosphorylated-mitogen-activated protein kinase (MAPK/ERK) kinase (P-MEK) proteins closely related to raf kinase inhibitor protein (RKIP) have gradually emerged in digestive tract tumors.
The expression of downstream proteins of the ERK/MAPK pathway combined with RKIP in gastrointestinal stromal tumor (GIST) is scarce.
To detect the expression of RKIP, P-ERK, and P-MEK proteins in GIST and to analyze their relationship with clinicopathological characteristics and prognosis of this disease. Try to establish a new prognosis evaluation model using RKIP and P-ERK in combination and analyze its prognosis evaluation efficacy.
This study will focus on this aspect and use immunohistochemistry methods, large sample survival analysis data, and the latest bioinformatics analysis techniques to search for answers to the problem.
Our experimental results showed that the expression of RKIP and P-ERK proteins in GIST was associated with tumor size, NIH 2008 staging, tumor invasion, and P-ERK expression was also related to mitotic count. The expression of the two proteins had a certain negative correlation.
The combined expression of RKIP and P-ERK proteins can serve as an independent risk factor for predicting the prognosis of GIST patients. The new risk assessment model incorporating RKIP and P-ERK has superior evaluation efficacy and is worth further practical application to validate.
As one of the hotspots, our study found that the combination of RKIP and P-ERK proteins can effectively indicate the prognosis of GIST and can be used to guide GIST targeted therapy. More researches are needed to focus on the application and exploration of RKIP and P-ERK proteins in tumor diagnosis and treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Chinese Society of Clinical Oncology, 202240816.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chmura SJ, United States; Kwon T, United States S-Editor: Li L L-Editor: Filipodia P-Editor: Cai YX
| 1. | Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ, Eisenberg BL, von Mehren M, Fletcher CD, Sandau K, McDougall K, Ou WB, Chen CJ, Fletcher JA. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24:4764-4774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 616] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 2. | Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, George S, Bello CL, Huang X, Baum CM, Casali PG. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1942] [Cited by in RCA: 1918] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 3. | Riller Q, Rieux-Laucat F. RASopathies: From germline mutations to somatic and multigenic diseases. Biomed J. 2021;44:422-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Yan ZP, Li JT, Zeng N, Ni GX. Role of extracellular signal-regulated kinase 1/2 signaling underlying cardiac hypertrophy. Cardiol J. 2021;28:473-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Fan HY, Tong C, Sun QY. [Mitogen-activated protein kinase (MAPK) Signaling Pathways: State-of-the-art]. Donwuxue Zazhi. 2002;37:98-102. [DOI] [Full Text] |
| 6. | Yeung K, Janosch P, McFerran B, Rose DW, Mischak H, Sedivy JM, Kolch W. Mechanism of suppression of the Raf/MEK/extracellular signal-regulated kinase pathway by the raf kinase inhibitor protein. Mol Cell Biol. 2000;20:3079-3085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 289] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Granovsky AE, Clark MC, McElheny D, Heil G, Hong J, Liu X, Kim Y, Joachimiak G, Joachimiak A, Koide S, Rosner MR. Raf kinase inhibitory protein function is regulated via a flexible pocket and novel phosphorylation-dependent mechanism. Mol Cell Biol. 2009;29:1306-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Wang Y, Chen JJ, Wang XF, Wang Q. Clinical and prognostic significance of Raf kinase inhibitory protein expression in gastrointestinal stromal tumors. World J Gastroenterol. 2018;24:2508-2517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Sun K, Lv H, Chen B, Nie C, Zhao J, Wang S, Wang J, Xu W, Chen X. Dawning precision treatment for gastric cancer: The latest biomarkers. J Transl Int Med. 2021;9:228-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Lorenz K, Rosner MR. Harnessing RKIP to Combat Heart Disease and Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Sugiura R, Satoh R, Takasaki T. ERK: A Double-Edged Sword in Cancer. ERK-Dependent Apoptosis as a Potential Therapeutic Strategy for Cancer. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 207] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 12. | Gilbert CJ, Longenecker JZ, Accornero F. ERK1/2: An Integrator of Signals That Alters Cardiac Homeostasis and Growth. Biology (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Gallo S, Vitacolonna A, Bonzano A, Comoglio P, Crepaldi T. ERK: A Key Player in the Pathophysiology of Cardiac Hypertrophy. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 191] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 14. | Yang K, Li Y, Lian G, Lin H, Shang C, Zeng L, Chen S, Li J, Huang C, Huang K, Chen Y. KRAS promotes tumor metastasis and chemoresistance by repressing RKIP via the MAPK-ERK pathway in pancreatic cancer. Int J Cancer. 2018;142:2323-2334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Bian Y, Jiang H, Zheng J, Shao C, Lu J. Basic Pancreatic Lesions: Radiologic-pathologic Correlation. J Transl Int Med. 2022;10:18-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 16. | Fujimori Y, Inokuchi M, Takagi Y, Kato K, Kojima K, Sugihara K. Prognostic value of RKIP and p-ERK in gastric cancer. J Exp Clin Cancer Res. 2012;31:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Yuan L, Yi HM, Yi H, Qu JQ, Zhu JF, Li LN, Xiao T, Zheng Z, Lu SS, Xiao ZQ. Reduced RKIP enhances nasopharyngeal carcinoma radioresistance by increasing ERK and AKT activity. Oncotarget. 2016;7:11463-11477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Zhang M, Zhang D, Fan Q. RKIP suppresses the proliferation and invasion ofchoriocarcinoma cells through inhibiting the MAPK signaling pathway. Int J Clin Exp Med. 2015;8:22183-22190. [PubMed] |
| 19. | Shapiro PS, Vaisberg E, Hunt AJ, Tolwinski NS, Whalen AM, McIntosh JR, Ahn NG. Activation of the MKK/ERK pathway during somatic cell mitosis: direct interactions of active ERK with kinetochores and regulation of the mitotic 3F3/2 phosphoantigen. J Cell Biol. 1998;142:1533-1545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 184] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Zecevic M, Catling AD, Eblen ST, Renzi L, Hittle JC, Yen TJ, Gorbsky GJ, Weber MJ. Active MAP kinase in mitosis: localization at kinetochores and association with the motor protein CENP-E. J Cell Biol. 1998;142:1547-1558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 176] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Yesilkanal AE, Rosner MR. Raf kinase inhibitory protein (RKIP) as a metastasis suppressor: regulation of signaling networks in cancer. Crit Rev Oncog. 2014;19:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Ziogas A, Lorenz IC, Moelling K, Radziwill G. Mitotic Raf-1 is stimulated independently of Ras and is active in the cytoplasm. J Biol Chem. 1998;273:24108-24114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Hayne C, Tzivion G, Luo Z. Raf-1/MEK/MAPK pathway is necessary for the G2/M transition induced by nocodazole. J Biol Chem. 2000;275:31876-31882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Boilly B, Vercoutter-Edouart AS, Hondermarck H, Nurcombe V, Le Bourhis X. FGF signals for cell proliferation and migration through different pathways. Cytokine Growth Factor Rev. 2000;11:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 210] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Schoppmann SF, Beer A, Nirtl N, Ba-Ssalamah A, Brodowicz T, Streubel B, Birner P. Downregulation of phosphatidylethanolamine binding protein 1 associates with clinical risk factors in gastrointestinal stromal tumors, but not with activation of the RAF-1-MEK-ETV1 pathway. Cancer Lett. 2013;335:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Wang A, Duan G, Zhao C, Gao Y, Liu X, Wang Z, Li W, Wang K, Wang W. Reduced RKIP expression levels are associated with frequent non-small cell lung cancer metastasis and STAT3 phosphorylation and activation. Oncol Lett. 2017;13:3039-3045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Martinho O, Gouveia A, Silva P, Pimenta A, Reis RM, Lopes JM. Loss of RKIP expression is associated with poor survival in GISTs. Virchows Arch. 2009;455:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Cessna H, Baritaki S, Zaravinos A, Bonavida B. The Role of RKIP in the Regulation of EMT in the Tumor Microenvironment. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 29. | Beach S, Tang H, Park S, Dhillon AS, Keller ET, Kolch W, Yeung KC. Snail is a repressor of RKIP transcription in metastatic prostate cancer cells. Oncogene. 2008;27:2243-2248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 30. | Skinner JJ, Wang S, Lee J, Ong C, Sommese R, Sivaramakrishnan S, Koelmel W, Hirschbeck M, Schindelin H, Kisker C, Lorenz K, Sosnick TR, Rosner MR. Conserved salt-bridge competition triggered by phosphorylation regulates the protein interactome. Proc Natl Acad Sci U S A. 2017;114:13453-13458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Lorenz K, Lohse MJ, Quitterer U. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature. 2003;426:574-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 283] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 32. | Maimari T, Krasel C, Bünemann M, Lorenz K. The N-termini of GRK2 and GRK3 simulate the stimulating effects of RKIP on β-adrenoceptors. Biochem Biophys Res Commun. 2019;520:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Robinson EL, Drawnel FM, Mehdi S, Archer CR, Liu W, Okkenhaug H, Alkass K, Aronsen JM, Nagaraju CK, Sjaastad I, Sipido KR, Bergmann O, Arthur JSC, Wang X, Roderick HL. MSK-Mediated Phosphorylation of Histone H3 Ser28 Couples MAPK Signalling with Early Gene Induction and Cardiac Hypertrophy. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Blay JY, Hindi N, Bollard J, Aguiar S Jr, Angel M, Araya B, Badilla R, Bernabeu D, Campos F, Caro-Sánchez CHS, Carvajal B, Carvajal Montoya A, Casavilca-Zambrano S, Castro-Oliden V, Chacón M, Clara M, Collini P, Correa Genoroso R, Costa FD, Cuellar M, Dei Tos AP, Dominguez Malagon HR, Donati D, Dufresne A, Eriksson M, Farias-Loza M, Fernandez P, Frezza AM, Frisoni T, Garcia-Ortega DY, Gelderblom H, Gouin F, Gómez-Mateo MC, Gronchi A, Haro J, Huanca L, Jimenez N, Karanian M, Kasper B, Lopes David BB, Lopez-Pousa A, Lutter G, Martinez-Said H, Martinez-Tlahuel J, Mello CA, Morales Pérez JM, Moura David S, Nascimento AG, Ortiz-Cruz EJ, Palmerini E, Patel S, Pfluger Y, Provenzano S, Righi A, Rodriguez A, Salas R, Santos TTG, Scotlandi K, Soule T, Stacchiotti S, Valverde C, Waisberg F, Zamora Estrada E, Martin-Broto J. SELNET clinical practice guidelines for soft tissue sarcoma and GIST. Cancer Treat Rev. 2022;102:102312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 35. | Chen T, Xu L, Ye L, Qiu H, Hu Y, Liu H, Zhou Z, Li G, Yu J. A new nomogram for recurrence-free survival prediction of gastrointestinal stromal tumors: Comparison with current risk classification methods. Eur J Surg Oncol. 2019;45:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Dz Chen J, Zhu Y, Wang Y. Emerging Noninvasive Neuromodulation Methods for Functional Gastrointestinal Diseases. J Transl Int Med. 2022;10:281-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 37. | Papale M, Vocino G, Lucarelli G, Rutigliano M, Gigante M, Rocchetti MT, Pesce F, Sanguedolce F, Bufo P, Battaglia M, Stallone G, Grandaliano G, Carrieri G, Gesualdo L, Ranieri E. Urinary RKIP/p-RKIP is a potential diagnostic and prognostic marker of clear cell renal cell carcinoma. Oncotarget. 2017;8:40412-40424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Bedri SK, Nilsson OB, Fink K, Månberg A, Hamsten C, Ayoglu B, Manouchehrinia A, Nilsson P, Olsson T, Hillert J, Grönlund H, Glaser A. Plasma protein profiling reveals candidate biomarkers for multiple sclerosis treatment. PLoS One. 2019;14:e0217208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Bryant KL, Stalnecker CA, Zeitouni D, Klomp JE, Peng S, Tikunov AP, Gunda V, Pierobon M, Waters AM, George SD, Tomar G, Papke B, Hobbs GA, Yan L, Hayes TK, Diehl JN, Goode GD, Chaika NV, Wang Y, Zhang GF, Witkiewicz AK, Knudsen ES, Petricoin EF 3rd, Singh PK, Macdonald JM, Tran NL, Lyssiotis CA, Ying H, Kimmelman AC, Cox AD, Der CJ. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat Med. 2019;25:628-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 526] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 40. | Zheng S, Shu Y, Lu Y, Sun Y. Chloroquine Combined with Imatinib Overcomes Imatinib Resistance in Gastrointestinal Stromal Tumors by Inhibiting Autophagy via the MAPK/ERK Pathway. Onco Targets Ther. 2020;13:6433-6441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |