Published online Jun 28, 2023. doi: 10.3748/wjg.v29.i24.3922
Peer-review started: February 1, 2023
First decision: April 14, 2023
Revised: April 27, 2023
Accepted: May 24, 2023
Article in press: May 24, 2023
Published online: June 28, 2023
Processing time: 147 Days and 1.2 Hours
Splenic vein thrombosis is a known complication of pancreatitis. It can lead to increased blood flow through mesenteric collaterals. This segmental hypertension may result in the development of colonic varices (CV) with a high risk of severe gastrointestinal bleeding. While clear guidelines for treatment are lacking, splenectomy or splenic artery embolization are often used to treat bleeding. Splenic vein stenting has been shown to be a safe option.
A 45-year-old female patient was admitted due to recurrent gastrointestinal bleeding. She was anemic with a hemoglobin of 8.0 g/dL. As a source of bleeding, CV were identified. Computed tomography scans revealed thrombotic occlusion of the splenic vein, presumably as a result of a severe acute pancreatitis 8 years prior. In a selective angiography, a dilated mesenterial collateral leading from the spleen to enlarged vessels in the right colonic flexure and draining into the superior mesenteric vein could be confirmed. The hepatic venous pressure gradient was within normal range. In an interdisciplinary board, transhepatic recanalization of the splenic vein via balloon dilatation and consecutive stenting, as well as coiling of the aberrant veins was discussed and successfully performed. Consecutive evaluation revealed complete regression of CV and splenomegaly as well as normalization of the red blood cell count during follow-up.
Recanalization and stenting of splenic vein thrombosis might be considered in patients with gastrointestinal bleeding due to CV. However, a multidisciplinary approach with a thorough workup and discussion of individualized therapeutic strategies is crucial in these difficult to treat patients.
Core Tip: Splenic vein thrombosis (SVT) is a complication of pancreatitis and can lead to development of varices with a high risk of gastrointestinal bleeding. While clear guidelines are lacking, splenectomy or splenic artery embolization are often employed. We present a rare case of recurrently bleeding colonic varices (CV) due to pancreatitis-induced (PI) SVT. In an interdisciplinary board, transhepatic recanalization of the splenic vein and consecutive stenting, as well as coiling of aberrant veins was decided and successfully performed. Follow-up revealed complete regression of varices. According to our knowledge, there is no case report in English literature describing stenting of PISVT in a patient with CV.
- Citation: Füssel LM, Müller-Wille R, Dinkhauser P, Schauer W, Hofer H. Treatment of colonic varices and gastrointestinal bleeding by recanalization and stenting of splenic-vein-thrombosis: A case report and literature review. World J Gastroenterol 2023; 29(24): 3922-3931
- URL: https://www.wjgnet.com/1007-9327/full/v29/i24/3922.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i24.3922
Gastrointestinal bleeding due to colonic varices (CV) is rare but can be severe[1,2]. CV may be caused by thrombosis of the splenic vein. In our patient this was due to an episode of acute pancreatitis. Moreover, treatment is challenging, and a multidisciplinary individualized approach is warranted in these patients. Cases are rare and clear guidelines for management are lacking, as the available data is heterogenous and case numbers for interventional recanalization are low. Therefore, we present a patient with CV due to pancreatitis-induced splenic vein thrombosis (PISVT) and summarize the clinical features, treatment options and available data in the current literature.
The splenic vein is located at the posterior surface of the pancreas and is fed by tributaries from the spleen, the inferior mesenteric vein, the short gastric veins, the left gastroepiploic vein and pancreatic veins[2]. Isolated obstruction can occur as a result of external compression due to malignancy, retroperitoneal disease or splenic artery aneurysms[2,3]. In addition, SVT can be associated with a variety of diseases shown in Table 1[2,4,5].
| Pancreatic diseases1 | Carcinoma |
| All forms of pancreatitis | |
| Pseudocysts | |
| Abscess | |
| Pancreas divisum | |
| Liver diseases | Liver cirrhosis |
| Budd-Chiari syndrome | |
| Coagulation disorders | Thrombocythemia |
| Deficiency of antithrombin III, Protein S or C | |
| Antiphospholipid syndrome | |
| Mutation of factor V | |
| Myeloproliferative disorders | |
| Inflammatory disorders | Autoimmune disease |
| Inflammatory bowel disease | |
| Intraabdominal inflammation | |
| Others | Prior abdominal surgery |
| Hormonal therapy | |
| Paroxysmal nocturnal hemoglobinuria | |
| Bechet’s disease | |
| Retroperitoneal fibrosis | |
| Tuberculous adenitis |
The predominant cause in approximately 50%-60% of isolated SVT is acute or chronic pancreatitis[5,6]. While Butler et al[7] yielded an overall incidence of PISVT of 14.1% in patients with pancreatitis (22.6% in acute and 12.4% in chronic pancreatitis), a meta-analysis including over 10000 patients reported the pooled prevalence of SVT in pancreatitis to be as high as 11.2%[7,8]. Thrombosis is a consequence of the associated inflammatory process of the pancreas, fostered by endothelial damage, the release of prothrombotic cytokines and vein compression due to edema, lymphadenopathy or pseudocysts causing stasis of blood flow[6]. However, it is still unclear if the risk of thrombosis correlates with the severity of pancreatitis[2,8]. Since PISVT is mainly diagnosed after the acute phase, recurrent indolent episodes of pancreatitis could play a major role in its development[6]. The diagnosis can be challenging as the accuracy of doppler sonography to diagnose SVT is low due to the small size, the location of the splenic vein and the presence of adjacent collaterals[6]. In addition, the incidence of compensatory splenomegaly in SVT was reported at 51.9%, rendering it unreliable for diagnosis[8]. Additionally, D-dimer levels can be elevated in SVT, but are also unspecific[4]. The diagnostic test of choice to assess the presence of SVT is late-phase celiac angiography [computed tomography (CT) or magnetic resonance imaging][6]. Ultimately, the gold standard to confirm SVT is selective angiography of the splenic artery with lacking drainage via the splenic vein[6]. Clinical symptoms of SVT are nonspecific and include abdominal pain and nausea[4]. Historically, patients with SVT were commonly described to present with acute bleeding, thereby overestimating the risk for hemorrhage, which has since been thought to be about 4%-17%[9,10]. Due to a broader availability and improvement of imaging, at present the majority of cases are diagnosed at an asymptomatic state[8].
PISVT can lead to increased blood flow through splenoportal, gastroepiploic or mesenteric collaterals creating a localized form of venous hypertension referred to as “segmental”, “sinistral” or “linear hypertension”[8]. This results in the development of varices in the area decompressing the corresponding segment and entails an increased risk of severe hemorrhage[2]. Frequently, the short gastric veins collateralize through portosystemic esophageal veins to the azygos system leading to esophageal varices[11]. Other pathways decompress the short gastric veins via the coronary vein into the portal vein or via the gastroepiploic arcade into the vena mesenterica superior creating gastric varices[11]. Another path runs along the left gastroepiploic vein to the left colic and inferior mesenteric vein[11]. Furthermore, collateral channels along intercostal and diaphragmatic veins draining into the vena cava inferior and a pathway via the adrenal vein into the renal vein have been described[11]. Segmental hypertension occurred in 12.5% in acute pancreatitis and in 2.7% in chronic pancreatitis[10,12]. Identified risk factors for the development of PISVT in pancreatitis were smoking, alcohol consumption, hypertriglyceridemia, diabetes, recurrent acute pancreatitis, infection of necrosis and pseudocysts[10,12]. Gastrointestinal varices are found in 53% of PISVT and are gastric in the majority (77.3%) of cases[8]. This is especially relevant as the rate of bleeding in patients with PISVT was reported to be 12.3%[8]. In case of varices the bleeding rate was at 19.1% and seemed to be higher in alcoholic than in non-alcoholic pancreatitis[12,13].
CV associated with PISVT are very rare, data is scarce and only a few case reports exist[1,14-17]. In these cases, splenectomy was performed to manage bleeding and decompress the varices. The incidence rate of CV in general is estimated at 0.07%[3,18]. Causes of CV include liver cirrhosis (as the most frequent etiology), congestive heart failure, porto-mesenteric thrombosis, post-operative adhesions, inherited vascular anomalies (e.g., Klippel-Trenaunay-Weber syndrome) and idiopathic varices[18,19]. Diagnosis is primarily established via colonoscopy, although selective mesenteric angiography is the gold standard, as varices can be missed when flattened with insufflation and in hypotensive patients, especially if active bleeding is present[3]. Ectopic varices are reported to be more prone to bleeding and can have a mortality rate as high as 40%[20]. CV are rarer than small bowel and rectal varices; in one recent study only 3.5% of ectopic varices were situated in the colon[18,21].
We present a 45-year-old female who was admitted to the hospital due to recurrent hematochezia.
She denied abdominal pain, weight loss, fever or night sweats and there was no history of malignancy or inflammatory bowel disease.
Patient history included alcohol misuse, bipolar disorder, arterial hypertension, cholecystolithiasis, pancreatic insufficiency and an episode of severe acute pancreatitis 8 years prior, which required intensive care treatment.
Family history for gastrointestinal disease, tumor or bleeding was negative.
On presentation, abdominal examination was unremarkable and vital signs were stable.
Blood work showed anemia with a hemoglobin of 8 g/dL (12-15.4 g/dL), a mean corpuscular volume of 70 fL (80-99 fL) and a mean corpuscular hemoglobin of 22 pg (27-33.5 pg) while coagulation parameters were within normal range.
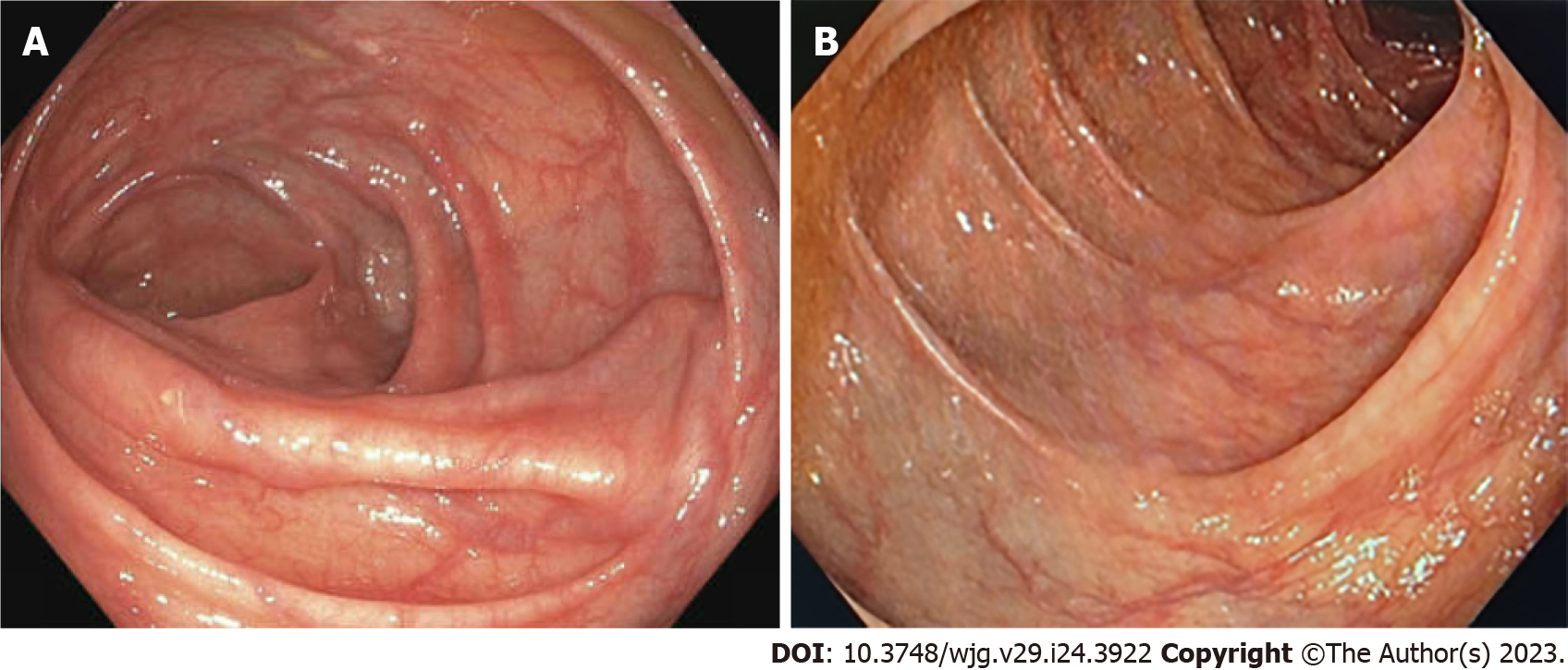
Gastroscopy revealed reflux esophagitis of grade A and a mild duodenitis. There was no evidence for varices or ulcers. During colonoscopy, largely dilated and tortuous varices spreading from the right transverse colon to just underneath the right colonic flexure were diagnosed (Figure 1). There was no active bleeding. A CT angiography confirmed an extensive venous convolute at the right colonic flexure and evidenced an aberrant mesenteric collateral coming from the splenic hilum as its feeding vein (Figure 2). The varices’ draining vein could be identified and lead to the superior mesenteric vein. In addition, splenomegaly (15 cm) and thrombotic occlusion of the splenic vein were diagnosed, while the portal vein and superior mesenteric vein were perfused regularly.
Selective angiography of the spleen via the femoral artery observed a long-segment occlusion of the splenic vein up to the confluence of the portal vein. It confirmed the findings of the CT angiography and noted that almost the entire venous drainage of the spleen occurred via mesenteric collaterals (Figure 2). Simultaneously, an arterio-portal fistula was ruled out. There was no evidence of liver cirrhosis or portal hypertension. Laboratory investigations showed transaminases, albumin and coagulation parameters within normal range. Platelets were slightly decreased due to splenomegaly. Transient elastography showed a value of 7.5 kPa and the hepatic venous pressure gradient was normal (3 mmHg).
Presumably, the splenic vein thrombosis was a result of the reported acute severe pancreatitis 8 years prior. Consequently, we postulated that PISVT prompted the formation of collaterals which lead to CV and gastrointestinal bleeding.
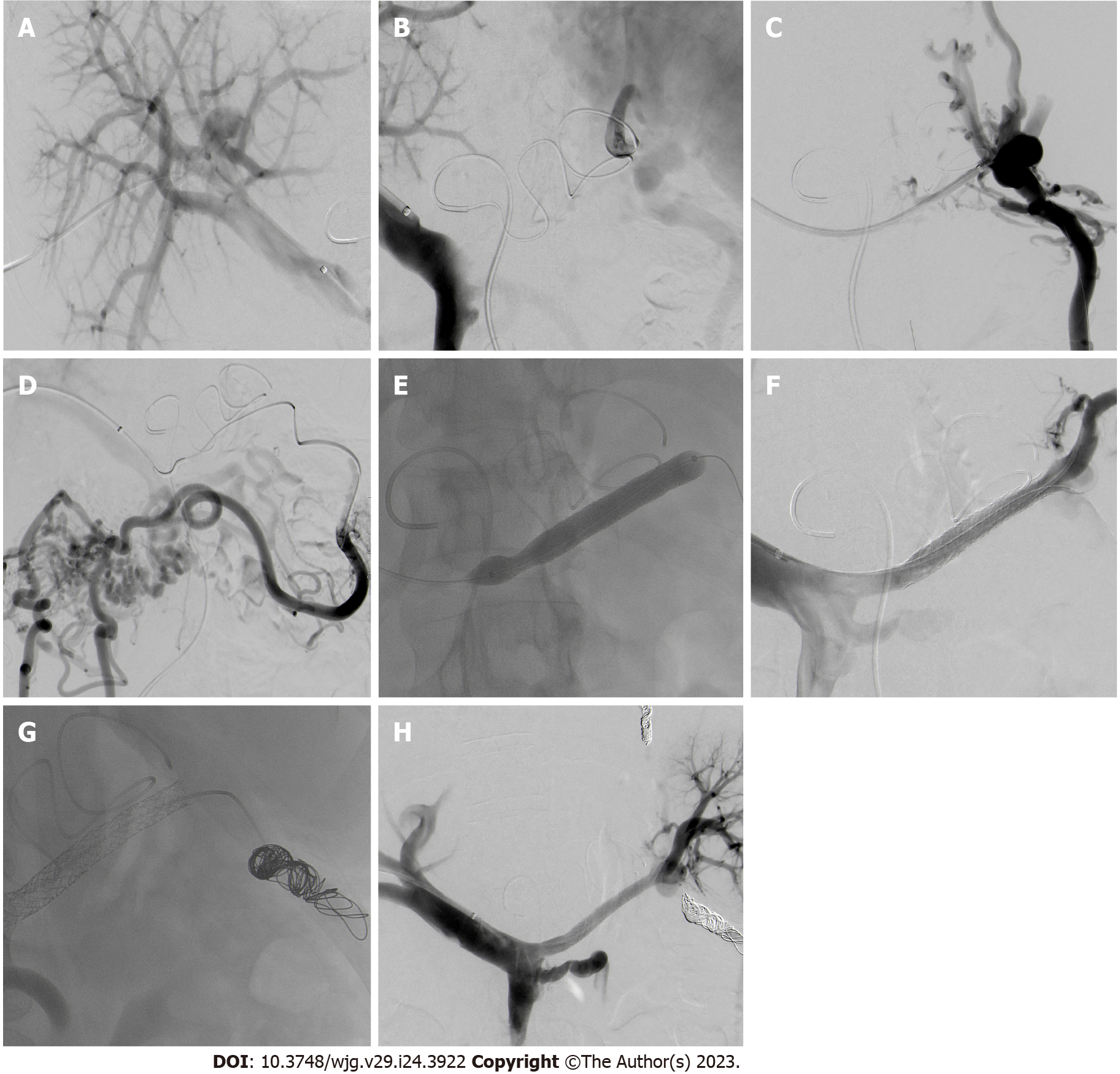
In an interdisciplinary board, interventional transhepatic recanalization of the splenic vein via balloon dilatation and consecutive stenting, as well as coiling of the aberrant mesenteric veins under general anesthesia was planned. The splenic artery was cannulated via the femoral artery and a microcatheter was used to reach the splenic hilus and monitor venous drainage during the procedure (Figure 3A). Subsequently, right portal vein access was obtained transhepatically, a 6-French vascular sheath (Biotronik®, Berlin, Germany) was placed and a contrast venogram showed the expected occlusion of the splenic vein measuring 8 cm (Figure 3B and C). The aberrant vein feeding the varices was contrasted (Figure 3D). The obstructed vein was successfully cannulated using a 5-MP catheter and a Progreat-microcatheter (Terumo Interventional Systems, Somerset, NJ, United States). After crossing the occlusion, balloon angioplasty with a 3 mm × 12 mm and a 5 mm × 40 mm percutaneous transluminal angioplasty balloon was performed and was followed by deployment of two overlapping stentgrafts (8 mm × 57mm and 8 mm × 37 mm, BeGraft, Bentley), achieving successful recanalization of the splenic vein (Figure 3E and F). A follow-up venography confirmed restoration of antegrade flow. Afterwards, the dilated mesenteric collateral vein leading from the splenic hilum to the colon was cannulated via stentgrafts and a distal and proximal embolization by the deployment of multiple coils (Boston Scientific Interlock®; Marlborough, MA, United States) was performed (Figure 3G). A small gastral aberrant vein was also coiled. Follow-up angiography demonstrated patent stentgrafts allowing complete drainage of the spleen through the splenic vein (Figure 3H). The transhepatic access tract was occluded by means of gelfoam slurry (EmboCubes®; Merit Medical, South Jordan, UT, United States) and the femoral access using AngioSeal® (Terumo Interventional Systems).
A consecutive Doppler examination revealed antegrade perfusion of the splenic vein and regression of splenomegaly (1 d post-recanalization 15 cm, after 4 d 13 cm). At follow-up 2 mo later, lab results found a normalization of red blood cell count (hemoglobin 12.1 g/dL) and a colonoscopy confirmed drastic regression of CV with few residual veins in the right colonic flexure (Figure 4A). One year post-intervention, hemoglobin was at 13.2 g/dL, the spleen measured 11.5 cm, the splenic vein stent was patent with orthograde perfusion on duplex sonography and residual veins had completely disappeared (Figure 4B).
Gastrointestinal bleeding due to CV caused by PISVT is rare and difficult to treat[1,14-17]. Time from the onset of pancreatitis to the detection of SVT is highly variable ranging between 10 d and 9 years[10]. In our patient, there was an 8 year gap between the episode of pancreatitis and diagnosis of PISVT.
Due to limited data, clear guidelines for the treatment of CV are lacking. The use of non-selective beta-blockers for bleeding prophylaxis has been recommended with a low quality of evidence[22,23]. Conservative treatment also includes iron supplements and avoiding constipation[24]. The use of anticoagulation (AC) in chronic SVT with collaterals is controversial and dependent on etiology, risk of recurrence, progression and hemorrhage[5]. Considering the low rate of recanalization as well as the substantial bleeding risk if varices are present, an individualized risk assessment is necessary[4,5,10]. In our case, AC was not viable due to ongoing intermittent bleeding. However, our patient had suffered from acute pancreatitis previously and AC might be beneficial in acute pancreatitis to avoid SVT[25]. In acute pancreatitis and PISVT, data on AC are conflicting regarding recanalization and bleeding rate[10,26-28].
Endoscopic therapies such as variceal ligation, injection of cyanoacrylate or sclerotherapy may be performed for bleeding control in localized varices, but success depends on the location and expansion of the varices[2,18,23]. In addition, these techniques do not treat the underlying cause, thereby resulting in high re-bleeding rates[2,23]. In the setting of portal hypertension, transjugular intrahepatic portosystemic shunt (TIPS) is an option and achieved 100% initial hemostasis in ectopic variceal bleeding, with a subsequent re-bleeding rate of 21%[29]. Nevertheless, TIPS is not expedient in segmental hypertension when portal pressure and hepatic function are normal[2,23]. Another option to manage CV is embolization of the feeding veins[23,29]. Complications are rare and include post-embolization syndrome, infection, vessel injury and thromboembolism[30]. Embolization represents another short-term therapy with a high rate of successful bleeding control, but does not influence portal pressure, thus entailing high re-bleeding rates[23].
Surgical treatment, as for example, colon resection, can be required in cases of uncontrolled bleeding or when extensive varices are present[24]. Surgical devascularization, shunt procedures or other local therapy depending on the underlying etiology have been reported[23,29]. Historically, the surgical treatment of choice for PISVT is splenectomy, which eliminates the collateral outflow[6,31]. In patients with hemorrhage unresponsive to conservative management or a history of bleeding, it is still the treatment of choice[2]. However, considering the reported lack of progression and rarity of hemorrhage in patients with asymptomatic varices, as well as operative risk, risk of postoperative infection and portal vein thrombosis, “prophylactic splenectomy” in patients without evidence of bleeding remains controversial[2,6,11,31]. Splenic artery embolization (SAE) resulting in splenic infarction represents an option, although the risk of splenic abscess and intervention associated morbidity is considerable[2,6].
Reports on splenic vein recanalization and thereby minimizing blood flow to collaterals in segmental hypertension are scarce[11]. A retrospective study of endovascular splenic vein recanalization in patients with SVT by a transjugular approach achieved success in 8 of 11 cases[32]. Another study reported effective splenic vein stenting in 3 patients using a transhepatic approach[33]. Recently, Liu et al[34] compared 9 patients with splenic vein stenting, 12 cases of splenectomy and 12 patients with conservative treatment in PISVT and there were no recurrent bleeding or major complications in the interventional group[34]. Stent patency was 100% and PISVT receded significantly[34]. Furthermore, a retrospective analysis comparing splenic vein stenting and SAE in SVT related bleeding found significantly less re-bleeding in the recanalization group[35].
In our patient, endoscopic treatment and TIPS were not viable options due to the localization, size and extension of the varices and absence of portal hypertension. Embolization alone, balloon-occluded retrograde transvenous obliteration (commonly referred to as BRTO) or segmental colonic resection were considered as short-term options, but were unlikely to prevent future bleeding because of potential collateral development. Moreover, splenectomy to alleviate the segmental hypertension was discussed, but as the patient was stable and preferred a less invasive alternative, we opted to perform transhepatic recanalization of the splenic vein and consecutive stenting, as well as coiling of the feeding veins. Splenic vein stenting has been shown to be a safe option[32,33]. According to our knowledge, there is no case report in the English literature describing recanalization and stenting of PISVT in a patient with CV. We found two case reports using recanalization and stenting in superior mesenteric vein thrombosis – one in hepatitis with CV and one in PISVT and associated duodenal varices with excellent results[18,36]. Also, there is a case report of successful splenic vein stenting in PISVT with gastric varices[11]. In the current case, we describe a successful transhepatic endovascular recanalization and stenting of a fully occluded splenic vein and embolization of aberrant veins in the setting of PISVT and CV with gastrointestinal bleeding. It can be argued, that coiling of the dilated aberrant veins leads to faster recompensation but may increase risk of splenic rupture in acute stent failure[35].
In summary, splenic vein recanalization seems to be an effective and safe procedure to resolve variceal complications in PISVT. An interdisciplinary discussion of potential therapeutic options is crucial as the available experience is important and an individualized approach to these patients is mandatory.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Austria
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ding WW, China; Protopapas AA, Greece S-Editor: Li L L-Editor: Filipodia P-Editor: Li L
| 1. | Kitagawa S, Sato T, Hirayama A. Colonic Varices Due to Chronic Pancreatitis: A Rare Cause of Lower Gastrointestinal Bleeding. ACG Case Rep J. 2015;2:168-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Köklü S, Coban S, Yüksel O, Arhan M. Left-sided portal hypertension. Dig Dis Sci. 2007;52:1141-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Dina I, Braticevici CF. Idiopathic colonic varices: case report and review of literature. Hepat Mon. 2014;14:e18916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Valeriani E, Riva N, Di Nisio M, Ageno W. Splanchnic Vein Thrombosis: Current Perspectives. Vasc Health Risk Manag. 2019;15:449-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 5. | Thatipelli MR, McBane RD, Hodge DO, Wysokinski WE. Survival and recurrence in patients with splanchnic vein thromboses. Clin Gastroenterol Hepatol. 2010;8:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Weber SM, Rikkers LF. Splenic vein thrombosis and gastrointestinal bleeding in chronic pancreatitis. World J Surg. 2003;27:1271-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Butler JR, Eckert GJ, Zyromski NJ, Leonardi MJ, Lillemoe KD, Howard TJ. Natural history of pancreatitis-induced splenic vein thrombosis: a systematic review and meta-analysis of its incidence and rate of gastrointestinal bleeding. HPB (Oxford). 2011;13:839-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 8. | Xu W, Qi X, Chen J, Su C, Guo X. Prevalence of Splanchnic Vein Thrombosis in Pancreatitis: A Systematic Review and Meta-Analysis of Observational Studies. Gastroenterol Res Pract. 2015;2015:245460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 9. | Heider TR, Azeem S, Galanko JA, Behrns KE. The natural history of pancreatitis-induced splenic vein thrombosis. Ann Surg. 2004;239:876-80; discussion 880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Li H, Yang Z, Tian F. Clinical Characteristics and Risk Factors for Sinistral Portal Hypertension Associated with Moderate and Severe Acute Pancreatitis: A Seven-Year Single-Center Retrospective Study. Med Sci Monit. 2019;25:5969-5976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | El Kininy W, Kearney L, Hosam N, Broe P, Keeling A. Recurrent variceal haemorrhage managed with splenic vein stenting. Ir J Med Sci. 2017;186:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Ru N, He CH, Ren XL, Chen JY, Yu FF, Yan ZJ, Guo JY, Zhu JH, Wang YC, Qian YY, Pan J, Hu LH, Li ZS, Zou WB, Liao Z. Risk factors for sinistral portal hypertension and related variceal bleeding in patients with chronic pancreatitis. J Dig Dis. 2020;21:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Anand A, Gunjan D, Agarwal S, Kaushal K, Sharma S, Gopi S, Mohta S, Madhusudhan KS, Singh N, Saraya A. Vascular complications of chronic pancreatitis: A tertiary center experience. Pancreatology. 2020;20:1085-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Burbige EJ, Tarder G, Carson S, Eugene J, Frey CF. Colonic varices. A complication of pancreatitis with splenic vein thrombosis. Am J Dig Dis. 1978;23:752-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Alarco HA, Gómez Rodríguez-Bethencourt MA, Díaz H, Pérez Palma J, Minguillón A, Bordallo A, González Hermoso F. [Colonic lesion caused by acute pancreatitis. Use of minimal catheter jejunostomy. Apropos of 6 cases]. Rev Esp Enferm Apar Dig. 1989;75:465-469. [PubMed] |
| 16. | Fracasso P, Caviglia R, Grassi A, Lapenta R, Stigliano V, Casole P, Casale V. [Colonic varices secondary to recurrent acute pancreatitis]. Minerva Gastroenterol Dietol. 1993;39:191-193. [PubMed] |
| 17. | Van Wijngaarden P, Van der Wiel HE, Tetteroo GW, Bode WA. A patient with gastric fundal varices and colonic varices due to splenic vein thrombosis. Neth J Med. 1997;51:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Naffouj S, Al-Shammari M, Salgia R. Treatment of colonic varices with a superior mesenteric venous stent: a case report describing a unique approach. Gastroenterol Rep (Oxf). 2021;9:597-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 19. | Krishna RP, Singh RK, Ghoshal UC. Recurrent lower gastrointestinal bleeding from idiopathic ileocolonic varices: a case report. J Med Case Rep. 2010;4:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Saad WE, Lippert A, Saad NE, Caldwell S. Ectopic varices: anatomical classification, hemodynamic classification, and hemodynamic-based management. Tech Vasc Interv Radiol. 2013;16:158-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Watanabe N, Toyonaga A, Kojima S, Takashimizu S, Oho K, Kokubu S, Nakamura K, Hasumi A, Murashima N, Tajiri T. Current status of ectopic varices in Japan: Results of a survey by the Japan Society for Portal Hypertension. Hepatol Res. 2010;40:763-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Simonetto DA, Singal AK, Garcia-Tsao G, Caldwell SH, Ahn J, Kamath PS. ACG Clinical Guideline: Disorders of the Hepatic and Mesenteric Circulation. Am J Gastroenterol. 2020;115:18-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 23. | Helmy A, Al Kahtani K, Al Fadda M. Updates in the pathogenesis, diagnosis and management of ectopic varices. Hepatol Int. 2008;2:322-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Akküçük S, Aydoğan A, Paltacı İ, Temiz M. Diffuse idiopathic varices in the colon characterized by lower gastrointestinal bleeding. Ulus Cerrahi Derg. 2014;30:109-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Zhou J, Zhang H, Mao W, Ke L, Li G, Ye B, Zhang J, Lin J, Gao L, Tong Z, Li W. Efficacy and Safety of Early Systemic Anticoagulation for Preventing Splanchnic Thrombosis in Acute Necrotizing Pancreatitis. Pancreas. 2020;49:1220-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Vadlamudi RS, Matli VVK, Thoguluva Chandrasekar V, Kalakonda A, Rawlins SR. Chemoprophylaxis to Prevent Deep Venous Thrombosis in Patients Hospitalized for Pancreatitis: Beneficial or Harmful? Cureus. 2021;13:e19645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 27. | Hajibandeh S, Hajibandeh S, Agrawal S, Irwin C, Obeidallah R, Subar D. Anticoagulation Versus No Anticoagulation for Splanchnic Venous Thrombosis Secondary to Acute Pancreatitis: Do We Really Need to Treat the Incidental Findings? Pancreas. 2020;49:e84-e85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Sissingh NJ, Groen JV, Koole D, Klok FA, Boekestijn B, Bollen TL, van Santvoort HC, Verdonk RC, Bonsing BA, van Eijck CHJ, van Hooft JE, Mieog JSD; Dutch Pancreatitis Study Group. Therapeutic anticoagulation for splanchnic vein thrombosis in acute pancreatitis: A systematic review and meta-analysis. Pancreatology. 2022;22:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Sarin SK, Kumar CKN. Ectopic varices. Clin Liver Dis (Hoboken). 2012;1:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (35)] |
| 30. | Ohs Z, Jones M, Sharma N, Loveridge K. Percutaneous Transhepatic Embolization of Ectopic Varices in a Patient With Portal Hypertension Presenting With Hemorrhagic Shock. Cureus. 2021;13:e18209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 31. | Pandey V, Patil M, Patel R, Chaubal A, Ingle M, Shukla A. Prevalence of splenic vein thrombosis and risk of gastrointestinal bleeding in chronic pancreatitis patients attending a tertiary hospital in western India. J Family Med Prim Care. 2019;8:818-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Luo X, Nie L, Wang Z, Tsauo J, Tang C, Li X. Transjugular endovascular recanalization of splenic vein in patients with regional portal hypertension complicated by gastrointestinal bleeding. Cardiovasc Intervent Radiol. 2014;37:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Stein M, Link DP. Symptomatic spleno-mesenteric-portal venous thrombosis: recanalization and reconstruction with endovascular stents. J Vasc Interv Radiol. 1999;10:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Liu J, Wang Q, Ding X, Liu Q, Huang W, Gu J, Wang Z, Wu W, Wu Z. The clinical applicability of percutaneous splenic vein stent implantation for pancreatic portal hypertension. BMC Gastroenterol. 2022;22:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 35. | Wei B, Zhang L, Tong H, Wang Z, Wu H. Retrospective Comparison of Clinical Outcomes Following Splenic Vein Stenting and Splenic Arterial Embolization in Sinistral Portal Hypertension-Related Gastrointestinal Bleeding. AJR Am J Roentgenol. 2021;216:1579-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Bhat AP, Davis RM, Bryan WD. A rare case of bleeding duodenal varices from superior mesenteric vein obstruction -treated with transhepatic recanalization and stent placement. Indian J Radiol Imaging. 2019;29:313-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |