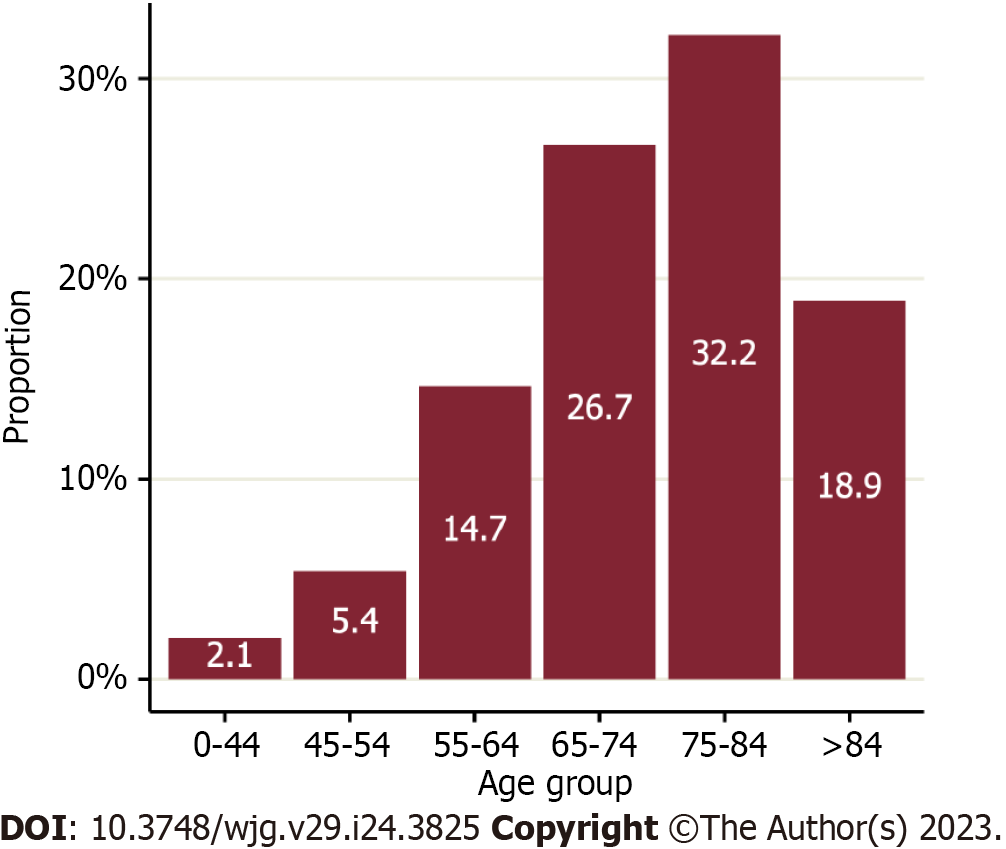Published online Jun 28, 2023. doi: 10.3748/wjg.v29.i24.3825
Peer-review started: March 20, 2023
First decision: April 27, 2023
Revised: May 5, 2023
Accepted: May 25, 2023
Article in press: May 25, 2023
Published online: June 28, 2023
Processing time: 99 Days and 20.1 Hours
Incidence of cholangiocarcinoma (CCA) is rising, with overall prognosis re-maining very poor. Reasons for the high mortality of CCA include its late presentation in most patients, when curative options are no longer feasible, and poor response to systemic therapies for advanced disease. Late presentation presents a large barrier to improving outcomes and is often associated with diagnosis via mergency presentation (EP). Earlier diagnoses may be made by Two Week Wait (TWW) referrals through General practitioner (GP). We hypothesise that TWW referrals and EP routes to diagnosis differ across regions in England.
To investigate routes to diagnosis of CCA over time, regional variation and influencing factors.
We linked patient records from the National Cancer Registration Dataset to Hospital Episode Statistics, Cancer Waiting Times and Cancer Screening Programme datasets to define routes to diagnosis and certain patient characteristics for patients diagnosed 2006-2017 in England. We used linear probability models to investigate geographic variation by assessing the proportions of patients diagnosed via TWW referral or EP across Cancer Alliances in England, adjusting for potential confounders. Correlation between the proportion of people diagnosed by TWW referral and EP was investigated with Spearman’s correlation coefficient.
Of 23632 patients diagnosed between 2006-2017 in England, the most common route to diagnosis was EP (49.6%). Non-TWW GP referrals accounted for 20.5% of diagnosis routes, 13.8% were diagnosed by TWW referral, and the remainder 16.2% were diagnosed via an ‘other’ or Unknown route. The proportion diagnosed via a TWW referral doubled between 2006-2017 rising from 9.9% to 19.8%, conversely EP diagnosis route declined, falling from 51.3% to 46.0%. Statistically significant variation in both the TWW referral and EP proportions was found across Cancer Alliances. Age, presence of comorbidity and underlying liver disease were independently associated with both a lower proportion of patients diagnosed via TWW referral, and a higher proportion diagnosed by EP after adjusting for other potential confounders.
There is significant geographic and socio-demographic variation in routes to diagnosis of CCA in England. Knowledge sharing of best practice may improve diagnostic pathways and reduce unwarranted variation.
Core Tip: We investigated changes to routes to diagnosis for cholangiocarcinoma patients across England’s 21 regional Cancer Alliances over a 12-year period, and factors associated with differences. We found almost half (49.6%) of 23632 patients in the study were diagnosed via emergency presentation, a route associated with late presentation, advanced disease and poorer outcomes. Those diagnosed by Two Week Wait referral through a primary care doctor, where earlier diagnoses are possible, increased from 9.8-19.8% from 2006-2017, still considered low. Significant regional variation was found, alongside the discovery that age, comorbidities and underlying liver disease had independent associations with their routes to diagnosis.
- Citation: Zalin-Miller A, Jose S, Knott C, Paley L, Tataru D, Morement H, Toledano MB, Khan SA. Regional variation in routes to diagnosis of cholangiocarcinoma in England from 2006 to 2017. World J Gastroenterol 2023; 29(24): 3825-3842
- URL: https://www.wjgnet.com/1007-9327/full/v29/i24/3825.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i24.3825
Cholangiocarcinoma (CCA) comprise a group of malignancies arising from the epithelium of the biliary tree within or outside the hepatic parenchyma. Globally CCA are the commonest biliary malignancy, and those arising within the liver parenchyma are the second most common primary hepatic malignancy[1]. CCA are sub-classified depending on their anatomical location within the biliary tree. Intrahepatic CCA (iCCA) arise within the hepatic parenchyma itself, anatomically above the second-order bile ducts. Extrahepatic CCA (eCCA) arise more distally and are further sub-divided into perihilar CCA and distal CCA, with the latter originating between the cystic duct and ampulla of Vater[1,2].
Studies over the past few decades have consistently reported an increasing incidence in CCA globally. These studies have utilised both international and national datasets, including data collections available through the World Health Organisation, United States cancer registries, and European cancer registries[3-6]. These studies indicate a long-running increase in the global incidence of CCA, together with a general trend of rising iCCA mortality rates but stable or decreasing eCCA mortality. The reasons for these trends remain unclear. The overall prognosis for all CCA is poor, with a 5-year overall survival of less than 10%[1]. Reasons for the high mortality of CCA include its late presentation in most patients, when the only curative option, surgery, is no longer feasible, and poor response to systemic therapies for advanced disease[2]. Late presentation therefore presents a large barrier to preserving effective/curative treatment options and improving outcomes. Overcoming late presentation is difficult due to: The disease’s largely sporadic nature, wherein most patients have no known pre-existing risk factors; a lack of effective diagnostic screening tools and an asymptomatic early disease phase. Late presentation is therefore often associated with diagnosis via an emergency route. It is well recognised the cancers with high rates of emergency presentation (EP) are associated with poorer outcomes[7]. This is one of the reasons behind the creation of the United Kingdom’s National Health Service Urgent Two Week Waitreferral system (TWW), whereby a primary care doctor (General practitioner, GP) can ask the hospital for an urgent appointment to investigate for the presence of cancer, depending on specified symptom and laboratory test criteria. However the role of the TWW referral system toward achieving earlier diagnosis and therefore better outcomes may be limited for cancers such as CCA that are characterised by vague and non-specific symptoms that fall outside of specified referral criteria, which has led to the recent roll out of rapid diagnostic centres to provide a way for GPs to directly request diagnostic investigations of such symptoms[8,9]. Variation in the rates of TWW referrals and EP has been shown across English regions for all cancers[10]. We hypothesise that TWW referrals and EP for CCA differ across regions in England. To our knowledge there are no published studies analysing the pattern of routes to diagnosis for CCA. The aims of this study were to examine routes to diagnosis for CCA at a national level in England, to ascertain if these have changed over time and if regional variations exist and to determine which factors might influence any variation.
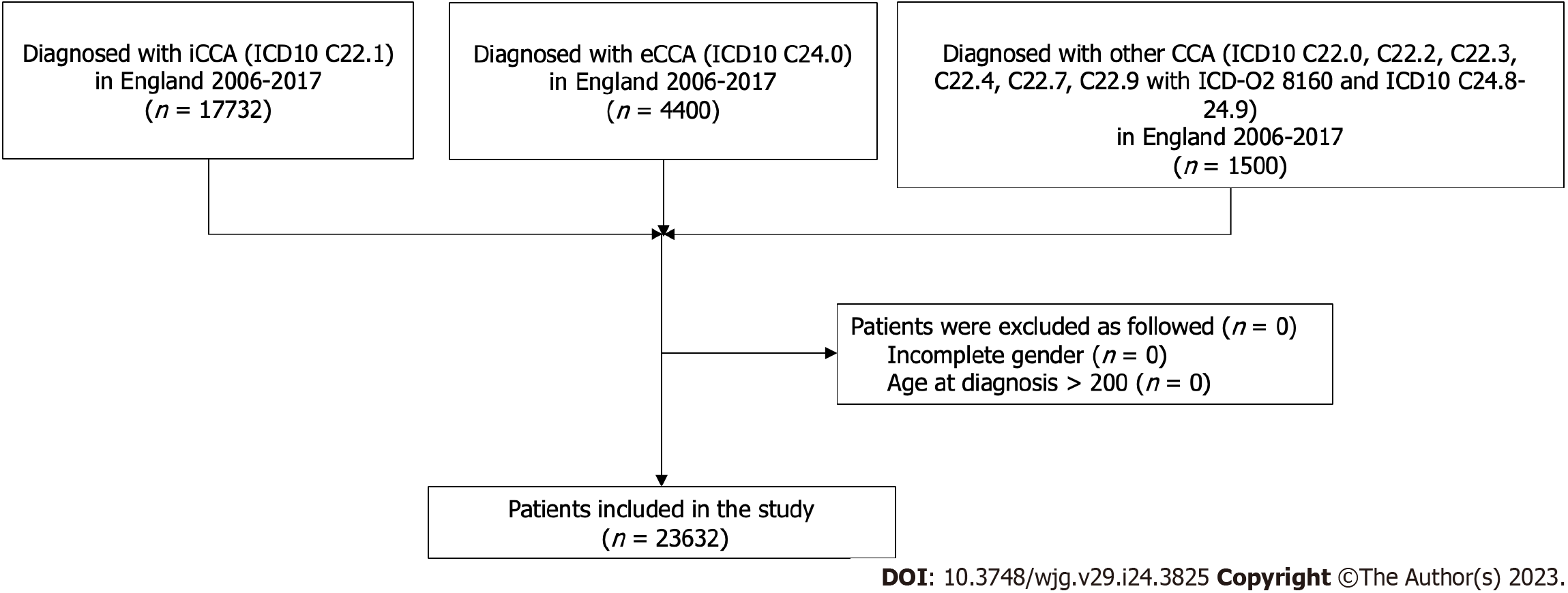
Data used were obtained from the National Cancer Registration Dataset (NCRD) [AV2018][11]. Linked patient records from Hospital Episode Statistics (HES), Cancer Waiting Times and Cancer Screening Programme datasets were used to define Routes to Diagnosis and certain patient characteristics[12]. A CCA cohort was defined based upon the topographical and histological International Classification of Disease (ICD) codes to define the following tumours: Intrahepatic iCCA ICD10 C22.1; eCCA ICD10 C24.0; other CCA ICD10 C220, C222, C223, C224, C227, C229 with ICD-O2 8160 and ICD10 C248-249 (Table 1). Malignant neoplasm of liver and intrahepatic bile ducts ICD10 C220, C222, C223, C224, C227, C229 with an ICD-O2 8160 morphology were included in the study to capture CCA tumours which may have been miscoded. Patients were selected if residents in England and diagnosed between 2006 and 2017. For patients with multiple CCA diagnoses during this period, the first registered tumour was selected. Further inclusion criteria recorded were male or female gender, and age at diagnosis between 0 and 200 years following the National Cancer Registration and Analysis Service’s Standard Operating Procedure[13].
| Sub-type | ICD10 topography code | ICDO2 histology code |
| Intrahepatic (iCCA) | C221 (Intrahepatic bile duct carcinoma) | All |
| Extrahepatic (eCCA) | C240 (Extrahepatic bile duct carcinoma) | All |
| CCA other | C248 (Overlapping lesion of biliary tract), C249 (Biliary tract, unspecified) | All |
| C220, C222, C223, C224, C227, C229 | 8160 |
Geographic variation in routes to diagnosis was analysed at the Cancer Alliance level according to 21 regions defined in 2020 for England[14]. The Cancer Alliance for each patient was assigned according to the main residence of the patient on the date of diagnosis. Patient characteristics identified a priori as possible confounding variables were: Gender (male/female); age at diagnosis (0-44/45-54/55-64/65-74/75-84/85+ years); income deprivation (quintiles)[15]; year of diagnosis; tumour sub-type (iCCA/eCCA/other); tumour morphology (adenocarcinoma/other); Charlson Comorbidity Index (CCI) (score 0/1/2/> 2)[16]; underlying liver disease (yes/no)[17]. Underlying liver disease was identified by searching diagnostic codes in HES Admitted Patient Care (APC) episodes from 5 years before to 1 year after diagnosis indictive of chronic hepatitis C or B virus, primary biliary cholangitis, autoimmune hepatitis, haemochromatosis, alcoholic liver disease, or non-alcoholic liver disease (NAFLD). NAFLD was defined as Fatty (change of) liver, not elsewhere classified, or by the presence of cirrhosis (defined using a published algorithm[17]) combined with obesity or diabetes without the presence of any other underlying liver disease.
Routes to diagnosis were defined based on an established algorithm[12] and categorised as follows: Urgent GP TWW referral for suspected cancer; Other GP referral; EP; Other (includes Outpatient, Inpatient and Death Certificate Only routes); Unknown. The proportion of people diagnosed via TWW referral and EP were the outcomes of interest for the main analyses.
The proportion of people diagnosed via each of five routes to diagnosis was described for all a priori patient and tumour characteristics. χ2 tests were used to assess the statistical significance of any unadjusted variation in proportions diagnosed via TWW referral (vs any other route) or EP (vs any other route).
Linear probability models were then performed to assess the proportions of patients diagnosed via TWW referral or EP after adjustment for potential confounders. For these, two binary outcome variables were created (TWW referral vs other diagnosis routes; EP vs other diagnosis routes) and three models run on each with increasing levels of covariate adjustment:
Model 1 (unadjusted): A model that included only Cancer Alliance as the independent variable.
Model 2 (demographic-adjusted): A model that included Cancer Alliance as well as patient age, gender and income deprivation quintile.
Model 3 (maximally-adjusted): A model that additionally included all patient and tumour characteristics.
Weighted effect coding was applied to the estimates generated by each linear probability model so that they became interpretable as percentage-point deviations from the sample mean[18]. Results from the linear probability models are presented as funnel plots with significance threshold lines denoting two and three SD from the sample mean, being approximately equivalent to 95.0% and 99.7% confidence intervals, respectively. The correlation between the proportion of people diagnosed by TWW referral and EP was investigated with Spearman’s correlation coefficient on the results from Model 3.
A sensitivity analysis was also run on both binary outcome variables, equal to Model 3 with additional adjustment for the stage at diagnosis. This analysis was applied to a subgroup of the cohort who were diagnosed between 2014-2017 (covering the year from which staging data completeness improved in the NCRD) and had a known stage at diagnosis. The stage at diagnosis was not included in the main analysis due to the high proportion of missing data (72.1%) for this variable. Model 3 was repeated in this subgroup to determine how reducing the cohort impacted on model estimates before the stage was additionally adjusted for in this group.
There were 23632 people diagnosed with CCA in England between 2006 and 2017 (Figure 1). Of these, 22.2% were aged under 65 years at diagnosis (Figure 2) and 51.5% were women. The majority were diagnosed with an iCCA (75.0%). Over a quarter (26.0%) had at least one comorbidity on the CCI and 7% were classified as having underlying liver disease. The largest proportion of diagnoses was situated in the West Midlands Cancer Alliance (10.9%), with the smallest in North Central London Cancer Alliance (1.7%) (Table 2).
| Characteristics | Patients, n (%) | |
| Total patients (n, %)1 | 23632 (100.0) | |
| Age at diagnosis (yr) | 0-44 | 488 (2.1) |
| 45-54 | 1282 (5.4) | |
| 55-64 | 3466 (14.7) | |
| 65-74 | 6315 (26.7) | |
| 75-84 | 7606 (32.2) | |
| > 84 | 4475 (18.9) | |
| Gender | Male | 11466 (48.5) |
| Female | 12166 (51.5) | |
| Yr of diagnosis | 2006 | 1585 (6.7) |
| 2007 | 1540 (6.5) | |
| 2008 | 1732 (7.3) | |
| 2009 | 1867 (7.9) | |
| 2010 | 1952 (8.3) | |
| 2011 | 1977 (8.4) | |
| 2012 | 1997 (8.5) | |
| 2013 | 2110 (8.9) | |
| 2014 | 2060 (8.7) | |
| 2015 | 2217 (9.4) | |
| 2016 | 2262 (9.6) | |
| 2017 | 2333 (9.9) | |
| Stage at diagnosis | 1 | 377 (1.6) |
| 2 | 873 (3.7) | |
| 3 | 430 (1.8) | |
| 4 | 4918 (20.8) | |
| Unstaged | 17034 (72.1) | |
| Tumour sub-type | CCA Other | 1500 (6.3) |
| eCCA | 4400 (18.6) | |
| iCCA | 17732 (75.0) | |
| Tumour morphology | Adenomas and adenocarcinomas | 21831 (92.4) |
| Other | 1801 (7.6) | |
| English Index of Multiple Deprivation, income component | Quintile 1 (least deprived) | 4491 (19.0) |
| Quintile 2 | 4966 (21.0) | |
| Quintile 3 | 4823 (20.4) | |
| Quintile 4 | 4742 (20.1) | |
| Quintile 5 (most deprived) | 4610 (19.5) | |
| Charlson Comorbidity Index | 0 | 17491 (74.0) |
| 1 | 2906 (12.3) | |
| 2 | 1630 (6.9) | |
| 3 + | 1605 (6.8) | |
| Underlying liver disease | No | 21967 (93.0) |
| Yes | 1665 (7.0) | |
| Cancer Alliance at diagnosis | Cheshire and Merseyside | 1218 (5.2) |
| East Midlands | 2164 (9.2) | |
| East of England - North | 1298 (5.5) | |
| East of England - South | 1352 (5.7) | |
| Greater Manchester | 1278 (5.4) | |
| Humber, Coast and Vale | 862 (3.6) | |
| Kent and Medway | 664 (2.8) | |
| Lancashire and South Cumbria | 853 (3.6) | |
| North Central London | 408 (1.7) | |
| North East London | 498 (2.1) | |
| North West and South West London | 1109 (4.7) | |
| Northern | 1835 (7.8) | |
| Peninsula | 950 (4.0) | |
| Somerset, Wiltshire, Avon and Gloucestershire | 1191 (5.0) | |
| South East London | 497 (2.1) | |
| South Yorkshire and Bassetlaw | 655 (2.8) | |
| Surrey and Sussex | 1354 (5.7) | |
| Thames Valley | 590 (2.5) | |
| Wessex | 1225 (5.2) | |
| West Midlands | 2573 (10.9) | |
| West Yorkshire and Harrogate | 1058 (4.5) | |
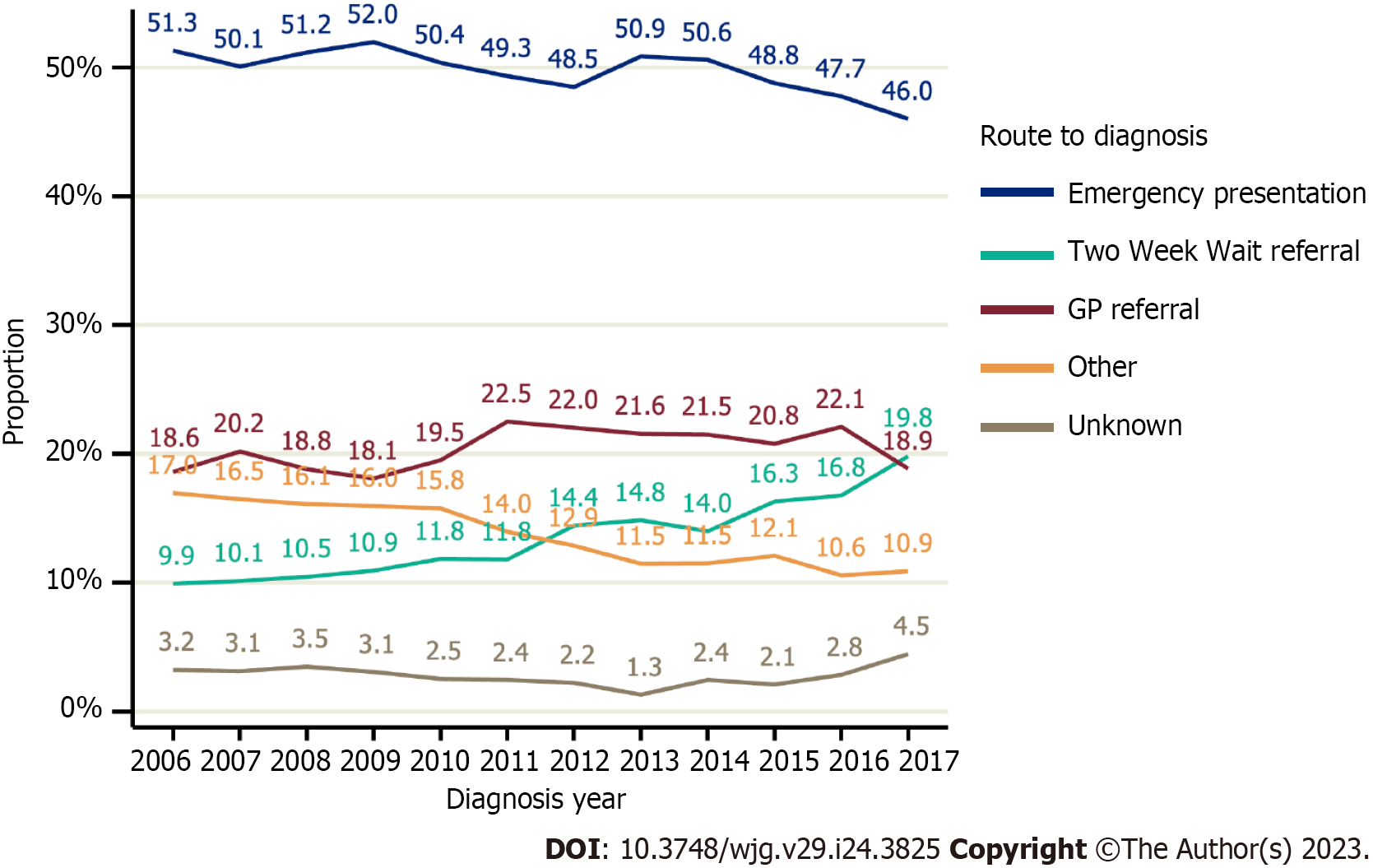
The most common route to diagnosis was EP (49.6%). Non-TWW GP referrals accounted for 20.5% of diagnosis routes, 13.8% were diagnosed by TWW referral, and the remainder 16.2% were diagnosed via an ‘other’ or Unknown route. The proportion of people diagnosed via a TWW referral doubled between 2006 and 2017, rising from 9.9% to 19.8% (Figure 3). Conversely, the proportion of people diagnosed via EP declined, falling from 51.3% to 46.0%, as did the proportion diagnosed via ‘other’ routes, from 17.0% to 10.9%.
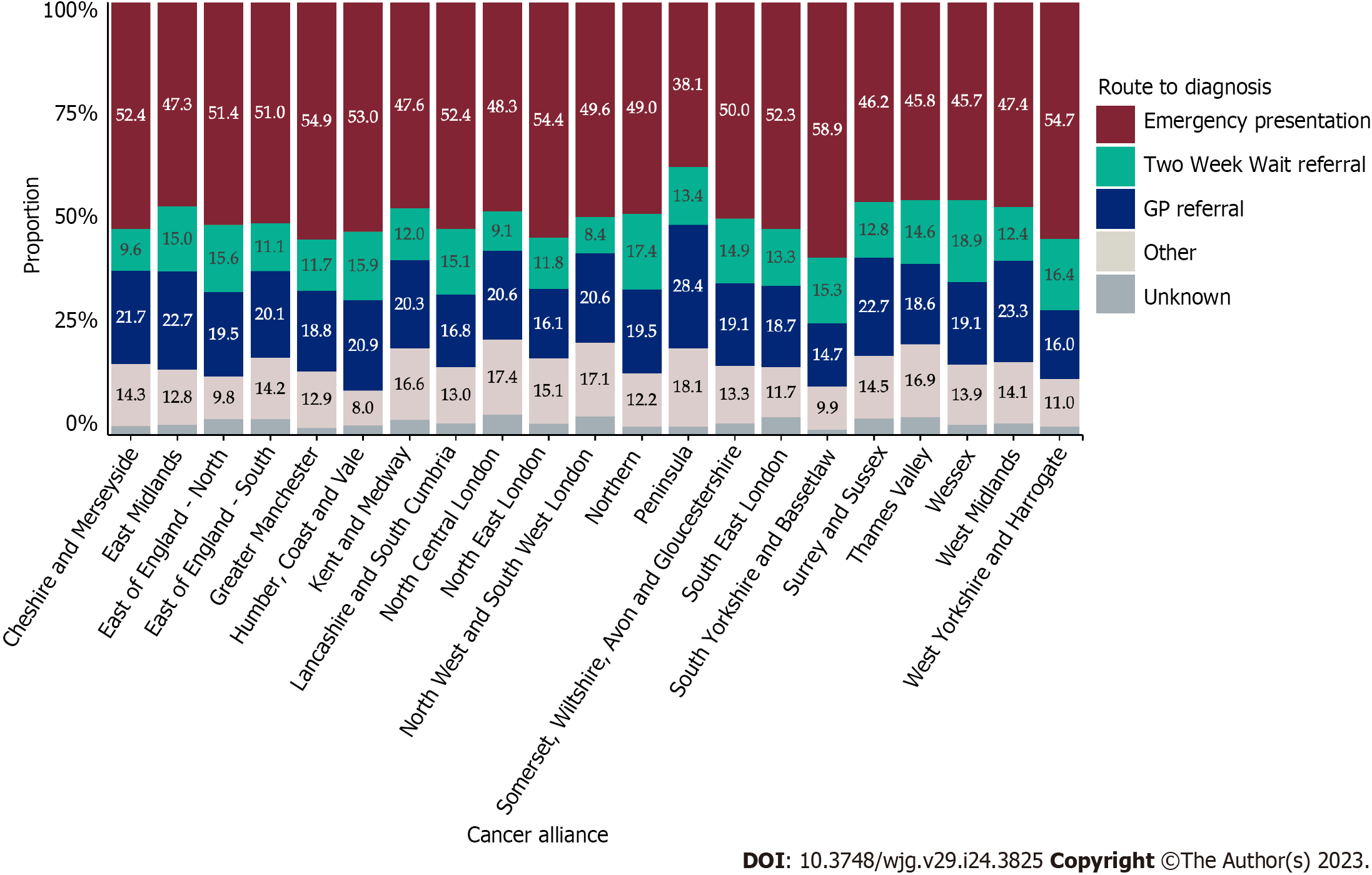
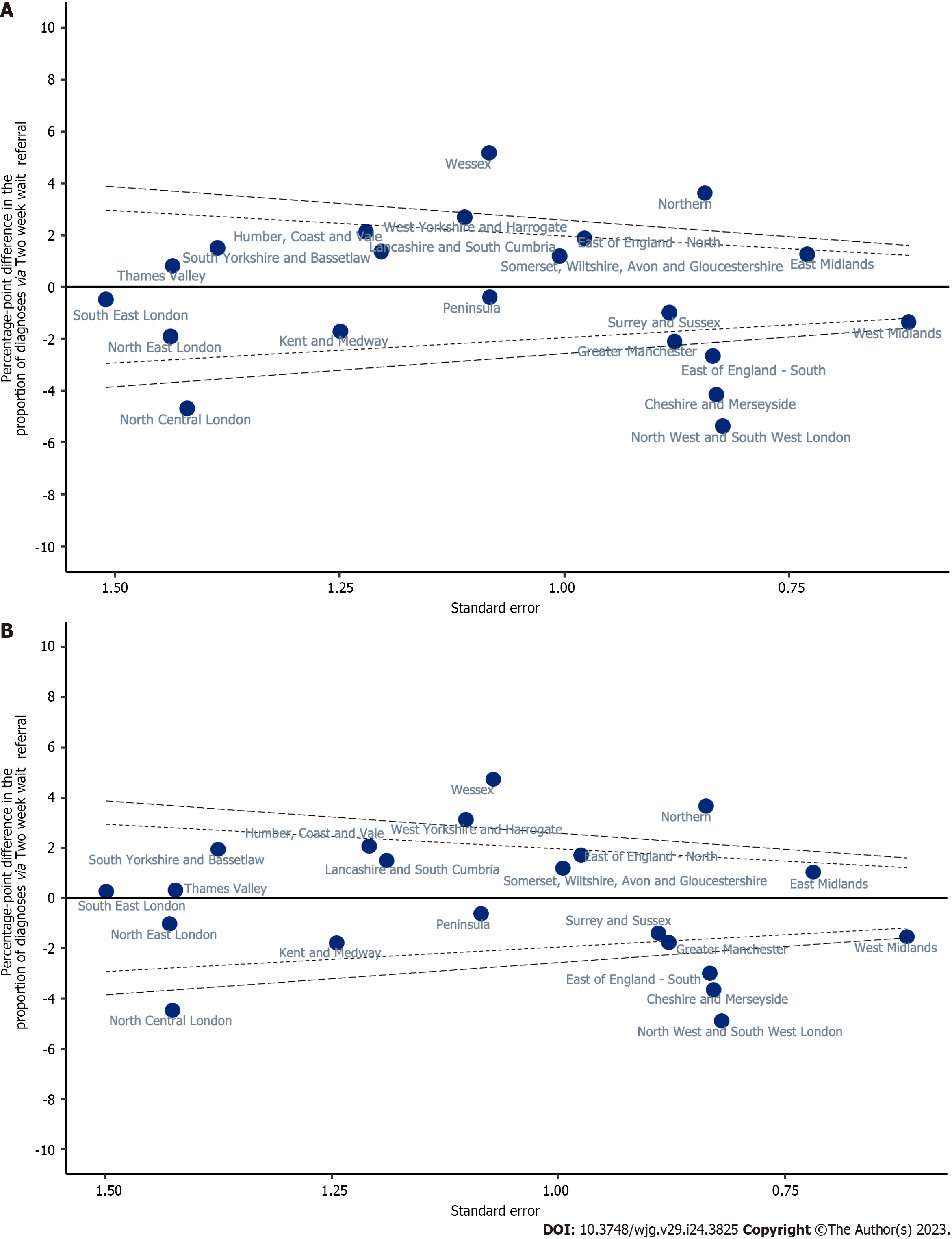
Variation in unadjusted proportions of patients diagnosed via a TWW referral was observed across the Cancer Alliances ranging from 8.4% to 18.9% (Supplementary Table 1; P < 0.001, Figure 4). In a linear probability model that included only Cancer Alliance, the proportion diagnosed via TWW referral was more than two SD higher than the sample mean in three Cancer Alliances (equating to statistically significant variation at the 5% significance level), but more than two SD lower than average for six Cancer Alliances (Table 3; Model 1 and Figure 5A). This finding remained present after adjustment for demographic factors (Table 3; Model 2) and adjustment for all patient and tumour characteristics being considered (Table 3; Model 3 and Figure 5B).
| Model 1: Unadjusted | Model 2: Demographic-adjusted1 | Model 3: Adjusted2 | ||||
| Estimate | 95%CI | Estimate | 95%CI | Estimate | 95%CI | |
| Population average (intercept) | 13.77 | 13.33-14.20 | 13.77 | 13.33-14.20 | 13.77 | 13.33-14.20 |
| Cheshire and Merseyside | -4.16 | -5.79--2.53 | -4.08 | -5.72--2.45 | -3.66 | -5.28--2.04 |
| East Midlands | 1.25 | -0.18-2.68 | 1.07 | -0.35-2.50 | 1.02 | -0.38-2.43 |
| East of England -North | 1.87 | -0.04-3.79 | 1.83 | -0.09-3.75 | 1.71 | -0.20-3.62 |
| East of England -South | -2.67 | -4.31--1.03 | -2.85 | -4.49--1.21 | -3.01 | -4.64--1.38 |
| Greater Manchester | -2.11 | -3.83--0.39 | -1.92 | -3.65--0.19 | -1.78 | -3.50--0.06 |
| Humber, Coast and Vale | 2.13 | -0.26-4.52 | 1.93 | -0.46-4.33 | 2.06 | -0.31-4.43 |
| Kent and Medway | -1.72 | -4.17-0.73 | -1.55 | -3.98-0.89 | -1.80 | -4.24-0.64 |
| Lancashire and South Cumbria | 1.36 | -1.00-3.72 | 1.37 | -0.99-3.72 | 1.49 | -0.84-3.82 |
| North Central London | -4.70 | -7.48--1.91 | -4.57 | -7.36--1.78 | -4.48 | -7.27--1.68 |
| North East London | -1.92 | -4.74-0.90 | -1.32 | -4.14-1.51 | -1.04 | -3.84-1.76 |
| North West and South West London | -5.38 | -6.99--3.77 | -5.15 | -6.76--3.53 | -4.91 | -6.51--3.30 |
| Northern | 3.62 | 1.97-5.27 | 3.6 | 1.94-5.25 | 3.66 | 2.02-5.30 |
| Peninsula | -0.40 | -2.52-1.73 | -0.39 | -2.52-1.74 | -0.64 | -2.76-1.49 |
| Somerset, Wiltshire, Avon and Gloucestershire | 1.18 | -0.79-3.15 | 1.03 | -0.94-2.99 | 1.18 | -0.76-3.13 |
| South East London | -0.49 | -3.44-2.47 | -0.02 | -2.97-2.93 | 0.27 | -2.67-3.20 |
| South Yorkshire and Bassetlaw | 1.50 | -1.21-4.22 | 1.82 | -0.89-4.54 | 1.93 | -0.76-4.63 |
| Surrey and Sussex | -0.99 | -2.72-0.74 | -1.20 | -2.94-0.54 | -1.41 | -3.15-0.34 |
| Thames Valley | 0.81 | -2.00-3.63 | 0.45 | -2.37-3.27 | 0.30 | -2.48-3.09 |
| Wessex | 5.17 | 3.05-7.30 | 4.89 | 2.76-7.02 | 4.73 | 2.63-6.83 |
| West Midlands | -1.37 | -2.58--0.16 | -1.30 | -2.51--0.09 | -1.55 | -2.76--0.35 |
| West Yorkshire and Harrogate | 2.68 | 0.50-4.86 | 2.91 | 0.73-5.08 | 3.12 | 0.96-5.28 |
| Age 0-44 | -8.44 | -10.44--6.43 | -7.76 | -9.77--5.75 | -8.76 | -10.78--6.74 |
| Age 45-54 | -1.13 | -2.90-0.65 | -0.84 | -2.62-0.93 | -1.37 | -3.14-0.40 |
| Age 55-64 | 1.12 | 0.03-2.21 | 1.25 | 0.16-2.33 | 1.03 | -0.06-2.13 |
| Age 65-74 | 2.32 | 1.57-3.08 | 2.29 | 1.53-3.05 | 2.16 | 1.41-2.91 |
| Age 75-84 | 0.42 | -0.22-1.06 | 0.33 | -0.31-0.97 | 0.56 | -0.08-1.20 |
| Age 85+ | -3.62 | -4.44--2.80 | -3.67 | -4.50--2.84 | -3.46 | -4.30--2.61 |
| Female | -0.17 | -0.60-0.26 | 0.04 | -0.39-0.46 | -0.27 | -0.70-0.16 |
| Male | 0.18 | -0.27-0.63 | -0.04 | -0.49-0.42 | 0.29 | -0.17-0.74 |
| Quintile 1 (least deprived) | 0.91 | -0.02-1.83 | 0.74 | -0.20-1.67 | 0.42 | -0.51-1.35 |
| Quintile 2 | 0.59 | -0.27-1.46 | 0.49 | -0.38-1.35 | 0.28 | -0.57-1.14 |
| Quintile 3 | 0.29 | -0.58-1.17 | 0.32 | -0.56-1.20 | 0.33 | -0.55-1.20 |
| Quintile 4 | -0.44 | -1.31-0.43 | -0.31 | -1.18-0.56 | -0.20 | -1.06-0.67 |
| Quintile 5 (most deprived) | -1.38 | -2.24--0.52 | -1.26 | -2.15--0.37 | -0.86 | -1.74-0.03 |
| 2006 | -3.86 | -5.30--2.42 | -4.4 | -5.84--2.97 | ||
| 2007 | -3.64 | -5.11--2.16 | -4.17 | -5.64--2.69 | ||
| 2008 | -3.31 | -4.72--1.91 | -3.90 | -5.29--2.51 | ||
| 2009 | -2.84 | -4.21--1.47 | -3.07 | -4.44--1.69 | ||
| 2010 | -1.93 | -3.31--0.55 | -2.15 | -3.53--0.77 | ||
| 2011 | -1.98 | -3.35--0.61 | -2.12 | -3.49--0.75 | ||
| 2012 | 0.66 | -0.82-2.13 | 0.73 | -0.72-2.18 | ||
| 2013 | 1.07 | -0.37-2.51 | 1.13 | -0.30-2.56 | ||
| 2014 | 0.22 | -1.21-1.65 | 0.54 | -0.88-1.96 | ||
| 2015 | 2.52 | 1.07-3.97 | 3.00 | 1.57-4.44 | ||
| 2016 | 2.99 | 1.54-4.44 | 3.39 | 1.95-4.82 | ||
| 2017 | 6.04 | 4.52-7.55 | 6.42 | 4.92-7.93 | ||
| Adenomas and adenocarcinomas | 0.20 | 0.08-0.32 | 0.04 | -0.08-0.16 | ||
| Other morphology | -2.44 | -3.86--1.02 | -0.49 | -1.99-1.02 | ||
| eCCA | -0.38 | -1.29-0.53 | -0.52 | -1.45-0.40 | ||
| iCCA | 0.12 | -0.13-0.37 | 0.07 | -0.19-0.33 | ||
| Other subtype | -0.30 | -1.97-1.38 | 0.72 | -0.97-2.42 | ||
| Charlson 0 | 1.64 | 1.40-1.87 | 1.68 | 1.44-1.92 | ||
| Charlson 1 | -2.93 | -4.00--1.85 | -2.88 | -3.95--1.81 | ||
| Charlson 2 | -4.87 | -6.23--3.51 | -5.03 | -6.40--3.67 | ||
| Charlson 3 + | -7.60 | -8.78--6.42 | -7.98 | -9.19--6.77 | ||
| Liver disease -No | 0.32 | 0.22-0.43 | 0.34 | 0.23-0.44 | ||
| Liver disease -Yes | -4.28 | -5.65--2.90 | -4.44 | -5.86--3.02 | ||
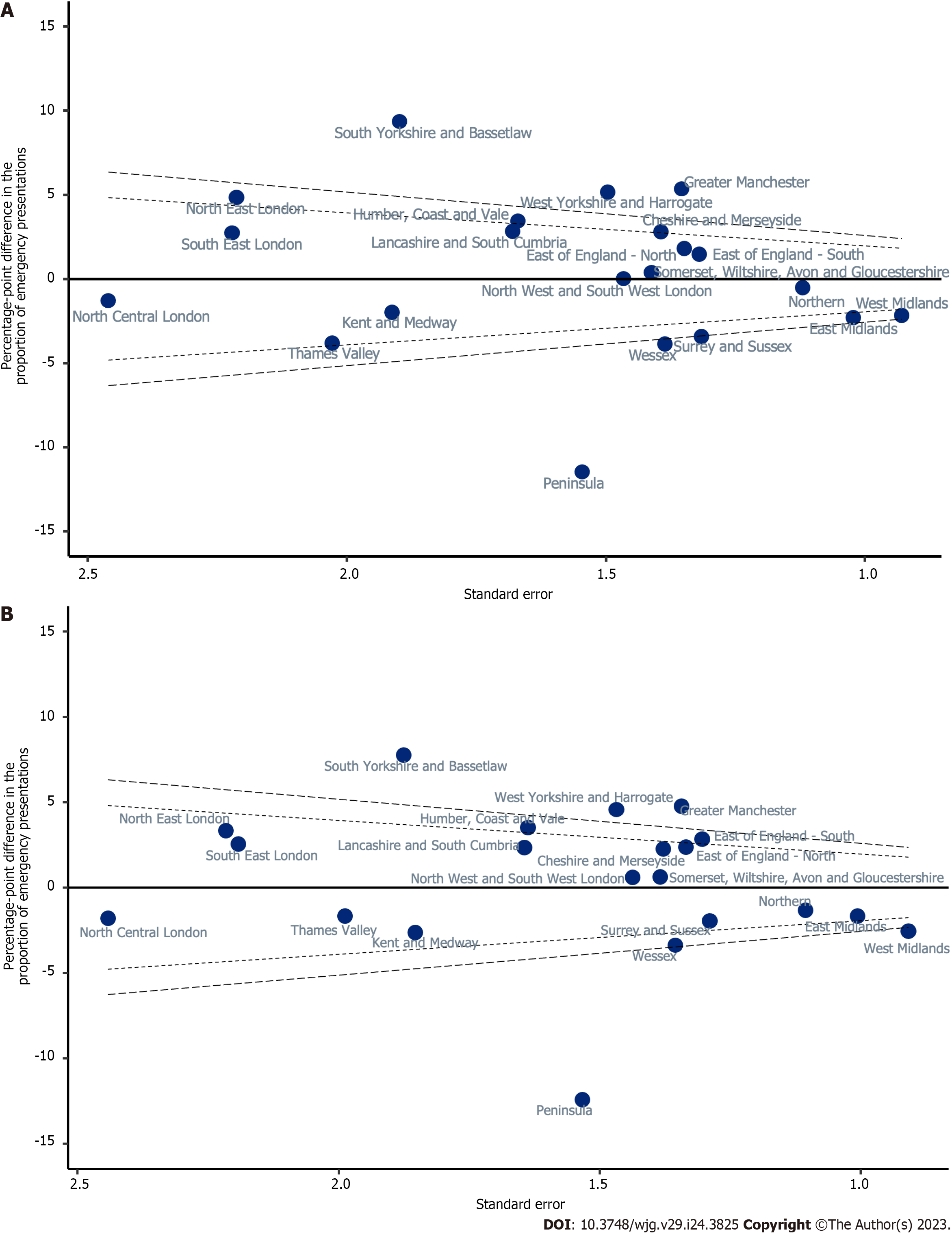
Variation in unadjusted proportions of patients diagnosed via EP was observed across the Cancer Alliances, ranging from 38.1% to 58.9% (Supplementary Table 1; P < 0.001, Figure 4). There was wider variation in the proportion diagnosed by EP across the nation than was observed for TWW referral route in Figure 4, with a larger number of Cancer Alliances showing statistically significant variation from the sample mean (Figure 6). In a linear probability model that included Cancer Alliance as the only independent variable, the proportion of patients diagnosed via EP was more than two SD below the sample mean for five Cancer Alliances. The proportion diagnosed via EP route was more than two SD higher than the sample mean for six Cancer Alliances (Table 4 and Figure 6A). In a model that additionally adjusted for demographics (Table 4; Model 2), four Cancer Alliances that varied significantly from the sample mean in unadjusted analyses were no longer outliers. However, one further Cancer Alliance became an outlier from the sample mean after this adjustment. Further adjustment for patient and tumour characteristics had no apparent effect on the variation observed relative to the demographic-adjusted model (Table 4; Model 3 and Figure 6B). Five Cancer Alliances had a proportion of EP that was more than two SD higher than the sample mean and three had a proportion of EP that was more than two SD lower.
| Model 1: Unadjusted | Model 2: Demographic-adjusted1 | Model 3: Adjusted2 | ||||
| Estimate | 95%CI | Estimate | 95%CI | Estimate | 95%CI | |
| Population average (intercept) | 49.57 | (48.94, 50.21) | 49.57 | (48.95, 50.20) | 49.57 | (48.95, 50.19) |
| Cheshire and Merseyside | 2.81 | (0.08, 5.54) | 2.39 | (-0.31, 5.10) | 2.26 | (-0.44, 4.96) |
| East Midlands | -2.30 | (-4.3, -0.29) | -1.76 | (-3.74, 0.22) | -1.69 | (-3.66, 0.29) |
| East of England -North | 1.81 | (-0.83, 4.46) | 1.79 | (-0.82, 4.40) | 2.35 | (-0.26, 4.97) |
| East of England -South | 1.46 | (-1.12, 4.05) | 2.55 | (0.00, 5.10) | 2.82 | (0.27, 5.38) |
| Greater Manchester | 5.36 | (2.70, 8.01) | 4.59 | (1.96, 7.22) | 4.75 | (2.12, 7.39) |
| Humber, Coast and Vale | 3.44 | (0.17, 6.72) | 3.57 | (0.37, 6.78) | 3.50 | (0.29, 6.71) |
| Kent and Medway | -1.98 | (-5.73, 1.77) | -2.78 | (-6.42, 0.85) | -2.66 | (-6.29, 0.97) |
| Lancashire and South Cumbria | 2.83 | (-0.46, 6.12) | 2.59 | (-0.63, 5.82) | 2.32 | (-0.90, 5.54) |
| North Central London | -1.29 | (-6.11, 3.53) | -1.97 | (-6.77, 2.83) | -1.82 | (-6.60, 2.97) |
| North East London | 4.85 | (0.51, 9.18) | 3.44 | (-0.89, 7.77) | 3.32 | (-1.02, 7.66) |
| North West and South West London | 0.02 | (-2.85, 2.90) | 0.47 | (-2.35, 3.28) | 0.58 | (-2.24, 3.40) |
| Northern | -0.53 | (-2.72, 1.67) | -1.26 | (-3.43, 0.91) | -1.34 | (-3.51, 0.82) |
| Peninsula | -11.47 | (-14.5, -8.44) | -12.11 | (-15.12, -9.09) | -12.44 | (-15.44, -9.44) |
| Somerset, Wiltshire, Avon and Gloucestershire | 0.39 | (-2.38, 3.15) | 1.32 | (-1.39, 4.03) | 0.61 | (-2.10, 3.32) |
| South East London | 2.74 | (-1.61, 7.10) | 2.78 | (-1.51, 7.07) | 2.53 | (-1.77, 6.82) |
| South Yorkshire and Bassetlaw | 9.36 | (5.64, 13.08) | 7.67 | (3.99, 11.34) | 7.74 | (4.06, 11.41) |
| Surrey and Sussex | -3.41 | (-5.99, -0.83) | -2.08 | (-4.61, 0.45) | -1.97 | (-4.49, 0.56) |
| Thames Valley | -3.81 | (-7.79, 0.17) | -1.23 | (-5.16, 2.70) | -1.67 | (-5.57, 2.22) |
| Wessex | -3.86 | (-6.58, -1.14) | -2.94 | (-5.61, -0.27) | -3.39 | (-6.04, -0.73) |
| West Midlands | -2.16 | (-3.98, -0.34) | -2.80 | (-4.58, -1.02) | -2.58 | (-4.36, -0.79) |
| West Yorkshire and Harrogate | 5.15 | (2.22, 8.09) | 4.33 | (1.45, 7.21) | 4.56 | (1.68, 7.43) |
| Age 0-44 | -5.72 | (-10.09, -1.35) | -6.46 | (-10.82, -2.10) | -5.66 | (-10.06, -1.27) |
| Age 45-54 | -8.39 | (-11.01, -5.76) | -8.83 | (-11.44, -6.23) | -8.16 | (-10.78, -5.53) |
| Age 55-64 | -10.85 | (-12.35, -9.35) | -10.98 | (-12.47, -9.49) | -10.46 | (-11.97, -8.95) |
| Age 65-74 | -6.82 | (-7.86, -5.77) | -6.61 | (-7.65, -5.57) | -6.32 | (-7.36, -5.28) |
| Age 75-84 | 3.66 | (2.74, 4.58) | 3.65 | (2.74, 4.56) | 3.35 | (2.44, 4.27) |
| Age 85+ | 14.83 | (13.56, 16.10) | 14.87 | (13.59, 16.14) | 14.28 | (12.98, 15.57) |
| Female | 1.91 | (1.29, 2.53) | 0.94 | (0.32, 1.55) | 1.11 | (0.49, 1.72) |
| Male | -2.02 | (-2.68, -1.37) | -0.99 | (-1.64, -0.34) | -1.17 | (-1.83, -0.52) |
| Quintile 1 (least deprived) | -4.93 | (-6.24, -3.62) | -4.91 | (-6.21, -3.60) | -4.61 | (-5.92, -3.31) |
| Quintile 2 | -2.69 | (-3.93, -1.46) | -2.65 | (-3.87, -1.43) | -2.50 | (-3.71, -1.28) |
| Quintile 3 | -0.66 | (-1.92, 0.60) | -0.44 | (-1.69, 0.80) | -0.41 | (-1.65, 0.83) |
| Quintile 4 | 3.11 | (1.84, 4.38) | 3.00 | (1.74, 4.25) | 2.86 | (1.61, 4.11) |
| Quintile 5 (most deprived) | 5.20 | (3.91, 6.49) | 5.02 | (3.70, 6.33) | 4.67 | (3.36, 5.99) |
| 2006 | 1.72 | (-0.66, 4.10) | 1.85 | (-0.49, 4.19) | ||
| 2007 | 0.49 | (-1.92, 2.91) | 1.40 | (-0.98, 3.79) | ||
| 2008 | 1.58 | (-0.68, 3.85) | 2.50 | (0.28, 4.71) | ||
| 2009 | 2.38 | (0.21, 4.56) | 2.47 | (0.33, 4.61) | ||
| 2010 | 0.79 | (-1.34, 2.91) | 0.64 | (-1.43, 2.71) | ||
| 2011 | -0.26 | (-2.37, 1.85) | -0.05 | (-2.13, 2.02) | ||
| 2012 | -1.10 | (-3.20, 1.00) | -1.38 | (-3.42, 0.66) | ||
| 2013 | 1.28 | (-0.76, 3.32) | 1.24 | (-0.74, 3.22) | ||
| 2014 | 1.01 | (-1.05, 3.07) | 0.32 | (-1.70, 2.33) | ||
| 2015 | -0.81 | (-2.79, 1.17) | -1.37 | (-3.31, 0.57) | ||
| 2016 | -1.83 | (-3.79, 0.13) | -2.22 | (-4.15, -0.30) | ||
| 2017 | -3.58 | (-5.50, -1.66) | -3.27 | (-5.16, -1.38) | ||
| Adenomas and adenocarcinomas | -0.56 | (-0.74, -0.37) | -0.38 | (-0.58, -0.19) | ||
| Other morphology | 6.73 | (4.52, 8.93) | 4.64 | (2.31, 6.97) | ||
| eCCA | -3.41 | (-4.74, -2.08) | -2.84 | (-4.16, -1.53) | ||
| iCCA | 0.80 | (0.43, 1.17) | 0.81 | (0.44, 1.18) | ||
| Other subtype | 0.56 | (-1.89, 3.01) | -1.26 | (-3.74, 1.22) | ||
| Charlson 0 | -2.09 | (-2.46, -1.71) | -1.28 | (-1.66, -0.90) | ||
| Charlson 1 | 3.97 | (2.27, 5.67) | 1.95 | (0.29, 3.62) | ||
| Charlson 2 | 5.21 | (2.88, 7.55) | 2.74 | (0.45, 5.02) | ||
| Charlson 3 + | 10.24 | (7.92, 12.56) | 7.63 | (5.31, 9.95) | ||
| Liver disease -No | 0.07 | (-0.11, 0.24) | -0.19 | (-0.36, -0.01) | ||
| Liver disease -Yes | -0.86 | (-3.18, 1.45) | 2.45 | (0.10, 4.70) | ||
There was no strong correlation (P = 0.2) between the proportion of people diagnosed via TWW referral and the proportion of EP across the Cancer Alliances; only one of three Cancer Alliances with a higher than average proportion of TWW referral routes had a lower than average proportion of EP. Two of six Cancer Alliances with a lower than average proportion of TWW referral routes had a higher than average proportion of EP.
A summary of all univariable associations between the route to diagnosis and patient and tumour characteristics is provided in Supplementary Table 1, with estimated proportions diagnosed via TWW referrals and EP in Tables 3 and 4. The lowest proportion diagnosed by TWW referral was observed in the 0-44 years age group [5.3%; unadjusted percentage point difference from average (pp) = -8.44 (95%CI: -10.44 to -6.43)], but the relationship with age was an inverted u-shape, with those aged over 84 years also having a lower proportion of TWW referral routes than average [unadjusted pp = -3.62 (95%CI: -4.44 to -2.80)]. Age remained associated with the proportion diagnosed via TWW referral in a model that included all other cofactors of interest (Model 3; Table 3). Likewise, the presence of comorbidity on the CCI and the presence of underlying liver disease were each independently associated with a lower proportion of people being diagnosed via a TWW referral (Model 3; Table 3).
A greater-than-average proportion of EP diagnosis routes was observed for females, those aged over 84 years, those who were more deprived, those with more comorbidities, intrahepatic tumour types and non-adenocarcinoma morphology (Supplementary Table 1; all P < 0.001). These associations remained present in an analysis that adjusted for all other factors of interest (Table 4; Model 3). Age above 84 [pp = 14.28 (95%CI: 12.98 to 15.57)] years and a CCI of 3 or more [pp = -7.98 (95%CI: -9.19 to -6.77)] showed the largest effect on the proportion of EP. Underlying liver disease was only associated with a higher proportion of EP after adjustment for other patients and tumour characteristics (Table 4).
A subgroup of 8872 people were diagnosed with CCA between 2014 and 2017, of whom 4832 (54.5%) had a known stage at diagnosis and were included in the sensitivity analysis. The proportion of people staged varied by Cancer Alliance, from 36.7% to 74.7% (P < 0.001). Those with unknown stage at diagnosis were more likely to be older, diagnosed in an earlier year of the study period, have a high comorbidity score and an ‘other’ tumour subtype or morphology (all P < 0.001). For both diagnosis routes, fewer Cancer Alliances showed significant variation from the sample mean than in the main results following adjustment to all a priori confounders (Tables 3 and 4; Model 3). Upon further adjustment for stage at diagnosis, the variation observed across the Cancer Alliances was not attenuated (results not shown).
This study examined changes to routes to diagnosis for CCA across all of England’s 21 regional Cancer Alliances over a 12-year period. We found significant variation in the proportion of patients diagnosed by EP and via TWW referral across regions. Exploration of factors contributing to regional differences revealed associations between diagnosis route and gender, the burden of comorbidity, deprivation, age at diagnosis and CCA sub-type. It is worth noting that although CCA is often deemed a cancer of the elderly, 22% of cases during the period under study were under 65 years old. The majority of CCA cases were recorded as intrahepatic, but due to the lack of historic codes specifically for peri-hilar CCA, there remains some controversy regarding the true proportional breakdown with nationally recorded numbers of the three anatomical variants of CCA[1,2]. The most common route to diagnosis was EP, in keeping with the typically late presentation of patients with CCA due to the lack of obvious early symptoms, a targetable screening population and accurate diagnostic screening tests.
The significant variation by Cancer Alliance in the proportions diagnosed via TWW referral and EP was found after controlling for a range of patient and tumour characteristics. This indicates there are marked differences in the routes that lead individuals to a diagnosis of CCA between Cancer Alliances in England, beyond what would be expected due to chance. The regional variation we found does not appear to be explained by case-mix, in so far as we could adjust for it. Although this variation is unlikely to be random, residual confounding could remain. For example, it was not possible to account for symptoms present prior to diagnosis or health seeking behaviour which might determine how and where people will present for care. Regions found to have higher than average EP or lower than average TWW may want to investigate why. It is possible that this reflects genuine differences in established diagnostic pathways from primary care to secondary care across regions, or different levels of expertise and knowledge of biliary tract cancers in primary care. There was more geographic variation in the proportion of EP routes for extrahepatic than for intrahepatic tumours. Possible reasons for why there might be more variation in diagnostic pathways could relate to symptom profile or other factors. It was not always the case that Cancer Alliances with significantly higher proportion of TWW have a lower proportion of EP. This suggests that for some areas ‘additional’ TWWs above average are in people who would otherwise have been diagnosed via other routes as well as via EP. Increases over time in the proportion of TWW were also not matched by decreases in EP alone, but also by decreases in the proportion diagnosed via ‘other’ routes. A lack of direct relationship between TWW and EP may suggest that a significant proportion of people who present as an emergency either would not meet the criteria for a TWW referral or do not interact with their GP at all prior to their diagnosis, limiting the potential for this diagnostic pathway to prevent EPs. The overall proportion of diagnoses via the TWW pathway did double from 9.8% to 19.8% during the study period, but this still remains low. Whilst consistent and high-quality implementation of the TWW pathway across all regions is vital to avoid disparities in care, there may also be a need to look to other techniques, particularly at primary care level, in order to further improve the early diagnosis rate specific for CCA in the UK. For example, there may be benefits for screening in patients known to be at high risk of CCA, such as patients with primary sclerosing cholangitis[19]; and the development and validation of novel diagnostic biomarkers remains an active and important area of CCA research[20].
As deprivation increased, so did the proportion diagnosed by EP, corresponding with a reduction in diagnoses by TWW referral. The association between deprivation and primary care services is complex and multi-factorial, relating both to levels of engagement with healthcare services and the availability and quality of primary care services in deprived areas[21-24]. CCA cases were also more likely to present via EP if they were female and the reasons for this are difficult to ascertain. That being female, deprived or elderly was associated with a higher risk of EP requires further exploration to ensure groups are not disadvantaged due to their inherent characteristics, which may not necessarily be specific to CCA.
As age at diagnosis increased, so did the proportion diagnosed by EP. Patients who are older may be more likely to excuse vague or non-specific symptoms of CCA as being down to ageing and be less likely to seek healthcare. Another possibility is that they may be less able to seek it. Similarly, CCA patients were less likely to present via the TWW pathway if they were over 84 years old, and had co-morbidities and/or underlying liver disease.
CCA cases were more likely to present via EP if the CCA was intrahepatic. This may be explained by the fact that iCCA more often presents with non-specific symptoms than eCCA, which can cause obstructive jaundice[1]. People with more specific symptoms may be more likely to seek healthcare sooner, potentially resulting in fewer diagnosed via EP.
Although a more limited cohort with staging data available was analysed (diagnosed 2014-2017), adjusting for stage at diagnosis did not explain geographic variation observed after controlling for other patient and tumour characteristics. The 2014-2017 cohort with complete staging data was shown to differ from the full 2006-2017 cohort, with a lower proportion diagnosed by EP, and a higher proportion diagnosed by TWW referral, which could suggest slightly better-managed diagnostic pathways in patients with complete staging data.
It is important to note that a large proportion of stage (72.1%) and performance status (89.4%) data is missing for CCA patients in the NCRD from 2006-2017, which is a limitation of this study. It is unknown if the stage of diagnosis is not known, not recorded, or missing only from the data feeds and submissions used to form the NCRD. Performance status was added to the Cancer Outcomes and Services data collection in 2013, so is only available for patients diagnosed after this. Missingness of performance status remains high (77.5%) even when restricting the cohort to 2013-2017. The completeness of CCA staging information was also variable across Cancer Alliances. Though stage completeness improved in more recent years (from 44% in 2014 to 64% in 2017), these are higher proportions of missing data compared to other tumour types. For example, 89% of colon cancers diagnosed in 2017 were staged, as were 81% of pancreas cancers[25]. Where stage at diagnosis was known, a high proportion were at an advanced disease stage. This reveals a need to publicise the value of this data and encourage the completion of these fields across all providers within Cancer Alliances, so future CCA research can monitor and adjust for these key prognostic factors for treatment options and patient outcomes.
Strengths of the study include that this was a population level analysis, and to our knowledge is the first study analysing routes to diagnosis for CCA across geographic regions and factors associated with this.
Our study does have some inherent limitations. Some of the observed geographic differences may be attributable to residual confounding that cannot be fully accounted for in the current analysis, rather than disparities in clinical practice. For example, it is not known what symptoms patients experienced leading up to their diagnosis of cancer. There is likely a significant role of patient health-seeking behaviour in these outcomes of interest particularly. It is not known from the data available whether individuals presented to their GP to seek advice prior to their diagnosis.
The study only included CCA patients diagnosed in England and therefore, without further research, it is unknown if our findings can be applied to other countries.
Regarding the identification of underlying liver disease, HES APC episodes from 5 years before to 1 year after diagnosis were searched for relevant diagnostic codes. The patient must therefore have had an inpatient episode where their underlying liver disease was documented. Patients may only spend time in the hospital towards the end of their disease and could be treated in outpatient or primary care prior to that. Therefore, the study is likely to have identified more serious cases of underlying liver disease only and may underestimate the burden by missing diagnoses or treatment exclusively documented in outpatient or primary care settings.
In conclusion, it would be preferable to reduce the amount of CCA presentations diagnosed by emergency and promote better managed diagnosis pathways earlier in the disease to improve outcomes. Whilst it is possible that the geographic variation observed may be explained by factors that we are unable to account for in the present analysis, these findings provide a signal that further research is necessary to identify and address the complex and multifactorial nature of the observed geographic differences, including potential differences in clinical practice, healthcare access, and socioeconomic factors across different regions in England. It is possible that variation in routes to the diagnosis of CCA by patient demographic factors could reflect genuine differences in practice and recognition of relevant cancer symptoms in a primary care setting, and that certain groups are disadvantaged when it comes to CCA diagnosis. Where variation exists, there may be opportunities for knowledge sharing of good practice to improve diagnostic pathways, such as education or awareness campaigns to increase public and clinician understanding of the common symptoms of CCA and increase diagnoses made by TWW urgent GP referrals. However, this may only improve things so far in a disease that often has no or non-specific symptoms, in which case there may also be a role for screening strategies and non-specific symptom diagnosis clinics.
Cholangiocarcinoma (CCA), a cancer with rising incidence and poor survival, is often diagnosed late. Treatment options are limited for late stage patients. The only curative treatment for CCA is surgery, which is not feasible in patients with advanced disease, and response to systemic therapies at later stage is poor.
Late stage diagnosis presents a large barrier to improving outcomes for patients with CCA. Being diagnosed via an emergency presentation (EP) route is associated with later stage diagnoses, whereas earlier diagnoses may be made via the Two Week Wait (TWW) referral route through primary care clinicians. Geographic variation in the proportion of patients diagnosed via these routes is important to understand and explore, as some regions may be diagnosing patients via better managed and less urgent routes at an earlier stage. Reasoning and learning behind such variation could be shared to improve CCA patients’ prognoses.
We aimed to investigate routes to diagnosis of CCA over time, regional variation across England and influencing factors to understand if TWW referrals and EP routes to diagnosis differ across regions in England as we hypothesised.
We conducted a retrospective cohort study including patients diagnosed with CCA from 2006-2017 in England. We linked electronic patient records to define routes to diagnosis and patient and tumour characteristics. We used linear probability models to investigate geographic variation in the proportions of patients diagnosed via TWW referral or EP across regions in England, adjusting for potential confounders.
Almost half of CCA patients in England from 2006-2017 were diagnosed via EP (49.6%), with just 13.8% diagnosed via TWW referrals. The proportion diagnosed via TWW referral doubled between 2006-2017 rising from 9.9% to 19.8%. Statistically significant variation in both the proportions diagnosed via a TWW referral and EP route was found across regions in England. Age, presence of comorbidities and underlying liver disease were independently associated with lower proportions of patients diagnosed via TWW referral and a higher proportion diagnosed via EP.
We found significant geographic and socio-demographic variation in routes to diagnosis of CCA in England.
There may be opportunities for knowledge sharing of good practice to improve diagnostic pathways where variation exists, such as education or awareness campaigns to increase public and clinician understanding of the common symptoms of CCA and increase diagnoses made by TWW urgent GP referrals. Further research is also warranted to identify and address the complex and multifactorial nature of the observed geographic differences, alongside exploring screening strategies and non-specific symptom diagnosis clinics as CCA often has no or non-specific symptoms.
This work uses data that has been provided by patients and collected by the NHS as part of their care and support. The data are collated, maintained and quality assured by the National Disease Registration Service, which is part of NHS England. Mireille B Toledano and Shahid Khan are additionally grateful for infrastructure support from the United Kingdom National Institutes for Health Research (NIHR) Biomedical Research Centre at Imperial College London. Mireille B Toledano’s Chair is supported in part by a donation from Marit Mohn to Imperial College London to support Population Child Health through the Mohn Centre for Children’s Health and Wellbeing.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee H, South Korea; Yildiz K, Turkey S-Editor: Li L L-Editor: A P-Editor: Zhang XD
| 1. | Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Rizvi S, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1555] [Cited by in RCA: 1539] [Article Influence: 307.8] [Reference Citation Analysis (0)] |
| 2. | Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, Teh BT, Wongkham S, Gores GJ. Cholangiocarcinoma. Nat Rev Dis Primers. 2021;7:65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 463] [Article Influence: 115.8] [Reference Citation Analysis (0)] |
| 3. | Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 359] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 4. | Bertuccio P, Bosetti C, Levi F, Decarli A, Negri E, La Vecchia C. A comparison of trends in mortality from primary liver cancer and intrahepatic cholangiocarcinoma in Europe. Ann Oncol. 2013;24:1667-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 5. | Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, La Vecchia C, Negri E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 422] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 6. | West J, Wood H, Logan RF, Quinn M, Aithal GP. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001. Br J Cancer. 2006;94:1751-1758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 186] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | McPhail S, Elliss-Brookes L, Shelton J, Ives A, Greenslade M, Vernon S, Morris EJ, Richards M. Emergency presentation of cancer and short-term mortality. Br J Cancer. 2013;109:2027-2034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 167] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 8. | Redaniel MT, Ridd M, Martin RM, Coxon F, Jeffreys M, Wade J. Rapid diagnostic pathways for suspected colorectal cancer: views of primary and secondary care clinicians on challenges and their potential solutions. BMJ Open. 2015;5:e008577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Dolly SO, Jones G, Allchorne P, Wheeler D, Ali S, Mukadam Y, Zheng S, Rahman L, Sindhar J, Moss CL, Harari D, Van Hemelrijck M, Cunliffe A, De Michele LV. The effectiveness of the Guy's Rapid Diagnostic Clinic (RDC) in detecting cancer and serious conditions in vague symptom patients. Br J Cancer. 2021;124:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | National Cancer Registration and Analysis Service. Routes to Diagnosis, 2018. [cited 13 May 2023]. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/routes-to-diagnosis/2018. |
| 11. | Henson KE, Elliss-Brookes L, Coupland VH, Payne E, Vernon S, Rous B, Rashbass J. Data Resource Profile: National Cancer Registration Dataset in England. Int J Epidemiol. 2020;49:16-16h. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 12. | Elliss-Brookes L, McPhail S, Ives A, Greenslade M, Shelton J, Hiom S, Richards M. Routes to diagnosis for cancer - determining the patient journey using multiple routine data sets. Br J Cancer. 2012;107:1220-1226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 386] [Cited by in RCA: 412] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 13. | National Cancer Registration and Analysis Service. National Cancer Registration and Analysis Service's Cancer Analysis System SOP#1 Counting cancer cases. [cited 13 May 2023]. Available from: https://www.cancerdata.nhs.uk/getdataout/GDO_0024/CAS_SOP_Counting_cancer_cases.pdf. |
| 14. | NHS England. Cancer Alliances - improving care locally. [cited 13 May 2023]. Available from: https://www.england.nhs.uk/cancer/cancer-alliances-improving-care-locally/. |
| 15. | Smith T, Noble M, Noble S, Wright G, McLennan D, Plunkett E. The English Indices of Deprivation 2015, Technical report, in Department for Communities and Local Government, 2015. [cited 13 May 2023]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/464485/English_Indices_of_Deprivation_2015_-_Technical-Report.pdf. |
| 16. | Crooks CJ, West J, Card TR. A comparison of the recording of comorbidity in primary and secondary care by using the Charlson Index to predict short-term and long-term survival in a routine linked data cohort. BMJ Open. 2015;5:e007974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Driver RJ, Balachandrakumar V, Burton A, Shearer J, Downing A, Cross T, Morris E, Rowe IA. Validation of an algorithm using inpatient electronic health records to determine the presence and severity of cirrhosis in patients with hepatocellular carcinoma in England: an observational study. BMJ Open. 2019;9:e028571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Te Grotenhuis M, Pelzer B, Eisinga R, Nieuwenhuis R, Schmidt-Catran A, Konig R. When size matters: advantages of weighted effect coding in observational studies. Int J Public Health. 2017;62:163-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Chascsa DM, Lindor KD. Cancer risk, screening and surveillance in primary sclerosing cholangitis. Minerva Gastroenterol Dietol. 2019;65:214-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Neal RD, Johnson P, Clarke CA, Hamilton SA, Zhang N, Kumar H, Swanton C, Sasieni P. Cell-Free DNA-Based Multi-Cancer Early Detection Test in an Asymptomatic Screening Population (NHS-Galleri): Design of a Pragmatic, Prospective Randomised Controlled Trial. Cancers (Basel). 14(19):4818.. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 104] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 21. | Maheswaran R, Pearson T, Jordan H, Black D. Socioeconomic deprivation, travel distance, location of service, and uptake of breast cancer screening in North Derbyshire, UK. J Epidemiol Community Health. 2006;60:208-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Parsons J, Bryce C, Atherton H. Which patients miss appointments with general practice and the reasons why: a systematic review. Br J Gen Pract. 2021;71:e406-e412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 23. | NHS Digital. A&E attendances twice as high for people in the most deprived areas as in the least deprived. [cited 13 May 2023]. Available from: https://digital.nhs.uk/news/2019/ae-attendances-twice-as-high-for-people-in-the-most-deprived-areas-as-in-the-least-deprived?msclkid=5453cc7ecfb611eca5d3a808deeb1be8. |
| 24. | The Health Foundation. Level or not? Comparing general practice in areas of high and low socioeconomic deprivation in England, 2021. [cited 13 May 2023]. Available from: https://reader.health.org.uk/Level-or-not. |
| 25. | National Disease Registration Service. Staging Data in England, 2020. [cited 13 May 2023]. Available from: https://www.cancerdata.nhs.uk/stage_at_diagnosis. |