Published online Jun 7, 2023. doi: 10.3748/wjg.v29.i21.3222
Peer-review started: December 28, 2022
First decision: January 10, 2023
Revised: January 23, 2023
Accepted: May 8, 2023
Article in press: May 8, 2023
Published online: June 7, 2023
Processing time: 155 Days and 3.1 Hours
Crohn’s disease (CD) is an inflammatory bowel disease characterized by immune-mediated flares affecting any region of the intestine alternating with remission periods. In CD, the ileum is frequently affected and about one third of patients presents with a pure ileal type. Moreover, the ileal type of CD presents epidemiological specificities like a younger age at onset and often a strong link with smoking and genetic susceptibility genes. Most of these genes are associated with Paneth cell dysfunction, a cell type found in the intestinal crypts of the ileum. Besides, a Western-type diet is associated in epidemiological studies with CD onset and increasing evidence shows that diet can modulate the composition of bile acids and gut microbiota, which in turn modulates the susceptibility of the ileum to inflammation. Thus, the interplay between environmental factors and the histological and anatomical features of the ileum is thought to explain the specific transcriptome profile observed in CD ileitis. Indeed, both immune response and cellular healing processes harbour differences between ileal and non-ileal CD. Taken together, these findings advocate for a dedicated therapeutic approach to managing ileal CD. Currently, interventional pharmacological studies have failed to clearly demonstrate distinct response profiles according to disease site. However, the high rate of stricturing disease in ileal CD requires the identification of new therapeutic targets to significantly change the natural history of this debilitating disease.
Core Tip: The ileum is most frequently affected by Crohn’s disease (CD). Ileal CD differs from other CD types in its epidemiology and natural history. Anatomical and histological features of the ileum provide the keys to understanding this distinct phenotype. Moreover, we discuss herein the crosstalk that occurs in the ileum between an individual and her/his environment and the clinical significance.
- Citation: Richard N, Savoye G, Leboutte M, Amamou A, Ghosh S, Marion-Letellier R. Crohn’s disease: Why the ileum? World J Gastroenterol 2023; 29(21): 3222-3240
- URL: https://www.wjgnet.com/1007-9327/full/v29/i21/3222.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i21.3222
Crohn’s disease (CD) is an inflammatory bowel disease (IBD) characterized by repetitive inflammatory flares, and often chronicity. Unlike ulcerative colitis (UC), the other main subtype of IBD, CD can affect any part of the digestive tract. The site of the disease is a critical biological aspect of CD whereas inflammatory, stricturing or penetrating behaviour is thought to be a reflection of disease progression[1].
Data on the epidemiology of IBD are provided by population-based studies showing an increasing incidence and prevalence of IBD in the West over the last 50 years[2,3]. In a systematic review pooling all epidemiological studies on IBD worldwide since 1990, the global prevalence of IBD is higher in Western countries (322 per 100000 in Germany) than in newly industrialized countries[4]. In newly industrialized countries like Asia and the Middle East, epidemiological studies report a rising incidence of CD[4,5]. The Montreal classification distinguishes CD involving the ileum, the colon and both the colon and the ileum[6]. About one third of patients with CD presents a disease involvement limited to the ileum and this proportion does not vary between ‘Western’ and newly industrialized countries[3,5,7]. Once the diagnosis of ileal CD is made, less than one fifth of patients will present colonic lesions over time[3]. In addition, ileal CD occurs in younger patients than colonic CD[1]. These epidemiological observations have led some experts to plead for personalized approaches to therapy based on the disease site.
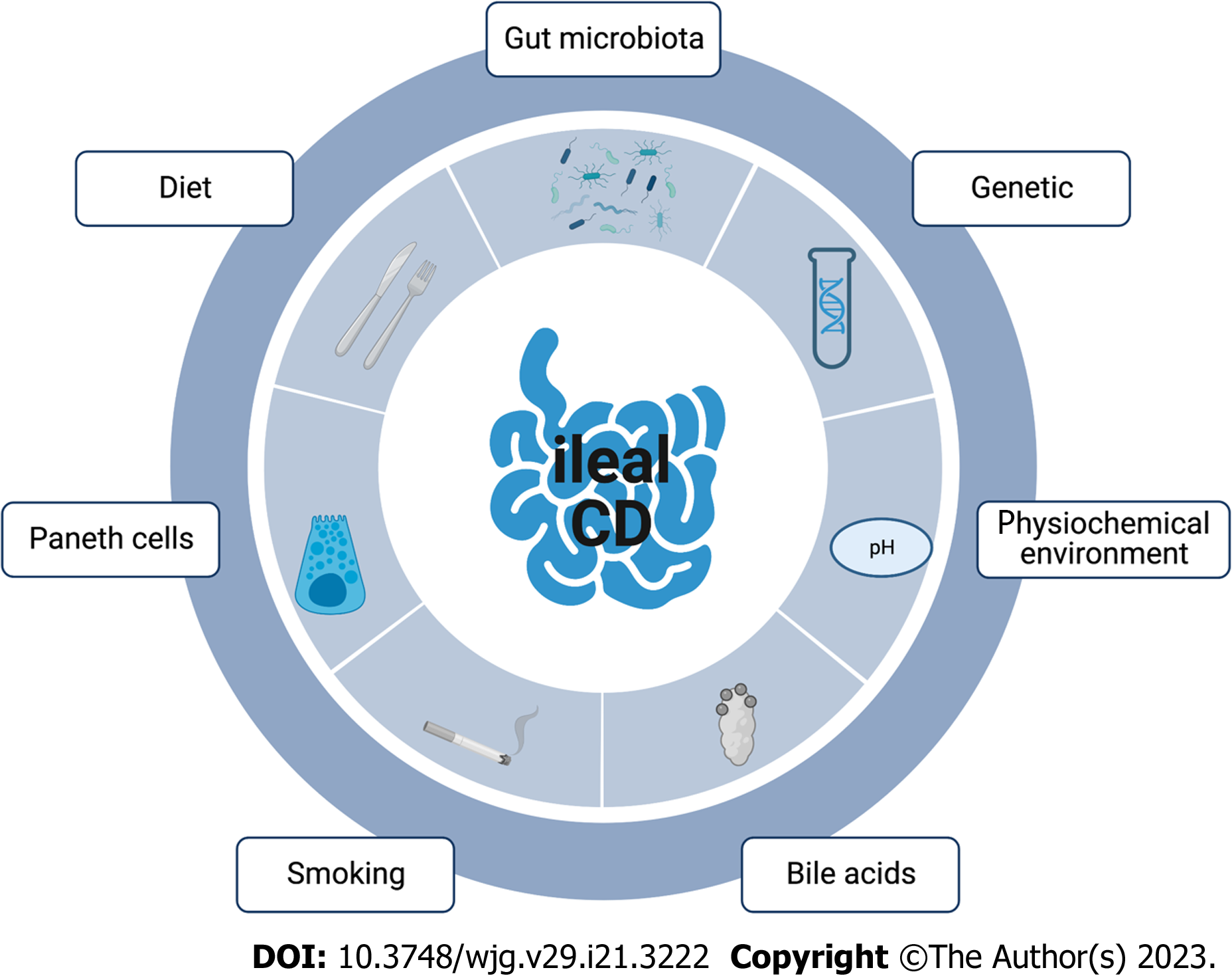
Even though CD pathogenesis remains elusive, current consensus considers CD a result of genetic, immunological and environmental factors[2]. Of relevance, the ileum is the site for the crosstalk of these multiple etiological factors in CD. In this review, we will depict the different physiopathological aspects of ileal CD and their clinical impact (Figure 1).
Genetic factors are involved in IBD physiopathology and genome-wide association studies have linked several genes with the site of the disease. In a large epidemiological study performed in more than 34000 patients with IBD across Europe, North America and Australia, susceptibility genes were determinants of the site of the disease whereas inherited genes showed a loose link with the inflammatory, penetrating or stricturing behaviour of the disease[1]. The genetic variants presented herein sum up the current state-of-the-art but new techniques such as genomic DNA are likely to provide new insights in the next few years.
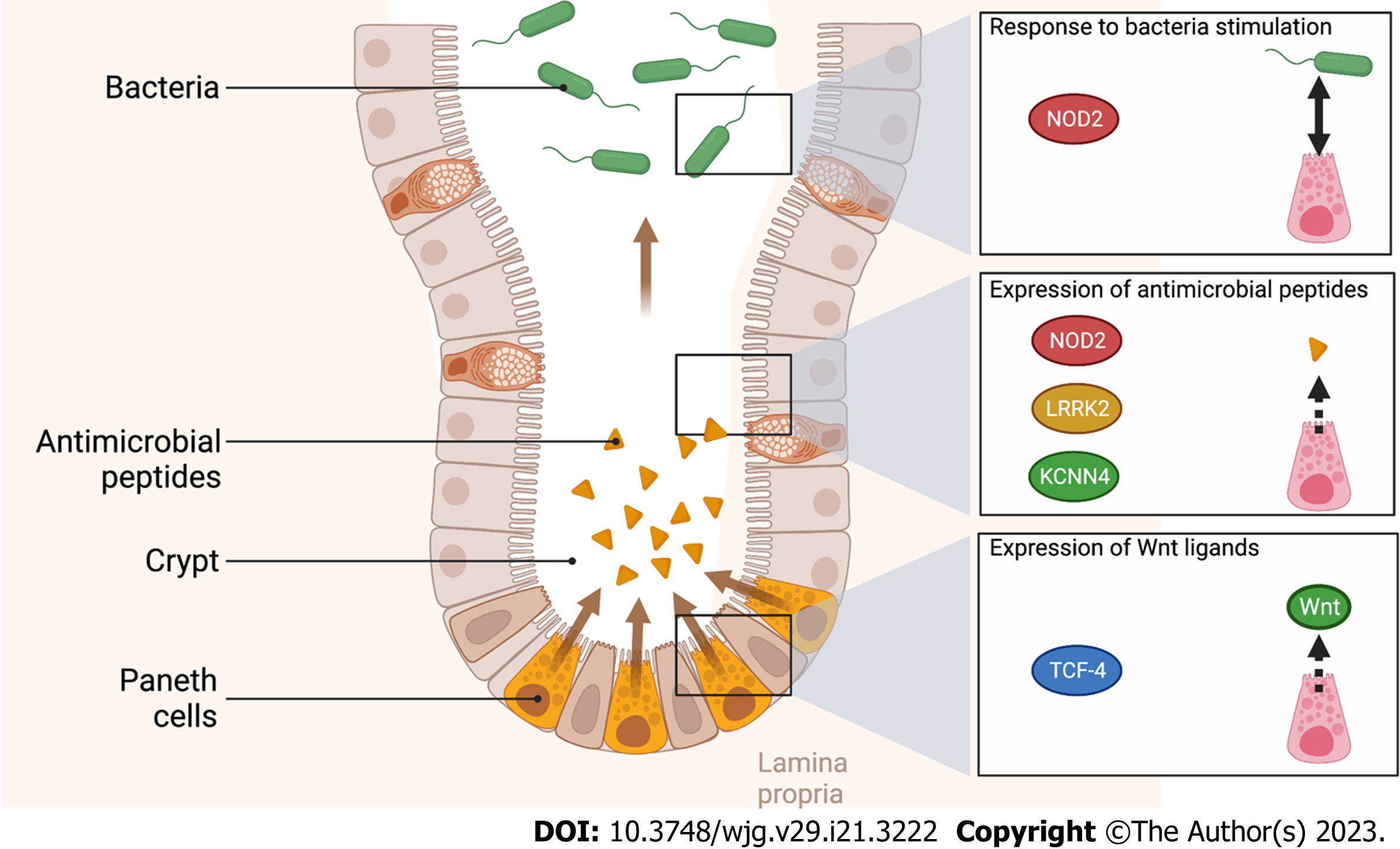
NOD2 is a sensor of the innate immune system, able to detect bacterial fragments, specifically muramyl dipeptide[8]. During in vitro differentiation of intestinal epithelial cells into Paneth cells, NOD2 signalling can modulate the expression of enteric antimicrobial peptides[9]. Even though intracellular pattern recognition receptor gene NOD2 is widely associated with CD risk[2], mutations in the NOD2 gene are strongly associated with ileal CD and are correlated with a younger age at diagnosis[1,10,11]. The specific association between NOD2 mutations and ileal CD is partially explained by its distribution along the gastrointestinal tract. Histologically, NOD2 is overexpressed in ileal crypts compared to colonic crypts[12].
Like NOD2, the LRRK2 gene is expressed in Paneth cells. LRRK2 gene is implicated in vesicular trafficking, cytoskeleton homeostasis and consequently in inflammation and immune response[13]. More specifically, LRRK2 is overexpressed in Paneth cells and its deficiency causes deprivation of lysozyme in Paneth cells[14]. In a case-control study, mutations of LRRK2 were associated with ileitis and an early onset of CD[15].
The expression of other genes involved in immune response is likewise implicated in ileal CD. The major histocompatibility complex is involved in the presentation of antigen in a large variety of cell types including T-cells. In a recent meta-analysis, several mutations of these genes were associated with CD especially in the Korean population but also in the European population[16]. Of note, single nucleotide polymorphisms of these genes were more common in patients with ileal CD compared to patients with colonic CD[1].
Among susceptibility genes identified in CD, the allele ATG16L1T300A was associated with impaired autophagy[17,18]. The expression of ATG16L1 was decreased in CD patients[19]. In 9-10-month-old mice, the specific deletion of Atg16L1 allele in intestinal epithelium cells (IEC) led to a transmural CD-like ileitis associated with endoplasmic reticulum stress[20]. Interestingly, NOD2 mutations are also associated with autophagy defects.
In IBD patients, the reduction of the Wnt-signalling pathway transcription factor Tcf-4 was associated with a predisposition for ileal CD[21,22]. A reduced expression of Tcf-4 resulted in a reduced expression of Paneth cell defensins[21]. In a murine Tcf-4 knockout model, a decreased expression of alpha-defensin levels and a reduced capacity of bacterial killing were observed[21].
Finally, in the array of genes implicated in the immune response mediated by Paneth cells, the conductance calcium-activated potassium channel protein (KCNN4) is part of the potassium pump in the human intestine[23]. The blocking of this calcium-activated potassium channel protein reduced mouse Paneth cell secretion in response to bacterial stimulation[24]. In human, mutation of the KCNN4 gene was associated with ileal CD in the Australian and New Zealand population[25].
In the susceptibility genes mentioned above, most genes are associated with Paneth cell dysfunction as illustrated in Figure 2. We will detail below the potential involvement of Paneth cells in ileal CD.
Beyond genetic factors, the histological and anatomical features of the ileum itself may partially explain the propensity of this specific part of the gastrointestinal tract to be affected by CD.
The ileum presents a unique chemical microenvironment. The intraluminal pH in the ileum is 7.4, the highest of the human digestive tract as a result of small bowel mucosal bicarbonate secretion[26]. Comparatively, the intraluminal pH in the caecum is lower, 6.5, due to the bacterial production of short fatty acids by colonic bacteria[26,27]. The functional characteristics of the digestive tract microbiota are modulated by pH level. In the environment of the ileum (pH = 7.4), short chain fatty acids increase the growth and the motility of pathobionts whereas, in the colonic environment (pH = 6.5), short chain fatty acids downregulate the virulence of gene expression of these strains[28].
From a clinical point of view, most of histological features encountered in ileal CD were also found in other diseases as backwash ileitis in UC for instance[29]. Although epithelioid granuloma is considered as the histological hallmark for the diagnosis of ileal CD, it is not a mandatory prerequisite[30]. In about one quarter of patients with ileal CD, pyloric gland metaplasia resulting from the expression of mucin genes normally specific to the stomach (MUC5AC and MUC6) were noted[30,31]. Although numerous other histological features are described in ileal CD, focal crypt irregularities are considered by expert consensus as one of the most reliable signs of CD[30].
From a biological point of view, the most noteworthy change is found in Peyer’s patches. Peyer’s patches are ileal immune structures characterized by a B-cell germinal centre surrounded by a T-cell interfollicular region. These mucosal-associated lymphoid tissues can act as “gateways” of the intestine. The epithelium and the underlying lymphoid follicle differ from the surrounding villus epithelium of the ileum. Indeed, the function of this follicle-associated epithelium consists in sampling and transporting luminal antigens through M cells and dendritic cells to CD4+ cells[32]. Early histological changes in Peyer’s patches were reported in ileal CD such as an increase in mast cells or erosive epithelial lesions[33,34]. Further, the increased number of glial cells in the Peyer’s patches of patients with ileal CD resulted in an enhanced intestinal permeability[34]. Together these phenomena may explain the increased vulnerability of the ileal mucosa to bacterial invasion in CD patients[35].
As mentioned in the previous section, the histological changes observed in ileal CD include glial cells and the enteric nervous system (ENS). In patients with ileal CD, both the submucous and the myenteric plexus present an overall increase in the number of neuronal cell bodies, enteroglia and interstitial cells of Cajal associated with an upregulation of apoptosis in enteric neurons and enteric glial cells[36,37]. To that extent, although functional evidence is lacking in the literature to fully support this hypothesis, the increased transit time observed in CD patients could be seen as a consequence of ultrastructural injury to interstitial cells of Cajal in the myenteric plexus[38,39].
Beyond the role of the ENS in intestinal mobility, the density of enteric glial cells conveys a higher risk of ileal CD recurrence after surgery. Thus, after ileocolonic resection for CD, inflammation in or around nerve bundles or enteric ganglia was reported in several clinical studies as a risk factor for CD recurrence[40-43]. In the uninflamed section from ileocolonic samples, the number of S100-positive enteric glial cells was enhanced in patients with relapsing disease unlike vasoactive intestinal polypeptide or substance P positive cells[44]. Furthermore, the ileum of CD patients harbours a different distribution of enteric glial cells with a higher density of these cells around Peyer’s patches. In parallel, the mediators of enteric glial cell increased the permeability of the ileal mucosa in CD patients whereas they decreased the permeability of the mucosa in non-IBD patients[34]. The importance of these findings on the natural history of CD remains to be determined. In particular, the effect of the modulation of the ENS in neuro-immune interplay needs to be investigated.
Paneth cells are mostly located in the ileum and nearly absent from the colon[45]. This cellular type is found between intestinal stem cells in the small intestinal crypts. Paneth cells produce not only antimicrobial peptides that regulate host-microbe interplay but also factors such as Wnt ligands modulating the activity of intestinal stem cells. Many of the ileal CD-associated mutations discussed before involve cellular pathways of Paneth cells.
Paneth cells are rich in mitochondria to sustain their energy-expending secretory functions. In SAMP1 mice, mice genetically predisposed to CD-like ileitis, the number of Paneth cells was decreased and abnormal Paneth cells were associated with disease progression[46,47]. Likewise, the number of Paneth cells is decreased in the small intestine of CD patients. This observation was made in several ethnic populations and particularly in paediatric cohorts[19,48,49]. Mucosal biopsies from adult CD showed ultrastructural abnormalities in mitochondria, especially in CD patients with inflammation (73.3%) but also in inactive CD patients (20.3%) which was not the case for goblet cells and enterocytes[50]. In patients with ileal CD, the count of abnormal Paneth cells correlated with disease activity and was predictive of recurrence after surgery[51]. Some authors hypothesised that this dysfunction in Paneth cells could result from mitochondrial impairment[50-52]. Besides, in mice, Paneth cell defects triggered by loss of prohibitin 1, a major component protein of the inner mitochondrial membrane implicated in cell respiration, caused ileitis[52]. This effect is mediated by oxidative stress. Furthermore, the use of a specific mitochondrial-targeted antioxidant (Mito-Tempo) ameliorates ileitis in mice. The use of the same mitochondrial-targeted antioxidant (Mito-Tempo) in human ileal biopsies of CD patients normalized the expression of 25% of altered CD genes, including genes implicated in antigen processing, lipid metabolism, apoptosis, and interleukin (IL)-17/IL-23 signalling[50].
As highlighted by research in immunological processes, Paneth cells are part of the crosstalk with the immune system to maintain intestinal microbial homeostasis and intestinal barrier[53,54]. When biopsies from CD patients were compared according to the site of inflammation in the gastrointestinal tract, differences in neutrophil activities were observed. For instance, matrix metalloproteinase-9 and myeloperoxidase were relatively less increased in ulcer edges of ileal CD compared to colonic CD, suggesting less neutrophilic degranulation in ileal CD[55].
Likewise, innate lymphoid cells (ILCs) are gaining interest as components of the immune system. Three groups have been individualized according to their properties: ILC1, ILC2 and ILC3. A switch was noted in the inflamed ileal mucosa of CD patients, from an ILC3 phenotype limiting commensal bacteria specific CD4+ T cell response to an ILC1 phenotype associated with interferon (IFN)-γ production[56]. The aryl hydrocarbon receptor (AhR), a ligand-dependent transcription factor, was involved in this process and downregulated in inflamed mucosa of IBD patients[57,58]. Pharmacological or genetic activation of the AhR enhanced ILC3 maintenance conferring a protection against pathogenic bacteria in mice and downregulated ILC2 maintenance implicated in immune response in worm[59]. AhR agonists arise from the environment, commensal flora and tryptophan metabolism[60]. In patients with ileal CD, impaired tryptophan metabolism was observed with decreased levels of kynurenine and expression of kynureninase[61].
Concordantly with a typical cellular immune response, ileal CD instigates a specific cytokine profile. Interferon lambda (IFNL) is secreted in response to microbial stimulation or to T-cell-mediated mucosal inflammation. IFNL was upregulated in the ileal mucosa of patients with CD and triggered ileitis in a murine model and induced Paneth cell depletion independently of tumour necrosis factor (TNF)[62]. The effect of IFNL in the ileum is mediated by the JAK-STAT pathway which represents a promising therapeutic target already being used in the clinic[62].
Besides the role played by IFNL, IL-22 contributes to the pathogenesis of ileal CD. IL-22 is produced by T-cells and ICL3 during IBD flares[63,64]. IL-22 is thought to assume an immunoregulative role as its inhibition by the IL-22 binding protein induced a severe inflammation in a rodent colitis model[64]. In the ileum, interestingly, the levels of IL-22 binding protein were specifically high in comparison with the colon[65]. This heterogenous distribution along the gastrointestinal tract may result from the infiltration of eosinophils in the ileum and the production of IL-22 binding protein[64,65].
If a high proportion of eosinophil cells was present in the ileum, an enhanced enteroendocrine cell activity was also found[66]. Additionally, terminal ileal chromogranin A cells and glucagon-like peptide 1 (GLP-1) positive L-cells were increased in ileal CD specifically[66]. However, studies on the serum levels of GLP-1 are rare and include small cohorts (< 20 patients). Data were inconclusive and showed similar serum levels of GLP-1 between patients with ileocolic CD and colonic CD but a higher serum level of GLP-1 in IBD compared to healthy controls[67,68]. Thus, no firm conclusion can be drawn regarding the nutritional and immune impact of this discovery.
Besides harbouring enteroendocrine cells, the ileum harbours oestrogen receptor subtype β, whether the ileum is affected by CD or not. The expression of subtype β was associated with a milder disease course in a cohort of 37 patients with ileal CD. Accordingly, the inflammation score was inversely correlated with the expression of oestrogen receptor β. Similarly, a higher expression of oestrogen receptor β was found in patients with non-stricturing non-penetrating disease[69]. In a chemical colitis model in rats, the activation of oestrogen receptor b reduced inflammation score as well as inflammatory pain and inhibited the ionotropic P2X3 receptor[70]. Notwithstanding this observation, female sex was not independently associated with ileal CD[1,71].
While ileitis is often studied from an inflammation perspective, little is published about cell repair. The gene encoding tumour progression locus-2 kinase, a proinflammatory enzyme, was associated in a genetically invalidated murine model with a surprising homeostatic role, modulating the effect of chemically induced colitis. Mechanistically, this gene does not directly impact the immune response but plays a critical role in intestinal myofibroblasts which contribute to the healing of intestinal epithelium. In response to inflammatory signals from the microenvironment, intestinal myofibroblasts trigger compensatory epithelial proliferation in the intestinal crypts[72].
The integration of the different pathways mentioned above in the pathogenesis of ileal CD is facilitated by multi-omic studies. Thus, differences in pathogenesis between the ileum and the colon are highlighted by these techniques. For instance, in CD patients, the metabolomic study of non-inflamed ileum and non-inflamed colon biopsies distinguish clearly different profiles. It is noteworthy that these differences were blurred in inflamed ileum and colon samples[73].
Although the physiopathology of ileal CD has been studied as a unique phenomenon, emerging evidence advocates for a more heterogeneous process in which physiopathological pathways differ from one patient to the other. Thus, transcriptomic data analysis from CD ileal tissue sample was able to identify subgroups of patients with distinct recurrence rates after surgery[74].
In a recent transcriptomic study of ileal mucosa samples, inflammatory genes (IL-6, IL-8, IL-1β) were upregulated whereas metabolic process genes were downregulated in ileal samples from CD patients compared to controls. Early post-operative recurrence of CD was associated with an overexpression of TNF-α, IFN-γ, IL-23A and IL-17A upregulation. In addition, using a regression model to predict post-operative recurrence of CD, mitochondrial dysfunction and JAK/STAT upregulation in the ileum were independently associated with post-operative recurrence[75]. Transcriptomic studies also provide new insights into cell population specificities in ileal CD. When mapping the cell type of the ileal mucosa of CD patients, tuft and BEST4+ cells were the cell types associated with CD, irrespectively of the treatment status[76]. Intestinal tuft cells are a rare cell type implicated in the defence against helminthic and protozoan infections[77]. BEST4+ cells represent about 1% of ileal epithelial cells and plausibly contribute to the mucus secretion of goblet cells[78]. Surprisingly in this ileal cell type study, immune compartment was slightly affected by CD despite the fact that treatment status in CD patients modified the epithelial and immune compartment[76].
Among the differentially expressed genes in ileal inflammatory response, activating transcription factor 4 (ATF4) is a transcription factor widely expressed in the human body, including the ileum. ATF4 downregulation in intestinal epithelial cells has been observed in active CD in patients and causes spontaneous enterocolitis in mice while altering ileal Paneth cell function. Furthermore, murine ATF4 deletion impaired the glutamine uptake of the intestinal epithelium and concordantly glutamine supplementation restored Paneth cell function and decreased intestinal inflammation[79]. A few years earlier, the inhibition of the ATF4 pathway demonstrated an altered autophagy in human intestinal epithelium in the presence of adherent-invasive Escherichia coli (AIEC). This pathological response resulted in the intracellular bacterial replication of AIEC and consequently in pro-inflammatory patterns[80].
The role of bile acids is largely reported in liver disease in which bile acids are inflammatory cues and treatment targets[81]. The involvement of the gut in the pathogenesis of inflammatory liver diseases and similarities in etiological factors has led to consideration of the role of bile acids in IBD[82].
Primary bile acids are produced in the liver and secreted through the biliary tree into the gastrointestinal tract. Most primary bile acids are reabsorbed by the ileum and hence recycled several times. A minority of primary bile acids are transformed into secondary bile acids by a narrow range of gut bacteria. The pool of bile acids influenced the composition of gut microbiota which in turn modulated the composition of the bile acid pool[83]. Thus, impaired bile acid pools in relation to impaired microbiota enzymatic activities have been described as contributing to the inflammatory loop of IBD[84]. Bile acid composition in the lumen of the ileum differs between CD and non-CD patients with a relative decrease of primary bile acids in the ileum of CD patients[85]. Nevertheless, this finding should be interpreted with caution as malabsorption of bile acids was documented as a consequence of ileal CD especially in patients with a history of ileal resection[86,87]. Moreover, in a cohort of 166 patients, enhanced primary biliary level in the stool was independently associated with ileitis[88]. In a multi-omics approach based on a stool collection of 200 IBD patients, ileal CD profile was characterized by increased primary and secondary bile acid levels and shifts in taxa in favour of bacteria associated with bile acid-rich environments (Gammaproteobacteria and Blautia sp.)[89]. Further, the level of secondary bile acids in patients with inflammation limited to the ileum tended to increase after biological treatment reaching a similar level with control subjects[90].
In addition, bile acids exert inflammatory modulating properties through the stimulation of farnesoid X receptor (FXR). In a murine model of chemically induced inflammation, the activation of FXR demonstrated anti-inflammatory effects through a reduction of epithelial hyperpermeability and of proinflammatory cytokine production[91]. Furthermore, the obstruction of bile flow in mice induced mucosal ileal injuries reversed by administration of bile acids. In detail, this result may be explained by the activation of FXR by bile acids which promoted enteroprotective genes and limited ileal bacterial overgrowth[92]. Furthermore, bile acid pool modulation directly affects the ileum and Paneth cells. According to a recent article, Paneth cell number was linked to diet and to microbiota by bile acid in obese CD and non-CD patients irrespectively of other risk alleles (ATG16L1 and NOD2)[93]. In fact, in mice fed with a high fat diet, a similar phenomenon was observed and notably, high fat diet alone in germ-free mice as well as microbiome transfer alone in mice fed with standard diet were unable to induce an alteration in Paneth cells. The reason for this phenomenon was also dependent on FXR activation by the bile acid pool. Thus, the conjunction of a high fat diet and Clostridium-mediated production of secondary bile acids may explain the role of both diet and microbiome in this mechanism[93].
Lastly, bile acids promoted the expression of long polar fimbriae favouring the interplay of these strains with Peyer’s patches and bacterial translocations[94]. For example, primary bile acid level was inversely correlated with the abundance of Faecalibacterium prausnitzii (F. prausnitzii) and its acetate and L-methionine producing enzyme[88]. In patients treated by surgery for ileal CD, bile acid metabolism specificities were associated with an ileal recurrence of CD[89].
Nevertheless, there is only limited evidence on the therapeutic role of bile acids in ileal CD. While several authors reported the protective role of ursodeoxycholic acid and of its precursor, the lithocholic acid, against chemically induced colitis in mice[95-97], there is a lack of data about the effect of oral supplementation with ursodeoxycholic acid in patients with IBD. Thus, only one small single-centre trial examined the effect of ursodeoxycholic acid in UC and none was performed in patients with CD[98].
An increasing number of authors have investigated the link between gut microbiota and CD. However, many of these studies focused on the analysis of DNA extracted from stool. This methodology shed light on the colonic microbiota of the colonic lumen but was unable to draw any conclusions on the microbiota associated specifically with the ileum. Moreover, in a systematic review, the study mucosa-associated microbiota was regarded as more relevant in the understanding of CD pathogenesis[99]. In addition, dysbiosis was described in some mice models as a by-stander of ileitis. For example, in genetically predisposed mice Atg16L1ΔIEC, dysbiosis was observed but litter cross-fostering predisposed to colitis but not ileitis[20].
Microbiota homeostasis plays a critical role in CD physiopathology[2]. Thus, mucosal immunity regulator molecules such as vitamin D receptors (VDR) have been associated with a susceptibility to bacterial and chemical colitis. The genetic inhibition of VDR in the Paneth cells of VDRΔPC mice resulted in a lower expression of lysozymes in Paneth cells[19]. Inhibition of VDR influences the response to pathogenic bacteria. VDRΔPC mice are not only more sensitive to bacterial infection but also to chemical damage. Conversely, this susceptibility in VDRΔPC mice was reduced in case of co-housing with non-VDRΔPC mice, indirectly suggesting a protective role of the microbiome[19]. In SAMP1/YitFcsJ (SAMP1) mice which develop spontaneous terminal ileitis, dysbiosis occurred during disease progression with a decrease in Lachnospiraceae and in Bacteroides. In the same animal model, α-defensins misfolding was associated with dysbiosis and even induced dysbiosis in wild-type mice[47].
In this regard, the barrier function of the ileum is also a major feature in the understanding of host-microbiota interplay. Accordingly, human β-defensin 3 peptide was decreased and redistributed to the basolateral surface of the ileal epithelium[100]. In parallel, increased enzyme indoleamine 2,3-dioxygenase 1 (IDO1) was found in patients with active CD. This enzyme is the first enzyme in tryptophan metabolism on the kynurenine pathway and is responsible for mucus layer thickening and mucus-associated modulation of microbiota. In a murine enterocolitis model, IDO1 upregulation reduced the abundance of enteropathogenic E. coli in the ileum. Likewise, IDO1 downregulated inflammation in response to chemical colitis in mice and augmented A. muciniphila and M. schaedleri abundance[101].
In the nineties, a French team identified a strain of Escherichia coli, AIEC, which was adherent to the ileum without harbouring virulence factor-encoding genes[102]. AIEC was shown to be associated with ileal CD in further studies and to be able to invade epithelial cells[102,103]. Interestingly, AIEC was able to induce granulomas in vitro, which is one of the main histologic features of CD[2,104]. AIEC is classically identified on ileal biopsy but a dedicated serology could also be informative and less invasive[105]. The invasive property of AIEC is favoured by the overexpression of the glycoprotein CEACAM6 in the ileal epithelium. The interaction of this glycoprotein with the bacterial adhesive factor FimH promoted AIEC-enterocyte interplay in the ileum[106]. Using this receptor, AIEC modulated the metabolism of the ileal epithelium and induced strong gut inflammation[107]. AIEC induced the expression of hypoxia inducible factor, overexpressed in the ileum of CD patients and promoted barrier defects in the intestinal epithelium paving the way for the onset of inflammation[108].
The relapse of CD in patients after surgical treatment may be used to understand the early steps in CD onset. In a recent prospective, multicentric cohort of patients with ileal resection, AIEC in the remaining ileum was associated with an early ileal lesion of CD recurrence. The presence of AIEC within the surgical ileal specimen was predictive of the endoscopic recurrence of CD[109].
As highlighted in a recently published article, factors influencing ileal susceptibility to AIEC were numerous and among them epigenetic regulators had a modulating effect on a large range of proteins[110]. For this reason, before considering a clinical application in ileal CD, further studies are needed to prevent possible adverse effects induced by the modulation of these epigenetic targets or to identify more specific genes associated with AIEC colonisation. Another promising approach would be the use of bacteriophages to target specifically AIEC in ileal disease[111]. To date, no bacteriophage has been approved for intestinal therapeutic use in human, either in the European Union or in the United States, despite numerous studies that have reported encouraging results in vivo. Therefore, data about the effect of bacteriophages on human microbiota are needed in the future[111].
In a stool based multi-omics analysis of 200 IBD patients, the abundance of a member of Firmicutes, F. prausnitzii, was highly discriminant of ileal CD compared to colonic CD[89]. Even in non-inflamed ileal samples of CD patients, the commensal bacterium F. prausnitzii was decreased in comparison with healthy controls which suggests a relevant causal link between this bacteria and CD onset[112]. Accordingly, a low level of F. prausnitzii in the ileal mucosa-associated microbiota was associated with a higher recurrence rate of CD after ileal resection, corroborating the putative role of F. prausnitzii in the pathogenesis of ileal CD[113]. In addition, a recent study confirmed the role of F. prausnitzii in ileitis after ileocolectomy, pointing to a possible association with a bile salt profile. Thus, elevated levels of primary bile acids were associated with a decreased abundance of F. prausnitzii. These two parameters were the only factors associated with ileitis in this cohort of 166 patients[88]. The administration of F. prausnitzii or of its supernatant in vitro and in vivo counterbalanced gut inflammation by blocking nuclear factor kB activation and IL-8 production[113]. Interestingly, in CD patients, F. prausnitzii was associated specifically with ileitis, irrespectively of their genetic background[89,114]. In an original work conducted in twins in whom biopsies were performed in the lower gastrointestinal tract, the abundance of F. prausnitzii was specifically decreased in ileal CD, compared to colonic CD or in healthy twins[114].
The study of microbiota in IBD is not limited to bacteria but also includes fungi. Fungal microbiota was suspected to be strongly involved in CD pathogenesis in particular because of the diagnosis value of anti-Saccharomyces cerevisiae antibodies in CD[2]. In a study of 168 ileal biopsies, mycobiota was modified in CD patients with an increased abundance of Malassezia and a decreased abundance of Saccharomyces. The increase of Malassezia was notably associated with a severe evolution of the disease during follow-up[115].
The fungus Debaryomyces hansenii was increased in CD patients with inflamed ileum compared to non-inflamed ileum. Moreover, oral gavage with Debaryomyces hansenii impaired crypt regeneration and wound healing after biopsy injury[116].
The microbiota bridges the gap between host susceptibility factors and its environment. Most environmental factors are identified by epidemiological studies and associated with the onset of CD or its recurrence after surgery. Few of them though can impede the natural history of CD once the disease is established.
Cigarette smoking is a well-established risk factor for developing CD[1-3]. In patients with CD, the relative statistical weight of smoking was larger than the relative weight of the genetic variants presented in the previous section[1]. Besides, cannabis is frequently used as a symptomatic treatment by patients with CD involving the ileum and is associated with tobacco[117]. Regarding cannabis use, in a double-blind, randomized, placebo-controlled trial, cannabis oil induced clinical improvement without any endoscopic change[118].
Regarding tobacco, in mice, cigarette smoke extract induced intestinal inflammation and morphometric changes in the ileal epithelium regardless of the route of administration (intragastric or intraperitoneal), advocating for a systemic effect[119]. Moreover, mice exposed to cigarette smoking were more likely to develop pathological inflammation in response to bacterial inflammation which could be the hallmark of an impaired expression of antimicrobial peptides[119]. Besides, smoking was associated with a reduced number of normal Paneth cells in the ileum, both in patients presenting a CD susceptibility allele (ATG16L1T300A) as well as in mice presenting this genetic susceptibility. This defect in the ileum was mediated by the Paneth cell apoptosis driven by the activation of peroxisome proliferator-activated receptor gamma (PPARγ) and prevented in mice by the use of anti-TNF-α drugs[120].
Moreover, smoking is associated with an upregulation of angiogenesis in smokers with CD compared to their non-smoking counterparts. Importantly, mice exposed to cigarette smoke for 8 wk presented mucosal tissue hypoxia associated with an increased expression of pro-inflammatory cytokines and of angiogenic factors whereas smoking cessation reversed this process in the ileal mucosa. In addition, cigarette smoke exposure was associated with an increased sensitivity to chemically induced colitis[121]. Although genes of hypoxia-inducible factor were overexpressed in the inflamed ileum and in the adjacent mesenteric tissue of CD patients, hypoxia in mice did not impact experimental ileitis[122-124]. All together, these results advocate for a specific impact of tobacco smoking in the pathogenesis of ileitis.
Evidence for other environmental factor is rare in the literature. Recently, serum levels of bisphenol A, a component used in the manufacture of various plastics, have been linked to inflammatory status in CD patients. Furthermore, patients with bacterial DNA translocation presented a higher serum level of bisphenol A associated with a reduced expression of tight junction genes[125].
Western diet is characterized by a low intake of fibres contrasting with a high intake of refined sugar, animal protein and total fat. Numerous links between diet and IBD have been established[126]. To that extent, diet modulates the microbiota associated with the ileal epithelium. Thus, AIEC was associated with hyperacetylated histone H3 in the ileal epithelium and consequently histone deacetylase enzymes controlled the entry of AIEC in IECs in a murine model. Interestingly, a high fat diet enhanced histone acetylation in mice compared to a standard diet[110]. Overweight (body mass index > 25 kg/m2) in IBD and in non-IBD patients was associated with Paneth cell defects in the ileal epithelium. This defect can be induced by Western diet in mice[93].
Regarding fat intake, the ratio between n-3 and n-6 polyunsaturated fatty acids (PUFAs) is unbalanced in favour of an excessive intake of n-6 PUFAs in Western diet. Oral feeding with n-3 PUFAs in SAMP1/Yit mice ameliorated the histological features of ileitis, and decreased addressin molecule expression (MAdCAM-1) as well as lymphocyte infiltration in the ileum[127]. To that extent, oral supplementation with linseed oil rich in α-linolenic acid (n-3 PUFAs), in a physically active murine model, reduced inflammation after oral challenge with AIEC[128]. However, oral n-3 PUFAs supplementation has not shown definitive results in patients with CD[129]. In all likelihood, n-3 PUFAs alone are unable to stop the inflammatory loop once started but remain putative candidates to prevent the onset of ileal CD.
Fibre intake was identified as a protective factor against CD onset but not UC[130]. Although epidemiological data were based on a 40-year-old population, fibre intake and more accurately inulin supplementation modulated PPARγ signalling pathway in pigs[131].
Vitamin D deficiency in IBD patients has been reported in numerous studies reviewed elsewhere as an explanation of the North-South gradient of IBD prevalence[132]. In mice fed with a vitamin D deficient diet, miR-142-3p expression was upregulated, culminating in a reduction of ATG16L1 and autophagy specifically in ileal Paneth cells. In a paediatric cohort of IBD patients, colonic samples displayed likewise an enhanced expression of miR-142-3p statistically associated with low serum vitamin D levels[133]. As discussed previously, VDR were overexpressed in the ileum and were involved in the adequate response to enteropathogens[19]. When associated with a high fat diet, vitamin D deficiency and genetic VDR inhibition led to defective Paneth cell defensins secretion and gut permeability driving endotoxemia and systemic inflammation[134].
Last, Western diet is also characterized by a high intake of dietary additives related to the consumption of ultra-processed food. To that extent, dietary emulsifiers carboxymethylcellulose and polysorbate-80 were associated with an increased virulence and enrichment of ileal pathobionts[135].
More generally speaking, diet in patients with ileal CD should be based on the guidelines of the European Society of Nutrition. The findings discussed above and others have led to recommendations for a balanced diet rich in fruit, vegetables and n-3 fatty acids, avoiding a restrictive diet[136]. As highlighted in this European consensus, good quality data regarding the effects of experimental diets are rare in the literature. In particular, there is a lack of randomized control trials to recommend a more specific diet in patients with active ileal CD[136].
The factors summarised in the previous paragraphs give rise to a distinctive natural history of ileal CD with specific clinical consequences and interventions necessary.
In CD, the shortest time from diagnosis to first surgery is in ileal CD compared to other disease sites. Thus, the median time to surgery in ileal CD is about 6 years. Thirty years after being diagnosed with ileal CD, almost all patients had undergone at least one surgery according to a broad international study[1]. At 12 mo after surgery, a recurrence was observed on colonoscopy, at and above this anastomosis in 73% of patients in absence of any treatment[137]. These data regarding recurrence rates published 30 years ago led to the development of preventive strategies after surgery.
In terms of behaviour, ileal CD was more likely than colonic CD to be complicated by penetrating or stricturing lesions[3]. These severe manifestations contribute to the high rate of surgery. Accordingly, strictures are challenging complications that are usually unresponsive to medical therapy in the absence of surgery[3]. Strictures result from the accumulation of fibrotic protein in the extracellular matrix produced by fibroblasts which are partially derived from epithelial cells via epithelial-mesenchymal transition (EMT)[138]. Accordingly, based on a comparative study of ileal versus colonic ulcers, EMT appeared to be a highly noticeable feature in ileal ulcers of CD unlike colonic ulcers of CD[55]. Ileal strictures were likely to present mesenteric fat wrapped around the stricture, known as creeping fat[139], which harbours viable bacterial translocation and leads to a pro-fibrosis M2-type microenvironment[140-143]. Remarkably, creeping fat associated with the ileum presented a 10-fold higher concentration of T-cells than colonic fat[144]. In addition, adipocyte hyperplasia was observed in ileal fat unlike colonic fat[144].
Ileal crypts have been reported in the colon of CD patients[12,45]. Recently, the presence of ectopic ileal crypts discriminated IBD subtype in patients with undetermined (unclassified) colitis. The presence of ileal metaplasia in the colon was strongly associated with a final diagnosis of colonic CD[145]. This interesting discovery could enhance understanding of ileal physiopathology, pivotal to understand CD.
In a specific statistical model based on HLA types and single nucleotide polymorphism, colonic CD appeared to be an intermediate between UC and ileal CD[1]. Consecutively, knowledge of ileal CD may pave the way to understanding other phenotypes of CD.
Logically, the specificities of ileal CD discussed in this review should lead to a dedicated treatment strategy. Nevertheless, current ECCO guidelines on the medical management of CD do not advocate for a specific treatment in ileal CD[146]. Indeed, studies are controversial. Some studies reported a lower rate of response to infliximab, an anti-TNF treatment[147,148]. Conversely, numerous other studies did not describe such a difference in response rates with anti-TNF[149]. This discrepancy may result from a bias such as the extent of the disease irrespectively of the site of the disease. Interventional trials specifically dedicated to the study of ileal CD treatment are henceforth required. In the literature, colonic CD is more likely to respond to treatment compared to ileocolonic CD and mucosal healing may be more difficult to achieve in ileal disease. Likewise, ileal stricturing CD may have lower response rates[149].
As ileal stricture may develop in spite ofCD treatment, an American team sought to determine the gene pattern associated with ileal stricturing in a paediatric CD cohort. This analysis identified a long-chain fatty acid, the eicosatetranoic acid as a possible antifibrotic tool[150]. In a follow-up study, the same team showed a decrease in fibrosis and an improvement in stiffness in human intestinal organoids exposed to eicosatetraynoic acid[151]. In parallel, butyrate, a short-chain fatty acid, also downregulated fibrosis according to the same protocol[151].
The histological and anatomical features of the ileum are direct answers to the question “why the ileum?”. Furthermore, the recent works presented in this review highlight the specific interplay between environmental factors, like smoking or diet, and the ileum. Currently, the prevailing requirement for surgery in a significant proportion of patients with ileal CD testifies to the urgent need for a dedicated pharmacological approach according to disease site. As the ileum is the site of interplay between host characteristics and environmental influences, a full understanding of the molecular crosstalk that occurs in the ileum is crucial to identify new therapeutic targets to significantly change the natural history of this debilitating disease.
Mathilde Leboutte was supported by a PhD from Normandie Region. Authors are grateful to Nikki Sabourin-Gibbs, CHU Rouen, for her help in editing the manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Castelucci P, Brazil; Leal RF, Brazil S-Editor: Fan JR L-Editor: A P-Editor: Cai YX
| 1. | Cleynen I, Boucher G, Jostins L, Schumm LP, Zeissig S, Ahmad T, Andersen V, Andrews JM, Annese V, Brand S, Brant SR, Cho JH, Daly MJ, Dubinsky M, Duerr RH, Ferguson LR, Franke A, Gearry RB, Goyette P, Hakonarson H, Halfvarson J, Hov JR, Huang H, Kennedy NA, Kupcinskas L, Lawrance IC, Lee JC, Satsangi J, Schreiber S, Théâtre E, van der Meulen-de Jong AE, Weersma RK, Wilson DC; International Inflammatory Bowel Disease Genetics Consortium, Parkes M, Vermeire S, Rioux JD, Mansfield J, Silverberg MS, Radford-Smith G, McGovern DP, Barrett JC, Lees CW. Inherited determinants of Crohn's disease and ulcerative colitis phenotypes: a genetic association study. Lancet. 2016;387:156-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 568] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 2. | Roda G, Chien Ng S, Kotze PG, Argollo M, Panaccione R, Spinelli A, Kaser A, Peyrin-Biroulet L, Danese S. Crohn's disease. Nat Rev Dis Primers. 2020;6:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 601] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 3. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1560] [Article Influence: 111.4] [Reference Citation Analysis (1)] |
| 4. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4097] [Article Influence: 512.1] [Reference Citation Analysis (110)] |
| 5. | Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, Li MF, Ng KK, Kamm MA, Studd C, Bell S, Leong R, de Silva HJ, Kasturiratne A, Mufeena MNF, Ling KL, Ooi CJ, Tan PS, Ong D, Goh KL, Hilmi I, Pisespongsa P, Manatsathit S, Rerknimitr R, Aniwan S, Wang YF, Ouyang Q, Zeng Z, Zhu Z, Chen MH, Hu PJ, Wu K, Wang X, Simadibrata M, Abdullah M, Wu JC, Sung JJY, Chan FKL; Asia-Pacific Crohn's and Colitis Epidemiologic Study (ACCESS) Study Group. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology. 2013;145:158-165.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 597] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 6. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, Jewell DP, Karban A, Loftus EV Jr, Peña AS, Riddell RH, Sachar DB, Schreiber S, Steinhart AH, Targan SR, Vermeire S, Warren BF. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2148] [Cited by in RCA: 2367] [Article Influence: 215.2] [Reference Citation Analysis (0)] |
| 7. | Park SJ, Kim WH, Cheon JH. Clinical characteristics and treatment of inflammatory bowel disease: a comparison of Eastern and Western perspectives. World J Gastroenterol. 2014;20:11525-11537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Grimes CL, Ariyananda Lde Z, Melnyk JE, O'Shea EK. The innate immune protein Nod2 binds directly to MDP, a bacterial cell wall fragment. J Am Chem Soc. 2012;134:13535-13537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 9. | Tan G, Li RH, Li C, Wu F, Zhao XM, Ma JY, Lei S, Zhang WD, Zhi FC. Down-regulation of human enteric antimicrobial peptides by NOD2 during differentiation of the paneth cell lineage. Sci Rep. 2015;5:8383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Cuthbert AP, Fisher SA, Mirza MM, King K, Hampe J, Croucher PJ, Mascheretti S, Sanderson J, Forbes A, Mansfield J, Schreiber S, Lewis CM, Mathew CG. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology. 2002;122:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 466] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 11. | Ahmad T, Armuzzi A, Bunce M, Mulcahy-Hawes K, Marshall SE, Orchard TR, Crawshaw J, Large O, de Silva A, Cook JT, Barnardo M, Cullen S, Welsh KI, Jewell DP. The molecular classification of the clinical manifestations of Crohn's disease. Gastroenterology. 2002;122:854-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 412] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 12. | Ogura Y, Lala S, Xin W, Smith E, Dowds TA, Chen FF, Zimmermann E, Tretiakova M, Cho JH, Hart J, Greenson JK, Keshav S, Nuñez G. Expression of NOD2 in Paneth cells: a possible link to Crohn's ileitis. Gut. 2003;52:1591-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 326] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 13. | Wallings R, Manzoni C, Bandopadhyay R. Cellular processes associated with LRRK2 function and dysfunction. FEBS J. 2015;282:2806-2826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 14. | Zhang Q, Pan Y, Yan R, Zeng B, Wang H, Zhang X, Li W, Wei H, Liu Z. Commensal bacteria direct selective cargo sorting to promote symbiosis. Nat Immunol. 2015;16:918-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 15. | Hui KY, Fernandez-Hernandez H, Hu J, Schaffner A, Pankratz N, Hsu NY, Chuang LS, Carmi S, Villaverde N, Li X, Rivas M, Levine AP, Bao X, Labrias PR, Haritunians T, Ruane D, Gettler K, Chen E, Li D, Schiff ER, Pontikos N, Barzilai N, Brant SR, Bressman S, Cheifetz AS, Clark LN, Daly MJ, Desnick RJ, Duerr RH, Katz S, Lencz T, Myers RH, Ostrer H, Ozelius L, Payami H, Peter Y, Rioux JD, Segal AW, Scott WK, Silverberg MS, Vance JM, Ubarretxena-Belandia I, Foroud T, Atzmon G, Pe'er I, Ioannou Y, McGovern DPB, Yue Z, Schadt EE, Cho JH, Peter I. Functional variants in the LRRK2 gene confer shared effects on risk for Crohn's disease and Parkinson's disease. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 273] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 16. | Jung S, Ye BD, Lee HS, Baek J, Kim G, Park D, Park SH, Yang SK, Han B, Liu J, Song K. Identification of Three Novel Susceptibility Loci for Inflammatory Bowel Disease in Koreans in an Extended Genome-Wide Association Study. J Crohns Colitis. 2021;15:1898-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J, Günther S, Prescott NJ, Onnie CM, Häsler R, Sipos B, Fölsch UR, Lengauer T, Platzer M, Mathew CG, Krawczak M, Schreiber S. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1519] [Cited by in RCA: 1455] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 18. | Murthy A, Li Y, Peng I, Reichelt M, Katakam AK, Noubade R, Roose-Girma M, DeVoss J, Diehl L, Graham RR, van Lookeren Campagne M. A Crohn's disease variant in Atg16 L1 enhances its degradation by caspase 3. Nature. 2014;506:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 307] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 19. | Lu R, Zhang YG, Xia Y, Zhang J, Kaser A, Blumberg R, Sun J. Paneth Cell Alertness to Pathogens Maintained by Vitamin D Receptors. Gastroenterology. 2021;160:1269-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 20. | Tschurtschenthaler M, Adolph TE, Ashcroft JW, Niederreiter L, Bharti R, Saveljeva S, Bhattacharyya J, Flak MB, Shih DQ, Fuhler GM, Parkes M, Kohno K, Iwawaki T, Janneke van der Woude C, Harding HP, Smith AM, Peppelenbosch MP, Targan SR, Ron D, Rosenstiel P, Blumberg RS, Kaser A. Defective ATG16L1-mediated removal of IRE1α drives Crohn's disease-like ileitis. J Exp Med. 2017;214:401-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 21. | Wehkamp J, Wang G, Kübler I, Nuding S, Gregorieff A, Schnabel A, Kays RJ, Fellermann K, Burk O, Schwab M, Clevers H, Bevins CL, Stange EF. The Paneth cell alpha-defensin deficiency of ileal Crohn's disease is linked to Wnt/Tcf-4. J Immunol. 2007;179:3109-3118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Koslowski MJ, Kübler I, Chamaillard M, Schaeffeler E, Reinisch W, Wang G, Beisner J, Teml A, Peyrin-Biroulet L, Winter S, Herrlinger KR, Rutgeerts P, Vermeire S, Cooney R, Fellermann K, Jewell D, Bevins CL, Schwab M, Stange EF, Wehkamp J. Genetic variants of Wnt transcription factor TCF-4 (TCF7L2) putative promoter region are associated with small intestinal Crohn's disease. PLoS One. 2009;4:e4496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci U S A. 1997;94:11651-11656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 462] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 24. | Ayabe T, Wulff H, Darmoul D, Cahalan MD, Chandy KG, Ouellette AJ. Modulation of mouse Paneth cell alpha-defensin secretion by mIKCa1, a Ca2+-activated, intermediate conductance potassium channel. J Biol Chem. 2002;277:3793-3800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Simms LA, Doecke JD, Roberts RL, Fowler EV, Zhao ZZ, McGuckin MA, Huang N, Hayward NK, Webb PM, Whiteman DC, Cavanaugh JA, McCallum R, Florin TH, Barclay ML, Gearry RB, Merriman TR, Montgomery GW, Radford-Smith GL. KCNN4 gene variant is associated with ileal Crohn's Disease in the Australian and New Zealand population. Am J Gastroenterol. 2010;105:2209-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Chikina A, Matic Vignjevic D. At the right time in the right place: How do luminal gradients position the microbiota along the gut? Cells Dev. 2021;168:203712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Nugent SG, Kumar D, Rampton DS, Evans DF. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut. 2001;48:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 485] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 28. | Zhang S, Dogan B, Guo C, Herlekar D, Stewart K, Scherl EJ, Simpson KW. Short Chain Fatty Acids Modulate the Growth and Virulence of Pathosymbiont Escherichia coli and Host Response. Antibiotics (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Goldstein N, Dulai M. Contemporary morphologic definition of backwash ileitis in ulcerative colitis and features that distinguish it from Crohn disease. Am J Clin Pathol. 2006;126:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Magro F, Langner C, Driessen A, Ensari A, Geboes K, Mantzaris GJ, Villanacci V, Becheanu G, Borralho Nunes P, Cathomas G, Fries W, Jouret-Mourin A, Mescoli C, de Petris G, Rubio CA, Shepherd NA, Vieth M, Eliakim R; European Society of Pathology (ESP); European Crohn's and Colitis Organisation (ECCO). European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013;7:827-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 466] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 31. | Buisine MP, Desreumaux P, Leteurtre E, Copin MC, Colombel JF, Porchet N, Aubert JP. Mucin gene expression in intestinal epithelial cells in Crohn's disease. Gut. 2001;49:544-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 135] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Kiyono H, Fukuyama S. NALT- vs Peyer's-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 587] [Cited by in RCA: 562] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 33. | Casado-Bedmar M, Heil SDS, Myrelid P, Söderholm JD, Keita ÅV. Upregulation of intestinal mucosal mast cells expressing VPAC1 in close proximity to vasoactive intestinal polypeptide in inflammatory bowel disease and murine colitis. Neurogastroenterol Motil. 2019;31:e13503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Biskou O, Meira de-Faria F, Walter SM, Winberg ME, Haapaniemi S, Myrelid P, Söderholm JD, Keita ÅV. Increased Numbers of Enteric Glial Cells in the Peyer's Patches and Enhanced Intestinal Permeability by Glial Cell Mediators in Patients with Ileal Crohn's Disease. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Keita ÅV, Alkaissi LY, Holm EB, Heil SDS, Chassaing B, Darfeuille-Michaud A, McKay DM, Söderholm JD. Enhanced E. coli LF82 Translocation through the Follicle-associated Epithelium in Crohn's Disease is Dependent on Long Polar Fimbriae and CEACAM6 expression, and Increases Paracellular Permeability. J Crohns Colitis. 2020;14:216-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 36. | Bassotti G, Villanacci V, Nascimbeni R, Cadei M, Fisogni S, Antonelli E, Corazzi N, Salerni B. Enteric neuroglial apoptosis in inflammatory bowel diseases. J Crohns Colitis. 2009;3:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 37. | Villanacci V, Bassotti G, Nascimbeni R, Antonelli E, Cadei M, Fisogni S, Salerni B, Geboes K. Enteric nervous system abnormalities in inflammatory bowel diseases. Neurogastroenterol Motil. 2008;20:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 38. | Tursi A, Brandimarte G, Giorgetti G, Nasi G. Assessment of orocaecal transit time in different localization of Crohn's disease and its possible influence on clinical response to therapy. Eur J Gastroenterol Hepatol. 2003;15:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Wang XY, Zarate N, Soderholm JD, Bourgeois JM, Liu LW, Huizinga JD. Ultrastructural injury to interstitial cells of Cajal and communication with mast cells in Crohn's disease. Neurogastroenterol Motil. 2007;19:349-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Decousus S, Boucher AL, Joubert J, Pereira B, Dubois A, Goutorbe F, Déchelotte PJ, Bommelaer G, Buisson A. Myenteric plexitis is a risk factor for endoscopic and clinical postoperative recurrence after ileocolonic resection in Crohn's disease. Dig Liver Dis. 2016;48:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Misteli H, Koh CE, Wang LM, Mortensen NJ, George B, Guy R. Myenteric plexitis at the proximal resection margin is a predictive marker for surgical recurrence of ileocaecal Crohn's disease. Colorectal Dis. 2015;17:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Lemmens B, de Buck van Overstraeten A, Arijs I, Sagaert X, Van Assche G, Vermeire S, Tertychnyy A, Geboes K, Wolthuis A, D'Hoore A, De Hertogh G, Ferrante M. Submucosal Plexitis as a Predictive Factor for Postoperative Endoscopic Recurrence in Patients with Crohn's Disease Undergoing a Resection with Ileocolonic Anastomosis: Results from a Prospective Single-centre Study. J Crohns Colitis. 2017;11:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 43. | Sokol H, Polin V, Lavergne-Slove A, Panis Y, Treton X, Dray X, Bouhnik Y, Valleur P, Marteau P. Plexitis as a predictive factor of early postoperative clinical recurrence in Crohn's disease. Gut. 2009;58:1218-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 44. | Li Y, Ge Y, Zhu W, Gong J, Cao L, Guo Z, Gu L, Li J. Increased enteric glial cells in proximal margin of resection is associated with postoperative recurrence of Crohn's disease. J Gastroenterol Hepatol. 2018;33:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Lala S, Ogura Y, Osborne C, Hor SY, Bromfield A, Davies S, Ogunbiyi O, Nuñez G, Keshav S. Crohn's disease and the NOD2 gene: a role for paneth cells. Gastroenterology. 2003;125:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 361] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 46. | Lee C, Hong SN, Kim ER, Chang DK, Kim YH. Depletion of Intestinal Stem Cell Niche Factors Contributes to the Alteration of Epithelial Differentiation in SAMP1/YitFcsJ Mice With Crohn Disease-Like Ileitis. Inflamm Bowel Dis. 2021;27:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 47. | Shimizu Y, Nakamura K, Yoshii A, Yokoi Y, Kikuchi M, Shinozaki R, Nakamura S, Ohira S, Sugimoto R, Ayabe T. Paneth cell α-defensin misfolding correlates with dysbiosis and ileitis in Crohn's disease model mice. Life Sci Alliance. 2020;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 48. | Liu TC, Gurram B, Baldridge MT, Head R, Lam V, Luo C, Cao Y, Simpson P, Hayward M, Holtz ML, Bousounis P, Noe J, Lerner D, Cabrera J, Biank V, Stephens M, Huttenhower C, McGovern DP, Xavier RJ, Stappenbeck TS, Salzman NH. Paneth cell defects in Crohn's disease patients promote dysbiosis. JCI Insight. 2016;1:e86907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 49. | Liu TC, Naito T, Liu Z, VanDussen KL, Haritunians T, Li D, Endo K, Kawai Y, Nagasaki M, Kinouchi Y, McGovern DP, Shimosegawa T, Kakuta Y, Stappenbeck TS. LRRK2 but not ATG16L1 is associated with Paneth cell defect in Japanese Crohn's disease patients. JCI Insight. 2017;2:e91917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Alula KM, Jackson DN, Smith AD, Kim DS, Turner K, Odstrcil E, Kaipparettu BA, Dassopoulos T, Venuprasad K, Feagins LA, Theiss AL. Targeting Mitochondrial Damage as a Therapeutic for Ileal Crohn's Disease. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 51. | Khaloian S, Rath E, Hammoudi N, Gleisinger E, Blutke A, Giesbertz P, Berger E, Metwaly A, Waldschmitt N, Allez M, Haller D. Mitochondrial impairment drives intestinal stem cell transition into dysfunctional Paneth cells predicting Crohn's disease recurrence. Gut. 2020;69:1939-1951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 52. | Jackson DN, Panopoulos M, Neumann WL, Turner K, Cantarel BL, Thompson-Snipes L, Dassopoulos T, Feagins LA, Souza RF, Mills JC, Blumberg RS, Venuprasad K, Thompson WE, Theiss AL. Mitochondrial dysfunction during loss of prohibitin 1 triggers Paneth cell defects and ileitis. Gut. 2020;69:1928-1938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 53. | Walker CR, Hautefort I, Dalton JE, Overweg K, Egan CE, Bongaerts RJ, Newton DJ, Cruickshank SM, Andrew EM, Carding SR. Intestinal intraepithelial lymphocyte-enterocyte crosstalk regulates production of bactericidal angiogenin 4 by Paneth cells upon microbial challenge. PLoS One. 2013;8:e84553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 54. | Matsuzawa-Ishimoto Y, Yao X, Koide A, Ueberheide BM, Axelrad JE, Reis BS, Parsa R, Neil JA, Devlin JC, Rudensky E, Dewan MZ, Cammer M, Blumberg RS, Ding Y, Ruggles KV, Mucida D, Koide S, Cadwell K. The γδ IEL effector API5 masks genetic susceptibility to Paneth cell death. Nature. 2022;610:547-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 55. | Pierre N, Salée C, Massot C, Blétard N, Mazzucchelli G, Smargiasso N, Morsa D, Baiwir D, De Pauw E, Reenaers C, Van Kemseke C, Loly JP, Delvenne P, Meuwis MA, Louis E. Proteomics Highlights Common and Distinct Pathophysiological Processes Associated with Ileal and Colonic Ulcers in Crohn's Disease. J Crohns Colitis. 2020;14:205-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Li J, Doty AL, Iqbal A, Glover SC. The differential frequency of Lineage(-)CRTH2(-)CD45(+)NKp44(-)CD117(-)CD127(+)ILC subset in the inflamed terminal ileum of patients with Crohn's disease. Cell Immunol. 2016;304-305:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Li J, Doty A, Glover SC. Aryl hydrocarbon receptor signaling involves in the human intestinal ILC3/ILC1 conversion in the inflamed terminal ileum of Crohn's disease patients. Inflamm Cell Signal. 2016;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, MacDonald TT, Pallone F, Monteleone G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237-248, 248.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 505] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 59. | Li S, Bostick JW, Ye J, Qiu J, Zhang B, Urban JF Jr, Avram D, Zhou L. Aryl Hydrocarbon Receptor Signaling Cell Intrinsically Inhibits Intestinal Group 2 Innate Lymphoid Cell Function. Immunity. 2018;49:915-928.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 60. | Rothhammer V, Borucki DM, Kenison JE, Hewson P, Wang Z, Bakshi R, Sherr DH, Quintana FJ. Detection of aryl hydrocarbon receptor agonists in human samples. Sci Rep. 2018;8:4970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Huhn M, Juan MHS, Melcher B, Dreis C, Schmidt KG, Schwiebs A, Collins J, Pfeilschifter JM, Vieth M, Stein J, Radeke HH. Inflammation-Induced Mucosal KYNU Expression Identifies Human Ileal Crohn's Disease. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Günther C, Ruder B, Stolzer I, Dorner H, He GW, Chiriac MT, Aden K, Strigli A, Bittel M, Zeissig S, Rosenstiel P, Atreya R, Neurath MF, Wirtz S, Becker C. Interferon Lambda Promotes Paneth Cell Death Via STAT1 Signaling in Mice and Is Increased in Inflamed Ileal Tissues of Patients With Crohn's Disease. Gastroenterology. 2019;157:1310-1322.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 63. | Geremia A, Arancibia-Cárcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, Travis SP, Powrie F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208:1127-1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 521] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 64. | Martin JC, Bériou G, Heslan M, Bossard C, Jarry A, Abidi A, Hulin P, Ménoret S, Thinard R, Anegon I, Jacqueline C, Lardeux B, Halary F, Renauld JC, Bourreille A, Josien R. IL-22BP is produced by eosinophils in human gut and blocks IL-22 protective actions during colitis. Mucosal Immunol. 2016;9:539-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 65. | Fantou A, Lagrue E, Laurent T, Delbos L, Blandin S, Jarry A, Beriou G, Braudeau C, Salabert N, Marin E, Moreau A, Podevin J, Bourreille A, Josien R, Martin JC. IL-22BP production is heterogeneously distributed in Crohn's disease. Front Immunol. 2022;13:1034570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 66. | Moran GW, Pennock J, McLaughlin JT. Enteroendocrine cells in terminal ileal Crohn's disease. J Crohns Colitis. 2012;6:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 67. | Keller J, Beglinger C, Holst JJ, Andresen V, Layer P. Mechanisms of gastric emptying disturbances in chronic and acute inflammation of the distal gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;297:G861-G868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Keller J, Binnewies U, Rösch M, Juul Holst J, Beglinger C, Andresen V, Layer P. Gastric emptying and disease activity in inflammatory bowel disease. Eur J Clin Invest. 2015;45:1234-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 69. | Li H, Chen H, Chen L, Shen D, Xu X. Expression of oestrogen receptor beta was negatively correlated with disease activity in patients with Crohn's disease involving the terminal ileum. Steroids. 2019;141:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Jiang Q, Li WX, Sun JR, Zhu TT, Fan J, Yu LH, Burnstock G, Yang H, Ma B. Inhibitory effect of estrogen receptor beta on P2X3 receptors during inflammation in rats. Purinergic Signal. 2017;13:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Subramanian S, Ekbom A, Rhodes JM. Recent advances in clinical practice: a systematic review of isolated colonic Crohn's disease: the third IBD? Gut. 2017;66:362-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 72. | Roulis M, Nikolaou C, Kotsaki E, Kaffe E, Karagianni N, Koliaraki V, Salpea K, Ragoussis J, Aidinis V, Martini E, Becker C, Herschman HR, Vetrano S, Danese S, Kollias G. Intestinal myofibroblast-specific Tpl2-Cox-2-PGE2 pathway links innate sensing to epithelial homeostasis. Proc Natl Acad Sci U S A. 2014;111:E4658-E4667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 73. | Santoru ML, Piras C, Murgia F, Leoni VP, Spada M, Murgia A, Liggi S, Lai MA, Usai P, Caboni P, Manzin A, Atzori L. Metabolic Alteration in Plasma and Biopsies From Patients With IBD. Inflamm Bowel Dis. 2021;27:1335-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | Potdar AA, Li D, Haritunians T, VanDussen KL, Fiorino MF, Liu TC, Stappenbeck TS, Fleshner P, Targan SR, McGovern DPB, Bilsborough J. Ileal Gene Expression Data from Crohn's Disease Small Bowel Resections Indicate Distinct Clinical Subgroups. J Crohns Colitis. 2019;13:1055-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Ngollo M, Perez K, Hammoudi N, Gorelik Y, Delord M, Auzolle C, Bottois H, Cazals-Hatem D, Bezault M, Nancey S, Nachury M, Treton X, Fumery M, Buisson A, Barnich N, Seksik P; REMIND Study Group Investigators, Shen-Orr SS, Le Bourhis L, Allez M. Identification of Gene Expression Profiles Associated with an Increased Risk of Post-Operative Recurrence in Crohn's Disease. J Crohns Colitis. 2022;16:1269-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 76. | Maddipatla SC, Kolachala VL, Venkateswaran S, Dodd AF, Pelia RS, Geem D, Yin H, Sun Y, Xu C, Mo A, Kosters A, Yang J, Matthews JD, Ghosn E, Kugathasan S, Qiu P. Assessing Cellular and Transcriptional Diversity of Ileal Mucosa Among Treatment-Naïve and Treated Crohn's Disease. Inflamm Bowel Dis. 2023;29:274-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 77. | Hendel SK, Kellermann L, Hausmann A, Bindslev N, Jensen KB, Nielsen OH. Tuft Cells and Their Role in Intestinal Diseases. Front Immunol. 2022;13:822867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 78. | Elmentaite R, Kumasaka N, Roberts K, Fleming A, Dann E, King HW, Kleshchevnikov V, Dabrowska M, Pritchard S, Bolt L, Vieira SF, Mamanova L, Huang N, Perrone F, Goh Kai'En I, Lisgo SN, Katan M, Leonard S, Oliver TRW, Hook CE, Nayak K, Campos LS, Domínguez Conde C, Stephenson E, Engelbert J, Botting RA, Polanski K, van Dongen S, Patel M, Morgan MD, Marioni JC, Bayraktar OA, Meyer KB, He X, Barker RA, Uhlig HH, Mahbubani KT, Saeb-Parsy K, Zilbauer M, Clatworthy MR, Haniffa M, James KR, Teichmann SA. Cells of the human intestinal tract mapped across space and time. Nature. 2021;597:250-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 394] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 79. | Hu X, Deng J, Yu T, Chen S, Ge Y, Zhou Z, Guo Y, Ying H, Zhai Q, Chen Y, Yuan F, Niu Y, Shu W, Chen H, Ma C, Liu Z, Guo F. ATF4 Deficiency Promotes Intestinal Inflammation in Mice by Reducing Uptake of Glutamine and Expression of Antimicrobial Peptides. Gastroenterology. 2019;156:1098-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 80. | Bretin A, Carrière J, Dalmasso G, Bergougnoux A, B'chir W, Maurin AC, Müller S, Seibold F, Barnich N, Bruhat A, Darfeuille-Michaud A, Nguyen HT. Activation of the EIF2AK4-EIF2A/eIF2α-ATF4 pathway triggers autophagy response to Crohn disease-associated adherent-invasive Escherichia coli infection. Autophagy. 2016;12:770-783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 81. | Shao JW, Ge TT, Chen SZ, Wang G, Yang Q, Huang CH, Xu LC, Chen Z. Role of bile acids in liver diseases mediated by the gut microbiome. World J Gastroenterol. 2021;27:3010-3021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 82. | Calzadilla N, Comiskey SM, Dudeja PK, Saksena S, Gill RK, Alrefai WA. Bile acids as inflammatory mediators and modulators of intestinal permeability. Front Immunol. 2022;13:1021924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 83. | Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15:111-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1355] [Cited by in RCA: 1253] [Article Influence: 179.0] [Reference Citation Analysis (0)] |
| 84. | Duboc H, Rajca S, Rainteau D, Benarous D, Maubert MA, Quervain E, Thomas G, Barbu V, Humbert L, Despras G, Bridonneau C, Dumetz F, Grill JP, Masliah J, Beaugerie L, Cosnes J, Chazouillères O, Poupon R, Wolf C, Mallet JM, Langella P, Trugnan G, Sokol H, Seksik P. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 654] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 85. | Bamba S, Inatomi O, Nishida A, Ohno M, Imai T, Takahashi K, Naito Y, Iwamoto J, Honda A, Inohara N, Andoh A. Relationship between the gut microbiota and bile acid composition in the ileal mucosa of Crohn's disease. Intest Res. 2022;20:370-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (1)] |
| 86. | Nyhlin H, Merrick MV, Eastwood MA. Bile acid malabsorption in Crohn's disease and indications for its assessment using SeHCAT. Gut. 1994;35:90-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 87. | Skouras T, Dodd S, Prasad Y, Rassam J, Morley N, Subramanian S. Brief report: length of ileal resection correlates with severity of bile acid malabsorption in Crohn's disease. Int J Colorectal Dis. 2019;34:185-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 88. | Battat R, Scherl EJ, Lukin D, Charilaou P, Mahtani P, Gerber J, Gandara JA; JRI Live Cell Bank, Dündar F, Zumbo P, Betel D, Guo CJ, Longman RS. Increased Primary Bile Acids with Ileocolonic Resection Impact Ileal Inflammation and Gut Microbiota in Inflammatory Bowel Disease. J Crohns Colitis. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 89. | Gonzalez CG, Mills RH, Zhu Q, Sauceda C, Knight R, Dulai PS, Gonzalez DJ. Location-specific signatures of Crohn's disease at a multi-omics scale. Microbiome. 2022;10:133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 90. | Roda G, Porru E, Katsanos K, Skamnelos A, Kyriakidi K, Fiorino G, Christodoulou D, Danese S, Roda A. Serum Bile Acids Profiling in Inflammatory Bowel Disease Patients Treated with Anti-TNFs. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S, Klomp LW, Siersema PD, Schipper ME, Danese S, Penna G, Laverny G, Adorini L, Moschetta A, van Mil SW. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 637] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 92. | Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103:3920-3925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 893] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 93. | Liu TC, Kern JT, Jain U, Sonnek NM, Xiong S, Simpson KF, VanDussen KL, Winkler ES, Haritunians T, Malique A, Lu Q, Sasaki Y, Storer C, Diamond MS, Head RD, McGovern DPB, Stappenbeck TS. Western diet induces Paneth cell defects through microbiome alterations and farnesoid X receptor and type I interferon activation. Cell Host Microbe. 2021;29:988-1001.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 94. | Chassaing B, Etienne-Mesmin L, Bonnet R, Darfeuille-Michaud A. Bile salts induce long polar fimbriae expression favouring Crohn's disease-associated adherent-invasive Escherichia coli interaction with Peyer's patches. Environ Microbiol. 2013;15:355-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 95. | Lajczak-McGinley NK, Porru E, Fallon CM, Smyth J, Curley C, McCarron PA, Tambuwala MM, Roda A, Keely SJ. The secondary bile acids, ursodeoxycholic acid and lithocholic acid, protect against intestinal inflammation by inhibition of epithelial apoptosis. Physiol Rep. 2020;8:e14456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 96. | Ward JBJ, Lajczak NK, Kelly OB, O'Dwyer AM, Giddam AK, Ní Gabhann J, Franco P, Tambuwala MM, Jefferies CA, Keely S, Roda A, Keely SJ. Ursodeoxycholic acid and lithocholic acid exert anti-inflammatory actions in the colon. Am J Physiol Gastrointest Liver Physiol. 2017;312:G550-G558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 97. | Martínez-Moya P, Romero-Calvo I, Requena P, Hernández-Chirlaque C, Aranda CJ, González R, Zarzuelo A, Suárez MD, Martínez-Augustin O, Marín JJ, de Medina FS. Dose-dependent antiinflammatory effect of ursodeoxycholic acid in experimental colitis. Int Immunopharmacol. 2013;15:372-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 98. | Wang Z, Chen J, Chen Z, Xie L, Wang W. Clinical effects of ursodeoxycholic acid on patients with ulcerative colitis may improve via the regulation of IL-23-IL-17 axis and the changes of the proportion of intestinal microflora. Saudi J Gastroenterol. 2021;27:149-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 99. | Pittayanon R, Lau JT, Leontiadis GI, Tse F, Yuan Y, Surette M, Moayyedi P. Differences in Gut Microbiota in Patients With vs Without Inflammatory Bowel Diseases: A Systematic Review. Gastroenterology. 2020;158:930-946.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 401] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 100. | Meisch JP, Nishimura M, Vogel RM, Sung HC, Bednarchik BA, Ghosh SK, Fu P, McCormick T, Weinberg A, Levine AD. Human β-defensin 3 peptide is increased and redistributed in Crohn's ileitis. Inflamm Bowel Dis. 2013;19:942-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 101. | Alvarado DM, Chen B, Iticovici M, Thaker AI, Dai N, VanDussen KL, Shaikh N, Lim CK, Guillemin GJ, Tarr PI, Ciorba MA. Epithelial Indoleamine 2,3-Dioxygenase 1 Modulates Aryl Hydrocarbon Receptor and Notch Signaling to Increase Differentiation of Secretory Cells and Alter Mucus-Associated Microbiota. Gastroenterology. 2019;157:1093-1108.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 102. | Darfeuille-Michaud A, Neut C, Barnich N, Lederman E, Di Martino P, Desreumaux P, Gambiez L, Joly B, Cortot A, Colombel JF. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology. 1998;115:1405-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 638] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 103. | Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, Bringer MA, Swidsinski A, Beaugerie L, Colombel JF. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1123] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 104. | Meconi S, Vercellone A, Levillain F, Payré B, Al Saati T, Capilla F, Desreumaux P, Darfeuille-Michaud A, Altare F. Adherent-invasive Escherichia coli isolated from Crohn's disease patients induce granulomas in vitro. Cell Microbiol. 2007;9:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 105. | Buisson A, Vazeille E, Fumery M, Pariente B, Nancey S, Seksik P, Peyrin-Biroulet L, Allez M, Ballet N, Filippi J, Yzet C, Nachury M, Boschetti G, Billard E, Dubois A, Rodriguez S, Chevarin C, Goutte M, Bommelaer G, Pereira B, Hebuterne X, Barnich N; CEALIVE & REMIND study group. Faster and less invasive tools to identify patients with ileal colonization by adherent-invasive E. coli in Crohn's disease. United European Gastroenterol J. 2021;9:1007-1018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 106. | Sivignon A, Chervy M, Chevarin C, Ragot E, Billard E, Denizot J, Barnich N. An adherent-invasive Escherichia coli-colonized mouse model to evaluate microbiota-targeting strategies in Crohn's disease. Dis Model Mech. 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 107. | Carvalho FA, Barnich N, Sivignon A, Darcha C, Chan CH, Stanners CP, Darfeuille-Michaud A. Crohn's disease adherent-invasive Escherichia coli colonize and induce strong gut inflammation in transgenic mice expressing human CEACAM. J Exp Med. 2009;206:2179-2189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 108. | Mimouna S, Gonçalvès D, Barnich N, Darfeuille-Michaud A, Hofman P, Vouret-Craviari V. Crohn disease-associated Escherichia coli promote gastrointestinal inflammatory disorders by activation of HIF-dependent responses. Gut Microbes. 2011;2:335-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 109. | Buisson A, Sokol H, Hammoudi N, Nancey S, Treton X, Nachury M, Fumery M, Hébuterne X, Rodrigues M, Hugot JP, Boschetti G, Stefanescu C, Wils P, Seksik P, Le Bourhis L, Bezault M, Sauvanet P, Pereira B, Allez M, Barnich N; Remind study group. Role of adherent and invasive Escherichia coli in Crohn's disease: lessons from the postoperative recurrence model. Gut. 2023;72:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 110. | Chervy M, Sivignon A, Dambrine F, Buisson A, Sauvanet P, Godfraind C, Allez M, Le Bourhis L; The Remind Group; Barnich N, Denizot J. Epigenetic master regulators HDAC1 and HDAC5 control pathobiont Enterobacteria colonization in ileal mucosa of Crohn's disease patients. Gut Microbes. 2022;14:2127444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 111. | Boucher D, Barnich N. Phage Therapy Against Adherent-invasive E. coli: Towards a Promising Treatment of Crohn's Disease Patients? J Crohns Colitis. 2022;16:1509-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 112. | Zakrzewski M, Simms LA, Brown A, Appleyard M, Irwin J, Waddell N, Radford-Smith GL. IL23R-Protective Coding Variant Promotes Beneficial Bacteria and Diversity in the Ileal Microbiome in Healthy Individuals Without Inflammatory Bowel Disease. J Crohns Colitis. 2019;13:451-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 113. | Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottière HM, Doré J, Marteau P, Seksik P, Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731-16736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2747] [Cited by in RCA: 3199] [Article Influence: 188.2] [Reference Citation Analysis (0)] |
| 114. | Willing B, Halfvarson J, Dicksved J, Rosenquist M, Järnerot G, Engstrand L, Tysk C, Jansson JK. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn's disease. Inflamm Bowel Dis. 2009;15:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 362] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 115. | Olaisen M, Richard ML, Beisvåg V, Granlund AVB, Røyset ES, Rué O, Martinsen TC, Sandvik AK, Sokol H, Fossmark R. The ileal fungal microbiota is altered in Crohn's disease and is associated with the disease course. Front Med (Lausanne). 2022;9:868812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 116. | Jain U, Ver Heul AM, Xiong S, Gregory MH, Demers EG, Kern JT, Lai CW, Muegge BD, Barisas DAG, Leal-Ekman JS, Deepak P, Ciorba MA, Liu TC, Hogan DA, Debbas P, Braun J, McGovern DPB, Underhill DM, Stappenbeck TS. Debaryomyces is enriched in Crohn's disease intestinal tissue and impairs healing in mice. Science. 2021;371:1154-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 117. | Martinho-Grueber M, Kapoglou I, Bravo F, Sarraj R, Benz E, Restellini S, Biedermann L, Rogler G, Vavricka SR, Schoepfer A, Maillard MH, Michetti P, Brunner F, Clair C, Barry MP, Pittet V, von Känel R, Juillerat P. Alcohol and cannabis consumption in patients with inflammatory bowel disease: prevalence, pattern of consumption and impact on the disease. Eur J Gastroenterol Hepatol. 2023;35:21-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 118. | Naftali T, Bar-Lev Schleider L, Almog S, Meiri D, Konikoff FM. Oral CBD-rich Cannabis Induces Clinical but Not Endoscopic Response in Patients with Crohn's Disease, a Randomised Controlled Trial. J Crohns Colitis. 2021;15:1799-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 119. | Berkowitz L, Pardo-Roa C, Salazar GA, Salazar-Echegarai F, Miranda JP, Ramírez G, Chávez JL, Kalergis AM, Bueno SM, Álvarez-Lobos M. Mucosal Exposure to Cigarette Components Induces Intestinal Inflammation and Alters Antimicrobial Response in Mice. Front Immunol. 2019;10:2289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 120. | Liu TC, Kern JT, VanDussen KL, Xiong S, Kaiko GE, Wilen CB, Rajala MW, Caruso R, Holtzman MJ, Gao F, McGovern DP, Nunez G, Head RD, Stappenbeck TS. Interaction between smoking and ATG16L1T300A triggers Paneth cell defects in Crohn's disease. J Clin Invest. 2018;128:5110-5122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 121. | Fricker M, Goggins BJ, Mateer S, Jones B, Kim RY, Gellatly SL, Jarnicki AG, Powell N, Oliver BG, Radford-Smith G, Talley NJ, Walker MM, Keely S, Hansbro PM. Chronic cigarette smoke exposure induces systemic hypoxia that drives intestinal dysfunction. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 122. | De Galan C, De Vos M, Hindryckx P, Laukens D, Van Welden S. Long-Term Environmental Hypoxia Exposure and Haematopoietic Prolyl Hydroxylase-1 Deletion Do Not Impact Experimental Crohn's Like Ileitis. Biology (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 123. | Knyazev E, Maltseva D, Raygorodskaya M, Shkurnikov M. HIF-Dependent NFATC1 Activation Upregulates ITGA5 and PLAUR in Intestinal Epithelium in Inflammatory Bowel Disease. Front Genet. 2021;12:791640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 124. | Zuo L, Li Y, Zhu W, Shen B, Gong J, Guo Z, Zhang W, Wu R, Gu L, Li N, Li J. Mesenteric Adipocyte Dysfunction in Crohn's Disease is Associated with Hypoxia. Inflamm Bowel Dis. 2016;22:114-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 125. | Linares R, Fernández MF, Gutiérrez A, García-Villalba R, Suárez B, Zapater P, Martínez-Blázquez JA, Caparrós E, Tomás-Barberán FA, Francés R. Endocrine disruption in Crohn's disease: Bisphenol A enhances systemic inflammatory response in patients with gut barrier translocation of dysbiotic microbiota products. FASEB J. 2021;35:e21697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 126. | Marion-Letellier R, Savoye G, Ghosh S. IBD: In Food We Trust. J Crohns Colitis. 2016;10:1351-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 127. | Matsunaga H, Hokari R, Kurihara C, Okada Y, Takebayashi K, Okudaira K, Watanabe C, Komoto S, Nakamura M, Tsuzuki Y, Kawaguchi A, Nagao S, Miura S. Omega-3 polyunsaturated fatty acids ameliorate the severity of ileitis in the senescence accelerated mice (SAM)P1/Yit mice model. Clin Exp Immunol. 2009;158:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 128. | Plissonneau C, Sivignon A, Chassaing B, Capel F, Martin V, Etienne M, Wawrzyniak I, Chausse P, Dutheil F, Mairesse G, Chesneau G, Boisseau N, Barnich N. Beneficial Effects of Linseed Supplementation on Gut Mucosa-Associated Microbiota in a Physically Active Mouse Model of Crohn's Disease. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 129. | MacLean CH, Mojica WA, Newberry SJ, Pencharz J, Garland RH, Tu W, Hilton LG, Gralnek IM, Rhodes S, Khanna P, Morton SC. Systematic review of the effects of n-3 fatty acids in inflammatory bowel disease. Am J Clin Nutr. 2005;82:611-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 130. | Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR, Fuchs CS, Willett WC, Richter JM, Chan AT. A prospective study of long-term intake of dietary fiber and risk of Crohn's disease and ulcerative colitis. Gastroenterology. 2013;145:970-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 453] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 131. | Schroyen M, Li B, Arévalo Sureda E, Zhang Y, Leblois J, Deforce D, Van Nieuwerburgh F, Wavreille J, Everaert N. Pre-Weaning Inulin Supplementation Alters the Ileal Transcriptome in Pigs Regarding Lipid Metabolism. Vet Sci. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 132. | Fletcher J, Cooper SC, Ghosh S, Hewison M. The Role of Vitamin D in Inflammatory Bowel Disease: Mechanism to Management. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 133. | McGillis L, Bronte-Tinkew DM, Dang F, Capurro M, Prashar A, Ricciuto A, Greenfield L, Lozano-Ruf A, Siddiqui I, Hsieh A, Church P, Walters T, Roth DE, Griffiths A, Philpott D, Jones NL. Vitamin D deficiency enhances expression of autophagy-regulating miR-142-3p in mouse and "involved" IBD patient intestinal tissues. Am J Physiol Gastrointest Liver Physiol. 2021;321:G171-G184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 134. | Su D, Nie Y, Zhu A, Chen Z, Wu P, Zhang L, Luo M, Sun Q, Cai L, Lai Y, Xiao Z, Duan Z, Zheng S, Wu G, Hu R, Tsukamoto H, Lugea A, Liu Z, Pandol SJ, Han YP. Vitamin D Signaling through Induction of Paneth Cell Defensins Maintains Gut Microbiota and Improves Metabolic Disorders and Hepatic Steatosis in Animal Models. Front Physiol. 2016;7:498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 135. | Viennois E, Bretin A, Dubé PE, Maue AC, Dauriat CJG, Barnich N, Gewirtz AT, Chassaing B. Dietary Emulsifiers Directly Impact Adherent-Invasive E. coli Gene Expression to Drive Chronic Intestinal Inflammation. Cell Rep. 2020;33:108229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 136. | Bischoff SC, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, Shamir R, Stardelova K, Wierdsma N, Wiskin AE, Forbes A. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin Nutr. 2020;39:632-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (1)] |
| 137. | Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99:956-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1221] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 138. | Rieder F, Fiocchi C, Rogler G. Mechanisms, Management, and Treatment of Fibrosis in Patients With Inflammatory Bowel Diseases. Gastroenterology. 2017;152:340-350.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 365] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 139. | Li XH, Feng ST, Cao QH, Coffey JC, Baker ME, Huang L, Fang ZN, Qiu Y, Lu BL, Chen ZH, Li Y, Bettenworth D, Iacucci M, Sun CH, Ghosh S, Rieder F, Chen MH, Li ZP, Mao R. Degree of Creeping Fat Assessed by Computed Tomography Enterography is Associated with Intestinal Fibrotic Stricture in Patients with Crohn's Disease: A Potentially Novel Mesenteric Creeping Fat Index. J Crohns Colitis. 2021;15:1161-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 140. | Huang L, Qian W, Xu Y, Guo Z, Yin Y, Guo F, Zhu W, Li Y. Mesenteric Adipose Tissue Contributes to Intestinal Fibrosis in Crohn's Disease Through the ATX-LPA Axis. J Crohns Colitis. 2022;16:1124-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 141. | Wang J, Lin S, Brown JM, van Wagoner D, Fiocchi C, Rieder F. Novel mechanisms and clinical trial endpoints in intestinal fibrosis. Immunol Rev. 2021;302:211-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 142. | Kredel LI, Batra A, Stroh T, Kühl AA, Zeitz M, Erben U, Siegmund B. Adipokines from local fat cells shape the macrophage compartment of the creeping fat in Crohn's disease. Gut. 2013;62:852-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 143. | Ha CWY, Martin A, Sepich-Poore GD, Shi B, Wang Y, Gouin K, Humphrey G, Sanders K, Ratnayake Y, Chan KSL, Hendrick G, Caldera JR, Arias C, Moskowitz JE, Ho Sui SJ, Yang S, Underhill D, Brady MJ, Knott S, Kaihara K, Steinbaugh MJ, Li H, McGovern DPB, Knight R, Fleshner P, Devkota S. Translocation of Viable Gut Microbiota to Mesenteric Adipose Drives Formation of Creeping Fat in Humans. Cell. 2020;183:666-683.e17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 280] [Article Influence: 56.0] [Reference Citation Analysis (1)] |
| 144. | Kredel LI, Jödicke LJ, Scheffold A, Gröne J, Glauben R, Erben U, Kühl AA, Siegmund B. T-cell Composition in Ileal and Colonic Creeping Fat - Separating Ileal from Colonic Crohn's Disease. J Crohns Colitis. 2019;13:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 145. | Rana T, Korolkova OY, Rachakonda G, Williams AD, Hawkins AT, James SD, Sakwe AM, Hui N, Wang L, Yu C, Goodwin JS, Izban MG, Offodile RS, Washington MK, Ballard BR, Smoot DT, Shi XZ, Forbes DS, Shanker A, M'Koma AE. Linking bacterial enterotoxins and alpha defensin 5 expansion in the Crohn's colitis: A new insight into the etiopathogenetic and differentiation triggers driving colonic inflammatory bowel disease. PLoS One. 2021;16:e0246393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 146. | Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, Adamina M, Armuzzi A, Bachmann O, Bager P, Biancone L, Bokemeyer B, Bossuyt P, Burisch J, Collins P, El-Hussuna A, Ellul P, Frei-Lanter C, Furfaro F, Gingert C, Gionchetti P, Gomollon F, González-Lorenzo M, Gordon H, Hlavaty T, Juillerat P, Katsanos K, Kopylov U, Krustins E, Lytras T, Maaser C, Magro F, Marshall JK, Myrelid P, Pellino G, Rosa I, Sabino J, Savarino E, Spinelli A, Stassen L, Uzzan M, Vavricka S, Verstockt B, Warusavitarne J, Zmora O, Fiorino G. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. J Crohns Colitis. 2020;14:4-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 991] [Cited by in RCA: 903] [Article Influence: 180.6] [Reference Citation Analysis (2)] |
| 147. | Vermeire S, Louis E, Carbonez A, Van Assche G, Noman M, Belaiche J, De Vos M, Van Gossum A, Pescatore P, Fiasse R, Pelckmans P, Reynaert H, D'Haens G, Rutgeerts P; Belgian Group of Infliximab Expanded Access Program in Crohn's Disease. Demographic and clinical parameters influencing the short-term outcome of anti-tumor necrosis factor (infliximab) treatment in Crohn's disease. Am J Gastroenterol. 2002;97:2357-2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 169] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 148. | Choi CH, Song ID, Kim YH, Koo JS, Kim YS, Kim JS, Kim N, Kim ES, Kim JH, Kim JW, Kim TO, Kim HS, Kim HJ, Park YS, Park DI, Park SJ, Song HJ, Shin SJ, Yang SK, Ye BD, Lee KM, Lee BI, Lee SY, Lee CK, Im JP, Jang BI, Jeon TJ, Cho YK, Chang SK, Jeon SR, Jung SA, Jeen YT, Cha JM, Han DS, Kim WH; IBD Study Group of the Korean Association for the Study of the Intestinal Diseases. Efficacy and Safety of Infliximab Therapy and Predictors of Response in Korean Patients with Crohn's Disease: A Nationwide, Multicenter Study. Yonsei Med J. 2016;57:1376-1385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 149. | Gisbert JP, Chaparro M. Predictors of Primary Response to Biologic Treatment [Anti-TNF, Vedolizumab, and Ustekinumab] in Patients With Inflammatory Bowel Disease: From Basic Science to Clinical Practice. J Crohns Colitis. 2020;14:694-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 190] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 150. | Haberman Y, Minar P, Karns R, Dexheimer PJ, Ghandikota S, Tegge S, Shapiro D, Shuler B, Venkateswaran S, Braun T, Ta A, Walters TD, Baldassano RN, Noe JD, Rosh J, Markowitz J, Dotson JL, Mack DR, Kellermayer R, Griffiths AM, Heyman MB, Baker SS, Moulton D, Patel AS, Gulati AS, Steiner SJ, LeLeiko N, Otley A, Oliva-Hemker M, Ziring D, Gokhale R, Kim S, Guthery SL, Cohen SA, Snapper S, Aronow BJ, Stephens M, Gibson G, Dillman JR, Dubinsky M, Hyams JS, Kugathasan S, Jegga AG, Denson LA. Mucosal Inflammatory and Wound Healing Gene Programs Reveal Targets for Stricturing Behavior in Pediatric Crohn's Disease. J Crohns Colitis. 2020;15:273-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 151. | Jurickova I, Bonkowski E, Angerman E, Novak E, Huron A, Akers G, Iwasawa K, Braun T, Hadar R, Hooker M, Han S, Cutler DJ, Okou DT, Kugathasan S, Jegga A, Wells J, Takebe T, Mollen KP, Haberman Y, Denson LA. Eicosatetraynoic Acid and Butyrate Regulate Human Intestinal Organoid Mitochondrial and Extracellular Matrix Pathways Implicated in Crohn's Disease Strictures. Inflamm Bowel Dis. 2022;28:988-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (1)] |