Published online May 14, 2023. doi: 10.3748/wjg.v29.i18.2733
Peer-review started: December 30, 2022
First decision: January 22, 2023
Revised: February 14, 2023
Accepted: April 4, 2023
Article in press: April 4, 2023
Published online: May 14, 2023
Processing time: 131 Days and 8.8 Hours
Ulcerative colitis (UC) and Crohn’s disease (CD) are part of Inflammatory Bowel Diseases (IBD) and have pathophysiological processes such as bowel necrosis and enteric neurons and enteric glial cells. In addition, the main inflammatory mediator is related to the tumor necrosis factor-alpha (TNF-α). TNF-α is a me-diator of the intestinal inflammatory processes, thus being one of the main cytokines involved in the pathogenesis of IBD, however, its levels, when measured, are present in the serum of patients with IBD. In addition, TNF-α plays an important role in promoting inflammation, such as the production of interleukins (IL), for instance IL-1β and IL-6. There are two receptors for TNF as following: The tumor necrosis factor 1 receptor (TNFR1); and the tumor necrosis factor 2 receptor (TNFR2). They are involved in the pathogenesis of IBD and their receptors have been detected in IBD and their expression is correlated with disease activity. The soluble TNF form binds to the TNFR1 receptor with, and its activation results in a signaling cascade effects such as apoptosis, cell proliferation and cytokine secretion. In contrast, the transmembrane TNF form can bind both to TNFR1 and TNFR2. Recent studies have suggested that TNF-α is one of the main pro-inflammatory cytokines involved in the pathogenesis of IBD, since TNF levels are present in the serum of both patients with UC and CD. Intravenous and subcutaneous biologics targeting TNF-α have revolutionized the treatment of IBD, thus becoming the best available agents to induce and maintain IBD remission. The application of antibodies aimed at neutralizing TNF-α in patients with IBD that induce a satisfactory clinical response in up to 60% of patients, and also induced long-term maintenance of disease remission in most patients. It has been suggested that anti-TNF-α agents inactivate the pro-inflammatory cytokine TNF-α by direct neutralization, i.e., resulting in suppression of inflammation. However, anti-TNF-α antibodies perform more complex functions than a simple blockade.
Core Tip: This review summarizes the role of Tumor Necrosis Factor-alpha (TNF-α) in promoting inflammation. Studies have suggested that TNF-α is one of the main pro-inflammatory cytokines involved in the pathogenesis of Inflammatory Bowel Diseases (IBD). In addition, the enteric nervous system is affected by IBD. There are two receptors for TNF: The tumor necrosis factor 1 receptor; and the tumor necrosis factor 2 receptor. They are involved in the pathogenesis of IBD. The application of antibodies aimed at neutralizing TNF-α in patients with IBD induce a satisfactory clinical recovery. This review addresses the main aspects of TNF-α in IBD and anti-TNF therapies.
- Citation: Souza RF, Caetano MAF, Magalhães HIR, Castelucci P. Study of tumor necrosis factor receptor in the inflammatory bowel disease. World J Gastroenterol 2023; 29(18): 2733-2746
- URL: https://www.wjgnet.com/1007-9327/full/v29/i18/2733.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i18.2733
Inflammatory bowel diseases (IBD) comprise ulcerative colitis (UC) and Crohn’s disease (CD), which are chronical and recurrent disorders that affect the gastrointestinal (GI) tract[1-4]. The etiology of IBD is not yet fully understood, but there are reports of a complex relationship between genetic[5,6], immunological and environmental factors[7-10], as well as gut microbiota[11-15]. There is an imbalance between anti- and pro-inflammatory cytokines that cause an exacerbated and inadequate immune response.
The enteric nervous system (ENS) is composed by intrinsic neurons, their axons and enteric glial cells, which constitute a complex of structures responsible for controlling motility of the GI tract, secretion of gastric acid, regulation of fluid movement through the epithelium, local blood flow control, and interactions with the endocrine and immune systems of the gut[16-18]. The ENS is affected by IBD which causes necrosis, apoptosis, degeneration of enteric ganglia and alterations on motility patterns[19-23].
Tumor necrosis factor-alpha (TNF-α) is a mediator in intestinal inflammatory processes, thus being one of the main cytokines involved in pathogenesis of IBD, with high levels in patients with IBD[24,25]. There are two receptors for TNF-α as following: The tumor necrosis factor receptor 1 (TNFR1); and the tumor necrosis factor receptor 2 (TNFR2). After TNF-α binding to these receptors, it activates signaling pathways for cell survival, death and differentiation[26]. An increase in TNF-α expression can lead to mucosal barrier defects in patients with IBD, which increases inflammation and worsens the prognosis[10].
Due to the TNF-α relation with IBD, biological drugs have been used to treat CD and UC with neutralizing monoclonal antibodies used for TNF-α target and blockade, which reduce the development of the inflammatory process and the activation of immune system cells[27]. Anti-TNF-α agents are indicated by various guidelines for the treatment of IBD[28,29] and the use of these agents induces a satisfactory clinical response, and long-term maintenance of disease remission in most patients[24,30]. This review aims to provide the main aspects of IBD and TNF-α relationship, elucidating the role of the TNF-α receptors, anti TNF-α therapy and some perspectives related to the involvement of the ENS.
The ENS is a complex of structures responsible for controlling motility of the GI tract, secretion of gastric acid, regulation of fluid movement through the epithelium, changes in local blood flow, and interactions with the endocrine and immune systems of the gut[16-18]. The ENS is located in the intestinal wall and is organized into two ganglionated plexuses: The myenteric plexus; and the submucosal plexus[16-18].
The myenteric plexus (Auerbach’s plexus) is situated between the longitudinal muscle layer and the circular muscle layer throughout the entire GI tract, from the esophagus to the rectum. It has three components as following: A primary plexus; a secondary plexus; and a tertiary plexus. The myenteric plexus is involved with reflex regulation of the contractile activities of the muscle layer[16,17]. The submucosal plexus (Meissner’s plexus) is predominantly found in the small and large intestines, with a smaller ganglion and finer interconnected fibers when compared to the myenteric plexus[16,17]. It has a direct role in controlling secretion and absorption through motor neurons that regulate the secretomotor and vasomotor activity of the mucosal layer[16,17].
Enteric neurons can be classified according to their function into motor neurons, interneurons, and Intrinsic Primary Afferent Neurons[16]. Motor neurons are further divided according to their chemical code into excitatory neurons (labeled by the enzyme choline acetyltransferase or by calretinin), inhibitory neurons (labeled by the enzyme neuronal nitric oxide synthase), and secretomotor/vasodilator neurons. Excitatory and inhibitory motor neurons are observed in the myenteric plexus and are involved in motility control, while secretomotor/vasodilator neurons are observed in the submucosal plexus and are responsible for innervating the mucosa and regulating secretion, absorption, and local blood flow[16,17].
Enteric glial cells are small, consisting of numerous processes that do not synthesize myelin, and are also essential for the organization and function of the ENS[31]. These cells have a star shape with numerous branches that surround the neurons. They also exhibit a series of voltage-dependent ion channels that are connected by gap junctions, giving the impression of an intimate and complex intertwining of neurons and glial cells. In addition to their role in supporting neurons, enteric glia cells regulate synaptic transmission, release cytokines, and mediate communication with the immune system[32-34].
Enteric glial cells are identified by immunohistochemical methods such as labeling of cells expressing glial fibrillary acidic protein (GFAP) and/or calcium-binding protein S100/S100β since mature enteric glial cells express these protein types[35,36]. GFAP expression is modulated by enteric glial cell proliferation, differentiation, and inflammation[22]. S100 is a calcium-binding protein that can be found in the nucleus or cytoplasm and acts to regulate the cytoskeleton structure and function as well as the calcium homeostasis in the cytoplasm[35,37,38]. Under inflammatory conditions, enteric glial cells can acquire new functional properties, and in patients with IBD there was an increase in GFAP expression in mucosal inflammation compared to non-inflamed areas[39].
The enteric plexuses are formation of cells that spread throughout the entire GI tract, however, differences in the density and size of enteric neurons and glial cells, as well as in ganglion morphology, may occur in the same segment of the GI tract in different species, or in different experimental models such as undernourishment protein and renutrition[40,41], obesity[42,43], ischemia and reperfusion[44-47], and intestinal inflammation[23,48-52].
About IBD specifically, they are classically classified into UC and CD, disorders that chronically and recurrently affect the GI tract[1-4]. Inflammatory reactions in the intestinal mucosa cause epithelial damage that compromises the intestinal barrier[53]. Although the etiology of IBD is not yet fully understood, they are usually triggered by a complex relationship between genetic[5,6], immunological and environmental factors[7-10], and gut microbiota itself[11-15]. There is, then, an imbalance between anti- and pro-inflammatory cytokines that cause an exacerbated and inadequate immune response. It is emphasized that, in colitis, there are alterations in the neuronal density such as imbalances of contractile and secretory functions associated with diarrhea due to intestinal inflammation[20,49,50,52].
CD is characterized by a segmental and transmural disorder that can affect the entire GI tract, and it could be noted there is also involvement of T-auxiliary cells (TCD4) in the pathogenesis of the disease[54,55]. In contrast, UC is characterized by continuous inflammation of the distal colon and a modi-fication of the cytokine profile coming from Th2 cells, involved in humoral immune response patterns[2,56]. The main clinical manifestations of IBD are abdominal pain, diarrhea, vomiting, weight loss, and the presence of blood in the stool[2,50] and, worryingly, it has been demonstrated that this occurrence has increased worldwide over the years[57-60].
The literature has shown that the ENS may be affected by the IBD, thus presenting necrosis, apoptosis and degeneration of enteric ganglia[19-23]. Similarly, alterations in neurotransmitters and neuropeptides of enteric neurons have been noted in these pathologies[19,48]. Furthermore, injuries in the ENS result in activation of enteric glial cells[61,62], and the onset and/or progression of IBD can be attributed to an immune-mediated damage of enteric glial cells[32]. This activation is pointed to as a signaling mechanism that results in subsequent neuronal death[63].
The diversity of neurotransmitters and receptors found in the ENS makes the intestine one of the main tissue choices for studies of neurotransmitter receptors in pharmacological tests. In turn, several ENS-related treatment strategies have been explored to alter gut function in an effort to improve symptoms, which include drugs targeting opioid, serotoninergic, dopaminergic, and cholinergic receptors[64].
Induced colitis models in the laboratory has been widely used to study intestinal inflammation, especially through dextran sulfate sodium and 2,4,6-trinitrobenzene sulfonic acid[65-69]. Despite this, the cell signaling mechanisms underlying neuronal and tissue damage are poorly understood[49,70], and new therapeutic approaches for IBD are emerging[71-74].
As expected, experimental animal models of colitis, enteric neuronal hyperplasia and hypoplasia may be associated with increased and reduced levels of TNFα production, respectively[75,76]. In addition, hypertrophy and hyperplasia of enteric neurons have been reported in IBD[77]. Although, the mechanism responsible for neuronal modulation of inflammation severity is still unclear, due to modulations of neuroimmune interactions, it is speculated that enteric neurons could produce and regulate cytokines involved in IBD[76].
Specific markers have been identified in GI tract when tissues are affected by injury or disease such as IBD. They are responsible for mucosal injury and tissue damage and consequently may trigger autoimmune disease - specific immune responses in UC[10]. The TNF-α, inducible nitric oxide synthase, heme oxygenase 1, arginase-1 or CD206 in higher levels can lead to massive tissue damage in the intestine[25,54,78].
TNF-α is a mediator in intestinal inflammatory processes, being one of the main cytokines involved in pathogenesis of IBD, since their levels, are frequently high in patients with IBD[24,25]. TNF-α was first described in 1975 by a group in Sloan-Kettering who identified TNF as a promising serum soluble activity[79]. Thus, TNF-α plays roles in promoting inflammation such as the production of interleukins (IL) such as IL-1β and IL-6[10,80]. In the central nervous system (CNS), TNF-α plays homeostatic roles by regulating crucial physiological processes, such as synaptic plasticity, learning and memory, sleep and food and water intake[81]. However, some findings demonstrated that overexpression of TNF in the CNS has negative effects on cognitive functions and TNF deficiency is related to learning processes and poor memory[82]. In addition, it may be involved in gliosis and tissue remodeling and in various processes about mechanisms subserving cognition, such as synaptic scaling, change in neurotransmitter metabolism, and the process of neurodevelopment[82,83]. This observation may pave the way for understand mechanisms of innate immunity and the pathogenesis of infectious diseases which are driven by the same cascade of pro-inflammatory cytokines[79].
TNF-α is mainly generated by macrophages and monocytes. However, other cells such as some subsets of T cells, NK cells, dendritic cells, B cells, cardiomyocytes, fibroblasts, and astrocytes are also low-level producers of this cytokine[84]. In pathological conditions, astrocytes and microglia release large amounts of TNF-α, seeing that the production of this cytokine is an important component of the neuroinflammatory response that is associated with various neurological disorders such as Alzheimer’s, Parkinson’s, multiple sclerosis and amyotrophic lateral sclerosis[81].
TNF-α operates significantly in the apoptotic phase increasing the expression of the TNF receptor-associated factor 2 (TRAF2)[53]. Furthermore, TNF-α activates mitogen-activated protein kinase and nuclear factor which contribute to cell differentiation and proliferation and increased expression of pro-inflammatory cytokines[10]. Two distinct forms of TNF-α were identified: 26kDa homotrimeric transmembrane precursor form (mTNF), which is cleaved by the TNF-α-converting enzyme, and matrix metalloproteinase (TACE/Adam17) to its next 17kDa TNF soluble form (sTNF) of monomers[85,86]. Both forms of TNF are biologically active and are signaled through two distinct receptors discussed below.
There are two receptors for TNF being: The 75 kDa TNFR1, ubiquitously expressed and has a cellular death domain; and the 55 kDa TNFR2 has been found in lymphocytes and endothelial cells. Interactions of TNF-α with its receptor activate signaling pathways for cell survival, death and differentiation that control immune function and disease, and various receptors pairs of ligands within TNF family molecules expressed by immune cells that play important roles in T cell Immunity[26].
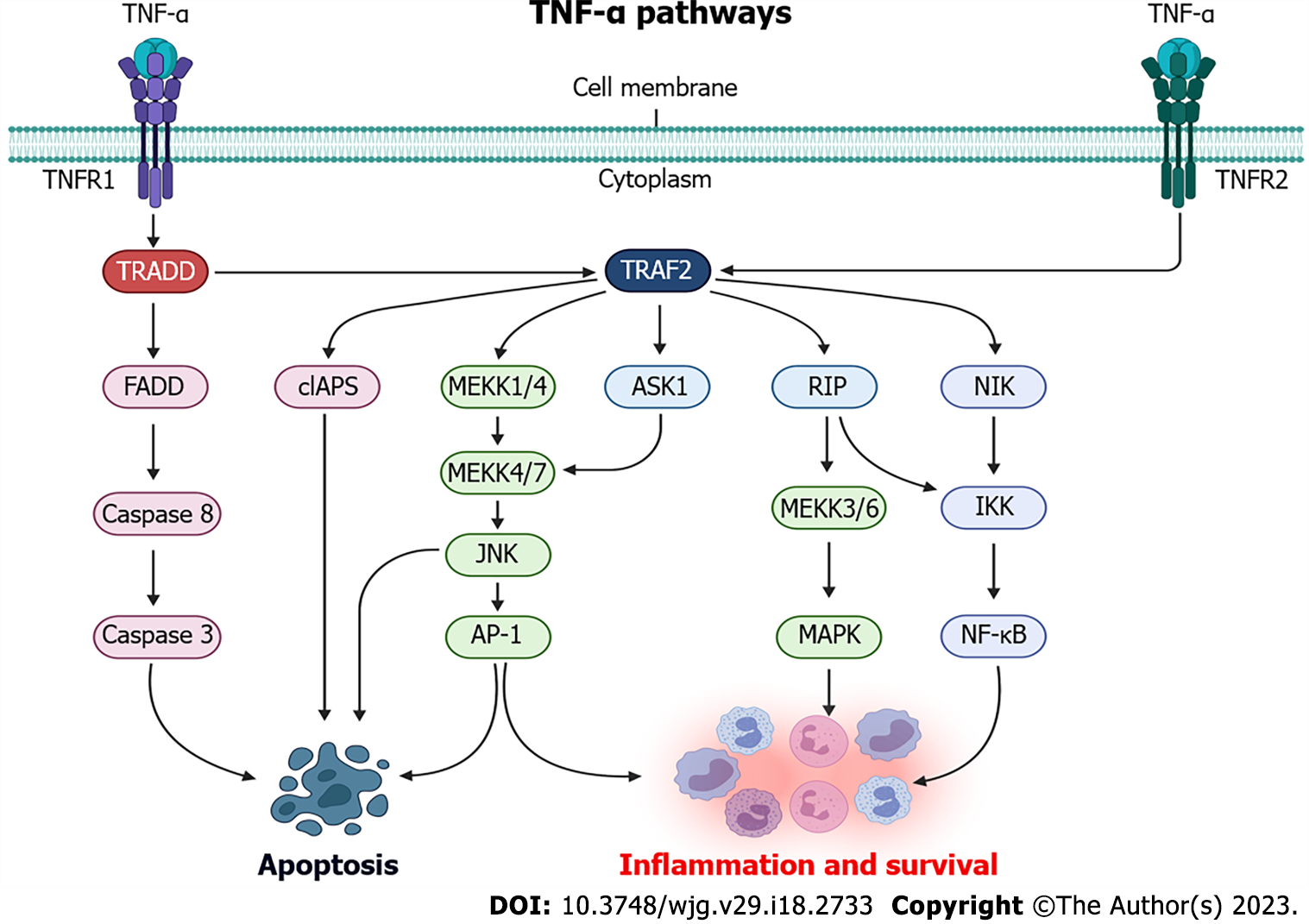
When TNF-α binds to TNFR1 and TNFR2, several intracellular pathways are activated, thus mediating cell death and/or survival response (Figure 1). When TNF-α binds to TNFR1, TNF receptor associated death domain protein (TRADD) is activated which, in turn, can induce the activation of three signaling pathways. In the first one, after TNFR1 activation, TRADD binds to FAS-associated death domain protein, which recruits caspase 8 proteins, culminating in the activation and cleavage of caspase 3, as well as leading to cell death by apoptosis[87]. The second TNFR1 pathway is related to the recruitment of TRAF2 and receptor-interacting protein (RIP) kinase via TRADD. TRAF2, in turn, recruits the IκB kinase (IKK) protein, which will be activated by RIP and will result in the phosphorylation of nuclear factor κB (NF-κB), which will mediate the transcription of proteins involved in the inflammation response and cell survival[87,88]. The third pathway resulting from TNFR1 activation is connected with activation of mitogen-activated protein kinase (MAPK) pathways via TRAF2, which activate MAPK kinase kinase 1/4 (MEKK1/4) and, upon phosphorylation, MEKK4/7 leads to activation of c-Jun N-terminal kinase, which is translocated to the nucleus and activate transcription factors such as activator protein 1 (AP-1), that can converge to activate the apoptotic and survival responses[89-91].
Although the pathways behind TNFR2 activation remains poorly understood, when the TNF-α binds to TNFR2, its activation is mediated by TRAF2, and TNFR2 has been widely known as a mediator of the activation of genes related to cell survival and proliferation[91-93]. After TNF-α binds to TNFR2, TRAF2 is activated which, through common signaling pathways to TNFR1 activation, can activate NF-κB through IKK, and AP-1 via MEKK, which can also be activated via apoptosis signal-regulating kinase 1[94]. Furthermore, activation of TRAF2 via TNFR2 can lead to the recruitment of cellular inhibitor of apoptosis (cIAPS), which will partially inhibit caspase activation and, for this reason, reduce apoptosis response[95]. When both TNFR1 and TNFR2 are activated together, cIAPS recruitment is reduced and the caspase activity, mainly mediated by TNFR1, is activated[91].
These receptors are involved in the pathogenesis of IBD and their expression is correlated with disease activity[96]. The sTNf binds selectively to the TNFR1 receptor, and its activation results in a signaling cascade with effects such as apoptosis, cell proliferation and cytokine secretion[24,97]. In contrast, the mTNF can bind both to TNFR1 and TNFR2[91]. TNFR1 signaling pathways deserve attention, due to cytotoxic effects triggered by activation of TNFR1 via sTNF binding and it could be noted that some aspects regarding TNFR2 function are still unclear[91].
Some studies pointed to the presence of a functional cross-talk between TNFR1 and TNFR2, whichever TNFR2 would act as an complement-dependent cytotoxic effect of TNFR1, thus being responsible for the inhibition of anti-apoptotic pathways and for the increase in the cytotoxicity triggered by TNFR1 when both receptors are co-expressed and activated[91,98,99]. This finding could be seen as a distinct situation of the classic phenomenon in which the balance between apoptotic and anti-apoptotic signals triggered by TNF-α determines the accuracy in cell signaling[91].
The TNFR2 pathway does not contain death domains and its stimulation can result in proliferation, migration and production of cytokines such as IL-1 and IL-6. This receptor not only activate an intracellular signaling pathway, but can also induce reverse signaling within the cell expressing TNF-α[100].
TNFR2 is also involved in several autoimmune diseases, playing a protective role or being involved in its development. Thus, TNFR2 is known to be involved in rheumatoid arthritis, CD, erythematosus systemic lupus, UC, scleroderma, among other diseases[10]. It was recognized that the expression of TNFR2 is more limited than that in TNFR1, i.e., this finding suggests that the sTNF-mediating signaling pathway via TNFR1 drives a predominantly pro-inflammatory program, whereas mTNF binding to TNFR2 primarily initiates immune modulation and tissue regeneration[84]. Thus, an increase in TNF-α expression can cause mucosal barrier defects in patients with IBD, exacerbating inflammation[10]. However, only a part of the role of TNF-α receptors in the pathogenesis of IBD is understood. It is known that it can be indicated the presence of TNFR2 in the CNS, i.e., in neurons of the cerebral cortex[83], and no data in the literature could identify the presence of TNFR2 in enteric neurons.
The immune response plays an important role in the initiation and maintenance of UC[101]. Cytokines play key roles in inflammatory processes, such as targeting cell signaling molecules during inflammation and UC pathogenesis through different roles, such as the production of inflammatory mediators and activation of inflammatory pathways[5,57,102,103]. They are also involved in several biological processes, such as cell activation, differentiation and central factors in the process of developing the inflammatory and immune response. In addition, studies provide evidence of their involvement in epithelial injury, and consequently, intestinal barrier injury and tissue damage[5,104,105].
The treatment approach in the control of inflamed GI tissue in IBD, including clinical, laboratory conditions, endoscopic and histological remission for a prolonged period, play an important role in its evolution and possibly modifying the natural course of the disease[9,106]. Recent studies have suggested that TNF-α is one of the main pro-inflammatory cytokines involved in the pathogenesis of IBD, as higher TNF-α levels are present in the serum of both patients with UC and CD[25].
For the treatment of IBD, different classes of drugs can be used, respecting the particularities of CD and UC, with the aim of alleviating the symptomatic crisis of patients, as well as inducing and maintaining remission of the disease[107,108]. Generally, the first treatment option for mild to moderate cases of UC and CD involves aminosalicylates, or 5-ASAs, and other treatments include the use of corticosteroids, immunosuppressants, and biological drugs[109,110].
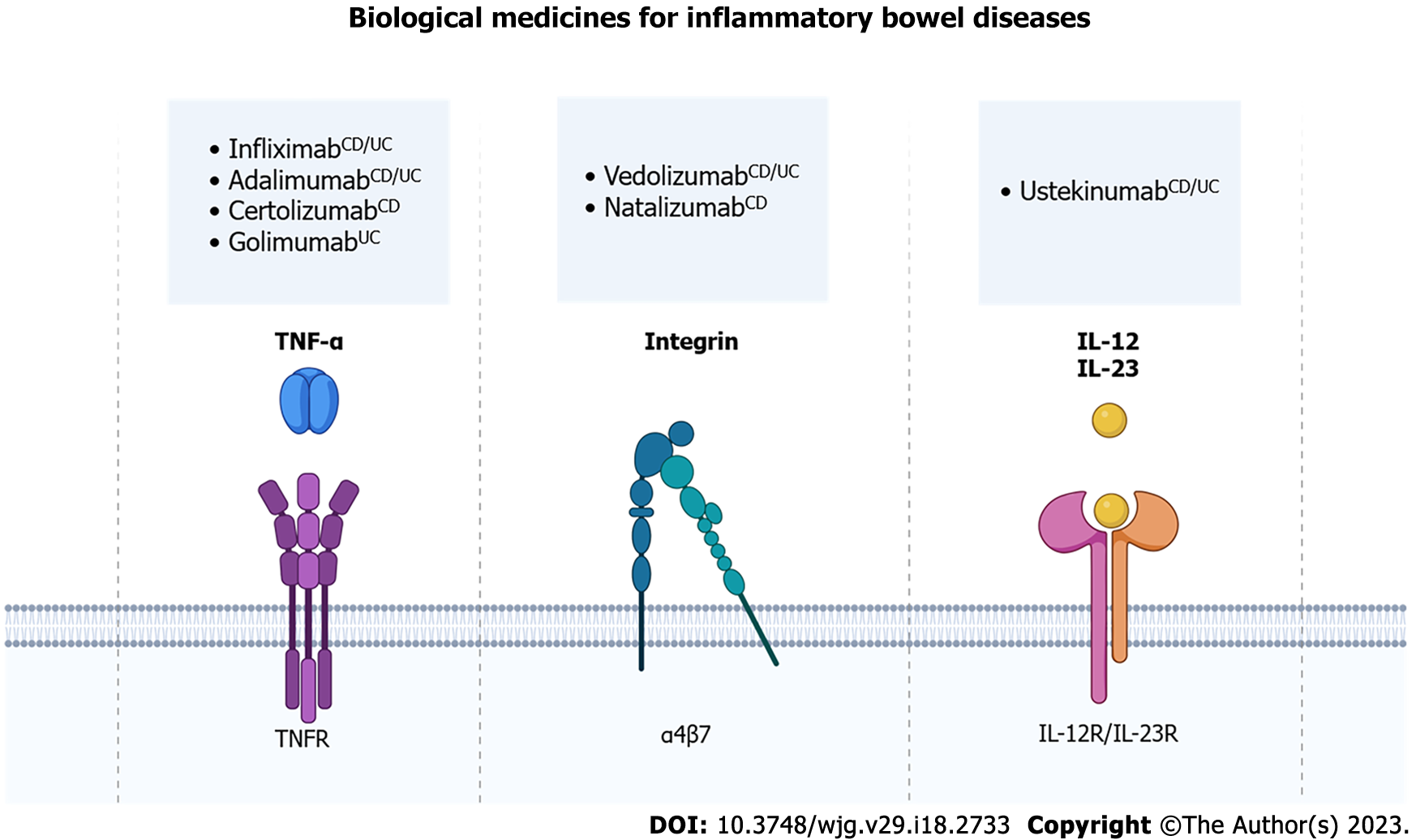
Biological drugs used to treat IBD are monoclonal antibodies that neutralize/block key targets in the development of intestinal[27]. There are biological drugs that target, for example, integrins, interleukins, and cytokines, such as TNF-α, which are related to the development of the inflammatory process and the activation of immune system cells (Figure 2)[111,112].
Currently, four anti-TNF-α agents are indicated by various guidelines for the treatment of IBD: Infliximab; adalimumab; certolizumab pegol; and golimumab[28,29]. Infliximab and adalimumab are indicated for the treatment of both UC and CD, while golimumab is indicated only for the treatment of UC and certolizumab pegol only for CD. American Gastroenterological Association (AGA) and American College of Gastroenterology (ACG) guidelines recommend the use of infliximab, ada-limumab, and certolizumab pegol to induce and maintain CD remission in moderate to severe patients unresponsive to corticosteroid or methotrexate treatment, and regarding UC, it could be noted that AGA and ACG recommend the use of infliximab, adalimumab and golimumab in patients who have not responded to conventional therapy[28,29]. These anti-TNF-α antibodies bind to TNF-α, blocking its harmful effects, such as NF-ĸB activation and increase of pro-inflammatory cytokines, that mediate intestinal inflammation[113,114].
Intravenous and subcutaneous biologics targeting TNF-α have revolutionized the treatment of IBD, becoming the best available agents, when conventional therapy does not work, to induce and maintain IBD remission[115-117]. The application of this type of antibodies in patients with IBD induces a satisfactory clinical response in up to 60% of patients, and induced long-term maintenance of disease remission in most patients[24,30] and also reduces colorectal cancer risks[118]. Despite this, some patients do not improve after the use of these antibodies, some relapses within the first year of treatment and, one alternative used for the management of these cases has been to change the anti-TNF-α used for another one, or even to increase the dosage used[119]. Other alternative option for anti-TNF-α non responsiveness is the use other biological medicines like anti-integrin or anti-interleukin therapies.
Anti-integrin therapy, such as vedolizumab, which can be used for UC and CD treatment, block the integrin α4β7, which is expressed in B and T lymphocytes and interact with mucosal addressin-cell adhesion molecule-1 on intestinal vessels[120-122]. So, vedolizumab blocks lymphocyte trafficking only in the gut, preventing a systemic immunosuppression[123]. Otherwise, natalizumab blocks the integrin α4β7 and α1β7 and blocks lymphocyte trafficking in other organs, for example the brain, which can lead to infections such as progressive multifocal leukoencephalopathy[124]. For this reason, the use of natalizumab is very carefully, considering risk-benefits factors, and it is not available in some countries. Other biological medicine is ustekinumab, an anti-interleukin agent for subunit p40 of IL-12 and IL-23, used for CD and UC. This biological medicine attenuates the immune cell activation by these interleukins and consequently reduces inflammatory response and pro-inflammatory signaling[125-127].
Limitations of therapy with biological drugs include the high cost of adherence to therapy, differences in legislation and availability of these drugs in different countries, and the meticulous risk-benefit analysis related to the choice over treatment alternatives. However, new strategies and medicines have constantly been studied at pre-clinical and clinical instances for treatment of IBD.
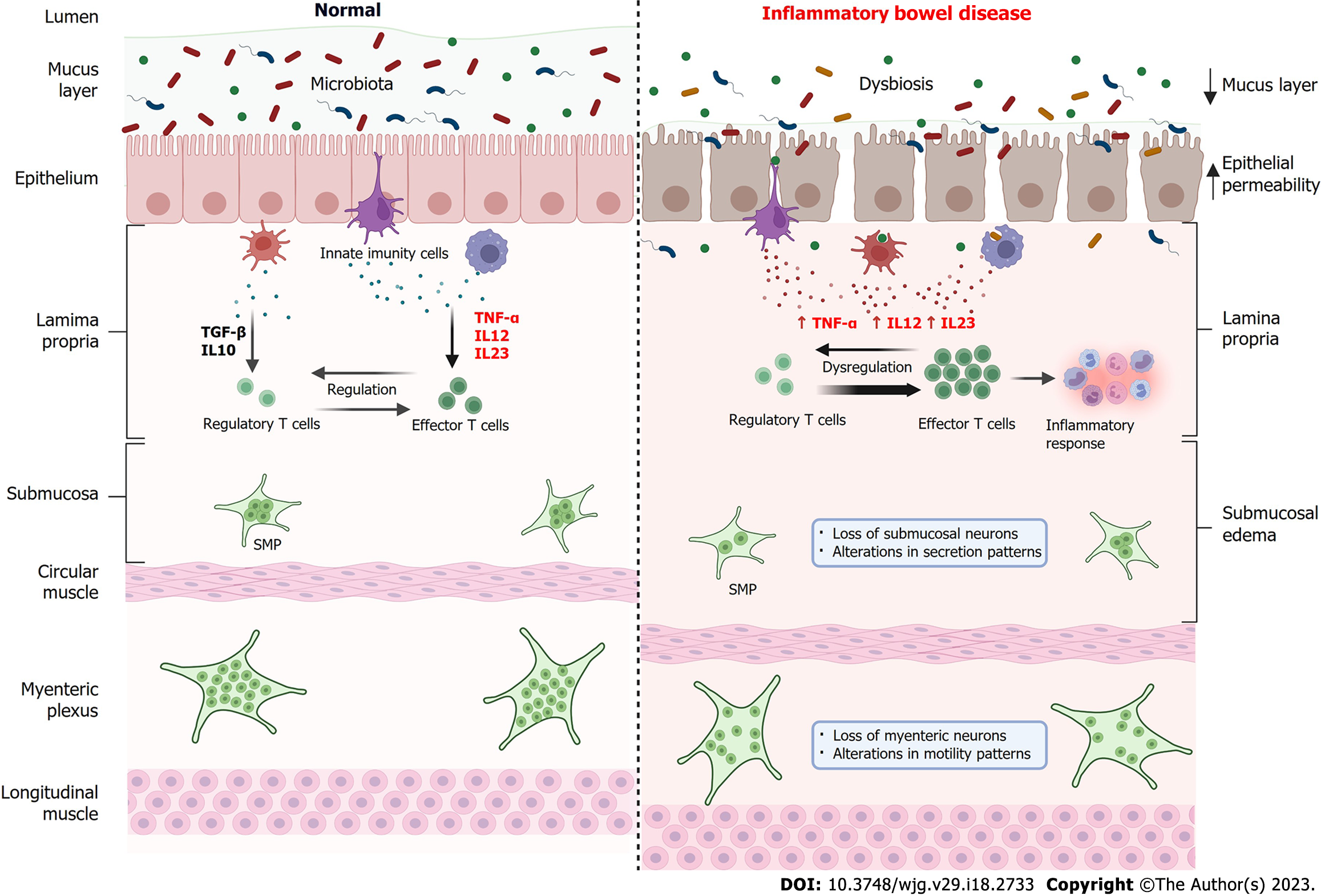
Although the involvement of TNF-α in the ENS is poorly described in the literature, it is reported that the ENS has TNF-α receptors and responds to the inflammatory stimulus, can lead to changes in motility patterns and fluid and electrolyte balance, a condition often found in patients with IBD[128]. When comparing the intestine, under normal conditions, and the intestine in IBD, some aspects must be considered (Figure 3). Under physiological conditions, large populations of microorganisms (bacteria, virus and fungi) inhabit the gut, which constitute the gut microbiota[129]. This microbiota establishes a symbiosis with the host[130]. The intestinal barrier, composed mainly by mucus layer and epithelial, is an intact and functional structure[131]. There is a balance between the levels of pro-inflammatory cytokines TNF-α, IL-12 and IL-23, and anti-inflammatory cytokines such as transforming growth factor-beta and IL-10 by innate immune cells, which leads to a balance between regulatory and effector T cells, inducing tolerance to microorganisms from the gut microbiota[130,132]. In physiological conditions, both submucosal plexus and myenteric plexus are functional and controls, respectively, fluid secretion and intestinal motility.
In IBD, there is an imbalance in the gut microbiota (dysbiosis), the intestinal barrier is compromised, with a reduction in the mucus layer, weakening of the intercellular junctions and consequently increased epithelial permeability and entry of microorganisms in the lamina propria[15,133]. Innate immune cells increase the secretion of pro-inflammatory cytokines such as TNF-α, IL-12 and IL-23, which leads to a dysregulation of the immune system, which increases the activity of effector T cells which, in turn, recruit cells for the inflammatory response[134]. Morphological findings include submucosal edema, as well as a reduction in the number of neurons in the SMP, causing changes in secretion patterns and loss of neurons in the myenteric plexus, thus changing the motility patterns[51,52,135].
Experimental UC has been shown to affect enteric neurons, causing changes in the number of enteric neurons and glia, as well as changes in intestinal motility and secretion patterns[50-52,136]. As the presence of TNFR1 and TNFR2 have been reported in the ENS, the use of anti-TNF-α agents can exert a series of beneficial effects on ENS levels, improving the inflammatory response, peristalsis and intestinal secretion patterns, relieving symptoms such as diarrhea, fecal bleeding and colic. However, despite the important approach to the ENS and its relationship with IBD, literature data on anti-TNF treatment with analysis focused on the ENS are scarce. Therefore, further studies on the role of mechanisms/signaling pathways of sTNF, mTNF, TNF-α and their receptors in enteric neurons in IBD are needed, since this approach can guide the choice of a more adequate, effective anti-TNF-α agent with lower chances of failure responsiveness.
This review provided main details about TNF-α relationship with IBD. The role of TNF receptors in the development of IBD is an issue that deserves attention and may be a key to the treatment of UC and CD. In addition, anti- TNF-α treatments have been very promising in the treatment of IBD unresponsive to conventional therapies. The relationship of the ENS with TNF-α and its response to anti- TNF-α treatment are important aspects to be addressed, as they may direct new therapies and reduce non-responsiveness to specific anti- TNF-α agents.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anatomy and morphology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xu B, China; Yoo JH, South Korea S-Editor: Chang KL L-Editor: A P-Editor: Chen YX
| 1. | Loddo I, Romano C. Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis. Front Immunol. 2015;6:551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 303] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 2. | Shapiro JM, Subedi S, LeLeiko NS. Inflammatory Bowel Disease. Pediatr Rev. 2016;37:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Shivashankar R, Lichtenstein GR. Mimics of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2018;24:2315-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Liu Y, Zhao J, Zhao Y, Zong S, Tian Y, Chen S, Li M, Liu H, Zhang Q, Jing X, Sun B, Wang H, Sun T, Yang C. Therapeutic effects of lentinan on inflammatory bowel disease and colitis-associated cancer. J Cell Mol Med. 2019;23:750-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | Ahmad T, Satsangi J, McGovern D, Bunce M, Jewell DP. Review article: The genetics of inflammatory bowel disease. Aliment Pharmacol Ther. 2001;15:731-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3351] [Article Influence: 186.2] [Reference Citation Analysis (11)] |
| 7. | Fiocchi C. Intestinal inflammation: A complex interplay of immune and nonimmune cell interactions. Am J Physiol. 1997;273:G769-G775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Ke P, Shao BZ, Xu ZQ, Chen XW, Liu C. Intestinal Autophagy and Its Pharmacological Control in Inflammatory Bowel Disease. Front Immunol. 2016;7:695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Argollo M, Fiorino G, Hindryckx P, Peyrin-Biroulet L, Danese S. Novel therapeutic targets for inflammatory bowel disease. J Autoimmun. 2017;85:103-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Tatiya-Aphiradee N, Chatuphonprasert W, Jarukamjorn K. Immune response and inflammatory pathway of ulcerative colitis. J Basic Clin Physiol Pharmacol. 2018;30:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 237] [Article Influence: 33.9] [Reference Citation Analysis (1)] |
| 11. | Kozik AJ, Nakatsu CH, Chun H, Jones-Hall YL. Comparison of the fecal, cecal, and mucus microbiome in male and female mice after TNBS-induced colitis. PLoS One. 2019;14:e0225079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Neurath MF. Host-microbiota interactions in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:76-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 13. | Tavakoli P, Vollmer-Conna U, Hadzi-Pavlovic D, Grimm MC. A Review of Inflammatory Bowel Disease: A Model of Microbial, Immune and Neuropsychological Integration. Public Health Rev. 2021;42:1603990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 14. | Vicentini FA, Keenan CM, Wallace LE, Woods C, Cavin JB, Flockton AR, Macklin WB, Belkind-Gerson J, Hirota SA, Sharkey KA. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome. 2021;9:210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 15. | Caetano MAF, Castelucci P. Role of short chain fatty acids in gut health and possible therapeutic approaches in inflammatory bowel diseases. World J Clin Cases. 2022;10:9985-10003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (5)] |
| 17. | Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 1066] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 18. | Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv Exp Med Biol. 2014;817:39-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 532] [Article Influence: 48.4] [Reference Citation Analysis (1)] |
| 19. | Boyer L, Ghoreishi M, Templeman V, Vallance BA, Buchan AM, Jevon G, Jacobson K. Myenteric plexus injury and apoptosis in experimental colitis. Auton Neurosci. 2005;117:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Linden DR, Couvrette JM, Ciolino A, McQuoid C, Blaszyk H, Sharkey KA, Mawe GM. Indiscriminate loss of myenteric neurones in the TNBS-inflamed guinea-pig distal colon. Neurogastroenterol Motil. 2005;17:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Lomax AE, Fernández E, Sharkey KA. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol Motil. 2005;17:4-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Cirillo C, Sarnelli G, Turco F, Mango A, Grosso M, Aprea G, Masone S, Cuomo R. Proinflammatory stimuli activates human-derived enteroglial cells and induces autocrine nitric oxide production. Neurogastroenterol Motil. 2011;23:e372-e382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Linden DR. Colitis is associated with a loss of intestinofugal neurons. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1096-G1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Billmeier U, Dieterich W, Neurath MF, Atreya R. Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J Gastroenterol. 2016;22:9300-9313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 140] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (6)] |
| 25. | Holtmann MH, Schütz M, Galle PR, Neurath MF. Functional relevance of soluble TNF-alpha, transmembrane TNF-alpha and TNF-signal transduction in gastrointestinal diseases with special reference to inflammatory bowel diseases. Z Gastroenterol. 2002;40:587-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 26. | So T, Ishii N. The TNF-TNFR Family of Co-signal Molecules. Adv Exp Med Biol. 2019;1189:53-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (1)] |
| 27. | de Mattos BR, Garcia MP, Nogueira JB, Paiatto LN, Albuquerque CG, Souza CL, Fernandes LG, Tamashiro WM, Simioni PU. Inflammatory Bowel Disease: An Overview of Immune Mechanisms and Biological Treatments. Mediators Inflamm. 2015;2015:493012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 28. | Feuerstein JD, Isaacs KL, Schneider Y, Siddique SM, Falck-Ytter Y, Singh S; AGA Institute Clinical Guidelines Committee. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology. 2020;158:1450-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 471] [Article Influence: 94.2] [Reference Citation Analysis (3)] |
| 29. | Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am J Gastroenterol. 2018;113:481-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 930] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 30. | Panaccione R, Colombel JF, Louis E, Peyrin-Biroulet L, Sandborn WJ. Evolving definitions of remission in Crohn's disease. Inflamm Bowel Dis. 2013;19:1645-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Kabouridis PS, Lasrado R, McCallum S, Chng SH, Snippert HJ, Clevers H, Pettersson S, Pachnis V. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron. 2015;85:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 262] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 32. | Bassotti G, Villanacci V, Fisogni S, Rossi E, Baronio P, Clerici C, Maurer CA, Cathomas G, Antonelli E. Enteric glial cells and their role in gastrointestinal motor abnormalities: Introducing the neuro-gliopathies. World J Gastroenterol. 2007;13:4035-4041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Clarke LE, Barres BA. Glia keep synapse distribution under wraps. Cell. 2013;154:267-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Jensen CJ, Massie A, De Keyser J. Immune players in the CNS: The astrocyte. J Neuroimmune Pharmacol. 2013;8:824-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 35. | Rühl A, Nasser Y, Sharkey KA. Enteric glia. Neurogastroenterol Motil. 2004;16 Suppl 1:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Hagström C, Olsson C. Glial cells revealed by GFAP immunoreactivity in fish gut. Cell Tissue Res. 2010;341:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Gonzalez-Martinez T, Perez-Piñera P, Díaz-Esnal B, Vega JA. S-100 proteins in the human peripheral nervous system. Microsc Res Tech. 2003;60:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Hsieh HL, Schäfer BW, Weigle B, Heizmann CW. S100 protein translocation in response to extracellular S100 is mediated by receptor for advanced glycation endproducts in human endothelial cells. Biochem Biophys Res Commun. 2004;316:949-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Pochard C, Coquenlorge S, Freyssinet M, Naveilhan P, Bourreille A, Neunlist M, Rolli-Derkinderen M. The multiple faces of inflammatory enteric glial cells: Is Crohn's disease a gliopathy? Am J Physiol Gastrointest Liver Physiol. 2018;315:G1-G11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 40. | Misawa R, Girotti PA, Mizuno MS, Liberti EA, Furness JB, Castelucci P. Effects of protein deprivation and re-feeding on P2X2 receptors in enteric neurons. World J Gastroenterol. 2010;16:3651-3663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Girotti PA, Misawa R, Palombit K, Mendes CE, Bittencourt JC, Castelucci P. Differential effects of undernourishment on the differentiation and maturation of rat enteric neurons. Cell Tissue Res. 2013;353:367-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Mizuno MS, Crisma AR, Borelli P, Castelucci P. Expression of the P2X₂ receptor in different classes of ileum myenteric neurons in the female obese ob/ob mouse. World J Gastroenterol. 2012;18:4693-4703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Mizuno MS, Crisma AR, Borelli P, Schäfer BT, Silveira MP, Castelucci P. Distribution of the P2X2 receptor and chemical coding in ileal enteric neurons of obese male mice (ob/ob). World J Gastroenterol. 2014;20:13911-13919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Palombit K, Mendes CE, Tavares-de-Lima W, Silveira MP, Castelucci P. Effects of ischemia and reperfusion on subpopulations of rat enteric neurons expressing the P2X7 receptor. Dig Dis Sci. 2013;58:3429-3439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Mendes CE, Palombit K, Tavares-de-Lima W, Castelucci P. Enteric glial cells immunoreactive for P2X7 receptor are affected in the ileum following ischemia and reperfusion. Acta Histochem. 2019;121:665-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Mendes CE, Palombit K, Vieira C, Silva I, Correia-de-Sá P, Castelucci P. The Effect of Ischemia and Reperfusion on Enteric Glial Cells and Contractile Activity in the Ileum. Dig Dis Sci. 2015;60:2677-2689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Palombit K, Mendes CE, Tavares-de-Lima W, Barreto-Chaves ML, Castelucci P. Blockage of the P2X7 Receptor Attenuates Harmful Changes Produced by Ischemia and Reperfusion in the Myenteric Plexus. Dig Dis Sci. 2019;64:1815-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2012;9:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 286] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 49. | Stavely R, Abalo R, Nurgali K. Targeting Enteric Neurons and Plexitis for the Management of Inflammatory Bowel Disease. Curr Drug Targets. 2020;21:1428-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 50. | da Silva MV, Marosti AR, Mendes CE, Palombit K, Castelucci P. Differential effects of experimental ulcerative colitis on P2X7 receptor expression in enteric neurons. Histochem Cell Biol. 2015;143:171-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Magalhães HIR, Castelucci P. Enteric nervous system and inflammatory bowel diseases: Correlated impacts and therapeutic approaches through the P2X7 receptor. World J Gastroenterol. 2021;27:7909-7924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (2)] |
| 52. | Evangelinellis MM, Souza RF, Mendes CE, Castelucci P. Effects of a P2X7 receptor antagonist on myenteric neurons in the distal colon of an experimental rat model of ulcerative colitis. Histochem Cell Biol. 2022;157:65-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Begue B, Wajant H, Bambou JC, Dubuquoy L, Siegmund D, Beaulieu JF, Canioni D, Berrebi D, Brousse N, Desreumaux P, Schmitz J, Lentze MJ, Goulet O, Cerf-Bensussan N, Ruemmele FM. Implication of TNF-related apoptosis-inducing ligand in inflammatory intestinal epithelial lesions. Gastroenterology. 2006;130:1962-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Ueno A, Ghosh A, Hung D, Li J, Jijon H. Th17 plasticity and its changes associated with inflammatory bowel disease. World J Gastroenterol. 2015;21:12283-12295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 55. | Deepak P, Fowler KJ, Fletcher JG, Bruining DH. Novel Imaging Approaches in Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2019;25:248-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Kaplan GG, Ng SC. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology. 2017;152:313-321.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 811] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 58. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4097] [Article Influence: 512.1] [Reference Citation Analysis (110)] |
| 59. | Quaresma AB, Kaplan GG, Kotze PG. The globalization of inflammatory bowel disease: The incidence and prevalence of inflammatory bowel disease in Brazil. Curr Opin Gastroenterol. 2019;35:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 60. | Kotze PG, Underwood FE, Damião AOMC, Ferraz JGP, Saad-Hossne R, Toro M, Iade B, Bosques-Padilla F, Teixeira FV, Juliao-Banos F, Simian D, Ghosh S, Panaccione R, Ng SC, Kaplan GG. Progression of Inflammatory Bowel Diseases Throughout Latin America and the Caribbean: A Systematic Review. Clin Gastroenterol Hepatol. 2020;18:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 61. | Eng LF, Yu AC, Lee YL. Astrocytic response to injury. Prog Brain Res. 1992;94:353-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 126] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | O'Callaghan JP. Quantitative features of reactive gliosis following toxicant-induced damage of the CNS. Ann N Y Acad Sci. 1993;679:195-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 63. | Giulian D, Li J, Leara B, Keenen C. Phagocytic microglia release cytokines and cytotoxins that regulate the survival of astrocytes and neurons in culture. Neurochem Int. 1994;25:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 137] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 64. | Spear ET, Mawe GM. Enteric neuroplasticity and dysmotility in inflammatory disease: key players and possible therapeutic targets. Am J Physiol Gastrointest Liver Physiol. 2019;317:G853-G861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Kawada M, Arihiro A, Mizoguchi E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J Gastroenterol. 2007;13:5581-5593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 128] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 66. | Brenna Ø, Furnes MW, Drozdov I, van Beelen Granlund A, Flatberg A, Sandvik AK, Zwiggelaar RT, Mårvik R, Nordrum IS, Kidd M, Gustafsson BI. Relevance of TNBS-colitis in rats: a methodological study with endoscopic, histologic and Transcriptomic [corrected] characterization and correlation to IBD. PLoS One. 2013;8:e54543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 67. | Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:15.25.1-15.25.14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 1321] [Article Influence: 120.1] [Reference Citation Analysis (1)] |
| 68. | Bramhall M, Flórez-Vargas O, Stevens R, Brass A, Cruickshank S. Quality of methods reporting in animal models of colitis. Inflamm Bowel Dis. 2015;21:1248-1259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 69. | Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1255] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 70. | Wan P, Liu X, Xiong Y, Ren Y, Chen J, Lu N, Guo Y, Bai A. Extracellular ATP mediates inflammatory responses in colitis via P2 × 7 receptor signaling. Sci Rep. 2016;6:19108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 71. | Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD, Pollack PF. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1598] [Cited by in RCA: 1620] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 72. | Sandborn WJ, Colombel JF, D'Haens G, Van Assche G, Wolf D, Kron M, Lazar A, Robinson AM, Yang M, Chao JD, Thakkar R. One-year maintenance outcomes among patients with moderately-to-severely active ulcerative colitis who responded to induction therapy with adalimumab: subgroup analyses from ULTRA 2. Aliment Pharmacol Ther. 2013;37:204-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 73. | Eser A, Colombel JF, Rutgeerts P, Vermeire S, Vogelsang H, Braddock M, Persson T, Reinisch W. Safety and Efficacy of an Oral Inhibitor of the Purinergic Receptor P2X7 in Adult Patients with Moderately to Severely Active Crohn's Disease: A Randomized Placebo-controlled, Double-blind, Phase IIa Study. Inflamm Bowel Dis. 2015;21:2247-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 74. | Liu Y, Liu X. Research progress of P2X7 receptor in inflammatory bowel disease. Scand J Gastroenterol. 2019;54:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Margolis KG, Stevanovic K, Karamooz N, Li ZS, Ahuja A, D'Autréaux F, Saurman V, Chalazonitis A, Gershon MD. Enteric neuronal density contributes to the severity of intestinal inflammation. Gastroenterology. 2011;141:588-598, 598.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 76. | Coquenlorge S, Duchalais E, Chevalier J, Cossais F, Rolli-Derkinderen M, Neunlist M. Modulation of lipopolysaccharide-induced neuronal response by activation of the enteric nervous system. J Neuroinflammation. 2014;11:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 77. | Geboes K, Collins S. Structural abnormalities of the nervous system in Crohn's disease and ulcerative colitis. Neurogastroenterol Motil. 1998;10:189-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 171] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 78. | Cipriani G, Gibbons SJ, Kashyap PC, Farrugia G. Intrinsic Gastrointestinal Macrophages: Their Phenotype and Role in Gastrointestinal Motility. Cell Mol Gastroenterol Hepatol. 2016;2:120-130.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 79. | Clark IA. How TNF was recognized as a key mechanism of disease. Cytokine Growth Factor Rev. 2007;18:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 193] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 80. | Osawa Y, Banno Y, Nagaki M, Brenner DA, Naiki T, Nozawa Y, Nakashima S, Moriwaki H. TNF-alpha-induced sphingosine 1-phosphate inhibits apoptosis through a phosphatidylinositol 3-kinase/Akt pathway in human hepatocytes. J Immunol. 2001;167:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 137] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 81. | Olmos G, Lladó J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm. 2014;2014:861231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 496] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 82. | Baune BT, Wiede F, Braun A, Golledge J, Arolt V, Koerner H. Cognitive dysfunction in mice deficient for TNF- and its receptors. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1056-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 83. | Richter F, Lütz W, Eitner A, Leuchtweis J, Lehmenkühler A, Schaible HG. Tumor necrosis factor reduces the amplitude of rat cortical spreading depression in vivo. Ann Neurol. 2014;76:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Yang S, Wang J, Brand DD, Zheng SG. Role of TNF-TNF Receptor 2 Signal in Regulatory T Cells and Its Therapeutic Implications. Front Immunol. 2018;9:784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 271] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 85. | Slevin SM, Egan LJ. New Insights into the Mechanisms of Action of Anti-Tumor Necrosis Factor-α Monoclonal Antibodies in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:2909-2920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 86. | Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1145] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 87. | Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1401] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 88. | Wajant H, Scheurich P. TNFR1-induced activation of the classical NF-κB pathway. FEBS J. 2011;278:862-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 219] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 89. | Gan Y, Long J, Zeng Y, Zhang Y, Tao Y. lncRNA IL-17RA-1 Attenuates LPS-Induced Sepsis via miR-7847-3p/PRKCG-Mediated MAPK Signaling Pathway. Mediators Inflamm. 2022;2022:9923204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 90. | Jurewicz A, Matysiak M, Tybor K, Selmaj K. TNF-induced death of adult human oligodendrocytes is mediated by c-jun NH2-terminal kinase-3. Brain. 2003;126:1358-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 91. | Cabal-Hierro L, Lazo PS. Signal transduction by tumor necrosis factor receptors. Cell Signal. 2012;24:1297-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 330] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 92. | MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal. 2002;14:477-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 453] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 93. | Wajant H, Henkler F, Scheurich P. The TNF-receptor-associated factor family: scaffold molecules for cytokine receptors, kinases and their regulators. Cell Signal. 2001;13:389-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 291] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 94. | Naudé PJ, den Boer JA, Luiten PG, Eisel UL. Tumor necrosis factor receptor cross-talk. FEBS J. 2011;278:888-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 95. | Zhao Y, Conze DB, Hanover JA, Ashwell JD. Tumor necrosis factor receptor 2 signaling induces selective c-IAP1-dependent ASK1 ubiquitination and terminates mitogen-activated protein kinase signaling. J Biol Chem. 2007;282:7777-7782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 96. | Walczak H. TNF and ubiquitin at the crossroads of gene activation, cell death, inflammation, and cancer. Immunol Rev. 2011;244:9-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 97. | Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A. 1998;95:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 346] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 98. | Weiss T, Grell M, Hessabi B, Bourteele S, Müller G, Scheurich P, Wajant H. Enhancement of TNF receptor p60-mediated cytotoxicity by TNF receptor p80: requirement of the TNF receptor-associated factor-2 binding site. J Immunol. 1997;158:2398-2404. [PubMed] |
| 99. | Fotin-Mleczek M, Henkler F, Samel D, Reichwein M, Hausser A, Parmryd I, Scheurich P, Schmid JA, Wajant H. Apoptotic crosstalk of TNF receptors: TNF-R2-induces depletion of TRAF2 and IAP proteins and accelerates TNF-R1-dependent activation of caspase-8. J Cell Sci. 2002;115:2757-2770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 199] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 100. | Eissner G, Kolch W, Scheurich P. Ligands working as receptors: reverse signaling by members of the TNF superfamily enhance the plasticity of the immune system. Cytokine Growth Factor Rev. 2004;15:353-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 216] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 101. | Kałużna A, Olczyk P, Komosińska-Vassev K. The Role of Innate and Adaptive Immune Cells in the Pathogenesis and Development of the Inflammatory Response in Ulcerative Colitis. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 106] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 102. | Shouval DS, Rufo PA. The Role of Environmental Factors in the Pathogenesis of Inflammatory Bowel Diseases: A Review. JAMA Pediatr. 2017;171:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 103. | Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S, Arumugam M, Kultima JR, Prifti E, Nielsen T, Juncker AS, Manichanh C, Chen B, Zhang W, Levenez F, Wang J, Xu X, Xiao L, Liang S, Zhang D, Zhang Z, Chen W, Zhao H, Al-Aama JY, Edris S, Yang H, Hansen T, Nielsen HB, Brunak S, Kristiansen K, Guarner F, Pedersen O, Doré J, Ehrlich SD; MetaHIT Consortium, Bork P, Wang J; MetaHIT Consortium. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol. 2014;32:834-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1198] [Cited by in RCA: 1405] [Article Influence: 127.7] [Reference Citation Analysis (0)] |
| 104. | Pawłowska-Kamieniak A, Krawiec P, Pac-Kożuchowska E. Interleukin 6: Biological significance and role in inflammatory bowel diseases. Adv Clin Exp Med. 2021;30:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 105. | Moldoveanu AC, Diculescu M, Braticevici CF. Cytokines in inflammatory bowel disease. Rom J Intern Med. 2015;53:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 106. | Nakase H, Sato N, Mizuno N, Ikawa Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun Rev. 2022;21:103017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 224] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 107. | Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014;7:113-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 337] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 108. | Song K, Wu D. Shared decision-making in the management of patients with inflammatory bowel disease. World J Gastroenterol. 2022;28:3092-3100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (7)] |
| 109. | Yamamoto-Furusho JK. [Treatment of inflammatory bowel disease]. Rev Gastroenterol Mex. 2012;77 Suppl 1:39-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 110. | Rolak S, Kane SV. Conventional Therapies for Crohn's Disease. Gastroenterol Clin North Am. 2022;51:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 111. | Moss AC. Optimizing the use of biological therapy in patients with inflammatory bowel disease. Gastroenterol Rep (Oxf). 2015;3:63-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 112. | Cheifetz AS, Abreu MT, Afif W, Cross RK, Dubinsky MC, Loftus EV Jr, Osterman MT, Saroufim A, Siegel CA, Yarur AJ, Melmed GY, Papamichael K. A Comprehensive Literature Review and Expert Consensus Statement on Therapeutic Drug Monitoring of Biologics in Inflammatory Bowel Disease. Am J Gastroenterol. 2021;116:2014-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 113. | D'Haens GR, van Deventer S. 25 years of anti-TNF treatment for inflammatory bowel disease: lessons from the past and a look to the future. Gut. 2021;70:1396-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 114. | Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-33; quiz 591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1194] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 115. | Griffiths OR, Landon J, Coxon RE, Morris K, James P, Adams R. Inflammatory bowel disease and targeted oral anti-TNFα therapy. Adv Protein Chem Struct Biol. 2020;119:157-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 116. | Zhu M, Ran Z. Clinical characteristics of ulcerative colitis in elderly patients. JGH Open. 2021;5:849-854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 117. | Little RD, Ward MG, Wright E, Jois AJ, Boussioutas A, Hold GL, Gibson PR, Sparrow MP. Therapeutic Drug Monitoring of Subcutaneous Infliximab in Inflammatory Bowel Disease-Understanding Pharmacokinetics and Exposure Response Relationships in a New Era of Subcutaneous Biologics. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 118. | Alkhayyat M, Abureesh M, Gill A, Khoudari G, Abou Saleh M, Mansoor E, Regueiro M. Lower Rates of Colorectal Cancer in Patients With Inflammatory Bowel Disease Using Anti-TNF Therapy. Inflamm Bowel Dis. 2021;27:1052-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 119. | Papamichael K, Gils A, Rutgeerts P, Levesque BG, Vermeire S, Sandborn WJ, Vande Casteele N. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis. 2015;21:182-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 120. | von Andrian UH, Engelhardt B. Alpha4 integrins as therapeutic targets in autoimmune disease. N Engl J Med. 2003;348:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 203] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 121. | Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, Tidswell M. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol. 1994;153:517-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 122. | Arihiro S, Ohtani H, Suzuki M, Murata M, Ejima C, Oki M, Kinouchi Y, Fukushima K, Sasaki I, Nakamura S, Matsumoto T, Torii A, Toda G, Nagura H. Differential expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in ulcerative colitis and Crohn's disease. Pathol Int. 2002;52:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 123. | Fedyk ER, Wyant T, Yang LL, Csizmadia V, Burke K, Yang H, Kadambi VJ. Exclusive antagonism of the α4 β7 integrin by vedolizumab confirms the gut-selectivity of this pathway in primates. Inflamm Bowel Dis. 2012;18:2107-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 124. | Ransohoff RM. Natalizumab and PML. Nat Neurosci. 2005;8:1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 125. | Guillo L, D'Amico F, Danese S, Peyrin-Biroulet L. Ustekinumab for Extra-intestinal Manifestations of Inflammatory Bowel Disease: A Systematic Literature Review. J Crohns Colitis. 2021;15:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 126. | Honap S, Meade S, Ibraheim H, Irving PM, Jones MP, Samaan MA. Effectiveness and Safety of Ustekinumab in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2022;67:1018-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 127. | Sands BE, Sandborn WJ, Panaccione R, O'Brien CD, Zhang H, Johanns J, Adedokun OJ, Li K, Peyrin-Biroulet L, Van Assche G, Danese S, Targan S, Abreu MT, Hisamatsu T, Szapary P, Marano C; UNIFI Study Group. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2019;381:1201-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 827] [Article Influence: 137.8] [Reference Citation Analysis (1)] |
| 128. | Chandrasekharan B, Jeppsson S, Pienkowski S, Belsham DD, Sitaraman SV, Merlin D, Kokkotou E, Nusrat A, Tansey MG, Srinivasan S. Tumor necrosis factor-neuropeptide Y cross talk regulates inflammation, epithelial barrier functions, and colonic motility. Inflamm Bowel Dis. 2013;19:2535-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 129. | Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes S, Mancabelli L, Lugli GA, Rodriguez JM, Bode L, de Vos W, Gueimonde M, Margolles A, van Sinderen D, Ventura M. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol Mol Biol Rev. 2017;81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1143] [Article Influence: 142.9] [Reference Citation Analysis (0)] |
| 130. | Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1428] [Cited by in RCA: 2246] [Article Influence: 280.8] [Reference Citation Analysis (1)] |
| 131. | Capaldo CT, Powell DN, Kalman D. Layered defense: how mucus and tight junctions seal the intestinal barrier. J Mol Med (Berl). 2017;95:927-934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 255] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 132. | Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers. 2017;5:e1373208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 626] [Article Influence: 78.3] [Reference Citation Analysis (1)] |
| 133. | Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018;50:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 1151] [Article Influence: 164.4] [Reference Citation Analysis (1)] |
| 134. | Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 1973] [Article Influence: 179.4] [Reference Citation Analysis (1)] |
| 135. | Bassotti G, Antonelli E, Villanacci V, Nascimbeni R, Dore MP, Pes GM, Maconi G. Abnormal gut motility in inflammatory bowel disease: an update. Tech Coloproctol. 2020;24:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 136. | da Silva MV, Marosti AR, Mendes CE, Palombit K, Castelucci P. Submucosal neurons and enteric glial cells expressing the P2X7 receptor in rat experimental colitis. Acta Histochem. 2017;119:481-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |