Published online May 14, 2023. doi: 10.3748/wjg.v29.i18.2704
Peer-review started: December 15, 2022
First decision: March 15, 2023
Revised: March 28, 2023
Accepted: April 17, 2023
Article in press: April 17, 2023
Published online: May 14, 2023
Processing time: 146 Days and 10.5 Hours
Diabetes, as a metabolic disorder, is accompanied with several gastrointestinal (GI) symptoms, like abdominal pain, gastroparesis, diarrhoea or constipation. Serious and complex enteric nervous system damage is confirmed in the background of these diabetic motility complaints. The anatomical length of the GI tract, as well as genetic, developmental, structural and functional differences between its segments contribute to the distinct, intestinal region-specific effects of hyperglycemia. These observations support and highlight the importance of a regional approach in diabetes-related enteric neuropathy. Intestinal large and microvessels are essential for the blood supply of enteric ganglia. Bidirectional morpho-functional linkage exists between enteric neurons and enteroglia, however, there is also a reciprocal communication between enteric neurons and immune cells on which intestinal microbial composition has crucial influence. From this point of view, it is more appropriate to say that enteric neurons partake in multidirectional communication and interact with these key players of the intestinal wall. These interplays may differ from segment to segment, thus, the microenvironment of enteric neurons could be considered strictly regional. The goal of this review is to summarize the main tissue components and molecular factors, such as enteric glia cells, interstitial cells of Cajal, gut vasculature, intestinal epithelium, gut microbiota, immune cells, enteroendocrine cells, pro-oxidants, antioxidant molecules and extracellular matrix, which create and determine a gut region-dependent neuronal environment in diabetes.
Core Tip: Diabetes-related intestinal motility disturbances result from multifactorial damage to the enteric nervous system. However, the diversity of the neuronal environment in different gut segments basically determines the regionality of diabetic enteric neuropathy. Therefore, in this review, we highlight the role of enteric glial cells, gut circulation, intestinal epithelium, gut microbiota, immune and enteroendocrine cells, pro-oxidants, antioxidant defence and extracellular matrix, which have great impact on the formation and maintenance of a region-specific enteric neuronal environment in diabetes.
- Citation: Bagyánszki M, Bódi N. Key elements determining the intestinal region-specific environment of enteric neurons in type 1 diabetes. World J Gastroenterol 2023; 29(18): 2704-2716
- URL: https://www.wjgnet.com/1007-9327/full/v29/i18/2704.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i18.2704
In the middle of last century, the general belief was that the neurons within the intestinal wall are parasympathetic neurons[1]. In recent decades, it has become evident that the neurons and glia cells of the gastrointestinal (GI) tract form a third, unique division of the nervous system besides the sympa
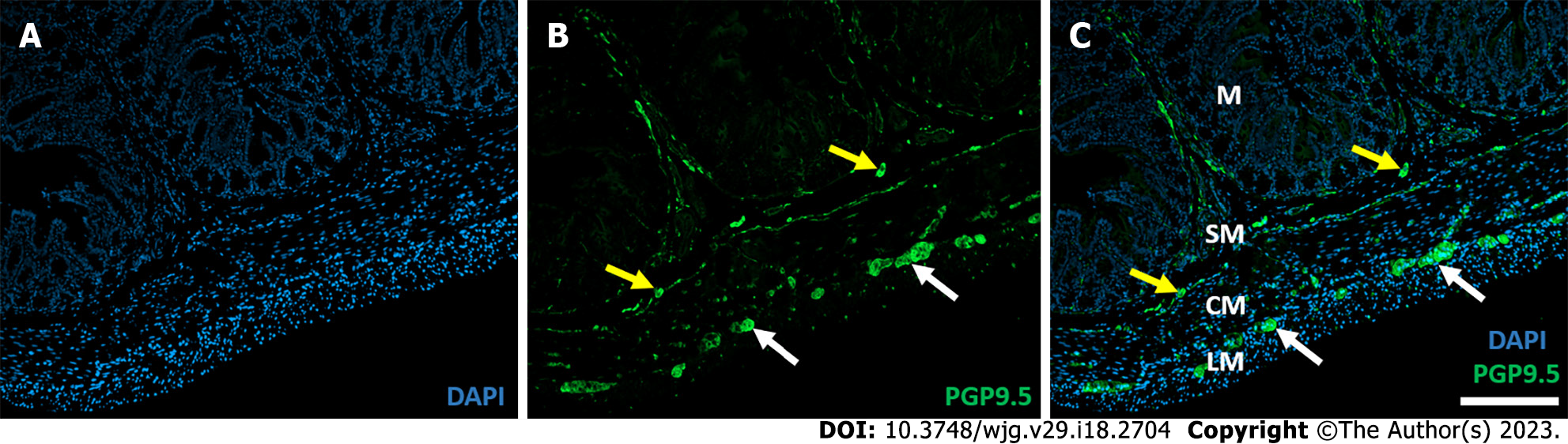
There is a large body of data on the structure and function of the ENS in physiological state, and it is clear that many pathological conditions strongly affect the enteric plexuses. Since the enteric plexuses are embedded in the histological layers of the intestinal wall (Figure 1), the projections of neurons and glia cells weave through the cross-section of the entire intestine, and because of the lack of blood-brain barrier in the periphery, the role of the environment surrounding the enteric ganglia and neuronal projections is increasingly evident in both health and diseases[2,8].
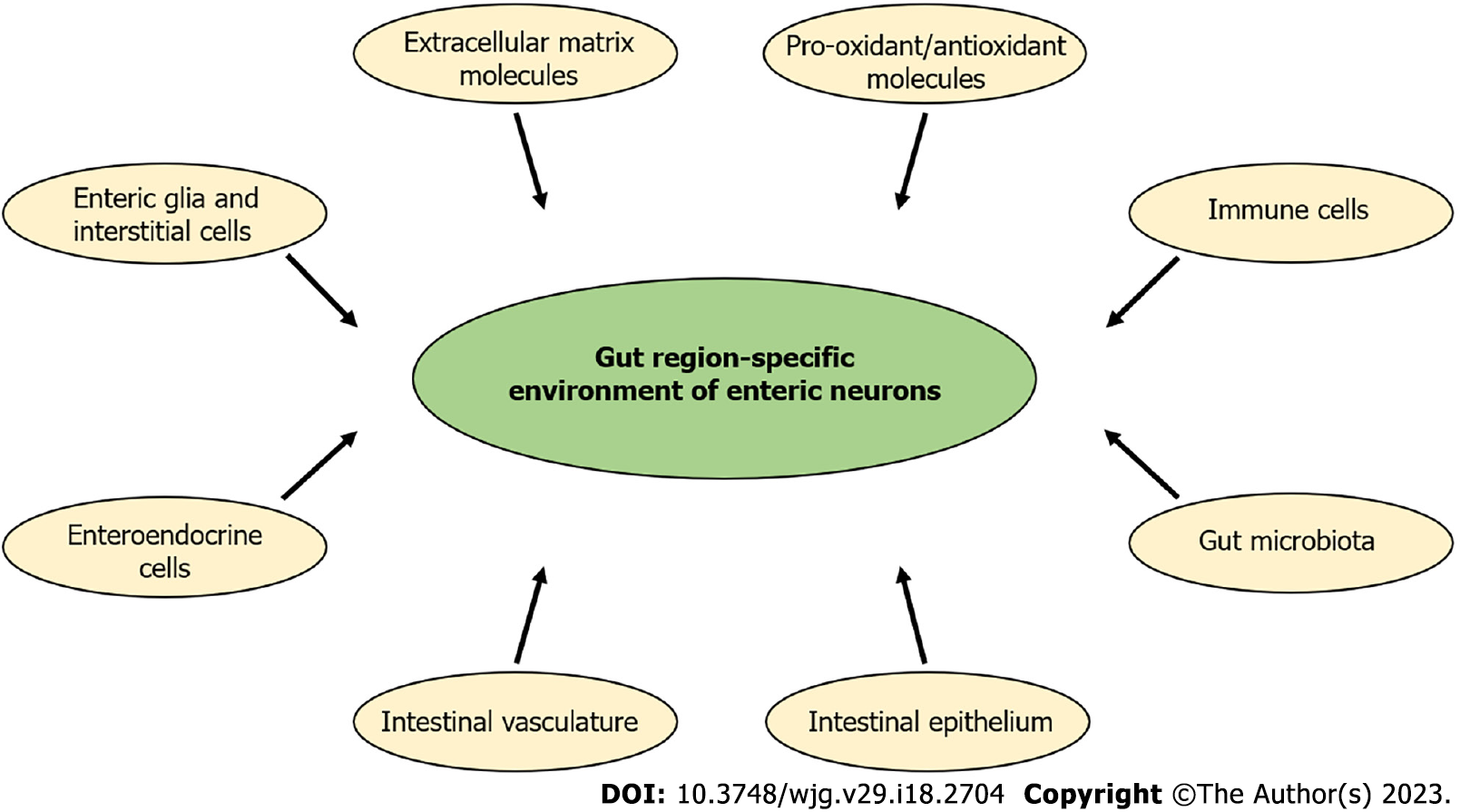
In this review, we provide a brief overview of the effects of type 1 diabetes (T1D) on the intestinal region-specific enteric neuronal environment. Unfortunately, the incidence of T1D is increasing and this incurable disease causes severe GI symptoms[8]. Chronic hyperglycemia influences the structural and functional features of the enteric neurons[9,10], it could change neurochemical code or even can cause neuronal cell death and thus lead to enteric neuropathy described by others and in our former review[11-13]. Hyperglycemia-related enteric neuropathy shows gut-region specific alterations. Therefore, the aim of this paper is to review the main environmental factors (Figure 2) in the intestinal tube from enteric glia cells (EGCs) to the luminal microbiota, which can play a crucial role in the region-specific damage of enteric neurons in the diabetic state. Some key factors like gut microbiota[14-16], GI immune[17-19] or epithelial cells[20-24] are highly emphasized in several papers, so here these are briefly summarized, while other, also critical components of the neuronal environment [e.g. EGCs, intestinal vasculature, pro-oxidant/antioxidant balance and extracellular matrix (ECM) molecules], are discussed in more detail.
EGCs are not only supporting their neighboring neurons, but also actively regulate GI barrier function, immune homeostasis, or gut motility[25,26]. They directly interact with numerous cells in the gut wall, like enterocytes, immune cells, muscle cells, enteric neurons and vasculature, and these cross-talks influence their survival and functions in different intestinal layers and gut segments in health and disease[27].
Based on cellular morphology and location within different anatomical layers, EGC subtypes are classified into mucosal, intramuscular, submucosal and myenteric glia cells[28,29]. Glial fibrillary acidic protein (GFAP), S100ß and Sox10 are among the main glial markers, but expression patterns of different EGCs inside and outside the ganglia can be varied and reflect dynamic gene regulation[30]. In summary, the variety of EGCs, as functional heterogeneity and phenotypic plasticity, fundamentally determine the cellular microenvironment within the gut wall[31,32].
Besides gut layer-dependent diversity, intestinal regional heterogeneity of EGCs is also demonstrated among GI segments. Unique developmental patterns derived from different enteric precursors and specialized functions of EGCs are associated with different GI regions (e.g. esophagus, stomach or intestine) and result in local environmental properties[32,33]. Different protein expression and transcriptional profiles of myenteric glia cells have observed in mouse ileum and colon[31,34]. EGCs of myenteric ganglia displayed region-dependent responses to neuromodulators and glial regulation of gut contractility was also region- and pathway-specific in the duodenum and colon[35].
Development and function of EGCs and enteric neurons are in close interdependence[36]. Different neurotransmitters can activate EGCs and glial derived neurotrophic factors are crucial for neuronal survival and maintenance[27,37]. EGCs could also act as a critical link in the communication of enteric nervous and immune systems through the modulation of macrophages[38]. Because of the close neuron-glia relationship, it would be beneficial to investigate the involvement of EGCs in diabetes in addition to gut region-specific diabetic neuronal damage[39].
In the duodenum of type 2 diabetic mice with high-fat diet, a decline in the mucosa-associated glial network density was observed, however, neither the glial density and ultrastructure nor the expression of S100ß, Sox10 and GFAP markers were changed in the EGCs of myenteric ganglia[40]. Meanwhile, in a distal direction, an intense reduction in the number of both the enteric neurons and S100-immunoreactive glia cells was seen in diabetic rat jejunum[41]. Expression of GFAP and neurotrophins, like glia cell-derived neurotrophic factor (GDNF) and neurotrophin-3 were decreased in the colon of diabetic rats[42]. Loss of enteric neurons and progressive decrease in GDNF expression was demonstrated with the course of diabetic state both in proximal and distal colon of Sprague-Dawley diabetic rats. Moreover, reduced Akt phosphorylation also accompanied these changes[43]. Also, hyperglycemia stimulated EGC apoptosis in culture by repressing the PI3K/Akt molecular pathway[44]. The down-regulation of the PI3K/Akt pathway, an important mediator of neuronal survival, is heavily involved in the diabetic damage of enteric neurons[45,46].
Interstitial cells of Cajal (ICCs) are pacemaker cells in GI motility that generate spontaneous and rhythmic slow waves to promote the spontaneous contractions of smooth muscles[47]. The delayed gastric emptying both in diabetic patients and diabetic animal models is associated with ICC depletion[10,48]. Furthermore, damage of ICCs contributes to impaired motility in other GI regions causing constipation[49,50].
Blood vessels enmeshing the small and large intestine are important in nutrient transport and also responsible in supplying enteric cells. Macro- and microvascular anatomy of the GI tract fundamentally determine its regionality. Extramural circulation of the duodenum arises from the coeliac trunk, the jejunum and ileum are supplied by branches of superior mesenteric artery, while different parts of large intestine are supplied by the superior or inferior mesenteric arteries[51]. The impairment of large mesenteric vessels has been described in T1D[52], and substantial heterogeneity of endothelial dysfunction of different large arteries has been observed in a type 2 diabetic animal model[53]. Besides the variability of diabetic macroangiopathy, the impact of diabetes on the intestinal microvasculature can also be region-dependent[54-56].
Investigation of small capillaries in the close vicinity of myenteric ganglia revealed their different susceptibility to diabetic damage along the duodenum-ileum-colon axis[54]. Structural changes such as thickening of the endothelial basement membrane, caveolar hypertrophy and tight junction opening were confirmed in the ileum and colon, whereas only junctional alterations were visible in the duodenal capillaries. In addition, a severely impaired regulation of vascular permeability was shown in ileal and colonic capillaries, while an accelerated, but well-balanced albumin transport was indicated in the duodenum. Immediate insulin treatment prevented most of the diabetes-related changes of the capillary endothelium in the ileum, but not in the colon[54]. Increased thickening of arteriolar wall representing microangiopathy in colonic submucosal vessels was also shown in diabetic patients[56].
Naturally, distinct degrees of capillary damage in different gut segments strongly determine a segment-specific cellular environment and contribute to the diabetic fate of the cells they supply[8]. Close interaction between the capillary endothelial cells and migrating neural crest-derived cells has already been observed in intestinal neurovascular development and has an important role in creating a favorable neuronal microenvironment[57]. Therefore, the diabetes-related regional capillary impairments may greatly contribute to region-dependent enteric neuropathy in T1D[8].
However, not only the structural complications in the vascular system, but also the circulating microparticles can impair the endothelial function in diabetes[58]. Different gut segments feature their distinct microbial compositions and metabolites. Imbalance in the microbial composition accompanying diabetes results in changes of metabolites production, like short-chain fatty acids, bile acids, or tryptophan catabolites[59,60]. Enhancement of gut permeability related to dysbiosis may allow not only the release of different metabolites and endotoxin but also bacterial translocation from the gut to the venous circulation. These elements as integral mediators significantly contribute to vascular inflammation and immune activation[59-62].
The lining of the GI tract is directly exposed to an ever-changing environment. This single layer of epithelial cells is crucial for preserving gut homeostasis and functions both as barrier and channel for the crosstalk between the GI immune cells and microbiota[23,63].
Epithelial tight junctions are the key components of the physical intestinal barrier along the GI tract[20]. Altered barrier function of enterocytes and colonocytes leads to several pathologic conditions, including obesity or diabetes[20,21,23]. The chronic hyperglycemia-related breakdown of barrier integrity leads to the systemic influx of microbial products and an enhanced incidence of enteric infection[64].
The intestinal epithelium also has an immunological role, as it contains pattern recognition receptors, such as the Toll-like receptors (TLRs)[20]. Recently, several studies demonstrated the expression of TLR4 in metabolic diseases[65-67]. When sensing microbial lipopolysaccharides of Gram-negative bacteria, TLR4 can activate pro-inflammatory pathways in the GI tract[16,22], thus TLRs may play a crucial role in diabetic enteropathy[65]. TLR4 not only affects ENS function, but also modulates neuro-immune interactions by mediating the effects of the intestinal microbiota[65].
In the last two decades it has become clear that the imbalance of microbial species due to a reduction in microbial diversity, known as dysbiosis, is associated with several pathological conditions like autoimmune diseases[68], cancers[69], arteriosclerosis[70] depression[71,72], neurodegenerative diseases[73], obesity or diabetes[74-76]. Dysbiosis contribute to the formation of a proinflammatory milieu and gut leakiness[77,78].
Decreased microbiota diversity has been observed in T1D. At the phyla level, the proportion of Firmicutes decreased in patients compared to the healthy individual group, while Bacteroidetes abundance increased[16,79].
It is also obvious that the composition of microbiota and the number of microbes is different along the GI tract and each segment contains unique microorganism communities[80,81]. Unfortunately, only a very few studies performed longitudinal comparisons, but results showed that the mode and severity of dysbiosis has also been distinct in different gut segments[82-85]. Besides a longitudinal variability, a horizontal gradient also exists in the gut, with oxygen, redox and mucus gradients from the mucosal surface to the lumen[80] and these variables can contribute to the differences of luminal and mucosal microbiota both in health and disease[83,84,86].
By now, a sufficient amount of evidence has been gathered which show that probiotics have a beneficial influence on diabetes-related dysbiosis[78], and a plethora of studies investigate the effects of prebiotics, synbiotics and fecal microbiota transplantation on hyperglycemia and other diabetes-associated symptoms. Among others, Roseburia intestinalis, Lactobacillus casei, Akkermansia muciniphila and Bacteroides fragilis have been shown to ameliorate glucose metabolism and insulin sensitivity[75]. In the last 20 years, considerable progress has been made and the intestinal microbiota most certainly represents a promising target for T1D prevention and therapy; however, numerous unresolved concerns require further in-depth investigation.
The GI tract is the largest immune organ in vertebrates, where the intestinal homeostasis is determined by the gut microbiota, intestinal epithelium and host immunity[21,87].
The complex and enormous amount of information available about the GI immune system is summarized in other reviews[17-19], here we would like to highlight only one aspect. In earlier studies, the GI immune system has been examined as small vs large intestine, based on obvious differences in structure and function. Recently it has become apparent that the immunological niches of the GI tract differ between more refined functional compartments, making it necessary to study them separately to understand the consequences on intestinal immune homeostasis[88].
Based on the review of Brown and Esterházy[88], the GI tube can be divided into the following five main parts: Proximal small intestine, gut-draining lymph nodes, distal small intestine, large intestine and mesentery. Each intestinal niche is influenced by a combination of intrinsic tissue properties, extrinsic environmental signals, and immune cell composition.
It would be beneficial if therapies would take into account the regionally different susceptibility of the GI tract to infections and diseases.
Enteroendocrine cells (EECs) not only play a role in humoral processes but also act as sensory cells in the GI mucosa next to the neurons and immune cells[89]. Therefore, microbial metabolites could stimulate or suppress hormone secretion by EECs, while endocrine and paracrine factors regulate GI functions and affect several metabolic processes in the body[90]. The diversity of EECs prompts the introduction of a new classification scheme. Earlier, EECs were classified based on producing a single hormone, but in the last decade it was shown that most EECs contain multiple hormones. Several hormones, like secretin and serotonin, are in separate storage vesicles at subcellular level[91]. The hormones produced by EECs might have a big potential in the future as novel microbiota-based therapies to alter metabolically active hormone levels, similarly to the use of the anorectic gut hormone, glucagon-like peptide 1, in the treatment of obesity and type 2 diabetes[90].
It is well established that EEC composition and proportion is different along the GI tract[92,93]. A recently published paper by Martin et al[93] has indicated that regional differences in nutrient sensing capability exist in mouse EECs. Colonic EECs has been shown to be more sensitive to glucose, while duodenal EECs to fructose and sucrose.
The intestinal redox state is critical in maintaining gut homeostasis and functional regulation. The maintenance of this delicate balance in redox state is influenced by the gut microbiota, immune cells and epithelium, which can all produce and respond to redox signals[94]. Numerous genera of bacteria has been identified as biomarkers for gut redox state[95]. Reactive sulfur species-producing bacterial families enhance the host’s antioxidant capacity[96], however, sulfur metabolism can be distracted by opportunistic pathogens[94]. Large differences can be observed in different GI segments regarding the quantity and composition of microbiota, intestinal pH or partial pressure of oxygen within the luminal-facing epithelium[80]. There is a richer and more diverse microbial community and deeper anaerobic state from proximal to distal parts of the gut, therefore, it is not surprising that oxidant and antioxidant mechanisms have also been linked to strict region-dependency in health and disease[97]. Foods containing numerous antioxidants, such as vitamins, carotenoids, flavonoids, polyphenols, bioactive peptides or others, however, also include a lot of pro-oxidant molecules[98-100] and all of these can modulate the composition of microbial communities[101]. Consumed and endogenously produced antioxidants have varied strategies at different levels to maintain optimal redox balance[102,103]. Still, the accumulation of reactive oxygen species and/or decrease in antioxidant defence contribute to serious imbalance of intestinal pro-oxidant/antioxidant milieu[104].
Higher mucosal vitamin E and carotenoid concentration, higher total antioxidant activity, superoxide dismutase and catalase activity, as well as glutathione level were observed in the duodenum compared to the ileum and colon of different animal species[105-107]. The presence of probiotic Lactobacillus species also reflects a highly beneficial cellular environment in the duodenum[108]. Moreover, in diabetic rats, an increased abundance of the genus Lactobacillus has been observed relative to controls[83], which can result in enhanced antioxidant capacity[109]. While no significant changes in peroxynitrite production has been observed, a robust increase of metallothionein 2 and elevated glutathione level has been found in the duodenum of diabetic rats[110], which may contribute to cell survival in this particular gut segments.
In contrast to the duodenum, diabetes increased lipid peroxidation and catalase activity, as well as the percentage of nitrotyrosine-immunoreactive myenteric neurons in the jejunum[111]. Decreased superoxide dismutase and increased myeloperoxidase enzyme concentrations were also demonstrated in the diabetic jejunum[112]. Enhanced lipid peroxidation and protein oxidation accompanied with significantly lower superoxide dismutase levels, catalase and glutathione levels were also observed in the diabetic ileum[113,114]. However, a great increase in the activity of the endogenous heme oxygenase system was shown in myenteric neurons of diabetic ileum[115], maybe as an effect of microbial changes[116]. In the colon of diabetic rats, the doubled peroxynitrite level, reduced superoxide dismutase activity and the induction of the endogenous heme oxigenase system emphasizes the observation that distal gut segments have greater susceptibility to the diabetic oxidative environment, which is in correlation with diabetic neuronal cell loss[97,110,115].
ECM structures composed of various proteins and polysaccharides are essential in the maintenance and well-regulated remodeling of tissues and have a key role in regulating different cellular events, like cell proliferation, differentiation or migration[117-119]. In the gut, numerous cells (e.g. epithelial, mesenchymal, stem cells) participate in the production of matrix molecules, and their precise composition is indispensable for the optimal cellular environment and normal intestinal function. Sensing the stiffness or the porosity of the ECM through specific receptors such as integrins, intestinal cells can change their intracellular state or dynamics[120,121].
Diabetes-related alterations of ECM is demonstrated in all parts of the gut, but with different extent in different regions and intestinal layers. In streptozotocin-induced diabetic rats, a significant increase in the amount of laminin-1 and fibronectin was observed in the small intestine by Western blotting and immunohistochemistry, and the strong labelling was restricted mainly to the intestinal smooth muscle and serous layers[122]. These hyperglycemia-mediated ECM accumulation was reversed by insulin treatment[122]. Additionally, in the distal colon, a marked increase of type 1 collagen was detected with no changes in type 3 and 4 collagen expression[123]. Besides of the well-marked pockets of collagen among the smooth muscle cells, formation of advanced glycation end-products was also observed in diabetic rats. Type 1 collagen deposits and glycation increase stiffness of the diabetic colon muscle, which contribute to limited colonic function[123]. There is a strict association between collagen content and mechanical properties, however, this varied in different parts of the small intestine [124]. Increased ECM deposition, as well as high levels of type 1 and 3 collagen and fibronectin mRNAs were also detected in diabetic colon mucosa[125]. The accumulation of ECM in the mucosa of the diabetic colon was associated with the deregulation of the transforming growth factor (TGF)-β1/Smad signaling pathway[125]. However, TGF-β can also influence deposition of matrix molecules by upregulating several ECM receptors[126].
Structural alterations of basement membranes as specialized ECM structures have been characterized in diabetes mellitus. Thickening of capillary basement membrane is among the first histological hallmarks of the disease. Capillaries located in gut smooth muscle in different gut segments displayed region-specific thickening of their basement membranes in T1D[54]. Additionally, significant increase in mRNA levels of different matrix scaffold proteins, like fibronectin or procollagen type 1, was observed in the aorta and mesenteric artery of type 2 diabetic Goto-Kakizaki rats[127]. Moreover, gene expression was restored in the mesenteric bed but not in the aorta using an endothelin-1 antagonist[127]. Basement membrane thickening of smooth muscle cells was also demonstrated in the small intestine[122] and colon[123]. Moreover, the basement membrane surrounding the myenteric ganglia was also thickened in diabetic rats with strict regionality in different gut segments[128].
ECM accumulation can be due to the enhanced synthesis of matrix components, but also their decreased degradation, which in turn leads to the imbalance of ECM dynamics[129]. Matrix metalloproteinases (MMPs) and their tissue specific inhibitors (TIMPs) mainly produced by macrophages, neutrophils or epithelial cells have an essential role in tissue remodeling as a response to intestinal inflammation[130,131]. Growing molecular evidence support that these proteolytic enzymes are also targets of diabetic damage. In the diabetic ileum, MMP9 expression decreased in myenteric ganglia, capillary endothelial cells and intestinal smooth muscle cells, while these values did not change in the duodenum, which is in perfect agreement with the regionally distinct thickening of the ganglionic basement membrane. However, a specific, but great induction was revealed in MMP9 and TIMP1 at the mRNA level both in duodenum and ileum homogenates of diabetics[128]. Increased early expression of MMP2 and MMP9 mRNAs and MMP1 later on was also demonstrated in the diabetic colon mucosa. On the other hand, increased TIMP1 and TIMP2 expression could be the result of decreased MMPs degrading activities here[125].
Because of its various functions, as food intake, mechanical and chemical breakdown, motility, absorption, regulation of blood flow, secretion, water reabsorption and immune functions, the GI tract has unique features. It has three detecting systems, which are more extensive than those of any other organ: (1) The ENS contains as many neurons as the spinal cord and different subpopulations of the EGCs cover all histological layers of the intestinal wall. Intrinsic primary neurons, interneurons and motoneurons can form local reflex circuits in the gut wall[1,2]; (2) There are more than 20 hormones produced by several types of EECs[89]; and (3) The GI tract is the largest immune organ with the cc. 70-80% of the body's immune cells[87,132].
In addition to the three systems listed above, other essential factors such as intestinal epithelial barrier, microcirculation of the gut wall, pro-oxidant/antioxidant milieu, ECM components and gut microbiota also play a crucial role in the formation of the enteric neuronal environment in both physiological and pathological states. Results demonstrated a clear relationship between intestinal microorganisms and the occurrence of T1D, but the correlation or causality remains an important question for several reasons. Altered gut microbiota-mediated redox imbalance and changes in cellular cross-talks may contribute to enteric neuropathy and also influence the function of gut-brain axis[15].
Considering these properties and the size of the GI tract, it is not surprising that it shows profound functional and structural differences along its length. When planning experiments, the gut should be regarded as a multiple organ, and in the case of illness, the applied therapies should take into account the intestinal segment-specific effects[88].
The authors sincerely thank to their PhD students, Abigél Egyed-Kolumbán, Afnan AL Doghmi, Bence
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Hungary
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Baccari MC, Italy; Castelucci P, Brazil; Sahin Y, Turkey S-Editor: Fan JR L-Editor: A P-Editor: Yu HG
| 1. | Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 573] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 2. | Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 1066] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 3. | Muhammad F, Fan B, Wang R, Ren J, Jia S, Wang L, Chen Z, Liu XA. The Molecular Gut-Brain Axis in Early Brain Development. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 4. | Mongardi Fantaguzzi C, Thacker M, Chiocchetti R, Furness JB. Identification of neuron types in the submucosal ganglia of the mouse ileum. Cell Tissue Res. 2009;336:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Qu ZD, Thacker M, Castelucci P, Bagyánszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 6. | Danan CH, Katada K, Parham LR, Hamilton KE. Spatial transcriptomics add a new dimension to our understanding of the gut. Am J Physiol Gastrointest Liver Physiol. 2023;324:G91-G98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Dharshika C, Gulbransen BD. Enteric Neuromics: How High-Throughput "Omics" Deepens Our Understanding of Enteric Nervous System Genetic Architecture. Cell Mol Gastroenterol Hepatol. 2023;15:487-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Bagyánszki M, Bódi N. Diabetes-related alterations in the enteric nervous system and its microenvironment. World J Diabetes. 2012;3:80-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 9. | Santhanam P, Marashdeh W, Solnes L. Functional Imaging of Evaluation of Diabetic Gastroparesis. Curr Diabetes Rev. 2018;14:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Selby A, Reichenbach ZW, Piech G, Friedenberg FK. Pathophysiology, Differential Diagnosis, and Treatment of Diabetic Diarrhea. Dig Dis Sci. 2019;64:3385-3393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. 2007;19:951-960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 12. | Yarandi SS, Srinivasan S. Diabetic gastrointestinal motility disorders and the role of enteric nervous system: current status and future directions. Neurogastroenterol Motil. 2014;26:611-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 13. | Bódi N, Szalai Z, Bagyánszki M. Nitrergic Enteric Neurons in Health and Disease-Focus on Animal Models. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Del Chierico F, Rapini N, Deodati A, Matteoli MC, Cianfarani S, Putignani L. Pathophysiology of Type 1 Diabetes and Gut Microbiota Role. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 15. | Shandilya S, Kumar S, Kumar Jha N, Kumar Kesari K, Ruokolainen J. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J Adv Res. 2022;38:223-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 146] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 16. | Ye J, Wu Z, Zhao Y, Zhang S, Liu W, Su Y. Role of gut microbiota in the pathogenesis and treatment of diabetes mullites: Advanced research-based review. Front Microbiol. 2022;13:1029890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Schill EM, Floyd AN, Newberry RD. Neonatal development of intestinal neuroimmune interactions. Trends Neurosci. 2022;45:928-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Sterling KG, Dodd GK, Alhamdi S, Asimenios PG, Dagda RK, De Meirleir KL, Hudig D, Lombardi VC. Mucosal Immunity and the Gut-Microbiota-Brain-Axis in Neuroimmune Disease. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Viola MF, Boeckxstaens G. Niche-specific functional heterogeneity of intestinal resident macrophages. Gut. 2021;70:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 20. | Aleman RS, Moncada M, Aryana KJ. Leaky Gut and the Ingredients That Help Treat It: A Review. Molecules. 2023;28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 82] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 21. | Lin PY, Stern A, Peng HH, Chen JH, Yang HC. Redox and Metabolic Regulation of Intestinal Barrier Function and Associated Disorders. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 22. | Singh N, Singh V, Rai SN, Mishra V, Vamanu E, Singh MP. Deciphering the gut microbiome in neurodegenerative diseases and metagenomic approaches for characterization of gut microbes. Biomed Pharmacother. 2022;156:113958. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Stolfi C, Maresca C, Monteleone G, Laudisi F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 167] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 24. | Van Spaendonk H, Ceuleers H, Witters L, Patteet E, Joossens J, Augustyns K, Lambeir AM, De Meester I, De Man JG, De Winter BY. Regulation of intestinal permeability: The role of proteases. World J Gastroenterol. 2017;23:2106-2123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 146] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (2)] |
| 25. | Liu C, Yang J. Enteric Glial Cells in Immunological Disorders of the Gut. Front Cell Neurosci. 2022;16:895871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 26. | Progatzky F, Pachnis V. The role of enteric glia in intestinal immunity. Curr Opin Immunol. 2022;77:102183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 27. | Grubišić V, Gulbransen BD. Enteric glia: the most alimentary of all glia. J Physiol. 2017;595:557-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 28. | Coelho-Aguiar Jde M, Bon-Frauches AC, Gomes AL, Veríssimo CP, Aguiar DP, Matias D, Thomasi BB, Gomes AS, Brito GA, Moura-Neto V. The enteric glia: identity and functions. Glia. 2015;63:921-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2012;9:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 286] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 30. | Grundmann D, Loris E, Maas-Omlor S, Huang W, Scheller A, Kirchhoff F, Schäfer KH. Enteric Glia: S100, GFAP, and Beyond. Anat Rec (Hoboken). 2019;302:1333-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 31. | Boesmans W, Lasrado R, Vanden Berghe P, Pachnis V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia. 2015;63:229-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 32. | Seguella L, Gulbransen BD. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat Rev Gastroenterol Hepatol. 2021;18:571-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 185] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 33. | Espinosa-Medina I, Jevans B, Boismoreau F, Chettouh Z, Enomoto H, Müller T, Birchmeier C, Burns AJ, Brunet JF. Dual origin of enteric neurons in vagal Schwann cell precursors and the sympathetic neural crest. Proc Natl Acad Sci U S A. 2017;114:11980-11985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 34. | Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, van der Zwan J, Häring M, Braun E, Borm LE, La Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S. Molecular Architecture of the Mouse Nervous System. Cell. 2018;174:999-1014.e22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1775] [Cited by in RCA: 1767] [Article Influence: 252.4] [Reference Citation Analysis (0)] |
| 35. | Seguella L, McClain JL, Esposito G, Gulbransen BD. Functional Intraregional and Interregional Heterogeneity between Myenteric Glial Cells of the Colon and Duodenum in Mice. J Neurosci. 2022;42:8694-8708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 36. | Boesmans W, Nash A, Tasnády KR, Yang W, Stamp LA, Hao MM. Development, Diversity, and Neurogenic Capacity of Enteric Glia. Front Cell Dev Biol. 2021;9:775102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 37. | Liu S. Neurotrophic factors in enteric physiology and pathophysiology. Neurogastroenterol Motil. 2018;30:e13446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Grubišić V, McClain JL, Fried DE, Grants I, Rajasekhar P, Csizmadia E, Ajijola OA, Watson RE, Poole DP, Robson SC, Christofi FL, Gulbransen BD. Enteric Glia Modulate Macrophage Phenotype and Visceral Sensitivity following Inflammation. Cell Rep. 2020;32:108100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 39. | Izbéki F, Wittman T, Rosztóczy A, Linke N, Bódi N, Fekete E, Bagyánszki M. Immediate insulin treatment prevents gut motility alterations and loss of nitrergic neurons in the ileum and colon of rats with streptozotocin-induced diabetes. Diabetes Res Clin Pract. 2008;80:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Stenkamp-Strahm C, Patterson S, Boren J, Gericke M, Balemba O. High-fat diet and age-dependent effects on enteric glial cell populations of mouse small intestine. Auton Neurosci. 2013;177:199-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | de Souza SR, de Miranda Neto MH, Martins Perles JV, Vieira Frez FC, Zignani I, Ramalho FV, Hermes-Uliana C, Bossolani GD, Zanoni JN. Antioxidant Effects of the Quercetin in the Jejunal Myenteric Innervation of Diabetic Rats. Front Med (Lausanne). 2017;4:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Liu W, Yue W, Wu R. Effects of diabetes on expression of glial fibrillary acidic protein and neurotrophins in rat colon. Auton Neurosci. 2010;154:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Du F, Wang L, Qian W, Liu S. Loss of enteric neurons accompanied by decreased expression of GDNF and PI3K/Akt pathway in diabetic rats. Neurogastroenterol Motil. 2009;21:1229-e114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Chen Y, Liu G, He F, Zhang L, Yang K, Yu H, Zhou J, Gan H. MicroRNA 375 modulates hyperglycemia-induced enteric glial cell apoptosis and Diabetes-induced gastrointestinal dysfunction by targeting Pdk1 and repressing PI3K/Akt pathway. Sci Rep. 2018;8:12681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Leinninger GM, Backus C, Uhler MD, Lentz SI, Feldman EL. Phosphatidylinositol 3-kinase and Akt effectors mediate insulin-like growth factor-I neuroprotection in dorsal root ganglia neurons. FASEB J. 2004;18:1544-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 46. | Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855-3862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 454] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 47. | Kishi K, Kaji N, Kurosawa T, Aikiyo S, Hori M. Hyperglycemia in the early stages of type 1 diabetes accelerates gastric emptying through increased networks of interstitial cells of Cajal. PLoS One. 2019;14:e0222961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Yamamoto T, Watabe K, Nakahara M, Ogiyama H, Kiyohara T, Tsutsui S, Tamura S, Shinomura Y, Hayashi N. Disturbed gastrointestinal motility and decreased interstitial cells of Cajal in diabetic db/db mice. J Gastroenterol Hepatol. 2008;23:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Gotfried J, Priest S, Schey R. Diabetes and the Small Intestine. Curr Treat Options Gastroenterol. 2017;15:490-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Jin B, Ha SE, Wei L, Singh R, Zogg H, Clemmensen B, Heredia DJ, Gould TW, Sanders KM, Ro S. Colonic Motility Is Improved by the Activation of 5-HT(2B) Receptors on Interstitial Cells of Cajal in Diabetic Mice. Gastroenterology. 2021;161:608-622.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 51. | Geboes K, Geboes KP, Maleux G. Vascular anatomy of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2001;15:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Lee JH, Bahk JH, Park SH, Huh J. The diabetes-induced functional and distributional changes of the alpha 1-adrenoceptor of the abdominal aorta and distal mesenteric artery from streptozotocin-induced diabetic rats. Korean J Anesthesiol. 2011;60:272-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Sallam NA, Laher I. Redox Signaling and Regional Heterogeneity of Endothelial Dysfunction in db/db Mice. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Bódi N, Talapka P, Poles MZ, Hermesz E, Jancsó Z, Katarova Z, Izbéki F, Wittmann T, Fekete É, Bagyánszki M. Gut region-specific diabetic damage to the capillary endothelium adjacent to the myenteric plexus. Microcirculation. 2012;19:316-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | De Las Casas LE, Finley JL. Diabetic microangiopathy in the small bowel. Histopathology. 1999;35:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Sasor A, Ohlsson B. Microangiopathy is common in submucosal vessels of the colon in patients with diabetes mellitus. Rev Diabet Stud. 2014;11:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Hatch J, Mukouyama YS. Spatiotemporal mapping of vascularization and innervation in the fetal murine intestine. Dev Dyn. 2015;244:56-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 58. | Ishida K, Taguchi K, Hida M, Watanabe S, Kawano K, Matsumoto T, Hattori Y, Kobayashi T. Circulating microparticles from diabetic rats impair endothelial function and regulate endothelial protein expression. Acta Physiol (Oxf). 2016;216:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 59. | Thenappan T, Khoruts A, Chen Y, Weir EK. Can intestinal microbiota and circulating microbial products contribute to pulmonary arterial hypertension? Am J Physiol Heart Circ Physiol. 2019;317:H1093-H1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 60. | Zhu Y, Shui X, Liang Z, Huang Z, Qi Y, He Y, Chen C, Luo H, Lei W. Gut microbiota metabolites as integral mediators in cardiovascular diseases (Review). Int J Mol Med. 2020;46:936-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 61. | Alarcón Yempén RE, Venzel R, Paulino Campos MC, de Oliveira LP, Lins RVD, Pessoni AM, Fanaro GB, de Oliveira Souza A, Calaza KDC, de Brito Alves JL, Cavalcanti-Neto MP. Gut microbiota: A potential therapeutic target for management of diabetic retinopathy? Life Sci. 2021;286:120060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Di Ciaula A, Baj J, Garruti G, Celano G, De Angelis M, Wang HH, Di Palo DM, Bonfrate L, Wang DQ, Portincasa P. Liver Steatosis, Gut-Liver Axis, Microbiome and Environmental Factors. A Never-Ending Bidirectional Cross-Talk. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 63. | Allaire JM, Crowley SM, Law HT, Chang SY, Ko HJ, Vallance BA. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018;39:677-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 596] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 64. | Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M, Ben-Zeev R, Lehavi-Regev D, Katz MN, Pevsner-Fischer M, Gertler A, Halpern Z, Harmelin A, Aamar S, Serradas P, Grosfeld A, Shapiro H, Geiger B, Elinav E. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359:1376-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 619] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 65. | Bódi N, Egyed-Kolumbán A, Onhausz B, Barta BP, Doghmi AA, Balázs J, Szalai Z, Bagyánszki M. Intestinal Region-Dependent Alterations of Toll-Like Receptor 4 Expression in Myenteric Neurons of Type 1 Diabetic Rats. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Sun Q, Zhang S, Zhang BY, Zhang Y, Yao L, Hu J, Zhang HH. microRNA-181a contributes to gastric hypersensitivity in rats with diabetes by regulating TLR4 expression. Mol Pain. 2023;19:17448069231159356. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 67. | Zeng F, Zheng J, Shen L, Herrera-Balandrano DD, Huang W, Sui Z. Physiological mechanisms of TLR4 in glucolipid metabolism regulation: Potential use in metabolic syndrome prevention. Nutr Metab Cardiovasc Dis. 2023;33:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 68. | Liu X, Cheng YW, Shao L, Sun SH, Wu J, Song QH, Zou HS, Ling ZX. Gut microbiota dysbiosis in Chinese children with type 1 diabetes mellitus: An observational study. World J Gastroenterol. 2021;27:2394-2414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (3)] |
| 69. | Zhang H, Chang Y, Zheng Q, Zhang R, Hu C, Jia W. Altered intestinal microbiota associated with colorectal cancer. Front Med. 2019;13:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 70. | Anto L, Blesso CN. Interplay between diet, the gut microbiome, and atherosclerosis: Role of dysbiosis and microbial metabolites on inflammation and disordered lipid metabolism. J Nutr Biochem. 2022;105:108991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 71. | Masanetz RK, Winkler J, Winner B, Günther C, Süß P. The Gut-Immune-Brain Axis: An Important Route for Neuropsychiatric Morbidity in Inflammatory Bowel Disease. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 72. | Simpson CA, Diaz-Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CSM. The gut microbiota in anxiety and depression - A systematic review. Clin Psychol Rev. 2021;83:101943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 532] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 73. | Kaviyarasan S, Chung Sia EL, Retinasamy T, Arulsamy A, Shaikh MF. Regulation of gut microbiome by ketogenic diet in neurodegenerative diseases: A molecular crosstalk. Front Aging Neurosci. 2022;14:1015837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 74. | Candon S, Perez-Arroyo A, Marquet C, Valette F, Foray AP, Pelletier B, Milani C, Ventura M, Bach JF, Chatenoud L. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS One. 2015;10:e0125448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 75. | Iatcu CO, Steen A, Covasa M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 207] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 76. | Kaiko GE, Stappenbeck TS. Host-microbe interactions shaping the gastrointestinal environment. Trends Immunol. 2014;35:538-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 77. | Ismail HM, Evans-Molina C. Does the Gut Microbiome Play a Role in Obesity in Type 1 Diabetes? Front Cell Infect Microbiol. 2022;12:892291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 78. | Megur A, Daliri EB, Baltriukienė D, Burokas A. Prebiotics as a Tool for the Prevention and Treatment of Obesity and Diabetes: Classification and Ability to Modulate the Gut Microbiota. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 79. | Pellegrini S, Sordi V, Bolla AM, Saita D, Ferrarese R, Canducci F, Clementi M, Invernizzi F, Mariani A, Bonfanti R, Barera G, Testoni PA, Doglioni C, Bosi E, Piemonti L. Duodenal Mucosa of Patients With Type 1 Diabetes Shows Distinctive Inflammatory Profile and Microbiota. J Clin Endocrinol Metab. 2017;102:1468-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 80. | de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71:1020-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 1249] [Article Influence: 416.3] [Reference Citation Analysis (0)] |
| 81. | Zheng SJ, Luo Y, Xiao JH. The Impact of Intestinal Microorganisms and Their Metabolites on Type 1 Diabetes Mellitus. Diabetes Metab Syndr Obes. 2022;15:1123-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Saffouri GB, Shields-Cutler RR, Chen J, Yang Y, Lekatz HR, Hale VL, Cho JM, Battaglioli EJ, Bhattarai Y, Thompson KJ, Kalari KK, Behera G, Berry JC, Peters SA, Patel R, Schuetz AN, Faith JJ, Camilleri M, Sonnenburg JL, Farrugia G, Swann JR, Grover M, Knights D, Kashyap PC. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun. 2019;10:2012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 83. | Wirth R, Bódi N, Maróti G, Bagyánszki M, Talapka P, Fekete É, Bagi Z, Kovács KL. Regionally distinct alterations in the composition of the gut microbiota in rats with streptozotocin-induced diabetes. PLoS One. 2014;9:e110440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 84. | Wirth R, Bódi N, Szalai Z, Chandrakumar L, Maróti G, Kovács LK, Bagi Z, Mezei D, Balázs J, Bagyánszki M. Perturbation of the mucosa-associated anaerobic gut microbiota in streptozotocin-induced diabetic rats. Acta Biol Szegediensis. 2021;65:75-84. [DOI] [Full Text] |
| 85. | Xu SS, Wang N, Huang L, Zhang XL, Feng ST, Liu SS, Wang Y, Liu ZG, Wang BY, Wu TW, Mu YL, Hou SH, Li K. Changes in the Mucosa-Associated Microbiome and Transcriptome across Gut Segments Are Associated with Obesity in a Metabolic Syndrome Porcine Model. Microbiol Spectr. 2022;10:e0071722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 86. | Zhang L, Wu W, Lee YK, Xie J, Zhang H. Spatial Heterogeneity and Co-occurrence of Mucosal and Luminal Microbiome across Swine Intestinal Tract. Front Microbiol. 2018;9:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 87. | Margolis KG, Gershon MD, Bogunovic M. Cellular Organization of Neuroimmune Interactions in the Gastrointestinal Tract. Trends Immunol. 2016;37:487-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 88. | Brown H, Esterházy D. Intestinal immune compartmentalization: implications of tissue specific determinants in health and disease. Mucosal Immunol. 2021;14:1259-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 89. | Furness JB, Kunze WA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol. 1999;277:G922-G928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 128] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 90. | Arora T, Vanslette AM, Hjorth SA, Bäckhed F. Microbial regulation of enteroendocrine cells. Med. 2021;2:553-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 91. | Fothergill LJ, Furness JB. Diversity of enteroendocrine cells investigated at cellular and subcellular levels: the need for a new classification scheme. Histochem Cell Biol. 2018;150:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 92. | Martin AM, Lumsden AL, Young RL, Jessup CF, Spencer NJ, Keating DJ. The nutrient-sensing repertoires of mouse enterochromaffin cells differ between duodenum and colon. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 93. | Martin AM, Lumsden AL, Young RL, Jessup CF, Spencer NJ, Keating DJ. Regional differences in nutrient-induced secretion of gut serotonin. Physiol Rep. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 94. | Campbell EL, Colgan SP. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2019;16:106-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 95. | Ma H, Zhang B, Hu Y, Wang J, Liu J, Qin R, Lv S, Wang S. Correlation Analysis of Intestinal Redox State with the Gut Microbiota Reveals the Positive Intervention of Tea Polyphenols on Hyperlipidemia in High Fat Diet Fed Mice. J Agric Food Chem. 2019;67:7325-7335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 96. | Uchiyama J, Akiyama M, Hase K, Kumagai Y, Kim YG. Gut microbiota reinforce host antioxidant capacity via the generation of reactive sulfur species. Cell Rep. 2022;38:110479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 97. | Bódi N, Bagyánszki M. Diabetic enteric neuropathy: Imbalance between oxidative and antioxidative mechanisms. Diabetes: Elsevier, 2020: 25-33. |
| 98. | Sotler R, Poljšak B, Dahmane R, Jukić T, Pavan Jukić D, Rotim C, Trebše P, Starc A. Prooxidant activities of antioxidants and their impact on health. Acta Clin Croat. 2019;58:726-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 99. | Sun C, Liu Y, Zhan L, Rayat GR, Xiao J, Jiang H, Li X, Chen K. Anti-diabetic effects of natural antioxidants from fruits. Trends Food Sci Tech. 2020;117. [RCA] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 100. | Surai KP, Surai PF, Speake BK, Sparks NHC. Antioxidant-prooxidant balance in the intestine: Food for thought 2. Curr Top Nutraceutical Res. 2004;2:27-46. |
| 101. | Riaz Rajoka MS, Thirumdas R, Mehwish HM, Umair M, Khurshid M, Hayat HF, Phimolsiripol Y, Pallarés N, Martí-Quijal FJ, Barba FJ. Role of Food Antioxidants in Modulating Gut Microbial Communities: Novel Understandings in Intestinal Oxidative Stress Damage and Their Impact on Host Health. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 102. | Bernardi S, Del Bo' C, Marino M, Gargari G, Cherubini A, Andrés-Lacueva C, Hidalgo-Liberona N, Peron G, González-Dominguez R, Kroon P, Kirkup B, Porrini M, Guglielmetti S, Riso P. Polyphenols and Intestinal Permeability: Rationale and Future Perspectives. J Agric Food Chem. 2020;68:1816-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 103. | Surai PF, Kochish II, Fisinin VI, Kidd MT. Antioxidant Defence Systems and Oxidative Stress in Poultry Biology: An Update. Antioxidants (Basel). 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 299] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 104. | Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int. 2014;2014:761264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 826] [Cited by in RCA: 989] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 105. | Blau S, Rubinstein A, Bass P, Singaram C, Kohen R. Differences in the reducing power along the rat GI tract: lower antioxidant capacity of the colon. Mol Cell Biochem. 1999;194:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 106. | Loguercio C, Di Pierro M. The role of glutathione in the gastrointestinal tract: a review. Ital J Gastroenterol Hepatol. 1999;31:401-407. [PubMed] |
| 107. | McLean JA, Karadas F, Surai PF, McDevitt RM, Speake BK. Lipid-soluble and water-soluble antioxidant activities of the avian intestinal mucosa at different sites along the intestinal tract. Comp Biochem Physiol B Biochem Mol Biol. 2005;141:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 108. | Wang Y, Wu Y, Wang Y, Xu H, Mei X, Yu D, Li W. Antioxidant Properties of Probiotic Bacteria. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 547] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 109. | Mikelsaar M, Zilmer M. Lactobacillus fermentum ME-3 - an antimicrobial and antioxidative probiotic. Microb Ecol Health Dis. 2009;21:1-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 110. | Jancsó Z, Bódi N, Borsos B, Fekete É, Hermesz E. Gut region-specific accumulation of reactive oxygen species leads to regionally distinct activation of antioxidant and apoptotic marker molecules in rats with STZ-induced diabetes. Int J Biochem Cell Biol. 2015;62:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 111. | Hermes-Uliana C, Frez FCV, Sehaber CC, Ramalho FV, de Souza Neto FP, Cecchini R, Guarnier FA, Zanoni JN. Supplementation with l-glutathione improves oxidative status and reduces protein nitration in myenteric neurons in the jejunum in diabetic Rattus norvegicus. Exp Mol Pathol. 2018;104:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 112. | Ferreira PEB, Beraldi EJ, Borges SC, Natali MRM, Buttow NC. Resveratrol promotes neuroprotection and attenuates oxidative and nitrosative stress in the small intestine in diabetic rats. Biomed Pharmacother. 2018;105:724-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 113. | Kochar NI, Umathe SN. Beneficial effects of L-arginine against diabetes-induced oxidative stress in gastrointestinal tissues in rats. Pharmacol Rep. 2009;61:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 114. | Shirpoor A, Ansari MH, Salami S, Pakdel FG, Rasmi Y. Effect of vitamin E on oxidative stress status in small intestine of diabetic rat. World J Gastroenterol. 2007;13:4340-4344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 115. | Chandrakumar L, Bagyánszki M, Szalai Z, Mezei D, Bódi N. Diabetes-Related Induction of the Heme Oxygenase System and Enhanced Colocalization of Heme Oxygenase 1 and 2 with Neuronal Nitric Oxide Synthase in Myenteric Neurons of Different Intestinal Segments. Oxid Med Cell Longev. 2017;2017:1890512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 116. | Onyiah JC, Sheikh SZ, Maharshak N, Otterbein LE, Plevy SE. Heme oxygenase-1 and carbon monoxide regulate intestinal homeostasis and mucosal immune responses to the enteric microbiota. Gut Microbes. 2014;5:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 117. | Ahmed M, Ffrench-Constant C. Extracellular Matrix Regulation of Stem Cell Behavior. Curr Stem Cell Rep. 2016;2:197-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 118. | Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2554] [Cited by in RCA: 2445] [Article Influence: 152.8] [Reference Citation Analysis (0)] |
| 119. | Mecham RP. The Extracellular Matrix: an Overview. Heidelberg: Springer Berlin, 2011. |
| 120. | Hageman JH, Heinz MC, Kretzschmar K, van der Vaart J, Clevers H, Snippert HJG. Intestinal Regeneration: Regulation by the Microenvironment. Dev Cell. 2020;54:435-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 121. | Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, Oyen ML, Cohen Stuart MA, Boehm H, Li B, Vogel V, Spatz JP, Watt FM, Huck WT. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11:642-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1208] [Cited by in RCA: 1141] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 122. | Sánchez SS, Genta SB, Aybar MJ, Honoré SM, Villecco EI, Sánchez Riera AN. Changes in the expression of small intestine extracellular matrix proteins in streptozotocin-induced diabetic rats. Cell Biol Int. 2000;24:881-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 123. | Siegman MJ, Eto M, Butler TM. Remodeling of the rat distal colon in diabetes: function and ultrastructure. Am J Physiol Cell Physiol. 2016;310:C151-C160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 124. | Storkholm JH, Villadsen GE, Jensen SL, Gregersen H. Mechanical properties and collagen content differ between isolated guinea pig duodenum, jejunum, and distal ileum. Dig Dis Sci. 1998;43:2034-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 125. | D'Arpino MC, Fuchs AG, Sánchez SS, Honoré SM. Extracellular matrix remodeling and TGF-β1/Smad signaling in diabetic colon mucosa. Cell Biol Int. 2018;42:443-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 126. | Weston BS, Wahab NA, Mason RM. CTGF mediates TGF-beta-induced fibronectin matrix deposition by upregulating active alpha5beta1 integrin in human mesangial cells. J Am Soc Nephrol. 2003;14:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 127. | Song W, Ergul A. Type-2 diabetes-induced changes in vascular extracellular matrix gene expression: relation to vessel size. Cardiovasc Diabetol. 2006;5:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 128. | Bódi N, Mezei D, Chakraborty P, Szalai Z, Barta BP, Balázs J, Rázga Z, Hermesz E, Bagyánszki M. Diabetes-related intestinal region-specific thickening of ganglionic basement membrane and regionally decreased matrix metalloproteinase 9 expression in myenteric ganglia. World J Diabetes. 2021;12:658-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 129. | Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 488] [Cited by in RCA: 453] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 130. | Gao Q, Meijer MJ, Kubben FJ, Sier CF, Kruidenier L, van Duijn W, van den Berg M, van Hogezand RA, Lamers CB, Verspaget HW. Expression of matrix metalloproteinases-2 and -9 in intestinal tissue of patients with inflammatory bowel diseases. Dig Liver Dis. 2005;37:584-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 131. | Medina C, Radomski MW. Role of matrix metalloproteinases in intestinal inflammation. J Pharmacol Exp Ther. 2006;318:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 132. | Silva LM, Kim TS, Moutsopoulos NM. Neutrophils are gatekeepers of mucosal immunity. Immunol Rev. 2023;314:125-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |