Published online May 7, 2023. doi: 10.3748/wjg.v29.i17.2628
Peer-review started: November 19, 2022
First decision: February 1, 2023
Revised: March 12, 2023
Accepted: April 10, 2023
Article in press: April 10, 2023
Published online: May 7, 2023
Processing time: 168 Days and 9.5 Hours
Inflammatory bowel diseases (IBD) are a worldwide health problem and mainly affect young people, consequently affecting the workforce. Available treatments are often associated with side effects, and new therapeutic options are needed. For centuries, plants have represented important substrates in the field of drug development. Lafoensia pacari (L. pacari) is a plant whose pharmaceutical potential has been described, and may have biological activity relevant to the treatment of IBD symptoms.
To investigate the activity of keto-alcoholic extracts of L. pacari with respect to ameliorating the inflammatory and nociceptive symptoms of acute experimental colitis in mice.
Keto-alcoholic extracts of L. pacari leaves and bark were administered to male and female Swiss mice weighing 25 g to 30 g (n = 8 male mice and n = 8 female mice). The effect of these extracts was observed in an acetic acid-induced acute experimental model of colitis with regard to antinociception/analgesia and inflammatory tissue damage. Recorded macroscopic indices included the Wallace score and the colon weight obtained using a precision scale. Mechanical hyperalgesia was determined using an electronic analgesimeter. Behavior related to overt pain was determined by quantifying the number of writhing instances within 20 min of administration of acetic acid. Molecular docking was performed using human and murine cyclooxygenase-2 (COX-2) with 3 flavonoids (ellagic acid, kaempferol, and quercetin) on the AutoDock Vina software. Analysis of variance followed by Tukey’s posttest was used with P < 0.05 indicating significance.
In this murine model of colitis, administration of extracts from L. pacari ameliorated acetic acid-induced writhing and colitis-associated inflammatory pain. These improvements may be attributable to the reduction in edema, inflammation (e.g., ulcers, hyperemia, and bowel wall damage), and the intensity of abdominal hyperalgesia. The keto-alcoholic extracts of L. pacari leaves and bark administered at a dose of either 100 mg/kg or 300 mg/kg significantly reduced the number of writhing events when compared to the negative control (P < 0.05). Additionally, extracts of L. pacari bark also performed better than Dipyrone. Leaf extracts administered at 10 mg/kg, 30 mg/kg, and 100 mg/kg and bark extracts administered at 30 mg/kg significantly reduced or prevented the development of edema in the colon of treated mice, while mesalazine did not. Moreover, using molecular docking, we observed that the flavonoids present in L. pacari extracts bind to COX-2, an event not unique to ellagic acid.
The results of this study demonstrate a potential novel application of L. pacari extracts for the reduction of inflammation and promotion of antinociception/analgesia as demonstrated by our findings in a murine model of colitis. These findings were also corroborated by in silico analyses, and suggest that L. pacari extracts may be a promising therapeutic agent in the treatment of IBD.
Core Tip: Keto-alcoholic extracts derived from the leaves and bark of Lafoensia pacari (L. pacari) demonstrate significant analgesic/antinociceptive activity in a murine acetic acid-induced pain model of colitis. These results can likely be extrapolated to humans, as the identities and structures of mouse and human cyclooxygenase-2 (COX-2) are similar. Furthermore, mouse and human COX-2 interacted in a similar fashion with L. pacari extract flavonoids when tested in silico. Extracts from both the leaves and bark of L. pacari were found to improve inflammation and pain symptoms in mice with acetic acid-induced acute colitis.
- Citation: Peiter GC, Moesch Queiroz TK, Michalkiewicz Jr EL, Chappuis RH, Luz JS, Casagrande Piovezani LH, Ferreira Silva C, Nozomi Tsutumi M, Fernandes Chaves A, Luiz RM, Façanha Wendel C, Zarpelon-Schutz AC, Teixeira KN. Lafoensia pacari alleviates intestinal damage by modulating cyclooxygenase-2: In silico and in vivo evaluation in a colitis model. World J Gastroenterol 2023; 29(17): 2628-2641
- URL: https://www.wjgnet.com/1007-9327/full/v29/i17/2628.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i17.2628
Inflammatory bowel diseases (IBD) represent a wide spectrum of pathologies that manifest with chronic inflammation of the digestive tract and in many cases may not have a defined etiology[1]. The major diseases under the umbrella of IBD are Crohn's disease and ulcerative colitis, which are both characterized by the presence of an inappropriate immune response against gastrointestinal microbiota and other antigens in the digestive tract lumen[2]. Individual genetic susceptibility and other external factors are also important in the pathogenesis of IBD[3].
IBD occurs worldwide and represents a serious health problem, as it affects young people, frequently relapses, and can often be clinically severe. As such, IBD commonly negatively impacts patient quality of life[4,5].
Conventional pharmacological treatment for IBD is associated with many adverse effects[6] and is often associated with long-term resistance[7]. These phenomena are observed with aminosalicylates, corticosteroids, immunosuppressants, immunomodulators, and antibiotics, all of which are commonly used for induction of remission and are also used for disease maintenance as these drugs reduce inflammatory lesions[8]. Therefore, the search for new drugs to treat IBD is critical. Research on medicinal plants could lead to the discovery of bioactives with anti-inflammatory properties that are capable of curbing the symptoms of IBD with the benefit of avoiding adverse reactions associated with current drugs. Lafoensia pacari (L. pacari) (Lythraceae), a tree from the Brazilian Cerrado popularly known as dedaleiro, mangava-brava, or pacari, is a fever reducer and promoter of healing according to folk knowledge, and is a candidate for an alternative IBD treatment. Pharmacological trials using extracts derived from the leaves and bark of this plant have demonstrated its antifungal, antimicrobial, and anti-free radical properties. L. pacari has also been used in the treatment of gastric ulcers and inflammation[9].
Preclinical models of intestinal inflammation are important tools in the study of bioactives, and can assist in the identification of compounds with therapeutic potential, including drugs used in the treatment of IBD such as mesalazine and sulfasalazine[10].
The most common IBD symptom is abdominal pain, and is even reported in individuals in remission. Yet, IBD-associated abdominal pain is undertreated and there is a scarcity of research on this common issue[11]. Therefore, a drug with antinociceptive (reducing pain via modulation of nociceptive pathways[12]) and anti-inflammatory activities would be ideal for IBD therapy.
In view of the colloquial sense of the effects of L. pacari, it is possible that its components may be able to help improve the symptomatology of IBD through its antinociceptive and anti-inflammatory actions. Here, we tested this hypothesis using an in vivo preclinical mouse model and in silico analysis of molecular docking to elucidate the bioactive potential of keto-alcoholic extracts of L. pacari leaves and bark.
Leaves and bark of L. pacari were collected in the cerrado of Presidente Olegário, Minas Gerais, Brazil (coordinates 18°24'56” S; 46°25'17” W) in November 2021. The samples were analyzed by a biologist from the Centro Universitário de Patos de Minas (Minas Gerais, Brazil). The material was processed according to a previously published protocol[13] with some modifications. Leaves and bark of L. pacari were sanitized, disinfected, washed, dried at 40 ºC for 72 h, and finally ground.
The keto-alcoholic extracts were prepared according to another previously published protocol[14] with modifications. The ground material was mixed in a 1:1 acetone/methanol solution in a 1:10 plant/solution ratio, sonicated (5 cycles of 5 min on/5 min off), incubated at room temperature for 24 h, filtered, and evaporated. The entire extraction process was carried out in the dark. For the in vivo experiments, the extracts were solubilized in dimethyl sulfoxide (DMSO) 2% (v/v) in saline [NaCl 0.9% (w/v)].
Keto-alcoholic extracts were submitted for total flavonoid content determination by spectrophotometry using a methanol solution of AlCl3 5% (w/v). Quercetin was used as the standard with results expressed as quercetin equivalents[15].
This study was approved by the Ethics Committee for Animal Use of the Setor de Ciências Biológicas at the Universidade Federal do Paraná, Paraná, Brazil (CEUA no. R.O. 07/2022). Male and female Swiss mice weighing 25 g to 30 g were used in the experiments. The animals were kept in the animal house of the Universidade Federal do Paraná - Toledo Campus in polypropylene boxes (41 cm × 33 cm × 16 cm) with pine shavings. Animals had access to rodent food and chlorinated drinking water ad libitum and were kept at a controlled temperature (22 ºC ± 2.0 ºC), in a 12 h light/dark cycle.
Female Swiss mice weighing 25 g to 30 g were used (n = 8 per group). The induction of nociceptive response was achieved by intraperitoneal administration of acetic acid [0.8% (v/v)] in saline (10 mL/kg). The noxious stimulus was given 30 min after administration via gavage of keto-alcoholic extracts of L. pacari leaves and bark (10 mg/kg, 30 mg/kg, 100 mg/kg, or 300 mg/kg), saline (negative control), dipyrone monohydrate 100 mg/kg (positive control), DMSO 2% (v/v), or ellagic acid (a flavonoid found in L. pacari) in DMSO 2% (v/v) in saline (1.6 mg/kg)[16]. The mice were individually housed in glass cylinders and writhing and paw extension events were recorded over a span of 20 min. The intensity of the contortional response was expressed by the cumulative number of contortions over this 20 min period[17,18].
The following experiment lasted for 14 h. After 24 h of solid fasting, male Swiss mice weighing 25 g to 30 g (n = 8 per group) were anesthetized with isoflurane (1.5% in 100% oxygen). At hour 0 and hour 6, the mice were pretreated with keto-alcoholic extracts of L. pacari leaves and bark (10 mg/kg, 30 mg/kg, 100 mg/kg, or 300 mg/kg), mesalazine 200 mg/kg (positive control), or saline (negative control). Mice in which colitis was not induced were used as controls. At hour 9, saline enema was performed on all animals. At hour 10, induction of experimental acute colitis was achieved with 7.5% (v/v) acetic acid administered rectally.
The intracolonic injection was performed with a polyethylene cannula measuring 3 cm in length[19]. At hour 12, treatment was administered using the same protocol as for pretreatment. At hour 13, the animals were subjected to mechanical hyperstimulation (Von Frey nociception test). At hour 14, the animals were euthanized by overdose of isoflurane followed by cervical dislocation.
The distal colon of each animal was collected and washed with saline to remove fecal debris. Macroscopic evaluation of each specimen was performed and the severity of lesions was rated using a numerical score (from 0 to 6)[20]. The presence of edema was also evaluated[19,21] by weighing a 2 cm colonic segment using a precision digital scale.
Visceral mechanical hyperalgesia was assessed using the electronic Von Frey test[22]. Mice were allocated to a box in a temperature-controlled room for at least 45 min prior to testing. Withdrawal reflexes were elicited with a portable force transducer (Digital Algesimeter EFF 310; Insight Ltda, São Paulo, Brazil) fitted to a 0.7 mm2 polypropylene tip. This apparatus was applied to the lower abdomen up to the mid-abdomen of each animal. After tip removal, the pressure intensity was automatically recorded (recording values from the average of 3 measurements).
The means of the values obtained for each group in each experiment were calculated and analyzed using Prism 9.0.1 software. One-way analysis of variance was used to compare groups according to treatment dose followed by Tukey's test. Significance was set at P < 0.05.
In order to better understand the results of the in vivo antinociceptive testing, an in silico molecular docking technique was used to evaluate the activity of flavonoids present in L. pacari[23] with respect to murine cyclooxygenase-2 (COX-2). The same simulations were performed with human COX-2 for extrapolation of the murine data. Primary sequences of both murine and human COX-2 were analyzed by global alignment using ClustalX 2.1 software[24] for obtaining identity data.
The structure files of the flavonoids (ligands) were obtained from the ZINC database (zinc.docking.org). The 2-dimensional ".sdf" files were converted into 3-dimensional ".pdb" files using the ViewerLite 5.0 software (Accelrys Inc., San Diego, CA, United States). The 3-dimensional structure of murine COX-2 (receptor) was obtained from the Protein Data Bank (PDB) (rcsb.org).
Molecular docking was performed using flexible ligandrigid receptor methodology[25]. For the execution of molecular docking, the AutoDock Tools 4[26] and AutoDock Vina v.1.2.0[27] programs were used. Preparation of molecules was performed, which included the detection of torsion points and calculation of the torsion angles of the ligands, besides the delimitation of the region where the docking was performed (Grid box).
The determination of total flavonoid content indicated 97.38 ± 0.0091 (97.38%) and 47.95 ± 0.0035 (47.95%) quercetin/g dry mass equivalents in L. pacari leaves or bark, respectively (i.e. 97.38 g and 47.95 mg of total flavonoids/g dry mass of leaves or bark, respectively).
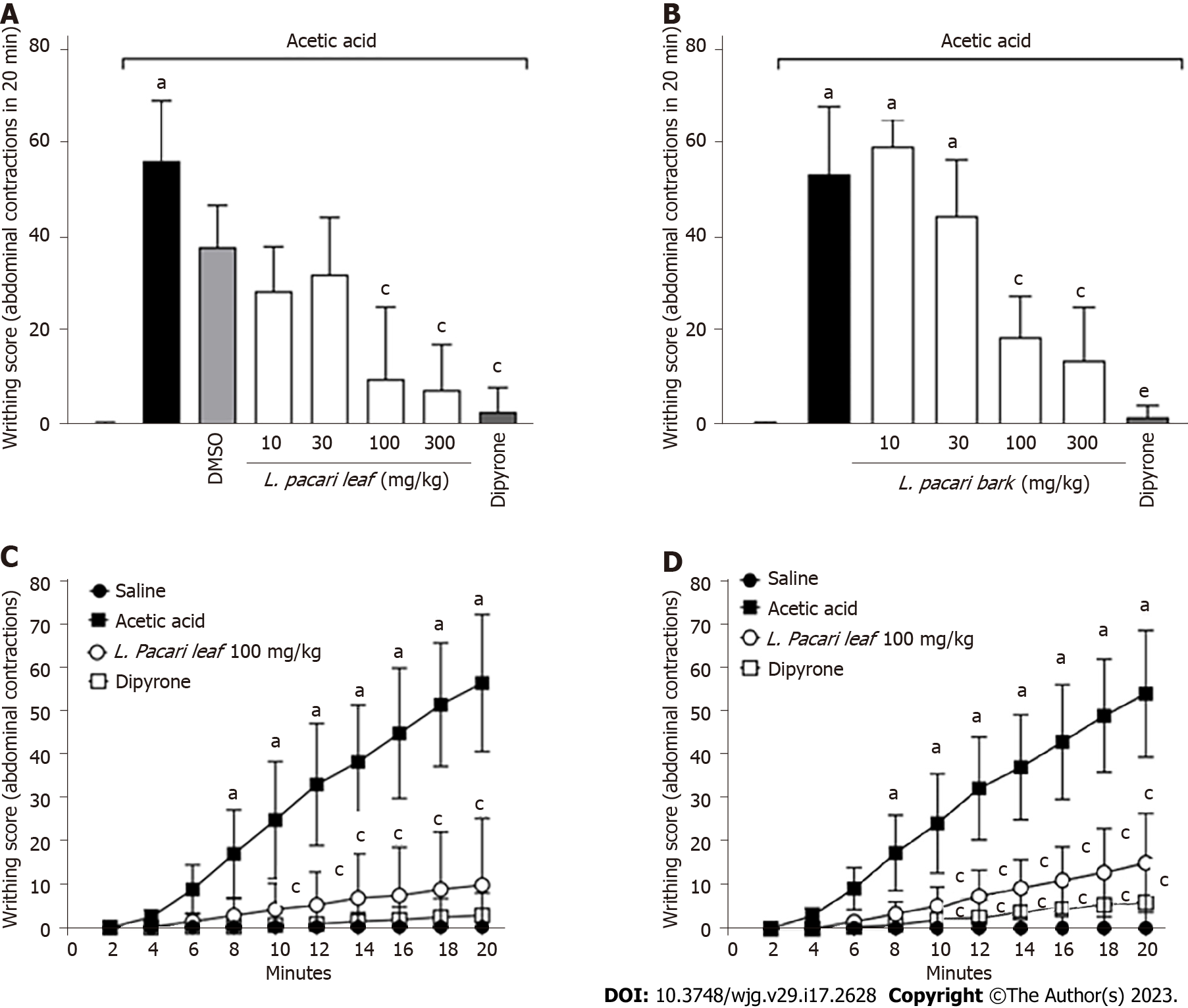
The writhing test revealed that DMSO 2% (v/v) did not influence the number of painful contortions when compared to the acetic acid noxious stimulus. A statistically significant decrease in the number of writhing events was observed in mice treated with dipyrone when compared to negative controls (Figure 1).
With respect to the keto-alcoholic extracts of L. pacari leaves, doses of 100 mg/kg and 300 mg/kg reduced the number of acetic acid-induced writhing events significantly as compared to negative controls, but positive controls (dipyrone-treated; Figure 1A). The keto-alcoholic extracts of L. pacari bark at 100 mg/kg and 300 mg/kg significantly reduced the number of acetic-acid induced writhing events as compared to negative controls and also when compared to dipyrone (Figure 1B).
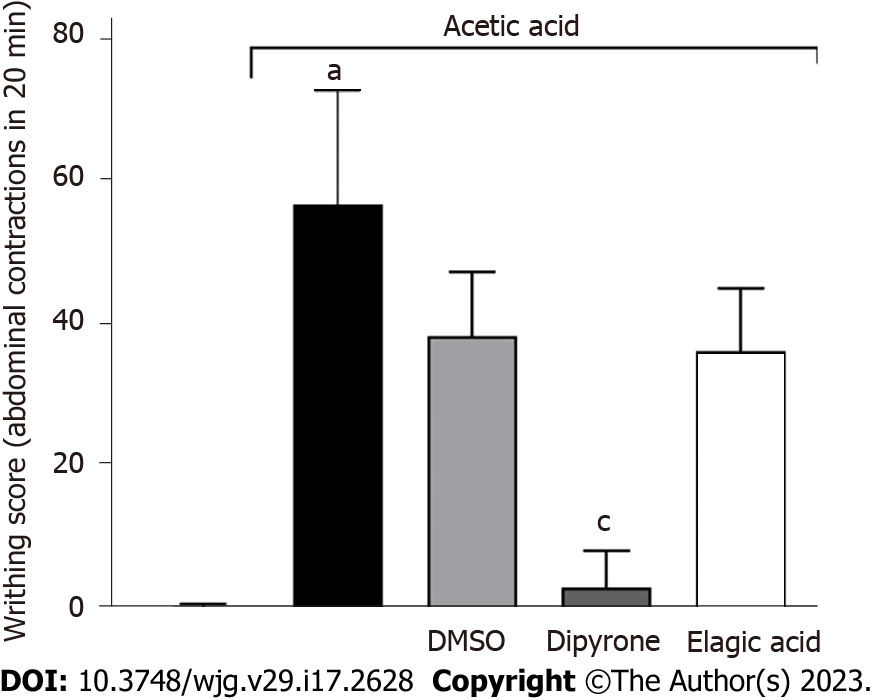
No statistically significant difference was observed between the antinociceptive potential of the low and high L. pacari leaves or bark extract doses (100 mg/kg and 300 mg/kg, respectively). The lower dose (100 mg/kg) was found to act in a time-dependent manner, exhibiting effects 10 min after administration (Figure 1C and D). Ellagic acid alone, administered at 1.6 mg/kg, did not significantly reduce the number of contortions (Figure 2).
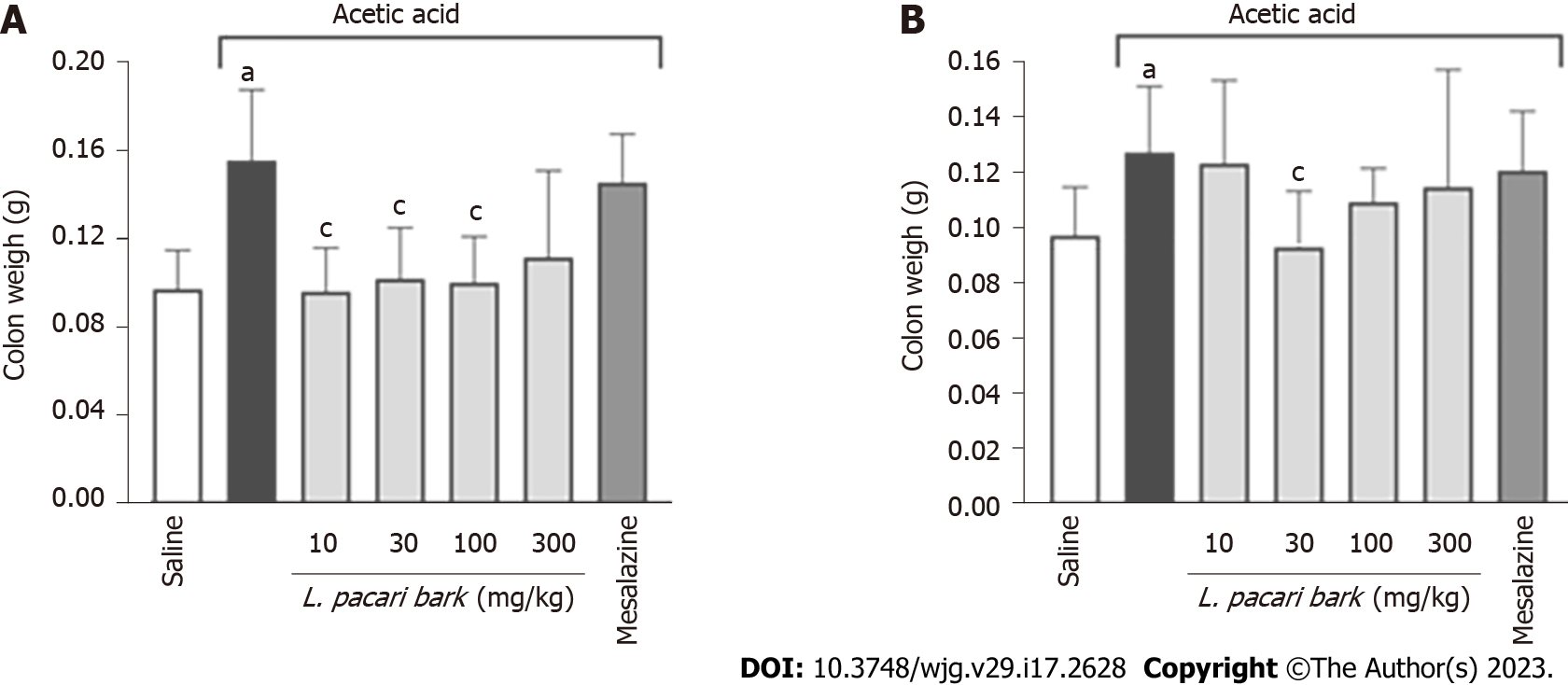
Colitis-induced edema was observed and quantified by weighing the removed colonic segment. Colon segment weight of colitis mice was higher than that of healthy mice with no colitis induction. Keto-alcoholic extracts of L. pacari leaves administered at doses of 10 mg/kg, 30 mg/kg, and 100 mg/kg significantly reduced or prevented the development of colonic edema in treated mice (Figure 3A); similar results were observed in mice treated with the keto-alcoholic extracts of L. pacari bark administered at 30 mg/kg (Figure 3B), as evidenced by the weight of colonic segments of these animals being similar to that of healthy mice. Treatment with mesalazine did not improve edema.
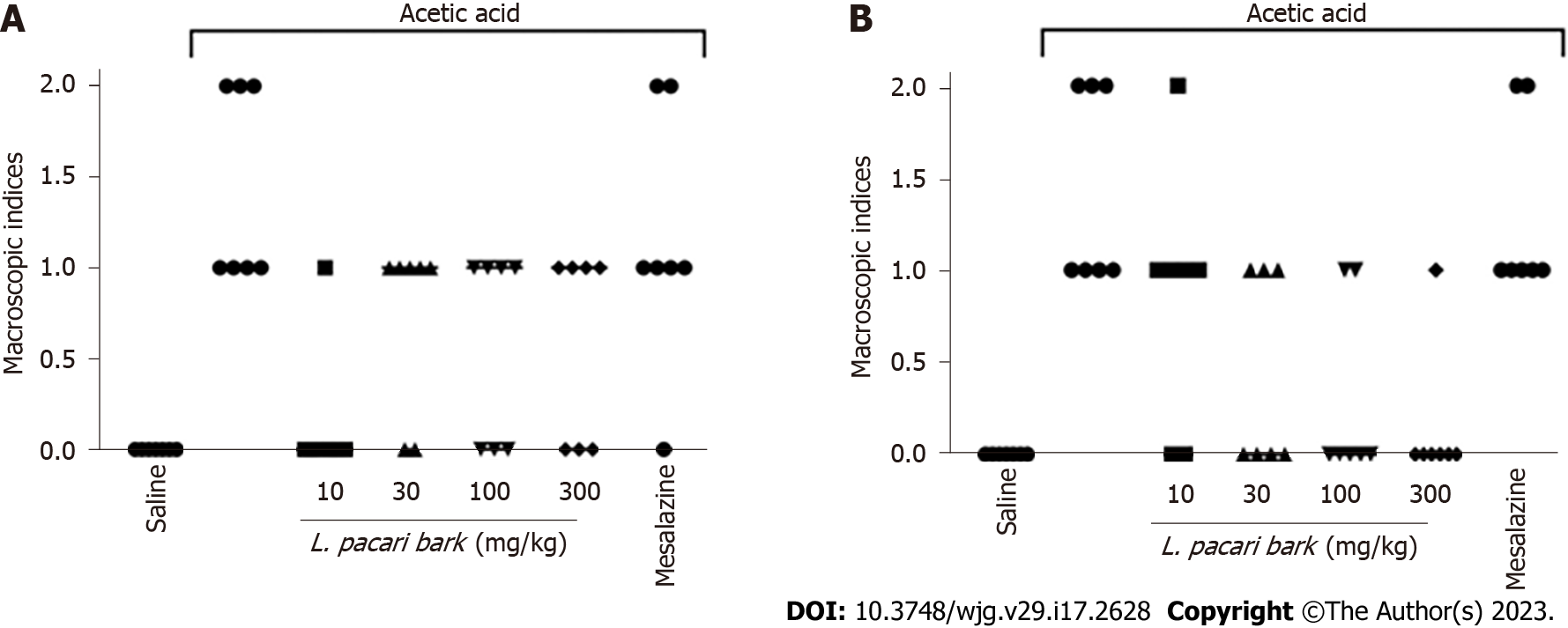
Numerical lesional severity scores as assigned by gross assessment of colonic segments ranged from 0 to 2 (0 = no lesion; 1 = hyperemia without ulceration; 2 = ulceration without intestinal wall hypertrophy). No control mice were found to have lesions (score 0), and untreated mice with induced colitis demonstrated scores of 1 or 2 (Figure 4). Gross assessment of colonic segments of mice treated with keto-alcoholic extracts of L. pacari bark at 30 mg/kg, 100 mg/kg, and 300 mg/kg had fewer lesions than untreated mice (Figure 4B); similar results were observed in mice treated with keto-alcoholic extracts of L. pacari leaves at 10 mg/kg (scores = 0 or 1; Figure 4A). In general, the keto-alcoholic extracts of L. pacari showed anti-inflammatory activity in the colon of animals with acute colitis. Mice treated with mesalazine showed intestinal lesions with scores of 1 or 2, suggesting poorer performance of this drug when compared to keto-alcoholic extracts of L. pacari leaves or bark.
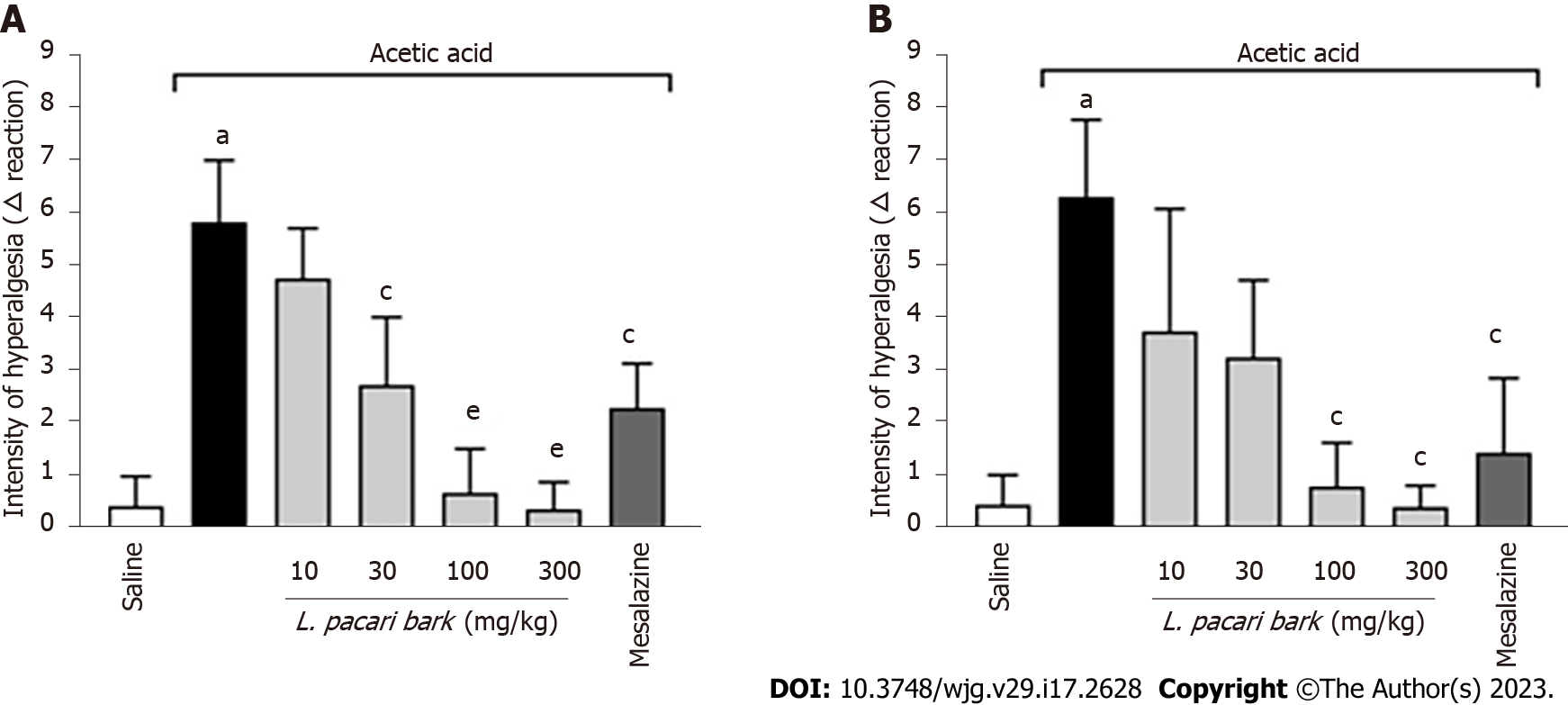
Results of the Von Frey test were expressed as the difference between the intensity required to elicit a positive response before and after the induction of acute colitis by acetic acid. Mice treated with the keto-alcoholic extract of L. pacari leaves at 30 mg/kg, 100 mg/kg, or 300 mg/kg or mesalazine showed greater analgesic effect when compared to untreated mice. The keto-alcoholic extract of the leaves administered at 100 mg/kg or 300 mg/kg performed better than the extract administered at 30 mg/kg or mesalazine (Figure 5A).
With respect to the keto-alcoholic extract of L. pacari bark, mice treated with 100 mg/kg or 300 mg/kg or mesalazine required a higher intensity of stimulus to elicit a positive response when compared to negative controls. No significant difference was observed among these 3 treatments, although both 100 mg/kg and 300 mg/kg doses of the extract trended toward higher analgesic activity when compared to mesalazine (Figure 5B).
The 3-dimensional structures of murine COX-2 (PDB: 3LN0) and human COX-2 (PDB: 5 LKQ) were selected from PDB; these were originally complexed to compound 5c-S and meclofenamic acid, respectively. These compounds were removed for analysis in this study. Human and murine COX-2 are homologous and have similar structures. The number of total residues in the primary sequence of both is 604, and the number of residues resolved by X-ray diffraction of human COX-2 and murine COX-2 is 551 and 587, respectively. After global alignment analysis, it was observed that these enzymes have 86.6% primary sequence identity.
Molecular docking simulations with COX-2 were performed with 3 flavonoids: ellagic acid (ZINC000003872446), kaempferol (ZINC000003869768) and quercetin (ZINC000003869685). It was observed that all flavonoids bind to chain A, the catalytic monomer of COX-2, with significant affinity (Table 1) and possibly, under physiological conditions, the interaction is stable and negatively affects enzyme activity.
| Flavonoid | Energy of affinity in kcal/mol | |
| Human COX-2 | Murine COX-2 | |
| Ellagic acid | -9.7 | -9.9 |
| Kaempferol | -9.9 | -8.8 |
| Quercetin | -10.1 | -9.5 |
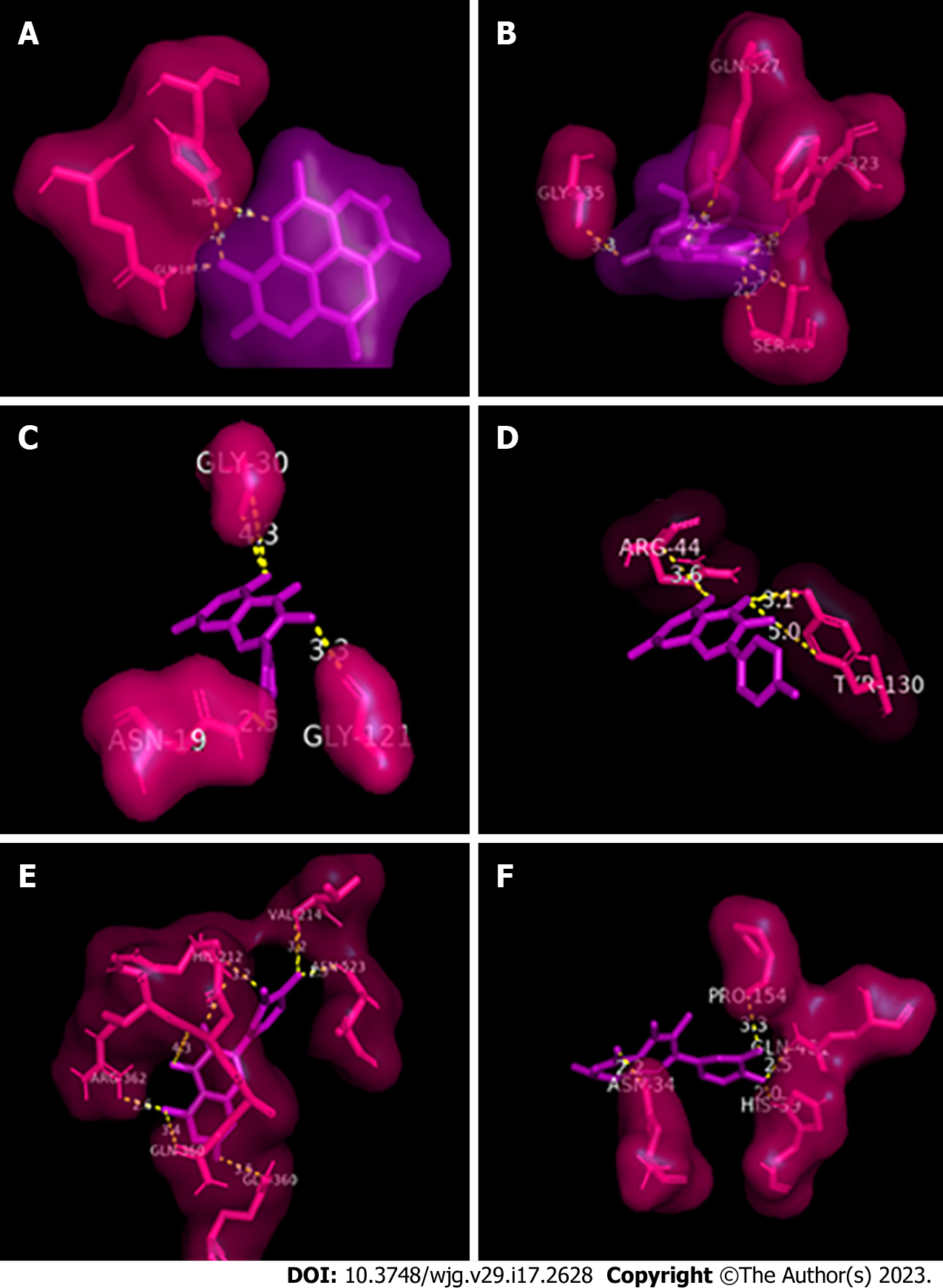
Ellagic acid was bound to 2 amino acid residues of murine COX-2 (3 bonds; 2.0-2.8 Å) and to 4 residues of human COX-2 (5 bonds; 2.1-3.3 Å; Figure 6A and B). Kaempferol bound to 3 residues of murine COX-2 (3 bonds; 2.5-4.3 Å) and 2 residues of human COX-2 (3 bonds; 3.1-5.0 Å; Figure 6C and D), and quercetin bound to 6 residues of murine COX-2 (8 bonds; 2.3-4.3 Å) and 4 residues of human COX-2 (4 bonds; 2.0-3.3 Å; Figure 6E and F). None of the flavonoids were bound to the prosthetic group (heme group) of COX-2. The values of the affinity energies are similar to each other, the most significant interactions were between ellagic acid and murine COX-2 and between quercetin and human COX-2.
Using extraction in acetone/methanol solution, 97.38 ± 0.0091 mg total flavonoids/g dry mass of the leaves and 47.95 ± 0.0035 mg total flavonoids/g dry mass of the bark of L. pacari were obtained; in crude hydroalcoholic and methanolic extracts of leaves, 15.7 ± 0.02 and 14.4 ± 0.04 equivalents of quercetin were obtained, respectively[28]. In another study[29] 13.61% ± 0.72 equivalents of quercetin was obtained from an aqueous extraction of the bark of L. pacari. Comparatively, the keto-alcoholic extraction was more efficient in obtaining total flavonoids from both leaves and bark of L. pacari.
The antinociceptive effect of the keto-alcoholic extracts of L. pacari leaves and bark was demonstrated by the writhing test, an assay of nociception using injection of a noxious agent[30]. When tissues and cells are exposed to a noxious stimulus, chemical mediators are released, which in turn stimulate C-type nerve fibers causing local pain. Acetic acid has properties that can cause pain, in addition to stimulating the release of cytokines (IL-1β, TNF- and IL-8) by macrophages and basophils present in the abdominal cavity[31]. The writhing test is considered a model for studying visceral pain[32]; keto-alcoholic extracts of L. pacari leaves and bark decreased the number of writhing events in this test, suggesting the efficacy of these extracts in treating this type of pain.
The extracts of L. pacari leaves and bark administered at 100 mg/kg and 300 mg/kg, reduced the number of painful contortions in the murine colitis model; however, there was no significant difference between these dosages in terms of analgesic effect. However, the extract administered at 300 mg/kg (the highest concentration used in this study) did not exert such a potent antinociceptive effect when compared to the positive control group (dipyrone-treated).
The aqueous extract of L. pacari bark administered at 0.5 g/kg and 1.0 g/kg had previously shown an antinociceptive effect in the writhing test[33]. In a study using the ethanolic extract of L. pacari leaves administered at 1.0 g/kg, the extract reduced the number of writhing events, especially when it was administered subcutaneously; however, this dose caused toxicity and death in treated animals[31]. Therefore, although the dose of 300 mg/kg is still below the accepted toxic value[31], in order to avoid undesirable adverse effects, the 100 mg/kg dose of keto-alcoholic L. pacari extract may be more appropriate.
The choice of oral administration of the extracts was based on the better viability and ease of administration considering future drug development from the plant's bioactive compounds. As we observed analgesic activity when extracts were administered orally, it seems that this route of administration is sufficient for absorption of the active compound at a therapeutic level.
In comparison, one study evaluated the analgesic and anti-inflammatory activity of total flavonoids from the leaves of Juniperus sabina, a plant commonly known as sabina native to Europe and Asia. This study indicated significant dose-dependent analgesic activity in the acetic acid-induced writhing test, with the highest dose (500 mg total flavonoids/kg animal mass) showing the greatest inhibition of writhing (22.02%)[34]. In the present study, the most effective inhibitory dose was 100 mg/kg of dry mass of L. pacari leaves and bark, which is equivalent to 9.738 mg/kg and 4.795 mg/kg total flavonoids, respectively.
Other studies have investigated the ethanolic extract of white willow as an anti-inflammatory and antinociceptive, with results similar to ours in which the plant extract was effective in an animal model, but the positive control treatment was more effective, even at lower concentrations[35]. Another study aimed to elucidate the effects of an ethanolic extract of L. pacari on acute peritonitis in mice[9], and demonstrated significant anti-inflammatory activity of this extract. This finding may be akin to the antinociceptive effect of the keto-alcoholic L. pacari extracts demonstrated in the present study.
Although this study does not present the rate of inhibition of writhing, the difference in concentration of total flavonoids used per body mass in each study suggests that flavonoids from L. pacari are more effective, as analgesia was observed at lower doses. One possible explanation for this observation may be the types of flavonoids present in each plant species analyzed, and those of L. pacari may act more synergistically to produce an analgesic effect.
Fractionation of the L. pacari bark extract leads to the isolation of ellagic acid as the main flavonoid compound, suggesting that this compound is responsible for the anti-inflammatory and anti-edema activity of the extract observed in mouse models of asthma. Ellagic acid was also identified in L. pacari leaves by high performance liquid chromatography[31]. Recently, evidence regarding the efficacy of ellagic acid in the treatment of acute visceral pain has been growing; its analgesic effects are attributed to the inhibition of COX, which synthesizes prostaglandins at sites of peripheral cell damage[36].
Ellagic acid is reported to have several benefits, such as anti-inflammatory, anticancer, and hepatoprotective actions, and is also a promising agent for the treatment of chronic diseases such as ulcerative colitis, Crohn’s disease, Alzheimer's disease, and diabetes[37]. However, according to the results obtained in this study, ellagic acid was not the only phenolic compound present in the extract of the L. pacari that may be responsible for the observed analgesic effect. In a statistical comparison, its effect resembles that of the 30 mg/kg dosage of leaf extract in the twitch test. The relatively low efficiency of ellagic acid compared to other doses of extract indicates that this flavonoid likely acts synergistically with other compounds present in the plant to confer a more robust analgesic pharmacological effect.
Regarding acute experimental colitis, the keto-alcoholic extracts of L. pacari leaves administered at 10 mg/kg, 30 mg/kg, or 100 mg/kg significantly reduced or prevented the development of colonic in treated mice. The same was observed in mice treated with the keto-alcoholic extract of L. pacari bark administered at 30 mg/kg as evidenced by similar colonic segment weights between treated and health mice.
Gross evaluation of colonic segments of mice revealed lower lesional severity scores in mice receiving keto-alcoholic extracts of L. pacari leaves and bark, with further reduction in the groups that received a higher dose. The mice that received mesalazine had similar lesional severity scores compared to the saline-treated group; this may be due to the fact that mesalazine typically used in the treatment of chronic inflammatory bowel disease[38,39], and we used an acute colitis model.
The macroscopic findings suggest that the keto-alcoholic extracts of L. pacari leaves and bark have an anti-inflammatory effect on the mucosa of the gastrointestinal tract and reduce tissue destruction in this model of acute colitis. In agreement with these findings, one study showed that a methanolic extract of the bark of L. pacari had a potent gastroprotective effect against ethanol-, indomethacin-, and stress-induced ulcers[40].
Another study showed that an ethanolic extract of L. pacari possesses healing properties when applied to skin wounds, accelerating healing when compared to controls. This is attributed to the inhibition of the proliferation and inflammation phases of the healing process, corroborating the findings in our acute colitis induction experiments, which showed reduced colonic tissue damage in mice treated with L. pacari extracts. These data suggest a negative modulation of tissue inflammatory processes[23].
The anti-inflammatory effects of extracts derived from L. pacari leaves have also been demonstrated in a murine model of asthma. One study showed a significant reduction in lung inflammation and tissue damage in animals that received the extract as evidenced by gross tissue damage score analysis[41]. These data corroborate the results of the present study, which also demonstrated the anti-inflammatory effects of keto-alcoholic extracts of L. pacari leaves and bark in reducing macroscopic damage to colonic tissue.
Both the Von Frey monofilament and the electronic Von Frey tests are used as quantitative sensory assays to explore mechanical hyperalgesia[42]. In this study, pain stimuli were administered to mice using the electronic Von Frey test, and the keto-alcoholic extracts of L. pacari leaves administered at 30 mg/kg, 100 mg/kg, or 300 mg/kg or mezalazine showed greater analgesic effect when compared to untreated mice. Among the treatments, the keto-alcoholic extract administered at 100 mg/kg or 300 mg/kg performed better than the extract at 30 mg/kg or mesalazine.
Regarding the keto-alcoholic extract of L. pacari bark, mice treated with the doses 100 mg/kg or 300 mg/kg or mesalazine required a higher intensity stimulus to induce a positive response when compared to the negative controls. No significant difference was observed among these 3 treatments, although the keto-alcoholic extract at 100 mg/kg or 300 mg/kg had greater analgesic effect as compared to mesalazine. It is important to emphasize that, since this is a study based on a model of acute colitis, the findings regarding mesalazine are consistent with what is expected, since this drug has a better effect with long-term use[43]. The Von Frey test was one of the first evoked stimulus tests to be developed and continues to be widely used today[44]. This test is an excellent tool for evaluating hyperalgesia, visceral pain, and inflammation[45].
Molecular docking is an important method to complement and help better understand results obtained by in vivo testing. In humans, despite being structurally a homodimer, COX-2 functions as a conformational heterodimer, with 1 monomer being catalytic and the other allosteric[46]. In our experiments, the catalytic monomer, composed of the A chain, was the interaction target of ellagic acid, kaempferol, and quercetin in both human and murine COX-2.
We observed that the evaluated flavonoids bind to COX-2, but also that the interactions are strong. Binding affinity is measured by the affinity energy, which when lower than -6.0 kcal/mol indicates interaction in a biological environment[47]. Although the values are expressive, they are all within a narrow range (-8.8 1 kcal/mol to 10.1 kcal/mol). Our data suggest that COX-2 inhibition after interaction with any of the flavonoids tested should not trigger events other than the expected inhibition of prostaglandin production and consequent decrease in pain sensation.
Our findings also demonstrate that ellagic acid is not the only compound of interest with respect to analgesia found in the keto-alcoholic extracts of L. pacari leaves and bark. The affinity energy evaluated by molecular docking represents the strength of interaction between 2 or more interacting molecules. The strength of interaction is favorable when it is negative, and the receptor/ligand interaction is greater when the energy is lower.
Corroborating our study, another demonstrated that quercetin is also effective in inhibiting hyperalgesia[48]. This flavonoid showed an analgesic effect in animal models of inflammatory pain, inhibiting hyperalgesia induced by mechanical or thermal stimuli, and reduced the pain behaviors of studied animals. In addition, it also inhibited the development of inflammation-related edema, cytokine production, myeloperoxidase activity, and cell migration[49].
Nonsteroidal anti-inflammatory agents (NSAIDs) are part of a heterogeneous group of compounds from different chemical classes, and have 3 main actions: Anti-inflammation, analgesia, and fever reduction. NSAIDs are the most commonly used therapeutic agents[50]. Although they are effective, their long-term use is limited in most patients, as they are associated with adverse gastrointestinal effects such as abdominal pain, ulcers, bleeding, and even gastric or duodenal perforation. These effects have been attributed to the low specificity of these compounds for COX-2[51]. Acetylsalicylic acid (ASA) promotes the irreversible inhibition of human COX activity by covalent binding to a serine residue within the active site.
In COX-1, ASA acetylates serine 530, preventing the binding of arachidonic acid to the enzyme's active site, thus suppressing the production of prostaglandins. In COX-2, ASA acetylates serine 516, blocking the activity of this isoform[50,51]. In our study, in silico analysis showed that these amino acid residues, which are key to the action of some analgesics, showed no direct interaction with the evaluated flavonoids, demonstrating that in this model there are other residues involved in COX-2 inhibition.
We demonstrated the anti-inflammatory effects of keto-alcoholic extracts of L. pacari leaves and bark in a murine acetic acid-induced colitis model. Both extracts inhibited colitis-induced tissue damage as assessed using the Wallace score and the development of colonic edema; this effect seems to be COX-2-dependent. The flavonoid content of L. pacari extracts has been used to treat many diseases, and shows anti-inflammatory, analgesic, and antioxidant properties. However, the effects of this plant on symptoms related to colitis have not been described. Using molecular docking, we showed a strong interaction between ellagic acid, kaempferol or quercetin with both human and murine COX-2; however, the inflammatory and antinociceptive effects of L. pacari are not solely attributable to ellagic acid. Taken together, these results suggest the potential of oral L. pacari extract administration in the treatment of inflammatory diseases such as colitis.
Therapeutic activities of Lafoensia pacari (L. pacari) have been reported in folk medicine, and this plant has been used as an antifungal, an anti-ulcer, an antibacterial, an anti-inflammatory, a fever reducer, in the treatment of pneumonia, and in the treatment of stomach pain. Despite the common use of this plant, the mechanisms by which it achieves medicinal effects have not been elucidated.
L. pacari is a tree found in Brazil thought to have beneficial pharmacological properties, with its main bioactive compounds belonging to the flavonoid class. One of the most studied flavonoids found in this plant is ellagic acid, which has been reported to have anti-inflammatory activity. However, L. pacari contains numerous flavonoids, and these compounds may also have similar activity to or act synergistically with ellagic acid.
We aimed to evaluate the activity of keto-alcoholic extracts of L. pacari leaves and bark, which contain a significant amount of flavonoids, with respect to the improvement of symptoms related to inflammatory bowel diseases (e.g., inflammation of the intestinal mucosa, edema, and abdominal pain).
The methodology used in this study was based on protocols already well established in the scientific literature, and, when necessary, some modifications were made. All experiments were performed with a number of mice that allowed for statistical analysis of the data and were performed in duplicate or triplicate depending on statistical requirements.
Our results corroborated those described by other studies with respect to the beneficial effects of L. pacari extracts; however, to our knowledge, we are the first to evaluate the therapeutic potential of these extracts in a murine model of acute colitis. Antinociception/analgesia in this murine model were observed with respect to treatment with extracts derived from L. pacari leaves and bark.
Our findings suggest that keto-alcoholic extracts of L. pacari leaves and bark are beneficial for the reduction of symptoms related to inflammatory bowel disease. These effects are attributed to the decrease or inhibition of inflammation in the intestinal mucosa. Regarding pain, bioinformatics techniques indicated that ellagic acid alone is not responsible for these effects, since other flavonoids interact and inhibit with significant affinity cyclooxygenase-2, an enzyme that produces pain mediators.
This study is part of a larger research project; follow-up experiments are planned using a rat model of chronic colitis, other antinociception tests. Future experiments will also investigate the histology of intestinal segments after induction and treatment of chronic colitis with L. pacari extracts.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Howarth GS, Australia; Petrovic S, Serbia; Wang WJ, China; Yao SK, China S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Yu HG
| 1. | Sairenji T, Collins KL, Evans DV. An Update on Inflammatory Bowel Disease. Prim Care. 2017;44:673-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 342] [Article Influence: 42.8] [Reference Citation Analysis (10)] |
| 2. | Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1352] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 3. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2746] [Article Influence: 119.4] [Reference Citation Analysis (2)] |
| 4. | Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014;7:113-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 332] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 5. | Figueroa C C, Quera P R, Valenzuela E J, Jensen B C. [Inflammatory bowel disease: experience of two Chilean centers]. Rev Med Chil. 2005;133:1295-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Carter MJ, Lobo AJ, Travis SP; IBD Section, British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5:V1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 772] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 7. | Cai Z, Wang S, Li J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front Med (Lausanne). 2021;8:765474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 263] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 8. | Stenson WF, Korzenik J. Inflammatory bowel disease. In: Yamada T, Alpers DH, Laine L, editors. Yamada's textbook of gastroenterology. 4th ed. Philadelphia: Lippincott Williams & Wilkins Publishers, 2003: 1699–759.. |
| 9. |
Albuquerque DA, Juliani JM, Santos JA, Hosida PY, Borges S, Borralho CT.
Effect of |
| 10. | Melo NM da C, Almeida MVS, Campos DM de O, Oliveira CBS de, Oliveira JIN. Animal models for inducing inflammatory bowel diseases: integrative review. Revista Ciências em Saúde. 2021;11:80-87. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Norton C, Czuber-Dochan W, Artom M, Sweeney L, Hart A. Systematic review: interventions for abdominal pain management in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Basbaum CB, Mann JK, Chow AW, Finkbeiner WE. Monoclonal antibodies as probes for unique antigens in secretory cells of mixed exocrine organs. Proc Natl Acad Sci U S A. 1984;81:4419-4423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. |
Albuquerque ER, Gossler SC, Piovezan M, Conto LC.
Extraction and determination of flavonoids, condensed and total tannins from pine nut bark ( |
| 14. |
Oliveira MA.
Extraction of polyphenols from cocoa seed ( |
| 15. | Dutra RP, Nogueira AMC, Marques RRO, Costa MCP, Ribeiro MNS. Avaliação farmacognóstica de geoprópolis de Melipona fasciculata Smith (tiúba) em municípios da Baixada maranhense, Brasil. Rev Bras Farmacogn. 2008;18:557-562. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Rogerio AP, Fontanari C, Melo MC, Ambrosio SR, de Souza GE, Pereira PS, França SC, da Costa FB, Albuquerque DA, Faccioli LH. Anti-inflammatory, analgesic and anti-oedematous effects of Lafoensia pacari extract and ellagic acid. J Pharm Pharmacol. 2006;58:1265-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Valério DA, Cunha TM, Arakawa NS, Lemos HP, Da Costa FB, Parada CA, Ferreira SH, Cunha FQ, Verri WA Jr. Anti-inflammatory and analgesic effects of the sesquiterpene lactone budlein A in mice: inhibition of cytokine production-dependent mechanism. Eur J Pharmacol. 2007;562:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Verri WA Jr, Cunha TM, Magro DA, Domingues AC, Vieira SM, Souza GR, Liew FY, Ferreira SH, Cunha FQ. Role of IL-18 in overt pain-like behaviour in mice. Eur J Pharmacol. 2008;588:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Guazelli CF, Fattori V, Colombo BB, Georgetti SR, Vicentini FT, Casagrande R, Baracat MM, Verri WA Jr. Quercetin-loaded microcapsules ameliorate experimental colitis in mice by anti-inflammatory and antioxidant mechanisms. J Nat Prod. 2013;76:200-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Wallace JL, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 312] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 21. | Colombo BB. A vimpocetina reduz a colite induzida por ácido acético em camundongos. Universidade Estadual de Londrina, 2017. |
| 22. | Cunha TM, Verri WA Jr, Vivancos GG, Moreira IF, Reis S, Parada CA, Cunha FQ, Ferreira SH. An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res. 2004;37:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 249] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 23. | Pereira LOM, Vilegas W, Tangerina MMP, Arunachalam K, Balogun SO, Orlandi-Mattos PE, Colodel EM, Martins DTO. Lafoensia pacari A. St.-Hil.: Wound healing activity and mechanism of action of standardized hydroethanolic leaves extract. J Ethnopharmacol. 2018;219:337-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876-4882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29832] [Cited by in RCA: 27618] [Article Influence: 986.4] [Reference Citation Analysis (0)] |
| 25. | Lengauer T, Rarey M. Computational methods for biomolecular docking. Curr Opin Struct Biol. 1996;6:402-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 415] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 26. | Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785-2791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19127] [Cited by in RCA: 16143] [Article Influence: 1008.9] [Reference Citation Analysis (0)] |
| 27. | Eberhardt J, Santos-Martins D, Tillack AF, Forli S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J Chem Inf Model. 2021;61:3891-3898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 2649] [Article Influence: 662.3] [Reference Citation Analysis (0)] |
| 28. | Burque RK, Francesconi LP, Victorino AT, Mascarenhas MA, Ceresér KM. Determination of phenolic and evaluation of antioxidant activity of Lafoensia pacari (Lythraceae). Revista Eletrônica de Farmácia. 2015;12:1-10. [DOI] [Full Text] |
| 29. |
Cunha GA, Bailão EFLC, Borges LL, Almeida LM.
Evaluation of the antioxidant potential of |
| 30. | Collier HO, Dinneen LC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968;32:295-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 636] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 31. | Guimarães HA, Nascimento MVM, Tavares A, Galdino PM, De Paula JR, Costa EA. Effects of ethanolic extract of Lafoensia pacari A. St.-Hil., Lythraceae (pacari), in pain and inflammation model. Brazilian J Pharm. 2010;20:328-333. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Silva ABL, Dias KS, Marques MS, Menezes IAC, Santos TC, Mello ICM, Lisboa ACCD, Cavalcanti SCH, Marçal RM, Antoniolli AR. Evaluation of the analgesic effect and acute toxicity of the aqueous extract of Hyptis fruticosa (Salmz. Ex Benth.). Brazilian J Pharm. 2006;16:475-479. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | de Matos LG, Santos L da R, Ferreira RN, Pontes IS, de Paula JR, Costa EA. Anti-Inflammatory, Antinociceptive, and Sedating Effects of Lafoensia pacari. Aqueous Extract. Pharm Biol. 2008;46:341-346. [DOI] [Full Text] |
| 34. | Zhao J, Maitituersun A, Li C, Li Q, Xu F, Liu T. Evaluation on Analgesic and Anti-Inflammatory Activities of Total Flavonoids from Juniperus sabina. Evid Based Complement Alternat Med. 2018;2018:7965306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Shara M, Stohs SJ. Efficacy and Safety of White Willow Bark (Salix alba) Extracts. Phytother Res. 2015;29:1112-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 36. | Naghizadeh B, Mansouri MT, Ghorbanzadeh B. Ellagic acid enhances the antinociceptive action of carbamazepine in the acetic acid writhing test with mice. Pharm Biol. 2016;54:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Derosa G, Maffioli P, Sahebkar A. Ellagic Acid and Its Role in Chronic Diseases. Adv Exp Med Biol. 2016;928:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Ministry of Health of Brazil. Department of Health Care: Clinical protocol and therapeutic guidelines for the treatment of Crohn’s disease. In: Diário Oficial da União. Portaria 858/02, 2002; 214: 77-82. |
| 39. | Ministry of Health of Brazil. Department of Health Care: Clinical protocol and therapeutic guidelines for the treatment of ulcerative colitis. In: Diário Oficial da União. Portaria 861/02, 2002; 214: 87-98. |
| 40. | Tamashiro Filho P, Sikiru Olaitan B, Tavares de Almeida DA, Lima JC, Marson-Ascêncio PG, Donizeti Ascêncio S, Rios-Santos F, Martins DT. Evaluation of antiulcer activity and mechanism of action of methanol stem bark extract of Lafoensia pacari A. St.-Hil. (Lytraceae) in experimental animals. J Ethnopharmacol. 2012;144:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Rogerio AP, Fontanari C, Borducchi E, Keller AC, Russo M, Soares EG, Albuquerque DA, Faccioli LH. Anti-inflammatory effects of Lafoensia pacari and ellagic acid in a murine model of asthma. Eur J Pharmacol. 2008;580:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 42. | Tena B, Escobar B, Arguis MJ, Cantero C, Rios J, Gomar C. Reproducibility of Electronic Von Frey and Von Frey monofilaments testing. Clin J Pain. 2012;28:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Sandborn WJ, Hanauer SB. Systematic review: the pharmacokinetic profiles of oral mesalazine formulations and mesalazine pro-drugs used in the management of ulcerative colitis. Aliment Pharmacol Ther. 2003;17:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 179] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 44. | Deuis JR, Dvorakova LS, Vetter I. Methods Used to Evaluate Pain Behaviors in Rodents. Front Mol Neurosci. 2017;10:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 776] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 45. | Laird JMA, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 284] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 46. | Dong L, Vecchio AJ, Sharma NP, Jurban BJ, Malkowski MG, Smith WL. Human cyclooxygenase-2 is a sequence homodimer that functions as a conformational heterodimer. J Biol Chem. 2011;286:19035-19046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Pantsar T, Poso A. Binding Affinity via Docking: Fact and Fiction. Molecules. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 314] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 48. | Lin CF, Leu YL, Al-Suwayeh SA, Ku MC, Hwang TL, Fang JY. Anti-inflammatory activity and percutaneous absorption of quercetin and its polymethoxylated compound and glycosides: the relationships to chemical structures. Eur J Pharm Sci. 2012;47:857-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Souto FO, Zarpelon AC, Staurengo-Ferrari L, Fattori V, Casagrande R, Fonseca MJ, Cunha TM, Ferreira SH, Cunha FQ, Verri WA Jr. Quercetin reduces neutrophil recruitment induced by CXCL8, LTB4, and fMLP: inhibition of actin polymerization. J Nat Prod. 2011;74:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Malta DJN. Síntese, elucidação estrutural e atividade biológica de novos derivados imidazolidinônicos. M.Sc. Thesis, Universidade Federal de Pernambuco, 2005. Available at: https://repositorio.ufpe.br/handle/123456789/40873. |
| 51. | Carvalho WA, Carvalho RD, Rios-Santos F. [Specific cyclooxygenase-2 inhibitor analgesics: therapeutic advances.]. Rev Bras Anestesiol. 2004;54:448-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |