Published online Apr 28, 2023. doi: 10.3748/wjg.v29.i16.2502
Peer-review started: January 9, 2023
First decision: February 15, 2023
Revised: February 21, 2023
Accepted: March 31, 2023
Article in press: March 31, 2023
Published online: April 28, 2023
Processing time: 105 Days and 3.3 Hours
Bacteremia, which is a major cause of mortality in patients with acute cholangitis, induces hyperactive immune response and mitochondrial dysfunction. Presepsin is responsible for pathogen recognition by innate immunity. Acylcarnitines are established mitochondrial biomarkers.
To clarify the early predictive value of presepsin and acylcarnitines as biomarkers of severity of acute cholangitis and the need for biliary drainage.
Of 280 patients with acute cholangitis were included and the severity was stratified according to the Tokyo Guidelines 2018. Blood presepsin and plasma acylcarnitines were tested at enrollment by chemiluminescent enzyme immu
The concentrations of presepsin, procalcitonin, short- and medium-chain acylcarnitines increased, while long-chain acylcarnitines decreased with the severity of acute cholangitis. The areas under the receiver operating characteristic curves (AUC) of presepsin for diagnosing moderate/severe and severe cholangitis (0.823 and 0.801, respectively) were greater than those of conventional markers. The combination of presepsin, direct bilirubin, alanine aminotransferase, temperature, and butyryl-L-carnitine showed good predictive ability for biliary drainage (AUC: 0.723). Presepsin, procalcitonin, acetyl-L-carnitine, hydroxydodecenoyl-L-carnitine, and temperature were independent predictors of bloodstream infection. After adjusting for severity classification, acetyl-L-carnitine was the only acylcarnitine independently associated with 28-d mortality (hazard ratio 14.396; P < 0.001) (AUC: 0.880). Presepsin concentration showed positive correlation with direct bilirubin or acetyl-L-carnitine.
Presepsin could serve as a specific biomarker to predict the severity of acute cholangitis and need for biliary drainage. Acetyl-L-carnitine is a potential prognostic factor for patients with acute cholangitis. Innate immune response was associated with mitochondrial metabolic dysfunction in acute cholangitis.
Core Tip: Acute cholangitis leads to sepsis and organ dysfunction because of biliary obstruction. Identification of predictive biomarkers for patients who require emergent biliary drainage and patients who may progress to systemic bloodstream infection at an early stage of the disease is a key imperative. Our study suggests that presepsin and acetyl-L-carnitine may serve as biomarkers to predict the severity of acute cholangitis and the need for biliary drainage. Innate immune response was associated with mitochondrial metabolic dysfunction.
- Citation: Zhang HY, Xiao HL, Wang GX, Lu ZQ, Xie MR, Li CS. Predictive value of presepsin and acylcarnitines for severity and biliary drainage in acute cholangitis. World J Gastroenterol 2023; 29(16): 2502-2514
- URL: https://www.wjgnet.com/1007-9327/full/v29/i16/2502.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i16.2502
Acute cholangitis refers to bacterial infection of the extra-hepatic biliary tract which typically occurs in association with bile duct obstruction caused by choledocholithiasis, malignant stricture, or primary sclerosing cholangitis. Approximately 20% to 71% of patients with acute cholangitis progress to bacteremia or sepsis, which may lead to life-threatening organ failure and death[1,2]. Early biliary drainage to remove biliary obstruction is one of the main emergency treatments for acute cholangitis. Therefore, the identification of predictive biomarkers for patients who require emergent biliary drainage and patients who maybe progress to systemic bloodstream infection at an early stage of the disease is a key imperative.
Bacteremia induces hyperactive immune response and mitochondrial dysfunction which alter metabolism[3]. Presepsin, a soluble leukocyte differentiation antigen 14 (CD14) subtype, is responsible for pathogen recognition by innate immunity[4]. Presepsin is a biomarker of systemic inflammation that can facilitate early diagnosis, risk-stratification, and prognostic assessment of patients with sepsis[5-7]. Carnitine is responsible for mitochondrial transport and β oxidation of fatty acids. L-carnitine and acylcarnitines are established mitochondrial biomarkers[8]. Preclinical and recent clinical studies have demonstrated the association of plasma carnitine or acylcarnitines with organ dysfunction and poor survival in sepsis[9-11]. However, it is unknown whether presepsin or specific acylcarnitine species can reflect severity of acute cholangitis and the timing of biliary drainage. Therefore, this study aimed to evaluate the value of presepsin as well as specific acylcarnitines as predictors of severity, bloodstream infection, biliary drainage and prognosis in patients with acute cholangitis.
A prospective observational study was conducted to clarify the predictive value of presepsin and acylcarnitines for severity and biliary drainage in patients with acute cholangitis. The primary outcomes were the abilities of presepsin and acylcarnitines to predict severe acute cholangitis compared with procalcitonin. The secondary outcomes included the value of presepsin and acylcarnitines to predict emergency biliary drainage, positive bloodstream infection, and prognosis of acute cholangitis. This study complied with the principles of the Declaration of Helsinki. The Beijing Friendship Hospital Ethics Committee approved the study protocol (No. 2018-P2-063-01). Patients were enrolled after providing written informed consent.
This was a single-center study conducted at the emergency department and emergency intensive care unit of Beijing Friendship Hospital, a National Clinical Research Center of Digestive Diseases. Between May 2019 and July 2021, consecutive adult patients who fulfilled the acute cholangitis criteria based on the Tokyo Guidelines 2018 (TG18) for acute cholangitis were enrolled[12]. The severity was stratified as mild, moderate, and severe according to TG18[12]. The exclusion criteria were as follows: (1) Patients with chronic kidney or liver disease who may have increased presepsin or acylcarnitine levels at baseline[13]; (2) HIV infection; (3) pregnant and lactating women; (4) patients with abdominal trauma or history of abdominal surgery in the past seven days; (5) incomplete data about the main study indices (presepsin, acylcarnitines, or blood culture results); and (6) patients who declined to participate.
We recorded demographic data, comorbidities, clinical and laboratory data, severity grading of acute cholangitis and biliary drainage data within 48 h after admission. Disease severity was assessed using the sequential organ failure assessment (SOFA) scores. The criteria for implementing biliary drainage were based on the American Society for Gastrointestinal Endoscopy (ASGE) guidelines for the management of cholangitis[14]. Blood samples of all patients and bile samples of patients who were subjected to endoscopic or percutaneous biliary drainage were cultured for aerobic and anaerobic bacteria. Pathogens in blood samples were identified by blood culture and metagenomic next generation sequencing (mNGS). Data for 28-d mortality were collected during follow-up.
Blood samples (5 mL) were collected immediately after admission and stored at 4 °C. 200 μL of these blood samples were extracted to detect presepsin concentration. The remaining blood samples were centrifuged for 10 min at 4500 g and the plasma sample was then stored at -80 °C within 24 h. For acylcarnitines detection, 50 μL plasma sample was drawn into a 2 mL centrifuge tube and mixed with 140 µL methanol and 10 µL internal standard (NSK-B-1) and further centrifuged for 5 minutes at 12000 g. 100 µL supernatant was transferred into 200 µL inner liner before analyses. For mNGS detection, 3 mL blood sample was drawn from patients and centrifuged at 4000 g for 10 min within 8 h after collection. DNA was extracted from plasma using a TIANamp Micro DNA Kit (Tiangen Biotech, Beijing, China, No. DP316) according to the manufacturer’s operating manual. The extracted DNA specimens were used for the construction of DNA libraries.
A chemiluminescent enzyme immunoassay was used to test presepsin concentration by a PATHFAST analyzer (Mitsubishi Chemical Medience Corporation, Tokyo, Japan). The detection range was 20 pg/mL to 200000 pg/mL. Information regarding conventional inflammatory biomarkers procalcitonin, C-reactive protein (CRP) and other indicators were obtained from the clinical laboratory data.
Plasma acylcarnitines at enrollment were determined by an ultra-high-performance liquid chromatography-mass system (UHPLC-MS, Supplementary file 1) using a Waters XEVO TQ-S Micro triple quadrupole mass spectrometer (Waters Corp, United States). The length of carbon chains was used to define short-chain (C ≤ 5), medium-chain (C6-10) and long-chain acylcarnitines (C ≥ 12) (Supple
mNGS testing of the blood samples was performed and analyzed by BGI-Shenzhen, as previously reported[15]. Briefly, the extracted DNA was fragmented to 300 bp. DNA libraries were constructed by end-repair, adapter ligation and PCR amplification using the PMseqTM high throughput gene detection kit for infectious pathogens (combined probe anchored polymerization sequencing method, BGI-Shenzhen, China, No. RM0438), according to the manufacturer's instructions (Supplementary file 2). Using bioinformatics analysis methods and pathogenic microorganism database, the types of pathogenic microorganisms obtained by sequencing were analyzed, and the detection results of each sample were obtained.
Factoring a two-sided α = 0.05, β = 0.2, and assuming 50% of patients with mild acute cholangitis[16], it was determined that 268 patients were required for enrollment, i.e., 134 with mild and moderate acute cholangitis and 134 with severe acute cholangitis. This study enrolled 387 patients to account for patients lost to follow-up and patients with incomplete data collection. Continuous variables with non-normal distribution were presented as median (25th to 75th percentile) and compared by Mann-Whitney U test or Kruskal-Wallis test. Comparisons between categorical variables were analyzed by Pearson χ2 test. Significant biomarkers and clinical variables associated with severity, biliary drainage, bloodstream infection, and 28-d mortality were identified by multivariate logistic regression models. The area under the receiver operating characteristic (ROC) curves were applied to examine the predictive accuracy of presepsin, acylcarnitines, and procalcitonin for severity and biliary drainage. The optimal cutoff levels determined by ROC curves and Youden index were used to dichotomize presepsin, acylcarnitines, procalcitonin, and other independent predictors. The area under the curve (AUC) comparisons were performed using MedCalc Version 13 software (Mariakerke, Belgium). Kaplan-Meier survival curves were established, and between-group differences in 28-d survival were assessed using the log-rank test. The Cox proportional hazard model was used to calculate the hazard ratio (HR) for 28-d mortality. Spearman rank correlation was performed for the correlation analysis. Two-sided P values < 0.05 were considered indicative of statistical significance. SPSS 25.0 software (SPSS, Chicago, IL, United States) was used for statistical analyses.
From May 2019 through July 2021, 387 patients with acute cholangitis were admitted to the emergency department or EICU. Data from 107 patients were not analyzed because 29 patients did not meet the inclusion criteria, 38 patients refused consent, 35 patients had incomplete main records, and 5 patients were lost to follow-up (Supplementary Figure 1). The remaining 280 patients were enrolled in this study and assigned to the mild group (n = 65), moderate group (n = 84), and severe group (n = 131) based on the TG18 criteria. The age, proportion of patients with biliary drainage, levels of temperature, white blood cell (WBC) count, total bilirubin, direct bilirubin and SOFA score, and 28-d mortality increased with the severity of acute cholangitis, and the differences among the three groups were significant (P < 0.05 for all) (Table 1).
| Variables | All cases (n = 280) | Mild (n = 65) | Moderate (n = 84) | Severe (n = 131) | P value |
| Demographic data, age (yr) | 74 (66, 84) | 69 (63, 81) | 79.5 (69, 87) | 74 (66, 84) | 0.001 |
| Male, n (%) | 166 (59.3) | 34 (52.3) | 53 (63.1) | 79 (60.3) | 0.392 |
| Comorbidities, n (%) | |||||
| CHD | 78 (27.9) | 19 (29.2) | 22 (26.2) | 37(28.2) | 0.911 |
| Heart failure | 23 (8.2) | 3 (4.6) | 5 (6.0) | 15 (11.5) | 0.173 |
| Hypertension | 87 (31.1) | 16 (24.6) | 32 (38.1) | 39 (29.8) | 0.192 |
| CVD | 33 (11.8) | 4 (6.2) | 10 (11.9) | 19 (14.5) | 0.233 |
| COPD | 10 (3.6) | 1 (1.5) | 2 (2.4) | 7 (5.3) | 0.313 |
| Diabetes mellitus | 64 (22.9) | 11 (16.9) | 18 (21.4) | 35 (26.7) | 0.286 |
| Biliary drainage, n (%) | 0.015 | ||||
| No | 104 (37.1) | 34 (52.3) | 27 (32.1) | 43 (35.0) | |
| ERCP/PTCD | 176 (62.9) | 31 (47.7) | 57 (67.9) | 88 (67.2) | |
| Infection data | |||||
| Temperature (℃) | 37.5 (36.7, 38.5) | 37.2 (36.5, 38.0) | 37.5 (36.6, 38.6) | 37.8 (36.8, 38.5) | 0.044 |
| WBC count (× 109/L) | 10.73 (7.37, 14.83) | 8.10 (6.47, 10.22) | 13.00 (8.61, 16.56) | 11.35 (7.89, 15.71) | < 0.001 |
| Liver function | |||||
| TBIL (μmol/L) | 98.69 (62.63, 142.32) | 69.26 (48.02, 103.37) | 114.40 (77.04, 164.67) | 103.99 (74.30, 139.53) | < 0.001 |
| DBIL (μmol/L) | 70.29 (42.68, 98.75) | 43.20 (26.53, 75.64) | 78.90 (58.09, 110.40) | 72.07 (44.85, 96.29) | < 0.001 |
| ALT (U/L) | 148.00 (80.00, 287.50) | 166.00 (63.50, 386.50) | 145.00 (84.62, 300.00) | 148.00 (77.00, 240.00) | 0.626 |
| AST (U/L) | 138.05 (82.50, 286.10) | 129.50 (69.60, 346.20) | 138.80 (96.10, 299.10) | 137.85 (72.03, 266.53) | 0.744 |
| SOFA score | 2 (1, 4) | 1 (1, 2) | 1 (1, 2) | 4 (3, 5) | < 0.001 |
| 28-d mortality, n (%) | 9 (3.2) | 0 (0.0) | 0 (0.0) | 9 (6.9) | 0.005 |
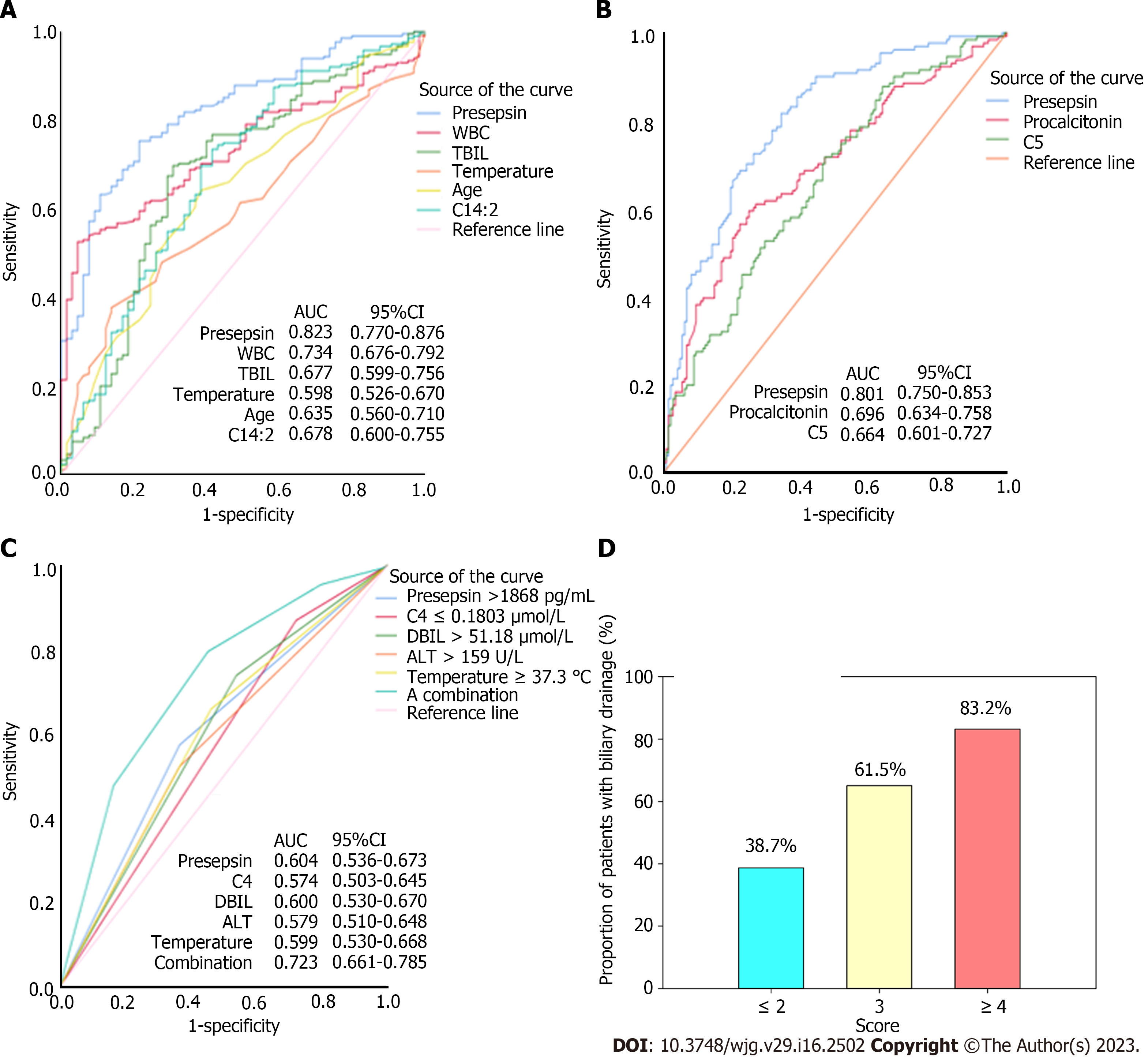
Compared with the mild group, presepsin, procalcitonin, and CRP levels were significantly higher in patients with moderate and severe acute cholangitis (P < 0.001, Table 2). Plasma short chain and medium chain acylcarnitines (C0, C2–C6, C8) increased, while long chain acylcarnitines (C12-C14, C18, C20, C22) decreased with the severity of acute cholangitis (P < 0.05, Table 2). Multivariate logistic regression showed that increased levels of presepsin, WBC, total bilirubin, temperature, and age, and decreased level of tetradecadienyl-L-carnitine (C14:2), but not procalcitonin and CRP levels, were independent predictors of moderate and severe patients, compared with mild patients (Table 3). The AUC of presepsin for predicting moderate/severe cholangitis was 0.823 (sensitivity 0.75; specificity 0.78; cutoff value 1519 pg/mL), which was higher than that of WBC (0.734; P = 0.0189), total bilirubin (0.677; P = 0.0004), temperature (0.598; P < 0.0001), age (0.635; P = 0.0002), and C14:2 (0.678; P = 0.0012) (Figure 1A). Compared with mild/moderate patients, presepsin, procalcitonin and valeryl-L-carnitine (C5) were independently associated with severe cholangitis (Table 3). The AUC of presepsin (0.801; sensitivity 0.82; specificity 0.66; cutoff value 1680 pg/mL) for severe cholangitis was significantly higher than that of procalcitonin (0.696, P = 0.0028) and C5 (0.664, P = 0.0008) (Figure 1B).
| Biomarkers | All cases (n = 280) | Mild (n = 65) | Moderate (n = 84) | Severe (n = 131) | P value |
| Presepsin (pg/mL) | 1864.00 (1169.75, 2765.75) | 1053.00 (576.50, 1505.50) | 1634.00 (1103.00, 2325.75) | 2536.00 (1812.00, 3730.00) | < 0.001 |
| Procalcitonin (ng/mL) | 11.32 (2.10, 40.53) | 3.57 (0.64, 10.87) | 10.70 (1.48, 31.00) | 27.43 (4.76, 54.32) | < 0.001 |
| CRP (mg/L) | 83.71 (44.23, 153.29) | 48.02 (17.00, 103.48) | 80.76 (48.39, 143.91) | 107.00 (61.18, 174.12) | < 0.001 |
| Acylcarnitines (μmol/L) | |||||
| C0 | 28.54 (19.60, 38.65) | 25.39 (18.72, 32.47) | 29.65 (19.96, 38.38) | 30.00 (21.19, 40.64) | 0.042 |
| C2 | 9.64 (6.16, 14.01) | 8.35 (5.23, 12.29) | 9.44 (6.67, 12.53) | 10.32 (6.43, 15.30) | 0.016 |
| C3 | 0.38 (0.26, 0.60) | 0.31 (0.21,0.43) | 0.35 (0.26, 0.53) | 0.46 (0.29, 0.71) | < 0.001 |
| C4 | 0.09 (0.06, 0.16) | 0.08 (0.06, 0.12) | 0.09 (0.05, 0.13) | 0.10 (0.07, 0.19) | 0.003 |
| C5 | 0.11 (0.07, 0.18) | 0.08 (0.06, 0.18) | 0.09 (0.06, 0.14) | 0.14 (0.08, 0.24) | < 0.001 |
| C6 | 0.11 (0.07,0.15) | 0.08 (0.06, 0.14) | 0.10 (0.06, 0.13) | 0.13 (0.08, 0.20) | < 0.001 |
| C8 | 0.13 (0.10, 0.22) | 0.12 (0.08, 0.19) | 0.13 (0.10, 0.20) | 0.14 (0.11, 0.23) | 0.037 |
| C12 | 0.09 (0.04, 0.18) | 0.14 (0.07, 0.28) | 0.09 (0.04, 0.17) | 0.08 (0.03, 0.13) | < 0.001 |
| C12:1 | 0.05 (0.03, 0.08) | 0.06 (0.04, 0.10) | 0.05 (0.03, 0.09) | 0.04 (0.02, 0.07) | 0.001 |
| C12DC | 0.0005 (0.0004, 0.0008) | 0.0006 (0.0005, 0.0009) | 0.0005 (0.0004, 0.0009) | 0.0004 (0.0003, 0.0007) | 0.041 |
| C13 | 0.03 (0.02, 0.07) | 0.04 (0.03, 0.07) | 0.03 (0.02, 0.08) | 0.02 (0.01, 0.05) | 0.008 |
| C14 | 0.02 (0.01, 0.03) | 0.03 (0.02, 0.04) | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.03) | 0.001 |
| C14:1 | 0.09 (0.04, 0.16) | 0.16 (0.07, 0.31) | 0.09 (0.05, 0.13) | 0.06 (0.03, 0.11) | < 0.001 |
| C14:2 | 0.07 (0.03, 0.14) | 0.12 (0.05, 0.22) | 0.06 (0.03, 0.13) | 0.05 (0.02, 0.10) | < 0.001 |
| C16 | 0.10 (0.06, 0.15) | 0.12 (0.08, 0.17) | 0.10 (0.06, 0.14) | 0.09 (0.05, 0.13) | 0.003 |
| C16:1 | 0.06 (0.03, 0.12) | 0.10 (0.05, 0.16) | 0.05 (0.03, 0.10) | 0.05 (0.02, 0.12) | 0.003 |
| C16:2 | 0.02 (0.002, 0.06) | 0.04 (0.01, 0.09) | 0.01 (0.002, 0.05) | 0.02 (0.002, 0.05) | 0.006 |
| C18:2 | 0.13 (0.07, 0.21) | 0.16 (0.09, 0.24) | 0.12 (0.07, 0.18) | 0.11 (0.05, 0.22) | 0.014 |
| C18 OH | 0.0004 (0.0002, 0.0009) | 0.004 (0.003, 0.0013) | 0.0006 (0.0002, 0.0010) | 0.0004 (0.0002, 0.0007) | 0.035 |
| C20 | 0.0010 (0.0004, 0.0018) | 0.0013 (0.0009, 0.0029) | 0.0013 (0.0005, 0.0020) | 0.0007 (0.0003, 0.0014) | 0.001 |
| C20:4 | 0.0013 (0.0007, 0.0023) | 0.0018 (0.0009, 0.0021) | 0.0018 (0.0008, 0.0037) | 0.0012 (0.0005, 0.0020) | 0.024 |
| C22 | 0.0009 (0.0003, 0.0015) | 0.0011 (0.0006, 0.0023) | 0.0010 (0.0003, 0.0018) | 0.0006 (0.0002, 0.0014) | 0.009 |
| Mild vs moderate/severe | Mild/moderate vs severe | |||
| Variables | OR (95%CI) | P value | OR (95%CI) | P value |
| Presepsin (pg/mL) | 1.001 (1.000-1.002) | < 0.001 | 1.000 (1.000-1.001) | < 0.001 |
| Procalcitonin (ng/mL) | 1.018 (0.995-1.041) | 0.134 | 1.021 (1.008-1.035) | 0.001 |
| WBC (× 109/L) | 1.149 (1.050-1.258) | 0.003 | 0.990 (0.945-1.037) | 0.676 |
| CRP (mg/L) | 1.001 (0.995-1.008) | 0.642 | 1.003 (0.999-1.008) | 0.104 |
| TBIL(μmol/L) | 1.008 (1.003-1.013) | 0.003 | 0.999 (0.996-1.002) | 0.504 |
| Temperature (℃) | 1.540 (1.046-2.267) | 0.029 | 1.118 (0.859-1.456) | 0.407 |
| Age (yr) | 1.051 (1.018-1.085) | 0.002 | 1.000 (0.977-1.022) | 0.973 |
| C14:2 (μmol/L) | 0.036 (0.002-0.663) | 0.025 | - | - |
| C5 (μmol/L) | - | - | 11.490(2.213-59.656) | 0.004 |
One hundred and seventy-six of 280 patients underwent biliary drainage. Compared with patients without biliary drainage, patients with biliary drainage had significantly increased temperature, levels of presepsin, total bilirubin, direct bilirubin, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (P < 0.05 for all), rather than procalcitonin (P = 0.199) and CRP (P = 0.410) levels. On multivariate logistic regression, high presepsin (OR 2.312, P = 0.004), direct bilirubin (OR 1.902, P = 0.027), ALT (OR 1.878, P = 0.022) and temperature (OR 2.108, P = 0.006), and low plasma butyryl-L-carnitine (C4) (OR 3.326, P = 0.001) were identified as independent predictors of biliary drainage (Table 4). The AUC of a combination of these five predictors was 0.723, which was significantly greater compared with presepsin (0.604, P = 0.0001), direct bilirubin (0.600, P < 0.0001), ALT (0.579, P < 0.0001), temperature (0.599, P = 0.0001), and C4 (0.574, P < 0.0001) alone (Figure 1C). In the model composed of these five factors (Table 4), a cutoff score of 3 was associated with 79.6% sensitivity, 54.8% specificity, 63.8% PPV, and 72.9% NPV for predicting biliary drainage (LR + 1.76 and LR– 0.37) (Figure 1D).
| Variables | B | SE | Wald | df | OR | 95%CI | P value | Score |
| Presepsin > 1868 (pg/mL) | 0.838 | 0.291 | 8.308 | 1 | 2.312 | 1.308-4.087 | 0.004 | 1 |
| C4 ≤ 0.1803 (μmol/L) | 1.202 | 0.347 | 12.017 | 1 | 3.326 | 1.686-6.561 | 0.001 | 1 |
| DBIL > 51.18 (μmol/L) | 0.643 | 0.290 | 4.912 | 1 | 1.902 | 1.077-3.358 | 0.027 | 1 |
| ALT > 159 (U/L) | 0.630 | 0.275 | 5.254 | 1 | 1.878 | 1.096-3.219 | 0.022 | 1 |
| Temperature ≥ 37.3 (℃) | 0.746 | 0.270 | 7.602 | 1 | 2.108 | 1.241-3.581 | 0.006 | 1 |
| Variables | B | SE | Wald | df | OR | 95%CI | P value |
| Presepsin (High vs low1) | 1.243 | 0.524 | 5.617 | 1 | 3.466 | 1.240-9.689 | 0.018 |
| Procalcitonin (High vs low2) | 1.400 | 0.401 | 12.208 | 1 | 4.054 | 1.849-8.889 | < 0.001 |
| C2 (High vs low3) | 1.313 | 0.464 | 7.998 | 1 | 3.716 | 1.496-9.229 | 0.005 |
| C3 (High vs low4) | 0.319 | 0.400 | 0.636 | 1 | 1.376 | 0.628-3.015 | 0.425 |
| C6 (High vs low5) | -0.450 | 0.436 | 1.065 | 1 | 0.638 | 0.271-1.499 | 0.302 |
| C12:1 OH (High vs low6) | 1.284 | 0.410 | 9.829 | 1 | 3.611 | 1.618-8.058 | 0.002 |
| Sex | 0.282 | 0.356 | 0.628 | 1 | 1.326 | 0.660-2.667 | 0.428 |
| Temperature (℃) | 0.513 | 0.174 | 8.714 | 1 | 1.671 | 1.188-2.350 | 0.003 |
| Severity grading | -0.071 | 0.285 | 0.062 | 1 | 0.932 | 0.533-1.628 | 0.804 |
| SOFA score | -0.062 | 0.081 | 0.587 | 1 | 0.940 | 0.802-1.101 | 0.444 |
As a substitute for the severity of acute cholangitis, blood infection was identified by blood culture and blood mNGS. Compared to patients with no blood infection (n = 188), patients with blood infection (n = 92) were more likely to require biliary drainage, and had significantly higher temperature, WBC, and SOFA scores (P < 0.05 for all, Supplementary Table 2). The proportion of male patients in the blood infection group was significantly lower than that in the group without blood infection. The positive rates of blood culture, blood mNGS, and bile culture were 29.3% (82/280), 66.7% (14/21), and 76.7% (135/176), respectively. The most common bacteria identified were Escherichia coli, Klebsiella pneumoniaeleisure and Enterococcus faecium. Blood infection positivity was associated with significantly higher level of presepsin (P = 0.001), procalcitonin (P < 0.001), acetyl-L-carnitine (C2, P = 0.009), propionyl-L-carnitine (C3, P = 0.035), hexanoyl-L-carnitine (C6, P = 0.036), and hydroxydodecenoyl-L-carnitine (C12:1 OH, P = 0.018).
We dichotomized presepsin, procalcitonin, C2, C3, C6, and C12:1 OH using the optimal cutoff value. After adjusting for sex, severity grading, and SOFA score, presepsin (OR 3.466, P = 0.018), procalcitonin (OR 4.054, P < 0.001), C2 (OR 3.716, P = 0.005), C12:1 OH (OR 3.611, P = 0.002), and temperature (OR 1.671, P = 0.003) were found to be independent predictors for bloodstream infection (Table 5). The AUC of presepsin for diagnosing blood infection was 0.610 (sensitivity 0.91; specificity 0.32; cut-off 1147.5 pg/mL), but there was no significant difference between presepsin and procalcitonin (AUC: 0.679), C2 (AUC: 0.599), C12:1 OH (AUC: 0.603), and temperature (AUC: 0.639) in this respect (Suppleme
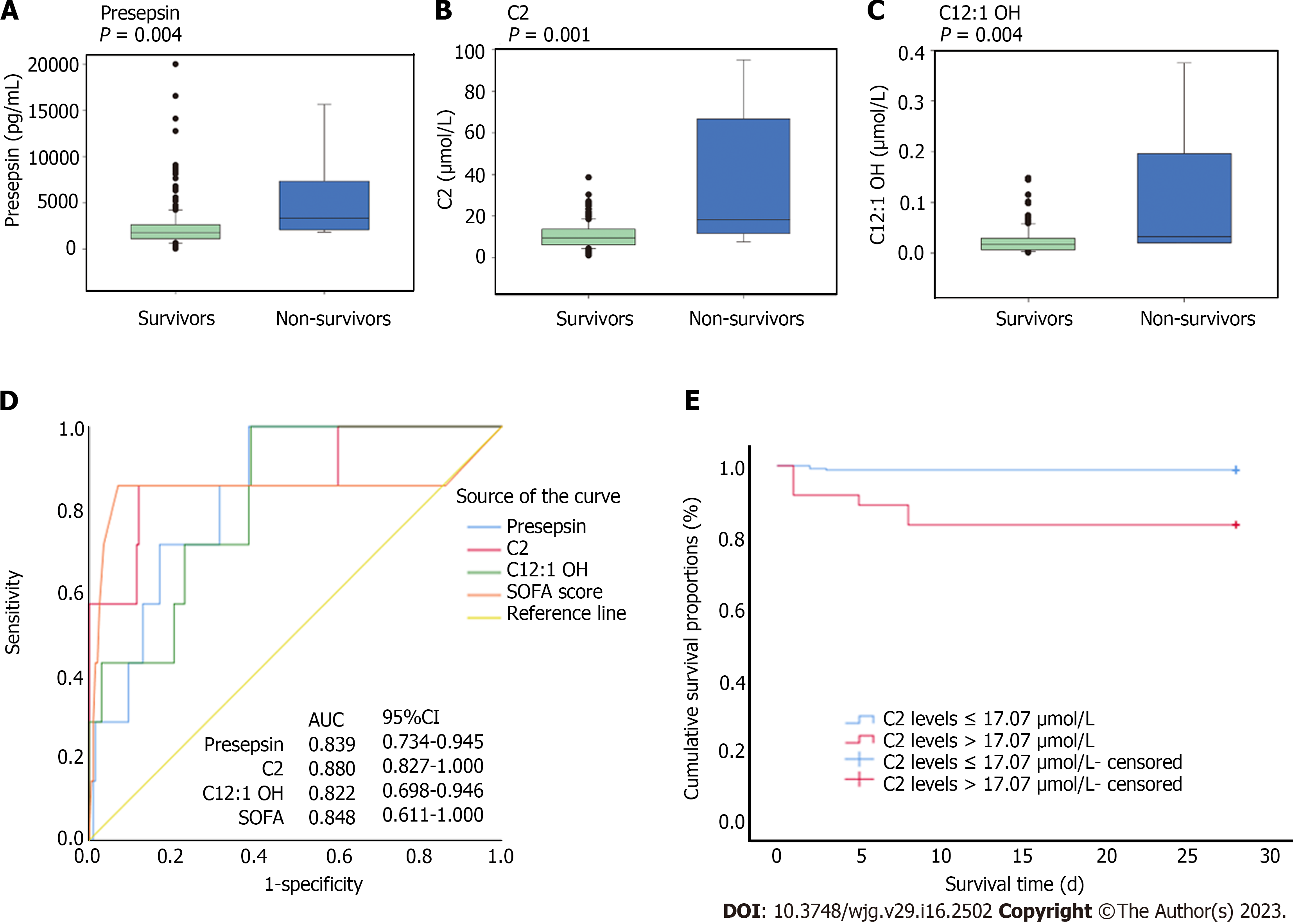
The 28-d mortality rate in this study was 3.2%. The characteristics of survivors and non-survivors are illustrated in Supplementary Table 4. Compared with patients who survived, those who died within 28 d had significantly increased presepsin (P = 0.004), C2 (P = 0.001), and C12:1 OH (P = 0.004) (Figure 2A-C), but not increased procalcitonin (P = 0.591) and CRP (P = 0.141). The AUC of presepsin (0.839), C2 (0.880), and C12:1 OH (0.822) for 28-d mortality was similar to that of SOFA score (0.848, P > 0.05 for all) (Figure 2D and Supplementary Table 5). After adjusting for severity of acute cholangitis by multivariate Cox proportional hazard models, high C2 (P = 0.004) was the only independent predictor of 28-d mortality, rather than the levels of presepsin (P=0.732), C12: 1 OH (P = 0.899), and SOFA score (P = 0.133) (Supplementary Table 6). Based on ROC curves for 28-d mortality and Youden index, a cutoff value of 17.07 μmol/L was used to dichotomize C2. Patients with high C2 Level had significantly higher 28-d mortality compared to those with low C2 Level (HR 14.396; 95%CI: 3.599-57.576; P < 0.001; Figure 2E).
Increased level of presepsin (r = 0.424, P < 0.001), procalcitonin (r = 0.357, P < 0.001), and C2 (r = 0.208, P < 0.001) showed a significant association with SOFA score. We confirmed that presepsin, but not procalcitonin, showed a significant positive correlation with total bilirubin (r = 0.290, P < 0.001), direct bilirubin (r = 0.304, P < 0.001), and C2 (r = 0.270, P < 0.001) (Supplementary Table 7).
The immunologic profile and mitochondrial function of patients with acute cholangitis are similar to those of septic patients. Thus, in this prospective study, we explored the value of presepsin and carnitine metabolites as biomarkers to predict severity, emergency biliary drainage, and prognosis of patients with acute cholangitis. Our results indicated that the ability of presepsin level to predict moderate/severe and severe cholangitis was superior to that of procalcitonin level. High presepsin, direct bilirubin, ALT, temperature, and low C4 were independent predictors of urgent biliary drainage, and the combination of these five predictors significantly improved the predictive accuracy. As a substitute for severity of acute cholangitis, blood infection was found to be independently associated with the biomarkers of presepsin, procalcitonin, C2, and C12:1 OH. High C2 was identified as the only independent predictor of 28-d mortality. Additionally, the positive correlation between presepsin and C2 reflected the association between innate immune response and mitochondrial fatty acid β-oxidation (FAO) impairment during the progression of acute cholangitis.
CD14 are expressed on the surface of innate immune cells and play a role in the activation of innate immune response after recognition of bacteria[4,17]. Presepsin (soluble CD14) has been confirmed as a marker of host response in sepsis patients. Increased presepsin was demonstrated to be associated with organ dysfunction, positive blood culture and mortality in sepsis[4]. This result was consistent with our finding wherein presepsin level was found to identify severe acute cholangitis and bloodstream infection. Animal model of acute obstructive cholangitis demonstrated infiltration of macrophages and neutrophils into the liver sinusoids and around the bile duct leading to coagulopathy[18]. The study of Guicciardi et al[19] revealed that macrophages contributed to the pathogenesis of sclerosing cholangitis. These findings suggested the activation of innate immune response in acute cholangitis. In addition, a recent study showed that the conventional septic biomarker procalcitonin which was produced by C cells of the thyroid gland predicted severe but not moderate/severe acute cholangitis with better accuracy than WBC and CRP[16]. Furthermore, our finding demonstrated the superior ability of presepsin to predict severe or moderate/severe cholangitis compared to procalcitonin and other markers. The AUC of presepsin was higher than that of other markers in predicting any severity of acute cholangitis. As a surrogate of severe acute cholangitis, predictors for positive bloodstream infection were explored. The most commonly identified bacteria in our study were Escherichia coli, Klebsiella pneumoniaeleisure, and Enterococcus faecium, which is consistent with the findings reported by An et al[20]. Similar to the study by Umefune et al[16] on the association between procalcitonin and positive blood culture in acute cholangitis, the current study found that presepsin, procalcitonin, C2, and C12:1 OH were independent predictors of positive blood infection.
Additionally, to facilitate early identification of patients who require emergency biliary drainage, we established a predictive model consisting of five factors including presepsin, direct bilirubin, ALT, temperature, and butyryl-L-carnitine (C4). Previous studies suggested that procalcitonin might be a decision-supporting biomarker for urgent biliary decompression even in cases that are not categorized as severe based on TG13[21,22]. However, there was no evidence in this study that procalcitonin, rather than presepsin, could independently predict biliary drainage. The results indicated superior ability of presepsin to reflect the degree of biliary obstruction compared to procalcitonin.
Acylcarnitines are recognized for facilitating FAO for energy production in mitochondria[23]. The blood concentrations of acylcarnitines, which represent a group of mitochondrial-derived metabolites, reflect disorders of long-chain FAO[24]. The production of acetylcarnitine (C2) represents metabolic flexibility in buffering the metabolic status between glucose oxidation and fat oxidation states[25]. Elevation in plasma concentration of C2 is a signal of metabolic inflexibility[8]. Mitochondrial metabolic dysfunction has been implicated as one of the potential causes of organ dysfunction in sepsis[26]. Metabolic flexibility was shown to be an important characteristic of patients with sepsis for survival[27]. Plasma C2 Level was shown to be associated with multiple organ dysfunction, extubation, and freedom from vasopressors, or mortality in patients with sepsis[10,11]. In several studies, plasma short chain and medium chain acylcarnitines (C2, C3, C4, C5, C6, C8, C10) were significantly increased in the non-survivors[11,28-30] and only C2 was associated with all of these indices and 28-d mortality in sepsis[12]. Similar to previous studies, our findings showed that concentrations of short- and medium-chain acylcarnitines increased with the severity and C2 was the only acylcarnitine implicated in 28-d mortality. Increased plasma C2 Level may indicate metabolic inflexibility of nonsurvivors with acute cholangitis. Inconsistent with the absence of long chain acetylcarnitine in sepsis studies, we found that concentrations of long chain acylcarnitines decreased with the severity of acute cholangitis, which might be due to impairment of long-chain FAO with disease progression.
Interestingly, in the current study, reduced butyryl-L-carnitine (C4) was found to be an independent predictor of biliary drainage. Butyrate, a short chain fatty acid, is produced in the bowel by bacterial fermentation of dietary fiber. C4, a butyrate ester of carnitine, is known to help maintain intestinal health and prevent intestinal inflammation[31]. C4 combined with presepsin, direct bilirubin, ALT, and temperature showed better predictive accuracy for emergency biliary drainage. The total score of this model was 5, and 83.2% of patients with score > 4 required biliary drainage (Figure 1D). Moreover, the association between C4 and SOFA score, presepsin, and procalcitonin (Supplementary Table 7) may be explained by the compensatory mechanism of intestinal health on intestinal inflammation in acute cholangitis. Furthermore, the association between C2 and inflammation, as well as the hepatic host response to bacteria leading to the accumulation of long-chain acylcarnitines and defective FAO[32], may explain why C2 and hydroxydodecenoyl-L-carnitine (C12:1 OH) were identified as independent predictors of bloodstream infection in acute cholangitis.
The association between innate immunity and FAO may explain the positive correlation between presepsin and acetylcarnitine. Recent evidence suggested that metabolic reprogramming including FAO was a prerequisite for the activation of macrophages and monocytes[33,34]. A study by Zhu et al[35] found that the rewiring of metabolic and mitochondrial bioenergetics by monocytes activated, deactivated and resolved acute inflammation in turn. During deactivation, the characteristics of lipid metabolic rewiring included increased acylcarnitines levels. The function of immunocytes depends on specific metabolic programs in mitochondria, including post-translational modifications (e.g., acetylation). In their in vitro and in vivo studies, Chi et al[36] found that histone deacetylase 3 couples mitochondria to deacetylate the FAO enzyme HADHA for NLRP3 inflammasome activation in macrophages.
Some limitations of this study should be considered. First, we did not analyze the dynamic changes in presepsin and acylcarnitines levels over time throughout the disease course. Second, the association of presepsin or acylcarnitines with chronic liver or kidney dysfunction was not assessed in this study. Third, due to the low mortality, a larger sample size was required to verify biomarkers that were associated with death. Fourth, blood mNGS was required for larger population size to improve the detection rate of positive bloodstream infection.
Our study identified presepsin as a specific biomarker to predict the severity and emergency biliary drainage of acute cholangitis compared to procalcitonin and other clinical parameters. Acetyl-L-carnitine might be a promising biomarker for predicting mortality in patients with acute cholangitis. Our findings clarify the association between innate immune responses and mitochondrial FAO impairment in acute cholangitis.
Acute cholangitis is potentially lethal when accompanied by sepsis because of biliary obstruction. It is necessary to identify predictive biomarkers for patients who require emergent biliary drainage and patients who maybe progress to systemic bloodstream infection at an early stage of the disease.
Bacteremia induces hyperactive immune response and mitochondrial dysfunction. Presepsin is responsible for pathogen recognition by innate immunity. Acylcarnitines are established mitochondrial biomarkers. However, it is unknown whether presepsin or specific acylcarnitine species can reflect the severity of acute cholangitis and the timing of biliary drainage.
To clarify the early predictive value of presepsin and acylcarnitines for severity and biliary drainage of acute cholangitis.
In this prospective observational study, 280 patients with acute cholangitis were included from May 2019 to July 2021. The severity was stratified as mild, moderate, and severe according to according to the Tokyo Guidelines 2018. Blood presepsin and plasma acylcarnitines were tested at enrollment by chemiluminescent enzyme immunoassay and ultra-high-performance liquid chromatography-mass spec
The concentrations of presepsin, procalcitonin, short- and medium-chain acylcarnitines increased, while long-chain acylcarnitines decreased with the severity of acute cholangitis. The areas under the receiver operating characteristic curves (AUC) of presepsin for diagnosing moderate/severe and severe cholangitis (0.823 and 0.801, respectively) were greater than those of conventional markers. The AUC of a combination of presepsin, direct bilirubin, alanine aminotransferase, temperature, and butyryl-L-carnitine for predicting biliary drainage was 0.723. Presepsin, procalcitonin, acetyl-L-carnitine, hydroxydodecenoyl-L-carnitine, and temperature were independent predictors of bloodstream infection. After adjusting for severity classification, acetyl-L-carnitine was the only acylcarnitine independently associated with 28-d mortality (hazard ratio 14.396; P < 0.001) (AUC: 0.880). Presepsin concentration showed positive correlation with direct bilirubin and acetyl-L-carnitine.
Presepsin may serve as a specific biomarker to predict the severity and biliary drainage of acute cholangitis. Acetyl-L-carnitine might be a promising prognostic factor for patients with acute cholangitis. Innate immune response was associated with mitochondrial metabolic dysfunction in acute cholangitis.
Prospective observational study reports the predictive value of presepsin and acylcarnitines for severity and biliary drainage of acute cholangitis. Future research should focus on the association between acylcarnitines and the changes of intestinal microflora and bacterial translocation in acute cholangitis.
We thank the emergency staff, gastroenterologists, hepatobiliary surgeons, radiologists, sonographers, and interventional physicians for their assistance; We express our gratitude to Zhang XX and Tan ZM for their help with patient recruitment; We also thank Wu SS for statistical analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kimura Y, Japan; Kitamura K, Japan S-Editor: Yan JP L-Editor: A P-Editor: Cai YX
| 1. | Lavillegrand JR, Mercier-Des-Rochettes E, Baron E, Pène F, Contou D, Favory R, Préau S, Galbois A, Molliere C, Miailhe AF, Reignier J, Monchi M, Pichereau C, Thietart S, Vieille T, Piton G, Preda G, Abdallah I, Camus M, Maury E, Guidet B, Dumas G, Ait-Oufella H. Acute cholangitis in intensive care units: clinical, biological, microbiological spectrum and risk factors for mortality: a multicenter study. Crit Care. 2021;25:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Khashab MA, Tariq A, Tariq U, Kim K, Ponor L, Lennon AM, Canto MI, Gurakar A, Yu Q, Dunbar K, Hutfless S, Kalloo AN, Singh VK. Delayed and unsuccessful endoscopic retrograde cholangiopancreatography are associated with worse outcomes in patients with acute cholangitis. Clin Gastroenterol Hepatol. 2012;10:1157-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Tappy L, Chioléro R. Substrate utilization in sepsis and multiple organ failure. Crit Care Med. 2007;35:S531-S534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Masson S, Caironi P, Fanizza C, Thomae R, Bernasconi R, Noto A, Oggioni R, Pasetti GS, Romero M, Tognoni G, Latini R, Gattinoni L. Circulating presepsin (soluble CD14 subtype) as a marker of host response in patients with severe sepsis or septic shock: data from the multicenter, randomized ALBIOS trial. Intensive Care Med. 2015;41:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 5. | Shozushima T, Takahashi G, Matsumoto N, Kojika M, Okamura Y, Endo S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J Infect Chemother. 2011;17:764-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 6. | Liu B, Chen YX, Yin Q, Zhao YZ, Li CS. Diagnostic value and prognostic evaluation of Presepsin for sepsis in an emergency department. Crit Care. 2013;17:R244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 7. | Ulla M, Pizzolato E, Lucchiari M, Loiacono M, Soardo F, Forno D, Morello F, Lupia E, Moiraghi C, Mengozzi G, Battista S. Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: a multicenter prospective study. Crit Care. 2013;17:R168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | McCann MR, George De la Rosa MV, Rosania GR, Stringer KA. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 191] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 9. | Lado-Abeal J, Martinez-Sánchez N, Cocho JA, Martín-Pastor M, Castro-Piedras I, Couce-Pico ML, Saha AK, López M. Lipopolysaccharide (LPS)-induced septic shock causes profound changes in myocardial energy metabolites in pigs. Metabolomics. 2018;14:131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Jennaro TS, Viglianti EM, Ingraham NE, Jones AE, Stringer KA, Puskarich MA. Serum Levels of Acylcarnitines and Amino Acids Are Associated with Liberation from Organ Support in Patients with Septic Shock. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Chung KP, Chen GY, Chuang TY, Huang YT, Chang HT, Chen YF, Liu WL, Chen YJ, Hsu CL, Huang MT, Kuo CH, Yu CJ. Increased Plasma Acetylcarnitine in Sepsis Is Associated With Multiple Organ Dysfunction and Mortality: A Multicenter Cohort Study. Crit Care Med. 2019;47:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, Hata J, Liau KH, Miura F, Horiguchi A, Liu KH, Su CH, Wada K, Jagannath P, Itoi T, Gouma DJ, Mori Y, Mukai S, Giménez ME, Huang WS, Kim MH, Okamoto K, Belli G, Dervenis C, Chan ACW, Lau WY, Endo I, Gomi H, Yoshida M, Mayumi T, Baron TH, de Santibañes E, Teoh AYB, Hwang TL, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Higuchi R, Kitano S, Inomata M, Deziel DJ, Jonas E, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 425] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 13. | Ackland GL, Prowle JR. Presepsin: solving a soluble (CD14) problem in sepsis? Intensive Care Med. 2015;41:351-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | ASGE Standards of Practice Committee; Buxbaum JL, Abbas Fehmi SM, Sultan S, Fishman DS, Qumseya BJ, Cortessis VK, Schilperoort H, Kysh L, Matsuoka L, Yachimski P, Agrawal D, Gurudu SR, Jamil LH, Jue TL, Khashab MA, Law JK, Lee JK, Naveed M, Sawhney MS, Thosani N, Yang J, Wani SB. ASGE guideline on the role of endoscopy in the evaluation and management of choledocholithiasis. Gastrointest Endosc. 2019;89:1075-1105.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 335] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 15. | Chen J, Zhao Y, Shang Y, Lin Z, Xu G, Bai B, Zheng J, Li P, Mao Y, Deng Q, Yu Z. The clinical significance of simultaneous detection of pathogens from bronchoalveolar lavage fluid and blood samples by metagenomic next-generation sequencing in patients with severe pneumonia. J Med Microbiol. 2021;70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Umefune G, Kogure H, Hamada T, Isayama H, Ishigaki K, Takagi K, Akiyama D, Watanabe T, Takahara N, Mizuno S, Matsubara S, Yamamoto N, Nakai Y, Tada M, Koike K. Procalcitonin is a useful biomarker to predict severe acute cholangitis: a single-center prospective study. J Gastroenterol. 2017;52:734-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med. 2000;343:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1517] [Cited by in RCA: 1450] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 18. | Higure A, Okamoto K, Hirata K, Todoroki H, Nagafuchi Y, Takeda S, Katoh H, Itoh H, Ohsato K, Nakamura S. Macrophages and neutrophils infiltrating into the liver are responsible for tissue factor expression in a rabbit model of acute obstructive cholangitis. Thromb Haemost. 1996;75:791-795. [PubMed] |
| 19. | Guicciardi ME, Trussoni CE, Krishnan A, Bronk SF, Lorenzo Pisarello MJ, O'Hara SP, Splinter PL, Gao Y, Vig P, Revzin A, LaRusso NF, Gores GJ. Macrophages contribute to the pathogenesis of sclerosing cholangitis in mice. J Hepatol. 2018;69:676-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 20. | An Z, Braseth AL, Sahar N. Acute Cholangitis: Causes, Diagnosis, and Management. Gastroenterol Clin North Am. 2021;50:403-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Shinya S, Sasaki T, Yamashita Y, Kato D, Yamashita K, Nakashima R, Yamauchi Y, Noritomi T. Procalcitonin as a useful biomarker for determining the need to perform emergency biliary drainage in cases of acute cholangitis. J Hepatobiliary Pancreat Sci. 2014;21:777-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Lee YS, Cho KB, Park KS, Lee JY, Lee YJ. Procalcitonin as a Decision-Supporting Marker of Urgent Biliary Decompression in Acute Cholangitis. Dig Dis Sci. 2018;63:2474-2479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Ruiz-Sala P, Peña-Quintana L. Biochemical Markers for the Diagnosis of Mitochondrial Fatty Acid Oxidation Diseases. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Merritt JL 2nd, Norris M, Kanungo S. Fatty acid oxidation disorders. Ann Transl Med. 2018;6:473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 25. | Bruls YM, de Ligt M, Lindeboom L, Phielix E, Havekes B, Schaart G, Kornips E, Wildberger JE, Hesselink MK, Muoio D, Schrauwen P, Schrauwen-Hinderling VB. Carnitine supplementation improves metabolic flexibility and skeletal muscle acetylcarnitine formation in volunteers with impaired glucose tolerance: A randomised controlled trial. EBioMedicine. 2019;49:318-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 26. | Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014;5:66-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 319] [Cited by in RCA: 410] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 27. | Eckerle M, Ambroggio L, Puskarich MA, Winston B, Jones AE, Standiford TJ, Stringer KA. Metabolomics as a Driver in Advancing Precision Medicine in Sepsis. Pharmacotherapy. 2017;37:1023-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Puskarich MA, Evans CR, Karnovsky A, Das AK, Jones AE, Stringer KA. Septic Shock Nonsurvivors Have Persistently Elevated Acylcarnitines Following Carnitine Supplementation. Shock. 2018;49:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Ferrario M, Cambiaghi A, Brunelli L, Giordano S, Caironi P, Guatteri L, Raimondi F, Gattinoni L, Latini R, Masson S, Ristagno G, Pastorelli R. Mortality prediction in patients with severe septic shock: a pilot study using a target metabolomics approach. Sci Rep. 2016;6:20391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 30. | Rogers AJ, McGeachie M, Baron RM, Gazourian L, Haspel JA, Nakahira K, Fredenburgh LE, Hunninghake GM, Raby BA, Matthay MA, Otero RM, Fowler VG, Rivers EP, Woods CW, Kingsmore S, Langley RJ, Choi AM. Metabolomic derangements are associated with mortality in critically ill adult patients. PLoS One. 2014;9:e87538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 31. | Srinivas SR, Prasad PD, Umapathy NS, Ganapathy V, Shekhawat PS. Transport of butyryl-L-carnitine, a potential prodrug, via the carnitine transporter OCTN2 and the amino acid transporter ATB(0,+). Am J Physiol Gastrointest Liver Physiol. 2007;293:G1046-G1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Schaarschmidt B, Vlaic S, Medyukhina A, Neugebauer S, Nietzsche S, Gonnert FA, Rödel J, Singer M, Kiehntopf M, Figge MT, Jacobsen ID, Bauer M, Press AT. Molecular signatures of liver dysfunction are distinct in fungal and bacterial infections in mice. Theranostics. 2018;8:3766-3780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Mussotter F, Potratz S, Budczies J, Luch A, Haase A. A multi-omics analysis reveals metabolic reprogramming in THP-1 cells upon treatment with the contact allergen DNCB. Toxicol Appl Pharmacol. 2018;340:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | van Teijlingen Bakker N, Pearce EJ. Cell-intrinsic metabolic regulation of mononuclear phagocyte activation: Findings from the tip of the iceberg. Immunol Rev. 2020;295:54-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 35. | Zhu X, Meyers A, Long D, Ingram B, Liu T, Yoza BK, Vachharajani V, McCall CE. Frontline Science: Monocytes sequentially rewire metabolism and bioenergetics during an acute inflammatory response. J Leukoc Biol. 2019;105:215-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 36. | Chi Z, Chen S, Xu T, Zhen W, Yu W, Jiang D, Guo X, Wang Z, Zhang K, Li M, Zhang J, Fang H, Yang D, Ye Q, Yang X, Lin H, Yang F, Zhang X, Wang D. Histone Deacetylase 3 Couples Mitochondria to Drive IL-1β-Dependent Inflammation by Configuring Fatty Acid Oxidation. Mol Cell. 2020;80:43-58.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |