Published online Apr 28, 2023. doi: 10.3748/wjg.v29.i16.2469
Peer-review started: November 15, 2022
First decision: February 2, 2023
Revised: February 11, 2023
Accepted: March 24, 2023
Article in press: March 24, 2023
Published online: April 28, 2023
Processing time: 160 Days and 10.6 Hours
There is paucity of data on outcomes of acute severe ulcerative colitis (ASUC) in older adults (≥ 60 years of age).
To assess steroid non-response rates during the index admission for ASUC in older adults. Secondary outcomes were response to medical rescue therapy and colectomy rates; at index admission, 3 and 12 mo.
This retrospective multicentre cohort study included ASUC admissions who received intravenous steroids between January 2013 and July 2020 at two tertiary hospitals. Electronic medical records were reviewed to collect clinical, biochemical, and endoscopic data. A modified Poisson regression model was used for analysis.
Of 226 ASUC episodes, 45 (19.9%) occurred in patients ≥ 60 years of age. Steroid non-response rates were comparable in older adults and patients < 60 years of age [19 (42.2%) vs 85 (47%), P = 0.618, crude risk ratio (RR) = 0.89 [95% confidence interval (CI): 0.61-1.30], adjusted RR = 0.99 (0.44-2.21). Rates of response to medical rescue therapy in older adults was comparable to the younger cohort [76.5% vs 85.7%, P = 0.46, crude RR = 0.89 (0.67-1.17)]. Index admission colectomy [13.3% vs 10.5%, P = 0.598, crude RR = 1.27 (0.53-2.99), adjusted RR = 1.43 (0.34-6.06)], colectomy at 3 mo [20% vs 16.6%, P = 0.66, crude RR = 1.18 (0.61-2.3), adjusted RR = 1.31 (0.32-0.53)] and colectomy at 12 mo [20% vs 23.2%, P = 0.682, crude RR = 0.85 (0.45-1.57), adjusted RR = 1.21 (0.29-4.97)], were similar between the two groups.
In older adults with ASUC, the steroid non-response rate, response to medical rescue therapy, and colectomy rate at index admission, 3 and 12 mo is similar to patients less than 60 years of age.
Core Tip: This is a retrospective study to assess the outcomes of older adults (≥ 60 years of age) hospitalised with acute severe ulcerative colitis (ASUC) as per Truelove and Witts’ criteria. We identified 45 episodes of ASUC in older adults and compared outcomes with 181 episodes of ASUC in patients < 60 years of age. Older adults with ASUC have similar steroid non-response rate, response to medical rescue therapy and colectomy rates up to 12 mo from index admission, when compared to patients less than 60 years of age.
- Citation: Subhaharan D, Ramaswamy PK, Willmann L, Moattar H, Bhullar M, Ishaq N, Dorrington A, Shukla D, McIvor C, Edwards J, Mohsen W. Older adults with acute severe ulcerative colitis have similar steroid non-response and colectomy rates as younger adults. World J Gastroenterol 2023; 29(16): 2469-2478
- URL: https://www.wjgnet.com/1007-9327/full/v29/i16/2469.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i16.2469
Ulcerative colitis (UC) is a chronic, relapsing-remitting, inflammatory disorder of the colon, resulting from numerous factors including genetic predisposition, environmental triggers, and gut microbiota[1,2]. Acute severe UC (ASUC), as defined by the Truelove and Witts criteria, occurs in 10%-25% at diagnosis and 20%-30% during the disease course of UC[3-5]. Intravenous corticosteroids (IVCS) remain the first-line therapy for ASUC, however infliximab (IFX) and ciclosporin (CsA) have been used as medical rescue therapy for those who are steroid-refractory[6-9].
Up to 20% of patients with UC have late-onset disease with their first flare occurring after the age of 60[10,11]. The basic principles of management of ASUC in older adults do not differ from younger patients[12]. However, there are unique challenges in managing inflammatory bowel disease (IBD) in older adults, including delay in diagnosis, misdiagnosis, and variable clinical presentations. Older adults may suffer from comorbidities, polypharmacy, complex drug-drug interactions, cognitive dysfunction, post-surgical complications, as well as social factors, which increase complexity in management of older adults with ASUC[13-17]. Studies have demonstrated higher treatment failure rates in elderly IBD patients who are commenced on their first anti-tumour necrosis factor agent[18]. In the setting of these factors, management decisions need to be patient-centred and individualised to minimise morbidity and mortality for older adults with ASUC.
Advanced age has not been shown to predict outcomes in ASUC[19]. However, in routine clinical practice, age is an important factor which is taken into consideration in the decision-making algorithm. As older adults are generally excluded from clinical trials, management decisions for these patients are often made by extrapolating data from a younger cohort of patients[11]. Moreover, short and long-term outcomes of ASUC in this cohort of patients are not well described. The primary outcome of the study was to assess steroid non-response rates during the index admission for ASUC in older adults. The secondary outcomes were response to medical rescue therapy and colectomy rates at index admission, 3 and 12 mo.
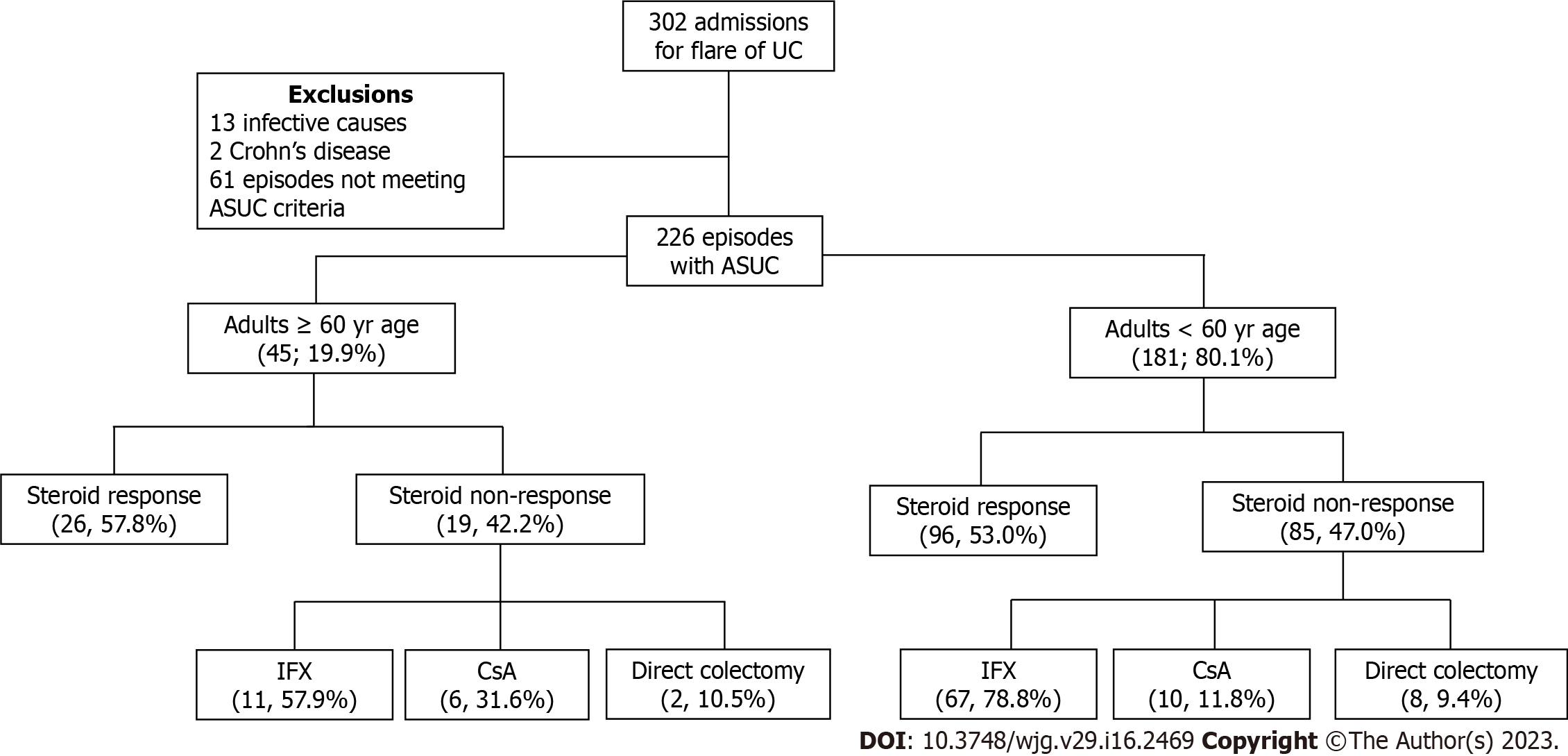
All consecutive admissions with a diagnosis of UC at two tertiary Australian hospitals, from January 2013 to July 2020 at Gold Coast University Hospital and from January 2018 to July 2020 at Logan Hospital, were identified using international classification of disease (ICD-10) codes (K51). Retrospective analysis identified adult patients (≥ 18 years of age) admitted for management of ASUC, as identified by Truelove and Witts criteria[3] (Figure 1). The study was approved by the Gold Coast Health Service Human Research Ethics Committee (Ref: LNR/2020/QGC/67173).
Inclusion was limited to patients with ASUC who received at least 3-5 d of IVCS (either hydrocortisone 400 mg/d or methylprednisolone 60 mg/d). Patients with a diagnosis of Crohn’s disease or positive stool cultures for other enteric pathogens were excluded. Patients with superimposed Clostridium difficile or cytomegalovirus infection were included in the final analysis. Demographic, clinical and laboratory results were collected. Endoscopic data was collected from procedure reports and images, and scored based on the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) score[20].
All patients received IVCS as per international guidelines[21]. The Oxford criteria was used to determine failure of IVCS therapy after 3-5 d[22]. Patients received IFX or CsA for medical rescue therapy at their treating physician’s preference. The standard dose IFX induction strategy utilised was 5 mg/kg at week 0, 2 and 6. Accelerated dose of IFX was defined as 10 mg/kg on day 0 followed by 5 mg/kg at week 2 and 6. The dose of IFX was determined by the treating physician based on clinical assessment of disease severity. CsA was dosed at 2 mg/kg body weight with a target trough level of 200-300 ng/mL at 48 h. In patients responding to medical rescue therapy, maintenance therapy was based on disease severity and prior treatment history as per the treating physician’s discretion.
UC: The diagnosis of UC was based on standard clinical, endoscopic, and histological criteria[23].
ASUC: The diagnosis of ASUC was based on Truelove and Witts criteria; defined as ≥ 6 bloody stool motions per day and one or more of the following: Haemoglobin < 10.5 g/dL, erythrocyte sedimentation rate ≥ 30 mm/hr or C-reactive protein ≥ 30 mg/L, temperature ≥ 37.8 °C, or heart rate ≥ 90 beats/min[3].
Disease extent: The maximum endoscopic extent at index colonoscopy according to the Montreal classification[24]. In patients with ASUC as their first presentation of disease, the extent was determined from the first available colonoscopy after discharge, or the surgical specimen if they underwent colectomy.
Older adults with ASUC: ASUC occurring in patients ≥ 60 years of age (irrespective of the age at diagnosis of UC).
Endoscopic severity: Defined by the UCEIS. The score (0-8) is calculated by the sum of three descriptors: Vascular pattern (scored 0-2), bleeding (scored 0-3), and erosions/ulcers (scored 0-3). It is assessed at the most severely affected area on flexible sigmoidoscopy[20].
Steroid non-response: Defined as failure to respond to IVCS as defined by the Oxford criteria[22], and receiving either medical or surgical rescue therapy.
IFX dosing: Standard dose strategy was defined as IFX 5 mg/kg at week 0, 2 and 6. Accelerated dose was defined as IFX 10 mg/kg on day 0 followed by 5 mg/kg at week 2 and 6.
Responder to medical rescue therapy: Defined as the patient being discharged from hospital on medical therapy after receiving inpatient medical rescue therapy, and avoiding colectomy during the admission.
The primary outcome was to assess steroid non-response rates during the index admission for ASUC in older adults. The secondary outcomes were response to medical rescue therapy and colectomy rates at index admission, 3 and 12 mo.
Descriptive statistics were used to describe the study cohort. Results were reported as median with interquartile range (IQR) for continuous variables, and frequencies with percentages for categorical variables. For comparison of variables, Fisher’s exact or Chi-square tests were used for categorical variables, and Wilcoxon Ranksum test for continuous variables. Continuous data was tested for normality using the Shapiro-Wilk test and a two-tailed P value of < 0.05 was considered statistically significant. A modified Poisson regression model was used to estimate risk differences (RDs) and RRs to evaluate the difference in clinical outcomes between the two groups. Kaplan-Meier plots and the Cox proportional hazards regression model were also used. A log-rank test was used to compare the curves of the Kaplan-Meier plots. Multiple imputations were performed to account for missing covariates. All analysis was performed using Stata15 (StataCorp LLC, College Station, Texas).
A total of 302 admissions for UC who received IVCS were identified, of which 76 were excluded. 226 episodes of ASUC were included in the analysis. 45 (19.9%) episodes of ASUC in older adults ≥ 60 years of age and 181 (80.1%) episodes in younger adults were identified (Figure 1). Median age of disease onset was 66.5 (IQR: 59-76) vs 27 (IQR: 21-37), P < 0.001. Disease duration was similar between the two groups (2.5 vs 2 years, P 0.94). 33 out of 45 (73.3%) episodes had their first presentation of UC after the age of 60 years. Median Charlson Comorbidity Index in older adults was 3 (IQR: 2-4). Smoking status, albumin and platelet count at admission were significantly different between the two groups. Current immunomodulator use, biologic use and oral steroid use at admission were similar between the two groups. Clinical, endoscopic, and biochemical parameters are provided in Table 1. Summary of primary and secondary outcomes are shown in Table 2.
| ≥ 60 yr (n = 45) | < 60 yr (n = 181) | P value | |
| Female, n (%) | 22 (48.9) | 88 (48.6) | 1 |
| Median age (yr) | 71 (63-77) | 32 (24-42) | < 0.0011 |
| Median disease duration (yr) | 2.5 (0-5) | 2 (0.1-6) | 0.941 |
| Index presentation of UC as ASUC (n, %) | 14 (31.1) | 45 (24.9) | 0.45 |
| Median follow up post admission for ASUC (wk) | 104 (20-160) | 74 (30-168) | 0.971 |
| Median symptom duration before admission (d) | 14 (7-24) | 14 (5-28) | 0.591 |
| Median length of stay (d) | 10 (7-19) | 9 (7-13.5) | 0.221 |
| Disease extent, n (%) | 0.072 | ||
| Left-sided colitis | 8 (17.8) | 54 (29.8) | |
| Pancolitis | 37 (82.2) | 127 (70.2) | |
| Toxic megacolon, n (%) | 0 | 4 (2.2) | 0.41 |
| Extraintestinal manifestations, n (%) | 2 (4.4) | 31 (17.1) | 0.02 |
| Superimposed clostridium difficile, n (%) | 3 (6.7) | 6 (3.3) | 0.26 |
| Smoking status, n (%) | 0.037 | ||
| Never | 21 (46.7) | 121 (66.9) | |
| Current | 6 (13.3) | 17 (9.4) | |
| Former | 18 (40.0) | 43 (23.8) | |
| 5-aminosalicyclate use, n (%) | 0.86 | ||
| Current | 24 (53.3) | 100 (55.2) | |
| Never | 14 (31.1) | 49 (27.1) | |
| Intolerant/ceased | 7 (15.6) | 32 (17.7) | |
| Current thiopurine use, n (%) | 6 (13.3) | 29 (16.0) | 0.29 |
| Current methotrexate use, n (%) | 1 (2.2) | 2 (1.1) | 0.16 |
| Anti-TNF antagonist use, n (%) | 0.74 | ||
| Current | 6 (13.3) | 23 (12.7) | |
| Never | 37 (82.2) | 134 (74.0) | |
| Intolerant | 1 (2.2) | 6 (3.3) | |
| Secondary loss of response | 1 (2.2) | 18 (9.9) | |
| Vedolizumab use, n (%) | 0.024 | ||
| Current | 8 (17.8) | 9 (5.0) | |
| Never | 35 (77.8) | 163 (90.0) | |
| Intolerant | 0 | 4 (2.2) | |
| Secondary loss of response | 2 (4.4) | 5 (2.8) | |
| Biologics on admission, n (%) | 14 (31.0) | 35 (19.4) | 0.11 |
| Oral steroids at admission, n (%) | 15 (33.3) | 78 (43.1) | 0.31 |
| Median admission UCEIS | 5.5 (5-7) | 6 (5-7) | 0.821 |
| Median serum albumin on day of admission (g/L) | 31 (27-34) | 33 (29-38) | 0.0051 |
| Median haemoglobin on day of admission (g/L) | 126 (111-135) | 124 (108-139) | 0.921 |
| Median platelet count on day of admission (units) | 333.5 (277-386) | 393 (293-500) | 0.0061 |
| Median CRP on day of admission (mg/L) | 69 (33-121) | 54 (30-99) | 0.341 |
| Median admission faeces calprotectin (mcg/g) | 2400 (1600-4600) | 2850 (1400-5300) | 0.481 |
| Median stool frequency on day of admission | 10 (7-15) | 10 (8-18) | 0.411 |
| ≥ 60 yr, n = 45 | < 60 yr, n = 181 | Crude RD (95%CI) | Crude RR (95%CI) | Adjusted RR1 (95%CI) | OR (95%CI) | |
| Primary outcome: Steroid non-response | 19 (42.2%) | 85 (47%) | -0.47 (-0.21 to 0.11) | 0.89 (0.61-1.30) | 0.99 (0.34-2.90) | 0.82 (0.43-1.58) |
| Response to medical rescue therapy | 13 (76.5%) | 66 (85.7%) | -0.09 (-0.31 to 0.12) | 0.89 (0.67-1.17) | - | 0.54 (0.16-1.85) |
| Colectomy same admission | 6 (13.3%) | 19 (10.5%) | 0.028 (-0.08 to 0.13) | 1.27 (0.53-2.99) | 1.43 (0.34-6.06) | 1.31 (0.50-3.41) |
| Colectomy at 3 mo | 9 (20.9%) | 30 (17.6%) | -0.03 (-0.10 to 0.16) | 1.18 (0.61-2.3) | 1.31 (0.32-5.30) | 1.23 (0.54-2.80) |
| Colectomy at 12 mo | 9 (24.3%) | 42 (28.8%) | -0.04 (-0.20 to 0.11) | 0.85 (0.45-1.57) | 1.2 (0.29-4.97) | 0.79 (0.35-1.80) |
Failure to IVCS therapy, as defined by the Oxford criteria[22], was similar between older and younger adults [19 (42.2%) vs 85 (47%), P = 0.618; crude RR = 0.89 (0.61-1.30), P = 0.34; adjusted RR = 0.99 (0.34-2.90), P = 0.175; odds ratio (OR) = 0.82 (0.43-1.58), P = 0.344; crude hazard ratio (HR) = 0.89 (0.556-1.455), P = 0.674]. In older adults, of the 19 episodes that failed IVCS, 17 (89.5%) episodes received medical rescue therapy (7 episodes IFX 5 mg/kg, 4 episodes IFX 10 mg/kg, 6 episodes CsA) and 2 (10.5%) patients proceeded directly to colectomy. Median time to initiation of rescue therapy was 4 d (IQR: 3-5 d). In patients < 60 years of age, of the 85 episodes that failed IVCS, 77 (90.6%) episodes received medical rescue therapy (45 episodes IFX 5 mg/kg, 22 episodes IFX 10 mg/kg, 10 episodes CsA) and 8 (9.4%) patients underwent direct colectomy. When the cut-off age was defined as 70 years, a significantly lower proportion of episodes failed IVCS [6/23 (26.1%) in ≥ 70 years vs 98/203 (48.3%) in < 70 years, P = 0.049; crude RD = -0.22 (-0.41 to -0.03); crude RR = 0.54 (0.27-1.09), P = 0.034; adjusted RR = 0.36 (0.08-1.49), P = 0.897; crude OR = 0.378 (0.143-1.00), P = 0.05].
Response to medical rescue therapy: In older adults, of the 17 episodes who received medical rescue therapy, 4 (23.5%) patients underwent colectomy during the index admission. In the younger cohort, of the 77 episodes who received medical rescue therapy, 10 (13%) patients underwent a colectomy during the index admission. The rates of response to medical rescue therapy in older adults were similar to the younger cohort [76.5% vs 85.7%, P = 0.46; crude RD = -0.092 (-0.31 to 0.12); crude RR = 0.89 (0.67-1.17), P = 0.27; crude OR = 0.54 (0.16-1.85)]. When the cut-off age was defined as 70 years, a lower proportion of episodes responded to medical rescue therapy [4/6 (66.7%) in ≥ 70 years vs 75/88 (85.2%) in < 70 years, P = 0.243; crude RD = -0.18 (-0.57 to 0.19); crude RR = 0.78 (0.44-1.38); crude OR = 0.34 (0.65-), P = 0.24].
Index admission colectomy: In older adults, 6 (13.3%) of 45 patients underwent colectomy during the index admission for ASUC compared to 19 (10.5%) of 191 patients in the younger cohort [crude RD = 0.028 (-0.08 to 0.13); crude RR = 1.27 (0.53-2.99), P = 0.376; adjusted RR = 1.43 (0.34-6.06), P = 0.71; crude OR = 1.31 (0.50-3.41); crude HR = 1.27 (0.47-3.39), P = 0.608]. When the cut-off age was defined as 70 years, a similar proportion of episodes underwent colectomy during the index admission [2/25 (8.7%) in ≥ 70 years vs 21/201 (8.7%) in < 70 years, P = 0.52; crude RD = -0.026 (-0.15 to 0.09); crude RR = 0.77 (0.19-3.04); adjusted RR = 0.91 (0.16-5.09), P = 0.52; crude OR = 0.74 (0-3.0), P = 0.52].
Colectomy at 3 mo: At 3 mo, 9 (20%) patients ≥ 60 years of age had undergone a colectomy, compared to 30 (17.6%) patients < 60 years of age [crude RD = -0.03 (-0.10 to 0.16); crude RR = 1.18 (0.61-2.3), P = 0.38; adjusted RR = 1.31 (0.32-0.53), P = 0.82; crude OR = 1.23 (0.54-2.80); crude HR = 1.21 (0.55-2.648, P = 0.620]. In older adults, of the 13 episodes which responded to medical rescue therapy, 1 patient with two episodes of ASUC within a three-month period of the index admission underwent a colectomy. When age cut-off was defined as 70 years, a lower proportion of episodes underwent colectomy at 3 mo [2/23 (8.7%) in ≥ 70 years vs 37/190 (19.5%) in < 70 years of age, P = 0.264; crude RD = -0.1.09 (-0.235 to 0.02); crude RR = 0.44 (0.11-1.73); adjusted RR = 0.72 (0.14-3.73), crude OR = 0.39 (0-1.58), P = 0.165].
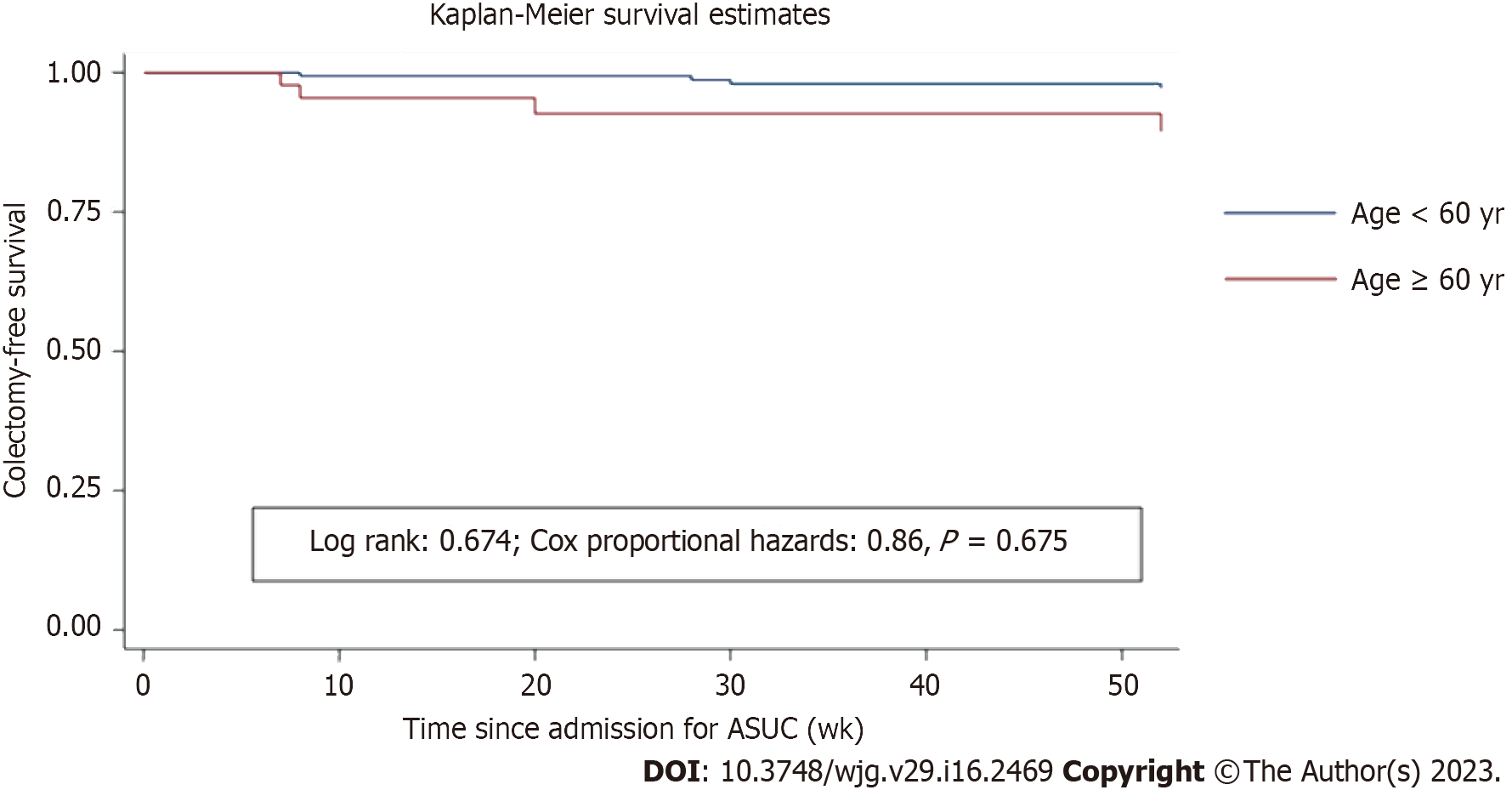
Colectomy at 12 mo: At 12 mo, 9 (24.3%) patients ≥ 60 years of age had undergone a colectomy, compared to 42 (28.8%) patients < 60 years of age [crude RD = -0.04 (-0.20 to 0.11); crude RR = 0.85 (0.45-1.57), P = 0.376; adjusted RR = 1.21 (0.29-4.97), P = 0.88; crude OR = 0.79 (0.35-1.80); crude HR = 0.86 (0.43-1.71), P = 0.69]. The Kaplan-Meier curve for colectomy-free survival is shown in Figure 2. When age cut-off was defined as 70 years, a lower proportion of episodes underwent colectomy at 12 mo [2/23 (8.7%) in ≥ 70 years vs 49/203 (24.1%) in < 70 years of age, P = 0.042; crude RD = -0.21 (-0.35 to -0.07); crude RR = 0.29 (0.07-1.14), P = 0.026; adjusted RR = 0.63 (0.11-3.41), P = 0.673; OR = 0.23 (0-0.92)].
Although the management of IBD in older adults remains a challenge, the basic treatment paradigms across all age groups are the same. This study is one of the largest studies describing outcomes of ASUC in older adults. It demonstrates that the rates of steroid non-response as well as short and long-term colectomy risk in older adults is comparable to those who are less than 60 years of age.
There is an increasing number of older adults with IBD, correlating with both the rising incidence of IBD and the ageing population[25]. The widely accepted definition of elderly-onset IBD is disease onset at age 60 years or older[25]. Hence, this study used 60 years as the cut-off age to define older adults. In this study, 20% of patients were over 60 years of age at the time of their ASUC presentation; 15% of patients (33 out of 226) had their initial diagnosis of UC after the age of 60. This is comparable to current data showing 10%-25% of IBD patients are diagnosed after the age of 60[25,26]. Previous studies have exhibited that older adults with UC are more likely to present with a severe initial episode, display proctocolitis or limited left-sided colitis, and develop toxic megacolon which is associated with high mortality[27,28]. In this study, 13 (28.9%) episodes had proctocolitis or limited left-sided colitis, and there were no episodes of toxic megacolon in older adults.
Traditionally, the Oxford index has been utilised to define steroid failure in patients with ASUC, and in this study the same definitions were applied[22]. Previous studies have shown that about 40% of patients with ASUC fail initial therapy with IVCS[29]. This study reconfirms that the rate of steroid failure is similar between older adults (42.2%) and the younger cohort of patients (47%). This is in contrast to a recently published multicentre Japanese study[30]. IVCS continue to be the first-line treatment option for older adults, although steroid-specific adverse effects are to be taken into consideration. Nevertheless, older adults with ASUC should not be undertreated, as poorly controlled disease and repeated courses of steroids induce undesirable outcomes. In this study, more than 75% of older adults responded to medical rescue therapy and avoided colectomy during admission for ASUC. The effectiveness of medical rescue therapy demonstrated in the current study is comparable to that demonstrated in larger randomised-controlled trials[31,32]. Of the episodes who responded to medical rescue therapy, only 1 patient had undergone a colectomy by 12 mo. Biologic agents in older adults with IBD were recently shown to have similar drug sustainability, effectiveness, and safety[33]. Older adults on IFX also have a similar risk of developing adverse effects and loss of response as younger patients[34]. Thus, medical rescue therapy can be offered judiciously to older adults.
This study has several strengths, foremost that it is one of the largest studies describing outcomes of ASUC in older adults. Although this was not a controlled trial, this cohort of patients was managed through two tertiary IBD subspeciality units which have defined treatment protocols for hospitalised ASUC patients consistent with international guidelines. Results are therefore generalisable to similar real-world clinical settings. The study has a few limitations. Firstly, the study is retrospective. Secondly, long-term safety of IFX and CsA were not studied systematically. The assessment of clinical response after initiation of rescue therapy with the Lichtiger score or Mayo score may have been beneficial. Finally, clinical and biochemical data at 12 mo may have also proved valuable for the analysis of the study.
Management of older adults with ASUC remains challenging. This study demonstrates that the rate of IVCS non-response in older adults with ASUC is similar to younger patients, and medical rescue therapy is equally effective. Clinical decisions for older adults with ASUC should still be determined by disease severity rather than chronological age alone.
The management of older adults with acute severe ulcerative colitis (ASUC) is uniquely challenging due to their numerous medical and social factors. Up to 20% of patients with ulcerative colitis have late-onset disease with their first flare occurring after the age of 60.
There is minimal data available on the outcomes of older adults with ASUC. Previous studies report higher treatment failure rates in older adults who are commenced on their first biologic. We planned this study to define both short and long term outcomes for this cohort and determine if they have similar outcomes compared to the younger cohort.
We aimed to determine the steroid non-response rates for older adults with ASUC during index admission. We also aimed to study their response to medical rescue therapy and colectomy rates up to 12 mo from initial presentation.
We conducted a retrospective cohort study investigating the short and long term outcomes among 226 ASUC episodes between January 2013 and July 2020 at two tertiary hospitals in Queensland, Australia. Clinical characteristics, laboratory parameters, and disease outcomes, including mortality, were compared between older and younger adults. A modified Poisson regression model was used for analysis.
The prevalence of older adults with ASUC was 19.9%. Steroid non-response rate in older adults were comparable to younger adults (42.2% vs 47%, P = 0.62). Response rates to medical rescue therapy was also comparable between the two groups (76.5% vs 85.7%, P = 0.46). Index admission colectomy (13.3% vs 10.5%, P = 0.60), colectomy at 3 mo (20% vs 16.6%, P = 0.66), and colectomy at 12 mo (20% vs 23.2%, P = 0.68) were also similar between the two groups.
Older adults with ASUC have similar outcomes compared to younger patients less than 60 years of age for rates of steroid non-response, medical rescue therapy, and colectomy at index admission, 3 and 12 mo.
Clinical decisions for older adults with ASUC remains to be a challenge however should still be determined by disease severity rather than chronological age alone. Future prospective studies will allow further improvement in their management.
Ms Elise Sawyer, Ms Rayshelle James, Ms Roz McLean, Mr Timothy Lyons, Ms Janine Meinig from the IBD Nursing Service.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pandey NM, India; Sahin Y, Turkey; Wu G, China S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
| 1. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2191] [Article Influence: 136.9] [Reference Citation Analysis (6)] |
| 2. | Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1339] [Cited by in RCA: 1375] [Article Influence: 80.9] [Reference Citation Analysis (1)] |
| 3. | Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1832] [Cited by in RCA: 1863] [Article Influence: 26.6] [Reference Citation Analysis (1)] |
| 4. | Burisch J, Katsanos KH, Christodoulou DK, Barros L, Magro F, Pedersen N, Kjeldsen J, Vegh Z, Lakatos PL, Eriksson C, Halfvarson J, Fumery M, Gower-Rousseau C, Brinar M, Cukovic-Cavka S, Nikulina I, Belousova E, Myers S, Sebastian S, Kiudelis G, Kupcinskas L, Schwartz D, Odes S, Kaimakliotis IP, Valpiani D, D'Incà R, Salupere R, Chetcuti Zammit S, Ellul P, Duricova D, Bortlik M, Goldis A, Kievit HAL, Toca A, Turcan S, Midjord J, Nielsen KR, Andersen KW, Andersen V, Misra R, Arebi N, Oksanen P, Collin P, de Castro L, Hernandez V, Langholz E, Munkholm P; Epi-IBD Group. Natural Disease Course of Ulcerative Colitis During the First Five Years of Follow-up in a European Population-based Inception Cohort-An Epi-IBD Study. J Crohns Colitis. 2019;13:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 5. | Domènech E, Mañosa M, Cabré E. An overview of the natural history of inflammatory bowel diseases. Dig Dis. 2014;32:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Truelove SC, Jewell DP. Intensive intravenous regimen for severe attacks of ulcerative colitis. Lancet. 1974;1:1067-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 336] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Lichtiger S, Present DH, Kornbluth A, Gelernt I, Bauer J, Galler G, Michelassi F, Hanauer S. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1217] [Cited by in RCA: 1171] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 8. | Järnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlén P, Grännö C, Vilien M, Ström M, Danielsson A, Verbaan H, Hellström PM, Magnuson A, Curman B. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005;128:1805-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 766] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 9. | Van Assche G, D'Haens G, Noman M, Vermeire S, Hiele M, Asnong K, Arts J, D'Hoore A, Penninckx F, Rutgeerts P. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003;125:1025-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 271] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Jeuring SF, van den Heuvel TR, Zeegers MP, Hameeteman WH, Romberg-Camps MJ, Oostenbrug LE, Masclee AA, Jonkers DM, Pierik MJ. Epidemiology and Long-term Outcome of Inflammatory Bowel Disease Diagnosed at Elderly Age-An Increasing Distinct Entity? Inflamm Bowel Dis. 2016;22:1425-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 11. | Charpentier C, Salleron J, Savoye G, Fumery M, Merle V, Laberenne JE, Vasseur F, Dupas JL, Cortot A, Dauchet L, Peyrin-Biroulet L, Lerebours E, Colombel JF, Gower-Rousseau C. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut. 2014;63:423-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 257] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 12. | Gisbert JP, Chaparro M. Systematic review with meta-analysis: inflammatory bowel disease in the elderly. Aliment Pharmacol Ther. 2014;39:459-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | Katz S, Surawicz C, Pardi DS. Management of the elderly patients with inflammatory bowel disease: practical considerations. Inflamm Bowel Dis. 2013;19:2257-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Cottone M, Kohn A, Daperno M, Armuzzi A, Guidi L, D'Inca R, Bossa F, Angelucci E, Biancone L, Gionchetti P, Ardizzone S, Papi C, Fries W, Danese S, Riegler G, Cappello M, Castiglione F, Annese V, Orlando A. Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 271] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 15. | Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, Hébuterne X, Cortot A, Bouhnik Y, Gendre JP, Simon T, Maynadié M, Hermine O, Faivre J, Carrat F; CESAME Study Group. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 801] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 16. | Kaplan GG, Hubbard J, Panaccione R, Shaheen AA, Quan H, Nguyen GC, Dixon E, Ghosh S, Myers RP. Risk of comorbidities on postoperative outcomes in patients with inflammatory bowel disease. Arch Surg. 2011;146:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Kaplan GG, McCarthy EP, Ayanian JZ, Korzenik J, Hodin R, Sands BE. Impact of hospital volume on postoperative morbidity and mortality following a colectomy for ulcerative colitis. Gastroenterology. 2008;134:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 240] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 18. | de Jong ME, Smits LJT, van Ruijven B, den Broeder N, Russel MGVM, Römkens TEH, West RL, Jansen JM, Hoentjen F. Increased Discontinuation Rates of Anti-TNF Therapy in Elderly Inflammatory Bowel Disease Patients. J Crohns Colitis. 2020;14:888-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Vavricka SR, Rogler G. Treatment of severe ulcerative colitis: differences in elderly patients? Dig Dis. 2009;27:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Travis SP, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, Feagan BG, Hanauer SB, Lichtenstein GR, Marteau PR, Reinisch W, Sands BE, Yacyshyn BR, Schnell P, Bernhardt CA, Mary JY, Sandborn WJ. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology. 2013;145:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 340] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 21. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1402] [Cited by in RCA: 1534] [Article Influence: 255.7] [Reference Citation Analysis (0)] |
| 22. | Ventham NT, Kalla R, Kennedy NA, Satsangi J, Arnott ID. Predicting outcomes in acute severe ulcerative colitis. Expert Rev Gastroenterol Hepatol. 2015;9:405-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, Mantzaris G, Reinisch W, Colombel JF, Vermeire S, Travis S, Lindsay JO, Van Assche G. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 638] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 24. | Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1970] [Cited by in RCA: 2334] [Article Influence: 122.8] [Reference Citation Analysis (2)] |
| 25. | Sturm A, Maaser C, Mendall M, Karagiannis D, Karatzas P, Ipenburg N, Sebastian S, Rizzello F, Limdi J, Katsanos K, Schmidt C, Jeuring S, Colombo F, Gionchetti P. European Crohn's and Colitis Organisation Topical Review on IBD in the Elderly. J Crohns Colitis. 2017;11:263-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Taleban S, Colombel JF, Mohler MJ, Fain MJ. Inflammatory bowel disease and the elderly: a review. J Crohns Colitis. 2015;9:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Sinclair TS, Brunt PW, Mowat NA. Nonspecific proctocolitis in northeastern Scotland: a community study. Gastroenterology. 1983;85:1-11. [PubMed] |
| 28. | Grimm IS, Friedman LS. Inflammatory bowel disease in the elderly. Gastroenterol Clin North Am. 1990;19:361-389. [PubMed] |
| 29. | Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007;5:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 448] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 30. | Okabayashi S, Yamazaki H, Tominaga K, Miura M, Sagami S, Matsuoka K, Yamaguchi Y, Noake T, Ozeki K, Miyazaki R, Kamano T, Fukuda T, Yoshioka K, Ando K, Fukuzawa M, Andoh A, Yamamoto Y, Hibi T, Kobayashi T; IBD Terakoya Group. Lower effectiveness of intravenous steroid treatment for moderate-to-severe ulcerative colitis in hospitalised patients with older onset: a multicentre cohort study. Aliment Pharmacol Ther. 2022;55:1569-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Laharie D, Bourreille A, Branche J, Allez M, Bouhnik Y, Filippi J, Zerbib F, Savoye G, Nachury M, Moreau J, Delchier JC, Cosnes J, Ricart E, Dewit O, Lopez-Sanroman A, Dupas JL, Carbonnel F, Bommelaer G, Coffin B, Roblin X, Van Assche G, Esteve M, Färkkilä M, Gisbert JP, Marteau P, Nahon S, de Vos M, Franchimont D, Mary JY, Colombel JF, Lémann M; Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012;380:1909-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 437] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 32. | Williams JG, Alam MF, Alrubaiy L, Arnott I, Clement C, Cohen D, Gordon JN, Hawthorne AB, Hilton M, Hutchings HA, Jawhari AU, Longo M, Mansfield J, Morgan JM, Rapport F, Seagrove AC, Sebastian S, Shaw I, Travis SP, Watkins A. Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (CONSTRUCT): a mixed methods, open-label, pragmatic randomised trial. Lancet Gastroenterol Hepatol. 2016;1:15-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 33. | Hahn GD, LeBlanc JF, Golovics PA, Wetwittayakhlang P, Qatomah A, Wang A, Boodaghians L, Liu Chen Kiow J, Al Ali M, Wild G, Afif W, Bitton A, Lakatos PL, Bessissow T. Effectiveness, safety, and drug sustainability of biologics in elderly patients with inflammatory bowel disease: A retrospective study. World J Gastroenterol. 2022;28:4823-4833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Calafat M, Mañosa M, Ricart E, Nos P, Iglesias-Flores E, Vera I, López-Sanromán A, Guardiola J, Taxonera C, Mínguez M, Martín-Arranz MD, de Castro L, de Francisco R, Rivero M, Garcia-Planella E, Calvet X, García-López S, Márquez L, Gomollón F, Barrio J, Esteve M, Muñoz F, Gisbert JP, Gutiérrez A, Hinojosa J, Argüelles-Arias F, Busquets D, Bujanda L, Pérez-Calle JL, Sicilia B, Merino O, Martínez P, Bermejo F, Lorente R, Barreiro-de Acosta M, Rodríguez C, Fe García-Sepulcre M, Monfort D, Cañete F, Domènech E; ENEIDA Study Group of GETECCU. Risk of Immunomediated Adverse Events and Loss of Response to Infliximab in Elderly Patients with Inflammatory Bowel Disease: A Cohort Study of the ENEIDA Registry. J Crohns Colitis. 2022;16:946-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |