Published online Apr 28, 2023. doi: 10.3748/wjg.v29.i16.2433
Peer-review started: December 11, 2022
First decision: January 11, 2023
Revised: January 26, 2023
Accepted: April 7, 2023
Article in press: April 7, 2023
Published online: April 28, 2023
Processing time: 134 Days and 1.9 Hours
Ferroptosis is an emerging novel form of non-apoptotic, regulated cell death that is heavily dependent on iron and characterized by rupture in plasma membrane. Ferroptosis is distinct from other regulated cell death modalities at the bioche
Core Tip: Gastrointestinal tumors contribute to the majority of cancer-related deaths. Ferroptosis is a novel form of non-apoptotic cell death that plays a vital role in reducing the invasiveness and metastasis of gastrointestinal tumors. Herein, we discussed the regulatory mechanisms involved in ferroptosis through the hallmark pathways of glutathione peroxidase 4, iron metabolism, lipid peroxidation, and redox signaling that provide a novel therapeutic approach for gastrointestinal cancers.
- Citation: Rabitha R, Shivani S, Showket Y, Sudhandiran G. Ferroptosis regulates key signaling pathways in gastrointestinal tumors: Underlying mechanisms and therapeutic strategies. World J Gastroenterol 2023; 29(16): 2433-2451
- URL: https://www.wjgnet.com/1007-9327/full/v29/i16/2433.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i16.2433
Cancer is a notorious disease that causes numerous disorders and deaths worldwide, particularly gastrointestinal (GI) cancers comprising esophageal cancer, gastric cancer (GC), liver cancer, pancreatic cancer, and colorectal cancer[1]. A recent report stated that there are approximately 4 million new cases each year, which is far greater than breast and lung cancers combined; thus, there is a need to improve the therapeutics of GI cancers[2]. Although there has been an improvement in the prognosis of GI cancers in the last decade due to development of intensive therapies, such as incorporating cytotoxic drugs and targeted therapies, GI cancers are one of the leading causes of cancer deaths in developed and developing countries[3-5]. With varying risk factors, incidence, prevalence and prognosis, early diagnosis with increased screening may facilitate therapies to fight against GI cancers.
Biomarkers, including epigenetic markers, also play an effective role in decreasing the progression of GI cancers by early diagnosis and decreasing risk assessment by predicting the tumor response from specific therapies in patients[6]. Apart from internal stimulation, external exposures, such as dietary intake, tobacco use, alcohol consumption, obesity, and pathogens, are considered to increase the risk of GI tumors[7]. Various improvements in the therapeutic aspects to decrease the burden of GI cancer, including the chemoprevention of these cancers by using antioxidants, have drawn much attention[8]. Murphy et al[9] estimated that by 2025, the pervasiveness of obesity will be increased in men and women by 18% and 21%, respectively, which could escalate the encumbrance of GI cancers worldwide[9].
The induction of cell death by ferroptosis has increased in the recent past. Cell death is an endogenous mechanism that regulates homeostasis, and it is perceived as a requisite bodily process. As an imperative system, it exterminates useless or unwanted cells and bolsters the defense system of the body. Many forms of cell death have been observed in the recent past, particularly accidental cell death and regulated cell death (RCD)[10,11]. Accidental cell death occurs suddenly in response to stress, heat shock, or mechanical nature, while RCD is orchestrated by exhibiting a precise signaling cascade provided by a defined group of effector molecules. Many forms of RCD have been observed, such as apoptotic, autophagic, pyroptotic, necrotic, entotic, and ferroptotic cell death[12].
Investigating RCD introduces a new path for developing cancer therapeutics. Among the various forms of RCD, ferroptosis, an iron dependent, non-apoptotic RCD, is fascinating because of its ability to manage cancer cells that develop resistance to apoptosis and drugs. Although a link between iron and lipid peroxidation has been found in cancer research, this form of RCD has a peculiar combination of morphological, biochemical, and genetic characteristics distinct from necrosis, apoptosis, and autophagy[13]. Dixon et al[14] proposed the term ferroptosis in 2012, and its emerging role in several other disease settings was observed[14]. Ferroptotic cell death is characterized by the occurrence of oxidative stress and membrane lipid peroxidation, thereby causing mitochondrial atrophy, increased density of the mitochondrial membrane, and membrane damage[15]. Therefore, this review summarizes the role of ferroptosis in regulating key cancer signaling and cell death pathways in GI tumors for better prognosis.
GI cancers contribute to significant morbidity and mortality rates worldwide[16] (Table 1), accounting for 26% of all cancer-related incidence and 35% of all cancer-related fatalities. It was noted that there is a two-fold increase in men compared to women. It has been reported that by 2040, there will be an increase in new cases and deaths from GI cancers by 58% and 73% to 7.5 million and 5.6 million, respectively[17]. Incidence and mortality rates of GI cancers worldwide is presented in Table 1[18-22].
While cell death modalities play a decisive role in eradication of tumor cells and maintenance of homeostasis, the role of RCD pathways, particularly ferroptosis, in tumor progression and metastasis of cancers has been the subject of interest for the last decade.
The various features of ferroptosis include the morphological, biochemical, and epigenetic alterations that describe this novel form of cell death.
Ferroptosis is a non-apoptotic form of cell death because it lacks the classical features of apoptosis, such as mitochondrial cytochrome-C release, caspase activation, and chromatin fragmentation[23]. In contrast, ferroptosis induces mitochondrial membrane disintegration, which results in cell enlargement, plasma membrane rupture, volume reduction, increased density, and disappearance of cristae observed under the electron microscope. These changes are potentially caused by permeability loss due to increased lipid peroxidation[24].
The main biochemical features of ferroptosis are iron accumulation, reactive oxygen species (ROS) production, and induction of lipid peroxidation. Iron is the most abundant metal and is essential to all life on earth. As an iron-dependent form of cell death, increased circulating iron leads to the Fenton reaction through activated iron-containing enzymes that act as biochemical markers of ferroptosis[25]. Intracellular iron and iron-containing enzymes are indispensable for the execution of ferroptosis. Molecular regulators associated with iron homeostasis, such as transferrin, lactotransferrin, transferrin receptor, nuclear receptor coactivator 4-dependent degradation of ferritin, iron regulatory protein, and iron responsive element regulatory network, facilitate ferroptotic cell death[26,27].
ROS are the major culprits in many disease settings. ROS can damage essential biomolecules such as DNA, protein, carbohydrates, and lipids, thereby causing denaturation, peptide s-s bond breaking, crosslinking, enzyme inhibition, and permeability changes in tissues and cells. Therefore, increased ROS promote several perturbances leading to altered cell death pathways as well as induction of ferroptosis[28].
Lipid peroxidation is a seminal process in which the generated free radicals (H2O2, super peroxides, peroxy radicals) target long-chain fatty acids[29]. Extensive lipid peroxidation affects the permeability, fluidity, and curvature of the membrane, thereby stimulating cell death by forming micelles and pores in the biological membrane. A close connection between lipid peroxidation and ferroptosis exists in cancers. Importantly, to overcome drug resistance in chemotherapy, ferroptosis plays a crucial role in tumor microenvironment, thereby controlling cell proliferation through redox signaling pathways[30].
Epigenetics is a genetic mechanism that can influence gene expression by DNA methylation, histone modifications, and the effects of noncoding RNA without altering the DNA sequence[31,32]. DNA methylation is an epigenetic modification process that uses DNA methyltransferases to covalently transfer the methyl group to the C-5 position in the cytosine ring of DNA[33]. It has been reported that hypermethylation of the CDH1 gene promoter could increase ferroptosis susceptibility in head and neck cancer cells[34]. Recently, epigenetic regulation through H2B monoubiquitination and p53 has been determined[35].
Histone acetylation is controlled by histone acetyltransferases, bromodomains (BRDs), and histone deacetylases (HDACs) to regulate ferroptosis. In the histone acetylation process, histone acetyltransferases play the writer role, BRDs the reader role, and HDACs the eraser role[36,37]. The tumor suppressive role of Tp53 is well known. For p53-induced ferroptosis, acetylation plays a crucial role by regulating solute carrier family 7 member 11 (SLC7A11) expression[38]. Acetylated and mutant p533KR suppresses SLC7A11 expression and inhibits cysteine uptake, which alleviates ferroptosis and lipid peroxidation by decreasing glutathione (GSH) synthesis[38,39]. BRD4 induces the expression of anti-ferroptotic genes, and it has been observed that the BRD4 inhibitor JQ1 induces ferroptosis by downregulating the expression of ferroptosis-related genes such as glutathione peroxidase 4 (GPX4), SLC7A11, and solute carrier family 3 member 2 in breast and lung cancer cells[40]. HDACs were initially identified as an eraser for removing acetyl groups from histones, but it was later discovered to be involved in many important biological functions. Recently, HDAC inhibitors have been endorsed as potential therapeutics for various cancers[41].
As a multifarious group of noncoding transcripts, noncoding RNAs, microRNAs (miRNAs), long noncoding RNAs (lncRNAs), and circular RNAs were first considered nonfunctional junk, but they participate in a broad domain of gene-regulatory pathways[42]. Beyond this, it has been suggested that circular RNAs play a role in ferroptosis by regulating multiple signaling pathways either directly or indirectly by acting on the key regulating factors and upstream targets of ferroptosis[43]. miRNAs tend to act as stimulants in ferroptosis by targeting ferroptosis-associated factors by downregulating the expression of activating transcription factor 4 (ATF4), which is a stress signal that tends to inhibit ferroptosis by miR-214-3p in hepatocellular carcinoma (HCC) by inducing SLC7A11 and several other miRNAs, including miR-101-3p, at high expression levels. They profoundly enhance the activity of nuclear factor kappa B, which regulates ferroptosis via GPX4 and prostaglandin endoperoxide synthase 2 in lung cancer, and miR-324-3p at high expression levels induces ferroptosis in lung adenocarcinoma by targeting GPX4[44]. Furthermore, miRNAs have also been found to have inhibitory effects on ferroptosis; therefore, they play diverse roles in ferroptosis. The alterations they cause to ferroptosis-associated factors make them a potential target in cancer therapeutics.
Over the past few years, several small molecules and plant-based compounds that target transporters or enzymes involved in ferroptosis have been described.
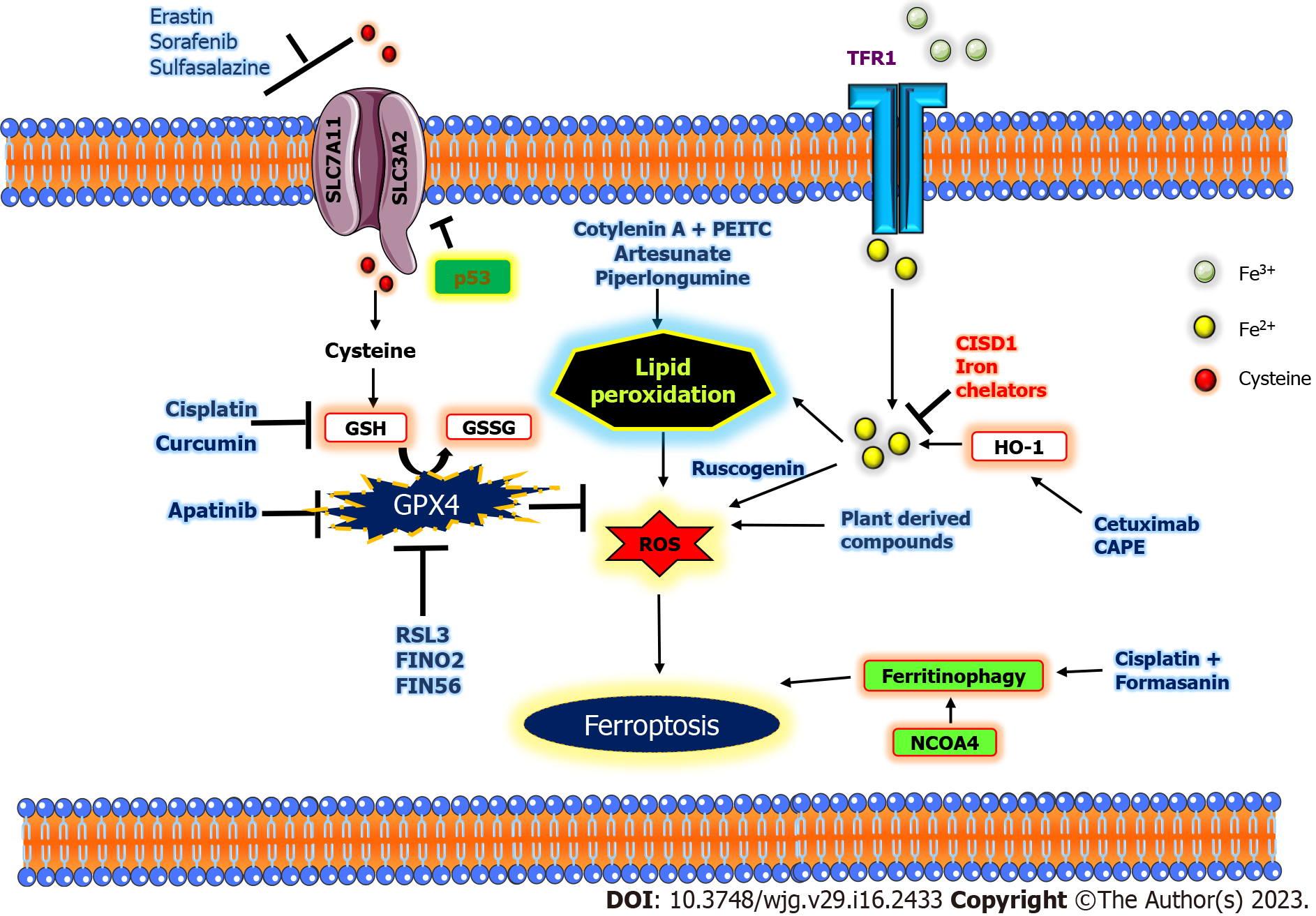
In principle, GSH synthesis and GPX4, a selenoenzyme, are the major regulators of ferroptosis. GSH is an important substrate for GPX4. Therefore, any depletion in GSH leads to substantial lipid peroxidation, leading to ferroptosis. By inhibiting GPX4, accumulation of lipid peroxidation takes place[45]. To produce GSH, system Xcˉ regulated cysteine activity is needed. Certain compounds, such as sorafenib, glutamate, and erastin, induce ferroptosis through inhibition of system Xcˉ. Ras-selective lethal 3 (RSL3), a known GPX4 inhibitor and other compounds containing electrophilic chloroacetamides, covalently binds and restricts selenocysteine activity inside the active site of GPX4 to initiate ferroptosis[46]. Other nitrile oxide electrophiles include ML210, JKE-1674, and JKE-1716, which attach to selenocysteine and cause ferroptosis[46,47]. By directly oxidizing lipids and indirectly impairing GPX4 action, FINO2 causes ferroptosis, and FIN56 drives the destruction of GPX4 to induce ferroptosis[48]. To induce ferroptosis, FINO2 harnesses either a direct or indirect iron oxide to induce suppression of GPX4 activity. Organic peroxides are compounds with multiple O2 bonds that are cleaved, resulting in the production of RO anions. These organic peroxides are often used as models to induce ROS. A commonly held view is that the lipid peroxide analogue tert-butyl hydroperoxide stimulates lipid peroxidation-dependent ferroptosis[49].
Excessive iron accumulation is a precursor of ferroptosis in a plethora of cell types. In vitro, hemoglobin causes ferroptosis, and in vivo, it causes intracerebral hemorrhage in other disease states[50-52]. Furthermore, the rise in cellular iron levels and consequent ferroptosis caused by pharmacological stimulation of selective autophagy through degradation of ferritin is known as ferritinophagy[53,54]. Apart from these, there are many other ferroptotic inducers; for example, in human pancreatic cancer cells, zalcitabine causes autophagy-dependent ferroptosis, pointing to a link between DNA sensor pathways, autophagy activation, and mitochondrial malfunction[55].
The inhibition of ferroptosis by small molecular compounds takes place through the following mediators.
Iron chelators: The important role of iron is to promote lipid peroxidation by either activating iron-containing lipid oxygenases (LOX) or nonenzymatic iron-mediated Fenton action[56-58]. Iron chelators like deferoxamine inhibit ferroptotic cell death. Iron chelators reduce H2O2-induced necrosis but not ferrostatin-1, suggesting that iron may be potentially involved in multiple cell death modalities[59,60].
Enzyme inhibitors: The enzyme inhibitor acyl-CoA synthetase long chain family member 4 (ACSL4) mediates addition of CoA to long-chain fatty acids, particularly arachidonic acid, and appears to be a crucial precursor to further arachidonate lipoxygenase-dependent lipid peroxidation[57]. ACSL4 and arachidonate lipoxygenase inhibitors are known to inhibit the ferroptotic cell death process by preventing LOX accumulation[57,61]. In addition, NAD phosphate oxidase inhibitors comprising of diphenylene iodonium and GKT137831 prevent erastin-induced ferroptosis[62].
Protein degradation inhibitors: GPX4 may be degraded by a variety of ferroptosis activators, which results in lipid peroxidation. However, FIN56 and erastin-induced GPX4 breakdown is inhibited by the molecular chaperone heat shock protein 90, 5-(tetradecyloxy)-2-furoic acid, and dopamine[63-65].
Other inhibitors: The mitogen-activated protein kinase kinase inhibitor, U0162 is commonly used to reduce ferroptosis owing to its broad antioxidant activity[66]. The strong inhibitory effects of exogenous monounsaturated fatty acids (MUFAs) or deuterated polyunsaturated fatty acids (PUFAs) on ferroptosis may be attributed to their displacement of PUFAs from phospholipids, which lowers the accumulation of lipid peroxidation[67].
A deeper knowledge of how ferroptosis is regulated by metabolic pathways, including iron, GSH, and lipid metabolism, has been the focus of research in proposing therapeutic drugs. In this context, several metabolic and cancer signaling pathways that connect ferroptosis have been described.
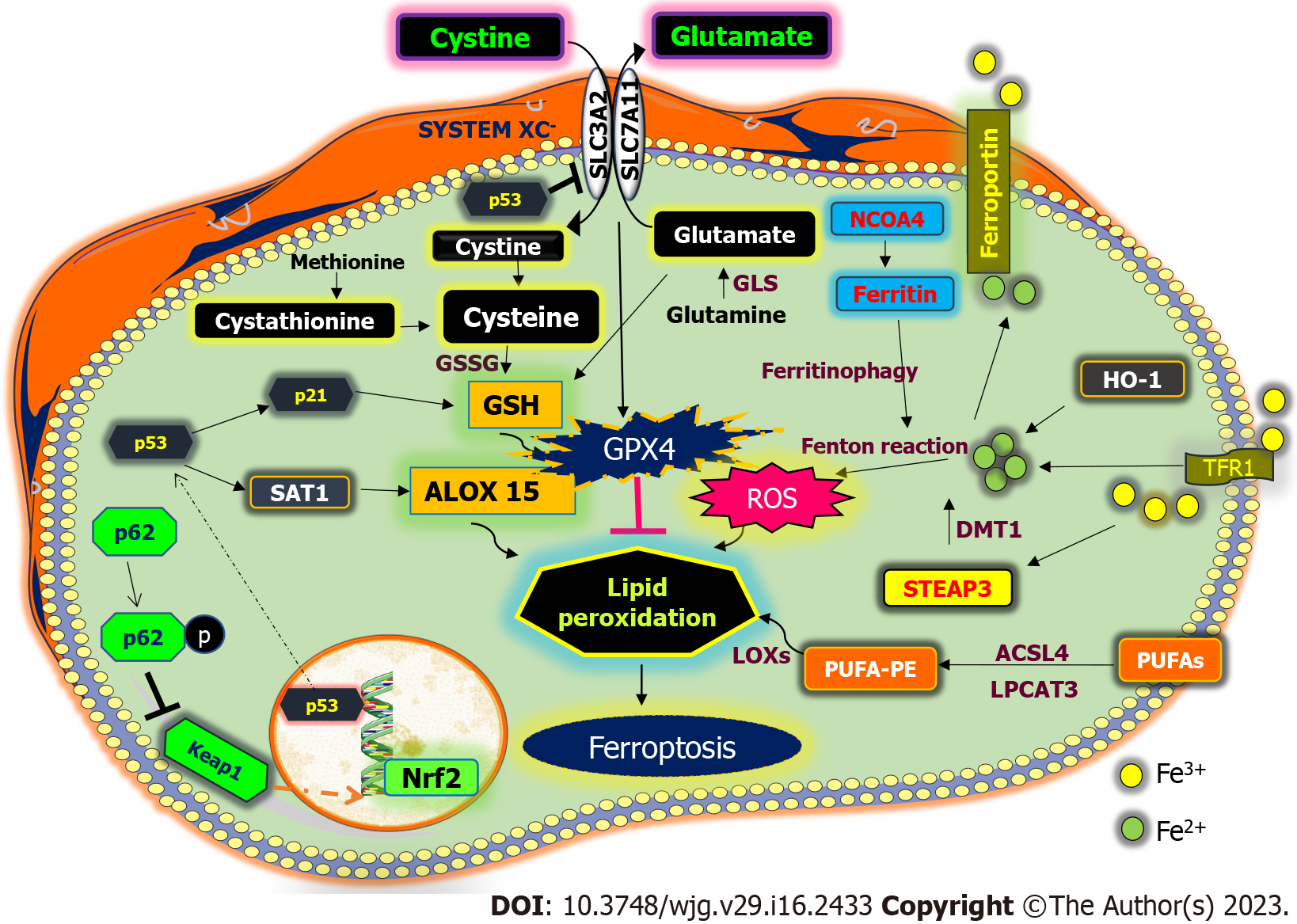
Ferroptosis is primarily caused by the inactivation of the cellular antioxidant system, particularly the system that is dependent on the antioxidant defense Xcˉ GSH-GPX4 that leads to toxic lipid ROS accumulation[68]. GSH peroxidases are pivotal enzymes that enable the reduction of peroxides via GSH. The system Xcˉ family mediates transport of cysteine and glutamate, where glutamate is exported outside the cell and cysteine is imported inside the cell, which initiates the production of GSH, hence inhibiting ferroptosis in cancer cells[69] (Figure 1). The GPX protein comprises different types, among which GPX4, a selenoprotein, is an elemental antioxidant mediator known for its capability of reducing large peroxides such as PUFAs[68]. It is primarily involved in the maintenance of lipid metabolism and defense against the accumulation of toxic lipid ROS. Since GPX4 activity is directly impacted by conditions that reduce intracellular cysteine and subsequently GSH levels and because GSH is the dominant antioxidant in mammalian cells, these conditions trigger ferroptosis[70]. Small molecules such as erastin can block GSH or GPX4 expression to activate ferroptosis. In many cells, the rate at which GSH is synthesized is limited by the internal reduction of cystine to cysteine[71].
The process in which free radicals and non-free radicals cleave the C-C double bonds in lipids such as PUFAs, phospholipids, glycolipids, and cholesterol is known as lipid peroxidation.
According to lipidomic research, arachidonic acid and adrenic acid are key driving factors in inducing ferroptosis. Elongation of very long-chain fatty acid protein 5 and fatty acid desaturase 1 are associated with the synthesis of fatty acids, where both enzymes are upregulated in mesenchymal GC cells, resulting in ferroptosis sensitivity, according to studies on the disease[72]. The process by which these PUFAs become coenzyme-A derivatives that are integrated into phospholipids triggers ferroptosis. Both autophagy and deubiquitylation can result in the reduction of intracellular GPX4[73]. Specialized sequences in GPX4 are distinctly recognized by heat shock protein 90 and facilitate the breakdown of GPX4 by chaperone-mediated autophagy[63]. On the other hand, the inhibition of chaperone-mediated autophagy by the mTOR pathway is relieved by its suppression, which may have caused GPX4 to degrade and cause ferroptosis[74].
Numerous investigations have revealed ACSL4 as a crucial sensitizer in ferroptosis machinery. ACSL4 mediates the esterification of PUFAs into phospholipids by adding CoA to the polyunsaturated bond of arachidonic acid[75] (Figure 1). Lysophosphatidylcholine acyltransferase 3, which is activated by ACSL4, contributes to ferroptotic lipid signaling by adding acyl groups to lysophospholipids, particularly to the phospholipids phosphatidylcholine and phosphatidylethanolamine[75]. Apart from PUFAs, MUFAs can promote ferroptosis resistance by initiating mutations in the PI3K-AKT-mTOR pathways, sterol regulatory element-binding protein 1 can mediate the production of MUFAs, which aids cancer cells in resisting ferroptosis[76]. MUFAs can modulate the hydroxycarboxylic acid receptor 1-sterol regulatory element-binding protein 1-stearyl-coenzyme A desaturase 1 pathway and lactate in the tumor microenvironment, which might increase the production of MUFAs and lower the expression of ACSL4 to avoid ferroptosis[77]. As functional lipids in ferroptosis, polyunsaturated ether phospholipids have a significant regulatory effect in changing cancer cells from a ferroptosis-sensitive state to a ferroptosis-resistant state[78]. Thus, lipid metabolism has a notable effect on the capability of cancer cells to induce ferroptosis.
To carry out ferroptosis and allow lipid peroxides to build up, iron is particularly necessary. The capacity of iron to catalyze numerous metabolic events and flip between the various ionic states depends largely on how it can absorb and give electrons[79]. In the Fenton reaction, Fe2+ is converted to Fe3+ in the presence of H2O2, and HO· is produced as a result of electron transfer to H2O2[80]. In contrast, the Haber-Weiss process could convert Fe3+ back to Fe2+ by reacting with O2, causing O2 to lose one electron and become O2-[81].
The transfer of iron takes place in the following ways. Transferrin synthesized by the liver as a chelator of Fe3+ ions. In contrast to apo-transferrin, which is sans iron transferrin that is not recognized by transferrin receptor 1 (TfR1) and not internalized, transferrin recognizes and binds to TfR1 when it reaches the cell membrane, which is then internalized by clathrin-mediated endocytosis[82]. Accumulating evidence suggests the role of iron metabolism in ferroptosis[83,84]. Fe3+ is liberated from the transferrin TfR1 complex due to the acidic pH of endocytic vesicles and is converted to Fe2+ by the six-transmembrane epithelial antigen of the prostate (STEAP) family. STEAP1 and STEAP2 are implicated in multiple human malignancies, including Ewing sarcoma bladder, ovary, colon, breast, prostate, pancreatic, and cervical cancer[85-87]. Malignant gliomas have high levels of STEAP3, which triggers the cancer epithelial-mesenchymal transition[88]. Under hypoxic circumstances, STEAP4 is activated, resulting in an imbalance of mitochondrial iron and increased ROS generation[89].
The intracellular labile iron pool is subsequently created by divalent metal transporter 1, which mediates the release of Fe2+ into the cytoplasm[90]. Apo-transferrin is released back to the plasma membrane post recycling, while TfR1 and apo-transferrin remain linked[91]. Fe2+ is then directly transported into cells by divalent metal transporter 1 during the conversion of Fe3+ to Fe2+ (Figure 1). Another mechanism includes the absorption of porphyrin-bound Fe2+ that contains hemoglobin, particularly in macrophages, and involvement of cell membrane receptors of iron-storing protein ferritin, such as scavenger receptor class A member 5, as observed in kidney and embryonic development, which absorbs iron to regulate the ferroptosis mechanism[92,93].
Ferritin, an iron-sequestering protein with 4500 iron atoms, plays a crucial role in iron transport, cellular multiplication, angiogenesis, and immune suppression[94]. By using NCOA4, ferritin can also be split up to liberate free iron, through “ferritinophagy”[95]. Ferroportin (FPN), recognized as the sole iron release pump that works with ceruloplasmin to export iron, is primarily in charge of moving Fe2+ out of cells[96]. Ceruloplasmin controls HepG2 and Hep3B cell iron regulation to prevent ferroptosis, and its loss causes a buildup of intracellular Fe2+ and lipid ROS and enhances ferroptotic death caused by erastin and RSL3[97]. Prominin-2 promotes the growth of multivesicular structures that comprise ferritin and exosomes that transfer iron from cells, enabling ferroptosis resistance in breast cancer[98]. FPN is severely reduced in many cancer types, suggesting that cancer cells may contain large amounts of iron[99]. Decreased FPN levels promote proliferation and epithelial-mesenchymal transition in triple-negative breast cancer cells[100]. Hepcidin, produced by tumors of the liver, promotes the breakdown of FPN and aids in the spread and development of cancer[101].
The unfolded protein response is activated in mammalian cells due to endoplasmic stress and has a two-dimensional functional role in cell survival and death[102]. Activating transcription factor 3 (ATF3) is a member of the ATF/cAMP-responsive element-binding protein family of transcription factors. It binds to the ATF/cAMP-responsive element-binding protein cis-regulatory element and coordinates gene expression. ATF3 has tumor suppressive roles and inhibits cancer malignancy in GI cancers[103]. The context-dependent role that ATF3 plays in cancer is likely due to complex protein-protein interaction networks in which ATF3 is involved.
Indeed, in addition to transcriptional regulation, ATF3 has been found to interact with many critical cellular proteins and regulate their functions. One of the well-characterized ATF3-binding proteins is wild-type p53, and ROS signals are necessary for this aberrant production of endoplasmic reticulum stress markers; however, the antioxidant N-acetyl L-cysteine prevents overexpression and consequent ferroptotic cellular death. Although the ATF3-TP53 complex helps to initiate DNA damage, TP53 is not necessary for ATF3-regulated suppression of SLC7A11 transcription[104]. Nuclear factor erythroid 2-related factor 2 (Nrf2) can, in contrast, regulate the expression of ATF3 by creating complex feedback loops for the activation of a number of transcription factors in coordinating the ferroptotic response[105].
ATF4 is a double-sided sword that plays a dual role in ferroptosis. In numerous cancer cells, including human glioblastoma as well as pancreatic cancer cell lines, the depletion of ATF4 increases erastin-induced or RSL3-induced ferroptosis, and the inhibition of ATF4 increases artesunate-induced ferroptosis in the DAUDI cell line[106,107]. The classes of genes targeted by ATF4, such as SLC7A11, heat shock protein 5 (HSPA5), and tribbles pseudokinase 3, may have an impact on this ATF4-dependent mediation of ferroptosis in various cancer types. ATF4 is inhibited by the erastin-induced overexpression of HSPA5, which results in the sequential breakdown of GPX4 and lipid peroxidation[108-112]. Thus, ATF3-dependent and/or ATF4-dependent pathway dysregulation might influence ferroptosis in a tumor-specific phenotype.
Binding to antioxidant response elements, Nrf2, a transcription factor from the Cap-N-Collar family, is essential in regulating antioxidant genes and maintaining redox homeostasis[113]. Nrf2 transcriptional activity is normally attached to Kelch-like ECH-associated protein 1 (Keap1), retaining it in the cytoplasm. Nrf2 separates from Keap1 during environmental conditions (such as oxidative stress) and subsequently translocates into the nucleus, where it stimulates the expression of ARE-dependent target genes[113]. Nrf2, however, depends on the expression of the target genes that are involved in the control of cell proliferation, migration, and death to have a double impact on carcinogenesis and tumor treatment[114].
Initially, researchers showed that ferroptosis resistance may be promoted by activating the Nrf2 pathway using a model of HCC. In human HCC cell lines, ferroptosis activators like erastin and sorafenib boost Nrf2 stability by inhibiting the development of the Nrf2-Keap1 complex. The autophagy receptor SQSTM1/p62 elevates Nrf2 expression by inactivating Keap1, which is another regulator of this process[115]. By boosting GSH production and function, Nrf2-dependent genes such as glutathione synthetase, GPX4, and SLC7A11 contribute to ferroptosis inhibition[115]. Control of NADPH synthesis, a vital electron donor required for reduction of oxidized substrates[116], which is also a ferroptosis sensitivity biomarker[117], is another way that Nrf2 intermediate metabolism is connected to the regulation of ferroptosis. Overall, Nrf2 is an important transcription factor that regulates ferroptosis.
p53, a tumor suppressor encoded by the TP53 gene, is involved in the mediation of DNA damage, oncogene activation, and hypoxia. p53 promotes ferroptosis by transcriptional or posttranscriptional pathways in addition to its impacts on apoptosis, autophagy, and cell cycle. p53 can both induce and inhibit ferroptosis in a context dependent manner. To cause ferroptotic cell death, p53 increases the production of spermidine/spermine N1-acetyltransferase 1, which sequentially causes increased 15-LOX expression responsible for oxidation of PUFAs (Figure 1)[118]. Simultaneously, p53 decreases the expression of ELAV-like RNA-binding protein and its action with LINC00336, by limiting the capability of cells to fight ferroptosis by increasing the activity of cystathionine β synthase (CBS)[119]. Additionally, p53 interaction with ubiquitin-specific protease 7, a deubiquitinating enzyme, facilitates its nuclear translocation by altering histones, favorably controlling ferroptosis[120]. Human colon cancer cells, such as HCT116 and SW48, are inhibited against ferroptosis by p53 in a transcription-independent manner[121] The p53-p21 axis prevents ferroptosis, allowing cancer cells to withstand stressful metabolic situations. Thus, this dual role of p53 in ferroptosis can be explored for therapeutic treatment of cancers.
Heme oxygenase-1 (HO-1) is a significant redox-mediating enzyme that is activated in reaction to oxidative stress, cellular stress, neurodegeneration, and other diseases. HO-1 has a dual personality. The use of HO-1 antagonist zinc protoporphyrin IX and HO-1 knockdown animals demonstrated that HO-1 increases erastin-induced ferroptosis[121]. Conversely, because HO-1 expression is knocked down, erastin and sorafenib more effectively limit cell proliferation in HCC caused by these drugs[121]. In addition, HO-1 mediates iron and ROS levels where Nrf2-derived HO-1 provides a cytoprotective effect by scavenging ROS when HO-1 is moderately active[121]. In contrast, given that cancer cells produce more HO-1 than normal cells, a high level of HO-1 activation may increase fragile Fe2+, resulting in an excess of ROS and eventual oxidative cell death[121]. Hence, the employment of ferroptosis by HO-1 activation can define the fate of cancer cells.
Sirtuins are NAD+-dependent deacetylases that are involved in DNA repair, cellular metabolism, and cancer development. As a key oxidizing agent, sirtuins tend to play a crucial role in regulating redox signaling pathways[122]. Seven sirtuins have been identified in mammals so far, and each family has been found to regulate cellular homeostasis. SIRT1 activation increases the antioxidant activity mediated by oxidative stress-related transcription factors[122]. Studies have found that increased expression of SIRT1 induces Nrf2-mediated antioxidant activity, thereby increasing GPX4 and GSH levels in HCC. Conversely, their downregulation by protocadherin 20 mediates ferroptotic cell death by lowering the expression of GPX4 and GSH and increasing intracellular iron levels and ROS[123]. SIRT6 promotes ferroptosis in pancreatic cells through upregulation of the ROS levels[124], and its downregulation causes GC cells to resist sorafenib-induced ferroptosis[125].
Hypoxia is a prominent factor involved in the progression and metastasis of numerous cancers. Prolyl hydroxylase hydroxylates the hypoxia inducible factor (HIF)-a subunits HIF-1α and HIF-2α under normoxic circumstances; subsequently, they are subjected to ubiquitin-mediated proteolysis and destruction. HIF target genes are activated in hypoxia as a result of HIF-1α and HIF-2α failing to hydroxylate and translocate into the nucleus[126]. Our laboratory’s previous studies found that under hypoxic conditions, hypoxia-inducible factors such as HIF-1α and HIF-2α are activated and stimulate the activation of matrix metalloproteinases 2 and 9, thereby increasing tumorigenesis[127]. Singhal et al[128] demonstrated the mechanism by which activation of HIF-2α raises cellular iron to accelerate ferroptosis or irreversible cysteine oxidation that causes cell death.
The leading cause of cancer deaths and incidence from GI tumors has become the most essential health concern, so improving the therapeutic aspects provides an important way to decrease the burden of GI cancers. Several studies have used ferroptotic cell death as a key modulator to inhibit the growth of GI cancers such as colorectal cancer, liver cancer, pancreatic cancer, GC, and esophageal cancer.
Recent studies by Wang et al[129] suggested that ferroptosis can have a potential modulatory role in the targeted killing of chemoresistant colon cancer cells, and ferroptosis-related genes could also be harnessed as biomarkers in colorectal cancer therapeutics. Genes involved in the colorectal cancer tumor microenvironment are intricately linked to ferroptosis, and some of them are involved in the lipid peroxidase and GPX/GSH enzyme system. Iron metabolism genes are also powerful prognostic markers in colorectal cancer; for example, elevated expression of thio-redoxin tumor suppressing protein is closely concomitant with iron accumulation in mitochondria[130]. The above findings clearly state that targeting ferroptosis-inducing genes could provide a novel avenue for treating colorectal cancer. p53 tumor-suppressing protein and heme oxygenase have been implicated as key factors in regulating ferroptosis in colorectal cancer.
HCC is the most prevalent type of liver cancer, accounting for approximately 90% of cases[131]. Multiple studies have reported that ferroptosis plays a role in ameliorating the burden of HCC progression. Sorafenib, an anticancer agent that is used for the treatment of advanced HCC, has been found to induce ferroptosis by initiating the translation of rapamycin kinase signaling pathway, thereby initiating ferroptosis. Iron chelators such as deferoxamine inhibit sorafenib-induced ferroptosis by reducing the oxidative stress created by sorafenib in HCC cells[132].
The finding of a potent inhibitor of CBS, which is the main source of cystathionine gamma lyase by metabolizing homocysteine to cystathionine to increase intracellular L-cysteine, called CH004, selectively inhibits CBS but not human cystathionine gamma lyase in both in vivo and in vitro experiments, thereby increasing lipid ROS and decreasing the viability of HepG2 cells, indicating their role in ferroptosis[133].
Modulating the function of key regulators of ferroptosis also plays a significant role in the induction of ferroptotic cell death in HCC cells. Nrf2 is a key regulator of the antioxidant response. It plays a negative regulatory role in ferroptosis by actuating p62-Keap1-Nrf2 pathway, which upregulates the expression of quinone oxidoreductase 1, HO-1, and ferritin heavy chain 1. Therefore, inhibiting the p62-Keap1-Nrf2 pathways by erastin and sorafenib significantly enhances the ferroptosis-mediated cell death of liver cancer cells[134].
The mitochondrial membrane protein CDGSH iron sulfur domain 1 serves as a target to treat diabetes using glitazone, but it acts as a negative regulator of ferroptosis by modulating mitochondrial iron uptake; therefore, the pharmacological or genetic inhibition of CDGSH iron sulfur domain 1 enhanced mitochondrial lipid peroxidation, increasing erastin-induced ferroptosis in liver cancer cells[135].
The highly reactive metabolite NAPQ1 from acetaminophen, an analgesic and antipyretic agent, is involved in the ferroptotic cell death of HepG2 cells by decreasing their viability through GSH depletion and GPX inhibition[136]. The expression of retinoblastoma protein (Rb) in HCC cells determines the susceptibility of cancer cells to sorafenib treatment and regulates ferroptosis. Louandre et al[137] showed that the decrease in the levels of Rb protein exhibits an increase in cell death when cells were treated with sorafenib compared with controls; thus, determining the Rb status of HCC patients treated with sorafenib will provide novel insight into the HCC treatment.
LncRNAs represent a vital class of molecules that have regulatory effects in both physiological and abnormal conditions, but lncRNA GABPB1-AS1 has a role in regulating oxidative stress, confirming its involvement in the ferroptosis-mediated cell death of HCC cells. The expression of this lncRNA was upregulated by erastin to inhibit the translation of GA binding protein transcription factor subunit beta 1, resulting in the rapid accumulation of ROS in HepG2 cells by decreasing the expression of the PRDX5 peroxidase gene[138].
Several molecules were demonstrated to induce ferroptosis in pancreatic cancer cells in which the first-line drug gemcitabine is used to treat advanced pancreatic cancer, but HSPA5 causes resistance to gemcitabine treatment by inhibiting ferroptosis. Therefore, genetically or pharmacologically inhibiting HSPA5 enhanced the sensitivity of gemcitabine to pancreatic ductal adenocarcinoma cells by inducing ferroptosis[139]. Inhibiting cytosolic aspartate aminotransferase, which is predominant for redox balance, represses the growth of pancreatic cancer cells by enhancing labile iron release, thereby inducing sensitivity to ferroptosis[140].
Several studies have shown that certain natural plant extracts possess potential anticancer effects by inducing ferroptosis in pancreatic cancer cells. A saponin called ruscogenin represses cell viability and induces ferroptotic cell death in a pancreatic cancer cell line by increasing the concentration of intracellular ferrous iron and ROS production; it also exerts anti-tumor effects in in vivo experiments with less toxicity[141].
The combinatorial regimen using plant derivatives also expresses effective anticancer effects in pancreatic cancer cells by inducing ferroptosis; cotylenin A (a plant growth regulator) in combination with phenethyl isothiocyanate, an anti-carcinogenic compound, stimulated ferroptotic cell death in PANC-1 cells (Figure 2)[142]. Artesunate, an antimalarial agent, induced ferroptosis in Kras-activated pancreatic ductal adenocarcinoma cell lines driven by ROS generation and lysosomal iron-dependent cell death without affecting normal pancreatic cell lines[143]. Piperlongumine alone, as well as in combination with cotylenin A and sulfasalazine, generates ferroptotic death of pancreatic cancer cells[144].
GC is a heterogeneous disease among GI cancers, with over 1 million new cases worldwide where surgery is the primary treatment to prevent progression[145]. The factors involved in PUFA biosynthesis play an essential role in inducing ferroptosis sensitivity in GC[72]. Apatinib, an anti-tumor agent, decreases the expression of GPX4 and results in apatinib-mediated ferroptotic cell death in GC cells[146]. GC cells resistant to sorafenib-induced ferroptosis treatment by silencing SIRT6, one of the sirtuin proteins that plays a vital role in the regulation of metabolism, DNA repair, and cancer development and is primarily located in the cell nucleus. SIRT6 sensitizes GC cells to sorafenib-induced ferroptosis by the Keap1/Nrf2/GPX4 signaling pathway[125,147]. The ingredient from the Chinese medicine tanshinone IIA, isolated from the rhizome of Saliva miltiorrhiza Bunge, exhibits an anticancer effect on GC cells by inducing ferroptosis and downregulating p53-mediated SLC7A11[148].
Targeting ferroptosis will provide new avenues for esophageal cancer diagnostics and treatment strategies. Sulfasalazine, a ferroptotic inducer, inhibits the progression of esophageal cancer, and plant-derived compounds such as oridonin, a diterpenoid, have also been reported to stimulate ferroptotic cell death in esophageal cancer cells[149].
Ferroptosis is intricately intertwined with other forms of cell death through various iron and lipid peroxidation proteins and several transcription factors. However, molecular mechanisms underlying the role of other forms of RCD remain poorly understood. For example, TP53, a critical mediator of tumor suppressive response involved in apoptosis, ferroptosis, and antioxidant response machinery like Nrf2, has a significant role in ferroptosis as well as autophagy. The molecular mechanisms associated with other forms of RCD pathways and ferroptosis are discussed herein.
Numerous studies have reported the interconnection between apoptosis and ferroptosis. Apoptosis, typically through p53, induces cell cycle arrest, thereby preventing tumorigenesis; likewise, TP53 is known to sensitize cells to ferroptosis, leading to a reduced tumor burden[150]. The widely known ferroptosis inducer erastin has the potential to induce the unfolded protein response and promote p53 expression through apoptotic markers PUMA, CHOP, and TRAIL, and TRAIL-dependent apoptosis implies an augmented link between apoptosis and ferroptosis[151]. A similar study on a metal-encapsulated p53 plasmid construct by Zheng et al[152] was found to release ions of iron, instigating the Fenton reaction and leading to ROS oxyradical overload, thereby leading to ferroptosis-dependent apoptosis in the liver. This consequently reduced the tumor burden and prevented metastasis in mice. An imbalance in the ferroptotic process is implicated in severely hindering apoptosis induction; for example, cancer cells subjected to ferroptosis by cysteine starvation were found to have reduced GSH levels but failed to induce caspase activation, which is seminal in apoptosis[153].
Autophagy-dependent ferroptosis is putatively ferritinophagy; under an excessive Fe2+ milieu, ferritin degradation is mediated by Atg5, an autophagy regulator protein. Ferritin is a seminal protein complex with light and heavy chain polypeptides (ferritin light chain 1 and ferritin heavy chain 2) predominantly controlling iron metabolism. Atg5 and Atg7 knockdown is implicated in preventing erastin-induced ferroptosis, facilitating tumorigenesis[154]. Similarly, BECN1 or Atg6 is known to induce ferroptosis by regulating glutamine and cysteine and inhibiting system Xcˉ through the BECN1-SLC7A11 complex in cancer cells. Additionally, studies have reported BECN1 facilitates lipid peroxidation through malondialdehyde (MDA) stress modulation[155]. Observations from our laboratory demonstrated that Eupatilin exhibits anticancer effects in part through regulation of autophagy-mediated ferroptosis (data not shown).
In summary, while apoptosis, ferroptosis, and autophagy are all different cellular pathways, they can be linked in the sense that autophagy can play dual roles by promoting apoptosis and protecting against ferroptosis. In contrast, both apoptosis and ferroptosis are forms of RCD mediated by different enzymes and signals that manifest different morphological outcomes.
The listed drugs have potential therapeutic applications and have been reported to regulate ferroptosis (Table 2)[156-164].
| GI cancer | Drugs | Pathway involved | Mode of action | Ref. |
| Colorectal cancer | Paclitaxel | p53, SLC7A11 | Lipid peroxidation by suppressing GPX4 expression | [156] |
| 5-fluorouracil | Lipocalin2 (LCN2), xCT | Stimulates GPX4 expression by diminishing circulating iron levels | [157] | |
| Cisplatin | Ferrostatin-1 | Conjugates with GPX and GSH modulation | [158] | |
| Cetuximab | GSH, GPX4, HO-1, SLC7A11, KRAS, RSL3 | Through β-elemene synergism by inducing iron dependent ROS, GSH accumulation, and EMT regulation | [159] | |
| Dihydro-artemisinin (DAT) | Iron metabolism, GPX4 | Sensitizes cells to ferroptosis by iron overload and lysosomal degradation | [160] | |
| Hepatocellular carcinoma | Artesunate | Ferritin | Elevates lysosomal degradation partially through autophagy along with sorafenib; it induces cathepsin activation | [161] |
| Sorafenib | Nrf2 | Through metallothionein-1 activation (MT-1G–Nrf2) axis | [162,125] | |
| Deferoxamine | Iron metabolism | Through peroxidation and iron storage depletion | [163] | |
| Gastric cancer | Cisplatin | Nrf2/Keap1-xCT | Through transcription factor ATF3 overexpression | [164] |
Although conventional drugs are implicated in inducing or modulating ferroptosis, resistance to these drugs supersedes the benefits. Therefore, the identification of compounds with neutral toxicity profiles and natural origins has garnered tremendous attention in ferroptosis and iron metabolism. The section below briefly discussed the role of natural compounds in ferroptosis.
Formasanin C (FC) is known to induce ferroptosis in p53-null and p53 wildtype cellular phenotypes of HCC, and FC treatment increases the mitochondrial morphology and membrane potential in HepG2 cells, as a hallmark of cells undergoing ferroptosis[165]. FC and cisplatin synergistic treatment is known to induce ferritinophagy and enhance therapeutic potential of cisplatin. Similarly, gallic acid (GA) is known to prompt lipid peroxidation and ferroptosis. Upon exposure to an Fe2+ chelator, GA activity is suppressed, which in part signifies its role in ferroptosis. GA exhibited anti-tumor effects in colorectal cancer by deterring GPX4 and elevating MDA expression[166]. Celastrol, a tri-terpenoid is reported to induce ROS production, thereby promoting ferroptosis in liver cancer cells. Structural protein activity revealed that celastrol directly binds to multiple orthologues of PDXs. PDX knockdown in turn elevates ROS production, ensuing ferroptosis[167]. Chen et al[168] reported that curcumin could induce ferroptosis in colorectal cancer by modulating expression of key ferroptotic markers Fer1, SLC7A11, GSH, MDA, and ROS through PI3K/mTOR pathway.
The mechanisms orchestrating ferroptosis has been the subject of interest in several cancers. Many investigators have contributed to identifying key molecules that regulate ferroptosis in GI tumors[169]. The above findings are helpful in understanding the mechanism of many synthetic and natural compounds in inducing iron-dependent cell death in GI cancers. There are ample avenues to further elucidate the mode of action and mechanistic aspects of how natural compounds could be synergistically used to induce ferroptosis.
The increasing incidence and mortality imposed by GI cancers, a dangerous malignancy, warrants novel therapeutic strategies. Ferroptosis, a form of non-apoptotic RCD, has been found to play a significant role in regulating the progression of GI cancers. This review delineated the major regulatory mechanisms involved in ferroptosis for better understanding to create a new opportunity for diagnosis and therapeutic intervention. The involvement of ferroptosis-associated factors and the effect of several drugs, including the first discovered ferroptotic inducers erastin, sorafenib, cisplatin, artesunate, piperlongumine, haloperidol, baicalein, bromelain, and saponins have been found to induce ferroptotic cancer cell death in GI cancers. Ferroptotic inducers synergized with various anticancer drugs in clinical trials have demonstrated effective therapeutic results in GI cancers. Thus, inducing ferroptosis may have significant potential for treating GI cancers and related malignancies. Overall, this review provides insights into the regulatory mechanisms involved in ferroptotic cell death for development of novel therapeutic strategies. The mechanism of ferroptosis with other RCD, such as autophagy and apoptosis, to induce cancer cell death may also provide new development in the therapeutic aspects of treating GI tumors. Therefore, further investigation of ferroptosis in GI cancers will improve the prognosis and therapeutic aspects of GI cancers.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feng S, China; Peng J, China; Zhang X, China S-Editor: Li L L-Editor: Filipodia P-Editor: Zhao S
| 1. | Sharma KL, Bhatia V, Agarwal P, Kumar A. Gastrointestinal Cancers: Molecular Genetics and Biomarkers. Can J Gastroenterol Hepatol. 2018;2018:4513860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Griffin-Sobel JP. Gastrointestinal Cancers: Screening and Early Detection. Semin Oncol Nurs. 2017;33:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Procaccio L, Schirripa M, Fassan M, Vecchione L, Bergamo F, Prete AA, Intini R, Manai C, Dadduzio V, Boscolo A, Zagonel V, Lonardi S. Immunotherapy in Gastrointestinal Cancers. Biomed Res Int. 2017;2017:4346576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Keighley MR. Gastrointestinal cancers in Europe. Aliment Pharmacol Ther. 2003;18 Suppl 3:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | S ST, Krishnan SK, Das P, Sudarshan KL, Kotian CM, Santhappan S, Vishwakarma MB, N S, Mathur P. Descriptive Epidemiology of Gastrointestinal Cancers: Results from National Cancer Registry Programme, India. Asian Pac J Cancer Prev. 2022;23:409-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Vedeld HM, Goel A, Lind GE. Epigenetic biomarkers in gastrointestinal cancers: The current state and clinical perspectives. Semin Cancer Biol. 2018;51:36-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Sonnenberg WR. Gastrointestinal Malignancies. Prim Care. 2017;44:721-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for preventing gastrointestinal cancers. Cochrane Database Syst Rev. 2008;CD004183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Murphy N, Jenab M, Gunter MJ. Adiposity and gastrointestinal cancers: epidemiology, mechanisms and future directions. Nat Rev Gastroenterol Hepatol. 2018;15:659-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 10. | Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, Alnemri ES, Altucci L, Andrews D, Annicchiarico-Petruzzelli M, Baehrecke EH, Bazan NG, Bertrand MJ, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Bredesen DE, Brenner C, Campanella M, Candi E, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, Di Daniele N, Dixit VM, Dynlacht BD, El-Deiry WS, Fimia GM, Flavell RA, Fulda S, Garrido C, Gougeon ML, Green DR, Gronemeyer H, Hajnoczky G, Hardwick JM, Hengartner MO, Ichijo H, Joseph B, Jost PJ, Kaufmann T, Kepp O, Klionsky DJ, Knight RA, Kumar S, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, López-Otín C, Lugli E, Madeo F, Malorni W, Marine JC, Martin SJ, Martinou JC, Medema JP, Meier P, Melino S, Mizushima N, Moll U, Muñoz-Pinedo C, Nuñez G, Oberst A, Panaretakis T, Penninger JM, Peter ME, Piacentini M, Pinton P, Prehn JH, Puthalakath H, Rabinovich GA, Ravichandran KS, Rizzuto R, Rodrigues CM, Rubinsztein DC, Rudel T, Shi Y, Simon HU, Stockwell BR, Szabadkai G, Tait SW, Tang HL, Tavernarakis N, Tsujimoto Y, Vanden Berghe T, Vandenabeele P, Villunger A, Wagner EF, Walczak H, White E, Wood WG, Yuan J, Zakeri Z, Zhivotovsky B, Melino G, Kroemer G. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22:58-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 618] [Cited by in RCA: 732] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 11. | Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1493] [Cited by in RCA: 1734] [Article Influence: 289.0] [Reference Citation Analysis (0)] |
| 12. | Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D'Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, García-Sáez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jäättelä M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, López-Otín C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Muñoz-Pinedo C, Nagata S, Nuñez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3672] [Cited by in RCA: 4480] [Article Influence: 640.0] [Reference Citation Analysis (0)] |
| 13. | Xu T, Ding W, Ji X, Ao X, Liu Y, Yu W, Wang J. Molecular mechanisms of ferroptosis and its role in cancer therapy. J Cell Mol Med. 2019;23:4900-4912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 455] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 14. | Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4711] [Cited by in RCA: 11571] [Article Influence: 890.1] [Reference Citation Analysis (1)] |
| 15. | Jiang M, Hu R, Yu R, Tang Y, Li J. A narrative review of mechanisms of ferroptosis in cancer: new challenges and opportunities. Ann Transl Med. 2021;9:1599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55721] [Article Influence: 7960.1] [Reference Citation Analysis (132)] |
| 17. | Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology. 2020;159:335-349.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1219] [Article Influence: 243.8] [Reference Citation Analysis (0)] |
| 18. | Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, Laversanne M, Ferlay J, Arnold M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates From GLOBOCAN 2020. Gastroenterology. 2022;163:649-658.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 538] [Article Influence: 179.3] [Reference Citation Analysis (0)] |
| 19. | Morgan E, Arnold M, Camargo MC, Gini A, Kunzmann AT, Matsuda T, Meheus F, Verhoeven RHA, Vignat J, Laversanne M, Ferlay J, Soerjomataram I. The current and future incidence and mortality of gastric cancer in 185 countries, 2020-40: A population-based modelling study. EClinicalMedicine. 2022;47:101404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 401] [Article Influence: 133.7] [Reference Citation Analysis (0)] |
| 20. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 1035] [Article Influence: 345.0] [Reference Citation Analysis (0)] |
| 21. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64220] [Article Influence: 16055.0] [Reference Citation Analysis (174)] |
| 22. | Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14:101174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 687] [Cited by in RCA: 1329] [Article Influence: 332.3] [Reference Citation Analysis (5)] |
| 23. | Chen X, Comish PB, Tang D, Kang R. Characteristics and Biomarkers of Ferroptosis. Front Cell Dev Biol. 2021;9:637162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 260] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 24. | Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 639] [Cited by in RCA: 2492] [Article Influence: 498.4] [Reference Citation Analysis (0)] |
| 25. | Sousa L, Oliveira MM, Pessôa MTC, Barbosa LA. Iron overload: Effects on cellular biochemistry. Clin Chim Acta. 2020;504:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Yu Y, Jiang L, Wang H, Shen Z, Cheng Q, Zhang P, Wang J, Wu Q, Fang X, Duan L, Wang S, Wang K, An P, Shao T, Chung RT, Zheng S, Min J, Wang F. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood. 2020;136:726-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 404] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 27. | Sharma A, Flora SJS. Positive and Negative Regulation of Ferroptosis and Its Role in Maintaining Metabolic and Redox Homeostasis. Oxid Med Cell Longev. 2021;2021:9074206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 28. | Park E, Chung SW. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10:822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 612] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 29. | Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2367] [Cited by in RCA: 3531] [Article Influence: 321.0] [Reference Citation Analysis (0)] |
| 30. | Yang F, Sun SY, Wang S, Guo JT, Liu X, Ge N, Wang GX. Molecular regulatory mechanism of ferroptosis and its role in gastrointestinal oncology: Progress and updates. World J Gastrointest Oncol. 2022;14:1-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (4)] |
| 31. | Cheng Y, He C, Wang M, Ma X, Mo F, Yang S, Han J, Wei X. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduct Target Ther. 2019;4:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 478] [Cited by in RCA: 689] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 32. | Logie E, Van Puyvelde B, Cuypers B, Schepers A, Berghmans H, Verdonck J, Laukens K, Godderis L, Dhaenens M, Deforce D, Vanden Berghe W. Ferroptosis Induction in Multiple Myeloma Cells Triggers DNA Methylation and Histone Modification Changes Associated with Cellular Senescence. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Jin B, Li Y, Robertson KD. DNA methylation: superior or subordinate in the epigenetic hierarchy? Genes Cancer. 2011;2:607-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 511] [Article Influence: 36.5] [Reference Citation Analysis (1)] |
| 34. | Pei Y, Qian Y, Wang H, Tan L. Epigenetic Regulation of Ferroptosis-Associated Genes and Its Implication in Cancer Therapy. Front Oncol. 2022;12:771870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Wang Y, Yang L, Zhang X, Cui W, Liu Y, Sun QR, He Q, Zhao S, Zhang GA, Wang Y, Chen S. Epigenetic regulation of ferroptosis by H2B monoubiquitination and p53. EMBO Rep. 2019;20:e47563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 36. | Marmorstein R, Zhou MM. Writers and readers of histone acetylation: structure, mechanism, and inhibition. Cold Spring Harb Perspect Biol. 2014;6:a018762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 435] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 37. | Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3359] [Cited by in RCA: 4003] [Article Influence: 285.9] [Reference Citation Analysis (0)] |
| 38. | Wei X, Yi X, Zhu XH, Jiang DS. Posttranslational Modifications in Ferroptosis. Oxid Med Cell Longev. 2020;2020:8832043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 39. | Wu J, Zhu S, Wang P, Wang J, Huang J, Wang T, Guo L, Liang D, Meng Q, Pan H. Regulators of epigenetic change in ferroptosisassociated cancer (Review). Oncol Rep. 2022;48. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 40. | Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17:2054-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 1245] [Article Influence: 249.0] [Reference Citation Analysis (0)] |
| 41. | Li G, Tian Y, Zhu WG. The Roles of Histone Deacetylases and Their Inhibitors in Cancer Therapy. Front Cell Dev Biol. 2020;8:576946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 190] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 42. | Zhang Z, Guo M, Li Y, Shen M, Kong D, Shao J, Ding H, Tan S, Chen A, Zhang F, Zheng S. RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy. 2020;16:1482-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 331] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 43. | Qi R, Bai Y, Wei Y, Liu N, Shi B. The role of non-coding RNAs in ferroptosis regulation. J Trace Elem Med Biol. 2022;70:126911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | Balihodzic A, Prinz F, Dengler MA, Calin GA, Jost PJ, Pichler M. Non-coding RNAs and ferroptosis: potential implications for cancer therapy. Cell Death Differ. 2022;29:1094-1106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 45. | Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2388] [Cited by in RCA: 5137] [Article Influence: 467.0] [Reference Citation Analysis (0)] |
| 46. | Eaton JK, Furst L, Ruberto RA, Moosmayer D, Hilpmann A, Ryan MJ, Zimmermann K, Cai LL, Niehues M, Badock V, Kramm A, Chen S, Hillig RC, Clemons PA, Gradl S, Montagnon C, Lazarski KE, Christian S, Bajrami B, Neuhaus R, Eheim AL, Viswanathan VS, Schreiber SL. Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat Chem Biol. 2020;16:497-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 295] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 47. | Eaton JK, Ruberto RA, Kramm A, Viswanathan VS, Schreiber SL. Diacylfuroxans Are Masked Nitrile Oxides That Inhibit GPX4 Covalently. J Am Chem Soc. 2019;141:20407-20415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 48. | Gaschler MM, Andia AA, Liu H, Csuka JM, Hurlocker B, Vaiana CA, Heindel DW, Zuckerman DS, Bos PH, Reznik E, Ye LF, Tyurina YY, Lin AJ, Shchepinov MS, Chan AY, Peguero-Pereira E, Fomich MA, Daniels JD, Bekish AV, Shmanai VV, Kagan VE, Mahal LK, Woerpel KA, Stockwell BR. FINO(2) initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol. 2018;14:507-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 548] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 49. | Wenz C, Faust D, Linz B, Turmann C, Nikolova T, Bertin J, Gough P, Wipf P, Schröder AS, Krautwald S, Dietrich C. t-BuOOH induces ferroptosis in human and murine cell lines. Arch Toxicol. 2018;92:759-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 50. | Hassannia B, Wiernicki B, Ingold I, Qu F, Van Herck S, Tyurina YY, Bayır H, Abhari BA, Angeli JPF, Choi SM, Meul E, Heyninck K, Declerck K, Chirumamilla CS, Lahtela-Kakkonen M, Van Camp G, Krysko DV, Ekert PG, Fulda S, De Geest BG, Conrad M, Kagan VE, Vanden Berghe W, Vandenabeele P, Vanden Berghe T. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J Clin Invest. 2018;128:3341-3355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 460] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 51. | Li Q, Han X, Lan X, Gao Y, Wan J, Durham F, Cheng T, Yang J, Wang Z, Jiang C, Ying M, Koehler RC, Stockwell BR, Wang J. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight. 2017;2:e90777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 532] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 52. | Baba Y, Higa JK, Shimada BK, Horiuchi KM, Suhara T, Kobayashi M, Woo JD, Aoyagi H, Marh KS, Kitaoka H, Matsui T. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2018;314:H659-H668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 272] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 53. | Mai TT, Hamaï A, Hienzsch A, Cañeque T, Müller S, Wicinski J, Cabaud O, Leroy C, David A, Acevedo V, Ryo A, Ginestier C, Birnbaum D, Charafe-Jauffret E, Codogno P, Mehrpour M, Rodriguez R. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat Chem. 2017;9:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 455] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 54. | Sui S, Zhang J, Xu S, Wang Q, Wang P, Pang D. Ferritinophagy is required for the induction of ferroptosis by the bromodomain protein BRD4 inhibitor (+)-JQ1 in cancer cells. Cell Death Dis. 2019;10:331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 168] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 55. | Li C, Zhang Y, Liu J, Kang R, Klionsky DJ, Tang D. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy. 2021;17:948-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 303] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 56. | Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayır H. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1637] [Cited by in RCA: 1974] [Article Influence: 246.8] [Reference Citation Analysis (0)] |
| 57. | Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113:E4966-E4975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 1603] [Article Influence: 178.1] [Reference Citation Analysis (0)] |
| 58. | Shah R, Shchepinov MS, Pratt DA. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent Sci. 2018;4:387-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 537] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 59. | Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Rådmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Förster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O'Donnell VB, Kagan VE, Schick JA, Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 2619] [Article Influence: 238.1] [Reference Citation Analysis (0)] |
| 60. | Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A, Stockwell BR. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136:4551-4556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 502] [Cited by in RCA: 823] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 61. | Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trümbach D, Mao G, Qu F, Bayir H, Füllekrug J, Scheel CH, Wurst W, Schick JA, Kagan VE, Angeli JP, Conrad M. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 2678] [Article Influence: 297.6] [Reference Citation Analysis (0)] |
| 62. | Liu J, Kang R, Tang D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022;289:7038-7050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 368] [Article Influence: 122.7] [Reference Citation Analysis (0)] |
| 63. | Wu Z, Geng Y, Lu X, Shi Y, Wu G, Zhang M, Shan B, Pan H, Yuan J. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc Natl Acad Sci U S A. 2019;116:2996-3005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 430] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 64. | Zhu S, Zhang Q, Sun X, Zeh HJ 3rd, Lotze MT, Kang R, Tang D. HSPA5 Regulates Ferroptotic Cell Death in Cancer Cells. Cancer Res. 2017;77:2064-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 420] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 65. | Wang D, Peng Y, Xie Y, Zhou B, Sun X, Kang R, Tang D. Antiferroptotic activity of non-oxidative dopamine. Biochem Biophys Res Commun. 2016;480:602-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 66. | Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol Cell. 2015;59:298-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 782] [Cited by in RCA: 1486] [Article Influence: 148.6] [Reference Citation Analysis (0)] |
| 67. | Magtanong L, Ko PJ, To M, Cao JY, Forcina GC, Tarangelo A, Ward CC, Cho K, Patti GJ, Nomura DK, Olzmann JA, Dixon SJ. Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem Biol. 2019;26:420-432.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 720] [Article Influence: 120.0] [Reference Citation Analysis (0)] |
| 68. | Forcina GC, Dixon SJ. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics. 2019;19:e1800311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 647] [Article Influence: 107.8] [Reference Citation Analysis (0)] |
| 69. | Liu MR, Zhu WT, Pei DS. System Xc(-): a key regulatory target of ferroptosis in cancer. Invest New Drugs. 2021;39:1123-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 70. | Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med. 2019;133:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 993] [Article Influence: 165.5] [Reference Citation Analysis (0)] |
| 71. | Wang L, Liu Y, Du T, Yang H, Lei L, Guo M, Ding HF, Zhang J, Wang H, Chen X, Yan C. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc(). Cell Death Differ. 2020;27:662-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 536] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 72. | Lee JY, Nam M, Son HY, Hyun K, Jang SY, Kim JW, Kim MW, Jung Y, Jang E, Yoon SJ, Kim J, Seo J, Min JK, Oh KJ, Han BS, Kim WK, Bae KH, Song J, Huh YM, Hwang GS, Lee EW, Lee SC. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc Natl Acad Sci U S A. 2020;117:32433-32442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 322] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 73. | Yang L, Chen X, Yang Q, Chen J, Huang Q, Yao L, Yan D, Wu J, Zhang P, Tang D, Zhong N, Liu J. Broad Spectrum Deubiquitinase Inhibition Induces Both Apoptosis and Ferroptosis in Cancer Cells. Front Oncol. 2020;10:949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 74. | Arias E, Koga H, Diaz A, Mocholi E, Patel B, Cuervo AM. Lysosomal mTORC2/PHLPP1/Akt Regulate Chaperone-Mediated Autophagy. Mol Cell. 2015;59:270-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 224] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 75. | Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 761] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 76. | Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci U S A. 2020;117:31189-31197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 636] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 77. | Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z, Cai K, Zhao Y, Luo Z. HCAR1/MCT1 Regulates Tumor Ferroptosis through the Lactate-Mediated AMPK-SCD1 Activity and Its Therapeutic Implications. Cell Rep. 2020;33:108487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 251] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 78. | Zou Y, Henry WS, Ricq EL, Graham ET, Phadnis VV, Maretich P, Paradkar S, Boehnke N, Deik AA, Reinhardt F, Eaton JK, Ferguson B, Wang W, Fairman J, Keys HR, Dančík V, Clish CB, Clemons PA, Hammond PT, Boyer LA, Weinberg RA, Schreiber SL. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature. 2020;585:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 533] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 79. | Hohenberger J, Ray K, Meyer K. The biology and chemistry of high-valent iron-oxo and iron-nitrido complexes. Nat Commun. 2012;3:720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 426] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 80. | Abe C, Miyazawa T. Current Use of Fenton Reaction in Drugs and Food. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 81. | Koppenol WH. The Haber-Weiss cycle--70 years later. Redox Rep. 2001;6:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 312] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 82. | Shi Z, Zhang L, Zheng J, Sun H, Shao C. Ferroptosis: Biochemistry and Biology in Cancers. Front Oncol. 2021;11:579286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 83. | Crielaard BJ, Lammers T, Rivella S. Targeting iron metabolism in drug discovery and delivery. Nat Rev Drug Discov. 2017;16:400-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 263] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 84. | Zhou L, Zhao B, Zhang L, Wang S, Dong D, Lv H, Shang P. Alterations in Cellular Iron Metabolism Provide More Therapeutic Opportunities for Cancer. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 85. | Whiteland H, Spencer-Harty S, Morgan C, Kynaston H, Thomas DH, Bose P, Fenn N, Lewis P, Jenkins S, Doak SH. A role for STEAP2 in prostate cancer progression. Clin Exp Metastasis. 2014;31:909-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 86. | Burnell SEA, Spencer-Harty S, Howarth S, Bodger O, Kynaston H, Morgan C, Doak SH. STEAP2 Knockdown Reduces the Invasive Potential of Prostate Cancer Cells. Sci Rep. 2018;8:6252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 87. | Wu YY, Jiang JN, Fang XD, Ji FJ. STEAP1 Regulates Tumorigenesis and Chemoresistance During Peritoneal Metastasis of Gastric Cancer. Front Physiol. 2018;9:1132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 88. | Han M, Xu R, Wang S, Yang N, Ni S, Zhang Q, Xu Y, Zhang X, Zhang C, Wei Y, Ji J, Huang B, Zhang D, Chen A, Li W, Bjerkvig R, Li X, Wang J. Six-Transmembrane Epithelial Antigen of Prostate 3 Predicts Poor Prognosis and Promotes Glioblastoma Growth and Invasion. Neoplasia. 2018;20:543-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 89. | Xue X, Bredell BX, Anderson ER, Martin A, Mays C, Nagao-Kitamoto H, Huang S, Győrffy B, Greenson JK, Hardiman K, Spence JR, Kamada N, Shah YM. Quantitative proteomics identifies STEAP4 as a critical regulator of mitochondrial dysfunction linking inflammation and colon cancer. Proc Natl Acad Sci U S A. 2017;114:E9608-E9617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 90. | Hubert N, Hentze MW. Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: implications for regulation and cellular function. Proc Natl Acad Sci U S A. 2002;99:12345-12350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 321] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 91. | Dautry-Varsat A, Ciechanover A, Lodish HF. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983;80:2258-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 863] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 92. | Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1209] [Cited by in RCA: 1321] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 93. | Li JY, Paragas N, Ned RM, Qiu A, Viltard M, Leete T, Drexler IR, Chen X, Sanna-Cherchi S, Mohammed F, Williams D, Lin CS, Schmidt-Ott KM, Andrews NC, Barasch J. Scara5 is a ferritin receptor mediating non-transferrin iron delivery. Dev Cell. 2009;16:35-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 268] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 94. | Bou-Abdallah F. The iron redox and hydrolysis chemistry of the ferritins. Biochim Biophys Acta. 2010;1800:719-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 95. | Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 728] [Cited by in RCA: 1428] [Article Influence: 129.8] [Reference Citation Analysis (0)] |
| 96. | Lei P, Bai T, Sun Y. Mechanisms of Ferroptosis and Relations With Regulated Cell Death: A Review. Front Physiol. 2019;10:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 386] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 97. | Shang Y, Luo M, Yao F, Wang S, Yuan Z, Yang Y. Ceruloplasmin suppresses ferroptosis by regulating iron homeostasis in hepatocellular carcinoma cells. Cell Signal. 2020;72:109633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 98. | Brown CW, Amante JJ, Chhoy P, Elaimy AL, Liu H, Zhu LJ, Baer CE, Dixon SJ, Mercurio AM. Prominin2 Drives Ferroptosis Resistance by Stimulating Iron Export. Dev Cell. 2019;51:575-586.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 428] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 99. | Guo W, Zhang S, Chen Y, Zhang D, Yuan L, Cong H, Liu S. An important role of the hepcidin-ferroportin signaling in affecting tumor growth and metastasis. Acta Biochim Biophys Sin (Shanghai). 2015;47:703-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 100. | Shan Z, Wei Z, Shaikh ZA. Suppression of ferroportin expression by cadmium stimulates proliferation, EMT, and migration in triple-negative breast cancer cells. Toxicol Appl Pharmacol. 2018;356:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 101. | Wang Y, Yu L, Ding J, Chen Y. Iron Metabolism in Cancer. Int J Mol Sci. 2018;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 194] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 102. | Almanza A, Carlesso A, Chintha C, Creedican S, Doultsinos D, Leuzzi B, Luís A, McCarthy N, Montibeller L, More S, Papaioannou A, Püschel F, Sassano ML, Skoko J, Agostinis P, de Belleroche J, Eriksson LA, Fulda S, Gorman AM, Healy S, Kozlov A, Muñoz-Pinedo C, Rehm M, Chevet E, Samali A. Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications. FEBS J. 2019;286:241-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 606] [Cited by in RCA: 665] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 103. | Hackl C, Lang SA, Moser C, Mori A, Fichtner-Feigl S, Hellerbrand C, Dietmeier W, Schlitt HJ, Geissler EK, Stoeltzing O. Activating transcription factor-3 (ATF3) functions as a tumor suppressor in colon cancer and is up-regulated upon heat-shock protein 90 (Hsp90) inhibition. BMC Cancer. 2010;10:668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 104. | Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, Stroedicke M, Zenkner M, Schoenherr A, Koeppen S, Timm J, Mintzlaff S, Abraham C, Bock N, Kietzmann S, Goedde A, Toksöz E, Droege A, Krobitsch S, Korn B, Birchmeier W, Lehrach H, Wanker EE. A human protein-protein interaction network: a resource for annotating the proteome. Cell. 2005;122:957-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1772] [Cited by in RCA: 1706] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 105. | Kim KH, Jeong JY, Surh YJ, Kim KW. Expression of stress-response ATF3 is mediated by Nrf2 in astrocytes. Nucleic Acids Res. 2010;38:48-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 106. | Wang N, Zeng GZ, Yin JL, Bian ZX. Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects ferroptosis in Burkitt's Lymphoma. Biochem Biophys Res Commun. 2019;519:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 107. | Chen D, Fan Z, Rauh M, Buchfelder M, Eyupoglu IY, Savaskan N. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene. 2017;36:5593-5608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 319] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 108. | Ye P, Mimura J, Okada T, Sato H, Liu T, Maruyama A, Ohyama C, Itoh K. Nrf2- and ATF4-dependent upregulation of xCT modulates the sensitivity of T24 bladder carcinoma cells to proteasome inhibition. Mol Cell Biol. 2014;34:3421-3434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 109. | Koppula P, Zhang Y, Shi J, Li W, Gan B. The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. J Biol Chem. 2017;292:14240-14249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 214] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 110. | Luo M, Wu L, Zhang K, Wang H, Zhang T, Gutierrez L, O'Connell D, Zhang P, Li Y, Gao T, Ren W, Yang Y. miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ. 2018;25:1457-1472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 347] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 111. | Chen MS, Wang SF, Hsu CY, Yin PH, Yeh TS, Lee HC, Tseng LM. CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2α-ATF4 pathway. Oncotarget. 2017;8:114588-114602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 220] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 112. | Lim JKM, Delaidelli A, Minaker SW, Zhang HF, Colovic M, Yang H, Negri GL, von Karstedt S, Lockwood WW, Schaffer P, Leprivier G, Sorensen PH. Cystine/glutamate antiporter xCT (SLC7A11) facilitates oncogenic RAS transformation by preserving intracellular redox balance. Proc Natl Acad Sci U S A. 2019;116:9433-9442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 242] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 113. | Divya T, Dineshbabu V, Soumyakrishnan S, Sureshkumar A, Sudhandiran G. Celastrol enhances Nrf2 mediated antioxidant enzymes and exhibits anti-fibrotic effect through regulation of collagen production against bleomycin-induced pulmonary fibrosis. Chem Biol Interact. 2016;246:52-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 114. | Rojo de la Vega M, Chapman E, Zhang DD. NRF2 and the Hallmarks of Cancer. Cancer Cell. 2018;34:21-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 986] [Cited by in RCA: 1178] [Article Influence: 168.3] [Reference Citation Analysis (0)] |
| 115. | Dodson M, de la Vega MR, Cholanians AB, Schmidlin CJ, Chapman E, Zhang DD. Modulating NRF2 in Disease: Timing Is Everything. Annu Rev Pharmacol Toxicol. 2019;59:555-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 366] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 116. | Wu KC, Cui JY, Klaassen CD. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol Sci. 2011;123:590-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 299] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 117. | Shimada K, Hayano M, Pagano NC, Stockwell BR. Cell-Line Selectivity Improves the Predictive Power of Pharmacogenomic Analyses and Helps Identify NADPH as Biomarker for Ferroptosis Sensitivity. Cell Chem Biol. 2016;23:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 241] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 118. | Ou Y, Wang SJ, Li D, Chu B, Gu W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci U S A. 2016;113:E6806-E6812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 586] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 119. | Wang M, Mao C, Ouyang L, Liu Y, Lai W, Liu N, Shi Y, Chen L, Xiao D, Yu F, Wang X, Zhou H, Cao Y, Liu S, Yan Q, Tao Y, Zhang B. Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ. 2019;26:2329-2343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 395] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 120. | Xu G, Wang H, Li X, Huang R, Luo L. Recent progress on targeting ferroptosis for cancer therapy. Biochem Pharmacol. 2021;190:114584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 121. | Chiang SK, Chen SE, Chang LC. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int J Mol Sci. 2018;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 332] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 122. | Singh CK, Chhabra G, Ndiaye MA, Garcia-Peterson LM, Mack NJ, Ahmad N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid Redox Signal. 2018;28:643-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 574] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 123. | Jun L, Chen W, Han L, Yanmin L, Qinglei Z, Pengfei Z. Protocadherin 20 promotes ferroptosis by suppressing the expression of Sirtuin 1 and promoting the acetylation of nuclear factor erythroid 2-related factor 2 in hepatocellular carcinoma. Int J Biochem Cell Biol. 2023;156:106363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 124. | Gong S, Xiong L, Luo Z, Yin Q, Huang M, Zhou Y, Li J. SIRT6 promotes ferroptosis and attenuates glycolysis in pancreatic cancer through regulation of the NF-κB pathway. Exp Ther Med. 2022;24:502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 125. | Cai S, Fu S, Zhang W, Yuan X, Cheng Y, Fang J. SIRT6 silencing overcomes resistance to sorafenib by promoting ferroptosis in gastric cancer. Biochem Biophys Res Commun. 2021;577:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 126. | Wang H, Cheng Y, Mao C, Liu S, Xiao D, Huang J, Tao Y. Emerging mechanisms and targeted therapy of ferroptosis in cancer. Mol Ther. 2021;29:2185-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 197] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 127. | Paneerselvam C, Ganapasam S. β-Escin alleviates cobalt chloride-induced hypoxia-mediated apoptotic resistance and invasion via ROS-dependent HIF-1α/TGF-β/MMPs in A549 cells. Toxicol Res (Camb). 2020;9:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 128. | Singhal R, Mitta SR, Das NK, Kerk SA, Sajjakulnukit P, Solanki S, Andren A, Kumar R, Olive KP, Banerjee R, Lyssiotis CA, Shah YM. HIF-2α activation potentiates oxidative cell death in colorectal cancers by increasing cellular iron. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 129. | Wang Y, Zhang Z, Sun W, Zhang J, Xu Q, Zhou X, Mao L. Ferroptosis in colorectal cancer: Potential mechanisms and effective therapeutic targets. Biomed Pharmacother. 2022;153:113524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 68] [Reference Citation Analysis (0)] |
| 130. | Karmi O, Sohn YS, Zandalinas SI, Rowland L, King SD, Nechushtai R, Mittler R. Disrupting CISD2 function in cancer cells primarily impacts mitochondrial labile iron levels and triggers TXNIP expression. Free Radic Biol Med. 2021;176:92-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 131. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3832] [Article Influence: 958.0] [Reference Citation Analysis (3)] |
| 132. | Song Y, Yang H, Lin R, Jiang K, Wang BM. The role of ferroptosis in digestive system cancer. Oncol Lett. 2019;18:2159-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 133. | Wang L, Cai H, Hu Y, Liu F, Huang S, Zhou Y, Yu J, Xu J, Wu F. A pharmacological probe identifies cystathionine β-synthase as a new negative regulator for ferroptosis. Cell Death Dis. 2018;9:1005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 134. | Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 1484] [Article Influence: 164.9] [Reference Citation Analysis (0)] |
| 135. | Yuan H, Li X, Zhang X, Kang R, Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun. 2016;478:838-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 366] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 136. | Lőrincz T, Jemnitz K, Kardon T, Mandl J, Szarka A. Ferroptosis is Involved in Acetaminophen Induced Cell Death. Pathol Oncol Res. 2015;21:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 137. | Louandre C, Marcq I, Bouhlal H, Lachaier E, Godin C, Saidak Z, François C, Chatelain D, Debuysscher V, Barbare JC, Chauffert B, Galmiche A. The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Lett. 2015;356:971-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 297] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 138. | Qi W, Li Z, Xia L, Dai J, Zhang Q, Wu C, Xu S. LncRNA GABPB1-AS1 and GABPB1 regulate oxidative stress during erastin-induced ferroptosis in HepG2 hepatocellular carcinoma cells. Sci Rep. 2019;9:16185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 139. | Chen G, Guo G, Zhou X, Chen H. Potential mechanism of ferroptosis in pancreatic cancer. Oncol Lett. 2020;19:579-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 140. | Kremer DM, Nelson BS, Lin L, Yarosz EL, Halbrook CJ, Kerk SA, Sajjakulnukit P, Myers A, Thurston G, Hou SW, Carpenter ES, Andren AC, Nwosu ZC, Cusmano N, Wisner S, Mbah NE, Shan M, Das NK, Magnuson B, Little AC, Savani MR, Ramos J, Gao T, Sastra SA, Palermo CF, Badgley MA, Zhang L, Asara JM, McBrayer SK, di Magliano MP, Crawford HC, Shah YM, Olive KP, Lyssiotis CA. GOT1 inhibition promotes pancreatic cancer cell death by ferroptosis. Nat Commun. 2021;12:4860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 169] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 141. | Song Z, Xiang X, Li J, Deng J, Fang Z, Zhang L, Xiong J. Ruscogenin induces ferroptosis in pancreatic cancer cells. Oncol Rep. 2020;43:516-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 142. | Kasukabe T, Honma Y, Okabe-Kado J, Higuchi Y, Kato N, Kumakura S. Combined treatment with cotylenin A and phenethyl isothiocyanate induces strong antitumor activity mainly through the induction of ferroptotic cell death in human pancreatic cancer cells. Oncol Rep. 2016;36:968-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 143. | Eling N, Reuter L, Hazin J, Hamacher-Brady A, Brady NR. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience. 2015;2:517-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 407] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 144. | Yamaguchi Y, Kasukabe T, Kumakura S. Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int J Oncol. 2018;52:1011-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 145. | Sexton RE, Al Hallak MN, Diab M, Azmi AS. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020;39:1179-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 451] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 146. | Zhao L, Peng Y, He S, Li R, Wang Z, Huang J, Lei X, Li G, Ma Q. Apatinib induced ferroptosis by lipid peroxidation in gastric cancer. Gastric Cancer. 2021;24:642-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 147. | Zhang C, Liu X, Jin S, Chen Y, Guo R. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer. 2022;21:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 761] [Article Influence: 253.7] [Reference Citation Analysis (0)] |
| 148. | Guan Z, Chen J, Li X, Dong N. Tanshinone IIA induces ferroptosis in gastric cancer cells through p53-mediated SLC7A11 down-regulation. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 162] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 149. | Wang Z, Wu S, Zhu C, Shen J. The role of ferroptosis in esophageal cancer. Cancer Cell Int. 2022;22:266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 150. | Liu J, Zhang C, Wang J, Hu W, Feng Z. The Regulation of Ferroptosis by Tumor Suppressor p53 and its Pathway. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 151. | Lee YS, Lee DH, Choudry HA, Bartlett DL, Lee YJ. Ferroptosis-Induced Endoplasmic Reticulum Stress: Cross-talk between Ferroptosis and Apoptosis. Mol Cancer Res. 2018;16:1073-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 282] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 152. | Zheng DW, Lei Q, Zhu JY, Fan JX, Li CX, Li C, Xu Z, Cheng SX, Zhang XZ. Switching Apoptosis to Ferroptosis: Metal-Organic Network for High-Efficiency Anticancer Therapy. Nano Lett. 2017;17:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 334] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 153. | Homma T, Kobayashi S, Conrad M, Konno H, Yokoyama C, Fujii J. Nitric oxide protects against ferroptosis by aborting the lipid peroxidation chain reaction. Nitric Oxide. 2021;115:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 154. | Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd, Kang R, Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1633] [Article Influence: 181.4] [Reference Citation Analysis (0)] |
| 155. | Kang R, Zhu S, Zeh HJ, Klionsky DJ, Tang D. BECN1 is a new driver of ferroptosis. Autophagy. 2018;14:2173-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 156. | Lv C, Qu H, Zhu W, Xu K, Xu A, Jia B, Qing Y, Li H, Wei HJ, Zhao HY. Low-Dose Paclitaxel Inhibits Tumor Cell Growth by Regulating Glutaminolysis in Colorectal Carcinoma Cells. Front Pharmacol. 2017;8:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 157. | Chaudhary N, Choudhary BS, Shah SG, Khapare N, Dwivedi N, Gaikwad A, Joshi N, Raichanna J, Basu S, Gurjar M, P K S, Saklani A, Gera P, Ramadwar M, Patil P, Thorat R, Gota V, Dhar SK, Gupta S, Das M, Dalal SN. Lipocalin 2 expression promotes tumor progression and therapy resistance by inhibiting ferroptosis in colorectal cancer. Int J Cancer. 2021;149:1495-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 158. | Guo J, Xu B, Han Q, Zhou H, Xia Y, Gong C, Dai X, Li Z, Wu G. Ferroptosis: A Novel Anti-tumor Action for Cisplatin. Cancer Res Treat. 2018;50:445-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 548] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 159. | Chen P, Li X, Zhang R, Liu S, Xiang Y, Zhang M, Chen X, Pan T, Yan L, Feng J, Duan T, Wang D, Chen B, Jin T, Wang W, Chen L, Huang X, Zhang W, Sun Y, Li G, Kong L, Li Y, Yang Z, Zhang Q, Zhuo L, Sui X, Xie T. Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics. 2020;10:5107-5119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 280] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 160. | Chen GQ, Benthani FA, Wu J, Liang D, Bian ZX, Jiang X. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. 2020;27:242-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 337] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 161. | Li ZJ, Dai HQ, Huang XW, Feng J, Deng JH, Wang ZX, Yang XM, Liu YJ, Wu Y, Chen PH, Shi H, Wang JG, Zhou J, Lu GD. Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma. Acta Pharmacol Sin. 2021;42:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 204] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 162. | Louandre C, Ezzoukhry Z, Godin C, Barbare JC, Mazière JC, Chauffert B, Galmiche A. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer. 2013;133:1732-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 441] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 163. | Yamada N, Karasawa T, Kimura H, Watanabe S, Komada T, Kamata R, Sampilvanjil A, Ito J, Nakagawa K, Kuwata H, Hara S, Mizuta K, Sakuma Y, Sata N, Takahashi M. Ferroptosis driven by radical oxidation of n-6 polyunsaturated fatty acids mediates acetaminophen-induced acute liver failure. Cell Death Dis. 2020;11:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 236] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 164. | Fu D, Wang C, Yu L, Yu R. Induction of ferroptosis by ATF3 elevation alleviates cisplatin resistance in gastric cancer by restraining Nrf2/Keap1/xCT signaling. Cell Mol Biol Lett. 2021;26:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 172] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 165. | Lin PL, Tang HH, Wu SY, Shaw NS, Su CL. Saponin Formosanin C-induced Ferritinophagy and Ferroptosis in Human Hepatocellular Carcinoma Cells. Antioxidants (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 166. | Hong Z, Tang P, Liu B, Ran C, Yuan C, Zhang Y, Lu Y, Duan X, Yang Y, Wu H. Ferroptosis-related Genes for Overall Survival Prediction in Patients with Colorectal Cancer can be Inhibited by Gallic acid. Int J Biol Sci. 2021;17:942-956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 167. | Luo P, Liu D, Zhang Q, Yang F, Wong YK, Xia F, Zhang J, Chen J, Tian Y, Yang C, Dai L, Shen HM, Wang J. Celastrol induces ferroptosis in activated HSCs to ameliorate hepatic fibrosis via targeting peroxiredoxins and HO-1. Acta Pharm Sin B. 2022;12:2300-2314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 193] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 168. | Chen M, Tan AH, Li J. Curcumin Represses Colorectal Cancer Cell Proliferation by Triggering Ferroptosis via PI3K/Akt/mTOR Signaling. Nutr Cancer. 2023;75:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 46] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 169. | Capelletti MM, Manceau H, Puy H, Peoc'h K. Ferroptosis in Liver Diseases: An Overview. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |